Abstract
Background
There is a lack of contemporary data on cardiogenic shock (CS) in‐hospital mortality trends.
Methods and Results
Patients with CS admitted January 1, 2004 to December 31, 2018, were identified from the US National Inpatient Sample. We reported the crude and adjusted trends of in‐hospital mortality among the overall population and selected subgroups. Among a total of 563 949 644 hospitalizations during the period from January 1, 2004, to December 30, 2018, 1 254 358 (0.2%) were attributed to CS. There has been a steady increase in hospitalizations attributed to CS from 122 per 100 000 hospitalizations in 2004 to 408 per 100 000 hospitalizations in 2018 (P trend<0.001). This was associated with a steady decline in the adjusted trends of in‐hospital mortality during the study period in the overall population (from 49% in 2004 to 37% in 2018; P trend<0.001), among patients with acute myocardial infarction CS (from 43% in 2004 to 34% in 2018; P trend<0.001), and among patients with non–acute myocardial infarction CS (from 52% in 2004 to 37% in 2018; P trend<0.001). Consistent trends of reduced mortality were seen among women, men, different racial/ethnic groups, different US regions, and different hospital sizes, regardless of the hospital teaching status.
Conclusions
Hospitalizations attributed to CS have tripled in the period from January 2004 to December 2018. However, there has been a slow decline in CS in‐hospital mortality during the studied period. Further studies are necessary to determine if the recent adoption of treatment algorithms in treating patients with CS will further impact in‐hospital mortality.
Keywords: cardiogenic shock, in‐hospital mortality, national trends
Subject Categories: Heart Failure, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- CS
cardiogenic shock
- MCS
mechanical circulatory support
- NCSI
National Cardiogenic Shock Initiative
- NIS
National Inpatient Sample
Clinical Perspective
What Is New?
Hospitalizations attributed to cardiogenic shock (CS) tripled in the period from January 2004 to December 2018.
There has been a steady decline in CS in‐hospital mortality in the United States during the study period from 1 in 2 in 2004 to about 1 in 3 in 2018.
What Are the Clinical Implications?
A slow decline in CS mortality has been observed during the study period.
Despite the currently available treatment strategies for CS, mortality among patients with CS continues to be substantial and warrants further research to improve CS outcomes.
Cardiogenic shock (CS) is a highly fatal condition and is a significant cause of morbidity and mortality.1 With the expansion in mechanical circulatory support (MCS) strategies, timely revascularization, and advances in intensive care, there has been a reduction in mortality among patients with CS.2, 3, 4, 5 Previous studies on mortality in patients with CS have been limited to multicenter registries from participating hospitals or single‐center studies, which may not represent national outcomes.2, 4, 6 Few studies used national databases to report in‐hospital mortality rates, but they used old data that may not represent the most recent trends.7, 8 This study's primary objective was to report the contemporary temporal trends in in‐hospital mortality among patients with CS. In addition, we explored the mortality trends based on demographic variables such as sex, race/ethnicity, etiology of CS, and regional variations in the trends of CS in‐hospital mortality.
METHODS
Study Data
The study was derived from National Inpatient Sample (NIS) data from January 1, 2004, to December 31, 2018. The NIS database is part of the Healthcare Cost and Utilization Project databases and is sponsored by the Agency for Healthcare Research and Quality.9 The NIS is the largest publicly available all‐payer administrative claims‐based database and contains patient discharges from 1000 hospitals in 45 states. It has clinical and resource use information on >7 million discharges annually. Weighted, it represents >35 million hospitalizations nationally on an annual basis. These data are stratified to represent 20% of US inpatient hospitalizations across different hospital and geographic regions (random sample).9 National databases have been used extensively to study national trends, disparities, and outcomes of different cardiac procedures.7, 10, 11, 12, 13, 14, 15 Because of the NIS database's deidentified nature and public availability, institutional review board approval and informed consent were not required for this study. Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the Healthcare Cost and Utilization Project at https://www.hcup‐us.ahrq.gov/tech_assist/centdist.jsp.
Study Population
Adult patients (≥18 years) admitted with CS from January 1, 2004 to December 31, 2018 were identified in the NIS using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) codes 78551 and R570, respectively. The ICD‐9‐CM and ICD‐10‐CM codes for CS used in the current study have been previously validated.16, 17 We excluded the following patients: (1) patients with missing mortality, age, or sex data, (2) patients aged <18 years, (3) patients who were admitted electively to the hospital, and (4) patients who were transferred out from the receiving hospital.
We have reported the trends of in‐hospital mortality in the following populations: (1) among all patients who received the diagnosis of CS during their hospitalization, (2) among patients with acute myocardial infarction (AMI) CS (AMI‐CS), and (3) among patients with non–AMI‐CS. The AMI‐CS cohort was identified by selecting patients with the principal diagnosis of AMI (ST‐segment–elevation myocardial infarction or non–ST‐segment–elevation myocardial infarction) and then identifying patients with a secondary code of CS. For the non–AMI‐CS cohort, we identified patients with CS who lacked a concomitant code for acute coronary syndrome as a primary or secondary diagnosis.
