Abstract
In the current era of precision medicine, there is renewed interest in radiopharmaceutical therapy and theranostics. The approval of somatostatin receceptor directed therapy and norepinephrine transporter targeted 131I-MIBG therapies by the FDA and the rapid progress of highly promising beta and alpha emitter tagged PSMA directed therapy of prostate cancer have stimulated clinically impactful changes in practice. Many novel strategies are being explored and novel radiopharmaceutical therapeutic agents including peptide based ligands as well as antibodies or antibody fragments are being developed preclinically or are in early phase clinical trials. While beta particle emitters have most commonly been used for targeted radiotherapy and radioimmunotargeting, there is an emerging interest in alpha emitters that cause greater density of ionization events leading to increased double-strand DNA damage and cluster breaks because of the high-energy particles within a shorter tissue range of penetration and thereby lower toxicity to adjacent normal tissues.
Radioimmunotargeting with antibodies is attractive for highly selective targeting of tumor antigens expressed on cancer cells. The vast development of immune therapies for cancer treatment in the past decade has also accelerated interest in exploring radiotargeted antibody agents for therapy. While high specificity of these antibodies is a distinct advantage, antibodies are limited by large size, long circulation time and slow biological clearance that can lead to larger radiation-absorbed doses to normal organs and blood. Several novel strategies such as multistep targeting or click chemistry mechanisms may be used to optimize the tissue doses, and these approaches appear promising. There is also major development in production of small molecule radioligands that have the advantage of fast clearance and rapid accumulation in target sites enabling early assessment and therapeutic tumor targeting. However, the renal clearance of smaller molecules can pose challenges as well due to radiation exposure to normal tissue and requires optimization of therapeutic doses.
This short review is focused on an overview of these novel targeted agents under development including preclinical studies and clinical trials; barring details on those that are discussed in other reviews in this issue. Finally we conclude with a consideration of what is needed for effective implementation of these novel agents and the future growth of this clinical therapy in the context of oncologic care.
Novel Radiotargeted Agents for Theranostics Under Development
Established clinical modalities such as CT, MRI and FDG-PET are powerful techniques for diagnosing and monitoring cancer; however, they are not ideal for stratifying patients for targeted therapies. Targeted molecular imaging agents are designed to fill this gap and several of these are also under preclinical investigation as targets for radiopharmaceutical therapies
CD38-Targeted Imaging and Therapy
An exciting emerging target in imaging and therapy is the cluster of differentiation 38 (CD38), one of the earliest cell surface molecules identified in the late 1970s. Initially considered an activation marker, CD38 was later recognized as a pleiotropic antigen that could serve as a multifunctional ectoenzyme as well as a receptor. Functionally, the extracellular enzymatic component of CD38 metabolizes nicotinamide adenine dinucleotide (NAD+) to induce Ca2+ signaling molecules, adenosine diphosphate ribose and cyclic adenosine diphosphate ribose at neutral pH. In acidic pH, CD38 metabolizes nicotinamide adenine dinucleotide phosphate (NADP+) to nicotinic acid adenine dinucleotide phosphate (NAADP). As a receptor, CD38 can mediate cell adhesion, differentiation, and proliferation, following ligation with agonistic mAbs or its natural ligand, CD31 (PECAM-1).
CD38 plays a critical role in malignancies such as B cell chronic lymphocytic leukemia and multiple myeloma (MM).1 The relatively high expression of CD38 on malignant plasma cells combined with its role in modulating intracellular signaling makes it an attractive target for imaging of diseases such as MM. Daratumumab is a CD38 targeted antibody that was approved by the FDA in 2015 and is most advanced clinically for targeting CD38 in patients with MM. It has demonstrated a favorable safety profile with encouraging results as a monotherapy or as part of a triplet myeloma treatment regimen.2
Ghai et al. were first to demonstrate PET imaging of CD38 on MM cells in preclinical mouse models using radiolabeled daratumumab, [89Zr]-DFO-daratumumab3 (Fig. 1); while Caserta et al. later performed PET imaging of MM with copper-64 [64Cu]-daratumumab.4 Radiolabeled daratumumab was also used to image lymphoma,5 and it has been explored as an imaging target in lung cancer.6
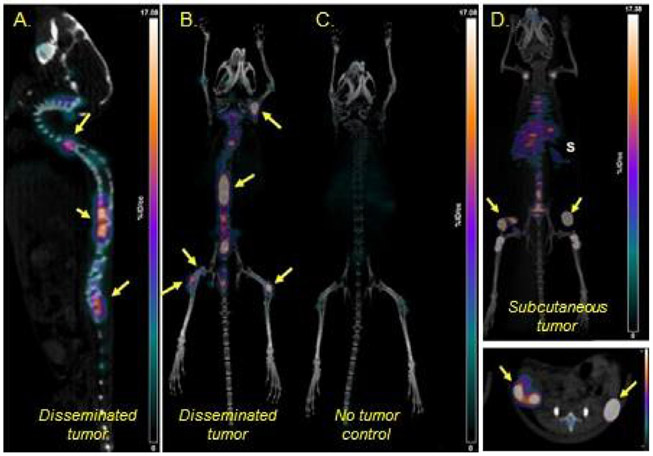
Figure 1.
(A-D) Representative maximum-intensity-projection (MIP) [89Zr]Zr-DFO-daratumumab PET/CT images of disseminated MM.1S-CG tumor–bearing mouse, nontumor control mouse and subcutaneous MM.1S-CG tumor–bearing SCID mice 7 days post injection of radiopharmaceutical. S = spleen; Yellow Arrows = tumors. Scale: 0-17 %ID/g. DFO, Desferoxamine, chelator for [89Zr]. (part of figure reprinted from JNM 2018, (ref. 3)).
89Zr-DFO-daratumumab is currently undergoing assessment in human clinical trials (NCT03665155). Preliminary results show disease sites well targeted (Fig. 2)7 and that 89Zr-DFO-daratumumab is an attractive candidate for directed therapy with alpha-emitters such as 225Ac. In mouse models mechanistic and feasibility studies of using 225Ac-daratumumab have demonstrated8 increased antitumor activity ~30-fold compared to 89Zr-DFO-daratumumab, without negatively impacting its inherent functional mechanistic properties.
