Abstract
Background
Despite its established effectiveness, adherence to cardiac rehabilitation remains suboptimal. The purpose of our study is to examine whether mobile technology improves adherence to cardiac rehabilitation and other outcomes.
Methods and Results
We identified all enrollees of the cardiac rehabilitation program at Boston Medical Center from 2016 to 2019 (n=830). Some enrollees used a mobile technology application that provided a customized list of educational content in a progressive manner, used the patient’s smartphone accelerometer to provide daily step counts, and served as a 2‐way messaging system between the patient and program staff. Adherence to cardiac rehabilitation was defined as the number of attended sessions and completion of the program. Enrollees had a mean age of 59 years; 32% were women, and 42% were Black. Using 3:1 propensity matching for age, sex, race/ethnicity, education, smoking status, transportation time, diagnosis, and baseline depression survey score, we evaluated change in exercise capacity, weight, functional capacity, and nutrition scores. Those in the mobile technology group (n=114) attended a higher number of prescribed sessions (mean 28 versus 22; relative risk, 1.17; 95% CI, 1.04–1.32; P=0.009), were 1.8 times more likely to complete the cardiac rehabilitation program (P=0.01), and had a slightly greater weight loss (pounds) following rehabilitation (−1.71; 95% CI, −0.30 to −3.11; P=0.02) as compared with those in the standard group (n=213); other outcomes were similar between the groups.
Conclusions
In a propensity‐matched, racially diverse population, we found that adjunctive use of mobile technology is significantly associated with improved adherence to cardiac rehabilitation and number of attended sessions.
Keywords: cardiac rehabilitation, mobile technology, outcomes research, prevention
Subject Categories: Quality and Outcomes, Secondary Prevention, Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- AHA
American Heart Association
- BMC
Boston Medical Center
- BDI
Beck Depression Inventory
- CR
cardiac rehabilitation
Clinical Perspective
What Is New?
Cardiac rehabilitation (CR) has been found to reduce mortality by up to 34%. However, despite these known benefits, only 14% to 30% of eligible patients eventually participate in CR.
New strategies are needed to promote participation in a CR program. Use of mobile technology is one such strategy that can influence health behaviors.
In a large propensity‐matched and racially diverse population, we found that adjunctive use of mobile technology is significantly associated with improved adherence to CR and number of attended sessions.
What Are the Clinical Implications?
The greater interaction, personalized guidance, communication, and feedback with the use of mobile technology may have led to increased well‐being and motivation for patients to complete the program.
Mobile technology use may help augment the design of optimal patient‐centered CR programs and potentially increase participation in CR, thereby improving clinical outcomes and mortality for those with ischemic heart disease.
Ischemic heart disease is a leading cause of death in the United States and worldwide.1, 2 Some patients with ischemic heart disease are referred to cardiac rehabilitation (CR), a multidisciplinary program that consists of individualized cardiovascular risk factor assessment and management, nutrition counseling, exercise training, and psychosocial support services.2, 3 CR has been found to reduce mortality by up to 34%1, 4 and is strongly endorsed by the American Heart Association (AHA), the American College of Cardiology, and the European Cardiology guidelines for patients after a myocardial infarction (MI), percutaneous coronary intervention, coronary artery bypass grafting, and new diagnosis of heart failure.5, 6, 7 In a sample of 30 161 elderly Medicare beneficiaries, Hammill et al8 found that there was a dose‐response relationship between the number of CR sessions attended and lower risk of death and MI at 4 years. Those who attended all 36 sessions had a 47% lower risk of death compared with those who attended 1 session.8
However, despite these known benefits, only 14% to 30% of eligible patients eventually participate in cardiac rehabilitation. Barriers to participation include difficulty in attending, low referral rates, and cost.6, 9 In addition, women and minorities are less likely to receive referral or physician instruction to CR.10 New strategies are needed to promote participation in a CR program.6, 7 Use of mobile technology is one such strategy that can influence health behaviors. Up to 96% of American adults use mobile phones, and 81% have smartphones.11 The AHA statement on mobile technologies in cardiovascular disease prevention highlights that mobile technology use can be effective in increasing physical activity, smoking cessation, weight loss, blood glucose management, and hypertension management.12 However, few prior studies have evaluated the use of mobile technology in CR settings. Thus, the purpose of our study is to examine whether the use of mobile technology improves adherence to CR and number of sessions attended and whether it improves other outcomes of cardiovascular disease prevention. A better understanding of this relation would help augment the design of optimal patient‐centered CR programs and potentially increase participation in CR, thereby improving clinical outcomes and mortality for those with ischemic heart disease.
Methods
The aggregate data that support the findings of this study are available from the corresponding author upon reasonable request.