In addition, as previous studies suggested regional variations and disparity based on sex and race in the management and outcomes of CS, we reported in‐hospital mortality trends among patients with CS stratified by sex, race/ethnicity, US regions, hospital bed size, and hospital teaching status.11, 18, 19 Furthermore, we reported the trend of mortality among patients who were treated with MCS (intra‐aortic balloon pump, percutaneous left ventricular assist devices, and extracorporeal membrane oxygenation) and among the patients with AMI‐CS who received revascularization (percutaneous coronary intervention or coronary artery bypass grafting).
In addition, and to study the difference in the treatment strategies for patients with AMI‐CS and non–AMI‐CS, the study period was divided into the following 3 different eras: (1) pre–percutaneous left ventricular assist device approval era (2004–2007); (2) percutaneous left ventricular assist device era (2008–2014), which corresponded to the US Food and Drug Administration approval of Impella (AbioMed, Danvers, MA) and the wide adoption of robust MCS in the management of CS; and (3) shock team era (2015–2018), during which an algorithmic approach for the management of CS was implemented and used by multiple institutions across the United States.2, 4, 20, 21, 22 We reported the treatment strategies for patients with AMI‐CS and non–AMI‐CS across the different eras.
Study End Points
The current study's primary end point was the trend of in‐hospital mortality during the study period. Furthermore, we studied the differences in the care of patients with AMI‐CS and non–AMI‐CS by reporting the percentage of patients treated with MCS, durable left ventricular support devices, mechanical ventilation, and renal replacement therapy across the different eras. The list of the ICD‐9‐CM and ICD‐10‐CM codes used in the current analysis is shown in Table S1.
Statistical Analysis
All variables are expressed as weighted national estimates. This was done following the survey analysis method by incorporating the HOSP_NIS as a clustering variable and accounting for the different strata in the NIS design using the NIS_STRATUM as recommended in the Agency for Healthcare Research and Quality methods series. For trend analysis, and because of the NIS design change following the year 2011, we followed the Agency for Healthcare Research and Quality methods to adjust for that by incorporating the correct sample weight.9 For trend analysis, we used the Mantel‐Haenszel χ2 test of linear association.
We used multilevel generalized structural equation modeling to adjust for selected covariates to report the adjusted mortality trends. Following that, we applied marginal standardization using the Stata postestimation margins command to estimate the predicted probability (adjusted rates) of in‐hospital mortality per year while accounting for the selected covariates. We used marginal standardization because it has been shown to be an appropriate method when making an inference to the overall population.23 Variables included in the regression models included demographics (age, sex, race/ethnicity), all Elixhauser comorbidities,24 and hospital characteristics (hospital region and bed size). We removed the corresponding covariate from the regression model when it was the subgroup of interest (eg, when conducting analysis stratified by sex, we removed sex from the covariate list). A P value of <0.05 was considered statistically significant. All statistical analyses were performed using Stata Statistical Software version 15.1 and SPSS version 26 (IBM Corp).
RESULTS
Among a total of 563 949 644 hospitalizations during the period from January 1, 2004, to December 30, 2018, 1 254 358 (0.2%) were attributed to CS. There has been a steady increase in hospitalizations attributed to CS from 122 per 100 000 hospitalizations in 2004 to 408 per 100 000 hospitalizations in 2018 (P trend<0.001). This increase was observed among AMI‐CS (from 44 per 100 000 hospitalizations in 2004 to 103 per 100 000 hospitalizations in 2018) and non–AMI‐CS (from 68 per 100 000 hospitalizations in 2004 to 258 per 100 000 hospitalizations in 2018) (Figure 1). This was accompanied by a decline in in‐hospital mortality during the study periods from 49% in 2004 to 37% in 2018 (P trend<0.001). The reduction in in‐hospital mortality was seen among both patients with AMI‐CS (from 44% in 2004 to 35% in 2018; P trend<0.001) and non–AMI‐CS (from 53% in 2004 to 36% in 2018; P trend<0.001). The results remained significant even after the adjustment for covariates with the overall in‐hospital mortality improving from 49% in 2004 to 37% in 2018 (P trend<0.001), AMI‐CS improving from 43% in 2004 to 34% in 2018 (P trend<0.001), and non–AMI‐CS improving from 52% in 2004 to 37% in 2018 (P trend<0.001) (Figure 2).
Figure 1. Prevalence of CS among hospitalizations during the study period.
