Abstract
Background
Patients with recurrent pericarditis (RP) may develop complications, multiple recurrences, or inadequate treatment response. This study aimed to characterize disease burden and unmet needs in RP.
Methods and Results
This retrospective US database analysis included newly diagnosed patients with RP with ≥24 months of continuous history following their first pericarditis episode. RP was defined as ≥2 pericarditis episodes ≥28 days apart. Some patients had ≥2 recurrences, while others had a single recurrence with a serious complication, ie, constrictive pericarditis, cardiac tamponade, or a large pericardial effusion with pericardiocentesis/pericardial window. Among these patients with multiple recurrences and/or complications, some had features relating to treatment history, including long‐term corticosteroid use (corticosteroids started within 30 days of flare, continuing ≥90 consecutive days) or inadequate treatment response (pericarditis recurring despite corticosteroids and/or colchicine, or other drugs [excluding NSAIDs] within 30 days of flare, or prior pericardiectomy). Patients (N=2096) had hypertension (60%), cardiomegaly (9%), congestive heart failure (17%), atrial fibrillation (16%), autoimmune diseases (18%), diabetes mellitus (21%), renal disease (20%), anxiety (21%), and depression (14%). Complications included pericardial effusion (50%), cardiac tamponade (9%), and constrictive pericarditis (4%). Pharmacotherapy included colchicine (51%), NSAIDs (40%), and corticosteroids (30%), often in combination. This study estimates 37 000 US patients with RP; incidence was 6.0/100 000/year (95% CI, 5.6‒6.3), and prevalence was 11.2/100 000 (95% CI, 10.6‒11.7).
Conclusions
Patients with RP may have multiple recurrences and/or complications, often because of inadequate treatment response and persistent underlying disease. Corticosteroid use is frequent despite known side‐effect risks, potentially exacerbated by prevalent comorbidities. Substantial clinical burden and lack of effective treatments underscore the high unmet need.
Keywords: database analysis, epidemiology, pericarditis, recurrent pericarditis
Subject Categories: Pericardial Disease, Complications, Mortality/Survival, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- RP
recurrent pericarditis
Clinical Perspective
What Is New?
This is the first study to characterize the burden of pericarditis with multiple recurrences and/or complication in the United States.
Over one fourth of patients with a first acute pericarditis episode experience at least 1 more episode within 2 years, and 15% experience either multiple recurrences, or 1 recurrence and a serious complication.
What Are the Clinical Implications?
While most patients experience resolution of acute pericarditis, there is a subset of patients who experience a high disease burden, partly as a result of persistent underlying disease.
Among this subset of patients, available treatments are insufficient, as evidenced by continued pericarditis recurrence and, in some cases, prolonged use of corticosteroids or the need for invasive surgical procedures such as pericardiectomy or pericardial window.
Acute pericarditis is often associated with severe symptoms, including sharp, pleuritic chest pain and dyspnea.1 While pericarditis epidemiology data are limited in the United States, about 5% of patients presenting in emergency departments with chest pain ultimately are identified as having an acute pericarditis episode.2, 3, 4 Acute pericarditis is diagnosed based on having at least 2 of the following: chest pain, pericardial friction/rub, electrocardiographic changes, and pericardial effusion.2, 3, 4 The etiology of pericarditis varies, and causes can include infection, systemic inflammatory diseases, cancer, and post‐cardiac injury syndromes. In developing countries, tuberculosis is often a cause, but up to 90% of cases are considered “idiopathic” in developed countries, and presumed to be viral or post‐viral.5 While the term “idiopathic” is commonly used in these presumed post‐viral cases without a clear underlying cause, there is a growing body of evidence that innate immune system‐mediated autoinflammation, specifically interleukin‐1 signaling, plays an important role in various inflammatory disorders, including recurrent pericarditis (RP) cases that are classified as “idiopathic”.6, 7
For most patients, pericarditis manifests as a single episode that lasts a few days to several weeks, and symptoms are resolved through conventional treatments such as NSAIDs with or without colchicine.2 There are, however, some patients who do not experience long‐term symptom resolution after an acute pericarditis episode. Up to 30% of patients experience recurrent pericarditis within 18 months when a subsequent episode occurs following a symptom‐free period of at least 4 weeks.3, 8, 9 Beyond the first recurrence, the clinical picture of RP varies broadly, with some patients responding well to conventional therapy and having no further recurrence. By contrast, 25% to 50% of patients who have experienced a first recurrence will experience additional recurrence, and among patients who have experienced ≥2 recurrences, 20% to 40% are expected to have subsequent episodes.3 Reasons for recurrence include incomplete or inadequate response to therapy and persistent underlying factors that drive pericardial inflammation, which appears to be primarily driven by autoinflammatory processes in patients with RP either without an underlying autoimmune etiology or in conjunction with other known causes, such as post‐cardiac injury syndromes.2, 6
Within the population of RP, some patients, including those with multiple recurrences or other serious complications, are at higher risk for adverse outcomes.3 Cremer et al have proposed a set of real–world clinical stages of pericarditis: acute, first recurrence, multiple recurrences, colchicine‐resistant or steroid‐dependent, and constrictive. While each stage is clearly defined clinically, the epidemiology of patients who experience multiple recurrences and/or recurrence plus complications is not well understood. Multiple recurrences and/or complications occur in up to one‐third of patients with acute pericarditis, leading to increased morbidity, prolonged disease duration, impaired physical and mental health‐related quality of life, and clinical complications.3, 10 Complications related to pericarditis include serious and potentially life‐threatening issues such as cardiac tamponade, constrictive pericarditis, or pericardial effusion.3, 4 Patients who experience multiple recurrences and/or have serious complications or comorbidities may utilize substantial healthcare resources without necessarily experiencing clinical improvement.4 Given the treatment challenges among these patients, additional research would be helpful in identifying patients who are at risk for recalcitrant and protracted pericarditis.3