Patient Population
Data collection and program components of the CR program at Boston Medical Center (BMC) have been previously described in detail.13 The BMC CR program was founded in 1985 and is located within the cardiovascular center at the hospital. Classes are held 5 days per week from 8 am to 4:30 pm. BMC is located in a busy urban area in the city of Boston at the crossroads of several different diverse communities. It is one of the core academic institutions of Boston University School of Medicine. Although it is a private institution, it is the largest safety net hospital in New England. The core components of the program include patient assessment, nutritional counseling, weight management, blood pressure management, lipid management, diabetes mellitus management, tobacco cessation, psychosocial management, physical activity counseling, and exercise training, as defined by the AHA statement on Core Components of Cardiac Rehabilitation Secondary Prevention Programs.3 Patient assessment includes evaluation of medical history and pertinent cardiovascular conditions, medications, physical examination, appropriate testing (such as a resting 12‐lead ECG), and a treatment plan for risk reduction strategies.
We identified all patients enrolled in the BMC CR program who underwent an initial medical history and physical examination including height and body weight. Weight (pounds) was measured using a balanced floor scale. All patients underwent exercise testing using a standardized ramp treadmill protocol.13, 14, 15 Peak metabolic equivalent of task was estimated using peak exercise work rate as calculated using the American College of Sports Medicine regression equation for treadmill exercise.14, 16 The same protocol was used for each patient at the initial exercise test and the follow‐up exercise test at the end of the cardiac rehabilitation program. Supervised exercise training was conducted at 60% to 85% of the heart rate reserve based on the patient’s initial exercise test and modified by perceived exertion for a total of 30 to 50 minutes.15 The patients performed exercise training 3 times a week using upper‐ and lower‐body training modalities (treadmill, cycle ergometer, rowing machine, and stair climber equipment) that were selected by the supervising exercise physiologist for each individual patient.
Nutritional counseling, performed by a registered dietitian, included individual assessment of dietary content and caloric intake and counseling sessions and interventions with specific modifications. In addition, weekly group nutritional counseling was also provided. Patients with diabetes mellitus, hypertension, and renal failure received additional specific recommendations, consistent with the AHA/American College of Cardiology recommendations.3 For weight management, the patients initially underwent a baseline assessment of weight and height, and subsequently short‐term and long‐term weight goals were established for those who were overweight or obese based on body mass index categories. Patients with hyperlipidemia were treated with pharmacologic therapies as needed, according to the lipid goals outlined by the American College of Cardiology/AHA guidelines.17, 18 Those who were current smokers were enrolled in smoking cessation counseling sessions at program entry with follow‐up sessions as needed. Follow‐up tests at the end of the program included a repeat exercise tolerance treadmill test, measurement of body weight, and fasting lipids.19
In a retrospective cohort analysis, we identified all enrollees of the 12‐week cardiac rehabilitation program at BMC from January 1, 2016, to December 31, 2019. We included all eligible patients who enrolled in the CR program at BMC from 2016 to 2019. Of 830 participants, 108 did not join any prescribed session; 76 were missing information on race, and 245 did not complete the Beck Depression Inventory (BDI) survey or other key covariates including education, leaving 401 for analysis. Of note, not all patients completed the BDI on follow‐up if they did not have any mental health conditions. Patients self‐selected into 1 of 2 groups: the standard CR group and those who used the Wellframe mobile technology as an adjunct to the standard CR program, the mobile technology CR group. The Wellframe mobile technology is further described in detail below. The Institutional Review Board at BMC approved the study. Chart review data collection was performed; thus, informed consent was waived.
Covariates
Covariates included enrollment date, age (years), sex, self‐described race/ethnicity as per the hospital patient registration system (Black, White, Hispanic, other: Asian, Pacific Islander, Native American), education (highest level attained; categories of less than high school, high school graduate, college graduate or higher), tobacco use (current), average transportation time to the BMC cardiac rehabilitation program (1‐way, in minutes), and qualifying diagnosis for CR–coronary artery disease post MI and percutaneous coronary intervention, coronary artery disease post coronary artery bypass grafting, heart failure, or valve surgery. Baseline BDI score, exercise capacity (peak metabolic equivalent of task level on the entry exercise tolerance test), low‐density lipoprotein (mg/dL, either measured directly by the laboratory or calculated using the Friedewald equation: low‐density lipoprotein cholesterol=total cholesterol – high‐density lipoprotein cholesterol – [triglycerides/5]), baseline weight, functional capacity score, and Rate Your Plate survey scores were also assessed.
The BDI is a well validated and widely used 21 item self‐reported rating survey that evaluates for symptoms of depression and severity.20, 21 The items include questions on symptoms such as feelings of hopelessness, irritability, and guilt, and physical symptoms such as fatigue. The patients are asked to choose 1 response to each item based on how they have felt over the past 2 weeks. The score ranges from 0 to 63, with a lower score indicating minimal depression (<9). The BDI has demonstrated high internal consistency, with an alpha coefficient of 0.9.21 The Rate Your Plate survey is a validated self‐reported food‐frequency questionnaire that consists of questions on categories of food consumed and allows for evaluation of dietary habits related to cardiovascular disease prevention.22 Initially developed in the 1980s at Brown University, it has subsequently been updated to reflect changes in the national dietary recommendations for Americans. The sum of points from all questions reflects an overall score (scale of 23–69), with a higher score indicating better diet quality. It also allows clinicians and dietitians to assess particular food groups for improvement. Functional health capacity was evaluated using the international Dartmouth Co‐op Functional Health Assessment/World Organization of National Colleges, Academies, and Academic Associations of General Practices/Family Physicians functional assessment charts.23 This tool is a self‐reported survey consisting of 6 charts that assess various domains including physical fitness, feelings, daily activities, social activities, and overall health. Each chart has a 5‐point scale, with each step of the scale illustrated by a drawing. Scores range from 6 to 30, with a lower score indicating better functional health.
