ABSTRACT
To compare the safety and immunogenicity of lyophilized PVRV under Zagreb and Essen regimen.
A post-marketing parallel control clinical trial was conducted. Totally 240 subjects were assigned to two groups randomly, immunized with lyophilized PVRV under Zagreb and Essen schedule. Solicited adverse events were observed after each dose and unsolicited adverse events were collected. Serum samples were collected on days 0, 7, 14, 42, 180 and 365 to be used to determine immunogenicity level. No severe adverse events (SAE) were observed. The incidence of adverse events under Zagreb and Essen were similar and there was no significant difference between the two groups and within all age groups. Fever and pain were the most frequently reported systemic and local adverse events (AEs) respectively. There were no differences in the GMT and the positive seroconversion rate between these two groups. All participants in the Zagreb group obtained protective effect on day 14, while 99.16% of the subjects obtained in the Essen group. Both groups showed similar enduring immunity. Immunizations under Zagreb and Essen regimens showed similar safety and immunogenicity. For lyophilized PVRV, Zagreb was non-inferior to Essen to patients of all age groups.
KEYWORDS: Human rabies vaccine, lyophilized, regimen, safety, immunogenicity, cost-effectiveness
Introduction
Rabies is a fatal zoonotic disease. Approximately 59000 human deaths and 3.7 million disability-adjusted life years (DALYs) lost are attributable to rabies every year.1 Most cases occur in Asia and China.2 Spatiotemporal distributions of human rabies in mainland China and hotspots were expanding northward and westward.3 From 2004 to 2014, areas reported rabies cases were expanded to central and northern provinces in China.4 Wuhan of Hubei province is a megacity with a population of nearly 15 million and it is one of the principal monitoring regions of human rabies epidemic in China, with a medium incidence of rabies.5 Prompt PEP (post-exposure prophylaxis) can prevent rabies from occurring. But in remote and rural areas, human rabies vaccines were not abundant and might lead to inadequate vaccinations. With vast territories, it is essential to better preserve and deliver vaccines. Lyophilized vaccines are alternative options. Lyophilized vaccines are easily transported and preserved for a relatively longer period than liquid vaccines. The most commonly used human rabies vaccine in China is the purified Vero cell vaccine.6 As a second-generation human rabies vaccine, PVRV was cheaper than HDCV and a previous study proved that freeze-dried vero cell vaccine was safe and effective.7 By January 2013, lyophilized purified vero rabies vaccines (PVRV) developed by a biotechnological manufacturer have been used for over 30 million person-times in China. About 22 million of them were vaccinated in the Essen regimen and 8 million or so were in the Zagreb regimen. Zagreb regimen is a simplified schedule and could lead to better compliance for patients. Besides, cost under the Zagreb regimen is 32 USD per patient cheaper than under the Essen schedule.8 However, the number of vaccinations under the Zagreb regimen is greatly lower than Essen. Besides, intradermal (ID) vaccination would greatly improve the affordability and accessibility of PEP, with increasing cost-effectiveness as throughput clinics increased, using 25–80% less vaccine compared to intramuscular (IM) administration.9 But ID was not a permitted option in China, where throughput clinics were scattered. Therefore, we conducted this study, in which ID was not be investigated, to explore the safety and immunogenicity of lyophilized PVRV under Zagreb and Essen course, to provide further evidence of the Zagreb option.
Methods
Study design and participants
This postmarketing randomized parallel-control clinical trial was conducted in Wuhan, China. Registered in China Drug Trial Registry (CTR20140353, search available at www.chinadrugtrials.org.cn), it was performed in accordance with the principles of Good Clinical Practices and Declaration of Helsinki. The sample size of this study was estimated according to the “Practical Manual of Sample Size Determination in Health Studies.” Preliminary clinical trial about liquid PVRV found that seroconversion rate on day 7 in Zagreb and Essen group was, respectively, 69.40% and 46.67%; therefore, the sample size was calculated according to the following formula:
| (1) |
in which, p1 = 0.694, p2 = 0.467, α was the probability of type I error and was set as 0.05, β was the probability of type II error and was set as 0.1.
