ABSTRACT
Human papillomavirus (HPV) infection is a well-known cause of cervical cancer. Therapeutic cancer vaccines are part of the current therapeutic options for HPV-associated cancers. Axalimogen filolisbac (ADXS11-001) is an immunotherapy based on live attenuated Listeria monocytogenes-listeriolysin O (Lm-LLO), designed by biological engineering to secrete an antigen-adjuvant fusion protein, composed of a truncated fragment of LLO fused to HPV. The proposed mechanism of action is that Lm-based vectors infect antigen-presenting cells (APC) and secrete HPV-LLO fusion proteins within the APC cytoplasm, these proteins are processed and presented to cytotoxic T lymphocytes (CTL), thus generating a new population of CTLs specific to HPV antigens. These HPV-specific CTLs destroy HPV infected cells. ADXS11-001 has demonstrated safety results in phase I–II studies in women with cervical cancer and is being assessed in clinical trials in patients with HPV-positive anal canal and head and neck cancers.
KEYWORDS: Immunotherapy, ADXS11-001, cervical cancer, listeria monocytogenes vaccine
Introduction
Cervical cancer represents the third cause of cancer in Mexican women, according to GLOBOCAN 2018.1 Currently, three types of prophylactic HPV vaccines (bivalent, quadrivalent and nonavalent) are effective in protecting against HPV infection in 90% of the cases, being the most effective intervention to prevent the development of cervical cancer.2 Despite advances in vaccination, screening and detection of cervical cancer early stages, a significant proportion of patients will be diagnosed at an advanced stage and will develop recurrence or persistence of the disease. For the latter group of patients, so far only chemotherapy and biological therapy are available; therefore, new therapeutic options are being explored. One of them is immunotherapy.3
The ability of the immune system to recognize and eliminate cancer cells is a well-defined process, called immune surveillance of tumors. Its failure allows cancer development and has motivated the search for strategies to restore effective immune response against neoplastic cells.4 One of these strategies includes the development of cancer vaccines. In general, these are composed of antigens found in tumor cells, often referred to as tumor-associated antigens, combined with adjuvants designed to induce an immune response. Antigen-specific T-cell responses induced by cancer vaccines have the potential to produce a more specific elimination of cancer cells than conventional chemotherapy, and also lead to lasting memory responses capable of challenging cancer recurrence.5,6
The use of live attenuated bacteria, designed by biological engineering, is an approach to cancer immunotherapy that is different from those based on synthetic chemistry, cellular therapy or antibody-based therapy. With the use of this new technological platform, activation of an immune response to live bacteria appears to be more complex and comprehensive. Axalimogen filolisbac is an immunotherapy based on live attenuated Listeria monocytogenes-listeriolysin O (Lm-LLO) that has been developed for the treatment of HPV-associated cancer types and designed with the purpose of stimulating the immune system to specifically respond against tumor dysplastic cells that express the tumor-associated antigen (HPV16 E7). The Lm-LLO immunotherapy generates strong innate and adaptive cellular immune responses against the bacterial antigen.7–10
Currently, this therapy is under development as single agent and in combination with other cancer 3treatments.
HPV as a target for immunotherapy
HPV infection is estimated to account for approximately 4.5% of all cancer cases worldwide. Infection with high-risk HPV subtypes generates almost 100% of cervical cancer cases, 90% of anal canal cancers, 40% of penile, vulvar and vagina cancers and around 12% of head and neck cancers, mainly in the oropharynx.11 High-risk HPV subtypes 16 and 18 are responsible for more than 70% of cervical cancer.12
HPV belongs to the papillomavirus family, composed of circular, non-enveloped double chain deoxyribonucleic acid (DNA) viruses that can infect stratified tissue. Its genome codifies for early proteins (E1, E2, E4, E5, E6, E7, and E8) and two structural proteins that appear later (L1 and L2). HPV infects cervical mucosa basal epithelial cells, which leads to low levels of expression of intracellular viral proteins. Viral DNA replicates after infection, and the production of viral proteins increases once HPV-infected cells leave the basal layer. Chronic infection is maintained in approximately 10% of women owing to the ability of HPV to evade the host immune response.13
HPV-induced cancer can occur when viral DNA is integrated into the host genome, typically with the removal of the E2, E4, E5, L1 and L2 genes. Loss of the E2 viral gene, a transcription inhibitor, leads to positive regulation of two oncoproteins, E6 and E7. Viral complexes of E6 with p53 tumor-suppressor and E7 with retinoblastoma tumor-suppressor (pRb) disrupt cell cycle regulation and cause genomic instability, which generates the neoplasm.14 HPV-associated cancers are a clear example where an infection with a virus (HPV) is the direct cause of the neoplasm. Immune response against HPV-infected cells that express the E6 and E7 proteins is sufficient to eliminate the presence of dysplastic or neoplastic tissue caused by this viral infection. Cytotoxic CD8 + T cells are responsible for the immune recognition of proteins expressed by HPV-infected and transformed cells; also, a higher ratio of CD8+/FOXP3 tumor infiltrating T cells counteract the tolerance developed toward transformed dysplastic cells.15,16
