Abstract
Context
Autism spectrum disorders (ASDs) are a group of conditions characterized by impaired social function and repetitive behaviors. Their etiology is largely unknown.
Objective
This work aims to examine the associations of maternal second-trimester and cord blood leptin and adiponectin levels with ASDs in offspring.
Methods
We used data from 1164 mother-child pairs enrolled in Project Viva, a prospective prebirth cohort. We used logistic regression analysis to examine the associations of leptin and adiponectin levels in maternal second-trimester blood and cord blood obtained at birth with ASDs. Additionally, we examined the association of maternal prepregnancy body mass index (BMI) as an exposure. Main outcome measures included doctor-diagnosed ASDs reported by mothers using questionnaires in midchildhood and early adolescence.
Results
The cumulative incidence of ASDs was 3.4%. Maternal prepregnancy BMI (per 5 points) was positively associated with ASDs in a logistic regression model adjusted for maternal race/ethnicity, education, smoking status and child sex (adjusted odds ratio [OR] 1.38; 95% CI, 1.06-1.79). Higher second-trimester adiponectin was associated with lower odds of ASD in offspring (unadjusted OR 0.49; 95% CI, 0.30-0.78; and OR 0.54; 95% CI, 0.32-0.91 after adjusting for maternal race/ethnicity, education, child sex, OR 0.55; 95% CI, 0.33-0.93 after adjusting for BMI, gestational weight gain, gestational diabetes, and smoking status). Maternal leptin and cord blood leptin and adiponectin levels were not associated with ASDs.
Conclusion
Prepregnancy BMI and adiponectin during pregnancy may be useful as a tool to monitor the risk of autism. Increasing adiponectin levels prenatally may play a role in the prevention of ASDs.
Keywords: autism spectrum disorder, pregnancy, BMI, leptin, adiponectin
Autism spectrum disorders (ASD) are a group of developmental disorders, clinically characterized by impairment of social interaction, and repetitive and restrictive behaviors (1). The prevalence of ASD in the United States is estimated to be 1 in 54 (~ 2%), and the incidence has been increasing substantially over the past decades (2). While the complete pathophysiology is not fully understood, there is growing evidence that autism risk is related to intrauterine factors such as maternal obesity, gestational weight gain (GWG), and maternal immune activation (3, 4). Because there is no known effective treatment for autism, efforts to identify and ameliorate risk factors are important.
The association of maternal prepregnancy body mass index (BMI) with ASD in the offspring has previously been reported (5, 6). A recent study showed that this association could be confounded by socioeconomic factors such as race, education, and smoking status (7), and the extent to which these associations are mediated by specific hormonal factors needs to be clarified.
The second trimester of pregnancy gestation is known as a period that fetus is extremely vulnerable to intrauterine infection and inflammation, and as the most important period in development of sulci and the formation of hippocampus (8-10). Whether maternal circulating hormone levels in the second trimester are associated with the development of ASD remains unknown.
Adipokines, hormones secreted by adipose tissue, are associated with adiposity and act as proinflammatory or anti-inflammatory cytokines (11). Leptin is an adipokine with appetite-suppressing and neuroendocrine effects acting via hypothalamic receptors (12, 13). Elevated leptin levels are associated with obesity and metabolic syndrome (13). Several cross-sectional studies in children have revealed that elevated circulating levels of leptin were positively associated with ASDs, but this may simply reflect fat mass (14, 15). Adiponectin is an adipokine and endogenous insulin sensitizer that enhances glucose uptake and fatty acid oxidation, which has anti-inflammatory properties (16, 17). The circulating levels of adiponectin are determined by genetic and environmental factors and are inversely proportional to the metabolically adverse central fat deposition (18). Adiponectin has anti-inflammatory effects and functions as an immune modulator, and its levels are associated with protection against metabolic syndrome and obesity-related comorbidities including neurocognitive dysfunction in adults (18-20). Previous cross-sectional studies have reported an inverse association of adiponectin with ASDs, but no prior study has evaluated this association prospectively (21, 22).
The purpose of this study was to examine the associations of 1) maternal second-trimester and cord blood leptin and adiponectin levels with ASDs and confirm the association of 2) maternal prepregnancy BMI with ASDs in children after adjusting for maternal race/ethnicity, education, smoking status, and child sex, using data from a well-established prebirth cohort with follow-up into adolescence. We hypothesized that higher leptin levels and lower adiponectin levels in maternal circulation in the second-trimester and in cord blood would be independently associated with the development of ASDs.
