Abstract
Background
Inhaled short acting beta2 adrenergic agonists and ipratropium bromide are both used in the treatment of acute exacerbations of chronic obstructive pulmonary disease.
Objectives
In patients with acute exacerbations of COPD to: 1. To assess the efficacy of short‐acting beta‐2 agonists against placebo ; 2. Compare the efficacy of short‐acting beta‐2 agonists and ipratropium.
Search methods
A comprehensive search of the literature was carried out of EMBASE, MEDLINE, CINAHL and the Cochrane COPD trials register was carried out using the terms: bronchodilator* OR albuterol OR metaproterenol OR terbutaline OR isoetharine OR pirbuterol OR salbutamol OR beta‐2 agonist.
Selection criteria
All trials that appeared to be relevant were assessed by two reviewers who independently selected trials for inclusion. Differences were resolved by consensus.
Data collection and analysis
All trials that appeared to be relevant were assessed by two reviewers who independently selected trials for inclusion. Differences were resolved by consensus. References listed in each included trial were searched for additional trial reports. Trials were combined using Review Manager using a fixed effects model. The size of the treatment effects were tested for heterogeneity.
Main results
We identified no placebo‐controlled comparisons of beta‐2 agonists. Three studies permitted comparison of ipratropium to an inhaled beta‐2 agonist. These studies included a total of 103 patients. The beta2‐agonists used were: fenoterol and metaproterenol. One study was a parallel group trial of regular therapy for seven days. The other two were cross over studies of single dose treatments, with efficacy measured 90 min post dose. There was no washout period between treatments.
Both treatments produced an improvement in forced expiratory volume (FEV1) after 90 min in the range 150‐250 ml. The was no difference between treatments, mean difference in FEV1 10 ml; 95% CI ‐220, 230 ml. In one small crossover study (n=10) there was a significant improvement in arterial PaO2 after 30 minutes with ipratropium (+5.8 mm Hg ± 3.0 (SEM)) compared to metaproterenol (‐6.2 ± 1.2 mm Hg), but this was not significant at 90 min. There were no data concerning respiratory symptoms. The crossover studies showed no evidence of an additive effect of the two treatments, although they were not designed specifically to test this. An update search conducted in February 2002 yielded one further excluded study.
Authors' conclusions
There are few controlled trial data concerning the use of inhaled beta2‐agonist agents in acute exacerbations of COPD and none that have compared these agents directly with placebo. None of the studies used the more modern beta2‐agonists used most widely in this setting (salbutamol and terbutaline). Beta2‐agonists and ipratropium both produce small improvements in FEV1, but beta2‐agonists may worsen PaO2 for a period. We could not draw conclusions concerning possible additive effects.
Plain language summary
Inhaled short‐acting beta2‐agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease
During an acute worsening of chronic obstructive pulmonary disease, there may be a worsening in airway function. Two different types of drugs may be given as inhaled therapy to improve this: anticholinergic drugs such as ipratropium and beta2‐agonists. These days the drugs of the latter type that are used for acute COPD are salbutamol and terbutaline, but neither of these drugs have been used in the only studies that we could find. We found only three small studies. Overall, both types of drug showed a small but worthwhile effect. There was no difference between them. Our review was not designed to test whether they would have had a greater effect if both were given at the same time.
Background
The natural history of moderate to severe COPD is punctuated by acute exacerbations in which worsening symptoms of dyspnea and an increase in amount or purulence of sputum may be accompanied by chest discomfort, fever and other constitutional symptoms. Acute exacerbations are often the reason that those with mild disease seek medical care. Treatment is often multifaceted with antibiotics, corticosteroids and bronchodilators.
Part of the dyspnoea during acute exacerbations is due to broncho‐constriction. To some extent the severity of the exacerbation is based on the degree of constriction/obstruction present. Along with airway inflammation, contraction of smooth muscle within the bronchial tree is responsible for this phenomenon. Short acting bronchodilators such as beta‐2 agonists, ipratropium bromide (an anticholinergic agent) and methylxanthines reduce airway smooth muscle constriction. These drugs may also have an effect on secretions, mucus production ciliary function.
Short acting beta2‐agonists and ipratropium are widely used in the management of acute exacerbations of COPD. This review was originally designed to assess the benefit of short‐acting beta2‐agonists alone vs placebo, but no trials of this kind were found, all compared a beta2‐agonist with ipratropium. For this reason the objective of the review was changed to compare these two classes of agent.
Objectives
To compare the effects of inhaled short‐acting beta‐2 agonists and ipratropium on lung function and dyspnoea in patients with an acute exacerbation of COPD.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials comparing inhaled short‐acting beta‐2 agonists with ipratropium. Results from patients with acute asthma were excluded. Studies of acute exacerbations in any setting were permitted, although we expected that most studies would be performed in the emergency room or within hospital.
Types of participants
Studies were included if the participants were adult patients with a known diagnosis of COPD and symptoms of an acute exacerbation, defined as increased shortness of breath, increased sputum production, or sputum purulence (the 'Winnipeg criteria' for defining/diagnosing acute exacerbations of COPD (Anthonisen 1987, Murphy 1992)).