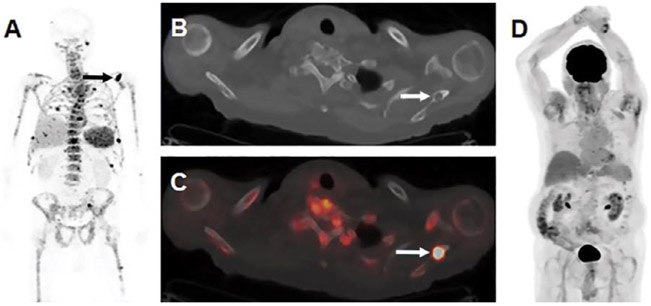
Figure 2.
Images show visualization of skeletal myeloma by using immuno-PET antibody composed of native daratumumab labeled with positron-emitting radionuclide zirconium 89 (89Zr) through chelator deferoxamine (DFO), or 89Zr-DFO-daratumumab, in an 80-year-old man with osseous myeloma. (A) Maximum intensity projection (MIP) image from 89Zr-DFO-daratumumab PET/CT demonstrates multiple foci of osseous avidity, including left scapular focus (arrow). (B) Axial CT and, (C) fused PET/CT images from 89Zr-DFO-daratumumab PET/CT demonstrate left scapular focus localizes to lytic osseous lesion at CT (arrows). (D) MIP image from fluorine 18 fluorodeoxyglucose PET/CT 1 week prior fails to identify lesions seen at 89Zr-DFO-daratumumab PET/CT. (reprinted from Radiology, 2020 (ref. 31).
While highly specific, antibodies are not always ideal for longitudinal imaging studies due to their long biological halflife. High-affinity peptide-based small molecule imaging agents are nonimmunogenic and display relatively quick uptake by the tumor tissue with rapid clearance from nontarget organs in vivo. The Shokeen Lab is developing new CD38 targeted peptide based PET imaging probes specific for enzymatically active CD38 conformation that will overcome the limitations of existing antibody based bioconjugates. These new functional probes will be used for defining basic cellular and molecular pathways as well as the spatial relationships that govern MM-associated CD38 expression and activity. This approach could be clinically transformative for identifying those patients that are most sensitive and most resistant to anti-CD38 monoclonal immunotherapies, as currently the success rate of CD38-targeted therapies is ~30%.
VLA-4 Targeted Imaging and Therapy
Integrins are transmembrane adhesion receptors representing a type I class of αβ-heterodimers that are activated via conformational changes in their extracellular domains in response to signaling events inside the cell. The transmembrane location of integrins on the cell surface connects the cytoskeleton with the microenvironment and serves to activate intracellular signaling pathways in response to environmental stimuli. Integrins play a prominent role in hematopoiesis, immune response, cell development, and trafficking in both normal and diseased cells. In cancer, inside-out and outside-in signaling modulates cell functions, including proliferation, polarity, gene expression, differentiation, and survival. There is evidence supporting the mechanistic role of integrin receptor adhesion molecules in facilitating the binding of cancer cells to the bone marrow stromal components and mediating survival and drug resistance. Very Late Antigen-4 (VLA-4, α4β1, and CD49d/CD29) is an adhesion receptor that participates in normal immune responses as well as cancer pathogenesis.9 VLA-4 is over-expressed in MM cells, and is strongly implicated in myeloma pathogenesis, such as cell-cell interactions, trafficking, proliferation, and drug resistance.10 High levels of VLA-4 correlate with drug resistance and poor prognosis in MM patients. The biological and clinical significance of VLA-4 in disease and its high expression in cancer cells makes it a valuable molecular imaging and therapeutic target.
In a mouse model of MM, Soodgupta et al. demonstrated VLA-4 targeted PET using the radiopharmaceutical, 64Cu-LLP2A.11 64Cu-LLP2A-PET/CT (static and dynamic) imaging was conducted in C57BL6/KaLwRij mice bearing murine 5TGM1-GFP syngeneic tumors generated after intravenous tail-vein injection. Semi-quantitative standard uptake values and the increased number of tumor lesions detected (qualitative visual assessment) by 64Cu-LLP2A/PET corresponded with increased tumor secreted monoclonal (M) protein (g/ dL) in tumor bearing mice over time (3.29 ± 0.58 g/dL at week 0 and 9.97 ± 1.52 g/dL at week 3) (Fig. 3).12 A first-in-human imaging trial is ongoing at Washington University to evaluate human dosimetry and safety of 64Cu-LLP2A (NCT03804424). Thus far, no adverse effects have been observed. The team is hoping to evaluate the tracer for its potential to guide therapies.
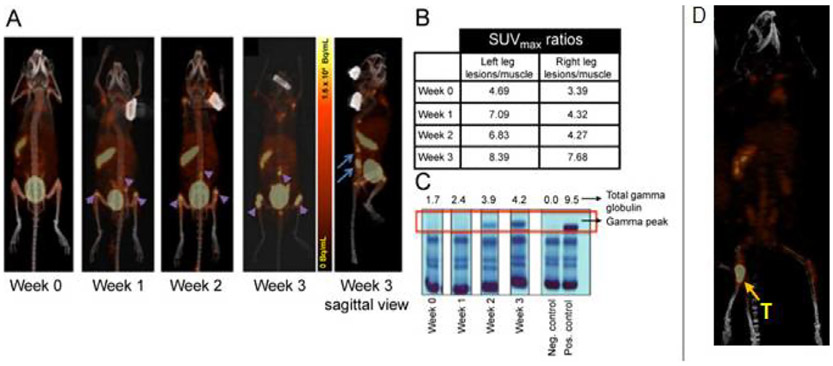
Figure 3.
(A) Representative images of a disseminated 5TGM1/KaLwRij mouse model imaged longitudinally with 64Cu-LLP2A/PET/CT. Sagittal view shows medullar lesions in the BM rich pelvic bone and spine (4 hours post radiotracer administration image, 1.85 MBq/mouse). (B) SUV ratios of the medullar tumor lesions to muscle. (C) Serum Protein Electrophoresis (SPEP) gel (g/dL) showed tumor progression over time. (D) In an orthotopic 5TGM1/KaLwRij intratibial mouse model, 64Cu-LLP2A selectively localized at the tumor site in the BM lesion (24 hours post radiotracer administration image, 1.85 MBq/mouse, Specific Activity: 45-57 MBq/nmol). (part of figure reprinted from JNM 2016, (ref. 12).
