Abstract
Context
Leptin is an adipokine that signals energy sufficiency. In rodents, leptin deficiency decreases energy expenditure (EE), which is corrected following leptin replacement. In humans, data are mixed regarding leptin-mediated effects on EE.
Objective
To determine the effects of metreleptin on EE in patients with lipodystrophy.
Design, setting, and patients
Nonrandomized crossover study of 25 patients with lipodystrophy (National Institutes of Health, 2013-2018).
Intervention
The initiation cohort consisted of 17 patients without prior exposure to metreleptin, studied before and after 14 days of metreleptin. The withdrawal cohort consisted of 8 previously metreleptin-treated patients, studied before and after 14 days of metreleptin withdrawal.
Main outcomes
24-h total energy expenditure (TEE), resting energy expenditure (REE), autonomic nervous system activity [heart rate variability (HrV)], plasma-free triiodothyronine (T3), free thyroxine (T4), epinephrine, norepinephrine, and dopamine.
Results
In the initiation cohort, TEE and REE decreased by 5.0% (121 ± 152 kcal/day; P = 0.006) and 5.9% (120 ± 175 kcal/day; P = 0.02). Free T3 increased by 19.4% (40 ± 49 pg/dL; P = 0.01). No changes in catecholamines or HrV were observed. In the withdrawal cohort, free T3 decreased by 8.0% (P = 0.04), free T4 decreased by 11.9% (P = 0.002), and norepinephrine decreased by 34.2% (P = 0.03), but no changes in EE, epinephrine, dopamine, or HrV were observed.
Conclusions
Metreleptin initiation decreased EE in patients with lipodystrophy, but no changes were observed after metreleptin withdrawal. Thyroid hormone was higher on metreleptin in both initiation and withdrawal cohorts. Decreased EE after metreleptin in lipodystrophy may result from reductions in energy-requiring metabolic processes that counteract increases in EE via adipose tissue-specific neuroendocrine and adrenergic signaling.
Keywords: lipodystrophy, leptin, energy expenditure, thyroid hormone, autonomic nervous system
Leptin is an adipocyte-derived hormone that signals overall energy sufficiency in the body (1) as well as short-term energy balance (2). In physiologic states of leptin deficiency (eg, weight loss) as well as pathologic states (eg, mutations of the leptin gene), low leptin is sensed by the hypothalamus as a starvation signal, leading to hyperphagia, which is reversed with leptin replacement. In rodents, leptin deficiency is associated with hypothermia and decreased energy expenditure (EE), which are restored to normal following leptin replacement (2). Leptin’s effect on EE is mediated through stimulation of sympathetic nervous system activity, leading to brown adipose tissue (BAT) thermogenesis and increased heart rate and blood pressure (3).
In humans, there are limited data to support a role of leptin in EE. This is not surprising given that maintenance of core body temperature via BAT is the major leptin-dependent effect on EE in rodents, but in large mammals, such as adult humans, BAT thermogenesis contributes little to body temperature regulation. Humans with congenital leptin deficiency are not hypothermic (4), and 1 study in healthy participants found that leptin replacement after short-term fasting did not alter EE (5). However, studies in 10% weight-reduced subjects suggested that leptin replacement to preweight loss levels restored the decline in EE associated with weight loss (6,7).
Given the uncertainty surrounding a role for leptin in human EE regulation, we conducted a study to investigate this question in patients with lipodystrophy. Lipodystrophy is characterized by partial or complete (generalized) deficiency of adipose tissue leading to chronic hypoleptinemia and subsequent hyperphagia. The excess caloric intake causes ectopic lipid deposition in muscle and liver and resultant insulin resistance, diabetes, hypertriglyceridemia, steatohepatitis, and other metabolic abnormalities (8,9). Administration of metreleptin, a recombinant analog of human leptin, ameliorates metabolic complications of leptin deficiency in patients with lipodystrophy, with greater benefits seen in more leptin deficient patients with generalized lipodystrophy (8-11).
Using lipodystrophy as a human model of leptin deficiency and replacement, we conducted a nonrandomized crossover study to determine the effects of metreleptin on EE during periods of constant and ad libitum food intake. We hypothesized that metreleptin treatment in patients with lipodystrophy would increase EE, neuroendocrine mediators of EE, sympathetic nervous system activity, and blood pressure.
Materials and Methods
The institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases approved this study. All patients or legal guardians for those under 18 years of age provided written informed consent before participation; patients <18 years provided written assent. This study was registered at www.clinicaltrials.gov (trial ID NCT01778556).
Study Population
Eligible patients were 14 to 70 years of age, had a clinical diagnosis of generalized or partial lipodystrophy, low endogenous leptin documented prior to study enrollment (females <12 ng/mL, males <8 ng/mL), and 1 or more metabolic abnormalities including diabetes mellitus defined by the 2007 American Diabetes Association criteria, insulin resistance (fasting insulin ≥ 30 µIU/mL), or hypertriglyceridemia (fasting triglyceride > 200 mg/dL). Two cohorts of patients were studied: leptin initiation (n = 17) and leptin withdrawal (n = 8). Leptin initiation patients had no prior exposure to metreleptin, and leptin withdrawal patients had taken a stable dose of exogenous metreleptin for a minimum of 4 months prior to enrollment. Exclusion criteria included HIV-associated lipodystrophy, active inflammatory disease or glucocorticoid use, and changes in diabetes or lipid-lowering medications within 6 weeks of enrollment. Because of the risk of worsening metabolic status with metreleptin withdrawal, additional exclusion criteria in the leptin-withdrawal cohort were age <18 years, hemoglobin A1c ≥ 9%, serum triglycerides 800 mg/dL, >1 lifetime episode of acute pancreatitis, or ≥1 episode of pancreatitis while on metreleptin, lipase greater than upper limit of normal at study entry or known presence of neutralizing antibodies to leptin.
Study Design
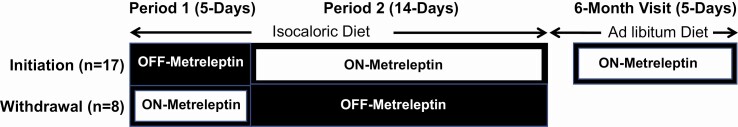
This was a prospective, nonrandomized, crossover study conducted from April 2013 to February 2018 (Fig. 1). Initiation patients were studied for the first 5 days without metreleptin (period 1) and then treated with metreleptin (5 mg subcutaneous injection every 12 h) for the next 14 days (period 2). Withdrawal patients were studied for the first 5 days on their home dose of metreleptin (period 1) and then withdrawn from metreleptin for the next 14 days (period 2). The study investigators and patients were not blinded to the intervention. All patients were hospitalized in the metabolic unit of the National Institutes of Health (NIH) Clinical Center and consumed a diet controlled for total calorie and macronutrient content (20 ± 5% protein, 25% ± 5% fat, 55 ± 5% carbohydrate). Caloric needs were estimated for weight maintenance using the Mifflin St. Jeor equations for males (for both male and female patients to account for the higher lean body mass observed in both men and women with lipodystrophy) with an activity factor of 1.3 (to account for low levels of activity in an inpatient setting). To assess metreleptin’s effects independent of energy intake, food intake was held constant during periods 1 and 2. Patients were instructed about the necessity of eating 100% of food given and not consuming any additional food. Any uneaten food was weighed, and any uneaten calories were reported, which demonstrated no differences in calorie or macronutrient intake in the on vs off leptin periods as previously published (12). The mean energy intake during periods 1 and 2 in the initiation cohort was 2373 ± 306 and 2355 ± 351, respectively (P = 0.48). The mean energy intake during periods 1 and 2 in the withdrawal cohort was 2347 ± 504 and 2403 ± 512, respectively (P = 0.24). Patients in the initiation cohort continued self-administered metreleptin treatment after discharge and were re-evaluated after 6 months of metreleptin treatment on an ad libitum diet. At the 6-month follow-up visit, during the 24-h chamber stay, patients received the same calorie and macronutrient-controlled diet that was given in periods 1 and 2.
Figure 1.
Study design schematic, nonrandomized, crossover study. Initiation patients (n = 17) were studied for the first 5 days without metreleptin (Period 1) and then treated with metreleptin for the next 14 days (Period 2). Withdrawal patients (n = 8) were studied for the first 5 days on their home dose of metreleptin (Period 1) and then withdrawn from metreleptin for the next 14 days (Period 2). Patients in the initiation cohort continued self-administered metreleptin treatment after discharge and were reevaluated after 6 months of metreleptin treatment on an ad libitum diet.
Patients continued their pre-admission diabetes and lipid-lowering medications throughout the study. However, in the initiation cohort, insulin and sulfonylurea doses were reduced as needed to minimize hypoglycemic events. None of the withdrawal patients were taking insulin.