Materials and Methods
Study Population
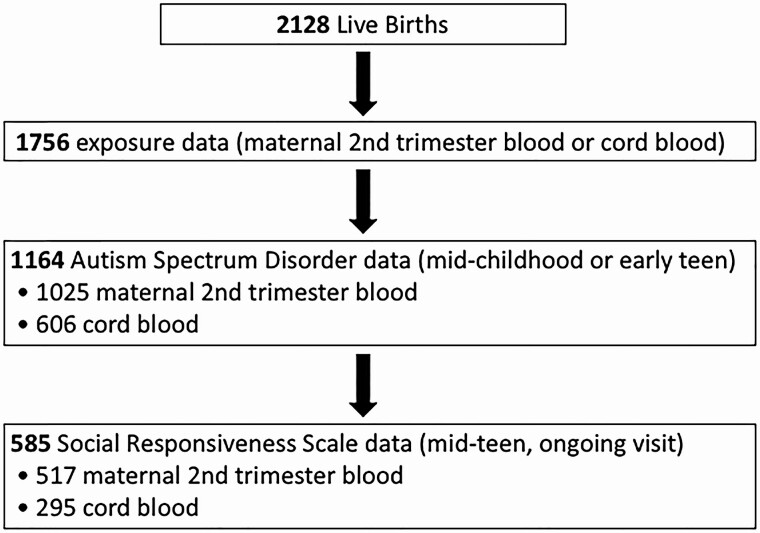
This is a prospective longitudinal cohort analysis of data from a prebirth cohort, Project Viva. We recruited pregnant women in 1999 to 2002 who attended their initial prenatal visit at Atrius Harvard Vanguard Medical Associates, a multispecialty group practice in eastern Massachusetts (23, 24). We performed in-person study visits with the mother at the end of the first and the second trimesters of pregnancy, and both with the mother and child immediately after delivery, during infancy, and in early childhood (median age 3.3 years), midchildhood (median age 7.7 years), and early adolescence (median 13.0 years) (23). We are currently conducting midteen visits (median age 17.4 years [range, 15.4-20.2 years], ongoing). We obtained written informed consent from all participating mothers at each in-person visit and assent from all participating children younger than 18 years. Adolescents age 18 years and older provided their own written informed consent. Out of 2128 live births, 1756 had maternal second-trimester (n = 1540) and/or infant cord blood (n = 881) exposures. We excluded from the present analysis 592 children who were missing ASD status, leaving 1164 mother-child pairs in our analysis sample. Of the 1164, 1025 had maternal second-trimester blood and 606 had infant cord blood (Fig. 1). The institutional review boards of participating institutions approved the study.
Figure 1.
Diagram of the study population.
Exposures
Circulating adipokines
We included as exposures the circulating levels of leptin and adiponectin in maternal second-trimester blood, and leptin and adiponectin in cord blood. As previously described, clinicians collected maternal blood during the second trimester of pregnancy and cord blood from the umbilical vein at the time of delivery of the infant, and whole blood was refrigerated, centrifuged, and plasma samples were stored in liquid nitrogen (−80 °C) (23, 24). Leptin and adiponectin were measured by radioimmunoassay (RIA, Linco Research Inc), as described previously (24, 25).
Maternal prepregnancy body mass index
Mothers reported their prepregnancy weight and height at the time of enrollment, and we calculated prepregnancy BMI as weight in kilograms kg divided by height in meters squared. We analyzed BMI both as a continuous variable and as a categorical variable (BMI < 25: normal; 25-< 30: overweight; ≥ 30: obesity).
Main Outcome
On the midchildhood (median age, 7.7 years) and early-teen questionnaires (median age, 13.0 years), we asked parents, “Have you ever been told by a health care professional, such as a doctor, physician assistant, or a nurse practitioner, that your child has autism or autism spectrum disorder (eg, Asperger syndrome, pervasive developmental disorder)?” We coded ASDs as “yes” if the parent reported yes on the midchildhood or early-teen questionnaire; we coded ASDs as “no” if the parent reported no on both questionnaires.
The social responsive scale (SRS) provides the quantitative measurement of social ability in children and adolescents ages 4 to 18 years from a set of 65 questions with evaluations of 5 domains, including social awareness, social cognition, social communication, social motivation, and restricted interests and repetitive behavior (26). Higher scores indicate more severe degree of social impairments (26). As of February 2020, mothers completed the SRS for 593 adolescents, of whom 531 had previously assayed maternal second-trimester blood and 295 had cord blood (see Fig. 1). We used sex-standardized SRS T scores for analysis.
Covariates
We collected information on sociodemographic and other variables including age, race and ethnicity, parity, education level, and pregnancy smoking status from questionnaires and interviews as described in previous reports (23). We calculated total GWG and GWG from prepregnancy to day 182 (up to second trimester, approximately when blood samples were collected) as previously reported (23). Gestational diabetes mellitus (GDM) screens were performed by a nonfasting 50-g glucose challenge test (GCT) between 26 and 28 weeks of pregnancy. For those participants who had abnormal GCT (> 140 mg/dL), a 3-hour fasting oral glucose tolerance test (GTT) was performed (27). Isolated hyperglycemia (IH) was diagnosed when GCT was abnormal but GTT was normal, impaired glucose tolerance (IGT) was diagnosed if there was one abnormal GTT result, and GDM was diagnosed when there were 2 or more abnormal GTT values (28).