Therefore, the sample size was estimated as followed,
Considering drop-outs, 240 participants were required for enrollment and equally divided into two groups. Participants were recruited at a ratio of 1:1:2:1:1 based on their age (≤10, 11–20, 21–50, 51–60, ≥61 years old). There was no special requirement for gender. Subjects were included if they were in good health condition, armpit temperature ≤37°C, have not received human rabies vaccines before, did not receive attenuated live vaccines within 4 weeks, did not receive other kinds of vaccines within 2 weeks, and had good compliance with the clinical trial protocol. Exclusion criteria were administration with rabies immunoglobulin (RIG), pregnancy or during the lactation period, allergic to any component in the vaccine of interest, convulsions, epilepsy, encephalopathy or other neurological symptoms, immunodeficiency, thrombocytopenia, any acute diseases within 1 week. According to Guidelines for The Classification of Adverse Reactions in Clinical Trials of Prophylactic Vaccines issued by the National Medical Products Administration of China, there were four kinds of adverse events (AEs), slight/mild (grade 1), moderate (grade 2), serious (grade 3) and severe (grade 4). A SAE was in accordance with grade 4 which was life-threatening, requiring emergency or hospitalization. Once SAE occurred and that was evidenced being relevant to vaccines, the participant would discontinue the study, turn to receive treatments and resume vaccinations after recovery from SAE, so they would be considered as drop-outs. After enrollment, each subject was assigned a unique number and a corresponding sealed envelope, in which detailed instructions for a specific regimen was provided. The unique number was generated by a random number table. Thus, participants were randomly allocated into Zagreb and Essen group. In this clinical trial, adverse events observers, laboratory personnel, and data analyzers were not aware of the specific allocation.
The study was approved by the Ethics Committee of Chaoyang Center for Disease Prevention and Control (Certificate No. WHCDPCIRB-20140828-1). Written informed consents were obtained from all participants.
Vaccine information and vaccination procedure
Vaccines in this study contained inactivated PV strain virus, which was serum type I rabies virus originated from the rabies virus strain “L. Pasteur 2061”. Stability tests proved that the vaccines were potent in 37°C condition in 4 weeks. Sterility tests and identification tests were also eligible. Lot certificate was provided by CFDA to confirm the eligibility. The potency of this lyophilized vaccine was determined by National Institutes for Food and Drug Control (NIFDC) with standard NIH test. All vaccines in this study came from the marketed batch, were not particularly manufactured for the clinical trial (batch number: 201204117). Each dose of vaccine was attached with a 0.5 ml vial of sterile water for injection.
Participants in the Zagreb group received the vaccines on day 0, 7, 21, and the Essen group on days 0, 3, 7, 14, 28, alternately on deltoid regions on the left or right arm. In the Zagreb regimen, two doses of vaccines were administered on day 0 and then each one on day 7 and 21, respectively. In the Essen group, each dose of vaccine was provided on days 0, 3, 7, 14, and 28.
Assessment of safety and immunogenicity
All participants were observed for 30 minutes after each vaccination for possible immediate adverse reactions. Any AEs were collected and recorded by observers. AEs were observed and collected during the whole study period and 28 days after the last vaccination. The severity of AEs was assessed according to Adverse Events Grading Guidelines for Preventive Vaccines Clinical Trials issued by China Food and Drug Administration.10 Face-to-face interviews were conducted by investigators on the very day of vaccinations. Structured individual diaries were provided to record solicited systemic and local adverse events. Systemic adverse events include fever, allergic reactions, headache, fatigue, nausea, vomiting, diarrhea, myalgia, cough, and so on. Local adverse reactions were pain, erythema, induration, swelling, rash, and itching.
Blood samples were collected before each single dose on day 0, 7, 14, 42, and on days 180, 365 to assess long-term immunity. For each subject, 3.0 mL blood was collected at each time point. Rabies virus neutralizing antibody (RVNA) titers were assessed with a rapid fluorescence focus inhibition test (RFFIT) by National Institutes for Food and Drug Control (NIFDC). In accordance with WHO criteria, RVNA ≥0.5IU/mL was considered as a protective immune response.11
Statistical analysis
Primary endpoints included circulating RVNA titers and seroconversion rate on days 7, 14 since the first vaccination, and incidence rate of adverse events within 28 days since the first dose. Secondary endpoints were RVNA titers and seroconversion rate on day 42, 180, 365. The safety analysis was assessed by the incidence and severity of systemic and local adverse events and SAEs of two regimen groups. Immunogenicity was analyzed by comparing the geometric mean titers (GMT) of these two groups through student’s t- and chi-square tests. Statistical analysis was performed by an independent statistical institution (School of Public Health, Huazhong University of Science and Technology). All tests were two-sided and α was set as 0.05.
