Abstract
Context
In a cross-sectional study, we found an association between type 2 diabetes mellitus (T2DM) and smaller bone area together with greater bone mineral density (BMD) at the total hip.
Objective
This work aims to investigate these associations longitudinally, by studying T2DM status (no T2DM n = 1521, incident T2DM n = 119, or prevalent T2DM n = 106) in relation to changes in total hip bone area and BMD.
Methods
In 3 cohorts, the Swedish Mammography Cohort Clinical (SMCC; n = 1060), Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS; n = 483), and Uppsala Longitudinal Study of Adult Men (ULSAM; n = 203), with repeat assessment of T2DM status and dual energy x-ray absorptiometry (DXA) measurements of total hip bone area and BMD on average 8 years apart, a linear regression model was used to assess the effect of T2DM status on change in bone area and BMD at the total hip.
Results
After meta-analysis, the change in bone area at the total hip was 0.5% lower among those with incident T2DM compared to those without T2DM (–0.18 cm2; 95% CI, –0.30 to –0.06). The change in bone area was similar among those with prevalent T2DM compared to those without (0.00 cm2; 95% CI, –0.13 to 0.13). For BMD, the combined estimate was 0.004 g/cm2 (95% CI, –0.006 to 0.014) among those with incident T2DM and 0.010 g/cm2 (95% CI, –0.000 to 0.020) among those with prevalent T2DM, compared to those without T2DM.
Conclusion
Those with incident T2DM have a lower expansion in bone area at the total hip compared to those without T2DM.
Keywords: type 2 diabetes mellitus, bone area, bone mineral density, longitudinal study
Several studies indicate that type 2 diabetes mellitus (T2DM) is associated with an increased risk of hip fracture both in men and women (1-3). Hip fractures are the most severe fragility fractures and are associated with high mortality during the first year after the injury, independent of comorbidity, lifestyle or genetic predisposition (4), and disability (5), thus posing an additional strain on the many complications associated with T2DM (6). Hip and other fragility fractures seen in older individuals are often the consequence of a fall (7, 8) and there is a greater risk of falling in those with T2DM (9). The risk for a fragility fracture following a fall typically increases with older age and lower bone mineral density (BMD) (10). However, paradoxically, the greater risk of hip fracture in those with T2DM persists despite a normal or even greater BMD (11).
Another important component of bone that increases the resistance to fracture is the size of the bone, as it affects the mechanical integrity of bone when a force is applied to it (12, 13). Specifically, bone strength is proportional to the fourth power of the radius and therefore even small changes in bone size can be of importance for fracture risk (14). A reduced bone width is associated with an increased risk of fracture in women and men (15, 16) and contributes to hip fracture risk independently of bone density (17). Studies using dual energy x-ray absorptiometry (DXA) bone area measures showed that intertrochanter and shaft outer diameter measurements and buckling ratios were identified as independent predictors of hip fracture (18), while bone cross-sectional area was lower in hip fracture cases (19). Associations between T2DM and smaller bone size have been reported at the tibia and radius (20, 21) and at the total hip in our previous study using 2 of the same cohorts included in the present analysis (22). These previous studies that have reported associations between T2DM and smaller bone area yet greater BMD (11, 22) have had cross-sectional designs, thereby limiting their ability to infer a causal effect of T2DM. Bone area expands via periosteal apposition throughout life as a compensatory mechanism for age-related loss in BMD (23, 24) to preserve bone strength and resistance to fragility fracture. The associations between T2DM and smaller bone area in previous cross-sectional studies may suggest that this expansion is lesser in those with T2DM, but it is unclear if this is a late feature of T2DM or if this occurs in parallel with the transition to T2DM.
Building on the reported cross-sectional associations (22), using 3 Swedish population based cohorts consisting both of men and women, we therefore aimed to investigate if the lesser expansion of the bone at the total hip is actually an effect of T2DM status by comparing those with no T2DM, those with incident T2DM, and those with prevalent T2DM in relation to change in bone area at the total hip. We also aimed to study T2DM status in relation to change in total hip BMD.
Materials and Methods
Study Population
We included women and men from 3 population-based cohorts based in central Sweden: the Swedish Mammography Cohort Clinical (SMCC), Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS), and Uppsala Longitudinal Study of Adult Men (ULSAM). Participants in each cohort underwent clinical examinations including DXA twice. Most participants were born in Sweden or in another Nordic country (98% in ULSAM). This study received ethical approval from regional ethical review boards, and each participant provided written informed consent.
Swedish Mammography Cohort Clinical
Between November 2003 and October 2009, a randomly selected subcohort (SMCC) of 5022 women living in Uppsala, Sweden, were selected from the Swedish mammography cohort (25). These women underwent DXA measurements, provided fasting blood samples for plasma glucose concentrations, had height and weight measurements taken, and completed a medical and lifestyle questionnaire that gathered information on education, physical activity, and dietary intake. Fasting plasma glucose concentrations were analyzed using 3 different methods depending on date of collection: a glucose dehydrogenase reagent (Bergman & Beving, instrument Advia 1650), the glucose oxidase method (Bayer, instrument Advia 1650), and the hexokinase method (Abbott, Abbott Architect). The same examination routines were repeated in the participants starting in 2015 and are currently ongoing. We used for the present analysis the first 1060 women with complete information on fasting glucose, diabetes status, and DXA measurements from both examinations. The SMCC is managed by the Swedish Infrastructure for Medical Population-based Life-course and Environmental Research (www.simpler4health.se).
Prospective Investigation in the Vasculature of Uppsala Seniors
Between 2001 and 2004, all 70-year-old residents of Uppsala, Sweden, were invited to participate in a health survey and clinical assessment (26). Of 2025 invited, 1016 (50.2%) participated in the baseline assessment and 838 had DXA measurements taken on average 2 years after baseline assessment at a mean age of 72 years. In the spring 2011 participants were invited for the 80 years of age reinvestigation and 604 attended. Both examinations consisted of anthropometrical measurements, fasting plasma glucose sampling, DXA, and a questionnaire that collected information on diabetes status, cardiovascular risk factors, dietary intake, education, and physical activity level. Fasting glucose concentrations were analyzed in whole blood using a HemoCue instrument (HemoCue) at Uppsala University Hospital (27). There were 483 men and women with complete information on fasting glucose, diabetes status, and DXA measurements from both examinations.
Uppsala Longitudinal Study of Adult Men
In 1970 all men born between 1920 and 1924, living in the county of Uppsala, Sweden, were invited to participate in a health survey (28). The men who participated were regularly reexamined, and the present analyses were based on the examinations taken between 2003 and 2005, when the mean age of the men was 82 years and then repeated in 2008 to 2009 at a mean age of 88 years. Both examinations consisted of anthropometrical measurements, fasting plasma glucose sampling, DXA measurements, and a detailed questionnaire regarding medical and social issues including diabetes status, dietary intake, and activities in daily life. Fasting plasma glucose concentrations (mmol/L) were measured by the glucose dehydrogenase method (Gluc-DH, Merck). There were 203 men with complete information on fasting glucose, diabetes status, and DXA measurements from both examinations.
Exposure: Incident and Prevalent Type 2 Diabetes Mellitus
We defined T2DM according to the World Health Organization and American Diabetes Association criteria using a fasting plasma glucose of 7.0 mmol/L or greater and/or self-reported diabetes with or without treatment with oral hypoglycemic agents or insulin. Fasting whole blood glucose samples taken from PIVUS were converted to plasma concentrations by multiplying by 1.11, as plasma glucose values are around 11% higher than those of whole blood (29). We were also able to combine the SMCC data set with the Swedish prescribed drug register and identify individuals with T2DM treatments (Anatomical Therapeutic Chemical Classification [ATC] codes: A10A and A10B) at either examination. Participants were thereafter categorized into “no T2DM” (those without T2DM at both the first and the second examination), “incident T2DM” (no T2DM at the first examination but T2DM at the second examination), or “prevalent T2DM” (T2DM at both examinations).
