ABSTRACT
Despite the availability of vaccines that efficiently reduce the severity of clinical symptoms, influenza viruses still cause substantial morbidity and mortality worldwide. In this regard, nasal influenza vaccines—because they induce virus-specific IgA—may be more effective than traditional parenteral formulations in preventing infection of the upper respiratory tract. In addition, the neuraminidase (NA) of influenza virus has shown promise as a vaccine antigen to confer broad cross-protection, in contrast to hemagglutinin (HA), the target of most current vaccines, which undergoes frequent antigenic changes, leading to vaccine ineffectiveness against mismatched heterologous strains. However, the usefulness of NA as an antigen for nasal vaccines is unclear. Here, we compared NA and HA as antigens for nasal vaccines in mice. Intranasal immunization with recombinant NA (rNA) plus adjuvant protected mice against not only homologous but also heterologous virus challenge in the upper respiratory tract, whereas intranasal immunization with rHA failed to protect against heterologous challenge. In addition, intranasal immunization with rNA, but not rHA, conferred cross-protection even in the absence of adjuvant in virus infection-experienced mice; this strong cross-protection was due to the broader capacity of NA-specific antibodies to bind to heterologous virus. Furthermore, the NA-specific IgA in the upper respiratory tract that was induced through rNA intranasal immunization recognized more epitopes than did the NA-specific IgG and IgA in plasma, again increasing cross-protection. Together, our findings suggest the potential of NA as an antigen for nasal vaccines to provide broad cross-protection against both homologous and heterologous influenza viruses.
IMPORTANCE Because mismatch between vaccine strains and epidemic strains cannot always be avoided, the development of influenza vaccines that induce broad cross-protection against antigenically mismatched heterologous strains is needed. Although the importance of NA-specific antibodies to cross-protection in humans and experimental animals is becoming clear, the potential of NA as an antigen for providing cross-protection through nasal vaccines is unknown. We show here that intranasal immunization with NA confers broad cross-protection in the upper respiratory tract, where virus transmission is initiated, by inducing NA-specific IgA that recognizes a wide range of epitopes. These data shed new light on NA-based nasal vaccines as powerful anti-influenza tools that confer broad cross-protection.
KEYWORDS: adjuvant, epitope, hemagglutinin, IgA, influenza virus, nasal vaccine, neuraminidase, upper respiratory tract, vaccine
INTRODUCTION
Despite continued development of vaccines, seasonal influenza viruses cause serious human morbidity and mortality worldwide (1). Most current vaccines against influenza viruses focus heavily on inducing neutralizing antibodies against hemagglutinin (HA) (2, 3) because of its crucial role in initiating virus entry into susceptible cells (4). However, the constant antigenic changes of HA drive the virus’s escape from selection by the immune response (1). Therefore, the antigenicity of the HAs in vaccine strains is often mismatched with that of circulating strains, thus decreasing vaccine effectiveness (5, 6). Influenza vaccines able to protect simultaneously against highly similar (homologous) strains and antigenically mismatched (heterologous) strains are urgently needed.
The neuraminidase (NA) of influenza virus is a tetrameric transmembrane surface protein with sialidase activity (7). NA has essential roles in the viral life cycle, from the point of first attachment to the final dispersal of nascent viral particles. In particular, by removing sialic acid residues in the host cell membrane, NA is responsible for the release of budding virus from infected cells (7). In addition, NA facilitates the transport of incoming virus through mucins by removing sialic acid moieties present as decoy receptors within the airways (7, 8). Thus, NA supports multiple rounds of infection by new viral progeny. Nevertheless, the potential utility of NA as a vaccine antigen has long been overlooked. In fact, licensed influenza vaccines are standardized according to a fixed amount of HA, whereas the amount of NA is not regulated, and conventional influenza split vaccines typically contain 2 to 3 times less NA than HA (9, 10). Consequently, in contrast to natural infection, many influenza vaccines fail to induce sufficient levels of anti-NA antibodies (11).
The selective pressure exerted by adaptive immune responses is lower against NA than HA; consequently, the amino acids at antigenic sites change more slowly in NA than HA (12). In addition, antibodies against NA are becoming recognized as important for protection against virus (7, 13–15). For example, some studies have demonstrated that anti-NA antibodies bind not only NA from homologous viruses but also NA from heterologous viruses and thereby confer broad cross-protection against heterologous virus challenge in mice (16–23). Furthermore, vaccination with NA provides broad cross-protection against virus challenge in mice, guinea pigs, and ferrets (24–29). In addition, increasing evidence has suggested that the titers of both anti-NA antibodies and NA-inhibiting antibodies are correlated with protection against influenza virus infection and disease in humans (30–32). Together, these reports indicate the benefits of developing vaccines using NA as a vaccine antigen to improve and broaden cross-protection against influenza virus.
In humans, infections with seasonal influenza viruses are initiated in the upper respiratory tract, where they cause relatively mild illness, whereas progression of infection to the lower respiratory tract often leads to pneumonia and more severe disease (33, 34). In addition, the upper respiratory tract is an important site in the transmission of virus via coughing, sneezing, or talking (1, 33–35). Therefore, developing vaccines that block the infection, generation, and expulsion of influenza viruses in the upper respiratory tract is critical. Because of their ability to induce antibodies in the upper respiratory tract, vaccines administered intranasally, i.e., “nasal vaccines,” are anticipated to be powerful tools for combating influenza viruses (36–38). In this regard, nasal vaccines induce not only antigen-specific IgG in blood but also antigen-specific IgA in the upper respiratory tract; in contrast, traditional, parenterally (e.g., intramuscularly or subcutaneously) administered vaccines do not induce antigen-specific IgA in the upper respiratory tract (36–38). Furthermore, compared with parenteral vaccines, nasal vaccines generated by using inactivated influenza virus more efficiently protect against influenza virus challenge in the upper respiratory tract of mice (39–41). However, only a few studies have assessed the potential of NA as a vaccine antigen for nasal vaccines (24, 29), and the utility of NA as an antigen for nasal vaccines and its superiority to HA in this context are unclear as yet.
In this study, we showed that a nasal vaccine using recombinant NA (rNA) from an H1N1 influenza virus as the antigen achieved broader cross-protection in the upper respiratory tract than an otherwise similar vaccine containing recombinant HA (rHA). Furthermore, IgA that was specific for NA in the upper respiratory tract and was induced by intranasal immunization recognized more epitopes than did NA-specific IgG in the plasma. Our data suggest the potential of NA as an antigen for nasal vaccines that achieve broad cross-protection against influenza viruses.
RESULTS
Generation and characterization of rNAs.
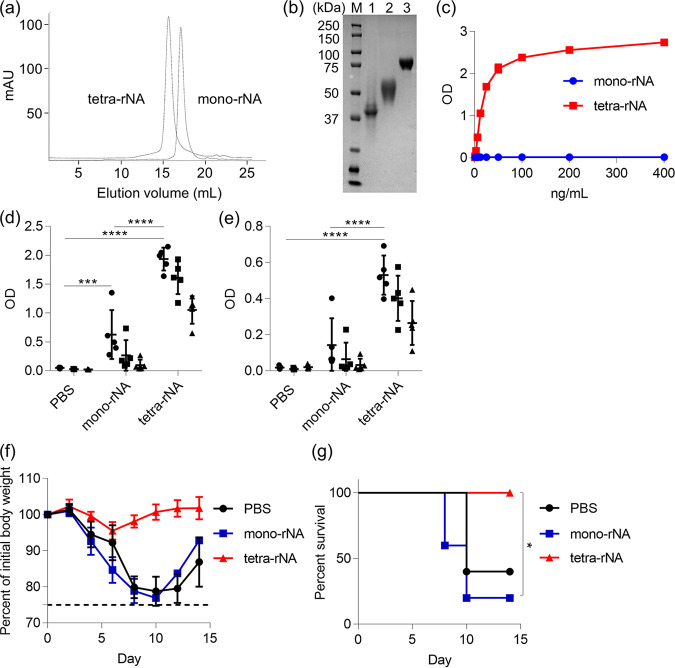
First, we compared the antigenicities of monomeric and tetrameric rNA proteins created from the ectodomain of NA from H1N1 A/California/07/2009 (Cal7); tetramerization of NA is required for its enzymatic activity (42). To obtain tetrameric rNA, we used the tetrabrachion domain from Staphylothermus marinus as a tetramerization motif; both rNAs were generated in mammalian cells and purified by using immobilized metal ion and size exclusion chromatography. In addition, trimeric rHA was generated and purified in the same way as rNA. We obtained about 5.4 mg monomeric rNA, 0.9 mg tetrameric rNA, and 8 mg rHA after purification from 1 liter of culture medium for mammalian cells. In size exclusion chromatography, tetrameric rNA had a shorter elution time than monomeric rNA, and both rNAs were eluted at the volume that was expected from their anticipated molecular weights (Fig. 1a). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) performed under reducing conditions showed that both monomeric rNA and tetrameric rNA migrated as single bands, with molecular masses of about 40 kDa and 50 kDa, respectively (Fig. 1b). Because of the size of the tetrabrachion domain, the molecular weight of tetrameric rNA was slightly greater than that of monomeric rNA (Fig. 1b). rHA migrated as a single band with a molecular mass of about 70 kDa (Fig. 1b). We then used an NA enzyme-linked lectin assay (ELLA) to determine the sialidase activity of both rNAs (Fig. 1c). Our tetrameric rNA had strong sialidase activity, as previously reported (43), but the monomeric rNA lacked enzymatic activity (Fig. 1c).
FIG 1.
