Abstract
Background
Prevalence of atrial fibrillation (AF) continues to increase and is associated with significant cardiovascular morbidity and mortality. To inform prevention strategies aimed at reducing the burden of AF, we sought to quantify trends in cardiovascular mortality related to AF in the United States.
Methods and Results
We performed serial cross‐sectional analyses of national death certificate data for cardiovascular mortality related to AF, whereby cardiovascular disease was listed as underlying cause of death and AF as multiple cause of death among adults aged 35 to 84 years using the Centers for Disease Control and Prevention's Wide‐Ranging Online Data for Epidemiologic Research. We calculated age‐adjusted mortality rates per 100 000 population and examined trends over time, estimating average annual percentage change using the Joinpoint Regression Program. Subgroup analyses were performed by race‐sex and across 2 age groups (younger: 35–64 years; older: 65–84 years). A total of 276 373 cardiovascular deaths related to AF were identified in the United States between 2011 and 2018 in decedents aged 35 to 84 years. Age‐adjusted mortality rate increased from 18.0 (95% CI, 17.8–18.2) to 22.3 (95% CI, 22.0–22.4) per 100 000 population between 2011 and 2018. The increase in age‐adjusted mortality rate (average annual percentage change) between 2011 and 2018 was greater among younger decedents (7.4% per year [95% CI, 6.8%–8.0%]) compared with older decedents (3.0% per year [95% CI, 2.6%–3.4%]).
Conclusions
Cardiovascular deaths related to AF are increasing, especially among younger adults, and warrant greater attention to prevention earlier in the life course.
Keywords: atrial fibrillation, cardiovascular deaths, United States
Subject Categories: Atrial Fibrillation, Epidemiology, Race and Ethnicity
Nonstandard Abbreviations and Acronyms
- AAMR
age‐adjusted mortality rate
- AAPC
average annual percentage change
- CDC
Centers for Disease Control and Prevention
- WONDER
Wide‐Ranging Online Data for Epidemiologic Research
Clinical Perspective
What Is New?
The present study elucidated, for the first time, that age‐adjusted cardiovascular mortality rates related to atrial fibrillation have increased since 2011 across sex‐race subgroups.
Age‐adjusted cardiovascular mortality rates related to atrial fibrillation were significantly higher in older decedents compared with younger decedents over years, although accelerating trends in age‐adjusted mortality rate were more pronounced in younger decedents (aged 35–64 years).
What Are the Clinical Implications?
These trends suggest that atrial fibrillation is a more common cause of death than previously recognized, and significant differences exist across race, sex, and age subgroups.
Greater attention to prevention, early recognition, and optimal management of atrial fibrillation is warranted to reverse these unfavorable trends.
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in the world, with an estimated lifetime risk of 21% to 33%.1 Prevalence of AF is expected to exceed 12 million people by 2030 in the United States in the context of the aging population as well as increasing burden of risk factors for AF (eg, obesity and diabetes mellitus).2, 3 Projected increases in AF burden are especially concerning given the associated cardiovascular morbidity and mortality related to AF.4, 5
Death rates attributed to total cardiovascular disease in the United States have been well described, with a pattern of decline in the first decade of the 21st century, followed by a plateau.6, 7 In contrast, focused investigation of cardiovascular death rates related to heart failure have demonstrated increases since 2011, with significant Black‐White disparities, especially in younger adults.8 The contemporary burden and trends in cardiovascular mortality related to AF and stratified by race‐sex and age subgroups have not been described. Therefore, using national mortality data, we sought to describe trends in cardiovascular mortality related to AF.