In additional analyses, we further classified those without T2DM at baseline and follow-up as having normal fasting glucose (NFG; fasting plasma glucose < 5.6 mmol/L) or impaired fasting glucose (IFG; fasting plasma glucose ≥ 5.6 and < 7.0 mmol/L), allowing study of the following categories (status at baseline to status at follow-up): NFG to NFG (reference category), NFG to IFG, NFG to T2DM, IFG to IFG, IFG to T2DM, and T2DM to T2DM.
Outcome: Bone Area and Bone Mineral Density of the Total Hip
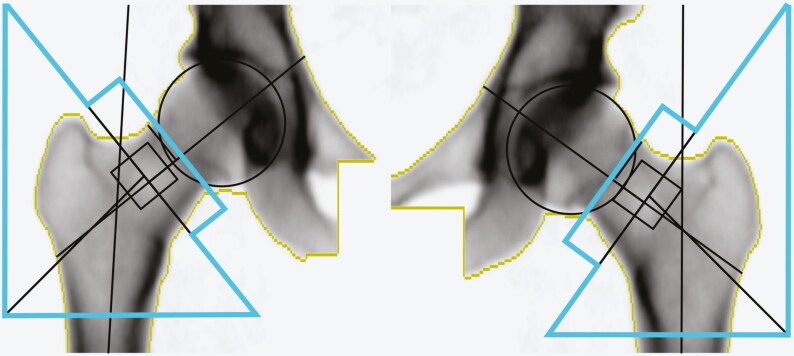
In all participants, measurements of bone area (cm2) and BMD (g/cm2) of the total hip were performed by the same experienced and accredited DXA x-ray nurse using the same DXA equipment (DXA, DPX Prodigy, Lunar Corp). For the DXA scan, the hip of each participant was set in a standard position by a fixed position of the foot, ankle, and knee to ensure that area did not vary because of a difference in rotation. The quality of each individual scan was manually checked by the x-ray nurse before it was accepted. The standard output from the DXA scanner provides bone mineral area and BMD of the femoral neck, Ward area, trochanter, and femoral shaft regions of interest (ROIs), as shown in Fig. 1 and described previously (22). These areas sum up to the total hip area ROI, shown as the total area within the blue lines in Fig. 1. The ROI was adjusted to the same location for each participant if needed (< 0.5% of scans). The precision error from the DXA was less than 1% for bone area and BMD at the total hip taken from triple measurements in 15 individuals. The long-term coefficient of variation (CV) since 2003 has been less than 1% for a spine phantom.
Figure 1.
Dual energy x-ray absorptiometry (DXA) image of dual femur and total hip in an adult woman. The total hip area region of interest is defined as the total area within the blue lines. This is the standard output from the Lunar Prodigy DXA scanner.
Other Dual X-Ray Absorptiometry Variables
Total body fat and lean mass measurements were taken from the total body DXA. The femoral neck diameter (mm) is a measurement of the average diameter of the femoral neck and the cross-sectional moment of inertia (CSMI, mm4) is a measure of the load-bearing capacity.
Physical Activity
Physical activity levels were obtained from questionnaires in all cohorts. In the SMCC physical activity was ascertained through a validated (30, 31) self-administered questionnaire. Participants specified time spent on “walking or bicycling” and time spent on “leisure-time exercise” during the last year. In PIVUS, participants were asked in a written questionnaire how many times a week they were engaged in heavy exercise, defined as exercise that produces sweat, such as running, playing tennis, skiing, skating, fast bicycling, for more than 30 minutes on a regular basis (32). In ULSAM, leisure-time physical activity was assessed from the questionnaire using 4 questions to establish the participants’ activity level in 4 categories from sedentary to highly active (33).
Charlson Comorbidity Index
The Charlson weighted comorbidity index (34), based on in-patient diagnosis codes from the Swedish National Patient Register and modified not to include diabetes, was available in the SMCC and ULSAM. In PIVUS, the Charlson comorbidity index was not available, so for additional adjustment for the overall health status of individuals we used self-reported cardiovascular disease diagnosis from the questionnaire.
Diet
In the SMCC, we calculated a modified Mediterranean diet score (mMED; range, 0-8 points) from a food-frequency questionnaire (FFQ) that was answered in 2008 by giving one point for intakes above the median for fruit and vegetables, legumes and nuts, nonrefined or high-fiber grains, fermented dairy products, and fish. One point was also given for intakes below the median of red and processed meat, use of olive or rapeseed oil for cooking or as dressing, and for moderate alcohol consumption (5-15 g of ethanol/day). This score was designed to represent the relative adherence to a traditional Mediterranean dietary pattern (35).
In the PIVUS and ULSAM, it was not possible to calculate an mMED. In the PIVUS, we calculated intakes of beta carotene (mg/day), zinc (mg/day), saturated fat (g/day), monounsaturated fat (g/day), and polyunsaturated fat (g/day) based on FFQ responses in 2001 to 2004. In the ULSAM, we combined FFQ information from 2003 to 2005 for the same variables and additionally on fruit and vegetable intake (servings per day).
Medicine Use
Information from the Swedish Prescribed Drug Registry was available from July 1, 2005, for the SMCC cohort. Use of medicines in the following ATC categories was indicated if at least 2 prescriptions were dispensed, either before the baseline (baseline use) or before the follow-up visit: C01 to C09 (cardiovascular drugs), C10 (lipid-lowering drugs), H03 (drugs affecting thyroid function), M05B (drugs affecting bone turnover), and G03C and G03F (estrogen-replacement drugs). The analysis adjusting for use of these medicines was restricted to those participating in the baseline examination after July 1, 2005 (n = 777).
Serum Concentrations of Vitamin D, Calcium, and Parathyroid Hormone
In the SMCC, serum samples from the baseline examination were analyzed for concentrations of vitamin D (nmol/L; determined by high-performance liquid chromatography at Vitas AS with CVs for interassay analyses between 3% and 6%), calcium (mmol/L; analyzed on an Architect C16000 [Abbott Laboratories] with the total CV 0.9% at 2.2 mmol/L and 1.2% at 2.9 mmol/L), and parathyroid hormone (PTH; pmol/L; analyzed on a Roche Cobas 8000 [e602 module, Roche Diagnostics]) using the PTH reagent kits; the total CV was 3% at 5.1 pmol/L and 3% at 17 pmol/L). The season of blood draw was defined as winter (December to February), spring (March to May), summer (June to August), and autumn (September to November). These concentrations and season of blood draw were added to the main model as additional adjustments (n = 1056).
Statistical Analyses
Descriptive characteristics are presented as means (SD) and frequencies (%). Analyses were performed using linear regression with T2DM status category (no T2DM [reference], incident T2DM and prevalent T2DM) as the independent variable and bone area or BMD at the total hip as the dependent variable in model 1 (adjusted for age, sex, time between examinations, and the DXA measurement under study taken from the first examination) and model 2 (multivariable adjusted). Additional confounders adjusted for in the multivariable-adjusted models included the continuous variables, height and BMI, and the categorical variables (obtained from questionnaires), smoking status (never smoker, former smoker, current smoker), either physical activity (1 lowest to 4 highest) in the SMCC and ULSAM or light exercise and heavy exercise in the PIVUS, education (≤ 7, 8-10, or ≥ 12 years) and either Charlson comorbidity index (SMCC and ULSAM) or cardiovascular disease diagnosis (yes or no) (PIVUS). All confounders were taken from the first examination and were selected based on the directed acyclic graph method (36).
In additional analyses using covariates as in model 2, we studied the association of our main exposure with change in femoral neck diameter and CSMI as outcome and used transitions between NFG, IFG, and T2DM as exposure with change in total hip bone area and density as outcomes.
In sensitivity analyses of our main results, we performed additional adjustments by adding covariates to model 2: a) dietary components (as described earlier), b) baseline total fat mass (g), lean mass (g), and the difference between total fat and lean mass between the first and second examinations, and in SMCC only: c) medicine use and d) serum concentrations of vitamin D, calcium, and PTH, and season of blood draw.
