Abstract
Background
Placental derived cell‐free DNA (cfDNA), widely utilized for prenatal screening, may serve as a biomarker for preeclampsia. To determine whether cfDNA parameters are altered in preeclampsia, we conducted a case‐control study using prospectively collected maternal plasma (n=20 preeclampsia, n=22 normal) using our in‐house validated prenatal screening assay.
Methods and Results
Isolated cfDNA was quantified, sequenced using Illumina NextSeq 500, and the placental‐derived fraction was determined. Clinical and test characteristics were compared between preeclampsia and controls, followed by comparisons within the preeclampsia cohort dichotomized by cfDNA concentration. Lastly, cfDNA parameters in preeclampsia were correlated with markers of disease severity. Maternal age, body mass index, gestational age at delivery, cesarean rate, and neonatal birthweight were expectedly different between groups (P≤0.05). The placental‐derived cfDNA fraction did not differ between groups (21.4% versus 16.9%, P=0.06); however, total cfDNA was more than 10 times higher in preeclampsia (1235 versus 106.5 pg/µL, P<0.001). This relationship persisted when controlling for important confounders (OR 1.22, 95% CI 1.04–1.43, P=0.01). The dichotomized preeclampsia group with the highest cfDNA concentration delivered earlier (33.2 versus 36.6 weeks, P=0.02) and had lower placental‐derived fractions (9.1% versus 21.4%, P=0.04). Among preeclampsia cases, higher total cfDNA correlated with earlier gestational age at delivery (P=0.01) and higher maximum systolic blood pressure (P=0.04).
Conclusions
At diagnosis, total cfDNA is notably higher in preeclampsia, whereas the placental derived fraction remains similar to healthy pregnancies. In preeclampsia, higher total cfDNA correlates with earlier gestational age at delivery and higher systolic blood pressure. These findings may indicate increased release of cfDNA from maternal tissue injury.
Keywords: cell‐free DNA, hypertension, preeclampsia, pregnancy
Subject Categories: High Blood Pressure, Hypertension, Preeclampsia
Nonstandard Abbreviations and Acronyms
- cfDNA
cell‐free deoxyribonucleic acid
- DBP
diastolic blood pressure
- SBP
systolic blood pressure
Clinical Perspective
What Is New?
Individuals with preeclampsia have significantly higher circulating total cell‐free DNA compared with gestational age matched controls.
This rise is not accompanied by changes in the fraction of cell‐free DNA derived from the placenta.
Increasing amounts of circulating cell‐free DNA correlates with markers of preeclampsia disease severity, including earlier gestational age at delivery and worse systolic blood pressure.
What Are the Clinical Implications?
The notable increase in circulating cell‐free DNA in preeclampsia suggests contribution from maternal sources.
These maternal sources likely represent release of cell‐free DNA from maternal tissue injury during preeclampsia.
Understanding and being able to evaluate maternal tissue injury at the time of preeclampsia has implications for management during pregnancy, and also for later‐life cardiovascular health.
Preeclampsia is a heterogenous disease with clinical variability and imprecise diagnostic criteria. Placental dysfunction is a hallmark of preeclampsia and likely plays a central role in disease pathophysiology. Placental‐derived cell‐free DNA (cfDNA) is released from trophoblasts into the maternal circulation via apoptosis and necrosis,1 and since 2011, placental‐derived cfDNA has been widely utilized to prenatally screen pregnancies for the common aneuploidies in a non‐invasive, yet highly accurate manner.2
Given its source and mechanism of release, placental‐derived cfDNA is an attractive target to better understand disorders of placental dysfunction such as preeclampsia and fetal growth restriction. Studies investigating whether the amount of placental‐derived cfDNA (or “fetal fraction”) obtained from first‐trimester samples is able to predict preeclampsia have yielded mixed and inconsistent results.3, 4, 5, 6, 7 Studies investigating whether the amount of placental‐derived cfDNA is altered at the time of preeclampsia diagnosis have utilized real‐time PCR targeting male‐specific loci (SRY or DYS14) for quantification,8 effectively excluding half of all pregnancies. Despite important methodologic restrictions, these studies suggest that both placental‐derived cfDNA and total cfDNA (maternal and placental sources) are altered at the time of preeclampsia diagnosis.8, 9, 10, 11, 12
In May of 2017, we became the first US academic institution to develop and clinically implement a cfDNA‐based non‐invasive prenatal screening assay using whole genome‐sequencing and an in‐house developed bioinformatics platform. Here we sought to quantify total cfDNA concentration and the placental‐derived fraction from maternal plasma at the time of preeclampsia diagnosis using this assay, which allows for inclusion of all pregnancies regardless of fetal sex, in conjunction with incorporation of detailed obstetric records. We hypothesized that both the total cfDNA concentration and the placental‐derived fraction will be increased at the time of preeclampsia diagnosis, and that this will correlate with markers of disease severity.
