Abstract
Introduction
The COVID-19 pandemic has challenged healthcare systems worldwide over the last few months, and it continues to do so. Although some restrictions are being removed, it is not certain when the pandemic is going to be definitively over. Pandemics can be seen as a highly complex logistic scenario. From this perspective, some of the indications provided for palliative radiotherapy (PRT) during the COVID-19 pandemic could be maintained in the future in settings that limit the possibility of patients achieving symptom relief by radiotherapy.
This paper has two aims: (1) to provide a summary of the indications for PRT during the COVID-19 pandemic; since some indications can differ slightly, and to avoid any possible contradictions, an expert panel composed of the Italian Association of Radiotherapy and Clinical Oncology (AIRO) and the Palliative Care and Supportive Therapies Working Group (AIRO-palliative) voted by consensus on the summary; (2) to introduce a clinical care model for PRT [endorsed by AIRO and by a spontaneous Italian collaborative network for PRT named “La Rete del Sollievo” (“The Net of Relief”)]. The proposed model, denoted “No cOmpRoMise on quality of life by pALliative radiotherapy” (NORMALITY), is based on an AIRO-palliative consensus-based list of clinical indications for PRT and on practical suggestions regarding the management of patients potentially suitable for PRT but dealing with highly complex logistics scenarios (similar to the ongoing logistics limits due to COVID-19).
Material and Methods
First, a summary of the available literature guidelines for PRT published during the COVID-19 pandemic was prepared. A systematic literature search based on the PRISMA approach was performed to retrieve the available literature reporting guideline indications fully or partially focused on PRT. Tables reporting each addressed clinical presentation and respective literature indications were prepared and distributed into two main groups: palliative emergencies and palliative non-emergencies. These summaries were voted in by consensus by selected members of the AIRO and AIRO-palliative panels. Second, based on the summary for palliative indications during the COVID-19 pandemic, a clinical care model to facilitate recruitment and delivery of PRT to patients in complex logistic scenarios was proposed. The summary tables were critically integrated and shuffled according to clinical presentations and then voted on in a second consensus round. Along with the adapted guideline indications, some methods of performing the first triage of patients and facilitating a teleconsultation preliminary to the first in-person visit were developed.
Results
After the revision of 161 documents, 13 papers were selected for analysis. From the papers, 19 clinical presentation items were collected; in total, 61 question items were extracted and voted on (i.e., for each presentation, more than one indication was provided from the literature). Two tables summarizing the PRT indications during the COVID-19 pandemic available from the literature (PRT COVID-19 summary tables) were developed: palliative emergencies and palliative non-emergencies. The consensus of the vote by the AIRO panel for the PRT COVID-19 summary was reached. The PRT COVID-19 summary tables for palliative emergencies and palliative non-emergencies were adapted for clinical presentations possibly associated with patients in complex clinical scenarios other than the COVID-19 pandemic. The two new indication tables (i.e., “Normality model of PRT indications”) for both palliative emergencies and palliative non-emergencies were voted on in a second consensus round. The consensus rate was reached and strong. Written forms facilitating two levels of teleconsultation (triage and remote visits) were also developed, both in English and in Italian, to evaluate the patients for possible indications for PRT before scheduling clinical visits.
Conclusion
We provide a comprehensive summary of the literature guideline indications for PRT during COVID-19 pandemic. We also propose a clinical care model including clinical indications and written forms facilitating two levels of teleconsultation (triage and remote visits) to evaluate the patients for indications of PRT before scheduling clinical visits. The normality model could facilitate the provision of PRT to patients in future complex logistic scenarios.
Keywords: Palliative Radiotherapy, COVID-19, Palliation, QoL, Consensus, Clinical Indication, Clinical Care Model, Guidelines
Introduction
The COVID-19 pandemic has challenged healthcare systems worldwide over the last few months, and this is still ongoing [1, 2]. Although some restrictions have been removed depending on the country, it is not certain when the pandemic is going to be over for certain. Radiation oncologists (ROs) will be forced to face the pandemic for an unknown time interval. Two main approaches have been adopted to control COVID-19: suppression and mitigation, the latter being the most frequently adopted [3]. The mitigation approach imposes the need for indications for how and when to delay or omit radiotherapy (RT). Alternatively, hypofractionated RT schedules, which adequately manage different clinical settings, have been proposed to reduce the number of interactions and physical contact in hospitals (for both patients and patients) while delivering effective treatments [4–7].
National and international guidelines and expert opinions about RT indications and prescriptions have been provided for primary malignancies (e.g., head and neck [6] or gastrointestinal [7]). More often, palliative RT (PRT) indications during the COVID-19 pandemic scenario are dealt with using reports focused on primary malignancies. To the best of our knowledge, very few guidelines have been specifically dedicated to PRT, and in some cases, these are limited to particularly relevant palliative presentations (e.g., bone metastases [8]). Although the level of priority of PRT has frequently been the object of discussion [3, 9–11], it remains one of the primary aims of RT. Once the COVID-19 pandemic has concluded, many of the RT indications currently modified due to pandemic issues could not be further considered for most primary tumors. Conversely, in some situations (e.g., patients admitted in hospice; patients living at high distance from an RT department; less-resourced developing countries), the issue of patients suitable for PRT but dealing with complex logistic settings and thus subject to limitations in their possibility to achieve symptom relief by PRT will surely persist. Therefore, some of the indications provided for PRT during the COVID-19 pandemic could be safely and effectively maintained in these peculiar settings (since they are currently clinically accepted).
This paper has two aims: (1) to provide a summary of the indications for PRT during the COVID-19 period. Since some published guidelines are slightly different, in order to harmonize the suggestions, an expert panel composed of the Italian Association of Radiotherapy and Clinical Oncology (AIRO) and the Palliative Care and Supportive Therapies Working Group (AIRO-palliative) voted by consensus on the summary. (2) To introduce a clinical care model for PRT [endorsed by AIRO and by a spontaneous Italian collaborative network for PRT named “La Rete del Sollievo” (“The Net of Relief”)]. The proposed model, denoted “No cOmpRoMise on quality of life by pALliative radIoTherapY" (NORMALITY), is based on an AIRO-palliative consensus-based list of clinical indications for PRT and on practical suggestions regarding the management of patients potentially suitable for PRT but dealing with highly complex logistics scenarios (similar to the ongoing logistics limits due to COVID-19).
Material and methods
The two aims of this project were handled separately and progressively. The first aim (1) was to summarize the PRT clinical indications during the COVID-19 pandemic from the available RT literature. In particular, we aimed to (1.1) provide a summary (PRT COVID-19 summary) of the indications and guidelines for PRT during the COVID-19 period and to (1.2) have the AIRO expert team group vote by consensus on the PRT COVID-19 summary.
Our second (2) aim was to create the NORMALITY clinical care model (“No cOmpRoMise on quality of life by pALliative radIoTherapY"). In particular, we aimed to (2.1) provide a set of PRT indications for patients dealing with complex logistic scenarios (strongly limiting their possibility of receiving PRT beyond its given clinical indication) in order to (2.2) provide practical advice and supportive materials to optimize the clinical management of these patients by RT departments.
Summarize the PRT clinical indications from the available COVID-19 RT literature
Create the PRT COVID-19 summary
A systematic literature search based on the PRISMA approach was performed by two radiation oncologists (ROs; RDF and VB). The search was performed using PubMed. We applied the following medical subject headings (MeSH) and keywords such as: “Radiotherapy,” “Radiation Therapy,” “Radiation Oncology,” “Palliative,” “Palliative Radiotherapy,” “COVID-19,” and “SARS-COV2.” The detailed Medline search strategy is reported in Appendix.
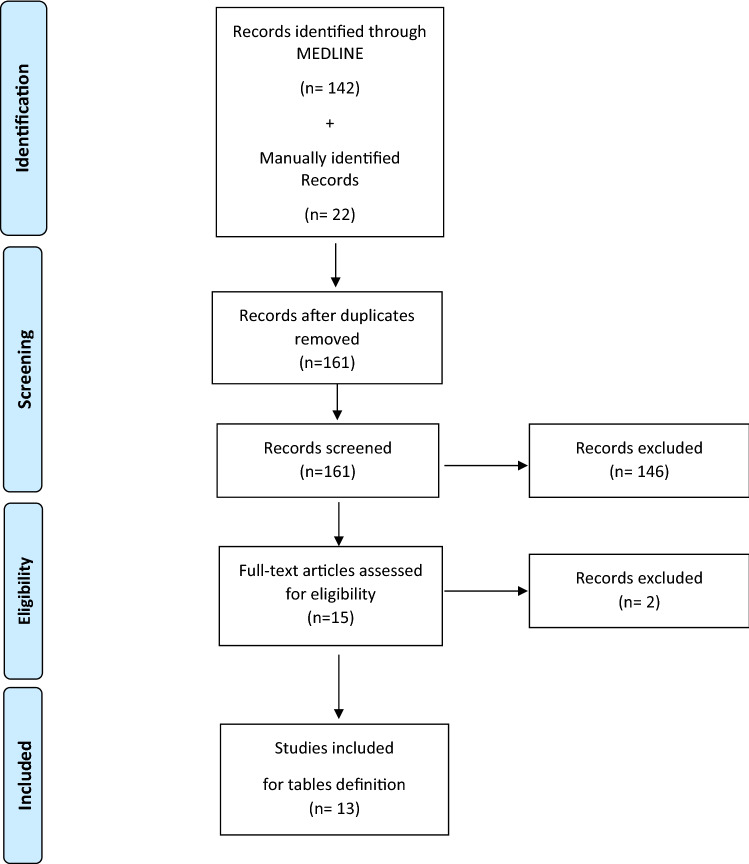
For the first literature search, other documents were added by a manual search performed by a third RO (SM). The review was strictly composed of full-text publications that were written in English and reported clinical indications for PRT to be applied during the period of the COVID-19 pandemic. The literature search was conducted on April 26, 2020. All types of publications were initially considered, including surveys, letters, and editorials, provided that the prescriptive indications for PRT were clearly reported. Papers reviewing literature or personal considerations and not directly addressing a prescriptive indication were excluded. Reports of congress abstracts and book chapters were excluded. Reports providing clinical PRT indications and those that did not undergo a peer-review process were also excluded. No specific time restrictions other than those implicitly related to the COVID-19 pandemic period were applied. An independent literature revision was made by a different RO who supervised the summary consolidation process (FC). Eligible citations were retrieved for full-text review. Figure 1 illustrates the PRISMA workflow.
Fig. 1.
PRISMA Literature Search
To homogenize the summary output, the clinical presentations discussed in the documents were collected into two main groups: emergencies and palliative non-emergencies. A retrospective collection of each clinical presentation was organized into these two groups within multiple clinical presentation subgroups (defined as “clinical presentation items,” CPI).
Three information categories were extracted from each selected document: (i) the main (preferable) PRT prescriptive indication; (ii) the alternative (secondary) PRT prescriptive indication; (iii) additional statements in the document per each subgroup clinical presentation not strictly indicating a PRT prescription but specifically aimed at the considered subgroup topic.
A document was excluded by a certain clinical presentation subgroup if none of the three categories were addressed, but possibly included for other subgroup topic indications.
A group of four separate ROs double-checked the different sections of the PRT COVID-19 summary to confirm the correspondence of the data extraction (FA, AT, GS, and AC).
AIRO expert team consensus vote on the PRT COVID-19 summary
An expert panel of 14 AIRO members, which was not involved in any of the previously described phases, was asked to vote on, in a single round, the consensus of each of the reported PRT indications. Consensus was addressed by four options: 1 = “strongly agree,” 2 = “agree,” 3 = “disagree,” and 4 = “strongly disagree”. Consensus was based on all of the indications from each paper (i.e., main + alternative PRT indication + additional statements), thus preventing experts from only agreeing or disagreeing with specific parts of the summarized papers.
For each paper reported in the summary, the results were analyzed by a single vote and coupled as either a “positive consensus vote” (i.e.: 1 + 2) or “negative consensus vote” (i.e.: 3 + 4).
As for other experiences [5], an agreement or disagreement threshold ≥ 66% was required for each item to reach a consensus and a threshold of ≥ 80% was required for a strong consensus.