Outcomes
Resting EE (REE) was measured at baseline (study entry) and at the end of periods 1 and 2 by indirect calorimetry using a ventilated hood connected to a metabolic cart (ParvoMedics TrueOne2400, Sandy, UT, USA). Measurements were taken upon the patient awakening after a minimum 8-h fast, resting in a supine position. Twenty-four-hour total EE (TEE) was measured at the end of periods 1 and 2 using a whole-room indirect calorimetry (metabolic) chamber. Time spent in physical activity during the 24-h metabolic chamber stay was assessed using a microwave detection system; periods of exercise were excluded, and the remaining data were normalized to a 24-h period. Twenty-four-hour urine samples were collected at the end of periods 1 and 2 in both initiation and withdrawal cohorts and at the 6-month time point in the initiation cohort. Samples were analyzed for excretion of glucose at these time points and EE from urinary glucose excretion was calculated using the Southgate and Durnin 3.75 conversion factor for monosaccharides (13). The sum of 24-h EE measured by indirect calorimetry in the metabolic chamber and urinary glucose excretion EE was calculated. Nonresting EE (nREE) was calculated as the difference between TEE and REE. Activity EE was calculated as the difference between nREE and thermic effect of feeding (TEF). EE during sleep (SEE) was calculated as the average EE during periods of minimal activity (microwave activity <2%) from 12 am to 6 am (14). TEF was calculated as the regression of EE against microwave activity (excluding periods of exercise and sleep), and subtracting the estimated REE during the calorimeter stay (1.1 * SEE) from the regression y-intercept (EE while awake, and activity is 0) (15). The respiratory quotient was computed as the ratio of the volume of carbon dioxide produced to the volume of oxygen consumed by patients during the 24-h stay. Exercise EE was measured using indirect calorimetry with a face mask connected to a metabolic cart (ParvoMedics TrueOne2400, Sandy, UT, USA) during a standardized cycle ergometer protocol. Following a 10-min warm-up period without resistance, patients pedaled at 60 rpm with the resistance adjusted to generate 10W of power for 4 min. The resistance was then increased such that the power generated was 25W for 4 min and then 50W for 4 min. Skeletal muscle work efficiency was calculated as the ratio of cycle power in kcal/min to change in EE above REE in kcal/min during exercise. A lower incremental increase in EE corresponds to greater muscle work efficiency (16).
Skin temperature was measured using wireless probes (iButtons, Maxim Inc, Sunnyvale, CA, USA) at 5 sites (deltoid, hand, pectoralis major, anterior thigh, and shin) according to the International Organization for Standardization (17). Skin temperature monitoring was performed throughout each 24-h stay in the metabolic chamber. Skin temperature was reported using the Ramanathan weighting system for mean surface temperature (18). Core body temperature was measured daily in the morning in the fasted state using a tympanic thermometer. The mean core body temperature during the last 2 days of periods 1 and 2 and the 6-month follow-up visit were reported.
Free-living physical activity (actigraphy) was quantified minute-by-minute using accelerometers (Actigraph wGT3X+, Actigraph Inc. Pensacola, FL, USA) at the patients’ hip and wrist. Analysis of mean hip and wrist accelerometer data was performed using data from 1 to 2 days at the end of each period to avoid days in which the accelerometer was worn for less than 10 h or when there were scheduled tests that required bedrest (such as liver biopsy, oral glucose tolerance testing, or hyperinsulinemic clamp) or days spent in the metabolic chamber. Wrist and hip activity were reported as the mean total count divided by total accelerometer wear time at the end of periods 1 and 2. There was insufficient actigraphy data for analysis at the 6-month time point; thus, only periods 1 and 2 are reported. Body fat percentage, fat mass, and lean body mass were measured using whole body dual energy x-ray absorptiometry at the end of periods 1 and 2 in both initiation and withdrawal cohorts and at the 6-month time point in the initiation cohort (iDXA, GE Healthcare, Madison WI, USA).
Glucose, insulin, C-peptide, hemoglobin A1c, triglycerides, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, total and free thyroxine (T4) and triiodothyronine (T3), thyroid-stimulating hormone (TSH), dopamine, and epinephrine were measured after an 8- to 12-h fast using the techniques of the NIH Clinical Center laboratory. In the withdrawal cohort, endogenous leptin in fasting serum samples were measured prior to metreleptin initiation by radioimmunoassay (MilliporeSigma) as previously reported (12). In both cohorts, leptin was measured by enzyme-linked immunosorbent assay in fasting EDTA plasma samples at the end of periods 1 and 2 and again at the 6-month time point of metreleptin treatment in the initiation cohort as previously reported (12).
Heart rate was measured and recorded over 24 h using a portable electrocardiogram Holter monitor (Evo Recorder, Spacelabs Healthcare, Snoqualmie, WA). Electrocardiogram waveform data was downloaded and analyzed for heart rate and heart rate variability (HrV) using time domain methods power spectrum analysis (19). The SD of the normal-to-normal interbeat intervals (SDNN) is a measure of total HrV. The very low frequency component (VLF) provides a measure of renin-angiotensin-aldosterone system effects on HrV. The low frequency power component (LF) provides a measure of both sympathetic and parasympathetic influence on HrV. The high frequency power component (HF) provides a measure of parasympathetic impact on HrV. The LF/HF ratio provides a measure of sympathetic influence on HrV.
Statistical Analysis
Outcomes are reported as mean ± SD or median (interquartile range: 25th, 75th percentile) based on data distribution. Skewed data were log transformed prior to analysis. Univariate comparisons for outcomes measured at 2 time points were conducted using paired t-test (for normally distributed variables) and Wilcoxon rank-signed test (for skewed variables). For all outcomes obtained at >2 time points, linear mixed effects models applying a Dunnett correction for multiple comparisons (comparing each follow-up time point to baseline) were used. P < 0.05 represented statistical significance. For models of EE, lean mass, fat mass, and physical activity (percentage of time active in the metabolic chamber) were included in linear-mixed effects models as covariates. For models of weighted skin temperature, room temperature was included as a covariate. All P-values are 2-sided. Analyses were conducted using GraphPad Prism, version 8.1 (GraphPad Software) and SAS Enterprise Guide, version 7.1 (SAS Institute).
Patients in the initiation and withdrawal cohorts were analyzed separately per protocol. To increase sample size, exploratory analyses were conducted combining period 1 initiation and period 2 withdrawal cohorts in the off metreleptin state (termed “OFF-metreleptin”), and period 2 initiation and period 1 withdrawal cohorts in the metreleptin treated state (termed “ON-metreleptin”). For measures of thyroid function (TSH, free and total T3 and T4), the primary analysis excluded patients on levothyroxine or any thyroid replacement as inclusion may bias results toward the null hypothesis. Further exploratory analyses were conducted in the initiation cohort in which patients were subgrouped by lipodystrophy type (generalized vs partial). For measures of autonomic nervous system tone assessed by HrV, sensitivity analyses were conducted excluding patients who had changes in medications with known or theoretical effects on heart variability (eg, selective serotonin reuptake inhibitors, benzodiazepines, etc.).
Power Calculations
This study, assuming a sample size of 10 patients in each group (initiation and withdrawal), had greater than 80% power to detect differences before vs after metreleptin treatment equal to or greater than those previously reported in the literature after metreleptin treatment for the following primary, secondary, and exploratory outcomes—primary outcomes: TEE (7) and total body insulin sensitivity (11); secondary outcomes: skeletal muscle work efficiency (7), fasting plasma glucose (20), and fasting triglycerides (20); and exploratory outcomes: epinephrine (7) and sympathetic nervous system tone (7). The study lacked adequate power to detect differences equal or greater than those previously reported in additional secondary and exploratory outcomes including REE (10), T3 (7), T4 (7), TSH (21), and norepinephrine (7). The study was not powered a priori for exploratory subgroup analysis of the generalized and partial lipodystrophy subgroups. Patients with missing data were excluded from analyses (ie, complete case analysis).
Results
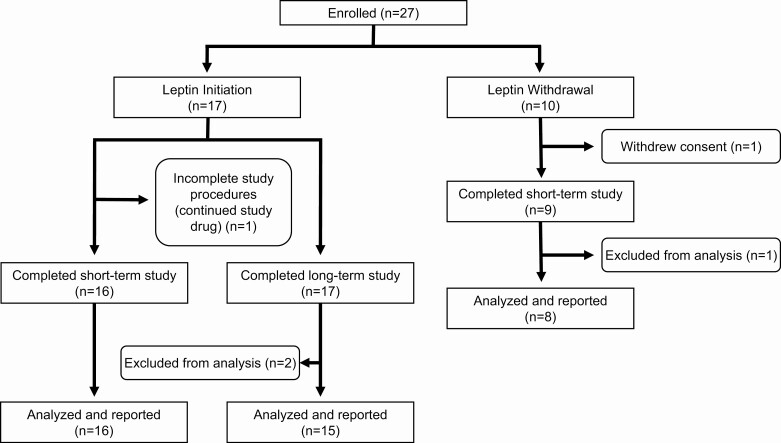
Patient disposition throughout the study is shown in Figure 2. Twenty-seven patients with generalized or partial lipodystrophy were enrolled in the study. Of the 17 patients who initiated metreleptin (initiation), 1 did not complete the study procedures for the short-term study (period 2) but was continued on metreleptin and re-evaluated at 6 months, and 1 completed the short-term study but was excluded from analysis of the 6-month data because of noncompliance with metreleptin. Eight patients who were taken off metreleptin for a 2-week period (withdrawal) completed the study and were analyzed. Table 1 presents baseline characteristics by cohort. The mean age of participants in the initiation and withdrawal cohorts was 29 and 25 years, respectively. Females represented 83% (n = 14) of the initiation cohort and 62.5% (n = 5) of the withdrawal cohort. Participants with generalized lipodystrophy accounted for 29.4% (n = 5) of the initiation cohort and 100% (n = 8) of the withdrawal cohort. The average weight in the initiation cohort was 70.1 ± 17.3 kg and 59.2 ± 17.0 kg in the withdrawal cohort. At baseline, the median (25th, 75th) endogenous leptin level in the initiation cohort was 3.9 ng/dL (0.6, 12.8; mean 8.4 ± 10.0 ng/dL) but was only 1.3 ng/dL (0.9, 1.5; mean 1.2 ± 0.5 ng/dL) in the withdrawal cohort prior to initiation of metreleptin. This difference in endogenous leptin levels between the 2 cohorts reflects lower leptin levels in patients with generalized lipodystrophy, who accounted for all participants in the withdrawal cohort.
