Abstract
Objective
Data quality (DQ) must be consistently defined in context. The attributes, metadata, and context of longitudinal real-world data (RWD) have not been formalized for quality improvement across the data production and curation life cycle. We sought to complete a literature review on DQ assessment frameworks, indicators and tools for research, public health, service, and quality improvement across the data life cycle.
Materials and Methods
The review followed PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Databases from health, physical and social sciences were used: Cinahl, Embase, Scopus, ProQuest, Emcare, PsycINFO, Compendex, and Inspec. Embase was used instead of PubMed (an interface to search MEDLINE) because it includes all MeSH (Medical Subject Headings) terms used and journals in MEDLINE as well as additional unique journals and conference abstracts. A combined data life cycle and quality framework guided the search of published and gray literature for DQ frameworks, indicators, and tools. At least 2 authors independently identified articles for inclusion and extracted and categorized DQ concepts and constructs. All authors discussed findings iteratively until consensus was reached.
Results
The 120 included articles yielded concepts related to contextual (data source, custodian, and user) and technical (interoperability) factors across the data life cycle. Contextual DQ subcategories included relevance, usability, accessibility, timeliness, and trust. Well-tested computable DQ indicators and assessment tools were also found.
Conclusions
A DQ assessment framework that covers intrinsic, technical, and contextual categories across the data life cycle enables assessment and management of RWD repositories to ensure fitness for purpose. Balancing security, privacy, and FAIR principles requires trust and reciprocity, transparent governance, and organizational cultures that value good documentation.
Keywords: data quality, DQ measures, DQ indicators, DQ assessment tools, data custodianship, data stewardship, literature review
INTRODUCTION
Background
Globally, the increasing use of electronic health records (EHRs), health information systems, and personalized health monitoring devices has led to repositories of large volumes of complex longitudinal real-world data (RWD). Extracting valid inferences from these RWD repositories requires critical thinking and informatics-based analytic tools. Artificial intelligence (AI) and deep machine learning algorithms are increasingly being harnessed to mine these repositories for research, population health, quality improvement, clinical decision support, and personalized medicine.1,2 Guiding principles have evolved for data custodians and data stewards to ethically manage these RWD repositories, including Privacy, Ethics, and Data Access frameworks3 and the FAIR principles of findability, accessibility, interoperability and reusability, for public good and scientific advancement.4 Improved access to interoperable data will accelerate systematic and innovative research using RWD to generate real-world evidence (RWE).
Examples of RWD repositories include PEDsNet in the United States5 and the My Health Record system6 in Australia. These datasets usually contain linked patient summary data uploaded from participating health services from diverse primary and secondary care settings, providing a clinician- and patient-centric view of longitudinal health data, collected at point of care in the real world. Relevant regulations enable the release of these RWD for research, public health, and quality improvement purposes. Similar RWD repositories exist globally, with different models of governance and provenance requirements of data custodians to ensure data quality (DQ) and fitness for purpose.
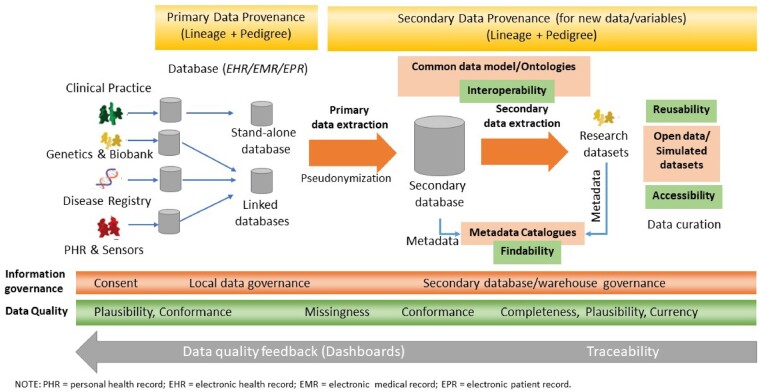
RWD is only as good as its quality. High quality data should be “intrinsically good, contextually appropriate, clearly represented and accessible to data consumers.”7 However, the DQ domain has lacked a commonly agreed terminology and clear conceptualization of the intrinsic and contextual determinants of DQ.8–10 This diversity reflects the semantic and syntactic heterogeneity in the data, metadata, databases, and diverse needs of the creators, custodians, informaticians, researchers, and users of data across the data production and curation life cycle (Figure 1).2,11–13
Figure 1.
The data production and curation life cycle with associated provenance, governance, and data quality assessment. EHR: electronic health record; EMR: electronic medical record; EPR: electronic patient record; PHR: personal health record.