NORMALITY (“No cOmpRoMise on quality of life by pALliative radIoTherapY") clinical care model
The spontaneous network named “La Rete del Sollievo” (www.laretedelsollievo.net) (i.e. Net of Relief, NOR), which is set up at the Department of Radiation Oncology of Fondazione Policlinico A. Gemelli IRRCS (Rome, Italy), promotes palliative radiation oncology clinical care models and shares research projects in collaboration with the AIRO-palliative panel. Under the endorsement of the AIRO, the NOS aimed to create a clinical care model for patients with an indication for PRT who are dealing with high complexity logistic scenarios that limit their possibility of receiving a regular PRT schedule.
Provide PRT indications (through consensus vote by the AIRO expert team)
From the evidence base of the PRT COVID-19 summary, a table of PRT indications for patients in complex logistic settings (potentially other than the COVID-19 pandemic) was set up for the NORMALITY clinical care model. The table “Normality model of PRT indications” followed the structure of the PRT COVID-19 summary. The AIRO expert panel voted in two rounds of consensus using the same previously described methodology. In this case, we also added the opportunity to provide comments and alternative indications for the first voting round only. After the revision of the first-round votes and comments, a final version of the table was voted on once more. Analysis of the consensus was performed as previously described. Only the results of the final consensus round were considered in the analysis.
Provide practical advice and supportive materials for the NORMALITY clinical care model
To establish the practicalities of the NORMALITY clinical care model, the definition of the workflow was addressed based on the available literature indications [9, 12], some currently ongoing practices among the Radiation Oncology departments of the AIRO experts involved in the project, and through discussion among the AIRO experts. The core concept was the advantage of a preliminary evaluation of the patient’s indications ahead of a live visit. During the live visit, the PRT prescription would be confirmed, potentially including (within the same day) the RT simulation and the first (or single) session administration. Moreover, two types of forms aiding practical patient management were prepared: one to perform the first general patient data collection and allow for triage in palliative settings, and the second to aid the offline evaluation of patients ahead of clinical visits.
Results
PRT COVID-19 summary + AIRO expert team consensus vote
From the search results of 161 documents, 13 papers were selected for data extraction [4–9, 13–19]. Globally, 19 clinical presentation items (CPIs) were identified. For “Emergencies,” the following CPIs were extracted from the literature and considered: metastatic epidural spinal cord compression (MESCC); hemostasis (including hemoptysis); and mediastinal syndrome.
For “Palliative Non-Emergencies,” the following CPIs were extracted from the literature and considered: painful bone metastasis; non-painful bone metastasis; bone oligometastases suitable for (stereotactic body radiation therapy) SBRT; retreatment of painful bone metastasis; adjuvant (post-surgery) bone metastasis radiotherapy; pain symptoms NOT associated with bone metastases; symptomatic hematological malignancies; other oligometastases suitable for SBRT (lung); other oligometastases suitable for SBRT (liver); other oligometastases suitable for SBRT (adrenal); other oligometastases suitable for SBRT (lymph-node asymptomatic); brain metastases (N° 1–4); brain metastases (N° 5–10); brain metastases (N° > 4), poor Karnofsky performance status (KPS), meningeal involvement; primary symptomatic brain tumor, poor KPS; and postoperative brain metastases.
In total, 61 question items to be voted on were extracted from the papers including the 19 CPIs. Table 1 presents the PRT COVID-19 summary for palliative emergencies. Table 2 presents the PRT COVID-19 summary for palliative non-emergencies, along with the consensus results. References cited by selected papers are also reported in the table, if specifically related to trials [5, 20–50]. The average agreement was over the agreement threshold, with only 10/61 question items from the different evidence having an agreement below 60% and five that were below 50%. The latter was related to single evidence regarding painful bone metastases, bone metastases suitable for SBRT, adjuvant treatment of bone metastases, other than pain symptoms related to lung primary, and SBRT for adrenal lesions.
Table 1.
PRT Covid-19 Summary: palliative emergencies
| Emergencies | ||||||
|---|---|---|---|---|---|---|
| Reference | Main Prescriptive Indication | Alternative | Additional Statement (if any) | % Consensus Vote* | ||
| A = Agreement (1 + 2) | ||||||
| D = Disagreement (3 + 4) | ||||||
| SA = Strong Agreement (1) | ||||||
| SD = Strong Disagreement (4) | ||||||
| E1 Metastatic Epidural Spinal Cord Compression (MESCC) | ||||||
| QE1a | [9] | 8 Gy/1fx8Gy [Maranzano [19]] | – | • Requires multidisciplinary discussion with neurosurgery, and evaluation of factors including degree of spinal cord compression and presence or absence of spinal instability | A = 100% [SA = 100%] | D = 0% [SD = 0%] |
| • Similar impact on OS and post-RT motor functions than multifractions | ||||||
| • Retreatment is safe | ||||||
| QE1b | Curigliano [16] | – | – | • RT is urgent | A = 100% [SA = 80%] | D = 0% [SD = 0%] |
| QE1c | Thureau [8] | 8 Gy/1fx8Gy | – | • Surgical treatment should theoretically be preferred if possible and for all pt with a life expectancy of more than few months | A = 70% [SA = 30%] | D = 30% [SD = 0%] |
| • Adjuvant RT after surgery for MESCC can be postponed for 4 to 12 weeks | ||||||
| • In cases where surgical treatment is contraindicated or not appropriate, RT should be arranged without delay | ||||||
| • The simplest conformal RT techniques should be used | ||||||
| • MESCC is likely the only instance justifying urgent management of a COVID + patient | ||||||
| QE1d | Simcock [14] | 6-10 Gy/1fx6-10 Gy [ICORG 05–03 [20], TROG 96.05 [21]] | – | • Prefer 3D | A = 80% [SA = 10%] | D = 20% [SD = 0%] |
| E2 Hemostasis (including Hemoptysis) | ||||||
| QE2a | Tchelebi [7] | • Esophageal cancer bleeding: 6–8 Gy/ 1fx 6-8 Gy | – | • Gastric cancer bleeding: RT should be strictly reserved for palliation of symptoms in pts with gastric cancer at the present time | A = 80% [SA = 20%] | D = 20% [SD = 0%] |
| • Gastric cancer bleeding: 6–8 Gy/ 1fx 6-8 Gy (with anti-emetic) | ||||||
| QE2b | [9] | • Pelvic malignancies bleeding: 14.8 Gy/4fx/3.7BID | – | Pelvic malignancies bleeding pt Covid + : Avoid BID | A = 80% [SA = 20%] | D = 20% [SD = 0%] |
| • Pelvic malignancies bleeding, pt Covid + : 20 Gy/5fx4Gy | ||||||
| QE2c | Wu [13] | Hemoptysis: 20 Gy/5fx4Gy | – | Palliative lung radiation should be deferred when possible, otherwise reserved for pt with life-threatening complications such as high-volume hemoptysis | A = 80% [SA = 30%] | D = 20% [SD = 10%] |
| • 17 Gy/2fx8.5 Gy§ | ||||||
| • 10 Gy/1fx10Gy | ||||||
| QE2d | Hahn et al. [63] | Pelvic bleeding: 8 Gy/1fx8Gy | – | – | A = 80% [SA = 40%] | D = 20% [SD = 0%] |
| QE2e | Combs [15] | Bleeding 8 Gy /1fx8Gy (not further specified) | – | – | A = 60% [SA = 30%] | D = 40% [SD = 0%] |
| QE2f | Thomson [6] |
H&N bleeding: o Scenario 1- Early Pandemic—Risk mitigation |
– | – | A = 70% [SA = 30%] | D = 30% [SD = 0%] |
| • 8 Gy/1fx8Gy | ||||||
| • 20 Gy/5fx4Gy | ||||||
| • 44.4 Gy/12fx3,7 Gy | ||||||
| o Scenario 2- Late Pandemic—Severe shortage of RT capacity | ||||||
| • 8 Gy/1fx8Gy | ||||||
| • 20 Gy/5fx4Gy | ||||||
| QE2g | Simcock [14] |
Esophageal bleeding: • 12 Gy/4fx3Gy BID [SHARON project [23]] |
Esophageal bleeding: 15 Gy/3fx5Gy [SHARON project]§ |
Esophageal bleeding: • Prefer 3D |
A = 80% [SA = 30%] | D = 20% [SD = 0%] |
| • 18 Gy/3fx6Gy Day (Q) 0, 7, 21 (weekly) (Adapted from other sites) [25] | Pelvic/GI bleeding: | |||||
| Pelvic/GI bleeding: | • Prefer 3D | |||||
| • 20-24 Gy/5-6fx4Gy | • Prefer 3D | |||||
| • 18 Gy/4fx4.5 Gy BID | ||||||
| [SHARON project [23]] | ||||||
| • 14.8 Gy/4fx3.7 Gy BID (Repeat q2-4 wks to total 44.4 Gy in 3 courses) [QUAD SHOT- RTOG 8502 [26, 27]] | ||||||
| • 18-24 Gy/3fx6-8 Gy Day 0, 7, 21 [25] | ||||||
| • 18-24 Gy/3fx6-8 Gy Day 0, 7, 21 [25] | ||||||
| E3 Mediastinal Syndrome | ||||||
| QE3a | Yerramilli [9] |
SVC syndrome Airway Obstruction: • 17 Gy/2fx8.5 Gy (each, weekly) [Sundstrom [31]] |
– | Multidisciplinary discussion may be recommended | A = 100% [SA = 70%] | D = 0% [SD = 0%] |
| • 20 Gy/5fx4Gy | ||||||
| QE3b | Guckenberger | o NSCLC-Early Phase of the COVID-19 pandemic (risk mitigation): | – | Order reported for “NSCLC Early Phase” follows the highest consensus reported in the paper | A = 80% [SA = 50%] | D = 30% [SD = 0%] |
| 2. 8–10 Gy/1fx 8–10 Gy 20 Gy/5fx 4 Gy | ||||||
| o NSCLC -Later phase of the COVID-19 pandemic: (lack of RT resources and need for patient triage) 8-10 Gy/1fx 8-10 Gy | ||||||
| QE3c | Wu [13] | Superior vena cava syndrome: | – | Palliative lung RT should be deferred when possible, otherwise reserved for patients with lifethreatening complications such as superior vena cava syndrome | A = 70% [SA = 40%] | D = 30% [SD = 0%] |
| • 17 Gy/2fx8.5 Gy§ | ||||||
| §(Authors do not specify in text/table but the reference report the schedule as “weekly”) [24] [Rodrigues] | ||||||
| • 10 Gy/1fx10Gy | ||||||
| SCV Syndrome/Lung | ||||||
| QE3d | Simcock [14] | Cancer: | – | Prefer 3D | A = 90% [SA = 30%] | D = 10% [SD = 0%] |
| • 8–10 Gy/1fx8-10 Gy | ||||||
| • 17 Gy/2fx8.5 Gy (weekly) [33] [MRC] | ||||||
§(Authors do not specify in text/table but the reference report the schedule as “weekly”) [Rodrigues [24]]
§ Note: the schedule reported in the paper do not corresponds to Sharon Project schedule
*Consensus Vote: 1 = Strongly Agree; 2 = Agree; 3 = Disagree; 4 = Strongly Disagree
MESCC Metastatic Epidural Spinal Cord Compression; fx fraction; OS overall Survival; RT Radiotherapy; pt patient; BID bis in die; Q schedule repetition interval; QoL quality of life; SBRT stereotactic body RT mets: metastases; wks weeks; PEG percutaneous endoscopic gastrostomy; WBRT whole brain RT; TMZ Temozolamide; mth months; IMRT-SIB Intensity modulated RT—Simultaneous integrated boost
Table 2.
