Abstract
Context
Features of Prader-Willi syndrome (PWS) overlap with features of growth hormone (GH) deficiency, like small hands and feet, short stature, increased body fat, and low muscle mass and strength. In children with PWS, GH treatment (GHt) improves physical health and cognition. GHt has become the standard of care in PWS children, but in adults this is not yet the case.
Objective
This work aims to provide an overview of the current knowledge on GHt in PWS adults.
Methods
Medline, Embase, and the Cochrane Central Register of Controlled Trials databases were searched. Study selection included randomized clinical trials (RCTs) and nonrandomized (un)controlled trials (NRCTs) that reported data for adults with PWS, who received GHt for at least 6 months. Data on body composition, body mass index (BMI), cardiovascular end points, bone, cognitive function, quality of life, and safety were extracted.
Results
Nine RCTs and 20 NRCTs were included. Body composition improved during 12 months of GHt with an increase in mean (95% CI) lean body mass of 1.95 kg (0.04 to 3.87 kg) and a reduction of mean (95% CI) fat mass of –2.23% (–4.10% to –0.36%). BMI, low-density lipoprotein cholesterol levels, fasting glucose levels, and bone mineral density did not change during GHt. There were no major safety issues.
Conclusion
GHt appears to be safe and improves body composition in adults with PWS. Because poor body composition is closely linked to the observed high incidence of cardiovascular morbidity in adults with PWS, improving body composition might reduce cardiovascular complications in this vulnerable patient group.
Keywords: Prader-Willi syndrome, growth hormone, body composition, cardiovascular
Prader-Willi syndrome (PWS) is a rare and complex developmental disorder caused by the lack of expression of paternally inherited genes on chromosome 15q11 to q13 (1). The incidence of PWS is around 1:16.000 to 21.000 live births (2, 3). PWS is associated with low muscle mass, intellectual disability, and hypothalamic dysfunction (1, 4). Hypothalamic dysfunction results in an insatiable appetite, disturbed thermoregulation, abnormal pain perception, and pituitary hormone deficiencies (1, 4, 5).
Mortality in adults with PWS is high (3%) (6, 7) compared with non-PWS adults with an intellectual disability (8). More than half of mortality is caused by cardiopulmonary pathology (7, 9).
Cardiovascular morbidity and mortality are the result of a complex interplay between hypotonia and hyperphagia that, combined with pituitary hormone deficiencies and a strong behavioral phenotype, results in a poor body composition of high fat and low muscle mass (10). This induces cardiovascular risk factors like morbid obesity, hypertension, hypercholesterolemia, and type 2 diabetes mellitus (T2DM). Therefore, improving body composition is key in preventing cardiovascular morbidity and mortality.
Theoretically, body composition could be improved by vigorous exercise. However, this is hard to achieve because of hypotonia and (severe) behavioral issues. Another promising way to improve body composition in adults with PWS is growth hormone (GH) treatment (GHt).
Features of PWS overlap with features of GH deficiency, like small hands and feet, short stature, increased body fat, and low muscle mass and strength (1). It is generally accepted that GH deficiency in adults with PWS is due to hypothalamic dysfunction (11). Burnett et al showed that individuals with PWS have prohormone convertase 1 deficiency (12). Because prohormone convertase 1 deficiency results in impaired prohormone processing, GH deficiency in individuals with PWS might be the result of impaired growth hormone–releasing hormone (GHRH) processing. The reported prevalence of GH deficiency in adults with PWS ranges between 0% and 38% (13, 14). However, this is probably an underestimation of the true prevalence as there are no adequate tests to confirm this diagnosis in patients with PWS (13, 15). The 2 provocative tests most often used to diagnose GH deficiency are the insulin tolerance test (ITT) and the GHRH-arginine test (16). However, the GHRH-arginine test is unreliable when GH deficiency is caused by hypothalamic dysfunction, which is the case in patients with PWS (17). The ITT is a sensitive and specific test for GH deficiency of hypothalamic origin (16), but it is often contraindicated in adults with PWS because of the presence of cardiovascular disease and epilepsy. Furthermore, for ITT, 2 different intravenous catheters are needed, which is often impossible in adults with PWS because of obesity and disturbed vascularization (18, 19). Moreover, hypoglycemia has to be induced, which is especially dangerous in patients with an intellectual disability. Additionally, the ITT may have some ethical concerns because most adults with PWS have an intellectual disability and therefore may not fully understand the purpose of the ITT and why they feel bad when they get hypoglycemic.
In children with PWS, several large placebo-controlled trials, cohort studies, and a recent meta-analysis have shown that GHt causes improvements in physical health and cognition, and might improve quality of life (QoL) (20-27). The improvements were seen both in PWS children with proven GH deficiency, as well as in PWS children without proven GH deficiency. This indicates that GHt is useful for individuals with PWS regardless of their GH status (28, 29).
GHt has become the standard of care in PWS children, even in those who do not have proven GH deficiency (28). However, for adults this is not the case.
In adults with PWS, GHt may have a beneficial effect on body composition by increasing lean body mass (LBM) and decreasing fat mass (FM) (30, 31). Furthermore, it may improve muscle strength, endurance, metabolic health, cognition, and QoL (31-35). GHt can potentially also decrease cardiovascular mortality by reducing the cardiovascular risk profile. However, there have been some concerns regarding the safety of GHt in adults with PWS (28).
Recently, the global community of PWS experts has advocated for approval of GHt in adults with PWS (36). Although several review articles on GHt in PWS have been published, a meta-analysis is crucial to stress the importance of GHt in adults with PWS.
In this systematic review and meta-analysis, we provide a concise overview of the current knowledge on effectiveness and safety of GHt in adults with PWS.
Materials and Methods
This systematic review and meta-analysis has been performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (37). The meta-analysis is registered in PROSPERO (International Prospective Register of Systematic Reviews)with number CRD42019140295.
Search Strategy
Two separate searches were conducted in the databases MEDLINE (via PubMed and ALL OVID), Embase, and the Cochrane Central Register of Controlled Trials. The full search strategy is included in Supplementary Table S1 (38). Data collection was finished by February 2020 and updated by December 2020. There were no restrictions on language or period. References of articles and relevant reviews identified through the search were further searched to find additional eligible studies.
All articles were evaluated independently by 3 reviewers, first by title and abstract, and then by full text. The full text of selected articles was assessed for data extraction and bias evaluation. A characterization matrix was constructed with the following variables: author, publication year, study design, country, age, number of participants, period, dosage of GHt/placebo, and outcome measurements.
Eligibility Criteria
We included all original research articles describing adults with genetically confirmed PWS, regardless of genetic subtype, with or without proven GH deficiency, who participated in GH studies performed in adulthood that were either RCTs (double-blinded or not) or nonrandomized (un)controlled studies (NRCTs; cohort and before-after studies). We excluded reviews, protocols, case reports, conference abstracts, data from unpublished research, and incomplete articles. We also excluded studies with fewer than 6 months of GHt. The analyzed outcome measurements are described in Table 1.
Table 1.
Analyzed outcome measurements
| Body composition: lean body mass in kilograms and total body fat mass in percentage as measured by dual-energy x-ray absorptiometry |
| Body mass index (in kg/m2) |
| Cardiovascular end points: low-density lipoprotein cholesterol levels in milligrams per deciliter, thromboembolisms, and cardiovascular function (defined as structural and functional cardiovascular features) |
| Bone: BMD in grams per centimeters squared (g/cm2) and BMD SDS, bone strength, and geometry, and markers of bone formation, bone resorption, and bone regulation. For readability, we use the term BMD both for BMD in g/cm2 and BMD SDS in the text. The distinction between the 2 is made in the tables in “Results.” |
| Cognitive function: all types of validated questionnaires (such as WISC-III, WAIS-III, Stanford-Binet test) |
| Quality of life: evaluated by different instruments |
| Adverse events: glucose metabolism (glycemia, HOMA-IR, glycated hemoglobin A1c), peripheral edema, sleep apnea, and mortality. |
Abbreviations: BMD, bone mineral density; HOMA-IR, homeostatic model assessment of insulin resistance; SDS, SD score; WAIS-III, Wechsler Adult Intelligence Scale; WISC-III, Wechsler Intelligence Scale for Children–III.
Study Selection and Data Extraction
Identification, screening, and eligibility assessments were performed independently by 3 reviewers (A.R., C.P., and K.P.) in an unblinded, standardized manner. Disagreements between reviewers were discussed with a fourth researcher (L.d.G.). Extraction of variables was performed independently by 2 researchers. The corresponding or principal study authors were contacted to clarify data if needed. In case different studies assessed the same outcome parameter in the same study population, the study with the most participants was selected for the analysis of that outcome parameter.
Risk of Bias and Data Quality Assessment
Risk of bias was evaluated separately by 2 reviewers (A.R. and C.P.). The Cochrane Collaboration Risk of Bias 2.0 tool was used for RCTs to evaluate study limitations (39). The ROBINS-I tool was used for NRCTs (40). The possibility of publication bias was assessed by evaluating a funnel plot (Begger test) for asymmetry. GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) were used to assess the data quality for each outcome and to draw conclusions about the quality of evidence.
Statistical Analysis
Data were analyzed with Cochrane Review Manager (version 5.4; The Nordic Cochrane Centre) and R version 3.6.0 (41, 42). Meta-analyses were performed separately for RCTs and NRCTs when data were available in at least 3 studies that investigated the same outcome and had a comparable study design. Data are presented as pooled mean difference and 95% CI. Inverse variance weighting was used to pool the data based on the number of participants, mean, and SD of the individual studies. Studies that did not report data in terms of means and their respective SDs were recalculated, if possible. Because of different lengths of follow-up, meta-analyses were only performed for outcomes at 12 months of follow-up. Outcomes at different follow-up times are shown in scatterplots in the supplementary data (38). Inconsistency (the proportion of total variation across studies due to heterogeneity) of effects across interventions were measured with the I2 statistic. To obtain adequate results, a random-effects model was used if I2 was greater than 50%, and a fixed-effects model was used if I2 was less than 50%. For the random-effects model, the between-study variance (τ 2) was estimated with the DerSimonian-Laird estimator.
P values less than .05 were considered statistically significant.
Results
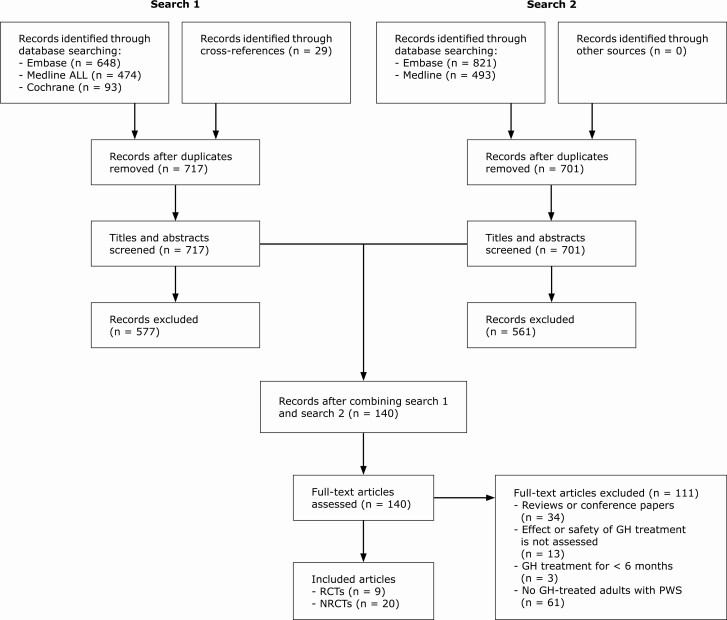
For search 1, we identified 1215 records through database screening and 29 records through other sources (cross-references). After deduplication, 717 records remained. For search 2, we identified 1314 records through database screening, of which 701 remained after deduplication. A.R. and K.P. evaluated the titles and abstracts of search 1 (717 records). C.P. evaluated the titles and abstracts of search 2 (701 records). After this, we combined search 1 and search 2 and assessed the full text of 140 articles. We excluded 111 articles that did not meet the eligibility criteria. We included 29 articles in this systematic review and meta-analysis, of which there were 9 RCTs and 20 NRCTs (Fig. 1). Study characteristics and outcomes of the included articles are described in Table 2.
Figure 1.
Flowchart of screened articles. Abbreviations: GH, growth hormone; NRCTs, nonrandomized (un)controlled studies; PWS, Prader-Willi syndrome; RCTs, randomized clinical trials.
