Abstract
Background
Maximal left atrial (LA) volume is reported by most echocardiography laboratories and is associated with clinical outcomes in patients with heart failure (HF). Recent studies suggest that minimal LA volume may better reflect left ventricular filling pressure and may be more prognostic than maximal LA volume. This study assessed the prognostic value of indexed minimal LA volume (LAVImin) in patients with HF with preserved ejection fraction.
Methods and Results
We assessed the relationship of LAVImin with a primary composite end point of cardiovascular death, aborted cardiac death, or HF hospitalization in 347 patients with HF with preserved ejection fraction enrolled from the Americas region in TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial). We compared LAVImin with indexed maximal LA volume with respect to their prognostic values. In addition, we assessed if LA functional parameters provide additional prognostic information over LAVImin. During a median follow‐up of 2.5 years, 107 patients (31%) experienced a primary composite end point. LAVImin was associated with increased risk of a primary composite outcome (hazard ratio [HR], 1.35; 95% CI, 1.12–1.61) and HF hospitalization alone (HR, 1.42; 95% CI, 1.17–1.71) after adjusting for clinical confounders and ejection fraction. In contrast, indexed maximal LA volume was not related to the primary composite outcome, but related to HF alone (HR, 1.25; 95% CI, 1.02–1.54). In comparison with indexed maximal LA volume, LAVImin was significantly more prognostic for primary composite outcome (P for comparison=0.032). Both LA emptying fraction and LA strain were prognostic of primary outcome independent of LAVImin (all P<0.05).
Conclusions
In patients with HF with preserved ejection fraction, LAVImin was more predictive of cardiovascular outcome than indexed maximal LA volume, suggesting this measure may be more physiologically relevant and might better identify patients at high risk for cardiovascular events. LA functional parameters provide prognostic information independent of LAVImin.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT00094302.
Keywords: cardiovascular outcomes, heart failure, left atrial volume, preserved ejection fraction
Subject Categories: Heart Failure, Echocardiography
Nonstandard Abbreviations and Acronyms
- GLS
global longitudinal strain
- HFpEF
heart failure with preserved ejection fraction
- LAEF
left atrial emptying fraction
- LAVImax
indexed maximal left atrial volume
- LAVImin
indexed minimal left atrial volume
- LAVmax
maximal left atrial volume
- LAVmin
minimal left atrial volume
- TOPCAT
Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial
Clinical Perspective
What Is New?
In patients with heart failure (HF) with preserved ejection fraction, minimal left atrial (LA) volume index was independently associated with worse clinical outcomes, even after adjusting for clinical predictors and left ventricular ejection fraction.
Minimal LA volume index was significantly more predictive of HF hospitalization, cardiovascular death, or resuscitated sudden death than maximal LA volume index among patients with HF with preserved ejection fraction.
Both LA emptying fraction and LA strain were prognostic of primary outcome and hospitalization for HF, independent of minimal LA volume index.
What Are the Clinical Implications?
Minimal LA volume may be more physiologically relevant than maximal LA volume and might better identify at high risk for cardiovascular events in patients with HF with preserved ejection fraction.
LA functional parameters provide prognostic information independent of minimal LA volume.
Left atrial (LA) remodeling is of particular interest in patients with heart failure (HF) with preserved ejection fraction (HFpEF) because it has been considered an indicator of left ventricular (LV) diastolic dysfunction and the chronicity of elevated LV filling pressure, and has been associated with adverse outcome.1, 2, 3Loss of atrial function has been related to greater adverse effects in patients with HFpEF than those with HF with reduced ejection fraction.4
LA size has been used as an indicator of the chronicity and burden of elevated LV filling pressure and as a predictor of cardiovascular events.5, 6 For this purpose, maximal LA volume (LAVmax) is currently reported by most clinical echocardiographic laboratories as a key cardiac structural assessment and is recommended as a component of the parameters for LV diastolic dysfunction in published guidelines.7, 8 However, the prognostic value of LAVmax in patients with HFpEF is controversial. Although several studies have reported the association between maximal LA size and clinical outcome,9, 10, 11 others have shown that LAVmax is not strongly associated with outcome.12, 13, 14LAVmax can be influenced by LV systolic function through systolic descent of the mitral plane. Recent studies have shown that minimal LA volume (LAVmin) is measured at LV end diastole when the LA is directly exposed to LV end‐diastolic pressure and thus may be more closely related to LV filling pressure and clinical outcome than LAVmax, suggesting that LAVmin might be a better marker for LA structural remodeling.15, 16, 17, 18, 19, 20 However, data on the prognostic value of LAVmin in patients with HFpEF are limited. We used data from the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial) to test the hypothesis that LAVmin would be better than the commonly used LAVmax in predicting outcome in patients with HFpEF.21 We also assessed if LA functional parameters provide additional prognostic information over LAVmin.
Methods
TOPCAT data are available to qualified researchers through the National Institutes of Health website (https://biolincc.nhlbi.nih.gov/studies/topcat/). The study was approved by an institutional review committee at each study center, and all subjects gave informed consent.