Results
Demographic characteristics
Two hundred and forty participants were included in this study. One hundred and twenty of which were assigned to Zagreb regimen and 120 to Essen regimen. In the safety analysis, 239 subjects completed all doses of vaccination and observations of adverse events. One subject in the age group (>60) in the Zagreb regimen did not vaccinate the 4th dose. In immunogenicity analysis, serum samples were collected on the day of the first vaccination and on days 7, 14, 42, 180, 365 post the first vaccination. A total of 240, 236, 237, 235, 216, and180 participants, respectively, completed the serum sample collections on these time points (Figure 1).
Figure 1.
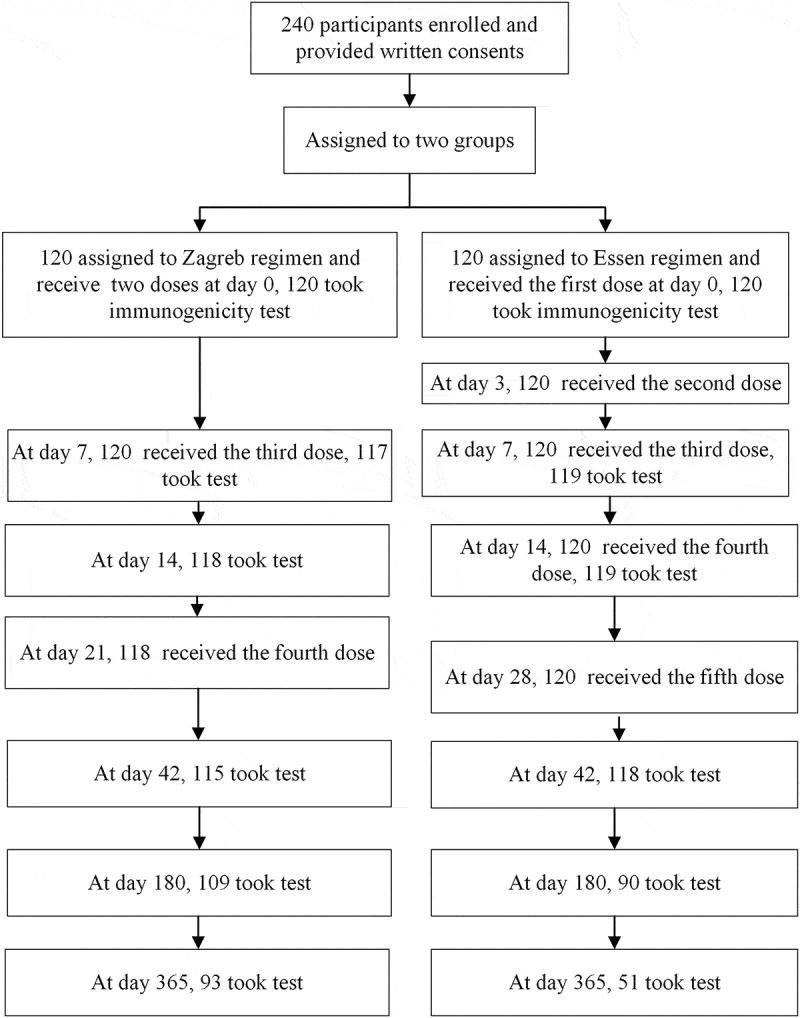
Flow chart of safety and immunogenicity analysis
The average age of the Zagreb group was 36.15 and 36.15 of the Essen group. There was not a statistically significant difference between the two groups (P = .988). The difference within age subgroups was also not statistically significant (Table 1). There were 48 males and 72 females in the Zagreb group, and 50 males and 70 females in the Essen group. Participants of the Zagreb and Essen groups were comparable.
Table 1.
Demographic characteristics of participants enrolled in Zagreb and Essen groups
| Groups | Age |
Gender |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zagreb |
Essen |
t | P | Zagreb |
Essen |
χ2 | P | |||||
| N | mean±sd | N | mean±sd | Male | Female | Male | Female | |||||
| ≤ 10 | 23 | 6.74 ± 1.71 | 17 | 7.18 ± 2.04 | 0.737 | 0.466 | 11 | 12 | 9 | 8 | 0.102 | 0.749 |
| 11–20 | 15 | 17.27 ± 2.55 | 24 | 17.54 ± 1.84 | 0.391 | 0.698 | 5 | 10 | 8 | 16 | 0.000 | 1.000 |
| 21–50 | 41 | 34.56 ± 9.07 | 40 | 35.00 ± 8.63 | 0.223 | 0.824 | 13 | 28 | 16 | 24 | 0.606 | 0.436 |
| 51–60 | 21 | 54.62 ± 2.80 | 17 | 54.18 ± 2.46 | 0.511 | 0.612 | 10 | 11 | 7 | 10 | 0.158 | 0.691 |
| >60 | 20 | 68.10 ± 4.48 | 22 | 66.86 ± 5.37 | 0.806 | 0.425 | 9 | 11 | 10 | 12 | 0.001 | 0.976 |
| Total | 120 | 36.15 ± 21.19 | 120 | 36.15 ± 21.36 | 0.015 | 0.988 | 48 | 72 | 50 | 70 | 0.069 | 0.793 |
Safety
Totally 75 (6.95%) solicited adverse events were recorded during the study and none unsolicited AEs were reported. Among the solicited AEs, 25 (5.22%) local events and 10 (2.09%) systemic events were from the Zagreb regimen; 32 (5.33%) local events and 8 (1.33%) systemic events were from the Essen regimen (Table 2). Most of the AEs were grade 1 which were slight and transient and most of them occurred at early doses. None of the SAEs was found during the study. The difference in incidence rate was not significant between the two groups (Table 2).
