Abstract
Context
Abnormalities in the hypothalamic-pituitary-adrenal (HPA) axis are frequent accompaniments of depression, and studies have documented the role of stress and stressful life events in the ontogeny of perimenopausal depressions (PMD). Because HPA axis function in women is further modulated both by aging and ovarian steroids, it is possible that a dysregulated HPA axis contributes to the increased risk of PMD.
Objective
We examined HPA axis function in perimenopausal women with and without depression using the combined dexamethasone–corticotropin-releasing hormone (Dex/CRH) test.
Methods
Dex/CRH tests were performed on 20 women with PMD and 20 women who were also perimenopausal but without current or past depression (control women). Main outcome measures were plasma levels of cortisol and adrenocorticotropin (ACTH) and 24-hour urinary free cortisol (UFC). Five women took chronic stable medications, otherwise all women were medically healthy, and both groups were comparable with respect to reproductive stage and age. Standardized symptom rating scales were administered to each woman prior to Dex/CRH testing.
Results
No group differences were present in either baseline or stimulated ACTH and cortisol secretion. Baseline plasma measures of estradiol, progesterone, and 24-hour UFC levels similarly did not differ in PMD and control women.
Conclusion
Despite reports of increased stress responsiveness in PMD, we observed no abnormalities of HPA axis activity associated with PMD compared with women without depression. These findings suggest that PMD is not uniformly associated with HPA dysregulation and could reflect underlying pathophysiologic processes that are distinct from women with nonreproductive-related depressions.
Keywords: cortisol, ACTH, perimenopause, depression, Dex/CRH test
Perimenopause is accompanied by an increased risk both for first-onset and recurrent depressions (1-5); however, the relevant biological substrates of risk for perimenopausal depression (PMD) remain to be characterized. Studies have demonstrated the role of estradiol in PMD: Estradiol therapy improves mood in PMD (6-9), and acute withdrawal of estradiol triggers a recurrence of depressive symptoms in women with but not those without PMD (10). However, the majority of women do not experience PMD, despite exposure to similar declines in estradiol secretion during reproductive aging (ie, during perimenopause), and, therefore, additional substrates of risk likely contribute to the susceptibility to experience PMD. Recently, dysregulation and sensitization of stress physiology (eg, glucocorticoid signaling pathway, catecholamine activity, and peripheral inflammatory pathways) have been suggested to be potential substrates of risk for PMD (11). Indeed, several factors could combine during perimenopause to dysregulate hypothalamic-pituitary-adrenal (HPA) axis and stress responsivity and increase the risk for depression. First, a role of antecedent stress and stressful life events has been well documented in the ontogeny of PMD (2, 9, 12-14). Furthermore, chronic and recurrent stress both can alter glucocorticoid receptor sensitivity and sensitize both peripheral and central tissue responsivity to stress (15-17) (as also reported in several other forms of affective illness unrelated to perimenopause). Second, changes in estradiol exposure similar to those occurring during perimenopause have been demonstrated to modify stress responsivity both in animal (18-22) and human studies (23). In women, for example, estradiol therapy attenuates HPA axis and catecholamine responsivity to psychological stress in perimenopausal women (23). Estradiol can act alone as well as synergistically with glucocorticoid signaling (17) to modify the impact of stress on several brain regions. Third, in women, both reproductive and chronologic aging interact to alter the HPA axis response to a variety of endocrine and stressful challenges, with older, postmenopausal women showing increased HPA axis responsivity (24-27). (As a caveat, these effects of aging can be modulated by environmental factors including exercise, medical comorbidity, and exposures to ovarian steroids) (28-34). Third, a recent study (albeit in male rodents) demonstrated the plausibility of prior exposure to stress (ie, social subordinate housing) in the programming of a differential behavioral response to changing levels of ovarian steroids, as previously demonstrated in PMD (10, 35). Thus, previous exposures to stress combined with the effects of aging and a changing pattern of estradiol secretion could dysregulate HPA and stress axis function to increase a perimenopausal woman’s susceptibility to develop depression.
Abnormalities of stress responsivity and HPA axis function are consistent accompaniments of depression (36-38) and also could serve to increase the risk for depression. During reproductive aging, declining secretion of ovarian estradiol and progesterone (along with the progesterone-derived neurosteroid metabolite allopregnanolone) and/or distressing vasomotor symptoms (and the accompanying sleep disturbances and heightened arousal) also could alter HPA axis function sufficiently to increase the risk for depression in some perimenopausal women (11, 39). Thus, HPA axis function in PMD needs to be compared with nondepressed women of comparable age and reproductive status to control for the impact of these factors on HPA axis function. However, to date, HPA axis function in women with well-documented PMD compared with controls has not been comprehensively examined.
In this study, we evaluated HPA axis function in well-characterized women with current episodes of PMD and asymptomatic perimenopausal controls by measuring 24-hour urinary free cortisol (UFC) and performing dexamethasone (Dex)-suppressed corticotropin-releasing hormone (CRH) stimulation (Dex/CRH) tests.
Materials and Methods
Participant Selection
Perimenopausal women between ages 40 and 60 years who reported the onset of depression during perimenopause, and who presented to the National Institute of Mental Health midlife clinic between 2007 and 2013, participated in this study. The study was conducted at an outpatient clinic within the National Institutes of Health (NIH) Clinical Center. Women reported the onset of depressive symptoms and accompanying personal distress or occupational impairment in the context of menstrual irregularity of at least 6 months but not more than 1 year of amenorrhea. All women were either self-referred in response to local advertisements or referred by their physicians. All women with PMD entering this study met the following criteria: 1) a current episode of minor (meeting 3-4 criteria symptoms) or major depression (of moderate severity or less on the Global Assessment of Functioning [GAF] scale [40] confirmed by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders-IV [SCID]) (40); 2) elevated plasma follicle-stimulating hormone (FSH) level greater than 14 IU/L on 3 of 4 visits obtained during a screening phase (plasma FSH levels of 14 IU/L represent 2 SD above the mean values for the early-follicular phase FSH levels in women of reproductive age, consistent with the 2001 Stages of Reproductive Aging Workshop [STRAW] criteria) (41); and 3) scores greater than 10 on one of the following scales: Center for Epidemiologic Studies Depression Scale (CESD) (42) or the Beck Depression Inventory (BDI) (43) consistently during the 2-month screening phase. Women were excluded if they had depression severity greater than moderate on the GAF scale, a medical illness (current or recent medical illness confirmed by medical history and physical examination in addition to laboratory tests), as were (with the exception of long-term stable doses of thyroid replacement) women receiving menopausal hormonal therapy or psychotropic medications.
