Abstract
Background
Atrial fibrillation (AF) is a major risk factor for mortality. The prevalence, clinical correlates, and prognostic impact of AF in Takotsubo syndrome (TTS) have not yet been investigated in a large patient cohort. This study aimed to investigate the prevalence, clinical correlates, and prognostic impact of AF in patients with TTS.
Methods and Results
Patients with TTS were enrolled from the International Takotsubo Registry, which is a multinational network with 26 participating centers in Europe and the United States. Patients were dichotomized according to the presence or absence of AF at the time of admission. Of 1584 patients with TTS, 112 (7.1%) had AF. The mean age was higher (P<0.001), and there were fewer women (P=0.046) in the AF than in the non‐AF group. Left ventricular ejection fraction was significantly lower (P=0.001), and cardiogenic shock was more often observed (P<0.001) in the AF group. Both in‐hospital (P<0.001) and long‐term mortality (P<0.001) were higher in the AF group. Multivariable Cox regression analysis revealed that AF was independently associated with higher long‐term mortality (hazard ratio, 2.31; 95% CI, 1.50–3.55; P<0.001). Among patients with AF on admission, 42% had no known history of AF before the acute TTS event, and such patients had comparable in‐hospital and long‐term outcomes compared with those with a history of AF.
Conclusions
In patients presenting with TTS, AF on admission is significantly associated with increased in‐hospital and long‐term mortality rates. Whether antiarrhythmics and/or cardioversion are beneficial in TTS with AF should thus be tested in a future trial.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01947621.
Keywords: atrial fibrillation, broken heart syndrome, outcome, Takotsubo syndrome
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- InterTAK Registry
International Takotsubo Registry
- TTS
Takotsubo syndrome
Clinical Perspective
What Is New?
The prevalence of atrial fibrillation (AF) on admission in patients with takotsubo syndrome (TTS) was 7%, and patients with TTS with AF on admission had more eventful in‐hospital courses and significantly higher mortality rates compared with those without AF.
42% of patients with TTS had no known history of AF before their TTS index event, suggesting that these patients may have developed new‐onset AF.
Patients with TTS with preexisting AF and newly diagnosed AF had comparable in‐hospital and long‐term outcomes.
What Are the Clinical Implications?
The presence of AF in TTS should alert clinicians to the prognostic implications of this rhythm.
Takotsubo syndrome (TTS) is characterized by acute left ventricular (LV) dysfunction.1 This syndrome has historically been considered a benign disease, as the LV dysfunction recovers spontaneously within a few weeks.2 However, recent data show that TTS is associated with a substantial risk of adverse events both during the acute phase and in the long‐term, with complication rates comparable with those of acute coronary syndrome (ACS).3, 4 The occurrence of complications in patients with TTS is associated with poor and even fatal outcomes. For example, patients with TTS can experience concurrent arrhythmias, severe heart failure with or without pulmonary edema, cardiogenic shock, and LV free‐wall rupture.5 A recent international consensus document on TTS reported that cardiac arrhythmias are a major determinant of clinical outcomes in patients with TTS.5
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, and its presence has been associated with a 5‐fold increase in the incidence of stroke and a 2‐fold increase in mortality.6, 7 AF among patients with TTS has been reported in several small studies, with a prevalence ranging from 5% to 25%.8, 9 Recent evidence suggested that patients with TTS with AF, including those with newly diagnosed AF at the time of TTS presentation, may have worse short‐term and long‐term mortality rates compared with those without AF.10, 11 However, the impact of a history of AF versus newly diagnosed AF in the context of an acute event has not been evaluated in a large cohort. To overcome this drawback, we analyzed data from the International Takotsubo Registry (InterTAK Registry), which is the largest available cohort of patients with TTS, to determine the prevalence, clinical correlates, and prognostic impact of AF in patients with TTS.
Methods
Study Population
The study design of the InterTAK Registry has been comprehensively outlined previously.3 The inclusion criteria were the Mayo Clinic Diagnostic Criteria:12 (1) transient LV wall motion abnormalities beyond a single epicardial coronary artery distribution territory; (2) absence of obstructive coronary artery disease or acute plaque rupture, which could explain the wall motion abnormalities; (3) evidence of new ECG abnormalities and/or elevation in cardiac troponin levels; and (4) absence of myocarditis. Exceptions to these criteria were3 patients who matched all other criteria but had wall motion abnormalities corresponding to the territory of a single coronary artery as well as those who died during the acute phase, before complete LV wall motion recovery. The authors declare that all supporting data are available within the article.
Data Collection
Data on the patients’ clinical characteristics were collected through a review of their medical records and included demographics, cardiovascular risk factors, comorbidities, triggering factors, symptoms at admission, laboratory profiles, ECG findings, imaging features, and management. Outcome data were collected from clinical visits, personal telephone interviews, or medical charts. AF was diagnosed based on guidelines of the European Society of Cardiology, which define AF as irregular RR intervals with no discernable, distinct P waves on admission ECG.13 Atrial flutter was considered equivalent to AF. Patients were dichotomized according to the presence or absence of AF as assessed using 12‐lead surface ECG on admission, and we compared clinical characteristics and in‐hospital and long‐term outcomes between the 2 groups. Furthermore, in a subanalysis, patients with AF on admission were categorized into 2 groups based on the presence or absence of known history of AF before TTS events, and baseline features and outcomes were compared. Patients without complete information for this categorization were excluded from this subanalysis.