METHODS
Study Population
Patients with preeclampsia in a singleton pregnancy were prospectively recruited at the time of clinical diagnosis as part of a prior study13 and as part of a currently ongoing study. Informed consent was obtained from all participants prior to sample collection. Maternal peripheral blood was collected at the time of preeclampsia diagnosis and before the onset of labor in acid citrate dextrose solution A‐vacutainer tubes and underwent processing within 24 hours. Maternal plasma was isolated and stored in −80°C. Gestational‐age matched controls were previously prospectively recruited14 and included healthy pregnant women carrying non‐anomalous singleton fetuses confirmed to have uncomplicated term deliveries. Samples were similarly collected, processed, and stored. There were no cases of suspected or confirmed fetal aneuploidy in our cohort. We included n=20 plasma samples from preeclampsia participants. These participants were selected for inclusion based on detailed clinical characterization confirming a preeclampsia diagnosis based on current guidelines15 and the availability of sufficient plasma. For the control population, 20 participants contributed n=22 plasma samples as 2 participants supplied 2 plasma samples, each drawn at different gestational ages, several weeks apart. Preeclampsia and normal outcomes were confirmed by detailed medical record review. Cases and controls were matched based on the gestational age at the time of sample collection. Frequency matching was utilized to ensure appropriate representation of cases and controls within gestational age categories. We chose to include n=20 participants in each group as prior studies have demonstrated statistically significant differences with smaller and mixed populations using an equally or less sensitive approach. This study was approved by the University of Washington and Fred Hutchinson Cancer Research Center Institutional Review Boards. Signed informed consent was obtained from each participant prior to enrollment.
Quantification of Total cfDNA
From maternal plasma, cfDNA was isolated and extracted using the Qiagen QiaSymphony Circulating DNA kit. Total cfDNA concentration was measured by Qubit fluorometry, a highly sensitive method for quantification of intact double‐stranded DNA (pg/µL). The Agilent TapeStation workflow, an automated electrophoresis system, was used to assess the size and integrity of the DNA and functions as a critical quality control step throughout next‐generation library preparation, hybridization capture, and sample pooling before sequencing.
Sequencing and Quantification of Placental‐Derived cfDNA
Following library preparation with KAPA HyperPrep for adapter and index ligation followed by Agencourt AMPureXP purification and amplification, libraries were pooled using an equimolar strategy. Libraries were sequenced using an Illumina NextSeq 500 High Output 75 cycle kit with a 37 bp paired‐end read configuration. On average, each cfDNA sample was sequenced to a depth of ≈20 million paired‐end reads, corresponding to an average genome depth of 0.5X. Reads were aligned to the human reference genome (hg19) with Bowtie (version 1.1.2), and run metrics are calculated with Picard (version 1.141). The placental‐derived fraction for each sample is calculated either by the percent of reads that align to the Y chromosome, or a custom bioinformatic algorithm based on the aggregate length distribution in sequencing reads for samples in which the Y chromosome is not present (ie, female fetuses).16 The 2 methods are not quantitatively compared for every case, but the data are qualitatively reviewed. The fragmentation size‐based method is consistently revalidated as the assay we utilized is validated and utilized clinically as a prenatal screen for fetal aneuploidy.
Statistical Analysis
Our primary outcome was total cfDNA concentration in preeclampsia and control samples. Fisher's exact and chi‐square analyses were used to compare categorical variables as appropriate. Mann Whitney U or t test was used to compare continuous variables as appropriate. Logistic regression was performed to evaluate the association of total cfDNA concentration and a diagnosis of preeclampsia, controlling for gestational age at sample collection and maternal body mass index (BMI), as these 2 factors are known to be critically associated with the placental‐derived fraction and total cfDNA in maternal plasma. Among those with preeclampsia, we dichotomized cases by total cfDNA concentration (<1000 and ≥1000 pg/µL) and compared clinical and test characteristics between these 2 groups. Lastly, we analyzed the relationship between total cfDNA concentration and the placental‐derived fraction in the preeclampsia group with surrogates of disease severity, including gestational age at delivery, maximum systolic blood pressure (SBP), and maximum diastolic blood pressure (DBP), using Pearson or Spearman's correlation as appropriate based on the normality of the data. A P value of <0.05 was considered statistically significant for all analyses. All statistical analyses were performed on Stata Statistical Software: Release 16 (2019, College Station, TX: StataCorp LLC).
RESULTS
Compared with normal controls, the preeclampsia participants were significantly younger (28.0 versus 34.5 years, P<0.01), had a higher BMI at delivery (32.5 versus 29.5 kg/m2, P=0.05), delivered at earlier gestational ages (34.1 versus 39.6 weeks, P<0.001), had a higher Cesarean delivery rate (75% versus 30%, P<0.01), and delivered smaller neonates (1845 versus 3600 g, P<0.001), as expected. There were no differences in maternal race, rates of nulliparity, or neonatal sex between the 2 groups (Table 1).
Table 1.