Diagnostic Evaluation
Prior to study entry, all women participated in a 6- to 8-week screening phase consisting of 4 clinic visits at 2-week intervals during which mood ratings were completed and blood samples were obtained to measure plasma levels of FSH. Additionally, all women received history and physical examinations, electrocardiogram, urinalysis, and blood laboratory assessments. During the screening phase, all participants completed the Midlife Daily Rating Form (DRF) (44), a 6-point, Likert-type scale modified to measure night time and daytime hot flashes, completed nightly to reflect a composite rating for the previous 24 hours; DRF scores range from 1 (symptoms not present) to 6 (symptoms present in the extreme). Additionally, the CESD and BDI were administered at each clinic visit.
Nondepressed perimenopausal women between ages 40 and 60 years were recruited to participate as a control group in this study. These control women participated in a screening procedure similar to the women with PMD. All women met the clinical and endocrine research criteria for perimenopause (as described earlier) in the absence of any significant current episodes of depression as determined by SCID interview (40). Additionally, all control women were subject to the same exclusion criteria as the women with PMD. On completion of this study, participants received compensation for their time as determined by the NIH Office of Healthy Volunteers.
Women meeting study criteria for PMD as well as nondepressed perimenopausal control women were further classified into subgroups based on the presence or absence of hot flashes (see subsequent sections).
This protocol was approved by the Central Neuroscience Intramural Research Board, and written informed consent was obtained from each woman prior to entering the study.
Study Design and Procedures
The combined Dex/CRH stimulation (45) test was performed during the early follicular phase (days 3-5) in women who were menstruating or randomly in those who were amenorrheic. All women were instructed to eat a light breakfast and then fast until after the procedure. Participants took oral dexamethasone, 1.5 mg, at 2300 hours before the scheduled procedure. On the procedure day, the women drank no caffeine, ate a light breakfast, and arrived fasting at 1330. Women were resting, supine, and awake during the procedure. Ovine CRH (100 µg/2 mL saline) was administered 60 minutes after intravenous line placement, with blood drawn at baseline (–15 and 0 minutes) prior to, and in intervals 15, 30, 45, 60, and 75 minutes after CRH administration. Urine collections for UFC were completed 48 hours prior to the Dex administration and brought to the clinic the day of testing.
Outcome Measures
The primary outcome measures were the plasma levels of adrenocorticotropin (ACTH) and cortisol before and after CRH stimulation and UFC levels. Additionally, we measured plasma levels of estradiol, progesterone, Dex, and cortisol-binding globulin (CBG) at baseline prior to CRH administration to characterize baseline measures that could contribute to differences in HPA axis response. The presence and absence of perimenopause-related depressive symptoms were monitored using the CESD (42) and BDI (43) to each woman prior to testing. The presence and severity of hot flashes were measured with a daily hot-flash rating scale (44) that was a modification of the DRF, a 21-item, self-report scale. The DRF rates the severity of symptoms on a 6-point scale (1 = no symptoms and 6 = extreme symptoms) and was used to confirm the presence of clinically significant hot flashes. Those women who had a weekly average hot flash symptom severity score of 2 or greater (minimal) during screening were classified as having clinically significant hot flashes. Additionally, each woman completed once, during the baseline evaluation, the GAF scale (40) (to assess functional disability) and the Childhood Trauma Questionnaire (CTQ) (46, 47) (to assess the presence or absence of early life trauma, which is reported to affect HPA activity) (29, 31).
Assay Methodology
Plasma hormone levels of estradiol, progesterone, ACTH, and cortisol were assayed by enzyme-linked immunosorbent assay (ALPCO Diagnostics kit, Core Laboratory, Johns Hopkins). Additionally, plasma was assayed for CBG (ALPCO Diagnostics kit, Core Laboratory, Johns Hopkins) and dexamethasone (high-performance liquid chromatography–tandem mass spectrometry [LC-MS/MS]; Esoterix). UFC was analyzed by turbulent flow and detection by LC-MS/MS (Department of Laboratory Medicine, NIH). Plasma levels of FSH were determined by chemiluminescent immunometric assay on Siemens Immulite 2000 XPi analyzer (Department of Laboratory Medicine, NIH).
Statistical Analysis
In the primary analysis of CRH-stimulated cortisol and ACTH, we obtained 6 repeated measures (t = 0 to t = 5: ie, 0 [average of –15 and –5 minutes before CRH], +15, +30, +45, +60, and +75 minutes, respectively) in each woman (PMD and control women). Linear mixed models were used to analyze the repeated measures data. To meet the model normality assumption, plasma levels of both cortisol and ACTH were transformed by square roots and natural log transformations, respectively. The unstructured variance-covariance matrix was used to account for the correlations between measures over time within the same participant. All models included the following fixed effects: 1) diagnostic group (PMD, control women); and 2) time (0-5); and the interaction of the two. Given the nonsignificant differences in plasma CBG levels, we repeated the same analysis using calculated free cortisol levels using the method described by Coolens et al (48). Additionally, areas under the curves (AUCs) both for ACTH and cortisol were determined by a baseline-corrected trapezoidal integration method and compared by Wilcoxon ranked sum tests because of the violation of the normality assumptions. UFC levels were analyzed in a similar manner. Although this study was not powered to adequately examine interactions, covariates were selected based on previous literature documenting the potential impact of these clinical variables on Dex/CRH stimulation response and 24-hour UFC levels and examined for possible interactions with diagnostic group. These variables were age, STRAW stage, duration of menopause transition, and body mass index; measures of the course and severity of PMD: depression subtype (major or minor depression), presence of a past major depressive episode (MDE), BDI, and CESD scores; history of early life trauma: CTQ scores; hot flashes (present or absent); and baseline biochemistry and hormone levels: plasma levels of FSH, estradiol, CBG, Dex, and UFC levels. The nonparametric alternative to the parametric analysis of covariance was applied in R (Package ARTool). In case of a significant interaction, we adjusted the P values as follows: individual time points (ie, average baseline [ie, average of t = –15 and 0) and t = +15 to t = +75): A total of 24 pairwise comparisons were performed (4 pairwise comparisons in 6 separate time points for each of the cortisol and ACTH values), and therefore, those comparisons with P greater than .002 (=0.05/24) were not considered statistically significant; AUC values: we employed a criterion P value of less than .0125 (ie, 0.05/4 pairwise comparisons) to suggest statistical significance.
Potential differences between women with PMD and controls in baseline clinical characteristics were compared by Wilcoxon ranked sum tests (continuous variables) or Fisher exact test (categorical variables) with SAS version 9.2 software (SAS Institute Inc).