The study protocol was reviewed by the local ethics committee or investigational review board at each collaborating site. Because of the partly retrospective nature of the study, the ethics committees of most study centers waived the need for informed consent. At centers in which the ethics committees or investigational review boards required informed consent or in which patients were included prospectively, formal written consent was obtained from the patients or their surrogates.
Statistical Analysis
Categorical variables are presented as frequencies (percentages), and continuous parameters are expressed as mean±standard deviation (SD) or as median (interquartile range [IQR]). Group comparisons were conducted using the chi‐square test or Fisher exact test for categorical variables, and the Student t test or Mann–Whitney U test for continuous variables. The Kaplan–Meier method was used to assess the long‐term mortality and major adverse cardiac and cerebrovascular event (a composite of a recurrence of TTS, myocardial infarction, stroke or transient ischemic attack, or death from any cause) rates, and the log‐rank test was used to compare Kaplan–Meier curves as well as a landmark analysis with a landmark set at 60 days. Cox regression analysis was conducted to determine the hazard ratio and 95% CI of AF on admission for long‐term outcomes. To account for possible differences in clinical characteristics and comorbidities between patients with and without AF, a multivariable adjustment analysis including covariates that had a significant difference in the baseline comparison was performed in a Cox regression model. The number of covariates was limited to 12 because of the number of events in the study cohort.14 Multiple imputations before multivariable Cox regression were used to complete missing data. Overall, data on covariates were 95.6% complete. All tests were 2‐sided, and P<0.05 indicated statistical significance. Statistical analyses were performed using SPSS version 25.0, and GraphPad version 7.0 was used for figure preparation.
Results
Patient Characteristics
Of a total of 1750 potentially eligible patients, 1584 patients with TTS with complete information on AF on admission were enrolled in the present study. Among these, 112 (7.1%) had AF on admission as determined by ECG. Median length of hospitalization was 6 days (interquartile range [IQR], 3–10 days) in the no AF cohort and 7 days (IQR, 4–13 days) in the cohort with AF. Compared with patients with TTS without AF, those with AF were less frequently women (84.8% versus 90.6%; P=0.046) and were significantly older (73.8±12.2 years versus 66.1±13.0 years; P<0.001). Physical triggering factors were more common among those with AF than among those without AF (44.6% versus 33.8%; P=0.020). A detailed description of triggering factors in patients with and without AF is shown in Figure S1. Notably, patients with AF more often had the apical TTS type (89.3% versus 81.4%; P=0.036), presented with significantly higher levels of B‐type natriuretic peptide, and had a lower LV ejection fraction (37.2±11.1% versus 41.3±11.8%; P=0.001) than patients without AF. In addition, C‐reactive protein level and white blood cell (WBC) counts on admission were higher in patients with TTS with AF than in patients with TTS without AF (Table 1). Preexisting comorbidities such as hypertension (86.1% versus 63.9%; P<0.001), diabetes mellitus (25.7% versus 13.0%; P<0.001), and coronary artery disease (22.4% versus 15.2%; P=0.049) were more common among those with AF than among those without AF. Angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers (52.2% versus 37.3%, P=0.005) and β‐blockers (48.9% versus 31.6%, P=0.001) had been more frequently prescribed to patients with AF before admission (Table 1).
Table 1.
Characteristics of TTS Patients with and without Atrial Fibrillation
| Atrial fibrillation | w/o Atrial fibrillation | P value | |
|---|---|---|---|
| N=112 | N=1472 | ||
| Demographics | |||
| Female sex, no./total no. (%) | 95/112 (84.8) | 1334/1472 (90.6) | 0.046 |
| Age, y | 73.8±12.2 (N=112) | 66.1±13.0 (N=1472) | <0.001 |
| Takotsubo Type, no./total no. (%) | |||
| Apical | 100/112 (89.3) | 1198/1472 (81.4) | 0.036 |
| Triggers, no./total no. (%) | |||
| Physical trigger | 50/112 (44.6) | 498/1472 (33.8) | 0.020 |
| Emotional trigger | 25/112 (22.3) | 430/1472 (29.2) | 0.12 |
| Symptoms on admission, no./total no. (%) | |||
| Chest pain | 71/100 (71.0) | 1061/1381 (76.8) | 0.19 |