Potential of recombinant tetrameric NA as a vaccine antigen. (a) Monomeric recombinant NA (rNA) and tetrameric rNA from Cal7 were generated in Expi293F cells and analyzed via size exclusion chromatography. (b) Purified rNAs and rHA were analyzed through SDS-PAGE followed by staining with Coomassie brilliant blue. M, marker; lane 1, monomeric rNA from Cal7; lane 2, tetrameric rNA from Cal7; lane 3, rHA from Cal7. (c) The sialidase activity of serially diluted rNAs was evaluated through enzyme-linked lectin assay (n = 3). (d to g) Mice (n = 5) were immunized subcutaneously with monomeric rNA from Cal7 (1 μg/mouse) plus alum or tetrameric rNA from Cal7 (1 μg/mouse) plus alum. At 7 days after final immunization, plasma levels of (d) tetrameric rNA-specific IgG and (e) Cal7 virus-specific IgG were evaluated by using ELISA. We used 160-fold (●), 800-fold (■), and 4,000-fold (▴) diluted plasma. At 10 days after final immunization, mice were challenged with Cal7 (homologous virus) and the percentage changes in (f) body weight and (g) survival were monitored. (d to f) Data are given as means ± SD. (d and e) Significant differences (***, P < 0.001; ****, P < 0.0001; Tukey’s test) were analyzed only for the 160-fold-diluted samples. (g) *, P < 0.05 according to comparison of Kaplan-Meier curves by using the log-rank test.
In this study, we used two influenza virus challenge models, in which virus was administered intranasally to mice to achieve either an upper respiratory tract infection (5 μl to the nares) or lower respiratory tract infection (30 μl to the nares). Prior to using the upper respiratory tract model, we confirmed that the virus strains we used were not detected in the lung and did not cause body weight loss after challenge (data not shown); then, as an indicator of vaccine efficacy, we used this model to measure the virus titer in nasal wash fluid after challenge. Using the lower respiratory tract model, we evaluated body weight loss and survival as indicators of vaccine efficacy. To assess the potential of each rNA as a vaccine antigen in vivo, we immunized mice subcutaneously with 1 μg/mouse of each Cal7-based rNA by using aluminum salts (alum) as an adjuvant. After the last immunization, we used enzyme-linked immunosorbent assay (ELISA) to analyze the levels of tetrameric rNA from Cal7-specific IgG (Fig. 1d) and Cal7 virus-specific IgG (Fig. 1e) in plasma. Immunization with tetrameric rNA induced significantly higher levels of rNA- and Cal7-specific IgG than did that with monomeric rNA (Fig. 1d and e). In addition, we challenged immunized mice with homologous Cal7 after the last immunization to induce a lower respiratory tract infection and then assessed their body weights (Fig. 1f) and survival (Fig. 1g). Mice treated with either phosphate-buffered saline (PBS; controls) or immunized with monomeric rNA rapidly lost body weight, and more than half of these mice died within 10 days after Cal7 challenge (Fig. 1f and g). In contrast, all of the mice immunized with tetrameric rNA survived without loss of body weight (Fig. 1f and g). These results suggest that the antigenicity of tetrameric rNA is stronger than that of monomeric rNA. Therefore, we focused on, and used, tetrameric rNA as a vaccine antigen for immunization and as a coating antigen for ELISA in subsequent experiments.
Potential of NA as an antigen for a parenteral vaccine.
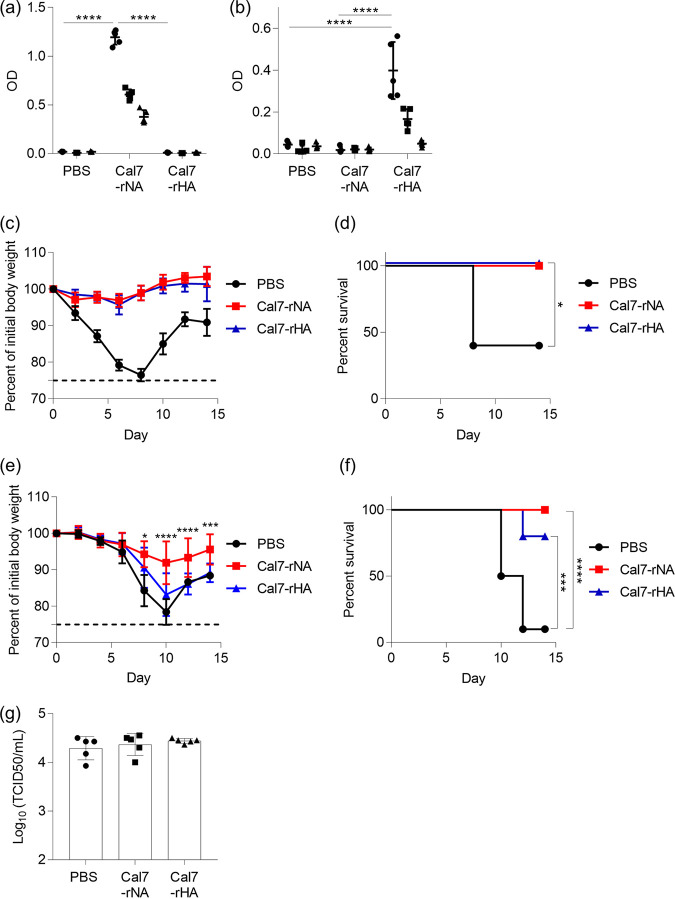
To compare the antigenicities of rNA and rHA, we immunized mice subcutaneously with 1 μg/mouse rNA from Cal7 (i.e., Cal7-rNA) or 1 μg/mouse rHA from Cal7 (i.e., Cal7-rHA) by using alum and used ELISA to examine the levels of Cal7-rNA- and Cal7-rHA-specific IgG in plasma (Fig. 2a and b). Immunization with Cal7-rNA induced high levels of Cal7-rNA-specific IgG (Fig. 2a), and immunization with rHA induced high levels of Cal7-rHA-specific IgG (Fig. 2b). After the final immunization, we challenged the immunized mice with homologous Cal7 to achieve lower respiratory tract infection (Fig. 2c and d). Neither the Cal7-rNA- nor Cal7-rHA-immunized mice lost weight, and all mice survived in both groups, whereas the PBS-treated control mice lost weight, and 60% of them died within 10 days after Cal7 challenge (Fig. 2c and d).
FIG 2.
Comparison of rNA and rHA from Cal7 as vaccine antigens via subcutaneous immunization. Mice were immunized subcutaneously with Cal7-rNA plus alum or Cal7-rHA (a to d, 1 μg/mouse; e to g, 10 μg/mouse) plus alum. Plasma levels of (a) Cal7-rNA- and (b) Cal7-rHA-specific IgG were evaluated by ELISA of 160-fold (●), 800-fold (■), and 4,000-fold (▴) diluted samples. At 10 days after the final immunization, mice were challenged with (c and d) Cal7 (homologous virus) or (e and f) NC20 (heterologous virus) to achieve lower respiratory tract infection. The percent changes in (c and e) body weight and (d and f) survival were monitored after challenge with viruses. (g) At 10 days after the final immunization, mice were challenged with Cal7 (homologous virus) to cause upper respiratory tract infection. At 3 days after challenge, virus titers in nasal wash samples were evaluated. n = 5 (a to d and g) or 10 (e and f) per group. (a to c, e, and g) Data are means ± SD. (a and b) Significant differences (****, P < 0.0001; Tukey’s test) were analyzed only for the 160-fold-diluted samples. (d) *, P < 0.05 versus PBS-treated control mice, according to comparison of Kaplan-Meier curves by using the log-rank test. (e) *, P < 0.05; ***, P < 0.001; and ****, P < 0.0001, between Cal7-rNA-immunized mice and Cal7-rHA-immunized mice, as indicated by using Tukey’s test. (f) ***, P < 0.001, and ****, P < 0.0001, according to comparison of Kaplan–Meier curves by using the log-rank test.
To compare the cross-protective activity of rNA and rHA, we immunized mice subcutaneously with 10 μg/mouse Cal7-rNA or Cal7-rHA by using alum and then challenged them with heterologous H1N1 A/New Caledonia/20/1999 (NC20) after the final immunization to achieve lower respiratory tract infection (Fig. 2e and f). All of the Cal7-rNA-immunized and 80% of the Cal7-rHA-immunized mice survived; in contrast, the PBS-treated control mice lost considerable weight, and only 10% of the PBS-treated control mice survived after NC20 challenge (Fig. 2e and f). Furthermore, weight loss in the Cal7-rNA-immunized mice was significantly milder than that in Cal7-rHA-immunized mice (Fig. 2e and f). To assess protection against influenza infection in the upper respiratory tract, mice underwent subcutaneous immunization with 10 μg/mouse Cal7-rNA or Cal7-rHA with alum (Fig. 2g) and then were challenged with homologous virus (i.e., Cal7). Virus titers in nasal wash fluid on day 3 after challenge were similar among Cal7-rNA-immunized, Cal7-rHA-immunized, and PBS-treated (control) mice (Fig. 2g). These results suggest that subcutaneous immunization of rNA induces stronger cross-protection than rHA during lower respiratory tract infection, but the two antigens did not induce protection during upper respiratory tract infection.
Superiority of NA as an antigen for nasal vaccines.
Many studies have instilled large volumes (i.e., 20 to 30 μl/mouse) of vaccine-containing solution as nasal vaccines. However, because vaccine solution is transferred not only to the upper respiratory tract but also to the lower respiratory tract under these conditions, the experimental scenario might not have accurately reflected the actual situation in humans. Therefore, to compare the potential of rNA and rHA as antigens for nasal vaccines, we intranasally administered rNA or rHA to each mouse in a total of 5 μl (2.5 μl for each nostril), in a model of a nasal vaccine. In addition, we used cyclic di-GMP (c-di-GMP), an intracellular receptor stimulator of interferon genes (STING) agonist, as an adjuvant, because STING agonists have been used as nasal vaccine adjuvants in many experiments (44–46).