Studies of patients receiving ventilatory assistance were excluded. While we assumed that most cases of exacerbation are due to infection we did not limit the target population to those patients for whom infection was the suspected etiology. We expect that patients with acute exacerbations of COPD may have various comorbidities (such as congestive heart failure or pulmonary embolus) or complications of COPD (e.g. pneumothorax) as the etiology of exacerbation of their symptoms. We required that studies in some way assured that patients with other reasons for these symptoms were not studied.
Types of interventions
Any studies employing short acting beta2‐agonists administered by inhalation via nebulizer or metered dose inhaler. Relevant agents include salbutamol (albuterol), metaproterenol, terbutaline, isoetharine, and pirbuterol.
Types of outcome measures
Outcome measures include: 1. Lung function measurements, forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC) or peak expiratory flow rate (PEFR) 2. Arterial blood gas measurements 3. Symptom scores The above short term outcomes will be assessed as post‐treatment data or change from pre‐ to post‐treatment in two time frames ‐ within 90 minutes and greater than 1 day.
Outcomes also to be assessed: 4. Health status or quality of life assessments 5. Functional capacity ‐ timed walking tests, endurance tests 6. Health services utilization measures, duration of hospitalization or time to emergency room discharge 7. Hospital readmission or return to emergency room 8. Mortality 9. Adverse drug reactions.
Search methods for identification of studies
The Cochrane Airways Group has developed a COPD‐RCT register through a comprehensive search of EMBASE, MEDLINE, and CINAHL. An initial search of the register was carried out using the search terms: bronchodilator* OR albuterol OR metaproterenol OR terbutaline OR isoetharine OR pirbuterol OR salbutamol. Concurrently, similar searches of MEDLINE and EMBASE were performed.
References listed in each included trial were searched for additional trial reports.
Data collection and analysis
All trials that appeared to be relevant were assessed by two reviewers who independently selected trials for inclusion. Differences were resolved by consensus.
All trials were entered and scored using the following principles: Grade A ‐ Double blind Grade B ‐ Single blind Grade C ‐ Obviously not blinded or not known
The internal validity of individual trials was assessed using the scale devised by Jadad (Jadad 1996), which will be operationalized as follows:
(1) Was the study described as randomized? (1=yes; 0=no) (2) Was the method of randomization well described and adequate? (0=not described; 1=described and adequate; ‐1=described, but not adequate) (3) Was the study described as double‐blind? (1=yes; 0=no) (4) Was the method of double‐blinding well described and adequate? (0=not described; 1=described and adequate; ‐1=described, but not adequate) (5) Was there a description of withdrawals and dropouts sufficient to determine the number of patients in each treatment group entering and completing the trial? (1=yes; 0=no).
The external validity of the trials was described using the following features: (1) Validity of the underlying COPD diagnosis: (a) Is COPD diagnosis based on spirometry‐based criteria (e.g. ATS (American 1995) or Siafakas (Siafakas 1995))? (b) Is the baseline stable FEV1 of study population described? (2) Validity of diagnosis of AECB (Acute exacerbation of chronic bronchitis): Does the definition of AECB include at least two of the following: increased sputum production, increased sputum volume, and increased dyspnea? (3) Characterization of severity of AECB: Does the study describe the severity of AECB at enrolment based upon at least two of the following domains: (a) Mental status change (b) Work of breathing (e.g. respiratory rate or use of accessory muscles)? (c) Ventilatory status (e.g. FEV1 or PEFR, O2 saturation or PaO2 and PaCO2)? (4) Duration of follow‐up: Is there an outcome assessment at 24 hours or longer?
Statistical considerations
Trials were combined using Review Manager 4.0.4 using a fixed effects model The size of the treatment effects were tested for heterogeneity. If significant heterogeneity were found, an attempt would be made to explain the differences based on the clinical characteristics of the included studies. Clinically dissimilar studies would not be combined. However, when a group of studies with heterogeneous results appeared clinically similar the study estimates were combined using a random effects model.
Planned subgroup analyses included:
1. A comparison of studies using concurrent medication vs those that did not. Such co‐interventions may include other bronchodilating agents, mucus clearing therapies or corticosteroids. 2. A comparison of studies by severity of exacerbation of included patients or degree of airways obstruction. 3. A comparison of studies utilizing delivery of bronchodilators by metered dose inhaler versus nebulizer.
Results
Description of studies
Results of the search
A combined database of 1343 entries was created from the results of MEDLINE and COPD‐RCT register literature searches. After excluding articles that focused on pediatric patients and/or asthma there were 430 entries for further evaluation. We then excluded papers where stable COPD patients were the study population. Twenty five papers were selected for full‐text evaluation for inclusion in the review. Of those 25 papers only three were RCTs that met the criteria for inclusion. (Note: we identified no randomised placebo‐controlled comparisons of beta‐2 agonists). Of the three studies one was a parallel group study of regular nebulised fenoterol vs ipratropium over 7 days (Backman 1985). The other two were single dose cross‐over studies with assessments at 90 mins, one using metaproterenol or ipratropium via a metered dose inhalers (Karpel 1990), the other using nebulised fenoterol or ipratropium (Rebuck 1987). An update search conducted in February 2002 yielded one further excluded study (see Characteristics of excluded studies).