Table 2.
Incidence rate of adverse events after each vaccination in Zagreb and Essen regimen
| Observations (%) | Zagreb |
Essen |
P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| D0 N = 240 |
D7 N = 120 |
D21 N = 119 |
D0 N = 120 |
D3 N = 120 |
D7 N = 120 |
D14 N = 120 |
D28 N = 120 |
||
| Local AEs | 25 (5.22) | 32 (5.33) | |||||||
| Total | 18 (7.50) | 6 (5.00) | 1 (0.84) | 14 (11.67) | 7 (5.83) | 7 (5.83) | 3 (2.50) | 1 (0.83) | 0.934 |
| Grade 1 | 17 (7.08) | 6 (5.00) | 1 (0.84) | 14 (11.67) | 7 (5.83) | 7 (5.83) | 3 (2.50) | 1 (0.83) | 0.812 |
| Grade 2 | 1 (0.42) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0.444 |
| Grade 3 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | – |
| Grade 4 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | – |
| Systemic AEs | 10 (2.09) | 8 (1.33) | |||||||
| Total | 10 (4.17) | 0 (0.00) | 0 (0.00) | 6 (5.00) | 2 (1.67) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0.336 |
| Grade 1 | 7 (2.92) | 0 (0.00) | 0 (0.00) | 5 (4.17) | 2 (1.67) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0.671 |
| Grade 2 | 2 (0.83) | 0 (0.00) | 0 (0.00) | 1 (0.83) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0.588 |
| Grade 3 | 1 (0.42) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0.444 |
| Grade 4 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | – |
Incidence rate of local AEs in Zagreb regimen was 6.52%, 5.00%, 5.49%, 4.76%, 3.80% in five age groups successively and in Essen group were 3.53%, 4.17%, 7.50%, 2.35%, 6.36%. Incidence rate of systemic AEs in Zagreb regimen was 5.43%, 3.33%, 0.61%, 1.19%, 1.27% in five age groups and 3.53%, 0, 1.50%, 2.35%, 0 in Essen group. Most of the local and systemic AEs were pain, induration and fever, headache (Table 3).
Table 3.
Incidence rate of adverse events in different age groups of Zagreb and Essen regimen
| Zagreb |
Essen |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age groups | ≤10 | 11–20 | 21–50 | 51–60 | >60 | ≤10 | 11–20 | 21–50 | 51–60 | >60 |
| N | 23 | 15 | 41 | 21 | 20 | 17 | 24 | 40 | 17 | 22 |
| Observations | 92 | 60 | 164 | 84 | 79* | 85 | 120 | 200 | 85 | 110 |
| Local AEs | ||||||||||
| Induration | 0 (0.00) | 1 (1.67) | 1 (0.61) | 0 (0.00) | 1 (1.27) | 0 (0.00) | 0 (0.00) | 1 (0.50) | 0 (0.00) | 0 (0.00) |
| Erythema | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Swelling | 0 (0.00) | 0 (0.00) | 1 (0.61) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (1.00) | 0 (0.00) | 0 (0.00) |
| Pain | 6 (6.52) | 2 (3.33) | 7 (4.27) | 4 (4.76) | 2 (2.53) | 3 (3.53) | 5 (4.17) | 12 (6.00) | 2 (2.35) | 7 (6.36) |
| Itching | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Total | 6 (6.52) | 3 (5.00) | 9 (5.49) | 4 (4.76) | 3 (3.80) | 3 (3.53) | 5 (4.17) | 15 (7.50) | 2 (2.35) | 7 (6.36) |
| Systemic AEs | ||||||||||
| Fever | 5 (5.43) | 2 (3.33) | 1 (0.61) | 0 (0.00) | 0 (0.00) | 3 (3.53) | 0 (0.00) | 2 (1.00) | 1 (1.18) | 0 (0.00) |
| Allergy | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.50) | 0 (0.00) | 0 (0.00) |
| Headache | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (1.19) | 1 (1.27) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (1.18) | 0 (0.00) |
| Dysphoria | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Loss of appetite | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Nausea | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Total | 5 (5.43) | 2 (3.33) | 1 (0.61) | 1 (1.19) | 1 (1.27) | 3 (3.53) | 0 (0.00) | 3 (1.50) | 2 (2.35) | 0 (0.00) |
*Note: One of the fourth dose was dropped out in Zagreb group.