Study Population
The TOPCAT randomized 3445 patients, aged ≥50 years, with symptomatic HF and an LV ejection fraction of ≥45% per local site reading to double‐blinded treatment with spironolactone or placebo. Eligible patients had a history of hospitalization within the previous 12 months for which HF was a major component (per site determination; not adjudicated by the clinical events adjudication committee), or elevated brain natriuretic peptide level ≥100 pg/mL or an NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) level ≥360 pg/mL within 60 days before randomization. Randomization was stratified according to the type of the inclusion criteria. Details of trial design, inclusion and exclusion criteria, and the main results have been previously reported.21, 22 The design and overall findings of the TOPCAT echocardiographic substudy have previously been described in detail.23
Echocardiographic Measurements
Standard echocardiographic and Doppler parameters were measured by dedicated analysts at the core laboratory, blinded to clinical information, as described previously, according to American Society of Echocardiography recommendations.23 Speckle‐tracking analysis for the LA and LV was performed using vendor‐independent software with algorithms designed for the LV (TomTec Imaging Systems, Unterschleissheim, Germany), as previously described in detail.24, 25 The LA and LV endocardial borders were traced at the end‐diastolic frame of the apical 2‐ and 4‐chamber views and manually adjusted as needed. Patients were excluded if they had inadequate image quality for deformational analysis, which was defined as a missing view, lack of a full cardiac cycle, non‐digital imaging and communications in medicine (DICOM) images, >2‐segment dropout, or significantly foreshortened images. Using speckle‐tracking echocardiography with electrocardiographic gating set from R wave to R wave, phasic LAV and LA strain were measured using apical 4‐ and 2‐chamber views. Maximal and minimal LA volumes were obtained from LA time‐volume curves (generated as part of the LA speckle‐tracking analysis) by calculating LA volume at each phase of the cardiac cycle using the Simpson method and were indexed to body surface area (LAVI). Analyses were performed on 1 cardiac cycle for patients in sinus rhythm and over 3 cardiac cycles for patients with atrial fibrillation (AF). LA emptying fraction (LAEF) was calculated as follows: [(LAVmax−LAVmin)/LAVmax]×100. LA passive (conduit function) and active (pump function) emptying fractions were additionally estimated.25 LA strain was assessed as a peak reservoir strain value during LV systole to estimate LA reservoir function. All LA strain analysis was performed by a single investigator. Intraobserver variability for LA volumes was assessed in a sample of 20 randomly selected TOPCAT echocardiographic studies. The coefficients of variation for LAVmin and LAVmax were 10% and 8%, respectively. Reproducibility measures for other key echocardiographic measures have been previously published.23, 24, 25
Of 935 patients in the TOPCAT echocardiography study, 278 (30%) had echocardiograms that were not in DICOM format, 191 (20%) did not have adequate image quality for LA speckle‐tracking analysis, and 1 had missing data for body surface area. Among the remaining 465 patients, we included the 347 (75%) who were enrolled in the Americas region because of the marked regional differences in patient characteristics and outcomes previously noted in TOPCAT.26
Outcomes
All events for cardiovascular death, aborted cardiac arrest, and hospitalization for HF were adjudicated by a centralized and independent adjudication committee, according to prespecified definitions.22 The primary outcome for the TOPCAT and for the present analysis was the composite of cardiovascular death, aborted cardiac arrest, or hospitalization for HF. Secondary outcomes assessed included cardiovascular death and HF hospitalization individually.
Statistical Analysis
Continuous variables were presented as mean and SD, and categorial variables were presented as count and proportion. Comparison of baseline characteristics between patients included in the TOPCAT and those included in this analysis was performed using χ2 test for categorial variables and a t test or Wilcoxon rank‐sum test for continuous variables, as specified. Clinical characteristics and echocardiographic measures were presented by terciles of indexed minimal LA volume (LAVImin), with P values for trend across the ordered groups calculated using linear regression or Wilcoxon rank‐sum test. We assessed the association of maximal and minimal LAVs with LA function, measured by LAEF and LA strain, using Spearman rank order correlation coefficient (ρ).
The association of each measure of LA structure and function with clinical outcome variables was assessed using a time‐to‐event analysis with Cox proportional hazard regression models. The multivariable models adjusted for demographic and clinical prognostic covariates, including age, sex, race, randomization strata, randomization treatment assignment, history of AF, heart rate, New York Heart Association class, history of stroke, creatinine, hematocrit, and core laboratory LV ejection fraction, as previously described.27 The proportional hazards assumption was tested for all analyses. Additional adjustment was made for LV mass, LV global longitudinal strain (GLS), or peak early mitral inflow velocity/peak early diastolic mitral annular velocity (E/e′), which are prognostically relevant in the TOPCAT echocardiography study, in the multivariate analysis.24, 27 To compare the prognostic value of LA measures in the adjusted models, we used Weibull survival models to estimate the hazard ratios (HRs) per SD of each predictor and tested the equality of the standardized values' coefficients. Continuous net reclassification improvement associated with LAVImin was assessed for the primary composite outcome and HF hospitalization at 5 years using time‐to‐event data.