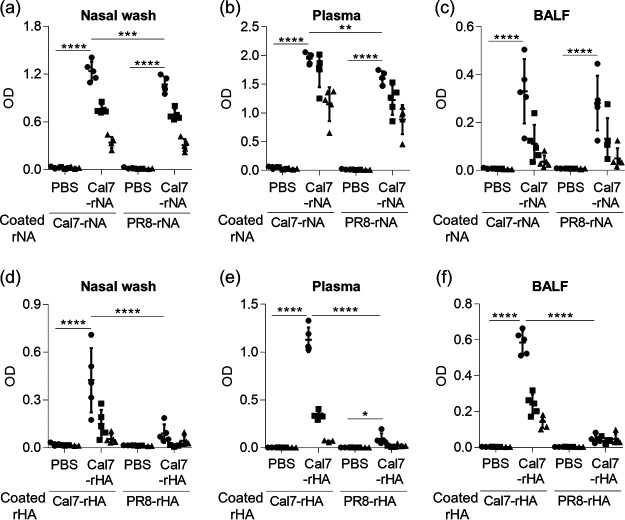
Mice were immunized intranasally with Cal7-rNA plus c-di-GMP (Fig. 3a to c) or Cal7-rHA plus c-di-GMP (Fig. 3d to f), and the levels of rNA- and rHA-specific IgA in nasal wash fluid, rNA- and rHA-specific IgG in plasma, and rNA- and rHA-specific IgG in bronchoalveolar lavage fluid (BALF) were analyzed by using ELISA after the last immunization (Fig. 3a to f). We used Cal7-rNA and PR8-rNA (Fig. 3a to c) and Cal7-rHA and PR8-rHA (Fig. 3d to f) as coating antigens for ELISA. Immunization with Cal7-rNA induced high levels of Cal7-rNA-specific antibody responses (Fig. 3a to c), and immunization with Cal7-rHA induced high levels of Cal7-rHA-specific antibodies (Fig. 3d to f). For example, Cal7-specific IgAs were observed in nasal washes from both Cal7-rNA- and Cal7-rHA-immunized mice (Fig. 3a and d). In addition, the level of antibodies specific for rNA from the heterologous virus H1N1 A/Puerto Rico/8/34 (PR8; i.e., PR8-rNA) was significantly higher in mice immunized with Cal7-rNA than in PBS-treated control mice (Fig. 3a to c). In contrast, PBS-treated control mice and Cal7-rHA-immunized mice had similar levels of PR8-rHA-specific IgA in nasal wash fluid and PR8-rHA-specific IgG in BALF (Fig. 3d and f), whereas Cal7-rHA-immunized mice had slightly (yet significantly) higher PR8-rHA-specific IgG in plasma than PBS-treated control mice (Fig. 3e). These results suggest that the binding capacity for heterologous antigen is broader for NA-specific antibodies than HA-specific antibodies.
FIG 3.
Antibody responses after intranasal immunization with rNA or rHA. Mice were immunized intranasally with Cal7-rNA (5 μg/mouse) plus c-di-GMP or Cal7-rHA (5 μg/mouse) plus c-di-GMP. (a to c) The levels of Cal7-rNA-specific and PR8-rNA-specific (a) IgA in nasal wash fluid, (b) IgG in plasma, and (c) IgG in BALF from Cal7-rNA-immunized mice were evaluated by using ELISA at 7 days after final immunization. (d to f) The levels of Cal7-rHA-specific and PR8-rHA-specific (d) IgA in nasal wash fluid, (e) IgG in plasma, and (f) IgG in BALF from Cal7-rHA-immunized mice were evaluated by using ELISA at 7 days after the final immunization. We used (a and d) 1-fold (●), 2-fold (■), and 4-fold (▴) dilutions of nasal wash samples, (b and e) 160-fold (●), 800-fold (■), and 4,000-fold (▴) dilutions of plasma samples, and (c and f) 5-fold (●), 25-fold (■), and 125-fold (▴) dilutions of BALF samples. (a to f) Data are means ± SD. n = 5 per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001; and ****, P < 0.0001, as indicated by using Tukey’s test.
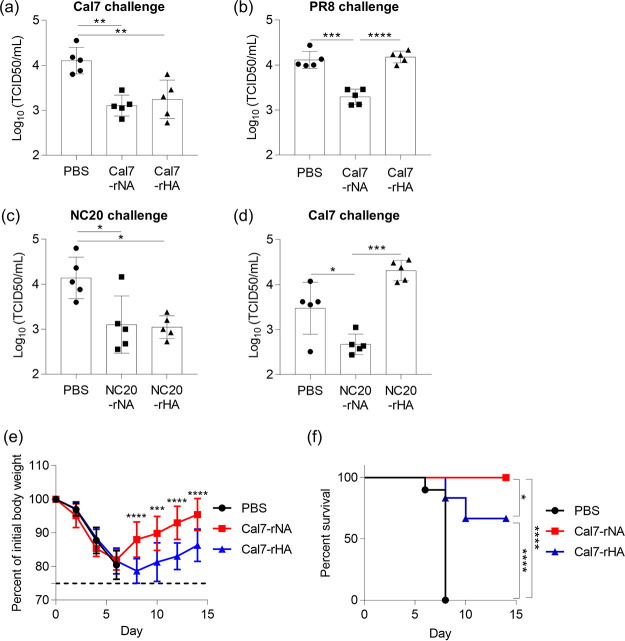
After the final immunization in our nasal vaccine model, we challenged immunized mice with Cal7 (i.e., homologous virus) or PR8 (i.e., heterologous virus) and determined the virus titers in nasal wash fluid on day 3 after challenge (Fig. 4a and b). The Cal7 virus titers in the Cal7-rNA- and Cal7-rHA-immunized mice were significantly lower than those in the PBS-treated control mice, and Cal7 virus titers did not differ between Cal7-rNA- and Cal7-rHA-immunized mice (Fig. 4a). In contrast, the titer of the heterologous PR8 virus was significantly lower in the Cal7-rNA-immunized mice than in both the PBS-treated controls and the Cal7-rHA-immunized mice, which had similar PR8 titers (Fig. 4b).
FIG 4.
Protective effects against influenza virus after intranasal immunization with rNA or rHA. Mice were immunized intranasally with (a, b, e, and f) Cal7-rNA plus c-di-GMP or Cal7-rHA (5 μg/mouse) plus c-di-GMP or (c and d) NC20-rNA plus c-di-GMP or NC20-rHA (5 μg/mouse) plus c-di-GMP. At 10 days after final immunization, mice were challenged with (a) homologous Cal7, (b) heterologous PR8, (c) homologous NC20, or (d) heterologous Cal7 to achieve upper respiratory tract infection. (a to d) At 3 days after challenge, virus titers in nasal wash samples were evaluated. (e and f) At 10 days after the final immunization, mice were challenged with Cal7 (homologous virus) to achieve lower respiratory tract infection. The percentage changes in (e) body weight and (f) survival were monitored after challenge with virus. n = 5 (a to d) or 10 (e and f) per group. (a to e) Data are means ± SD. (a to d) *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, according to Tukey’s test. (e) ***, P < 0.001, and ****, P < 0.0001, between Cal7-rNA-immunized mice and Cal7-rHA-immunized mice, as indicated by using Tukey’s test. (f) *, P < 0.05, and ****, P < 0.0001, according to comparison of Kaplan-Meier curves by using the log-rank test.
Next, to assess the versatility of rNA as an antigen for nasal vaccines, we immunized mice intranasally with NC20-rNA plus c-di-GMP or NC20-rHA plus c-di-GMP. We then challenged the immunized mice with homologous NC20 or heterologous Cal7 in our upper respiratory infection model and measured the virus titers in nasal wash fluid on day 3 after challenge (Fig. 4c and d). Consistent with the results in Fig. 4a, homologous NC20 titers were significantly lower in the NC20-rNA- and NC20-rHA-immunized mice than in the PBS-treated control mice, with similar NC20 virus titers in NC20-rNA- and NC20-rHA-immunized mice (Fig. 4c). Furthermore, titers of the heterologous Cal7 virus were significantly lower in the NC20-rNA-immunized mice than in both the PBS-treated controls and NC20-rHA-immunized mice, which had similar Cal7 titers (Fig. 4d). Collectively, these results suggest that rNA has potential superior to that of rHA as an antigen for nasal vaccines.
We then examined whether intranasal immunization with rNA provides protection in the lower respiratory tract as it does in the upper respiratory tract. To this end, we immunized mice intranasally with Cal7-rNA plus c-di-GMP or Cal7-rHA plus c-di-GMP and then challenged them with homologous Cal7 after the final immunization to achieve lower respiratory tract infection. The weight loss in the Cal7-rNA-immunized mice was significantly milder than that in Cal7-rHA-immunized mice, and the survival rate was significantly higher in Cal7-rNA-immunized mice than in Cal7-rHA-immunized mice (Fig. 4e and f). These results suggest that rNA-based nasal vaccine provides protection superior to that of rHA-based nasal vaccine in both the upper and lower respiratory tracts.
Nasal vaccine for virus infection-experienced mice by using rNA without adjuvant.
Almost all human adults have preexisting antibodies to influenza viruses owing to previous exposure to seasonal influenza viruses (11, 30, 47). Therefore, we examined whether rNA induces antibody responses even in the absence of adjuvants in this situation. On 30 and 51 days after naive mice had been infected with Cal7 to achieve upper respiratory tract infection, they were immunized intranasally with Cal7-rNA or Cal7-rHA without adjuvant. At 58 days after Cal7 infection, the levels of Cal7-rNA-specific IgA in nasal wash fluid (Fig. 5a) and Cal7-rNA-specific IgG in plasma (Fig. 5b) were significantly higher in Cal7-infected mice than uninfected control mice, whereas the levels of Cal7-rHA-specific IgA in nasal wash fluid (Fig. 5c) and Cal7-rHA-specific IgG in plasma (Fig. 5d) did not differ between uninfected control mice and Cal7-infected mice. Subsequent immunization with rNA—but not rHA—enhanced these antibody responses in Cal7-infected mice (Fig. 5a to d). After the Cal7-infected mice were immunized with Cal7-rNA or Cal7-rHA, they were challenged with heterologous PR8 to achieve upper respiratory tract infection. At day 3 after challenge, PR8 virus titers were significantly lower in unimmunized preinfected mice than in mice without preinfection (Fig. 5e). In addition, PR8 virus titers were significantly lower in Cal7-rNA-immunized preinfected mice than in unimmunized preinfected mice, whereas PR8 virus titers were similar between Cal7-rHA-immunized preinfected mice and unimmunized preinfected mice (Fig. 5e). These data suggest that intranasal immunization with rNA, but not rHA, without adjuvant enhances NA-specific antibody responses and provides cross-protection in virus-experienced mice.