Study Population Demographics
| Characteristic | Normal (n=20) | Preeclampsia (n=20) | P Value |
|---|---|---|---|
| Maternal age, y* | 34.5 (32.0–38.5) | 28.0 (23.5–35.0) | <0.01 |
| White race† | 17 (85.0) | 12 (60.0) | 0.07 |
| BMI at delivery, kg/m2 * | 29.5 (26.7–36.4) | 32.5 (30.9–41.3) | 0.05 |
| Nulliparity† | 10 (50) | 11 (55) | 0.75 |
| Gestational age at delivery, wk* | 39.6 (38.5–40.7) | 34.1 (31.4–36.6) | <0.001 |
| Cesarean delivery† | 6 (30.0) | 15 (75.0) | <0.01 |
| Neonatal birthweight, g* | 3600 (3347–4094) | 1845 (1288–2670) | <0.001 |
| Fetal female sex‡ | 8 (40.0) | 6 (30.0) | 0.51 |
Data expressed as median (interquartile range) or n (%). BMI indicates body mass index.
Mann‐Whitney U test.
Fisher's exact test.
Chi‐square test.
There was no difference in the mean gestational age at draw between the 2 groups (34.1 weeks in normal versus 33.1 weeks in preeclampsia, P=0.37), confirming appropriate matching between samples (Table 2). There was no significant difference in the placental‐derived fraction of cfDNA between normal and preeclampsia participants (20.0% versus 14.3%, P=0.06) (Table 2, Figure 1). In contrast, significantly higher total cfDNA concentration was noted in the preeclampsia group compared with controls (1235 versus 106.5 pg/µL, P<0.001) (Table 2, Figure 2). Given the variation in total cfDNA concentration values, we chose to evaluate cfDNA concentration in units of 10 for the logistic regression analysis to improve clinical interpretation of results. After controlling for gestational age at sample draw and maternal BMI, both considered to be critically important confounders, the association between higher total cfDNA concentration in preeclampsia persisted (OR 1.22, 95% CI 1.04–1.43, P=0.01). As such, for every 10 unit increase in total cfDNA concentration, the odds of preeclampsia increased by a factor of 1.22. There are 2 notable outliers in our preeclampsia cohort with markedly elevated total cfDNA concentrations (10 100 and 11 500 pg/µL). Removal of these 2 outliers did not change our findings with respect to differences in total cfDNA between preeclampsia and controls (696 versus 106.5 pg/µL, P<0.001) or the multivariable logistic analysis evaluating the likelihood of preeclampsia diagnosis with higher total cfDNA concentrations (OR 1.22, 95% CI 1.04–1.43, P=0.01).
Table 2.
cfDNA Metrics in Normal and Preeclampsia Samples
| Characteristic | Normal (n=22) | Preeclampsia (n=20) | P Value |
|---|---|---|---|
| Gestational age at draw, wk* | 34.1±4.6 | 33.1±4.0 | 0.37 |
| Placental‐derived fraction (%)† | 20.0 (16.8–27.2) | 14.3 (8.3–23.5) | 0.06 |
| Total cfDNA concentration, pg/µL† | 106.5 (53–187) | 1235 (419–2955) | <0.001 |
Data expressed as mean±standard deviation or median (interquartile range). cfDNA indicates cell‐free DNA.
Student t test.
Mann‐Whitney U test
Figure 1. Placental‐derived cell‐free DNA (cfDNA) fraction in all samples characterized by pregnancy status.
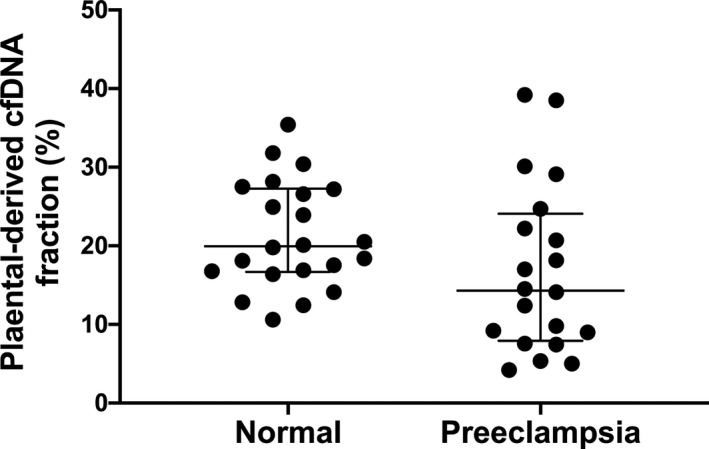
The line represents the median placental‐derived cfDNA fraction and interquartile range.
Figure 2. Total cell‐free DNA (cfDNA) concentration in all samples characterized by pregnancy status.
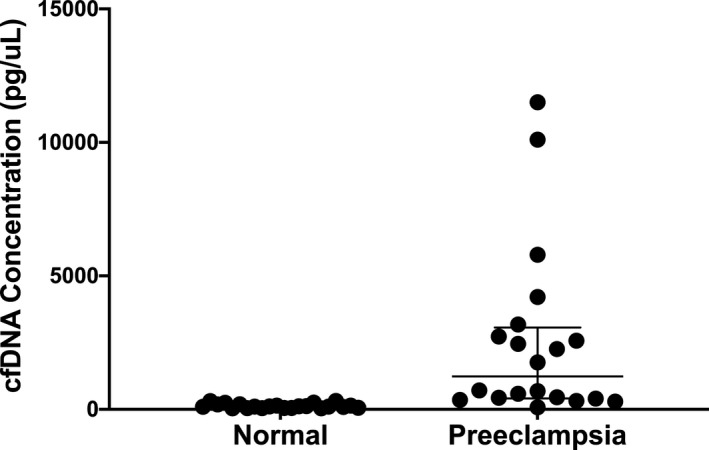
The line represents the median total cfDNA concentration and interquartile range.