Listeria monocytogenes as a mediator of immune response
Listeria monocytogenes (Lm) has numerous characteristics that make it an attractive vector for cancer immunotherapy and is the most commonly used bacterial vector as the basis of immunotherapeutic vaccines. Lm is a gram-positive, facultative, intracellular anaerobic bacterium that is associated with opportunistic foodborne diseases in susceptible hosts.17
During active infection, phagocytic cells, such as macrophages and dendritic cells, recognize Lm through pattern recognition receptors (PRR), and phagocytize the bacterium. Once Lm is internalized, it evades the phagosome by secreting the listeriolysin O (LLO) toxin, a virulence factor that destroys the phagosome membrane, to allow its escape from the phagosome toward the cytoplasm, where it rapidly grows and spreads to other cells using actin polymerizing protein (ActA) to promote its own motility. ActA is an abundant surface protein that is overregulated more than 200 times during intracellular bacterial growth.18 ActA is activated in the host cell cytosol after prfA allosteric activation, which subsequently regulates host actin polymerization. Once in the cell membrane, Lm forms a protuberance that is internalized by an adjacent macrophage, thus spreading the infection. Appropriate LLO and ActA regulation by prfA is critical for Lm pathogenesis.19
Once the bacterium successfully infects the host, innate immune responses are rapidly and gradually activated. Innate immune cells recognize Lm through the PRRs toll like receptors (TLR)-2 and TLR-5 (Figure 1). Primary myeloid differentiation response protein 88 (MyD88) is important in the signal transduction following the interaction of TLRs with the pathogen, it leads to the activation of the transcription factor Nuclear Factor κB (NF-κB), which is required for macrophage activation and synthesis of proinflammatory cytokines, mainly tumor necrosis factor-α (TNF-α), IL-1β, IL-6 and IL-12. In response to IL-12, NK cells produce interferon-γ (IFN-γ) which plays an important role in host defense and enhances anti-tumor and antiviral effects of cytotoxic T cells. Antigen presenting cells alert the adaptive immune response of the presence of Lm through the major histocompatibility complex (MHC) molecules by two different routes. Lm antigens from bacteria that do not escape the phagosome, are presented in MHC II molecules, with subsequent activation of CD4+ helper T cells, while Lm antigens derived from bacteria that manage to escape the phagosome are presented in MHC I molecules and activate CD8+ cytotoxic T cells (Figure 1).18,21,22
Figure 1.
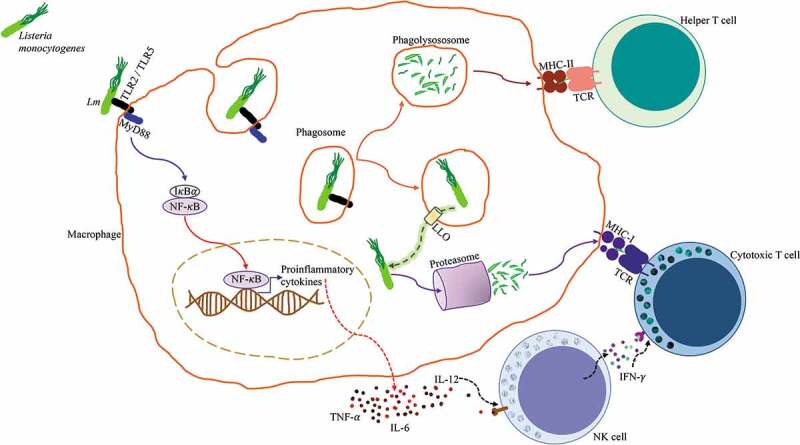
Antigen processing and presentation of Listeria monocytogenes. Lm is identified by a macrophage through pattern recognition receptors TLR2/TLR5, these initiate the MyD88 signaling pathway that leads to the activation of NF-κB, which promotes the transcription of pro-inflammatory genes and subsequent production of proinflammatory cytokines TNF-α, IL-6, and IL-12. Lm is endocytosed by the macrophage and contained in a phagosome. The phagosome fuses with a lysosome to form a phagolysosome where Lm is killed and digested, antigenic peptides derived from the digested bacteria are loaded into MHC class II molecules and presented to CD4+ Helper T cells. Alternatively, Lm has the ability to express the pore-forming listeriolysin O (LLO) toxin that perforates the phagosome and allows it to escape into the cytosol. Once in the cytosol, Lm secretes proteins that are degraded by the proteasome, these antigenic peptides are loaded into MHC class I molecules and presented to CD8+ Cytotoxic T cells. NK cells respond to IL-12 by producing IFN-γ, which enhances Cytotoxic T cell activation.20
Listeria monocytogenes has been identified as a potential vector to explore therapeutic vaccines. The usefulness of the Lm vector is achieved through its genetic recombination with a truncated, non-hemolytic form of LLO, which eliminates Lm cytolytic activity and associated cellular toxicity, while preserving the significant immunogenic and adjuvant properties of the pathogen.23
Listeria monocytogenes and its products as agents for cancer immunotherapy
Listeriolysin O is a 529-amino acid protein that is secreted by Lm and as previously mentioned, serves to evade the phagosome. The fusion of tumor antigens to the first 420 amino acid sequence of LLO, which excludes the hemolytic domain, helps to facilitate secretion of the antigen,20,24 increase antigen presentation25 and stimulate maturation of dendritic cells,26 as shown in Figure 2. In addition, LLO can act as a potent adjuvant when incorporated into DNA vaccines27 or viral vaccines28 (Figure 2).
Figure 2.