FIG 5.
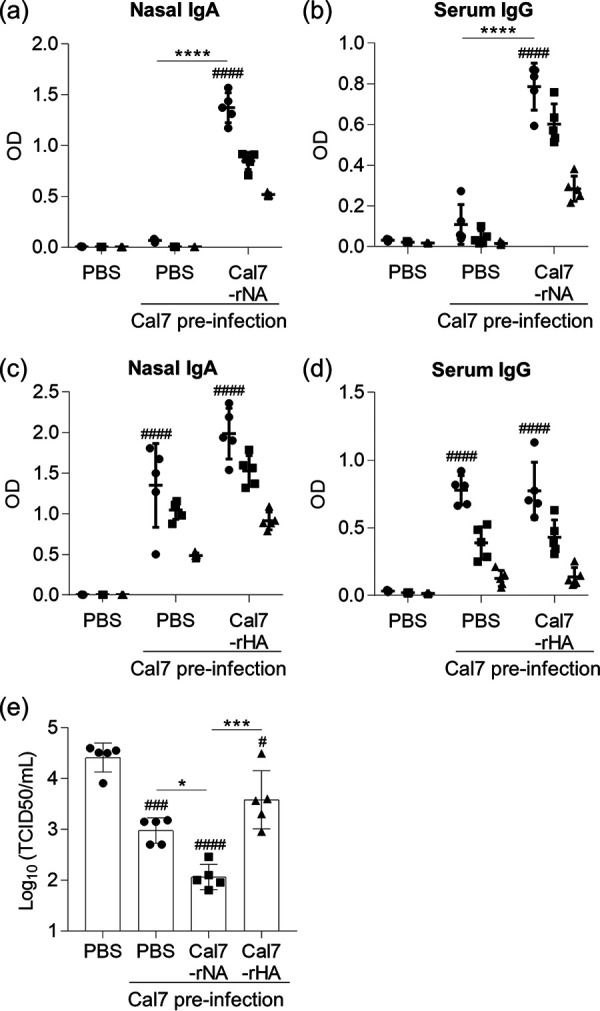
Intranasal immunization of preinfected mice by using NA without adjuvant. (a to e) Naive mice were infected intranasally with Cal7. On days 30 and 51 after exposure, mice were intranasally immunized with Cal7-rNA or Cal7-rHA (5 μg/mouse) without adjuvant. The levels of (a) Cal7-rNA-specific IgA in nasal wash fluid, (b) Cal7-rNA-specific IgG in plasma, (c) Cal7-rHA-specific IgA in nasal wash fluid, and (d) Cal7-rHA-specific IgG in plasma were evaluated by using ELISA at 7 days after final immunization. We used (a and c) 1-fold (●), 2-fold (■), and 4-fold (▴) dilutions of nasal wash samples and (b and d) 160-fold (●), 800-fold (■), and 4,000-fold (▴) fold dilutions of plasma samples. (e) At 10 days after final immunization, mice were challenged with heterologous virus (PR8) to achieve upper respiratory tract infection; virus titers in nasal wash samples were evaluated at 3 days after challenge. (a to e) Data are means ± SD. n = 5 per group. ##, P < 0.01; ###, P < 0.001; and ####, P < 0.0001, versus uninfected control group as indicated by Tukey’s test. *, P < 0.05; ***, P < 0.001; and ****, P < 0.0001, according to Tukey’s test.
Broad epitope recognition by NA-specific IgA in nasal wash fluid.
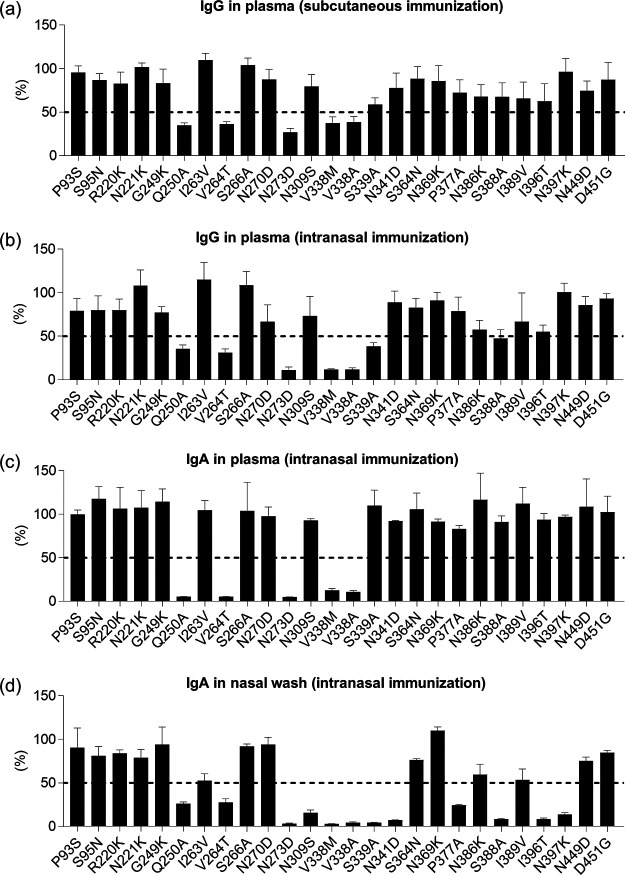
To clarify why intranasal immunization with rNA induces strong cross-protection, we sought to identify the epitopes recognized by the antibodies induced by rNA immunization. To this end, we used mutant rNAs based on the Cal7 NA sequence, each of which bore one of the 26 single-amino-acid escape mutations in common antibody-binding sites on NA (11). Mutant rNAs were generated in mammalian cells, purified by using immobilized metal ion and size exclusion chromatography, and used as coating antigens for ELISA. We confirmed that each mutant rNA had the same elution time as wild-type rNA in size exclusion chromatography (data not shown), indicating that these mutant rNAs were tetrameric. By using ELISA, we examined the levels of mutant-rNA-specific IgA and IgG in samples from mice immunized with wild-type Cal7-rNA (Fig. 6). Mutant rNAs that reduced the ELISA signal by 50% relative to that for wild-type rNA were defined as mutant rNAs carrying epitopes recognized by antibodies. The IgG in plasma from mice subcutaneously immunized with rNA plus alum showed >50% weaker binding to the Q250A, V264T, N273D, V338M, and V338A mutant rNAs than to wild-type rNA (Fig. 6a). We observed the same trends for IgG (Fig. 6b) and IgA (Fig. 6c) in plasma from mice immunized intranasally with Cal7-rNA plus c-di-GMP. In contrast, IgA in nasal wash fluid from mice immunized intranasally with Cal7-rNA plus c-di-GMP showed >50% weaker binding than wild-type rNA, not only to Q250A, V264T, N273D, V338M, V338A, and S339A mutant rNAs but also to N309S, N341D, P377A, S388A, I396T, and N397K mutant rNAs (Fig. 6d). These results indicate that NA-specific IgA in nasal wash fluid recognizes more epitopes than do NA-specific IgG and IgA in plasma.
FIG 6.
Identification of epitopes recognized by anti-NA antibodies. Levels of mutant rNA-specific (a) IgG in plasma from mice immunized subcutaneously with Cal7-rNA plus alum and of mutant rNA-specific (b) IgG in plasma, (c) IgA in plasma, and (d) IgA in nasal wash fluid from mice immunized intranasally with Cal7-rNA plus c-di-GMP were evaluated by using ELISA. The ELISA signals for mutant rNAs relative to that for wild-type rNA are shown as percentages. Data are means ± SD.
DISCUSSION
In assessing the potential of NA as an antigen in nasal vaccines to confer broad cross-protection against influenza viruses, we first showed that monomeric rNA lacks sialidase activity and the ability to induce NA-specific antibody responses (Fig. 1). Whether the structure of monomeric rNA is the same as that of the protomer of tetrameric rNA is unclear. In addition, compared with monomeric antigen, multimerization of antigen generally enhances B-cell-receptor cross-linking, which is followed by strong B-cell activation and an increased antigen-specific antibody response (48). Therefore, as we showed, tetramerization of rNA is required to induce appropriate immune responses.
We also demonstrated that subcutaneous immunization with either rHA or rNA provides full protection against homologous virus challenge and that rNA induces cross-protection against heterologous virus in the lower respiratory tract more efficiently than does rHA (Fig. 2a to f). However, a subcutaneous dose of 10 μg rNA was required to protect against heterologous virus challenge, although 1 μg rNA achieved complete protection against homologous virus. In addition, even when high antigen doses were administered subcutaneously, the protective efficacy against heterologous virus was lower than that against homologous virus (Fig. 2c to f). These results are consistent with those of another report, which showed that challenge with high-titer heterologous virus diminished the protective efficacy induced by NA immunization (25). Therefore, it might be difficult to induce full protection against heterologous virus challenge through subcutaneous immunization with rNA.