Included studies
Three studies met the eligibility criteria of this review.
All of the studies were small, with a total of 103 patients included.
Risk of bias in included studies
All three studies included in this review were randomized, controlled, double‐blind trials. Rebuck 1987 used a computer‐generated randomization schedule, but Backman 1985 and Karpel 1990 did not explain the methods used to randomise patients.
Two studies (Rebuck 1987, Karpel 1990) used the ATS diagnostic criteria for COPD (American 1995). Backman 1985 used the British MRC criteria. Only Karpel 1990 provided the criteria used to diagnose exacerbations. Severity of disease was assessed using FEV1 and/or PEFR at the time of entry into the study. Spirometry was also the used to evaluate the outcomes of treatment.
The following parameters were used to measure efficacy: change in FEV1, PEFR, PaO2, and PaCO2. Because all the patients were admitted to hospitals for care of severe exacerbations the patient population tended to include those patients with more severe disease (i.e. FEV1<1 litre). There was also a lack of standardization of co‐interventions in the studies. All allowed the use of additional medications, such as corticosteroids, theophylline, and antibiotics; however, use of these interventions was left to the discretion of the admitting physician.
Most studies did not report precisely the statistical information we required ‐ namely the variance of the change in outcomes from pre‐ to post‐treatment. We had to estimate the variance of the changes based on the sum of the pre‐and post‐treatment variances.
Effects of interventions
There were small mean improvements in FEV1 over the first 90 minutes of treatment with both beta2‐agonists and ipratropium. These were in the range 150‐250 ml. There was no difference in the size of the response between the two treatments, mean difference: 10 ml; 95% CI ‐220 to 230 ml (Figure 1). There were limited data available for short‐term differences in change in PEFR and long‐term differences in change in FEV1 and PEFR.
1.
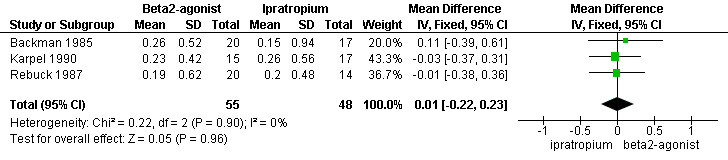
Forest plot of comparison: 1 Beta2‐Agonist vs. Ipratropium, outcome: 1.1 Change in FEV1 (L) over 90 minutes.
Data on changes in blood gases were limited. Only two of the three studies (Backman 1985, Karpel 1990) reported such changes. Neither found any differences in short‐ or long‐term PaO2 between treatments, but only one (Karpel 1990) provided actual data for interpretation. They found an increase of PaO2, in those subjects using ipratropium bromide and a decrease in those using metaproterenol at 60 minutes, that was statistically significant. However, levels had returned to baseline for both study groups at 90 minutes.
Symptom or dyspnoea scores were not reported and there were no data on length of hospital stay.
Adverse drug reactions were reported by a minority of subjects in all 3 studies. Backman 1985 had one person in each group describe an unexplained "strange feeling" after using ipratropium bromide or fenoterol. Dry mouth and tremor were the most commonly reported reactions by Karpel 1990 and Rebuck 1987. One subject in each group experienced dry mouth in the study conducted by Karpel 1990; Rebuck 1987 reports 4 patients in the beta‐2 agonist group, and 1 in the ipratropium bromide group for the same reaction. Tremor occurred in similar numbers with 2 in each of the groups of Karpel 1990 and 3 each for those reported by Rebuck 1987.
Discussion
Few studies of inhaled beta‐2 agonists have been performed in populations with acute exacerbations of COPD. The studies available describe the short‐ and intermediate‐term impact on spirometric function. The studies show that there is no difference in efficacy between beta‐2 agonists and ipratropium bromide. All of these studies were small, and none were powered to test equivalence rather than superiority (more participants are required for equivalence studies). In addition since we had to estimate the variance of the changes based on the sum of the pre‐and post‐treatment variances, this may have inflated the size of the estimated variance for the change in measurement used in the analysis and decreased the power of the analysis to detect a difference. Despite all of these considerations, it unlikely that there is a difference in the relatively efficacy of these two treatments that is clinically worthwhile. In all outcomes, the differences between treatments was very close to zero, with wide confidence intervals around these estimates.
It should be noted that all drug doses were at or above what is considered standard and acceptable practice. The trials identified for inclusion in this review were not designed to test for an additive effect between ipratropium and beta2‐agonists. Overall, patients appeared to have achieved maximal bronchodilation with the first drug given, since no further broncho‐dilatation was seen with the second agent, as exemplified by Karpel 1990. However one of these studies did find that in patients with acute asthma, a combination of ipratropium and fenoterol did produce a greater effect than either drug alone.