Figure 1 summarizes the data life cycle along with the required primary and secondary data provenance, governance and DQ assessment at various points in the life cycle. DQ assessment must be conducted systematically at all stages and at various points in the life cycle, including data creation and collection to the extract, transform, and load process; data processing and annotation with metadata; and curation, visualization, and sharing.2 Traceability is important to ensure that DQ feedback can be provided to data stewards and custodians at each point in the life cycle to address and improve DQ.14 Some categories of DQ may be more important or easier to assess and manage at different points of the life cycle. The distribution of data and its quality attributes along the life cycle (green bar in the bottom third of Figure 1) illustrates how context and plausibility may be more important to know and easier to manage at the data source.2,15
In 2016, an international DQ research collaboration reviewed the literature and developed and published a harmonized framework and terminology to assess the intrinsic quality of a dataset.15 This framework defined 3 DQ categories—conformance, completeness, and plausibility—and 2 DQ assessment contexts—verification and validation. Conformance and plausibility categories are further divided into subcategories. Data may be verified with organizational data or validated against an accepted gold standard, depending on proposed context and uses. The coverage of this harmonized intrinsic DQ framework (HIDQF) and terminology was validated by successfully aligning to multiple published DQ terminologies.15
The use and evolution of the HIDQF since 2016 has included the development of indicators and tools to support the intrinsic DQ assessment of RWD repositories for research, public health, and quality improvement purposes.11,16–19 These developments have neither explicitly nor systematically addressed the contextual and process categories that are vital to DQ assessment and management in RWD production and curation life cycles. The senior authors (S.-T.L., M.G.K., S.d.L.) therefore guided the integration of the HIDQF and data life cycle framework as the conceptual starting point for this literature review to identify practical and potential gaps in the assessment and management of DQ.
Objective
We sought to conduct a literature review on DQ assessment frameworks, indicators, and tools for research, public health, and quality improvement, incorporating the additional perspective of the full data production and curation life cycle.
Materials and METHODS
Developing the conceptual framework for the review and synthesis
Figure 1 illustrate some of the included intrinsic DQ categories and subcategories from the HIDQF, along with the FAIR guiding principles for the secondary use of data, and their relevance and importance at various points in the data life cycle. The Privacy, Ethics, and FAIR principles are implicit in and central to the governance and provenance concepts in Figure 1. The elements of this conceptual framework guided the search strategy and criteria for inclusion of articles and DQ concepts.
Conducting the literature review
Guided by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, the published literature—including reviews, conference articles and original research articles—from 2009 onward was examined for research on DQ frameworks, indicators ,and tools. While the focus is health, we were also interested in similar work reported in the social and physical sciences. As such, the databases used were Cinahl, Embase, Scopus, ProQuest, Emcare, PsycINFO, Compendex, and Inspec. Embase was used instead of PubMed, which is an interface to search MEDLINE, because it covers all the journals that are in MEDLINE, as well as covers 2900 additional unique journals and conference abstracts that are not in MEDLINE. Embase is indexed with the Emtree terminology, which includes all the MeSH (Medical Subject Headings) terms used in MEDLINE. Gray literature sources included:
Australian Institute of Health and Welfare (https://www.aihw.gov.au/)
Australian Digital Health Agency (https://www.digitalhealth.gov.au/)
Australian Bureau of Statistics (ABS) (https://www.aihw.gov.au/)
Australian Department of Health (https://www.health.gov.au/)
Irish Health Information Quality Authority (https://www.hiqa.ie/)
World Health Organization Institutional Repository for Information Sharing (https://apps.who.int/iris/browse).
Search strategy
This study built on our previous DQ literature reviews.8,15 The full search strategy is available as Supplementary Appendix 1. The search syntax in Scopus is presented as an example (Box 1):
Box 1. Search Syntax in Scopus
SYNTAX 1
ALL ((((data PRE/0 (assessment OR accuracy OR quality OR completeness OR conformance OR validation OR verification OR plausibility)) PRE/5 (tool* OR framework OR software)) AND (“electronic medical record*” OR “electronic health record*” OR “my health record” OR “electronic patient record*” OR “personal health record*”))) AND PUBYEAR > 2009 AND (LIMIT-TO (SUBJAREA,”MEDI”) OR LIMIT-TO (SUBJAREA,”COMP”) OR LIMIT-TO (SUBJAREA,”ENGI”) OR LIMIT-TO (SUBJAREA,”NURS”
) OR LIMIT-TO (SUBJAREA,”BUSI”) OR LIMIT-TO (SUBJAREA,”DECI”)) AND (LIMIT-TO (DOCTYPE,”ar”) OR LIMIT-TO (DOCTYPE,”cp”) OR LIMIT-TO (DOCTYPE,”re”)) AND (LIMIT-TO (LANGUAGE,”English”))
SYNTAX 2
ALL ((((tool* OR framework OR software) PRE/5 (data PRE/0 (assessment OR accuracy OR quality OR completeness OR conformance OR validation OR verification OR plausibility))) AND (“electronic medical record*” OR “electronic health record*” OR “my health record” OR “electronic patient record*” OR “personal health record*”))) AND PUBYEAR > 2009 AND (LIMIT-TO (SUBJAREA,”MEDI”) OR LIMIT-TO (SUBJAREA,”COMP”) OR LIMIT-TO (SUBJAREA,”ENGI”) OR LIMIT-TO (SUBJAREA,”NURS”
) OR LIMIT-TO (SUBJAREA,”BUSI”) OR LIMIT-TO (SUBJAREA,”DECI”)) AND (LIMIT-TO (DOCTYPE,”ar”) OR LIMIT-TO (DOCTYPE,”cp”) OR LIMIT-TO (DOCTYPE,”re”)) AND (LIMIT-TO (LANGUAGE,”English”))
Note: Search was run in 2 syntax to cover tool/framework group occurring in front of group 1.