Currently, there are no US treatment guidelines for pericarditis or RP; standard therapy consists of NSAIDs or aspirin with or without colchicine. Corticosteroids and other off‐label immunosuppressants are used in patients with continued recurrence and inadequate response to conventional therapy.3, 4 European guidelines indicate that corticosteroids should generally be avoided, as their use has been associated with increased risk of recurrence, particularly if the dose is tapered rapidly.4 In the most severe cases, pericardiectomy may be performed, although it does not always prevent recurrence.3, 4 Among the patients with RP with multiple recurrences and/or complications, disease burden is substantial and treatment options are currently inadequate.3
There is currently a lack of detailed information on the epidemiology of RP among patients with multiple recurrences and/or complications, in whom the unmet need is presumably the highest. A better understanding of the disease persistence and risk of recurrence and other complications in various subgroups of patients with pericarditis would help to clarify the burden of disease in this patient population and could assist in treatment planning. To better characterize the unmet needs among patients with RP, particularly in the United States where there are no clinical guidelines to inform treatment decisions, we conducted a retrospective healthcare claims database analysis focused on idiopathic/post‐viral etiologies of the inflammatory phenotype. Our objectives were to estimate recurrence burden and comorbid conditions, describe real‐world treatment patterns, and determine the incidence and prevalence of patients with multiple recurrences and recurrence plus complication in the United States. We sought to better understand the clinical and treatment experience of the subset of patients with acute pericarditis who do not achieve long‐term symptom resolution or who require chronic corticosteroid treatment, and to determine how their experiences aligned with the pericarditis stages proposed by Cremer et al.3
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Setting
This retrospective analysis used fully adjudicated medical and pharmacy claims from the PharMetrics Plus database, which includes 90 million commercially insured US patients with dates of service from January 1, 2013 to March 31, 2018. The data capture all claims submitted on behalf of a patient under the insurer that is included in the database. Each record includes anonymized patient identifiers, patient demographics, type, and specialty of healthcare provider (based on National Provider Identifier number and primary specialty), site of care (based on provider location codes), procedures (based on common procedural terminology codes), and service dates associated with a given claim. Dispensed prescription drugs (but not over‐the‐counter drugs) are identified using a National Drug Code, while diagnoses are coded using the International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD‐9‐CM and ICD‐10‐CM) systems. Institutional review board approval and informed consent for de‐identified data were not required.
Participants
To be eligible for inclusion, newly diagnosed patients with pericarditis were identified based on the following criteria: (1) at least 12 months without a claim for pericarditis or pericardiectomy (the "washout period"), followed by (2) at least 1 relevant ICD‐9 or ICD‐10 code (Table 1) for an office visit, emergency department visit, or hospitalization (the "index episode"); (3) at least 24 months of continuous insurance coverage captured in the database following the index episode (the "observation period"). The relevant codes were identified through a comprehensive literature search, recognizing that there is not a specific code that covers all idiopathic/post‐viral etiologies. The codes were selected to include patients with idiopathic or post‐viral etiology, where the treatment objective is to treat the inflammation, rather than patients with a known cause for pericarditis, where treatment approaches generally are tailored to the specific etiology. While the codes were purposely broad enough to include all possible idiopathic/post‐viral etiologies, it is possible that the codes captured some patients outside of the specific target population. For example, code I30.8 may include patients with other etiologies, and code I30.1 could include bacterial pericarditis. Code I31.9 could include pericardial conditions other than pericarditis, such as atypical chest pain or pericardial cyst/mass. Nonetheless, by excluding codes that are specific for pericarditis secondary to other conditions, such as I32 (pericarditis in diseases classified elsewhere), the selected codes would be expected to primarily capture idiopathic/post‐viral etiologies.
Table 1.
Pericarditis Coding
| ICD‐9 | ICD‐10 | ||
|---|---|---|---|
| Code | Description | Code | Description |
| 420.91 | Acute idiopathic pericarditis | I30.0 | Acute nonspecific idiopathic pericarditis |
| 420.99 | Other acute pericarditis | I30.8 | Other forms of acute pericarditis |
| 420.90 | Acute pericarditis unspecified | I30.9 | Acute pericarditis, unspecified |
| I30.1 | Infective pericarditis | ||
| 423.90 | Unspecified disease of pericardium | I31.9 | Disease of pericardium, unspecified |
Excluded codes include I31.0 (chronic adhesive pericarditis), I31.1 (chronic constrictive pericarditis), I32 (pericarditis in diseases classified elsewhere; includes bacterial [I32.0], other infectious/parasitic diseases [I32.1], other diseases: rheumatoid, systemic lupus erythematosius, uremic [I32.8]). ICD‐9 indicates International Classification of Diseases, Ninth Revision; and ICD‐10, International Classification of Diseases, Tenth Revision.
Statistical Analysis
Recurrent pericarditis was defined as any claim with a relevant ICD code for pericarditis occurring at least 28 days following the index episode, and subsequent recurrences were defined as additional claims that were separated by at least 28 days from the previous episode. In addition to identifying patients with multiple recurrences, we identified those who experience RP with a serious complication such as constrictive pericarditis, cardiac tamponade, or a pericardial effusion that necessitated surgical intervention defined by a procedural code (common procedural terminology) for pericardiocentesis or a pericardial window (Table 2). The data collection period was 24 months following the index episode. This study did not capture the specific indication that led to a procedure being performed.
Table 2.
Serious Complication Coding
| Type | Code | Description |
|---|---|---|
| ICD‐CM | 423.3, I31.4 | Cardiac tamponade |
| ICD‐CM | 423.2, I31.1 | Constrictive pericarditis |
| ICD‐CM | 423.9, I31.3 | Large pericardial effusion |
| ICD‐CM; CPT | 37.0; 33 010 | Pericardiocentesis |
| CPT | 33 025 | Pericardial window |
| ICD‐CM; CPT | 37.31; 33 030, 33 031 | Pericardiectomy |
CPT indicates common procedural terminology; ICD‐CM , International Classification of Diseases ‐ Clinical Modification.