Authors' conclusions
Implications for practice.
In acute exacerbations of COPD, short‐acting beta‐2 agonists and ipratropium appear to produce short‐term improvements of FEV1 and PEFR of very similar magnitude. The average improvement in FEV1 in response to a single dose of either drug lay in the range 150‐250 ml. The trials included in the review provide no evidence for a synergistic effect between these two agents, although they were not designed to address this issue directly.
Implications for research.
Few studies of bronchodilating drugs have been performed in populations with acute exacerbation of COPD. Most studies included one or more additional medications, usually a corticosteroid, an antibiotic, or both, which may confound the results. Much needs to be done ‐ ideally, a large, multi‐centre RCT is needed with strict control of co‐interventions in order to determine the true effect of beta‐2 agonists and ipratropium on the short and long term outcomes of patients with acute exacerbations of COPD. Studies should measure both the objective changes (i.e. FEV1, PEFR, PaO2) and the more subjective patient‐oriented outcomes (quality of life, dyspnoea, symptom scores). This may not be possible because of ethical constraints in trying to perform research on therapies that have already become a part of standard and accepted patient care.
Tests of an additive or synergistic effect of these drugs need to be designed specifically to address this issue.
What's new
| Date | Event | Description |
|---|---|---|
| 30 July 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 5 October 2000 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to acknowledge the following persons, without whom this project would not be possible: Mrs Karen Blackhall for obtaining all articles reviewed for this analysis; Dr. John White for his editing skills, and Mr. Steve Milan for his patience.
Data and analyses
Comparison 1. Beta2‐Agonist vs. Ipratropium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 (L) over 90 minutes | 3 | 103 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.22, 0.23] |
| 2 Change in PEFR (l/min) over 90 minutes | 2 | 71 | Mean Difference (IV, Fixed, 95% CI) | 5.18 [‐43.92, 54.28] |
| 3 Change in FEV1 (L) over 7 days | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.46, 0.60] |
| 4 Change in PEFR (l/min) over 7 days | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐26.60 [‐104.95, 51.75] |
| 5 Change in pO2 (mm Hg) within 30 minutes | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐12.00 [‐18.34, ‐5.66] |
| 6 Change in pCO2 (mm Hg) within 30 minutes | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.84 [‐9.97, 6.29] |
1.1. Analysis.
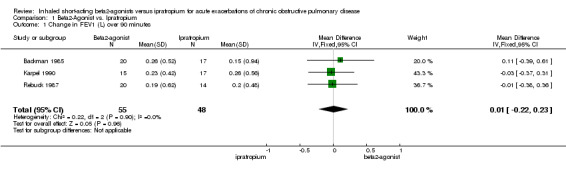
Comparison 1 Beta2‐Agonist vs. Ipratropium, Outcome 1 Change in FEV1 (L) over 90 minutes.
1.2. Analysis.
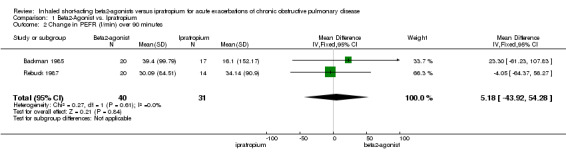
Comparison 1 Beta2‐Agonist vs. Ipratropium, Outcome 2 Change in PEFR (l/min) over 90 minutes.
1.3. Analysis.
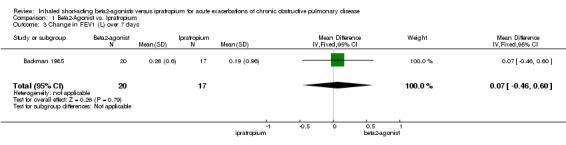
Comparison 1 Beta2‐Agonist vs. Ipratropium, Outcome 3 Change in FEV1 (L) over 7 days.
1.4. Analysis.
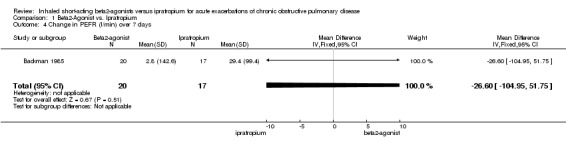
Comparison 1 Beta2‐Agonist vs. Ipratropium, Outcome 4 Change in PEFR (l/min) over 7 days.
1.5. Analysis.
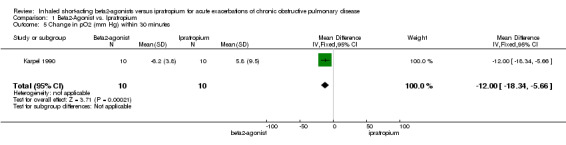
Comparison 1 Beta2‐Agonist vs. Ipratropium, Outcome 5 Change in pO2 (mm Hg) within 30 minutes.