PRT Covid-19 Summary: palliative non-emergencies
| Palliative (Non-Emergencies) | ||||||
|---|---|---|---|---|---|---|
| Reference | Main Prescriptive Indication | Alternative | Additional Statement (if any) | % Consensus Vote* | ||
| A = Agreement (1 + 2) | ||||||
| D = Disagreement (3 + 4) | ||||||
| SA = Strong Agreement (1) | ||||||
| SD = Strong Disagreement (4) | ||||||
| P1 Painful bone metastasis | ||||||
| QP1a | Thureau [8] | 8 Gy/1fx8Gy | 6-10 Gy/1 fx6-10 Gy | • Adapt the medical treatment as much as possible and avoid palliative RT in pt controlled by level 1 to 3 oral analgesics | A = 70% [SA = 40%] | D = 30% [SD = 0%] |
| • Palliative RT remains an important option for patients experiencing significant pain, diminished QoL and reduced autonomy by bone metastases, especially if it enables a reduction in the need for daily nursing care | ||||||
| • The simplest conformal | ||||||
| RT techniques should be used | ||||||
| • Other that 8 Gy should be avoided | ||||||
| QP1b | Simcock [14] | 8 Gy/1fx8Gy | – | • Evaluate Omission | A = 40% [SA = 10%] | D = 60% [SD = 20%] |
| • If RT is for symptom relief then it is best to ensure that all other options have been fully explored e.g. maximizing analgesia or bisphosphonates in the case of bone pain | ||||||
| QP1c | Combs [15] | • 8 Gy/1fx8Gy | – | – | A = 60% [SA = 30%] | D = 40% [SD = 0%] |
| • 20 Gy/ 5 fx4Gy | ||||||
| • 21 Gy/ 3 fx7Gy | ||||||
| QP1d | Yerramilli [9] | 8 Gy/1fx8Gy | – | • If pts have life expectancy of days to weeks: refer to Best supportive Care | A = 50% [SA = 10%] | D = 50% [SD = 20%] |
| • If pts have life expectancy of longer than weeks, but not emergency: delay RT | ||||||
| • pt with less urgent symptoms (able to wait planning) single-fraction SBRT may be considered | ||||||
| QP1e | Curigliano [16] | – | – | • Advanced breast cancer (ABC): RT is urgent if pts not responding to pharmaceutical interventions | A = 80% [SA = 20%] | D = 20% [SD = 10%] |
| P3 Bone Oligometastases Suitable for SBRT | ||||||
| QP3a | Thureau [8] | – | Single fraction (16 to 24 Gy) SBRT for Retreatment of Symptomatic MESCC | • Evidence for using SBRT in oligometastatic is too low to be considered in the current situation | A = 90% [SA = 30%] | D = 10% [SD = 0%] |
| • It is often possible to postpone this treatment for a few weeks, especially for hormone sensitive tumors | ||||||
| QP3b | Simcock [14] | – | – | • Omit RT | A = 20% [SA = 0%] | D = 80% [SD = 80%] |
| QP3c | Combs [15] | SBRT 1–5 fx (not further specified) | – | – | A = 80% [SA = 10%] | D = 20% [SD = 10%] |
| P4 Retreatment of painful bone metastasis | ||||||
| QP4a | Thureau [8] | 8 Gy/1fx8Gy | – | • Waiting a minimum of 6 weeks after completion of the initial RT | A = 90% [SA = 50%] | D = 10% [SD = 0%] |
| • The simplest conformal RT techniques should be used | ||||||
| P5 Adjuvant (post-surgery) bone metastasis radiotherapy | ||||||
| QP5a | Thureau [8] | 30 Gy/10 fx3Gy | 20 Gy/4 or 5fx 5 or 4 Gy | • RT may be postponed or performed secondarily in case of progressive post-operative signs | A = 80% [SA = 50%] | D = 20% [SD = 0%] |
| QP5b | Simcock [14] | – | 20 Gy/4or5fx 5or4Gy | • Omit RT | A = 40% [SA = 0%] | D = 60% [SD = 20%] |
| P6 Pain NOT associated to Bone Mets (e.g.: direct infiltration, primary pancreatic; H&N; Lymph-node infiltrating surrounding structures, etc.) | ||||||
| QP6a | Thomson [6] | H&N: If restricted RT department resources single fraction could be used: 8 Gy/1fx8Gy | H&N: If restricted RT department resources: 20 Gy/5fx4Gy | • Symptomatic benefit and chance of cure are two of the top three factors determining which patients should start RT within 1–3 wks | A = 70% [SA = 30%] | D = 30% [SD = 0%] |
| • Do not postpone RT initiation of HNSCC radiotherapy by more than 4–6 wks | ||||||
| • If Covid + pt delay RT until clinical recover | ||||||
| • Use a more hypofractionated schedule if restricted RT department resources | ||||||
| QP6b | Combs [15] | H&N: | – | – | A = 60% [SA = 20%] | D = 40% [SD = 10%] |
| 14 Gy/4fx 3.5 Gy BID (repeated Q 4 weeks interval × 2 times) [QUAD SHOT- RTOG 8502 [26, 27]] | ||||||
| QP6c | Hahn et al. [63] | H&N + Gyn + Melanoma: | – | – | A = 80% [SA = 40%] | D = 20% [SD = 0%] |
| • 8 Gy/1fx8Gy | ||||||
| • 18–24/3fx6-8 Gy Q 0–7-(21 if needed) [25, 29, 36] | ||||||
| QP6d | Tchelebi [7] | • Pain by primary Esophageal + HCC: 6–8/1fx6-8 Gy | – | – | A = 80% [SA = 20%] | D = 20% [SD = 0%] |
| • Pain by primary Pancreas: 8–10/1fx8-10 Gy | ||||||
| QP6e | Rathod [18] | SCLC/NSCLC: | – | – | A = 90% [SA = 20%] | D = 10% [SD = 0%] |
| • 8–10 Gy/1fx8-10 Gy [IAEA [37]] | ||||||
| • 16 Gy/2fx 8 Gy (1 week apart) [IAEA [37]] | ||||||
| P7 Other than Pain symptoms NOT associated to Bone Mets (e.g.: obstruction, etc.) | ||||||
| QP7a | Thomson [6] | (H&N) If restricted RT department resources single fraction could be used: 8 Gy/1fx8Gy | (H&N) If restricted RT department resources: 20 Gy/5fx4Gy | • Do not postpone RT initiation of HNSCC RT by more than 4–6 wks | A = 60% [SA = 30%] | D = 40% [SD = 0%] |
| • If Covid + pt: delay RT until clinical recover | ||||||
| • Use a more hypofractionated schedule if restricted RT department resources | ||||||
| QP7b | Combs [15] | H&N: | – | – | A = 70% [SA = 20%] | D = 30% [SD = 0%] |
| 14 Gy/4fx 3.5 Gy BID (repeated Q4 weeks interval × 2 times) [QUAD SHOT- RTOG 8502 [26, 27]] | ||||||
| QP7c | Simcock [14] | H&N: | H&N: 18or24Gy/3fx6or8 (1 fx/week) Prefer 3D or IMRT | – | A = 70% [SA = 10%] | D = 30% [SD = 0%] |
| • 36 Gy/5fx6Gy | ||||||
| (2 fx/week) | ||||||
| • 30 Gy/6fx6Gy | ||||||
| (2 fx/week) [HYPO trial [38]] | ||||||
| QP7d | Hahn et al. [63] | H&N + Gyn + Melanoma: | – | – | A = 60% [SA = 40%] | D = 40% [SD = 0%] |
| • 8 Gy/1fx8Gy 18–24 Gy/3fx6-8 Gy Q 0–7-(21 if needed) | ||||||
| QP7e | Simcock [14] | Esophageal dysphagia: | Esophageal dysphagia: 15 Gy/3fx5Gy [SHARON project]§ § Note: the schedule reported in the paper do not corresponds to Sharon Project schedule | – | A = 60% [SA = 0%] | D = 40% [SD = 0%] |
| • 12 Gy/4fx3GyBID [SHARON project [23]] | ||||||
| • 18 Gy/3fx6 (1 fx/week) | ||||||
| QP7f | Tchelebi [7] | Esophageal Dysfagia:20 Gy/5fx4Gy | – | • RT is preferred over either an esophageal stent or percutaneous endoscopic gastrostomy (PEG) tube placement in order to avoid consumption of limited operative supplies and aerosolization of the virus secondary to intubation | A = 90% [SA = 50%] | D = 10% [SD = 0%] |
| QP7g | Tchelebi [7] | Pancreas Symptomatic (non-pain): 8–10 Gy/1fx8-10 Gy | – | – | A = 70% [SA = 20%] | D = 30% [SD = 0%] |
| QP7h | Rathod [18] | SCLC/NSCLC: | – | – | A = 70% [SA = 30%] | D = 30% [SD = 0%] |
| • 8-10 Gy/1fx 8–10 Gy | ||||||
| • 16 Gy/2 fx 8 Gy (1 week apart) [IAEA [37]] | ||||||
| QP7i | Guckemberger [5] | NSCLC Early Phase (Risk Mitigation): | – | • NSCLC Early Phase (Risk Mitigation): do not postpone RT of 4–6 weeks | A = 70% [SA = 20%] | D = 30% [SD = 0%] |
| 1.17 Gy/2 fx 8.5 Gy § | • Postpone or interrupt RT if pts is or became Covid + | |||||
| • Order for “NSCLC Early Phase” follows the highest consensus reported in the paper | ||||||
| 2.8–10 Gy/1fx 8–10 Gy | ||||||
| 3.20 Gy/5fx 4 Gy | ||||||
| NSCLC Later Phase (Lack of RT Resources): | ||||||
| • 8–10 Gy/1fx 8–10 Gy | ||||||
| QP7j | Wu [13] | – | – | • Lung tumors: palliative lung radiation should be deferred | A = 20% [SA = 0%] | D = 80% [SD = 20%] |
| P8 Symptomatic Haematological Malignancies (non-emergencies) | ||||||
| QP8a | Yahalom 4 | • Symptomatic aggressive NHL (no chemo options) Life expectancy > 3 months 25 Gy/5fx5Gy | – | • Consider omitting RT when the risk of severe outcomes from COVID-19 infection (aged ≥ 60 years and/or presence of serious underlying health conditions) outweigh the benefit of RT; where alternatives can be offered e.g. optimizing pain control | A = 100% [SA = 40%] | D = 0% [SD = 0%] |
| • Symptomatic aggressive NHL (no chemo options) Life expectancy < 3 months: 8 Gy/1fx8Gy | ||||||
| • Symptomatic multiple myeloma (No cord compression): 8 Gy/1fx8Gy | ||||||
| • Symptomatic multiple myeloma (Cord compression): 20 Gy/5fx4Gy | ||||||
| • Symptomatic indolent lymphoma (No cord compression): 4 Gy/1fx4Gy | ||||||
| • Symptomatic indolent lymphoma (Cord compression): 20 Gy/5fx4Gy | ||||||
| • Myeloid sarcoma/leukemia -Cranial leptomeningeal disease: 8 Gy/2fx4Gy | ||||||
| • Myeloid sarcoma/leukemia—Focal leptomeningeal spine disease, and symptomatic chloroma outside the CNS: 12 Gy/3fx4Gy | ||||||
| QP8b | Simcock [14] | Palliative Lymphoma, low grade: 4 Gy/1 fx4Gy | – | – | A = 90% [SA = 40%] | D = 10% [SD = 0%] |
| P9 Other Oligometastases Suitable for SBRT (Lung) | ||||||
| QP9a | Combs [15] | – | – | SBRT 1–5 fx (not further specified) | A = 50% [SA = 20%] | D = 50% [SD = 0%] |
| P10 Other Oligometastases Suitable for SBRT (Liver) | ||||||
| QP10a | Combs [15] | – | – | SBRT 1–5 fx (not further specified) | A = 50% [SA = 20%] | D = 50% [SD = 0%] |
| QP10b | Tchelebi [7] | Colorectal Primary | – | – | A = 60% [SA = 30%] | D = 40% [SD = 0%] |
| • For small, non-central lesions: 16–30 Gy in 1fx | ||||||
| • For lesions near the biliary tree: 48–60 in 3–5 fx | ||||||
| P11 Other Oligometastases Suitable for SBRT (Adrenal) | ||||||
| QP11a | Combs [15] | – | – | SBRT 1–5 fx (not further specified) | A = 40% [SA = 30%] | D = 60% [SD = 0%] |
| P12 Other Oligometastases Suitable for SBRT (Lymph-node asymptomatic) | ||||||
| QP12a | Combs [15] | – | – | SBRT 1–5 fx (not further specified) | A = 50% [SA = 30%] | D = 50% [SD = 0%] |
| P13 Brain metastases (N° 1–4) | ||||||
| QP13a | Yerramilli [9] | SRS (not further specified) | • In pt with good performance SRS for all or dominant lesion cause of morbidity | A = 80% [SA = 30%] | D = 20% [SD = 0%] | |
| • To delay or avoid whole brain | ||||||
| QP13b | Combs [15] | 1–10 Brain Mets with good performance status: “single fraction” | A = 60% [SA = 10%] | D = 40% [SD = 10%] | ||
| • 18 Gy/1fx18Gy | ||||||
| • 20 Gy/1f × 20 Gy | ||||||
| QP13c | Simcock [14] | 1–3 Brain Mets, good KPS, no extracranial disease 15–20 Gy/1fx15-20 Gy | • SRS | A = 100% [SA = 60%] | D = 0% [SD = 0%] | |