Consensus process
Two reviewers independently screened the titles and abstracts for studies of DQ frameworks, indicators, or assessment tools. A third reviewer was involved if consensus was not reached. Following consensus, full-text appraisal defined the final set of included reviews, conference articles, and original research articles.
Data were extracted by combinations of 2 authors, including details of the following:
DQ framework, categories, subcategories, indicators and assessment tools;
setting and purpose (research, population/public health, clinical/managerial quality improvement); and
methodology (machine learning, statistics, models, ontologies), including innovative concepts and processes.
The relevance and importance of all the DQ concepts extracted were qualitatively assessed and, using an iterative consensus process, categorized according the HIDQF and data life cycle conceptual frameworks. Final inclusion was determined following discussion, including consideration of the frequency of separate articles that addressed them, and consensus by the UNSW team and, subsequently, all authors. The full data extraction matrix is available as Supplementary Appendix 2.
The synthesis aimed to map the different terminologies used to describe the same or similar DQ concepts. Commonalities in the key features and purpose of DQ assessment across the data life cycle, including the indicators and tools used, were identified and agreed. The implications for the HIDQF specifically and DQ assessment generally were examined.
RESULTS
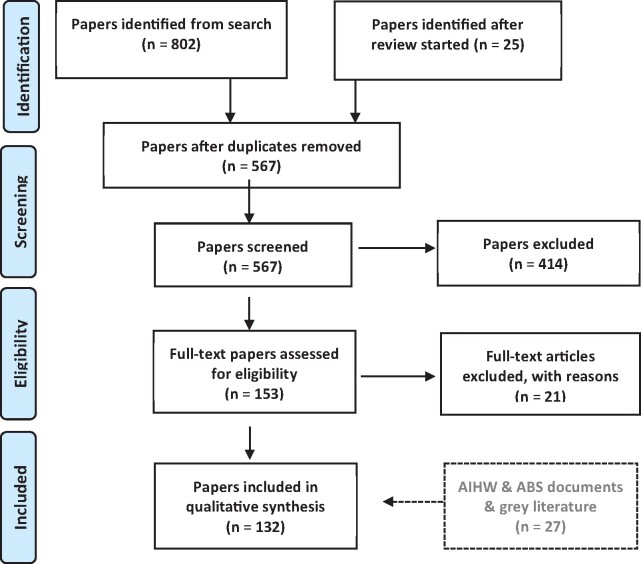
Figure 2 summarizes the literature review process and articles included and excluded at all stages of the review, using the PRISMA guidelines.
Figure 2.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram for data quality (DQ) literature review.
Articles that were published after the review began included the Computer Methods and Programs in Biomedicine Special Issue on Data Quality.20 Relevant documents from the gray literature were also included as they became available and eligible.
The complete list of articles reviewed is available as Supplementary Appendix 3.