1.6. Analysis.
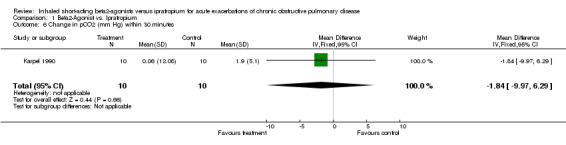
Comparison 1 Beta2‐Agonist vs. Ipratropium, Outcome 6 Change in pCO2 (mm Hg) within 30 minutes.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Backman 1985.
| Methods | Design: SPPG
Location: Finland
Assessment period: 7 days External validity: 2/5 COPD dx‐ COPD bl‐ AECB dx‐ AECB sev+ Tx duration+ Internal validity: 3/5 r, db, dd |
|
| Participants | N = 40
Trial included both pts with acute asthma (n = 150) and pts with acute exacerbation of COPD (n = 52). Most results were reported separately for the two types of patients.
Setting: Hospitalized pts FEV1 Inclusion (COPD): Chronic bronchitis, according to criteria of the British Medical Council [no ref given] Inclusion (AcEx): Acute exacerbation of chronic bronchitis (no diagnostic criteria given) Exclusion: N/S Smoking history: N/S Baseline stable FEV1: N/S FEV1 before treatment on day 1: Ipratropium, 0.73 ± 0.35 (SD) L; fenoterol, 0.93 ± 0.56 L (n.s.; p‐value not reported) Age: Range: 48‐77 Sex: 36 M, 4 F Race: N/S Dropouts: 1/40 pts (2.5%), treatment group N/S |
|
| Interventions | Experimental: Nebulized ipratropium 0.2 mg, 3x/day Control: Nebulized fenoterol 0.5 mg, 3x/day Co‐interventions: Pts with signs of an acute infection (fever, pneumonic infiltration, and positive sputum bacteriology) were treated with antibiotics before the start of the trial; pts taking beta2‐receptor stimulants (n = 16) stopped them at least 8 hrs before starting the trial; all pts received daily physical treatment; otherwise, pts continued their usual regimens, which included oral theophyllamine (n = 7), combination treatment with ephedrine and hydroxyzine (n = 4), prednisolone (n = 2), and no continuous treatment (n = 7) |
|
| Outcomes | FEV1: Measured on the 1st and 7th day immediately before and 5, 10, 15, 30, 60, 120, and 240 min after the morning study drug administration. Investigators compared mean FEV1 scores for identical timepoints on days 1 and 7. Blood gases: Arterial PaO2 and PaCO2 measured on 1st and 7th day. Investigators analyzed change from day 1 to 7. There were no significant differences between the two treatment groups for any of the outcomes measured (no between‐group p‐values reported). Dropouts: 1/40 pts (2.5%), treatment group N/S |
|
| Notes | RESULTS: FEV1 improvements were small with both interventions. In the ipratropium group (n = 17), mean scores at 15 min (p < 0.02) and 30 min (p < 0.05) post‐treatment were significantly higher on the 7th day than on the 1st; no significant differences were noted between the 1st and 7th day at 0, 5, 10, 60, 120, or 240 min. In the fenoterol group (n = 20), mean scores at 60 min post‐treatment were significantly higher on the 7th day than on the 1st (p < 0.05); otherwise, no significant differences were observed between the 1st and 7th day. Mean pre‐treatment FEV1 scores on days 1 and 7 were: (a) Ipratropium (n = 17): Day 1, 0.73 ± 0.35 (SD) L; Day 7, 0.82 ± 0.34 L (b) Fenoterol (n = 20): Day 1, 0.93 ± 0.56 L; Day 7, 1.02 ± 0.66 L Blood gases/oximetry: Investigators stated that there were no significant changes in PaO2 or PaCO2 from day 1 to day 7 in either group, but did not report any data. Adverse events: 1/19 pts (5%) taking ipratropium and 1/20 (5%) taking fenoterol complained of a "strange feeling" Two pts in the ipratropium group who did not withdraw prematurely were unable to perform spirometry and so were not included in the FEV1 analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Karpel 1990.