| Syncope | 5/103 (4.9) | 104/1374 (7.6) | 0.31 |
| Cardiac biomarkers on admission, median (IQR) | |||
| Troponin, factor increase in ULN* | 5.60 (1.73–18.08) N=98 | 8.00 (2.50–24.29) N=1207 | 0.10 |
| Creatine kinase, factor increase in ULN | 0.82 (0.45–1.69) N=79 | 0.86 (0.54–1.46) N=1033 | 0.58 |
| BNP, factor increase in ULN† | 10.27 (0.42–36.21) N=40 | 5.25 (1.76–13.79) N=384 | 0.001 |
| Inflammatory markers on admission, median (IQR) | |||
| CRP, mg/L | 7.10 (2.00–47.05) N=85 | 3.90 (1.20–10.35) N=968 | 0.001 |
| WBC, 103/µL | 10.71 (8.10–13.79) N=96 | 9.68 (7.50–12.57) N=1257 | 0.033 |
| ECG on admission, no./total no. (%) | |||
| ST‐segment elevation | 50/112 (44.6) | 640/1466 (43.7) | 0.84 |
| T‐wave inversion | 43/112 (38.4) | 605/1466 (41.3) | 0.55 |
| QTc, ms | 453.2±60.9 (N=81) | 457.9±48.6 (N=1072) | 0.51 |
| Hemodynamics, mean±SD (N) | |||
| Heart rate, beats/min | 94.5±23.0 (N=96) | 86.5±21.1 (N=1245) | <0.001 |
| Systolic blood pressure, mm Hg | 132.1±30.6 (N=92) | 131.1±28.7 (N=1250) | 0.75 |
| Diastolic blood pressure, mm Hg | 77.6±17.9 (N=92) | 76.6±16.9 (N=1232) | 0.60 |
| Left ventricular ejection fraction, %‡ | 37.2±11.1 (N=101) | 41.3±11.8 (N=1360) | 0.001 |
| Left ventricular end‐diastolic pressure, mm Hg | 21.5±7.6 (N=66) | 21.5±7.9 (N=890) | >0.99 |
| Cardiovascular risk factors/history, no./total no. (%) | |||
| Hypertension | 93/108 (86.1) | 918/1437 (63.9) | <0.001 |
| Diabetes mellitus | 28/109 (25.7) | 187/1436 (13.0) | <0.001 |
| Current smoking | 13/104 (12.5) | 278/1402 (19.8) | 0.07 |
| Hypercholesterolemia | 34/108 (31.5) | 450/1431 (31.4) | 0.99 |
| Coexisting medical condition, no./total no. (%) | |||
| Coronary artery disease§ | 24/107 (22.4) | 207/1359 (15.2) | 0.049 |
| Cancer (total) | 17/99 (17.2) | 227/1360 (16.7) | 0.90 |
| COPD or asthma | 9/105 (8.6) | 234/1415 (16.5) | 0.032 |
| Hyperthyroidism | 8/106 (7.5) | 84/1426 (5.9) | 0.45 |
| Hypothyroidism | 10/106 (9.4) | 169/1426 (11.9) | 0.46 |
| Medication on admission, no./total no. (%) | |||
| ACE inhibitor or ARB | 48/92 (52.2) | 448/1200 (37.3) | 0.005 |
| Beta‐blocker | 45/92 (48.9) | 379/1200 (31.6) | 0.001 |
| Statin | 16/90 (17.8) | 212/1169 (18.1) | 0.90 |
| Aspirin | 36/90 (40.0) | 399/1169 (34.1) | 0.26 |
| Vitamin K antagonist | 21/90 (23.3) | 33/1169 (2.8) | <0.001 |
| Acute cardiac care, no./total no. (%) | |||
| Intra‐aortic balloon pump | 7/111 (6.3) | 34/1466 (2.3) | 0.011 |
| Cardiopulmonary resuscitation | 22/111 (19.8) | 107/1466 (7.3) | <0.001 |
| Invasive or noninvasive ventilation | 30/111 (27.0) | 218/1466 (14.9) | 0.001 |
| Catecholamine use | 23/111 (20.7) | 158/1466 (10.8) | 0.002 |
| In‐hospital complications, no./total no. (%) | |||
| Cardiogenic shock | 22/110 (20.0) | 127/1451 (8.8) | <0.001 |
| Death | 16/112 (14.3) | 46/1472 (3.1) | <0.001 |
| Hospitalization (days), median (IQR) | 7 (4–13) N=79 | 6 (3–10) N=1006 | 0.021 |
| 5‐year outcome, no./total no. (%) | |||
| MACCE | 34/112 (30.4) | 201/1472 (13.7) | <0.001 |
| Death | 28/112 (25.0) | 121/1472 (8.2) | <0.001 |
ACE indicates angiotensin‐converting‐enzyme; ARB, angiotensin‐receptor blocker; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; CRP, c‐reactive protein; ECG, electrocardiogram; IQR, interquartile range; MACCE, major adverse cardiac and cerebrovascular events; QTc, QT interval corrected for heart rate; SD, standard deviation; TTS, takotsubo syndrome; ULN, upper limit of the normal; and WBC, white blood cell count.
Including upper limits of the normal range for troponin T, high‐sensitivity troponin T, and troponin I.
Including upper limits of the normal range for brain natriuretic peptide and the N‐terminal of prohormone brain natriuretic peptide.
Data obtained during catheterization or echocardiography; if both results were available data from catheterization were used.
Coexisting coronary artery disease during acute hospitalization.
In‐Hospital Management and Outcomes
Acute cardiac care measures such as intra‐aortic balloon pump insertion (6.3% versus 2.3%; P=0.011), cardiopulmonary resuscitation (19.8% versus 7.3%; P<0.001), invasive or noninvasive ventilation (27.0% versus 14.9%; P=0.001), or catecholamine administration (20.7% versus 10.8%; P=0.002) were more often required in patients with AF than in patients without AF in relation to a significantly higher incidence of cardiogenic shock (20.0% versus 8.8%; P<0.001; Table 1). Moreover, in‐hospital mortality was significantly higher among patients with AF compared with those without AF (14.3% versus 3.1%; P<0.001).