Patients with recurrent pericarditis with multiple recurrences and/or complication were also assessed based on treatment history, to identify those with long‐term corticosteroid use (where corticosteroids were started within 30 days of a pericardial flare, and treatment was continued for at least 90 consecutive days) and those considered to have inadequate treatment response, with a new episode (ie, recurrence) despite corticosteroids and/or colchicine, or other drugs (excluding NSAIDs) such as anakinra or azathioprine within 30 days of flare, or prior pericardiectomy.
Calculations of Disease Persistence, Incidence, and Prevalence
Annual disease persistence was calculated by determining the portion of patients with recurrence in the full calendar years following the index episode. This was calculated in patients with at least 3 years of continuous history following their index episode. To calculate the incidence and prevalence of RP, age‐specific incidence of the initial pericarditis recurrence was calculated by dividing the number of patients experiencing their first recurrence by the enrolled population in the Pharmetrics Plus database. The incidence rates were then standardized using the 2018 US Census to estimate the size of incident population across the United States. Age categories used for projection were 0 to 17, 18 to 39, 40 to 64, and ≥65 years. Prevalence was calculated by estimating the number of existing patients with ongoing recurrence from prior years and adding the total to the incident patient population. This approach assumed that the incident population for each of the prior years was the same as the size of the incident population for the current year and that recurrence rates remained constant over the analysis horizon. It was also assumed that all recurrences occurred in the successive year; thus, the calculation represents the upper bound of the RP population. Point prevalence estimates were calculated based on the incident and prevalent populations using the 2018 US Census.
Results
A total of 7502 patients with pericarditis were identified with at least 2 years of continuous history following their index episode; data from the 24 months following the index episode were used in the analysis. Forty‐five percent of these patients were female sex, and the median age was 52 years (range 2–83 years).
Cohorts of Interest
Out of the 7502 patients, 2096 (27.9%) were identified as having at least 1 recurrence in the 2‐year observation period (Figure 1). Similar to what was observed among all patients with an index episode, among the 2096 patients with RP, 48% were female sex, and the median age was 52 years (age range, 2–83; interquartile range, 39–59). Of these, 994 patients (13.2% of acute pericarditis population; 47.4% of RP population) had ≥2 recurrences. Of the 2096 patients: 57 (3%) had long‐term corticosteroid use; 505 (24%) had inadequate treatment response; 432 (21%) had multiple recurrences, and 144 (7%) had 1 recurrence plus a serious complication. The remaining patients (n=960, [46%]) had 1 recurrence without complication.
Figure 1. Included patient distribution by clinical history.
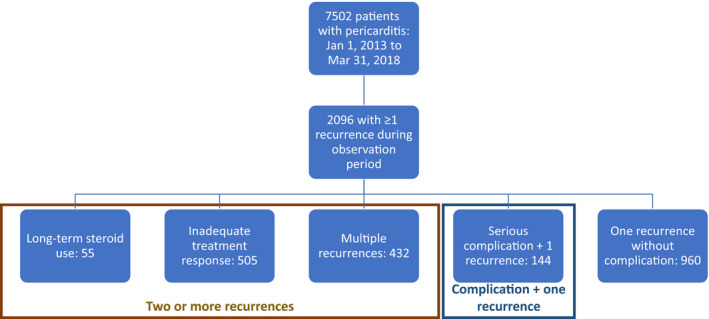
Recurrence Burden and Disease Persistence
The majority of the 2096 patients experienced 1 or 2 recurrences during the observation period, but a small subset (1% of patients; n=26) experienced ≥10 recurrences during the observation period. Figure 2 shows the distribution of the number of recurrences per patient. Patients may have had additional episodes after the observation period that were not captured in this study.
Figure 2. Distribution of recurrences among study population during the observation period.
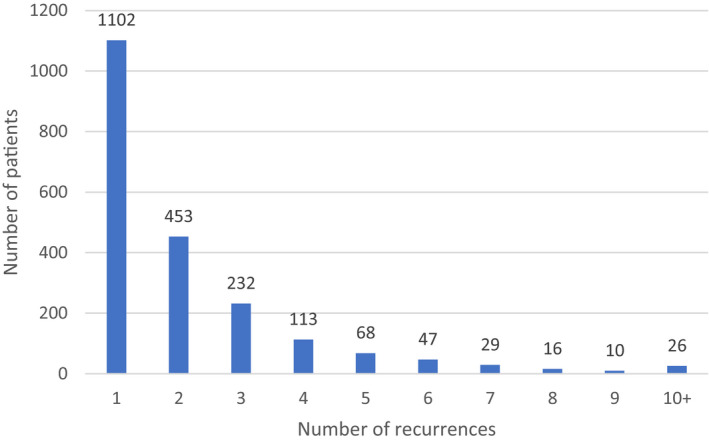
Among the 2096 patients with RP (27.9% of all newly diagnosed pericarditis patients), the mean number of recurrences was 2.16 (SD, 1.95; range, 1–22) during the observation period. Nearly half (994 of 2096, or 47%) of patients who had 1 recurrence experienced a second recurrence, and 54% (541 of 994) of those with a second recurrence had a third recurrence. The likelihood of an additional recurrence generally increased with each subsequent recurrence: 36 of the 52 patients (69%) with 8 recurrences experienced a ninth recurrence.
Refractory and corticosteroid‐dependent patients experienced the most frequent recurrences. The annual recurrence rate was ≥3 per year in 6.1% of patients with RP and reached 15% among those with inadequate treatment response and 42% among those with long‐term corticosteroid use, suggesting that the corticosteroid‐dependent patients had more severe disease.