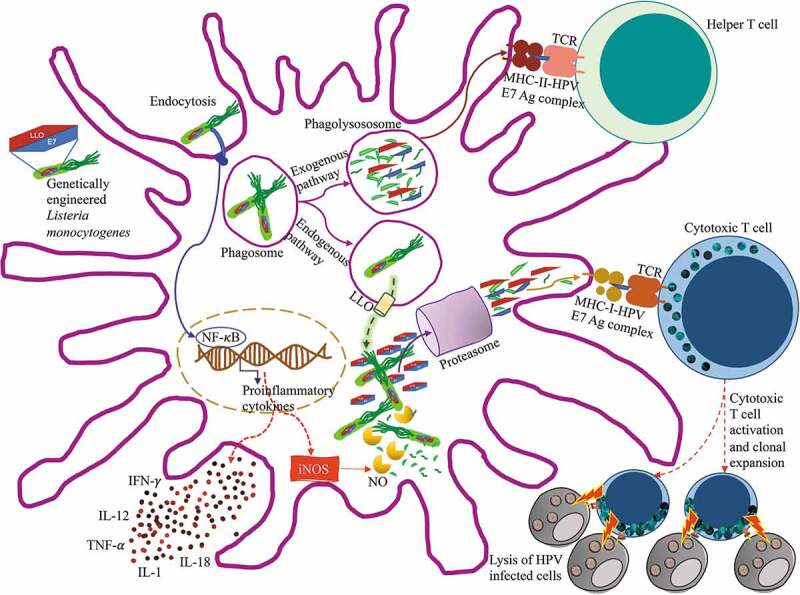
Antigen processing and presentation of ADXS11-001 Lm LLO-HPV E7 fusion protein
ADXS11-001 is Listeria monocytogenes genetically engineered to produce the LLO-(HPV) E7 fusion protein for immunotherapy. After injection in vivo, these Lm are recognized and endocytosed by dendritic cells. The bacterium stimulates both arms of the adaptive immune system because it is processed by both, the endogenous and exogenous pathways for antigen presentation. HPV E7 antigens from the bacteria processed by the phagolysosome are presented in MHC class II molecules to helper T cells. Fusion proteins LLO-HPV E7 from the bacteria that escaped to the cytosol are processed through the proteasome and HPV E7 antigens are presented in MHC class I molecules to cytotoxic T cells. This process generates and activates HPV specific cytotoxic T cell clones that will identify and destroy HPV+ infected cells. Lm also causes a strong innate immune response that leads to the generation of numerous mediators, including nitric oxide (NO), which participates in the destruction of bacteria, and the production of cytokines, such as TNF-α, IL-1, IL-12, IL-18 and IFN-γ, which enhance T cell activation. Adapted from Wallecha A 7
Preclinical studies have demonstrated that Lm-LLO-E7 stimulates the production of a wide array of pro-inflammatory cytokines by dendritic cells, such as interleukin-2 (IL-2), IL-12, TNF-α and IFN-γ; in addition, it promotes dendritic cell maturation, activates CD4 + T cell-mediated adaptive immune response, induces tumor antigen-specific cytotoxic CD8 + T cells, disrupts immune tolerance, maintains protective immunity and blocks tumor recurrence. In addition, LLO is capable of inducing the production of chemokines and costimulatory molecules that are crucial to the development of potent immune responses.29,30
In 2001, Gun et al. prepared two recombinant Lm strains, one expressing the HPV-16 E7 protein without attempting to modify the LLO molecule (Lm-E7), and the second expressed E7 as a non-hemolytic LLO-bound fusion protein (Lm-LLO-E7). Both strains induced qualitatively different T-cell immune responses that correlated with their ability to induce HPV-associated tumor regression in mice. Lm-LLO-E7, but not Lm-E7, caused the regression, mediated by CD8 + T cells, of E7-expressing tumors in a syngeneic mouse model. Antitumor response to Lm-LLO-E7, but not to Lm-E7, was significantly reduced with CD4 + T cell depletion, which indicates that helper T cell activation is necessary for an adequate antitumor cytotoxic response.24
ADXS11-001 mechanism of action
ADXS11-001 (Axalimogen filolisbac, or AXAL) is an attenuated strain of the Lm bacterium designed to express the truncated LLO (tLLO) protein, fused with the HPV 16 E7 antigen. It was created by biological engineering from the XFL-7 strain that lacks the prfA gene, which is not necessary for in vitro culture and makes it non-virulent. The XFL-7 strain was modified with the pGG55 plasmid, which contains the tLLO-E7 fusion protein in addition to a mutated copy of the prfA gene, to partially restore the XFL-7, necessary to retain the plasmid in vivo.10 In addition, the tLLO-E7 fusion protein promotes specific immune responses to antigen E7.31
When ADXS11-001 is delivered in the bloodstream, APCs in the spleen phagocytize the bacteria. Once inside the phagosome, ADXS11-011 secretes LLO to evade the phagolysosome, when it reaches the cytosol, antigen tLLO-E7 is processed through the proteasome. The resulting peptides form complexes with MHC class I molecules, which are presented to antigen specific CD8+ cytotoxic T cells. The bacteria unable to exit the phagolysosome is processed through the exogenous pathway, the peptides form complexes with MHC class II molecules and are presented to antigen specific CD4+ helper T cells. Helper T cells become activated and secrete cytokines that modulate the immune response toward a cytotoxic Th1 response. As a result, cytotoxic T cells seek and infiltrate the tumor, which allows them to attack and destroy HPV+ cancer cells (Figure 2).10
ADXS11-0001 in cervical cancer
ADXS11-001 has been explored as a specific immunotherapy in cervical cancer. The results of the first clinical use of the vaccine were shown in a phase I study. The study assessed the safety of the vaccine in 15 patients with advanced carcinoma of the cervix. Patients were assigned to three doses (1 x 109 colony forming units (CFUs), 3.3 × 109 CFUs or 1 × 1010 CFUs), with an interval of 3 weeks between them. All patients developed fever, 60% experienced vomiting, 53.3% had chills, headache and anemia, 46.7% had nausea and tachycardia, and 26.7% suffered musculoskeletal pain. Grade 2 adverse events (AEs) were experienced by 87% of patients and 40% had grade 3 AEs, with no vaccine-related grade 4 AEs being observed. All patients experienced an increase in body temperature during the first hours after administration of the vaccine and, in some cases, a decrease in blood pressure and tachycardia was also observed, although in most cases these AEs disappeared 12 hours after injection. Among the patients who received the highest dose of 1 × 1010 CFUs, 3 experienced dose-limiting toxicity, which consisted of grade 2 hypotension that required therapeutic intervention; therefore, they withdrew from the study. Overall, the ADXS11-001 vaccine was safe and well tolerated.31