Results
Participant Characteristics
Twenty women with PMD and 20 control women completed the Dex/CRH procedure (Table 1). Nineteen women with PMD met the SCID criteria for a current major depression and 1 met the criteria for a minor depression. PMD and control women were similar in most baseline demographic and clinical characteristics, including plasma measures of estradiol, progesterone, and FSH. As expected, women with PMD had higher symptom severity scores (CESD, BDI, CTQ) and greater disability ratings on the GAF; 12 women with PMD reported a past major depression compared with none of controls. Fourteen women with PMD and 7 control women reported hot flashes. All were medication free with the exception of 5 women: Three were on thyroid supplementation (PMD [n = 2], control women [n = 1]), 2 women with PMD were on antilipid medications (one of whom was also on thyroid supplementation), and 1 control was on a diuretic (hydrochlorothiazide) for high blood pressure. These women had all been on stable doses of their nonstudy medications for greater than 2 months, and all women had normal laboratory values and physical exam results.
Table 1.
Baseline clinical characteristics in women with perimenopausal depression (n = 20) and control women (n = 20)
| Asymptomatic controls (n = 20) | Perimenopausal depression (n = 20) | ||||
|---|---|---|---|---|---|
| Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | P | |
| Age, y | 50.5 (50.0-52.2) | 51.3 (2.3) | 50.5 (47.7-53.0) | 50.4 (3.4) | .34 |
| BMI | 24.5 (22.4-27.6) | 25.8 (5.7) | 25.6 (23.8-29.2) | 26.4 (4.3) | .34 |
| Duration of MT, mo | 24.0 (12.0-35.2) | 23.5 (11.5) | 24.0 (12.0-36.0) | 24.8 (12.4) | .74 |
| GAF | 90.0 (90.0-95.0) | 91.5 (3.1) | 61 (60.0-65.0) | 62.4 (5.7) | .001 |
| BDI | 0.0 (0.0-2.0) | 1.2 (1.9) | 17.5 (12.7-20.0) | 18.3 (9.0) | .001 |
| CESD | 0.0 (0.0-2.0) | 1.2 (1.8) | 25.0 (15.0-36.0) | 26.2 (11.8) | .001 |
| CTQ | 29.5 (28.0-32.7) | 31.4 (6.4) | 44.0 (35.0-51.0) | 45.5 (18.7) | .01 |
| Plasma FSH, IU/L | 48.5 (40.2-73.9) | 54.9 (27.7) | 52.6 (28.9-73.2) | 51.2 (24.7) | .86 |
| Plasma estradiol, pg/mL | 41.8 (9.0-84.7) | 56.0 (57.0) | 45.2 (28.9-85.8) | 68.5 (61.4) | .40 |
| Plasma progesterone, ng/mL | 0.2 (0.1-0.4) | 0.3 (0.2) | 0.2 (0.2-0.3) | 0.3 (0.1) | .72 |
| Asymptomatic controls (n = 20) | Perimenopausal depression (n = 20) | ||||
| No. (%) | No. (%) | P | |||
| STRAW stage | .39 | ||||
| –2 | 1 (5) | 1 (5) | |||
| –1 | 18 (90) | 17 (85) | |||
| 1 | 1 (5) | 2 (10) | |||
| Hot flashes, present | 7 (38.9) | 14 (73.7) | .07 | ||
| Race | .48 | ||||
| Black | 5 (25) | 3 (18.8) | |||
| Asian | 2 (10) | 0 | |||
| White | 12 (60) | 11 (68.8) | |||
| Hispanic | 1 (5) | 2 (10) | |||
| Thyroid Tx | 1 (5) | 2 (10) | .99 | ||
| Past MDE | 0 | 12 (60) | .001 |
Wilcoxon rank sum test was used for the continuous variables; Fisher exact test was used to compare the categorical variables between groups.
Perimenopausal depression (PMD) and control women were similar in most baseline demographic and clinical characteristics with the exceptions that women with PMD had higher symptom severity scores and greater disability ratings on the GAF. All were medication free with the exception of 5 women: 3 on thyroid supplementation (PMD [n = 2], control women [n = 1]), 2 women with PMD were on antilipid medications (one of whom was also on thyroid supplementation), and 1 control was on a diuretic (hydrochlorothiazide) for high blood pressure.
Abbreviations: BDI, Beck Depression Inventory; BMI, body mass index; CESD, Center for Epidemiologic Studies Depression Scale; CTQ, Childhood Trauma Questionnaire; FSH, follicle-stimulating hormone; GAF, Global Assessment of Functioning; IQR, interquartile range; MDE, major depressive episode; MT duration, duration of menopause transition (self-report); STRAW, Stages of Reproductive Aging Workshop; Tx, treatment.
Main Effects of Diagnosis on Hypothalamic-Pituitary-Adrenal Axis Function
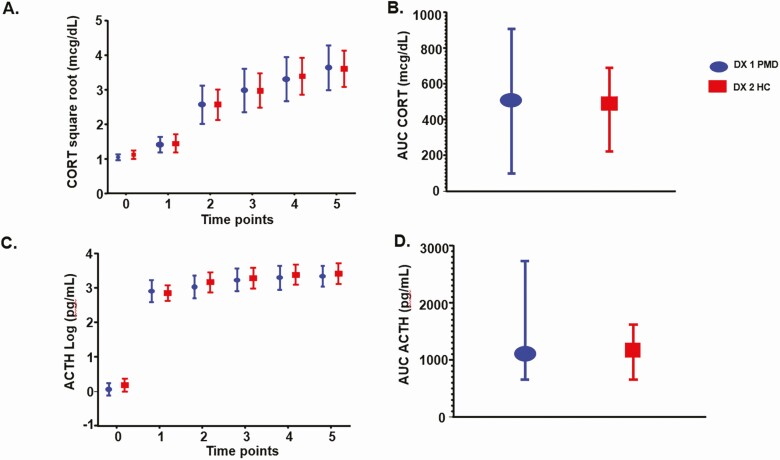
We observed no difference between women with PMD and control women in any measure of HPA axis function including the following: 1) individual time points in cortisol (effect of diagnosis: P = .94, diagnosis by time: P = .82) and ACTH (effect of diagnosis: P = .72, diagnosis by time: P = .05); 2) AUCs of cortisol (P = .94) and ACTH (P = .87) after CRH administration; and 3) baseline measures of 24-hour UFCs (P = .96), CBG (P = .06), and plasma Dex levels (P = .76). Finally, a repeat analysis employing free cortisol levels showed similar nonsignificant differences across diagnoses in the free cortisol response to Dex/CRH testing (Table 2, Fig. 1).
Table 2.