| P14 Brain metastases (N° 5–10) | ||||||
| QP14a | Simcock [14] | Palliation WBRT: 20 Gy/5fx4Gy [RTOG QUARTZ [43]] | – | • 3D WBRT | A = 100% [SA = 30%] | D = 0% [SD = 0%] |
| • A routine option in UK, Europe, Asia, Canada, and Australia. Established in RTOG dose escalation studies | ||||||
| QP14b | Combs [15] | 1–10 Brain Mets with good performance status: “single fraction” | • SRS | A = 50% [SA = 20%] | D = 50% [SD = 10%] | |
| • 18 Gy/1fx18Gy | ||||||
| • 20 Gy/1f × 20 Gy | ||||||
| P15 Brain metastases (N° > 4), poor KPS, meningeal involvement | ||||||
| QP15a | Yerramilli [9] | Multiple brain metastases or leptomeningeal disease WB: | – | • For patients with urgent indications, progressive neurologic symptom | A = 90% [SA = 40%] | D = 10% [SD = 0%] |
| • 20 Gy/5fx4Gy | • For patients in whom longer term survival is expected, in order limit neurocognitive complications | |||||
| • 30 Gy/10fx3Gy | • In patients with limited prognosis, the QUARTZ study demonstrated similar rates of overall survival and QoL with steroids and best supportive care alone | |||||
| QP15b | Combs [15] | Life expectancy > 3 mth: WBRT 20 Gy/5fx4 Gy | – | Poor performance status Evaluate BSC with critical view of steroids [RTOG QUARTZ10] | A = 100% [SA = 50%] | D = 0% [SD = 0%] |
| QP15c | Curigliano [16] | – | – | RT is urgent for the following situations: Treatment of brain and leptomeningeal metastases | A = 80% [SA = 20%] | D = 20% [SD = 10%] |
| QP15d | Simcock [14] | Brain metastasis Palliation, poor Prognosis: 12 Gy/2fx6Gy | – | CNS mets from NSCLC needing WBRT: | A = 70% [SA = 10%] | D = 30% [SD = 0%] |
| • Best supportive care including steroids | ||||||
| • Omit RT | ||||||
| [RTOG QUARTZ [43]] | ||||||
| Brain Mets Palliation, poor Prognosis: | ||||||
| • Prefer 3D | ||||||
| P16 Primary symptomatic Brain tumor, poor KPS | ||||||
| QP16a | Combs [15] | Glioblastoma KPS < 60: 25 Gy/5fx5Gy; (No TMZ) [Roa 2004 [44]] | – | • Glioblastoma KPS < 50; age > 70yy TMZ mono (MGMT methylated) or BSC [Malmstrom [45]] | A = 90% [SA = 30%] | D = 10% [SD = 0%] |
| QP16b | Simcock [14] | GBM, poor KPS: Age ≥ 50, KPS 50–70, or Age ≥ 65 KPS 50–100: 25 Gy/5fx5Gy No TMZ | – | • GBM, poor KPS Age ≥ 50, KPS 50–70, or Age ≥ 65 KPS 50–100: Prefer 3D; CTV 2 cm margin as per EORTC | A = 90% [SA = 30%] | D = 10% [SD = 0%] |
| • Glioblastoma Age > 60, methylated TMZ only Standard RT associated with poor outcomes | ||||||
| QP16c | Noticewala [19] | GMB, very poor PS KPS < 50: | – | • GMB, very poor PS KPS < 50: Alternatively, consider: Best supportive care or TMZ with omission of RT | A = 100% [SA = 10%] | D = 0% [SD = 0%] |
| • 34 Gy/10fx3.4 Gy [Malmstrom [45]] | • Recurrent GBM: not generally recommend re-irradiation Systemic therapies if considered reasonable. Therapies may include, but are not limited to temozolomide, bevacizumab, lomustine, and others | |||||
| • 25 Gy/5fx5Gy [Roa 2015 [46]] | ||||||
| P17 Postoperative Brain Mets | ||||||
| QP17a | Combs [15] | Postoperative SRS of resection cavity: | – | *The dose depends on target diameter: | A = 90% [SA = 40%] | D = 10% [SD = 0%] |
| • 35 Gy/7fx5Gy or | • < 2.0 cm | |||||
| • 20-24 Gy/1fx20-24 Gy* [Brown [47]] or | • 2 ≤ 2.9 cm #The dose depends on target size (in cc): | |||||
| • 16 Gy/1fx16Gy | • ≤ 10 cc | |||||
| • 14 Gy/1fx14Gy | • 10.1–15 cc | |||||
| • 12 Gy/1fx12Gy [Mahajan [48]]# | • > 15 cc | |||||
§(Authors do not specify in text/table but the reference report the schedule as “weekly”) [MRC [32]]
*Consensus Vote: 1 = Strongly Agree; 2 = Agree; 3 = Disagree; 4 = Strongly Disagree
MESCC Metastatic Epidural Spinal Cord Compression; fx fraction; OS: overall Survival; RT Radiotherapy; pt patient; BID bis in die; Q schedule repetition interval; QoL quality of life; SBRT stereotactic body RT; mets metastases; wks weeks; PEG percutaneous endoscopic gastrostomy; WBRT whole brain RT; TMZ Temozolamide; mth months; IMRT-SIB Intensity modulated RT—Simultaneous integrated boost
NORMALITY (“No cOmpRoMise on quality of life by pALliative radIoTherapY") clinical care model
The NORMALITY clinical care model aims to make the stays in RT departments of patients dealing with complex logistic settings (e.g., in home care or hospice, living a long distance from the closest RT department) as short as possible. Ideally, patients should receive clinical visits, simulations, and single (or first) PRT delivery on the same day. Single-fraction PRT should be preferred whenever possible, unless the risk of unacceptable toxicity cannot be avoided. This was realized in some fast-track or rapid-response RT programs [12, 51–53]. The integration proposed to such acknowledged care models is to prepare ahead of patient arrival via least two levels of teleconsultation (triage and remote visits). The first level (triage) aims to enable the triage of patients possibly requiring PRT through a simplified information collection method that can be performed by a clinician or a qualified nurse. The triage can subsequently require a remote visit. This second-level contact with the patient (remote visit) involves a single or repeated remote visit, with potentially more in-depth information collected by the RO in order to administer the PRT prescription. If imaging evaluation is needed, the caregiver can be asked to acquire imaging, or alternatively (depending privacy rules), sharing through a computer network could be considered. Teleconsultation for triage and remote visits can be done by interactive and video calls, but also through phone calls that can effectively respond to such needs [12]. Figure 2 represents a form in the English version that facilitates the first triage (the Italian version is shown in Fig. 3). Figure 4 represents a form (in the English version) to facilitate remote visits (the Italian version is shown in Fig. 5). The summary of the PRT indications (i.e.: PRT Normality model summary) for this peculiar setting (not belonging to regular PRT) with the relative consensus is reported in Table 3 for palliative emergencies and Table 4 for palliative non-emergencies. With respect to the PRT COVID-19 summary, in PRT Normality model summary 20 CPIs were identified and one additional subtype clinical presentation was included: non-painful bone metastases. Thirty subtype indications for the 20 CPIs were summarized. The average agreement (“agree” + “strongly agree”) was over the strong agreement threshold (i.e.: 80%), ranging from 82 to 100% among the 30 topic items, of which the inner rate of agreement of the first vote ranged from 33 to 92%.
Fig. 2.
Triage application form for Palliative Radiation Therapy (English Version)
Fig. 3.
Triage application form for Palliative Radiation Therapy (Italian Version)
Fig. 5.
Form for remote-visit for Palliative Radiation Therapy (Italian Version)
Table 3.
PRT Normality Model Summary—Normality model PRT indications: palliative emergencies
| Emergencies | ||||||
|---|---|---|---|---|---|---|
| Reference | Main Prescriptive Indication | Alternative | Additional Statement (if any) | % Consensus Vote* | ||
| A = Agreement (1 + 2) | ||||||
| D = Disagreement (3 + 4) | ||||||
| SA = Strong Agreement (1) | ||||||
| SD = Strong Disagreement (4) | ||||||
| E1 Metastatic Epidural Spinal Cord Compression (MESCC) | ||||||
| QE1e | AIRO Pall | 8 Gy/1fx8Gy (Maranzano [19]) | Preferable for alternative: | BID option can be considered balancing pt and department’s logistic, being suitable for hospitalized pt but not limited to those only | A = 100% [SA = 92%] | D = 0% [SD = 0%] |
| • 20 Gy/5fx4Gy | ||||||
| Secondary alternative option: | ||||||
| • 20 Gy/4fx5GyBID [SHARON project [22, 23]] | ||||||
| • 6 Gy/1fx6Gy | ||||||
| E2 Hemostasis (including Hemoptysis) | ||||||
| QE2h | AIRO Pall | H&N cancer bleeding: | Preferable for alternative: | – | A = 100% [SA = 42%] | D = 0% [SD = 0%] |
| • 20 Gy/5fx4Gy | • 20 Gy/4fx5Gy BID | |||||
| [SHARON project [28]] | ||||||
| Secondary alternative option: | ||||||
| • 44.4 Gy/12fx3,7 Gy | ||||||
| • 8 Gy/1fx8Gy | ||||||
| QE2i | AIRO Pall | Esophageal cancer bleeding: | 20 Gy/5fx4Gy | – | A = 100% [SA = 42%] | D = 0% [SD = 0%] |
| • 6 Gy/1fx6Gy | ||||||
| • 8 Gy/1fx8Gy | ||||||
| • 12 Gy/4fx3Gy BID [SHARON project [23]] | ||||||
| QE2j | AIRO Pall | Gastric cancer bleeding: | 20 Gy/5fx4Gy | – | A = 84% [SA = 42%] | D = 16% [SD = 0%] |
| • 6 Gy/1fx6Gy | ||||||
| • 8 Gy/1fx8Gy (with anti-emetic) | ||||||
| QE2l | AIRO Pall | Pelvic malignancies bleeding: 8 Gy/1fx8Gy | Preferable for alternative: | BID option can be considered balancing pt and department’s logistic, being suitable for hospitalized pt but not limited to those only | A = 100% [SA = 50%] | D = 0% [SD = 0%] |
| • 24 Gy/3fx8Gy Day 0, 7, 21 [29] | ||||||
| • 18 Gy/4fx4.5 Gy BID [SHARON project [30]] | ||||||
| Secondary alternative option: | ||||||
| • 18 Gy/3fx6Gy (Day 0, 7, 21) | ||||||
| • 20 Gy/5fx4Gy | ||||||
| • 24 Gy/6fx4Gy | ||||||
| QE2m | AIRO Pall | Hemoptysis: | • 20 Gy/5fx4Gy | – | A = 92% [SA = 75%] | D = 8% [SD = 0%] |
| • 17 Gy/2fx8.5 Gy (weekly) | ||||||
| E3 Mediastinal Syndrome | ||||||
| QE3e | AIRO Pall | Superior vena cava syndrome: | Preferable for alternative: | BID option can be considered balancing pt and department’s logistic, being suitable for hospitalized pt but not limited to those only | A = 100% [SA = 75%] | D = 0% [SD = 0%] |
| • 17 Gy/2fx8.5 Gy weekly [MRC] [32, 33] | • 8 Gy/1fx8Gy | |||||
| • 20 Gy/5fx4Gy | Secondary alternative option: | |||||
| • 20 Gy/4fx5Gy BID [SHARON project [34]] | ||||||
*Consensus Vote: 1 = Strongly Agree; 2 = Agree; 3 = Disagree; 4 = Strongly Disagree
MESCC Metastatic Epidural Spinal Cord Compression; fx fraction; OS: overall Survival; RT Radiotherapy; pt patient; BID bis in die; Q schedule repetition interval; QoL quality of life; SBRT stereotactic body RT; mets metastases; wks weeks; PEG percutaneous endoscopic gastrostomy; WBRT whole brain RT; TMZ Temozolamide; mth months; IMRT-SIB Intensity modulated RT—Simultaneous integrated boost
Table 4.