Table 2.
Characteristics of included articles
| Author | Country | Age, mean ± SD, y | No. of participants (GH/placebo) | Naive or previously treated with GH | GH dosage | Study period | Outcomes | |
|---|---|---|---|---|---|---|---|---|
| RCTs | ||||||||
| Höybye et al (2003)a (43) | Sweden | Range, 17-32 | 17 (9/8) | GH naive | 0.26 mg/d for 1 mo followed by 0.53 mg/d for 5 mo and individually titrated for open-label part | 6 mo RCT + 6 or 12 mo open-label GHt | LBM, FM, BMI, WHR, OGTT, HOMA-IR, HbA1c, IGF-1, IGFBP-1, glucose, insulin, leptin, lipids, adverse events | |
| Höybye et al (2005)a (32) | Sweden | 25b (range, 17-37) | 19 (10/9) | GH naive | 0.26 mg/d for 1 mo followed by 0.53 mg/d for 5 mo and individually titrated for open-label part | 6 mo RCT + 12 mo open-label GHt | Neuropsychological tests, mental health, and psychosocial adjustment | |
| Sode-Carlsen et al (2010) (30) | Scandinavia | 28.7 ± 6.7 | 46 (24/22) | No GH for 12 mo | 0.3 or 0.4 mg/d for 1 mo followed by 0.6 or 0.8 mg/d for 11 mo | 12 mo | LBM, FM, body water, VF, SF, thigh fat, thigh muscle, BMI, waist circumference, OGTT, HOMA-IR, HbA1c, IGF-1, IGFBP-1, glucose, insulin, lipids, PEF, physical performance, adverse events | |
| Jørgensen et al (2013)c (44) | Scandinavia | 28.5 ± 6.7 | 42 (20/18)d | No GH for 12 mo | 0.6 or 0.8 mg/d for 12 mo and individually titrated for open-label part | 12 mo RCT + 24 mo open-label GHt | LBM, FM, IGF-1, BMD, markers of bone formation and resorption | |
| Kuppens et al (2016a)e (45) | NL | 17.8 (15.7-18.5)f | 25 (25/25) | Previous GH for at least 24 mo and on GH at time of inclusion | 0.67 mg/m2/d | 12-mo crossover | LBM, FM, IGF-1, cognitive function (TIQ, VIQ, PIQ) | |
| Kuppens et al (2016b)e (46) | NL | 17.2 ± 1.8 | 27 (27/27) | Previous GH for at least 24 mo and on GH at time of inclusion | 0.67 mg/m2/d | 12mo crossover | LBM, FM, BMI, IGF-1, IGFBP-3, glucose, insulin, adverse events, BP | |
| Kuppens et al (2017)e (33) | NL | 17.2 ± 1.8 | 27 (27/27) | Previous GH for at least 24 mo and on GH at time of inclusion | 0.67 mg/m2/d | 12-mo crossover | Lipids, OGTT, IGF-1, IGFBP-3, BP, MS | |
| Donze et al (2018)e (61) | NL | 17.2 ± 1.8 | 27 (27/27) | Previous GH for at least 24 mo and on GH at time of inclusion | 0.67 mg/m2/d | 12-mo crossover | BMD, BMAD | |
| Donze et al (2019)e (65) | NL | 17.2 ± 1.8 | 27 (27/27) | Previous GH for at least 24 mo and on GH at time of inclusion | 0.67 mg/m2/d | 12-mo crossover | AHI, CAI, OAI, OSA | |
| NRCTs | ||||||||
| Höybye et al (2007)a (47) | Sweden | 31b | 10 (6/4) | GH naive | Individually titrated (0.2-0.5 mg/d) | 5.1 yb (range, 4.8-6.3 y) | LBM, FM, BMI, OGTT, IGF-1, glucose, insulin, HOMA-IR | |
| Bertella et al (2007)g (34) | Italy | 27.08 ± 4.55 | 13 (13/0) | 5 previously treated with GH. GH was ceased 1-4 y before inclusion | 0.04 mg/kg/wk for 1 mo followed by 0.08 mg/kg/wk for 11 mo and individually titrated for 12 mo | 24 mo | BMI, IGF-1, QoL, degree of wellbeing | |
| Marzullo et al (2007)g (48) | Italy | 26.9 ± 1.2 | 13 (13/0) | 5 previously treated with GH. GH was ceased 1-4 y before inclusion | Individually titrated (mean ± SD: 0.96 ± 0.04 mg/d at 12 mo) | 12 mo | LBM, FM, VF, SF, APD, WHR, BMI, IGF-I, HOMA-IR, glucose, insulin, lipids, BP, CRP, LVPWT, IVST, LVM, LVEDD, E/A, PASP, DT, LVEJ, RVEJ, PFR, HR | |
| Gondoni et al (2008)g (49) | Italy | 26.4 ± 4.4 | 12 (12/0) | 5 previously treated with GH. GH was ceased at least 1 y before inclusion | Individually titrated (mean ± SD: 0.065 ± 0.01 mg/kg/wk at 12 mo) | 12 mo | LBM, FM, WHR, BMI, IGF-1, HOMA-IR, glucose, insulin, physical performance, adverse events, BP, HR | |
| Mogul et al (2008) (50) | USA | 30.5 ± 1.5h | 38 (38/0) | No GH for 12 months | Individually titrated (median 0.6 mg/d; range, 0.4-1.0 mg/d) | 12 mo | LBM, FM, BMI, waist circumference, HOMA-IR, HbA1c, IGF-1, glucose, insulin, lipids, T3, T4, FT4, TSH, adverse events, BP, MS | |
| Sode-Carlsen et al (2011)i (51) | Scandinavia | 29.5 (range, 16-42) | 43 (43/0) | No GH for 12 mo. 21 received GH in study by Sode-Carlsen et al (2010) | Individually titrated (mean 0.61 mg/d; range, 0.2-1.6 mg/d) | 24 mo | LBM, FM, body water, VF, SF, thigh fat, thigh muscle, BMI, waist circumference, OGTT, HOMA-IR, HbA1c, IGF-I, glucose, insulin, lipids, PEF, adverse events, BP | |
| Tsuchiya et al (2011) (66) | Japan | 19b (range, 10 mo-53 y) | 65 (31/34) | NA | NA | NA | DM | |
| Butler et al (2013) (52) | USA | 32.3 ± 11.1 | 11 (11/0) | GH naive | 0.0125 mg/kg/d | 12 mo | LBM, FM, BMI, waist circumference, hip circumference, HOMA-IR, IGF-1, glucose, insulin, lipids, FT4, TSH, physical activity, BMD, QoL, energy intake, energy expenditure, metabolic rate, RQ, RMR, adverse events | |
| Jørgensen et al (2014)c (53) | Scandinavia | 28.6 ± 6.5 | 39 (39/0) | No GH for 12 mo | 0.6 or 0.8 mg/d for 12 mo and individually titrated for open-label part | 12 mo RCT + 24 mo open-label GHt | LBM, FM, VF, SF, waist circumference, OGTT, HOMA-IR, HbA1c, IGF-1, glucose, insulin, leptin, adiponectin, triglycerides, OCN, CRP | |
| Khare et al (2014) (62) | USA | Range, 0.2-47 | 79 (46/33) | NA | NA | Mean 2.5 y (range, 0.5-6.5 y) | BMD | |
| Lafortuna et al (2014)g (35) | Italy | 26.1 ± 5.4 | 15 (15/0) | 8 previously treated with GH. GH was ceased 2-4 y before inclusion | Individually titrated (mean ± SD: 0.027 ± 0.012 mg/kg/wk at 24 mo) | 24 mo | LBM, FM, BMI, WHR, waist circumference, HOMA-IR, HbA1c, IGF-1, glucose, insulin, lipids, CRP, skeletal muscle characteristics and strength, physical performance | |
| Höybye (2015)j (58) | Sweden | 36 ± 9 | 10 (10/0) | 5 previously treated with GH | Individually titrated (mean ± SD: 0.4 ± 0.1 mg) | Mean ± SD: 15 ± 4 y | LBM, FM, BMI, TBW, HbA1c, IGF-1, lipids, BP, testosterone, adverse events | |
| Longhi et al (2015) (54) | Italy | 29.4 ± 8.6 | 41 (23/18) | Previously treated with GH | N/A | Mean ± SD: 7.2 ± 2.5 y | LBM, FM, BMI, BMD, BMC, bone geometry, bone strength | |
| Marzullo et al (2015)g (55) | Italy | 26.4 ± 3.7 | 9 (9/0) | 4 previously treated with GH. GH was ceased 1-4 y before inclusion. All received GH in study by Marzullo et al (2007) | Individually titrated (mean ± SD: 0.40 ± 0.11 mg/d at 48 mo) | 48 mo | LBM, FM, VF, SF, APD, BMI, WHR, HOMA-IR, glucose, insulin, lipids, BP, CRP, LVPWT, IVST, LVM, LVEDD, LVEDV, E/A, DT, LVEF, RVEF, PFR, HR, adverse events | |
| Fintini et al (2016) (67) | Italy | 28.3 ± 7.2 | 145 (20/125) | 9 previously treated with GH | Individually titrated (mean ± SD: 0.07 ± 0.08 mg/kg/wk) | Mean ± SD: 47.4 ± 43.8 mo | OGTT | |
| Brunetti et al (2018) (63) | Italy | 29.5 ± 7.2 | 14 (6/8) | NA | Individually titrated (mean: 0.23 mg/d) | NA | RANKL, OPG, sclerostin | |
| Butler et al (2019) (64) | USA | 11.60 (range: 3.25 – 38) | 56 (30/26) | NA | NA | Mean ± SD: 2.5 ± 1.9 y | Cognitive function | |
| Manzardo et al (2019) (60) | USA | 21.0 ± 14 | 1067 (765/302) | NA | NA | NA | Thromboembolisms | |
| Damen et al (2020a)k (56) | NL | 19.0 (17.5-20.7)f | 43 (43/0) | Previous GH for at least 5 y during childhood and at least 3 y after attainment of AH | Individually titrated (median 0.38 mg/m2/d; IQR 0.33-0.45 mg/m2/d) | 36 mo | LBM, FM, BMI, IGF-1, glucose, insulin, adverse events, BP | |
| Damen et al (2020b)k (57) | NL | 19.0 (17.5-20.7)f | 43 (43/0) | Previous GH for at least 5 y during childhood and at least 3 y after attainment of AH | Start dose: 0.33 mg/m2/d. Individually titrated from here on | 36 mo | OGTT, HOMA-IR, glucose, insulin, lipids, BP, MS |
Abbreviations: AH, adult height; AHI, apnea hypopnea index; APD, abdominal anteroposterior diameter; BMAD, bone mineral apparent density; BMC, bone mineral content; BMD, bone mineral density; BMI, body mass index; BP, blood pressure; CAI, central apnea index; CRP, C-reactive protein; DM, diabetes mellitus; DT, deceleration time; E/A, early-to-late mitral peak flow velocity; FM, fat mass; FT4, free thyroxine; GH, growth hormone; GHt, growth hormone treatment; HbA1c, glycated hemoglobin A1c; HOMA-IR; homeostatic model assessment of insulin resistance; HR, heart rate; IGF-1, insulin-like growth factor 1; IGFBP-1, insulin-like growth factor binding protein 1; IGFBP-3, insulin-like growth factor binding protein 3; IQR, interquartile range; IVST, interventricular septum thickness; LBM, lean body mass; LVEDD, left ventricle end-diastole diameter; LVEDV, left ventricular end-diastole volume; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; LVPWT, left ventricular posterior wall thickness; MS, metabolic syndrome; NA, not available; NRCT, nonrandomized (un)controlled study; OAI, obstructive apnea index; OCN, osteocalcin; OGTT, oral glucose tolerance test; OPG, osteoprotegerin; OSA, obstructive sleep apnea; PASP, pulmonary artery systolic pressure; PEF, peak expiratory flow; PFR, peak filling rate; PIQ, performance intelligence quotient; QoL, quality of life; RANKL, receptor activator of nuclear factor-κB ligand; RCT, randomized clinical trial; RMR, resting metabolic rate; RQ, respiratory quotient; RVEF, right ventricular ejection fraction; SF, subcutaneous abdominal fat; TBW, total body water; TIQ, total intelligence quotient; TSH, thyroid stimulating hormone; T3, total triiodothyronine; T4, total thyroxine; USA, United States of America; VF, visceral abdominal fat; VIQ, verbal intelligence quotient; WHR, waist-hip ratio.
a These studies contain the same study population.
b Expressed as median.
c This is a subgroup analysis of the Scandinavian study by Sode-Carlsen et al (2010) (30) and Sode-Carlsen et al (2011) (51).
d Thirty-eight participants completed the study. It is unclear to which group these participants were randomly assigned.
e These studies contain the same study population.
f Expressed as median (interquartile range).
g These studies contain (partly) the same study population.
h Expressed as mean ± SE.
i This study is a follow-up study by Sode-Carlsen et al (2010) (30).
j This study contains only men.
k These studies contain the same study population.