Wellframe Mobile Application
Wellframe (wellframe.com) provides a framework for digital care management of patients through a patented technology platform. The Wellframe mobile application features an interactive daily checklist of written and video‐based educational and support materials that have been developed from evidence‐based, peer‐reviewed guidelines and literature as well as 2‐way, Health Insurance Portability and Accountability Act–compliant messaging between the patients and the CR program staff. The content materials provided by the Wellframe application were reviewed and standardized for each patient. Educational supportive material tailored to their specific needs was provided (ie smoking cessation materials for patients who actively smoke or recently quit smoking cigarettes; diabetes mellitus content for patients with diabetes mellitus; importance of weight change for patients who are overweight and obese; and heart failure education for patients with heart failure). In addition, customized material and videos were created by the BMC program staff to supplement the standard content of the Wellframe application. These were delivered via the Wellframe application in a staged, progressive manner to support the patient’s treatment plan. This material is designed to reinforce the education provided by staff, covering topics ranging from understanding the physiology of the heart to goal setting and habit formation, safe exercise, and healthy eating. The Wellframe application also delivered functional capacity, depression, and nutrition surveys in digital format that are routinely provided to patients in paper form. Content materials and videos were provided in both English and Spanish but were not available for other languages. A screenshot of the main Wellframe application dashboard on the computer screen as well as the interface on a smartphone is shown in Figure 1.
Figure 1.
A dashboard of the Wellframe application on the computer screen and the smartphone interface is shown.
The CR staff monitored each patient’s progress on a daily basis through a real‐time Web‐based dashboard. This allowed staff to respond to patient questions; monitor whether the patient had opened the reading and video material that was sent via the Wellframe application; monitor physical activity via the application, which uses the patient’s smartphone accelerometer to provide daily step counts; and provide support and encouragement that promotes adherence to the patient’s individualized treatment plan.
After a 4‐month pilot and customization phase, consecutive patients who enrolled in the BMC CR program between January 1, 2016, and December 31, 2019, were provided verbal and written information regarding the Wellframe application, and how it would be used to supplement the standard CR program. Patients who spoke either English or Spanish, had suitable smartphones, and elected to use them as part of their CR program were provided with the Wellframe application and instructions for its use. All other patients received the standard CR program without the Wellframe application. To minimize selection bias between those patients using the Wellframe application and those in the standard program, we used propensity score matching to estimate the program effect on outcomes.
Outcomes
Our primary outcome of interest was completion of the CR program, defined as (1) percentage of enrollees who completed the CR program as prescribed and (2) number of sessions of CR attended. The number of sessions needed to complete the program was defined for each patient depending on individual clinical needs. Secondary outcomes included change in exercise capacity (metabolic equivalent of task), change in functional capacity assessment scores, change in BDI score, change in Rate Your Plate nutrition score, and change in weight. The change accounted for the difference between the initial time point (entry in the CR program) and the end of the prescribed program (at completion or last session attended).
Statistical Analysis
Baseline characteristics of patients in the mobile technology CR and standard CR groups were compared using the chi‐square test for categorical variables or the Student’s t‐test for continuous variables. We conducted propensity score–matched analyses to account for measured confounding by demographics, comorbid conditions, medications, lifestyle factors, and healthcare use factors previously mentioned. Propensity score variables included age, sex, race/ethnicity, education level, tobacco use, transportation time, qualifying diagnosis for CR, and the baseline BDI score. Propensity scores were computed using a logistic regression model with the mobile technology CR group as the dependent variable and the baseline characteristics as covariates. We set a caliper of 0.2 SD on the logit of the propensity score to conduct greedy matching (ie, nearest‐neighbor matching). Each patient was selected once at most (ie, without replacement) with each patient in the mobile technology CR group having 1 to a maximum of 3 matched patients in the standard CR group. To verify the balance between the 2 groups, we estimated the standardized differences for the baseline covariates before and after matching (illustrated in Figure 2). Standardized differences of <10.0% for a given covariate indicate a relatively small imbalance.24, 25
Figure 2.