To assess whether the relationship between LA structure and function and risk of clinical outcomes was significantly modified by AF status, Cox proportional hazards models were built, including LA parameter, AF status, and an interaction term between the 2 in unadjusted models. As a sensitivity analysis, we checked the association of indexed maximal LA volume (LAVImax), which was assessed by conventional volumetric measurement with clinical outcomes. Two‐sided P<0.05 was considered statistically significant. Statistical analyses were performed using STATA 14.0 (Stata Corp, College Station, TX) and R software.
Results
Baseline Characteristics
Compared with TOPCAT participants from the Americas not included in our analysis, the 347 patients included had similar baseline characteristics, except they were less often White race (Table S1). Patients with larger LAVImin were older and had a history of AF more frequently (Table 1). Larger LAVImin was associated with greater LAVImax and worse LA function, assessed by both LAEF and LA strain. LAVImin was closely associated with LAVImax (ρ=0.80; P<0.001). LAVImin was more strongly related to LAEF and LA strain than LAVImax (both P for comparison <0.001; Figure 1). Greater LAVImin was also associated with greater LV mass, worse LV systolic function, higher E/e′, worse right ventricular systolic function, and more significant mitral regurgitation.
Table 1.
Baseline Characteristics According to Tercile of LAVImin
| Characteristics |
Tercile 1, <20.7 mL/m2 (n=116) |
Tercile 2, 20.7–31.5 mL/m2 (n=116) | Tercile 3, ≥31.5 mL/m2 (n=115) | P for Trend |
|---|---|---|---|---|
| Age, y | 67.3±9.6 | 70.4±10.2 | 74.8±8.7 | <0.001 |
| Female sex, n (%) | 63 (54) | 62 (53) | 61 (53) | 0.85 |
| White race, n (%) | 76 (66) | 83 (72) | 92 (80) | 0.014 |
| Body mass index, kg/m2 | 35.0±6.8 | 34.4±8.4 | 31.4±6.9 | <0.001 |
| Medical history, n (%) | ||||
| Hypertension | 110 (95) | 105 (91) | 103 (90) | 0.14 |
| Diabetes mellitus | 59 (51) | 56 (49) | 43 (37) | 0.040 |
| AF | 20 (17) | 41 (36) | 83 (72) | <0.001 |
| Paroxysmal | 14 (12) | 26 (22) | 19 (17) | |
| Persistent/permanent | 6 (5) | 15 (13) | 64 (56) | |
| MI | 19 (16) | 28 (24) | 21 (18) | 0.72 |
| NYHA functional class, n (%) | 0.49 | |||
| I/II | 81 (71) | 70 (60) | 76 (67) | |
| III/IV | 33 (29) | 46 (40) | 38 (33) | |
| Heart rate, bpm | 70±11 | 69±11 | 69±12 | 0.41 |
| eGFR, mL/min per 1.73 m2 | 66±23 | 64±23 | 62±19 | 0.15 |
| eGFR <60 mL/min per 1.73 m2, n (%) | 57 (49) | 58 (50) | 55 (48) | 0.84 |
| Echocardiographic measurement | ||||
| LVEDV, mL | 93±28 | 104±40 | 95±31 | 0.63 |
| LVESV, mL | 35±14 | 44±24 | 41±17 | 0.031 |
| LVMI, mg/m2 | 100±29 | 111±31 | 117±34 | <0.001 |
| LAVImax, mL/m2 | 41±10 | 51±10 | 69±19 | <0.001 |
| LVEF, % | 63±7 | 59±8 | 58±9 | <0.001 |
| LV GLS, % | −17.2±2.9 | −15.7±3.6 | −14.0±3.2 | <0.001 |
| E wave, cm/s | 81±29 | 95±29 | 102±27 | <0.001 |
| A wave, cm/s | 81±22 | 74±25 | 66±29 | <0.001 |
| E/A ratio | 1.0±0.4 | 1.4±0.7 | 1.8±0.8 | <0.001 |
| DT, ms | 208±55 | 207±61 | 184±51 | 0.001 |
| e′ (average), cm/s | 7.4±3.0 | 6.8±2.2 | 7.9±2.9 | 0.24 |
| E/e′ (average) | 12.5±5.4 | 15.8±6.6 | 15.6±7.4 | 0.001 |
| TR jet velocity, m/s | 2.8±0.4 | 2.9±0.5 | 2.9±0.5 | 0.14 |
| RVFAC, % | 0.51±0.07 | 0.50±0.08 | 0.46±0.08 | <0.001 |
| LAEF, % | 61±9 | 48±10 | 35±9 | <0.001 |
| LA reservoir strain, % | 30±8 | 23±7 | 15±6 | <0.001 |
| ≥Moderate MR, n (%) | 2 (3) | 15 (15) | 18 (18) | 0.002 |
Data are given as mean±SD, unless otherwise indicated. A, peak late diastolic mitral inflow velocity; AF indicates atrial fibrillation; bpm, beats per minute; DT, deceleration time; E, peak early mitral inflow velocity; e′, peak early diastolic mitral annular velocity; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; LA, left atrial; LAEF, LA emptying fraction; LAVImax, indexed maximal LA volume; LAVImin, indexed minimal LA volume; LV, left ventricular; LVEDV, LV end‐diastolic volume; LVEF, LV ejection fraction; LVESV, LV end‐systolic volume; LVMI, LV mass index; MI, myocardial infarction; MR, mitral regurgitation; NYHA, New York Heart Association; RV FAC, right ventricular fractional area change; and TR, tricuspid regurgitation.