Body Composition
Randomized clinical trials
Five RCTs assessed LBM, 5 FM, and 3 body mass index (BMI) (30, 43-46). Kuppens et al (2016a) (45) and Kuppens et al (2016b) (46) assessed the same study population, as did Sode-Carlsen et al (2010) (30) and Jørgensen et al (2013) (44). Therefore, only Kuppens et al (2016b) and Sode-Carlsen et al (2010) are included in the analysis. The open-label part of the study by Höybye et al (2003) (43) is included in the meta-analyses of the NRCTs. Meta-analyses for LBM, FM, and BMI were not possible because of extreme heterogeneity in study design and reported data among the RCTs. Their findings are summarized in Table 3. All participants in the study by Kuppens et al (2016b) were on GHt at the time of inclusion, whereas the other studies included only adults with PWS who had not been treated with GH for at least 12 months.
Table 3.
Results of studies that assessed the efficacy of growth hormone treatment on body composition in adults with Prader-Willi syndrome
| Author | Outcome parameter | Result | Limitations/remarks | |
|---|---|---|---|---|
| Lean body mass | ||||
| RCTs | Höybye et al (2003) (43) | LBM, kg | Compared to placebo, LBM did not change during 6 mo of GH treatment (P = .07). 12 mo of open-label GH treatment resulted in an LBM decrease of 2.2 kg (P < .05). Significant differences were only seen in methylation-positive participants. | Baseline differences between GH-treated and placebo-treated participants. Only 11 of 17 participants had a confirmed diagnosis of PWS (methylation-positive). No information about blinding. |
| Sode-Carlsen et al (2010)a (30) | LBM, kg | Compared to placebo, LBM increased by 2.25 kg (95% CI, 0.72 to 3.77; P = .005) during 12 mo of GH treatment. | None | |
| Kuppens et al (2016b)b (46) | Relative change in LBM, kg; LBMlimb, kg; LBMtrunk, kg | LBM decreased by 2.0% (–0.9 kg; P = .07) during 12 mo of placebo. LBMlimb and LBMtrunk decreased by 3.0% (P = .004) and 1.0% during placebo. Compared to placebo, LBM increased with 3.5% (1.5 kg; P = .005) during 12 mo of GH treatment. LBMlinb and LBMtrunk increased by 4.7% (0.8 kg; P < .001) and 2.5% (0.6 kg; P = .09) during GH vs placebo. | All participants were on GH treatment at time of inclusion. | |
| Fat mass | ||||
| RCTs | Höybye et al (2003) (43) | Change in FM, % | Compared to placebo, FM decreased during 6 mo of GH treatment (P < .05). 12 mo of open-label GH treatment resulted in an FM decrease of 2.5% (P < .01). Significant differences were only seen in methylation-positive participants. | Baseline differences between GH-treated and placebo-treated participants. Only 11 of 17 participants had a confirmed diagnosis of PWS (methylation-positive). No information about blinding. |
| Sode-Carlsen et al (2010)a (30) | FM, kg | Compared to placebo, FM decreased by 4.20 kg (95% CI, –6.40 to –2.00; P < .001) during 12 mo of GH treatment. | None | |
| Kuppens et al (2016b)b (46) | Relative change in FM, kg; FMlimb, %; FMtrunk, % | FM increased by 21.5% (4.1 kg; P < .001) during 12 mo of placebo. FMlimb and FMtrunk increased by 17.3% and 15.6% (P < .01 for both) during placebo. Compared to placebo, FM decreased by 17.3% (–2.9 kg; P = .004) during 12 mo of GH treatment. FMlinb and FMtrunk decreased by 17.1% (P < .001) and 5.6% (P = .007) during GH vs placebo. | All participants were on GH treatment at time of inclusion. | |
| Body mass index | ||||
| RCTs | Höybye et al (2003) (43) | BMI, kg/m2 | BMI did not change during 12 mo of open-label GH treatment (P = .79). | Baseline differences between GH-treated and placebo-treated participants. Only 11 of 17 participants had confirmed diagnosis of PWS (methylation-positive). No information about blinding. |
| Sode-Carlsen et al (2010) (30) | BMI, kg/m2 | Compared to placebo, BMI decreased by 0.61 kg/m2 (95% CI, –1.69 to 0.46; P = .26). However, baseline BMI was already lower because the majority of participants were living in special PWS group homes with extensive control of calorie intake and exercise. | None | |
| Kuppens et al (2016b) (46) | Relative change in BMI | BMI increased by 5.8% during 12 mo of placebo. Compared to placebo, BMI decreased by 2.5% (–0.6; P = .052) during 12 mo of GH treatment. | All participants were on GH treatment at time of inclusion. |
Abbreviations: BMI, body mass index; FM, total fat mass; GH, growth hormone; LBM, total lean body mass; PWS, Prader-Willi syndrome.
a The same outcomes in this population were assessed by Jørgensen et al (2013) (44).
b The same outcomes in this population were assessed by Kuppens et al (2016a) (45).
All studies found improvements in body composition with a reduction of FM (range, 2.90- to 4.20-kg reduction) and an increase in LBM (range, 1.50- to 2.25-kg increase). BMI slightly decreased in 2 studies, although not significantly.
Nonrandomized (un)controlled studies
Twelve NRCTs studied LBM, FM, and BMI (34, 35, 47-58). Bertella et al (2007) (34), Marzullo et al (2007) (48), Gondoni et al (2008) (49), Lafortuna et al (2014) (35), and Marzullo et al (2015) (55) (partly) assessed the same study population, as did Damen et al (2020a) (56) and Damen et al (2020b) (57), and Sode-Carlsen et al (2011) (51) and Jørgensen et al (2014) (53). Therefore, only Lafortuna et al (2014), Damen et al (2020a), and Jørgensen et al (2014) are included in the meta-analyses. Höybye et al (2007) (47) contained the same study population as the open-label part of Höybye et al (2003) (43) and was therefore excluded from the meta-analyses. Because Höybye (2015) (58) and Longhi et al (2015) (54) were cross-sectional studies, these studies were also excluded from the meta-analyses.
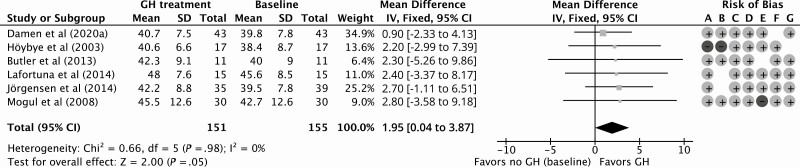
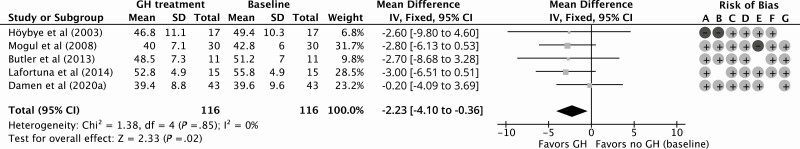
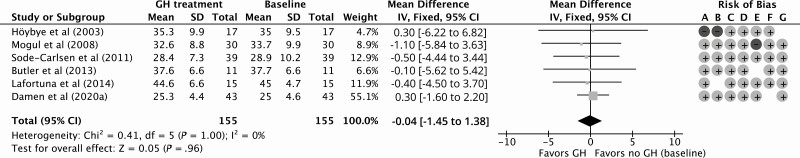
GH treatment resulted in a significant improvement of 1.95 kg (95% CI, 0.04 to 3.87 kg) in LBM compared to baseline for 12 months of follow-up with a low quality of evidence according to GRADE considerations (Fig. 2; Table 4). Compared to baseline, the percentage of FM decreased significantly by –2.23% (95% CI, –4.10% to –0.36%) during 12 months of GHt, with a low quality of evidence (Fig. 3; Table 4). BMI decreased nonsignificantly by –0.04 kg/m2 (95% CI, –1.45 to 1.38 kg/m2) during 12 months of GHt compared to baseline, with a low quality of evidence (Fig. 4; Table 4). Changes in LBM, FM, and BMI at different follow-up times compared to baseline are shown in Supplementary Fig. S1 (38).
Figure 2.
Forest plot of the effect of 12 months of growth hormone treatment on lean body mass (kg) in nonrandomized (un)controlled studies. Abbreviations: GH, growth hormone; IV, inverse variance. Risk of bias legend: A, confounding; B, selection; C, intervention; D, intervention deviation; E, missing data; F, outcome; G, reported results.
Table 4.
GRADE of evidence
| No. of participants, No. of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence | Mean difference (95% CI) with GH |
|---|---|---|---|---|---|---|---|
| Lean body mass, kg | |||||||
| 151, 6 observational studies | Not serious | Not serious | Not serious | Not serious | None | ⊕⊕◯◯ Low | MD 1.95 higher (0.04 higher to 3.87 higher) |
| Fat mass, % | |||||||
| 116, 5 observational studies | Not serious | Not serious | Not serious | Not serious | None | ⊕⊕◯◯ Low | MD 2.23 lower (4.10 lower to 0.36 lower) |
| Body mass index, kg/m2 | |||||||
| 155, 6 observational studies | Not serious | Not serious | Not serious | Not serious | None | ⊕⊕◯◯ Low | MD 0.04 lower (1.45 lower to 1.38 higher) |
| LDL cholesterol, mg/dL | |||||||
| 136, 5 observational studies | Not serious | Not serious | Not serious | Not serious | None | ⊕⊕◯◯ Low | MD 3.52 lower (10.86 lower to 3.82 higher) |
| Fasting glucose, mmol/L | |||||||
| 151, 6 observational studies | Not serious | Not serious | Serious | Not serious | None | ⊕◯◯◯ Very low | MD 0.20 higher (0.01 lower to 0.28 higher) |
| Glycated hemoglobin A1c, mmol/mol | |||||||
| 80, 3 observational studies | Not serious | Not serious | Serious | Not serious | None | ⊕◯◯◯ Very low | MD 0.78 higher (4.04 lower to 5.60 higher) |
| HOMA-IR index | |||||||
| 151, 6 observational studies | Not serious | Serious | Serious | Not serious | None | ⊕◯◯◯ Very low | MD 0.48 higher (0.23 higher to 0.73 higher) |
Abbreviations: GH, growth hormone; HOMA-IR, homeostatic model assessment of insulin resistance; LDL, low-density lipoprotein; MD, mean difference.
Figure 3.
Forest plot of the effect of 12 months of growth hormone treatment on fat mass (%) in nonrandomized (un)controlled studies. Abbreviations: GH, growth hormone; IV, inverse variance. Risk of bias legend: A, confounding; B, selection; C, intervention; D, intervention deviation; E, missing data; F, outcome; G, reported results.
Figure 4.
Forest plot of the effect of 12 months of growth hormone treatment on body mass index in nonrandomized (un)controlled studies. Abbreviations: GH, growth hormone; IV, inverse variance. Risk of bias legend: A, confounding; B, selection; C, intervention; D, intervention deviation; E, missing data; F, outcome; G, reported results.
Longhi et al (2015) compared LBM, FM, and BMI between GH-treated and GH-untreated adults with PWS in a cross-sectional study (54). They found an improved body composition in GH-treated adults compared to GH-untreated adults, with an increase in LBM (mean ± SD: 44.6 ± 8.3 kg in GH-treated vs 37.5 ± 8.1 kg in GH-untreated; P = .01) and a reduction of FM (mean ± SD: 50.1 ± 6.3% in GH-treated vs 51.8 ± 6.7% in GH-untreated; P > .05). BMI was slightly higher in GH-treated adults compared to GH-untreated adults (mean ± SD: 41.6 ± 10.8 kg/m2 vs 41.1 ± 12.4 kg/m2; P > .05). Höybye (2015) showed that long-term GHt for 15 years resulted in a mean ± SD LBM of 71.1 ± 8.4%, mean ± SD FM of 28.6 ± 8.5%, and mean ± SD BMI of 30.6 ± 7.7 kg/m2 (58). Jørgensen et al (2014) reported FM only in kilograms and was therefore excluded from the meta-analysis for FM (%) (53). They found a significant reduction of FM after 12 months of GHt (mean ± SD: 27.5 ± 13.4 kg at 12 months vs 29.6 ± 12.5 kg at baseline; P < .05).