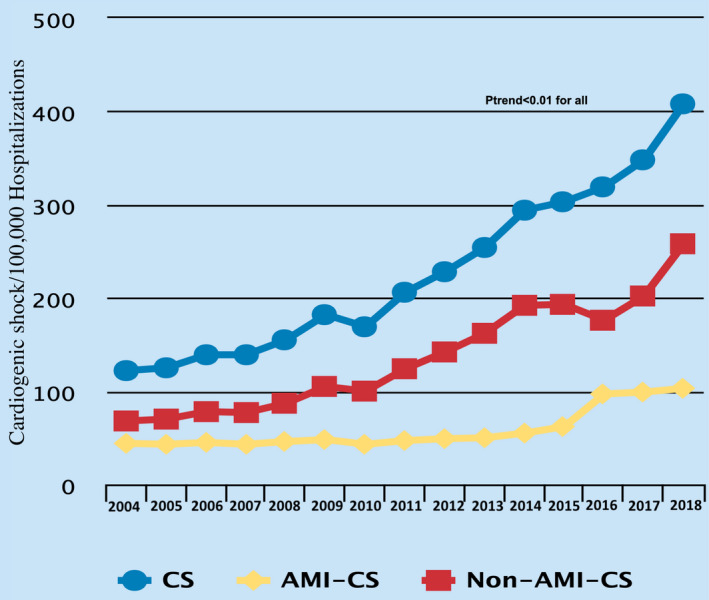
AMI‐CS indicates acute myocardial infarction cardiogenic shock; CS, cardiogenic shock; and non–AMI‐CS, non–acute myocardial infarction cardiogenic shock.
Figure 2. Trends in in‐hospital mortality among patients with CS during the study period.
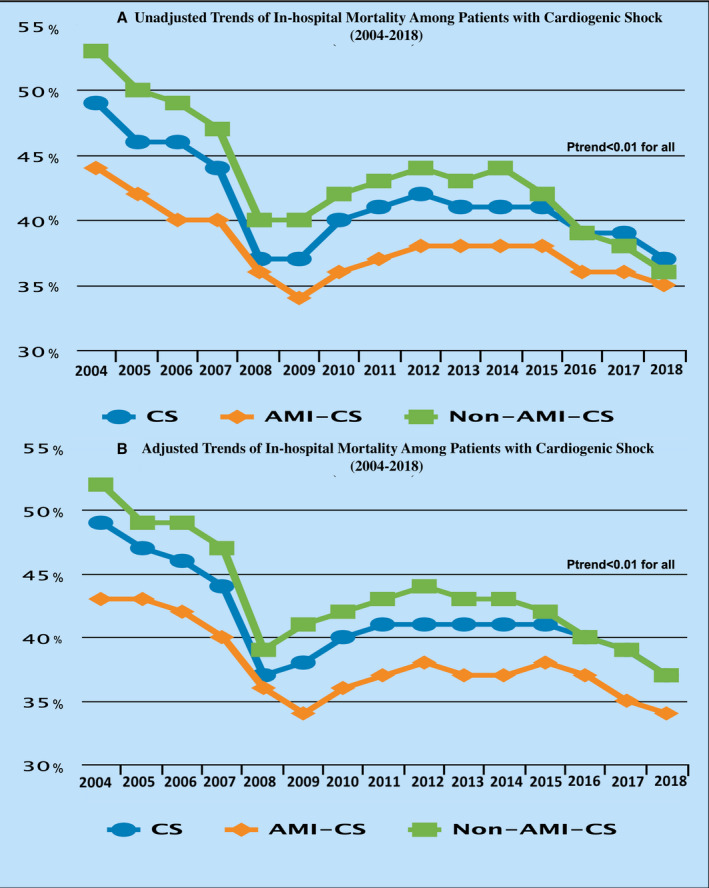
A, Unadjusted trends. B, Adjusted trends. AMI‐CS indicates acute myocardial infarction cardiogenic shock; CS, cardiogenic shock; and non–AMI‐CS, non–acute myocardial infarction cardiogenic shock.
In the unadjusted analysis, a consistent reduction of in‐hospital mortality was seen among men (from 47% in 2004 to 35% in 2018; P trend<0.001) and women (from 51% in 2004 to 40% in 2018; P trend<0.001) (Figure 3). Furthermore, the trends showed a consistent reduction in in‐hospital mortality among White patients (from 50% in 2004 to 37% in 2018; P trend<0.001), Black patients (from 49% in 2004 to 36% in 2018; P trend<0.001), Hispanic patients (from 49% in 2004 to 35% in 2018; P trend<0.001), and Asian or Pacific Islander patients (from 54% in 2004 to 39% in 2018; P trend<0.001) (Figure 4). In addition, there has been a consistent drop in the in‐hospital mortality among patients with CS in different regions of the United States: Northeast from 53% in 2004 to 39% in 2018, Midwest from 46% in 2004 to 36% in 2018, South from 47% in 2004 to 37% in 2018, and West from 50% for 2004 to 37% for 2018 (P trend<0.001 for all) (Figure 5). Furthermore, a consistent trend of reduction in mortality was seen among hospitals of different sizes and regardless of the hospital teaching status, MCS use, or revascularization (Figures S1 through S4).
Figure 3. Trends in in‐hospital mortality among patients with cardiogenic shock during the study period stratified by sex.