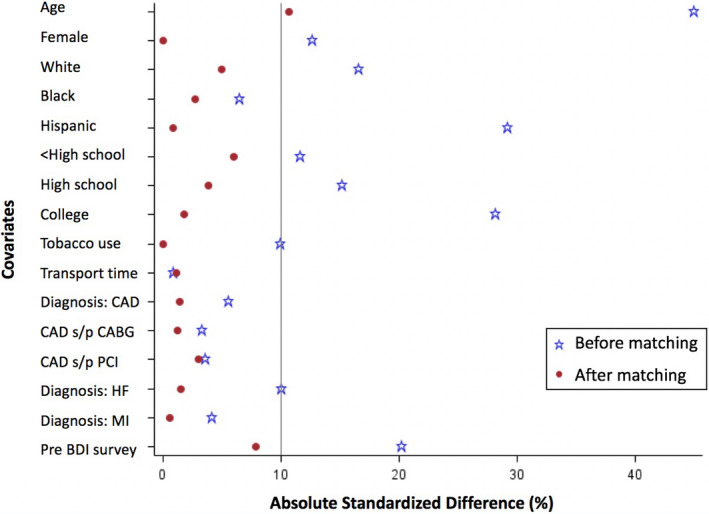
This figure illustrates the standardized differences for the baseline covariates before and after propensity matching.
BDI indicates Beck Depression Inventory; CAD, coronary artery disease; CABG, coronary artery bypass grafting; HF, heart failure; MI, myocardial infarction; and PCI, percutaneous coronary intervention.
We analyzed the matched data using the generalized estimating equations model to determine the program effect on each outcome. To test the robustness of our findings, we performed a sensitivity analysis using the propensity score as a covariate in a regression model. All analyses were conducted using 2‐sided tests, with an alpha of <0.05 considered as statistically significant. Analyses were conducted using SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
A total of 401 patients met criteria for inclusion in the study (280 in the standard CR group and 121 in the mobile technology CR group). After matching on the propensity score, the total was 327 (213 in the standard CR group, and 114 in the mobile technology CR group). Baseline characteristics of the CR program participants are shown by group in Table 1 before and after matching. To verify the balance between the 2 groups with propensity matching, the standardized differences for the baseline covariates before and after matching are shown in Figure 2. In the propensity score–matched group, enrollees had a mean age of 59 years; 32% were women, and 42% were Black. Most had a qualifying diagnosis of coronary artery disease status following MI or percutaneous coronary intervention. Patients attended the CR program 3 days per week over a 12‐week period. The mean number of sessions prescribed for the standard CR group was 33, versus 34 for the mobile technology group; the median was 36 sessions for both groups.
Table 1.
Baseline Characteristics of Cardiac Rehabilitation Program Participants According to Mobile Technology Use
| Before Matching | After Matching | |||
|---|---|---|---|---|
| Standard Group (n=280) | Mobile Technology Group (n=121) | Standard Group (n=213) | Mobile Technology Group (n=114) | |
| Age, y, mean±SD | 60±11 | 55±12 | 60±11 | 56±11 |
| Women, n (%) | 95 (34) | 34 (28) | 64 (30) | 32 (28) |
| Self‐described race/ethnicity, n (%) | ||||
| White | 100 (36) | 53 (44) | 85 (40) | 49 (43) |
| Black | 120 (43) | 48 (40) | 89 (42) | 45 (40) |
| Hispanic | 26 (9) | 3 (3) | 8 (4) | 3 (3) |
| Other (Asian/Pacific Islander, Native American) | 34 (12) | 17 (14) | 31 (15) | 17 (15) |
| Education, n (%) | ||||
| Less than high school | 16 (6) | 4 (3) | 7 (3) | 4 (4) |
| High school graduate | 125 (45) | 45 (37) | 89 (42) | 43 (38) |
| College graduate or higher | 87 (31) | 54 (45) | 82 (39) | 49 (43) |
| Unknown | 52 (19) | 18 (15) | 35 (16) | 18 (16) |
| Current smoker, n (%) | 24 (9) | 6 (5) | 12 (6) | 6 (5%) |
| Transportation time, min, median (IQR)* | 25 (15–40) | 30 (15‐45) | 25 (15–40) | 30 (15–45) |
| Diagnosis, n (%) | ||||
| CAD status post MI and/or PCI | 187 (67) | 75 (62) | 142 (67) | 72 (63) |
| CAD status post CABG | 45 (16) | 18 (15) | 34 (16) | 18 (16) |
| Heart failure | 22 (8) | 13 (11) | 19 (9) | 11 (10) |
| Valve surgery | 26 (9) | 15 (12) | 18 (9) | 13 (11) |
| BDI score, median (IQR)* | 5 (2–11) | 5 (3–8) | 4 (2–9) | 5 (3–9) |
| Exercise capacity, mean±SD (METs) | 7.3±2.9 | 8.0±3.2 | 7.5±2.8 | 7.8±3.1 |
| LDL (mg/dL), median (IQR)* | 87 (68–117) | 99 (66–130) | 86 (66–117) | 99 (69–130) |
| Weight (pounds), mean±SD | 187±38 | 200±41 | 188±36 | 202±40 |
| Functional health capacity score, median (IQR)* | 13 (10–17) | 13 (10–16) | 13 (10–16) | 13 (10–16) |
| Rate Your Plate score, mean±SD | 53±9 | 52±11 | 53±9 | 52±11 |
Column percentages are shown. Mean and SD are reported for continuous variables, while frequency and percentage are presented for categorical variables. BDI indicates Beck Depression Inventory; CABG, coronary artery bypass grafting; CAD, coronary artery disease; IQR, interquartile range, LDL, low density lipoprotein cholesterol; METs, metabolic equivalents; MI, myocardial infarction; and PCI, percutaneous coronary intervention.