Figure 1. Relationship between left atrial (LA) volume and function.
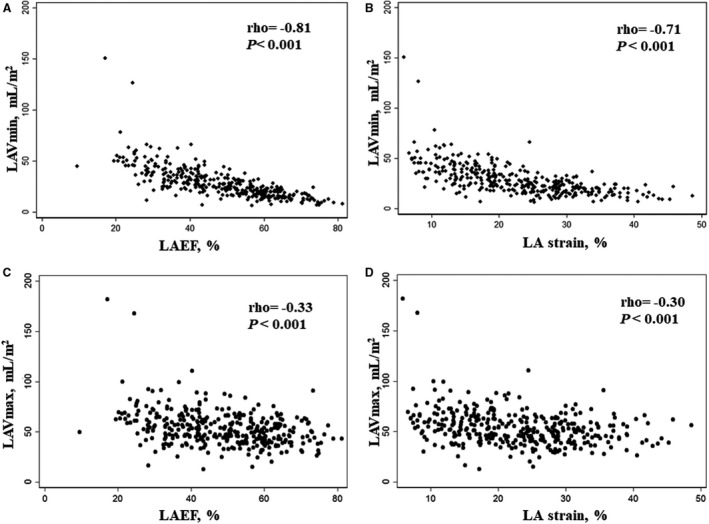
(A–B) Relations of LAVmin with LAEF or LA strain. (C–D) Relations of LAVmax with LAEF or LA strain. LAEF indicates LA emptying fraction; LAVmax, maximal LA volume; and LAVmin, minimal LA volume.
Prognostic Value of LA Volumes and LA Function
During a median follow‐up of 2.5 years (interquartile range, 1.4–3.8 years), 107 participants (31%) experienced a primary composite end point of cardiovascular death, aborted cardiac death, or HF hospitalization. Among them, 46 patients (13%) died as a result of cardiovascular cause and 81 patients (23%) were hospitalized for HF. Minimal LAVI was associated with higher risk of the primary composite outcome (HR, 1.23 [95% CI, 1.05–1.44] per 1‐SD increase; P=0.011) and of HF hospitalization alone (HR, 1.30 [95% CI, 1.10–1.52] per 1‐SD increase; P=0.002), but not of cardiovascular death alone (HR, 1.16 [95% CI, 0.87–1.56] per 1‐SD increase; P=0.31; Table 2). These associations persisted without appreciable attenuation after adjusting for age, sex, race, randomization strata, randomized treatment assignment, history of AF, heart rate, New York Heart Association class, history of stroke, creatinine, hematocrit, and LV ejection fraction (adjusted HR, 1.35 [95% CI, 1.12‐.1.61] per 1‐SD increase; P=0.001 for primary composite outcome; adjusted HR, 1.42 [95% CI, 1.17–1.71] per 1‐SD increase; P<0.001 for HF hospitalization; Table 2). In contrast, LAVImax was not related to the primary composite end point of cardiovascular death, aborted cardiac death, or HF hospitalization and cardiovascular death (adjusted HR, 1.18 [95% CI, 0.97–1.44] per 1‐SD increase; P=0.09 for primary composite outcome; adjusted HR, 1.04 [95% CI, 0.73–1.47] per 1‐SD increase; P=0.84 for cardiovascular death), but was related to hospitalization for HF alone (adjusted HR, 1.25 [95% CI, 1.02–1.54] per 1‐SD increase; P=0.034) in both unadjusted and adjusted models. LAVImin was more prognostic for the primary composite outcome than LAVImax (P=0.032), but not for individual components of primary outcome (P=0.06 for HF hospitalization; P=0.12 for cardiovascular death). To avoid the effect of obesity, the relationship of LAVmin with clinical outcomes was additionally assessed and LAVmin showed comparable predictive value with LAVImin (adjusted HR, 1.37 [95% CI, 1.16–1.62] per 1‐SD increase; P<0.001 for primary composite outcome; adjusted HR, 1.16 [95% CI, 0.81–1.66] per 1‐SD increase; P=0.41 for cardiovascular death; adjusted HR, 1.43 [95% CI, 1.20–1.69] per 1‐SD increase; P<0.001 for HF hospitalization). The association of LAEF and LA strain with primary composite end point and HF hospitalization was of marginal significance in unadjusted analysis, and was significant after adjustment for baseline characteristics, randomization strata, and treatment assignment. LAEF had a comparable HR of a composite outcome (P for comparison=0.58) and HF hospitalization (P for comparison=0.39) with LA strain.