Methods
This study uses completely deidentified publicly available data from the multiple cause of death files obtained from the Centers for Disease Control and Prevention`s (CDC's) Wide‐Ranging Online Data for Epidemiologic Research (WONDER), which captures all deaths occurring in the United States.9 Each death certificate includes a single underlying cause of death, multiple causes of death, up to 20 contributing causes of death, and demographic data, such as age, sex, race, and ethnicity. The underlying cause of death is based on the World Health Organization criteria as the disease that directly led to or initiated the sequence of events leading directly to death, and additional causes of death that contributed are noted. The contributing cause of death on the death certificate is defined by the CDC as any other diseases or conditions that contributed to death. Causes of death in CDC WONDER are classified according to International Classification of Diseases, Tenth Revision (ICD‐10), for the entire study period. This study was determined to be exempt from review by the institutional review board at Northwestern University, Feinberg School of Medicine, because of the deidentified nature of the data.
Cardiovascular mortality statistics related to AF were queried from CDC WONDER for deaths between January 1, 2011, and December 31, 2018, based on previous studies establishing an inflection point in cardiovascular mortality after 2011.6, 7 Cardiovascular deaths related to AF were ascertained, with cardiovascular disease (ICD‐10 codes: I00–I78) as underlying or primary cause of death, and AF (ICD‐10 code: I48) was listed as a contributing cause of death in the multiple cause of death files.10 Although ICD‐10 code was implemented in billing and care of patients at the end of 2015, the World Health Organization authorized the publication of ICD‐10 in 1999. This was implemented for mortality coding and classification for cause of death on death certificates in the United States beginning in 1999 and spanning the entire study period.7, 8, 11 Specifically, this included all deaths where AF was the underlying cause of death as well as deaths in which AF may have contributed to a cardiovascular cause of death but was not considered the proximate cause of death to more broadly quantify the burden of cardiovascular mortality related to AF. The use of the multiple cause of death files to identify underlying and contributing causes of death is consistent with prior publications.8, 11 We excluded deaths that occurred <35 years of age given the concern for associated congenital heart disease and those >85 years of age given poor demonstrated reliability of underlying cause of death reporting, particularly among cardiovascular causes in this age range.10
Statistical Analysis
Age‐group specific crude mortality rates and age‐adjusted mortality rates (AAMRs) per 100 000 population were calculated by using yearly population estimates standardized to the year 2000 US population.6, 9 We used the year 2011 to 2018 population estimates (denominators) that were bridged‐race postcensal estimates of the July 1 resident population in accordance with CDC recommendations for AAMR calculations. We examined trends over time, estimating average annual percentage change (AAPC) using Joinpoint Regression Program (National Cancer Institute),12 which calculates AAPC with 95% CIs. Subgroup analyses were performed to compare AAMRs by race‐sex group (Black and White men and women) and across 2 age groups (younger: 35–64 years; older 65–84 years). Finally, we also evaluated changes in AAMR in the following major cardiovascular disease categories as underlying cause of death considered to be associated with AF: hypertensive heart disease (ICD codes: I10–I15), ischemic heart disease (ICD codes: I20–I25), and cerebrovascular disease (ICD codes: I60–I69). As heart failure is not routinely considered an underlying cause of death in death certificate reporting, we did not investigate heart failure as an underlying cause of death related to AF specifically in this study. We also performed a secondary analysis to compare trends in AAMR for AF as the underlying cause of death compared with the broader definition, including all cardiovascular deaths related to AF. A 2‐sided P<0.05 was considered statistically significant. Data visualization was made using statistical software (StataCorp 2019; Stata Statistical Software: Release 16.1; College Station, TX: StataCorp LLC).
Results
A total of 276 373 cardiovascular deaths related to AF were identified between 2011 and 2018. Age‐group specific crude mortality rates by the years of study are summarized in Table S1. The crude mortality rate from cardiovascular deaths related to AF substantially increased in older age groups across study years. In addition, the crude mortality rate from cardiovascular deaths related to AF has increased in the same age group since 2011. Overall, AAMR from cardiovascular deaths related to AF increased from 18.0 per 100 000 (95% CI, 17.8–18.2) in 2011 to 22.3 per 100 000 (95% CI, 22.1–22.5) in 2018. A significant inflection point was not observed between 2011 and 2018 (Figure 1). AAPC of cardiovascular mortality rates related to AF was 3.4% per year (95% CI, 3.0%–3.7%) between 2011 and 2018 (Table S2).