Hypothalamic-pituitary-adrenal axis measurements in women with perimenopausal depression (n = 20) and control women (n = 20)
| Asymptomatic controls (n = 20) | Perimenopausal depression (n = 20) | ||||
|---|---|---|---|---|---|
| Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | P | |
| Cortisol, mcg/dL | |||||
| –15 min | 1.1 (0.9-1.5) | 1.3 (0.8) | 1.1 (0.9-1.5) | 1.1 (0.4) | .63 |
| –5 min | 1.0 (1.0-1.6) | 1.3 (0.8) | 1.1 (0.9-1.5) | 1.1 (0.4) | .72 |
| Baseline AVG | 1.1 (0.9-1.6) | 1.3 (0.8) | 1.1 (0.9-1.5) | 1.1 (0.4) | .59 |
| 15 min | 1.9 (1.1-2.3) | 2.4 (2.4) | 1.7 (1.0-3.6) | 2.3 (1.6) | .96 |
| 30 min | 7.3 (3.7-9.3) | 7.5 (5.4) | 7.2 (1.4-15.3) | 8.1 (6.6) | .98 |
| 45 min | 10.1 (3.8-12.8) | 10.0 (7.0) | 9.5 (2.0-16.7) | 10.8 (8.8) | .91 |
| 60 min | 13.2 (7.0-17.2) | 12.9 (8.5) | 11.9 (3.1-20.3) | 12.9 (9.9) | .83 |
| 75 min | 14.1 (7.9-18.5) | 14.4 (8.5) | 14.7 (4.6-24.0) | 15.3 (10.6) | .90 |
| AUC | 503.5 (219.8-686.2) | 510.6 (366.2) | 489.9 (95.9-907.7) | 549.4 (466.0) | .94 |
| Delta max | 13.5 (7.7-17.2) | 13.3 (8.4) | 13.2 (3.9-23.5) | 14.5 (11.1) | .94 |
| ACTH, pg/mL | |||||
| –15 min | 6.9 (4.7-7.8) | 7.1 (2.9) | 5.7 (5.0-7.2) | 6.4 (2.2) | .50 |
| –5 min | 6.4 (5.3-8.1) | 6.6 (2.0) | 5.7 (5.0-6.2) | 5.9 (1.2) | .27 |
| Baseline avg | 6.4 (5.4-7.6) | 6.8 (2.2) | 5.9 (5.2-7.5) | 6.1 (1.4) | .41 |
| 15 min | 16.6 (12.2-22.7) | 20.0 (11.6) | 18.8 (10.4-29.8) | 23.7 (20.3) | .87 |
| 30 min | 22.1 (16.0-26.7) | 30.2 (24.9) | 19.5 (12.4-42.4) | 26.7 (18.6) | .45 |
| 45 min | 24.7 (16.9-32.9) | 34.9 (31.0) | 21.4 (15.5-52.0) | 33.0 (24.7) | .73 |
| 60 min | 27.8 (19.4-39.0) | 37.4 (33.5) | 23.9 (19.2-50.5) | 35.3 (26.1) | .68 |
| 75 min | 28.7 (22.7-40.2) | 38.4 (28.7) | 26.5 (19.3-45.5) | 34.8 (23.4) | .58 |
| AUC | 1106.3 (643.4-1612.3) | 1657.0 (1555.4) | 1039.7 (652.7-2726.0) | 1627.4 (1339.7) | .87 |
| Delta max | 23.9 (16.9-30.5) | 35.5 (33.5) | 20.4 (14.4-51.5) | 34.0 (27.9) | .65 |
| Dexamethasone, mcg/dL | 108.0 (85.2-218.0) | 155.8 (97.2) | 129.5 (106.7-183.7) | 152.9 (87.2) | .76 |
| CBG, mcg/mL | 51.2 (48.4-60.5) | 55.6 (9.8) | 64.2 (54.2-70.2) | 62.8 (11.8) | .06 |
| UFC, mcg/24 h | 12.4 (10.6-17.5) | 16.0 (10.2) | 14.0 (9.7-19.4) | 15.5 (8.0) | .96 |
| Urine creatinine, mg/d | 58.0 (40.5-73.0) | 68.3 (49.2) | 66.0 (43.5-91.5) | 69.7 (31.4) | .47 |
Values of cortisol and ACTH listed in table are nontransformed. Wilcoxon rank sum test was used for the continuous variables.
No significant differences were observed between women with perimenopausal depression (PMD) and control women in any measure of hypothalamic-pituitary-adrenal axis function including individual time points in cortisol and ACTH, AUCs, and delta max of cortisol and ACTH after corticotropin-releasing hormone administration, as well as baseline measures of 24-hour UFCs, CBG, and plasma dexamethasone levels.
Estradiol (conversion to SI pmol/L—3.671); progesterone (conversion to SI nmol/liter 3.18); ACTH (conversion to SI pmol/L—0.22); cortisol (conversion to SI nmol/L—27.59), UFC (conversion to SI nmol/d—2.76), CBG (conversion to SI nmol/L—17.18).
Abbreviations: ACTH, adrenocorticotropin; AUC, area under the curve; avg, average; CBG, cortisol-binding globulin; Delta max, difference between maximum stimulated and baseline values; IQR, interquartile range; UFC, 24-hour urinary free cortisol.
Figure 1.
A and B, effects of diagnosis on stimulated cortisol (CORT); and C and D, adrenocorticotropin (ACTH) during dexamethasone (DEX)-suppressed corticotropin-releasing hormone (CRH)-stimulation tests in women with perimenopausal depression (n = 20) and control women (n = 20). No significant differences were observed between women with perimenopausal depression (PMD) and control women in the individual time points in cortisol (effect of diagnosis: P = .94, effect of time: P < .001, diagnosis by time interaction: P = .82) and ACTH (effect of diagnosis: P = .72, effect of time; P < .001, diagnosis by time interaction: P = .05) after CRH administration (A and C, respectively). Similarly, areas under the curve (AUCs) of cortisol (P = .94) and ACTH (P = .87) after CRH administration were not significantly different (B and D, respectively). Values for cortisol are square root–transformed and those for ACTH are log-transformed. Values in A and B are least squares means ± 95% CI; values in C and D are median and interquartile range. For the repeated measures (baseline average to 75 minutes), linear mixed models were used on the square root–transformed CORT and log-transformed ACTH; the P values were obtained for each time point in the model. For all other variables, the Wilcoxon rank sum test was used.
