Abstract
Background
Breastfeeding in infancy is associated with lower cardiovascular disease risk in adulthood; however, the amount of breastfeeding required to achieve this benefit is unknown.
Methods and Results
In the CHILD (Canadian Healthy Infant Longitudinal Development) Cohort Study, we analyzed 2382 children with complete data on early life feeding and blood pressure. Infant feeding was documented from hospital records in the first few days of life and reported by mothers throughout infancy. Blood pressure was measured at 3 years of age. Analyses controlled for birth weight, gestational age, socioeconomic status, maternal body mass index, and other potential confounders. We found that nearly all children (2333/2382; 97.9%) were ever breastfed, of whom 98 (4.2%) only briefly received breast milk during their birth hospitalization (“early limited breastfeeding”). At 3 years of age, blood pressure was higher in children who were never breastfed (mean systolic/diastolic 103/60 mm Hg) compared with those who were ever breastfed (99/58 mm Hg), including those who received only early limited breastfeeding (99/57 mm Hg). These differences in systolic blood pressure persisted in adjusted models (ever breastfed: −3.47 mm Hg, 95% CI, −6.14 to −0.80; early limited breastfeeding: −4.24 mm Hg, 95% CI, −7.45 to −1.04). Among breastfed children, there was no significant dose‐response association according to the duration or exclusivity of breastfeeding. Associations were not mediated by child body mass index.
Conclusions
Although the benefits of sustained and exclusive breastfeeding are indisputable, this study indicates any breastfeeding, regardless of duration or exclusivity, is associated with lower blood pressure at 3 years of age. Further research examining the bioactive components of early breast milk, underlying mechanisms, and long‐term associations is warranted.
Keywords: blood pressure, breastfeeding, colostrum, epidemiology, hypertension, pediatrics
Subject Categories: Cardiovascular Disease, Diet and Nutrition, Epidemiology, Pediatrics
Clinical Perspective
What is New?
This is the first prospective study to evaluate the associations of breastfeeding in the first days of life and blood pressure in early childhood.
The results show that breastfeeding ‐ even if limited to the first few days of life (“early limited breastfeeding”) ‐ is associated with lower blood pressure at the age of 3 years, independent of many potential maternal and infant confounders.
What Are the Clinical Implications?
The observed difference in blood pressure at the age of 3 years between early limited breastfed and never breastfed infants may be clinically relevant because blood pressure tracks from early childhood to adulthood.
Supporting breastfeeding initiation could positively impact cardiovascular health later in life.
These findings emphasize the need for immediate postpartum lactation support in clinical settings to facilitate provision of colostrum and breastfeeding initiation.
The prevalence of hypertension is increasing worldwide. It is expected to reach 1.5 billion globally by 2025,1, 2 causing increased rates of coronary heart disease, stroke, and mortality.3, 4 A growing body of evidence suggests risk factors for poor cardiovascular health (including high blood pressure), track from childhood to adulthood, and are influenced by early life exposures.5, 6 For example, studies in both humans and animals show that preterm birth, low birth weight, and early life nutrition are all associated with hypertension risk in adulthood.
7, 8, 9 Breastfeeding provides critical nutrients and bioactive factors that may have beneficial effects on cardiovascular development during early life;10 however, there is conflicting evidence on the associations of breastfeeding and blood pressure in children.11, 12, 13, 14, 15, 16 Two previous meta‐analyses, including studies from both developed and developing countries, showed that despite the heterogeneity between studies, blood pressure was consistently lower among individuals who had been breastfed.17, 18 In contrast, a more recent larger meta‐analysis of 43 studies found no consistent long‐term association between breastfeeding and blood pressure.19
The inconsistency between previous studies on breastfeeding and blood pressure may be related to the use of different breastfeeding definitions (eg, ever versus never, any versus none, or more versus less at various different time points, with or without consideration of exclusivity or complementary feeding).11, 12, 13, 14, 15, 16 Notably, previous studies have not typically documented or specifically addressed breastfeeding in the first days of life, yet this early milk (colostrum) is especially rich in growth factors20, 21, 22 immunologic components,23 and stem cells.24 This is especially important during the COVID‐19 pandemic where the support to successfully start and establish breastfeeding may be limited because of early hospital discharge and reduced in‐home postpartum support from public health nurses.
We explored the associations of breastfeeding exclusivity and duration (including “early limited breastfeeding” in the first days of life) with blood pressure at 3 years of age among Canadian children in the CHILD (Canadian Healthy Infant Longitudinal Development) Cohort Study.
Methods
The data that support the findings of this study are available from the CHILD Cohort Study’s National Coordinating Centre (child@mcmaster.ca) upon reasonable request.
Design and Study Population
This study was embedded in the CHILD Cohort Study, a general population birth cohort recruited from 4 sites in Canada.25 Women with singleton pregnancies from Vancouver, Edmonton, Manitoba, and Toronto were enrolled between 2008 and 2012 and remained eligible if they delivered a healthy infant >34 weeks gestation (N=3455). Child blood pressure was measured during a clinical assessment at 3 years. For the present study, 2382 children had breastfeeding information and blood pressure measurements available (Figure S1). Written informed consent was obtained from the participant’s caregivers. This study was approved by the Human Research Ethics Boards at McMaster University and the Universities of Manitoba, Alberta, and British Columbia and the Hospital for Sick Children.
Infant Feeding Assessment
Information on infant feeding in hospital was obtained by nursing staff and validated by chart review for a subset of Manitoba participants. Information on subsequent feeding was collected from questionnaires completed by caregivers at the ages of 3, 6, 12, 18, and 24 months. These data were used to define breastfeeding in the first days of life as none (no initiation of breastfeeding at all), “early limited breastfeeding” (breastfeeding limited to the hospital stay), or “sustained” (breastfeeding initiated and continued after hospital discharge). Among breastfed children, we categorized breastfeeding duration into the following groups: <3 months; 3 to <6 months; 6 to <12 months, and ≥12 months. Breastfeeding exclusivity at 3 months was categorized as follows: exclusive (breast milk only, without any formula, other milk, solid foods, or fluids since birth), partial (breast milk plus any formula, other milk, solid foods, or fluids); or none (no breast milk).