Table 2.
Association of LA Structure and Function With Cardiovascular Outcomes in Univariate and Multivariate Analyses
| Variable | Event/No. |
LAVImax per 1‐SD Increase |
LAVImin per 1‐SD Increase |
LAEF per 1‐SD Decrease |
LA Reservoir Strain per 1‐SD Decrease |
||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Primary | |||||||||
| Unadjusted | 107/347 | 1.18 (0.99–1.41) | 0.06 | 1.23 (1.05–1.44) | 0.011 | 1.20 (0.99–1.46) | 0.06 | 1.17 (0.97–1.42) | 0.11 |
| Adjusted | 103/331 | 1.18 (0.97–1.44) | 0.09 | 1.35 (1.12–1.61) | 0.001 | 1.63 (1.22–2.18) | 0.001 | 1.54 (1.17–2.03) | 0.002 |
| Cardiovascular death | |||||||||
| Unadjusted | 46/347 | 1.10 (0.81–1.51) | 0.61 | 1.16 (0.87–1.56) | 0.31 | 1.09 (0.82–1.47) | 0.55 | 1.14 (0.85–1.53) | 0.39 |
| Adjusted | 44/331 | 1.04 (0.73–1.47) | 0.84 | 1.19 (0.84–1.69) | 0.33 | 1.26 (0.82–1.93) | 0.29 | 1.36 (0.90–2.05) | 0.14 |
| HF hospitalization | |||||||||
| Unadjusted | 81/347 | 1.26 (1.06–1.51) | 0.010 | 1.30 (1.10–1.52) | 0.002 | 1.33 (1.06–1.66) | 0.013 | 1.27 (1.01–1.59) | 0.041 |
| Adjusted | 78/331 | 1.25 (1.02–1.54) | 0.034 | 1.42 (1.17–1.71) | <0.001 | 2.04 (1.45–2.86) | <0.001 | 1.83 (1.31–2.54) | <0.001 |
SD for LAVImax=17.6 mL/m2, SD for LAVImin=15.4 mL/m2, SD for LAEF=14.2%, and SD for LA strain=8.9%; adjusted for age, sex, race, randomization strata, randomized treatment assignment, history of atrial fibrillation, heart rate, New York Heart Association class, history of stroke, creatinine, hematocrit, and left ventricular ejection fraction. HF indicates heart failure; HR, hazard ratio; LA, left atrial; LAEF, LA emptying fraction; LAVImax, indexed maximal LA volume; and LAVImin, indexed minimal LA volume.
Table 3 showed the association of LA structure and function after additional adjusting for LV mass index, LV GLS, or E/e′, which are prognostically relevant in the TOPCAT, in the multivariable models. The association of LAVImin with the primary composite outcome remained significant after additional adjustment for LV mass or LV GLS, but not after further adjustment for E/e′. LAVImin was significantly related to HF hospitalization when additionally adjusted for LV mass, LV GLS, or E/e′. Similar findings were observed for LAEF. LA strain remained significantly associated with the primary composite end point and HF hospitalization alone in multivariable analysis only after additional adjustment for LV mass index, but not independent of LV GLS or E/e′. Both LAEF and LA strain were prognostic of primary outcome and hospitalization for HF, independent of LAVImax or LAVImin. However, LAVImin was not associated with clinical outcomes independent of LAEF or LA strain. When additionally adjusted for mitral regurgitation, which can affect LA volume and function, LAVImin, LAEF, and LA strain were significantly related to the primary outcome and HF hospitalization. We performed sensitivity analyses using LAVImax based on volumetric assessment and did not find any significant association with primary composite outcome, cardiovascular death, and HF hospitalization. The continuous net reclassification improvement improved around 0.2 but not of statistical significance with the addition of LAVImin to clinical predictors alone and in combination with LVMI or LV GLS for primary composite outcome and HF hospitalization alone (Table S2).
Table 3.