DQ frameworks
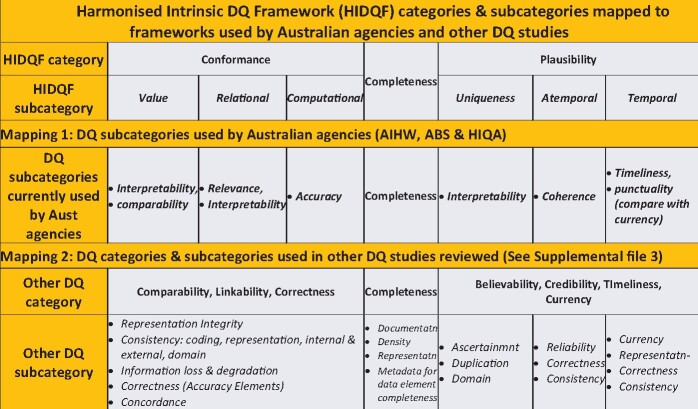
Figure 3 summarizes the existing HIDQF and mapped its categories and subcategories to those used by the Australian Institute of Health and Welfare, Australian Bureau of Statistics, and Irish Health Information Quality Authority (Mapping 1), and other published DQ studies as listed in Supplementary Appendix 3 (Mapping 2). It was not possible to map all the diverse DQ concepts used by Australian agencies and reported in the included DQ studies to the HIDQF subcategories. The ABS DQ Framework lists 7 dimensions, equivalent to categories in HIDQF: institutional environment, relevance, timeliness, accuracy, coherence, interpretability and accessibility.21 This framework underpins the ABS DQ declaration or statement.22
Figure 3.
Existing harmonized intrinsic data quality (DQ) framework (HIDQF) categories and subcategories mapped to frameworks used by Australia agencies and other DQ studies. ABS: Australian Bureau of Statistics; AIHW: Australian Institute of Health and Welfare; HIQA: Irish Health Information Quality Authority.
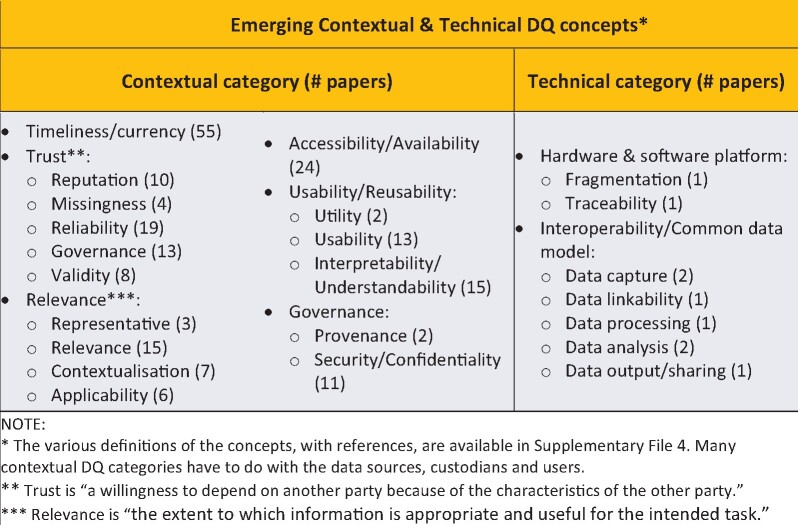
A number of concepts that may determine the DQ of a RWD repository were identified from the 120 included articles (Figure 4), including timeliness or currency (55 articles), accessibility (18 articles), relevance or irrelevance (15 articles), reliability (13 articles), usability (13 articles), interpretability (10 articles), reputation/believability (10 articles) articles, validity (8 articles), contextualization (7 articles), and applicability (6 articles). The definitions of these concepts, with references, are available in Supplementary Appendix 4.
Figure 4.
Emerging contextual and technical data quality (DQ) concepts.
DQ concepts also emerged from a number of articles reporting on DQ research and development associated with interoperability and common data models.23 The extrinsic DQ concepts were categorized as organizational (eg, reputation and governance), functional (eg, timeliness and usability), and technical (eg, interoperability). The “trust” construct24 is a good example of the utility of the DQ across the life cycle framework: in addition to intrinsic DQ concepts, contextual concepts such as reputation (trusted source), missingness (absence of data or fields), usability, accessibility, and relevance (to intended task) are important contextual determinants of DQ (Figure 4).
A use case of this additional contextual and technical categories is when RWD become dated or fall outside the regulatory requirements for storage, as in data sunsetting. The “sunset data” can be identified and addressed through a combination of temporal plausibility (intrinsic DQ) and provenance and governance provisions (contextual DQ) and computational (technical DQ) aspects in the extended HIDQF.
Indicators and measures of DQ
Many published DQ indicators and measures found were relevant and mappable to indicators for the HIDQF categories and subcategories. The DQ indicators and assessment tools appeared to be robust, with testing and validation in the open source domain, including those developed by the Observational Health Data Science and Informatics (OHDSI) collaborative. 25
Conformance of data was measured by a value for homogeneity and compliance to metadata, data model, specifications, and standards. Examples of conformance measures would be a ratio of known and unknown data types or calculated as [Total Semantically Consistent Rows] divided by [Total Rows].
Plausibility was measured as a believable unique value, range, or pattern at one time point or over many time points. Examples of temporal plausibility would be measures of single or multisite temporal patterns and variability, including probabilistic variability, over time.