Long‐Term Outcomes
Patients with AF had significantly worse long‐term major adverse cardiac and cerebrovascular events and mortality rates compared with those without AF (Table 1). In addition, cumulative incidence curves showed a substantially higher mortality rate in patients with AF than in those without AF within the first 60 days (P<0.001; Figure 1). After 60 days and up to 5 years, a significant difference of mortality rates between patients with and without AF still existed (P=0.006; Figure 1). The 5‐year mortality rate was substantially higher albeit not statistically significant in patients with AF and malignancy than in the counterparts without AF but with malignancy (35.3% versus 13.2%; P=0.19). A multivariable analysis demonstrated that AF on admission was independently associated with long‐term mortality (hazard ratio, 2.31; 95% CI, 1.50–3.55; P<0.001; Figure 2). A comparison of AF subtypes revealed that 5‐year mortality rates were 12.4%, 20.0%, 33.3% in patients with paroxysmal, persistent, or permanent AF, respectively.
Figure 1. Short‐term and long‐term mortality rates of patients with TTS with and without atrial fibrillation.
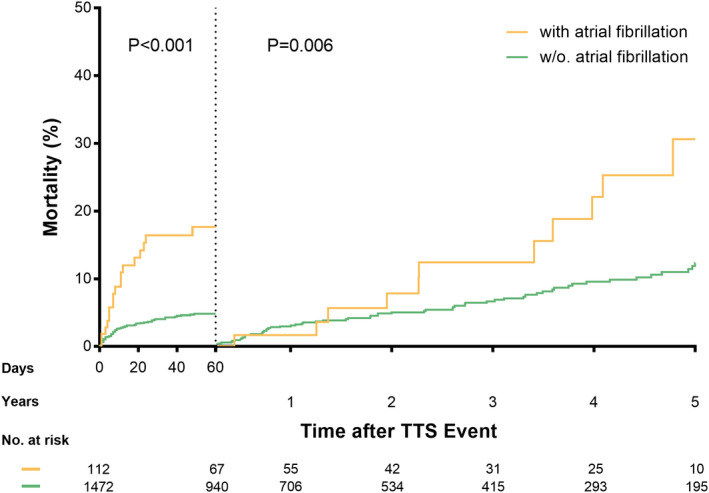
Cumulative incidence curves with a landmark set at 60 days show a higher mortality rate in patients with TTS with atrial fibrillation compared with patients with TTS without atrial fibrillation both in the short term (P<0.001) and long term (P=0.006). TTS indicates Takotsubo syndrome.
Figure 2. Multivariable Cox regression analysis of patients with TTS.
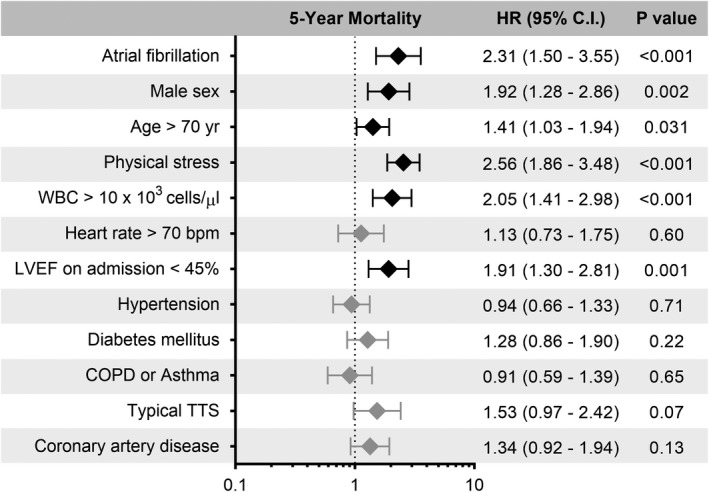
Multivariable Cox regression revealed that atrial fibrillation was independently associated with worse long‐term mortality rates. Male sex, age>70 years, physical trigger, WBC>10×103 cells/µL, and LVEF<45% also emerged as independent predictors of worse long‐term mortality rates in TTS. Errors bars represent 95% CI. Black rhombi indicate statistical significance; gray rhombi indicate no statistical significance. Bpm indicates beats per minute; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; LVEF, left ventricular ejection fraction; TTS, Takotsubo syndrome; and WBC, white blood cell.
Comparison of a History of AF Versus Newly Diagnosed AF
Of the patients with AF on admission, previous AF episodes were identified in 58% (48/83) of patients, whereas in the remaining 42% with AF the episode was considered the first in the patients’ history. Main patient characteristics between the 2 groups are summarized in Table 2. No significant differences in clinical characteristics were observed except for heart rate on admission (87.0±23.6 bpm versus 103.4±15.9 bpm; P=0.002). There were no significant differences on cardiovascular risk factors and coexisting medical conditions. Of the patients with preexisting AF, 57.1% were on oral anticoagulation therapy upon presentation, whereas 1 patient with newly diagnosed AF was prescribed such an agent before admission. Of note, acute intensive care measures including intra‐aortic balloon pump, catecholamine use, invasive or noninvasive ventilation, and cardiopulmonary resuscitation were required equally often in patients with preexisting or newly diagnosed AF. Furthermore, in‐hospital mortality rates were not statistically significant different between the 2 groups (18.8% versus 14.3%; P=0.59). Cumulative incidence curves showed that there were no significant differences on long‐term major adverse cardiac and cerebrovascular events (P=0.53) or mortality (P=0.48) between patients with preexisting AF or newly diagnosed AF (Table 2).