Association of LA Structure and Function With Primary Composite Outcome and HF Hospitalization in Multivariable Analysis
| Variable | Event/No. |
LAVImax per 1‐SD Increase |
LAVImin per 1‐SD Increase |
LAEF per 1‐SD Decrease |
LA Reservoir Strain per 1‐SD Decrease |
||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Primary | |||||||||
| Model 1 | 103/331 | 1.18 (0.97–1.44) | 0.09 | 1.35 (1.12–1.61) | 0.001 | 1.63 (1.22–2.18) | 0.001 | 1.54 (1.17–2.03) | 0.002 |
| Model 1+LVMI | 103/329 | 1.11 (0.89–1.38) | 0.37 | 1.30 (1.06–1.58) | 0.011 | 1.58 (1.18–2.11) | 0.002 | 1.47 (1.11–1.94) | 0.007 |
| Model 1+LV GLS | 87/285 | 1.19 (0.96–1.47) | 0.12 | 1.30 (1.05–1.60) | 0.015 | 1.49 (1.05–2.11) | 0.024 | 1.29 (0.92–1.82) | 0.15 |
| Model 1+E/e′ | 87/270 | 1.13 (0.90–1.42) | 0.29 | 1.24 (0.98–1.57) | 0.07 | 1.26 (0.89–1.79) | 0.19 | 1.19 (0.86–1.64) | 0.29 |
| Model 1+LAVImax | 103/331 | 1.58 (1.17–2.13) | 0.003 | 1.49 (1.13–1.98) | 0.005 | ||||
| Model 1+LAVImin | 103/331 | … | … | … | … | 1.45 (1.02–2.06) | 0.037 | 1.37 (1.01–1.87) | 0.044 |
| Model 1+LAEF | 103/331 | 1.09 (0.89–1.34) | 0.39 | 1.17 (0.91–1.49) | 0.23 | ||||
| Model 1+LA reservoir strain | 103/331 | 1.11 (0.91–1.35) | 0.30 | 1.21 (0.96–1.51) | 0.10 | ||||
| HF hospitalization | |||||||||
| Model 1 | 78/331 | 1.25 (1.02–1.54) | 0.034 | 1.42 (1.17–1.71) | <0.001 | 2.04 (1.45–2.86) | <0.001 | 1.83 (1.31–2.54) | <0.001 |
| Model 1+LVMI | 78/329 | 1.16 (0.91–1.48) | 0.23 | 1.36 (1.10–1.67) | 0.004 | 1.96 (1.39–2.75) | <0.001 | 1.72 (1.23–2.40) | 0.002 |
| Model 1+LV GLS | 66/285 | 1.26 (1.01–1.58) | 0.043 | 1.37 (1.11–1.71) | 0.004 | 1.82 (1.22–2.74) | 0.004 | 1.48 (0.99–2.22) | 0.06 |
| Model 1+E/e′ | 67/270 | 1.17 (0.91–1.51) | 0.22 | 1.31 (1.02–1.67) | 0.035 | 1.55 (1.03–2.33) | 0.034 | 1.32 (0.90–1.94) | 0.15 |
| Model 1+LAVImax | 78/331 | 1.95 (1.37–2.78) | <0.001 | 1.74 (1.24–2.44) | 0.001 | ||||
| Model 1+LAVImin | 78/331 | … | … | … | … | 1.79 (1.21–2.65) | 0.004 | 1.58 (1.10–2.25) | 0.013 |
| Model 1+LAEF | 78/331 | 1.11 (0.89–1.38) | 0.36 | 1.14 (0.87–1.50) | 0.33 | ||||
| Model 1+LA reservoir strain | 78/331 | 1.14 (0.92–1.41) | 0.25 | 1.22 (0.96–1.55) | 0.96 | ||||
SD for LAVImax=17.6 mL/m2, SD for LAVImin=15.4 mL/m2, SD for LAEF=14.2%, and SD for LA strain=8.9%. Model 1 included age, sex, race, randomization strata, randomized treatment assignment, history of atrial fibrillation, heart rate, New York Heart Association class, history of stroke, creatinine, hematocrit, and left ventricular ejection fraction. E indicates peak early mitral inflow velocity; e′, peak early diastolic mitral annular velocity; GLS, global longitudinal strain; HF, heart failure; HR, hazard ratio; LA, left atrial; LAEF, LA emptying fraction; LAVImax, indexed maximal LA volume; LAVImin, indexed minimal LA volume; LV, left ventricular; and LVMI, LV mass index.
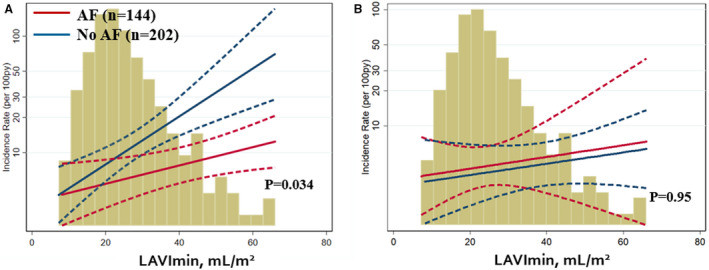
Prognostic Value of LA Structure and Function in Patients With AF
In unadjusted model, history of AF significantly modified the relationship between LAVImin and HF hospitalization (P for interaction=0.034; Figure 2). The association between LAVImin and HF hospitalization was greater in magnitude in patients without history of AF (HR, 1.95 [95% CI, 1.40–2.72]; P<0.001) than those with history of AF (HR, 1.26 [95% CI, 1.01–1.57]; P=0.038). History of AF did not modify the relationship of LAVImin with the primary composite outcome or with cardiovascular death alone (P for interaction=0.42 for primary outcome; P for interaction=0.95 for cardiovascular death). The relationships between LAEF or LA strain and clinical outcomes were not modified by history of AF. In contrast, the presence of AF rhythm at the time of echocardiographic examination did not modify the relationship between any LA measures and outcomes. The type of AF did not significantly modify the relationship between LAVImin and clinical outcomes (Table S3).