Particularly among patients with high recurrence burden, the time between recurrences was relatively short. Within the observation window, the time to recurrence ranged from 4 to 104 weeks, with the means plotted in Figure 3. Recurrence was defined as a new claim separated from the previous one by at least 28 days, so the minimum time to recurrence was, by definition, 28 days, ie, 4 weeks. Patients with ≥6 recurrences had a mean of <3 months between episodes.
Figure 3. Mean time to recurrence of pericarditis within 2‐year observation window.
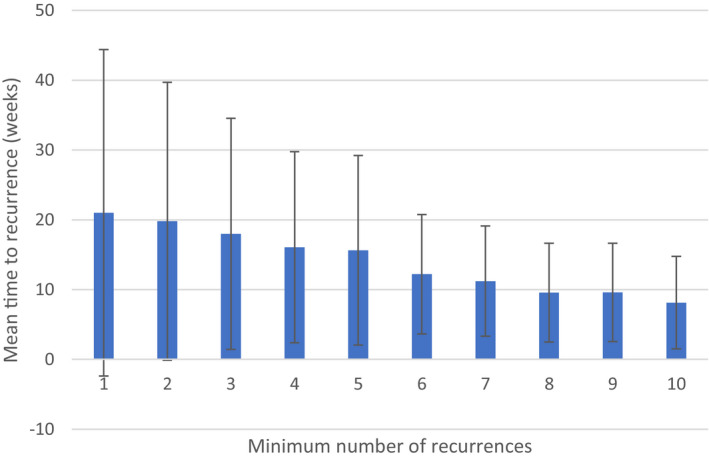
Error bars represent SD.
Most of the patients with recurrent had their first recurrence within 1 year of the index episode: overall, 28% of patients (N=2096) had at least one recurrence, with 23% of patients having a recurrence in the first year. A subset of patients went on to experience multiple recurrences over more than 1 year during the follow‐up period. Among the patients who had a Year 1 recurrence, 41% experienced a recurrence in the second calendar year following the index episode. Among those with a Year 2 recurrence, 53% went on to experience a recurrence in the third year.
Comorbidities
Identified patients had, in addition to recurrent pericarditis, a range of comorbidities. As shown in Table 3, patients also had hypertension (60%), cardiomegaly (9%), congestive heart failure (17%), atrial fibrillation (16%), autoimmune diseases (18%), diabetes mellitus (21%), renal disease (20%), anxiety (21%), and depression (14%). Among patients who had autoimmune conditions, the most common were systemic lupus, systemic sclerosis, or Sjogren syndrome and rheumatoid arthritis.
Table 3.
Comorbidities, Including Selected Autoimmune Comorbidities, n (%)
| Comorbidity | All (n=2096) |
|---|---|
| Autoimmune diseases | 18% |
| Systemic lupus, systemic sclerosis or Sjogren syndrome* | 45% |
| Rheumatoid arthritis* | 47% |
| Various necrotizing vasculopathies (eg, giant cell arteritis)* | 4% |
| Behcets* | 1% |
| Sarcoidosis* | 6% |
| Mediterranean fever* | 1% |
| Enteritis* | 9% |
| Ulcerative enterocolitis* | 12% |
| Anxiety | 21% |
| Atrial fibrillation | 16% |
| Cardiomegaly | 9% |
| Depression | 14% |
| Hypertensive diseases | 60% |
| Congestive heart failure | 17% |
| Diabetes mellitus | 21% |
| Renal diseases | 20% |
This table represents the percentage of patients with each underlying autoimmune condition out of the patients identified as having an autoimmune disease. Columns can add up to over 100%, as patients may have multiple conditions.
Complications and Procedures
Complications and procedures were relatively uncommon in the overall pericarditis population but increased in frequency among patients with RP (Table 4). In the overall pericarditis population, the most common complication was pericardial effusion (18.1%; any severity as coding does not distinguish by severity), and cardiac tamponade occurred in 5.1% of patients. Among patients with recurrences, these rates were higher: 49.7% for pericardial effusion and 8.9% for cardiac tamponade. The rates of complications were comparable among patients with and without autoimmune comorbidities.
Table 4.
Complications (2‐Year Cohort)
| Complications | Patients With Pericarditis (n=7502) | Patients With Recurrent Pericarditis (n=2096) |
|---|---|---|
| Pericardial effusion, n (%) | 1358 (18.1) | 1041 (49.7) |
| Cardiac tamponade, n (%) | 385 (5.1) | 186 (8.9) |
| Constrictive pericarditis, n (%) | 129 (1.7) | 82 (3.9) |
Pericardiectomy was performed on 1% of the 2096 patients with RP. Notably, this study found that 18% of pericardiectomy procedures performed in the overall population were associated with pericarditis. Pericardiocentesis and pericardial window were 2 clinical procedures that were used more often than pericardiectomy to treat recurrences (Table 5).
Table 5.
Procedures (2‐Year Cohort)
| Procedures | Patients With Pericarditis (n=7502) | Patients With Recurrent Pericarditis (n=2096) |
|---|---|---|
| Pericardiocentesis, n (%) | 285 (3.8) | 139 (6.6) |
| Pericardial window, n (%) | 296 (3.9) | 147 (7.0) |
| Pericardiectomy, n (%) | 37 (0.5) | 22 (1.0) |
Prescription Drug Use for RP
Commonly recognized drug treatments for RP were prescribed for over half of patients experiencing an episode: for the first episode, 54% of patients received pharmacotherapy; the proportion was 71% for a second episode, 63% for a third or fourth episode, and 56% for a fifth episode (Table 6). Patients received a variety of treatments for multiple recurrences. Colchicine was a component of treatment for 1065 patients, ie, 51% overall, and 79% of those receiving drug therapy. NSAIDs alone or in combination with corticosteroids and/or colchicine were prescribed to 833 patients (40% overall, 62% of drug‐treated patients), although additional patients may have used over‐the‐counter NSAIDs. Corticosteroids were a component of therapy for 620 patients (30% overall and 46% of drug‐treated patients). Nine percent overall received another drug, including azathioprine; none received anakinra.