The coverage of these mapped indicators, with some examples, are shown in Supplementary Appendix 5. A high-level summary of these indicators is also included in Figure 6.
Figure 6.
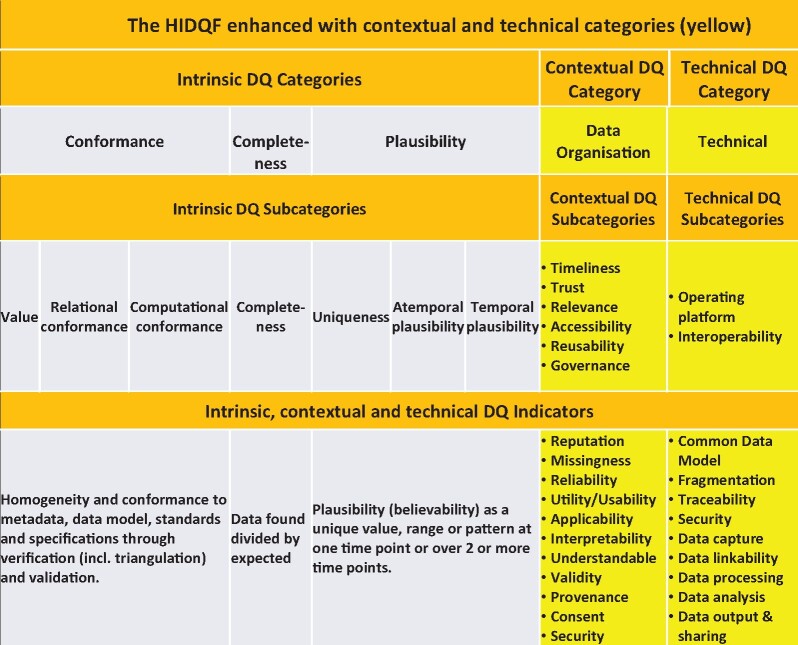
The harmonized intrinsic data quality (DQ) framework (HIDQF) enhanced with contextual and technical categories.
The DQ indicators not completely covered by the HIDQF were categorized as potentially intrinsic (eg, conciseness or objectivity), contextual (eg, applicability or understandability), or technical (eg, Euclidian distance or correct location or format after migration of records). Our approach to “context” was realist and includes places, people, time, institutions, resources available (or not), social relationships, rules, norms, and expectations that constitute them.26
These indicators are summarized in Supplementary Appendix 6.
DQ assessment tools
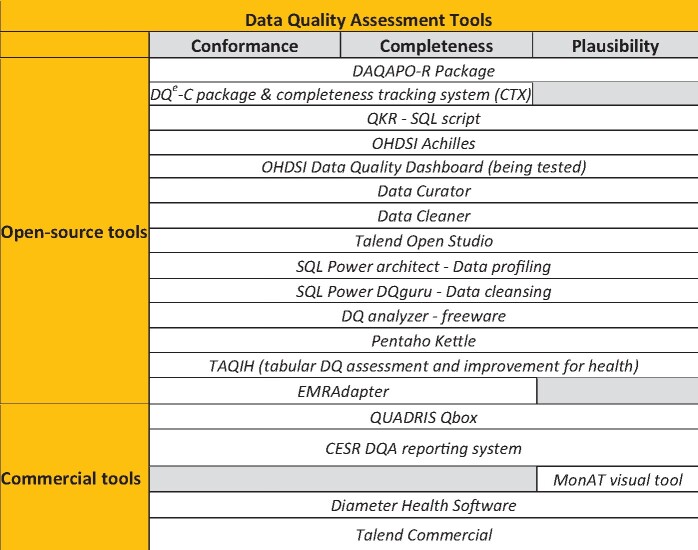
Figure 5 lists the currently available open source and commercial DQ assessment tools with an indication of the HIDQF category they addressed. This review included only open source tools, based on a qualitative examination of the logic and innovativeness of the approach and whether they have been field-tested (not many have been systematically evaluated for processes and outcomes). The included tools mainly addressed intrinsic DQ indicators because they are relatively easy to compute as DQ rules. The Quality Knowledge Repository was developed to store DQ Concepts and their methods of computation across information quality (IQ) domains with relationships across domains determined by a DQ meta-model. This knowledge repository has been leveraged into a service-oriented architecture to perform as a scalable and reproducible framework for DQ assessments of disparate data sources.18 Web-based tools such as the TAQIH (tabular DQ assessment and improvement for health) have been developed to conduct exploratory data analyses to improve completeness, accuracy, redundancy, and readability.27
Figure 5.
Data quality assessment tools.