Figure 2.
Study flow chart. Twenty-seven patients with generalized or partial lipodystrophy were enrolled in the study. Of the 17 patients who initiated metreleptin (initiation), 1 did not complete the study procedures for the short-term study (period 2) but was continued on metreleptin and re-evaluated at 6 months, and 1 completed the short-term study but was excluded from analysis of the 6-month data because of noncompliance with metreleptin. Eight patients who were taken off metreleptin for a 2-week period (withdrawal) completed the study and were analyzed.
Table 1.
Baseline characteristics of 25 patients with lipodystrophy who initiated or withdrew from metreleptin
| Clinical values | Initiation (n = 17) | Withdrawal (n = 8) |
|---|---|---|
| Type of lipodystrophy (generalized/partial) | (5/12) | (8/0) |
| Subtype of lipodystrophy | 5 CGL, 12 FPL | 7 CGL, 1 AGL |
| Sex (male/female) | (3/14) | (3/5) |
| Age (years) | 29 ± 16 | 25 ± 6 |
| Race/ethnicity | 9 White, 6 Hispanic, 1 Asian, 1 Other | 4 White, 2 African American, 2 Hispanic |
| Weight (kg) | 70.1 ± 17.3 | 59.2 ± 17.0 |
| Endogenous leptin level (ng/dL)a | 3.9 [0.6,12.8] | 1.3 [0.9,1.5] |
| Duration of metreleptin treatment prior to study (year) | 0 | 7.7 ± 4.7 |
| Patients on insulin (%) | 71% | 0 |
| Insulin dose (units/day, insulin users only) | 193 ± 130 | 0 |
| Noninsulin diabetes medications per subject (n) | 1.3 ± 1.1 | 0.4 ± 0.5 |
| Lipid medications per subject (n) | 1.8 ± 1.0 | 0.4 ± 0.7 |
Abbreviations: AGL, acquired generalized lipodystrophy; CGL, congenital generalized lipodystrophy; FPL, familial partial lipodystrophy.
a In the withdrawal cohort, endogenous leptin in fasting serum samples was measured prior to metreleptin initiation by radioimmunoassay. In the initiation cohort, endogenous leptin was measured by enzyme-linked immunosorbent assay.
Metreleptin Administration Improved Metabolic Abnormalities of Lipodystrophy Associated With Leptin Deficiency
As previously reported in an overlapping cohort of participants, Table 2 describes metabolic characteristics of both initiation and withdrawal cohorts. In the initiation cohort, comprised of metreleptin-naïve patients, plasma leptin concentrations were 3.9 ng/dL, 66.7 ng/dL, and 135 ng/dL at baseline, 2 weeks and 6 months following metreleptin treatment (P < 0.0001). In the withdrawal cohort, plasma leptin concentration was highest at baseline on metreleptin at 43.7 ng/dL and decreased to 0.5 ng/dL following 2 weeks of metreleptin withdrawal (P < 0.0001).
Table 2.
Metabolic characteristics and body composition in patients with lipodystrophy before and after initiation or withdrawal of metreleptin
| Initiation | Withdrawal | ||||||
|---|---|---|---|---|---|---|---|
| Baseline (n = 17) | 2 weeks (n = 16) | 6 months (n = 15) | P | Baseline (n = 8) | 2 weeks (n = 8) | P | |
| Leptin (ng/dL) | 3.9 (0.6,12.8) | 66.7 (51,85.1) | 135 (117.3,173.4) | <0.00012w,6m | 43.7 (16.6,96.6) | 0.5 (0.2,1.8) | <0.0001 |
| Total cholesterol (mg/dL) | 230 ± 113 | 166 ± 47 | 157 ± 53 | 0.042w,6m | 129 ± 32 | 123 ± 29 | 0.3 |
| Triglycerides (mg/dL) | 488 (231 1116) | 300 (164 600) | 248 (118 497) | 0.022w,6m | 137 (78 215) | 145 (99 361) | 0.3 |
| Glucose (mg/dL) | 156 ± 44 | 147 ± 47 | 130 ± 38 | 0.016m | 97 ± 18 | 105 ± 33 | 0.6 |
| Insulin sensitivitye (mg/kgFFM/min) | 4.9 ± 3.1 | 5.6 ± 2.4a | 8.2 ± 3.9b | 0.0042w,6m | 10.9 ± 4.1 | 6.4 ± 1.8 | 0.01 |
| Urinary glucose excretion (g/24 h) | 5.3 (0.2,10.3) | 1.8 (0.2,7.2) | 0.3 (0.1,2.1)c | 0.0076m | 0.2 (0.1,0.7) | 0.2 (0.05,2.4) | 0.56 |
| Lean body mass (kg) | 51 ± 9 | 51 ± 10a | 49 ± 10 | 0.0056m | 52 ± 16 | 52 ± 15 | 0.98 |
| Fat mass (kg) | 17 ± 11 | 17 ± 11a | 14 ± 11d | 0.0092w | 4.1 ± 1.2 | 4.2 ± 0.9 | 0.76 |
| Body fat (%) | 22 ± 11 | 22 ± 11a | 19 ± 11d | 0.12 | 7.1 ± 1.7 | 7.2 ± 1.3 | 0.75 |
Data show the mean ± SD for normally distributed metrics and median (interquartile range: 25th, 75th) for nonnormally distributed metrics. Patients in the initiation cohort have data prior to initiation of metreleptin (baseline), and after 2 weeks and 6 months of metreleptin administration. Patients in the withdrawal cohort have data during metreleptin treatment, and after 2 weeks of metreleptin withdrawal. +Overall P-value (P) for linear mixed-effects regression is shown. 2wP < 0.05 for 2 weeks vs baseline. 6mP < 0.05 for 6 months vs baseline.
a n=15.
b n=14.
c n=13.
d n=12.
e Insulin sensitivity measured as glucose disposal during a hyperinsulinemic, euglycemic clamp.
Total cholesterol, triglycerides, fasting glucose, and urinary glucose excretion decreased after initiation of metreleptin for 2 weeks and 6 months but were unchanged following 2 weeks of metreleptin withdrawal. Insulin sensitivity as measured by euglycemic, hyperinsulinemic clamp increased after initiation of metreleptin for 2 weeks and 6 months and decreased after 2 weeks of metreleptin withdrawal.
Metreleptin Initiation Decreased 24-h, Resting, and Sleep Energy Expenditure but Had No Effect on Muscle Work Efficiency, Core Body Temperature, Thermic Effect of Feeding, or Weighted Skin Temperature
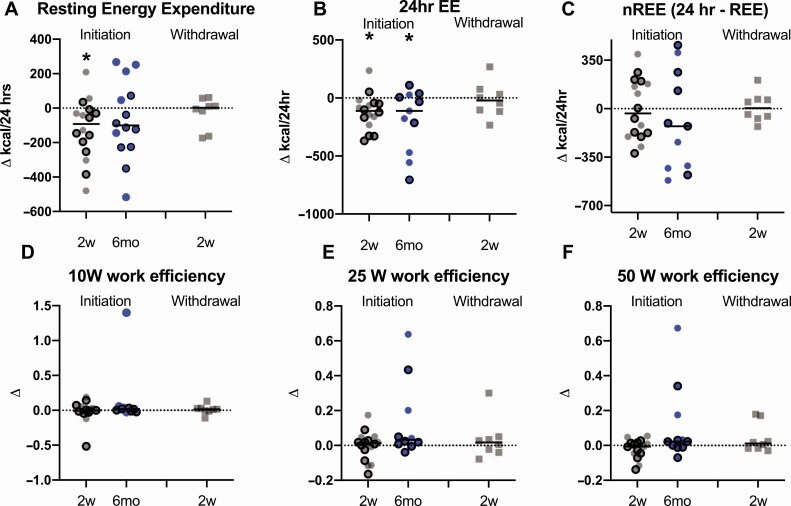
Table 3 and Figure 3 depict changes in EE and skeletal muscle work efficiency after initiation or withdrawal of metreleptin treatment. As previously reported in an overlapping cohort of patients, following metreleptin administration in metreleptin-naïve patients, there was a 5.0% (121 ± 152 kcal/day; P = 0.006) reduction in 24-h EE (TEE) at 2 weeks and 7.9% (190 ± 272 kcal/day; P = 0.04) reduction in 24-h EE at 6 months compared to baseline. The reduction in 24-h EE remained significant at 2 weeks compared to baseline after accounting for changes in EE due to urinary glucose excretion, lean body mass, fat mass, and physical activity [percentage of time active in the metabolic chamber; least mean square (LMS) difference: −103; P = 0.043) and at 6 months (LMS difference: −141; P = 0.045). In an exploratory subgroup analysis of metreleptin-naïve patients stratified by lipodystrophy type (partial vs generalized), metreleptin administration decreased 24-h EE in patients with generalized lipodystrophy (n = 5), but not partial lipodystrophy (n = 12). There was no significant change in 24-h EE in the withdrawal cohort. In the combined cohort, patients had lower 24-h EE in the ON-metreleptin state compared to the OFF-metreleptin state.
Table 3.