We obtained infant birth weight from hospital medical records. We obtained gestational age in days as previously reported (29). We calculated sex-specific birthweight for gestational age z-scores and BMI z-scores from US national reference data (30, 31).
Statistical Analysis
We first examined bivariate associations of maternal and infant/child characteristics with ASD status by t tests and chi-square tests. We natural–log-transformed hormone levels to achieve normality. Next, we performed unadjusted and multivariable-adjusted logistic regression analyses to examine the associations of second-trimester and cord blood hormone levels with ASDs. We adjusted models for potential confounders, which were selected a priori, including maternal race/ethnicity, education, pregnancy smoking status, prepregnancy BMI and GWG (for the second-trimester hormones, GWG up to the second trimester; for cord blood, total GWG until delivery), gestational glucose status (GDM or IGT vs normal or IH), and child sex.
We also performed logistic regression analysis to evaluate the associations of maternal prepregnancy BMI with ASDs. We performed both unadjusted analyses and adjusted analyses models adjusted for maternal race/ethnicity, education, pregnancy smoking status, and child sex. Next, in the subset of participants on whom we collected the SRS (n = 585), we performed unadjusted and multivariable-adjusted linear regression analysis to examine the associations of second-trimester and cord blood biomarkers with SRS T scores. We used the same covariates that we used for the ASD outcome (except we did not adjust for sex since SRS T scores are sex specific). We performed all analyses using SAS 9.4.
Results
Characteristics of the Study Population
Among the 1164 children included in the analysis, parents reported doctor-diagnosed ASDs for 39 (3.4%). The cumulative incidence of ASDs was higher among boys (32/595 = 5.4%) vs girls (7/569 = 1.2%), as expected. Compared with mothers of children without ASDs, mothers of children with ASDs were less likely to be White (64% vs 72%) and to be college graduates (59% vs 71%), and more likely to have overweight/obesity (62% vs 34%) and to have GDM or IGT (21% vs. 8%) (Table 1).
Table 1.
Characteristics of the study population, overall and by autism spectrum disorder status (N = 1164)
| ASD in childhood | P | |||
|---|---|---|---|---|
| Overall | Yes | No | ||
| n = 1164 | n = 39 | n = 1125 | ||
| Mother | ||||
| Maternal age at enrollment, y | 32.3 (5.0) | 32.8 (3.9) | 32.3 (5.1) | .39 |
| Parity | ||||
| Nulliparous | 573 (49) | 23 (59) | 550 (49) | .22 |
| Multiparous | 591 (51) | 16 (41) | 575 (51) | |
| Prepregnancy BMI | 24.7 (5.1) | 27.0 (5.6) | 24.6 (5.1) | .004 |
| Prepregnancy BMI category, % | .003 | |||
| < 25 | 747 (64) | 15 (38) | 732 (65) | |
| 25-< 30 | 252 (22) | 14 (36) | 238 (21) | |
| ≥ 30 | 161 (14) | 10 (26) | 151 (13) | |
| Total gestational wt gain, kg | 15.5 (5.4) | 13.9 (5.7) | 15.6 (5.4) | .07 |
| Glucose status, % | .02 | |||
| Normal | 965 (83) | 27 (71) | 938 (84) | |
| IH | 102 (9) | 3 (8) | 99 (9) | |
| IGT | 32 (3) | 2 (5) | 30 (3) | |
| GDM | 60 (5) | 6 (16) | 54 (5) | |
| Race/ethnicity, % | .42 | |||
| Black | 158 (14) | 8 (21) | 150 (13) | |
| White | 830 (71) | 25 (64) | 805 (72) | |
| Other | 174 (15) | 6 (15) | 168 (15) | |
| Education ≥ college grad, % | .10 | |||
| No | 338 (29) | 16 (41) | 322 (29) | |
| Yes | 824 (71) | 23 (59) | 801 (71) | |
| Pregnancy smoking status, % | .59 | |||
| Never | 822 (71) | 25 (64) | 797 (71) | |
| Former | 227 (20) | 10 (26) | 217 (19) | |
| During pregnancy | 110 (9) | 4 (10) | 106 (9) | |
| Mode of delivery, cesarean, % | 252 (22) | 12 (31) | 240 (21) | .16 |
| Second-trimester leptin, ng/mL | 23.0 (18.8) | 27.6 (18.7) | 22.9 (18.8) | .16 |
| Second-trimester adiponectin, μg/mL | 14.3 (6.8) | 11.9 (7.7) | 14.4 (6.8) | .04 |
| Child | ||||
| Sex, % | < .001 | |||
| Boy | 595 (51) | 32 (82) | 563 (50) | |
| Girl | 569 (49) | 7 (18) | 562 (50) | |
| Gestational age, wk | 39.6 (1.6) | 39.1 (2.2) | 39.6 (1.6) | .17 |
| Birth wt for gestational age z score | 0.22 (0.96) | 0.22 (0.85) | 0.22 (0.96) | .99 |
| Cord blood leptin, ng/mL | 8.9 (6.6) | 7.2 (5.1) | 8.9 (6.6) | .28 |
| Cord blood adiponectin, μg/mL | 28.7 (6.7) | 29.7 (6.4) | 28.7 (6.7) | .54 |
| SRS T score in midteens | 47.3 (8.0) | 60.8 (13.8) | 46.8 (7.3) | < .001 |
| SRS, total raw score, in midteens | 24.8 (19.8) | 60.3 (34.6) | 23.5 (17.9) | < .001 |
Data presented as mean (SD) or N (%). P values are from t test for continuous variables and chi-square for categorical variables.
