Abstract
Background
Prenatal diagnosis of congenital heart disease has been associated with early‐term delivery and cesarean delivery (CD). We implemented a multi‐institutional standardized clinical assessment and management plan (SCAMP) through the University of California Fetal‐Maternal Consortium. Our objective was to decrease early‐term (37–39 weeks) delivery and CD in pregnancies complicated by fetal congenital heart disease using a SCAMP methodology to improve practice in a high‐risk and clinically complex setting.
Methods and Results
University of California Fetal‐Maternal Consortium site‐specific management decisions were queried following SCAMP implementation. This contemporary intervention group was compared with a University of California Fetal‐Maternal Consortium historical cohort. Primary outcomes were early‐term delivery and CD. A total of 496 maternal–fetal dyads with prenatally diagnosed congenital heart disease were identified, 185 and 311 in the historical and intervention cohorts, respectively. Recommendation for later delivery resulted in a later gestational age at delivery (38.9 versus 38.1 weeks, P=0.01). After adjusting for maternal age and site, historical controls were more likely to have a CD (odds ratio [OR],1.8; 95% CI, 2.1–2.8; P=0.004) and more likely (OR, 2.1; 95% CI, 1.4–3.3) to have an early‐term delivery than the intervention group. Vaginal delivery was recommended in 77% of the cohort, resulting in 61% vaginal deliveries versus 50% in the control cohort (P=0.03). Among pregnancies with major cardiac lesions (n=373), vaginal birth increased from 51% to 64% (P=0.008) and deliveries ≥39 weeks increased from 33% to 48% (P=0.004).
Conclusions
Implementation of a SCAMP decreased the rate of early‐term deliveries and CD for prenatal congenital heart disease. Development of clinical pathways may help standardize care, decrease maternal risk secondary to CD, improve neonatal outcomes, and reduce healthcare costs.
Keywords: cesarean, fetal CHD, obstetrics, prenatal congenital heart disease, SCAMP
Subject Categories: Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- ANT
antenatal testing
- CD
cesarean delivery
- IOL
induction of labor
- SCAMP
standardized clinical assessment and management plan
- UCfC
University of California Fetal Consortium
Clinical Perspective
What Is New?
Implementing a standardized clinical assessment and management plan for prenatal congenital heart disease focused on vaginal birth and term delivery (>39 weeks' gestation) lowered cesarean delivery and early‐term (37 0/7–38 6/7 weeks') delivery across diverse multi‐institutional referral hospitals.
Both cesarean delivery and early‐term delivery are associated with adverse maternal and neonatal outcomes and increased hospital costs.
What Are the Clinical Implications?
Standardized clinical assessment and management plan methodology can improve clinical practice in a high‐risk and clinically complex setting, including pregnancy complicated by prenatally diagnosed congenital heart disease.
Congenital heart disease (CHD) is the most common cause of congenital malformations occurring in ~1% of newborns and is a significant cause of infant morbidity and mortality.1, 2 The prenatal diagnosis and the survival of neonates with CHD has greatly improved over the past decades.3 However, the rate of detection of prenatal CHD has remained low.4 Today, prenatal detection of CHD occurs through clinical risk assessment and standard of care 2‐dimensional Doppler ultrasounds and echocardiography. Prenatal detection of critical or complex CHD reduces morbidity (Morris et al 2014, Tworetzky et al 2001) and facilitates delivery coordination and surgical planning.5 Unfortunately, a prenatal CHD diagnosis has also been associated with lower birth weights and earlier gestational age at birth,6, 7 which have been linked to decreased survival and reduced neurodevelopmental outcomes in these neonates.8, 9 Our initial work through the University of California Fetal Consortium (UCfC) found similar results with prenatally diagnosed infants being born earlier. In addition, we identified a higher rate of cesarean deliveries (CD) in women whose fetus had a prenatal diagnosis of CHD10 without a clear clinical indication. Thus, there is a need to provide quality improvement interventions in routine clinical practice in order to maximize maternal and neonatal outcomes in this population.