Initial evaluation of VLA-4 targeted imaging and treatment in metastatic melanoma has also shown potential.13 More recently, VLA-4 targeted imaging and targeted radionuclide therapy in metastatic melanoma tumors was evaluated.14 In mice bearing B16F10 mouse melanoma tumors, the efficacy of 177Lu-LLP2A alone and combined with immune checkpoint inhibitors showed enhanced survival in the targeted radionuclide therapy plus immune checkpoint inhibitor group. Roxin et al developed an elegant approach to imaging VLA-4 in murine melanoma models using 18F-Labeled Radiotracer: [18F]DOTA-AMBF3-LLP2A.15
CXCR-4 Targeted Imaging and Therapy
Significant advances have been made in the imaging and therapy of the C-X-C chemokine receptor 4, CXCR-4. In cancer pathogenesis, CXCR-4 is implicated in survival, proliferation and metastasis. CXCR-4 imaging has been demonstrated in acute lymphoblastic and myeloid leukemia (ALL, AML),16 invasive breast cancer17 and MM.18 CXCL-12 is the natural ligand for CXCR-4; and the CXCR-4/CXCL-12 axis is an attractive theranostic target. Plerixafor is a clinically used CXCR-4 antagonist that can disrupt the CXCR-4/CXCL-12 axis. The radiolabeled analogs of CXCR-4 antagonists have been used for imaging and beta-therapy. There are ongoing early phase 1 [68Ga]Pentixafor/PET clinical trials in MM (NCT03436342) and lymphoma (NCT03335670) patient populations. The first-in-human experience with [177Lu]/[90Y]Pentixather in MM patient population has shown promising results.
Nontraditional Approaches to Classical Radiation Therapy
An exciting new emerging domain in systemic radiation therapy is Cherenkov radiation mediated therapy. Cherenkov luminescence results from the interaction of a charged particle such as a β particle traveling faster than the phase velocity of light within a dielectric medium. Imaging and therapeutic studies using Cherenkov radiation have recently demonstrated feasibility in preclinical settings.19 Clinically, the noninvasive use of Cherenkov luminescence imaging has been piloted in patients with lymphoma, leukemia and metastatic lymph nodes (NCT01664936). These developments hold the promise of synergistic effects of Cherenkov radiation emitting therapeutic radionuclides combined with systemic therapies at safe and effective levels in a wider patient population.
Small Molecules in Clinical Development
Marked advances have been made in the development of small molecules for targeted radiopharmaceutical therapy. The positive and relatively low risk experience with 177Lu-dotatate has paved the way for rapid acceptance of additional agents in the clinic. The authors have chosen a few to highlight.
Fibroblast Activation Protein Theranostic Agents
Activated fibroblasts express fibroblast activation protein (FAP) on their cell surfaces as compared with resting fibroblasts.20 These cells are found in the context of wound healing, active fibrosis and in the tumor associated stroma of a large number of epithelial tumors in adults.21 The degree of presence of FAP in cancers is thought to be directly related to prognosis, suggesting that these cells play a major role in cancer development and growth.22 Recently, much work has been done in exploring this target as both an imaging agent and a therapeutic agent.
FAP imaging has been performed with targeted antibodies and small molecules for various pathologies including arthritis, atherosclerotic plaques and tumors.22-24 An anti-FAP antibody labeled with 131I has been trialed as a therapeutic agent in metastasized FAP positive cancers.25 131I-sibrotuzumab was slow to clear from the blood pool but did effectively accumulate in neoplastic lesions larger than 1.5cm. It is worth noting that imaging with 131I is suboptimal due to high energy gamma photons that result in low resolution images. Hence, PET radiopharmaceuticals would allow for improved detection of smaller lesions with high resolution.
Haberkorn et al. developed a small molecule agent modeled after a FAP specific inhibitor (FAPI) which is internalized, and were able to demonstrate promising uptake on diagnostic scans in animals and human subjects.22 The 68Ga FAPI agent showed excellent uptake in tumors in 3 patients with different cancers (lung, breast and pancreas) with relatively low background uptake in healthy tissue (Fig. 4). The agent is rapidly cleared by the kidneys without significant retention in the parenchyma.
Figure 4. Targeting the disease with FAPI04 (ref.26).
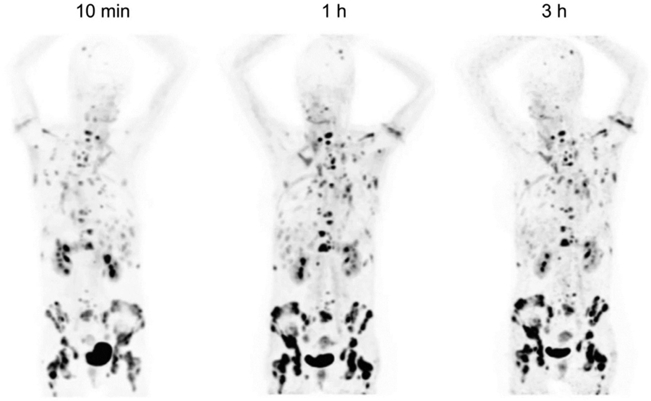
68Ga-FAPI-04 images of a patient with metastasized breast cancer shows excellent uptake in tumor with low uptake in normal tissue. Thomas Lindner et al. J Nucl Med 2018;59:1415-1422 (26).