Abbreviations: ASD, autism spectrum disorder; BMI, body mass index; IH, isolated hyperglycemia; IGT, impaired glucose tolerance, GDM, gestational diabetes mellitus; SRS, social responsiveness scale.
Maternal prepregnancy body mass index and autism spectrum disorders
Maternal prepregnancy BMI was associated with a higher risk of autism. For each 5-point increase in maternal BMI, the odds ratio (OR) (95% CI) for ASDs was 1.42 (95% CI, 1.11-1.82) in the unadjusted model and 1.38 (95% CI, 1.06-1.79) in the model adjusted for maternal race/ethnicity, education, pregnancy smoking status, and child sex. When we used maternal BMI as a categorical exposure, there was a stepwise increase in the OR of ASDs: Compared with the reference group (BMI < 25), the adjusted OR (95% CI) was 2.54 (95% CI, 1.19-5.43) for overweight (BMI ≥ 25 and < 30) and 3.16 (95% CI, 1.34-7.44) for obesity (BMI ≥ 30, Table 2).
Table 2.
Associations of maternal and infant weight-related characteristics with autism spectrum disorders in childhood
| Unadjusted | Adjusteda | |
|---|---|---|
| OR (95% CI) | ||
| Mothers | ||
| Prepregnancy BMI, per 5 | 1.42 (1.11-1.82) | 1.38 (1.06-1.79) |
| Prepregnancy BMI category | ||
| < 25 | 1.0 (reference) | 1.0 (reference) |
| 25-< 30 | 2.87 (1.37, 6.03) | 2.54 (1.19-5.43) |
| ≥ 30 | 3.23 (1.42-7.33) | 3.16 (1.34-7.44) |
| Infants | ||
| Birth wt for gestational age z score | 1.00 (0.72-1.40) | 1.05 (0.75-1.47) |
Values with P < .05 are presented in bold.
Abbreviations: BMI, body mass index; OR, odds ratio.
a Models were adjusted for maternal race/ethnicity, education, smoking status, and child sex.
Associations of leptin and adiponectin with autism spectrum disorders
The mean (SD) concentration of leptin in maternal second-trimester plasma tended to be somewhat higher in the ASD group compared to the non-ASD group (27.6 [18.7] ng/mL vs 22.9 [18.8] ng/mL, P = .16). On the other hand, the mean second-trimester adiponectin was significantly lower in mothers of children with ASDs compared to those without ASDs (11.9 [7.7] μg/mL vs 14.4 [6.8] μg/mL, P = .04) (see Table 1).
There was no significant positive association of maternal second-trimester leptin levels with ASDs (unadjusted OR 1.52; 95% CI, 0.88-2.63). This association remained nonsignificant after adjusting for maternal race/ethnicity, education and child sex (OR 1.39; 95% CI, 0.79-2.45), and after adjusting for maternal prepregnancy BMI, GWG, maternal gestational glucose status, and smoking status (OR 1.15; 95% CI, 0.56-2.38) (Table 3).
Table 3.
Associations of second-trimester and cord blood biomarkers with autism spectrum disorder in childhood (logistic regression)
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| OR (95% CI) | |||
| Log-transformed exposures | |||
| Mothers | |||
| Second-trimester leptin, ng/mL | 1.52 (0.88-2.63) | 1.39 (0.79-2.45) | 1.15 (0.56-2.38) |
| Second-trimester adiponectin, μg/mL | 0.49 (0.30-0.78) | 0.54 (0.32-0.91) | 0.55 (0.33-0.93) |
| Infants | |||
| Cord blood leptin, ng/mL | 0.85 (0.46-1.56) | 0.96 (0.50-1.83) | 0.73 (0.38-1.40) |
| Cord blood adiponectin, ug/mL | 1.86 (0.29-11.92) | 1.97 (0.30-13.01) | 2.15 (0.28-16.32) |
Values with P < .05 are presented in bold.
Model 1: unadjusted; model 2: adjusted for maternal race/ethnicity, education, and child sex; model 3: adjusted for prepregnancy body mass index, gestational weight gain, gestational glucose status, and smoking status.
Abbreviation: OR, odds ratio.
Importantly, higher second-trimester adiponectin was associated with lower odds of ASDs in the offspring (unadjusted OR 0.49; 95% CI, 0.30-0.78). The association remained significant after adjusting for maternal race/ethnicity, education, and child sex (OR 0.54; 95% CI, 0.32-0.91; model 2), and after adjusting for maternal prepregnancy BMI, GWG, maternal gestational glucose status, and smoking status (OR 0.55; 95% CI, 0.33-0.93; model 3) (see Table 3).