To build upon our previous work, the UCfC sought to identify the triggers that lead to increased early‐term delivery and CD across a large multicenter cohort using clinical registry data. standardized clinical assessment and management plans (SCAMPs) are a quality improvement initiative designed to eliminate unnecessary resource utilization, decrease practice variation, and improve patient outcomes.11 SCAMPs are assessment and management algorithms associated with incremental improvements in healthcare delivery that promote high‐quality care in a heterogenous patient populations, as in our cohort.11, 12 Unlike a clinical practice guideline, SCAMPs allow for iterative improvements to the algorithms as they relate to diversions and outcome data. We aimed to develop and implement a multi‐institutional fetal CHD SCAMP across the UCfC to decrease the number of early‐term deliveries and the number of CDs in women with a prenatal diagnosis of fetal CHD.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. The UCfC comprises the 5 University of California campuses affiliated with university medical centers that offer prenatal diagnosis and treatment (UCfC: UC Davis, UC Irvine, UC Los Angeles, UC San Diego, and UC San Francisco). The UCfC was established to better study pregnancies affected with maternal and fetal diseases, including CHD and to define treatment practices within our health system. This multi‐institution consortium developed a SCAMP for pregnant women with a prenatal diagnosis of fetal CHD, based on current practices and best available evidence. Suggested SCAMP guidelines for delivery timing and mode were made and included (1) routine delivery and/or induction at ≥ 39 0/7 weeks (earlier if required for any other medically indicated obstetrical reason) and (2) planned vaginal delivery with CD only for obstetrical indications (consider CD for a fetus with complete heart block or if delivery coordination is needed for CHD lesions with expected hemodynamic instability at the time of placental separation and requires immediate intervention). Women carrying a singleton fetus with a diagnosis of CHD starting at 32 weeks' gestation with plans to deliver at 1 of the 5 UCfC sites during the study period of May 2018 to December 2019 were included as the intervention cohort. A preimplementation cohort from a previous study of similar women served as the historical control cohort with an enrollment period of 2011 to 2013.10
A multi‐institutional retrospective study review board reliance registry provided approval for the study (institutional review board #10‐04093) and informed consent was waived. Patients were identified and a delivery planning worksheet (Data S1) that included the recommendations was reviewed and completed at the time of the maternal fetal medicine visit in the third trimester. The delivery planning worksheet included estimated gestational age, CHD diagnosis, CHD diagnostic category, the presence of a known genetic abnormality or extracardiac anomaly, genetic counseling, and whether antenatal testing (ANT) was planned and if so, what testing would be done, at what gestational age, and at what frequency. The form included questions such as whether or not delivery was planned for <39 weeks and if so, the reason, as well as the planned mode of delivery and the reason for CD. The worksheet was primarily completed by the maternal fetal medicine physician seeing the patient that day.
Before implementation (rollout) of the SCAMP, representative maternal fetal medicine physicians and pediatric cardiologists at each of the sites provided feedback on the form to ensure relevance and feasibility at their institution. When site champions from both specialties at all intuitions agreed, the SCAMP was rolled out and updates on progress were discussed quarterly with all site champions. How each site collected and ensured that the SCAMP was followed was left to the discretion of the site champions (maternal fetal medicine and pediatric cardiology). The project champions (Y. A. and W. H.) sent regular updates and reminders to all team members. The SCAMP was modified to include elements of ANT and genetic counseling after the data were reviewed in year 1.
All fetal CHD lesions were included to encompass minor and major fetal CHD. The CHD lesions were categorized based on predicted risk of hemodynamic instability in the delivery room or first days of life, based on the American Heart Association guidelines13 and included type (1) CHD without predicted risk of hemodynamic instability in the delivery room or first days of life (ventricular septal defects, complete atrioventricular canal), (2) CHD with minimal risk of hemodynamic instability in the delivery room but requires postnatal catheterization/surgery (ductal‐dependent lesions), (3) CHD with likely hemodynamic instability in the delivery room requiring immediate specialty care for stabilization (d‐transposition of the great arteries), and (4) CHD with expected hemodynamic instability with placental separation requiring immediate catheterization/surgery to improve survival (hypoplastic left heart syndrome with restrictive atrial septum, obstructed total anomalous pulmonary venous return).
Maternal and neonatal data were gathered by chart review after delivery at each site directly. All data were collected and stored in a Health Insurance Portability and Accountability Act‐compliant database through Research Electronic Data Capture.14 Maternal fetal medicine physicians and pediatric cardiologists at each site were asked to provide information regarding site‐specific standard management practices at their site. In order to preserve site confidentiality, sites were de‐identified in the results. Data were chart abstracted for review. The primary outcomes were delivery <39 weeks and CD.