*Median and interquartile range are shown for skewed variables.
We conducted 3:1 propensity score matching for age, sex, race/ethnicity, education, smoking status, transportation time to CR center, qualifying diagnosis, and baseline BDI score. We found that those in the mobile technology CR group attended a higher number of prescribed sessions (mean, 28 versus 22; relative risk, 1.17; 95% CI, 1.05–1.31; P=0.009), were 1.6 times more likely to complete the CR program (P=0.046), and had a slightly greater weight loss (pounds) after rehabilitation: −2.19 (95% CI, −0.47 to −3.74; P=0.01) as compared with those in the standard CR group; other outcomes were similar between the groups (Table 2). In total, the percentage of completion was 68.4% for the standard CR group and 80.2% for the mobile technology CR group (on the basis of the number of sessions attended). In sensitivity analysis using the propensity score as a covariate and adjusting for baseline covariates, the findings were similar (Table 3). Those in the mobile technology CR group were 1.8 times (95% CI, 1.14–2.94; P=0.01) more likely to complete the CR program.
Table 2.
Effect Estimates and 95% CIs of Propensity Score–Matched Models (n=327)
| Outcome |
Model 1† Beta Estimate (95% CI) |
P Value |
Model 2‡ Beta Estimate (95% CI) |
P Value |
|---|---|---|---|---|
| ∆ BDI score | −0.46 (−1.90 to 0.99) | 0.536 | −0.28 (−1.48 to 0.91) | 0.642 |
| ∆ Exercise capacity (METs) | −0.55 (−1.28 to 0.18) | 0.141 | −0.34 (−1.04 to 0.36) | 0.343 |
| ∆ LDL | 1.73 (−19.5 to 22.9) | 0.873 | 5.10 (−4.36 to 14.5) | 0.291 |
| ∆ Weight | −2.10 (−3.74 to −0.47) | 0.012 | −1.77 (−3.39 to −0.15) | 0.032 |
| ∆ Functional health capacity score | 0.94 (−0.15 to 2.02) | 0.090 | 0.66 (−0.20 to 1.51) | 0.132 |
| ∆ Rate Your Plate score | 1.54 (−1.24 to 4.31) | 0.278 | 0.60 (−1.53 to 2.72) | 0.584 |
| Sessions attended as prescribed* |
RR (95% CI): 1.17 (1.05 to 1.31) |
0.005 | ||
| Rehabilitation program completed |
OR (95% CI): 1.64 (1.01 to 2.70) |
0.046 | ||
∆=refers to change before and after the cardiac rehabilitation program comparing the mobile technology cardiac rehabilitation group to the standard cardiac rehabilitation group. The generalized estimating equation model was applied for matched propensity score pairs and weighted by the matching weight. The reference group is the standard cardiac rehabilitation group. BDI indicates Beck Depression Inventory; METs, metabolic equivalents; LDL, low‐density lipoprotein cholesterol; OR, odds ratio; and RR, relative risk.
Negative binomial regression applied with total session prescribed as offset.
Model 1 adjusts for the propensity score.
Model 2 adjusts for the propensity score and baseline values.
Table 3.
Sensitivity Analysis Adjusting for Baseline Values and Propensity Scores as Covariates Using a Regression Model (n=401)
| Outcome | Beta Estimate (95% CI) | P Value |
|---|---|---|
| ∆ BDI score | −0.09 (−1.47 to 1.29) | 0.896 |
| ∆ Exercise capacity (METs) | −0.44 (−1.13 to 0.24) | 0.240 |
| ∆ LDL | 5.01 (−3.71 to 13.72) | 0.258 |
| ∆ Weight | −1.71 (−0.30 to −3.11) | 0.017 |
| ∆ Functional health capacity score | 0.47 (−0.71 to 1.64) | 0.434 |
| ∆ Rate Your Plate score | −0.11 (−2.13 to 1.91) | 0.917 |
| Sessions attended as prescribed* |
RR (95% CI): 1.17 (1.04 to 1.32) |
0.009 |
| Rehabilitation program completed |
OR (95% CI): 1.82 (1.14 to 2.94) |
0.012 |
∆=refers to the change in outcome before and after the cardiac rehabilitation program comparing the mobile technology cardiac rehabilitation group to the standard cardiac rehabilitation group. The reference group is the standard cardiac rehabilitation group. BDI indicates Beck Depression Inventory; METs, metabolic equivalents; LDL, low‐density lipoprotein cholesterol; OR, odds ratio; and RR, relative risk.
Negative binomial regression applied with total session prescribed as offset.
