Abstract
Objective
We compared neonatal immunity after vaccination against SARS-CoV-2 during pregnancy to that achieved after maternal infection.
Study design
We tested cord blood from women infected with SARS-CoV-2 during pregnancy (group 1, n = 29), women who were vaccinated during pregnancy (group 2, n = 29) and from women not infected and not vaccinated (Group 3, n = 21) for titers of antibodies to both SARS-CoV-2 spike and ‘N’ proteins.
Results
Seventy-nine women were included: Antibodies against SARS-CoV-2 spike protein were detected in all samples from Group 1 and 2. Antibodies to the ‘N’ protein were detected in 25/29 samples in Group 1. None of the samples from Group 3 had antibodies to either protein. Mean titers of SARS-CoV-2 antibodies were significantly higher in Group 2 than in Group 1 (p < 0.05).
Conclusions
Neonates born to mothers vaccinated during pregnancy have higher antibody titers and may therefore have more prolonged protection than those born to women infected during pregnancy.
Subject terms: Viral infection, Preventive medicine
Introduction
The novel coronavirus (SARS-CoV-2) pandemic, declared in March 2020, has been responsible for more than 3 million deaths globally [1]. Several vaccines against the SARS-CoV-2 virus have been developed. In December 2020, the United States Food and Drug Administration (FDA) granted an emergency use authorization for SARS-CoV-2 vaccines developed by Pfizer and Moderna for individuals over the age of sixteen [2]. Both vaccines are based on the production of the SARS-CoV-2 Spike protein (‘S’ protein) via mRNA to “educate” the immune system and produce immunoglobulins (IgG) antibodies against the virus [3]. Due to the lack of clinical data regarding the safety and efficacy of these vaccines in pregnant and lactating women, in January 2021 the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine advised to allow pregnant women to decide whether they be vaccinated after counselling about the risks and benefits of vaccination during pregnancy [4]. Since then, studies have shown that mRNA SARS-CoV-2 vaccines are safe and effective in pregnant women and provide the same level of immunity as to they do in the general population [5].
There is limited data regarding the effect on neonatal immunity to SARS-CoV-2 in women vaccinated during pregnancy. Researchers showed that maternal IgG antibodies that were produced in response to vaccination of pregnant women against influenza and pertussis crossed the placenta and significantly decreased neonatal morbidity and mortality due to these infections [6, 7]. Similarly, Flannery et al. reported that neonates born to mothers infected by SARS-CoV-2 during pregnancy were born with IgG antibodies to SARS-CoV-2 as a result of transfer across the placenta [8]. Additional publications regarding transplacental transfer of maternal SARS-CoV-2–specific antibodies to newborns after vaccination of the mother are limited to case reports [5, 9].
We studied cord blood for the presence of IgG to both SARS-CoV-2 ‘S’ protein and the Nucleocapsid Protein (‘N’ protein). Anti ‘S’ protein antibodies can be detected in both infected and vaccinated women. Nucleocapsid Protein of SARS-CoV-2 is located in the viral core, therefore IgG antibodies against the ‘N’ protein are detectable only in the serum of infected women; these antibodies may disappear over a period of 18 months [10].
The aim of our study was to compare the titers of IgG antibodies to SARS-CoV-2 in umbilical cord blood in women who received the SARS-CoV-2 BNT162b2 mRNA vaccine during gestation and in women who were infected with SARS-CoV-2 during gestation.
Methods
This was a cohort study of women who delivered singleton livebirths at the Mayanei Hayeshua Medical Center in Bnei Brak, Israel between February 28th and March 8th, 2021. At the time of delivery, umbilical venous blood was routinely collected for neonatal blood typing and Coombs’s testing. Using the umbilical cord blood that remained, we tested for the presence of IgG antibodies to both the SARS-CoV-2 spike protein and the ‘N’ protein in a proportion of the samples that were available. Maternal sera was also analyzed at the time of delivery for the presence of IgG antibodies to both the SARS-CoV-2 ‘S’ protein and the ‘N’ protein. We then correlated maternal and matching neonatal antibody titers. The study population was divided into three groups: Group 1 included 29 women who were infected with SARS-CoV-2 during pregnancy. This included women who had a positive RT-PCR test during pregnancy or those found to have positive serology at delivery. Group 2 included 29 women who were vaccinated with two doses of the BNT162b2- mRNA SARS-CoV-2 Pfizer vaccine in the third trimester of pregnancy. There was a 3-week interval between the two doses in line with the guidelines of the Israeli ministry of Health [11].
Group 3 included 21 women who were not vaccinated and had no evidence of SARS-CoV-2 infection during pregnancy (negative serology and negative RT-PCR test).