The ACHILLES (Automated Characterization of Health Information at Large-scale Longitudinal Evidence Systems) tool was developed and maintained by the OHDSI collaborative to conduct DQ checks on databases that are based on or mapped to the Observational Medical Outcomes Partnership Common Data Model (CDM).25 The Achilles DQ rules have been well tested, including a published application to 24 large healthcare datasets across 7 different organizations in America, Europe, and Asia. This highlighted at least 12 DQ rule violations in 10 of the 24 datasets and violations of the full set of 71 DQ rules in at least 1 dataset.23 This CDM-enabled rapid comparisons of the DQ of multiple international datasets reduced the data model variations and increased the robustness and generalizability of the methods and outputs. These findings also highlighted the importance of interoperability, a FAIR guiding principle,4 as a core component of DQ assessment of RWD repositories that integrate data from multiple EHRs and health information systems. The OHDSI Data Quality Dashboard (https://ohdsi.github.io/DataQualityDashboard/) has been developed based on the HIDQF and Achilles. It is a potential mechanism to standardize DQ assessment of RWD within a CDM.
These tools do not appear to be used widely as only a small number of included studies reported on the quality of the underpinning dataset. There were no DQ statements that met, for example, the criteria promoted in the ABS DQ declaration checklist.21
DISCUSSION
We found a diverse range of DQ assessment frameworks, indicators, measures, tools, and use cases across the informatics, health services, and population health domains. These use cases highlighted information flow, business processes, and data elements; relevance to user and system requirements; and uses and challenges to DQ across the data life cycle (Figure 1). The diversity of frameworks, indicators, and tools presented different data and DQ needs depending on whether the purpose was for patient-level prediction or population-level estimation, or to answer questions of varying complexity in terms of time, place, and target population.
The majority of the concepts identified could be mapped to the HIDQF categories and subcategories. It was difficult to map some extracted concepts, such as conciseness, objectivity, ease of manipulation, status, content, and presentation to the HIDQF. This was mainly because the measures were mostly qualitative and more contextual than intrinsic. The common practice has been to use contextual DQ concepts qualitatively on a case-by-case basis as, for example, in the case of the ABS DQ statement and declaration.22
Much of the gray literature, especially those published by government agencies such as the ABS (Figure 3) and the WHO, 13 continue to describe DQ with a mix of intrinsic and contextual categories in a nonstandardized or systematic manner. For example, timeliness can refer to when data were collected, made findable, or accessible; or missingness of data or data fields can occur during collection, extraction, or visualization. A fundamental challenge is trust, which we have developed as a DQ construct (Figure 4).24 While contextual DQ dimensions are not easily quantifiable or measurable, they are important to meaningfully assess and manage DQ across the data life cycle.
The diversity found emphasizes the need for an extended approach to DQ that standardizes conventions to describe and assesses contextual and technical as well as intrinsic DQ categories in RWD repositories across the data life cycle. Because the repositories are ever-changing in content and purpose, and ever-extending in duration, DQ assessment models will need to consider continuous DQ assessment for periods longer than years or decades. These contextual elements may potentially be addressed with a common metadata model or an enhanced CDM or a combination of both.
The literature reflected an increasing recognition that a CDM is useful to constrain and, over time, overcome the inherent interoperability challenge with aggregating and integrating different RWD repositories. At the very least, it will improve the reuse of the data in one by another RWD repository. Interoperability standards are important to ensure that RWD collected as part of clinical care are captured, represented, curated, and shared appropriately and accurately among users in the primary and secondary health services in an integrated health neighborhood.28 The benefits to integrated care will be further enhanced if and when research datasets created from the secondary use of RWD are also interoperable across domains and disciplines. Transparent, comprehensive reporting of DQ features directly aligns with the Privacy, Ethics, and FAIR guiding principles by engendering trust among stakeholders of RWD repositories.2,29,30
The CDM-based ACHILLES23 approach to DQ assessment is being enhanced through a service-oriented architecture-based Open Quality and Analytics Framework.18 The Open Quality and Analytics Framework includes a DQ metamodel, a federated data integration platform to support semantically consistent metadata-centric querying of heterogeneous data sources, and a visualization meta-framework to store visualizations for different DQ concepts, indicators, and measures. This supports the inclusion of a technical category in the HIDQF.
This review suggests that RWD repositories require a DQ assessment framework that includes new or expanded intrinsic, technical, and contextual categories. The context needs to address best practice across micro-, meso-, and macro-organizations as well as the health system across the data life cycle. To support this role, we recommend that the current HIDQF be enhanced with 2 categories—technical and contextual. Regulatory, organizational, and with increasing use, other subcategories can be included in the contextual category.