Cord blood levels of leptin and adiponectin were not associated with ASDs (Table 3).
Associations of leptin and adiponectin with the social responsiveness scale
The mean (SD) SRS T score was 47.3 (8.0); a higher SRS means poorer social responsiveness. Among children with ASDs, SRS T score was 60.8 (13.8) vs 46.8 (7.3) among children without with ASDs (P = .0002) (see Table 1).
The levels of maternal second-trimester leptin showed a trend for a positive association with SRS T score in an unadjusted linear regression model (β = 0.82; 95% CI, –0.20 to 1.85). However, this association was null after adjusting for maternal race/ethnicity and education (β = 0.44; 95% CI, –0.57 to 1.45; model 2), and after adjusting for prepregnancy BMI, GWG, GDM status, and smoking status (β = 0.22; 95% CI, –1.11 to 1.56; model 3) (Table 4).
Table 4.
Associations of second trimester and cord blood biomarkers with social responsive scale (SRS) T scores in mid-teens (linear regression) in total population with available SRS T scores
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| β (95% CI) | |||
| Log-transformed exposures | |||
| Mothers | |||
| Second-trimester leptin, ng/mL (n = 515) | 0.82 (–0.20 to 1.85) | 0.44 (–0.57 to 1.45) | 0.22 (–1.11 to 1.56) |
| Second-trimester adiponectin, μg/mL (n = 517) | –1.64 (–2.83 to –0.45) | –0.62 (–1.85 to 0.60) | –1.46 (–2.75 to –0.17) |
| Infants | |||
| Cord blood leptin, ng/mL (n = 294) | –0.23 (–1.32 to 0.86) | –0.30 (–1.37 to 0.76) | –0.20 (–1.34 to 0.94) |
| Cord blood adiponectin, ug/mL (n = 295) | 1.82 (–1.09 to 4.72) | 3.05 (–0.48 to 6.57) | 2.42 (–0.62 to 5.46) |
Model 1: unadjusted; model 2: adjusted for maternal race/ethnicity, education, and child sex; model 3: adjusted for prepregnancy body mass index, gestational weight gain, maternal gestational diabetes status, and smoking status.
Maternal second-trimester adiponectin had a significant inverse association with SRS T score in an unadjusted linear regression model (β = –1.64; 95% CI, –2.83 to –0.45). Since a higher SRS means poorer social responsiveness, this means that higher adiponectin levels were associated with better social function. When the model was adjusted for prepregnancy BMI, GWG, maternal GDM status, and smoking status, the inverse association between maternal adiponectin and SRS T score remained significant (β = –1.46; 95% CI, –2.75 to –0.17; model 3). This association was weakened but remained in the same direction when adjusted for maternal race/ethnicity and education (β = –0.62; 95% CI, –1.85 to 0.60; model 2) (see Table 4).
Cord blood leptin was not associated with SRS T score (unadjusted β = –0.23; 95% CI, –1.32 to 0.86; model adjusted for maternal race/ethnicity, and education (model 2) β = –0.30; 95% CI, –1.37 to 0.76; model adjusted for prepregnancy BMI, GWG, maternal GDM status, and smoking status (β = –0.20; 95% CI, –1.34 to 0.94; model 3).
Cord adiponectin had a trend for a positive association with SRS (unadjusted β = 1.82; 95% CI, –1.09 to 4.72) that did not reach statistical significance. This positive association was somewhat strengthened but remained null when we adjusted for maternal race/ethnicity and education (β = 3.05; 95% CI, –0.48 to 6.57; model 2, and prepregnancy BMI, GWG, maternal GDM status, and smoking status (β = 2.42; 95% CI, –0.62 to 5.46; model 3).
In an additional analysis in the subset of children without ASDs, the association of maternal and cord blood leptin and adiponectin with SRS T scores remained similar to the results in the whole population, with the exception that the inverse association between maternal second-trimester adiponectin and SRS T score remained significant in a model adjusted for race/ethnicity (β = –1.23; 95% CI, –2.41 to –0.05; model 2) (Table 5).
Table 5.
Associations of second-trimester and cord blood biomarkers with social responsive scale (SRS) T scores in midteens (linear regression) in children without diagnosis of autism spectrum disorder with available SRS T scores
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| β (95% CI) | |||
| Log-transformed exposures | |||
| Mothers | |||
| Second-trimester leptin, ng/mL (n = 500) | 0.65 (–0.29 to 1.60) | –0.27 (–1.51 to 0.96) | –0.23 (–1.46 to 1.00) |
| Second-trimester adiponectin, μg/mL (n = 502) | –1.51 (–2.61 to –0.40) | –1.23 (–2.41 to –0.05) | –1.30 (–2.48 to –0.11) |
| Infants | |||
| Cord blood leptin, ng/mL (n = 284) | 0.04 (–0.99 to 1.08) | –0.14 (–1.21 to 0.92) | 0.03 (–1.05 to 1.11) |
| Cord blood adiponectin, μg/mL (n = 284) | 1.19 (–1.57 to 3.96) | 1.10 (–1.70 to 3.89) | 1.80 (–1.09 to 4.70) |
Model 1: unadjusted; model 2: adjusted for maternal race/ethnicity, education, and child sex; model 3: adjusted for prepregnancy body mass index, gestational weight gain, maternal gestational diabetes status, and smoking status.