Child Blood Pressure
At the age of 3 years, blood pressure was measured with the child sitting comfortably and quietly, with their feet on the floor and back and right arm supported. The cuff size that fit the upper arm circumference was selected. Using the validated automatic sphygmanometer the Carescape Dinamap machine, blood pressure was measured at the right brachial artery. The measurement was repeated if systolic blood pressure was higher than 105 mm Hg. Children with blood pressure readings greater or less than ±4 SD (N=44) where excluded from the analyses. We calculated Z scores and percentiles for individual systolic and diastolic blood pressure values using normative values from the “Fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents” from the National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents.26, 27
Covariates
Maternal age, race/ethnicity (classified as White versus non‐White [eg, Asian, First Nation or Other ethnic groups]), education level (completion of postsecondary degree), smoking during pregnancy (any or none), and stress during pregnancy (classified in 3 groups: never or almost never, sometimes, and often) were self‐reported at enrollment during the second or third trimester of pregnancy.25 Preeclampsia and delivery mode were extracted from medical records. Maternal prepregnancy body mass index (BMI) was calculated from measured height and self‐reported prepregnancy weight, validated against health records in a subset.28 Infant sex, gestational age and weight at birth, and length of stay in the hospital were obtained from medical records. At 3 years of age, child height was determined in a standing position to the nearest millimeter without shoes by a Harpenden stadiometer, weight was measured using a calibrated scale, and BMI Z scores were calculated using the World Health Organization reference standard.29 Child screen time (hours per day) including watching TV/DVDs, using a computer, tablet, mobile phone, or playing video games30 and sugar‐sweetened beverage intake (servings per day) at the age of 3 years were assessed using questionnaires completed by caregivers.
Statistical Analysis
We compared characteristics between children with and without blood pressure measurements and between different breastfeeding categories using chi‐square tests for categorical variables, ANOVA for continuous variables with a constant variance, and Kruskal‐Wallis tests for nonnormally distributed variables. The association of breastfeeding (ever, early limited, or sustained versus never) and child blood pressure (systolic and diastolic) was explored using 4 multivariable linear regression models: (1) a basic model, adjusted for child’s sex and age at blood pressure measurement; (2) a confounder model, which additionally adjusted for covariates selected based on their associations with blood pressure in our analysis or in previous studies and confounders factors or proxies of unmeasured variables that could be the cause of breastfeeding exposure (eg, length of hospital stay after delivery, maternal age, race/ethnicity, education as an indicator of socioeconomic status), or of blood pressure outcomes (eg, child screen time and sugar‐sweetened beverages intake at 3 years of age), or both (eg, maternal prepregnancy BMI, smoking during pregnancy) and are not instrumental or mediating variables (eg, BMI, which was not a mediator in our analyses);31 (3) a birth model, which included gestational age and weight at birth (confounders related mainly to blood pressure) in addition to the confounder model; and (4) a final childhood BMI model (fully adjusted model), which included child current BMI in addition to the birth model. Rapid infant weight gain (defined as an increase in weight‐for‐length Z score >1 from birth to 12 months) was also assessed as a potential covariate. Maternal preeclampsia was not included in the models, as there were less than 10 cases for each of the breastfeeding categories. The same modeling strategy was applied to further assess breastfeeding duration and exclusivity among breastfed infants. Thereafter, we examined the potential mediating role of child current BMI in the association of breastfeeding and blood pressure using the PROCESS mediation package (version 3) in SPSS.32
To assess whether associations differed by child sex, maternal race/ethnicity, or birth weight, we evaluated the statistical interaction by including the product term for each of these covariates with breastfeeding variables in the adjusted regression models. In a sensitivity analysis, we restricted our analysis to the Manitoba site (N=776), where hospital feeding data were validated by chart review (99% agreement) and had the largest number of never breastfed infants (N=33). In another sensitivity analysis, to account for the uneven group sizes, we restricted our study population to the infants who were never breastfed or received limited breastfeeding in the first few days of life (N=147) and compared those 2 groups. Furthermore, we performed another sensitivity analysis to determine the impact of misclassification error in maternal education (proxy of socioeconomic status) by adding a small amount of noise (shifting 10% of the education labels up or down by 1 category), or a moderate amount of noise (shifting 20% of the labels up or down 1 category). To reduce potential bias associated with missing covariate data (ranging from 0.5%–15%), missing values of covariates (maternal age, race/ ethnicity, educational level, smoking, and prepregnancy body mass index, infant gestational age and weight at birth, length of stay in the hospital after delivery and child body mass index, screen time, and sugar‐sweetened beverage intake), were multiple imputed (n=5 imputations), according to the Fully Conditional Specification method (predictive mean matching), assuming no monotone missing pattern. In the imputation model, we additionally included the following as predictor variables: mother’s smoking postnatally, paternal race, paternal education, household income, parity, birth length, birth head circumference, frozen food intake at 3 years of age, and the studied determinants and outcomes. We report the pooled effect estimates after the multiple imputation procedure.33 Subject characteristics before and after imputation are given in Table S1, where no substantiative changes are observed. Statistical analyses were performed using SPSS version 24.0 (SPSS Inc., Chicago, IL, USA).
Results
Overall, 2333/2382 children (98%) were ever breastfed, of whom 98 (4%) only briefly received breast milk in the first days of life during their birth hospital stay (early limited breastfeeding). Among breastfed children, 78% were breastfed for 6 months or more, and 62% were exclusively breastfed for at least 3 months. On average, mothers who never breastfed were younger, more likely to smoke, and less likely to have a postsecondary degree compared with those who breastfed briefly or for a sustained period (Table 1). Infant birth characteristics, length of hospital stay after delivery, height, and BMI at 3 years of age were similar across these 3 groups. At 3 years of age, the mean (±SD) systolic blood pressure was 99 (±9) mm Hg (Z score = 0.72; 70th percentile), and mean diastolic blood pressure was 57 (±7) mm Hg (Z score =0.86; 77th percentile). Children who attended the 3‐year follow‐up assessment (N=2382) were more likely to be exclusively breastfed for at least 3 months than children who did not attend (N=883) (Table S2).
Table 1.