In 2016, Ghamande et al. published results of a phase I study that assessed high-dose ADXS11-001 in patients with persistent, recurrent cervical cancer not eligible for surgical treatment or radiotherapy. The study evaluated safety and tolerability as the primary objective. Patients received ADXS11-001 every 3 weeks for 12 weeks, with a 3 + 3 design, two dosages were staggered, 5 × 109 (level 1) and 1 × 1010 (level 2). Ten patients were enrolled and nine were treated. Dose-limiting toxicity was lower than 33% for level 2. Most common AEs were chills, vomiting, hypotension, tachycardia, fever and nausea. The authors concluded that the 1 × 1010 dosage was well tolerated.32
In the American Society of Clinical Oncology (ASCO) 2016 meeting, preliminary results of the GOG/NRG-0265 phase II trial were presented. This is a study with a single treatment arm that was designed to assess the safety and efficacy of the ADXS11-001 vaccine in patients with persistent or recurrent cervical cancer. The study was planned to recruit 67 patients who were to receive ADXS11-001 at a dosage of 1 × 109 CFUs once every 4 weeks for 3 doses. It is a two-stage study, with only 24 patients being treated on stage 2. Six-month overall survival (OS) was 42%, with a median of 4.8 months (3.8 – NR) and median persistence free survival (PFS) of 2.6 months (2.0–3.2 months). In those who received three doses, the 6-month survival rate was 67%. AEs were experienced by 92%, 15% were G3 and 4% G4. Treatment-related AEs included nausea, vomiting, chills, fatigue and fever, with G3 hypotension and cytokine-release syndrome. The authors concluded that ADXS11-001 is well tolerated and shows activity in patients previously treated for recurrent disease.33
Recently, Basu et al.34 published the results of a phase II study that assessed the safety and efficacy of the treatment with ADXS11-001 alone or in combination with cisplatin in patients with recurrent or refractory cervical cancer of squamous histology. A total of 109 patients were treated and 69 were assessed for response. One third of the patients withdrew the informed consent in both groups. The dosage used for the ADXS11-001 arm was 1 × 109 CFUs for 3 doses (day 1, 29 and 57). The combination dose was ADXS11-001 on day 1 followed at 4 weeks by five doses of cisplatin 40 mg/m2 weekly, followed by another dose of ADXS11-001. Fifty-six patients were included in the monotherapy arm and 54 in the combination arm. Each patient received oral ampicillin (500 mg every 6 hours for 7 days) or trimeptroprim/sulfamethoxazole in case of penicillin allergy, starting 72 hours after each ADXS11-001 dose. Mean OS was similar between treatments, with 8.28 months (95% CI: 5.85–10.5) for ADXS11-001 alone and 8.78 months (95% CI: 7.4–13.3) for the combination. The 12 month and 18-month OS rates were 30.9% versus 38.9%, and 23.6% versus 25.9% for each group, respectively (34.9% at 12 months and 24.8% at 18 months, combined). Fifteen patients continued the follow-up after 18 months, out of which 12 (11%) reached a 24-month OS. ORR was 17.1% for the group of patients treated with ADXS11-001 and 14.7% for ADXS11-001 plus cisplatin group. Disease control rate was 62.9% and 58.8%, respectively, and mean duration of disease control was 5.2 months (4.8 vs. 5.6 months). Mean PFS for patients treated with ADXS11-001 was 6.08 months (95% CI: 5.88–9.36) and 6.44 months (95% CI: 4.17–8.94) for those treated with ADXS11-001 plus cisplatin (p = .75). The group treated with the combination had more AEs than the group treated with monotherapy (429 vs 275 AEs), possibly owing to cisplatin-associated AEs. Most AEs were mild or moderate (80.4%) and unrelated to the study drug (76.6%). Drug-related AEs were reported by 46.3% of patients treated with ADXS11-001 plus cisplatin and by 36.4% of those treated with ADXS11-001, with chills and fever being the most common in both groups. The incidence of treatment-related serious AEs was 7.3% in patients treated with ADXS11-001 and 3.8% in those treated with ADXS11-001 plus cisplatin.34 Seventeen serious AEs (10%) occurred in 49 patients (45%). One patient treated with the combination reported pyrexia. Two patients (3.6%) of the monotherapy arm reported three AEs that were considered related to the drug: cytokine-release syndrome in one patient and abdominal pain and bacterial peritonitis with septicemia in one patient (fatal).34
Currently, the AIM2CERV phase III study is comparing disease-free survival of patients treated with ADXS11-001 versus placebo, after concomitant chemo-radiotherapy treatment in individuals showing a complete response to the treatment (ClinicalTrials.gov – NCT02853604). The ADXS11-001 dosage is administered at 1 × 109 CFUs every 4 weeks for 3 doses (week 1, 4 and 7) for the first three months, and then every 8 weeks (weeks 15, 23, 31, 39 and 47) for a total of 5 doses or until disease progression, with the latter being called the maintenance phase. Patients receive oral treatment with ampicillin or trimetroprim/sulfamethoxazole for approximately 1 year. Unlike previous clinical trials, this study will assess patients with locally advanced high-risk cervical cancer.35 The study started its recruitment in 2016 and the results are expected by 2021.
The studies carried out in cervical cancer are shown in Table 1.31–34,36,37
Table 1.