Several iterations of the FAPI molecular imaging agent have been developed. FAPI-01 was quickly deiodinated resulting in short resident time within the tumor cells. FAPI-02 when linked with a DOTA showed a significant improvement in tumor residence when compared with FAPI-01.22,26 Ultimately, FAPI-04 iteration of the compound was found to have a higher tumor uptake and retention time without increasing exposure to normal tissue26 Kratochwil et al. looked at multiple tumors and assessed the standard uptake values on PET/CT in lesions after injection of FAPI. The highest values were seen in sarcomas, esophageal, breast, cholangiocarcinoma and lung cancer while the lowest values were seen in pehochromocytoma, differentiated thyroid and gastric cancer.27
The DOTA construct of the FAPI agents allows for the synthesis of therapeutic agents in particular 90Y and 177Lu. Lindner et al. trialed a 90Y FAPI-04 agent in a breast cancer patient with bone metastases which resulted in reduction in opioid medication administration for pain.26 Even with FAPI-04 the radiopharmaceutical residence time in tumor tissue is not optimal and improved iterations of the agent which result in longer retention in tumor tissue are likely to yield better results.28
Neurotensin Theranostic Agents
Neurotensin is a peptide that is present in the central nervous system, GI tract and the heart serving various roles ranging from pain processing to intestinal motility to name a few.29-31 Neurotensin receptors are also found in several malignancies including small cell and non-small cell lung cancer, colorectal cancer, breast and pancreatic cancer.30,32 Neurotensin binding may augment tumor growth. 177Lu-3BP-227 a neurotensin receptor 1 (NSTR1) antagonist therapy agent was administered to 6 patients with advanced pancreatic cancer. Five out of 6 patients demonstrated significant radiopharmaceutical uptake in tumor lesions.33 Phase I/II trials are currently underway to determine optimal safety and optimal dose with a phase II trial evaluating drug response in patients with pancreatic and colorectal cancers (NCT03525392).
Gastrin Peptide Receptor
Gastrin-releasing peptide receptors are highly expressed in several solid tumors including prostate, breast and small cell lung cancer.34 Radioantagonists of the gastrin-releasing peptide receptors are emerging theranostic treatment modalities in solid cancers.35 Phase I/IIa clinical trials are underway to determine the safety, tolerability, dosimetry and anti-tumor activity of 177LuNeoB in patients with GRPR positive solid tumors (NCT03872778).
Radiolabeled Antibodies
Prostate Cancer
Several theranostic agents are under investigation for prostate cancer. Novel targets beyond the PSMA directed radioligand therapy include antibodies to PSMA or human kallekrien receptor (HK2). In contrast to ligands, the biodistribution of antibodies is low in organs such as the salivary glands, kidneys and small bowel. Monoclonal antibody J591 is an anti PSMA antibody that targets the external domain of prostate-specific membrane antigen (PSMA). PET imaging with 89Zr-J591 PET imaging shows excellent uptake in bone and soft tissue, and targeted therapy with 90Y and 177Lu-J591 is feasible.36 89Zr- J591 PET imaging can be used as a theranostic companion diagnostic to select patients for 177Lu/225Ac-J591 therapy and for dosimetry.
In phase 1 trials in metastatic castrate resistant prostate cancer, 90Y-J591 and 177Lu-J591 have been administered in escalating doses. Dose limiting toxicity of 90Y-J591 was noted at 20 mCi/m2 (0.74 GBq/m2) dose related to hematologic toxicity and maximum tolerated dose was 17.5 mCi/m2 (0.65 GBq/m2).37 A phase 2 trial in 47 patients with mCRPC included single treatment with 177Lu-J591 at dose of 65 mCi/m2 [2.4 GBq/m2] in 15 patients and at 2.6 GBq/m2 (70 mCi/m2) dose in 32 patients.28 Toxicity was primarily hematologic.38 A total of 10.6% experienced ≥50% decline in PSA, 36.2% experienced ≥30% decline, and 59.6% experienced any PSA decline following their single treatment while a significantly larger number of patients experienced ≥30% PSA declines (46.9% vs 13.3%) and longer survival (21.8 vs 11.9 months) at the higher dose level of 2.6 GBq/m2 (70 mCi/m2) as compared to the lower dose. Hematological toxicities were manageable and not limiting at these doses. Partial radiographic response was noted in 1 of 12 patients with measurable disease and 8 had stable disease. Myelosuppression was dose-limiting at higher levels. A combination study of 177Lu radiolabeled monoclonal antibody HuJ591 (177Lu-J591) and ketoconazole in patients with prostate cancer is underway (NCT00859781). PSMA-directed beta or alpha emitters delivered via antibodies may have a lower rate of xerostomia or nausea in comparison to those delivered by small molecules.
Alpha therapy with 225Ac-J591 in a phase 1 dose-escalation trial in 22 patients with progressive mCRPC have been treated with a single-dose of 225Ac-J591 at 7 dose levels, ranging between 13.3 and 93.3 kBq/Kg (NCT03276572). Preliminary results are favorable and show that 225Ac-J591 is well tolerated with early evidence of clinical activity in a heavily pretreated population that also includes patients who have received prior 177Lu-PSMA therapy.39 Imaging was not required for eligibility though 68Ga-PSMA11 PET was performed prior to treatment. Patients had PSA ranging from 4.8 to 7168.4 ng/dL and disease extent included 82% bone, 36% lymph node, 9% liver metastasis. Overall toxicity was low; 1 patient developed DLT (grade 4 anemia and thrombocytopenia). Recommend phase 2 dose is 93.3 KBq/Kg . Overall early results suggest low side effects, especially xerostomia, and may be advantageous.39
A phase 1 study with an alpha particle emitting thorium-227 (227Th) radiolabeled antibody-chelator conjugate, BAY 2315497 is underway in patients with metastatic castration-resistant prostate cancer (NCT03724747). BAY 2315497 is a PSMA specific humanized IgG1 monoclonal antibody conjugated with an octadentate 3,2-HOPO chelator (BAY 1903150), which is conjugated with the antibody to form the antibody-chelator conjugate (BAY 2287409). Patients with refractory metastatic castration resistant prostate cancer or those who are not eligible for other therapies are eligible and receive escalating doses of cold and 227Th radiolabeled antibody. Alpha emission of 227Th have high linear energy transfer, with a range of <100 μm with daughter decays including radium 223 with overall 5 alpha particles and 2 beta particles emissions that induce clustered double-strand DNA breaks, resulting in severe cell damage and cell death. BAY 2315497, as an antibody, is unlikely to bind to the salivary glands, as has been shown with other antibody-based therapy such as J591.