Subject Characteristics According to Category of Breastfeeding (N=2382) in the CHILD Cohort Study
|
Never Breastfed N=49 (2.1%) |
Early Limited Breastfeeding N=98 (4.1%) |
Continued Breastfeeding N=2235 (93.8%) |
P Value | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age, y | 29.8 (5.5) | 30.3 (5.1) | 32.6 (4.5) | <0.001§ |
| Race/ethnicity, White (%) | 35 (71.4) | 76 (77.6) | 1661 (74.3) | 0.73 |
| Postsecondary degree (%) | 24 (49.0) | 55 (56.1) | 1765 (79.0) | <0.001§ |
| Smoking during pregnancy (%) | 14 (28.6) | 15 (15.3) | 165 (7.4) | <0.001§ |
| Prepregnancy body mass index, kg/m2 | 24.4 (16.8–49.6) | 26.3 (18.6–42.4) | 23.0 (18.3–38.9) | <0.001§ |
| Preeclampsia (%) | 2 (4.0) | 2 (2.0) | 82 (3.6) | … |
| Infant characteristics at birth | ||||
| Cesarian section delivery (%) | 14 (30.4) | 23 (23.5) | 551 (25.0) | 0.66 |
| Girls (%) | 27 (55.1) | 47 (48.0) | 1055 (47.2) | 0.55 |
| Gestational age, wks | 39.1 (1.2) | 39.0 (1.4) | 39.2 (1.4) | 0.49 |
| Weight, grams | 3532 (532) | 3480 (522) | 3451 (477) | 0.42 |
| Length of stay in the hospital, d | 3.0 (2.9–3.7) | 3.0 (2.9–3.4) | 3.0 (2.9–3.6) | 0.65 |
| Child characteristics | ||||
| Age, mo | 36.0 (2.0) | 36.5 (1.7) | 36.4 (2.1) | 0.47 |
| Screen time, h | 2.0 (0.3–5.8) | 2.0 (0.2–5.2) | 1.6 (0–4.4) | <0.001§ |
| Sugar‐sweetened beverages, n per d | 0.1 (0–2.5) | 0.4 (0–3.77) | 0.2 (0–2.6) | 0.06 |
| Body mass index, Z score* | 0.7 (1.1) | 0.7 (1.1) | 0.6 (1.1) | 0.27 |
| Height, cm | 96.7 (3.2) | 95.5 (9.0) | 95.6 (7.4) | 0.61 |
| SBP, mm Hg | 103 (10) | 99 (10) | 99 (9) | 0.02§ |
| SBP – Z score† | 1.0 | 0.7 | 0.7 | 0.02§ |
| SBP percentiles‡ | 78 | 69 | 70 | 0.02§ |
| DBP, mm Hg | 60 (7) | 57 (6) | 58 (7) | 0.08 |
| DBP – Z score† | 1.0 | 0.8 | 0.9 | 0.07 |
| DBP percentiles‡ | 80 | 76 | 77 | 0.09 |
Values are means (SD), numbers (%), or medians (95% range) for variables with skewed distribution based on the imputed data. Differences in maternal, infant and childhood characteristics were evaluated using ANOVA tests for continuous variables with a constant variance, Kruskal‐Wallis for nonnormally distributed variables, and chi‐square tests for categorical variables. CHILD indicates Canadian Healthy Infant Longitudinal Development Cohort Study; DBP, diastolic blood pressure; and SBP, systolic blood pressure.
BMI Z scores based on World Health Organization reference standards.
Z scores of systolic and diastolic blood pressure are calculated using normative values from the “Fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents” from the National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents.
Blood pressure percentiles for age, sex, and height.
P‐Values < 0.05.
Compared with never breastfed children, those who were ever breastfed had lower systolic blood pressure (−3.65 mm Hg (95% CI, −6.23 to −1.07)) (Table 2, Figure 1), and systolic blood pressure Z scores (−0.31, 95% CI, −0.56 to −0.06) (Table 3). This association was independent of potential confounding factors (maternal age, race/ethnicity, smoking, education, prepregnancy BMI, duration of hospital stay after delivery, and child screen time and sugar‐sweetened beverages intake) (Table S3); birth factors (gestational age and weight at birth) (Table S3); and current child BMI (fully adjusted model: −3.47 mm Hg (95% CI, −6.14 to −0.80)). Similar patterns of association were observed for diastolic blood pressure, but these did not reach statistical significance (fully adjusted model: −1.66 mm Hg; 95% CI, −3.54 to 0.21). We found no evidence of mediation by current child BMI (Figure 2) or confounding by maternal stress during pregnancy (not shown), and no evidence of interaction by sex, maternal race/ethnicity, or birth weight (P>0.10, not shown). Among breastfed children (N=2333), we did not observe any evidence of “dose response” according to breastfeeding duration or exclusivity at 3 months (Table 2).
Table 2.
Associations of Infant Feeding With Blood Pressure at the Age of 3 Years in the CHILD Cohort Study
|
Systolic Blood Pressure Models mm Hg (95% CI) |
Diastolic Blood Pressure Models mm Hg (95% CI) |
|||
|---|---|---|---|---|
| Basic Model | Fully Adjusted | Basic Model | Fully Adjusted | |
| Breastfeeding (N=2382) | ||||
| Never (N=49) | 0.00 (Reference) | 0.00 (Reference) | ||
| Ever (N=2333) |
−3.65* (−6.23 to −1.07) |
−3.47* (−6.14 to −0.80) |
−1.76* (−3.02to −0.49) |
−1.66 (−3.54 to 0.21) |
| Early limited (n=98) |
−4.10* (−7.32 to −0.89) |
−4.24* (−7.45 to −1.04) |
−2.52* (−4.69 to −0.34) |
−2.62* (−4.87 to −0.37) |
| Sustained (n=2235) |
−3.63* (−6.25 to −1.02) |
−3.40* (−6.08 to −0.73) |
−1.72 (−3.60 to 0.16) |
−1.59 (−3.46 to 0.29) |
| Breastfeeding duration (N=2333)† | ||||
| >0–2.9 mo (n=261) | 0.00 (Reference) | 0.00 (Reference) | ||
| 3–5.9 mo (n=242) |
0.02 (−1.63 to 1.66) |
0.18 (−1.47 to 1.83) |
0.63 (−0.52 to 1.78) |
0.73 (−0.43 to 1.89) |
| 6–11.9 mo (n=719) |
−0.52 (−1.20 to 0.16) |
−0.18 (−1.53 to 1.18) |
−0.16 (−1.15 to 0.83) |
0.02 (−0.93 to 0.97) |
| ≥12 mo (n=1111) |
−0.32 (−0.97 to 0.32) |
0.18 (−1.15 to 1.51) |
0.38 (−0.07 to 0.83) |
0.62 (−0.31 to 1.55) |
| Breastfeeding exclusivity at 3 mo (N=2329)† | ||||
| No (n=261) | 0.00 (Reference) | 0.00 (Reference) | ||
| Partial (n=605) |
−0.11 (−1.27 to 1.48) |
0.36 (−1.04 to 1.75) |
0.76 (−0.16 to 1.68) |
0.93 (−0.05 to 1.90) |
| Exclusive (n=1463) |
−0.53 (−1.16 to 0.10) |
−0.10 (−1.39 to 1.19) |
0.01 (−0.43 to 0.45) |
0.18 (−0.73 to 1.08) |
Values are regression coefficients (95% CI) based on linear regression models. Estimates are based on multiple imputed data. Basic model is adjusted for child’s sex and age at visit. Fully adjusted model is adjusted for maternal age, prepregnancy body mass index, race/ethnicity, educational level, smoking during pregnancy, and length of stay in the hospital at delivery, and child’s sex, gestational age and weight at birth, current age, screen time, sugar‐sweetened beverage intake, and child current body mass index. CHILD indicates Canadian Healthy Infant Longitudinal Development Cohort Study.
P values <0.05.
Analysis of breastfeeding duration excludes 49 children who were never breastfed; analysis of breastfeeding exclusivity further excludes 4 breastfed children missing data on exclusivity. Table S3 shows intermediate models adjusted for different subgroups of covariates.