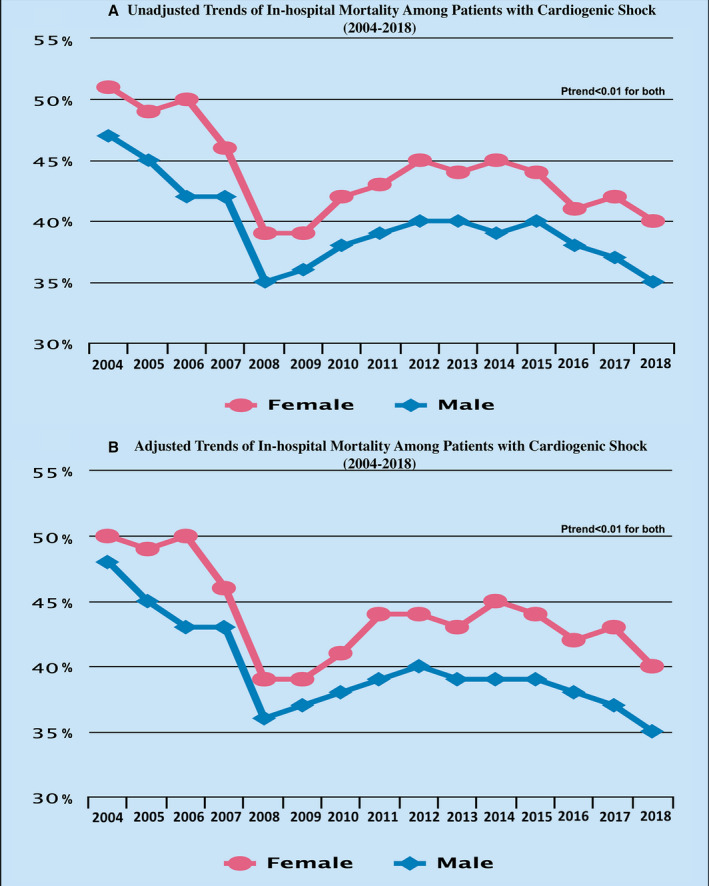
A, Unadjusted trends. B, Adjusted trends.
Figure 4. Trends in in‐hospital mortality among patients with cardiogenic shock during the study period stratified by race/ethnicity.
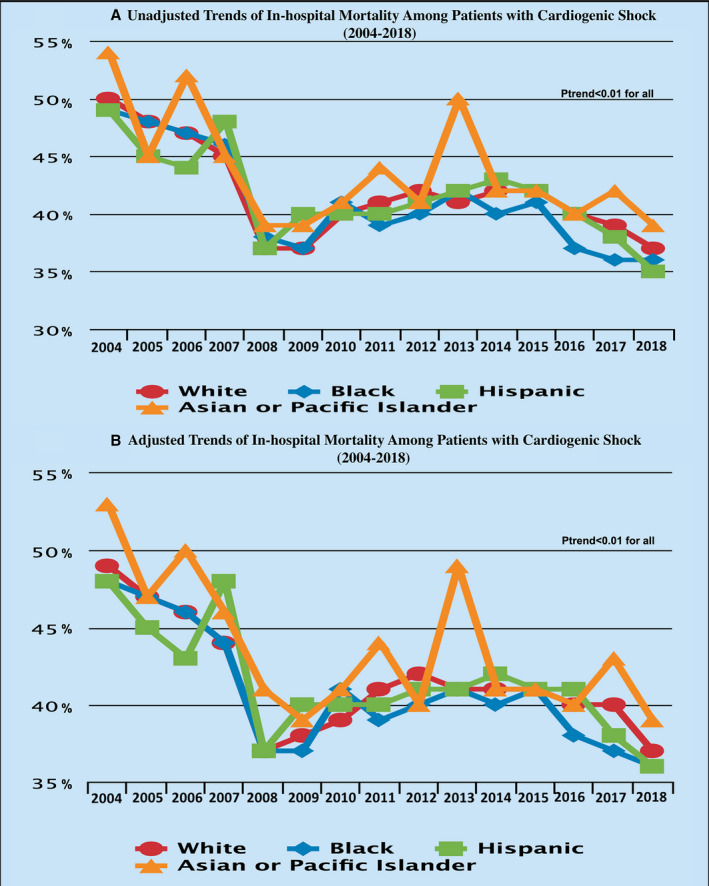
A, Unadjusted trends. B, Adjusted trends.
Figure 5. Trends in in‐hospital mortality among patients with cardiogenic shock during the study period stratified region.