Statistical Analysis
Outcomes were compared between the contemporary intervention cohort (2018–2019) and the historical control cohort (2011–2013). Because the historical control cohort included only cases of CHD that required surgical intervention within 30 days of delivery, a subanalysis of outcomes was performed that included only the cases of major CHD (type 2–4) from both cohorts. Associations between categorical variables were assessed with chi‐square tests (or Fisher's exact) and t tests (or Wilcoxon rank‐sum) for continuous variables. Multivariable analyses were conducted with logistic regressions on the 2 primary outcomes, controlling for variables significant different between the intervention and control cohorts. Outcomes were also compared within each of the 5 sites. All tests were 2 sided with an alpha of 0.05 for statistical significance. All analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
We identified 496 maternal–fetal dyads with prenatally diagnosed fetal CHD identified, 185 in the historical control cohort and 311 in the contemporary intervention cohort. The control group was younger (≥35: 32% versus 42%, P=0.0001) and more likely to come from sites 4 and 5 (82% versus 53%, P<0.0001) (Table 1).
Table 1.
Characteristics by Cohort (N=496)
| Total | Historical Control Cohort | Intervention Cohort | P Value | |
|---|---|---|---|---|
| N=496 | n=185 | n=311 | ||
| % (n) | % (n) | % (n) | ||
| Maternal age, y, median (IQR) | 32 (28–37) | 32 (26–36) | 33 (28–37) | 0.004* |
| ≤20 | 4% (18) | 8% (15) | 1% (3) | 0.0001* |
| 21–34 | 58% (278) | 60% (110) | 57% (168) | |
| ≥35 | 38% (181) | 32% (59) | 42% (122) | |
| Gravidity, median (IQR) | 2 (1, 3) | 2 (1, 4) | 2 (1, 3) | 0.604* |
| Parity, median (IQR) | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) | 0.201* |
| 0 | 41% (193) | 46% (84) | 37% (109) | 0.224 |
| 1 | 28% (133) | 24% (43) | 31% (90) | |
| 2 | 18% (88) | 18% (32) | 19% (56) | |
| ≥3 | 13% (62) | 12% (24) | 13% (38) | |
| Site/Campus | ||||
| Site 1 | 13% (67) | 9% (16) | 16% (51) | <0.0001 |
| Site 2 | 12% (59) | 4% (8) | 16% (51) | |
| Site 3 | 11% (53) | 5% (9) | 15% (44) | |
| Site 4 | 37% (182) | 41% (76) | 34% (106) | |
| Site 5 | 27% (135) | 41% (76) | 19% (59) | |
Abbreviation: IQR indicates interquartile range.
Wilcoxon rank‐sum or Fisher's exact test.
Among the intervention cohort, 98% resulted in a live birth. Of CHD types, 35% were low risk CHD (type 1) and 3% unknown (Table 2). The majority (62%) were considered major CHD lesions—49% type 2, minimal risk of hemodynamic instability: 8% type 3, likely risk of hemodynamic instability, and 5% type 4, expected risk of hemodynamic instability. An extracardiac anomaly was present in 21% of the cases and known genetic abnormality in 11% of the cases.
Table 2.
SCAMP Implementation Characteristics (n=311)
| Intervention Cohort n=311; % (n) | |
|---|---|
| CHD category | |
| Low risk | 35% (110) |
| High risk | |
| Minimal risk of hemodynamic instability | 49% (153) |
| Likely hemodynamic instability | 8% (26) |
| Expected hemodynamic instability | 5% (14) |
| Unknown | 3% (8) |
| Genetic counseling recommended (n=293) | |
| Yes | 80% (234) |
| No | 20% (59) |
| Known genetic abnormality (n=297) | |
| Yes | 11% (32) |
| No | 89% (265) |
| Presence of extracardiac anomaly (n=297) | |
| Yes | 21% (62) |
| No | 79% (235) |
| Genetic counseling recommended after CHD diagnosis (n=211) | |
| Yes | 87% (183) |
| No | 13% (28) |
| Planned antenatal testing (n=293) | |
| Yes | 80% (234) |
| No | 20% (59) |
| Timing of gestational age initiated antenatal testing (n=232) | |
| ≤32 wk | 68% (157) |
| 33–34 wk | 20% (47) |
| >34 wk | 12% (28) |
| Frequency of antenatal testing (n=234) | |
| Twice weekly | 67% (157) |
| Weekly | 32% (75) |
| Other | 1% (2) |
| Reason for antenatal testing (n=231) | |
| Fetal cardiac disease only | 55% (128) |
| Other indication only | 14% (32) |
| Both cardiac disease and other indication | 31% (71) |
| Mode or timing of delivery change based on antenatal testing (n=231) | |
| Yes | 11% (25) |
| No | 89% (206) |
| Live birth (n=301) | |
| Yes | 98% (296) |
| No | 2% (5) |
CHD indicates congenital heart disease; and SCAMP, standardized clinical assessment and management plan.