Figure 2. Prognostic impact of indexed minimal left atrial volume (LAVImin) on heart failure hospitalization (A) and cardiovascular death (B), according to history of atrial fibrillation (AF).
Discussion
In this study, larger LAVImin was associated with higher rates of the primary end point and HF hospitalization alone, even after adjustment for clinical and conventional echocardiographic measures in patients with HFpEF. LAVImin was better than LAVImax in predicting clinical outcomes in this population. Although both LAEF and LA strain related to LAVImin more closely than LAVImax, they provide prognostic information independent of LAVImin.
Maximum LA size has been used as one of the principal components in assessing diastolic function and can be prognostic because it reflects persistent elevation of LV filling pressure. Among several measures of LA size, LAVmax has been suggested as an important surrogate for the severity and chronicity of LV diastolic dysfunction and is prognostic in a variety of cardiovascular diseases.7, 8 Despite numerous studies demonstrating the prognostic utility of LA size using LAVmax, recent studies have reported that LAVmin is better in reflecting LV filling pressure and prognosticating clinical outcomes than LAVmax.15, 16, 17, 18, 19, 28 The LA can be stretched by LV longitudinal systolic function through systolic descent of the mitral annular plane toward LV apex, which can influence LAVmax. LAVmin is measured when the LA is more directly exposed to LV pressure at end diastole. It has been demonstrated to be a better correlate of LV diastolic dysfunction and to have a stronger association with NT‐proBNP than LAVmax.15, 16 LAVmin predicted AF development better,29, 30 and in a prospective study of 547 participants, LAVmin was superior to LAVmax for predicting newly developed AF or atrial flutter.29 Prior data have demonstrated that LAVmin was more prognostic for predicting cardiovascular events than LAVmax in a community cohort or in patients with cardiovascular disease.17, 18, 31 However, data on its utility in HFpEF are limited. In patients with HFpEF, LA remodeling is important in both making the diagnosis and assessing prognosis.1, 2, 3, 32 In a small cohort of 40 patients with HFpEF, LAVmin was shown to have the strongest association with HF hospitalization.33 Our study included larger number of patients with prospective follow‐up and found that LAVImin was more prognostic than LAVImax in this population. However, LAVImin did not remain a significant predictor for the primary composite outcome after adjusting for E/e′, suggesting that LAVImin and E/e′ might both be indirect measures of filling pressure and thus not independent. In our data, LAVImin provided statistically insignificant improvement in predicting clinical outcomes beyond clinical predictors alone and in combination with other echocardiographic parameters for primary composite outcome and HF hospitalization alone. Given that the magnitude of continuous net reclassification improvement was around 0.2, statistical power might be limited by a relatively small number of events.
In our study, both LAEF and LA strain, reflecting LA reservoir function, provided prognostic information, but the prognostic values of LA strain were attenuated by LV GLS more prominently than LAEF. LA reservoir strain is known to be dependent on LV systolic function, partly because it is influenced by movement of atrioventricular junction.34 LA reservoir strain can be reduced in the case of normal LA pressure if LV systolic function is reduced. This is similar to the results from the prior study, which investigated the prognostic values of LA function in the TOPCAT cohort, who were in sinus rhythm at the time of echocardiography,25 whereas our current study included the patients who enrolled in the Americas region, irrespective of rhythm at the time of echocardiography. Another study suggested that LAEF, based on volumetric measurement, might have low sensitivity to detect subtle LA dysfunction compared with LA strain in patients with LV diastolic dysfunction.35 However, our current study demonstrated that LA strain had similar prognostic value to LAEF. In HFpEF, both parameters might have similar magnitude of predictive values for adverse clinical outcome. Although LA reservoir function assessed by LAEF and LA strain had stronger association with LAVImin than LAVImax, both LA strain and LAEF were predictive of clinical outcome independent of LAVImin. However, LAVImin was not associated with clinical outcomes independent of LA functional parameters. In HFpEF, LA dilatation with sustained LA pressure will lead to LA dysfunction. Applying Frank‐Starling mechanism to LA mechanics, LA contractility would be expected to increase with increases of LA size in response to LA myocardial stretch, but it will start to decrease after a certain point in the setting of severe LA enlargement. Thus, the assessment of LA function can be expected to provide additional prognostic information in addition to LA volume. Until LA functional assessment becomes more widely available, LAVImin may provide more information without additional analysis or dedicated software compared with LAVImax, which is most commonly used in clinical practice.