Testing for IgG antibodies to the SARS-CoV-2 ‘S’ protein and ‘N’ protein was performed on these 83 cord blood samples using the Elecsys® Anti-SARS-CoV-2 S immunoassay [12].
The primary outcome was the presence and titers of antibodies in cord blood in these three groups. Secondary outcomes regarding neonatal outcomes were defined as: neonatal infection with SARS-CoV-2, Neonatal Intensive Care Unit (NICU) admissions, Respiratory Distress Syndrome (RDS), sepsis and neonatal death.
Demographic data were collected from electronic medical records. This included maternal age, gestational age at delivery, pre-term delivery (delivery < 37 weeks), mode of delivery, PCR confirmed SARS-CoV-2 infection during pregnancy, SARS-CoV-2 vaccination status, neonatal birth weight, RDS, NICU admission, sepsis, and death.
The cord blood and the peripheral blood samples were analyzed using Elecsys® Anti-SARS-CoV-2 S uses immunoassay (Roche Diagnostics) for the in vitro quantitative determination of antibodies (including IgG) to the SARS-CoV-2 ‘S’ protein receptor binding domain in human serum and plasma. The assay uses a recombinant protein representing the nucleocapsid (‘N’) antigen in a double-antigen sandwich assay format, which favors detection of high affinity antibodies against SARS-CoV-2 [12]. Although the test is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection it was approved by the Israeli ministry of health for detection of antibodies to the SARS-CoV-2 spike protein receptor binding domain in both recovered and vaccinated patients.
Statistical analyses were performed using IBM SPSS statistics version 25.0.
Demographic data and mean neonatal antibody titers were compared among the groups using one sided ANOVA tests.
Maternal and neonatal outcomes were analyzed using the Chi squared test.
Maternal IgG concentrations were correlated with their neonatal IgG concentrations using SPSS Paired Samples T-Test.
Pearson r2 analysis was used to compare the correlation between the maternal and neonatal antibody titers.
Differences were considered significant at P < 0.05.
The study was approved by the ethics committee of Mayenei Hayeshuah Medical Center.
Results
Between February 28th and March 8th, 2021 there were 181 singleton livebirths in our institution. We collected 83 cord blood samples and divided them into three groups: Group 1 included 29 samples (37%) from women who were infected with SARS-CoV-2 during pregnancy. Twelve had RT-PCR confirmed Covid-19 infection: three were infected in the first trimester, three in the second trimester and six in the third trimester. The other 17 had no clinical signs of SARS-CoV-2 infection during pregnancy and had a positive serologic test on admission. None of the 17 women had active SARS-CoV-2 infection at the time of delivery. Group 2 included 29 samples (37%) from women who were vaccinated against SARS-CoV-2. Group 3 included 21 women (34%) and served as controls. Four women were excluded; three of them contracted SARS-CoV-2 infection during pregnancy and were later vaccinated prior to delivery. Two had antibody titer of 250 U/ml and one woman had a titer of 27.6 U/ml. The fourth woman was excluded since she had received only a single dose of the vaccine; her titer was 250 U/ml. Aside from slight differences in age and a higher parity in Group 2, there were no significant differences in the demographic characteristics among the three groups (Table 1).
Table 1.
Demographic data.
| Group 1 (n = 29) | Group 2 (n = 29) | Group 3 (n = 21) | p value | |
| Mean maternal age (years) | 30.1 | 32.5 | 28.5 | 0.03a |
| Mean parity | 3.3 | 5.2 | 2.7 | <0.05a |
| Mean gestational age at delivery (weeks) | 39.5 | 39.3 | 38.8 | 0.29 |
| Number of pre-term delivery | 0 | 0 | 0 | n/a |
| Mode of delivery, NVD, No. (%) | 24(82.8) | 26(89.7) | 18(85.7) | 0.86 |
| Mean neonatal antibody titer U/ml | 83.7 | 225.5 | n/a | <0.05a |
| Mean birth weight (grams) | 3311.9 | 3382.1 | 3159.8 | 0.46 |
Continuous parameters were analyzed by one sided ANOVA test. Chi-square analysis was used to compare mode of delivery.
No number, NVD Normal vaginal delivery.
aClinical parameters did not differ among the groups, except for parity and maternal age which was significantly higher in the vaccinated group (group 2), as compared to the other two groups (ANOVA; P = 0.002 and P = 0.03 respectively). Mean neonatal antibody level were significantly higher in the vaccinated group (group 2).