Replication of influenza virus in the upper respiratory tract—especially the nasal respiratory epithelium—is crucial for airborne transmission of influenza viruses (35). Therefore, vaccines that prevent the generation of influenza viruses in the upper respiratory tract and their expulsion from those tissues are essential for reducing the rapid spread and continued circulation of these viruses in humans. In the current study, we showed that subcutaneous immunization with rNA or rHA failed to protect against even homologous virus challenge in the upper respiratory tract (Fig. 2g). In contrast, unlike intranasal administration of rHA, intranasal immunization with rNA provided superior cross-protection in the upper respiratory tract by inducing NA-specific IgA (Fig. 4). In particular, the protection conferred in the upper respiratory tract via intranasal immunization with rNA was as effective as that against homologous virus challenge (Fig. 4). We showed here that nasal rNA vaccine induced high titers of the NA-specific IgA in nasal wash fluid to both homologous and heterologous NAs, whereas titers of HA-specific IgAs to heterologous HA were much lower than those to homologous HA (Fig. 3a and d). Consequently, we attribute the strong cross-protection in NA-immunized mice to the broader binding capacity of NA-specific antibodies compared with HA-specific antibodies. One study showed that, compared with intramuscular immunization, nasal immunization with rNA more efficiently prevented the interhost transmission of influenza B virus from vaccinated guinea pigs to unvaccinated animals (29). Therefore, as an antigen for nasal vaccines, NA has the potential to confer strong cross-protection and thus limit influenza virus infection and transmission in the population. Previous reports have shown that parenteral immunization of mice with NA confers cross-protection against challenge with heterologous virus but not heterosubtypic virus (25, 49). Further investigation is needed to establish whether intranasal immunization with rNA provides cross-protection against heterosubtypic viruses in the upper respiratory tract.
In the absence of the sialidase activity of NA, influenza virus tends to aggregate, and this may decrease transmissibility (7, 50). In addition, inhibition of the sialidase activity of NA blocks the transport of the virus through mucus and the release of virus from the host cell (7, 8). However, the low sensitivity of the ELLA may have prevented us from observing any NA-inhibitory activity of NA-specific IgA in the upper respiratory tract. Further investigation is needed to examine the NA-inhibition activity of NA-specific IgA against both homologous NA and heterologous NA and to assess the involvement of NA-inhibitory activity of NA-specific IgA in cross-protection in the upper respiratory tract.
We wondered why intranasal immunization with rNA induces strong cross-protection in the upper respiratory tract, whereas subcutaneous immunization with rNA achieves only weak cross-protection. We found that NA-specific IgA in nasal wash fluid recognizes more epitopes in NA than do NA-specific IgA and IgG in plasma (Fig. 6). Therefore, the broad binding capacity of the NA-specific IgA in the upper respiratory tract might contribute to the extensive cross-protection at this site. Previous studies have revealed antibody-recognized amino acid residues in NA that are important for conferring cross-protection (16, 18). For example, by using monoclonal antibodies with differing reactivity, one group showed that highly cross-reactive antibodies recognized residues 273, 309, 338, and 339 of H1N1 NA and that such antibodies conferred cross-protection against challenge with several types of H1N1 in mice (16). Another report showed that Cal7-NA-specific antibodies recognized residues 364, 369, and 397, which are located within a polypeptide chain that encircles the enzyme active-site pocket (18). Among these previously identified residues, only our NA-specific IgA in nasal wash fluid recognized residues 309, 396, and 397, whereas residues 273, 338, and 339 were recognized not only by NA-specific IgA in nasal wash fluid but also by NA-specific IgA and IgG in plasma (Fig. 6). Furthermore, our results showed that NA-specific IgA in nasal wash fluid also recognized residues 377 and 388, which have not been mentioned previously in this regard (Fig. 6). Therefore, further investigation is needed to elucidate whether residues 309, 377, 388, 396, and 397 contribute to the broad cross-protective capacity of IgA in the upper respiratory tract.
It is interesting that NA-specific IgA in nasal wash fluid recognized a broader range of epitopes than did NA-specific IgA in plasma (Fig. 6). Secretory IgA antibodies at mucosal surfaces typically are present in polymeric forms, such as dimers and tetramers, whereas plasma IgA is primarily present as a monomer (51). The stronger virus-binding and -neutralizing activities of polymeric IgA compared with monomeric IgA are due to increased avidity (51–54). In addition, influenza virus–specific polymeric IgA has been suggested to have stronger cross-protective activity than monomeric IgA and IgG (39, 55–57), although the precise mechanism is unclear. Furthermore, a recent study demonstrated that polymerization of IgA enhances reactivity against viruses when the monomeric form of the IgA has low affinity (58). Considering these facts, IgA specific for residues 309, 377, 388, 396, and 397 present in the upper airways might show enhanced affinity due to polymerization, whereas monomeric plasma IgA specific for these residues expresses low affinity. Future studies that address the affinity to NA and cross-reactive activity of IgG, monomeric IgA, and polymeric IgA monoclonal antibodies specific for these residues will be useful for understanding the mechanism underlying the cross-protective capacity of IgA in the upper respiratory tract.
We also showed that intranasal immunization with rNA provides protection in the lower respiratory tract (Fig. 4e and f). However, unlike subcutaneous immunization (Fig. 2c and d), intranasal immunization with rNA was associated with body weight loss in mice even after homologous virus challenge (Fig. 4e and f). In our lower respiratory tract mouse model, viruses disseminate throughout the respiratory tract (including both upper and lower respiratory tracts); conversely, in humans, viruses are amplified in the upper respiratory tract and then migrate to the lower respiratory tract. Therefore, we consider that this lower respiratory tract mouse model is not optimal for evaluating the efficacy of nasal vaccine in the lower respiratory tract. In addition, H1N1 viruses such as those we used (i.e., Cal7 and PR8) do not disseminate efficiently from the upper respiratory tract to the lower respiratory tract after infection in mice (59). In contrast, specific H3N2 strain, X31 strain, efficiently migrates into the lower respiratory tract of mice after upper respiratory tract infection (60). Therefore, it will be important in the future to use this H3N2 strain to evaluate the protective efficacy of rNA nasal vaccine in the lower respiratory tract.
Many split vaccines and inactivated whole-virus vaccines only poorly induce anti-NA antibodies in humans. In fact, in one study, administering subunit or split vaccines to humans resulted in antibody responses directed predominantly to HA, although natural human influenza infection induces a variety of broadly reactive antibodies directed to NA (11). A predominant immune response in favor of HA over NA might result from insufficient content or structural integrity of NA when both proteins are in close association, as is the case in current split vaccines and whole-virion vaccines (7). Adding rNA to split vaccines or inactivated whole-virion vaccines for intranasal administration is one potential approach to enhancing NA-specific antibody responses to these formulations.
In our current study, we used c-di-GMP as an adjuvant for intranasal immunization, because intranasal inoculation of rNA in the absence of adjuvant did not induce any antibody responses in naive mice (data not shown). In general, several types of adjuvants, including c-di-GMP, CpG oligonucleotides, and poly(I·C), have been used in animal studies of nasal vaccines and induce strong antigen-specific antibody responses, including IgA, in the upper respiratory tract (61, 62). However, given the lack of adjuvants approved for nasal vaccines in humans, nasal vaccines that induce sufficient antibody responses even in the absence of adjuvants are needed. In this study, we showed that intranasal inoculation with rNA of mice that had experienced previous influenza infection efficiently enhanced antibody responses against NA, even in the absence of adjuvant, and improved cross-protection (Fig. 5). Using antigen only (without adjuvant) can boost B-cell and memory T-cell responses, although it is insufficient to prime naive mice (63, 64). However, in humans, almost all adults have been infected with, and mount immune responses to, influenza viruses (11, 30, 47). Therefore, rNA alone (in the absence of adjuvants) likely can be used as an antigen in nasal vaccines to boost antibody responses specific to NA in human adults, who likely have already been primed through infection or vaccination.
In addition to recombinant protein, several types of NA-based formulations have been developed as parenteral vaccines to provide cross-protection. For example, virus-like particles that express NA, NA mRNAs, and virus vectors expressing NA all induce anti-NA antibodies in mice (24, 65–67). Furthermore, recent reports have demonstrated the usefulness of a reverse-genetics approach to generating influenza virus carrying a modified NA (68, 69). For example, one group was able to improve anti-NA immune responses by using modified influenza virus particles that expressed increased surface amounts of NA and decreased HA (69). In another study, extending the stalk domain of the NA protein on the surface of virus particles improved its immunogenicity without affecting the immunogenicity of HA (68). Applying these NA-associated strategies to nasal influenza vaccine candidates likely will improve their efficacy.
MATERIALS AND METHODS
Influenza viruses.
H1N1 influenza A virus strain A/Puerto Rico/8/34 (PR8) was kindly provided by Yasuyuki Gomi (Research Foundation for Microbial Diseases, Osaka University, Japan). A/California/7/2009 (Cal7) was kindly provided Hideki Asanuma (National Institute of Infectious Diseases, Japan), and A/New Caledonia/20/1999 (NC20) was kindly provided by Ritsuko Kubota-Koketsu (Research Institute for Microbial Diseases, Osaka University, Japan).
Expression and purification of rHAs and rNAs.
The sequences for HAs and NAs were derived from Cal7 (GenBank accession number ACV82259.1 for HA and MN596847.1 for NA) and NC20 (GenBank accession number AAP34324.1 for HA and AJ518092.1 for NA). The cDNA of the ectodomain of HA (amino acids 1 to 523) with a C-terminal hexahistidine tag (His tag) or of NA (amino acids 71 to 469) with an N-terminal His tag was cloned into the pcDNA3.1 expression plasmid (Thermo Fisher Scientific, Hampton, NH, USA). The sequence of foldon (GYIPEAPRDGQAYVRKDGEWVLLSTFL) from fibritin of bacteriophage T4 was inserted at the C terminus of HA for generating trimeric rHA. The sequence of the tetrabranchion tetramerization domain (GSIINETADDIVYRLTVIIDDRYESLKNLITLRADRLEMIINDNVSTILASG) from the bacterium Staphylothermus marinus was inserted at the N terminus of NA for generating tetrameric rNA. All secreted soluble rHAs and rNAs were generated by using the Expi293 expression system (Thermo Fisher Scientific) as described previously (43, 70, 71). For size exclusion chromatography, the Akta explorer chromatography system with a Superose 6 Increase 10/300 GL column (GE Healthcare, Chicago, IL, USA) was used. For SDS-PAGE, purified proteins were mixed 1:1 (vol/vol) in sample buffer solution (Nacalai Tesque, Kyoto, Japan) containing 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA) and heated at 95°C for 5 min before being loaded onto a 10% Mini-Protean TGX precast protein gel (Bio-Rad, Hercules, CA, USA). After electrophoresis, gels were stained with Coomassie brilliant blue according to standard protocols.