In Pearson correlation analyses, as expected, second-trimester leptin and adiponectin were inversely correlated (r = –0.13, P < .0001), and by contrast, cord blood leptin and adiponectin were positively correlated (r = 0.38, P < .001, Table 6).
Table 6.
Pearson correlations (r) between log-transformed second-trimester and cord blood hormone levels, prepregnancy body mass index (BMI), gestational weight gain, birth weight for gestational age z score, and BMI z score
| Second-trimester, mother | Cord blood, infant | Mother | Infant | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Leptin | Adiponectin | Leptin | Adiponectin | Prepregnancy BMI | GWG | BW/GA z | BMI z | ||
| Second-trimester leptin | r | 1.00 | |||||||
| P | |||||||||
| N | (n = 1022) | ||||||||
| Second-trimester adiponectin | r | -0.13 | 1.00 | ||||||
| P | < .0001 | ||||||||
| N | (n = 1022) | (n = 1025) | |||||||
| Cord blood leptin | r | 0.14 | –0.13 | 1.00 | |||||
| P | .002 | .005 | |||||||
| N | (n = 460) | (n = 461) | (n = 597) | ||||||
| Cord blood adiponectin | r | 0.05 | 0.05 | 0.38 | 1.00 | ||||
| P | .29 | .27 | < .0001 | ||||||
| N | (n = 466) | (n = 467) | (n = 597) | (n = 606) | |||||
| Prepregnancy BMI | r | 0.55 | –0.31 | 0.12 | 0.01 | 1.00 | |||
| P | < .0001 | < .0001 | .004 | .76 | |||||
| N | (n = 1019) | (n = 1022) | (n = 594) | (n = 603) | (n = 1180) | ||||
| Total gestational wt gain | r | 0.19 | 0.10 | 0.03 | –0.02 | -0.25 | 1.00 | ||
| P | < .0001 | .002 | .40 | .70 | < .0001 | ||||
| N | (n = 1010) | (n = 1013) | (n = 587) | (n = 596) | (n = 1168) | (n = 1168) | |||
| Birth wt for GA z score | r | 0.07 | –0.09 | 0.37 | 0.11 | 0.09 | 0.17 | 1.00 | |
| P | .03 | .003 | < .0001 | .01 | .001 | < .0001 | |||
| N | (n = 1021) | (n = 1024) | (n = 597) | (n = 606) | (n = 1179) | (n = 1167) | (n = 1183) | ||
| BMI for GA z score | r | 0.10 | –0.10 | 0.40 | 0.16 | 0.08 | 0.15 | 0.79 | 1.00 |
| P | .01 | .02 | < .0001 | < .0001 | .03 | < .0001 | < .0001 | ||
| N | (n = 591) | (n = 594) | (n = 339) | (n = 342) | (n = 670) | (n = 663) | (n = 672) | (n = 672) | |
Abbreviations: BMI, body mass index; BW, birth weight; GA, gestational age; GWG, gestational weight gain.
Discussion
In an established large, prospective, prebirth cohort, we found an inverse association of circulating maternal adiponectin during the second trimester with ASDs in their children independent of maternal BMI and early pregnancy GWG. In addition, higher maternal second-trimester adiponectin was associated with better social responsiveness (lower SRS T score). We also confirm that maternal prepregnancy BMI had a dose-related association with the development of ASDs independent of race/ethnicity, education, smoking status, and child sex. This is the first report on the association of maternal circulating adipokines during pregnancy and the development of ASDs in their children in a longitudinal perinatal cohort. And this finding adds important information on the effect of the prenatal environment in the development of ASDs.
The potential mechanism underlying the association between adiponectin and autism is the role of adiponectin as an immune modulator (32). With evidence from animal studies suggesting maternal inflammatory activation is associated with the development of autism (33, 34), these findings suggest that the anti-inflammatory effect of adiponectin could play a significant protective role in the development of ASDs. Adiponectin is detected not only in the circulation but also in cerebrospinal fluid both in mice and humans and has been reported to play a key role in the immune function of the central nervous system (35-37). Adiponectin receptors (Adipo R1 and Adipo R2) are found in the hypothalamus, and Adipo R2 is also reported in arcuate neurons and glial cells (36, 37). Furthermore, there is growing evidence that adiponectin has neuroprotective effects in the setting of neurodegenerative diseases such as Alzheimer disease (20). Suppression of adiponectin receptors (AdipoR1 knockout mice) induces neurodegeneration and metabolic dysfunction in an animal study (38).