Adherence to the SCAMP in the intervention cohort was good. Notably, genetic counseling was routinely recommended in this population as wasANT (both 80%). Among those who underwent ANT, 68% started at 32 weeks' gestation or less and 12% after 34 weeks. 67% were recommended twice weekly ANT. Abnormal ANT led to a modification in intended delivery plan in 11% (n=25) (Table 2).
Planned delivery at ≥39 weeks was 88% but ultimately only 48% of the cases delivered at a gestational age of ≥39 weeks (Figure 1). The remaining 158 (52%) of deliveries occurred at <39 weeks' gestation for the following indications: 58 (37%) because of spontaneous labor at the center, 49 (31%) maternal indication, 39 (25%) noncardiac fetal indication, such as nonreassuring fetal heart rate tracing and fetal growth restriction, and 12 (7%) for other reasons. The other indications for delivery before 39 weeks' gestation included “lives too far away for to allow spontaneous labor” in 4 cases, maternal request because of poor prognosis in 3 cases (including 2 with Trisomy 18), coordinated delivery required for CHD diagnosis in 3 cases (including hypoplastic left heart syndrome with a restrictive atrial septum), maternal chronic pain syndrome in 1 case, and undocumented reason in 1 case. Notably, inductions occurring at <39 weeks for obstetrical maternal indications included hypertensive diseases of pregnancy (gestational hypertension, preeclampsia, and hemolysis elevated liver enzymes low platelets) and were not associated with type of cardiac lesion (P=0.32, Table S1). Similarly, fetal growth restriction inductions were not associated with CHD lesion (P=0.89, Table S2) and coordinated to optimize medically indicated early‐term delivery.
Figure 1. Standardized clinical assessment and management pathway (SCAMP) for fetal congenital heart disease (CHD) decreased early‐term delivery and cesarean delivery.
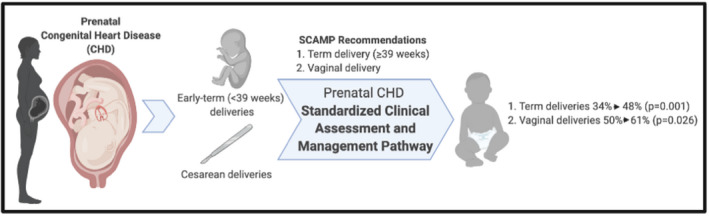
Among the intervention cohort, vaginal delivery was recommended in 234 (77%) of the cases and occurred in 185 (61%) of cases. Of those that underwent vaginal delivery, 72 (39%) awaited spontaneous labor and 112 (61%) underwent induction (Table 3). The remaining 23% of the cases were recommended to undergo a planned CD.
Table 3.
Planned and Actual Delivery Characteristics by Cohort (N=496)
| Total | Historical control cohort | Intervention cohort† | P value | |
|---|---|---|---|---|
| N=496 | n=185 | n=311 | ||
| % (n) | % (n) | % (n) | ||
| Planned mode of delivery* | ||||
| Vaginal delivery | 75% (363) | 71% (129) | 77% (234) | 0.162 |
| Spontaneous labor | 21% (101) | 24% (44) | 19% (57) | |
| Induction of labor | 54% (262) | 47% (85) | 58% (177) | |
| Cesarean delivery | 25% (122) | 29% (52) | 23% (70) | |
| Actual mode of delivery | ||||
| Vaginal delivery | 57% (276) | 50% (91) | 61% (185) | 0.026 |
| Cesarean delivery | 43% (210) | 50% (90) | 39% (120) | |
| Planned gestational age at delivery | ||||
| <39 wk | 21% (101) | 37% (66) | 12% (35) | <0.0001 |
| ≥39 wk | 79% (373) | 63% (114) | 88% (259) | |
| Actual gestational age at delivery, median (IQR) | 38.4 (37.0, 39.1) | 38.1 (37.0, 39.0) | 38.9 (37.1, 39.1) | 0.011‡ |
| <39 wk | 57% (280) | 67% (122) | 52% (158) | 0.001 |
| ≥39 wk | 43% (207) | 34% (60) | 48% (147) | |
Vaginal delivery includes spontaneous labor and induction of labor. No difference between spontaneous labor and induction of labor (P=0.055).