The phase III registration study of a 177Lu-PSMA-directed therapy and a second randomized phase II trial have completed accrual (VISION NCT03511664 and TheraP NCT03392428). 177Lu-PSMA data have shown a response rate of approximately 50% in mCRPC40 and while alpha labeled 225Ac-PSMA ligand treatment may be beneficial in those who did not respond or progressed following Lu-177 PSMA, side effects of xerostomia appears limiting. In a study with 225Ac-PSMA-617 after 177Lu-PSMA failure, xerostomia occurred after the first cycle and 6 of 23 patients discontinued 225Ac-PSMA-617 therapy despite good tumor control.41 Alternate treatment options are therefore needed and antibody 227Th BAY 2315497 PSMA directed alpha therapy may provide an option in such heavily pretreated patients.
Another approach that is being explored is the combination of PSMA directed therapy with poly[adenosine diphosphate ribose] polymerase (PARP) inhibitors (PARPi’s), that are emerging as a useful therapy of mCRPC. Studies have shown that prolonged and durable responses could be achieved with PARPi’s even in patients who have received multiple prior effective therapies for mCRPC.42 Since these agents inhibit the PARP-mediated DNA repair and since PARP inhibitors may also increase tumor sensitivity to DNA-damaging agents, a combination with alpha radiation that also causes potent DNA damage and cleaves dsDN, is likely to enhance the independent anticancer effects of either agent. This may be an important strategy in patients who have DNA repair defects as well as those resistant to hormone therapy and with unselected genomic profiles.
A novel target for prostate cancer is Human Kallikrein-Peptidase 2 (hK2), which is a trypsin-like antigen produced by columnar epithelial cells of the prostate gland. hK2 is largely expressed in normal prostate with higher expression in prostate cancer and negligible expression in other normal tissues. It is driven by androgen receptor signaling identical to prostate specific antigen (PSA)43 but the circulating levels of hK2 are very low and hK2 independently improved discrimination of severity of disease by 2%.44 The expression of hK2 is highly specific for prostate adenocarcinoma and increases with disease progression, hK2-targeted therapies seem attractive.
A humanized antibody (h11B6) that is specific for hK2, has been developed and demonstrated selective binding to the active form of hK2. In preclinical models, 111 In-1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) h11B6 showed high specific targeting of both primary and metastatic prostate cancer.45 An initial phase 0 trial of 111In-DOTA-h11B6 imaging in prostate cancer is underway (NCT04116164). Alpha emitting 225Ac-DOTA-h11B6 as a therapeutic agent has been developed and therapeutic efficacy has been demonstrated in a preclinical model of prostate adenocarcinoma46 and a therapeutic study in human subjects is planned.
Her2 Directed Antibodies
Other agents for specific targeting of malignancies includes Her2 directed antibodies. Trastuzumab is a IgG1 isotype humanized monoclonal antibody that is directed against the extracellular region of HER2 that is mainstay in treatment of HER2-positive breast cancer. Imaging with 89Zr-trastuzumab PET CT has shown specific localization in Her-2 expressing breast and gastroesophageal tumors that could allow for patient selection and predicting outcomes of HER2 directed therapy.47,48 Radiolabeled antibodies to Her2 can be useful to deliver radiation therapy to Her2 positive tumors. A radiolabeled 227Th conjugated to a humanized anti HER2 antibody (BAY 2701439) that consists of a HER2-targeted humanized monoclonal antibody (mAb) (BAY 2635190), conjugated with the octadentate 3,2-HOPO chelator (BAY 1903150) has been developed by Bayer. Preclinical studies have shown comparable binding affinity and specific internalization between trastuzumab and BAY 2701439 in gastric, breast cancer, and T-DM1-resistant breast cancer models. A phase I study in Her-2 positive tumors including breast, gastric and gastroesopheageal tumors as well as other cancers that have HER2 is being initiated with aims to establish the safety, feasibility and pharmacokinetics of the 227Th radiolabeled antibody (NCT04147819)
Mesothelin Directed Antibodies
Another alpha targeted therapeutic antibody is mesothelin-thorium-227 conjugate (MSLN-TTC). Mesothelin is overexpressed in mesothelioma, ovarian, lung, breast, and pancreatic cancer ranging between 80% and 100% of MSLN expression in these tumor types. A Phase I study in patients with malignant pleural or peritoneal epithelioid mesothelioma or serous ovarian cancer is underway (NCT03507452).
Targeted Antibodies in Renal Cancer
Radiolabeled antibody against CAIX, G250 (chimeric cG250; Girentuximab) is internalized by CAIX antigen-expressing clear cell renal cell carcinoma (ccRCC). Initial experience with (131I)-cG250 therapy in patients with progressive ccRCC administered at 2220 MBq/m2 and with retreatment with 75% of the first dose given after 3 months showed stabilization of disease in 4 of 16 patients (25%) treated at the optimal dose level at 6 months.49 Subsequently, 177Lu- girentuximab has been administered;50 the maximum tolerated dose was 2405 MBq/m2 and the majority of the patients 17 of 23 [74%] showed disease stabilization. Multiple treatments were possible with 56% of patients able to receive 2 doses and 17% of patients able to receive 3 treatments. A phase 2 study in 14 patients resulted in stable disease in 57% of subjects at 3 months after a single treatment and a median PFS of 8.1 months.51 Toxicity is low; myelotoxicity including thrombocytopenia was seen with other nonhematologic grade 1-3 toxicities such as fatigue, anorexia, vomiting, nausea, and diarrhea. Further studies of 177Lu-Girentuximab in ccRCC are planned.
Novel Agents for Hematologic Malignancies
Hematologic malignancies are well suited for radioimmuno-targeted therapy, especially with alpha emitters, as the disease involves marrow and therefore higher radiation doses to marrow are desirable especially in a marrow ablative setting. Bismuth-213-labeled anti-CD-33 antibody (213Bi-HuM195) has been studied for the treatment of leukemia. However, the short half-life of the isotope 213Bi (~45 min) limits its practical use.52 A phase 1 study of Actinium-225 (225Ac)lintuzumab in advanced myeloid leukemia demonstrated safety in an early phase study53 and is now being evaluated in phase 1/2 studies involving myeloma (NCT02998047), AML in older patients (NCT0257596), and in patients with relapsed or refractory AML in combination with chemotherapy (NCT03441048).