Figure 1. Trends in age‐adjusted mortality rates in cardiovascular deaths related to atrial fibrillation in all decedents aged 35 to 84 years between 2011 and 2018.
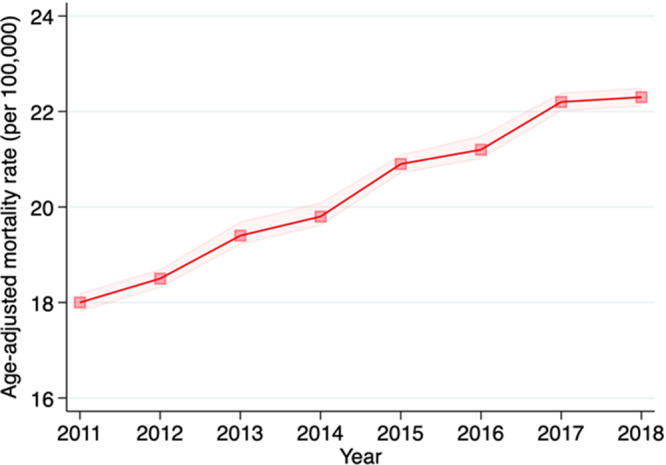
Mortality rates per 100 000 with 95% CI (dotted line) are shown.
A similar trend in AAMR was observed across race‐sex subgroups (Figure 2). Overall, AAMR was highest in White men, followed by Black men, White women, and Black women, across the study period. The acceleration of AAMR was most pronounced in Black men, with the increase of AAPC from 5.2% (95% CI, 4.5%–6.0%) per year, and White men, from 4.4% (95% CI, 4.1%–4.8%) per year, whereas a gradual acceleration of AAMR was observed in White women, from 2.0% (95% CI, 1.6%–2.5%) per year, and Black women, from 3.0% (95% CI, 2.2%–3.8%) per year (Table S2).
Figure 2. Trends in age‐adjusted mortality rates in cardiovascular deaths related to atrial fibrillation in race‐sex groups between 2011 and 2018.
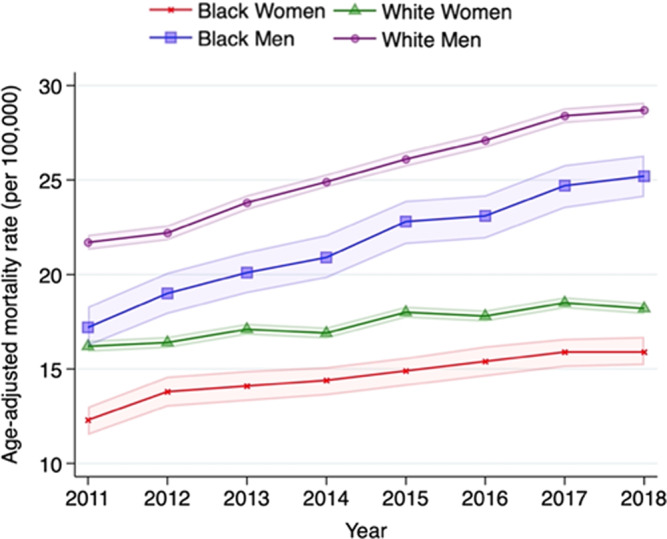
Mortality rates per 100 000 with 95% CI (dotted line) are shown by race and sex.
Younger decedents (aged 35–64 years) accounted for 2927 (10.8%) in 2011 and 4907 (12.1%) cardiovascular deaths related to AF in 2018. AAMR in the younger group was markedly lower than that in the older group (aged 65–84 years; Figure 3A and Table S2). AAPC was twice as high in the younger group (7.4% per year [95% CI, 6.8%–8.0%]) compared with the older group (3.0% per year [95% CI, 2.6%–3.4%]) after 2011. Among younger decedents, AAMR was higher in Black men and women compared with White men and women, respectively, between 2011 and 2018 (Figure 3B and Table S2). Among older decedents, AAMR was higher in White men and women compared with Black men and women between 2011 and 2018 (Figure 3A and Table S2).