Table 2.
Comparison between TTS Patients with Preexisting and Newly Diagnosed AF
| Preexisting AF | Newly diagnosed AF | P value | |
|---|---|---|---|
| N=48 | N=35 | ||
| Demographics | |||
| Female sex, no./total no. (%) | 44/48 (91.7) | 30/35 (85.7) | 0.48 |
| Age, y | 76.8±9.2 (N=48) | 73.8±11.8 (N=35) | 0.20 |
| Takotsubo Type, no./total no. (%) | |||
| Apical | 42/48 (87.5) | 32/35 (91.4) | 0.7 |
| Triggers, no./total no. (%) | |||
| Physical trigger | 24/48 (50.0) | 17/35 (48.6) | 0.90 |
| Emotional trigger | 9/48 (18.8) | 8/35 (22.9) | 0.65 |
| Symptoms on admission, no./total no. (%) | |||
| Chest pain | 29/41 (70.7) | 21/32 (65.6) | 0.64 |
| Syncope | 1/46 (2.2) | 2/32 (6.3) | 0.57 |
| Cardiac biomarkers on admission, median (IQR) | |||
| Troponin, factor increase in ULN* | 11.50 (3.05–25.78) N=41 | 5.35 (1.03–16.93) N=32 | 0.17 |
| Creatine kinase, factor increase in ULN | 0.80 (0.47–1.69) N=32 | 0.89 (0.43–1.67) N=26 | 0.62 |
| BNP, factor increase in ULN† | 10.23 (2.32–25.52) N=15 | 10.08 (5.49–37.81) N=15 | 0.49 |
| Inflammatory markers on admission, median (IQR) | |||
| CRP, mg/L | 9.30 (1.40–47.28) N=36 | 3.99 (1.95–18.20) N=26 | 0.64 |
| WBC, 103/µL | 11.35 (10.08–15.70) N=39 | 9.95 (7.60–11.60) N=31 | 0.05 |
| ECG on admission, no./total no. (%) | |||
| ST‐segment elevation | 19/48 (39.6) | 18/35 (51.4) | 0.28 |
| T‐wave inversion | 18/48 (37.5) | 14/35 (40.0) | 0.82 |
| QTc, ms | 451.0±63.0 (N=36) | 454.5±70.4 (N=25) | 0.84 |
| Hemodynamics, mean±SD (N) | |||
| Heart rate, beats/min | 87.0±23.6 (N=39) | 103.4±15.9 (N=30) | 0.002 |
| Systolic blood pressure, mm Hg | 134.1±28.8 (N=38) | 125.4±30.6 (N=30) | 0.23 |
| Diastolic blood pressure, mm Hg | 79.6±18.3 (N=38) | 74.9±17.5 (N=30) | 0.29 |
| Left ventricular ejection fraction, %‡ | 37.6±10.9 (N=44) | 37.4±10.8 (N=32) | 0.93 |
| Left ventricular end‐diastolic pressure, mm Hg | 22.0±8.5 (N=30) | 20.5±7.1 (N=20) | 0.51 |
| Cardiovascular risk factors/history, no./total no. (%) | |||
| Hypertension | 41/47 (87.2) | 31/34 (91.2) | 0.73 |
| Diabetes mellitus | 14/47 (29.8) | 7/33 (21.2) | 0.39 |
| Current smoking | 7/45 (15.6) | 5/31 (16.1) | 0.95 |
| Hypercholesterolemia | 15/47 (31.9) | 9/33 (27.3) | 0.66 |
| Coexisting medical condition, no./total no. (%) | |||
| Coronary artery disease§ | 8/47 (17.0) | 10/33 (30.3) | 0.16 |
| Cancer (total) | 9/45 (20.0) | 4/30 (13.3) | 0.55 |
| COPD or asthma | 5/47 (10.6) | 4/30 (13.3) | 0.73 |
| Medication on admission, no./total no. (%) | |||
| ACE inhibitor or ARB | 21/41 (51.2) | 13/27 (48.1) | 0.80 |
| Beta‐blocker | 20/41 (48.8) | 14/27 (51.9) | 0.80 |
| Statin | 10/41 (24.4) | 5/27 (18.5) | 0.57 |
| Aspirin | 21/41 (51.2) | 12/26 (46.2) | 0.69 |
| Oral anticoagulants | 24/42 (57.1) | 1/32 (3.1) | <0.001 |
| Acute cardiac care, no./total no. (%) | |||
| Intra‐aortic balloon pump | 4/47 (8.5) | 2/35 (5.7) | >0.99 |
| Cardiopulmonary resuscitation | 10/47 (21.3) | 6/35 (17.1) | 0.64 |
| Invasive or noninvasive ventilation | 16/47 (34.0) | 7/35 (20.0) | 0.16 |
| Catecholamine use | 10/47 (21.3) | 8/35 (22.9) | 0.86 |
| In‐hospital complications, no./total no. (%) | |||
| Cardiogenic shock | 13/46 (28.3) | 5/35 (14.3) | 0.13 |
| Death | 9/48 (18.8) | 5/35 (14.3) | 0.59 |
| 5‐year outcome, no./total no. (%) | |||
| MACCE | 17/48 (35.4) | 11/35 (31.4) | 0.53 |
| Death | 15/48 (31.3) | 9/35 (25.7) | 0.48 |
ACE indicates angiotensin‐converting‐enzyme; AF, atrial fibrillation; ARB, angiotensin‐receptor blocker; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; CRP, c‐reactive protein; ECG, electrocardiogram; IQR, interquartile range; MACCE, major adverse cardiac and cerebrovascular events; QTc, QT interval corrected for heart rate; SD, standard deviation; TTS, takotsubo syndrome; ULN, upper limit of the normal; and WBC white blood cell count.