When sum is <311, clinical observation missing.
Wilcoxon rank‐sum or Fisher's exact test.
There was a total of 185 vaginal deliveries (61%) and 120 CDs (39%). Reasons for planned CD included repeat CD 27.5% (n=33), malpresentation 15% (n=18), arrest of dilation 6.7% (n=8), arrest of descent 3.3% (n=4), nonreassuring fetal heart tracing 30.8% (n=37), fetus with complete heart block and inability to monitor during labor in 5% (n=6), coordination of delivery needed owing to CHD diagnosis in 3.3% (n=4), and other in 5% (n=6). Induction of labor (IOL) occurred in 58% of pregnancies and resulted in CD in 32% of cases.
Of those who underwent vaginal delivery, 72 (39%) had spontaneous labor and 112 (61%) underwent IOL. Of those with planned IOL, 38 (32%) had a CD. The reasons for this included 2 because of malpresentation, 10 arrest of dilation or descent, 24 r nonreassuring fetal status, 1 living too far away from the delivery center, and 1 complete heart block in the fetus. The remaining 70 (23%) of the cases were recommended to undergo a planned CD, nearly all of whom had a prior CD delivery.
When comparing the cohorts, 88% in the intervention cohort were recommended delivery ≥39 weeks versus 63% in the control cohort (P<0.0001) (Table 3). The recommendation for 39‐week delivery resulted in a higher median gestational age at delivery in the intervention cohort (38.9 [interquartile range 37.1–39.1] versus 38.1 [interquartile range 37.0–39.0] weeks, P=0.01). More vaginal deliveries were also observed in the intervention group than the historical cohort (61% versus 50%, P=0.03, Figure 1). After adjusting for maternal age and site, historical controls were 1.8 times more likely to have a CD (odds ratio [OR], 1.8; 95% CI, 2.1–2.8; P=0.004) than the intervention group and 2.1 times more likely (OR, 2.1; 95% CI, 1.4–3.3) to have an early‐term delivery than the intervention group (Table 4).
Table 4.
Logistic Regressions for Mode of Delivery and Gestational Age at Delivery
|
Unadjusted Model N=487 |
Adjusted Model N=468 |
|||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Cesarean delivery | ||||
| Historical control | 1.5 (1.0–2.2) | 0.0259 | 1.8 (1.2–2.8) | 0.004 |
| Maternal age, y | ||||
| 21–34 | 1.2 (0.4–3.2) | 0.6583 | ||
| ≥35 | 1.4 (0.5–3.8) | |||
| Site | ||||
| Site 1 | 1.6 (0.9–3.0) | 0.007 | ||
| Site 2 | 3.4 (1.7–6.8) | |||
| Site 3 | 2.1 (1.1–3.9) | |||
| Site 5 | 1.7 (1.0–2.7) | |||
| Gestational age, <39 wk | ||||
| Historical control | 1.9 (1.3–2.8) | 0.0011 | 2.1 (1.4–3.3) | 0.0003 |
| Maternal age, y | ||||
| 21–34 | 1.8 (0.7–4.8) | 0.172 | ||
| ≥35 | 2.3 (0.8–6.4) | |||
| Site | ||||
| Site 1 | 1.1 (0.6–2.0) | 0.239 | ||
| Site 2 | 1.7 (0.8–3.4) | |||
| Site 3 | 0.6 (0.3–1.2) | |||
| Site 5 | 0.9 (0.6–1.4) | |||
OR indicates odds ratio.
Full model: adjusting for maternal age (referent: ≤20 years), and site (referent: Site 4).
Among pregnancies with major cardiac lesions (type 2, 3, 4) (n=374), vaginal birth increased from 51% to 64% with SCAMP (P=0.008) and those delivering ≥39 weeks increased from 33% to 48% (P=0.004) (Table 5).
Table 5.
Mode of Delivery and Gestational Age at Delivery Among High‐Risk CHD* (N=374)
| Total High‐Risk Cohort | Historical Control High‐Risk Cohort | Intervention High‐Risk Cohort | P Value | |
|---|---|---|---|---|
| N=374 | n=181 | n=193 | ||
| % (n) | % (n) | % (n) | ||
| Actual delivery mode | ||||
| Vaginal birth | 58% (215) | 51% (91) | 64% (124) | 0.008 |
| Cesarean delivery | 42% (158) | 49% (89) | 36% (69) | |
| Gestational age at delivery | ||||
| <39 wk | 59% (222) | 67% (121) | 52% (101) | 0.004 |
| ≥39 wk | 41% (152) | 33% (60) | 48% (92) | |
CHD indicates congenital heart disease.