The different availability of covariates made pooling the cohorts inappropriate, so analyses were therefore performed separately in each cohort and the resulting estimates (β coefficients and SEs) were combined in a meta-analysis using the metan package for Stata. The resulting estimates for change in bone area and BMD were quantified by calculating the weighted average percentage change from the baseline measurement. However, since pooling was possible to perform adjusting for covariates in model 1, we performed an additional analysis with the pooled cohorts (adjusting for covariates in model 1 and the cohort) to provide a pooled estimate and an overall Wald test. We also tested for interaction between T2DM status and age in the SMCC by adding in an interaction term in the primary analysis for bone area. To help explain the results of the primary analysis, we conducted descriptive analyses: In each of the cohorts we described the fasting glucose distribution at the first examination in those individuals with incident T2DM and compared the difference in weight (kg) between the second and first examinations by T2DM status.
Analyses were performed in complete data sets without missing data on covariates. All statistical analyses were performed on resources provided by the Swedish National Infrastructure for Computing’s support for sensitive data (SNIC-SENS) through the Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX) using StataMP 15 (Stata Corp). The study and all cohorts were approved by the ethics committee of the University of Uppsala and the participants gave written informed consent.
Results
The baseline characteristics of members of each cohort are displayed by T2DM category with defined incident cases of T2DM in the SMCC (n = 64), PIVUS (n = 38), and ULSAM (n = 25) in Table 1 and in Supplementary Table 1 (37). The SMCC consisted of women only and the ULSAM men only, whereas the PIVUS comprised men and women alike, with a mean baseline age ranging from 64 to 82 years across all participants. The women in the SMCC had the highest levels of education, whereas the men from ULSAM were less physically active. In the ULSAM we saw a lower percentage of those with prevalent T2DM having a Charlson comorbidity index of 2 or more. Comparing those without T2DM with those with incident T2DM and prevalent T2DM across all 3 cohorts, we see that those with incident and prevalent diabetes have higher levels of BMI yet lower levels of physical activity and education.
Table 1.
Population characteristics at baseline
| SMCC | PIVUS | ULSAM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No T2DM | Incident T2DM | Prevalent T2DM | No T2DM | Incident T2DM | Prevalent T2DM | No T2DM | Incident T2DM | Prevalent T2DM | |
| No. (%) | 950 (89.6) | 64 (6.0) | 46 (4.3) | 415 (85.9) | 30 (6.2) | 38 (7.8) | 156 (76.8) | 25 (12.3) | 22 (10.8) |
| Women, No. (%) | 950 (100) | 64 (100) | 46 (100) | 200 (48.2) | 17 (56.7) | 21 (55.3) | 0 (0) | 0 (0) | 0 (0) |
| Age, y | 65.3 (5.1) | 64.4 (4.8) | 65.9 (5.3) | 70.1 (0.2) | 70.1 (0.1) | 70.1 (0.1) | 81.6 (1.0) | 81.4 (0.8) | 81.8 (1.0) |
| Time between examinations, y | 11.4 (0.7) | 11.4 (0.7) | 11.4 (0.7) | 8.2 (0.7) | 8.3 (0.8) | 8.3 (0.8) | 5.0 (0.2) | 5.0 (0.2) | 5.1 (0.2) |
| Height, cm | 163 (6) | 162 (6) | 163 (6) | 168 (10) | 170 (9) | 169 (9) | 173 (6) | 173 (5) | 174 (5) |
| Weight, kg | 67.7 (11.6) | 74.5 (14.3) | 74.9 (13.7) | 75.1 (13.1) | 81.3 (14.6) | 80.9 (12.9) | 77.6 (10.2) | 79.8 (9.0) | 84.4 (13.6) |
| BMI | 25.6 (4.2) | 28.5 (5.2) | 28.2 (4.8) | 26.6 (3.7) | 28.4 (4.9) | 28.4 (4.2) | 25.9 (3.0) | 26.7 (2.9) | 27.8 (4.4) |
| Smoking status, No. (%) | |||||||||
| Nonsmoker | 516 (54.3) | 35 (54.7) | 22 (47.8) | 216 (52.0) | 12 (40.0) | 17 (44.7) | 67 (42.9) | 9 (36.0) | 12 (54.5) |
| Former smoker | 367 (38.6) | 23 (35.9) | 21 (45.7) | 163 (39.3) | 14 (46.7) | 19 (50.0) | 81 (51.9) | 15 (60.0) | 10 (45.5) |
| Current smoker | 67 (7.1) | 6 (9.4) | 3 (6.5) | 36 (8.7) | 4 (13.3) | 2 (5.3) | 8 (5.1) | 1 (4.0) | 0 (0.0) |
| Physical activity, No. (%) | |||||||||
| 1 (Lowest) | 145 (15.3) | 18 (28.1) | 13 (28.3) | 11 (7.1) | 4 (16.0) | 1 (4.5) | |||
| 2 | 206 (21.7) | 10 (15.6) | 12 (26.1) | 56 (35.9) | 9 (36.0) | 9 (40.9) | |||
| 3 | 339 (35.7) | 26 (40.6) | 16 (34.8) | 75 (48.1) | 11 (44.0) | 11 (50.0) | |||
| 4 (Highest) | 260 (27.4) | 10 (15.6) | 5 (10.9) | 14 (9.0) | 1 (4.0) | 1 (4.5) | |||
| Light exercise, No. (%) | |||||||||
| 0 (Lowest) | 34 (8.2) | 2 (6.7) | 4 (10.5) | ||||||
| 1 | 29 (7.0) | 0 (0.0) | 4 (10.5) | ||||||
| 2 | 65 (15.7) | 8 (26.7) | 2 (5.3) | ||||||
| 3 | 73 (17.6) | 5 (16.7) | 9 (23.7) | ||||||
| 4 (Highest) | 214 (51.6) | 15 (50.0) | 19 (50.0) | ||||||
| Heavy exercise, No. (%) | |||||||||
| 0 (lowest) | 274 (66.0) | 25 (83.3) | 26 (68.4) | ||||||
| 1 | 68 (16.4) | 2 (6.7) | 9 (23.7) | ||||||
| 2 | 40 (9.6) | 3 (10.0) | 3 (7.9) | ||||||
| 3 | 19 (4.6) | 0 (0.0) | 0 (0.0) | ||||||
| 4 (Highest) | 14 (3.4) | 0 (0.0) | 0 (0.0) | ||||||
| Education, No. (%) | |||||||||
| ≤ 7 y | 382 (40.2) | 31 (48.4) | 21 (45.7) | 213 (51.3) | 16 (53.3) | 24 (63.2) | 82 (52.6) | 13 (52.0) | 9 (40.9) |
| 8-10 y | 79 (8.3) | 6 (9.4) | 3 (6.5) | 85 (20.5) | 7 (23.3) | 6 (15.8) | 28 (17.9) | 8 (32.0) | 7 (31.8) |
| ≥ 12 y | 489 (51.5) | 27 (42.2) | 22 (47.8) | 117 (28.2) | 7 (23.3) | 8 (21.1) | 46 (29.5) | 4 (16.0) | 6 (27.3) |
| Charlson comorbidity index, No. (%) | |||||||||
| 0 | 884 (93.1) | 58 (90.6) | 37 (80.4) | 87 (55.8) | 14 (56.0) | 15 (68.2) | |||
| 1 | 48 (5.1) | 6 (9.4) | 6 (13.0) | 34 (21.8) | 5 (20.0) | 4 (18.2) | |||
| 2+ | 18 (1.9) | 0 (0) | 3 (6.5) | 35 (22.4) | 6 (24.0) | 3 (13.6) | |||
| Cardiovascular disease diagnosis, No. (%) | |||||||||
| No | 369 (88.9) | 25 (83.3) | 35 (92.1) | ||||||
| Yes | 46 (11.1) | 5 (16.7) | 3 (7.9) | ||||||
Values are mean (SD) unless otherwise stated.
Abbreviations: BMI, body mass index; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors; SMCC, Swedish Mammography Cohort Clinical; T2DM, type 2 diabetes mellitus; ULSAM, Uppsala Longitudinal Study of Adult Men.
The average time between the baseline and the second examination was 9.8 years, with 11 years in the SMCC, 8 years in the PIVUS, and 5 years in the ULSAM. The absolute values and change of area and BMD at the total hip at baseline and follow-up by T2DM status are presented in Table 2, and corresponding measures of femoral neck diameter and CSMI are presented in Supplementary Table 2 (37).