Figure 1. Any breastfeeding (even early limited breastfeeding during the birth hospital stay) is associated with lower blood pressure at age 3 years in the CHILD Cohort Study.
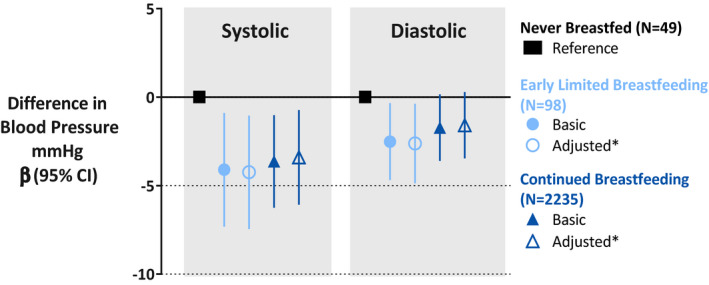
Values are regression coefficients (95% CI) based on multiple linear regression models. Basic model is adjusted for child’s age at visit and sex. *Adjusted model is fully adjusted body mass index model (see Table 2) adjusted for maternal age, prepregnancy body mass index, race/ethnicity, educational level, smoking during pregnancy, length of stay in the hospital at delivery, gestational age and weight at birth, and child’s sex, current age, screen time, sugar‐sweetened beverage intake, and current body mass index. Estimates are based on imputed data.
Table 3.
Associations of Infant Feeding With Blood Pressure Z Scores at the Age of 3 Years in the CHILD Cohort Study
|
Systolic Blood Pressure Models †Z score (95% CI) |
Diastolic Blood Pressure Models †Z score (95% CI) |
|||
|---|---|---|---|---|
| Basic Model | Fully Adjusted | Basic Model | Fully Adjusted | |
| Breastfeeding (N=2382) | ||||
| Never (N=49) | 0.00 (Reference) | 0.00 (Reference) | ||
| Ever (N=2333) |
−0.32* (−0.57 to −0.07) |
−0.31* (−0.56 to −0.06) |
−0.13 (−0.30 to 0.04) |
−0.13 (−0.30 to 0.04) |
| Early limited (n=98) |
−0.37* (−0.67 to −0.07) |
−0.40* (−0.70 to −0.10) |
−0.21 (−0.41 to −0.001) |
−0.22* (−0.43 to −0.02) |
| Sustained (n=2235) |
−0.31* (−0.56 to −0.07) |
−0.31* (−0.56 to −0.06) |
−0.13 (−0.30 to 0.04) |
−0.12 (−0.29 to 0.05) |
| Breastfeeding duration (N=2333)‡ | ||||
| >0–2.9 mo (n=261) | 0.00 (Reference) | 0.00 (Reference) | ||
| 3–5.9 mo (n=242) |
0.02 (−0.18 to 0.13) |
−0.002 (−0.16 to 0.15) |
0.05 (−0.06 to 0.15) |
0.06 (−0.05 to 0.17) |
| 6–11.9 mo (n=719) |
−0.05 (0.17 to 0.08) |
−0.01 (−0.14 to 0.12) |
−0.003 (−0.09 to 0.08) |
0.02 (−0.07 to 0.10) |
| ≥12 mo (n=1111) |
0.01 (−0.11 to 0.13) |
0.06 (−0.06 to 0.19) |
0.06 (−0.02 to 0.14) |
0.09* (0.01 to 0.18) |
| Breastfeeding exclusivity at 3 mo (N=2329)‡ | ||||
| No (n=261) | 0.00 (Reference) | 0.00 (Reference) | ||
| Partial (n=605) |
−0.003 (−0.13 to 0.13) |
0.02 (−0.11 to 0.15) |
0.09 (−0.001 to 0.17) |
0.10* (0.01 to 0.19) |
| Exclusive (n=1463) |
−0.02 (−0.13 to 0.10) |
0.03 (−0.10 to 0.15) |
0.02 (−0.06 to 0.10) |
0.04 (−0.05 to 0.12) |
Values are regression coefficients (95% CI) based on linear regression models. Blood pressure values are expressed in † Z scores based on the “Fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents” from the National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents.” Estimates are based on multiple imputed data. Basic model is adjusted for child’s age at visit, sex. Fully adjusted model is adjusted for maternal age, prepregnancy body mass index, ethnicity, educational level, smoking during pregnancy, and length of stay in the hospital at delivery, and child’s sex, gestational age and weight at birth, current age, screen time, sugar‐sweetened beverage intake, and child current body mass index. CHILD indicates Canadian Healthy Infant Longitudinal Development Cohort Study. ‡ Analysis of breastfeeding duration excludes 49 children who were never breastfed; analysis of breastfeeding exclusivity further excludes 4 breastfed children missing data on exclusivity.
P values <0.05.
Figure 2. Child BMI does not mediate the association of breastfeeding and blood pressure.
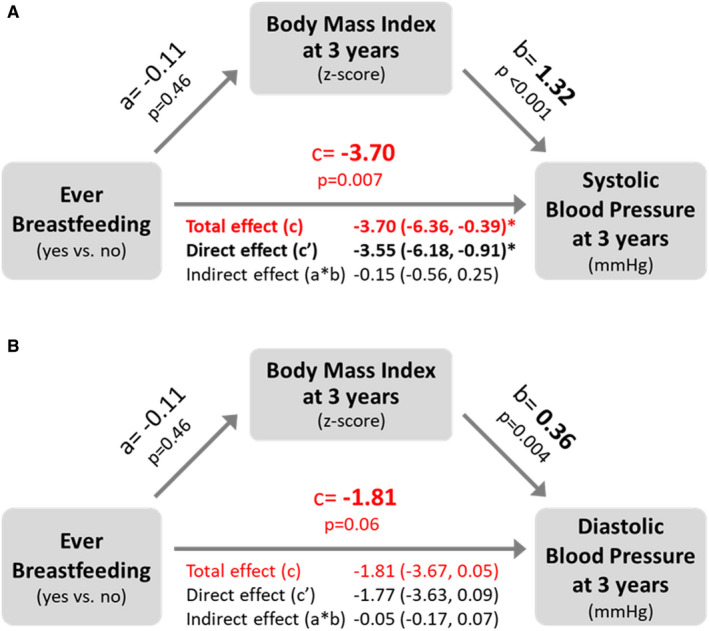
Values are beta estimates and 95% CIs from the mediation analyses of BMI on the association of ever breastfeeding with child (A) systolic and (B) diastolic blood pressure at 3 years of age. a: effect of ever breastfeeding (exposure) on BMI (mediator); b: effect of BMI (mediator) on blood pressure (outcome); c′: direct effect of ever breastfeeding on blood pressure when taking BMI into account; c: total effect of ever breastfeeding on blood pressure accounting for the mediation effect of BMI on blood pressure (sum of direct effect (c’) and indirect effect (a*b)). *P value <0.05.BMI indicates body mass index.