| Methods | Design: CrOv Dates: N/S Location: US Assessment period: 90 min for each phase of crossover External validity: 3/5 COPD dx+ COPD bl‐ AECB dx+ AECB sev+ Tx duration‐ Internal validity: 5/5 r+, db+, dd |
|
| Participants | N = 32 Setting: ED and pulmonary clinic Inclusion (COPD): Chronic bronchitis or emphysema as defined by the ATS Committee on Diagnostic Standards for Nontuberculous Respiratory Disease (1962) Inclusion (AcEx): Increasing dyspnea and/or change in sputum production newly occurring in previous 24 hrs; FEV1 < 60% of predicted Exclusion: Asthma; baseline pulmonary function tests which failed to show persistent airway obstruction; any acute concomitant medical problem; respiratory acidosis with pH < 7.3; need for continuous oxygen therapy; need for medications other than those included in study protocol; use of inhaled b‐agonist or ipratropium in previous 6 hrs Smoking history: N/S Baseline stable FEV1: N/S FEV1 at admission: Ipratropium first: 0.62 ± 0.08 (SEM) L; metaproterenol first: 0.69 ± 0.06 L Age: 61.6 Sex: N/S Race: N/S |
|
| Interventions | Experimental: Ipratropium, single dose of 3 puffs (54 mg) administered by MDI Control: Metaproterenol, single dose of 3 puffs (1.95 mg) administered by MDI Co‐interventions: None Crossover design: Pts treated with one of the two study meds initially (phase 1), then crossed over to treatment with the other after 90 min (phase 2); no washout; no information on possible carry‐over effect |
|
| Outcomes | FEV1: Measured at entry and at 30, 60, and 90 min after administration of study med in both phases of crossover; investigators analyzed change in mean FEV1 from 0 to 90 min in phase 1 Blood gases: Recorded only for the first 20 pts randomized into the study; measured at entry and at 30, 60, and 90 min after administration of study med in phase 1 only; investigators analyzed mean changes in PaO2, PaCO2, and pH from 0‐30 min and 0‐90 min Percentage of pts admitted to hospital |
|
| Notes | RESULTS: In phase 1 of the trial, mean FEV1 (± SEM) improved significantly in both treatment groups from 0‐90 min. For ipratropium (n = 17), scores improved 25%, from 0.62 ± 0.08 to 0.88 ± 0.11 (p< 0.01). For metaproterenol (n = 15), the improvement was 18%, from 0.69 ± 0.06 to 0.92 ± 0.09 (p< 0.01). There was no significant difference between the two groups for this outcome (p > 0.05). For phase 2 of the trial, investigators reported only that there was no further improvement in FEV1 in either group. Blood gases: At 30 min, there was a significant (p < 0.05) rise in PaO2 with ipratropium (n = 10) (5.8 ± 3.0 [SEM]), and a significant (p < 0.05) decline in PaO2 with metaproterenol (n = 10) (‐6.2 ± 1.2). At 90 min, these changes were no longer significant. There were no other significant changes in PO2, PCO2, or pH during phase 1. Percentage of pts admitted to hospital: 3/17 pts (18%) from the ipratropium‐first group, 2/15 pts (13%) from the metaproterenol‐first group. Adverse events: Recorded for phase 1 only. Ipratropium: Nervousness (2 pts), tremors (2), dry mouth (1), palpitations (1), headache (1). Metaproterenol: Palpitations (3 pts), nervousness (3), tremors (2), dry mouth (1), headache (1). Dropouts: 0 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Third party randomisation |
Rebuck 1987.
| Methods | Design: SPPG Dates: N/S Location: Canada Assessment period: 90 min External validity: 2/5 COPD dx+ COPD bl‐ AECB dx‐ AECB sev+ Tx duration‐ Internal validity: 5/5 r+, db+, dd |
|
| Participants | N = 52 with acute exacerbation of COPD (see Notes) Setting: 4 EDs Inclusion (COPD): ATS criteria (1962) Inclusion (AcEx): Age > 18 yrs; FEV1 < 70% of predicted Exclusion: Complicating medical illnesses (e.g., pneumonia, pulmonary edema, acute myocardial infarction, frequent ventricular ectopic beats); pregnancy or breastfeeding; use of nebulized bronchodilator in previous 6 hrs; need for medications other than those specified in study protocol Smoking history: N/S Baseline stable FEV1: N/S FEV1 at admission: 0.67 ± 0.29 (SD) L (28% of predicted) (COPD pts only) Age: 66.2 (COPD pts only) Sex: 28 M, 23 F Race: N/S |
|
| Interventions | Experimental: Nebulized ipratropium 0.5 mg + fenoterol 1.25 mg, single dose Control 1: Nebulized ipratropium 0.5 mg, single dose Control 2: Nebulized fenoterol 1.25 mg, single dose Co‐interventions: Aminophylline IV or corticosteroids IV could be used at discretion of attending physician; all other drugs proscribed |
|
| Outcomes | FEV1: Mean change from pre‐treatment to 45 and 90 min | |
| Notes | RESULTS