Table 2.
Population bone area and bone mineral density at the total hip at baseline, follow-up, and the change between examinations
| Total hip | SMCC | PIVUS | ULSAM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No T2DM | Incident T2DM | Prevalent T2DM | No T2DM | Incident T2DM | Prevalent T2DM | No T2DM | Incident T2DM | Prevalent T2DM | |
| Bone area at baseline, cm2 | 32.44 (2.19) | 32.31 (2.13) | 32.62 (1.94) | 35.71 (3.72) | 35.90 (3.66) | 36.12 (3.58) | 39.35 (2.77) | 38.58 (1.98) | 39.36 (2.18) |
| Bone area at follow-up, cm2 | 32.43 (2.20) | 32.13 (1.99) | 32.66 (1.96) | 35.87 (3.69) | 35.99 (3.62) | 36.26 (3.53) | 39.52 (2.74) | 38.63 (1.99) | 39.53 (2.19) |
| Bone area difference, cm2 | –0.01 (0.61) | –0.18 (0.60) | 0.04 (0.64) | 0.16 (0.56) | 0.09 (0.44) | 0.13 (0.84) | 0.17 (1.28) | 0.04 (0.66) | 0.17 (0.87) |
| BMD at baseline, g/cm2 | 0.897 (0.131) | 0.926 (0.118) | 0.972 (0.151) | 0.947 (0.161) | 1.012 (0.140) | 1.034 (0.140) | 0.946 (0.155) | 1.016 (0.167) | 1.061 (0.135) |
| BMD at follow-up, g/cm2 | 0.897 (0.131) | 0.934 (0.128) | 0.981 (0.174) | 0.929 (0.164) | 1.000 (0.142) | 1.022 (0.150) | 0.951 (0.150) | 0.998 (0.175) | 1.065 (0.139) |
| BMD difference, g/cm2 | 0.000 (0.086) | 0.008 (0.068) | 0.009 (0.111) | –0.018 (0.037) | –0.012 (0.034) | –0.012 (0.029) | 0.005 (0.062) | –0.019 (0.042) | 0.004 (0.061) |
Values are mean (SD) unless otherwise stated.
Abbreviations: BMD, body mineral density; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors; SMCC, Swedish Mammography Cohort Clinical; T2DM, type 2 diabetes mellitus; ULSAM, Uppsala Longitudinal Study of Adult Men.
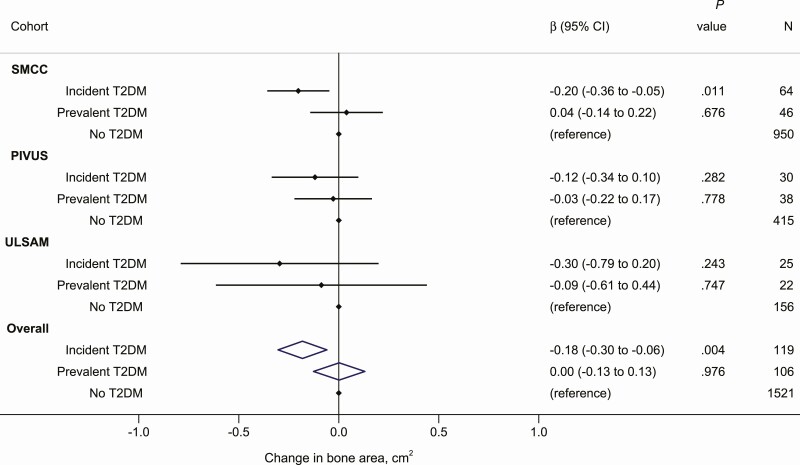
The total hip bone area was larger in men (39.26 cm2 in ULSAM and 38.74 cm2 in PIVUS men) than in women (32.44 cm2 in SMCC and 32.86 cm2 in PIVUS women). The associations between diabetes status and change in total hip bone area and density are shown in Supplementary Table 3 (37) (model 1) and in Figs. 2 and 3 (model 2). In the fully adjusted model and compared with individuals without T2DM at both examinations (“no T2DM” group), the change in bone area in those with incident T2DM was lower by 0.6% (–0.20 cm2 [–0.36 to –0.05]), 0.3% (–0.12 cm2 [–0.34 to 0.10]), and 0.8% (–0.30 cm2 [–0.79 to 0.20]) in the SMCC, PIVUS and ULSAM, respectively (Fig. 2). Combining the estimates in a meta-analysis, the change in bone area at the total hip was 0.5% lower among those with incident T2DM compared with those without T2DM (–0.18 cm2 [–0.30 to –0.06]). The corresponding pooled estimate comparing those with prevalent T2DM to those without T2DM indicated a difference of –0.01% (–0.002 cm2 [–0.13 to 0.13]) in bone area at the total hip (see Fig. 2). The heterogeneity test from the meta-analyses showed little variation between each cohort (Cochran Q = 0.60, P = .742 for incident T2DM and Q = 0.36, P = .836 for prevalent T2DM compared to no T2DM). From the primary analysis of bone area, the estimate for the interaction terms between T2DM status and age in the SMCC was close to 0 (P = .73).
Figure 2.
Change in bone area at the total hip (cm2) in those with incident and prevalent T2DM relative to those without T2DM in each of the cohorts and as meta-analyzed overall estimates. Estimates were adjusted for age, sex, time between baseline and follow-up examinations, baseline bone area at the total hip, height, body mass index, education, smoking status, physical activity, and comorbidity (Charlson comorbidity index in SMCC and ULSAM and cardiovascular disease diagnosis in PIVUS). PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors; SMCC, Swedish Mammography Cohort Clinical; T2DM, type 2 diabetes mellitus; ULSAM, Uppsala Longitudinal Study of Adult Men.
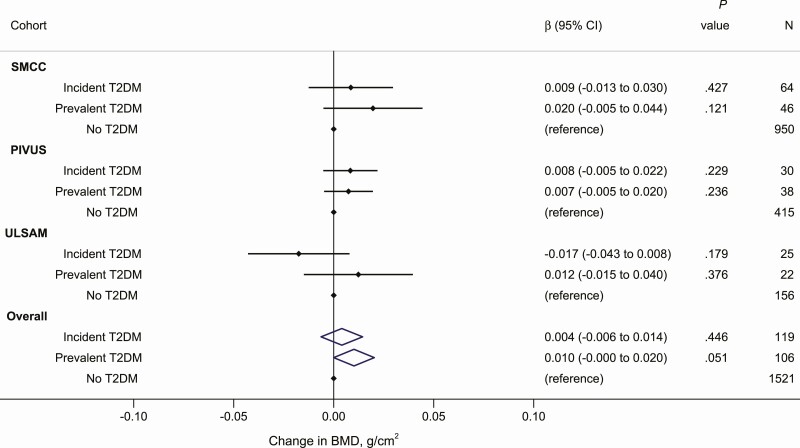
Figure 3.
Change in bone mineral density (BMD) at the total hip (g/cm2) in those with incident and prevalent T2DM relative to those without T2DM in each of the cohorts and as meta-analyzed overall estimates. Estimates were adjusted for age, sex, time between baseline and follow-up examinations, baseline BMD at the total hip, height, body mass index, education, smoking status, physical activity, and comorbidity (Charlson comorbidity index in SMCC and ULSAM and cardiovascular disease diagnosis in PIVUS). PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors; SMCC, Swedish Mammography Cohort Clinical; T2DM, type 2 diabetes mellitus; ULSAM, Uppsala Longitudinal Study of Adult Men.
When those with incident T2DM were compared with those without T2DM with respect to change in total hip BMD, results varied across cohorts (Fig. 3): BMD was higher by 1.0% (0.009 g/cm2 [–0.013 to 0.030]), higher by 0.8% (0.008 g/cm2 [–0.005 to 0.022]) and lower by 1.8% (–0.017 g/cm2 [–0.043 to 0.008]) in the SMCC, PIVUS, and ULSAM, respectively. The meta-analysis estimate indicated an increased BMD of 0.4% (0.004 g/cm2 [–0.006 to 0.014]) among those with incident T2DM compared to those without T2DM. The corresponding pooled estimate comparing those with prevalent T2DM to those without T2DM indicated a larger change in BMD of 1.1% (0.010 g/cm2 [–0.000 to 0.020]). The heterogeneity test from the meta-analyses of change in total hip BMD showed little variation between each cohort (Cochran Q = 3.32, P = .190 for incident T2DM and Q = 0.78, P = .676 for prevalent T2DM compared to no T2DM).