Immunogenicity
Participants’ average GMT at baseline was lower than 0.5 IU/mL and between two regimens, there was no significant difference of baseline GMT overall (P = .585) or within age groups (Figure 2). GMT gradually increased and reached the peak on day 14 (Figure 2). At every single time point, there was no difference between the two groups. Also, in each age group, there was no significant statistical difference of GMT between the two regimen groups, except for age 21–50 on day 7. All participants of the Zagreb group and 99.16% of the Essen group obtained positive seroconversion on day 14. On day 42, the positive seroconversion rate in both groups was 100%. There was no significant difference in seroconversion rate between the two groups (Table 4).
Figure 2.
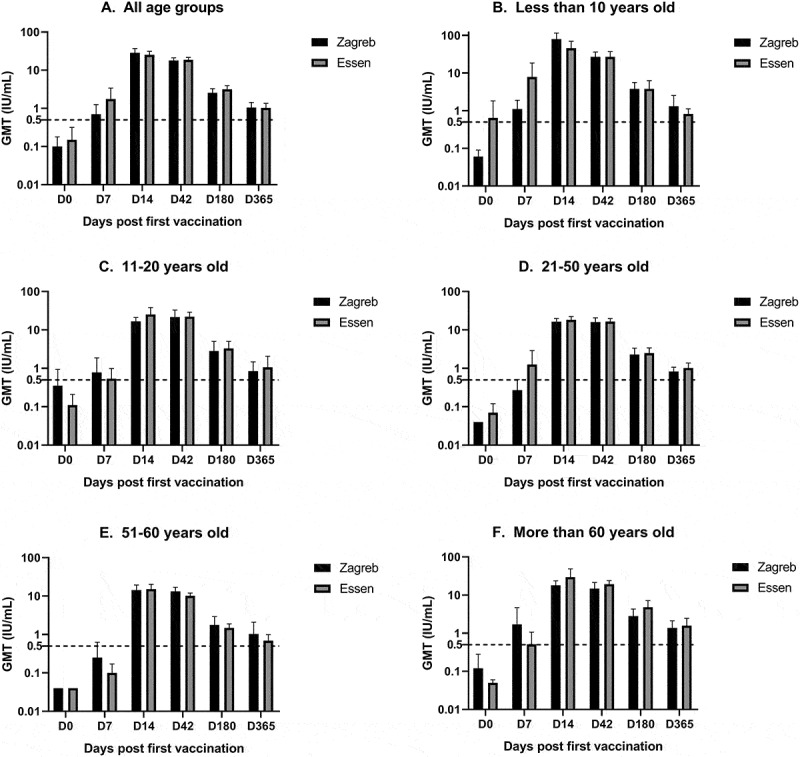
Geometric titers (GMT) of RVNA in patients with different age spans under Zagreb and Essen regimen. Dash line of 0.5 IU/mL is the protective immunity level
Table 4.
Percentage of participants’ GMT ≥0.5 IU/mL on days 0, 7, 14, 42, 180, and 365 in two groups
| Zagreb |
Essen |
Difference (%) | P | |||
|---|---|---|---|---|---|---|
| Observations | Positive (%) | Observations | Positive (%) | |||
| D 0 | 120 | 2 (1.67) | 120 | 3 (2.50) | 0.83 | 1.000 |
| D 7 | 117 | 17 (14.53) | 119 | 19 (15.97) | 1.44 | 0.759 |
| D 14 | 118 | 118 (100.00) | 119 | 118 (99.16) | −0.84 | 1.000 |
| D 42 | 115 | 115 (100.00) | 118 | 118 (100.00) | 0.00 | 1.000 |
| D 180 | 109 | 92 (84.40) | 107 | 90 (84.11) | −0.29 | 0.953 |
| D 365 | 93 | 57 (61.29) | 87 | 51 (58.62) | −2.67 | 0.715 |
To assess the duration of immunogenicity, immunogenicity tests were also evaluated at half- and 1-year intervals. The results indicated that average GMT remained at an adequate protective effect within 1 year (Figure 2), but the positive seroconversion rate decreased (Table 4).