Dichotomization of the preeclampsia cohort based on total cfDNA concentration resulted in n=10 in each the “low” cfDNA group (<1000 pg/µL) and the “high” cfDNA group (≥1000 pg/µL). As expected, there was a significant difference in median total cfDNA concentration between groups (2955 pg/µL for the “high” group versus 419 pg/µL for the “low” group, P<0.001). The “high” cfDNA group also had significantly lower placental‐derived cfDNA fractions (9.1% versus 21.4%, P=0.04) and delivered at earlier gestational ages (32.8 versus 36.4 weeks, P=0.03) (Table 3). There was no significant difference in the BMI at delivery, fetal sex, incidence of intrauterine growth restriction, maximum SBP, or maximum DBP between groups (Table 3).
Table 3.
Clinical and cfDNA Metrics Among the Preeclampsia Cases Dichotomized by Total cfDNA Concentration
| Characteristic | Low cfDNA Concentration (n=10) | High cfDNA Concentration (n=10) | P Value |
|---|---|---|---|
| Total cfDNA concentration, pg/µL* | 419 (314–585) | 2955 (2450–5790) | <0.001 |
| Placental‐derived fraction (%)* | 21.4 (12.4–30.1) | 9.1 (7.4–12.0) | 0.04 |
| Gestational age at draw, wk* | 36.4 (33.7–37.0) | 32.8 (30.2–33.4) | 0.03 |
| Gestational age at delivery, wk* | 36.6 (34.7–37.1) | 33.2 (30.4–33.4) | 0.02 |
| BMI at delivery, kg/m2 * | 32.1 (31.2–38.5) | 33.2 (30.9–41.3) | 0.76 |
| Female fetal sex† | 3 (30) | 3 (30) | 1.0 |
| Fetal growth restriction (%)† | 1 (10.0) | 5 (50.0) | 0.14 |
| Maximum SBP, mm Hg* | 149 (144–159) | 164 (157–180) | 0.06 |
| Maximum DBP, mm Hg* | 99.5 (83–103) | 105.5 (94–112) | 0.16 |
Data expressed as median (interquartile range) or n (%). BMI indicates body mass index; cfDNA, cell‐free DNA; DBP, diastolic blood pressure; and SBP, systolic blood pressure.
Mann‐Whitney U test.
Fisher's exact test.
Given the strong association of total cfDNA concentration with preeclampsia, we investigated correlations between total cfDNA concentration and surrogates of disease severity amongst the preeclampsia participants. There was a negative correlation between cfDNA concentration and gestational age at delivery (r s=−0.57, P=0.01) (Figure 3) and a modest positive correlation with maximum SBP (r s=0.46, P=0.04) (Figure 4). There was no significant correlation with maximum DBP (r s=0.42, P=0.06). We also investigated whether the placental‐derived cfDNA fraction correlated with surrogates of disease severity among the preeclampsia participants. Placental‐derived cfDNA fraction modestly negatively correlated with maximum SBP (r=−0.46, P=0.04) (Figure 5), but not with maximum DBP (r=−0.21, P=0.4). Because the placental‐derived cfDNA fraction is likely to be significantly affected by the gestational age at draw, and the interval between draw and delivery was short in our preeclampsia population (median 1 day, mean 2.3 days), we did not evaluate correlations between placental‐derived cfDNA fraction and gestational age at delivery. Review of the sequencing results did not identify any notable copy number alterations between the 2 groups.
Figure 3. Correlation of total cell‐free DNA (cfDNA) concentration and gestational age at delivery in preeclampsia.
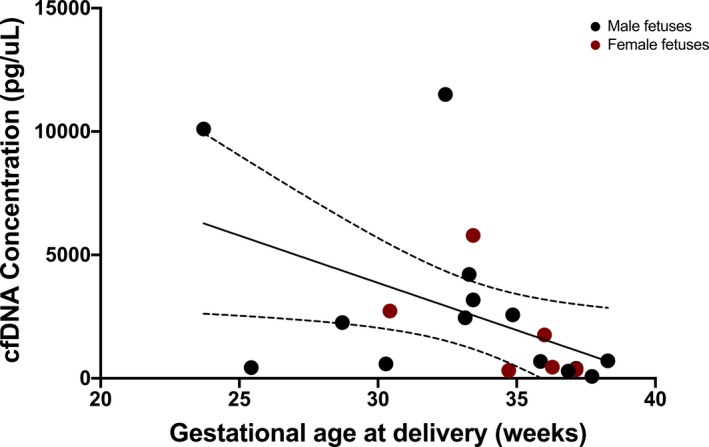
Correlation between total cfDNA concentration and gestational age at delivery in the preeclampsia cohort (r s=−0.57, P=0.01). The dotted lines represent the 95% CI.