High risk defined as ductal‐dependent fetal heart disease and fetal heart disease potentially needing or expected to need invasive cardiac intervention in the immediate neonatal period.
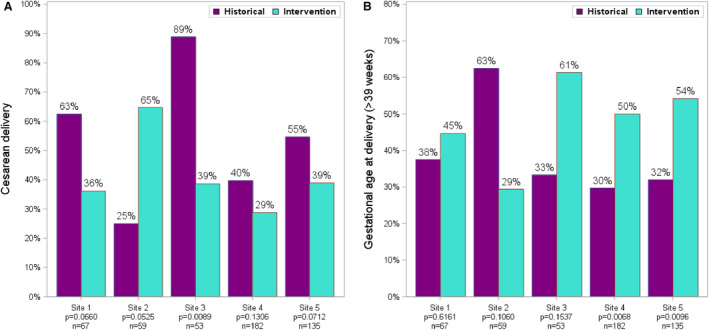
We also assessed site‐specific adherence to the SCAMP recommendations at each of the 5 sites individually by comparing gestational age and mode of delivery with both historical and intervention groups. At the 2 highest volume sites there was a significant increase in delivery ≥39 weeks, but the significance of CD was seen only in the aggregate data not at each site when we compared mode of delivery and gestational age of delivery with both historical and the intervention group (Figure 2).
Figure 2. Cesarean delivery changes by site between historical and intervention cohorts (A) and term delivery by site between historical and interventional cohorts (B).
DISCUSSION
We demonstrate that the implementation of a fetal CHD SCAMP in a multi‐institutional cohort was associated with a significant decrease in the rate of early‐term (<39 weeks) deliveries and CD for cases of prenatally suspected major and minor fetal CHD. We report on a group of patients with fetal CHD from throughout the state of California across multiple University of California academic medical centers who historically delivered suspected fetal CHD cases earlier and via CD more often and at a higher rate than would be expected for fetuses without CHD.
Development of clinical pathways incorporating these practices may help standardize care, decrease maternal risk secondary to CD, may improve neonatal outcomes, and reduce healthcare costs associated with delivery for both mother and newborn. Birth weight and gestational age at delivery have been reported to be inversely associated with hospital length of stay and perioperative complications8; better neurologic outcomes are seen in more mature infants as well. In our contemporary cohort, only 12% of deliveries were planned to deliver <39 weeks' gestation and the majority of those who delivered early were becaise pf spontaneous labor or a maternal indication for earlier delivery. The rate of CDs in our contemporary cohort also decreased, with the majority undergoing CD owing to obstetrical indications, such as repeat CD and malpresentation and unrelated to fetal CHD, with only 3.3% because of the need for a coordinated delivery because of CHD. Furthermore, the rate of CD was significantly lower in the groups with major fetal CHD compared with the historical cohort (36% versus 49%, P=0.008). It has been well documented that mode of delivery itself in fetal CHD does not improve neonatal outcome.15, 16 Both spontaneous labor and induction of labor for a trial of labor are safe for fetal CHD.10 Thus, normal spontaneous vaginal delivery or IOL is safe and the preferred mode of delivery. CD is well known to be associated with increased short‐ and long‐term maternal morbidities17 including adverse future reproductive outcomes such as invasive placental disease and uterine rupture.
Delivery planning for this complex group of patients is likely influenced by the goal of ensuring that the neonate is located at, or in close proximity to, a center that can perform specific neonatal cardiac interventions. In the state of California, many patients travel long distances to obtain care in tertiary level medical centers such as our UC medical centers and affiliated hospitals. Thus, planning IOL at 39 weeks' gestation allows for controlled‐term delivery near a tertiary center. In our cohort, IOL was planned in 58% of prenatally diagnosed mothers and carried out in 35% of the SCAMP cohort; delivery was still at the tertiary center in nearly all. Our data demonstrate that 38 (32%) of the planned for IOLs had a CD, with the majority (63%) of those owing to nonreassuring fetal status.