Recently, genetic variations in the neuronal growth regulator (NEGR)-1 gene, and PTB (polypyrimidine tract-binding protein)-2 gene have been reported both in ASDs and obesity (39), and epigenetic modifications including DNA methylation in brain tissue have been reported to be associated with ASDs (40). It is not known whether these genetic and epigenetic changes are associated with changes of adiponectin levels, therefore, further investigation is warranted.
Our study is in agreement with a recent study that reported that cord leptin is not associated with ASDs (41). This was expected given that brain development had largely been completed by the time cord blood levels were measured as well as the fact that the neonatal brain is not directly exposed to cord blood hormone levels. It is also noticeable that the maternal adipokines and cord blood adipokines did not show the same associations with ASDs. Unlike in adults in whom adiponectin levels are inversely associated with metabolic syndrome and low-grade inflammation, it has been reported that cord adiponectin is positively (not inversely) correlated with infant body weight and BMI, which serve as surrogates for fat mass (24). We confirm these associations that add validity to our study findings.
A previous meta-analysis of 6 longitudinal cohorts reported that maternal overweight and obesity are risk factors for autism in children (6). Windham et al reported a positive association of maternal prepregnancy BMI with autism in a California cohort, but in a model adjusted for socioeconomic factors such as race, education, and smoking status, this association became nonsignificant (7). We confirm these findings; in our study, the dose-dependent association between maternal BMI and ASDs in children remained significant even after adjustment for maternal race/ethnicity, education, smoking status, and child sex, a finding that also adds validity to our findings. Maternal obesity is known to be associated with increased levels of circulatory proinflammatory cytokines including interleukin-6 and transforming growth factor-β, and ASDs are associated with maternal immune activation (42, 43). Therefore, we speculate that maternal immune activation and inflammation in obesity could be the underlying mechanism behind this positive association of maternal BMI and ASDs in the offspring.
A strength of our study is the longitudinal assessment of study variables, including adipokines, in the context of a well-characterized cohort of mother-child pairs. Limitations include the fact that we collected the SRS only in a subset of participants as well as the relatively small absolute numbers of children with ASDs; however, the cumulative incidence was similar to national estimates. We used parent report of doctor-diagnosed ASDs from specific questionnaires and did not have direct access to individual medical records to confirm the diagnoses.
In summary, our study suggests that maternal adiponectin levels measured in the second trimester of pregnancy are inversely and independently associated with development of ASDs in their children. Adiponectin during pregnancy maybe useful as a tool to monitor the risk of autism, and future clinical trials need to explore whether raising adiponectin levels in mothers may prevent ASDs in their children.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health (K24DK081913 Award to C.M.S.) and a gift from the Zervas family to Beth Israel Deaconess Medical Center. Project Viva is supported by the National Institutes of Health (grant Nos. R01 HD 034568 to E.O. and UH3 OD023286 to E.O.).
Author Contributions: K.E.J. conceptualized the study and drafted the manuscript. S.R.S. performed data analysis and drafted the manuscript. E.O. participated in critical discussion on methodology, supervised data analysis, and finalized the manuscript. C.S.M. conceptualized the study, supervised data analysis, finalized the manuscript, had full access to all the data in the study, and had final responsibility for the decision to submit for publication.
Glossary
Abbreviations
- ASD
autism spectrum disorder
- BMI
body mass index
- SRS
social responsiveness scale
- GWG
gestational weight gain
- GCT
glucose challenge test
- GTT
glucose tolerance test
- GDM
gestational diabetes mellitus
- IH
isolated hyperglycemia
- IGT
impaired glucose tolerance.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Restrictions apply to all the availability of data generated or analyzed during this study to preserve patient confidentiality.