Notably, even early limited breastfeeding (breastfeeding limited to the first days of life in hospital) was significantly associated with lower systolic and diastolic blood pressure at 3 years (fully adjusted BMI model: −4.24 mm Hg (95% CI, −7.45 to −1.04) and −2.62 mm Hg (95% CI, −4.87 to −0.37), respectively, compared with never breastfeeding) (Table 2, Figure 1). These associations were also significant when we evaluated the Z scores of systolic (−0.40; 95% CI, −0.70 to −0.10) and diastolic (−0.22, 95% CI, −0.43 to −0.02) blood pressure (Table 3). The results were similar in a sensitivity analysis limited to the Manitoba study site where 743 of 776 children (96%) were ever breastfed and 6% received early limited breastfeeding, although not all associations reached statistical significance because of the smaller sample size (Figure S2). The associations of early limited breastfeeding with blood pressure remained significant even when we restricted our study population to the 147 infants that were never breastfed or received early limited breastfeeding (Table S4). Lastly, the simulation analyses to determine the impact of misclassification error in maternal education (proxy of socioeconomic status) showed no substantive changes in the effect estimates (data not shown).
Discussion
Main Findings
In this large prospective cohort study, we observed that any breastfeeding, including early limited breastfeeding in the first days of life, was associated with lower blood pressure at 3 years of age. These associations were independent of several maternal, birth, and childhood factors and were observed regardless of breastfeeding duration and exclusivity. These early subclinical differences could have long‐term health implications because blood pressure is known to track throughout life5 and even relatively small elevations could predispose young children to hypertension later in adulthood. Despite the observational nature of our findings, they emphasize the importance of early breastfeeding initiation, even if exclusive or sustained breastfeeding is not possible.
Interpretation of Main Findings
Conflicting evidence is reported on the associations of breastfeeding and blood pressure in later life. A secondary analysis of the PROBIT (Promotion of Breastfeeding Intervention Trial) study, a cluster randomized trial in Belarus that successfully increased breastfeeding duration34 among mothers who initiated breastfeeding, found no effect on blood pressure at 16 years of age.11 Similarly, in a recent meta‐analysis of 43 studies,19 no associations were reported between breastfeeding and blood pressure in large studies (>1000 participants). In contrast, 2 earlier meta‐analyses concluded that breastfeeding may lead to reductions of up to 2 mm Hg in systolic and 0.5 mm Hg in diastolic blood pressure, although these associations were primarily observed in smaller studies (<1000 participants).17, 18 One recent large observational study that was not included in the meta‐analyses found that, among 1509 participants from the Dutch PIAMA (Prevention and Incidence of Asthma and Mite Allergy) birth cohort, children who received any breastfeeding had 2.29 mm Hg lower systolic and 1.19 mm Hg lower diastolic blood pressure at the age of 12 years, independent of breastfeeding duration.35 Similarly, in our population of 2382 Canadian children, we observed that ever breastfeeding was associated with 3.47 mm Hg lower systolic blood pressure at 3 years of age, regardless of exclusivity or duration.
A novel aspect of our study is the assessment of infant feeding in the first days of life. Feeding data from hospital records are not typically captured in birth cohort studies, and to our knowledge, early limited breastfeeding has not been studied in relation to cardiovascular health later in childhood. Using this unique information, we observed that infants who received even a relatively small amount of their mother’s early breast milk (colostrum) had lower blood pressure at 3 years of age, regardless of breastfeeding duration or exclusivity. This finding could explain the null results from the PROBIT study, which excluded dyads who did not initiate breastfeeding in hospital (so there was no “never breastfed” group for comparison).
Our results for early limited breastfeeding might also partly explain the conflicting results in previous studies where hospital feeding was not considered, potentially leading to substantial misclassification of “ever breastfeeding.” For example, in our CHILD cohort, 98/2382 (4%) of mothers who reported “never breastfeeding” actually provided early limited breastfeeding during the neonatal period according to their hospital records. The extent of this misclassification likely varies substantially between studies because lactation support and formula supplementation policies vary greatly between institutions and have changed over time.36, 37 Such misclassification would not affect results if sustained breastfeeding is required to affect the outcome of interest (as shown for many outcomes including infections,38 asthma,39 and obesity19, 40), but if early limited breastfeeding is sufficient (as suggested by our current results for blood pressure), then misclassifying infants who briefly received breast milk as “never breastfed” could significantly bias results toward the null. Thus, our results highlight the importance of accurately capturing and considering early feeding exposures.
Notably, the lack of a “dose response” according to breastfeeding duration or exclusivity in our study is consistent with some prior studies13, 38 and highlights another potential reason for the heterogeneity and overall null findings in previous meta‐analyses: if any breastfeeding is sufficient to influence blood pressure, then no difference would be expected when comparing “more versus less” breastfeeding, as many studies have done, including PROBIT.18 Although a dose response or “biological gradient” is among the classic criteria for establishing causality in epidemiology,41 it is not a requirement. In fact, the presence or absence of a dose response in a potentially causal association could shed light on the underlying biological mechanism. For example, whereas the dose‐dependent association of prolonged breastfeeding with reduced gastrointestinal infections suggests protection through continuous exposure to maternal antibodies and other immunomodulatory factors in breast milk, the association of any breastfeeding (even early limited breastfeeding) with cardiovascular health points to a different mechanism, as discussed below. We are confident that, when they exist, dose effects of breastfeeding are observable in our study population because we have previously found inverse linear relationships between breastfeeding duration and infant BMI Z score40 as well as wheezing rates.42
There are several potential biological mechanisms that could explain why consuming early breast milk (colostrum) during the first days of life might positively influence cardiovascular health. First, early breastfeeding strongly affects the colonization and composition of the intestinal microbiota.43, 44 We have previously shown that early feeding exposures in the hospital are associated with subtle microbiota differences.45 An altered intestinal microbiota may be involved in atherogenic processes later in life.46 In addition, nutrients and bioactive components that are enriched in colostrum such as stem cells47 and vascular endothelial growth factor48 might influence cardiovascular development and have long‐lasting developmental benefits. Although not yet studied in infants, stem cells administered to adults can produce angiogenic and anti‐inflammatory factors, improve blood flow, and increase elasticity of blood vessels.49 In addition, colostrum is rich in high concentrations of n‐3 long‐chain polyunsaturated fatty acids, which are important structural components of the vascular endothelium.50 A recent study showed that infants receiving human milk high in n‐3 long‐chain polyunsaturated fatty acids had lower blood pressure at 12 years of age, independent of child’s current n‐3 long‐chain polyunsaturated fatty acids status.10 Experimental studies of other dietary exposures further support the long‐term impact of infant nutrition on blood pressure; for example, a randomized trial showed that sodium restriction in newborns caused a 2 mm Hg reduction in systolic blood pressure in infancy,51 which persisted into adolescence.9 We could not address these potential mechanisms in our observational study, but our hypothesis‐generating results can inform future research on this topic.