Cesarean birth is associated with a longer length of stay and higher hospital costs.17 Delivery mode may affect total length of hospital stay for the neonate requiring cardiac surgery as well. This was demonstrated in the previous paper from the consortium10 that found there was a trend toward shorter length of stay in neonates born by vaginal delivery compared with those born by CD. We therefore speculate that investing in temporary relocation of some mothers to within close proximity of tertiary centers at least 1 week before the estimated date of delivery and allowing for spontaneous onset of labor rather than IOL or planned CD may be more cost effective and beneficial to both the mother and fetus.
Because this was a large multicenter quality improvement effort, there are some limitations that result from multicenter efforts including variability in practice patterns that may not be accounted for. Additionally, the delivery care recommendations were suggested but with no obligation for adherence. At these large centers, there are multiple teams of providers and those making the recommendations may not always be those managing the patient at delivery. Although we adjusted for fetal CHD category, there may have been differences in CHD diagnosis severity or illness severity would not be accounted for using this methodology. In addition, it is plausible that the increased prevalence of extracardiac anomalies, which could imply syndromic etiology to fetal CHD, could influence delivery outcomes and the slightly higher prevalence of genetic anomalies influence our outcomes. Another limitation in our study was the gap between the historical cohort and the onset of the SCAMP. Though the SCAMP was incorporated in 2018, it is highly probable that we started changing practice in a nonuniform way between the publication of the historical cohort and the introduction of the SCAMP.
A larger sample size is needed to assess whether these factors play a significant role in maternal–neonatal outcomes in the context of fetal CHD. Our data reflect perinatal outcomes among patients diagnosed and treated at tertiary medical centers and thus may not be applicable to practices in a community‐based model. The current analysis focuses on live born infants with CHD and did not include an analysis of planned or actual delivery patterns among those with a fetal or perinatal demise. We also did not include a contemporary cohort of postnatally diagnosed neonates with CHD and therefore cannot speak to whether our observed rates of CD and delivery <39 weeks, which are still higher than what would be expected for the general population, might be further modifiable using a more rigorous and proscriptive SCAMP methodology. Interestingly, in over 10% of cases, delivery timing or mode were modified as a result of ANT. We find this intriguing and suspect it may be a factor leading to a portion of the identified early‐term delivery or CD, but this would need to be studied on a larger scale and is the basis for SCAMP modification and future focus, as SCAMPs allow for iterative improvements to the algorithms as they relate to diversions and outcome data. What remains uncertain is the exact utility of ANT in the setting of fetal CHD. We noted significant variability in the gestational age in which ANT is performed, as well as the frequency of surveillance. Future efforts of the working group hope to standardize ANT across the sites and incorporate that into the delivery care pathway in an effort to achieve improved data‐driven care.
There is ample evidence in the literature to conclude that by decreasing early‐term delivery and CD, both maternal and neonatal outcomes will improve and hospital costs and resource use will decrease. The UCfC is currently investigating these outcomes in the CHD cohort. Additionally, it is likely that socioeconomic status plays a role in both pregnancy and neonatal outcomes in this cohort, which was not collected for this analysis. Future studies assessing the impact of socioeconomic status on pregnancy and neonatal outcomes in the context of CHD will be performed.
CONCLUSIONS
Implementation of a fetal CHD delivery planning SCAMP in a multi‐institutional cohort significantly decreased the rate of early‐term deliveries and CD for fetal CHD. Development of clinical pathways incorporating best practices may help standardize care, decrease maternal risk secondary to CD, may improve neonatal outcomes, and reduce healthcare costs associated with perinatal management of pregnancies complicated by fetal CHD.
Sources of Funding
Y. Afshar is supported by the Reproductive Scientist Development Program (RSDP) by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K12 HD000849) and the American College of Obstetricians and Gynecologists, as part of the RSDP.
Disclosures
None.
Supporting information
Data S1
Tables S1–S2
Acknowledgments
We thank present and past members of the University of California Fetal–Maternal Consortium. Figure 1 created with BioRender.com
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021598
For Sources of Funding and Disclosures, see page 9.