Interaction Effects of Clinical Characteristics on Hypothalamic-Pituitary-Adrenal Axis Function
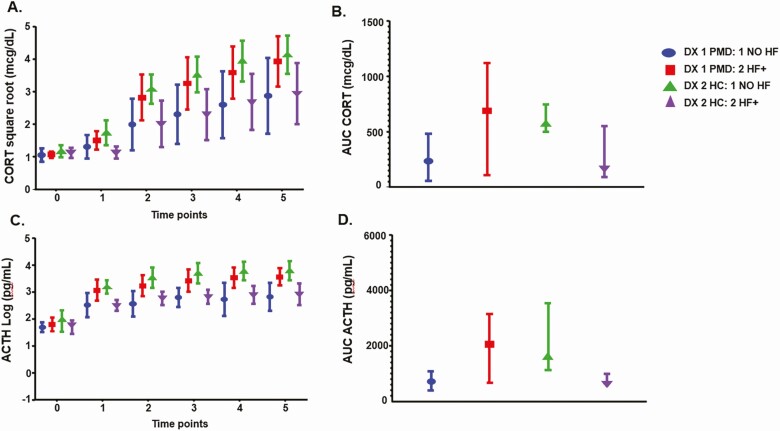
No interaction effects between diagnosis and any of the clinical characteristics (listed in Table 1) were observed in either the cortisol and ACTH response to CRH or 24-hour UFC values. The presence of hot flashes did not significantly affect any differences between women with PMD and controls in either the 24-hour UFC levels or the patterns of secretion of cortisol or ACTH after CRH administration (see legend to Fig. 2). There was an interaction between diagnosis (PMD vs control women) and the presence of hot flashes in the individual time points and in the AUC ACTH after CRH administration that in control women only reflected a decreased ACTH response to CRH administration in control women with hot flashes compared to those without (see legend to Fig. 2B and 2D for P values).
Figure 2.
A and B, the interactive effects of hot flushes on stimulated cortisol (CORT); and C and D, adrenocorticotropin (ACTH) during dexamethasone (DEX)-suppressed corticotropin-releasing hormone (CRH)-stimulation tests in women with perimenopausal depression (n = 20) and control women (n = 20). There was a significant interaction between diagnosis (perimenopausal depression [PMD] vs control women) and the presence or absence of hot flashes (HF) in the individual time points after CRH administration both for plasma CORT and ACTH levels (diagnosis by HF interaction: cortisol [P = .008], ACTH [P = .0002]; diagnosis by HF by time interaction: CORT [P = .08], ACTH [P = .03]), as well as the areas under the curve (AUCs; diagnosis by HF interaction: CORT [P = .02], ACTH [P = 0.001]). There also were no significant main or interactive effects of HFs and diagnosis in urinary free CORT (UFC) levels (main effect of HF: P = .82; HF by Dx interaction: P = .83). This study was not powered to evaluate diagnosis by HF interactions, and 14 of the 20 women with PMD, but only 7 of 20 control women reported HFs. A total of 24 post hoc pairwise comparisons were performed (4 pairwise comparisons in 6 separate time points for each of the CORT and ACTH values). Thus, those comparisons with P greater than .002 (=0.05/24) should not be considered significant. In women with PMD, the presence of HF did not alter the CORT response to CRH, but was accompanied by an increased ACTH response to CRH, although none of the post hoc P values met the threshold (P < .002) to be considered significant (t = 15, 30, 45, 60, and 75 minutes: CORT—P values = .39, .12, .12, .13, and .13; ACTH—P values = .07, .03, .03, .34, and .02). Second, in control women, in contrast to the women with PMD, the presence of HFs was accompanied by a decreased CORT and ACTH response to CRH administration (t = 15, 30, 45, 60, and 75 minutes: CORT—P values = .006, .02, .01, .02, and .04; ACTH—P values = .0001, .002, .0005, .0007, and .003) with the qualifier that none of the CORT values met significance at P less than .002. Comparing across diagnoses within each HF group, in those women with HFs, women with PMD had increased CORT and ACTH compared with control women—although none of the P values met the criterion for significance of P less than .002 (t = 15, 30, 45, 60, and 75 minutes: CORT—P values = .03, .11, .09, .13, and .11; ACTH—P values = .01, .05, .09, .02, and .02). In those women without HFs, women with PMD had decreased CORT and ACTH compared with control women—although none of the P values met the criterion for significance of P less than .002 (t = 15, 30, 45, 60, and 75 minutes: CORT— P values = .10, .02, .03, .03, and .06; ACTH—P values = .01, .003, .001, .005, and 0.004). Post hoc testing of the areas under the curve (AUCs) CORT and ACTH showed a similar pattern of effects. A total of 4 pairwise comparisons were performed for each of the CORT and ACTH values, and, therefore, those comparisons with P greater than .0125 (=0.05/4) should not be considered significant. Women with PMD did not have significantly different AUCs of cortisol or ACTH (P = .13 and .16, respectively) regardless of the presence of absence of HFs. Control women with HFs had decreased AUCs of CORT and ACTH compared to those without HFs (P = .03 and .02, respectively), although none of the P values met the criterion for significance of P less than .0125. Additionally, in the women with no HFs, women with PMD had decreased AUC ACTH compared with control women (P = .03), again not meeting the criterion of a P equal to .0125 level of significance. Otherwise there were no significant effects of HFs in control women (HF present—PMD vs control women: CORT AUC P = .15, ACTH AUC P = .07; no HFs—PMD vs control women: CORT AUC P = .12). Values for CORT are square root–transformed and those for ACTH are log-transformed. Values in A and B are least squares means ± 95% CI, values in C and D are median and interquartile range. For the repeated measures (baseline average to 75 minutes), linear mixed models were used on the square root–transformed CORT and log-transformed ACTH; the P values were obtained for each time point in the model. For all other variables, the Wilcoxon rank sum test was used.
Discussion
Stress and stressful life events are frequent antecedents of PMD, and dysregulation of the HPA axis, in addition to being a common accompaniment of depression, could also serve as an underlying substrate of vulnerability for PMD (45, 49-51) through interactions with sex steroid signaling. Stress responsivity in perimenopausal women can also be affected both by chronologic and reproductive aging. No study to date has employed standardized measures of HPA axis function in women with well-characterized depression during perimenopause and asymptomatic, perimenopausal control women.
We employed 2 measures of HPA axis function, both widely used in psychiatry and endocrinology, and failed to identify differences between women with PMD and control women in either their cortisol and ACTH responses to Dex/CRH challenge or their 24-hour UFC secretion. All women met strict criteria for perimenopausal reproductive status as well as standardized diagnostic criteria for a clinically significant major or minor depression. Our data strongly suggest that the abnormalities in HPA axis function documented in many men and women with depressive illness are not present in PMD. Thus, a possible pathophysiologic role for altered HPA axis function in PMD is not supported by these data. In control women, we did identify that hot flashes were associated with a blunted ACTH response to Dex/CRH, but hot flashes had no effect on UFCs, nor did hot flashes robustly affect HPA axis function in women with PMD (albeit with the caveats that this study was not powered to identify the impact of hot flashes, and the sample sizes of women with and without hot flashes were small). These findings are important since the absence of an abnormal HPA axis (Dex/CRH response) distinguishes PMD from several other more traditional mood disorders, arguing against a role of HPA axis dysregulation in the pathophysiology of PMD. Finally, these findings suggest that although antecedent stressful life events are relevant in PMD (2, 12-14) and abnormal stress responsivity is a feature of PMD (52, 53), these phenomena are not occurring in the context of an abnormal regulation of HPA axis function.