Table 6.
Drug Treatment by Pericarditis Recurrence
| Treatment | Recurrent Pericarditis Episode | |||||
|---|---|---|---|---|---|---|
| 1 (n=2096) | 2 (n=994) | 3 (n=541) | 4 (n=309) | 5 (n=196) | All Episodes (n=2096) | |
| Total treated patients, n (%) | 1134 (54) | 710 (71) | 343 (63) | 196 (63) | 110 (56) | 1351 (64) |
| Corticosteroid only, n (%) | 137 (6) | 129 (13) | 63 (12) | 37 (12) | 23 (12) | 328 (16) |
| Corticosteroid+NSAID, n (%) | 39 (2) | 22 (2) | 10 (2) | 4 (1) | 3 (2) | 77 (4) |
| Colchicine only, n (%) | 258 (12) | 220 (22) | 88 (16) | 55 (18) | 28 (14) | 485 (23) |
| Colchicine+corticosteroid, n (%) | 73 (3) | 48 (5) | 25 (5) | 16 (5) | 11 (6) | 126 (6) |
| Colchicine+NSAID | 298 (14) | 75 (8) | 32 (6) | 11 (4) | 3 (2) | 365 (17) |
| Colchicine+corticosteroid+NSAID | 56 (3) | 21 (2) | 10 (2) | 3 (1) | 4 (2) | 89 (4) |
| NSAID only | 183 (9) | 93 (9) | 42 (8) | 18 (6) | 9 (5) | 302 (14) |
| Another drug* | 90 (4) | 102 (10) | 73 (13) | 52 (17) | 29 (15) | 183 (9) |
| No prescribed drug | 962 (46) | 284 (29) | 198 (37) | 113 (37) | 86 (44) | 745 (36) |
Other drugs included intravenous immunoglobulin, azathioprine, methotrexate, and cyclosporine.
The most commonly prescribed treatments for a first episode were colchicine alone (12%), or with NSAIDs (14%). Second, third, fourth, and fifth episodes were most commonly treated with colchicine alone, corticosteroids alone, or another drug (not colchicine, corticosteroid, or NSAID; included intravenous immunoglobulin, azathioprine, methotrexate, and cyclosporine). The percentage of patients receiving something other than corticosteroids, NSAIDs, or colchicine, alone or in combination, rose from 4% in the first episode up to 17% in the fourth episode and 15% in the fifth episode. In general, patients with autoimmune comorbidities were more likely to receive 1 of these different drugs (eg, intravenous immunoglobulin, azathioprine, methotrexate, and cyclosporine) than were those without autoimmune comorbidities.
Incidence and Prevalence
Among the 7502 patients with at least 2 years of continuous history following their index episode, 2241 patients had 3 years of continuous history following the index episode and were used to calculate annual disease persistence for RP of primarily “idiopathic” or post‐viral etiology. Based on the claims analysis, RP incidence was estimated at 0.001% for ages 0 to 17 years, 0.005% for ages 18 to 39 years, 0.008% for ages 40 to 64 years, and 0.01% for ages ≥65 years. Standardizing to the 2018 US Census leads to incidence estimates of 400 patients ages 0 to 17 years; 5000 patients ages 18 to 39 years; 8000 patients ages 40 to 64 years, and 6000 patients ages ≥65 years, for a total of about 20 000 patients.
To account for patients with pericarditis recurring after an index episode in the years before the study period, we used the disease persistence estimates to calculate expected recurrences from the prior 7 years. It was assumed that the incident population for each of the prior years was the same as the size of the incident population for the current year, and that recurrence rates remained constant over the analysis horizon. It was also assumed that all recurrences occurred in the successive year; thus, the calculation represents the upper bound of the RP population. The sum of ongoing patients with RP from the aforementioned cohorts in the prior 7 years is about 17 000, leading to a total estimate of 37 000 patients estimated to have RP in the United States (Table 7). This translates to an RP incidence of 6.0 (95% CI, 5.6–6.3) per 100 000 persons per year, and a prevalence of 11.2 (95% CI, 10.6–11.7) per 100 000 population.
Table 7.