PRT Normality Model Summary—Normality model PRT indications: palliative non-emergencies
| Palliative (Non-emergencies) | ||||||
|---|---|---|---|---|---|---|
| Reference | Main Prescriptive Indication | Alternative | Additional Statement (if any) | % Consensus Vote* | ||
| A = Agreement (1 + 2) | ||||||
| D = Disagreement (3 + 4) | ||||||
| SA = Strong Agreement (1) | ||||||
| SD = Strong Disagreement (4) | ||||||
| P1 Painful bone metastasis | ||||||
| QP1f | AIRO Pall | 8 Gy/1fx8Gy | Preferable for alternative: | • SHARON project as useful option for painful complicated lesions (i.e.: extraosseous disease, impending fracture, pathological fracture); see also “Section E1” | A = 100% [SA = 75%] | D = 0% [SD = 0%] |
| • 20 Gy/4fx5GyBID [SHARON project [22]] | • BID option can be considered balancing pt and department’s logistic, being suitable for hospitalized pt but not limited to those only | |||||
| Secondary alternative Option: | • For extreme clinical settings of extensive bone involvement, or retreatment/ pain refractory to pain killers: caution consider “Half-body RT” (i.e.: lumbar + bony pelvis + femurs—15 Gy/4fx3.75 Gy BID [SHARON project [35]] | |||||
| • 20 Gy/5fx4Gy | ||||||
| P2 Non-painful bone metastasis | ||||||
| QP2b | AIRO Pall | – | – | • Consider to delay RT or evaluate SBRT (depending if oligometastatic and on the basis of prognostic score and impending fracture risk) | A = 100% [SA = 75%] | D = 0% [SD = 0%] |
| • Consider RT if impending fracture: if “Yes”, see E1 + P1 + P3 | ||||||
| P3 Bone Oligometastases Suitable for SBRT | ||||||
| QP3d | AIRO Pall | SBRT 1–5 fx (BED 50-60 Gy if not compromising spinal cord constraints) | Single fraction (16 to 24 Gy) SBRT for Retreatment of Symptomatic MESCC | • Apply validated prognostic score before clinical indication | A = 100% [SA = 58%] | D = 0% [SD = 0%] |
| • Consider SBRT in case of future risk of MESCC or fracture | ||||||
| • Alternatively, consider delay or avoid SBRT, and/or non-SBRT RT indications | ||||||
| P4 Retreatment of painful bone metastasis | ||||||
| QP4b | AIRO Pall | 8 Gy/1fx8Gy | SBRT Single fraction (16 to 24 Gy) SBRT for Retreatment of Symptomatic MESCC | • Waiting a minimum of 6 weeks after completion of the initial RT | A = 92% [SA = 42%] | D = 8% [SD = 0%] |
| • For highly selected clinical settings of extensive bone involvement, or retreatment/ pain refractory to pain killers: cautiously consider “Half-body RT” (i.e.: lumbar + bony pelvis + femurs—15 Gy/4fx3.75 Gy BID) [SHARON project [35]] | ||||||
| P5 Adjuvant (post-surgery) bone metastasis radiotherapy | ||||||
| QP5c | AIRO Pall | 30 Gy/10 fx3Gy | • 20 Gy/4fx 5 Gy | • Apply a validated prognostic score for RT to evaluate if expected survival < 3/3–6/ > 6 mth | A = 100% [SA = 75%] | D = 0% [SD = 0%] |
| • 20 Gy/5fx 4 Gy | • RT may be postponed in case of asymptomatic pt | |||||
| • RT may be performed secondarily in case of progressive post-operative signs | ||||||
| • If Adjuvant RT have been indicated after surgery for MESCC: it should not be postponed over 3–4 weeks | ||||||
| P6 Pain NOT associated to Bone Mets (e.g.: direct infiltration, primary pancreatic; H&N; Lymph-node infiltrating surrounding structures, etc.) | ||||||
| QP6f | AIRO Pall | H&N: | H&N Preferable for alternative: | BID options (Sharon, QUAD Shot) can be considered balancing pt and department’s logistic, being suitable for hospitalized pt but not limited to those only | A = 100% [SA = 83%] | D = 0% [SD = 0%] |
| • 20 Gy/5fx4Gy | • 20 Gy/4fx5GyBID [SHARON project [28]] | |||||
| • 14 Gy/4fx 3.5 Gy BID (repeated Q4 weeks interval × 2 times) [QUAD SHOT- RTOG 8502 [26, 27] | ||||||
| Secondary alternative option: | ||||||
| • 8 Gy/1fx8Gy | ||||||
| • 24/3fx8Gy Q 0–7-21 (weekly) [25, 29, 36] | ||||||
| • 18/3fx6Gy Q 0–7-21 (weekly) | ||||||
| QP6g | AIRO Pall | Pain by primary Gyn + Melanoma + Esophageal + HCC + Pancreas + SCLC/NSCLC: | o Gyn + Melanoma: | – | A = 92% [SA = 33%] | D = 8% [SD = 0%] |
| • 8 Gy/1fx8Gy | • 24/3fx8Gy Q 0–7-21 | |||||
| • 18/3fx6Gy | ||||||
| Q 0–7-21 | ||||||
| o Gyn 18 Gy/4fx4.5GyBID [SHARON project [30]] | ||||||
| o SCLC/NSCLC [IAEA [37]]: | ||||||
| • 16 Gy/2fx 8 Gy (1 week apart) | ||||||
| o Pancreas | ||||||
| 10 Gy/1fx10Gy | ||||||
| P7 Other than Pain symptoms NOT associated to Bone Mets (e.g.: obstruction, etc.) | ||||||
| QP7k | AIRO Pall | H&N: | H&N Preferable for alternative: | • BID options (Sharon, QUAD Shot) can be considered balancing pt and department’s logistic, being suitable for hospitalized pt but not limited to those only | A = 100% [SA = 67%] | D = 0% [SD = 0%] |
| • 20 Gy/5fx4Gy | • 20 Gy/4fx5GyBID [SHARON project [28]] | |||||
| • 14 Gy/4fx 3.5 Gy BID (repeated Q4 weeks interval × 2 times) [QUAD SHOT- RTOG 8502 [26, 27]] | ||||||
| Secondary alternative option: | ||||||
| • 8 Gy/1fx8Gy | ||||||
| • 24/3fx8Gy Q 0–7-21 (weekly) [25, 29, 36] | ||||||
| • 18/3fx6Gy Q 0–7-21 (weekly) | ||||||
| • 30 Gy/6fx6Gy (2 fx/week) [HYPO trial [38]] | ||||||
| QP7l | AIRO Pall | Gyn + Melanoma: | • Gyn + Melanoma: 24 Gy/3fx8Gy Q 0–7-21 | – | A = 92% [SA = 42%] | D = 8% [SD = 0%] |
| • 8 Gy/1fx8Gy | • Gyn 18 Gy/4fx4.5GyBID [SHARON project [30]] | |||||
| QP7m | AIRO Pall | Esophageal dysphagia: | • 12 Gy/4fx3GyBID [SHARON project [23]] | • BID option can be considered balancing pt and department’s logistic, being suitable for hospitalized pt but not limited to those only | A = 100% [SA = 67%] | D = 0% [SD = 0%] |
| • 20 Gy/5fx4Gy | • Consider either esophageal stent or percutaneous endoscopic gastrostomy (PEG) tube placement | |||||
| QP7n | AIRO Pall | Pancreas Symptomatic (non-pain): 10 Gy/1fx10Gy | Pancreas Symptomatic (non-pain): 8 Gy/1fx8Gy | – | A = 83% [SA = 33%] | D = 17% [SD = 0%] |
| QP7o | AIRO Pall | SCLC: 16 Gy/2 fx 8 Gy (1 week apart) [IAEA [37]] | SCLC Preferable for alternative: | – | A = 100% [SA = 58%] | D = 0% [SD = 0%] |
| • 8 Gy/1fx 8 Gy | ||||||
| • 20 Gy/4fx5Gy BID [SHARON project [34]] | ||||||
| Secondary alternative option: | ||||||
| • 10 Gy/1fx 10 Gy [IAEA [37]] | ||||||
| QP7p | AIRO Pall | NSCLC: | NSCLC Preferable for alternative: | • Order reported for main indication (17 Gy) follows the highest consensus reported in ESTRO-ASTRO Consensus (Guckemberger et al. [5]) | A = 100% [SA = 58%] | D = 0% [SD = 0%] |
| 1.17 Gy/2fx 8.5 Gy (1 week apart) [32, 33] | • 20 Gy/5fx 4 Gy | |||||
| 2.8 Gy/1fx 8 Gy | • 20 Gy/4fx5Gy BID [SHARON project [34]] | |||||
| Secondary alternative option: | ||||||
| • 10 Gy/1fx 10 Gy [IAEA [37]] | ||||||
| P8 Symptomatic Haematological Malignancies (non-emergencies) | ||||||
| QP8c | AIRO Pall | Accordingly to Yahalom [4]: | Accordingly to Yahalom [4] | Accordingly to Yahalom [4] | A = 100% [SA = 92%] | D = 0% [SD = 0%] |
| • Symptomatic aggressive NHL (no chemo options) Life expectancy > 3 months 25 Gy/5fx5Gy | ||||||
| • Symptomatic aggressive NHL (no chemo options) Life expectancy < 3 months: 8 Gy/1fx8Gy | ||||||
| • Symptomatic multiple myeloma (No cord compression): 8 Gy/1fx8Gy | ||||||
| • Symptomatic multiple myeloma (Cord compression): 20 Gy/5fx4Gy | ||||||
| • Symptomatic indolent lymphoma (No cord compression): 4 Gy/1fx4Gy | ||||||
| • Symptomatic indolent lymphoma (Cord compression): 20 Gy/5fx4Gy | ||||||
| • Myeloid sarcoma/leukemia -Cranial leptomeningeal disease: 8 Gy/2fx4Gy | ||||||
| • Myeloid sarcoma/leukemia—Focal leptomeningeal spine disease, and symptomatic chloroma outside the CNS: 12 Gy/3fx4Gy | ||||||
| P9 Other Oligometastases Suitable for SBRT (Lung) | ||||||
| QP9b | AIRO Pall | Oligometastases (1–3): | – | • Consider for pt at prognosis > 6mth (by validated prognostic score) | A = 100% [SA = 58%] | D = 0% [SD = 0%] |
| • Lesion ≤ 3 cm surrounded by lung parenchyma: 54 Gy/3fx18Gy | • Consider for pt with disease-free interval ≥ 6 mth | |||||
| • Lesion near chest wall or size > 3 cm: 55 Gy/5fx11Gy | • Biologic effective dose > 100 Gy (if not compromising OAR constraints—AAPM) | |||||
| • Lesion within 2 cm of mediastinum or brachial plexus: 60 Gy/8fx7.5 Gy [SabrComet [39]; SabrComet3 [40]] | ||||||
| P10 Other Oligometastases Suitable for SBRT (Liver) | ||||||
| QP10c | AIRO Pall | BED ≥ 100 (if not compromising liver parenchyma and other constraints according to AAPM) | o For small, non-central lesions: | • Consider for pt at prognosis > 6mth (by validated prognostic score) | A = 100% [SA = 75%] | D = 0% [SD = 0%] |
| • 50 Gy/5fx10Gy | • Consider for pt with disease-free interval ≥ 6 mth | |||||
| • 54 Gy/3fx18Gy (every second day) [SabrComet3 [40]] | ||||||
| o For lesions near the biliary tree: 54 Gy/6fx9Gy | ||||||
| P11 Other Oligometastases Suitable for SBRT (Adrenal) | ||||||
| QP11b | AIRO Pall | • 40 Gy/5fx8Gy [SabrComet3 [40]] | 36 Gy/3fx12Gy | • SBRT 1–5 fx | A = 100% [SA = 50%] | D = 0% [SD = 0%] |
| • 35 Gy/5fx7Gy | • Consider for pt at prognosis > 6mth (by validated prognostic score) | |||||
| • Consider for pt with disease-free interval ≥ 6 mth | ||||||
| • Evaluate constraints as per AAPM | ||||||
| P12 Other Oligometastases Suitable for SBRT (Lymph-node asymptomatic) | ||||||
| QP12b | AIRO Pall | • 40 Gy/5fx8Gy [SabrComet3 [40]] | 36 Gy/3fx12Gy | • SBRT 1–5 fx | A = 100% [SA = 50%] | D = 0% [SD = 0%] |
| • 35 Gy/5fx7Gy | • Consider for pt at prognosis > 6mth (by validated prognostic score) | |||||
| • Consider for pt with disease-free interval ≥ 6 mth | ||||||
| • Evaluate constraints as per AAPM [41] | ||||||
| P13 Brain metastases (N° 1–4) | ||||||
| QP13d | AIRO Pall | 1–4 Brain Mets, good KPS, no extracranial disease | – | • SRS | A = 100% [SA = 58%] | D = 0% [SD = 0%] |
| • 15 Gy/1fx15Gy | ||||||
| lesion > 3 cm ≤ 4 cm [42] | ||||||
| • 18 Gy/1fx18Gy | ||||||
| lesion > 2 cm ≤ 3 cm [42] | ||||||
| • 21 Gy/1fx21Gy lesion ≤ 2 cm | ||||||
| • 24 Gy/1fx24Gy lesion ≤ 2 cm [42] [RTOG 9508] | ||||||
| P14 Brain metastases (N° 5–10) | ||||||
| QP14c | AIRO Pall | 5–10 Brain Mets good KPS, no extracranial disease: | 5–10 Brain Mets, good KPS, no extracranial disease: Preferable for Alternative: Palliation WBRT IMRT-SIB (SIB40 + 30)Gy/10fx(SIB4 + 3)Gy Secondary Option: Palliation WBRT | • SRS (if single fraction adopted) | A = 82% [SA = 58%] | D = 8% [SD = 0%] |
| • 18 Gy/1fx18Gy | • 30 Gy/10fx3Gy | |||||
| • 15–20 Gy/1fx15-20 Gy | ||||||
| P15 Brain metastases (N° > 4), poor KPS, meningeal involvement | ||||||
| QP15e | AIRO Pall | Brain metastasis Palliation, poor Prognosis leptomeningeal disease: 20 Gy/5fx4Gy | Life expectancy > 3 mth: 30 Gy/10fx3Gy | – | A = 92% [SA = 75%] | D = 8% [SD = 0%] |
| P16 Primary symptomatic Brain tumor, poor KPS | ||||||
| QP16d | AIRO Pall | Glioblastoma | • 34 Gy/10fx3.4 Gy [Malmstrom [45]] | A = 100% [SA = 58%] | D = 0% [SD = 0%] | |