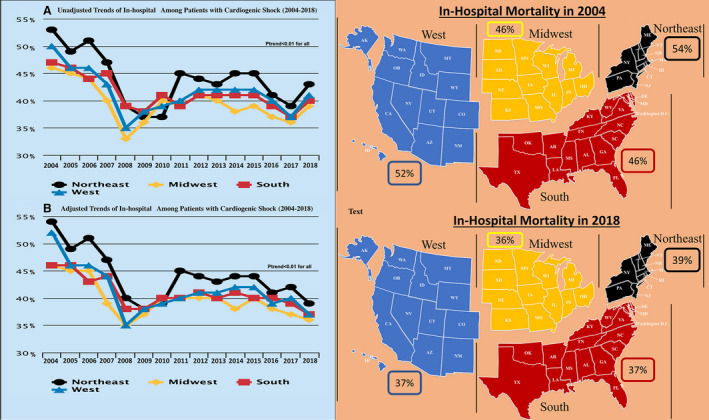
A, Unadjusted trends. B, Adjusted trends.
The results remained significant after adjusting for covariates, with a reduction of in‐hospital mortality among men (from 48% in 2004 to 35% in 2018, P trend<0.001), women (from 50% in 2004 to 40% in 2018, P trend<0.001), White patients (from 49% in 2004 to 37% in 2018; P trend<0.001), Black patients (from 48% in 2004 to 36% in 2018; P trend<0.001), Hispanic patients (from 48% in 2004 to 36% in 2018; P trend<0.001), and Asian or Pacific Islander patients (from 53% in 2004 to 39% in 2018; P trend<0.001) (Figures 3 and 4). In addition, a consistent reduction in in‐hospital mortality was seen among different regions of the United States during the study period: Northeast from 54% in 2004 to 39% in 2018 (P trend<0.001), Midwest from 46% in 2004 to 36% in 2018 (P trend<0.001), South from 46% in 2004 to 37% in 2018 (P trend<0.001), and West from 52% in 2004 to 37% in 2018 (P trend<0.001) (Figure 5). Furthermore, a consistent trend of reduction in mortality was seen among hospitals of different sizes and regardless of the hospital teaching status, MCS use, or revascularization (Figures S1 through S4). Trends in using different treatment strategies in the AMI‐CS and non–AMI‐CS cohorts during the study period are shown in Table S2.
DISCUSSION
In this contemporary observational study using a nationally representative sample of the US population, we report several significant findings. First, CS hospitalizations have almost tripled during the study period. Second, we report a decreasing trend in in‐hospital mortality among patients admitted with CS. Third, the decreasing trends of in‐hospital mortality were consistent across all subgroups analyzed, including men and women, AMI‐CS and non‐AMI CS, and across different racial/ethnic groups. Fourth, there was a decreasing temporal trend in CS in‐hospital mortality across all US regions and regardless of hospital size or teaching status.
This is the most extensive study using a nationally representative sample of the US population to analyze the temporal trends in CS hospitalization incidence and in‐hospital mortality. Prior investigations of temporal trends in CS morality were limited to registry data contributed by few select hospitals and do not necessarily reflect the national trends in incidence of outcomes of CS.25, 26 Our findings corroborate and expand on the results from prior single‐center studies, multicenter studies, and registries.25, 26 In a large registry spanning >20 years from Switzerland, the authors reported an increase in CS admission among patients with AMI‐CS in the period from 1997 to 2017 by more than double from 2.5% to 4.6%.27 Similarly, in a recent retrospective report from the Mayo Clinic cardiac intensive care unit, which included >12 000 patients, the authors reported an increase in CS incidence by almost 4‐fold from 5.7% in 2007 to 2009 to 19.4% in 2016 to 2018.25 We postulate that the observed rise in CS hospitalizations is attributed to several factors. There has been an increasing awareness about the importance of the appropriate and timely diagnosis of CS and the inception of shock teams and shock algorithms; these may have collectively led to the recognition of more patients with CS compared with the early years.2, 6 Moreover, studies have suggested a shift in the epidemiological risk factors of cardiovascular disease with a higher burden of classical risk factors such as obesity, diabetes mellitus, and hypertension; all are also linked to coronary artery disease and heart failure, which are the major etiologies of CS.26
Consistent with previous studies, we report a decline in CS in‐hospital mortality during the study period.2, 26, 28, 29 A reduction in in‐hospital mortality was seen in the early years of the study (2004–2008) and among the AMI‐CS and non–AMI‐CS cohorts. It is important to note that there have been no changes in the administrative coding algorithms used to identify patients with CS during this period. The adoption of early revascularization and the proliferation of cardiac catheterization laboratories contributed to the early drop in mortality among the AMI‐CS cohort.30 For the non‐AMI cohort, the reduction in in‐hospital mortality during the early years can be explained by the increased awareness about the importance of early diagnosis of CS, advances in critical care management, and the proliferation of cardiac intensive care units.30, 31, 32, 33, 34 In addition, more robust contemporary percutaneous MCS platforms were introduced into the US market between 2006 and 2008, which coincides with a major drop in CS‐related mortality among the AMI and non‐AMI cohorts (Figure 2). Although these devices have not been shown to improve survival in randomized clinical trials independently, there has been increasing evidence supporting the implementation of CS treatment algorithms that promote early diagnosis, early revascularization when appropriate, use of invasive hemodynamic data, and early deployment of MCS.3, 21, 35, 36 Further refinements in CS treatment strategies, including shock teams and care algorithms, and collaborative efforts such as the NCSI (National Cardiogenic Shock Initiative) may be responsible for more recent reductions in CS mortality.2, 6, 15, 21