Discussion
In a large propensity score–matched and racially diverse population, we found that adjunctive use of mobile technology is significantly associated with improved adherence to CR and number of attended sessions. The greater interaction, personalized guidance, communication, and feedback may have led to increased well‐being and motivation for patients to complete the program. In a recent study evaluating national adherence to CR among Medicare beneficiaries, only about one‐fourth (24.4%) of the 366 000 Medicare fee‐for‐service beneficiaries eligible for outpatient CR participated in the program. Among those who participated, only 26.9% completed a full course of prescribed sessions. In our study, the completion rate as prescribed was 82% in the mobile technology CR group and 67% in the standard CR group.26
These findings persisted in sensitivity analysis in which we included the propensity score as a variable in regression modeling. In addition, we also found a statistically significant weight loss in the mobile technology CR group (2.2 pounds more) as compared with the standard CR group, although clinically a minor change. The AHA presidential advisory emphasizes the need for CR programs to improve adherence, access to care, and outcomes for patients with cardiovascular disease.6 The current rate of CR remains shockingly low, as only an estimated 14% to 30% of eligible patients participate in CR.6, 9 Our study demonstrates that mobile technology supported CR is a practical and effective addition to standard CR that can improve adherence. Prior studies have found that women and minorities are less likely to receive referral or physician instruction to CR.10 In our cohort, women made up 30% of the all the patients enrolled in the CR program. However, nearly 60% of our patients enrolled in the CR program were non‐White and included individuals who self‐identified as Black, Hispanic, Asian/Pacific Islander, or Native American.
Prior observational studies have found mobile technology to be a useful and feasible adjunct in the CR setting. A nonrandomized study of 30 participants in Poland, followed over 8 weeks in which participants performed several home exercise sessions using mobile applications as an adjunct to in‐person rehabilitation services, an improvement in exercise capacity was noted (17.6±16% in the intervention group versus 11.5±36% in the control group), and there was no change in blood pressure.27 In a nonrandomized feasibility study, Widmer et al28 tested a digital health intervention (that tracked lifestyle habits and formed actionable tasks for users to improve their health) as an adjunct to CR at the Mayo Clinic in 19 patients. Their results demonstrated significant reductions in blood pressure, weight, body mass index, total cholesterol, low‐density lipoprotein cholesterol, triglycerides, exercise capacity, and quality‐of‐life scores. In a feasibility study including 26 patients in Boston, Massachusetts, a mobile smartphone application that provided personalized educational material was found to have a favorable impact on compliance and resulted in enhanced patient perceptions of CR care.29 In this feasibility study, 83% of patients reported a positive or very positive experience, and 93% of patients said that the application made it easier to adhere to CR activities. Providers reported that the mobile application improved communication, patient participation, and program efficiency.29 Similarly, a nonrandomized feasibility study in the Veterans Affairs healthcare system in Atlanta, Georgia (n=18) that evaluated a smartphone‐enabled home‐based CR program found the mobile technology to be useful.30 Participants had improved exercise capacity and mean resting systolic blood pressure.30 An observational study of 34 patients who completed a telemonitored exercise‐based CR intervention in Denmark demonstrated an improvement in muscle endurance and quality of life at 12‐month follow up.31
Few prior studies have evaluated mobile technology use in CR in a randomized trial or propensity score–matched study. In an unblinded randomized controlled trial in Australia of 60 patients following MI, Varnfield et al32 compared an entirely home‐based smartphone‐supported technology service with a standard CR program. The home‐based program included health and exercise monitoring, educational and motivational material, and weekly mentoring sessions. They found higher adherence (94% versus 68%) and completion (80% versus 47%) in the smartphone‐supported home‐based group as compared with the standard CR group.32 Improvements in 6‐minute walk tests were similar between the 2 groups. The Heart Cycle Trial, a prospective randomized trial that evaluated a smartphone‐guided training system, which included a sensor that monitored breathing rate and an ECG transmitted online to a medical team in Australia, versus standard CR found an improvement in exercise capacity and peak oxygen consumption after 6 months (n=118 initially, with a total of 54 participants who withdrew or could not participate because of technical problems).33 Another pilot randomized trial in Sydney, Australia, evaluated a smartphone‐based adjunct to standard CR in 66 patients. Patients were given a smartphone with a preinstalled application for CR, a portable blood pressure monitor, and a weight scale.34 Similar to our study, they found an improvement in adherence to CR. The completion rate for the mobile technology intervention group was 88% versus 67% for the standard CR group.34
Our study has limitations. As with other observational population studies, residual confounding from factors not accounted for cannot be entirely excluded. We attempted to account for several confounding variables including demographic, clinical, psychosocial, and functional variables in a propensity score–matched design. However, the merits of the generated propensity scores were limited to our measured covariates. Lipids were not measured at entry of CR for all patients if not clinically indicated. Some patients had lipid blood tests performed earlier according to appropriate clinical indications. Thus, we were not able to adequately evaluate change in low‐density lipoprotein levels at entry and completion. Also, several patients had not completed the BDI survey (since it was not clinically indicated for every patient), which slightly limited our sample size. Additionally, differences in culture and particularly socioeconomic status may in part account for differences between those who chose to use the mobile application and those who did not. We did not have information on socioeconomic status to measure these differences. Despite these limitations, our study has several strengths. To our knowledge, this is the first propensity score–matched study with a large sample size and a racially diverse cohort of patients that examines the association of mobile technologies with adherence to CR programs in the United States. Further strengths include a large sample size, which allows for adequate power to answer our clinical question. Our participants include various racial/ethnic groups, including Black and Hispanic individuals, and those with varying levels of education, and thus our findings may be generalizable to these populations as well. Importantly, we matched for several anthropometric, demographic, psychosocial, and functional variables that have been previously identified as barriers to CR program completion.