FEV1: Each of the three treatment regimens produced a significant improvement in FEV1 from 0 to 90 min (p < 0.05). There was no significant difference among the three treatments for this outcome (p < 0.2). Mean improvement scores (± SEM) were reported in graphic form only. Pre‐treatment and 90‐min mean FEV1 scores (± SD) for the three groups were: Fenoterol: Pre‐: 0.69 ± 0.30; 90‐min: 0.88 ± 0.44 Ipratropium: Pre‐: 0.69 ± 0.26; 90‐min: 0.89 ± 0.41 Ip + Fen: Pre‐: 0.63 ± 0.31; 90‐min: 0.80 ± 0.40 Adverse events: Pts were questioned about specific AEs. Results were not reported separately for pts with asthma and COPD. The most commonly reported AEs were dry mouth (10.6% of pts taking combination treatment, 19.1% taking fenoterol alone, 7.4% taking ipratropium alone); tremor (16.7% of pts taking combination treatment, 13.2% taking fenoterol alone, 2.9% taking ipratropium alone); and bad taste (8.4% overall). Eye irritation, sweating, and dizziness were each reported by < 3% of pts overall. Dropouts: 1/52 pts (2%), from ipratropium group. No significant difference in the proportion of COPD pts receiving aminophylline IV and cortico‐steroids IV in the three treatment groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Third party randomisation |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cydulka 1995 | Study is not designed to measure the effects of a beta‐2 agonist. |
| Emerman 1997 | Study is not designed to measure the effects of a beta‐2 agonist. |
| Ikeda 1998 | Nonsystematic review of the literature |
| Koutsogiannis 2000 | The study was not included because although all patients were having COPD exacerbations, they were given both salbutamol and ipratropium at time=0 in the study. They then received either salbutamol, ipratropium or both at 15 minutes and 30 minutes after that. The initial medications given make it impossible to determine the effect on FEV‐1 of either drug alone. |
| Lloberes 1988 | Patient population considered immediately recovered from an exacerbation of COPD. |
| Lu 1997 | A non‐systematic review of the literature on the use of bronchodilators in COPD treatment (stable and exacerbations) |
| Madison 1998 | A non‐systematic review of the literature on COPD treatment (stable and exacerbations) |
| Matera 1996 | Population studied were stable COPD patients |
| Moayyedi 1995 | Study is designed to measure the effects of ipratropium not a beta‐2 agonist. |
| Muir 1999 | An expert review of COPD treatment |
| Musil 1996 | Study is not designed to measure the effects of a beta‐2 agonist. |
| Musil 1997 | 20 patients with COPD and asthma exacerbations treated ‐ data not subdivided by underlying diagnosis |
| Musil 1998 | Dosing study of an ipratropium/phenoterol combination product |
| Naberan 1998 | Systematic review ( no meta‐analysis) with expert opinion treatment recommendations |
| Patrick 1990 | Study is designed to measure the effects of ipratropium not a beta‐2 agonist. |
| Perri 1985 | A randomized study to evaluate the effects of an inhaled steroid in combination with a beta‐2 agonist. |
| Phanareth 1997 | Telephone survey to determine actual treatment regimens prescribed to patients with COPD and asthma |
| Rees 1998 | Nonsystematic review/ expert opinion |
| Shrestha 1991 | Study is designed to measure the effects of ipratropium not a beta‐2 agonist. |
| Wortmann‐Schmid 1999 | A review of the ATS guidelines for treatment |
| Zehner 1995 | 83 patients with either asthma or COPD exacerbations ‐ unable to reliably determine the specific underlying disease in each patient so the results are pooled. |
Sources of support
Internal sources
NHS Research and Development, UK.
External sources
Agency for Health Care Policy and Research (Contract No. 290‐97‐0014), USA.
Agency for Healthcare Research & Quality ‐ National Research Service Award, USA.
Durham Veterans Affairs Hospital, USA.
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Backman 1985 {published data only}
- Backman R, Hellstrom P‐E. Femoterol and Ipratropium bromide in respirator treatment of patients with chronic bronchitis. Current Therapeutic Research, Clinical & Experimental 1985;38(1):135‐40. [Google Scholar]
Karpel 1990 {published data only}
- Karpel JP, Pesin J, Greenberg D, Gentry E. A comparison of the effects of ipratropium bromide and metaproterenol sulfate in acute exacerbations of COPD. Chest 1990;98(4):835‐9. [DOI] [PubMed] [Google Scholar]
Rebuck 1987 {published data only}
- Rebuck AS, Chapman KR, Abboud R, et al. Nebulized anticholinergic and sympathomimetic treatment of asthma and chronic obstructive airways disease in the emergency room. American Journal of Medicine 1987;82(1):59‐64. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cydulka 1995 {published data only}
- Cydulka RK, Emerman CL. Effects of combined treatment with glycopyrrolate and albuterol in acute exacerbation of chronic obstructive pulmonary disease. Annals of Emergency Medicine 1995;25(4):470‐3. [DOI] [PubMed] [Google Scholar]
Emerman 1997 {published data only}
- Emerman CL, Cydulka RK. Effect of different albuterol dosing regimens in the treatment of acute exacerbation of chronic obstructive pulmonary disease. Annals of Emergency Medicine 1997;29(4):474‐8. [DOI] [PubMed] [Google Scholar]
Ikeda 1998 {published data only}
- Ikeda A, Nishimura K, Izumi T. Pharmacological treatment in acute exacerbations of chronic obstructive pulmonary disease. Drugs & Aging 1998;12(2):129‐37. [DOI] [PubMed] [Google Scholar]
Koutsogiannis 2000 {published data only}