Our findings contrast to the conclusions of the study by Wayangankar et al, who reported an increasing trend of in‐hospital mortality among patients with CS during the period from 2005 to 2013.37 However, significant differences between the 2 studies need to be noted. First, Wayangankar et al used the NCDR (National Cardiovascular Data Registry CathPCI Registry), in contrast to the NIS used in the current study. Second, Wayangankar et al only included patients with AMI‐CS who were treated with percutaneous coronary intervention. Third, Wayangankar et al excluded patients who had symptoms for >24 hours (n=17 791).37
It is important to note that the only randomized trial to date that has shown a reduction in mortality among patients with CS is the SHOCK (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock) trial, which showed that compared with medical stabilization alone, emergency revascularization was associated with a significant reduction in mortality.30 On the other hand, although randomized clinical trials on MCS did not show a mortality benefit, these trials had several limitations and enrolled the patients who were sickest in whom shock had progressed from a reversible hemodynamic phase to an irreversible hemometabolic phase.36, 38 Recognition of this fact led multiple investigators to develop a CS treatment algorithm aimed at early recognition of CS, early revascularization, early use of invasive hemodynamic monitoring, and early deployment of MCS.2, 4 After the encouraging results of incorporating the CS treatment algorithm in the Detroit Cardiogenic Shock Initiative in which the investigators could reduce in‐hospital mortality to 24%, a similar treatment algorithm has been adopted at the national level under the currently active NCSI. Early reports from the NCSI indicate a reduction in mortality to 28% in patients with AMI‐CS.2 The mortality benefit of implementing a bundled approach with shock teams, early invasive hemodynamic monitoring, and early MCS need confirmation in randomized controlled trials. Despite the strategies mentioned previously, mortality among patients with CS continues to be substantial, thereby prompting further research to improve CS outcomes.
Study Limitations
Several limitations of the current analysis need to be acknowledged. First, as the present study uses billing codes, a reasonable concern would be that the increase in the prevalence may be attributed to more generous application of billing codes. Although this may have amplified any real change in the disease prevalence, we believe that there was an increase in the true prevalence of CS based on the reports from other studies of similar trends and the increased number of patients receiving MCS, heart transplantations, and durable left ventricular support devices over time.7, 8, 39 Moreover, the codes used to identify CS in the current analysis have been validated in previous studies. Furthermore, we used a hard clinical end point for the outcomes (death), which is less prone to coding errors.16, 17 Second, because of the data set's inherent limitation, we do not have hemodynamic, metabolic, or clinical data, which are vital in diagnosing and staging CS. Similarly, data on hospitals' adoption of shock teams and the algorithmic approach for the management of CS are lacking. Third, the database does not have (present on admission) indicators, so for some comorbidities, it is difficult to differentiate between chronic conditions and new complications attributed to the CS admission. Fourth, given the large sample size, small non–clinically significant changes could meet the statistical significance criteria. However, we report a statistically and clinically significant drop in in‐hospital mortality during the study period.
CONCLUSIONS
Hospitalizations attributed to CS have tripled in the period from January 2004 to December 2018. However, there has been a steady decline in CS in‐hospital mortality in the United States during the study period from 1 in 2 in 2004 to about 1 in 3 in 2018.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S2
Figures S1–S4
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021061
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1.Reyentovich A, Barghash MH, Hochman JS. Management of refractory cardiogenic shock. Nat Rev Cardiol. 2016;13:481–492. DOI: 10.1038/nrcardio.2016.96. [DOI] [PubMed] [Google Scholar]
- 2.Basir MB, Kapur NK, Patel K, Salam MA, Schreiber T, Kaki A, Hanson I, Almany S, Timmis S, Dixon S, et al. Improved outcomes associated with the use of shock protocols: updates from the national cardiogenic shock initiative. Catheter Cardiovasc Interv. 2019;93:1173–1183. DOI: 10.1002/ccd.28307. [DOI] [PubMed] [Google Scholar]