Discussions
Rabies is a deadly disease with nearly 100% mortality. Prompt and properly administered PEP can prevent and control it. Human rabies vaccine immunization is an important step of PEP to elicit enduring immunity. The feasibility of PEP depends on costs, number of doses, time, and compliance.2 Vaccinations under the Essen schedule were widely used in China for postexposed patients. Zagreb regimen was recommended by the WHO expert committee in 199212 and was approved for PVRV in China in 2010.8 Advisory Committee on Immunization Practices (ACIP) from the United States also recommended a 2-1-1 dose schedule with HDCV or PCECV since 2010.13 With three clinic visits and four doses, the Zagreb schedule is more timesaving and cost-effective for patients compared with the Essen regimen, and may lead to better compliance. A previous study stated that expenditure of rabies vaccine immunizations almost took up 1/10 of the annual revenue of ordinary rural residents.14 Compliance with vaccinations was directly affected by costs.15 Low economic status and accessibility were associated with vaccinations delay in India.16 Advisory Committee on Immunization Practices (ACIP) from the United States has recommended four-dose course for HDCV and PCECV since 2010 and has estimated that switching from five-dose schedule to five-dose schedule would save 16.6 USD million costs or so to the U.S. health-care system, assuming 100% compliance.13 Individual costs for human rabies vaccinations via Zagreb and Essen regimens in China were, respectively, 482.2RMB ($72) and 694RMB ($104).8,17 The estimated nationwide and annual costs for Zagreb and Essen regimen were 6.746 billion and 9.709 billion RMB.17 Whereas this regimen was not widely used in China, though previous studies found that both regimens performed well in safety and immunogenicity. Intradermal immunizations were also cost-effective and recommended by the WHO position paper, yet it was always not permitted to be applied in China, so this study did not include this regimen. In this study, we aimed to further compare the safety, immunogenicity, and duration of immunity of lyophilized PVRV under Zagreb and Essen procedures, with a population of a large age span and would provide evidence for reasonably using the Zagreb schedule.
Regarding safety, no SAEs occurred in this study, especially some SAEs that occurred in previous case reports like acute disseminated encephalomyelitis,18 severe allergic reaction,19 severe Henoch Schönlein purpura,20 and anaphylaxis.21 Most of the AEs were slight and moderate (Grade 1 and 2). Only one systemic AE which was grade 3 occurred under the Zagreb regimen on day 0. There was no difference between the two regimens on the total incidence of AEs and severity of AEs in this study. The most frequent local AEs were pain; several induration and swelling were observed. The most usually observed systemic AEs were fever, and one case occurred allergy, three cases occurred headache. There was no significant difference between Zagreb and Essen regimen on the incidence of AEs. The results of safety analysis in this study were similar to previous research.8,22
In immunogenicity analysis, the baseline GMT of participants in the two groups was similar, which indicated that they were comparable. There was no statistical difference on baseline GMT between the two groups at age groups. Compared to participants aged 11–20 in this study, GMT in children aged 6–17 on day 14 and on day 42 was higher in a previous study,23 but in adults aged 18–50 and more than 51, GMT on days 14 and 42 were higher in this study than previous literature.23,24 GMT in healthy adults received PCECV (purified chick embryo cell vaccine) on days 7 and 14 were similar with this study.25 On day 0, the positive seroconversion rate was 1.67% and 2.50% at the four-dose group and five-dose group. On day 7, the seroconversion rate increased to 14.53% and 15.97% relatively and there was no difference between the two groups, which was consistent with previous studies.8,24 Yet there was a discrepancy with other research, in which seroconversion rate on day 7 in Zagreb and Essen group was nearly 70% and 46.7%.14 The seroconversion rate on day 7 in this study was obviously lower than that in the preliminary clinical trial and some other studies. These differences between this study and previous studies were possibly because of the participants’ variation and the difference of vaccine potency from different batches. In order to meet the Chinese Pharmacopoeia’s demand that human rabies vaccine production potency is supposed to above 4.0 IU/mL and remain above 2.5 IU/mL before expiring date,26 and potency of many vaccines manufactured was much higher than the standard in varying degrees.