Figure 4. Correlation of total cell‐free DNA (cfDNA) concentration and maximum systolic blood pressure in preeclampsia.
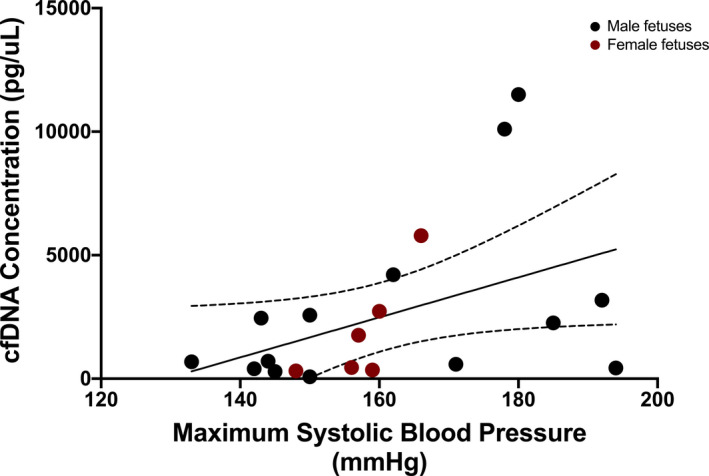
Correlation between total cfDNA concentration and maximum systolic blood pressure in the preeclampsia cohort (r s=0.46, P=0.04). The dotted lines represent the 95% CI.
Figure 5. Correlation of placental‐derived cell‐free DNA (cfDNA) fraction and maximum systolic blood pressure in preeclampsia.
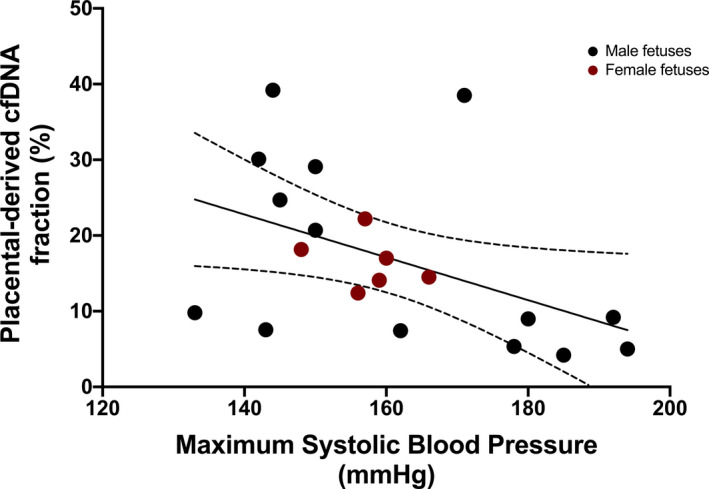
Correlation between placental‐derived cfDNA fraction and maximum systolic blood pressure in the preeclampsia cohort (r=−0.46, P=0.04). The dotted lines represent the 95% CI.
DISCUSSION
In this study of prospectively collected maternal plasma, we demonstrate that at the time of preeclampsia diagnosis, total cfDNA concentration is significantly higher compared with controls whereas the placental‐derived fraction is not statistically different. Notably, the median cfDNA concentration in the preeclampsia cohort was more than 10 times higher than the control group. When focusing on the preeclampsia cohort only, those participants with the highest total cfDNA concentrations demonstrated a lower placental fraction, suggesting dilution from maternal sources, and earlier gestational age at delivery, compared with those with lower total cfDNA concentrations. In the preeclampsia cases, we also found that higher total cfDNA concentration correlated with earlier gestational age at delivery and higher maximum SBP, 2 critical and universally recognized markers of disease severity.
Generated via a clinically utilized platform, our findings are in line with prior investigations that have used research laboratory methodologies. Much of this prior work, however, has focused on the placental‐derived component, rather than total cfDNA, using samples from varied points in gestation.9, 10, 12 Interestingly, while our study shows no statistical difference in the placental‐derived fraction of cfDNA between groups, the absolute amount of placental cfDNA is likely increased in preeclampsia as the total amount of cfDNA is increased. Although others have noted increased placental‐derived and total cfDNA in severe phenotypes of preeclampsia,10, 17 our study is unique in its correlation to clinically relevant parameters of disease severity, notably gestational age at delivery and SBP. One study correlated the amount of cfDNA in patients with preeclampsia with elevated serum AST and ALT, but did not find a correlation with maternal blood pressure and gestational age at delivery, although this is likely due to differences in patient population as their preeclampsia cohort delivered at a median gestational age of 38 weeks.18 Interestingly, the 2 preeclampsia participants in our study with the highest total cfDNA concentration were noted to have clinically severe disease: one with superimposed preeclampsia, severe fetal growth restriction, and significant renal dysfunction; the second with preeclampsia with severe features due to severe range blood pressures and severe fetal growth restriction, resulting in delivery at 23‐weeks gestation.