No associations were found between diabetes status and change in femoral neck diameter (Supplementary Table 3 and Supplementary Fig. 1) (37) or change in CSMI (see Supplementary Table 3 and Supplementary Fig. 2) (37).
Exploring the association of transitions between NFG and IFG and T2DM with change in total hip bone area and density, the precision was lower because of smaller groups. The largest changes in bone area compared to those having NFG at both examinations were seen among those transitioning to T2DM, both from NFG and from IFG at baseline (–0.19 cm2 [–0.39 to 0.02] for NFG-T2DM and –0.20 cm2 [–0.35 to –0.04] for IFG-T2DM (Supplementary Fig. 3) (37). The largest change in BMD was seen among those transitioning from NFG to T2DM (Supplementary Fig. 4) (37).
In a sensitivity analysis of total hip bone area and BMD, further adjustment for dietary components (Supplementary Figs. 5 and 6) (37) and for total fat mass, total lean mass, and the difference in total fat mass and lean mass between the first and second examinations (Supplementary Figs. 7 and 8) (37) did not greatly alter the results. In subsamples of the SMCC, information on prescribed drugs (n = 777) and concentrations of calcium, vitamin D, and PTH (n = 1056) at baseline were available, as shown in Supplementary Table 4 (37). Additional adjustments for these factors did not alter the interpretation of the results (Supplementary Figs. 9 and 10) (37).
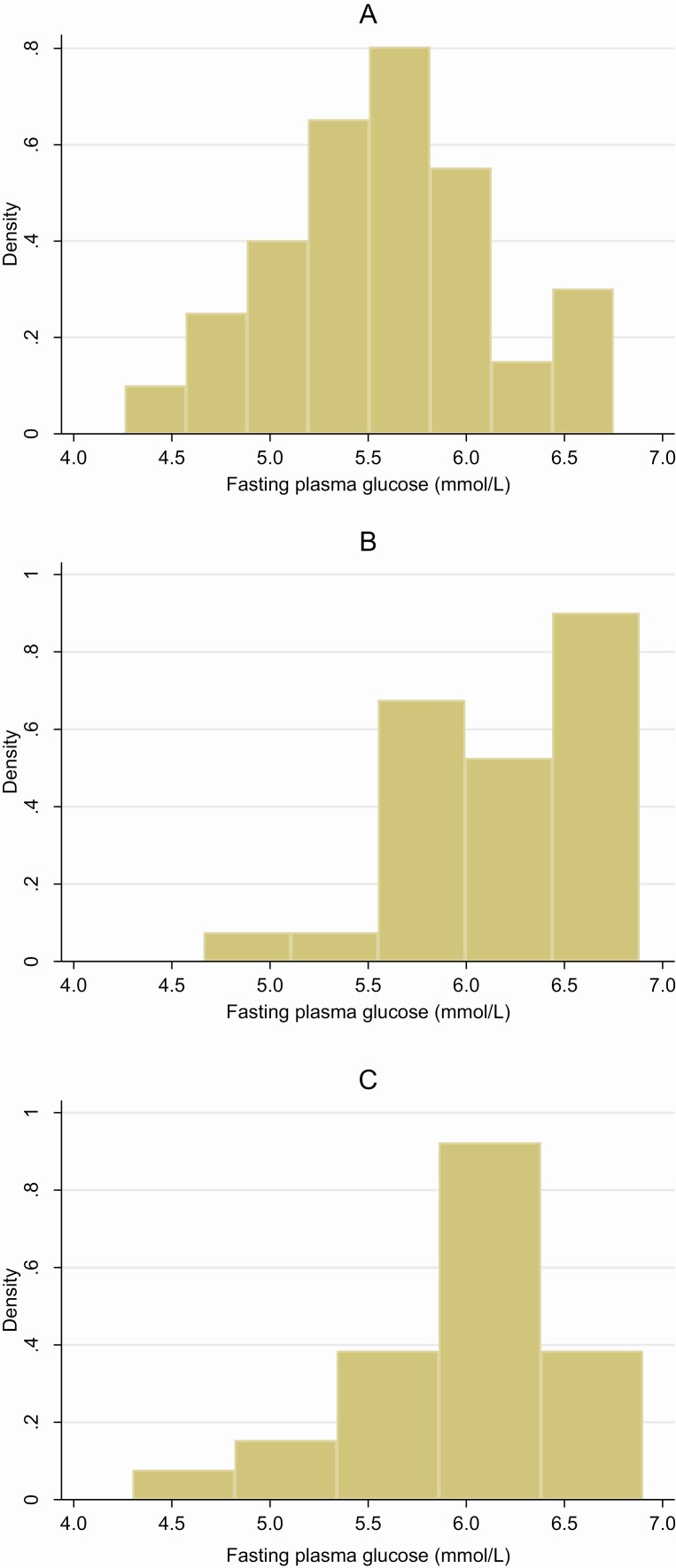
Even if the meta-analysis tests for heterogeneity were not statistically significant, there were some differences between the cohorts. When comparing incident T2DM with no T2DM, the difference in hip bone area change was smaller in the PIVUS (see Fig. 2) and the difference in BMD change was above zero in the SMCC and PIVUS but below zero in the ULSAM (Fig. 3). To help explain these differences, we plotted histograms of the fasting glucose distribution from the first examination in those with incident T2DM in each cohort (Fig. 4A-4C). Fasting glucose levels in the PIVUS and ULSAM were skewed to the right and toward higher levels of fasting glucose, whereas the fasting glucose levels for the SMCC had a normal distribution with a lower average. Also, among participants with incident T2DM, we calculated the weight differences between the first and second examinations in each cohort. Individuals from the SMCC, PIVUS, and ULSAM had a mean (SD) difference in weight of +0.1 (0.9) kg, +0.2 (5.0) kg, and –2.7 (5.0) kg, respectively (see Supplementary Table 1) (37).
Figure 4.
The distribution of fasting plasma glucose from the first examination in those with incident type 2 diabetes mellitus (T2DM). A, Swedish Mammography Cohort Clinical (SMCC). B, Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS). C, Uppsala Longitudinal Study of Adult Men (ULSAM).
Discussion
Our longitudinal analyses based on 3 Swedish cohorts consisting both of women and men is the first to show that incident T2DM is associated with a decrease in bone area expansion at the total hip compared to those without T2DM. We did not find an association between incident T2DM and change in BMD. There was also no association between prevalent T2DM and change in bone area or BMD.
These results add to the previously seen cross-sectional association between incident T2DM and lower bone area at the total hip in the same populations (22), suggesting that the smaller area at the total hip seen among those with T2DM may be an early effect of T2DM rather than a late complication. The average differences in total hip bone area were similar for those who transitioned to T2DM from NFG and from IFG, with less difference among those transitioning from NFG to IFG. The length of the period between disease onset and diagnosis where glucose control probably is poor may vary and influence the results. Poor glucose control in patients with diabetes was in a cross-sectional setting associated with higher BMD and narrower femoral neck diameter (38) but we unfortunately lacked such information in our study.
In this study we were able to combine 3 separate cohorts consisting both of older women and men based in central Sweden. Individuals from each cohort were measured longitudinally at 2 examination points, allowing us to investigate changes in bone characteristics over time and to define T2DM status at each examination. To the best of our knowledge, our previous cross-sectional study (22) was the first to analyze the association between T2DM and bone area at the hip, and this study is the first to examine this association over time to measure the change in total hip bone area and BMD. In our previous cross-sectional analysis in the same populations (SMCC n = 4713 and ULSAM n = 452), we observed an association between T2DM and lower bone area measures (22). Men and women with T2DM, compared to those with NFG levels and without T2DM, had lower bone area at the total hip of 1.7% and 1.0%, respectively. Other previous cross-sectional studies analyzing the association between prevalent T2DM and bone size focused on peripheral sites such as the tibia and radius using high-resolution peripheral quantitative computed tomography. T2DM was associated with a smaller cross-sectional area at the tibia and radius (men and women, n = 1069) (20), smaller cortical area of the tibia (25 postmenopausal women with T2DM and 25 controls) (21), and total area at the tibia (hypogonadal men, n = 105) (39). These studies were all cross-sectional designs without repeat bone measurements over time, limiting the ability to determine causality.