References
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. DOI: 10.1016/S0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.van der Linde D , Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos‐Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta‐analysis. J Am Coll Cardiol. 2011;58:2241–2247. DOI: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 3.Khoshnood B, De Vigan C, Vodovar V, Goujard J, Lhomme A, Bonnet D, Goffinet F. Trends in prenatal diagnosis, pregnancy termination, and perinatal mortality of newborns with congenital heart disease in France, 1983–2000: a population‐based evaluation. Pediatrics. 2005;115:95–101. DOI: 10.1542/peds.2004-0516. [DOI] [PubMed] [Google Scholar]
- 4.Quartermain MD, Pasquali SK, Hill KD, Goldberg DJ, Huhta JC, Jacobs JP, Jacobs ML, Kim S, Ungerleider RM. Variation in prenatal diagnosis of congenital heart disease in infants. Pediatrics. 2015;136:e378–e385. DOI: 10.1542/peds.2014-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinto NM, Morris SA, Moon‐Grady AJ, Donofrio MT. Prenatal cardiac care: goals, priorities & gaps in knowledge in fetal cardiovascular disease: perspectives of the Fetal Heart Society. Prog Pediatr Cardiol. 2020;59: 101312. DOI: 10.1016/j.ppedcard.2020.101312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kipps AK, Feuille C, Azakie A, Hoffman JI, Tabbutt S, Brook MM, Moon‐Grady AJ. Prenatal diagnosis of hypoplastic left heart syndrome in current era. Am J Cardiol. 2011;108:421–427. DOI: 10.1016/j.amjcard.2011.03.065. [DOI] [PubMed] [Google Scholar]
- 7.Levy DJ, Pretorius DH, Rothman A, Gonzales M, Rao C, Nunes ME, Bendelstein J, Mehalek K, Thomas A, Nehlsen C, et al. Improved prenatal detection of congenital heart disease in an integrated health care system. Pediatr Cardiol. 2013;34:670–679. DOI: 10.1007/s00246-012-0526-y. [DOI] [PubMed] [Google Scholar]
- 8.Costello JM, Pasquali SK, Jacobs JP, He X, Hill KD, Cooper DS, Backer CL, Jacobs ML. Gestational age at birth and outcomes after neonatal cardiac surgery: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Circulation. 2014;129:2511–2517. DOI: 10.1161/CIRCULATIONAHA.113.005864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goff DA, Luan X, Gerdes M, Bernbaum J, D'Agostino JA, Rychik J, Wernovsky G, Licht DJ, Nicolson SC, Clancy RR, et al. Younger gestational age is associated with worse neurodevelopmental outcomes after cardiac surgery in infancy. J Thorac Cardiovasc Surg. 2012;143:535–542. DOI: 10.1016/j.jtcvs.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peyvandi S, Nguyen TATT, Almeida‐Jones M, Boe N, Rhee L, Anton T, Sklansky M, Tarsa M, Satou G, Moon‐Grady AJ. Timing and mode of delivery in prenatally diagnosed congenital heart disease‐ an analysis of practices within the University of California Fetal Consortium (UCfC). Pediatr Cardiol. 2017;38:588–595. DOI: 10.1007/s00246-016-1552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rathod RH, Farias M, Friedman KG, Graham D, Fulton DR, Newburger JW, Colan S, Jenkins K, Lock JE. A novel approach to gathering and acting on relevant clinical information: SCAMPs. Congenit Heart Dis. 2010;5:343–353. DOI: 10.1111/j.1747-0803.2010.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farias M, Jenkins K, Lock J, Rathod R, Newburger J, Bates DW, Safran DG, Friedman K, Greenberg J. Standardized clinical assessment and management plans (SCAMPs) provide a better alternative to clinical practice guidelines. Health Aff. 2013;32:911–920. DOI: 10.1377/hlthaff.2012.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donofrio MT, Moon‐Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, Cuneo BF, Huhta JC, Jonas RA, Krishnan A, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129:2183–2242. DOI: 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. DOI: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parikh LI, Grantz KL, Iqbal SN, Huang CC, Landy HJ, Fries MH, Reddy UM. Neonatal outcomes in fetuses with cardiac anomalies and the impact of delivery route. Am J Obstet Gynecol. 2017;217:469.e1–469.e12. DOI: 10.1016/j.ajog.2017.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trento LU, Pruetz JD, Chang RK, Detterich J, Sklansky MS. Prenatal diagnosis of congenital heart disease: impact of mode of delivery on neonatal outcome. Prenat Diagn. 2012;32:1250–1255. DOI: 10.1002/pd.3991. [DOI] [PubMed] [Google Scholar]
- 17.American College of Obstetricians, Gynecologists, Society for Maternal‐Fetal Medicine , Caughey AB, Cahill AG, Guise JM, Rouse DJ. Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210:179–193. DOI: 10.1016/j.ajog.2014.01.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S2