As all prior studies examining cfDNA parameters in preeclampsia have utilized real time quantitative PCR to quantify placental‐derived cfDNA, they were limited by inclusion of only pregnancies carrying male fetuses. We have been able to overcome this limitation by utilizing bioinformatic approaches that estimate the placental‐derived fraction regardless of fetal sex and which have been validated and are currently utilized clinically for non‐invasive prenatal screening.16 Fetal sex is recognized as an important risk factor for preeclampsia and there is growing speculation that placentation and maternal adaptation to pregnancy may be influenced by fetal sex.19, 20 Thus, representation of both fetal sexes when investigating pathophysiologic processes at the maternal‐fetal interface are necessary. There were no differences in fetal sex or based on fetal sex in our population, however, larger studies are needed to determine its potential implications on cfDNA metrics in maternal plasma. It is important to note that maternal BMI influences the placental‐derived cfDNA fraction in maternal plasma.21, 22 As data regarding its effect on total cell‐free DNA, particularly in the third trimester, are currently emerging, we included this in our logistic model because it was different between our 2 groups. Importantly, with inclusion into our model, total cfDNA concentration remained significantly associated with a diagnosis of preeclampsia. Secondly, BMI at delivery did not differ among the preeclampsia participants dichotomized by total cfDNA concentration (Table 3), suggesting that this parameter alone did not contribute to the cfDNA concentration in the “high” group. Although BMI was ascertained at the time of delivery, the interval from sample collection to delivery in the preeclampsia cohort was minimal (mean 2.3 days).
Prior studies have suggested higher placental‐derived cfDNA quantities in maternal plasma in the setting of preeclampsia11, 12; however, these studies were restricted to male fetuses, utilized alternative approaches (PCR for Y chromosome material), and were not able to universally determine the contributing fraction. It is possible that our sample size precluded detection of a significant difference in the placental‐derived fraction, however, given methodological differences between our and other studies (sequencing versus PCR), direct comparisons are challenging. As the total cfDNA concentration was higher in preeclampsia, the absolute amount contributed by the placenta is likely also higher, despite no statistical difference in the fraction. The sample size of the current study did not allow for nuanced correlation between total cfDNA concentration and specific hypertensive disease phenotypes. Additionally, while we present novel findings, our data do not delineate mechanisms.
In healthy non‐pregnant individuals, cfDNA originates from hematopoietic lineages and to a lesser degree from vascular endothelial cells, neurons, and hepatocytes.23 Although our study and others indicate that the placental‐derived amount of cfDNA increases with preeclampsia, we demonstrate that the increase in maternal sources of cfDNA is also significant. We hypothesize that the increase in total cfDNA concentration in the preeclampsia group is related to maternal tissue injury and the subsequent release of cfDNA from relevant organs, such as the endothelium, liver, and/or kidneys. Recent advances in the field of genomics have allowed for the identification of tissue‐of‐origin of circulating cfDNA.24, 25 Future investigations examining the exact origin of maternal cfDNA can further our understanding of preeclampsia pathophysiology and determine if the sources of cfDNA correlate with disease phenotype given the especially heterogeneous presentation of preeclampsia. In addition to increased production, the level of extracellular DNA depends on clearance mechanisms that are found in the “home” tissue and organs such as liver, spleen, and kidney.23 Given the systemic multiorgan impact of preeclampsia, the elevation in cfDNA may not be solely due to release from damaged tissue, but also due to a decrease in its clearance.
Further research is needed to elucidate the function that both placental and maternal cfDNA play in the disease process of preeclampsia. It has been suggested that placental cfDNA increases 3 weeks before the clinical manifestation of preeclampsia and that this rise is attributed to accelerated apoptosis of trophoblastic cells.26 Free DNA is immunostimulatory and there is speculation that placental cfDNA in particular may contribute to the initiation of the systemic inflammatory syndrome of preeclampsia.27 In fact, 2 recent studies demonstrated that placental cfDNA from patients with preeclampsia can induce an inflammatory response in vitro on trophoblast cell culture.28, 29 Lastly, it is hypothesized that the preeclampsia pregnancy itself may result in enduring damage to the maternal system contributing to later‐life cardiovascular disease.30, 31 Understanding disease pathology and discrete sites of maternal tissue injury at the time of preeclampsia presentation may improve our current and long‐term management of this at‐risk, yet poorly understood population.
In summary, we found that total cfDNA concentration is higher in preeclampsia compared with healthy controls, while the placental‐derived fraction was similar between these groups. Among the preeclampsia group, those with the highest amount of total cfDNA had a significantly lower placental‐derived fraction suggesting possible dilution due to maternal sources. Importantly, we were able to correlate total cfDNA with key markers of disease severity, including gestational age at delivery and maximum SBP. Further research is needed to determine the maternal sources of the increased cfDNA in preeclampsia and how both placental‐ and maternal‐derived cfDNA contribute to the pathophysiology of this globally important disease to catalyze the development of appropriate interventions.
Sources of Funding
The study was funded by the National Institutes of Health (K08HD067221, K08HL150169, and R01HL11737) and by a pilot grant from the Brotman Baty Institute. The funders had no involvement in study design, in the collection, analysis, or interpretation of data, or in the writing of the report or decision to submit the article for publication.
Disclosures
None.