Clinical trials with ADX11-001 in cervical cancer
| Type of cancer |
Trial Phase | Author | Indication | Vaccine alone or in combination | Treatment regimen | Estimated N | Completion | Efficacy | Safety |
|---|---|---|---|---|---|---|---|---|---|
| Cervix | I | Maciag PC (31) | Recurrent/metastatic | Monotherapy | Staggered dose 3 5-subject dose cohorts: 1 x 109, 3.3 × 109 or 1 × 1010 CFU |
15/20 | 2009 | PR: 1 patient SD: 7 patients PD: 5 patients |
Pyrexia (100%) Vomiting (60%) Myalgia (57%) Cold, headache and anemia (53%) Nausea and tachycardia (49%) DLT: 1 × 109 CFU |
| I–II | Ghamande SA (32) NCT021 64461 |
Persistent/recurrent/metastatic | Monotherapy | Single group Staggered dose 5 x 109 CFU 5 x 1010 CFU ADXS11-001 every 3 wk x 2 doses |
25 | Dec-18 | - | Common TrAEs: Cold or vomiting Hypotension Tachycardia Fever Nausea |
|
| II | Huh WK (33) GOG 0265 NCT012 66460 |
Persistent/recurrent metastatic | Monotherapy | Single group: 1 x 109 CFU ADXS11-001 every 4 wk x 3 doses |
67 | Oct-18 | 1-year OS: 38.5% PFS: 3.1 m OS: 7.7 m PR: 1 patient SD: 9 patients |
TrAEs 38%: Vomiting Cold Fatigue Fever |
|
| II | Basu P (34) CTRI/2010/091/001232 |
Persistent/recurrent | ADXS11-001 ± cisplatin | Two groups: Monotherapy: ADXS11-001 (1 x 109 CFU) x 1 cycle (3 applications on days 1, 29 and 57) Combination: ADXS11-001 (1 x 109 CFU) x 1 application on day 1, followed 4 wk later by 5 doses/wk of cisplatin (40 mg/m2) |
110 | 2017 | 12-m OS: 34.9% 18-m OS: 24.8% RR: 11% (6 CR, 6 PR) SD: 35 patients |
TrAEs: 46% serious AEs G3: 7.3% |
|
| III | Herzog T (36) AIM2CERV NCT028 53604 |
High-risk, locally advanced | Monotherapy | ADXS11-001 (1 x 109 CFU) every 4 wk x 3 doses (wk 1, 4 and 7), then every 8 wk x 5 doses (wk 15, 23, 31, 39 and 46) | 450 | Jun-21 | Pending | Pending | |
| Cervix or head and neck | I/II | Cohen EEW (37) NCT022 91055 |
Persistent/recurrent metastatic | ADXS11-001 ± Durvalumab | Phase I: ADXS11-001 at 1 × 109 doses + Durvalumab 3 mg/kg (3 + 3 design for the staggered dose) Phase II: ADXS11-001 at 1 × 109 CFU doses + Durvalumab 10 mg/kg |
66 | Dec-19 | Pending | Pending |
CFU: colony forming units; CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; RR: response rates; DLT: dose limiting toxicity; AEs: adverse events; TrAEs: treatment-related adverse events; OS: overall survival; PFS: progression-free survival; wk: week; m: months.
ADXS11-001 for HPV-associated cancers
ADXS11-001 effects and safety have also been explored in early clinical trials in other neoplasms, such as head and neck, anal canal and lung cancers, which differ in regimen, dosage and application intervals. The summary of these studies is shown in Table 2.38–41 Of note, Sacco JJ, et al. reported a potential adverse event of attenuated Lm vectors with a case of systemic listeriosis during a phase I trial of ADXS11-001 (axalimogene filolisbac) in HPV-positive oropharyngeal cancer (OPC); although they did not confirm the sequence identity of the pathogen grown in blood culture to ADXS11-001, the chronology of events and pattern of antibiotic resistance point to the fact that systemic listeriosis was caused by the study vaccine.41
Table 2.
Clinical trials with ADX11-001 in HPV-associated cancers
| Type of cancer |
Trial Phase | Author | Indication | Vaccine alone or in combination | Treatment regimen | Estimated N | Completion | Efficacy | Safety |
|---|---|---|---|---|---|---|---|---|---|
| Head and neck | I | Sacco JJ (41) REALISTIC- NCT015 98792 NCT020 02182 |
Oropharyngeal cancer | Monotherapy | Staggered dose 3.3 x 108 CFU 1 x 109 CFU 3.3 x 109 CFU |
36 | Nov-14 | No results | Interrupted |
| II | Krupar R (38) | Resectable, treatment naïve, stage II–IV oropharyngeal cancer | ADXS11-001 + robotic trans-oral surgery | ADXS11-001 at 1 × 109 CFU doses every 3 wk for 6 wk (total of 2 doses) prior to surgery | 25 | Aug-19 | Pending | Pending | |
| Anal canal | I/II | Safran H (39) NCT016 71488 |
Locally advanced | ADXS11-001 + CT/RT (mitomycin, 5FU, IMRT) | ADXS11-001 at 1 × 109 CFU doses 4 doses: 1 to 14 d prior to CT/RT; 10 to 28 d after CT/RT; subsequent doses 3 and 4 at 28-d intervals Mitomycin 10 mg/m2 d 1 and 29 5FU 1 mg/m2 continuous infusion d 1–4 and 29–32 |
25 | Feb-18 | CR 9 patients 89% disease free at 42 m |
G3 AEs in 2 patients: Cold (2) Back pain (1) Hyponatremia (1) |
| II | Fakin M (40) NCT023 99813 |
Persistent/recurrent metastatic | Monotherapy | ADXS11-001 at 1 × 109 CFU doses every 3 wk in 9-wk cycles for ≤ 2 years or until discontinuation | 55 | Mar-22 | Pending | Pending | |
| Lung | II | NCT025 31854 | HPV+ NSCLC on induction treatment | Maintenance pemetrexed ± ADXS11-001 | Maintenance pemetrexed Maintenance pemetrexed + ADXS11-001 |
124 | Mar-19 | Pending | Pending |
CFU: colony forming units; CR: complete response; AEs: adverse events; CT/RT: chemo-radiotherapy; IMRT: intensity-modulated radiotherapy; 5-FU: 5-fluoruracil; NSCLC: non-small cell lung cancer; HPV: human papillomavirus; d: day; wk: week; m: month.