The effect size observed in our study (3.5 mm Hg higher blood pressure in never‐breastfed children) is substantial, and could be clinically significant. For context, this difference is more than double the estimated impact of a full SD increase in current BMI (1.3 mm Hg in the same statistical model). Studies in adults have estimated that a 2 mm Hg population‐wide reduction in blood pressure could reduce the prevalence of hypertension by 17% and the number of strokes by 15%,52 which equates to preventing 2000 strokes annually in the United Kingdom.53 Given that blood pressure tracks from childhood to adulthood,5 the observed 3.5 mm Hg reduction in systolic blood pressure associated with early limited breastfeeding suggests that supporting breastfeeding initiation could have substantial impact on cardiovascular disease prevention later in life.
Strengths and Limitations
Our study has several strengths including the prospective design within a large general population birth cohort and the collection of breastfeeding data from hospital records, which is rarely captured in other studies. Furthermore, we assessed blood pressure in early childhood, before the onset of many traditional cardiovascular disease risk factors (eg, smoking and alcohol consumption).
A limitation of our study is that we did not collect at least 2 repeated blood pressure measurements as recommended by the 2017 Hypertension Clinical Practice Guidelines.54 Blood pressure has a large within‐participant variation and is prone to measurement error. Also, a sizable proportion (26%) were missing blood pressure data. Children without blood pressure data were less likely to be exclusively breastfed, and their mothers were less educated and more likely to smoke. This potential selection bias, as well as the overall high socioeconomic status and high breastfeeding initiation rates in the CHILD cohort, could limit the generalizability of our results. Further, we had a relatively small number of children who were never breastfed (n=49) because of the high breastfeeding initiation rates in our population, which limited our statistical power in comparisons involving this group. It will therefore be important to replicate our findings in other populations. Many factors influence the decision to breastfeed and several are not captured in our study. For example, we did not collect information about maternal breastfeeding intentions before delivery or reasons for not breastfeeding (eg, lack of family or society support, incorrect or lack of information about breastfeeding, prior unsuccessful breastfeeding experiences); however, we deem it unlikely that these factors would affect the observed associations of breastfeeding and blood pressure. Finally, we had limited power to assess the role of gestational hypertensive disorders (eg, only 2 cases of preeclampsia in each of the never breastfed and early limited breastfed groups). Although we adjusted for a large number of potential confounders (which notably had very little impact on our effect estimates), residual confounding is still possible, as in any observational study.
Conclusions
In the prospective CHILD Cohort Study, we observed that children who were ever breastfed had lower blood pressure at the age of 3 years, even if they only briefly received early limited breastfeeding in the first few days of life. Although the clinical relevance of these associations remains to be determined, these early differences in blood pressure could translate into meaningful reductions in the risk of cardiovascular disease later life and associated healthcare cost savings. Further research is warranted to confirm and understand the effect of early breastfeeding on long‐term cardiovascular health.
Perspectives
Our findings have potentially important implications for healthcare practice and policy. They emphasize the importance of antenatal education and immediate postpartum lactation support to facilitate breastfeeding initiation and provision of colostrum. They are especially relevant to hospitals implementing cost containment strategies that could impede breastfeeding initiation, such as early postpartum discharge (often less than 24 hours following a vaginal delivery) and/or elimination of lactation support services on postpartum units. Our results suggest the short‐term savings from these practices could be greatly outweighed by the long‐term costs from resulting cardiovascular health deficits later in life.
Sources of Funding
This research was supported, in part, by the Canada Research Chairs program. The Canadian Institutes of Health Research and the Allergy, Genes and Environment (AllerGen) Network of Centres of Excellence provided core funding for the CHILD Cohort Study. Additional support has been provided by Research Manitoba, the Healthy Child Manitoba Office, the Manitoba Children’s Hospital Research Foundation, Health Canada, Environment Canada, and the Canada Mortgage and Housing Corporation. KM gratefully acknowledges the support of Canadian Lung Association. MBA holds a Tier 2 Canada Research Chair in the Developmental Origins of Chronic Disease. These entities had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; or decision to submit the articlefor publication.
Disclosures
MBA receives research funding from Canadian Institutes of Health Research, Research Manitoba, the Canada Foundation for Innovation, the Bill and Melinda Gates Foundation, the Manitoba Children’s Hospital Foundation, Prolacta Biosciences, and the Garfield G. Weston Foundation. MBA serves as Secretary to the International Society for Research on Human Milk and Lactation and is a member of the National Academy of Sciences, Engineering and Medicine Committee on Scanning New Evidence on the Nutrient Content of Human Milk. MBA regularly speaks at conferences and workshops on infant nutrition, some sponsored by Medela, the Milk Mob, and Prolacta Biosciences; she has contributed without remuneration to online courses on breast milk and the infant microbiome produced by Microbiome Courses. These entities had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; or decision to submit the articlefor publication.
Supporting information
Tables S1–S4
Figures S1–S2
Acknowledgments
We are grateful to all the families who took part in this study and the whole CHILD team, which includes interviewers, nurses, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, and receptionists at the following institutions: McMaster University, University of Manitoba, University of Alberta, University of Toronto, and University of British Columbia. We thank Ms. Deborah Chan for reviewing the medical charts and Dr. Robert Balshaw, Ms. Stephanie Goguen, and Ms. Myrtha Reina Vargas for statistical advice.
Author Contributions: KM and MBA designed and managed this project. The CHILD Study Founding Director (MRS) and site leaders (ABB, PS, TJM, PJM and SET) conceived the CHILD cohort design, managed study recruitment, and oversaw clinical assessments of study participants. KM conducted all the statistical analyses, interpreted the data, and drafted the article. MBA oversaw data analyses and provided feedback in data interpretation and the article. All authors provided feedback and approved the final version. KM and MBA have full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019067
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Kozeta Miliku, Email: milikuk@mcmaster.ca.
Meghan B. Azad, Email: meghan.azad@umanitoba.ca.