For Sources of Funding and Disclosures, see page 8.
REFERENCES
- 1.Lo YMD, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, Wainscoat JS. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–487. DOI: 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- 2.Norton ME, Jacobsson BO, Swamy GK, Laurent LC, Ranzini AC, Brar H, Tomlinson MW, Pereira L, Spitz JL, Hollemon D, et al. Cell‐free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589–1597. DOI: 10.1056/NEJMoa1407349. [DOI] [PubMed] [Google Scholar]
- 3.Bender W, Koelper N, Sammel M, Dugoff L. Association of fetal fraction of cell‐free DNA and hypertensive disorders of pregnancy. Am J Perinatol. 2019;36:311–316. DOI: 10.1055/s-0038-1667374. [DOI] [PubMed] [Google Scholar]
- 4.Ashoor G, Syngelaki A, Poon LCY, Rezende JC, Nicolaides KH. Fetal fraction in maternal plasma cell‐free DNA at 11–13 weeks’ gestation: relation to maternal and fetal characteristics: fetal fraction in maternal plasma cell‐free DNA. Ultrasound Obstet Gynecol. 2013;41:26–32. DOI: 10.1002/uog.12331. [DOI] [PubMed] [Google Scholar]
- 5.Gerson KD, Truong S, Haviland MJ, O’Brien BM, Hacker MR, Spiel MH. Low fetal fraction of cell‐free DNA predicts placental dysfunction and hypertensive disease in pregnancy. Pregnancy Hypertens. 2019;16:148–153. DOI: 10.1016/j.preghy.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rolnik DL, O’Gorman N, Fiolna M, van den Boom D , Nicolaides KH, Poon LC. Maternal plasma cell‐free dna in the prediction of pre‐eclampsia. Ultrasound Obstet Gynecol. 2015;45:106–111. DOI: 10.1002/uog.14671. [DOI] [PubMed] [Google Scholar]
- 7.Martin A, Krishna I, Martina B, Samuel A. Can the quantity of cell‐free fetal DNA predict preeclampsia: a systematic review: cell‐free fetal DNA and preeclampsia prediction. Prenat Diagn. 2014;34:685–691. DOI: 10.1002/pd.4416. [DOI] [PubMed] [Google Scholar]
- 8.Sifakis S, Zaravinos A, Maiz N, Spandidos DA, Nicolaides KH. First‐trimester maternal plasma cell‐free fetal DNA and preeclampsia. Am J Obstet Gynecol. 2009;201:472.e1–7. DOI: 10.1016/j.ajog.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Lo YD, Leung TN, Tein MS, Sargent IL, Zhang J, Lau TK, Haines CJ, Redman CW. Quantitative abnormalities of fetal DNA in maternal serum in preeclampsia. Clin Chem. 1999;45:184–188. DOI: 10.1093/clinchem/45.2.184. [DOI] [PubMed] [Google Scholar]
- 10.Zhong XY, Laivuori H, Livingston JC, Ylikorkala O, Sibai BM, Holzgreve W, Hahn S. Elevation of both maternal and fetal extracellular circulating deoxyribonucleic acid concentrations in the plasma of pregnant women with preeclampsia. Am J Obstet Gynecol. 2001;184:414–419. DOI: 10.1067/mob.2001.109594. [DOI] [PubMed] [Google Scholar]
- 11.Rafaeli‐Yehudai T, Imterat M, Douvdevani A, Tirosh D, Benshalom‐Tirosh N, Mastrolia SA, Beer‐Weisel R, Klaitman V, Riff R, Greenbaum S, et al. Maternal total cell‐free DNA in preeclampsia and fetal growth restriction: evidence of differences in maternal response to abnormal implantation. PLoS One. 2018;13:e0200360. DOI: 10.1371/journal.pone.0200360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seval MM, Karabulut HG, Tükün A, Koç A. Cell free fetal DNA in the plasma of pregnant women with preeclampsia. Clin Exp Obstet Gynecol. 2015;42:787–791. [PubMed] [Google Scholar]
- 13.Gammill HS, Aydelotte TM, Guthrie KA, Nkwopara EC, Nelson JL. Cellular fetal microchimerism in preeclampsia. Hypertension. 2013;62:1062–1067. DOI: 10.1161/HYPERTENSIONAHA.113.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shree R, Harrington WE, Kanann SB, Forsyth A, Cousin E, Lopez A, Lee Nelson J, Gammill HS. Fetal microchimerism by mode of delivery: a prospective cohort study. BJOG. 2019;126:24–31. DOI: 10.1111/1471-0528.15432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1–e25. [DOI] [PubMed] [Google Scholar]
- 16.Yu SCY, Chan KCA, Zheng YWL, Jiang P, Liao GJW, Sun H, Akolekar R, Leung TY, Go A, van Vugt JMG , et al. Size‐based molecular diagnostics using plasma DNA for noninvasive prenatal testing. Proc Natl Acad Sci USA. 2014;111:8583–8588. DOI: 10.1073/pnas.1406103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swinkels DW, de Kok JB , Hendriks JCM, Wiegerinck E, Zusterzeel PLM, Steegers EAP. Hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome as a complication of preeclampsia in pregnant women increases the amount of cell‐free fetal and maternal DNA in maternal plasma and serum. Clin Chem. 2002;48:650–653. DOI: 10.1093/clinchem/48.4.650. [DOI] [PubMed] [Google Scholar]