Other therapeutic vaccines against HPV-associated cervical cancer
Unlike prophylactic vaccines, whose main mechanism of action is mediated by antibodies that neutralize viral particles, therapeutic vaccines can induce a cytotoxic immune response targeted against HPV-infected cells.42 The sustained expression of E6 and E7 oncoproteins is essential for the induction and maintenance of the malignant phenotype of cancer cells, making them the most promising targets for the development of therapeutic vaccines against HPV infection and related diseases.42,43
Currently, there are several therapeutic vaccines that are under development and being tested in preclinical and clinical trials. These therapeutic vaccines include live vectors, inactivated viruses, live attenuated bacteria, peptides and proteins, nucleic acids, and cellular vaccines using dendritic cells loaded with antigen. It is important to emphasize that to assess the efficacy and safety of therapeutic vaccines, it is essential to demonstrate both their immunogenicity and safety through clinical trials.42
The main aim of therapeutic vaccines is to eliminate the precancerous lesions and the persistent infection caused by HPV. Listeria monocytogenes, Lactobacillus, and Salmonella are the most common bacteria-based vectors for tumor immunotherapy.44 The potent immune response induced by the vectors instead of the HPV antigen remains an obstacle in the use of live vector vaccines. Regarding clinical studies that tested DNA-based vaccines, the VGX-3100 is the first therapeutic vaccine that has shown efficacy against HPV 16 and 18-associated cervical intraepithelial neoplasia (CIN) 2 or 3.45 The therapeutic HPV peptide-based vaccine HPV16-SLP generated a specific immune response against HPV, showing promising results with few side effects.46
Currently, the effectiveness of prophylactic vaccines has been studied in patients with preexisting HPV infection or with premalignant lesions; however, the benefit of prophylactic vaccines in the management of infection and pre-invasive lesions is difficult to assess, results are conflicting, and only marginal benefits have been shown.42,47–50
Conclusions
In Mexico, cervical cancer remains a public health problem. Its high frequency at advanced stages, its high recurrence rate and its close relationship with the human papillomavirus forces the search for new therapeutic strategies for patients affected by this disease.
Among them, immune surveillance modulation through tumor-associated antigens and/or live attenuated bacteria appears to be a promising current focus of attention. ADXS11-001 is an immunotherapy consisting of live attenuated Lm-LLO-(HPV16-E7), which is optimal to elicit an adequate immune response. It has been explored in initial clinical trials in cervical cancer and other HPV-associated malignancies.
So far, immunotherapy with ADXS11-001 appears to be well tolerated, with manageable toxicity, and shows activity in heavily pretreated cervical cancer populations; however, its true role in cervical cancer global treatment is yet to be defined.
Recommendation
There are no phase III studies assessing the efficacy of ADXS11-001 in persistent/recurrent/metastatic cervical cancer; therefore, there can be no recommendation for standard use. The purpose of the phase III study in locally advanced cervical cancer is to compare ADXS11-001 to placebo, administered following concurrent chemotherapy and radiation therapy with curative intent, assessing disease-free survival in this population. Their results will eventually establish a recommendation on the use of ADXS11-001 in patients with high risk locally advanced cervical cancer. Also, the immunogenicity of the vaccine in this population and the potential adverse events that could develop are still unknown. Equally, the benefit in response rates, overall survival and progression-free survival are still under study. It is important to emphasize that this therapeutic vaccine can only be applied to patients with cervical cancer for research purposes under a clinical trial.
Declaration of interest
Authors declare they have no conflict of interest.
References
- 1.International Agency for Research on Cancer . Mexico source: globocan 2018. Glob Cancer Obs 2019;283:2018–19. [Google Scholar]
- 2.Wang R, Pan W, Jin L, Huang W, Li Y, Wu D, Gao C, Ma D, Liao S.. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 2020;471:88–102. doi: 10.1016/j.canlet.2019.11.039. [DOI] [PubMed] [Google Scholar]
- 3.Eskander RN, Tewari KS. Immunotherapy : an evolving paradigm in the treatment of advanced cervical cancer. Clin Ther. 2015;37(1):20–38. doi: 10.1016/j.clinthera.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauer P, Chow MY, Loessner MJ, Portnoy DA, Calendar R. Construction, characterization, and use of two listeria monocytogenes site-specific phage integration vectors. J Bacterology. 2002;184(15):4177–86. doi: 10.1128/JB.184.15.4177-4186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le DT, Dubensky TW Jr, Brockstedt DG. Clinical development of listeria monocytogenes–based immunotherapies. Semin Oncol. 2012;39(3):311–22. doi: 10.1053/j.seminoncol.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallecha A, French C, Petit R, Singh R, Amin A, Rothman J. Lm-LLO-based immunotherapies and HPV-associated disease. J Oncol. 