Energy expenditure and neuroendocrine mediators of energy expenditure in patients with lipodystrophy before and after initiation or withdrawal of metreleptin
| Initiation | Withdrawal | Combined | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 17) | 2 weeks (n = 16) | 6 months (n = 15) | P* | Baseline (n = 8) | 2 weeks (n = 8) | P | Off (n = 25) | On (n = 24) | P | |
| REE (kcal/24 h) | 1805 ± 332 | 1688 ± 318 | 1726 ± 309c | 0.032w | 1565 ± 406 | 1538 ± 362 | 0.8 | 1719 ± 357 | 1647 ± 346 | 0.05 |
| 24-h EE (kcal/24 h) | 2402 ± 383 | 2272 ± 396 | 2191 ± 444e | 0.0032w,6m | 2225 ± 504 | 2209 ± 467 | 0.7 | 2340 ± 412 | 2256 ± 424 | 0.03 |
| 24-h EE + urinary glucose excretion EE (kcal/24 h) | 2424 ± 380 | 2294 ± 405 | 2200 ± 446e | 0.0022w,6m | 2227 ± 504 | 2214 ± 470 | 0.8 | 2357 ± 414 | 2272 ± 431 | 0.03 |
| nREE (kcal/24 h) | 585 ± 183a | 584 ± 251 | 502 ± 243e | 0.6 | 660 ± 137 | 670 ± 143g | 1 | 614 ± 172 | 609 ± 220 | 0.9 |
| Sleep EE (kcal/24 h) | 1858 ± 270a | 1758 ± 242 | 1726 ± 312e | 0.0022w,6m | 1767 ± 383 | 1814 ± 307g | 0.3 | 1814 ± 307 | 1761 ± 288 | 0.1 |
| TEF (kcal/24 h) | 356 ± 132a | 350 ± 200 | 312 ± 192e | 0.4 | 324 ± 179 | 363 ± 126g | 0.3 | 363 ± 126 | 342 ± 190 | 0.4 |
| TEF as % of energy intake | 15 ± 5a | 14 ± 7a | 14 ± 9j | 0.9 | 13 ± 6 | 16 ± 3 | 0.3 | 15 ± 4g | 14 ± 7 | 0.3 |
| AEE (kcal/24 h) | 238 ± 178a | 234 ± 303a | 190 ± 267e | 1 | 335 ± 105 | 293 ± 133 | 0.5 | 256 ± 163g | 268 ± 256 | 0.5 |
| 24-h RQ | 0.86 ± 0.04 | 0.86 ± 0.04 | 0.86 ± 0.03e | 1 | 0.89 ± 0.04 | 0.86 ± 0.05 | 0.4 | 0.86 ± 0.05 | 0.87 ± 0.04 | 0.5 |
| 10W work efficiency | 0.12 (0.10, 0.15) | 0.12 (0.09, 0.23) | 0.14 (0.11, 0.16) k | 0.5 | 0.11 (0.09,0.13) | 0.12 (0.09,0.15) | 0.7 | 0.12 (0.10,0.15) | 0.11 (0.09,0.18) | 0.6 |
| 25W work efficiency | 0.20 (0.18, 0.26) | 0.22 (0.18, 0.31) | 0.24 (0.22, 0.50) k | 0.3 | 0.22 (0.16,0.23) | 0.20 (0.17,0.27) | 0.6 | 0.20 (0.18,0.26) | 0.22 (0.18,0.27) | 0.9 |
| 50W work efficiency | 0.25 (0.23, 0.28)a | 0.27 (0.24, 0.30) | 0.27 (0.25,0.42) j | 0.1 | 0.25 (0.22,0.28) | 0.26 (0.23,0.35) | 0.4 | 0.26 (0.23,0.29)g | 0.27 (0.24,0.29) | 0.2 |
| Dopamine (pg/mL) | 32 ± 19a | 26 ± 6b | 24 ± 1m | 0.2 | 24 ± 0 | 30 ± 10 | 0.5 | 31 ± 17g | 25 ± 5o | 0.06 |
| Epinephrine (pg/mL) | 19 ± 1a | 19 ± 0b | 19 ± 0.4m | 0.6 | 24 ± 15 | 28 ± 18 | 0.3 | 22 ± 11g | 21 ± 9o | 0.1 |
| Norepinephrine (pg/mL) | 262 ± 450a | 207 ± 189b | 233 ± 124m | 0.7 | 191 ± 70 | 112 ± 47 | 0.03 | 212 ± 371g | 201 ± 156o | 0.8 |
| Free T3 (pg/mL) | 248 (200 270)c | 295 (259 315)d | 276 (248 300)m | 0.0062w, 6m | 295 (267 331) | 265 (237 323) | 0.008 | 253 (212 281)h | 295 (266 320)i | 0.0002 |
| TSH (μIU/mL) | 2.1 ± 0.8c | 2.3 ± 0.7d | 1.8 ± 0.9e | 0.3 | 2.1 ± 1.0 | 2.5 ± 1.4 | 0.07 | 2.3 ± 1.0h | 2.2 ± 0.8i | 0.8 |
| Free T4 (ng/dL) | 1.0 ± 0.2c | 1.1 ± 0.2d | 1.1 ± 0.2e | 0.2 | 1.2 ± 0.2 | 1.0 ± 0.2 | 0.002 | 1.0 ± 0.2h | 1.1 ± 0.2i | 0.0004 |
| T3 (pg/dL) | 91 ± 21c | 101 ± 16d | 98 ± 14e | 0.3 | 112 ± 26 | 107 ± 35 | 0.4 | 97 ± 28h | 105 ± 21i | 0.052 |
| Core body temperature (°C) | 36.5 ± 0.3 | 36.5 ± 0.3 | 36.5 ± 0.3c | 0.4 | 36.6 ± 0.3 | 36.8 ± 0.4 | 0.8 | 36.6 ± 0.4 | 36.5 ± 0.5 | 0.4 |
| Weighted skin temperature (°C) | 32.5 ± 0.8 | 32.8 ± 0.5d | 33 ± 0.4k | 0.7 | 33.3 ± 0.6m | 33.3 ± 0.7 | 0.8 | 32.8 ± 0.9i | 32.9 ± 0.6p | 0.07 |
| Wrist activity (counts/min) | 1437 ± 850c | 1714 ± 620c | NA | 0.3 | 1204 ± 535m | 1205 ± 380m | 1 | 1367 ± 738n | 1553 ± 629p | 0.3 |
| Hip activity (counts/min) | 388 ± 264c | 537 ± 370d | NA | 0.1 | 293 ± 137m | 269 ± 48m | 0.7 | 352 ± 227n | 464 ± 334n | 0.1 |
Data show the mean ± SD for normally distributed metrics and median (interquartile range: 25th, 75th) for nonnormally distributed metrics. Patients in the initiation cohort have data prior to initiation of metreleptin (baseline) and after 2 weeks and 6 months of metreleptin administration. Patients in the withdrawal cohort have data during metreleptin treatment (baseline) and after 2 weeks of metreleptin withdrawal. The combined cohort includes baseline and 2-week data in both groups, categorized by on or off metreleptin status. 2wP < 0.05 for baseline vs 2 weeks. 6mP < 0.05 for baseline vs 6 months.
Abbreviations: AEE, activity energy expenditure; EE, energy expenditure; NA, not available; nREE, nonresting energy expenditure (equal to 24-h EE-REE); REE, resting energy expenditure; RQ, respiratory quotient; TEF, thermic effect of feeding.
a n = 16.
b n = 15.
c n = 14.
d n = 13.
e n = 12.
f n = 9.
g n = 24.
h n = 22.
i n = 21.
j n = 11.
k n = 10.
l n = 3.
m n = 6.
n n = 20.
o n = 23.
p n = 19.
q Overall P-value for linear mixed-effects regression is shown.
Figure 3.
Effects of metreleptin initiation or withdrawal on change in energy expenditure and skeletal muscle work efficiency in patients with lipodystrophy. Resting energy expenditure (A) decreased 2 weeks (2w; gray circles) after metreleptin initiation, but did not change from baseline to 6 months (6mo; blue circles) after metreleptin initiation or 2 weeks after metreleptin withdrawal (gray squares). Twenty-four-hour energy expenditure (B) decreased from baseline to 2 weeks and 6 months after metreleptin initiation but did not change after 2 weeks of metreleptin withdrawal. There were no changes in nonresting energy expenditure (C) or skeletal muscle work efficiency at 10 Watts (D), 25 Watts (E), and 50 Watts (F) from baseline to 2 weeks or 6 months following metreleptin initiation, or 2 weeks after metreleptin withdrawal (kcal/24 h). Patients with generalized lipodystrophy are shown as circles or squares without borders and those with partial lipodystrophy as circles with black borders. Comparisons were made using Wilcoxon rank-signed test and paired t-test for nonnormally and normally distributed data, respectively. Linear mixed effects models applying a Dunnett correction for multiple comparisons were used for outcomes measured at >2 time points. P < 0.05 represented statistical significance. P-values are 2-sided.
In the initiation cohort, REE decreased by 5.9% (120 ± 175 kcal/day; P = 0.02) from baseline to 2 weeks but returned to baseline levels at 6 months. After accounting for changes in lean body mass and fat mass, the decline in REE remained significant from 2 weeks compared to baseline and remained nonsignificant at 6 months. There was no significant change in REE in the withdrawal cohort. In the combined cohort, patients had lower REE in the ON-metreleptin state compared to the OFF-metreleptin state. In the initiation cohort, there was a 4.6% (91 ± 116 kcal/day; P = 0.009) reduction in SEE at 2 weeks and 7.3% (141 ± 176 kcal/day; P = 0.02) reduction at 6 months compared to baseline. The reduction in SEE remained significant at 2 weeks after accounting for lean mass, fat mass, and sleep motion percentage (LMS difference: −104; P = 0.007), but not at 6 months (LMS difference: −115; P = 0.07). nREE, respiratory quotient, activity EE, TEF, core body temperature, and weighted skin temperature did not significantly change in the initiation, withdrawal, or combined cohorts at any time point. Similarly, muscle work efficiency during exercise testing at all work levels (10W, 25W, or 50W) was unchanged by metreleptin in any group (Fig. 3). In subgroup analyses of generalized vs partial lipodystrophy in the initiation cohort, there was an increase of ~0.6°C in weighted skin temperature following 2 weeks and 6 months of metreleptin administration compared to baseline in patients with generalized lipodystrophy that persisted after accounting for room temperature. There was a decrease in TEF at 6 months compared to baseline in patients with generalized lipodystrophy; however, this association did not persist after accounting for changes in body composition.