Cardiovascular End Points (Lipids, Cardiac Function, and Thromboembolisms)
Randomized clinical trials
Three RCTs studied low-density lipoprotein (LDL) cholesterol levels in adults with PWS (30, 33, 43). However, a meta-analysis was not possible for LDL cholesterol because of extreme heterogeneity in study design and reported data. Study findings are summarized in Table 5. Only Sode-Carlsen et al (2010) reported a significant decrease in LDL cholesterol during 12 months of GHt compared to placebo (30). The other studies found similar LDL cholesterol levels in adults who received GHt and in adults who received placebo (33, 43).
Table 5.
Results of studies that assessed the cardiovascular impact of growth hormone treatment in adults with Prader-Willi syndrome
| Author | Outcome parameter | Result | Limitations/remarks | |
|---|---|---|---|---|
| Low-density lipoprotein cholesterol | ||||
| RCTs | Höybye et al (2003) (43) | LDLc, mmol/L | Compared to placebo, LDLc did not change during 6 mo of GH treatment (P = .35). At 6 mo of GH treatment mean ± SE LDLc was 2.8 ± 0.2 mmol/L compared to 2.9 ± 0.2 mmol/L at 6 mo of placebo. | Baseline differences between GH-treated and placebo treated participants. Only 11 of 17 participants had a confirmed diagnosis of PWS (methylation-positive). No information about blinding. |
| Sode-Carlsen et al (2010) (30) | Change in LDLc, mmol/L | Compared to placebo, LDLc decreased with 0.027 mmol/L (95% CI, –0.53 to –0.01 mmol/L; P = .047) during 12 mo of GH treatment. | None | |
| Kuppens et al (2017) (33) | LDLc, mmol/L | Compared to placebo, LDLc did not change during 12 mo of continuation of GH (P = .71). After 12 mo of GH continuation or placebo, mean (95% CI) LDLc was 2.8 mmol/L (2.6 to 3.0 mmol/L) in both groups. | All participants were on GH treatment at time of inclusion. | |
| Thromboembolisms | ||||
| NRCTs | Manzardo et al (2019) (60) | Thromboembolisms | GH treatment, either current or past, reduces the risk of developing thromboembolisms (OR = 0.2; 95% CI, 0.1 to 0.39). | Baseline differences in age and presence of multidisciplinary care between GH-treated and GH-untreated participants, possibly related to occurrence of thromboembolisms. Children and adults included. GH treatment could be current or past. |
| Cardiac function | ||||
| NRCTs | Marzullo et al (2015)a (55) | DBP, SBP, echocardiography (LVPWT, IVST, LVEDD, LVMFM, LVEF, E/A, deceleration time), radionuclide angiography (LVEFra, RVEFra, LV-PFRra) | DBP and SBP did not change during 48 mo of GH treatment. Although LVPWT and IVST did not change during GH treatment, mean ± SE LVEDD and mean ± SE LVMFM increased after 12 mo of GH treatment (47.1 ± 1.2 after 12 mo vs 43.0 ± 1.4 at baseline for LVEDD, P < .05; 2.8 ± 0.1 after 12 mo vs 2.4 ± 0.1 at baseline for LVMFM, P < .05) and after 48 mo of GH treatment (46.2 ± 1.2 after 48 mo for LVEDD, P > .05; 2.8 ± 0.1 after 48 mo for LVMFM, P > .05). There was a positive correlation between LVMFM and LBM (r = 0.62; P = .001). LVEF decreased at 12 mo and 48 mo of GH treatment (P = .03 and P = .02, respectively), compared to baseline, but remained > 55% during the whole study period. E/A ratio (diastolic function) did not change during GH treatment. Deceleration time decreased after 12 mo and 48 mo of GH treatment, compared to baseline (P = .02 and P = .04, respectively). LVEFra slightly decreased during 12 and 48 mo of GH treatment (69.3 ± 6.7 at 12 mo and 67.9 ± 1.4 at 48 mo vs 72.1 ± 2.2 at baseline, P < .01 for both). RVEFra did not change during GH treatment. LV-PFRra decreased during GH treatment, although not significantly. | Only participants who concluded the whole study period of 48 mo are included. |
Abbreviations: DBP, diastolic blood pressure; E/A, early-to-late mitral peak flow velocity; GH, growth hormone; IVST, interventricular septum thickness; LBM, lean body mass; LDLc, low-density lipoprotein cholesterol; LVEDD, left ventricular end-diastole diameter; LVEF, left ventricular ejection fraction; LVEFra, left ventricular ejection fraction as assessed by radionuclide angiography; LVMFM, left ventricular mass indexed by percentage fat body mass; LV-PFRra, left ventricular peak filling rate as assessed by radionuclide angiography; LVPWT; left ventricular posterior wall thickness; NRCT, nonrandomized (un)controlled trial; OR, odds ratio; PWS, Prader-Willi syndrome; RCT, randomized clinical trial; RVEFra, right ventricular ejection fraction as assessed by radionuclide angiography; SBP, systolic blood pressure.
a This study is a follow-up study by Marzullo et al (2007) (48).
Nonrandomized (un)controlled studies
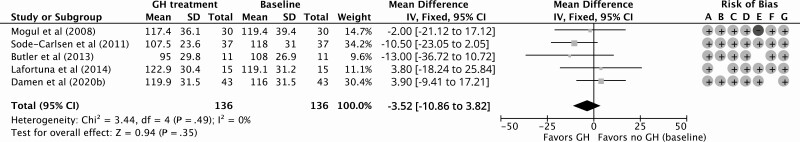
Eight NRCTs studied LDL cholesterol levels, 2 cardiac function, and 1 thromboembolism (35, 48, 50-52, 55, 57, 58). Because Marzullo et al (2015) (55), Lafortuna et al (2014) (35), and Marzullo et al (2007) (48) assessed the same study population, only Lafortuna et al (2014) is included in the meta-analysis. Höybye (2015) (58) produced a cross-sectional study and therefore was excluded from the meta-analysis. Twelve months of GHt resulted in a nonsignificant decrease of –3.52 mg/dL (95% CI, –10.86 to 3.82 mg/dL) in LDL cholesterol levels compared to baseline (Fig. 5). The risk of bias and grade of certainty were low (see Table 4). Change in LDL cholesterol levels at different follow-up times compared to baseline is shown in Supplementary Fig. S2 (38). Höybye (2015) reported a mean ± SD LDL cholesterol level of 2.4 ± 0.8 mmol/L for PWS adults who were treated with GH for a mean duration of 15.4 years (58). The findings on cardiac function and thromboembolisms are summarized in Table 5. Marzullo et al (2015) found that 48 months of GHt increased the left ventricular mass with preservation of systolic and diastolic function (59). Furthermore, Manzardo et al (2019) reported that GHt might protect against the development of thromboembolisms (60).
Figure 5.
Forest plot of the effect of 12 months of growth hormone treatment on low-density lipoprotein cholesterol levels (mg/dL) in nonrandomized (un)controlled studies. Abbreviations: GH, growth hormone; IV, inverse variance. Risk of bias legend: A, confounding; B, selection; C, intervention; D, intervention deviation; E, missing data; F, outcome; G, reported results.
Bone
Randomized clinical trials
Bone mineral density (BMD) was assessed in 2 RCTS (44, 61). The findings of these RCTs are summarized in Table 6. None of the studies that assessed the efficacy of 12 months of GHt on BMD found any significant difference when compared with placebo. Jørgensen et al (2013) also assessed the relation between GHt and bone formation and resorption markers (see Table 6) (44). They found an increase of the bone formation markers PINP (N-terminal propeptide of type I procollagen) and osteocalcin during 12 months of GHt.
Table 6.
Results of studies that assessed the efficacy or effectiveness of growth hormone treatment on bone in adults with Prader-Willi syndrome
| Author | Outcome parameter | Result | Limitations/remarks | |
|---|---|---|---|---|
| Bone mineral density | ||||
| RCTs | Jørgensen et al (2013) (44) | Change in BMDTS SDS (% ± SD); change in BMDLS SDS, % ± SD; change in BMDhip SDS, % ± SD | 12 mo of GH treatment resulted in a decrease of –1.0 ± 3.2% in BMDTS and –0.4 ± 8.0% in BMDhip. Placebo for 12 mo resulted in a decrease of –0.2 ± 2.1% in BMDTS and –1.8 ± 4.8% in BMDhip. Differences between GH treatment and placebo were nonsignificant (P > .05 for both). For BMDLS, there was a significant difference between 12 mo of GH treatment and placebo (–2.1 ± 3.4% and 1.9 ± 3.4%, respectively; P < .01). Continuous GH treatment for 24 mo did not result in any changes in BMDTS, BMDLS, or BMDhip (P > .05 for all). | Participants who dropped out were excluded from analyses. |
| Donze et al (2018)a (61) | BMDTB SDS, BMADLS SDS, BMDTB, g/cm2, BMADLS, g/cm2 | No significant difference in BMDTB SDS and BMADLS SDS between 12 mo of GH treatment and placebo (P = .51 and P = .37, respectively). BMDTB and BMADLS also did not significantly differ between 12 mo of GH treatment and placebo (data not shown). Independent of GH treatment or placebo, BMDTB SDS did not change during study (24 mo; P = .20), but BMADLS SDS decreased significantly compared to baseline (P < .01). | All participants were on GH treatment at time of inclusion. | |
| NRCTs | Butler et al (2013) (52) | BMDTB, g/cm2; mean ± SD | During 12 mo of GH treatment, BMDTB decreased from 1.14 ± 0.05 to 1.12 ± 0.05 (P > .05). | No information on missing data. |
| Khare et al (2014) (62) | BMDLS SDS | Individuals > 15 y who were treated with GH had a higher BMDLS SDS than individuals > 15 y who were never treated with GH (P = .02). | Children and adults were included. Unclear whether “on GH treatment” group also contains participants who received only GH in the past. | |
| Longhi et al (2015) (54) | BMC, g; BMDTB, g/cm2; BMDLS, g/cm2; BMDFN, g/cm2 | Individuals who were treated with GH (either in the past or at time of assessment) had a higher BMC than individuals never treated with GH (2184 ± 278 g and 1743 ± 601 g, respectively; P = .003). No differences between GH-treated and GH-untreated individuals for BMDTB (1.11 ± 0.09 and 1.15 ± 0.12, respectively; P > .05), BMDLS (1.02 ± 0.12 and 1.05 ± 0.16, respectively; P > .05), and BMDFN (0.86 ± 0.13 and 0.91 ± 0.16 respectively, P > .05). | GH treatment could either be current or past (during childhood). No information on missing data. | |
| Markers of bone formation, bone resorption, and bone regulatory markers | ||||
| RCTs | Jørgensen et al (2013) (44) | PINP, µg/L; osteocalcin, µg/L; NTX, nM | 12 mo of GH treatment resulted in a significant increase of PINP and osteocalcin (P < .001 and P < .05, respectively). NTX did not change during GH treatment (P > .05). | Participants who dropped out were excluded from analyses. |
| NRCTs | Brunetti et al (2018) (63) | RANKL, pg/mL; OPG, pg/mL; sclerostin, pg/mL | Multiple regression analysis showed GH treatment results in decreased levels of RANKL (ρ = –0.551; P < .0001) and increased levels of OPG (ρ = 0.392; P < .0001) when adjusted for age. No correlation between sclerostin and GH treatment (P > .05). | No information on participant selection. |
| Bone geometry and bone strength | ||||
| NRCTs | Longhi et al (2015) (54) | Outer diameter, mm; inner diameter, mm; MI; CSA, mm2; CA, mm2; BBRI, mm3. All parameters were evaluated at level of second metacarpal bone at its narrowest site. | Individuals who were treated with GH (either past or at time of assessment) had, compared to GH-untreated individuals, a bigger outer diameter (7.8 ± 0.7 and 7.2 ± 0.5, respectively; P = .004) and inner diameter (3.8 ± 0.8 and 3.3 ± 0.6, respectively; P = .000), and consequently a higher CSA (49.2 ± 8.9 and 40.4 ± 6.6, respectively; P = .005), CA (36.9 ± 6.5 and 31.9 ± 4.9, respectively; P = .02), MA (12.3 ± 4.8 and 8.9 ± 3.1; P = .03), and BBRI (467.3 ± 123.6 and 357.3 ± 74.2, respectively; P = .008). No difference in MI between GH-treated and GH-untreated individuals (0.6 ± 0.1 and 0.5 ± 0.1, respectively; P > .05). | GH treatment could either be current or past (during childhood). No information on missing data. |
Abbreviations: BBRI, bending breaking resistance index (bone strength); CA, cortical area; CSA, total cross-sectional area; BMADLS, lumbar spine bone mineral apparent density; BMC, bone mineral content; BMDFN, femoral neck bone mineral density; BMDhip, total hip bone mineral density; BMDLS, lumbar spine bone mineral density; BMDTS, total body bone mineral density; GH, growth hormone; MA, medullary endocortical area; MI, metacarpal index; NRCTs, nonrandomized (un)controlled studies; NTX, cross-linked n-telopeptides of type I collagen; OPG; osteoprotegerin; PINP, N-terminal propeptide of type I procollagen; RANKL, receptor activator of nuclear factor-κB ligand; RCTs, randomized clinical trials; SDS, SD scores (Z score).
a This study compares continuation of GH treatment with cessation of GH treatment, and restart of GH treatment with cessation of GH treatment.
b Bone formation markers: PINP, osteocalcin. Bone resorption marker: NTX. Bone regulatory markers: RANKL, OPG, sclerostin.