ELLA.
To determine the sialidase activity of rNAs, ELLA was performed according to previously published protocols (43). Briefly, ELISA plates (Corning, Corning, NY, USA) were coated overnight at 4°C with 25 μg/ml fetuin (Sigma-Aldrich, St. Louis, MO, USA) in carbonate buffer. Fetuin-coated plates were washed by using PBS containing 0.05% Tween 20, after which serial dilutions of each rNA were added to plate wells. Plates were incubated at 37°C for 16 h and washed by using PBS containing 0.05% Tween 20; 1 μg/ml peanut agglutinin-horseradish peroxidase conjugate (Sigma-Aldrich) was then added. After incubation of the plates at room temperature for 1 h, the color reaction was developed with tetramethyl benzidine (Nacalai Tesque), stopped with 2 N H2SO4, and measured as the optical density at 450 (OD450) on a microplate reader (Power Wave HT, BioTek, Winooski, VT, USA).
Mice.
C57BL/6J mice were purchased from SLC (Shizuoka, Japan). Mice were housed in a room with a 12-h/12-h light/dark cycle (lights on, 8:00 a.m.; lights off, 8:00 p.m.) and had unrestricted access to food and water. All animal experiments were performed in accordance with Osaka University’s institutional guidelines for the ethical treatment of animals (protocol numbers H26-11-0 and R01-15-0).
Immunization.
For subcutaneous immunization, mice were inoculated at the base of the tail on days 0 and 21 with rHA (1 or 10 μg/mouse) or rNA (1 or 10 μg/mouse) plus Alhydrogel 2% (InvivoGen, San Diego, CA, USA) as alum adjuvant. For intranasal immunization, anesthetized mice were intranasally inoculated on days 0 and 21 with rHA (5 μg/mouse) plus c-di-GMP (2 μg/mouse; InvivoGen) or rNA (5 μg/mouse) plus c-di-GMP (2 μg/mouse; InvivoGen) in a total volume of 5 μl (2.5 μl to each nostril). On day 28, we obtained samples of plasma, nasal wash fluid, and BALF, which were stored at −30°C until analysis. Nasal washes were collected by gentle flushing of the nasal passage with 400 μl of PBS. BALF was collected by subjecting the lung to lavage with 1 ml of PBS; BALF was centrifuged at 600 × g for 5 min and the supernatant used for ELISA.
Antibody responses.
To detect rHA-, rNA-, or Cal7-specific IgG and IgA, ELISA plates were coated overnight at 4°C with rHA or rNA (1 μg/ml for plasma and BALF; 10 μg/ml for nasal wash fluid) in carbonate buffer, or Cal7 (1 μg/ml) in PBS. Coated plates were then incubated with 1% Block Ace (DS Pharma Biomedical, Osaka, Japan) for 2 h at room temperature. Plasma, BALF, and nasal wash fluid samples were diluted serially with 0.4% Block Ace, and these dilutions were added to the antigen-coated plates. After incubation for 2 h at room temperature, sample-containing plates were incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Merck Millipore, Darmstadt, Germany) or HRP-conjugated goat anti-mouse IgA (SouthernBiotech, Birmingham, AL, USA) for 1 h at room temperature or with biotin-conjugated goat anti-mouse IgA (SouthernBiotech) for 2 h at room temperature, after which HRP-conjugated streptavidin (Thermo Fisher Scientific) was added. The color reaction was developed with tetramethyl benzidine (Nacalai Tesque), stopped with 2 N H2SO4, and measured as the OD450 to OD570 on a microplate reader (Power Wave HT, BioTek).
Infection.
Immunized mice were challenged with influenza virus on day 31 after the initial immunization (day 0). For upper respiratory tract infection, anesthetized mice were challenged intranasally with 3.0 × 104 50% tissue culture infective doses (TCID50) of Cal7, 1.2 × 103 TCID50 of PR8, or 9.6 × 104 TCID50 of NC20 in 5 μl of PBS (2.5 μl to each nostril). After 3 days, nasal washes were collected by using 400 μl of PBS, and the virus titers in nasal wash fluid were evaluated by using MDCK cells as described previously (72). For lower respiratory tract infection, anesthetized mice were challenged intranasally with 3.0 × 104 TCID50 of Cal7 or 4.8 × 103 TCID50 of NC20 in 30 μl of PBS (15 μl to each nostril). Body weights and survival rates of the challenged mice were monitored every 2 or 3 days for a total of 14 days after challenge. The humane endpoint was set at 25% body weight loss relative to that on day 0. We defined the day on which the mice weighed less than 75% of their day 0 body weight as the day of death.
Subsequent immunization after Cal7 infection.
Anesthetized naive mice were infected intranasally with 3.0 × 102 TCID50 of Cal7 in a volume of 5 μl of PBS (2.5 μl to each nostril). On days 30 and 51 postinfection, mice were intranasally immunized with Cal7-rHA (5 μg/mouse) or Cal7-rNA (5 μg/mouse) without c-di-GMP in a total volume of 5 μl (2.5 μl to each nostril) under anesthetic. Ten days after final immunization, anesthetized mice were challenged intranasally with 1.2 × 103 TCID50 of PR8 in a volume of 5 μl of PBS (2.5 μl to each nostril). Three days after PR8 challenge, nasal washes were collected by using 400 μl of PBS, and the virus titers in nasal wash fluid were evaluated by using MDCK cells as described previously (72).
Epitope mapping of antibodies by using mutant recombinant NAs.
By using the Expi293 expression system (Thermo Fisher Scientific) as described above, we generated 26 mutant rNAs (P93S, P95N, R220K, N221K, G249K, Q250A, I263V, V264T, S266A, N270D, N273D, N309S, V338M, V338A, S339A, N341D, S364N, N369K, P377A, N386K, S388A, I389V, I396T, N397K, N449D, and D451G mutants) as described previously (43, 70, 71). To detect mutant rNA-specific IgG and IgA through ELISA, we used 1-fold-diluted nasal wash samples for detecting rNA-specific IgA, 5-fold-diluted plasma samples for detecting rNA-specific IgA, and 800-fold-diluted plasma samples for detecting rNA-specific IgG.
Statistical analyses.
Statistical analyses were performed by using Prism (GraphPad Software, San Diego, CA, USA). All data are presented as means with standard deviations (SD). Significant differences were determined by using Tukey’s test. A P value of 0.05 or less was considered to indicate statistical significance.
ACKNOWLEDGMENTS
We thank Yasuyuki Gomi (Research Foundation for Microbial Diseases, Osaka University) for providing H1N1 influenza A virus A/Puerto Rico/8/34, Hideki Asanuma (National Institute of Infectious Diseases, Japan) for providing H1N1 influenza A virus A/California/7/2009, and Ritsuko Kubota-Koketsu (Research Institute for Microbial Diseases, Osaka University) for providing H1N1 influenza A virus A/New Caledonia/20/1999.
This study was supported by grants from the Japan Society for the Promotion of Science (JSPS KAKENHI grant numbers JP17H04009, JP18K19401, and JP20H03404 to Y. Yoshioka) and the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research [BINDS]) from AMED under grant number JP20am0101070 (support number 1737).
Y. Yamamoto and Y. Yoshioka are employed by the Research Foundation for Microbial Diseases (Osaka University). The other authors have no conflicts of interests to declare.
Contributor Information
Yasuo Yoshioka, Email: y-yoshioka@biken.osaka-u.ac.jp.
Stacey Schultz-Cherry, St. Jude Children's Research Hospital.