Figure 3. Trends in age‐adjusted mortality rates in cardiovascular deaths related to atrial fibrillation, stratified by race‐sex and age groups (younger: 35–64 years; older: 65–84 years), between 2011 and 2018.
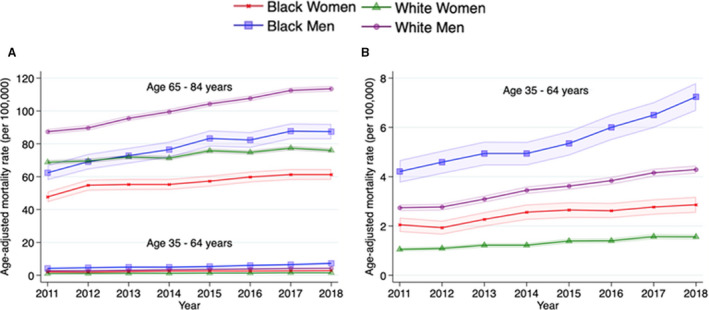
A, Trends stratified by race‐sex across 2 age groups. B, Magnified figure for younger age subgroup. Mortality rates per 100 000 with 95% CI (dotted line) are shown by race and sex.
The Table and Table S3 demonstrate the trends in AAMR in deaths related to AF stratified by specific leading causes of cardiovascular mortality as the underlying cause of death between 2011 and 2018. The leading underlying cause of death listed was ischemic heart disease (36.8% in 2011, 36.0% in 2014, and 36.2% in 2018) followed by cerebrovascular disease (14.8% in 2011, 13.0% in 2014, and 12.5% in 2018), which together accounted for ≈50% of total cardiovascular deaths related to AF. AAMR increased between 2011 and 2018 in deaths attributable to ischemic heart disease related to AF. The same increasing trend in AAMR was observed over the past 7 years in AF‐related deaths attributable to hypertensive heart disease. In contrast, AAMR for cerebrovascular deaths related to AF was stagnant over the past 7 years.
Table 1.
Trends in AAMRs and AAPC for Cardiovascular Mortality Related to AF by Leading CVD Subtype as Underlying Cause of Death, 2011 to 2018
| CVD Category | 2011 | 2014 | 2018 | 2011–2018 | |||
|---|---|---|---|---|---|---|---|
| No. (%) | AAMR (95% CI) | No. (%) | AAMR (95% CI) | No. (%) | AAMR (95% CI) | AAPC (95% CI) | |
| Overall | 27 850 (100) | 18.0 (17.8–18.2) | 32 929 (100) | 19.8 (19.6–20.1) | 41 862 (100) | 22.3 (22.1–22.5) | 3.4 (3.0–3.7) |
| HHD | 2059 (7.4) | 1.31 (1.25–1.37) | 2560 (7.8) | 1.53 (1.47–1.59) | 4132 (9.9) | 2.21 (2.14–2.28) | 8.1 (7.2–9.0) |
| IHD | 10 245 (36.8) | 6.61 (6.48–6.73) | 11 867 (36.0) | 7.14 (7.01–7.26) | 15 139 (36.2) | 8.05 (7.92–8.18) | 2.9 (2.3–3.5) |
| Stroke | 4120 (14.8) | 2.70 (2.61–2.78) | 4292 (13.0) | 2.60 (2.52–2.68) | 5223 (12.5) | 2.82 (2.74–2.90) | 1.2 (0.7–1.7) |
The AAMR indicates rate per 100 000 population, directly standardized to the 2000 US Census population. Each CVD category was defined on the basis of the following International Classification of Diseases, Tenth Revision (ICD‐10), codes: HHD (I10–I15), IHD (I20–I25), and stroke (I60–I69). AAMR indicates age‐adjusted mortality rate; AAPC, average annual percentage change; AF, atrial fibrillation; CVD, cardiovascular disease; HHD, hypertensive disease; and IHD, ischemic heart disease.