There were no cases of neonatal SARS-CoV-2 infections, neonatal sepsis, NICU admissions, RDS, sepsis or neonatal death. All umbilical cord samples from groups 1 and 2 were seropositive for the SARS-CoV-2 IgG ‘S’ protein (100%). Twenty-five were also positive for the N protein. Of the four who were not, three had asymptomatic infection and one had a clinical infection that was confirmed by a positive RT-PCR test during the first trimester. The mean titers of SARS-CoV-2 ‘S’ antibodies in these four samples were lower than the titers in the rest of the group (33.9 U/ml compared with 91.6 U/ml) but this difference did not reach statistical significance (p = 0.41). None of the samples from group 2 were positive for the SARS-CoV-2 ‘N’ protein. None of the samples from Group 3 were seropositive for antibodies to the SARS-CoV-2 IgG ‘S’ or ‘N’ protein. The mean antibody titer of group 1 was 83.7 ± 91.6 u/ml compared with 224.7 ± 64.3 U/ml in group 2, a statistically significant difference (p < 0.05).
Maternal SARS-CoV-2 antibody titers at delivery were available for 39 women: 13 in group 1, 17 in group 2, and 9 women from the control group (Group 3, who were all seronegative). There was a strong correlation between the maternal antibody titers and the titers found in the corresponding 30 cord blood samples (p < 0.05, R2 = 0.94, Fig. 1). The mean maternal and cord blood titers were 157 ± 112.8 u/ml and 150.2 ± 113.2 u/ml, respectively.
Fig. 1. Correlation between the maternal and neonatal antibody titers.
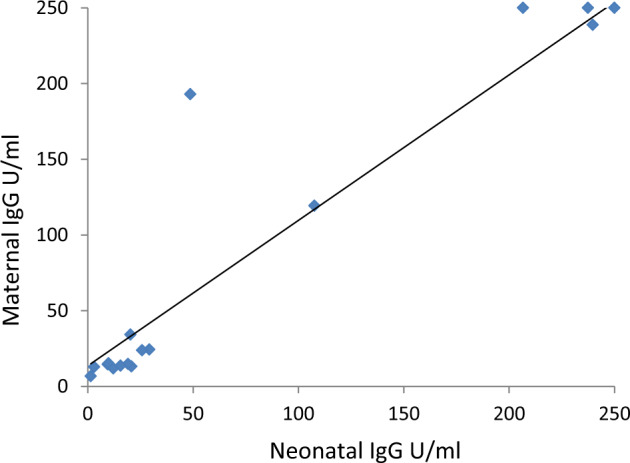
Maternal-fetal serological correlation of IgG for ‘S’ protein. Correlations between fetal and 30 maternal Antibodies were analyzed by Linear Regression test. Each dot represents data from a single pair of maternal and neonatal ‘S’ protein antibodies titer; the linear regression line is marked in black, with its 95% CI (dotted lines). R2 = 0.94, p < 0.05.
Discussion
Our results support the current recommendation for pregnant women to receive the SARS-CoV-2 vaccination [4].
We report that vaccination during gestation results in a robust IgG antibody response in the mother and that this response is significantly greater than the immune response in women who contracted SARS-CoV-2 infection during pregnancy. Given the higher antibody titers found among women who were vaccinated, we can speculate that boosting immunity during pregnancy translates into measurable serological benefits and might determine milder courses of the neonatal disease.
It is likely that this immunity is transient in both groups; among the women who contracted SARS-CoV-2 infection during pregnancy, as cord blood samples from four women were seronegative for the ‘N’ Protein. These women most likely contracted infection during the early stage of pregnancy. The implications of declining antibody titers in both those who contracted SARS-CoV-2 infection and those vaccinated against this virus are not clear since other immune mechanisms such as cellular mediated immunity and rapid boosting can afford sufficient protection against virus infection. However, immunization during pregnancy is important since the transplacental transfer of antibodies is the only means of protection from SARS-CoV-2 available to newborns. The magnitude of antibody titer is probably directly correlated to the duration of postnatal protection. We assume that the higher antibody titers detected in cord blood of babies born to mothers vaccinated during the third trimester, compared with the lower titers found in samples from those born to mothers who were previously infected with SARS-CoV-2, result in a longer protection period during the first months of life. These findings can be used to encourage pregnant women to vaccinate against SARS-CoV-2. Our findings also support the recent recommendation by health organizations to vaccinate people previously infected with SARS-CoV-2 to boost their immunity [13] This recommendation is the subject of debate when pertaining to pregnant women and is supported by the findings of the current study. However, the decision whether or not to vaccinate pregnant women who had previously contracted SARS-CoV-2 infection in order to enhance the protection of their babies (similarly to the practice of protection against infantile pertussis) [7] also depends on the local epidemiology of SARS-CoV-2 infection: this measure may be considered in communities where there is a high rate of SARS-CoV-2 infections.