Metreleptin Administration Increased Bioactive Thyroid Hormone but Had Inconsistent Effects on Catecholamines
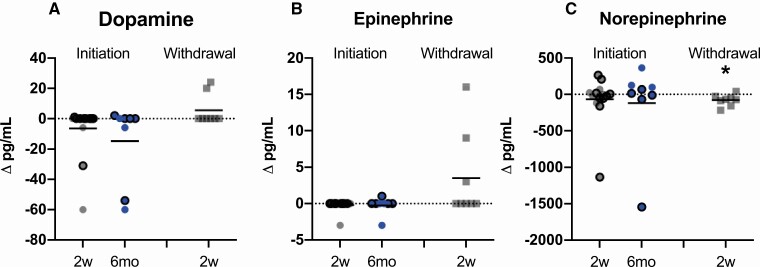
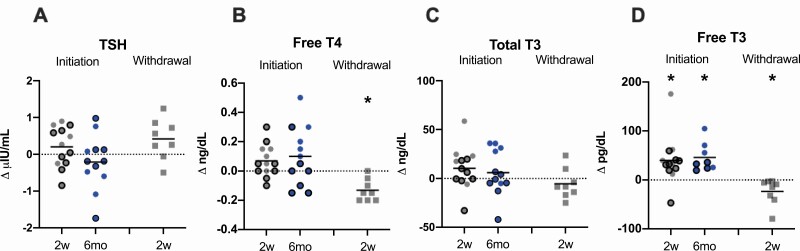
Catecholamines, except for norepinephrine, did not significantly change in the initiation, withdrawal, or combined cohorts or in the initiation cohort subgroups of generalized and partial lipodystrophy at any time point. Norepinephrine levels decreased after 2 weeks of metreleptin withdrawal compared to baseline in the withdrawal cohort only (Fig. 4). Free T3 was higher 2 weeks and 6 months after metreleptin initiation compared to baseline in the initiation cohort; however, free T4 was unchanged. Similarly, in the withdrawal cohort, free T3 and free T4 decreased following 2 weeks of metreleptin withdrawal. In the combined cohort, free T3 and free T4 were higher in the ON-metreleptin condition. There was no effect of metreleptin on TSH in the initiation, withdrawal, or combined cohorts (Fig. 5). In the subgroup analyses of generalized and partial lipodystrophy in the initiation cohort, there was an increase in free T4 in patients with generalized lipodystrophy and increase in free T3 in patients with partial lipodystrophy following metreleptin administration.
Figure 4.
Effects of metreleptin initiation or withdrawal on catecholamines in patients with lipodystrophy. (A) Dopamine and (B) epinephrine did not change from baseline to 2 weeks (2w; gray circles) or 6 months (6mo; blue circles) after metreleptin initiation or 2 weeks after metreleptin withdrawal (2w; gray squares). (C) Norepinephrine did not change from baseline to 2 weeks or 6 months after metreleptin initiation but decreased 2 weeks following metreleptin withdrawal. Patients with generalized lipodystrophy are shown as circles or squares without borders and those with partial lipodystrophy as circles with black borders. Comparisons were made using Wilcoxon rank-signed test and paired t-test for nonnormally and normally distributed data, respectively. Linear mixed effects models applying a Dunnett correction for multiple comparisons were used for outcomes measured at >2 time points. P < 0.05 represented statistical significance. P-values are 2-sided.
Figure 5.
Effects of metreleptin initiation or withdrawal on thyroid hormones in patients with lipodystrophy. (A) Thyroid stimulating hormone (TSH) did not change from baseline to 2 weeks (2w; gray circles) or 6 months (6mo; blue circles) after metreleptin administration or after 2 weeks (2w; gray squares) of metreleptin withdrawal. (B) Free thyroxine (T4) did not change from baseline to 2 weeks or 6 months after metreleptin administration but decreased after 2 weeks of metreleptin withdrawal. (C) Total triiodothyronine (T3) did not change at any time point from baseline following metreleptin initiation or withdrawal. (D) Free triiodothyronine (T3) increased from baseline to 2 weeks and 6 months after metreleptin initiation and decreased 2 weeks after metreleptin withdrawal. Patients with generalized lipodystrophy are shown as circles or squares without borders and those with partial lipodystrophy as circles with black borders. Comparisons were made using Wilcoxon rank-signed test and paired t-test for nonnormally and normally distributed data, respectively. Linear mixed effects models applying a Dunnett correction for multiple comparisons were used for outcomes measured at >2 time points. P < 0.05 represented statistical significance. P-values are 2-sided.
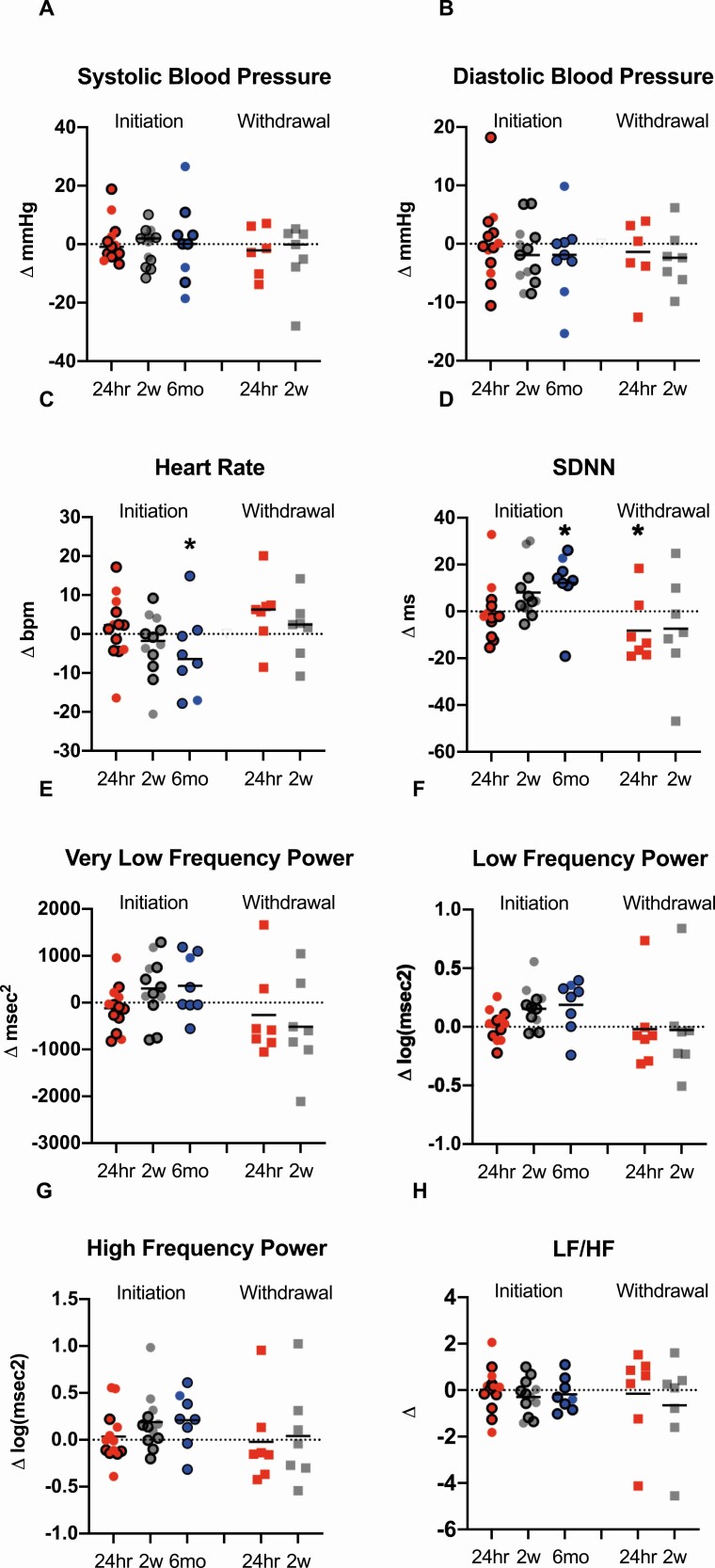
Metreleptin Administration Had No Effect on Blood Pressure or Cardiac Sympathetic Nervous System Tone, but Increased Overall Heart Rate Variability
Table 4 and Figure 6 describe blood pressure, heart rate and HrV after initiation or withdrawal of metreleptin. Neither systolic nor diastolic blood pressure were influenced by the presence or absence of metreleptin. Changes in heart rate, driven predominantly by the waking component of heart rate, decreased after 6 months of metreleptin treatment compared to baseline in the initiation cohort. HrV, assessed as SDNN, increased at 6 months following metreleptin treatment in the initiation cohort and decreased 24 h after metreleptin withdrawal. In the combined cohort, SDNN was higher in participants in the ON-metreleptin state. There was no change in HF (reflecting parasympathetic activity), or LF/HF ratio (reflecting sympathetic activity) in the initiation or withdrawal cohorts at any time point. In the combined cohort, VLF, LF, and HF were higher in the ON-metreleptin state compared to OFF-metreleptin; these differences were restricted to waking hours. In the subgroup analyses of generalized and partial lipodystrophy in the initiation cohort, an initial decrease in VLF after 24 h followed by an increase after 2 weeks of metreleptin treatment was seen in patients with partial lipodystrophy. There was an increase in VLF at 6 months in patients with generalized lipodystrophy. A sensitivity analysis excluding patients who had changes in medications with known or theoretical effects on heart variability (eg, selective serotonin reuptake inhibitors, benzodiazepines, etc.) demonstrated an increase in waking SDNN, LF, and HF after 2 weeks of metreleptin administration, similar to the primary analyses including all participants.