Conclusions
We found that adjunctive use of mobile technology is significantly associated with improved adherence to CR and the number of attended sessions in a large sample and racially diverse population. Improved adherence to CR and empowering patients to take an active role in monitoring their health may lead to improved clinical outcomes and mortality. Randomized trials with a longer‐term follow‐up are needed to confirm these findings on a larger scale and assess the potential benefits of mobile technology–supported CR on secondary prevention of cardiovascular disease and the alleviated burden on our healthcare system.
Sources of Funding
None.
Disclosures
None.
For Sources of Funding and Disclosures, see page 9.
References
- 1.Heron M. Deaths: leading causes for 2009. Natl Vital Stat Rep. 2012;61:1–94. [PubMed] [Google Scholar]
- 2.Beatty AL, Fukuoka Y, Whooley MA. Using mobile technology for cardiac rehabilitation: a review and framework for development and evaluation. J Am Heart Assoc. 2013;2:e000568. DOI: 10.1161/JAHA.113.000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JM, Franklin B, Sanderson B, Southard D. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007;115:2675–2682. DOI: 10.1161/CIRCULATIONAHA.106.180945. [DOI] [PubMed] [Google Scholar]
- 4.Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K, Thompson DR, Taylor RS. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;7:CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piepoli MF, Corrà U, Benzer W, Bjarnason‐Wehrens B, Dendale P, Gaita D, McGee H, Mendes M, Niebauer J, Zwisler A‐DO, et al. Secondary prevention through cardiac rehabilitation: from knowledge to implementation. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2010;17(1):1–17. DOI: 10.1097/HJR.0b013e3283313592. [DOI] [PubMed] [Google Scholar]
- 6.Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, Tomaselli GF, Yancy CW. American Heart Association Science Advisory and Coordinating Committee. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 2011;124:2951–2960. DOI: 10.1161/CIR.0b013e31823b21e2. [DOI] [PubMed] [Google Scholar]
- 7.Thomas RJ, Balady G, Banka G, Beckie TM, Chiu J, Gokak S, Ho PM, Keteyian SJ, King M, Lui K, et al. 2018 ACC/AHA clinical performance and quality measures for cardiac rehabilitation: a report of the American College of Cardiology/American Heart Association Task Force on performance measures. J Am Coll Cardiol. 2018;71:1814–1837. DOI: 10.1016/j.jacc.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long‐term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation. 2010;121:63–70. DOI: 10.1161/CIRCULATIONAHA.109.876383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson L, Leclerc J, Erskine Y, Linden W. Getting the most out of cardiac rehabilitation: a review of referral and adherence predictors. Heart. 2005;91:10–14. DOI: 10.1136/hrt.2004.045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mochari H, Lee JR, Kligfield P, Mosca L. Ethnic differences in barriers and referral to cardiac rehabilitation among women hospitalized with coronary heart disease. Prev Cardiol. 2006;9:8–13. DOI: 10.1111/j.1520-037X.2005.3703.x. [DOI] [PubMed] [Google Scholar]
- 11.Pew Research Center Mobile Fact Sheet February (2019). Pew Research Center. https://www.pewresearch.org/internet/fact‐sheet/mobile/ Accessed August 2, 2020.