Estimated Ongoing Recurrent Pericarditis Population by Annual Cohort
| Current Y | 1 Y Prior | 2 Y Prior | 3 Y Prior | 4 Y Prior | 5 Y Prior | 6 Y Prior | 7 Y Prior | |
|---|---|---|---|---|---|---|---|---|
| Current y | ≈20 000 | |||||||
| Cohort‐1 | ≈8000 | ≈20 000 | ||||||
| Cohort‐2 | ≈4000 | ≈8000 | ≈20 000 | |||||
| Cohort‐3 | ≈2000 | ≈4000 | ≈8000 | ≈20 000 | ||||
| Cohort‐4 | ≈1000 | ≈2000 | ≈4000 | ≈8000 | ≈20 000 | |||
| Cohort‐5 | <1000 | ≈1000 | ≈2000 | ≈4000 | ≈8000 | ≈20 000 | ||
| Cohort‐6 | <500 | <1000 | ≈1000 | ≈2000 | ≈4000 | ≈8000 | ≈20 000 | |
| Cohort‐7 | <500 | <500 | <1000 | ≈1000 | ≈2000 | ≈4000 | ≈8000 | ≈20 000 |
| Total | ≈17 000 | ≈17 000 | ≈16 000 |
Discussion
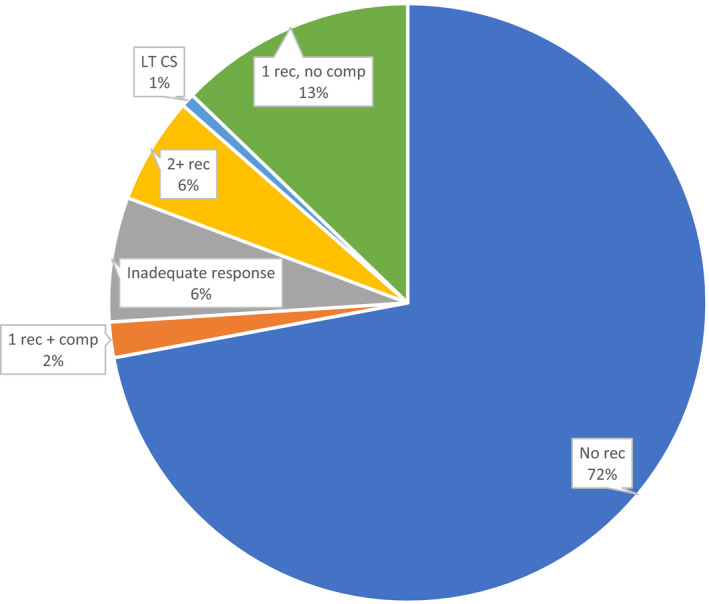
This is the first study to use a large, nationally representative data source, and broad eligibility criteria to establish the burden of RP in patients with multiple recurrences and/or complication—which accounts for a small portion of the overall acute pericarditis patient population—in the United States. Our findings are generalizable to commercially insured adults with RP, and further demonstrate that, for a subset of patients experiencing pericarditis, the disease becomes persistent and burdensome, in some cases with patients experiencing multiple recurrences for at least several years despite the use of conventional therapies. In this study, about 20% of newly diagnosed patients with pericarditis experienced a serious, and in some cases, life‐threatening, medical complication. Moreover, among patients with an index episode, 27.9% had a recurrence and/or another complication within 24 months, and 15.1% had either multiple recurrences or 1 recurrence plus a serious complication (Figure 4). This finding suggests that, while most patients experience resolution of acute pericarditis, there is a subset of patients who experience a high disease burden, possibly because of a combination of inadequate response to conventional treatments and persistent underlying disease.
Figure 4. Complications including recurrences among patients with an index pericarditis episode (n=7502). LT CS indicates long‐term corticosteroids.
Published literature generally states that 15% to 30% of patients experience recurrence within 18 months of the first acute pericarditis episode.3 The results of this US study were near the top of this range, with 27.9% of patients having a recurrence during the data collection period, with the vast majority of first recurrences within 12 months of the initial episode. The rate of second recurrences in this study was 47.4% and increased with each successive recurrence; this finding was higher than the 20% to 40% subsequent recurrence rate reported in the literature.3
In the patients with RP identified in this study, the clinical picture was heterogeneous: about 45% of patients had a single recurrence during the observation period without additional complications, but 55% had a more clinically challenging situation: inadequate treatment response/refractory status or multiple recurrences, and less commonly long‐term corticosteroid use or recurrence with a serious complication. Among patients who had autoimmune conditions, the most common were systemic lupus, systemic sclerosis, or Sjogren syndrome, and rheumatoid arthritis, which are known to be associated with an increased incidence of pericarditis.3
Overall, about one third of patients with RP are not prescribed any pharmacologic treatment, perhaps attributable to the use of over‐the‐counter anti‐inflammatory drugs and pain medication, modest expectations of patient benefit or to limited awareness of the condition and how to appropriately manage it. Lacking a defined treatment pathway, some US clinicians may choose not to prescribe a therapy without a clear benefit for a condition expected to be self‐limited. Among the two thirds of patients who do receive pharmacotherapy, treatments include colchicine, corticosteroids, NSAIDs, or other medications; as the number of recurrences increases, physicians may escalate treatment and prescribe other off‐label therapies. While some patients experience resolution of a pericarditis episode after treatment with existing options, there is a subset of patients for whom available treatments are insufficient, as evidenced by their continued recurrence and, in some cases, the need for invasive surgical procedures such as pericardiectomy or pericardial window. Although this study did not capture the specific indications for pericardiectomy, in general, it is used to manage symptoms of right‐sided heart failure in constrictive pericarditis (more common) or to relieve pain in patients with RP who do not experience symptom resolution with pharmacologic treatment (less common).3 Other patients require long‐term corticosteroid treatment, which is associated with well‐documented cardio‐renal, endocrine and infectious adverse effects, with the risk increasing with both dose and duration of therapy.11, 12 In addition to the risk of adverse events, among the cohort of patients on long‐term corticosteroid therapy, pericarditis was not well managed: the mean annual recurrence rate in this cohort was 5.7 (SD, 3.49), which was 2.6 times that of the overall recurrent pericarditis population. Likewise, the annual recurrence rate was ≥3 per year in 6.1% of patients with RP; this rate increased to 42% among those with long‐term corticosteroid use.
These outcomes suggest that patients treated long‐term with corticosteroids had more severe disease than the overall RP population. While it is possible that some patients were on long‐term corticosteroids to manage an underlying autoimmune disease rather than for pericarditis, patients in the long‐term corticosteroid cohort had to be corticosteroid‐free for at least 60 days before corticosteroid initiation, suggesting that they were not on chronic corticosteroid therapy for autoimmune diseases. The frequent use of corticosteroids in RP is of concern, even more so when taking into consideration the estimated prevalence of comorbidities, such as hypertension and diabetes mellitus, that carry increased risk of adverse effects.