Nonrandomized (un)controlled studies
Three NRCTs assessed BMD (52, 54, 62). Their findings are described in Table 6. None of the studies reported significant differences in BMD during 12 months of GHt. Two studies looked at differences in BMD between GH-naive individuals and individuals who were treated with GH (either in the past or at the time of assessment). One of the studies, by Khare et al (62), found that GH-treated individuals had a higher BMD than GH-untreated individuals, whereas Longhi et al (54) reported no differences in BMD between GH-treated and GH-untreated individuals. Longhi et al also studied the effect of GHt on bone geometry and bone strength (see Table 6) (54). They found that GH-treated individuals had a better bone geometry and higher bone strength than GH-untreated individuals. One study assessed the relation between GHt and bone regulatory markers (see Table 6) (63). They reported that GHt results in decreased levels of RANKL (receptor activator of nuclear factor-κB ligand) and increased levels of the regulatory marker OPG (osteoprotegerin).
Cognition and Quality of Life
Randomized clinical trials
Two RCTs assessed cognition, and one assessed QoL (Table 7) (32, 45). Compared to placebo, Höybye et al (2005) found that GHt had a positive effect on subtests for executive functions (trail making) and mental speed (reaction time) during 6 months of GHt (32). However, continuation of GHt for 12 months (open-label) resulted in significant improvements only in coding and mental speed (reaction time). After 18 months of open-label GHt, significant improvements in block design, and subtests for mental speed (reaction time) and motor speed and motor fluency (finger tapping) were reported. Kuppens et al (2016a) found that cessation of GHt did not result in cognitive deterioration (45). For QoL, Höybye et al (2005) found no effect of GHt (32). However, they showed that individuals with PWS already had a very positive self-image before GHt, which makes the potential effect of GHt on QoL much smaller.
Table 7.
Results of studies that assessed the efficacy or effectiveness of growth hormone treatment on cognition and quality of life in adults with Prader-Willi syndrome
| Author | Outcome parameter | Result | Limitations/remarks | |
|---|---|---|---|---|
| Cognition | ||||
| RCTs | Höybye et al (2005) (32) | SPIQ, block design test, coding test, Bender gestalt test, draw a man test, verbal fluency tests of semantic categories and phonological issues, trail making test (TMT A; TMT B), reaction-time (right; left), finger tapping (right; left), Luria word learning and retention testa | Significant improvements in TMT B (P = .01), reaction right (P = .01) and reaction left (P = .02) after 6 mo of GH treatment in GH-treated group. No significant changes in SPIQ, TMT A, tapping right, tapping left, word learning, and word retention after 6 mo. In the placebo group, there was a significant impairment in tapping left (P = .02) after 6 mo. After 12 mo of GH treatment (open-label part of study), there were significant improvements only in coding (P = .04) and reaction right (P = .04). 18 mo of GH treatment (12 mo of open-label) resulted in significant improvements in block design (P = .03), reaction right (P = .04), reaction left (P = .01), and tapping right (P = .03). | Baseline differences between GH-treated and placebo-treated participants. Only 13 of 19 participants had confirmed diagnosis of PWS (methylation-positive). |
| Kuppens et al (2016a) (45) | TIQ, VIQ, PIQb | There were no significant differences in TIQ, VIQ, and PIQ between 12 mo of GH treatment and placebo (P = 0.83, P = .486 and P = .32, respectively). | All participants were on GH treatment at time of inclusion. | |
| NRCTs | Butler et al (2019) (64) | Vocabulary, pattern analysis, quantitative, bead memory, total test score (Stanford-Binet test; mean ± SD)c | Vocabulary IQ insignificantly higher in GH-treated individuals compared to GH-untreated individuals in children-based UCI cohort (44.03 ± 8.94 vs 37.52 ± 10.66; P = .02). No differences for subtests pattern analysis, quantitative, bead memory, and for total test score (P > .37 for all). | No information on selection of participants. Children and adults were included. GH treatment could either be current or past. |
| Quality of life | ||||
| RCTs | Höybye et al (2005) (32) | Self-developed questionnaires that included domains related to mental functions, emotional status, physical status, and social behavior. Q1 (self-evaluation), Q2 (parent/caregivers), Q3 (possible changes after discontinuation of GH) | For Q1, no significant differences on mental, emotional, somatic, and social domain after 6, 12 (open-label part of study), and 18 mo (12 mo of open-label) of GH treatment. Participants already reported very positive self-image at baseline. Discontinuation of GH resulted in impaired social capacity after 3 (P = .03) and 6 mo (P = .045), and impaired physical status (P < .01) and decreased overall capacity (P < .01) after 6 mo. | Baseline differences between GH-treated and placebo-treated participants. Only 13 of 19 participants had confirmed diagnosis of PWS (methylation-positive). Used questionnaires are not validated. |
| NRCTs | Bertella et al (2007) (34) | SF-36 (mean ± SD), PGWBI (mean ± SD)d | 6 mo of GH treatment resulted in significant improvements in the subscales anxiety, depression, positive well-being and total scale of the PGWBI (self-evaluation) and in the subscales physical functioning, bodily pain, general mental health and total scale of SF-36 (self-evaluation; P < .05 for all). Same improvements were seen after 12 mo of GH treatment (P < .0292 for all) and after 24 mo of GH treatment (P < .03 for all). Subscale general health of the PGWBI significantly improved only after 24 mo of GH treatment (P = .01). For parents/caregivers questionnaires, there were significant improvements in subscales anxiety, depression, self-control, and total scale of the PGWBI after 6 mo of GH treatment (P < .02 for all). After 12 mo of GH treatment, there were also significant improvements in subscale general health of PGWBI and in subscales vitality and total scale of SF-36 (P < .03 for all). All these improvements were also seen after 24 mo of GH treatment (P < .02 for all), except for subscale vitality of SF-36. | Only participants with a Mini-Mental State Examination score > 24 and IQ score ≥ 45 were included. Used questionnaires were not validated for individuals with an intellectual disability. |
| Butler et al (2013) (52) | QoL-AGHDA, SF-36d | No differences after 12 mo of GH treatment compared to baseline for all subscales of SF-36. | No information on missing data. Used questionnaires were not validated for individuals with an intellectual disability. |
Abbreviations: GH, growth hormone; IQ, intelligence quotient; NRCT, nonrandomized (un)controlled study; PGWBI, Psychological General Well-Being Index; PIQ, performance IQ; Q1, questionnaire 1; Q2, questionnaire 2; Q3, questionnaire 3; QoL-AGHDA, Quality of Life–Assessment of Growth Hormone Deficiency in Adults; SF-36, 36-Items Short Form Health Survey; RCT, randomized clinical trial; SPIQ, speedy performance test of IQ; TIQ, total IQ; TMT A, trail making test A (digits); TMT B, trail making test B (digits and letters); UCI, University of California, Irvine; VIQ, verbal IQ.
a The block design test and coding test are from Wechsler Intelligence Scale–III for Children (WISC-III). Executive functions were tested with the verbal fluency tests and trail making test. Mental speed was tested with the reaction-time task. To measure motor speed and motor fluency, the finger tapping task was used. The Luria word learning and retention test was used to measure learning and memory capacity.
b TIQ in patients older than 16 years was assessed with 11 subscales of Wechsler Adult Intelligence Scale third edition (WAIS-III). TIQ in patients age 16 years or younger was assessed with 10 subscales of WISC-III. VIQ subtests were vocabulary, similarities, arithmetic, digit span, information, and comprehension for patients older than 16 years, and information, similarities, arithmetic, vocabulary, and comprehension for patients age 16 years or younger. PIQ subtests were picture completion, coding, block design, matrix reasoning, and picture arrangement for patients older than 16 years, and picture completion, coding, picture arrangement, block design, and visual puzzles for patients age 16 years or younger.
c Vocabulary, pattern analysis, quantitative, and bead memory are subtests of the Stanford-Binet test.
d The SF-36 and the PGWBI both are self-administered questionnaires. A copy was also given to the parent/caregiver. Subscales of the PGWBI were anxiety, depression, positive well-being, self-control, general health, vitality, and total scale (self-calculated by the authors). Subscales of the SF-36 were physical functioning, role limitation because of physical problems, bodily pain, general health, vitality, social functioning, role limitation because of emotional problems, general mental health, and total scale.
Nonrandomized (un)controlled studies
One NRCT assessed cognition, and 2 NRCTs QoL (see Table 7) (34, 52, 64). Butler et al (2019) reported higher vocabulary IQ in GH-treated individuals compared to GH-untreated individuals (64). For QoL, one study found a positive effect of GHt (34), whereas the other study found no effect of GHt (52).
General Safety and Effects on Glucose Metabolism
General safety
Five RCTs and 14 NRCTs assessed safety during GHt (30, 33, 35, 43, 46-53, 55-58, 65-67). The reported adverse events are described in Table 8. Marzullo et al (2007) (48), Gondoni et al (2008) (49), Lafortuna et al (2014) (35), and Marzullo et al (2015) (55) (partly) assessed the same study population, as did Damen et al (2020a) (56) and Damen et al (2020b) (57), and Sode-Carlsen et al (2011) (51) and Jørgensen et al (2014) (53). Therefore, only Lafortuna et al (2014), Damen et al (2020b), and Jørgensen et al (2014) are included in the meta-analyses. Höybye et al (2007) (47) contained the same study population as the open-label part of Höybye et al (2003) (43) and was therefore excluded from the meta-analyses. Because Höybye (2015) (58) was a cross-sectional study, this study was also excluded from the meta-analyses.
Table 8.