Iomab-B (apamistimab—I-131, or 131-1 BC8) is a 131I-radiolabeled anti-CD45 antibody consisting of the BC8 murine recombinant monoclonal antibody (mAb) and the radioactive isotope iodine 131 (I-131, 131I) that targets protein tyrosine phosphatase receptor type C antigen (CD45). CD45 is expressed on all nucleated hematopoietic cells, but not red blood cells and platelets. Iomab-B has been combined with low-dose total body irradiation and fludarabine in patients with acute myeloid leukemia or myelodysplastic syndrome and also been investigated as reduced-intensity conditioning therapy prior to allogeneic hematopoietic cell transplantation.54
In 46 patients with AML 131-I apamistamab was administered (dose range 102-298 mCi) estimated to deliver 5.3-19.0 Gy to bone marrow and 5.25 Gy to liver that resulted in median time to neutrophil and platelet engraftment of about 21 days.55 Iomab-B is currently being investigated as targeted conditioning prior to hematopoietic cell transplantation (HCT) in a phase III SIERRA trial in patients ≥55 years-old with relapsed/refractory acute myeloid leukemia (AML) prior to HCT (NCT02665065). For Iomab-ACT, a lower nonmyeloablative dose (50-100 mCi) of Iomab-B is used to generate transient lymphodepletion in contrast to the higher myeloablative doses (median of approximately 600 mCi) administered prior to HCT in the SIERRA trial.56
In 51 patients treated with 131-I apamistamab, median dose was 616 mCi corresponding to median radiation dose of 15.5 delivered to marrow.57 The therapeutic dose is individualized based on dosimetry assessment a week prior with an upper limit of 24 Gy liver exposure. All 51 patients treated with a therapeutic dose of Iomab-B including both randomized to Iomab-B arm or crossing over after failure of conventional therapy, successfully engrafted despite high median blast counts. 131-I apamistamab was well tolerated overall, with one grade 3 and no grade 4 infusion reactions and transplant-related mortality was low.57 Further enrollment in the SIERRA study is ongoing. Studies with alpha emitter 211At-BC8-B10 monoclonal antibody (antiCD45) in AML are ongoing (NCT03670966 and NCT04083183).
Other Antibody Targets
Other novel agents being evaluated in early-phase studies include 225Ac-FPI-1434, which targets the insulin-like growth factor 1 receptor (IGF1R) signaling pathway in patients with advanced refractory solid tumors (NCT03746431).
Building a Theranostic Center
Given the increasing spectrum of radiopharmaceuticals that are, or are likely to be, clinically available as well as those under development that involve a broader range of alpha and beta emitting agents, there is a recognizable need for centers to develop infrastructure to handle such diverse treatments for a larger number of patients. While some therapies are easy to administer and may not require intense resources, such as Radium-223; 177Lu-Dotatate and radiolabeled antibodies may require a dedicated suitably designed space, a robust team and well-organized workflow between various specialties including physicians and support staff. Being able to promptly introduce these emerging agents into the clinic in a safe and efficacious way requires a multidisciplinary team, capital investment and preparation. It is important that this occurs in parallel to the scientific development of these promising new agents. The goal ultimately is to treat patients with precision to improve patient outcomes.
In the US, many of us have had the experience of building a new or enhanced theranostic service following FDA approval of Radium-223 and 177Lu-Dotatate therapeutic agents. Many clinics saw rapid rise in volume within a few months of FDA approval. This has laid the foundation for many centers to introduce other agents into the clinic. To do this, one must bring all stakeholders to the table. Investing personnel and financial resources is a key component of building a successful theranostic center. This requires buy in from hospital administration to provide for properly shielded space that may require building or refurbishing the facility suitable for performing treatments with high radiation doses in a safe manner. An upfront investment in personnel and equipment will be required. Rapid incorporation of the radiopharmaceutical therapies into the clinical paradigm is crucial in gaining clinical success with the arsenal of therapeutic agents in the pipeline. This is particularly important now as it is widely believed that 177Lu-PSMA will soon receive FDA approval and the number of patients that might benefit from this agent is magnitudes larger than the neuroendocrine tumor population receiving 177Lu-Dotatate. This requires frequent interaction with the administration for resource planning and efficient implementation.
Financial planning is important for developing a sound business plan and to insure proper billing and payment for these new agents. Discussions with facilities personnel should occur early in the process to plan and insure that proper space is available for consults, patient examination and treatments. Treatment needs depending on the approved agents may be significantly different.
Nuclear medicine, radiation oncology, surgery and medical oncology physicians as well as radiation safety officers and other support staff such as nursing, technologists, medical physicists and radiopharmacists are key members of the team and coordinate the administration of the RPT. Staff must be adequately trained as new radiopharmaceuticals are incorporated into the workflow. This includes schedulers who may be asked questions by our patients regarding the procedure they are being scheduled for. Nurses and technologists are on the frontlines of the delivery of these agents. Kasi et al. detailed the processes that they incorporated to develop a successful theranostic service line.58 Ultimately, success hinges on the proper training and education of all members of the team. Communication between various departments, services and teams is extremely important for coordinating the care and providing treatment in a timely and efficient manner.
A key step in ensuring success is developing close working relationships with the referring clinicians, surgical oncologists and oncologists. Partnering with our colleagues at tumor boards and on multidisciplinary teams is essential to assist with clinical integration of these agents into the pattern of clinical care. It is important that the referral mechanisms are fluid and occur with ease without adding extra work onto our referrers. Frequent communications between teams about patients as well as directly with the patients is vital to our role as providers of radiopharmaceutical therapy and for management of their disease in follow-up as opposed to serving only as a consultant when needed. It is important that the physicians delivering this therapy take ownership of their patients, their side effects and responses to the treatment. We should develop close rapport with our patients and provide continued care, so we are an integral part of their oncologic care team. This will allow for us to establish and grow radiopharmaceutical therapies. By making these treatments available and providing excellent care for the patients, we will not only impact patient outcomes but may also provide greater satisfaction to us as physicians and scientists who are integral to the discovery, development and translation of these highly effective therapies.
Footnotes
Conflicts of Interest: N. Pandit-Taskar is a consultant, receives honoraria or serves on the advisory board for Actinium Pharma, Progenics, Medimmune/Astrazeneca, Illumina, Ymabs and conducted research supported by Imaginab, Janssen and Regeneron.L. Solnes is a consultant for Progenics and conducts research supported by Progenics and AAA.M. Shokeen is co-founder of Sarya, LLC.