REFERENCES
- 1.Krammer F, Smith GJD, Fouchier RAM, Peiris M, Kedzierska K, Doherty PC, Palese P, Shaw ML, Treanor J, Webster RG, Garcia-Sastre A. 2018. Influenza. Nat Rev Dis Primers 4:3. 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krammer F, Palese P. 2015. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov 14:167–182. 10.1038/nrd4529. [DOI] [PubMed] [Google Scholar]
- 3.Wei CJ, Crank MC, Shiver J, Graham BS, Mascola JR, Nabel GJ. 2020. Next-generation influenza vaccines: opportunities and challenges. Nat Rev Drug Discov 19:239–252. 10.1038/s41573-019-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouvier NM, Palese P. 2008. The biology of influenza viruses. Vaccine 26(Suppl 4):D49–D53. 10.1016/j.vaccine.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osterholm MT, Kelley NS, Sommer A, Belongia EA. 2012. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 12:36–44. 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman RK, Nowalk MP, Chung J, Jackson ML, Jackson LA, Petrie JG, Monto AS, McLean HQ, Belongia EA, Gaglani M, Murthy K, Fry AM, Flannery B, US Flu VE Investigators. 2016. 2014-2015 influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis 63:1564–1573. 10.1093/cid/ciw635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wohlbold TJ, Krammer F. 2014. In the shadow of hemagglutinin: a growing interest in influenza viral neuraminidase and its role as a vaccine antigen. Viruses 6:2465–2494. 10.3390/v6062465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen M, Zhang XQ, Senaati HP, Chen HW, Varki NM, Schooley RT, Gagneux P. 2013. Influenza A penetrates host mucus by cleaving sialic acids with neuraminidase. Virol J 10:321. 10.1186/1743-422X-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couch RB, Atmar RL, Keitel WA, Quarles JM, Wells J, Arden N, Nino D. 2012. Randomized comparative study of the serum antihemagglutinin and antineuraminidase antibody responses to six licensed trivalent influenza vaccines. Vaccine 31:190–195. 10.1016/j.vaccine.2012.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Getie-Kebtie M, Sultana I, Eichelberger M, Alterman M. 2013. Label-free mass spectrometry-based quantification of hemagglutinin and neuraminidase in influenza virus preparations and vaccines. Influenza Other Respir Viruses 7:521–530. 10.1111/irv.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YQ, Wohlbold TJ, Zheng NY, Huang M, Huang Y, Neu KE, Lee J, Wan H, Rojas KT, Kirkpatrick E, Henry C, Palm AE, Stamper CT, Lan LY, Topham DJ, Treanor J, Wrammert J, Ahmed R, Eichelberger MC, Georgiou G, Krammer F, Wilson PC. 2018. Influenza infection in humans induces broadly cross-reactive and protective neuraminidase-reactive antibodies. Cell 173:417–429.E410. 10.1016/j.cell.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandbulte MR, Westgeest KB, Gao J, Xu X, Klimov AI, Russell CA, Burke DF, Smith DJ, Fouchier RA, Eichelberger MC. 2011. Discordant antigenic drift of neuraminidase and hemagglutinin in H1N1 and H3N2 influenza viruses. Proc Natl Acad Sci USA 108:20748–20753. 10.1073/pnas.1113801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krammer F, Fouchier RAM, Eichelberger MC, Webby RJ, Shaw-Saliba K, Wan H, Wilson PC, Compans RW, Skountzou I, Monto AS. 2018. NAction! How can neuraminidase-based immunity contribute to better influenza virus vaccines? mBio 9:e02332-17. 10.1128/mBio.02332-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang YH, Seong BL. 2019. The quest for a truly universal influenza vaccine. Front Cell Infect Microbiol 9:344. 10.3389/fcimb.2019.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krammer F, Li L, Wilson PC. 2019. Emerging from the shadow of hemagglutinin: neuraminidase is an important target for influenza vaccination. Cell Host Microbe 26:712–713. 10.1016/j.chom.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Wan H, Gao J, Xu K, Chen H, Couzens LK, Rivers KH, Easterbrook JD, Yang K, Zhong L, Rajabi M, Ye J, Sultana I, Wan XF, Liu X, Perez DR, Taubenberger JK, Eichelberger MC. 2013. Molecular basis for broad neuraminidase immunity: conserved epitopes in seasonal and pandemic H1N1 as well as H5N1 influenza viruses. J Virol 87:9290–9300. 10.1128/JVI.01203-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan H, Yang H, Shore DA, Garten RJ, Couzens L, Gao J, Jiang L, Carney PJ, Villanueva J, Stevens J, Eichelberger MC. 2015. Structural characterization of a protective epitope spanning A(H1N1)pdm09 influenza virus neuraminidase monomers. Nat Commun 6:6114. 10.1038/ncomms7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang L, Fantoni G, Couzens L, Gao J, Plant E, Ye Z, Eichelberger MC, Wan H. 2016. Comparative efficacy of monoclonal antibodies that bind to different epitopes of the 2009 pandemic H1N1 influenza virus neuraminidase. J Virol 90:117–128. 10.1128/JVI.01756-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wohlbold TJ, Podolsky KA, Chromikova V, Kirkpatrick E, Falconieri V, Meade P, Amanat F, Tan J, tenOever BR, Tan GS, Subramaniam S, Palese P, Krammer F. 2017. Broadly protective murine monoclonal antibodies against influenza B virus target highly conserved neuraminidase epitopes. Nat Microbiol 2:1415–1424. 10.1038/s41564-017-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walz L, Kays SK, Zimmer G, von Messling V. 2018. Neuraminidase-inhibiting antibody titers correlate with protection from heterologous influenza virus strains of the same neuraminidase subtype. J Virol 92:e01006-18. 10.1128/JVI.01006-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilchuk IM, Bangaru S, Gilchuk P, Irving RP, Kose N, Bombardi RG, Thornburg NJ, Creech CB, Edwards KM, Li S, Turner HL, Yu W, Zhu X, Wilson IA, Ward AB, CroweJE, Jr.. 2019. Influenza H7N9 virus neuraminidase-specific human monoclonal antibodies inhibit viral egress and protect from lethal influenza infection in mice. Cell Host Microbe 26:715–728.E718. 10.1016/j.chom.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stadlbauer D, Zhu X, McMahon M, Turner JS, Wohlbold TJ, Schmitz AJ, Strohmeier S, Yu W, Nachbagauer R, Mudd PA, Wilson IA, Ellebedy AH, Krammer F. 2019. Broadly protective human antibodies that target the active site of influenza virus neuraminidase. Science 366:499–504. 10.1126/science.aay0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendez-Legaza JM, Ortiz de Lejarazu R, Sanz I. 2019. Heterotypic neuraminidase antibodies against different A(H1N1) strains are elicited after seasonal influenza vaccination. Vaccines (Basel) 7:30. 10.3390/vaccines7010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Easterbrook JD, Schwartzman LM, Gao J, Kash JC, Morens DM, Couzens L, Wan H, Eichelberger MC, Taubenberger JK. 2012. Protection against a lethal H5N1 influenza challenge by intranasal immunization with virus-like particles containing 2009 pandemic H1N1 neuraminidase in mice. Virology 432:39–44. 10.1016/j.virol.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wohlbold TJ, Nachbagauer R, Xu H, Tan GS, Hirsh A, Brokstad KA, Cox RJ, Palese P, Krammer F. 2015. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. mBio 6:e02556-14. 10.1128/mBio.02556-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu WC, Lin CY, Tsou YT, Jan JT, Wu SC. 2015. Cross-reactive neuraminidase-inhibiting antibodies elicited by immunization with recombinant neuraminidase proteins of H5N1 and pandemic H1N1 influenza A viruses. J Virol 89:7224–7234. 10.1128/JVI.00585-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith GE, Sun X, Bai Y, Liu YV, Massare MJ, Pearce MB, Belser JA, Maines TR, Creager HM, Glenn GM, Flyer D, Pushko P, Levine MZ, Tumpey TM. 2017. Neuraminidase-based recombinant virus-like particles protect against lethal avian influenza A(H5N1) virus infection in ferrets. Virology 509:90–97. 10.1016/j.virol.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mooney AJ, Gabbard JD, Li Z, Dlugolenski DA, Johnson SK, Tripp RA, He B, Tompkins SM. 2017. Vaccination with recombinant parainfluenza virus 5 expressing neuraminidase protects against homologous and heterologous influenza virus challenge. J Virol 91:e01579-17. 10.1128/JVI.01579-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMahon M, Kirkpatrick E, Stadlbauer D, Strohmeier S, Bouvier NM, Krammer F. 2019. Mucosal immunity against neuraminidase prevents influenza B virus transmission in guinea pigs. mBio 10:e00560-19. 10.1128/mBio.00560-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Couch RB, Atmar RL, Franco LM, Quarles JM, Wells J, Arden N, Nino D, Belmont JW. 2013. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J Infect Dis 207:974–981. 10.1093/infdis/jis935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monto AS, Petrie JG, Cross RT, Johnson E, Liu M, Zhong W, Levine M, Katz JM, Ohmit SE. 2015. Antibody to influenza virus neuraminidase: an independent correlate of protection. J Infect Dis 212:1191–1199. 10.1093/infdis/jiv195. [DOI] [PubMed] [Google Scholar]
- 32.Memoli MJ, Shaw PA, Han A, Czajkowski L, Reed S, Athota R, Bristol T, Fargis S, Risos K, Powers JH, DaveyRT, Jr, Taubenberger JK. 2016. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. mBio 7:e00417-16. 10.1128/mBio.00417-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Riel D, den Bakker MA, Leijten LM, Chutinimitkul S, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. 2010. Seasonal and pandemic human influenza viruses attach better to human upper respiratory tract epithelium than avian influenza viruses. Am J Pathol 176:1614–1618. 10.2353/ajpath.2010.090949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuiken T, Riteau B, Fouchier RA, Rimmelzwaan GF. 2012. Pathogenesis of influenza virus infections: the good, the bad and the ugly. Curr Opin Virol 2:276–286. 10.1016/j.coviro.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Richard M, van den Brand JMA, Bestebroer TM, Lexmond P, de Meulder D, Fouchier RAM, Lowen AC, Herfst S. 2020. Influenza A viruses are transmitted via the air from the nasal respiratory epithelium of ferrets. Nat Commun 11:766. 10.1038/s41467-020-14626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azegami T, Yuki Y, Kiyono H. 2014. Challenges in mucosal vaccines for the control of infectious diseases. Int Immunol 26:517–528. 10.1093/intimm/dxu063. [DOI] [PubMed] [Google Scholar]