In secondary analyses, trends in AAMR between 2011 and 2018 for deaths attributable to AF as an underlying cause demonstrated qualitatively similar patterns to all cardiovascular deaths related to AF, overall and across race‐sex groups (Figures 4 and 5A, 5B).
Figure 4. Trends in age‐adjusted mortality rates of deaths attributable to atrial fibrillation as the underlying cause of death in decedents aged 35 to 84 years between 2011 and 2018.
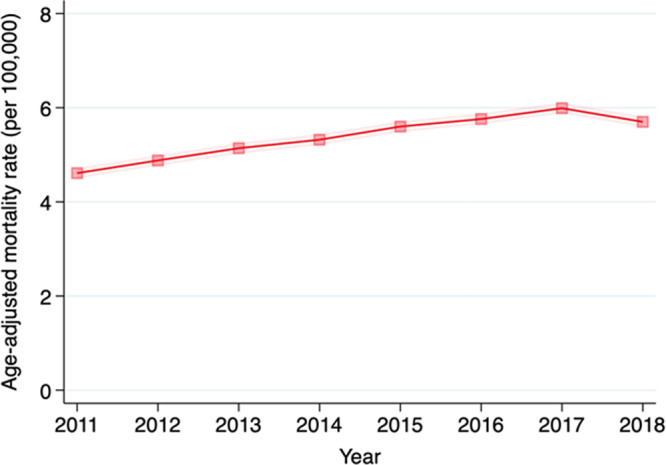
Mortality rates per 100 000 with 95% CI (dotted line) are shown.
Figure 5. Trends in age‐adjusted mortality rates of deaths attributable to atrial fibrillation as the underlying cause of death, stratified by race‐sex and age groups, between 2011 and 2018.
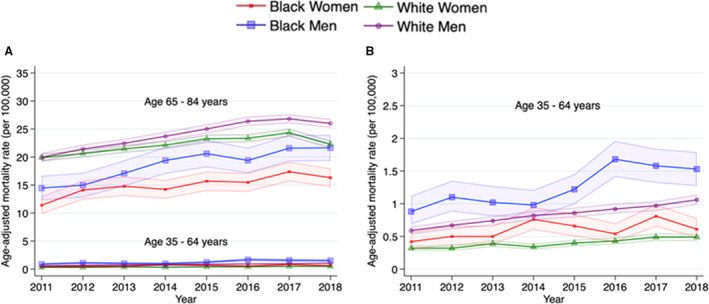
A, Trends stratified by race‐sex across 2 age groups. B, Magnified figure for younger age subgroup. Mortality rates per 100 000 with 95% CI (dotted line) are shown by race and sex.