2012;2012:542851. doi: 10.1155/2012/542851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahabi V, Reyes-Reyes M, Wallecha A, Rivera S, Paterson Y, MacIag P. Development of a Listeria monocytogenes based vaccine against prostate cancer. Cancer Immunol Immunother. 2008;57(9):1301–13. doi: 10.1007/s00262-008-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallecha A, Wood L, Pan ZK, Maciag PC, Shahabi V, Paterson Y. Listeria monocytogenes-derived listeriolysin O has pathogen-associated molecular pattern-like properties independent of its hemolytic ability. Clin Vaccine Immunol. 2013;20(1):77–84. doi: 10.1128/CVI.00488-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4(1):9. doi: 10.1186/s40661-017-0046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plummer M, De Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Heal. 2016;4(9):e609–16. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 12.Galani E, Christodoulou C. Human papilloma viruses and cancer in the post-vaccine era. Clin Microbiol Infect. 2009;15(11):977–81. doi: 10.1111/j.1469-0691.2009.03032.x. [DOI] [PubMed] [Google Scholar]
- 13.Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 14.Bodily J, Laimins LA. Persistence of human papillomavirus infections: keys to malignant progression. Trends Microbiol. 2012;19(1):33–39. doi: 10.1016/j.tim.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Øvestad IT, Gudlaugsson E, Skaland I, Malpica A, Kruse AJ, Janssen EAM, Baak JPA. Local immune response in the microenvironment of CIN2-3 with and without spontaneous regression. Mod Pathol. 2010;23(9):1231–40. doi: 10.1038/modpathol.2010.109. [DOI] [PubMed] [Google Scholar]
- 16.Patel S, Chiplunkar S. Host immune responses to cervical cancer. Curr Opin Obstet Gynecol. 2009;21(1):54–59. doi: 10.1097/GCO.0b013e32831a9890. [DOI] [PubMed] [Google Scholar]
- 17.Renier S, Hébraud M, Desvaux M. Molecular biology of surface colonization by Listeria monocytogenes: an additional facet of an opportunistic Gram-positive foodborne pathogen. Environ Microbiol. 2011;13(4):835–50. doi: 10.1111/j.1462-2920.2010.02378.x. [DOI] [PubMed] [Google Scholar]
- 18.Pamer EG. Inmune Responses to Listeria Monocytogenes. Nat Rev. 2004;4:812–23. [DOI] [PubMed] [Google Scholar]
- 19.Reniere ML, Whiteley AT, Portnoy DA. An In Vivo Selection Identifies Listeria monocytogenes genes required to sense the intracellular environment and activate virulence factor expression. PLOS Pathog. 2016Jul14;12(7):e1005741. doi: 10.1371/journal.ppat.1005741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flickinger JC Jr, Rodeck U, Snook AE. Listeria monocytogenes as a vector for cancer immunotherapy : current understanding and progress. vaccines. 2018;6(3):48. doi: 10.3390/vaccines6030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothman J, Paterson Y. Live-attenuated Listeria -based immunotherapy. Expert Rev Vaccines. 2013;12(5):493–504. doi: 10.1586/erv.13.34. [DOI] [PubMed] [Google Scholar]
- 22.Zenewicz LA, Shen H. Innate and adaptive immune responses to Listeria monocytogenes : a short overview. Microbes Infect. 2007;9:1208–15. doi: 10.1016/j.micinf.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cory L, Chu C. ADXS-HPV: a therapeutic Listeria vaccination targeting cervical cancers expressing the HPV E7 antigen. Hum Vaccin Immunother. 2014;10(11):3190–95. doi: 10.4161/hv.34378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167:6471–79. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 25.Sewell DA, Shahabi V, Gun G 3rd, Pan ZK, Dominiecki ME, Paterson Y. Recombinant Listeria Vaccines containing PEST sequences are potent immune adjuvants for the tumor-associated antigen human papillomavirus-16 E7. Cancer Res. 2004;64:8821–25. doi: 10.1158/0008-5472.CAN-04-1958. [DOI] [PubMed] [Google Scholar]
- 26.Peng X, Hussain SF, Paterson Y, Alerts E. The ability of two Listeria monocytogenes vaccines targeting human papillomavirus-16 E7 to induce an antitumor response correlates with myeloid dendritic cell function. J Immunol. 2004;172:6030–38. doi: 10.4049/jimmunol.172.10.6030. [DOI] [PubMed] [Google Scholar]
- 27.Peng X, Treml J, Paterson Y. Adjuvant properties of listeriolysin O protein in a DNA vaccination strategy. Cancer Immunol Immunother. 2014;56(6):797–806. doi: 10.1007/s00262-006-0240-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamikanra A, Pan ZK, Isaacs SN, Wu T, Paterson Y. Regression of established human papillomavirus type 16 (hpv-16) immortalized tumors in vivo by vaccinia viruses expressing different forms of HPV-16 E7 correlates with enhanced CD8 (+) T-cell responses that home to the tumor site. J Virol. 2001;75(20):9654–64. doi: 10.1128/JVI.75.20.9654-9664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun R, Liu Y. Listeriolysin O as a strong immunogenic molecule for the development of new anti-tumor vaccines. Hum Vaccin Immunother. 2013;9(5):1058–68. doi: 10.4161/hv.23871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freeman MM, Ziegler HK. Simultaneous Th1-type cytokine expression is a signature of peritoneal CD4 + lymphocytes responding to infection with Listeria monocytogenes. J Immunol. 2005;175:394–403. doi: 10.4049/jimmunol.175.1.394. [DOI] [PubMed] [Google Scholar]