Our understanding of the underlying biology assessed by the 24-hour UFC and the Dex/CRH stimulation test has been informed by studies both in Cushing syndrome and depressive disorders. The 24-hour UFC is an integrated measure of daily cortisol secretion, and, therefore, assesses the possibility of episodic alterations in the secretion of cortisol that otherwise could be missed using single time point measures. Studies report up to 40% of patients with an MDE show elevated UFC levels similar to those reported in Cushing syndrome—albeit in contrast to Cushing syndrome the circadian pattern of plasma cortisol secretion is better maintained in an MDE (54). The Dex/CRH test was first employed in depressive disorders (51, 55) to improve the sensitivity of the traditional Dex-suppression test in depression (51) and is widely used to evaluate the biology of depression as well as several other medical and psychiatric disorders (45, 50, 51, 55-57). In some institutions, the Dex/CRH test is reported to exhibit 80% sensitivity in an MDE, predict clinical response to antidepressant medications, and reflect an underlying biology of an MDE that could be targeted by novel treatments. However, the Dex/CRH test is not specific and is reported to be abnormal in a wide range of psychiatric conditions and to change with normal aging (45, 50, 51). Despite these widespread reports of abnormalities of Dex/CRH-stimulated cortisol and ACTH secretion, surprisingly, we observed no difference in PMD in either 24-hour UFC or Dex/CRH-stimulated cortisol/ACTH compared with controls. It is possible that the normal age-related increase in HPA axis response to Dex/CRH obscured the identification of potential differences between women with PMD and controls since the axes of both groups of women could be at threshold levels of activation, and, therefore were unable to be further stimulated by CRH stimulation. Nonetheless, the pathophysiologic relevance of the HPA axis modulation by age and declining ovarian steroid secretion in PMD remains unclear (11). Some studies have reported altered adrenal hormone function in PMD (58, 59), and alterations in salivary cortisol secretion have been observed to accompany variability in estradiol secretion and depressed mood in PMD (13, 53). We were unable to directly compare DEX/CRH responsivity to Trier stress response (53) because of our measurement of blood cortisol but not salivary cortisol. Thus, it is possible that alterations in HPA axis function could be an early pathophysiologic event in women at risk for developing PMD or that subtle alterations of the HPA axis in PMD are not detected by our testing but are relevant in the context of other predispositions to develop depression.
Hot flashes and the attendant sleep disturbance and autonomic arousal are common accompaniments of PMD and potentially could affect stress responsivity. The physiology of a hot flash remains poorly characterized; however, the sympathetic overactivity, temperature dysregulation, and feelings of anxiety during a hot flash, as well as the anecdotal clinical observation of the experience of “flushing” in some people after CRH administration suggest the possible involvement of abnormal secretion of CRH in hot flashes (60, 61). Indeed, some studies have reported abnormalities of cortisol (salivary, urinary, and hair measures) in women with hot flashes (62-65). In women with PMD, hot flashes did not affect responses to Dex/CRH stimulation, whereas in control women, the presence of hot flashes was associated with decreased secretion of ACTH after CRH administration (with the caveat that our sample contained a relatively small proportion [7/20] of control women with hot flashes). Hot flashes did not affect 24-hour UFC values in either group. Thus, overall cortisol secretion and HPA axis responsivity were similar in women with PMD and controls regardless of the presence of hot flashes. Finally, we found no evidence that CTQ scores, STRAW stage, past history of MDE, or any of the other clinical variables affected the ACTH and cortisol responses to Dex/CRH in either diagnostic group.
Although the Dex/CRH effectively assesses HPA axis function (45, 51, 66), it does not sample all sources of HPA axis regulation. Notably, the results of Dex/CRH testing are less able to detect the role of several brain areas (eg, prefrontal cortex, bed nucleus of the stria terminalis, amygdala) affecting the hypothalamus, which are targeted both by environmental stressors and some pharmacologic probes. Reproductive steroids, which are known to regulate the HPA axis, modulate neuronal and cellular functions in these brain areas and work synergistically with glucocorticoids to modulate the impact of stress (16, 17). Additionally, recurrent or chronic stress can sensitize peripheral tissue (eg, inflammatory mediators) and central nervous system responsivity to stress in the absence of ongoing HPA axis dysfunction (15). Consequently, our results do not preclude the presence in PMD of an abnormal HPA axis response to environmental stressors, which have been shown to sensitize the behavioral/affective response to changes in reproductive steroids (35).
The findings of this study are limited by the small sample size. We were powered to detect a moderate to large effect size; however, a larger sample size could detect smaller effect sizes that are unlikely to be of clinical significance. Additionally, the sample size was not sufficient to confidently examine either the impact of hot flashes or the interactions between hot flashes and depression on measures of HPA axis function. Plasma CBG levels were nonsignificantly higher (P = .06) in women with PMD compared with controls and could reflect a type 2 error. Nonetheless, a reanalysis with calculated free cortisol levels showed similar findings as those performed with total cortisol levels. Higher plasma CBG levels could be accompanied by less free cortisol in the blood and, therefore, lower UFC values in PMD and less negative feedback inhibition on the HPA axis associated with increased ACTH and cortisol responsivity in PMD. However, since the UFC and Dex/CRH findings did not differ between groups, our data suggest that the axis is functioning similarly in both groups, so if there is increased CBG in the PMD group, the axis has responded appropriately to maintain free cortisol, GC feedback, and CRH secretion. Finally, although the majority of women with PMD met the criteria for major depression, the absence of differences in HPA axis function in PMD compared with nonreproductive depression could nonetheless relate to the severity of depression; that is, in PMD, most women are able to function as outpatients.
These data are important for several reasons. First, methodologically, these data emphasize that the alterations in salivary cortisol measures previously described in PMD do not necessarily translate to observable alterations in HPA axis function, although it is true that 24-hour UFC and Dex/CRH stimulation might be limited in their ability to evaluate the regulation of the HPA axis from higher cortical centers (67). Second, mechanistically, our data and those of others strongly suggest that reproductive endocrine-related mood disorders are distinguished from nonreproductive depression by relatively normal HPA axis function. Finally, despite evidence of an otherwise normal HPA axis (and ostensibly functional glucocorticoid receptors) in PMD, it is possible that prior stressful life events could affect the susceptibility to depression during the menopause transition without sustained evidence of HPA axis dysregulation (29, 31).
Acknowledgments
The authors wish to thank the following: Karla Thompson for her clinical support and study management, Linda Schenkel for her support in data management, and Leslie Smith, BS, for her research assistance.