References
- 1.Morandi F, Horenstein AL, Costa F, Giuliani N, Pistoia V, Malavasi F: CD38: A target for immunotherapeutic approaches in multiple myeloma. Frontiers in Immunology 9, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKeage K: Daratumumab: first global approval. Drugs 76:275–281, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Ghai A, Maji D, Cho N, et al. : Preclinical development of CD38-targeted [(89)Zr]Zr-DFO-daratumumab for Imaging multiple myeloma. J Nucl Med 59:216–222,2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caserta E, Chea J, Minnix M, et al. : Copper 64-labeled daratumumab as a PET/CT imaging tracer for multiple myeloma. Blood 131:741–745, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang L, Jiang D, England CG, et al. : ImmunoPET imaging of CD38 in murine lymphoma models using 89Zr-labeled daratumumab. European journal of nuclear medicine and molecular imaging 45:1372–1381, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehlerding EB, England CG, Jiang D, et al. : CD38 as a PET imaging target in lung cancer. Molecular Pharmaceutics 14:2400–2406, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulaner GA, Sobol NB, O'Donoghue JA, et al. : CD38-targeted immuno-PET of multiple myeloma: from xenograft models to first-in-human imaging. Radiology 295:606–615, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawicki W, Allen KJH, Jiao R, et al. : Daratumumab-(225)Actinium conjugate demonstrates greatly enhanced antitumor activity against experimental multiple myeloma tumors. Oncoimmunology 8, 2019:1607673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlesinger M, Bendas G: Contribution of very late antigen-4 (VLA-4) integrin to cancer progression and metastasis. Cancer and Metastasis Reviews 34:575–591, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Sanz-Rodríguez F, Teixidó J: VLA-4-dependent myeloma cell adhesion. Leuk Lymphoma 41:239–245, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Soodgupta D, Hurchla MA, Jiang M, et al. : Very late antigen-4 (alpha(4) beta(1) Integrin) targeted PET imaging of multiple myeloma. PLoS One 8:e55841, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soodgupta D, Zhou H, Beaino W, et al. : Ex vivo and in vivo evaluation of overexpressed VLA-4 in multiple myeloma using LLP2A imaging agents. J Nucl Med 57:640–645, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaino W, Nedrow JR, Anderson CJ: Evaluation of (68)Ga- and (177) Lu-DOTA-PEG4-LLP2A for VLA-4-targeted PET imaging and treatment of metastatic melanoma. Mol Pharm 12:1929–1938, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi J, et al. : Combined VLA-4-Targeted Radionuclide Therapy and Immunotherapy in a Mouse Model of Melanoma. J Nucl Med 59:1843–1849,2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roxin Á, Zhang C, Huh S, et al. : A metal-free DOTA-conjugated 18F-labeled radiotracer: [18F]DOTA-AMBF3-LLP2A for imaging VLA-4 over-expression in murine melanoma with improved tumor uptake and greatly enhanced renal clearance. Bioconjugate Chemistry 30:1210–1219, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Habringer S, Lapa C, Herhaus P, et al. : Dual targeting of acute leukemia and supporting niche by CXCR4-directed theranostics. Theranostics 8:369–383, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vag T, Steiger K, Rossmann A, et al. : PET imaging of chemokine receptor CXCR4 in patients with primary and recurrent breast carcinoma. EJNMMI Res 8:90, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrmann K, Schottelius M, Lapa C, et al. : First-in-human experience of CXCR4-directed endoradiotherapy with 177Lu- and 90Y-labeled pentixather in advanced-stage multiple myeloma with extensive intra- and extramedullary disease. J Nucl Med 57:248–251, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Grimm J: High-resolution cherenkov tomography in vivo. Nature Biomedical Engineering 2:205–206, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Hamson EJ, Keane FM, Tholen S, Schilling O, Gorrell MD: Understanding fibroblast activation protein (FAP): substrates, activities, expression and targeting for cancer therapy. Proteomics Clin Appl 8:454–463, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Garin-Chesa P, Old LJ, Rettig WJ: Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci U S A 87:7235–7239, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loktev A, Lindner T, Mier W, et al. : A Tumor-imaging method targeting cancer-associated fibroblasts. J Nucl Med 59:1423–1429, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laverman P, van der Geest T, Terry SY, et al. : Immuno-PET and immuno-SPECT of rheumatoid arthritis with radiolabeled anti-fibroblast activation protein antibody correlates with severity of arthritis. J Nucl Med 56:778–783, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Meletta R, Muller Herde A, Chiotellis A, et al. : Evaluation of the radiolabeled boronic acid-based FAP inhibitor MIP-1232 for atherosclerotic plaque imaging. Molecules 20:2081–2099, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng JD, Weiner LM: Tumors and their microenvironments: tilling the soil. Commentary re: Scott AM et al. , A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin. Cancer Res 9:1639–1647, 2003. Clin Cancer Res. 2003;9(5):1590-1595 [PubMed] [Google Scholar]
- 26.Lindner T, Loktev A, Altmann A, et al. : Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med 59:1415–1422, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Kratochwil C, Flechsig P, Lindner T, et al. : (68)Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med 60:801–805, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langbein T, Weber WA, Eiber M: Future of theranostics: an outlook on precision oncology in nuclear medicine. J Nucl Med 60:13S–19S, 2019 [DOI] [PubMed] [Google Scholar]
- 29.St-Gelais F, Jomphe C, Trudeau LE: The role of neurotensin in central nervous system pathophysiology: what is the evidence? J Psychiatry Neurosci 31:229–245, 2006 [PMC free article] [PubMed] [Google Scholar]
- 30.Osadchii OE: Emerging role of neurotensin in regulation of the cardiovascular system. Eur J Pharmacol 762:184–192, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Qiu S, Pellino G, Fiorentino F, et al. : A Review of the Role of Neurotensin and Its Receptors in Colorectal Cancer. Gastroenterol Res Pract 2017, 2017:6456257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgat C, Mishra AK, Varshney R, Allard M, Fernandez P, Hindie E: Targeting neuropeptide receptors for cancer imaging and therapy: perspectives with bombesin, neurotensin, and neuropeptide-Y receptors. J Nucl Med 55:1650–1657, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Baum RP, Singh A, Schuchardt C, et al. : (177)Lu-3BP-227 for neurotensin receptor 1-targeted therapy of metastatic pancreatic adenocarcinoma: first clinical results. J Nucl Med 59:809–814, 2018 [DOI] [PubMed] [Google Scholar]