Reported adverse events during growth hormone treatment in adults with Prader-Willi syndrome
| Outcome parameter | Result | |
|---|---|---|
| RCTs | ||
| Sleep apnea | Donze et al (2019) reported GH treatment did not increase AHI, CAI, or OAI (P > .35) compared to placebo in young adults previously treated with GH (65). Also after stratification for sex and genotype, differences in AHI, CAI and OAI remained nonsignificant (P > .23 for sex; P > .14 for genotype). Compared to baseline (regardless of GH treatment or placebo) AHI, CAI, and OAI did not change during study period (P > .18). Regardless of GH treatment or placebo, 2 participants fulfilled criteria of obstructive sleep apnea. | |
| Peripheral edema | In the study by Höybye et al (2003), 3 participants developed edema during GH treatment, which reversed in 2 participants after reducing GH dose (43). The other participant had chronic edema and needed intensified diuretic treatment after 10 mo of GH treatment. Sode-Carlsen et al (2010) reported progression of pretibial edema in 7 participants treated with GH, compared to 5 participants treated with placebo (30). Five other GH-treated participants reported to have less pretibial edema after 12 mo of GH treatment compared to baseline. In the placebo group, 4 participants had progression of preexisting pretibial edema. | |
| Glucose metabolism | ||
| OGTT | OGTT was studied in 3 RCTs (30, 43). In the study by Höybye et al (2003), 5 participants had IGT after 12 mo of GH treatment compared to 1 at baseline (43). According to Sode-Carlsen et al (2010), 7 participants developed IGT during 12 mo of GH treatment, compared to 2 participants during placebo treatment (30). Kuppens et al (2017) reported no differences in OGTT between GH treatment and placebo for 12 mo (33). | |
| Diabetes mellitus | Presence of diabetes mellitus was studied in 3 RCTs (30, 33, 43). Sode-Carlsen et al (2010) reported 1 participant withdrew from GH treatment within first 6 mo because of progression of preexistent diabetes mellitus (30). In the other studies, none of the participants developed diabetes mellitus (33, 43). | |
| Fasting glucose | Kuppens et al (2016b) (46) and Sode-Carlsen et al (2010) (30) studied glucose levels. Only Kuppens et al (2016b) found an increase in fasting glucose levels during 12 mo of GH treatment compared to placebo (mean difference: 0.2 mmol/L; P = .01). However, glucose levels remained within reference range. | |
| HbA1c, mmol/mol | No differences in HbA1c reported (30). | |
| HOMA-IR index | No differences in HOMA-IR index reported (30). | |
| Metabolic syndrome | No differences in the presence of metabolic syndrome were reported (33). | |
| Others | Sode-Carlsen et al (2010) reported 1 participant experienced a few isolated instances of headache and nausea within 2 mo of treatment (30). In the study by Kuppens et al (2016b), 7 participants had a viral respiratory tract infection during GH treatment, compared to 10 participants during placebo (46). | |
| NRCTs | ||
| Sleep apnea | Höybye (2015) reported that 1 individual developed sleep apnea during 14 y of GH treatment (58). | |
| Peripheral edema | Gondoni et al (2008) (49) and Bertella et al (2007) (34) reported transient edema in 3 participants within the first month of GH treatment, which reversed in all participants after temporary reducing the GH dose. In the study by Mogul et al (2008), 5 participants had mild progression of preexisting ankle edema and 1 participant withdrew from the study because of myalgias associated with lower leg swelling (50). Sode-Carlsen et al (2011) reported that 2 participants developed discrete pretibial edema, whereas 4 participants had regression of pretibial edema (51). In the study by Butler et al (2013), one patient developed mild lower leg edema within the first month of GH treatment, which stabilized during the rest of the treatment period (52). | |
| Glucose metabolism | ||
| OGTT | OGTT was studied in 4 NRCTs (47, 51, 57, 67). Höybye et al (2007) found no differences in OGTT between baseline and 6 y of GH treatment (47). In the study by Sode-Carlsen et al (2011), 5 participants developed IGT during 2 y of GH treatment (51). However, in 3 participants with preexisting IGT the glucose tolerance normalized during GH treatment. Fintini et al (2016) found 15% of adults who were currently on GH treatment developed altered glucose tolerance, compared to 39.6% of adults who never received GH treatment (67). Damen et al (2020b) showed that the prevalence of IGT did not change significantly during 3 y of GH treatment (57). | |
| Diabetes mellitus | Presence of diabetes mellitus was studied in 9 NRCTs (35, 47, 50-53, 57, 58, 66). In the study by Sode-Carlsen et al (2011), 3 patients with preexisting IGT developed diabetes mellitus during 2 y of GH treatment (51). Part of this study population was also analyzed by Jørgensen et al (2014) (53). They reported that 1 individual developed diabetes mellitus during 2 years of GH treatment. Tsuchiya et al (2011) showed that the prevalence of diabetes mellitus was higher in GH-untreated individuals than in GH-treated individuals (41.2% in GH-untreated vs 9.7% in GH-treated; P < .05) (66). In the study by Damen et al (2020b), 1 participant developed diabetes mellitus during 3 y of GH treatment (57). In the study by Höybye (2015), 4 participants developed diabetes mellitus during 15 y of GH treatment. However, all were probably due to increase in weight as a result of nonadherence to lifestyle advice (58). In the other studies, none of the participants developed diabetes (35, 47, 50, 52). | |
| Fasting glucose | 12 mo of GH treatment resulted in a nonsignificant increase of 0.20 mmol/L (95% CI, –0.01 to 0.41 mmol/L) in fasting glucose levels compared to baseline. | |
| HbA1c, mmol/mol | 12 mo of GH treatment resulted in a nonsignificant increase of 0.78 mmol/mol (95% CI, –4.04 to 5.60 mmol/mol) compared to baseline. | |
| HOMA-IR index | 12 mo of GH treatment resulted in a significant increase of 0.48 (95% CI, 0.23 to 0.73) compared to baseline. | |
| Metabolic syndrome | Presence of metabolic syndrome variables did not significantly change during GH treatment (50, 57). | |
| Others | Sode-Carlsen et al (2011) reported headache in 2 participants and carpal tunnel syndrome in 1 participant (51). Höybye (2015) reported renal insufficiency in 1 participant (58). |
Abbreviations: AHI, apnea-hypopnea index; CAI, central apnea index; GH, growth hormone; HbA1c, glycated hemoglobin A1c; HOMA-IR, homeostatic model assessment of insulin resistance; IGT, impaired glucose tolerance; NRCTs, nonrandomized (un)controlled studies; OAI, obstructive apnea index; OGTT, oral glucose tolerance test; RCTs; randomized clinical trials.
Effects on glucose metabolism
Because GH counteracts the effects of insulin on glucose and lipid metabolism, one of the major concerns when starting GHt is impaired glucose tolerance. Therefore, we address this side effect separately.
Randomized clinical trials
Two RCTs studied fasting glucose levels, 1 glycated hemoglobin A1c (HbA1c), and 1 homeostatic model assessment of insulin resistance (HOMA-IR) index during GHt (30, 46). Only Kuppens et al (2016b) found an increase in fasting glucose levels during 12 months of GHt compared to placebo (mean difference: 0.2 mmol/L; P = .01) (46). However, glucose levels remained within the reference range. The other RCT did not find any significant differences in fasting glucose, HbA1c, and HOMA-IR index during GHt (30).
Nonrandomized (un)controlled studies
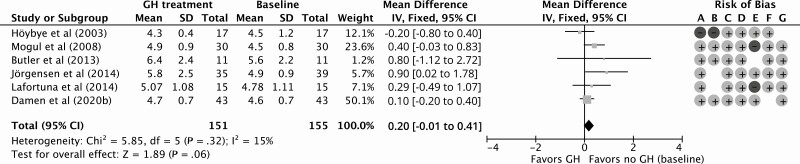
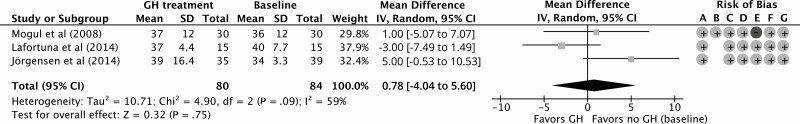
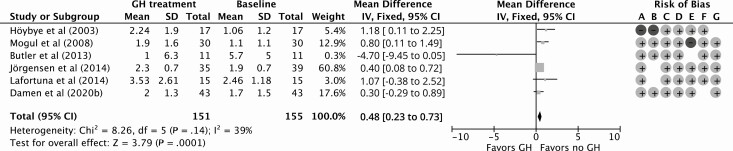
Eleven NRCTs studied fasting glucose levels, 5 HbA1c, and 10 HOMA-IR index during GHt (35, 47-53, 55-57). Fasting glucose levels and HOMA-IR index were also studied in the open-label part of the study by Höybye et al (2003) (43). GHt resulted in a nonsignificant increase of 0.20 mmol/L (95% CI, –0.01 to 0.41 mmol/L) in fasting glucose levels compared to baseline for 12 months of follow up (Fig. 6). Compared to baseline, HbA1c remained similar during 12 months of GHt (increase of 0.78 mmol/mol; 95% CI, –4.04 to 5.60 mmol/mol; Fig. 7). Höybye (2015) reported that HbA1c levels remained within the reference range (mean ± SD: 41 ± 12 mmol/L; reference range, 27 to 42 mmol/L) after GHt for 15 years (58). For HOMA-IR, 12 months of GHt resulted in a significant increase of 0.48 (95% CI: 0.23 to 0.73) compared to baseline (Fig. 8). Changes in fasting glucose, HbA1c, and HOMA-IR at different follow-up times compared to baseline are shown in Supplementary Fig. S3 (38).
Figure 6.
Forest plot on the effect of 12 months of growth hormone treatment on fasting glucose levels (mmol/L) in nonrandomized (un)controlled studies. Abbreviations: GH, growth hormone; IV, inverse variance. Risk of bias legend: A, confounding; B, selection; C, intervention; D, intervention deviation; E, missing data; F, outcome; G, reported results.
Figure 7.
Forest plot on the effect of 12 months of growth hormone treatment on glycated hemoglobin A1c (mmol/mol) in nonrandomized (un)controlled studies. Abbreviations: GH, growth hormone; IV, inverse variance. Risk of bias legend: A, confounding; B, selection; C, intervention; D, intervention deviation; E, missing data; F, outcome; G, reported results.
Figure 8.
Forest plot on the effect of 12 months of growth hormone treatment on homeostatic model assessment of insulin resistance in nonrandomized (un)controlled studies. Abbreviations: GH, growth hormone; IV, inverse variance. Risk of bias legend: A, confounding; B, selection; C, intervention; D, intervention deviation; E, missing data; F, outcome; G, reported results.
Discussion
We performed a meta-analysis and systematic review of studies that investigated the effect of GHt in adults with PWS. Our results confirm that GHt during adulthood can improve body composition by increasing LBM and decreasing FM. We found that GHt is safe for adults with PWS.
Contrary to LBM and FM, BMI did not change during GHt. The most probable explanation is the increase in LBM along with a reduction of FM, which might result in similar weight before and after GHt and hence no change in BMI, despite the improved body composition. Another possible explanation for this is that most participants were already on caloric restriction prior to inclusion and remained on this restriction during the study period. Studies that evaluated the effects of cessation of GHt were not included in this systematic review and meta-analysis. These effects were studied by Koizumi et al (2018) (68) and Oto et al (2014) (69), who reported an increase in FM and BMI, respectively, after cessation of GHt for 6 months. Butler et al (2013) also showed an increase in FM and BMI and reduction of LBM after 12 months of GHt cessation (52). Only Höybye (2015) (58) compared GH-naive participants and participants who continued GHt started during infancy, childhood, or adolescence. They showed that after a mean duration of 15.4 years of GHt, the effects on LBM, FM, BMI, and LDL cholesterol were similar between the groups. For other studies, differences between GH-naive and GH nonnaive participants could not be evaluated separately. The potential effect of GHt might be smaller in individuals who were previously treated with GH. However, improvements in body composition were seen both in studies that included only GH-naive participants and in studies that included participants who were previously treated with GH. This suggests that both groups benefit from GHt.
Improvements in body composition would be expected to increase exercise capacity, with increased muscle strength and physical fitness. This was shown by Sode-Carlsen et al (2010 and 2011) (30, 51) and Lafortuna et al (2014) (35), who studied muscle volume and strength. They found that 24 months of GHt significantly improved muscle volume, quality, and strength. Furthermore, Gondoni et al (2008) (49) and Lafortuna et al (2014) (35), who measured exercise tolerance with a motorized treadmill stress test in partly the same population, showed that exercise tolerance also improved during GHt for 12 and 24 months, respectively. Butler et al (2013) used accelerometers to measure the intensity and duration of exercise per day (52). They found that the number and duration of moderate to vigorous bouts of exercise per day improved during 12 months of GHt. These studies indicate that GHt improves exercise capacity. However, only a small number of studies used standardized tests to measure muscle strength and exercise tolerance. Therefore, more research is needed to confirm the effects of GHt on exercise capacity.
LDL cholesterol did not change during GHt. With respect to cardiac function, Marzullo et al (2015) reported that 4 years of GHt increased the left ventricular mass with preservation of systolic and diastolic function (55). However, more research is needed and close monitoring of the cardiac function is indicated. Since reduction of cardiovascular risk due to improvements in body composition by GHt takes many years, long-term studies are needed to confirm the impact of GHt on cardiovascular risk. Manzardo et al (2019) reported that individuals with PWS who had been treated with GH had less often thromboembolisms than individuals who had never been treated with GH (60). This suggests that GHt might protect against the development of thromboembolisms. However, it must be noted that the GH-untreated group consisted of older participants than the GH-treated group. In addition, participants in the GH-untreated group less frequently received multidisciplinary care. Because both factors can influence the occurrence of thromboembolisms, the observed effect of GHt on the development of thromboembolisms might be spurious.