Financial Support: This work was supported by the Intramural Research Program of the National Institute of Mental Health (NIMH Protocol 88-M-0131, NIMH Project Nos. ZIAMH002537 and NCT00001231).
Glossary
Abbreviations
- ACTH
adrenocorticotropin
- AUC
area under the curve
- BDI
Beck Depression Inventory
- CBG
cortisol-binding globulin
- CESD
Center for Epidemiologic Studies Depression Scale
- CRH
corticotropin-releasing hormone
- CTQ
Childhood Trauma Questionnaire
- Dex
dexamethasone
- DRF
Daily Rating Form
- FSH
follicle-stimulating hormone
- GAF
Global Assessment of Functioning
- HPA
hypothalamic-pituitary-adrenal
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- MDE
major depressive episode
- NIH
National Institutes of Health
- PMD
perimenopausal depression
- SCID
Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders-IV
- STRAW
Stages of Reproductive Aging Workshop
- UFC
urinary free cortisol.
Additional Information
Disclosures: This work was written as part of Peter J. Schmidt’s official duties as a Government employee. None of the other authors have any disclosures to report.
Data Availability
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will, on request, detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61(1):62-70. [DOI] [PubMed] [Google Scholar]
- 2. Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard Study of Moods and Cycles. Arch Gen Psychiatry. 2006;63(4):385-390. [DOI] [PubMed] [Google Scholar]
- 3. Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375-382. [DOI] [PubMed] [Google Scholar]
- 4. Freeman EW, Sammel MD, Boorman DW, Zhang R. The longitudinal pattern of depressive symptoms around natural menopause. JAMA Psychiatry. 2014;71(1):36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: the Study of Women’s Health Across the Nation (SWAN). J Affect Disord. 2007;103(1-3):267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183(2):414-420. [DOI] [PubMed] [Google Scholar]
- 7. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58(6):529-534. [DOI] [PubMed] [Google Scholar]
- 8. Joffe H, Petrillo LF, Koukopoulos A, et al. Increased estradiol and improved sleep, but not hot flashes, predict enhanced mood during the menopausal transition. J Clin Endocrinol Metab. 2011;96(7):E1044-E1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Xia K, Schmidt PJ, Girdler SS. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmidt PJ, Ben Dor R, Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gordon JL, Girdler SS, Meltzer-Brody SE, et al. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. Am J Psychiatry. 2015;172(3):227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmidt PJ, Murphy JH, Haq N, Rubinow DR, Danaceau MA. Stressful life events, personal losses, and perimenopause-related depression. Arch Womens Ment Health. 2004;7(1):19-26. [DOI] [PubMed] [Google Scholar]
- 13. Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Leserman J, Girdler SS. Estradiol variability, stressful life events, and the emergence of depressive symptomatology during the menopausal transition. Menopause. 2016;23(3):257-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bromberger JT, Schott LL, Kravitz HM, et al. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women’s Health Across the Nation (SWAN). Arch Gen Psychiatry. 2010;67(6):598-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rohleder N. Acute and chronic stress induced changes in sensitivity of peripheral inflammatory pathways to the signals of multiple stress systems—2011 Curt Richter Award Winner. Psychoneuroendocrinology. 2012;37(3):307-316. [DOI] [PubMed] [Google Scholar]
- 16. McEwen BS, Bowles NP, Gray JD, et al. Mechanisms of stress in the brain. Nat Neurosci. 2015;18(10):1353-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41(1):3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McEwen BS, Alves SE, Bulloch K, Weiland NG. Ovarian steroids and the brain: implications for cognition and aging. Neurology. 1997;48(5 Suppl 7):S8-S 15. [DOI] [PubMed] [Google Scholar]
- 19. Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. Estrogen alters behavior and forebrain c-fos expression in ovariectomized rats subjected to the forced swim test. Proc Natl Acad Sci U S A. 1998;95(23):13941-13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lund TD, Hinds LR, Handa RJ. The androgen 5α-dihydrotestosterone and its metabolite 5α-androstan-3β, 17β-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor β-expressing neurons in the hypothalamus. J Neurosci. 2006;26(5):1448-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-beta agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 2009;150(4):1817-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5α-androstane-3β,17β-diol. Horm Behav. 2008;53(5):741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Komesaroff PA, Esler MD, Sudhir K. Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal women. J Clin Endocrinol Metab. 1999;84(2):606-610. [DOI] [PubMed] [Google Scholar]
- 24. Seeman TE, Robbins RJ. Aging and hypothalamic-pituitary-adrenal response to challenge in humans. Endocr Rev. 1994;15(2):233-260. [DOI] [PubMed] [Google Scholar]
- 25. Greenspan SL, Rowe JW, Maitland LA, McAloon-Dyke M, Elahi D. The pituitary-adrenal glucocorticoid response is altered by gender and disease. J Gerontol. 1993;48(3):M72-M77. [DOI] [PubMed] [Google Scholar]
- 26. Seeman TE, Singer B, Wilkinson CW, McEwen B. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology. 2001;26(3):225-240. [DOI] [PubMed] [Google Scholar]
- 27. Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29(1):83-98. [DOI] [PubMed] [Google Scholar]
- 28. Traustadóttir T, Bosch PR, Matt KS. The HPA axis response to stress in women: effects of aging and fitness. Psychoneuroendocrinology. 2005;30(4):392-402. [DOI] [PubMed] [Google Scholar]
- 29. Heim C, Newport DJ, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284(5):592-597. [DOI] [PubMed] [Google Scholar]
- 30. von Bardeleben U, Holsboer F. Effect of age on the cortisol response to human corticotropin-releasing hormone in depressed patients pretreated with dexamethasone. Biol Psychiatry. 1991;29(10):1042-1050. [DOI] [PubMed] [Google Scholar]
- 31. Newport DJ, Heim C, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal responses to standard and low-dose dexamethasone suppression tests in adult survivors of child abuse. Biol Psychiatry. 2004;55(1):10-20. [DOI] [PubMed] [Google Scholar]
- 32. Carroll BJ. Are SSRI antidepressants clinically homogeneous? Lancet. 1994;344(8921):550. [DOI] [PubMed] [Google Scholar]
- 33. Patacchioli FR, Simeoni S, Monnazzi P, Pace M, Capri O, Perrone G. Menopause, mild psychological stress and salivary cortisol: influence of long-term hormone replacement therapy (HRT). Maturitas. 2006;55(2):150-155. [DOI] [PubMed] [Google Scholar]