Discussion
We found that the overall AAMR for cardiovascular deaths related to AF increased between 2011 and 2018, with varying race‐sex patterns in younger and older decedents. These findings build on and expand prior reports of increases in AF mortality between 1980 and 2014 in the United States by updating contemporary trends through 2018, examine the broader context of all cardiovascular deaths related to AF, and describe differences by age, sex, and race.13
Further studies are warranted to investigate the causes of the increase in cardiovascular mortality related to AF. Observed increases in cardiovascular deaths attributable to AF are increasing at a faster relative rate in younger adults (aged 35–64 years) compared with older adults (aged 65–84 years). This increase has led to a 1.6‐fold increase in AAMR between 2011 and 2018 in younger adults. These findings documenting higher relative changes in younger adults are of significant concern in the context of a decline in life expectancy in the United States observed since 2014 for the first time in several decades. This change in life expectancy has been attributed, in part, to increase in midlife cardiovascular mortality.14 Although our findings of trends in mortality statistics cannot confer causation, we and others have hypothesized that the growing burden of risk factors, such as obesity, diabetes mellitus, and hypertension, in younger adults is likely driving a greater proportion of the changing burden of AF mortality.15, 16, 17 In addition, although increases in AAMR were relatively greater in younger adults, the absolute burden of deaths was higher in those aged >65 years, suggesting that the aging population may also be contributing to the total contemporary burden of cardiovascular mortality related to AF. Although emerging technology (eg, AF‐sensing wearables coupled with artificial intelligence algorithms) for increasing screening, detection, and awareness of AF may improve early detection, it is unclear if this will translate into changes in population‐level mortality related to AF. It is possible that earlier detection may result in increased awareness without translation to earlier intervention or outcomes.18
Consistent with the higher prevalence of AF in individuals of European descent, cardiovascular mortality related to AF was higher in older White men and women compared with Black men and women.19 Given prior work documenting disproportionately higher burden of AF risk factors (eg, obesity, hypertension, and diabetes mellitus) in Black adults, it is possible that our findings and those of others documenting lower prevalence of AF in Black adults may be related to underdetection or undetermined factors.17 Disparities in diagnosis, awareness, and treatment of AF have been reported and may explain, in part, the increased mortality in Black men and women with AF.20, 21 Although rates in younger decedents were lower overall, the opposite trend was observed, with higher AAMR in cardiovascular deaths related to AF in Black men and women aged <65 years compared with White men and women of the same age range, which suggests greater case fatality rates in this high‐risk, vulnerable population.13, 22
Contributors toward the increase in mortality may be classified into 2 major categories: (1) a change in the prevalence of underlying disease and/or (2) case fatality rate. Changes in prevalence are particularly susceptible to a change in screening, awareness, and/or disease detection capabilities. In particular, temporal changes in physicians' perception of the mortality risk related to AF may influence death certificate coding. In addition, recent advances in novel diagnostic and therapeutic approaches to management of AF are likely to have led to decreased overall case fatality rate in AF attributable to earlier detection of milder or asymptomatic cases of AF. In fact, direct oral anticoagulants, first introduced in 2010, have demonstrated reduction in the risk of all‐cause mortality by 10% compared with warfarin in meta‐analyses of randomized controlled trials.23 Despite these advances, a recent longitudinal cohort study (Framingham Heart Study) failed to show any improvement in survival after diagnosis of AF over time.24 This suggests that a greater prevalence of AF may play a more important role in the recent AAMR acceleration in cardiovascular deaths related to AF compared with case fatality rate. We also demonstrated similar patterns when investigating AF as the underlying cause of death, suggesting that the higher awareness of AF in the clinical setting alone is not the only driver of observed trends in the broader cardiovascular mortality related to AF end point.
Finally, there may be some degree of inaccuracy of International Classification of Diseases, Ninth Revision (ICD‐9), or ICD‐10 codes for AF. However, we used the same methodologic approach as in a prior publication examining earlier trends of AF, by Vasan et al.13 Furthermore, the positive predictive value of ICD‐9 or ICD‐10 codes for identifying AF ranged from 70% to 96% (median, 89%) in a systematic review using administrative data25 and 97% (95% CI, 92%–99%) in a study using administrative data in Italy.26
Strengths of the study include up‐to‐date trends of cardiovascular mortality related to AF between 2011 and 2018 and the use of a national data set capturing all death certificates in the United States. In addition, we leveraged the multiple cause of death files to allow broader estimation of burden of cardiovascular mortality related to AF compared with prior studies.13, 27 This is especially important as AF is infrequently listed as an underlying cause of death but may contribute to or cause fatality through other cardiovascular subtypes, such as ischemic heart disease and cerebrovascular disease. We also had a large enough sample size to examine trends in race‐sex and age subgroups in cardiovascular death related to AF. Limitations of this study include use of death certificates to identify cause of death, which may be subject to misclassification or miscoding, especially related to multiple causes of death.28 We cannot exclude the possibility that AF was a concomitant diagnosis at the time of death and did not contribute to cause of death; however, we extracted contributing causes from the multiple cause of death files that are explicitly defined by the CDC as causes, diseases, or conditions that contributed to the cause of death. Given CDC WONDER does not have available data on important covariates, such as duration of AF diagnosis, subtypes of AF (paroxysmal or persistent), and treatment for AF, we were unable to definitively identify the cause for these trends, but rather we quantify national comprehensive estimates to direct future studies on individual‐level data. Finally, we cannot infer or adjudicate causality between AF and the underlying cause of cardiovascular death because of the cross‐sectional ecologic nature of the study. However, CDC WONDER is the most comprehensive source of vital statistics and allowed us to investigate nationwide trends in cardiovascular deaths, where AF was listed as a cause of death and, more important, similar patterns were observed whether AF was listed as a contributing or as the underlying cause of death.