The estimated sample size based on the seroconversion rate on day 7 (14.53% vs. 15.97%) and formula (1) is 13084 in each group, which reminds us in the future study, it is needed to increase the sample size. In another study about purified chick embryo cell vaccine, there was also a difference of seroconversion between Zagreb and Essen on day 7, approximately 71% and 57%.22 On day 14, a 100% positive rate was achieved under the Zagreb regimen and nearly reached under the Essen group. On day 42, the positive rate in both groups reached 100%. To assess the immunity durability, antibodies tests were also performed at the half- and one-year interval. About 84.40% and 84.11% of the participants remained positive in half year. The positive rate decreased relatively to 61.29% and 58.62% in one year. Previous study documented that if the patient is exposed afterward, one booster vaccination is enough to trigger expected protective immunity.27
The safety and immunogenicity of lyophilized PVRV between the two regimens were similar in this study. Considering that the Zagreb schedule is a convenient approach for patients and may make good compliance, it could be a promising option for clinics to promote using the Zagreb regimen.
There were several limitations in this study. China did not import the same kind of vero cell rabies vaccines, comparisons of safety and immunogenicity between different vaccines were not performed in this study. The sample size was not too large so rare AEs were not observed.
Acknowledgments
All authors highly appreciate each subject’s participation, NIFDC for laboratory tests and School of Public Health of Huazhong University of Science and Technology for data analysis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, et al. Estimating the global burden of endemic canine rabies. Plos Neglect Trop D. 2015;9(4):e0003709. doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Rabies vaccines: WHO position paper-April 2018. [DOI] [PubMed]
- 3.Yao H, Yang Y, Liu K, Li X, Zuo S, Sun R, Fang L-Q, Cao W-C.. The spatiotemporal expansion of human rabies and its probable explanation in Mainland China, 2004-2013. Plos Neglect Trop D. 2015;9(2):e0003502. doi: 10.1371/journal.pntd.0003502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou H, Vong S, Liu K, Li Y, Mu D, Wang L, Yin W, Yu H. Human rabies in China, 1960-2014: a descriptive epidemiological study. Plos Neglect Trop D. 2016;10(8):e0004874. doi: 10.1371/journal.pntd.0004874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang Y, Chen L, Liu MQ, Zhu ZG, Zhu ZR, Hu Q. Comparison of safety and immunogenicity of PVRV and PCECV immunized in patients with WHO category II animal exposure: a study based on different age groups. PLoS Negl Trop Dis. 2014;8(12):e3412. doi: 10.1371/journal.pntd.0003412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Zhang X, Song Q, Tang K. Promising rabies vaccine for postexposure prophylaxis in developing countries, a purified vero cell vaccine produced in China. Clin Vaccine Immunol. 2010;17(4):688–90. doi: 10.1128/CVI.00433-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Sun M, Zhang X, Suo L, Xu R, Zou Y, Zuo L-B, Qi H. Safety and immunogenicity of two freeze-dried Vero cell rabies vaccines for human use in post-exposure prophylaxis. Vaccine. 2011;29(15):2679–81. doi: 10.1016/j.vaccine.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 8.Shi N, Zhang Y, Zheng H, Zhu Z, Wang D, Li S, Li Y, Yang L, Zhang J, Bai Y, et al. Immunogenicity, safety and antibody persistence of a purified vero cell cultured rabies vaccine (Speeda) administered by the Zagreb regimen or Essen regimen in post-exposure subjects. Hum Vacc Immunother. 2017;13(6):1338–45. doi: 10.1080/21645515.2017.1279770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hampson K, Cleaveland S, Briggs D. Evaluation of cost-effective strategies for rabies post-exposure vaccination in low-income countries. PLoS Negl Trop Dis. 2011;5(3):e982. doi: 10.1371/journal.pntd.0000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.China Food and Drug Administration . The standard guidelines for adverse reactions grading of vaccine clinical trials. 2005.