Including upper limits of the normal range for troponin T, high‐sensitivity troponin T, and troponin I.
Including upper limits of the normal range for brain natriuretic peptide and the N‐terminal of prohormone brain natriuretic peptide.
Data obtained during catheterization or echocardiography; if both results were available data from catheterization were used.
Coexisting coronary artery disease during acute hospitalization.
Discussion
In this of patients with TTS, we investigated the impact of AF on clinical outcomes and found the following: (1) patients with TTS with AF on surface ECG at admission had a more eventful in‐hospital course and significantly higher mortality rates compared with those without AF; (2) AF on admission was independently associated with a higher long‐term mortality rate; (3) approximately one third of patients had no known history of AF before their TTS index event, suggesting that these patients may have developed new‐onset AF; and finally (4) patients with TTS with preexisting AF and newly diagnosed AF had comparable in‐hospital and long‐term outcomes.
Among the 1584 patients with TTS, the prevalence of AF on admission was 7.1%. Previous studies reported a prevalence ranging between 5% and 25% among patients with TTS.8, 9, 10, 15 In our study, fewer than a half of the patients (42%) who presented with AF during the index TTS event did not have a prior history of AF. This is higher than the results of Jesel et al, who reported an incidence of newly diagnosed AF or atrial flutter among patients with TTS of 24.8%.10 Meanwhile, Stiermaier et al showed the total prevalence of AF in patients with TTS was 25%, of whom 7.2% had new episodes of AF during their hospital stay.8 This indicates that in the majority of patients with TTS, new‐onset AF is likely related to TTS. In this context, it is of interest that patients with AF more commonly presented with apical ballooning and a lower LV ejection fraction, both conditions known to be associated with LV increased filling pressures and as a consequence increased left atrial pressures. Patients with TTS with AF also showed higher levels of inflammatory markers, including elevated C‐reactive protein and WBC levels, than those without AF in the present study. This is consistent with the study by Jesel et al, who also showed higher peak C‐reactive protein and WBC levels among patients with TTS with newly diagnosed AF compared with those without atrial arrhythmia.10 These findings suggest that inflammation plays a key role in AF during an acute TTS event. In this regard, recently published data on 56 patients with TTS has shown that increased serum interleukin (IL)‐6 and IL‐10 admission levels are associated with a higher risk of adverse events during follow‐up.16 On the other hand, it has been suggested that activation of systemic inflammation may trigger atrial electrical remodeling, and it has been discussed whether direct cytokine‐mediated effects on connexins are one of the underlying mechanisms. During acute or chronic inflammation, functional changes of atria might be documented by downregulating of cardiac connexins via increased IL‐6.17 Indeed, there is substantial evidence demonstrating that TTS and AF might be associated with inflammation,18, 19 although the precise mechanisms are unclear.
Acute AF is known to impair hemodynamics. Thus, it is of note that patients with TTS with AF on admission more often developed cardiogenic shock and required more commonly acute cardiac care measures including catecholamine administration and intra‐aortic balloon pump insertion compared with those without AF. In addition, in‐hospital mortality was significantly higher among patients with TTS with AF than among those without it. Our findings in our large TTS cohort are in line with observations in patients with ACS, in whom AF is associated with a higher rate of complications such as cardiogenic shock and life‐threatening ventricular tachyarrhythmias and poorer overall outcomes.20 The observed association between the presence of AF on admission and mortality in patients with TTS is likely related to the adverse hemodynamic effects of AF, such as loss of atrial contraction and the resultant loss of atrioventricular synchronicity, rhythm irregularity, and rapid ventricular rates. All of these aspects contribute further to the reduced LV ejection fraction and cardiac output. In addition, there is evidence demonstrating that TTS and AF might be associated with inflammation.18 Interestingly, patients with TTS with AF had a greater degree of inflammatory markers (C‐reactive protein and WBC) than those without AF in the present study. Therefore, it is conceivable that a strong inflammatory response might lead to a more eventful outcome in patients with TTS with AF. Furthermore, AF is known to increase the risk of cardioembolic events in patients with TTS.21 Such risk might be more pronounced in patients with TTS given the severe hypokinesia/akinesia of the ventricular wall in apical ballooning during the acute phase of the disease.
Besides ACS and TTS reported here, AF has been reported as a predictor of poor long‐term prognosis in patients with other cardiac conditions such as dilated cardiomyopathy and congenital heart disease.22, 23 Furthermore, in patients with chronic coronary syndromes, AF has been associated with significantly higher rates of thromboembolic stroke, heart failure, and long‐term mortality, regardless of the time of AF onset. Interestingly, although global LV ejection fraction is normalized within a few weeks in TTS, we demonstrated by landmark analysis that the long‐term mortality rate was still significantly higher in the AF group than in the non‐AF group after excluding the strong influence of the acute phase. In addition, AF emerged as an independent predictor of long‐term mortality in TTS.