The results of studies assessing the effect of GHt on bone suggest that, regardless of the effect on BMD, there might be a positive effect on bone geometry, bone strength, and the maintenance of bone mass (44, 54, 63). However, it must be noted that most studies did not correct for sex hormone replacement. As sex hormones are known to play an important role in the development and maintenance of bone, this might have given spurious results (70). Additionally, in most studies participants were treated with GH for 12 or 24 months. Only Longhi et al (2015) had a longer follow-up time of mean ± SD 7.2 ± 2.5 years (54). Although they did not find any difference in BMD between GH-treated and GH-untreated individuals, we cannot rule out that a longer duration of GHt may improve BMD. Therefore, more long-term research is needed to elucidate the role of GHt on bone, with correction for sex hormone replacement.
The studies that assessed the effect of GHt on cognition and QoL were not conclusive. Some studies found improvements in cognition and QoL, whereas others did not (32, 34, 45, 52, 64). However, different instruments were used across the studies, which makes it hard to compare the results. Furthermore, most studies were small and short-term studies, which might result in less potential for improvements in cognition and QoL. A study by Dykens et al (2017) among children and young adults with PWS showed that IQ might improve if GHt is started early (age < 1 year), in a period of important brain development, and with longer duration of treatment (71). For QoL, a major drawback is that there are no validated instruments to quantify QoL in individuals with an intellectual disability, which makes it impossible to reliably assess the effect of GHt on QoL in adults with PWS. Another limitation is that questionnaires were repeatedly administered, which might give spurious results because participants and/or the proxy can remember the questionnaires, which can influence their answers, regardless of GHt.
GHt was safe both for GH-naive adults with PWS and for adults who were previously treated with GH. Few adverse events were reported. Our results revealed that GHt does not increase the risk of glucose metabolism deterioration. No significant increase in HbA1c or fasting glucose was found, not even after 3 years of GHt (57). HbA1c even remained within the reference range after 15 years of GHt (58). A small significant increase in HOMA-IR was seen during 12 months of GHt. However, HOMA-IR levels remained below the threshold for insulin resistance (2.77) (72) in all studies, except for that by Lafortuna et al (2014), who reported elevated HOMA-IR in 5 participants at baseline and 6 participants at 12 months of GHt (35). Marzullo et al (2015), who assessed the same study population, showed that HOMA-IR levels were, cumulatively, subordinated to BMI (ρ = 0.70, P < .0001) and FM% (ρ = 0.41, P < .05) (55). Furthermore, HOMA-IR levels did not change even during 3 years of GHt (57). Only 8 participants developed T2DM during GHt in the selected articles, of whom 3 had preexistent impaired glucose tolerance (51, 57, 58). Cross-sectional studies by Tsuchiya et al (2011) (66) and Fintini et al (2016) (67) corroborate these findings. Fintini et al (2016) showed that of 110 GH-treated participants (40 previously and 70 currently treated) only 13 (11.8%) developed altered glucose metabolism and just 2 T2DM (67). Furthermore, the prevalence of altered glucose metabolism was statistically higher in participants who never received GHt (39.6%) than in GH-treated (15%) participants. This was also seen for the prevalence of T2DM (41.2% in GH-untreated and 9.7% in GH-treated individuals), as showed by Tsuchiya et al (2011) (66). Based on these findings, we may speculate that impaired glucose metabolism and insulin resistance during GHt could be mainly due to weight gain rather than to GH administration. This was also corroborated by Höybye (2015), who showed that all participants who developed T2DM increased in weight because of nonadherence to lifestyle advice (58). The beneficial effect of GHt on maintaining a healthier body composition might counterbalance the potential effect of GH treatment on glucose homeostasis.
Several systematic reviews on GHt in adults with PWS have been published in recent years. The most recent systematic review by Frixou et al (2020) included an overview of the effects of GHt on body composition, cardiovascular end points, and bone (73). Similarly to our findings, they concluded that GHt improves body composition and is safe in adults with PWS. In addition to the systematic review by Frixou et al (2020), we also present data on cognition and QoL. There was no recent meta-analysis on GHt in adults with PWS. The most recent meta-analysis, showing positive effects on body composition and maintenance of BMI, was published in 2012 (31). Because important new data have been published since then, we felt it was important to conduct a new meta-analysis. Furthermore, we provide an extensive overview of the safety and reported adverse events.
However, some limitations remain. Only a small number of studies (9 RCTs, 20 NRCTs) assessed the effects of GHt in adults with PWS. Therefore, meta-analysis was not possible for the RCTs. Besides, few data were available for the effects of GHt on bone, cognition, QoL, and cardiovascular end points. Last, most of the included studies did not differentiate between individuals with proven GH deficiency and individuals without proven GH deficiency. Therefore, subgroup analyses were not possible.
In conclusion, the results of the present meta-analysis confirm that GHt improves body composition in adults with PWS, without safety concerns. Because poor body composition plays a key role in the high cardiovascular morbidity of adults with PWS, improving body composition might reduce cardiovascular complications in this vulnerable patient group.
Acknowledgments
The authors wish to thank Wichor Bramer and Maarten F. M. Engel from the Erasmus MC Medical Library for developing and updating the search strategy. The authors also wish to thank Anja J. Ruten-Budde from the Erasmus MC Biostatistics department for her statistical advice.
Author Contributions: A.R. and C.d.G.B.P. selected and reviewed the literature, extracted the data, performed the statistical analysis, and wrote the first draft of the manuscript. K.P. screened titles and abstracts for selection of the literature. W.M.B. provided expert knowledge on performing meta-analysis. L.d.G. was responsible for the clinical interpretation of the data. All authors were involved in data interpretation, revision of the manuscript, and final approval of the manuscript.
Glossary
Abbreviations
- BMD
bone mineral density
- BMI
body mass index
- FM
fat mass
- GH
growth hormone
- GHRH
growth hormone–releasing hormone
- GHt
growth hormone treatment
- HbA1c
glycated hemoglobin A1c
- HOMA-IR
homeostatic model assessment of insulin resistance
- ITT
insulin tolerance test
- LBM
lean body mass
- LDL
low-density lipoprotein
- NRCT
nonrandomized (un)controlled trial
- PWS
Prader-Willi syndrome
- QoL
quality of life
- RCT
randomized clinical trial
- T2DM
type 2 diabetes mellitus
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14(1):10-26. [DOI] [PubMed] [Google Scholar]
- 2. Lionti T, Reid SM, White SM, Rowell MM. A population-based profile of 160 Australians with Prader-Willi syndrome: trends in diagnosis, birth prevalence and birth characteristics. Am J Med Genet A. 2015;167A(2):371-378. [DOI] [PubMed] [Google Scholar]
- 3. Bar C, Diene G, Molinas C, Bieth E, Casper C, Tauber M. Early diagnosis and care is achieved but should be improved in infants with Prader-Willi syndrome. Orphanet J Rare Dis. 2017;12(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Swaab DF. Prader-Willi syndrome and the hypothalamus. Acta Paediatr Suppl. 1997;423:50-54. [DOI] [PubMed] [Google Scholar]
- 5. Holm VA, Cassidy SB, Butler MG, et al. Prader-Willi syndrome: consensus diagnostic criteria. Pediatrics. 1993;91(2):398-402. [PMC free article] [PubMed] [Google Scholar]
- 6. Whittington JE, Holland AJ, Webb T, Butler J, Clarke D, Boer H. Population prevalence and estimated birth incidence and mortality rate for people with Prader-Willi syndrome in one UK Health Region. J Med Genet. 2001;38(11):792-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butler MG, Manzardo AM, Heinemann J, Loker C, Loker J. Causes of death in Prader-Willi syndrome: Prader-Willi Syndrome Association (USA) 40-year mortality survey. Genet Med. 2017;19(6):635-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hosking FJ, Carey IM, Shah SM, et al. Mortality among adults with intellectual disability in England: comparisons with the general population. Am J Public Health. 2016;106(8):1483-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pacoricona Alfaro DL, Lemoine P, Ehlinger V, et al. Causes of death in Prader-Willi syndrome: lessons from 11 years’ experience of a national reference center. Orphanet J Rare Dis. 2019;14(1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pellikaan K, Rosenberg AGW, Kattentidt-Mouravieva AA, et al. Missed diagnoses and health problems in adults with Prader-Willi syndrome: recommendations for screening and treatment. J Clin Endocrinol Metab. 2020;105:e4671-e4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tauber M, Hoybye C. Endocrine disorders in Prader-Willi syndrome: a model to understand and treat hypothalamic dysfunction. Lancet Diabetes Endocrinol. 2021;9(4):235-246. [DOI] [PubMed] [Google Scholar]
- 12. Burnett LC, LeDuc CA, Sulsona CR, et al. Deficiency in prohormone convertase PC1 impairs prohormone processing in Prader-Willi syndrome. J Clin Invest. 2017;127(1):293-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donze SH, Damen L, van Alfen-van der Velden JAEM, et al. Prevalence of growth hormone (GH) deficiency in previously GH-treated young adults with Prader-Willi syndrome. Clin Endocrinol (Oxf). 2019;91(1):118-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grugni G, Marzullo P. Diagnosis and treatment of GH deficiency in Prader-Willi syndrome. Best Pract Res Clin Endocrinol Metab. 2016;30(6):785-794. [DOI] [PubMed] [Google Scholar]
- 15. Höybye C, Tauber M, Angulo MA, et al. ; Clinical & Scientific Advisory Board of The International Prader-Willi Syndrome Organisation . Letter regarding “Prevalence of growth hormone deficiency in previously GH-treated young adults with Prader-Willi syndrome” by Donze et al. Clin Endocrinol (Oxf). 2019;91(4):578-579. [DOI] [PubMed] [Google Scholar]
- 16. Glynn N, Agha A. Diagnosing growth hormone deficiency in adults. Int J Endocrinol. 2012;2012:972617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schilbach K, Olsson DS, Boguszewski MCS, Bidlingmaier M, Johannsson G, Jørgensen JL. Biomarkers of GH action in children and adults. Growth Horm IGF Res. 2018;40:1-8. [DOI] [PubMed] [Google Scholar]
- 18. Patel S, Harmer JA, Loughnan G, Skilton MR, Steinbeck K, Celermajer DS. Characteristics of cardiac and vascular structure and function in Prader-Willi syndrome. Clin Endocrinol (Oxf). 2007;66(6):771-777. [DOI] [PubMed] [Google Scholar]
- 19. Legrand R, Tobias JD. Anesthesia and Prader-Willi syndrome: preliminary experience with regional anesthesia. Paediatr Anaesth. 2006;16(7):712-722. [DOI] [PubMed] [Google Scholar]
- 20. Lindgren AC, Hagenäs L, Müller J, et al. Growth hormone treatment of children with Prader-Willi syndrome affects linear growth and body composition favourably. Acta Paediatr. 1998;87(1):28-31. [DOI] [PubMed] [Google Scholar]
- 21. Bakker NE, Kuppens RJ, Siemensma EP, et al. Eight years of growth hormone treatment in children with Prader-Willi syndrome: maintaining the positive effects. J Clin Endocrinol Metab. 2013;98(10):4013-4022. [DOI] [PubMed] [Google Scholar]