- 31.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine : a Phase I safety study of Lm -LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975–83. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 32.Ghamande SA, Platt D, Wheatley D, Rungruang BJ. Phase I study evaluating high-dose treatment with ADXS11-001, a Listeria monocytogenes - listeriolysin O (Lm -LLO) immunotherapy, in women with cervical cancer. J Clin Oncol. 2015;33(suppl):abstractTPS3096. doi: 10.1200/jco.2015.33.15_suppl.tps3096. [DOI] [Google Scholar]
- 33.Huh WK, Brady WE, Moore KN, Lankes HA, Bradley J. A phase 2 study of live-attenuated listeria monocytogenes cancer immunotherapy (ADXS11-001) in the treatment of persistent or recurrent cancer of the cervix (GOG-0265). J Clin Oncol. 2014;32(suppl):abstractTPS5617. doi: 10.1200/jco.2014.32.15_suppl.tps5617. [DOI] [Google Scholar]
- 34.Basu P, Mehta A, Jain M, Gupta S, Nagarkar RV, John S, Petit R. A randomized phase 2 study of ADXS11-001 Listeria monocytogenes Y Listeriolysin O immunotherapy with or without cisplatin in treatment of advanced cervical cancer. Int J Gynecol Cancer. 2018;28(4):764–72. doi: 10.1097/IGC.0000000000001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11–001. Gynecol Oncol Res Pract. 2017;4(1):10. doi: 10.1186/s40661-017-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herzog T, Backes FJ, Copeland L, Del M, Estevez P, Hare TW, Huh W, Kim B, Moore K, Oaknin A, et al. AIM2CERV: a randomized phase 3 study of adjuvant AXAL immunotherapy following chemoradiation in patients who have high-risk locally advanced cervical cancer (HRLACC). Immunother Cancer. 2016;4(suppl):abstractP140. [Google Scholar]
- 37.Cohen EEW, Moore KN, Slomovitz BM, Chung CH, Anderson ML, Morris SR, Mauro D, Burtness B. Phase I/II study of ADXS11-001 or MEDI4736 immunotherapies alone and in combination, in patients with recurrent/metastatic cervical or human papillomavirus (HPV) -positive head and neck cancer. J Immunother Cancer. 2015;3(Suppl2):abstractP147. doi: 10.1186/2051-1426-3-S2-P147. [DOI] [Google Scholar]
- 38.Krupar R, Imai N, Miles B, Genden E, Misiukiewicz K, Saenger Y, Demicco E, Patel J, Herrera PC, Parikh F, et al. HPV E7 antigen-expressing Listeria-based immunotherapy (ADXS11-001) prior to robotic surgery for HPV-positive oropharyngeal cancer enhances HPV- specific T cell immunity. Cancer Res. 2016;76(14suppl):abstract LB–095. [Google Scholar]
- 39.Safran H, Leonard K, Perez K, Vrees M, Klipfel A, Schechter S, Oldenburg N, Roth L, Shah N, Rosati K, et al. Tolerability of ADXS11-001 Lm -LLO Listeria-based immunotherapy with mitomycin, fluorouracil, and radiation for anal cancer. Int J Radiat Oncol Biol Phys. 2019;100(5):1175–78. doi: 10.1016/j.ijrobp.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Fakih M, Neil BHO, Chiorean EG, Hochster HS, Mauro DJ, More S. Phase II study of ADXS11-001 in patients with persistent/recurrent, locoregional or metastatic squamous cell carcinoma of the anorectal canal. J Clin Oncol. 2016;34(4suppl):abstractTPS786. doi: 10.1200/jco.2016.34.4_suppl.tps786. [DOI] [Google Scholar]
- 41.Sacco JJ, Evans M, Harrington KJ, Man S, Powell N, Shaw RJ, Jones TM. Systemic listeriosis following vaccination with the attenuated Listeria monocytogenes therapeutic vaccine, ADXS11-001. Hum Vaccin Immunother. 2016;12(4):1085–86. doi: 10.1080/21645515.2015.1121338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castro-Eguiluz D, Barquet-Muñoz SA, Arteaga-Gómez AC, Salcedo Hernández RA, Rodríguez-Trejo A, Gallardo-Rincón D, Serrano-Olvera JA, Aranda-Flores C. Therapeutic use of human papillomavirus vaccines in cervical lesions. Rev Invest Clin. 2020;72(4):239–49. doi: 10.24875/RIC.20000059. [DOI] [PubMed] [Google Scholar]
- 43.Grabowska AK, Kaufmann AM, Riemer AB. Identification of promiscuous HPV16-derived T helper cell epitopes for therapeutic HPV vaccine design. Int J Cancer. 2015;136:212–24. doi: 10.1002/ijc.28968. [DOI] [PubMed] [Google Scholar]
- 44.Yang A, Farmer E, Wu TC, Hung CF. Perspectives for therapeutic HPV vaccine development. J Biomed Sci. 2016;23:75. doi: 10.1186/s12929-016-0293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trimble CL, Morrow MP, Kraynyak KA, Shen X, Dallas M, Yan J, Edwards L, Parker RL, Denny L, Giffear M, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386(10008):2078–88. doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Vos Van Steenwijk PJ, Ramwadhdoebe TH, Löwik MJG, Van Der Minne CE, Berends-van Der Meer DMA, Fathers LM, Valentijn ARPM, Oostendorp J, Fleuren GJ, Hellebrekers BWJ, et al. A placebo-controlled randomized HPV16 synthetic long-peptide vaccination study in women with high-grade cervical squamous intraepithelial lesions. Cancer Immunol Immunother. 2012;61(9):1485–92. doi: 10.1007/s00262-012-1292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, Tay EH, García P, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev Res. 2009;2(10):868–78. doi: 10.1158/1940-6207.CAPR-09-0031. [DOI] [PubMed] [Google Scholar]
- 48.Thiers BH. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. Yearb Dermatology Dermatologic Surg. 2011;2008:148–49. doi: 10.1016/S0093-3619(08)70789-5. [DOI] [Google Scholar]
- 49.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, Schiller JT, Gonzalez P, Dubin G, Porras C, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. J Am Med Assoc. 2007;298(7):743–53. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 50.Arbyn M, Xu L, Simoens C, Martin-Hirsch PPL. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;(5):Art. No.: CD009069. doi: 10.1002/14651858.CD009069.pub. [DOI] [PMC free article] [PubMed] [Google Scholar]