- 11.WHO Position Paper on rabies vaccines. Wkly Epidemiol Rec. 2010;85:309–20. [Google Scholar]
- 12.WHO Expert Committee on Rabies . Guide for post-exposure treatment. Eighth report. WHO technical report 824. Geneva Switzerland: World Health Organization; 1992. [Google Scholar]
- 13.Rupprecht C, Briggs D, Brown C, Franka R, Katz SL, Kerr HD. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the advisory committee on immunization practices. MMWR Recommendation Rep. 2010;59:1–9. [PubMed] [Google Scholar]
- 14.Liu H, Huang G, Tang Q, Li J, Cao S, Fu C, Cao Q, Liu B, Pan H, Wang M, et al. The immunogenicity and safety of vaccination with purified Vero cell rabies vaccine (PVRV) in China under a 2-1-1 regimen. Hum Vaccin. 2014;7(2):220–24. doi: 10.4161/hv.7.2.14003. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Luo F, Feng Z, Li L, Bai Y, Ai X, Ma J, Zhang Z, Shi N. Immunogenicity and safety of purified vero cell rabies vaccine (PVRV) produced by Liaoning Cheng Da Co. under Zagreb 2-1-1 or 5-dose Essen regimen in Chinese adults aged 50 and above. Hum Vaccin Immunother. 2017;13(1):144–50. doi: 10.1080/21645515.2016.1230260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph J, Khan NS, Khan AM, Rajoura OP. Determinants of delay in initiating post-exposure prophylaxis for rabies prevention among animal bite cases: hospital based study. Vaccine. 2013;32(1):74–77. doi: 10.1016/j.vaccine.2013.10.067. [DOI] [PubMed] [Google Scholar]
- 17.Wang CL, Zhang XW, Yu YX. Study on the compliance and economic cost of rabies vaccination. Chin J Vacc Immunization. 2010;16(3):254–57. [PubMed] [Google Scholar]
- 18.Peng J, Chen L, Zhu Z, Zhu Z, Hu Q, Fang Y. Effect of Corticosteroids on RVNA production of a patient with acute disseminated encephalomyelitis following rabies vaccination as well as administration of HRIG. Hum Vacc Immunother. 2015;10(12):3622–26. doi: 10.4161/21645515.2014.979621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang Y, Liu M, Chen L, Zhu Z, Zhu Z, Hu Q. Rabies post-exposure prophylaxis for a child with severe allergic reaction to rabies vaccine. Hum Vacc Immunother. 2016;12(7):1802–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu ZG, Zheng Y, Lu S, Hu Q, Fang Y. Rabies post-exposure prophylaxis for a male with severe Henoch Schonlein purpura following rabies vaccination. Hum Vaccin Immunother. 2018;14(11):2666–68. doi: 10.1080/21645515.2018.1486354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang S, Zhu Z, Cai L, Zhu Z, Zhang M, Hu Q, Fang Y. Analysis on the risks of severe adverse events in rabies post-exposure prophylaxis and appropriate decision-making procedure. Hum Vacc Immunother. 2018;15(9):2121–25. doi: 10.1080/21645515.2018.1533779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Q, Liu M, Zhu Z, Zhu Z, Lu S. Comparison of safety and immunogenicity of purified chick embryo cell vaccine using Zagreb and Essen regimens in patients with category II exposure in China. Hum Vacc Immunother. 2014;10(6):1645–49. doi: 10.4161/hv.28420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li R, Li Y, Wen S, Wen H, Nong Y, Mo Z, Xie F, Pellegrini M. Immunogenicity and safety of purified chick-embryo cell rabies vaccine under Zagreb 2-1-1 or 5-dose Essen regimen in Chinese children 6 to 17 years old and adults over 50 years: a randomized open-label study. Hum Vaccin Immunother. 2015;11(2):435–42. doi: 10.4161/21645515.2014.994460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma J, Wang H, Li J, Chang L, Xie Y, Liu Z, Zhao Y, Claudius M. A randomized open-labeled study to demonstrate the non-inferiority of purified chick-embryo cell rabies vaccine administered in the Zagreb regimen (2-1-1) compared with the Essen regimen in Chinese adults. Hum Vaccin Immunother. 2014;10(10):2805–12. doi: 10.4161/21645515.2014.972773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahendra BJ, Narayana DA, Agarkhedkar S, Ravish HS, Harish BR, Agarkhedkar S, Madhusudana SN, Belludi A, Ahmed K, Jonnalagedda R, et al. Comparative study on the immunogenicity and safety of a purified chick embryo cell rabies vaccine (PCECV) administered according to two different simulated post exposure intramuscular regimens (Zagreb versus Essen). Hum Vaccin Immunother. 2015;11(2):428–34. doi: 10.4161/21645515.2014.995059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chineses Pharmacopoeia Commission . Pharmacopoeia of people’s Republic of China. Beijing: China Medical Science and Technology Press; 2010. [Google Scholar]
- 27.Zhang X, Zhu Z, Wang C. Persistence of rabies antibody 5 years after postexposure prophylaxis with vero cell antirabies vaccine and antibody response to a single booster dose. Clin Vaccine Immunol. 2011;18(9):1477–79. doi: 10.1128/CVI.05090-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