| • 25 Gy/5fx5Gy [Roa 2015 [46]] | ||||||
| P17 Postoperative Brain Mets | ||||||
| QP17b | AIRO Pall | Postoperative SRS of resection cavity: | • 15-18 Gy/1fx15-18 Gy [Kepka [49]] | **The dose depends on target diameter (in cm): | A = 92% [SA = 50%] | D = 8% [SD = 0%] |
| • 20-24 Gy/1fx20-24 Gy [Brown [47]]** | • 25 Gy/5fx5Gy cavities larger than 5 cm [Kepka [49]] | • < 2.0 cm | ||||
| • 16 Gy/1fx16Gy | • ≥ 2 ≤ 2.9 cm ##The dose depends on target size (in cc): | |||||
| • 14 Gy/1fx14Gy | • ≤ 10 cc | |||||
| • 12 Gy/1fx12Gy [Mahajan [48]]## | • 10.1–15 cc | |||||
| Postoperative fractionated SRT of resection cavity: | • > 15 cc | |||||
| • 35-25 Gy/5fx7-5 Gy | ||||||
*Consensus Vote: 1 = Strongly Agree; 2 = Agree; 3 = Disagree; 4 = Strongly Disagree
MESCC Metastatic Epidural Spinal Cord Compression; fx fraction; OS: overall Survival; RT Radiotherapy; pt patient; BID bis in die; Q schedule repetition interval; QoL quality of life; SBRT stereotactic body RT; mets metastases; wks weeks; PEG percutaneous endoscopic gastrostomy; WBRT whole brain RT; TMZ Temozolamide; mth months; IMRT-SIB Intensity modulated RT—Simultaneous integrated boost
The latest versions of such materials can also be retrieved in the following section of the “La Rete del Sollievo” (NOS) website (http://www.gemelliart.it/laretedelsollievo/retedelsollievo-modelliassistenziali/). An interactive list of Italian RT departments providing different palliative services endorsed by the AIRO can also be downloaded from this website.
Discussion
Our paper aims to deal with three main issues regarding PRT: how to choose a PRT prescription during the COVID-19 pandemic; highlight the priority of administering PRT for patients both during the COVID-19 pandemic and in the future; and how to manage the risk of underuse of PRT in the future in patients dealing with complex logistic scenarios (particularly after the emergency pandemic experience, which suggests that different approaches to PRT are preferable if the RT department is inaccessible).
How to choose a PRT prescription during the COVID-19 pandemic? The COVID-19 pandemic challenges healthcare systems worldwide [1, 54]. Some authors described that two phases are possibly expected from an RO’s perspective: an early phase in which the department’s human resources are not limited and potentially all treatments could be provided for patients; and a second phase (“late phase or scenario”) in which the infection spread could limit the global amount of actually deliverable RT treatments [5]. To address this scenario, hypofractionated RT schedules have been proposed to reduce the number of contacts while effectively treating patients [4–7]. A recent survey reported by Jereczek-Fossa et al. confirmed that in a highly impacted country like Italy, 73.6% of RT departments shifted to adapted hypofractionated RT schedules [55]. To the best of our knowledge, most guidelines have not focused on PRT, apart from one addressing bone metastases [8]; thus, an RO needing to prescribe PRT during the COVID-19 pandemic would find indications distributed across different papers. The proposed PRT COVID-19 summary (Tables 1 and 2) could aid ROs during this period. The limitation that there are papers missing in our literature search because they were published after our search was conducted exists; however, the average support the summary provides for readers is not compromised and the expert consensus vote poses additional utility, offering an overview perspective for interpreting other similar indications.
What is the priority of administering PRT for our patients, both during the COVID-19 pandemic and in the future? Prioritization of PRT has been an object of discussion during the COVID-19 pandemic period [3, 9]. Yerramilli et al. suggested prioritizing PRT only for emergencies, providing a triage model to check for the need for PRT [9], although some authors have raised concerns over this [10]. Conversely, Tagliaferri et al. suggested a more inclusive triage-based patient selection strategy, possibly providing PRT even to COVID-19 positive patients, despite the consideration of dealing with highly aggressive diseases such as melanoma [56]. Neither considered the different phases of infection spread within the RT department. Van de Haar et al. [3] suggested multiple phases detailing the steps of expectable crisis from the clinical perspective of RT, surgical, and medical oncology departments, but still did not indicate if or how passing through one of the mentioned level of crisis to another would change the priority list that puts PRT at level four of five in their paper. A few authors [5, 6] indicated that different RT and PRT schedules are deliverable in the early or later (more complex) phases (both are included in Tables 1 and 2).
We substantially agreed with the average concern of treating patients during the COVID-19 period. Moreover, most of the indications provided in the early pandemic were unaware of the possible consequent scenarios, thus preparing ahead for the worst possible scenario. A wide range of consequences have been described for RT departments, ranging from compromised [57] to more manageable [58]. In our opinion (when indicated), PRT should remain one of the highest priority treatments from the perspective of ROs. For the two major oncological aims (cure and palliation), the pursued outcomes, as measured by the most appropriate endpoint (i.e., overall survival (OS) for cure and quality of life (QoL) for palliation, respectively), are equivalent from the patient’s perspective. Until we are not forced to restrict the delivery of RT to our patients due to the risk of infective spread, palliative and curative settings should be equally prioritized [11]. To put this into context, consider an example on bone-related pain control: if RT is not administered when indicated, the possibly needed dose escalation of medical analgesic therapy can determine side effects affecting QoL, despite controlling pain levels (besides the cost-effective impact on health services by increased drug administration). Separate administration of either palliative RT or medical analgesic therapy should not be considered equivalent by ROs; the concomitant integration of both with modulation over time should be the gold standard.
How to manage the risk of underuse of PRT in the future in patients dealing with complex logistic scenarios? In the future, when the COVID-19 pandemic is over, the issue of patients suitable for PRT who are dealing with complex logistic settings and who are at risk of losing their chance of receiving relief by PRT will surely persist. Looking at the current experience of emergency departments, we are afraid that PRT could be replaced by medical or different alternatives if it is not easily and logistically manageable. Some of the indications provided for PRT during COVID-19 pandemic can be safely and effectively maintained in these peculiar settings. The NORMALITY clinical care model aims to enhance the chance that these patients receive acceptable compromises, aiming for an efficient PRT schedule. The combination of clinical visits, simulation, and RT delivery on the same day is a well-known practice that has diffused over several RT centers for at least 30 years. The Rapid Response Radiotherapy Program (RRRP) was proposed in the literature in the 1990s by the Canadian group Chow et al. [51–53]. Similarly, the Vancouver Rapid Access (VARA) for incurable lung cancer was presented by Lefresne et al. [59], as well as the rapid multidisciplinary management of bone metastases described by Donato et al. [60]. The positive impact of the described “advanced practice radiation therapists” on workflows was also explored, for instance, by Job et al. [61, 62]. Our model integrates such experiences while focusing on patients with complex logistics, proposing the set of normality model PRT indications for such peculiar settings, as summarized in Tables 3 and 4. If appropriately selected for patients, treatment alternatives such as single fraction treatments applied in emergencies as suggested by Maranzano et al. [20], the use of single repeated schedules as per the “0–7–21” PRT schedule proposed by Nguyen et al. [26], or the bis-in-die (BID) schedules advised by both the “Quad Shot RTOG 8502–QUAD SHOT” report created by Spanos et al. [27, 28] and the “Sharon project” for multiple palliative settings [31], can be highly useful. Moreover, our NORMALITY model suggests and offers forms to facilitate the enhancement of preliminary teleconsultations before the first clinical visits of the patients (Figs. 2 and 4). This is in line with the literature acknowledging the efficacy of phone calls [12] and the renewed indication for teleconsultation during the COVID-19 period [9, 14]. Some issues remain unaddressed, including the management of patients strictly requiring hospital admittance and the role of technology in balancing urgent palliative patients’ needs. Clearly, improving such settings will require multidisciplinary collaboration among operators with different specializations and backgrounds dealing with palliation and oriented to facilitate each other’s respective roles and peculiarities.
Fig. 4.
Form for remote-visit for Palliative Radiation Therapy (English Version)
Conclusion
We provide a comprehensive summary of the literature guideline indications for PRT during the COVID-19 pandemic along with the respective reference and consensus evaluation voted by the AIRO panel. We also propose a clinical care model (based on the clinical guideline indications provided during the COVID-19 pandemic) including clinical indications and written forms facilitating two levels of teleconsultation (triage and remote visits) in order to evaluate patients for indications for PRT ahead of planning live clinical visits. The normality model could facilitate the provision of PRT to patients dealing with future complex logistic scenarios.
Acknowledgements
The Authors thank: The Scientific Committee and Board of the AIRO for the critical revision of the paper; the colleagues (and friends) of the AIRO that have strongly supported La Rete del Sollievo since its birth. One name for all: we want to renew the never-forgotten affection to the memory of Mauro Trovò; the friends of the Gigi Ghirotti Foundation (http://www.fondazioneghirotti.it) for their dedication to preventing patients from “feeling abandoned and lonely” in face of their illness; Professor Numa Cellini, for tenaciously spreading the relevance of paying sincere attention to the person beyond the patient.