In conclusion, this study demonstrated, for the first time, that cardiovascular deaths and death rates related to AF have increased since 2011, especially among younger decedents (aged 35–64 years). These trends suggest that AF is a more common cause of death than previously recognized, and significant differences exist across race, sex, and age subgroups. Greater attention to prevention and early recognition of AF is warranted to reverse these unfavorable trends.
Sources of Funding
This work was supported by grants from the American Heart Association (No. 19TPA34890060) to Dr Khan. Research reported in this publication was supported, in part, by the National Institutes of Health's National Center for Advancing Translational Sciences (grant No. KL2TR001424) to Khan. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by a grant from the American Heart Association (No. 18SFRN43110170) to Dr Tanaka.
Disclosures
None.
Supporting information
Tables S1–S3
(J Am Heart Assoc. 2021;10:e020163. DOI: 10.1161/JAHA.120.020163.)
For Sources of Funding and Disclosures, see page 6.
See Editorial by Kanagasundram and Stevenson
REFERENCES
- 1.Mou L, Norby FL, Chen LY, O'Neal WT, Lewis TT, Loehr LR, Soliman EZ, Alonso A. Lifetime risk of atrial fibrillation by race and socioeconomic status: ARIC study (Atherosclerosis Risk in Communities). Circ Arrhythm Electrophysiol. 2018;11:e006350. DOI: 10.1161/CIRCEP.118.006350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. DOI: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. DOI: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 4.Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta‐analysis. BMJ. 2016;354:i4482. DOI: 10.1136/bmj.i4482. [DOI] [PubMed] [Google Scholar]
- 5.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. DOI: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 6.Sidney S, Quesenberry CP, Jaffe MG, Sorel M, Nguyen‐Huynh MN, Kushi LH, Go AS, Rana JS. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 2016;1:594–599. DOI: 10.1001/jamacardio.2016.1326. [DOI] [PubMed] [Google Scholar]
- 7.Shah NS, Lloyd‐Jones DM, O'Flaherty M, Capewell S, Kershaw KN, Carnethon M, Khan SS. Trends in cardiometabolic mortality in the United States, 1999–2017. JAMA. 2019;322:780–782. DOI: 10.1001/jama.2019.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glynn P, Lloyd‐Jones DM, Feinstein MJ, Carnethon M, Khan SS. Disparities in cardiovascular mortality related to heart failure in the United States. J Am Coll Cardiol. 2019;73:2354–2355. DOI: 10.1016/j.jacc.2019.02.042. [DOI] [PubMed] [Google Scholar]
- 9.Center for Disease Control and Prevention National Center for Health Statistics . CDC wonder: multiple cause of death 1999–2018. Center for Disease Control and Prevention; February 2020. Available at https://wonder.cdc.gov/mcd‐icd10.html. Accessed June 24, 2020.