Our study is among the first that evaluated antibody titers in cord blood of newborns born to women who were vaccinated during pregnancy and compared them to titers in babies born to women who contracted SARS-CoV-2 infection during pregnancy. The limitations of our study include the relatively small cohort size, the low availability of the samples adequate for quantification of anti- SARS-CoV-2 antibodies and the lack of correlation to the presence of antibodies in breastmilk. Another limitation was that data regarding the timing of SARS-CoV-2 infection was available only for 12 of the 29 women in Group 1 (17 women were interviewed and had no known SARS-CoV-2 infection). We were unable to assess the latency period between acute infection to the appearance of antibodies, the highest antibodies level and the rate of decline. However, since the majority of women in group 1 (17 of 29) had asymptomatic infections, it is unlikely this information can be determined without periodically testing a very large cohort of pregnant women.
Conclusions
Titers of IgG antibodies against SARS-CoV-2 in umbilical cord blood samples were significantly higher in a cohort of newborns born to women vaccinated against SARS-CoV-2 during their third trimester than among those born to women who contracted SARS-CoV-2 during pregnancy. Cord blood antibody concentrations correlated with maternal antibody concentrations. These findings demonstrate that boosting immunity during pregnancy confers serological benefits to the offspring. Due to the small number of subjects and the short follow-up period, this study should be considered a primarily hypothesis generating; larger studies and longer follow up are required in order to recommend a clinical practice such as vaccination during pregnancy.
Author contributions
Concept and design: K-L, ES. Acquisition, analysis or interpretation of the data: K-L, ML, RC, HS, SC, AH, ABC, IK, JBL, RN. Drafting and paper: K-L, ML, RN, ES. Critical revision of the paper for importent intellectual content: ML, K-L, SC, RN, RC, JBL, ES. Statistical anlysis: RC. Supervision: K-L, ES, RN.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: Table 1 has been corrected.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12/7/2021
A Correction to this paper has been published: 10.1038/s41372-021-01272-7
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Commissioner O of the. Moderna COVID-19 Vaccine. FDA. Published online April 9, 2021. Accessed May 26, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine.
- 3.Commissioner O of the. Pfizer-BioNTech COVID-19 Vaccine. FDA. Published online May 19, 2021. Accessed May 26, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine.
- 4.ACOG and SMFM Joint Statement on WHO Recommendations Regarding COVID-19 Vaccines and Pregnant Individuals. Accessed May 26, 2021. https://www.acog.org/en/news/news-releases/2021/01/acog-and-smfm-joint-statement-on-who-recommendations-regarding-covid-19-vaccines-and-pregnant-individuals.
- 5.Gill L, Jones CW. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibodies in Neonatal Cord Blood After Vaccination in Pregnancy. Obstet Gynecol. 2021;137:894–96. doi: 10.1097/AOG.0000000000004367. [DOI] [PubMed] [Google Scholar]
- 6.Quach THT, Mallis NA, Cordero JF. Influenza vaccine efficacy and effectiveness in pregnant women: systematic review and Meta-analysis. Matern Child Health J. 2020;24:229–40. doi: 10.1007/s10995-019-02844-y. [DOI] [PubMed] [Google Scholar]
- 7.Munoz FM, Bond NH, Maccato M, Pinell P, Hammill HA, Swamy GK, et al. Safety and Immunogenicity of Tetanus Diphtheria and Acellular Pertussis (Tdap) Immunization during Pregnancy in Mothers and Infants: a randomized clinical trial. JAMA. 2014;311:1760. doi: 10.1001/jama.2014.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flannery DD, Gouma S, Dhudasia MB, Mukhopadhyay S, Pfeifer MR, Woodford EC, et al. Assessment of Maternal and Neonatal Cord Blood SARS-CoV-2 Antibodies and Placental Transfer Ratios. JAMA Pediatr. 2021;175:594–600. doi: 10.1001/jamapediatrics.2021.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atyeo C, Pullen KM, Bordt EA, Fischinger S, Burke J, Michell A, et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell. 2021;184:628–42.e10. doi: 10.1016/j.cell.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Lu S, Li H, Wang Y, Lu Z, Liu Z, et al. Viral and Antibody Kinetics of COVID-19 Patients with Different Disease Severities in Acute and Convalescent Phases: a 6-Month Follow-Up Study. Virol Sin. 2020;35:820–9. doi: 10.1007/s12250-020-00329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.COVID Live Update: 168,678,835 Cases and 3,502,484 Deaths from the Coronavirus - Worldometer. Accessed May 26, 2021. https://www.worldometers.info/coronavirus/.
- 12.Higgins V, Fabros A, Kulasingam V. Quantitative measurement of anti-SARS-CoV-2 antibodies: analytical and clinical evaluation. J Clin Microbiol. 2021;59:e03149–20. doi: 10.1128/JCM.03149-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl J Med. 2021;384:1372–4. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]