Appendix
Medline search strategy
(("radiotherapy"[Subheading] OR "radiotherapy"[All Fields] OR "radiotherapy"[MeSH Terms]) OR ("radiotherapy"[Subheading] OR "radiotherapy"[All Fields] OR ("radiation"[All Fields] AND "therapy"[All Fields]) OR "radiation therapy"[All Fields] OR "radiotherapy"[MeSH Terms] OR ("radiation"[All Fields] AND "therapy"[All Fields]) OR "radiation therapy"[All Fields]) OR ("radiation oncology"[MeSH Terms] OR ("radiation"[All Fields] AND "oncology"[All Fields]) OR "radiation oncology"[All Fields]) OR Palliative[All Fields] OR (Palliative[All Fields] AND ("radiotherapy"[Subheading] OR "radiotherapy"[All Fields] OR "radiotherapy"[MeSH Terms]))) AND (("COVID-19"[All Fields] OR "COVID-2019"[All Fields] OR "severe acute respiratory syndrome coronavirus 2"[Supplementary Concept] OR "severe acute respiratory syndrome coronavirus 2"[All Fields] OR "2019-nCoV"[All Fields] OR "SARS-CoV-2"[All Fields] OR "2019nCoV"[All Fields] OR (("Wuhan"[All Fields] AND ("coronavirus"[MeSH Terms] OR "coronavirus"[All Fields])) AND (2019/12[PDAT] OR 2020[PDAT]))) OR SARS-COV2[All Fields]).
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 Cases from the chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Haar J, Hoes LR, Coles CE, Seamon K, Fröhling S, Jäger D, Valenza F, de Braud F, De Petris L, Bergh J, Ernberg I, Besse B, Barlesi F, Garralda E, Piris-Giménez A, Baumann M, Apolone G, Soria JC, Tabernero J, Caldas C, Voest EE. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26(5):665–671. doi: 10.1038/s41591-020-0874-8. [DOI] [PubMed] [Google Scholar]
- 4.Yahalom J, Dabaja BS, Ricardi U, Ng A, Mikhaeel NG, Vogelius IR, Illidge TM, Qi S, Wirth A, Specht L. ILROG emergency guidelines for radiation therapy of hematological malignancies during the COVID-19 pandemic. Blood. 2020 doi: 10.1182/blood.2020006028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guckenberger M, Belka C, Bezjak A, Bradley J, Daly ME, DeRuysscher D, Dziadziuszko R, Faivre-Finn C, Flentje M, Gore E, Higgins KA, Iyengar P, Kavanagh BD, Kumar S, Le Pechoux C, Lievens Y, Lindberg K, McDonald F, Ramella S, Rengan R, Ricardi U, Rimner A, Rodrigues GB, Schild SE, Senan S, Simone Ii CB, Slotman BJ, Stuschke M, Videtic G, Widder J, Yom SS, Palma D. Practice recommendations for lung cancer radiotherapy during the COVID-19 pandemic: an ESTRO-ASTRO consensus statement. Radiother Oncol. 2020 doi: 10.1016/j.radonc.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson DJ, Palma D, Guckenberger M, Balermpas P, Beitler JJ, Blanchard P, Brizel D, Budach W, Caudell J, Corry J, Corvo R, Evans M, Garden AS, Giralt J, Gregoire V, Harari PM, Harrington K, Hitchcock YJ, Johansen J, Kaanders J, Koyfman S, Langendijk JA, Le QT, Lee N, Margalit D, Mierzwa M, Porceddu S, Soong YL, Sun Y, Thariat J, Waldron J, Yom SS. Practice recommendations for risk-adapted head and neck cancer radiation therapy during the COVID-19 pandemic: an ASTRO-ESTRO consensus statement. Int J Radiat Oncol Biol Phys. 2020 doi: 10.1016/j.ijrobp.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tchelebi LT, Haustermans K, Scorsetti M, Hosni A, Huguet F, Hawkins MA, Dawson LA, Goodman KA. Recommendations for the use of radiation therapy in managing patients with gastrointestinal malignancies in the era of COVID-19. Radiother Oncol. 2020;148:194–200. doi: 10.1016/j.radonc.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thureau S, Faivre JC, Assaker R, Biver E, Confavreux CB, Debiais F, Duterque-Coquillaud M, Giammarile F, Heymann D, Lecouvet FE, Morardet L, Paycha F, Body JJ, Vieillard MH. Adapting palliative radiation therapy for bone metastases during the Covid-19 pandemic: GEMO position paper. J Bone Oncol. 2020 doi: 10.1016/j.jbo.2020.100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yerramilli D, Xu AJ, Gillespie EF, Shepherd AF, Beal K, Gomez D, Yamada J, Tsai CJ, Yang TJ. Palliative radiotherapy for oncologic emergencies in the setting of COVID-19: approaches to balancing risks and benefits. Adv Radiat Oncol. 2020 doi: 10.1016/j.adro.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kate J (2020) 'Brutal' Plan to Restrict Palliative Radiation During Pandemic. www.medscape.com. https://www.medscape.com/viewarticle/928185_print.
- 11.Cellini F, Manfrida S, Gambacorta MA, Vincenzo V. Prioritization on palliative radiotherapy during the COVID-19 pandemic (and beyond) Radiother Oncol. 2020;150:181–182. doi: 10.1016/j.radonc.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow E, Hoskin P, Mitera G, Zeng L, Lutz S, Roos D, Hahn C, van der Linden Y, Hartsell W, Kumar E, International Bone Metastases Consensus Working P Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys. 2012;82(5):1730–1737. doi: 10.1016/j.ijrobp.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Wu AJ, Rimner A, Shepherd AF, Gelblum DY, Shaverdian N, Yorke E, Simone CB, 2nd, Gomez DR. Thoracic radiation therapy during COVID-19: provisional guidelines from a comprehensive cancer center within a pandemic epicenter. Adv Radiat Oncol. 2020 doi: 10.1016/j.adro.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simcock R, Thomas TV, Estes C, Filippi AR, Katz MA, Pereira IJ, Saeed H. COVID-19: global radiation oncology's targeted response for pandemic preparedness. Clin Transl Radiat Oncol. 2020;22:55–68. doi: 10.1016/j.ctro.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Combs SE, Belka C, Niyazi M, Corradini S, Pigorsch S, Wilkens J, Grosu AL, Guckenberger M, Ganswindt U, Bernhardt D. First statement on preparation for the COVID-19 pandemic in large German speaking university-based radiation oncology departments. Radiat Oncol. 2020;15(1):74. doi: 10.1186/s13014-020-01527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curigliano G, Cardoso MJ, Poortmans P, Gentilini O, Pravettoni G, Mazzocco K, Houssami N, Pagani O, Senkus E, Cardoso F, editorial board of The B Recommendations for triage, prioritization and treatment of breast cancer patients during the COVID-19 pandemic. Breast. 2020;52:8–16. doi: 10.1016/j.breast.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn E, Livergant J, Millar BA, Ringash J, Wong R, Dawson LA, Warde P, Cummings B, Barry A. Comments on the publication by Yerramilli et al titled "palliative radiotherapy for oncologic emergencies in the setting of COVID-19: approaches to balancing risks and benefits". Adv Radiat Oncol. 2020 doi: 10.1016/j.adro.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rathod S, Dubey A, Bashir B, Sivananthan G, Leylek A, Chowdhury A, Koul R. Bracing for impact with new 4R's in the COVID-19 pandemic- a provincial thoracic radiation oncology consensus. Radiother Oncol. 2020 doi: 10.1016/j.radonc.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noticewala Sonal SLEB, Bishop Andrew J, Chung C, Ghia Amol J, Grosshans D, McGovern S, de la Cruz AP, Wang C, Woodhouse Kristina D, Yeboa Debra N, Prabhu Sujit S, Weathers S-P, Das P, Koong Albert C, McAleer Mary F, Li J. Radiation for Glioblastoma in the Era of COVID-19: Patient Selection and Hypofractionation to Maximize Benefit and Minimize Risk. Adv Radiat Oncol. 2020 doi: 10.1016/j.adro.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maranzano E, Trippa F, Casale M, Costantini S, Lupattelli M, Bellavita R, Marafioti L, Pergolizzi S, Santacaterina A, Mignogna M, Silvano G, Fusco V. 8Gy single-dose radiotherapy is effective in metastatic spinal cord compression: results of a phase III randomized multicentre Italian trial. Radiother Oncol. 2009;93(2):174–179. doi: 10.1016/j.radonc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Lee KA, Dunne M, Small C, Kelly PJ, McArdle O, O'Sullivan J, Hacking D, Pomeroy M, Armstrong J, Moriarty M, Clayton-Lea A, Parker I, Collins CD, Thirion P. (ICORG 05–03): prospective randomized non-inferiority phase III trial comparing two radiation schedules in malignant spinal cord compression (not proceeding with surgical decompression); the quality of life analysis. Acta Oncol. 2018;57(7):965–972. doi: 10.1080/0284186X.2018.1433320. [DOI] [PubMed] [Google Scholar]
- 22.Roos DE, Turner SL, O'Brien PC, Smith JG, Spry NA, Burmeister BH, Hoskin PJ, Ball DL, Trans-Tasman Radiation Oncology Group T Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone metastases (Trans-Tasman Radiation Oncology Group, TROG 96.05) Radiother Oncol. 2005;75(1):54–63. doi: 10.1016/j.radonc.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Capuccini J, Macchia G, Farina E, Buwenge M, Genovesi D, Caravatta L, Nguyen NP, Cammelli S, Cilla S, Wondemagegnhu T, Uddin A, Aziz Sumon M, Cellini F, Valentini V, Deodato F, Morganti AG. Short-course regimen of palliative radiotherapy in complicated bone metastases: a phase i–ii study (SHARON Project) Clin Exp Metastasis. 2018;35(7):605–611. doi: 10.1007/s10585-018-9931-9. [DOI] [PubMed] [Google Scholar]
- 24.Deressa BT, Tigeneh W, Bogale N, Buwenge M, Morganti AG, Farina E. Short-course 2-dimensional radiation therapy in the palliative treatment of esophageal cancer in a developing country: a phase II study (Sharon Project) Int J Radiat Oncol Biol Phys. 2020;106(1):67–72. doi: 10.1016/j.ijrobp.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues G, Videtic GM, Sur R, Bezjak A, Bradley J, Hahn CA, Langer C, Miller KL, Moeller BJ, Rosenzweig K, Movsas B. Palliative thoracic radiotherapy in lung cancer: an American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1(2):60–71. doi: 10.1016/j.prro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen NT, Doerwald-Munoz L, Zhang H, Kim DH, Sagar S, Wright JR, Hodson DI. 0–7-21 hypofractionated palliative radiotherapy: an effective treatment for advanced head and neck cancers. Br J Radiol. 2015;88(1049):20140646. doi: 10.1259/bjr.20140646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spanos W, Jr, Guse C, Perez C, Grigsby P, Doggett RL, Poulter C. Phase II study of multiple daily fractionations in the palliation of advanced pelvic malignancies: preliminary report of RTOG 8502. Int J Radiat Oncol Biol Phys. 1989;17(3):659–661. doi: 10.1016/0360-3016(89)90120-x. [DOI] [PubMed] [Google Scholar]
- 28.Spanos WJ, Jr, Perez CA, Marcus S, Poulter CA, Doggett RL, Steinfeld AD, Grigsby PW. Effect of rest interval on tumor and normal tissue response–a report of phase III study of accelerated split course palliative radiation for advanced pelvic malignancies (RTOG-8502) Int J Radiat Oncol Biol Phys. 1993;25(3):399–403. doi: 10.1016/0360-3016(93)90059-5. [DOI] [PubMed] [Google Scholar]
- 29.Farina E, Capuccini J, Macchia G, Caravatta L, Nguyen NP, Cammelli S, Farioli A, Zanirato Rambaldi G, Cilla S, Wondemagegnhu T, Uddin A, Sumon MA, Genovesi D, Buwenge M, Cellini F, Valentini V, Deodato F, Morganti AG. Phase I-II study of short-course accelerated radiotherapy (SHARON) for palliation in head and neck cancer. Anticancer Res. 2018;38(4):2409–2414. doi: 10.21873/anticanres.12491. [DOI] [PubMed] [Google Scholar]