- 18.Lazar L, Rigó J, Nagy B, Balogh K, Makó V, Cervenak L, Mézes M, Prohászka Z, Molvarec A. Relationship of circulating cell‐free DNA levels to cell‐free fetal DNA levels, clinical characteristics and laboratory parameters in preeclampsia. BMC Med Genet. 2009;10:120. DOI: 10.1186/1471-2350-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burton GJ, Redman CW, Roberts JM, Moffett A. Pre‐eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. DOI: 10.1136/bmj.l2381. [DOI] [PubMed] [Google Scholar]
- 20.Broere‐Brown ZA, Adank MC, Benschop L, Tielemans M, Muka T, Gonçalves R, Bramer WM, Schoufour JD, Voortman T, Steegers EAP, et al. Fetal sex and maternal pregnancy outcomes: a systematic review and meta‐analysis. Biol Sex Differ. 2020;11:26. DOI: 10.1186/s13293-020-00299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinnings SL, Geis JA, Almasri E, Wang H, Guan X, McCullough RM, Bombard AT, Saldivar J‐S, Oeth P, Deciu C. Factors affecting levels of circulating cell‐free fetal DNA in maternal plasma and their implications for noninvasive prenatal testing. Prenat Diagn. 2015;35:816–822. DOI: 10.1002/pd.4625. [DOI] [PubMed] [Google Scholar]
- 22.Rolnik DL, Yong Y, Lee TJ, Tse C, McLennan AC, da Silva CF . Influence of body mass index on fetal fraction increase with gestation and cell‐free DNA test failure. Obstet Gynecol. 2018;132:436–443. DOI: 10.1097/AOG.0000000000002752. [DOI] [PubMed] [Google Scholar]
- 23.Kustanovich A, Schwartz R, Peretz T, Grinshpun A. Life and death of circulating cell‐free DNA. Cancer Biol Ther. 2019;20:1057–1067. DOI: 10.1080/15384047.2019.1598759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snyder MW, Kircher M, Hill AJ, Daza RM, Shendure J. Cell‐free DNA Comprises an in vivo nucleosome footprint that informs its tissues‐of‐origin. Cell. 2016;164:57–68. DOI: 10.1016/j.cell.2015.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun K, Jiang P, Cheng SH, Cheng THT, Wong J, Wong VWS, Ng SSM, Ma BBY, Leung TY, Chan SL, et al. Orientation‐aware plasma cell‐free DNA fragmentation analysis in open chromatin regions informs tissue of origin. Genome Res. 2019;29:418–427. DOI: 10.1101/gr.242719.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine RJ, Qian C, LeShane ES, Yu KF, England LJ, Schisterman EF, Wataganara T, Romero R, Bianchi DW. Two‐stage elevation of cell‐free fetal DNA in maternal sera before onset of preeclampsia. Am J Obstet Gynecol. 2004;190:707–713. DOI: 10.1016/j.ajog.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Hartley JDR, Ferguson BJ, Moffett A. The role of shed placental DNA in the systemic inflammatory syndrome of preeclampsia. Am J Obstet Gynecol. 2015;213:268–277. DOI: 10.1016/j.ajog.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 28.Li N, He F, Gao H, Ge Y, Fan X, Zhang J, Qi H, Ren L. Elevated cell‐free fetal DNA contributes to placental inflammation and antiangiogenesis via AIM2 and IFI16 during pre‐eclampsia. J Cell Physiol. 2020;235:9577–9588. DOI: 10.1002/jcp.29766. [DOI] [PubMed] [Google Scholar]
- 29.Ozeki A, Tani K, Takahashi H, Suzuki H, Nagayama S, Hirashima C, Iwata H, Kuwayama T, Ohkuchi A, Shirasuna K. Preeclamptic patient‐derived circulating cell‐free DNA activates the production of inflammatory cytokines via toll‐like receptor 9 signaling in the human placenta. J Hypertens. 2019;37:2452–2460. DOI: 10.1097/HJH.0000000000002208. [DOI] [PubMed] [Google Scholar]
- 30.Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in pregnancy and later cardiovascular risk: common antecedents? Circulation. 2010;122:579–584. DOI: 10.1161/CIRCULATIONAHA.110.943407. [DOI] [PubMed] [Google Scholar]
- 31.Lawlor DA, Emberson JR, Ebrahim S, Whincup PH, Wannamethee SG, Walker M, Smith GD; British Women’s Heart and Health Study, British Regional Heart Study . Is the association between parity and coronary heart disease due to biological effects of pregnancy or adverse lifestyle risk factors associated with child‐rearing? Findings from the British Women’s Heart and Health Study and the British Regional Heart Study. Circulation. 2003;107:1260–1264. DOI: 10.1161/01.CIR.0000053441.43495.1A. [DOI] [PubMed] [Google Scholar]