We also demonstrated that mortality differs depending on the AF subtype, that is, patients with paroxysmal AF had the lowest mortality rates. Similarly, Link et al showed that paroxysmal AF is associated with a more favorable outcome than persistent or permanent AF.24 Thus, all patients with TTS with AF on admission need to be managed appropriately according to the established guidelines for AF.25
The prevalence of AF in TTS is comparable with that of AF in ACS,26 which implies they may share a common pathophysiological mechanism. Indeed, heart failure, regardless of etiology, is associated with sympathetic activation that can be detrimental for the atrial substrate. This activation likely results from the neuro‐hormonal system and catecholaminergic activation as well as mechanical stretching of cardiomyocytes and ionic channel dysfunction. During ventricular overload, cell membrane stretching may activate certain ion channels in the cardiomyocytes and alter their electrical activity, resulting in mechano‐electrical feedback that increases the risk of arrhythmia.27, 28 Also, human cardiomyocytes from induced pluripotent stem cells modeling TTS show increased late sodium current and decreased transient outward current.29 Such an elevated late sodium current is observed in both TTS and ACS and may be triggered by a prolongation of repolarization.29, 30 However, further investigation into the underlying pathophysiological cascades of TTS and ACS is required.
Limitations
Because the InterTAK Registry is an in part retrospective registry, recall bias cannot be fully excluded. The definition of the presence of AF was based on ECG on admission, and the duration of AF was not assessed. The power to account for confounding factors is limited, and some residual selection bias might be present. Finally, we cannot rule out that AF was the primary cause of transient tachycardia‐induced cardiomyopathy rather than an incidental correlate of TTS.
Conclusions
Patients with TTS with AF on surface ECG on admission had a more eventful in‐hospital course and significantly higher mortality rates than patients with TTS without AF. Furthermore, AF was statistically significantly associated with a higher long‐term mortality. Although some patients with AF on admission had no known prior history of AF, they had comparable in‐hospital and long‐term outcomes compared with those with preexisting AF. Thus, the presence of AF on admission should alert clinicians to the prognostic implications of this rhythm. Adequate AF management as part of the overall TTS treatment strategy may improve outcomes of patients with TTS with AF.
Sources of Funding
Dr Templin has been supported by the H.H. Sheikh Khalifa bin Hamad Al‐Thani Research Programme. The International Takotsubo Registry is supported by the Biss Davies Charitable Trust.
Disclosures
No disclosures have been reported for any aspects of the submitted work.
Supporting information
Figure S1
This manuscript was sent to N.A. Mark Estes, III, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1.Kato K, Lyon AR, Ghadri JR, Templin C. Takotsubo syndrome: aetiology, presentation and treatment. Heart. 2017;103:1461–1469. DOI: 10.1136/heartjnl-2016-309783. [DOI] [PubMed] [Google Scholar]
- 2.Sato H. Tako‐tsubo‐like left ventricular dysfunction due to multivessel coronary spasm. In: Kodama K, Haze K, Hori M, eds. Clinical Aspect of Myocardial Injury: From Ischemia to Heart Failure. Tokyo: Kagakuhyoronsha Publishing Co; 1990:56–64 (Article in Japanese). [Google Scholar]
- 3.Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–938. DOI: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 4.Ghadri JR, Kato K, Cammann VL, Gili S, Jurisic S, Di Vece D, Candreva A, Ding KJ, Micek J, Szawan KA, et al. Long‐term prognosis of patients with takotsubo syndrome. J Am Coll Cardiol. 2018;72:874–882. DOI: 10.1016/j.jacc.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Ghadri J‐R, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, et al. International expert consensus document on takotsubo syndrome (part II): diagnostic workup, outcome, and management. Eur Heart J. 2018;39:2047–2062. DOI: 10.1093/eurheartj/ehy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S , Despres JP, Fullerton HJ, Howard VJ, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: The Framingham heart study. Circulation. 1998;98:946–952. DOI: 10.1161/01.CIR.98.10.946. [DOI] [PubMed] [Google Scholar]
- 8.Stiermaier T, Santoro F, Eitel C, Graf T, Möller C, Tarantino N, Guastafierro F, Di Biase M, Thiele H, Brunetti ND, et al. Prevalence and prognostic relevance of atrial fibrillation in patients with takotsubo syndrome. Int J Cardiol. 2017;245:156–161. DOI: 10.1016/j.ijcard.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 9.El‐Battrawy I, Lang S, Ansari U, Behnes M, Hillenbrand D, Schramm K, Fastner C, Zhou X, Bill V, Hoffmann U, et al. Impact of concomitant atrial fibrillation on the prognosis of takotsubo cardiomyopathy. Europace. 2017;19:1288–1292. DOI: 10.1093/europace/euw293. [DOI] [PubMed] [Google Scholar]