- 22. de Lind van Wijngaarden RF, Siemensma EP, Festen DA, et al. Efficacy and safety of long-term continuous growth hormone treatment in children with Prader-Willi syndrome. J Clin Endocrinol Metab. 2009;94(11):4205-4215. [DOI] [PubMed] [Google Scholar]
- 23. Carrel AL, Myers SE, Whitman BY, Allen DB. Growth hormone improves body composition, fat utilization, physical strength and agility, and growth in Prader-Willi syndrome: a controlled study. J Pediatr. 1999;134(2):215-221. [DOI] [PubMed] [Google Scholar]
- 24. Siemensma E, Tummers-de Lind VWR, Festen DAM, et al. Beneficial effects of growth hormone treatment on cognition in children with Prader-Willi syndrome: a randomized controlled trial and longitudinal study. Horm Res Paediatr 2012;97(7):2307-2314. [DOI] [PubMed] [Google Scholar]
- 25. de Lind van Wijngaarden RF, Cianflone K, Gao Y, Leunissen RW, Hokken-Koelega AC. Cardiovascular and metabolic risk profile and acylation-stimulating protein levels in children with Prader-Willi syndrome and effects of growth hormone treatment. J Clin Endocrinol Metab. 2010;95(4):1758-1766. [DOI] [PubMed] [Google Scholar]
- 26. de Lind van Wijngaarden RF, Festen DA, Otten BJ, et al. Bone mineral density and effects of growth hormone treatment in prepubertal children with Prader-Willi syndrome: a randomized controlled trial. J Clin Endocrinol Metab. 2009;94(10):3763-3771. [DOI] [PubMed] [Google Scholar]
- 27. Passone CGB, Franco RR, Ito SS, et al. Growth hormone treatment in Prader-Willi syndrome patients: systematic review and meta-analysis. BMJ Paediatr Open. 2020;4(1):e000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deal CL, Tony M, Höybye C, Allen DB, Tauber M, Christiansen JS; 2011 Growth Hormone in Prader-Willi Syndrome Clinical Care Guidelines Workshop Participants . Growth Hormone Research Society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab. 2013;98(6):E1072-E1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Donze SH, Hokken-Koelega ACS. Reply to commentary on ‘Prevalence of growth hormone (GH) deficiency in previously GH treated young adults with Prader-Willi syndrome’. Clin Endocrinol (Oxf). 2019;91(4):579-580. [DOI] [PubMed] [Google Scholar]
- 30. Sode-Carlsen R, Farholt S, Rabben KF, et al. One year of growth hormone treatment in adults with Prader-Willi syndrome improves body composition: results from a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2010;95(11):4943-4950. [DOI] [PubMed] [Google Scholar]
- 31. Sanchez-Ortiga R, Klibanski A, Tritos NA. Effects of recombinant human growth hormone therapy in adults with Prader-Willi syndrome: a meta-analysis. Clin Endocrinol (Oxf). 2012;77(1):86-93. [DOI] [PubMed] [Google Scholar]
- 32. Höybye C, Thorén M, Böhm B. Cognitive, emotional, physical and social effects of growth hormone treatment in adults with Prader-Willi syndrome. J Intellect Disabil Res. 2005;49(Pt 4):245-252. [DOI] [PubMed] [Google Scholar]
- 33. Kuppens RJ, Bakker NE, Siemensma EPC, Donze SH, Stijnen T, Hokken-Koelega ACS. Metabolic health profile in young adults with Prader-Willi syndrome: results of a 2-year randomized, placebo-controlled, crossover GH trial. Clin Endocrinol (Oxf). 2017;86(2):297-304. [DOI] [PubMed] [Google Scholar]
- 34. Bertella L, Mori I, Grugni G, et al. Quality of life and psychological well-being in GH-treated, adult PWS patients: a longitudinal study. J Intellect Disabil Res. 2007;51(Pt 4):302-311. [DOI] [PubMed] [Google Scholar]
- 35. Lafortuna CL, Minocci A, Capodaglio P, et al. Skeletal muscle characteristics and motor performance after 2-year growth hormone treatment in adults with prader-willi syndrome. J Clin Endocrinol Metab. 2014;99(5):1816-1824. [DOI] [PubMed] [Google Scholar]
- 36. Höybye C, Holland AJ, Driscoll DJ; Clinical and Scientific Advisory Board of The International Prader-Willi Syndrome Organisation . Time for a general approval of growth hormone treatment in adults with Prader-Willi syndrome. Orphanet J Rare Dis. 2021;16(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-34. [DOI] [PubMed] [Google Scholar]
- 38. Rosenberg AGW, Passone CdGB, Pellikaan K, et al. Supplementary data for Growth hormone treatment for adults with Prader-Willi syndrome—a meta-analysis. Uploaded April 14, 2021. http://hdl.handle.net/1765/135239. Accessed April 14, 2021.
- 39. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 40. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. The Cochrane Collaboration. Review Manger (RevMan) [Computer program], version 5.4. 2020. [Google Scholar]
- 42. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2017. https://www.R-project.org/. [Google Scholar]
- 43. Höybye C, Hilding A, Jacobsson H, Thorén M. Growth hormone treatment improves body composition in adults with Prader-Willi syndrome. Clin Endocrinol (Oxf). 2003;58(5):653-661. [DOI] [PubMed] [Google Scholar]
- 44. Jørgensen AP, Ueland T, Sode-Carlsen R, et al. Two years of growth hormone treatment in adults with Prader-Willi syndrome do not improve the low BMD. J Clin Endocrinol Metab. 2013;98(4):E753-E760. [DOI] [PubMed] [Google Scholar]
- 45. Kuppens RJ, Mahabier EF, Bakker NE, Siemensma EP, Donze SH, Hokken-Koelega AC. Effect of cessation of GH treatment on cognition during transition phase in Prader-Willi syndrome: results of a 2-year crossover GH trial. Orphanet J Rare Dis. 2016;11(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kuppens RJ, Bakker NE, Siemensma EP, et al. Beneficial effects of GH in young adults with Prader-Willi syndrome: a 2-year crossover trial. J Clin Endocrinol Metab. 2016;101(11):4110-4116. [DOI] [PubMed] [Google Scholar]
- 47. Höybye C. Five-years growth hormone (GH) treatment in adults with Prader-Willi syndrome. Acta Paediatr. 2007;96(3):410-413. [DOI] [PubMed] [Google Scholar]
- 48. Marzullo P, Marcassa C, Campini R, et al. Conditional cardiovascular response to growth hormone therapy in adult patients with Prader-Willi syndrome. J Clin Endocrinol Metab. 2007;92(4):1364-1371. [DOI] [PubMed] [Google Scholar]
- 49. Gondoni LA, Vismara L, Marzullo P, Vettor R, Liuzzi A, Grugni G. Growth hormone therapy improves exercise capacity in adult patients with Prader-Willi syndrome. J Endocrinol Invest. 2008;31(9):765-772. [DOI] [PubMed] [Google Scholar]
- 50. Mogul HR, Lee PD, Whitman BY, et al. Growth hormone treatment of adults with Prader-Willi syndrome and growth hormone deficiency improves lean body mass, fractional body fat, and serum triiodothyronine without glucose impairment: results from the United States multicenter trial. J Clin Endocrinol Metab. 2008;93(4):1238-1245. [DOI] [PubMed] [Google Scholar]
- 51. Sode-Carlsen R, Farholt S, Rabben KF, et al. Growth hormone treatment for two years is safe and effective in adults with Prader-Willi syndrome. Growth Horm IGF Res. 2011;21(4):185-190. [DOI] [PubMed] [Google Scholar]
- 52. Butler MG, Smith BK, Lee J, et al. Effects of growth hormone treatment in adults with Prader-Willi syndrome. Growth Horm IGF Res. 2013;23(3):81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jørgensen AP, Ueland T, Sode-Carlsen R, et al. Glucose homeostasis in adults with Prader-Willi syndrome during treatment with growth hormone: results from a 12-month prospective study. Growth Horm IGF Res. 2014;24(1):16-21. [DOI] [PubMed] [Google Scholar]
- 54. Longhi S, Grugni G, Gatti D, et al. Adults with Prader-Willi syndrome have weaker bones: effect of treatment with GH and sex steroids. Calcif Tissue Int. 2015;96(2):160-166. [DOI] [PubMed] [Google Scholar]
- 55. Marzullo P, Marcassa C, Minocci A, et al. Long-term echocardiographic and cardioscintigraphic effects of growth hormone treatment in adults with Prader-Willi syndrome. J Clin Endocrinol Metab. 2015;100(5):2106-2114. [DOI] [PubMed] [Google Scholar]
- 56. Damen L, Donze SH, Kuppens RJ, et al. Three years of growth hormone treatment in young adults with Prader-Willi syndrome: sustained positive effects on body composition. Orphanet J Rare Dis. 2020;15(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Damen L, Grootjen LN, Donze SH, et al. Three years of growth hormone treatment in young adults with Prader-Willi Syndrome previously treated with growth hormone in childhood: effects on glucose homeostasis and metabolic syndrome. Clin Endocrinol (Oxf). 2020;93(4):439-448. [DOI] [PubMed] [Google Scholar]
- 58. Höybye C. Growth hormone treatment of Prader-Willi syndrome has long-term, positive effects on body composition. Acta Paediatr. 2015;104(4):422-427. [DOI] [PubMed] [Google Scholar]
- 59. Marzullo P, Marcassa C, Campini R, et al. The impact of growth hormone/insulin-like growth factor-I axis and nocturnal breathing disorders on cardiovascular features of adult patients with Prader-Willi syndrome. J Clin Endocrinol Metab. 2005;90(10):5639-5646. [DOI] [PubMed] [Google Scholar]
- 60. Manzardo AM, Heinemann J, McManus B, Loker C, Loker J, Butler MG. Venous thromboembolism in Prader–Willi syndrome: a questionnaire survey. Genes (Basel). 2019;10(7):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Donze SH, Kuppens RJ, Bakker NE, van Alfen-van der Velden JAEM, Hokken-Koelega ACS. Bone mineral density in young adults with Prader-Willi syndrome: a randomized, placebo-controlled, crossover GH trial. Clin Endocrinol (Oxf). 2018;88(6):806-812. [DOI] [PubMed] [Google Scholar]
- 62. Khare M, Gold JA, Wencel M, et al. Effect of genetic subtypes and growth hormone treatment on bone mineral density in Prader-Willi syndrome. J Pediatr Endocrinol Metab. 2014;27(5-6):511-518. [DOI] [PubMed] [Google Scholar]
- 63. Brunetti G, Grugni G, Piacente L, et al. Analysis of circulating mediators of bone remodeling in Prader-Willi syndrome. Calcif Tissue Int. 2018;102(6):635-643. [DOI] [PubMed] [Google Scholar]
- 64. Butler MG, Matthews NA, Patel N, et al. Impact of genetic subtypes of Prader-Willi syndrome with growth hormone therapy on intelligence and body mass index. Am J Med Genet Part A. 2019;179(9):1826-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Donze SH, de Weerd AW, van den Bossche RAS, Joosten KFM, Hokken-Koelega ACS. Sleep-related breathing disorders in young adults with Prader-Willi syndrome: a placebo-controlled, cross-over GH trial. J Clin Endocrinol Metab. 2019;104(9):3931-3938. [DOI] [PubMed] [Google Scholar]
- 66. Tsuchiya T, Oto Y, Ayabe T, Obata K, Murakami N, Nagai T. Characterization of diabetes mellitus in Japanese Prader-Willi syndrome. Clin Pediatr Endocrinol. 2011;20(2):33-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fintini D, Grugni G, Bocchini S, et al. ; Genetic Obesity Study Group of the Italian Society of Pediatric Endocrinology and Diabetology (ISPED) . Disorders of glucose metabolism in Prader-Willi syndrome: results of a multicenter Italian cohort study. Nutr Metab Cardiovasc Dis. 2016;26(9):842-847. [DOI] [PubMed] [Google Scholar]
- 68. Koizumi M, Ida S, Shoji Y, Nishimoto Y, Etani Y, Kawai M. Visceral adipose tissue increases shortly after the cessation of GH therapy in adults with Prader-Willi syndrome. Endocr J. 2018;65(11):1127-1137. [DOI] [PubMed] [Google Scholar]
- 69. Oto Y, Tanaka Y, Abe Y, et al. Exacerbation of BMI after cessation of growth hormone therapy in patients with Prader-Willi syndrome. Am J Med Genet A. 2014;164A(3):671-675. [DOI] [PubMed] [Google Scholar]
- 70. Compston JE. Sex steroids and bone. Physiol Rev. 2001;81(1):419-447. [DOI] [PubMed] [Google Scholar]
- 71. Dykens EM, Roof E, Hunt-Hawkins H. Cognitive and adaptive advantages of growth hormone treatment in children with Prader-Willi syndrome. J Child Psychol Psychiatry. 2017;58(1):64-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders: the Bruneck Study. Diabetes. 1998;47(10):1643-1649. [DOI] [PubMed] [Google Scholar]
- 73. Frixou M, Vlek D, Lucas-Herald AK, Keir L, Kyriakou A, Shaikh MG. The use of growth hormone therapy in adults with Prader-Willi syndrome: a systematic review. Clin Endocrinol (Oxf). 2021;94(4):645-655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.