Contrasting to our previous study of prevalent T2DM, we did not find a strong association between incident T2DM and an increase in BMD at the total hip. Results from multiple studies show an association between T2DM and greater BMD (11, 38). However, again, these studies used cross-sectional designs. Why we did not see an association between incident T2DM and greater BMD may be explained by the effects of body weight on BMD, the shorter diabetes duration, and by the weight change in each cohort. In the studied cohorts, BMD increased in the PIVUS, which was the only cohort in which body weight also increased. Body weight and BMI explained 8.9% to 19.8% of total variance in all measured BMD sites in women and 2.8% to 6.9% of the total variance in the femur and spine BMD in men (40). Total body fat has been suggested as the most significant predictor of BMD independent of the skeletal load-bearing although the mechanisms are not clear (41). However, adjustment for lean and fat mass did not influence the estimates to a large extent in the present study. These results thus suggest that the larger BMD seen among those with T2DM is not an effect of the diabetes itself but rather an effect of the higher weight associated with the disease in combination with disease duration.
A smaller bone area is associated with an increased risk of fracture at the hip and at other fragility fracture sites, as reported by several studies (15, 16, 18, 19, 42). This may be due to the consequential effects of lower bone size on bone strength. Bone size is considered a determinant of bone strength (43) independent of BMD, and small differences in area can lead to large differences in strength and resistance to fracture (44). Indices of femoral neck strength have been shown to be inversely associated with incident hip fracture risk in older White women (17). To compensate for the age-related loss in BMD, bone area increases over life in both sexes by approximately 15% at central sites and by approximately 16% at peripheral sites (45). This increase in area via periosteal apposition partly offsets endosteal bone loss and losses in BMD in order to preserve bone strength (23), but less so in women than in men, which can partially explain why women fracture more than men (46). If T2DM reduces this expansion in bone area at the total hip, it may help explain the paradox of greater hip fracture risk in individuals with T2DM despite a greater BMD. No clear associations were seen between diabetes status and change in femoral neck diameter and the CSMI. This may be because the measurement sites of the femoral neck diameter and CSMI are not in regions containing periosteum, where age-related bone expansion occurs.
Strengths of our study include the longitudinal design with repeat DXA measurements, the definition of T2DM using a combination of fasting glucose samples, register, and self-reported diagnosis and medication use (self-reported and in SMCC also from the Swedish prescribed drug register), and that all DXA measurements in all cohorts were performed on the same DXA scanner operated by the same x-ray nurse. When meta-analyzed together, we have a study population with 2 different DXA measurements. Our 3 cohorts included women and men of different ages, and we were able to see a consistent direction of estimate in all of them, which increases our generalizability, although this may be limited to Swedish populations.
A main limitation of our study is that DXA is a 2-dimensional measurement technique set at a specific ROI for the total hip bone area and BMD. Using this method we were unable to distinguish between cortical and trabecular bone, and we were also unable to measure the microarchitecture and quality of bone mineralization (47). To improve on the 2-dimensional methods, 3-dimensional imaging techniques (48) have been developed. However, the most commonly used peripheral quantitative computed tomography is a technique used to measure peripheral sites such as the tibia and radius. A DXA measurement of the total hip area may be more relevant for hip fracture risk, the most devastating fracture (4). Other potential limitations with the DXA measure may be due to the influence of body weight on the distance between the DXA bed and the bone under measure, and DXA measurements have been suggested to be elevated with increased body fat, which may overestimate BMD in obese individuals (49). However, the Lunar Prodigy DXA used in the presents study corrects the scans to the actual effective object plane to limit the impact of these differences and also limits the effect of tissue thickness (50, 51). Magnification errors seen with ordinary fan-beam DXA that directly affect bone area (50) are reduced by the Lunar Prodigy by using a narrow fan beam along the axis. We have also shown in our previous study (22) that when we restricted our analysis to women who were overweight, we found the same direction of association between T2DM and bone area compared to those who were below the BMI threshold of overweight, which was also the case when we adjusted for height and weight individually, suggesting that the change in bone area is consistent in individuals with a similar body stature. Differences in height between 5 and 15 cm can result in an uncertainty of 1% for area measurements (50); therefore, we included height in our statistical models. Body weight may also influence the individual’s position on the DXA scanner and therefore the area. To limit the influence of measurement error on our results, the same DXA-accredited x-ray nurse performed all measurements in all cohorts using the same DXA equipment and a standard position for each participant that was checked before accepting each scan, which ensured ROI consistency.
We were also limited in our measurement of T2DM, relying mostly on self-report and a single measurement of fasting glucose. Using information on 2-hour glucose after an oral glucose tolerance test in the second examination of the ULSAM reclassified 6 of 156 (3.8%) individuals from no T2DM to T2DM. Adding glycated hemoglobin A1c to the diagnostic criteria did not change diabetes status at either examination in the ULSAM. Although self-reported diabetes has a high validity (52), there may still be some misclassification. However, this would likely bias associations toward the null. Unfortunately, we had no information on the exact timing of diabetes diagnosis or level, thus limiting our ability to investigate diabetes duration as an exposure, which may be of importance since duration of T2DM may lead to further health complications (53). With this in mind we also had a small population with regards to incident T2DM cases (n = 119) and prevalent T2DM cases (n = 106), which would also hinder an analysis into the effects of diabetes duration on bone health even if we had the relevant exposure information.
The overall results are largely similar to those in the SMCC. In the older cohorts (PIVUS and ULSAM), the age-related expansion in bone may already have taken place and the effect of diabetes might therefore be smaller in these older age groups (23, 54). The follow-up time was also shorter in these cohorts, and a shorter follow-up time may limit our ability to see larger changes in bone size. Even a small difference during an average of 10 years may have a greater clinical significance for hip fracture risk over longer periods of time, although bone area will be only one aspect of fracture risk (55). It has been suggested that a lower bone turnover rate may contribute to the development of cortical porosity or microcracks (38), which may be more abundant in women (56). We have previously shown that fasting glucose concentrations are associated with lower bone turnover (22). Inadequate glucose control among those with T2DM was associated with thicker cortices (38) and T2DM was related with cortical porosity (48). We were in the present study not able to break down these different aspects of bone or determine whether changes in bone area have an impact on fracture risk.
The repeat examinations are naturally and by design restricted to those participants who are alive, still living in the region, and still well enough to be able to consider participating in both examinations. This will influence the generalizability of our results but may also introduce selection bias. However, adjustment for covariates that are associated with the selection will limit the selection bias (57). Several of the factors in our multivariable-adjusted model and in the sensitivity analyses are likely to be not only confounders but also related to reasons for nonparticipation.
In the sensitivity analysis we further adjusted for dietary patterns and certain dietary intakes. In the SMCC we were able to adjust for an mMED (58), while in the PIVUS and ULSAM we adjusted for fat quality, individual vitamins, and fruit and vegetable intake. We did this because systematic reviews and meta-analyses have provided evidence that saturated fatty acids are positively (59) whereas n-3 fatty acids (60) are inversely related with hip fracture risk. There is also evidence that the Mediterranean diet is associated with hip fracture risk (58) and T2DM risk (61). In our analyses these additional adjustments did not greatly affect the estimates. In the ULSAM we saw a lower percentage of those with prevalent T2DM having a Charlson comorbidity index of 2 or more; this may be explained by the extensive medical advice and intervention the men received in the ULSAM. The men in this cohort were followed over a long period of time and reexamined several times, and if health conditions were discovered they received medical help. Adjustment for body fat, lean mass, and changes in these during follow-up did not change the interpretation of our results. Neither did additional adjustments in the SMCC for the use of hormone replacement therapy and cardiovascular, lipid-lowering, thyroid, and bone metabolism–influencing drugs at baseline or follow-up, or baseline concentrations of calcium, vitamin D, and PTH. The results thus seem fairly robust in these cohorts although we cannot exclude the possibility of residual or unmeasured confounding.