- 37.Yusuf H, Kett V. 2017. Current prospects and future challenges for nasal vaccine delivery. Hum Vaccin Immunother 13:34–45. 10.1080/21645515.2016.1239668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ainai A, Suzuki T, Tamura SI, Hasegawa H. 2017. Intranasal administration of whole inactivated influenza virus vaccine as a promising influenza vaccine candidate. Viral Immunol 30:451–462. 10.1089/vim.2017.0022. [DOI] [PubMed] [Google Scholar]
- 39.Tumpey TM, Renshaw M, Clements JD, Katz JM. 2001. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol 75:5141–5150. 10.1128/JVI.75.11.5141-5150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ichinohe T, Tamura S, Kawaguchi A, Ninomiya A, Imai M, Itamura S, Odagiri T, Tashiro M, Takahashi H, Sawa H, Mitchell WM, Strayer DR, Carter WA, Chiba J, Kurata T, Sata T, Hasegawa H. 2007. Cross-protection against H5N1 influenza virus infection is afforded by intranasal inoculation with seasonal trivalent inactivated influenza vaccine. J Infect Dis 196:1313–1320. 10.1086/521304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ichinohe T, Ainai A, Tashiro M, Sata T, Hasegawa H. 2009. PolyI:polyC12U adjuvant-combined intranasal vaccine protects mice against highly pathogenic H5N1 influenza virus variants. Vaccine 27:6276–6279. 10.1016/j.vaccine.2009.04.074. [DOI] [PubMed] [Google Scholar]
- 42.Dai M, Guo H, Dortmans JC, Dekkers J, Nordholm J, Daniels R, van Kuppeveld FJ, de Vries E, de Haan CA. 2016. Identification of residues that affect oligomerization and/or enzymatic activity of influenza virus H5N1 neuraminidase proteins. J Virol 90:9457–9470. 10.1128/JVI.01346-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prevato M, Ferlenghi I, Bonci A, Uematsu Y, Anselmi G, Giusti F, Bertholet S, Legay F, Telford JL, Settembre EC, Maione D, Cozzi R. 2015. Expression and characterization of recombinant, tetrameric and enzymatically active influenza neuraminidase for the setup of an enzyme-linked lectin-based assay. PLoS One 10:e0135474. 10.1371/journal.pone.0135474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen AC, Wilk MM, Misiak A, Borkner L, Murphy D, Mills KHG. 2018. Sustained protective immunity against Bordetella pertussis nasal colonization by intranasal immunization with a vaccine-adjuvant combination that induces IL-17-secreting TRM cells. Mucosal Immunol 11:1763–1776. 10.1038/s41385-018-0080-x. [DOI] [PubMed] [Google Scholar]
- 45.Luo J, Liu XP, Xiong FF, Gao FX, Yi YL, Zhang M, Chen Z, Tan WS. 2019. Enhancing immune response and heterosubtypic protection ability of inactivated H7N9 vaccine by using STING agonist as a mucosal adjuvant. Front Immunol 10:2274. 10.3389/fimmu.2019.02274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Li P, Yu Y, Fu Y, Jiang H, Lu M, Sun Z, Jiang S, Lu L, Wu MX. 2020. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science 367:eaau0810. 10.1126/science.aau0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Couch RB. 2008. Seasonal inactivated influenza virus vaccines. Vaccine 26(Suppl 4):D5–D9. 10.1016/j.vaccine.2008.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veneziano R, Moyer TJ, Stone MB, Wamhoff EC, Read BJ, Mukherjee S, Shepherd TR, Das J, Schief WR, Irvine DJ, Bathe M. 2020. Role of nanoscale antigen organization on B-cell activation probed using DNA origami. Nat Nanotechnol 15:716–723. 10.1038/s41565-020-0719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Z, Kadowaki S, Hagiwara Y, Yoshikawa T, Matsuo K, Kurata T, Tamura S. 2000. Cross-protection against a lethal influenza virus infection by DNA vaccine to neuraminidase. Vaccine 18:3214–3222. 10.1016/s0264-410x(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 50.Palese P, Tobita K, Ueda M, Compans RW. 1974. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology 61:397–410. 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- 51.Gianchecchi E, Manenti A, Kistner O, Trombetta C, Manini I, Montomoli E. 2019. How to assess the effectiveness of nasal influenza vaccines? Role and measurement of sIgA in mucosal secretions. Influenza Other Respir Viruses 13:429–437. 10.1111/irv.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Renegar KB, SmallPA, Jr, Boykins LG, Wright PF. 2004. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol 173:1978–1986. 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki T, Kawaguchi A, Ainai A, Tamura S, Ito R, Multihartina P, Setiawaty V, Pangesti KN, Odagiri T, Tashiro M, Hasegawa H. 2015. Relationship of the quaternary structure of human secretory IgA to neutralization of influenza virus. Proc Natl Acad Sci USA 112:7809–7814. 10.1073/pnas.1503885112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terauchi Y, Sano K, Ainai A, Saito S, Taga Y, Ogawa-Goto K, Tamura SI, Odagiri T, Tashiro M, Fujieda M, Suzuki T, Hasegawa H. 2018. IgA polymerization contributes to efficient virus neutralization on human upper respiratory mucosa after intranasal inactivated influenza vaccine administration. Hum Vaccin Immunother 14:1351–1361. 10.1080/21645515.2018.1438791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takada A, Matsushita S, Ninomiya A, Kawaoka Y, Kida H. 2003. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine 21:3212–3218. 10.1016/s0264-410x(03)00234-2. [DOI] [PubMed] [Google Scholar]
- 56.Maurer MA, Meyer L, Bianchi M, Turner HL, Le NPL, Steck M, Wyrzucki A, Orlowski V, Ward AB, Crispin M, Hangartner L. 2018. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep 23:90–99. 10.1016/j.celrep.2018.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okuya K, Yoshida R, Manzoor R, Saito S, Suzuki T, Sasaki M, Saito T, Kida Y, Mori-Kajihara A, Kondoh T, Sato M, Kajihara M, Miyamoto H, Ichii O, Higashi H, Takada A. 2020. Potential role of nonneutralizing IgA antibodies in cross-protective immunity against influenza A viruses of multiple hemagglutinin subtypes. J Virol 94:e00408-20. 10.1128/JVI.00408-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saito S, Sano K, Suzuki T, Ainai A, Taga Y, Ueno T, Tabata K, Saito K, Wada Y, Ohara Y, Takeyama H, Odagiri T, Kageyama T, Ogawa-Goto K, Multihartina P, Setiawaty V, Pangesti KNA, Hasegawa H. 2019. IgA tetramerization improves target breadth but not peak potency of functionality of anti-influenza virus broadly neutralizing antibody. PLoS Pathog 15:e1007427. 10.1371/journal.ppat.1007427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edenborough KM, Gilbertson BP, Brown LE. 2012. A mouse model for the study of contact-dependent transmission of influenza A virus and the factors that govern transmissibility. J Virol 86:12544–12551. 10.1128/JVI.00859-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pizzolla A, Nguyen THO, Smith JM, Brooks AG, Kedzieska K, Heath WR, Reading PC, Wakim LM. 2017. Resident memory CD8(+) T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol 2:eaam6970. 10.1126/sciimmunol.aam6970. [DOI] [PubMed] [Google Scholar]
- 61.Riese P, Sakthivel P, Trittel S, Guzman CA. 2014. Intranasal formulations: promising strategy to deliver vaccines. Expert Opin Drug Deliv 11:1619–1634. 10.1517/17425247.2014.931936. [DOI] [PubMed] [Google Scholar]
- 62.Takaki H, Ichimiya S, Matsumoto M, Seya T. 2018. Mucosal immune response in nasal-associated lymphoid tissue upon intranasal administration by adjuvants. J Innate Immun 10:515–521. 10.1159/000489405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koyama S, Aoshi T, Tanimoto T, Kumagai Y, Kobiyama K, Tougan T, Sakurai K, Coban C, Horii T, Akira S, Ishii KJ. 2010. Plasmacytoid dendritic cells delineate immunogenicity of influenza vaccine subtypes. Sci Transl Med 2:25ra24. 10.1126/scitranslmed.3000759. [DOI] [PubMed] [Google Scholar]
- 64.Sato K, Takahashi Y, Adachi Y, Asanuma H, Ato M, Tashiro M, Itamura S. 2019. Efficient protection of mice from influenza A/H1N1pdm09 virus challenge infection via high avidity serum antibodies induced by booster immunizations with inactivated whole virus vaccine. Heliyon 5:e01113. 10.1016/j.heliyon.2018.e01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones FR, Gabitzsch ES, Xu Y, Balint JP, Borisevich V, Smith J, Smith J, Peng BH, Walker A, Salazar M, Paessler S. 2011. Prevention of influenza virus shedding and protection from lethal H1N1 challenge using a consensus 2009 H1N1 HA and NA adenovirus vector vaccine. Vaccine 29:7020–7026. 10.1016/j.vaccine.2011.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim KH, Lee YT, Park S, Jung YJ, Lee Y, Ko EJ, Kim YJ, Li X, Kang SM. 2019. Neuraminidase expressing virus-like particle vaccine provides effective cross protection against influenza virus. Virology 535:179–188. 10.1016/j.virol.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freyn AW, Ramos da Silva J, Rosado VC, Bliss CM, Pine M, Mui BL, Tam YK, Madden TD, de Souza Ferreira LC, Weissman D, Krammer F, Coughlan L, Palese P, Pardi N, Nachbagauer R. 2020. A multi-targeting, nucleoside-modified mRNA influenza virus vaccine provides broad protection in mice. Mol Ther 28:1569–1584. 10.1016/j.ymthe.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Broecker F, Zheng A, Suntronwong N, Sun W, Bailey MJ, Krammer F, Palese P. 2019. Extending the stalk enhances immunogenicity of the influenza virus neuraminidase. J Virol 93:e00840-19. 10.1128/JVI.00840-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng A, Sun W, Xiong X, Freyn AW, Peukes J, Strohmeier S, Nachbagauer R, Briggs JAG, Krammer F, Palese P. 2020. Enhancing neuraminidase immunogenicity of influenza A viruses by rewiring RNA packaging signals. J Virol 94:e00742-20. 10.1128/JVI.00742-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei CJ, Xu L, Kong WP, Shi W, Canis K, Stevens J, Yang ZY, Dell A, Haslam SM, Wilson IA, Nabel GJ. 2008. Comparative efficacy of neutralizing antibodies elicited by recombinant hemagglutinin proteins from avian H5N1 influenza virus. J Virol 82:6200–6208. 10.1128/JVI.00187-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawai A, Yamamoto Y, Yoshioka Y. 2020. Vaccine effect of recombinant single-chain hemagglutinin protein as an antigen. Heliyon 6:e04301. 10.1016/j.heliyon.2020.e04301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shibuya M, Aoshi T, Kuroda E, Yoshioka Y. 2020. Murine cross-reactive nonneutralizing polyclonal IgG1 antibodies induced by influenza vaccine inhibit the cross-protective effect of IgG2 against heterologous virus in mice. J Virol 94:e00323-20. 10.1128/JVI.00323-20. [DOI] [PMC free article] [PubMed] [Google Scholar]