Table 4.
Blood pressure, heart rate, and heart rate variability metrics in patients with lipodystrophy before and after initiation or withdrawal of metreleptin
| Initiation | Withdrawal | Combined | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 14) | 24 h (n = 13) | 2 weeks (n = 13) | 6 months (n = 10) | P * | Baseline (n = 7) | 24 h (n = 7) | 2 weeks (n = 7) | P * | Off (n = 21) | On (n = 20) | P | |
| Systolic BP (mmHg) | 135 ± 14 | 136 ± 14 | 133 ± 12 | 132 ± 18 | 0.9 | 133 ± 14 | 126 ± 10f | 129 ± 8 | 0.4 | 133 ± 12 | 133 ± 13 | 0.6 |
| Diastolic BP (mmHg) | 77 ± 8 | 77 ± 10 | 75 ± 7 | 73 ± 9 | 0.7 | 73 ± 6 | 71 ± 3f | 70 ± 8 | 0.6 | 75 ± 8 | 75 ± 7 | 0.8 |
| Heart rate (beats/ min) | 84 ± 15a | 85 ± 13 | 81 ± 12b | 75 ± 13c | 0.002 | 73 ± 7 | 79 ± 8 | 75 ± 6 | 0.07 | 81 ± 13e | 78 ± 11g | 0.2 |
| SDNN (msec) | 68 ± 27a | 69 ± 29 | 76 ± 27b | 76 ± 21c | 0.0026m | 95 ± 18 | 87 ± 25 | 87 ± 29 | 0.0324hr | 75 ± 28e | 84 ± 27g | 0.02 |
| VLF (msec2) | 1576 ± 1063a | 1525 ± 1166 | 1845 ± 1330b | 1977 ± 1129 c | 0.03 | 2612 ± 748 | 2346 ± 1074 | 2096 ± 961 | 0.07 | 1758 ± 1034e | 2174 ± 1263g | 0.03 |
| LF (msec2) | 504 (346 1468)a | 517 (343 1760) | 677 (452 2755)b | 878 (634 1644) c | 0.026m | 1570 (586 1728) | 1455 (300 1917) | 1424 (347 1715) | 0.1 | 665 (357 1574)e | 679 (563 2112)g | 0.003 |
| HF (msec2) | 257 (121 969)a | 189 (92 901) | 488 (144 1463)b | 265 (133 1101) c | 0.08 | 473 (309 1492) | 643 (116 1461) | 609 (155 2645) | 0.1 | 293 (150 1426)e | 473 (203 1492)g | 0.004 |
| LF/HF | 3.3 ± 1.7a | 3.3 ± 1.7 | 3.0 ± 1.6b | 3.8 ± 1.8c | 0.5 | 3.7 ± 2.1 | 3.5 ± 1.4 | 3.0 ± 1.4 | 0.2 | 3.2 ± 1.5e | 3.2 ± 1.8g | 0.6 |
| Sleeping heart rate (beats/min) | 75 ± 14a | 73 ± 13 | 71 ± 12b | 66 ± 10d | 0.1 | 68 ± 12 | 65 ± 10 | 66 ± 7 | 0.7 | 72 ± 13e | 70 ± 12g | 0.5 |
| Sleeping SDNN (msec) | 81 ± 41a | 79 ± 38 | 87 ± 36b | 87 ± 17d | 0.4 | 101 ± 25 | 109 ± 53 | 100 ± 37 | 0.7 | 88 ± 40e | 92 ± 32g | 0.5 |
| Sleeping VLF (msec2) | 2015 ± 1636a | 1727 ± 1056 | 2076 ± 1311b | 2273 ± 690d | 0.2 | 3636 ± 1619 | 3312 ± 2089 | 2710 ± 1405 | 0.4 | 2259 ± 1559e | 2651 ± 1588g | 0.2 |
| Sleeping LF (msec2) | 762 (370 1815)a | 529 (325 2856) | 838 (445.9 3424)b | 833 (636 2878)d | 0.1 | 1974 (586 2391) | 2469 (383 2888) | 2110 (432 2851) | 0.6 | 1138 (438 2354)e | 1024 (586 2407)g | 0.2 |
| Sleeping HF (msec2) | 366 (143 1331)a | 408 (123 1611) | 688 (149 1986)b | 412 (159 1505)d | 0.1 | 822 (245 1653) | 1282 (162 2952) | 1224 (209 4112) | 0.7 | 466 (193 1922)e | 822 (212 1653)g | 0.6 |
| Sleeping LF/HF | 2.8 ± 1.8a | 2.5 ± 2.2 | 2.5 ± 2.0b | 3.6 ± 2.4d | 0.6 | 3.4 ± 2.8 | 2.3 ± 1.2 | 2.3 ± 1.2 | 0.4 | 2.6 ± 1.6e | 2.8 ± 2.3g | 1 |
| Waking heart rate (beats/min) | 87 ± 14a | 89 ± 13 | 84 ± 12b | 76 ± 13c | 0.00046m | 75 ± 6 | 83 ± 8 | 77 ± 6 | 0.1 | 84 ± 13e | 80 ± 11g | 0.1 |
| Waking SDNN (msec) | 63 ± 23a | 65 ± 23 | 74 ± 28b | 75 ± 22c | 0.0026mo | 93 ± 17 | 81 ± 21 | 84 ± 27 | 0.029 24hr | 71 ± 26e | 81 ± 26g | 0.03 |
| Waking VLF (msec2) | 1366 ± 926a | 1375 ± 1002 | 1871 ± 1510b | 1937 ± 1252c | 0.6 | 2316 ± 823 | 2092 ± 886 | 1920 ± 902 | 0.8 | 1560 ± 934e | 2035 ± 1292g | 0.1 |
| Waking LF (msec2) | 456 (326 1369)a | 514 (290 1416) | 669 (386 2548)b | 905 (592 1487)c | 0.0042w, 6mo | 1349 (609 1516) | 1362 (342 1772) | 1027 (392 1525) | 0.8 | 555 (346 1424)e | 750 (512 1684)g | 0.01 |
| Waking HF (msec2) | 214 (99 870)a | 141 (84 713) | 312 (123 1346)b | 252 (133 1024)c | 0.016mo | 376 (328 1318) | 474 (104 1069) | 439 (140 1752) | 0.7 | 229 (115 1291)e | 341 (198 1318)g | 0.2 |
| Waking LF/HF | 3.4 ± 1.8a | 3.5 ± 1.7 | 3.1 ± 1.6b | 3.8 ± 1.7c | 0.1 | 3.8 ± 2.0 | 3.9 ± 1.6 | 3.3 ± 1.5 | 0.2 | 3.4 ± 1.6e | 3.4 ± 1.7g | 0.5 |
Data show the mean ± SD for normally distributed metrics and median (interquartile range) for nonnormally distributed metrics. Patients in the initiation cohort have data prior to initiation of metreleptin (baseline) and after 2 weeks and 6 months of metreleptin administration. Patients in the withdrawal cohort have data during metreleptin treatment (baseline) and after 2 weeks of metreleptin withdrawal. The combined cohort includes baseline and 2 week data in both groups, categorized by on or off metreleptin status.
Abbreviations: BP, blood pressure; HF, high-frequency component is associated with parasympathetic regulation; LF, low-frequency component is associated with both sympathetic and parasympathetic regulation; LF/HF ratio is associated with sympathetic regulation; SDNN, standard deviation of the normal-to-normal interbeat interval is a measure of overall heart rate variability; VLF, very low frequency component of heart rate variability power spectrum is associated with renin/angiotensin regulation of heart rate variability. *Overall P-value for linear mixed-effects regression is shown. 24hrP < 0.05 for baseline vs 24 h. 2wP < 0.05 for baseline vs 2 weeks. 6mP < 0.05 for baseline vs 6 months.
a n=13.
b n=12.
c n=8.
d n=7.
e n=20.
f n=6.
g n=19.
Figure 6.
Effects of metreleptin initiation or withdrawal on blood pressure and heart rate variability metrics in patients with lipodystrophy. Systolic blood pressure (A) and diastolic blood pressure (B) did not change from baseline to 24 h (24hr; red circles), 2 weeks (2w; gray circles) or 6 months (6mo; blue circles) after metreleptin administration or after 24 h (24hr; red squares) or 2 weeks (2w; gray squares) following metreleptin withdrawal. Heart rate (C) did not change from baseline to 24 h or 2 weeks after metreleptin initiation but decreased at 6 months. No changes in heart rate were observed at any time point following metreleptin withdrawal. (D) SD of the normal-to-normal distance (SDNN) increased (associated with decreased cardiovascular disease risk) after 6 months of metreleptin initiation and decreased (associated with increased cardiovascular disease risk) after 24 h of metreleptin withdrawal. (E) The very low frequency (VLF) component of heart rate variability, a measure of renin-angiotensin-aldosterone system effects on heart rate variability, (F) low frequency power component (LF), a measure of both sympathetic and parasympathetic influence on heart rate variability, and (G) high frequency power component (HF), a measure of parasympathetic impact on heart rate variability, did not change from baseline to 24 h, 2 weeks, or 6 months after metreleptin initiation or after 24 h and 2 weeks following metreleptin withdrawal. (H) The LF/HF ratio, a measure of sympathetic influence on heart rate variability, did not change at any time point following metreleptin administration or withdrawal. Patients with generalized lipodystrophy are shown as circles or squares without borders and those with partial lipodystrophy as circles with black borders. Comparisons were made using Wilcoxon rank-signed test and paired t-test for normally and nonnormally distributed data. Linear mixed effects models applying a Dunnett correction for multiple comparisons were used for outcomes measured at >2 time points. P < 0.05 represented statistical significance. P-values are 2-sided.