- Koutsogiannis Z, Kelly AM. Does high dose ipratropium bromide added to salbutamol improve pulmonary function for patients with chronic obstructive airways disease in the emergency department?. Australian & New Zealand Journal of Medicine 2000;30:38‐40. [DOI] [PubMed] [Google Scholar]
Lloberes 1988 {published data only}
- Lloberes P, Ramis L, Montserrat JM, et al. Effect of three different bronchodilators during an exacerbation of chronic obstructive pulmonary disease. European Respiratory Journal 1988;1:536‐9. [PubMed] [Google Scholar]
Lu 1997 {published data only}
- Lu CC. Bronchodilator therapy for chronic obstructive pulmonary disease. Respirology 1997;2(4):317‐22. [DOI] [PubMed] [Google Scholar]
Madison 1998 {published data only}
- Madison JM, Irwin RS. Chronic obstructive pulmonary disease. Lancet 1998;352(9126):467‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matera 1996 {published data only}
- Matera MG, Caputi M, Cazzola M. A combination with clinical recommended dosages of salmeterol and ipratropium is not more effective than salmeterol alone in patients with chronic obstructive pulmonary disease. Respiratory Medicine 1996;90(8):497‐9. [DOI] [PubMed] [Google Scholar]
Moayyedi 1995 {published data only}
- Moayyedi P, Congleton J, Page RL, Pearson SB, Muers MF. Comparison of nebulised salbutamol and ipratropium bromide with salbutamol alone in the treatment of chronic obstructive pulmonary disease. Thorax 1995;50(8):834‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muir 1999 {published data only}
- Muir JF, Cuvelier A. Nebulization with bronchodilating agents in bronchial obstruction. Revue Des Maladies Respiratoires 1999;16(SUPPL. 3):3S41‐3. [PubMed] [Google Scholar]
Musil 1996 {published data only}
- Musil J, Vondra V. Combined inhalation therapy with bronchodilator solutions in airways obstruction (a contribution or risk?). Studia Pneumologica Et Phtiseologica 1996;Vol 56(6):243‐7. [Google Scholar]
Musil 1997 {published data only}
- Musil J, Vondra V. Do we have to fear inhalation of salbutamol solution in severe acute bronchial obstruction?. Prakticky Lekar 1997;77(1):29‐31. [Google Scholar]
Musil 1998 {published data only}
- Musil J, Hirsch V, Vondra V, Reisova M. What is the adequate dose in combined inhalation treatment of patients with exacerbations of chronic obstructive pulmonary disease?. Vnitrni Lekarstvi 1998;44(7):415‐7. [PubMed] [Google Scholar]
Naberan 1998 {published data only}
- Naberan K. [Bronchodilator treatment of COPD. Article in Spanish]. Atencion Primaria 1998;21(2):109‐13. [PubMed] [Google Scholar]
Patrick 1990 {published data only}
- Patrick DM, Dales RE, Stark RM, Laliberte G, Dickinson G. Severe exacerbations of COPD and asthma. Incremental benefit of adding ipratropium to usual therapy. Chest 1990;98(2):295‐7. [DOI] [PubMed] [Google Scholar]
Perri 1985 {published data only}
- Perri G, Giovannini M, Spada E. Salbutamol plus beclomethasone dipropionate (Ventolin Flogo) vs. fenoterol (Dosberotec) in chronic obstructive lung disease therapeutic strategy: a 4‐week clinical trial. International Journal of Clinical Pharmacology, Therapy, & Toxicology 1985;23(5):274‐8. [PubMed] [Google Scholar]
Phanareth 1997 {published data only}
- Phanareth K, Hansen EF, Laursen LC. Treatment of severe acute exacerbation of asthma and chronic obstructive pulmonary disease. Ugeskrift for Laeger 1997;159(47):6985‐91. [PubMed] [Google Scholar]
Rees 1998 {published data only}
- Rees PJ. Bronchodilators in the therapy of chronic obstructive pulmonary disease. European Respiratory Monograph 1998;3(7):135‐49. [Google Scholar]
Shrestha 1991 {published data only}
- Shrestha M, O'Brien T, Haddox R, Gourlay HS, Reed G. Decreased duration of emergency department treatment of chronic obstructive pulmonary disease exacerbations with the addition of ipratropium bromide to beta‐agonist therapy. Annals of Emergency Medicine 1991;20(11):1206‐9. [DOI] [PubMed] [Google Scholar]
Wortmann‐Schmid 1999 {published data only}
- Wortmann‐Schmid B. Exacerbation of chronic obstructive bronchitis. Therapiewoche Schweiz 1999;15(2):74‐5. [Google Scholar]
Zehner 1995 {published data only}
- Zehner WJ Jr, Scott JM, Iannolo PM, Ungaro A, Terndrup TE. Terbutaline vs albuterol for out‐of‐hospital respiratory distress: randomized, double‐blind trial. Academic Emergency Medicine 1995;2(8):686‐91. [DOI] [PubMed] [Google Scholar]
Additional references
American 1995
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 1995;152(5 Pt 2):S77‐121. [MEDLINE: ] [PubMed] [Google Scholar]
Anthonisen 1987
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Annals of Internal Medicine 1987;106:196‐204. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Murphy 1992
- Murphy TF, Sethi S. Bacterial infection in chronic obstructive pulmonary disease. American Review of Respiratory Disease 1992;146(4):1067‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Siafakas 1995
- Siafakas NM, Vermeire P, Pride NB, Paoletti P, Gibson J, Howard P, et al. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task Force. European Respiratory Journal 1995;8(8):1398‐420. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