Before this study, the incidence and prevalence of RP among patients with multiple recurrences and/or complication in the United States were not well established. A literature‐based calculation estimated the RP prevalence in the United States to be between 23 016 and 64 291.13 Based on our claims analysis, an incidence of ≈20 000 cases per year and a prevalence of ≈37 000 total cases is estimated in the United States. This result, which is in line with the literature‐based estimate, establishes RP as a rare condition based on Food and Drug Administration guidance.14
As noted by Brucato et al, the presumption of post‐viral etiology provides limited insight on the actual underlying pathophysiology, and may overemphasize the initial viral infection, which does not correlate to the severity of pericarditis and is not the treatment target.6 Other researchers have proposed that autoinflammation and persistent antiviral response may be contributing factors in recurrent and complicated cases.15, 16 Referring to pericarditis as idiopathic may be somewhat naïve, in that there is a growing understanding of the role of cytokine‐driven autoimmune and autoinflammatory processes in the evolution of acute and recurrent pericarditis.6, 7 The effectiveness of the recently Food and Drug Administration‐approved interleukin‐1 blocker, rilonacept, a potentially steroid‐sparing treatment option indicated for the treatment of RP and reduction in risk of recurrence in adults and pediatric patients aged ≥12 years,17 supports the putative role of the proinflammatory cytokine, interleukin‐1, in maintaining the autoinflammatory state that results from pericardial tissue damage. Blockade of the interleukin‐1 signaling pathway by rilonacept substantially reduced the risk of pericarditis recurrence by 96% (hazard ratio, 0.04; P<0.0001) and led to a rapid resolution of pericarditis episodes in a phase 3 randomized‐withdrawal trial.18
Limitations
The most significant limitations in our methods relate to the limited duration of follow‐up. We studied a defined period and required a 12‐month washout period before the first pericarditis‐related claim, and then required at least a 24‐month observation period after the index episode. While this approach helped to ensure that the index episode was a first episode rather than a recurrent episode, and to provide sufficient follow‐up to capture recurrences, the overall observation period was necessarily of limited duration. Patients with RP with multiple recurrences and/or complications may have episodes and outcomes of interest over a longer timeframe; within our observation period, the time to recurrence ranged from 4 to 104 weeks. It is possible that our findings were biased towards patients with more frequent recurrences or complications, as we would have missed those with recurrences that happened >24 months after the index episode. Nonetheless, our methods appropriately identified a population of interest: patients with pericarditis episodes occurring in rapid succession.
The 24‐month observation period also means that we were unable to identify longer‐term complications and sequelae to the events that were identified in the study. For example, constrictive pericarditis can be reversible, caused by acute inflammation of the pericardial tissue, or it can in rare cases be chronic, resulting from changes to the tissue after a prolonged period of inflammation. If patients in this study developed chronic constriction, it may have happened beyond the data collection period.3
There is also a possibility that the lack of specificity in coding may have inflated the number of distinct pericarditis episodes in some patients. There may be an insufficient distinction between new episodes versus follow‐up healthcare use for a sustained single episode. Future research to better understand the range of duration of pericarditis episodes would help to further establish the clinical, economic, and health‐related quality of life burden in patients with recurrent pericarditis. Another limitation related to coding is that NSAID usage is probably undercounted in this study because of the availability of over‐the‐counter NSAIDs that would not normally be tracked in a claims database. It is probable that some proportion of patients received NSAIDs in combination with the prescription therapies that we identified in this study. A general limitation of prescription tracking in database studies is the possibility that some patients did not fill or fully adhere to prescriptions that were captured in the database. Moreover, prescribing patterns evolve over time, and patterns seen in a study that covers a 5‐year period in the past may not fully reflect current practices.
This study focused on idiopathic and presumed post‐viral etiology, which accounts for the majority of acute pericarditis cases in the United States, but there is not a specific set of codes for idiopathic/viral etiology. The codes selected were therefore not exclusive for idiopathic and viral cases. It is possible that some of the included patients had a different etiology. Overall, 18% of patients with multiple recurrences or recurrence with a complication had autoimmune diseases, but the study did not establish whether autoimmune disorders were contributing factors to pericarditis in these patients. Also, the claims analyzed were from a commercial database and did not include US patients covered by non‐commercial plans.
Conclusions
In the United States, RP of idiopathic or post‐viral etiology has a prevalence of about 37 000 cases, of which approximately half are expected to develop a complication or require a procedure within 2 years of diagnosis. This poses a substantial clinical and likely health‐related quality of life burden to patients, some of whom experience multiple recurrences per year. Current prescription drug therapies, while effective in many patients, are inadequate in others because of incomplete symptom resolution, recurrence, and/or associated risks of potentially serious adverse effects, particularly in the case of long‐term corticosteroid use. Other patients receive no pharmacotherapy and continue to experience recurrence. This underscores the need for treatments that are proven to be safe and effective in adequately controlled clinical trials. Some patients ultimately require highly invasive procedures such as pericardiectomy, which are associated with risks and do not fully resolve symptoms in all patients. This study, to our knowledge, the first of its kind to assess the real‐world burden of RP with multiple recurrences or with complications in the United States, used a large, nationally representative data source to demonstrate that there are patients whose pericarditis is not adequately managed by existing treatment options. These findings are generalizable to commercially insured adults with RP. Recognition of these patients, who remain at risk for unresolved symptoms, complications, and/or chronic corticosteroid use, can inform risk assessment and help to identify patients with substantial, likely autoinflammatory, disease burden who may not benefit from conventional treatment approaches. Additional research is warranted to further understand the impact on patient and caregiver health‐related quality of life, healthcare resource usage, treatment patterns, and long‐term outcomes.
Sources of Funding
This project was funded by Kiniksa Pharmaceuticals, Ltd.