- 10.Lloyd‐Jones DM, Martin DO, Larson MG, Levy D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med. 1998;129:1020–1026. DOI: 10.7326/0003-4819-129-12-199812150-00005. [DOI] [PubMed] [Google Scholar]
- 11.Shah NS, Lloyd‐Jones DM, Kandula NR, Huffman MD, Capewell S, O'Flaherty M, Kershaw KN, Carnethon MR, Khan SS. Adverse trends in premature cardiometabolic mortality in the United States, 1999 to 2018. J Am Heart Assoc. 2020;9:e018213. DOI: 10.1161/JAHA.120.018213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Cancer Institute Division of Cancer Control and Population Sciences . Joinpoint regression program, version 4.7.0.0. Division of Cancer Controls & Population Sciences, National Cancer Institute. Available at: https://surveillance.cancer.gov/joinpoint/. Accessed June 24, 2020.
- 13.Vasan RS, Zuo Y, Kalesan B. Divergent temporal trends in morbidity and mortality related to heart failure and atrial fibrillation: age, sex, race, and geographic differences in the United States, 1991–2015. J Am Heart Assoc. 2019;8:e010756. DOI: 10.1161/JAHA.118.010756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta NK, Abrams LR, MyrskyläM.US life expectancy stalls due to cardiovascular disease, not drug deaths. Proc Natl Acad Sci USA. 2020;117:6998–7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005–2016. JAMA Pediatr. 2020;174:e194498. DOI: 10.1001/jamapediatrics.2019.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon‐Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315:2292–2299. DOI: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2018;15:230–240. DOI: 10.1038/nrcardio.2017.154. [DOI] [PubMed] [Google Scholar]
- 18.Wasserlauf J, You C, Patel R, Valys A, Albert D, Passman R. Smartwatch performance for the detection and quantification of atrial fibrillation. Circ Arrhythm Electrophysiol. 2019;12:e006834. DOI: 10.1161/CIRCEP.118.006834. [DOI] [PubMed] [Google Scholar]
- 19.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. DOI: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 20.Tedla YG, Schwartz SM, Silberman P, Greenland P, Passman RS. Racial disparity in the prescription of anticoagulants and risk of stroke and bleeding in atrial fibrillation patients. J Stroke Cerebrovasc Dis. 2020;29:104718. DOI: 10.1016/j.jstrokecerebrovasdis.2020.104718. [DOI] [PubMed] [Google Scholar]
- 21.Ugowe FE, Jackson LR, Thomas KL. Racial and ethnic differences in the prevalence, management, and outcomes in patients with atrial fibrillation: a systematic review. Heart Rhythm. 2018;15:1337–1345. DOI: 10.1016/j.hrthm.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Murphy SL, Xu J, Kochanek KD, Curtin SC, Arias E. Deaths: final data for 2015. Natl Vital Stat Rep. 2017;66:1–75. [PubMed] [Google Scholar]
- 23.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. DOI: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 24.Vinter N, Huang Q, Fenger‐Grøn M, Frost L, Benjamin EJ, Trinquart L. Trends in excess mortality associated with atrial fibrillation over 45 years (Framingham Heart Study): community based cohort study. BMJ. 2020;370:m2724. DOI: 10.1136/bmj.m2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cozzolino F, Montedori A, Abraha I, Eusebi P, Grisci C, Heymann AJ, Lombardo G, Mengoni A, Orso M, Ambrosio G. A diagnostic accuracy study validating cardiovascular ICD‐9‐CM codes in healthcare administrative databases: the Umbria Data‐Value Project. PLoS One. 2019;14:e0218919. DOI: 10.1371/journal.pone.0218919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roth GA, Dwyer‐Lindgren L, Bertozzi‐Villa A, Stubbs RW, Morozoff C, Naghavi M, Mokdad AH, Murray CJL. Trends and patterns of geographic variation in cardiovascular mortality among US counties, 1980–2014. JAMA. 2017;317:1976–1992. DOI: 10.1001/jama.2017.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coady SA, Sorlie PD, Cooper LS, Folsom AR, Rosamond WD, Conwill DE. Validation of death certificate diagnosis for coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. J Clin Epidemiol. 2001;54:40–50. DOI: 10.1016/S0895-4356(00)00272-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