- 30.Yan J, Milosevic M, Fyles A, Manchul L, Kelly V, Levin W. A hypofractionated radiotherapy regimen (0–7-21) for advanced gynaecological cancer patients. Clin Oncol (R Coll Radiol) 2011;23(7):476–481. doi: 10.1016/j.clon.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Farina E, Macchia G, Siepe G, Zamagni A, Buwenge M, Scirocco E, Cellini F, Deressa BT, Tigeneh W, Uddin K, Sumon MA, Caravatta L, Genovesi D, Mauro FA, Cammelli S, Cilla S, Morganti AG, Deodato F. Palliative short-course radiotherapy in advanced pelvic cancer: a phase II study (SHARON Project) Anticancer Res. 2019;39(8):4237–4242. doi: 10.21873/anticanres.13585. [DOI] [PubMed] [Google Scholar]
- 32.Sundstrom S, Bremnes R, Aasebo U, Aamdal S, Hatlevoll R, Brunsvig P, Johannessen DC, Klepp O, Fayers PM, Kaasa S. Hypofractionated palliative radiotherapy (17 Gy per two fractions) in advanced non-small-cell lung carcinoma is comparable to standard fractionation for symptom control and survival: a national phase III trial. J Clin Oncol. 2004;22(5):801–810. doi: 10.1200/JCO.2004.06.123. [DOI] [PubMed] [Google Scholar]
- 33.Macbeth FR, Bolger JJ, Hopwood P, Bleehen NM, Cartmell J, Girling DJ, Machin D, Stephens RJ, Bailey AJ. Randomized trial of palliative two-fraction versus more intensive 13-fraction radiotherapy for patients with inoperable non-small cell lung cancer and good performance status. Medical Research Council Lung Cancer Working Party. Clin Oncol. 1996;8(3):167–175. doi: 10.1016/s0936-6555(96)80041-0. [DOI] [PubMed] [Google Scholar]
- 34.A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party (1992). Br J Cancer 65 (6):934–941. doi:10.1038/bjc.1992.196 [DOI] [PMC free article] [PubMed]
- 35.Farina E, Macchia G, Buwenge M, Siepe G, Zamagni A, Cammelli S, Cilla S, Wondemagegnhu T, Woldemariam AA, Uddin A, Sumon MA, Cellini F, Deodato F, Morganti AG. Radiotherapy in palliation of thoracic tumors: a phase I-II study (SHARON project) Clin Exp Metastasis. 2018;35(8):739–746. doi: 10.1007/s10585-018-9942-6. [DOI] [PubMed] [Google Scholar]
- 36.Zamagni A, Buwenge M, Macchia G, Siepe G, Cilla S, Cellini F, Arcelli A, Farina E, Deodato F, Cammelli S, Morganti AG. Accelerated middle half body radiotherapy in bone metastases from prostate cancer: a phase I study (SHARON Project) Anticancer Res. 2019;39(9):5065–5069. doi: 10.21873/anticanres.13699. [DOI] [PubMed] [Google Scholar]
- 37.Johanson CR, Harwood AR, Cummings BJ, Quirt I. 0–7-21 radiotherapy in nodular melanoma. Cancer. 1983;51(2):226–232. doi: 10.1002/1097-0142(19830115)51:2<226::aid-cncr2820510210>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 38.Jeremic B, Fidarova E, Sharma V, Faheem M, Ameira AA, Nasr Ben Ammar C, Frobe A, Lau F, Brincat S, Jones G. The international atomic energy agency (IAEA) randomized trial of palliative treatment of incurable locally advanced non small cell lung cancer (NSCLC) using radiotherapy (RT) and chemotherapy (CHT) in limited resource setting. Radiother Oncol. 2015;116(1):21–26. doi: 10.1016/j.radonc.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Porceddu SV, Rosser B, Burmeister BH, Jones M, Hickey B, Baumann K, Gogna K, Pullar A, Poulsen M, Holt T. Hypofractionated radiotherapy for the palliation of advanced head and neck cancer in patients unsuitable for curative treatment–"Hypo Trial". Radiother Oncol. 2007;85(3):456–462. doi: 10.1016/j.radonc.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 40.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP, Schellenberg D, Ahmad B, Griffioen G, Senthi S, Swaminath A, Kopek N, Liu M, Moore K, Currie S, Bauman GS, Warner A, Senan S. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 41.Olson R, Mathews L, Liu M, Schellenberg D, Mou B, Berrang T, Harrow S, Correa RJM, Bhat V, Pai H, Mohamed I, Miller S, Schneiders F, Laba J, Wilke D, Senthi S, Louie AV, Swaminath A, Chalmers A, Gaede S, Warner A, de Gruijl TD, Allan A, Palma DA. Stereotactic ablative radiotherapy for the comprehensive treatment of 1–3 Oligometastatic tumors (SABR-COMET-3): study protocol for a randomized phase III trial. BMC Cancer. 2020;20(1):380. doi: 10.1186/s12885-020-06876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, Keall P, Lovelock M, Meeks S, Papiez L, Purdie T, Sadagopan R, Schell MC, Salter B, Schlesinger DJ, Shiu AS, Solberg T, Song DY, Stieber V, Timmerman R, Tome WA, Verellen D, Wang L, Yin FF. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37(8):4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 43.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ., Jr Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 44.Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, McColl E, Moore B, Brisbane I, Ardron D, Holt T, Morgan S, Lee C, Waite K, Bayman N, Pugh C, Sydes B, Stephens R, Parmar MK, Langley RE. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388(10055):2004–2014. doi: 10.1016/S0140-6736(16)30825-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roa W, Brasher PM, Bauman G, Anthes M, Bruera E, Chan A, Fisher B, Fulton D, Gulavita S, Hao C, Husain S, Murtha A, Petruk K, Stewart D, Tai P, Urtasun R, Cairncross JG, Forsyth P. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–1588. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 46.Malmstrom A, Gronberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, Abacioglu U, Tavelin B, Lhermitte B, Hegi ME, Rosell J, Henriksson R, Nordic Clinical Brain Tumour Study G Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 47.Roa W, Kepka L, Kumar N, Sinaika V, Matiello J, Lomidze D, Hentati D, Guedes de Castro D, Dyttus-Cebulok K, Drodge S, Ghosh S, Jeremic B, Rosenblatt E, Fidarova E. International atomic energy agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2015;33(35):4145–4150. doi: 10.1200/JCO.2015.62.6606. [DOI] [PubMed] [Google Scholar]
- 48.Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, Carrero XW, Barker FG, 2nd, Deming R, Burri SH, Menard C, Chung C, Stieber VW, Pollock BE, Galanis E, Buckner JC, Asher AL. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P, Settle S, Prabhu SS, Lang FF, Levine N, McGovern S, Sulman E, McCutcheon IE, Azeem S, Cahill D, Tatsui C, Heimberger AB, Ferguson S, Ghia A, Demonte F, Raza S, Guha-Thakurta N, Yang J, Sawaya R, Hess KR, Rao G. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1040–1048. doi: 10.1016/S1470-2045(17)30414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kepka L, Tyc-Szczepaniak D, Bujko K, Olszyna-Serementa M, Michalski W, Sprawka A, Trabska-Kluch B, Komosinska K, Wasilewska-Tesluk E, Czeremszynska B. Stereotactic radiotherapy of the tumor bed compared to whole brain radiotherapy after surgery of single brain metastasis: results from a randomized trial. Radiother Oncol. 2016;121(2):217–224. doi: 10.1016/j.radonc.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Danjoux C, Chow E, Drossos A, Holden L, Hayter C, Tsao M, Barnes T, Sinclair E, Farhadian M. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Supportive care in cancer : Official J Multinational Ass Supportive Care in Cancer. 2006;14(1):38–43. doi: 10.1007/s00520-005-0822-7. [DOI] [PubMed] [Google Scholar]
- 52.McDonald R, Lechner B, Pulenzas N, Bedard G, Wong E, Holden L, Tsao M, Barnes E, Szumacher E, Fenton G, Chow E, Popovic M, Danjoux C. Student accomplishments in the rapid response radiotherapy program: a 10-year review. J Cancer Educ. 2015;30(4):693–698. doi: 10.1007/s13187-014-0748-1. [DOI] [PubMed] [Google Scholar]
- 53.Razvi Y, Chan S, Zhang L, Tsao M, Barnes E, Danjoux C, Sousa P, Zaki P, McKenzie E, DeAngelis C, Chow E. A review of the Rapid Response Radiotherapy Program in patients with advanced cancer referred for palliative radiotherapy over two decades. Supportive care in cancer : Official J Multinational Ass Supportive Care in Cancer. 2019;27(6):2131–2134. doi: 10.1007/s00520-018-4474-9. [DOI] [PubMed] [Google Scholar]
- 54.Mauri D, Kamposioras K, Tolia M, Alongi F, Tzachanis D, International Oncology P, European Cancer Patient Coalition c Summary of international recommendations in 23 languages for patients with cancer during the COVID-19 pandemic. Lancet Oncol. 2020 doi: 10.1016/S1470-2045(20)30278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jereczek-Fossa BA, Pepa M, Marvaso G, Bruni A, di Monale EBMB, Catalano G, Filippi AR, Franco P, Gambacorta MA, Genovesi D, Iati G, Magli A, Marafioti L, Meattini I, Merlotti A, Mignogna M, Musio D, Pacelli R, Pergolizzi S, Tombolini V, Trovo M, Ricardi U, Magrini SM, Corvo R, DonatoAiro V. COVID-19 outbreak and cancer radiotherapy disruption in italy: Survey endorsed by the italian association of radiotherapy and clinical oncology (AIRO) Radiother Oncol. 2020 doi: 10.1016/j.radonc.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tagliaferri L, Di Stefani A, Schinzari G, Fionda B, Rossi E, Del Regno L, Gentileschi S, Federico F, Valentini V, Tortora G, Peris K, Gemelli Skin-Cancer Multidisciplianry Tumour B Skin cancer triage and management during COVID-19 pandemic. J Eur Acad Dermatol Venereol: JEADV. 2020 doi: 10.1111/jdv.16529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gemici C, Yaprak G. Covid-19 outbreak in a major radiation oncology department; which lessons should be taken? Radiother Oncol. 2020 doi: 10.1016/j.radonc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sriwijitalai W, Wiwanitkit V. COVID-19, radiotherapy and cancer. Radiother Oncol. 2020 doi: 10.1016/j.radonc.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lefresne S, Berthelet E, Cashman R, Levy K, Liu M, Carolan H, McKenzie M, Kostuik P, Olson R. The Vancouver rapid access clinic for palliative lung radiation, providing more than just rapid access. Supportive care in cancer: Official J Multinational Ass Supportive Care in Cancer. 2015;23(1):125–132. doi: 10.1007/s00520-014-2345-6. [DOI] [PubMed] [Google Scholar]
- 60.Donato V, Cianciulli M, Crescenzi M, Monaco A, Caruso C, Morrone A. Rapid palliative radiotherapy unit: multidisciplinary management of bone metastases. Radiol Med. 2012;117(6):1071–1078. doi: 10.1007/s11547-012-0834-6. [DOI] [PubMed] [Google Scholar]
- 61.Job M, Holt T, Bernard A. Reducing radiotherapy waiting times for palliative patients: the role of the Advanced Practice Radiation Therapist. J Med Radiat Sci. 2017;64(4):274–280. doi: 10.1002/jmrs.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Job M, Holt T, Bernard A. An evaluation of an advanced practice role in palliative radiation therapy. J Med Radiat Sci. 2019;66(2):96–102. doi: 10.1002/jmrs.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hahn E, Livergant J, Millar B-A, Ringash J, Wong R, Dawson LA, Warde P, Cummings B, Barry A. In Regard to Yerramilli et al’s “Palliative Radiotherapy for Oncologic Emergencies in the Setting of COVID-19: Approaches to Balancing Risks and Benefits”. Adv Radiat Oncol. 2020;5(4):595–596. doi: 10.1016/j.adro.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]