Discussion
This study demonstrates that treatment with metreleptin reduces EE in patients with lipodystrophy. Several studies in leptin-deficient rodents have shown increased EE following leptin replacement (1,2). Small mammals (eg, rodents), unlike large mammals (eg, humans), have a large surface area to volume ratio resulting in higher energy expended toward heat production to maintain core body temperature (22). To produce heat, rodents depend on thermogenesis mediated by BAT, which is 100-fold greater in relative mass compared to humans (22). In contrast, patients with congenital leptin deficiency are not hypothermic (4); furthermore, leptin replacement had no effect on EE in patients with diverse causes of hypoleptinemia, including acute (5,23) or chronic caloric restriction (24,25), hypothalamic amenorrhea (23,26), and congenital leptin deficiency (27), as well as obesity without leptin deficiency (24,28,29). Altogether this evidence suggests that although leptin is critical in regulating core body temperature (and hence EE) in rodents, its effects on EE in humans may be too small to be detected. Although the majority of human data supported a null effect of leptin on EE, a single study showed increased EE with metreleptin administration (6,7). In this study, conducted in healthy patients with hypoleptinemia due to 10% weight reduction, metreleptin treatment to achieve preweight loss plasma leptin concentrations led to increased EE, thyroid hormone, epinephrine, and sympathetic nervous system tone—consistent with rodent studies.
Surprisingly, our study of patients with lipodystrophy treated with metreleptin showed a 5.0% to 5.9% reduction in EE (driven predominantly by decreased REE), increased bioactive thyroid hormone and no effect on sympathetic nervous system activity. The results of our study deviate from existing literature and suggest that the mechanisms by which leptin regulates EE vary in different clinical contexts (Table 5). In lipodystrophy, adipose tissue deficiency causes low plasma leptin, excess caloric intake, insulin resistance, steatohepatitis, and hypertriglyceridemia. The metabolic abnormalities seen in lipodystrophy are associated with increases in energy requiring metabolic processes, including increased de novo lipogenesis (DNL) (30) and increased gluconeogenesis (GNG) (31). As previously reported, in patients with lipodystrophy, metreleptin decreases DNL and improves insulin sensitivity, which could lead to decreased GNG. It is possible that, in this unique cohort of patients, reductions in the energetic processes of DNL and GNG after metreleptin administration may offset the increased EE via increased thyroid hormones or sympathetic nervous system activity after metreleptin administration. Metreleptin also decreased urinary glucose excretion; however, the decrease in EE after metreleptin persisted even after accounting for this.
Table 5.
Differences in metabolic, neuroendocrine and autonomic responses to leptin replacement in various models of leptin deficiency
| 10% weight loss (7) | Lipodystrophy | Congenital Leptin Deficiency (34,42) | Hypothalamic Amenorrhea (23,43) | Acute Starvation (72-h fast) (5,23) | Chronic Starvation (24,25) | Obesity (24,28,29) | |
|---|---|---|---|---|---|---|---|
| Energy Expenditure (TEE) | ↑ | ↓ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Skeletal muscle work efficiency | ↓ | ↔ | — | — | — | — | — |
| Thyroid hormone | ↑ | ↑ | ↑ | ↑/↔ | ↑ | — | — |
| Epinephrine | ↑ | ↔ | ↓ | — | ↔ | — | — |
| Sympathetic nervous system | ↑ | ↔ | ↑ | — | ↔ | — | — |
In rodents, leptin deficiency is associated with decreased thyroid hormone, uncoupling protein 1 messenger RNA expression, and EE, which are restored with leptin replacement (32). Thyroid hormone increases EE through increased adrenergic signaling in BAT (33). Thus, the effect of leptin on EE in rodents is likely mediated by thyroid hormone action in BAT via adrenergic signaling. The relevance of thyroid hormone signaling on BAT thermogenesis has also been shown in humans. Studies have shown increased adipose tissue and skeletal muscle glucose uptake after administration of thyroid hormone or in endogenous hyperthyroid states, supporting the notion that elevations in thyroid hormone increase EE through promotion of cellular metabolism in tissues such as the heart, skeletal muscle, and adipose tissue (33). Additionally, replacing leptin to preweight loss concentrations restored EE, thyroid hormone, and sympathetic tone (7), supporting the hypothesis that leptin may increase EE through increased thyroid hormone and subsequent activation of the sympathetic nervous system in humans. Consistent with prior studies, our findings confirm that metreleptin administration in patients with lipodystrophy increased thyroid hormone, despite a reduction in EE and null effect on sympathetic tone. Additionally, although there was no change in core body temperature following metreleptin administration, there was an increase in weighted skin temperature in a subgroup analysis of patients with generalized lipodystrophy following metreleptin treatment, which could be related to increased thyroid hormone.
The effect of leptin on EE through alterations in thyroid hormone is thought to be mediated by sympathetic nervous system activity. In rodents, leptin increases mean arterial pressure by increasing sympathetic nervous system activity (3). Furthermore, hyperleptinemic states, such as obesity, are associated with high blood pressure, increased REE, activation of the renin-angiotensin-aldosterone system, and increased sympathetic tone, suggesting an association between high leptin, increased sympathetic tone and hypertension (3). In addition, leptin-deficient states, such as congenital leptin deficiency, have been associated with diminished sympathetic tone, supporting the link between leptin and sympathetic nervous system activity (34). Likewise, metreleptin replacement to achieve preweight loss plasma leptin in 10% weight-reduced healthy humans increased sympathetic tone, decreased parasympathetic tone, and increased epinephrine, providing causal evidence that leptin increases sympathetic nervous system activity in the transition from relative leptin deficiency to leptin sufficiency in humans. In contrast to existing literature, the current study of patients with lipodystrophy shows that metreleptin administration had no effect on cardiac sympathetic tone, catecholamines, or blood pressure, despite elevations in thyroid hormone. Altogether, the lack of an effect of metreleptin on sympathetic nervous system activity despite elevations in thyroid hormone in patients with lipodystrophy suggest that the effects of leptin on EE mediated through thyroid hormone stimulation of sympathetic nervous system activity may require the presence of normally functioning adipose tissue. This hypothesis is supported by a rodent study comparing mice with hypoleptinemia (ob/ob) due to leptin gene mutation vs hypoleptinemia from generalized lipodystrophy (A-ZIP/F1 mice) (35,36). This study showed that both ob/ob and lipodystrophic mice experience reduced EE and increased torpor with caloric restriction. However, whereas leptin administration reversed these phenomena in ob/ob mice, neither leptin nor thyroid hormone administration reversed hypothermia and torpor in lipodystrophic mice. This suggests that torpor is not only dependent on the presence of hypoleptinemia but also leptin-independent adipose tissue signaling and is consistent with a model in which leptin’s effects to increase EE via increased thyroid hormone requires presence of adipose tissue. Furthermore, if we presume that humans with lipodystrophy have a partial or complete loss of brown fat, then the effect of thyroid hormone action to stimulate EE in brown fat via sympathetic nervous system signaling would be diminished. Other explanations for the null effect of metreleptin on sympathetic nervous system activity in this study include the use of noninvasive and less sensitive techniques for measuring markers of sympathetic tone (eg, use of HrV analysis) compared to pharmacologic blockade (gold standard), measurement of autonomic inputs only to the heart, and measurement of catecholamines using assays with a high lower limit of detection. Despite the low-sensitivity catecholamine assays, it is notable that, among patients with detectable levels of norepinephrine, norepinephrine decreased after metreleptin withdrawal. The norepinephrine data are consistent with rodent and human studies showing lower norepinephrine during states of leptin deficiency (7,37-39). The decrease in heart rate and increase in SDNN after 6 months of metreleptin treatment is consistent with previous studies (40) and suggests that long-term metreleptin therapy may be associated with improved cardiovascular health in patients with lipodystrophy.
The strength of this study is the use of human models deficient in both leptin and adipose tissue, which provides unique insight not only into the effects of leptin deficiency and replacement but also biological actions of leptin that may be mediated through adipose tissue. Prior studies have been limited to rodent models with leptin gene mutations and human models of leptin deficiency post-weight loss or congenital leptin deficiency. The inclusion of both metreleptin initiation and withdrawal cohorts helped to illustrate effects showing opposite directions of change after initiation and withdrawal of metreleptin, which strengthened the biological plausibility of our findings. Although the majority of metreleptin effects were only observed in the initiation cohort, the thyroid hormone effects were observed in both initiation and withdrawal cohorts. A limitation of this study was the small sample size; however, lipodystrophy is rare disease. Although we were able to detect reductions in plasma leptin, thyroid hormone and norepinephrine following 2 weeks of metreleptin withdrawal, there was no significant effect of metreleptin withdrawal on EE or metabolic parameters other than insulin sensitivity. The lack of changes in the withdrawal cohort may be explained by the duration of metreleptin treatment [mean ± SD, 7.7 ± 4.7 years (range: 0.9-14.5)] and normal metabolic profile at the time of study enrollment, suggesting that 2 weeks of metreleptin withdrawal may be insufficient to detect changes in metabolic parameters in the withdrawal cohort. When comparing the ON- vs OFF-metreleptin periods in the combined initiation and withdrawal cohorts, we were able to detect differences in EE, suggesting that the null effects on EE observed following metreleptin withdrawal could also be due to small sample size. In this study, the lack of an effect of metreleptin on sympathetic nervous system activity may be explained by our assessment of autonomic nervous system activity using spectral analysis of HrV, as opposed to pharmacologic blockade used by Rosenbaum et al (7). Spectral analysis of HrV has several limitations in that it only detects a small fraction of total autonomic input (ie, cardiac), is highly variable from person to person, and cannot fully discriminate sympathetic and parasympathetic tone (41). Finally, we measured plasma catecholamines using an assay with a high lower limit of detection as opposed to urinary catecholamine excretion in Rosenbaum et al (7); thus, small changes in plasma catecholamine concentrations after metreleptin initiation or withdrawal were not captured and may explain the inconsistent effects on catecholamines observed in this study.