- 34.Panigone S, Nunn AD: Lutetium-177-labeled gastrin releasing peptide receptor binding analogs: a novel approach to radionuclide therapy. Q J Nucl Med Mol Imaging 50:310–321, 2006 [PubMed] [Google Scholar]
- 35.Maina T, Nock BA, Kulkarni H, Singh A, Baum RP: Theranostic prospects of gastrin-releasing peptide receptor-radioantagonists in oncology. PET Clin 12:297–309,2017 [DOI] [PubMed] [Google Scholar]
- 36.Pandit-Taskar N, O'Donoghue JA, Beylergil V, et al. : (8)(9)Zr-huJ591 immuno-PET imaging in patients with advanced metastatic prostate cancer. Eur J Nucl Med Mol Imaging 41:2093–2105, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vallabhajosula S, Goldsmith SJ, Kostakoglu L, Milowsky MI, Nanus DM, Bander NH: Radioimmunotherapy of prostate cancer using Y-90- and Lu-177-labeled J591 monoclonal antibodies: Effect of multiple treatments on myelotoxicity. Clinical Cancer Research 11:7195S–7200S, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Tagawa ST, Milowsky MI, Morris M, et al. : Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res 19:5182–5191, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tagawa ST OJ, Thomas C, Fernandez E, Niaz MJ, Vallabhajosula S, Vlachostergios P, Molina AM, Sternberg C, Singh S, Patel A, Tan A, Babich J, Nanus DM, Ballman K, Bander NH: Phase I dose-escalation study of prostate-specific membrane antigen (PSMA)-targeted alpha emitter 225Ac-J591 for progressive metastatic castration-resistant prostate cancer (mCRPC). In: In: Proceedings of the 105th Annual Meeting of the American Association for Cancer Research. 2020;April 24-29, San Diego, CA. Philadelphia (PA): AACR; 2020. [Google Scholar]
- 40.Hofman MS, Violet J, Hicks RJ, et al. : [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol 19:825–833, 2018 [DOI] [PubMed] [Google Scholar]
- 41.Kratochwil C, Bruchertseifer F, Rathke H, et al. : Targeted alpha-therapy of metastatic castration-resistant prostate cancer with (225)Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med 58:1624–1631,2017 [DOI] [PubMed] [Google Scholar]
- 42.Mateo J, Carreira S, Sandhu S, et al. : DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med 373:1697–1708, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darson MF, Pacelli A, Roche P, et al. : Human glandular kallikrein 2 (hK2) expression in prostatic intraepithelial neoplasia and adenocarcinoma: a novel prostate cancer marker. Urology 49:857–862, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Vickers A, Vertosick EA, Sjoberg DD, et al. : Value of intact prostate specific antigen and human kallikrein 2 in the 4 kallikrein predictive model: an individual patient data meta-analysis. J Urol 199:1470–1474,2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timmermand OV, Ulmert D, Evans-Axelsson S, et al. : Preclinical imaging of kallikrein-related peptidase 2 (hK2) in prostate cancer with a (111)In-radiolabelled monoclonal antibody, 11B6. EJNMMI Res 4:51, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ulmert D, Solnes L, Thorek D: Contemporary approaches for imaging skeletal metastasis. Bone Res 3:15024, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dehdashti F, Wu N, Bose R, et al. : Evaluation of [(89)Zr]trastuzumab-PET/CT in differentiating HER2-positive from HER2-negative breast cancer. Breast Cancer Res Treat 169:523–530, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez-Vega F, Hechtman JF, Castel P, et al. : EGFR and MET amplifications determine response to her2 inhibition in ERBB2-amplified esophagogastric cancer. Cancer Discov 9:199–209, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brouwers AH, Mulders PF, de Mulder PH, et al. : Lack of efficacy of two consecutive treatments of radioimmunotherapy with 131I-cG250 in patients with metastasized clear cell renal cell carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 23:6540–6548, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Stillebroer AB, Boerman OC, Desar IM, et al. : Phase 1 radioimmunotherapy study with lutetium 177-labeled anti-carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma. Eur Urol 64:478–485, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Muselaers CH, Boers-Sonderen MJ, van Oostenbrugge TJ, et al. : Phase 2 study of lutetium 177-labeled anti-carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma. Eur Urol 69:767–770, 2016 [DOI] [PubMed] [Google Scholar]
- 52.Rosenblat TL, McDevitt MR, Mulford DA, et al. : Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin Cancer Res 16:5303–5311, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jurcic JG, Rosenblat TL. Targeted alpha-particle immunotherapy for acute myeloid leukemia. Am Soc Clin Oncol Educ Book. 2014:e126–131. [DOI] [PubMed] [Google Scholar]
- 54.Pagel JM, Gooley TA, Rajendran J, et al. : Allogeneic hematopoietic cell transplantation after conditioning with 131I-anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood 114:5444–5453, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pagel JM, Appelbaum FR, Eary JF, et al. : 131I-anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood 107:2184–2191, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agura E, Gyurkocza B, Nath R, et al. : Targeted conditioning of iomab-B (131I-anti-CD45) prior to allogeneic hematopoietic cell transplantation versus conventional care in relapsed or refractory acute myeloid leukemia (AML): preliminary feasibility and safety results from the prospective. randomized phase 3 sierra trial. Blood 132,2018. 1017–1017 [Google Scholar]
- 57.Gyurkocza B, Nath R, Stiff PJ, et al. : Targeted conditioning with anti-CD45 iodine (131I) apamistamab [iomab-B] leads to high rates of allogeneic transplantation and successful engraftment in older patients with active, relapsed or refractory (rel/ref) AML after failure of chemotherapy and targeted agents: preliminary midpoint results from the prospective, randomized phase 3 sierra trial. Biology of Blood and Marrow Transplantation 26:S32–S33, 2020 [Google Scholar]
- 58.Kasi PM, Maige CL, Shahjehan F, et al. : A care process model to deliver (177)lu-dotatate peptide receptor radionuclide therapy for patients with neuroendocrine tumors. Front Oncol 8:663, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]