Figure 6 summarizes the enhanced HIDQF with proposed categories and indicators. The HIDQF needs to be enhanced because DQ assessment is highly context sensitive and dependent on the purpose. The needs and requirements of a range of diverse stakeholders across the RWD production and curation life cycle are central to DQ assessment and management of the data errors and problems found.
There is sufficient research and development of intrinsic DQ indicators to provide a library from which data custodians can choose to adopt or adapt to meet their needs. However, for the enhanced HIDQF, novel quantitative and qualitative DQ measures and metadata will need to be developed to meaningfully characterize trust, relevance, accessibility, reusability, and governance as well as the operating platforms and interoperability.
CONCLUSION
An enhanced HIDQF, combined with continuous quality improvement protocols across the RWD life cycle, is recommended to ensure that RWD can be assessed and managed, including consideration of the context, to be fit for purpose. It can lead to more meaningful and useful standardized DQ statements about RWD repositories or specific datasets. Data custodians and researchers must routinely report the DQ—a DQ statement—as well as the actual and potential impacts of the results of data-driven research and development. This is especially important with AI and deep machine learning being the inevitable future. The enhanced DQ assessment framework will determine fitness for purpose through assessment of intrinsic DQ, contextual DQ (timeliness, trustworthiness, relevance, accessibility, reusability, governance), and technical DQ (traceability and interoperability).
The CDM-based ACHILLES DQ assessment tool highlighted the importance of CDMs as a strategy to reduce variations in DQ assessments of distributed RWD datasets. Interoperability (eg, conformance to a semantic and syntactic standard) is an integral concept in the DQ framework. More research is required in this space.
Trust and willingness to share data for public good and scientific advancement is a core requirement. DQ assessment, management, visualization, and sharing requires an optimal balance of privacy and security arrangements with adherence to FAIR principles. An ethical and secure framework based on public good is essential to produce a data asset that is fit for purpose.
Comprehensive DQ assessment requires a culture of reciprocity, transparency, and interoperability across the data production and curation life cycle. Effective DQ assessment is underpinned by rigorous documentation at point of care, good management, and appropriate governance across the RWD production and curation life cycle.
FUNDING
This work was supported by the Australian Institute of Health and Welfare.
AUTHOR CONTRIBUTIONS
S-TL, MK, and SdL conceptualized the combined HIDQF and life cycle framework and guided the literature review. All authors contributed substantially to the review process; screening of abstracts and full texts, data extraction, synthesis, analysis and interpretation; and iterative enhancement of conceptual framework. S-TL initiated and completed the manuscript. All authors reviewed and contributed to the intellectual content, approved the final draft and agreed to be accountable for the accuracy and integrity of the work.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
DATA AVAILABILITY STATEMENT
All the data has been made available through the article and online Supplementary Appendices.
Supplementary Material
ACKNOWLEDGMENTS
We thank UNSW Sydney research librarian Ms Monica O’Brien for guidance on the search strategy.
CONFLICT OF INTEREST STATEMENT
The authors have no competing interests to declare.
References
- 1.Liyanage H, Liaw S-T, Jonnagaddala J, et al. Artificial intelligence in primary health care: perceptions, issues, and challenges. Yearb Med Inform 2019; 28 (1): 41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liaw S, Liyanage H, Kuziemsky C, et al. Ethical use of electronic health record data and artificial intelligence: recommendations of the primary care informatics working group of the international medical informatics association. Yearb Med Inform 2020; 29 (1): 51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liyanage H, Liaw ST, Di Iorio CT, et al. Building a privacy, ethics, and data access framework for real world computerised medical record system data: a Delphi study. Contribution of the Primary Health Care Informatics Working Group. Yearb Med Inform 2016; 25 (1): 138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkinson MD, Dumontier M, Aalbersberg IJ, et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data 2016; 3 (1): 160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qualls LG, Phillips TA, Hammill BG, et al. Evaluating foundational data quality in the national patientcentered clinical research network (PCORnet ®). EGEMS (Wash DC) 2018; 6 (1): 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Australian Digital Health Agency. Commonwealth of Australia. My Health Record - Practice Incentive Program Digital Health Incentive; 2016. https://www.myhealthrecord.gov.au/for-healthcare-professionals/what-is-my-health-record. Accessed January 17, 2020.