- 12.Burke LE, Ma J, Azar KMJ, Bennett GG, Peterson ED, Zheng Y, Riley W, Stephens J, Shah SH, Suffoletto B, et al. Current science on consumer use of mobile health for cardiovascular disease prevention. Circulation. 2015;132:1157–1213. DOI: 10.1161/CIR.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bader DS, Maguire TE, Spahn CM, O'Malley CJ, Balady GJ. Clinical profile and outcomes of obese patients in cardiac rehabilitation stratified according to National Heart, Lung, and Blood Institute criteria. J Cardiopulm Rehabil. 2001;21:210–217. DOI: 10.1097/00008483-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 14.McInnis KJ, Bader DS, Pierce GL, Balady GJ. Comparison of cardiopulmonary responses in obese women using ramp versus step treadmill protocols. Am J Cardiol. 1999;83:289–A7. DOI: 10.1016/S0002-9149(98)00843-1. [DOI] [PubMed] [Google Scholar]
- 15.Adams BJ, Carr JG, Ozonoff A, Lauer MS, Balady GJ. Effect of exercise training in supervised cardiac rehabilitation programs on prognostic variables from the exercise tolerance test. Am J Cardiol. 2008;101:1403–1407. DOI: 10.1016/j.amjcard.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Whaley MH (ed). American College of Sports Medicine Guidelines for Exercise Testing and Prescription. 7th Ed. New York: Lippincott, Williams & Wilkins; 2006. [Google Scholar]
- 17.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, et al. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;2014:2889–934. DOI: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S , Faiella‐Tommasino J, Forman DE, et al. 2018 American Heart Association Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285–e350. DOI: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Banzer JA, Maguire TE, Kennedy CM, O'Malley CJ, Balady GJ. Results of cardiac rehabilitation in patients with diabetes mellitus. Am J Cardiol. 2004;93:81–84. DOI: 10.1016/j.amjcard.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. DOI: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA. Manual for the Beck Depression Inventory. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 22.Gans KM, Sundaram SG, McPhillips JB, Hixson ML, Linnan L, Carleton RA, et al. Rate Your Plate: an eating pattern assessment and educational tool used at cholesterol screening and education programs. Journal of Nutrition Education. 1993;25:29–36. DOI: 10.1016/S0022-3182(12)80186-5. [DOI] [Google Scholar]
- 23.Nelson EC, Wasson JH, Johnson DJ, Hays RD. Dartmouth COOP Functional Health Assessment Charts: brief measures for clinical practice. http://www.dartmouth.edu/~coopproj/more_coop.html Accessed August 1, 2020.
- 24.D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med. 1998;17:2265–2281. DOI: . [DOI] [PubMed] [Google Scholar]
- 25.Pattanayak CW, Rubin DB, Zell ER. Métodos de puntuación de propensión para crear una distribución equilibrada de las covariables en los estudios observacionales [Propensity score methods for creating covariate balance in observational studies]. Rev Esp Cardiol. 2011;64:897–903. DOI: 10.1016/j.recesp.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Ritchey MD, Maresh S, McNeely J, Shaffer T, Jackson SL, Keteyian SJ, Brawner CA, Whooley MA, Chang T, Stolp H, et al. Tracking cardiac rehabilitation participation and completion among medicare beneficiaries to inform the efforts of a national initiative. Circ Cardiovasc Qual Outcomes. 2020;13:e005902. DOI: 10.1161/CIRCOUTCOMES.119.005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korzeniowska‐Kubacka I, Dobraszkiewicz‐Wasilewska B, Bilińska M, Rydzewska E, Piotrowicz R. Two models of early cardiac rehabilitation in male patients after myocardial infarction with preserved left ventricular function: comparison of standard out‐patient versus hybrid training programmes. Kardiol Pol. 2011;69:220–226. [PubMed] [Google Scholar]
- 28.Widmer RJ, Allison TG, Lerman LO, Lerman A. Digital health intervention as an adjunct to cardiac rehabilitation reduces cardiovascular risk factors and rehospitalizations. J Cardiovasc Transl Res. 2015;8:283–292. DOI: 10.1007/s12265-015-9629-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forman DE, LaFond K, Panch T, Allsup K, Manning K, Sattelmair J. Utility and efficacy of a smartphone application to enhance the learning and behavior goals of traditional cardiac rehabilitation: a feasibility study. J Cardiopulm Rehabil Prev. 2014;34:327–334. DOI: 10.1097/HCR.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 30.Harzand A, Witbrodt B, Davis‐Watts ML, Alrohaibani A, Goese D, Wenger NK, Shah AJ, Zafari AM. Feasibility of a smartphone‐enabled cardiac rehabilitation program in male veterans with previous clinical evidence of coronary heart disease. Am J Cardiol. 2018;122:1471–1476. DOI: 10.1016/j.amjcard.2018.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laustsen S, Oestergaard LG, van Tulder M , Hjortdal VE, Petersen AK. Telemonitored exercise‐based cardiac rehabilitation improves physical capacity and health‐related quality of life. J Telemed Telecare. 2020;26:36–44. DOI: 10.1177/1357633X18792808. [DOI] [PubMed] [Google Scholar]
- 32.Varnfield M, Karunanithi M, Lee CK, Honeyman E, Arnold D, Ding H, Smith C, Walters DL. Smartphone‐based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart. 2014;100:1770–9. DOI: 10.1136/heartjnl-2014-305783. [DOI] [PubMed] [Google Scholar]
- 33.Skobel E, Knackstedt C, Martinez‐Romero A, Salvi D, Vera‐Munoz C, Napp A, Luprano J, Bover R, Glöggler S, Bjarnason‐Wehrens B, et al. Internet‐based training of coronary artery patients: the Heart Cycle Trial. Heart Vessels. 2017;32:408–418. DOI: 10.1007/s00380-016-0897-8. [DOI] [PubMed] [Google Scholar]
- 34.Rosario MBD, Lovell NH, Fildes J, Holgate K, Yu J, Ferry C, Schreier G, Ooi SY, Redmond SJ. Evaluation of an mHealth‐based adjunct to outpatient cardiac rehabilitation. IEEE J Biomed Health Inform. 2018;22:1938–1948. DOI: 10.1109/JBHI.2017.2782209. [DOI] [PubMed] [Google Scholar]