- 3.Garan AR, Kanwar M, Thayer KL, Whitehead E, Zweck E, Hernandez‐Montfort J, Mahr C, Haywood JL, Harwani NM, Wencker D, et al. Complete hemodynamic profiling with pulmonary artery catheters in cardiogenic shock is associated with lower in‐hospital mortality. JACC Heart Fail. 2020;8:903–913. DOI: 10.1016/j.jchf.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Tehrani BN, Truesdell AG, Sherwood MW, Desai S, Tran HA, Epps KC, Singh R, Psotka M, Shah P, Cooper LB, et al. Standardized team‐based care for cardiogenic shock. J Am Coll Cardiol. 2019;73:1659–1669. DOI: 10.1016/j.jacc.2018.12.084. [DOI] [PubMed] [Google Scholar]
- 5.Alkhouli M, Osman M, Elsisy MFA, Kawsara A, Berzingi CO. Mechanical circulatory support in patients with cardiogenic shock. Curr Treat Options Cardiovasc Med. 2020;22:4. DOI: 10.1007/s11936-020-0804-6. [DOI] [PubMed] [Google Scholar]
- 6.Taleb I, Koliopoulou AG, Tandar A, McKellar SH, Tonna JE, Nativi‐Nicolau J, Alvarez Villela M, Welt F, Stehlik J, Gilbert EM, et al. Shock team approach in refractory cardiogenic shock requiring short‐term mechanical circulatory support: a proof of concept. Circulation. 2019;140:98–100. DOI: 10.1161/CIRCULATIONAHA.119.040654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan MZ, Munir MB, Khan MU, Osman M, Agrawal P, Syed M, Ghaffar YA, Alharbi A, Khan SU, Balla S. Trends, outcomes, and predictors of revascularization in cardiogenic shock. Am J Cardiol. 2020;125:328–335. DOI: 10.1016/j.amjcard.2019.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah M, Patnaik S, Patel B, Ram P, Garg L, Agarwal M, Agrawal S, Arora S, Patel N, Wald J, et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non‐infarction related cardiogenic shock in the United States. Clin Res Cardiol. 2018;107:287–303. DOI: 10.1007/s00392-017-1182-2. [DOI] [PubMed] [Google Scholar]
- 9.HCUP National Readmission Database (NRD) . Healthcare Cost and Utilization Project (HCUP). Agency Healthcare Research Quality R M. Available at: https://www.hcup‐us.ahrq.gov/nrdoverview.jsp. Accessed May 2, 2021. [Google Scholar]
- 10.Khan MZ, Syed M, Osman M, Faisaluddin M, Sulaiman S, Farjo PD, Khan MU, Agrawal P, Alharbi A, Khan SU, et al. Contemporary trends and outcomes in patients with ST‐segment elevation myocardial infarction and end‐stage renal disease on dialysis: insight from the National Inpatient Sample. Cardiovasc Revasc Med. 2020;21:1474–1481. DOI: 10.1016/j.carrev.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osman M, Syed M, Abdul Ghaffar Y, Patel B, Abugroun A, Kheiri B, Kawsara A, Kadiyala M, Balla S, Daggubati R. Gender‐based outcomes of impeller pumps percutaneous ventricular assist devices. Catheter Cardiovasc Interv. 2021;97:E627–E635. DOI: 10.1002/ccd.29222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osman M, Patel B, Munir MB, Kawsara A, Kheiri B, Balla S, Daggubati R, Michos ED, Alkhouli M. Sex‐stratified analysis of the safety of percutaneous left atrial appendage occlusion. Catheter Cardiovasc Interv. 2021;97:885–892. DOI: 10.1002/ccd.29282. [DOI] [PubMed] [Google Scholar]
- 13.Osman M, Al‐Hijji MA, Kawsara A, Patel B, Alkhouli M. Comparative outcomes of mitral valve in valve implantation versus redo mitral valve replacement for degenerated bioprotheses. Am J Cardiol. 2020;132:175–176. DOI: 10.1016/j.amjcard.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osman M, Benjamin MM, Balla S, Kheiri B, Bianco C, Sengupta PP, Daggubati R, Malla M, Liu SV, Mamas M, et al. Index admission and thirty‐day readmission outcomes of patients with cancer presenting with STEMI. Cardiovasc Revasc Med. 2021;21:S1553‐8389(21)00203‐7. DOI: 10.1016/j.carrev.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osman M, Balla S, Dupont A, O’Neill W, Basir MB. Invasive hemodynamic monitoring in cardiogenic shock. Am J Cardiol. 2021;150:128–129. DOI: 10.1016/j.amjcard.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Lauridsen MD, Gammelager H, Schmidt M, Nielsen H, Christiansen CF. Positive predictive value of International Classification of Diseases, 10th revision, diagnosis codes for cardiogenic, hypovolemic, and septic shock in the Danish National Patient Registry. BMC Med Res Methodol. 2015;15:23. DOI: 10.1186/s12874-015-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambert L, Blais C, Hamel D, Brown K, Rinfret S, Cartier R, Giguère M, Carroll C, Beauchamp C, Bogaty P. Evaluation of care and surveillance of cardiovascular disease: can we trust medico‐administrative hospital data? Can J Cardiol. 2012;28:162–168. DOI: 10.1016/j.cjca.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Joyce DL, Conte JV, Russell SD, Joyce LD, Chang DC. Disparities in access to left ventricular assist device therapy. J Surg Res. 2009;152:111–117. DOI: 10.1016/j.jss.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 19.Osman M, Balla S, Patibandla S, Kheiri B, Caccamo M, Bianco C, Sokos G. Regional variation in the adoption of invasive hemodynamic monitoring for cardiogenic shock in the United States. Am J Cardiol. 2021;148:174–175. DOI: 10.1016/j.amjcard.2021.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Available at: http://www.abiomed.com/products/impella‐2‐5/ IAAo. Accessed February 22, 2021.