- 7.Wang R, Strong D, Guarascio L.. Beyond accuracy: what data quality means to data consumers. J Manage Inf Syst 1996; 12 (4): 5–33. [Google Scholar]
- 8.Liaw S, Rahimi A, Ray P, et al. Towards an ontology for data quality in integrated chronic disease: a realist review of the literature. Int J Med Inform 2013; 82 (1): 10–24. [DOI] [PubMed] [Google Scholar]
- 9.Weiskopf NG, Weng C.. Methods and dimensions of electronic health record data quality assessment: enabling reuse for clinical research. J Am Med Inform Assoc 2013; 20 (1): 144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saez C, Martinez-Miranda J, Robles M, et al. Organizing data quality assessment of shifting biomedical data. Stud Health Technol Inform 2012; 180: 721–5. [PubMed] [Google Scholar]
- 11.Huser V, Kahn MG, Brown JS, et al. Methods for examining data quality in healthcare integrated data repositories. Biocomputing 2018; 23: 628–33. [PubMed] [Google Scholar]
- 12.World Health Organization. Data Quality Review: A Toolkit for Facility Data Quality Assessment. Module 1. Framework and Metrics. Geneva, Switzerland: WHO Press; 2017.
- 13.World Health Organization Western Pacific Regional Office. Improving Data Quality: A Guide for Developing Countries. Manila, the Phillippines: WHO Press; 2003. [Google Scholar]
- 14.Taggart J, Liaw S-T, Yu H.. Structured data quality reports to improve EHR data quality. Int J Med Inform 2015; 84 (12): 1094–8. [DOI] [PubMed] [Google Scholar]
- 15.Kahn MG, Callahan TJ, Barnard J, et al. A harmonized data quality assessment terminology and framework for the secondary use of electronic health record data. EGEMS (Wash DC) 2016; 4 (1): 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huser V, Li X, Zhang Z, et al. Extending Achilles heel data quality tool with new rules informed by multi-site data quality comparison. Stud Health Technol Inform 2019; 264: 1488–9. [DOI] [PubMed] [Google Scholar]
- 17.Khare R, Utidjian L, Ruth BJ, et al. A longitudinal analysis of data quality in a large pediatric data research network. J Am Med Inform Assoc 2017; 24 (6): 1072–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajan NS, Gouripeddi R, Mo P, et al. Towards a content agnostic computable knowledge repository for data quality assessment. Comput Methods Progr Biomed 2019; 177: 193–201. [DOI] [PubMed] [Google Scholar]
- 19.Henley-Smith S, Boyle D, Gray K.. Improving a secondary use health data warehouse: proposing a multi-level data quality framework. EGEMS (Wash DC) 2019; 7 (1): 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sáez C, Liaw S-T, Kimura E, Coorevits P, Garcia-Gomez JM.. Guest editorial: Special issue in biomedical data quality assessment methods. Comput Methods Programs Biomed 2019; 181: 104954. [DOI] [PubMed] [Google Scholar]
- 21.Australian Bureau of Statistics. The ABS Data Quality Framework; 2015. https://www.abs.gov.au/websitedbs/D3310114.nsf//home/ABS+Data+Quality+Statement+Checklist#:∼:text=Checklist%20of%20questions%20to%20generate,quality%20of%20the%20specific%20data. Accessed August 30, 2020.
- 22.Lee G, Allen B. Educated use of information about data quality. In: Proceedings of the International Statistical Institute 53rd Session; 2001.
- 23.Huser V, DeFalco FJ, Schuemie M, et al. Multisite evaluation of a data quality tool for patient-level clinical data sets. EGEMS (Wash DC) 2016; 4 (1): 1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKnight DH, Carter M, Thatcher JB, et al. Trust in a specific technology: An investigation of its components and measures. ACM Trans Manage Inf Syst 2011; 2 (2): 1–25. [Google Scholar]
- 25.Hripcsak G, Duke JD, Shah NH, et al. Observational Health Data Sciences and Informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform 2015; 216: 574–8. [PMC free article] [PubMed] [Google Scholar]
- 26.RAMESES II project team. What realists mean by context; or, Why nothing works everywhere or for everyone. http://www.ramesesproject.org/media/RAMESES_II_Context.pdf. Accessed September 10, 2020.
- 27.Álvarez Sánchez R, Beristain Iraola A, Epelde Unanue G, et al. TAQIH, a tool for tabular data quality assessment and improvement in the context of health data. Comput Methods Programs Biomed 2019; 181: 104824. [DOI] [PubMed] [Google Scholar]
- 28.Liaw S, de Lusignan S.. An ‘integrated health neighbourhood’ framework to optimise the use of EHR data. J Innov Health Inform 2016; 23 (3): 547–54. [DOI] [PubMed] [Google Scholar]
- 29.Kahn MG, Brown JS, Chun AT, et al. Transparent reporting of data quality in distributed data networks. EGEMS (Wash DC) 2015; 3 (1): 7–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown JS, Kahn M, Toh S.. Data quality assessment for comparative effectiveness research in distributed data networks. Med Care 2013; 51: S22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data has been made available through the article and online Supplementary Appendices.