References
- 1.Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood‐pressure‐related disease, 2001. Lancet. 2008;371:1513–1518. DOI: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 2.Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, Farzadfar F, Stevens GA, Lim SS, Riley LM, et al. National, regional, and global trends in systolic blood pressure since 1980: Systematic analysis of health examination surveys and epidemiological studies with 786 country‐years and 5.4 million participants. Lancet. 2011;377:568–577. DOI: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- 3.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration . Age‐specific relevance of usual blood pressure to vascular mortality: A meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. DOI: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 4.McCarron P, Smith GD, Okasha M, McEwen J. Blood pressure in young adulthood and mortality from cardiovascular disease. Lancet. 2000;355:1430–1431. DOI: 10.1016/S0140-6736(00)02146-2. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: A systematic review and meta‐regression analysis. Circulation. 2008;117:3171–3180. DOI: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lever AF, Harrap SB. Essential hypertension: A disorder of growth with origins in childhood? J Hypertens. 1992;10:101–120. DOI: 10.1097/00004872-199202000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Bertagnolli M, Luu TM, Lewandowski AJ, Leeson P, Nuyt AM. Preterm birth and hypertension: is there a link? Curr Hypertens Rep. 2016;18:28. DOI: 10.1007/s11906-016-0637-6. [DOI] [PubMed] [Google Scholar]
- 8.Lucas A. Programming by early nutrition: An experimental approach. J Nutr. 1998;128:401S–406S. DOI: 10.1093/jn/128.2.401S. [DOI] [PubMed] [Google Scholar]
- 9.Geleijnse JM, Hofman A, Witteman JC, Hazebroek AA, Valkenburg HA, Grobbee DE. Long‐term effects of neonatal sodium restriction on blood pressure. Hypertension. 1997;29:913–917. DOI: 10.1161/01.HYP.29.4.913. [DOI] [PubMed] [Google Scholar]
- 10.van Rossem L , Wijga AH, de Jongste JC , Koppelman GH, Oldenwening M, Postma DS, Abrahamse‐Berkeveld M, van de Heijning B , Brunekreef B, Smit HA. Blood pressure in 12‐year‐old children is associated with fatty acid composition of human milk: The prevention and incidence of asthma and mite allergy birth cohort. Hypertension. 2012;60:1055–1060. DOI: 10.1161/HYPERTENSIONAHA.112.197830. [DOI] [PubMed] [Google Scholar]
- 11.Martin RM, Kramer MS, Patel R, Rifas‐Shiman SL, Thompson J, Yang S, Vilchuck K, Bogdanovich N, Hameza M, Tilling K, et al. Effects of promoting long‐term, exclusive breastfeeding on adolescent adiposity, blood pressure, and growth trajectories: A secondary analysis of a randomized clinical trial. JAMA pediatrics. 2017;171:e170698. DOI: 10.1001/jamapediatrics.2017.0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin RM, Ness AR, Gunnell D, Emmett P, Davey SG. Does breast‐feeding in infancy lower blood pressure in childhood? The avon longitudinal study of parents and children (alspac). Circulation. 2004;109:1259–1266. DOI: 10.1161/01.CIR.0000118468.76447.CE. [DOI] [PubMed] [Google Scholar]
- 13.de Jonge LL , van Osch‐Gevers L , Geelhoed JJ, Hofman A, Steegers EA, Helbing WA, Jaddoe VW. Breastfeeding is not associated with left cardiac structures and blood pressure during the first two years of life. The generation r study. Early Hum Dev. 2010;86:463–468. DOI: 10.1016/j.earlhumdev.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Owen CG, Whincup PH, Cook DG. Breast‐feeding and cardiovascular risk factors and outcomes in later life: Evidence from epidemiological studies. Proc Nutr Soc. 2011;70:478–484. DOI: 10.1017/S0029665111000590. [DOI] [PubMed] [Google Scholar]
- 15.Hosaka M, Asayama K, Staessen JA, Ohkubo T, Hayashi K, Tatsuta N, Kurokawa N, Satoh M, Hashimoto T, Hirose T, et al. Breastfeeding leads to lower blood pressure in 7‐year‐old japanese children: Tohoku study of child development. Hypertens Res. 2013;36:117–122. DOI: 10.1038/hr.2012.128. [DOI] [PubMed] [Google Scholar]
- 16.Whincup PH, Cook DG, Shaper AG. Early influences on blood pressure: A study of children aged 5–7 years. BMJ. 1989;299:587–591. DOI: 10.1136/bmj.299.6699.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin RM, Gunnell D, Smith GD. Breastfeeding in infancy and blood pressure in later life: systematic review and meta‐analysis. Am J Epidemiol. 2005;161:15–26. DOI: 10.1093/aje/kwh338. [DOI] [PubMed] [Google Scholar]
- 18.Owen CG, Whincup PH, Gilg JA, Cook DG. Effect of breast feeding in infancy on blood pressure in later life: Systematic review and meta‐analysis. BMJ. 2003;327:1189–1195. DOI: 10.1136/bmj.327.7425.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horta BL, Loret de Mola C, Victora CG. Long‐term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: A systematic review and meta‐analysis. Acta Paediatr. 2015;104:30–37. DOI: 10.1111/apa.13133. [DOI] [PubMed] [Google Scholar]
- 20.Prosser CG. Insulin‐like growth factors in milk and mammary gland. J Mammary Gland Biol Neoplasia. 1996;1:297–306. DOI: 10.1007/BF02018082. [DOI] [PubMed] [Google Scholar]
- 21.Loui A, Eilers E, Strauss E, Pohl‐Schickinger A, Obladen M, Koehne P. Vascular endothelial growth factor (vegf) and soluble vegf receptor 1 (sflt‐1) levels in early and mature human milk from mothers of preterm versus term infants. J Hum Lact. 2012;28:522–528. DOI: 10.1177/0890334412447686. [DOI] [PubMed] [Google Scholar]
- 22.Read LC, Upton FM, Francis GL, Wallace JC, Dahlenberg GW, Ballard FJ. Changes in the growth‐promoting activity of human milk during lactation. Pediatr Res. 1984;18:133–139. DOI: 10.1203/00006450-198402000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Hurley WL, Theil PK. Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011;3:442–474. DOI: 10.3390/nu3040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassiotou F, Beltran A, Chetwynd E, Stuebe AM, Twigger A‐J, Metzger P, Trengove N, Lai CT, Filgueira L, Blancafort P, et al. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem cells (Dayton, Ohio). 2012;30:2164–2174. DOI: 10.1002/stem.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subbarao P, Anand SS, Becker AB, Befus AD, Brauer M, Brook JR, Denburg JA, HayGlass KT, Kobor MS, Kollmann TR, CHILD Study Investigators , et al. The Canadian Healthy Infant Longitudinal Development (CHILD) Study: Examining developmental origins of allergy and asthma. Thorax. 2015;70:998–1000. DOI: 10.1136/thoraxjnl-2015-207246. [DOI] [PubMed] [Google Scholar]
- 26.National High Blood Pressure Education Program Working Group on High Blood Pressure in C, Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 27.Falkner B, Daniels SR. Summary of the fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Hypertension. 2004;44:387–388. DOI: 10.1161/01.HYP.0000143545.54637.af. [DOI] [PubMed] [Google Scholar]
- 28.Azad MB, Sharma AK, de Souza RJ , Dolinsky VW, Becker AB, Mandhane PJ, Turvey SE, Subbarao P, Lefebvre DL, Sears MR, et al. Association between artificially sweetened beverage consumption during pregnancy and infant body mass index. JAMA pediatrics. 2016;170:662–670. DOI: 10.1001/jamapediatrics.2016.0301. [DOI] [PubMed] [Google Scholar]
- 29.Onis M. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006;450:76–85. DOI: 10.1111/j.1651-2227.2006.tb02378.x. DOI: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 30.Tamana SK, Ezeugwu V, Chikuma J, Lefebvre DL, Azad MB, Moraes TJ, Subbarao P, Becker AB, Turvey SE, Sears MR, et al. Screen‐time is associated with inattention problems in preschoolers: results from the CHILD birth cohort study. PLoS One. 2019;14:e0213995. DOI: 10.1371/journal.pone.0213995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VanderWeele TJ. Principles of confounder selection. Eur J Epidemiol. 2019;34:211–219. DOI: 10.1007/s10654-019-00494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayes AF. Introduction to mediation, moderation, and conditional process analysis. Second edition. A regression‐based approach. ISBN 9781462534654. 2017.