In conclusion, this study demonstrated that, like other leptin deficient models, metreleptin administration in patients with lipodystrophy increased thyroid hormone. However, in contrast to other models, metreleptin decreased EE and did not increase sympathetic tone. These findings suggest that leptin’s effects to increase EE via increased thyroid hormone and sympathetic tone require the presence of functional adipose tissue. Moreover, the decrease in EE following metreleptin administration in lipodystrophy may provide insight into the energetic cost of gluconeogenesis and de novo lipogenesis in patients with insulin resistance.
Acknowledgments
This work was supported by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases and the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, Genentech, the American Association for Dental Research, the Colgate-Palmolive Company, and other private donors.
Financial Support: This study was supported by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases (grant numbers ZIA DK075084-08, ZIA DK071013).
Clinical Trial Information: NCT01778556.
Author Contributions: AG and EQ analyzed data and wrote the manuscript. AV and BM reviewed manuscript. RJ Brychta, JC, MS, CM, and KC conducted experiments and reviewed manuscript. RJ Brown designed the study, conducted experiments, acquired data, analyzed data, and wrote the manuscript.
Glossary
Abbreviations
- BAT
brown adipose tissue
- EE
energy expenditure
- HF
high frequency
- HrV
heart rate variability;
- LF
low frequency
- LMS
least mean square
- nREE
nonresting energy expenditure
- REE
resting energy expenditure
- SDNN
standard deviation of the normal-to-normal interbeat interval
- SEE
sleep energy expenditure
- TEE
24-h total energy expenditure
- TEF
thermic effect of feeding
- VLF
very low frequency.
Additional Information
Disclosures: Metreleptin for the study was donated by Aegerion Pharmaceuticals.
Data Availability
Some or all datas sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763-770. [DOI] [PubMed] [Google Scholar]
- 2. Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540-543. [DOI] [PubMed] [Google Scholar]
- 3. Pandit R, Beerens S, Adan RAH. Role of leptin in energy expenditure: the hypothalamic perspective. Am J Physiol Regul Integr Comp Physiol. 2017;312(6):R938-R947. [DOI] [PubMed] [Google Scholar]
- 4. Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903-908. [DOI] [PubMed] [Google Scholar]
- 5. Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111(9):1409-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87(5):2391-2394. [DOI] [PubMed] [Google Scholar]
- 7. Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115(12):3579-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diker-Cohen T, Cochran E, Gorden P, Brown RJ. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab. 2015;100(5):1802-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Safar Zadeh E, Lungu AO, Cochran EK, et al. The liver diseases of lipodystrophy: the long-term effect of leptin treatment. J Hepatol. 2013;59(1):131-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moran SA, Patten N, Young JR, et al. Changes in body composition in patients with severe lipodystrophy after leptin replacement therapy. Metabolism. 2004;53(4):513-519. [DOI] [PubMed] [Google Scholar]
- 11. Petersen KF, Oral EA, Dufour S, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109(10):1345-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown RJ, Valencia A, Startzell M, et al. Metreleptin-mediated improvements in insulin sensitivity are independent of food intake in humans with lipodystrophy. J Clin Invest. 2018;128(8):3504-3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Southgate DA, Durnin JV. Calorie conversion factors. An experimental reassessment of the factors used in the calculation of the energy value of human diets. Br J Nutr. 1970;24(2):517-535. [DOI] [PubMed] [Google Scholar]
- 14. Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78(6):1568-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schutz Y, Bessard T, Jéquier E. Diet-induced thermogenesis measured over a whole day in obese and nonobese women. Am J Clin Nutr. 1984;40(3):542-552. [DOI] [PubMed] [Google Scholar]
- 16. Rosenbaum M, Vandenborne K, Goldsmith R, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R183-R192. [DOI] [PubMed] [Google Scholar]
- 17. International Organization for Standardization. Ergonomics—Evaluation of Thermal Strain by Physiological Measurements. ISO 9886:(E)2004. International Organization for Standardization; 2004. [Google Scholar]
- 18. Ramanathan NL. A new weighting system for mean surface temperature of the human body. J Appl Physiol. 1964;19:531-533. [DOI] [PubMed] [Google Scholar]
- 19. Hirsch J, Leibel RL, Mackintosh R, Aguirre A. Heart rate variability as a measure of autonomic function during weight change in humans. Am J Physiol. 1991;261(6 Pt 2):R1418-R1423. [DOI] [PubMed] [Google Scholar]
- 20. Ebihara K, Kusakabe T, Hirata M, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab. 2007;92(2):532-541. [DOI] [PubMed] [Google Scholar]
- 21. Musso C, Cochran E, Javor E, Young J, Depaoli AM, Gorden P. The long-term effect of recombinant methionyl human leptin therapy on hyperandrogenism and menstrual function in female and pituitary function in male and female hypoleptinemic lipodystrophic patients. Metabolism. 2005;54(2):255-263. [DOI] [PubMed] [Google Scholar]
- 22. Celi FS, Le TN, Ni B. Physiology and relevance of human adaptive thermogenesis response. Trends Endocrinol Metab. 2015;26(5):238-247. [DOI] [PubMed] [Google Scholar]
- 23. Chrysafi P, Perakakis N, Farr OM, et al. Leptin alters energy intake and fat mass but not energy expenditure in lean subjects. Nat Commun. 2020;11(1):5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hukshorn CJ, Saris WH. Leptin and energy expenditure. Curr Opin Clin Nutr Metab Care. 2004;7(6):629-633. [DOI] [PubMed] [Google Scholar]
- 25. Hukshorn CJ, Westerterp-Plantenga MS, Saris WH. Pegylated human recombinant leptin (PEG-OB) causes additional weight loss in severely energy-restricted, overweight men. Am J Clin Nutr. 2003;77(4):771-776. [DOI] [PubMed] [Google Scholar]
- 26. Chou SH, Chamberland JP, Liu X, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci U S A. 2011;108(16):6585-6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110(8):1093-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hukshorn CJ, Saris WH, Westerterp-Plantenga MS, Farid AR, Smith FJ, Campfield LA. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J Clin Endocrinol Metab. 2000;85(11):4003-4009. [DOI] [PubMed] [Google Scholar]
- 29. Westerterp-Plantenga MS, Saris WH, Hukshorn CJ, Campfield LA. Effects of weekly administration of pegylated recombinant human OB protein on appetite profile and energy metabolism in obese men. Am J Clin Nutr. 2001;74(4):426-434. [DOI] [PubMed] [Google Scholar]
- 30. Baykal AP, Parks EJ, Shamburek R, et al. Leptin decreases de novo lipogenesis in patients with lipodystrophy. JCI Insight. 2020;5(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sekizkardes H, Chung ST, Chacko S, et al. Free fatty acid processing diverges in human pathologic insulin resistance conditions. J Clin Invest. 2020;130(7):3592-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Commins SP, Watson PM, Padgett MA, Dudley A, Argyropoulos G, Gettys TW. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology. 1999;140(1):292-300. [DOI] [PubMed] [Google Scholar]
- 33. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. von Schnurbein J, Manzoor J, Brandt S, et al. Leptin is not essential for obesity-associated hypertension. Obes Facts. 2019;12(4):460-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gavrilova O, Leon LR, Marcus-Samuels B, et al. Torpor in mice is induced by both leptin-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 1999;96(25):14623-14628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Skowronski AA, Ravussin Y, Leibel RL, LeDuc CA. Energy homeostasis in leptin deficient Lepob/ob mice. Plos One. 2017;12(12):e0189784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Satoh N, Ogawa Y, Katsuura G, et al. Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes. 1999;48(9):1787-1793. [DOI] [PubMed] [Google Scholar]
- 38. Leigh FS, Kaufman LN, Young JB. Diminished epinephrine excretion in genetically obese (ob/ob) mice and monosodium glutamate-treated rats. Int J Obes Relat Metab Disord. 1992;16(8):597-604. [PubMed] [Google Scholar]
- 39. Folgueira C, Beiroa D, Porteiro B, et al. Hypothalamic dopamine signaling regulates brown fat thermogenesis. Nat Metab. 2019;1(8):811-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hart J. Association between heart rate variability and manual pulse rate. J Can Chiropr Assoc. 2013;57(3):243-250. [PMC free article] [PubMed] [Google Scholar]
- 41. Hirsch J, Mackintosh RM. Measuring activity of the autonomic nervous system in humans. Obes Res. 2003;11(1):2-4. [DOI] [PubMed] [Google Scholar]
- 42. Galgani JE, Greenway FL, Caglayan S, Wong ML, Licinio J, Ravussin E. Leptin replacement prevents weight loss-induced metabolic adaptation in congenital leptin-deficient patients. J Clin Endocrinol Metab. 2010;95(2):851-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351(10):987-997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datas sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.