Disclosures
Allan Klein received research grants from Kiniksa Corporation and served as an advisory board member for Kiniksa and Sobi. Paul Cremer served as an advisory board member for Sobi. Apostolos Kontzias served as a consultant for Novartis, Horizon, Sobi, and Kiniksa. Ryan Tubman and Mike Roy are employees of Clearview Health Partners. Matthew Magestro and Michelle Lim‐Watson are employees of Kiniksa Pharmaceuticals Corporation. Muhammad Furqan has no disclosures to report.
Acknowledgments
Writing and editorial support were provided by Karen Sandman (Purple Squirrel Economics).
(J Am Heart Assoc. 2021;10:e018950. DOI: 10.1161/JAHA.120.018950.)
This work was presented at the American College of Epidemiology Annual Meeting, September 7 to 10, 2019, in Pasadena, CA.
For Sources of Funding and Disclosures, see page 11.
References
- 1.Imazio M, Gaita F. Diagnosis and treatment of pericarditis. Heart. 2015;101:1159–1168. DOI: 10.1136/heartjnl-2014-306362. [DOI] [PubMed] [Google Scholar]
- 2.Adler Y, Charron P, Imazio M, Badano L, Barón‐Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) endorsed by: the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2015;36:2921–2964. DOI: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cremer PC, Kumar A, Kontzias A, Tan CD, Rodriguez ER, Imazio M, Klein AL. Complicated pericarditis: understanding risk factors and pathophysiology to inform imaging and treatment. J Am Coll Cardiol. 2016;68:2311–2328. DOI: 10.1016/j.jacc.2016.07.785. [DOI] [PubMed] [Google Scholar]
- 4.Khandaker MH, Espinosa RE, Nishimura RA, Sinak LJ, Hayes SN, Melduni RM, Oh JK. Pericardial disease: diagnosis and management. Mayo Clin Proc. 2010;85:572–593. DOI: 10.4065/mcp.2010.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imazio M, Gaita F, LeWinter M. Evaluation and treatment of pericarditis: a systematic review. JAMA. 2015;314:1498–1506. DOI: 10.1001/jama.2015.12763. [DOI] [PubMed] [Google Scholar]
- 6.Brucato A, Imazio M, Cremer PC, Adler Y, Maisch B, Lazaros G, Gattorno M, Caforio ALP, Marcolongo R, Emmi G, et al. Recurrent pericarditis: still idiopathic? The pros and cons of a well‐honoured term. Intern Emerg Med. 2018;13:839–844. DOI: 10.1007/s11739-018-1907-x. [DOI] [PubMed] [Google Scholar]
- 7.Cantarini L, Lopalco G, Selmi C, Napodano S, De Rosa G, Caso F, Costa L, Iannone F, Rigant D. Autoimmunity and autoinflammation as the yin and yang of idiopathic recurrent acute pericarditis. Autoimmun Rev. 2015;14:90–97. DOI: 10.1016/j.autrev.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Imazio M, Bobbio M, Cecchi E, Demarie D, Pomari F, Moratti M, Ghisio A, Belli R, Trinchero R. Colchicine as first‐choice therapy for recurrent pericarditis: results of the CORE (COlchicine for REcurrent pericarditis) Trial. Arch Intern Med. 2005;165:1987–1991. DOI: 10.1001/archinte.165.17.1987. [DOI] [PubMed] [Google Scholar]
- 9.Imazio M, Brucato A, Cemin R, Ferrua S, Maggiolini S, Beqaraj F, Demarie D, Forno D, Ferro S, Maestroni S, et al. A randomized trial of colchicine for acute pericarditis. N Engl J Med. 2013;369:1522–1528. DOI: 10.1056/NEJMoa1208536. [DOI] [PubMed] [Google Scholar]
- 10.LeWinter M, Kontzias A, Lin D, Cella D, DerSarkissian M, Zhou M, Duh MS, Lim‐Watson M, Magestr M. Burden of recurrent pericarditis on health‐related quality of life. Am J Cardiol. 2021;141:113–119. DOI: 10.1016/j.amjcard.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Dauphin C, Merlin E, Chalard A. Recurrent pericarditis: current challenges and future prospects. Res Rep Clin Cardiol. 2016;7:99–108. [Google Scholar]
- 12.Roxane Laboratories, Inc. PredniSONEReform prescribing information. November 2012. [Google Scholar]
- 13.Kiniksa Pharmaceuticals, Ltd . Orphan Drug Designation Amendment Application for Rilonacept. Lexington, MA, 9 September 2019. [Google Scholar]
- 14.US FDA . Orphan drugs. Available at: https://www.fda.gov/media/83372/download. Accessed February 6, 2020. [Google Scholar]
- 15.Pankuweit S, Stein A, Karatolios K, Richter A, Ruppert V, Maisch B. Viral genomes in the pericardial fluid and in peri‐ and epicardial biopsies from a German cohort of patients with large to moderate pericardial effusions. Heart Fail Rev. 2013;18:329–336. DOI: 10.1007/s10741-013-9375-x. [DOI] [PubMed] [Google Scholar]
- 16.Maisch B, Rupp H, Ristic A, Pankuweit S. Pericardioscopy and epi‐ and pericardial biopsy—a new window to the heart improving etiological diagnoses and permitting targeted intrapericardial therapy. Heart Fail Rev. 2013;18:317–328. DOI: 10.1007/s10741-013-9382-y. [DOI] [PubMed] [Google Scholar]
- 17.Arcalyst (Rilonacept) Prescribing Information. London, UK: Kiniksa Pharmaceuticals (UK), Ltd.; 2021. [Google Scholar]
- 18.Klein AL, Imazio M, Cremer P, Brucato A, Abbate A, Fang F, Insalaco A, LeWinter M, Lewis BS, Lin D, et al. Phase 3 trial of interleukin‐1 trap rilonacept in recurrent pericarditis. N Engl J Med. 2021;384:31–41. DOI: 10.1056/NEJMoa2027892. [DOI] [PubMed] [Google Scholar]