The present study adds further evidence to the possible effect of T2DM on bone area at the total hip. Further studies are needed to replicate these findings in other populations before we can establish a true causal effect of T2DM on bone area. We recently showed that genetically elevated glucose concentrations resulted in a smaller bone area of the total hip, using a mendelian randomization design (62). Further work could attempt to study the duration of IFG and T2DM in relation to bone size. Our results, however, indicate that the largest difference is seen after the transition to T2DM, both from NFG and IFG. Clinicians should be aware of this association between T2DM and smaller bone area and that the increased fracture risk in those with T2DM may in part be explained by a reduction in the expansion of bone area; therefore, prevention of T2DM would have an impact on fractures caused by the small area at the total hip. Future studies and cohorts could also consider the role of antidiabetic medication on bone aspects (63). There are yet to be clinical trials or epidemiological studies assessing such use of medical treatments on bone area. The study population in the present population was too small to investigate the role of different antidiabetic drugs on bone measurements; for example, only one woman in SMCC initiated treatment with thiazolidinediones during follow-up (no users at baseline).
In conclusion, the results of our longitudinal study suggest that older men and women with incident T2DM have a lesser increase in bone area at the total hip compared to those without T2DM over time. We found no association between incident T2DM and change in BMD at the total hip compared to those without T2DM. A reduced bone expansion at the hip in those with T2DM may help to explain why those with T2DM have an increased risk of hip fracture.
Acknowledgments
We acknowledge the national research infrastructure SIMPLER for providing facilities and experimental support, and we would like to thank Anna-Karin Kolseth for her assistance.
Financial Support: This work was supported by the Swedish Research Council (grant Nos. 2015-03257, 2017-00644 and 2017-06100), the Swedish Council for Working Life and Social Research, and an Uppsala County Council institutional grant “Avtal om Läkarutbildning och Forskning” (Agreement concerning Cooperation on Medical Education and Research). SIMPLER receives funding through the Swedish Research Council (under grant No. 2017-00644 to Uppsala University and K.M.). The computations were performed on resources provided by SNIC-SENS (www.snic.se) through the UPPMAX (under project Nos. SIMP2019016 and SENS2018565). SNIC is financially supported by the Swedish Research Council. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Glossary
Abbreviations
- ATC
Anatomical Therapeutic Chemical Classification
- BMD
bone mineral density
- BMI
body mass index
- CSMI
cross-sectional moment of inertia
- CV
coefficient of variation
- DXA
dual energy x-ray absorptiometry
- FFQ
food-frequency questionnaire
- IFG
impaired fasting glucose
- mMED
modified Mediterranean diet score
- NFG
normal fasting glucose
- PIVUS
Prospective Investigation of the Vasculature in Uppsala Seniors
- PTH
parathyroid hormone
- ROI
region of interest
- SMCC
Swedish Mammography Cohort Clinical
- SNIC
Swedish National Infrastructure for Computing
- T2DM
type 2 diabetes mellitus
- ULSAM
Uppsala Longitudinal Study of Adult Men
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Lipscombe LL, Jamal SA, Booth GL, Hawker GA. The risk of hip fractures in older individuals with diabetes: a population-based study. Diabetes Care. 2007;30(4):835-841. [DOI] [PubMed] [Google Scholar]
- 2. Nicodemus KK, Folsom AR; Iowa Women’s Health Study . Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care. 2001;24(7):1192-1197. [DOI] [PubMed] [Google Scholar]
- 3. Fan Y, Wei F, Lang Y, Liu Y. Diabetes mellitus and risk of hip fractures: a meta-analysis. Osteoporos Int. 2016;27(1):219-228. [DOI] [PubMed] [Google Scholar]
- 4. Michaëlsson K, Nordström P, Nordström A, et al. Impact of hip fracture on mortality: a cohort study in hip fracture discordant identical twins. J Bone Miner Res. 2014;29(2):424-431. [DOI] [PubMed] [Google Scholar]
- 5. Dyer SM, Crotty M, Fairhall N, et al. ; Fragility Fracture Network (FFN) Rehabilitation Research Special Interest Group . A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr. 2016;16:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cusick M, Meleth AD, Agrón E, et al. ; Early Treatment Diabetic Retinopathy Study Research Group . Associations of mortality and diabetes complications in patients with type 1 and type 2 diabetes: Early Treatment Diabetic Retinopathy Study report no. 27. Diabetes Care. 2005;28(3):617-625. [DOI] [PubMed] [Google Scholar]
- 7. Cummings SR, Nevitt MC. Non-skeletal determinants of fractures: the potential importance of the mechanics of falls. Study of Osteoporotic Fractures Research Group. Osteoporos Int. 1994;4(Suppl 1):67-70. [DOI] [PubMed] [Google Scholar]
- 8. Yang Y, Hu X, Zhang Q, Zou R. Diabetes mellitus and risk of falls in older adults: a systematic review and meta-analysis. Age Ageing. 2016;45(6):761-767. [DOI] [PubMed] [Google Scholar]
- 9. Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care. 2002;25(10):1749-1754. [DOI] [PubMed] [Google Scholar]
- 10. Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20(7):1185-1194. [DOI] [PubMed] [Google Scholar]
- 11. Ma L, Oei L, Jiang L, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27(5):319-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turner CH. Biomechanics of bone: determinants of skeletal fragility and bone quality. Osteoporos Int. 2002;13(2):97-104. [DOI] [PubMed] [Google Scholar]
- 13. Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14(Suppl 3):S13-S18. [DOI] [PubMed] [Google Scholar]
- 14. Hart NH, Nimphius S, Rantalainen T, Ireland A, Siafarikas A, Newton RU. Mechanical basis of bone strength: influence of bone material, bone structure and muscle action. J Musculoskelet Neuronal Interact. 2017;17(3):114-139. [PMC free article] [PubMed] [Google Scholar]
- 15. Ahlborg HG, Nguyen ND, Nguyen TV, Center JR, Eisman JA. Contribution of hip strength indices to hip fracture risk in elderly men and women. J Bone Miner Res. 2005;20(10):1820-1827. [DOI] [PubMed] [Google Scholar]
- 16. Seeman E, Duan Y, Fong C, Edmonds J. Fracture site-specific deficits in bone size and volumetric density in men with spine or hip fractures. J Bone Miner Res. 2001;16(1):120-127. [DOI] [PubMed] [Google Scholar]
- 17. Karlamangla AS, Barrett-Connor E, Young J, Greendale GA. Hip fracture risk assessment using composite indices of femoral neck strength: the Rancho Bernardo study. Osteoporos Int. 2004;15(1):62-70. [DOI] [PubMed] [Google Scholar]
- 18. LaCroix AZ, Beck TJ, Cauley JA, et al. Hip structural geometry and incidence of hip fracture in postmenopausal women: what does it add to conventional bone mineral density? Osteoporos Int. 2010;21(6):919-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaptoge S, Beck TJ, Reeve J, et al. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures. J Bone Miner Res. 2008;23(12):1892-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Samelson EJ, Demissie S, Cupples LA, et al. Diabetes and deficits in cortical bone density, microarchitecture, and bone size: Framingham HR-pQCT study. J Bone Miner Res. 2018;33(1):54-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shu A, Yin MT, Stein E, et al. Bone structure and turnover in type 2 diabetes mellitus. Osteoporos Int. 2012;23(2):635-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mitchell A, Fall T, Melhus H, Wolk A, Michaëlsson K, Byberg L. Type 2 diabetes in relation to hip bone density, area, and bone turnover in Swedish men and women: a cross-sectional study. Calcif Tissue Int. 2018;103(5):501-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. Bone loss and bone size after menopause. N Engl J Med. 2003;349(4):327-334. [DOI] [PubMed] [Google Scholar]