- 33.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338: b2393. DOI: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kramer MS, Chalmers B, Hodnett ED, Sevkovskaya Z, Dzikovich I, Shapiro S, Collet J‐P, Vanilovich I, Mezen I, Ducruet T, et al. Promotion of breastfeeding intervention trial (probit): a randomized trial in the republic of belarus. JAMA. 2001;285:413–420. DOI: 10.1001/jama.285.4.413. [DOI] [PubMed] [Google Scholar]
- 35.Pluymen LPM, Wijga AH, Gehring U, Koppelman GH, Smit HA, van Rossem L . Breastfeeding and cardiometabolic markers at age 12: A population‐based birth cohort study. Int J Obes (Lond). 2019;43:1568–1577. DOI: 10.1038/s41366-018-0317-5. [DOI] [PubMed] [Google Scholar]
- 36.Perez‐Escamilla R, Martinez JL, Segura‐Perez S. Impact of the baby‐friendly hospital initiative on breastfeeding and child health outcomes: a systematic review. Matern Child Nutr. 2016;12:402–417. DOI: 10.1111/mcn.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen T, Dennison BA, Fan W, Xu C, Birkhead GS. Variation in formula supplementation of breastfed newborn infants in new york hospitals. Pediatrics. 2017;140. DOI: 10.1542/peds.2017-0142. [DOI] [PubMed] [Google Scholar]
- 38.Victora CG, Bahl R, Barros AJD, Franca GVA, Horton S, Krasevec J, Murch S, Sankar MJ, Walker N, Rollins NC, et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–490. DOI: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 39.Klopp A, Vehling L, Becker AB, Subbarao P, Mandhane PJ, Turvey SE, Lefebvre DL, Sears MR, Azad MB. Modes of infant feeding and the risk of childhood asthma: A prospective birth cohort study. J Pediatr. 2017;190:192–199.e192. [DOI] [PubMed] [Google Scholar]
- 40.Azad MB, Vehling L, Chan D, Klopp A, Nickel NC, McGavock JM, Becker AB, Mandhane PJ, Turvey SE, Moraes TJ, et al. Infant feeding and weight gain: separating breast milk from breastfeeding and formula from food. Pediatrics. 2018;142. DOI: 10.1542/peds.2018-1092. [DOI] [PubMed] [Google Scholar]
- 41.Hill AB. The environment and disease: Association or causation? 1965. J R Soc Med. 2015;108:32–37. DOI: 10.1177/0141076814562718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azad MB, Vehling L, Lu Z, Dai D, Subbarao P, Becker AB, Mandhane PJ, Turvey SE, Lefebvre DL, Sears MR. Breastfeeding, maternal asthma and wheezing in the first year of life: A longitudinal birth cohort study. Eur Respir J. 2017;49:1602019. DOI: 10.1183/13993003.02019-2016. [DOI] [PubMed] [Google Scholar]
- 43.Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, Sears MR, Mandhane PJ, Turvey SE, Subbarao P, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2016;123:983–993. DOI: 10.1111/1471-0528.13601. [DOI] [PubMed] [Google Scholar]
- 44.Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva‐Datchary P, Li Y, Xia Y, Xie H, Zhong H, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:690–703. DOI: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Forbes JD, Azad MB, Vehling L, Tun HM, Konya TB, Guttman DS, Field CJ, Lefebvre D, Sears MR, Becker AB, et al. Association of exposure to formula in the hospital and subsequent infant feeding practices with gut microbiota and risk of overweight in the first year of life. JAMA Pediat. 2018;172:e181161. DOI: 10.1001/jamapediatrics.2018.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komaroff AL. The microbiome and risk for atherosclerosis. JAMA. 2018;319:2381–2382. DOI: 10.1001/jama.2018.5240. [DOI] [PubMed] [Google Scholar]
- 47.Hassiotou F, Hartmann PE. At the dawn of a new discovery: the potential of breast milk stem cells. Advances in nutrition (Bethesda, Md.). 2014;5:770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Godhia ML, Patel N. Colostrum ‐ its composition, benefits as a nutraceutical: A review. Curr Res Nutr Food Sci. 2013;1(1):37–47. DOI: 10.12944/CRNFSJ.1.1.04. [DOI] [Google Scholar]
- 49.Klunnyk MO, Sych NS, Matiyashchuk IG, Sinelnyk AA, Ivankova O, Demchuk M, Skalozub M, Sorochynska K. Use of stem cell suspensions containing separated fetal stem cells in complex treatment of patients with essential hypertension. J Stem Cell Res Ther. 2016;6:366. DOI: 10.4172/2157-7633.1000366. [DOI] [Google Scholar]
- 50.Koletzko B, Rodriguez‐Palmero M, Demmelmair H, Fidler N, Jensen R, Sauerwald T. Physiological aspects of human milk lipids. Early Hum Dev. 2001;65(Suppl):S3–S18. DOI: 10.1016/S0378-3782(01)00204-3. [DOI] [PubMed] [Google Scholar]
- 51.Hofman A, Hazebroek A, Valkenburg HA. A randomized trial of sodium intake and blood pressure in newborn infants. JAMA. 1983;250:370–373. DOI: 10.1001/jama.1983.03340030030023. [DOI] [PubMed] [Google Scholar]
- 52.Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155:701–709. DOI: 10.1001/archinte.1995.00430070053006. [DOI] [PubMed] [Google Scholar]
- 53.Stamler R. Implications of the intersalt study. Hypertension. 1991;17:I16–20. DOI: 10.1161/01.HYP.17.1_Suppl.I16. [DOI] [PubMed] [Google Scholar]
- 54.Flynn JT, Falkner BE. New clinical practice guideline for the management of high blood pressure in children and adolescents. Hypertension. 2017;70:683–686. DOI: 10.1161/HYPERTENSIONAHA.117.10050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S2