- 34. Lee EE, Nieman LK, Martinez PE, Harsh VL, Rubinow DR, Schmidt PJ. ACTH and cortisol response to Dex/CRH testing in women with and without premenstrual dysphoria during GnRH agonist-induced hypogonadism and ovarian steroid replacement. J Clin Endocrinol Metab. 2012;97(6):1887-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Löfgren M, Johansson M, Strömberg J, Meyerson B, Bäckström T. The influence of social subordinate housing on the withdrawal effects from progesterone and estradiol in male rats. Gen Comp Endocrinol. 2012;177(1):62-69. [DOI] [PubMed] [Google Scholar]
- 36. Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160(1):1-12. [DOI] [PubMed] [Google Scholar]
- 37. Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31(9):464-468. [DOI] [PubMed] [Google Scholar]
- 38. Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358(1):55-68. [DOI] [PubMed] [Google Scholar]
- 39. Joffe H, de Wit A, Coborn J, et al. Impact of estradiol variability and progesterone on mood in perimenopausal women with depressive symptoms. J Clin Endocrinol Metab. 2020;105:e642-e650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. First MB, Spitzer RL, Gibbon M, Williams JBW.. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-IP). Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 41. Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertil Steril. 2001;76(5):874-878. [DOI] [PubMed] [Google Scholar]
- 42. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401. [Google Scholar]
- 43. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-571. [DOI] [PubMed] [Google Scholar]
- 44. Endicott J, Nee J, Cohen J, Halbreich U. Premenstrual changes: patterns and correlates of daily ratings. J Affect Disord. 1986;10(2):127-135. [DOI] [PubMed] [Google Scholar]
- 45. Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994;28(4):341-356. [DOI] [PubMed] [Google Scholar]
- 46. Bernstein DP, Stein JA, Newcomb MD, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27(2):169-190. [DOI] [PubMed] [Google Scholar]
- 47. Scher CD, Stein MB, Asmundson GJ, McCreary DR, Forde DR. The Childhood Trauma Questionnaire in a community sample: psychometric properties and normative data. J Trauma Stress. 2001;14(4):843-857. [DOI] [PubMed] [Google Scholar]
- 48. Coolens JL, Van Baelen H, Heyns W. Clinical use of unbound plasma cortisol as calculated from total cortisol and corticosteroid-binding globulin. J Steroid Biochem. 1987;26(2):197-202. [DOI] [PubMed] [Google Scholar]
- 49. Kunugi H, Ida I, Owashi T, et al. Assessment of the dexamethasone/CRH test as a state-dependent marker for hypothalamic-pituitary-adrenal (HPA) axis abnormalities in major depressive episode: a multicenter study. Neuropsychopharmacology. 2006;31(1):212-220. [DOI] [PubMed] [Google Scholar]
- 50. Sher L. Combined dexamethasone suppression-corticotropin-releasing hormone stimulation test in studies of depression, alcoholism, and suicidal behavior. ScientificWorldJournal. 2006;6:1398-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ising M, Künzel HE, Binder EB, Nickel TF, Modell S, Holsboer F. The combined dexamethasone/CRH test as a potential surrogate marker in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(6):1085-1093. [DOI] [PubMed] [Google Scholar]
- 52. Gordon JL, Eisenlohr-Moul TA, Rubinow DR, Schrubbe L, Girdler SS. Naturally occurring changes in estradiol concentrations in the menopause transition predict morning cortisol and negative mood in perimenopausal depression. Clin Psychol Sci. 2016;4(5):919-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gordon JL, Peltier A, Grummisch JA, Sykes Tottenham L. Estradiol fluctuation, sensitivity to stress, and depressive symptoms in the menopause transition: a pilot study. Front Psychol. 2019;10:1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carroll BJ, Curtis GC, Davies BM, Mendels J, Sugerman AA. Urinary free cortisol excretion in depression. Psychol Med. 1976;6(1):43-50. [DOI] [PubMed] [Google Scholar]
- 55. Holsboer F, von Bardeleben U, Wiedemann K, Müller OA, Stalla GK. Serial assessment of corticotropin-releasing hormone response after dexamethasone in depression. Implications for pathophysiology of DST nonsuppression. Biol Psychiatry. 1987;22(2):228-234. [DOI] [PubMed] [Google Scholar]
- 56. Yanovski JA, Cutler GB Jr, Chrousos GP, Nieman LK. Corticotropin-releasing hormone stimulation following low-dose dexamethasone administration. A new test to distinguish Cushing’s syndrome from pseudo-Cushing’s states. JAMA. 1993;269(17):2232-2238. [PubMed] [Google Scholar]
- 57. Yanovski JA, Cutler GB Jr, Chrousos GP, Nieman LK. The dexamethasone-suppressed corticotropin-releasing hormone stimulation test differentiates mild Cushing’s disease from normal physiology. J Clin Endocrinol Metab. 1998;83(2):348-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schmidt PJ, Murphy JH, Haq N, Danaceau MA, St Clair L. Basal plasma hormone levels in depressed perimenopausal women. Psychoneuroendocrinology. 2002;27(8):907-920. [DOI] [PubMed] [Google Scholar]
- 59. Morrison MF, Freeman EW, Lin H, Sammel MD. Higher DHEA-S (dehydroepiandrosterone sulfate) levels are associated with depressive symptoms during the menopausal transition: results from the PENN Ovarian Aging Study. Arch Womens Ment Health. 2011;14(5):375-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schürmeyer TH, Schulte HM, Avgerinos PC, et al. Pharmacology of ovine and human CRH. Horm Metab Res Suppl. 1987;16:24-30. [PubMed] [Google Scholar]
- 61. Dunlop BW, Betancourt Y, Binder EB, et al. Tolerability of the dexamethasone-corticotropin releasing hormone test in major depressive disorder. J Psychiatr Res. 2011;45(1):24-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gibson CJ, Thurston RC, Matthews KA. Cortisol dysregulation is associated with daily diary-reported hot flashes among midlife women. Clin Endocrinol (Oxf). 2016;85(4):645-651. [DOI] [PubMed] [Google Scholar]
- 63. Gerber LM, Sievert LL, Schwartz JE. Hot flashes and midlife symptoms in relation to levels of salivary cortisol. Maturitas. 2017;96:26-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Katainen R, Kalleinen N, Teperi S, et al. The relationship between diurnal cortisol secretion and climacteric-related symptoms. Maturitas. 2018;115:37-44. [DOI] [PubMed] [Google Scholar]
- 65. Reed SD, Newton KM, Larson JC, et al. Daily salivary cortisol patterns in midlife women with hot flashes. Clin Endocrinol (Oxf). 2016;84(5):672-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sher L, Oquendo MA, Burke AK, Cooper TB, Mann JJ. Combined dexamethasone suppression-corticotrophin-releasing hormone stimulation test in medication-free major depression and healthy volunteers. J Affect Disord. 2013;151(3):1108-1112. [DOI] [PubMed] [Google Scholar]
- 67. Gold PW. The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry. 2015;20(1):32-47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will, on request, detail the restrictions and any conditions under which access to some data may be provided.