- 21.Tehrani BN, Truesdell AG, Psotka MA, Rosner C, Singh R, Sinha SS, Damluji AA, Batchelor WB. A standardized and comprehensive approach to the management of cardiogenic shock. JACC Heart Fail. 2020;8:879–891. DOI: 10.1016/j.jchf.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy B, Bastien O, Bendjelid K, Cariou A, Chouihed T, Combes A, Mebazaa A, Megarbane B, Plaisance P, Ouattara A, et al. Experts’ recommendations for the management of adult patients with cardiogenic shock. Ann Intensive Care. 2015;5:52. DOI: 10.1186/s13613-015-0052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43:962–970. DOI: 10.1093/ije/dyu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. DOI: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Jentzer JC, van Diepen S, Barsness GW, Katz JN, Wiley BM, Bennett CE, Mankad SV, Sinak LJ, Best PJ, Herrmann J, et al. Changes in comorbidities, diagnoses, therapies and outcomes in a contemporary cardiac intensive care unit population. Am Heart J. 2019;215:12–19. DOI: 10.1016/j.ahj.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Jentzer JC, Ahmed AM, Vallabhajosyula S, Burstein B, Tabi M, Barsness GW, Murphy JG, Best PJ, Bell MR. Shock in the cardiac intensive care unit: changes in epidemiology and prognosis over time. Am Heart J. 2020;232:94–104. DOI: 10.1016/j.ahj.2020.10.054. [DOI] [PubMed] [Google Scholar]
- 27.Hunziker L, Radovanovic D, Jeger R, Pedrazzini G, Cuculi F, Urban P, Erne P, Rickli H, Pilgrim T. Twenty‐year trends in the incidence and outcome of cardiogenic shock in AMIS plus registry. Circ Cardiovasc Interv. 2019;12:e007293. DOI: 10.1161/CIRCINTERVENTIONS.118.007293. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez GA, Lemor A, Blumer V, Rueda CA, Zalawadiya S, Stevenson LW, Lindenfeld J. Trends in utilization and outcomes of pulmonary artery catheterization in heart failure with and without cardiogenic shock. J Card Fail. 2019;25:364–371. DOI: 10.1016/j.cardfail.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST‐elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3:e000590. DOI: 10.1161/JAHA.113.000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625–634. DOI: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 31.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Baldwin JT, Young JB. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–156. DOI: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Lampropulos JF, Kim N, Wang Y, Desai MM, Barreto‐Filho JAS, Dodson JA, Dries DL, Mangi AA, Krumholz HM. Trends in left ventricular assist device use and outcomes among Medicare beneficiaries, 2004–2011. Open Heart. 2014;1:e000109. DOI: 10.1136/openhrt-2014-000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheldon WC. Trends in cardiac catheterization laboratories in the United States. Catheter Cardiovasc Interv. 2001;53:40–45. DOI: 10.1002/ccd.1127. [DOI] [PubMed] [Google Scholar]
- 34.Morrow DA. Trends in cardiac critical care: reshaping the cardiac intensive care unit. Circ Cardiovasc Qual Outcomes. 2017;10:e004010. DOI: 10.1161/CIRCOUTCOMES.117.004010. [DOI] [PubMed] [Google Scholar]
- 35.Saxena A, Garan AR, Kapur NK, O’Neill WW, Lindenfeld J, Pinney SP, Uriel N, Burkhoff D, Kern M. Value of hemodynamic monitoring in patients with cardiogenic shock undergoing mechanical circulatory support. Circulation. 2020;141:1184–1197. DOI: 10.1161/CIRCULATIONAHA.119.043080. [DOI] [PubMed] [Google Scholar]
- 36.Van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. DOI: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 37.Wayangankar SA, Bangalore S, McCoy LA, Jneid H, Latif F, Karrowni W, Charitakis K, Feldman DN, Dakik HA, Mauri L, et al. Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: a report from the CathPCI Registry. JACC Cardiovasc Interv. 2016;9:341–351. DOI: 10.1016/j.jcin.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 38.Ouweneel DM, Schotborgh JV, Limpens J, Sjauw KD, Engström AE, Lagrand WK, Cherpanath TGV, Driessen AHG, de Mol B, Henriques JPS. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta‐analysis. Intensive Care Med. 2016;42:1922–1934. DOI: 10.1007/s00134-016-4536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawson CA, Zaccardi F, Squire I, Ling S, Davies MJ, Lam CSP, Mamas MA, Khunti K, Kadam UT. 20‐year trends in cause‐specific heart failure outcomes by sex, socioeconomic status, and place of diagnosis: a population‐based study. Lancet Public Health. 2019;4:e406–e420. DOI: 10.1016/S2468-2667(19)30108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S4