References
- 1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association;2013. [Google Scholar]
- 2. Christensen DL, Baio J, Van Naarden Braun K, et al. , Centers for Disease Control and Prevention . Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Edlow AG. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat Diagn. 2017;37(1):95-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bilbo SD, Block CL, Bolton JL, Hanamsagar R, Tran PK. Beyond infection—maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp Neurol. 2018;299(Pt A):241-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardner RM, Lee BK, Magnusson C, et al. Maternal body mass index during early pregnancy, gestational weight gain, and risk of autism spectrum disorders: results from a Swedish total population and discordant sibling study. Int J Epidemiol. 2015;44(3):870-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Y, Tang S, Xu S, Weng S, Liu Z. Maternal body mass index and risk of autism spectrum disorders in offspring: a meta-analysis. Sci Rep. 2016;6:34248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Windham GC, Anderson M, Lyall K, et al. Maternal pre-pregnancy body mass index and gestational weight gain in relation to autism spectrum disorder and other developmental disorders in offspring. Autism Res. 2019;12(2):316-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Z, Hou Z, Lin X, et al. Development of the fetal cerebral cortex in the second trimester: assessment with 7T postmortem MR imaging. AJNR Am J Neuroradiol. 2013;34(7):1462-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ge X, Shi Y, Li J, et al. Development of the human fetal hippocampal formation during early second trimester. Neuroimage. 2015;119:33-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farnaroff AA, Martin RT.. Fanaroff and Martin’s Neonatal-Perinatal Medicine. 10th ed.Philadelphia, PA: Elsevier; 2014. [Google Scholar]
- 11. Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci. 2010;1212:E1-E19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425-432. [DOI] [PubMed] [Google Scholar]
- 13. Mantzoros CS, Magkos F, Brinkoetter M, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301(4):E567-E584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodrigues DH, Rocha NP, Sousa LF, Barbosa IG, Kummer A, Teixeira AL. Changes in adipokine levels in autism spectrum disorders. Neuropsychobiology. 2014;69(1):6-10. [DOI] [PubMed] [Google Scholar]
- 15. Ashwood P, Kwong C, Hansen R, et al. Brief report: plasma leptin levels are elevated in autism: association with early onset phenotype? J Autism Dev Disord. 2008;38(1):169-175. [DOI] [PubMed] [Google Scholar]
- 16. Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18(6):1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fang H, Judd RL. Adiponectin regulation and function. Compr Physiol. 2018;8(3):1031-1063. [DOI] [PubMed] [Google Scholar]
- 18. Yang WS, Lee WJ, Funahashi T, et al. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86(8):3815-3819. [DOI] [PubMed] [Google Scholar]
- 19. Spranger J, Kroke A, Möhlig M, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361(9353):226-228. [DOI] [PubMed] [Google Scholar]
- 20. Ng RCL, Chan KH. Potential neuroprotective effects of adiponectin in Alzheimer’s disease. Int J Mol Sci. 2017;18(3):592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lisik MZ, Gutmajster E, Sieroń AL. Plasma levels of leptin and adiponectin in fragile X syndrome. Neuroimmunomodulation. 2016;23(4):239-243. [DOI] [PubMed] [Google Scholar]
- 22. Fujita-Shimizu A, Suzuki K, Nakamura K, et al. Decreased serum levels of adiponectin in subjects with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(3):455-458. [DOI] [PubMed] [Google Scholar]
- 23. Oken E, Baccarelli AA, Gold DR, et al. Cohort profile: project viva. Int J Epidemiol. 2015;44(1):37-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics. 2009;123(2):682-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li LJ, Rifas-Shiman SL, Aris IM, et al. Associations of maternal and cord blood adipokines with offspring adiposity in project viva: is there an interaction with child age? Int J Obes (Lond). 2018;42(4):608-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Constantino JN, Gruber CP.. Social Responsiveness Scale. 2nd ed. Torrance, CA: ; Western Psychological Services; 2012. [Google Scholar]
- 27. ACOG practice bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49-e64. [DOI] [PubMed] [Google Scholar]
- 28. Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, Oken E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens. 2009;22(2):215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perng W, Hajj H, Belfort MB, et al. Birth size, early life weight gain, and midchildhood cardiometabolic health. J Pediatr. 2016;173:122-130.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;( 314):1-27. [PubMed] [Google Scholar]
- 32. Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323(2):630-635. [DOI] [PubMed] [Google Scholar]
- 33. Pendyala G, Chou S, Jung Y, et al. Maternal immune activation causes behavioral impairments and altered cerebellar cytokine and synaptic protein expression. Neuropsychopharmacology. 2017;42(7):1435-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim S, Kim H, Yim YS, et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017;549(7673):528-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yokota T, Oritani K, Takahashi I, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96(5):1723-1732. [PubMed] [Google Scholar]
- 36. Guillod-Maximin E, Roy AF, Vacher CM, et al. Adiponectin receptors are expressed in hypothalamus and colocalized with proopiomelanocortin and neuropeptide Y in rodent arcuate neurons. J Endocrinol. 2009;200(1):93-105. [DOI] [PubMed] [Google Scholar]
- 37. Beall C, Hanna L, Ellacott KLJ. CNS targets of adipokines. Compr Physiol. 2017;7(4):1359-1406. [DOI] [PubMed] [Google Scholar]
- 38. Kim MW, Abid NB, Jo MH, Jo MG, Yoon GH, Kim MO. Suppression of adiponectin receptor 1 promotes memory dysfunction and Alzheimer’s disease-like pathologies. Sci Rep. 2017;7(1):12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grove J, Ripke S, Als TD, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51(3):431-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dall’Aglio L, Muka T, Cecil CAM, et al. The role of epigenetic modifications in neurodevelopmental disorders: a systematic review. Neurosci Biobehav Rev. 2018;94:17-30. [DOI] [PubMed] [Google Scholar]
- 41. Raghavan R, Zuckerman B, Hong X, et al. Fetal and infancy growth pattern, cord and early childhood plasma leptin, and development of autism spectrum disorder in the Boston Birth Cohort. Autism Res. 2018;11(10):1416-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab. 2002;87(9):4231-4237. [DOI] [PubMed] [Google Scholar]
- 43. Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010;24(6):2104-2115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to all the availability of data generated or analyzed during this study to preserve patient confidentiality.