- 10.Jesel L, Berthon C, Messas N, Lim HS, Girardey M, Marzak H, Marchandot B, Trinh A, Ohlmann P, Morel O. Atrial arrhythmias in takotsubo cardiomyopathy: incidence, predictive factors, and prognosis. Europace. 2019;21:298–305. DOI: 10.1093/europace/euy147. [DOI] [PubMed] [Google Scholar]
- 11.Prasitlumkum N, Kittipibul V, Limpruttidham N, Rattanawong P, Chongsathidkiet P, Boondarikpornpant T. The presence of atrial fibrillation in takotsubo cardiomyopathy is predictive of mortality: systematic review and meta‐analysis. Ann Noninvasive Electrocardiol. 2019;24.e12566 DOI: 10.1111/anec.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (tako‐tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. DOI: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 14.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. DOI: 10.1016/S0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 15.Song BG, Hahn J‐Y, Cho SJ, Park YH, Choi SM, Park JH, Choi S‐H, Choi J‐H, Park SW, Lee SH, et al. Clinical characteristics, ballooning pattern, and long‐term prognosis of transient left ventricular ballooning syndrome. Heart Lung. 2010;39:188–195. DOI: 10.1016/j.hrtlng.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Santoro F, Tarantino N, Ferraretti A, Ieva R, Musaico F, Guastafierro F, Di Martino L, Di Biase M, Brunetti ND. Serum interleukin 6 and 10 levels in takotsubo cardiomyopathy: Increased admission levels may predict adverse events at follow‐up. Atherosclerosis. 2016;254:28–34. DOI: 10.1016/j.atherosclerosis.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Jalloul Y, Refaat MM. Il‐6 rapidly induces reversible atrial electrical remodeling by downregulation of cardiac connexins. J Am Heart Assoc. 2019;8:e013638. DOI: 10.1161/JAHA.119.013638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230–243. DOI: 10.1038/nrcardio.2015.2. [DOI] [PubMed] [Google Scholar]
- 19.Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79:495–502. DOI: 10.1253/circj.CJ-15-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabre P, Roger VL, Murad MH, Chamberlain AM, Prokop L, Adnet F, Jouven X. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta‐analysis. Circulation. 2011;123:1587–1593. DOI: 10.1161/CIRCULATIONAHA.110.986661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitsuma W, Kodama M, Ito M, Kimura S, Tanaka K, Hoyano M, Hirono S, Aizawa Y. Thromboembolism in takotsubo cardiomyopathy. Int J Cardiol. 2010;139:98–100. DOI: 10.1016/j.ijcard.2008.06.089. [DOI] [PubMed] [Google Scholar]
- 22.Wu MH, Lu CW, Chen HC, Chiu SN, Kao FY, Huang SK. Arrhythmic burdens in patients with tetralogy of fallot: a national database study. Heart Rhythm. 2015;12:604–609. DOI: 10.1016/j.hrthm.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 23.Aleksova A, Merlo M, Zecchin M, Sabbadini G, Barbati G, Vitrella G, Di Lenarda A, Sinagra G. Impact of atrial fibrillation on outcome of patients with idiopathic dilated cardiomyopathy: data from the heart muscle disease registry of trieste. Clin Med Res. 2010;8:142–149. DOI: 10.3121/cmr.2010.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Link MS, Giugliano RP, Ruff CT, Scirica BM, Huikuri H, Oto A, Crompton AE, Murphy SA, Lanz H, Mercuri MF, et al. Stroke and mortality risk in patients with various patterns of atrial fibrillation: results from the engage AF‐TIMI 48 trial (effective anticoagulation with factor XA next generation in atrial fibrillation‐thrombolysis in myocardial infarction 48). Circ Arrhythm Electrophysiol. 2017;10. DOI: 10.1161/CIRCEP.116.004267. [DOI] [PubMed] [Google Scholar]
- 25.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H‐C, Heidbuchel H, Hendriks J, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. DOI: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 26.Rubenstein JC, Cinquegrani MP, Wright J. Atrial fibrillation in acute coronary syndrome. J Atr Fibrillation. 2012;5:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravelli F. Mechano‐electric feedback and atrial fibrillation. Prog Biophys Mol Biol. 2003;82:137–149. DOI: 10.1016/S0079-6107(03)00011-7. [DOI] [PubMed] [Google Scholar]
- 28.Kamkin A, Kiseleva I, Wagner KD, Leiterer KP, Theres H, Scholz H, Gunther J, Lab MJ. Mechano‐electric feedback in right atrium after left ventricular infarction in rats. J Mol Cell Cardiol. 2000;32:465–477. DOI: 10.1006/jmcc.1999.1091. [DOI] [PubMed] [Google Scholar]
- 29.El‐Battrawy I, Zhao Z, Lan H, Schünemann J‐D, Sattler K, Buljubasic F, Patocskai B, Li X, Yücel G, Lang S, et al. Estradiol protection against toxic effects of catecholamine on electrical properties in human‐induced pluripotent stem cell derived cardiomyocytes. Int J Cardiol. 2018;254:195–202. DOI: 10.1016/j.ijcard.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Wang H‐M, Wang Y‐Z, Zhang Y‐Y, Jin X‐X, Zhao Y, Wang J, Sun Y‐L, Xue G‐L, Li P‐H, et al. Increment of late sodium currents in the left atrial myocytes and its potential contribution to increased susceptibility of atrial fibrillation in castrated male mice. Heart Rhythm. 2017;14:1073–1080. DOI: 10.1016/j.hrthm.2017.01.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