- 24. Seeman E. Periosteal bone formation—a neglected determinant of bone strength. N Engl J Med. 2003;349(4):320-323. [DOI] [PubMed] [Google Scholar]
- 25. Michaëlsson K, Wolk A, Byberg L, Mitchell A, Mallmin H, Melhus H. The seasonal importance of serum 25-hydroxyvitamin D for bone mineral density in older women. J Intern Med. 2017;281(2):167-178. [DOI] [PubMed] [Google Scholar]
- 26. Lind L, Fors N, Hall J, Marttala K, Stenborg A. A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Arterioscler Thromb Vasc Biol. 2005;25(11):2368-2375. [DOI] [PubMed] [Google Scholar]
- 27. Kumar J, Ingelsson E, Lind L, Fall T. No evidence of a causal relationship between plasma homocysteine and type 2 diabetes: a mendelian randomization study. Front Cardiovasc Med. 2015;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Michaëlsson K, Lithell H, Vessby B, Melhus H. Serum retinol levels and the risk of fracture. N Engl J Med. 2003;348(4):287-294. [DOI] [PubMed] [Google Scholar]
- 29. Fogh-Andersen N, D’Orazio P, Kuwa K, Külpmann WR, Mager G, Larsson L. Recommendation on reporting results for blood glucose (from an IFCC stage 1 document) IFCC scientific division working group on selective electrodes. EJIFCC. 2000;12(4):114-116. [PMC free article] [PubMed] [Google Scholar]
- 30. Norman A, Bellocco R, Bergström A, Wolk A. Validity and reproducibility of self-reported total physical activity—differences by relative weight. Int J Obes Relat Metab Disord. 2001;25(5):682-688. [DOI] [PubMed] [Google Scholar]
- 31. Orsini N, Bellocco R, Bottai M, et al. Validity of self-reported total physical activity questionnaire among older women. Eur J Epidemiol. 2008;23(10):661-667. [DOI] [PubMed] [Google Scholar]
- 32. Lind L, Carlsson AC, Siegbahn A, Sundström J, Ärnlöv J. Impact of physical activity on cardiovascular status in obesity. Eur J Clin Invest. 2017;47(2):167-175. [DOI] [PubMed] [Google Scholar]
- 33. Byberg L, Zethelius B, McKeigue PM, Lithell HO. Changes in physical activity are associated with changes in metabolic cardiovascular risk factors. Diabetologia. 2001;44(12):2134-2139. [DOI] [PubMed] [Google Scholar]
- 34. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. [DOI] [PubMed] [Google Scholar]
- 35. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599-2608. [DOI] [PubMed] [Google Scholar]
- 36. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37-48. [PubMed] [Google Scholar]
- 37. Mitchell A, Fall T, Melhus H, Lind L, Michaëlsson K, Byberg L. Supplementary data for: Type 2 diabetes and change in total hip bone area and bone mineral density in elderly Swedish men and women. Uppsala University Publications DiVA 2021. Deposited May 20, 2021. http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-442793 [DOI] [PMC free article] [PubMed]
- 38. Oei L, Zillikens MC, Dehghan A, et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam study. Diabetes Care. 2013;36(6):1619-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Colleluori G, Aguirre L, Dorin R, et al. Hypogonadal men with type 2 diabetes mellitus have smaller bone size and lower bone turnover. Bone. 2017;99:14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res. 1993;8(5):567-573. [DOI] [PubMed] [Google Scholar]
- 41. Reid IR, Ames R, Evans MC, et al. Determinants of total body and regional bone mineral density in normal postmenopausal women—a key role for fat mass. J Clin Endocrinol Metab. 1992;75(1):45-51. [DOI] [PubMed] [Google Scholar]
- 42. Black DM, Bouxsein ML, Marshall LM, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group . Proximal femoral structure and the prediction of hip fracture in men: a large prospective study using QCT. J Bone Miner Res. 2008;23(8):1326-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cheng XG, Lowet G, Boonen S, et al. Assessment of the strength of proximal femur in vitro: relationship to femoral bone mineral density and femoral geometry. Bone. 1997;20(3):213-218. [DOI] [PubMed] [Google Scholar]
- 44. Beck TJ, Oreskovic TL, Stone KL, et al. Structural adaptation to changing skeletal load in the progression toward hip fragility: the study of osteoporotic fractures. J Bone Miner Res. 2001;16(6):1108-1119. [DOI] [PubMed] [Google Scholar]
- 45. Riggs BL, Melton Iii LJ III, Robb RA, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19(12):1945-1954. [DOI] [PubMed] [Google Scholar]
- 46. Seeman E. Estrogen, androgen, and the pathogenesis of bone fragility in women and men. Curr Osteoporos Rep. 2004;2(3):90-96. [DOI] [PubMed] [Google Scholar]
- 47. Hamann C, Kirschner S, Günther KP, Hofbauer LC. Bone, sweet bone—osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol. 2012;8(5):297-305. [DOI] [PubMed] [Google Scholar]
- 48. Burghardt AJ, Issever AS, Schwartz AV, et al. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95(11):5045-5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kremer R, Gilsanz V. Fat and bone: an odd couple. Front Endocrinol (Lausanne). 2015;6:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mazess RB, Hanson JA, Payne R, Nord R, Wilson M. Axial and total-body bone densitometry using a narrow-angle fan-beam. Osteoporos Int. 2000;11(2):158-166. [DOI] [PubMed] [Google Scholar]
- 51. Bazzocchi A, Ponti F, Albisinni U, Battista G, Guglielmi G. DXA: technical aspects and application. Eur J Radiol. 2016;85(8):1481-1492. [DOI] [PubMed] [Google Scholar]
- 52. Jackson JM, DeFor TA, Crain AL, et al. Validity of diabetes self-reports in the Women’s Health Initiative. Menopause. 2014;21(8):861-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151-S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Szulc P, Delmas PD. Bone loss in elderly men: increased endosteal bone loss and stable periosteal apposition. The prospective MINOS study. Osteoporos Int. 2007;18(4):495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Davison KS, Siminoski K, Adachi JD, et al. Bone strength: the whole is greater than the sum of its parts. Semin Arthritis Rheum. 2006;36(1):22-31. [DOI] [PubMed] [Google Scholar]
- 56. Norman TL, Wang Z. Microdamage of human cortical bone: incidence and morphology in long bones. Bone. 1997;20(4):375-379. [DOI] [PubMed] [Google Scholar]
- 57. Nohr EA, Liew Z. How to investigate and adjust for selection bias in cohort studies. Acta Obstet Gynecol Scand. 2018;97(4):407-416. [DOI] [PubMed] [Google Scholar]
- 58. Byberg L, Bellavia A, Larsson SC, Orsini N, Wolk A, Michaëlsson K. Mediterranean diet and hip fracture in Swedish men and women. J Bone Miner Res. 2016;31(12):2098-2105. [DOI] [PubMed] [Google Scholar]
- 59. Mozaffari H, Djafarian K, Mofrad MD, Shab-Bidar S. Dietary fat, saturated fatty acid, and monounsaturated fatty acid intakes and risk of bone fracture: a systematic review and meta-analysis of observational studies. Osteoporos Int. 2018;29(9):1949-1961. [DOI] [PubMed] [Google Scholar]
- 60. Sadeghi O, Djafarian K, Ghorabi S, Khodadost M, Nasiri M, Shab-Bidar S. Dietary intake of fish, n-3 polyunsaturated fatty acids and risk of hip fracture: a systematic review and meta-analysis on observational studies. Crit Rev Food Sci Nutr. 2019;59(8):1320-1333. [DOI] [PubMed] [Google Scholar]
- 61. InterAct Consortium; Romaguera D, Guevara M, Norat T, et al. Mediterranean diet and type 2 diabetes risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study: the InterAct project. Diabetes Care. 2011;34(9):1913-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mitchell A, Larsson SC, Fall T, Melhus H, Michaëlsson K, Byberg L. Fasting glucose, bone area and bone mineral density: a Mendelian randomisation study. Diabetologia. 2021;64(6):1348-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Paschou SA, Dede AD, Anagnostis PG, Vryonidou A, Morganstein D, Goulis DG. Type 2 diabetes and osteoporosis: a guide to optimal management. J Clin Endocrinol Metab. 2017;102(10):3621-3634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.