In our study, LAVImin was shown to have stronger prognostic value in predicting HF hospitalization for patients without history of AF than in those with history of AF. Measurement robustness of LAV because of beat‐to‐beat variation in AF rhythm may not explain all the reasons why LAVImin showed less robustness for prediction of clinical outcomes in those with history of AF, because the presence of AF rhythm at the time of echocardiographic study did not have any interaction with LA parameters with respect to clinical outcomes in our study. The relationship between AF and LA structural remodeling can be more complex, and LA size may be a less reliable parameter for LV diastolic function and LV filling pressure in patients with AF.7 Although causal relationships between AF and LA size cannot be clarified from the cross‐sectional design of this study, LA enlargement can be related to multiple factors in AF and LA can enlarge with replacement fibrosis because of atrial cardiomyopathy regardless of LA filling pressure.36 In this circumstances, LA function might be a more robust prognosticator than LA size. Impairment of LA function can be present even when the LA is not enlarged in patients with AF.37 Given that AF can come directly from cardiomyocyte abnormalities, LA functional change can occur irrespective of LA structural remodeling, and importance of LA volume might be less pronounced in patients with AF compared with those without AF.36, 38
Several limitations of this study should be noted. First, LA volume can often be underestimated by 2‐dimensinal echocardiography, even in the dedicated views for LA, because it is easy to have foreshortened views of LA cavity, and the geometric assumptions involved in LA volume measurements may not always be appropriate for remodeled LAs. Three‐dimensional echocardiography is more accurate and reproducible than 2‐dimensional echocardiographic measurements.18, 39 Second, we used LA volumes from speckle‐tracking methods in our study. Although it cannot be directly transferred to the LA volumes based on volumetric measurements, previous studies have shown a good correlation between speckle‐tracking–derived LA volume and manually traced LA volume.40 Third, we assessed only a subset of the patients who enrolled in the overall TOPCAT, whereas the 347 patients included had similar baseline characteristics, except radial difference, compared with TOPCAT participants from America. Our findings may not extrapolate to the overall TOPCAT population, and to patients with HFpEF in the community, given the inclusion and exclusion criteria of the TOPCAT; however, the TOPCAT inclusion/exclusion criteria were broad and are similar to patients with HFpEF in community‐based studies.23 Finally, we did not assess the association of change of LA remodeling with clinical outcomes or impact of treatment with spironolactone versus placebo on changes of LA measures in this current analysis. Our findings should be validated in larger cohorts, and further research would be necessary to investigate the effect of changing LV filling pressure on LAVmin and the relationship of changes in LA structural and function remodeling to clinical outcomes. Adequate age‐ and sex‐specific normal reference values (from large population‐based studies) need to be defined for this measure to have utility in clinical practice.
Conclusions
In patients with HFpEF, LAVImin was more predictive of cardiovascular death, aborted cardiac arrest, or HF hospitalization than LAVImax, suggesting it might be more useful in identifying patients at higher risk for cardiovascular events and might play a role as a potential therapeutic target and an end point for evaluation of HFpEF therapies. LA functional parameters provide prognostic information independent of LAVImin.
Sources of Funding
Dr Shin was supported by Inha University Research grant (INHA61667).
Disclosures
Dr S.J. Shah has received research grants from the National Institutes of Health (NIH), Actelion, AstraZeneca, Corvia, and Novartis; and has served as a consultant or an Advisory Board member for Abbott, Actelion, AstraZeneca, Amgen, Bayer, Boehringer Ingelheim, Cardiora, Coridea, CVRx, Eisai, Ionis, Ironwood, Merck, MyoKardia, Novartis, Pfizer, Sanofi, Shifamed, Tenax, and United Therapeutics. Dr Zile has received grants and personal fees from Novartis, CVRx, and Medtronic; and has received personal fees from Abbott, Boston Scientific, EBR, Endotronics, Ironwood, Merck, Myokardia, and V Wave. Dr Pfeffer has received consulting fees from Amgen, AstraZeneca, Bayer, DalCor Pharma UK, Genzyme, Lilly, Medicines Company, MedImmune, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Salix, Sanderling, Sanofi, Takeda, Teva, Thrasos, and Vericel; and has received research grant support from Amgen, Celladon, Novartis, and Sanofi. The Brigham and Women's Hospital has patents for the use of inhibitors of the renin‐angiotensin system in selected survivors of myocardial infarction with Novartis Pharmaceuticals, on which Dr Pfeffer is a coinventor. His share of the licensing agreement is irrevocably transferred to charity. Dr A.M. Shah has received grants from NIH/National Heart, Lung, and Blood Institute (NHLBI); has received research support from Novartis through Brigham and Women's Hospital; and has received consulting fees from Philips Ultrasound and Bellerophon Therapeutics. Dr Solomon has received research grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Celladon, Gilead, GSK, Ionis Pharmaceutics, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Novartis, Sanofi Pasteur, and Theracos; and has consulted for Alnylam, Amgen, AstraZeneca, Bayer, BMS, Corvia, Gilead, GSK, Ironwood, Merck, Novartis, Pfizer, Takeda, and Theracos. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019545
For Sources of Funding and Disclosures, see page 10.
References
- 1.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. DOI: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. DOI: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 3.Rossi A, Gheorghiade M, Triposkiadis F, Solomon SD, Pieske B, Butler J. Left atrium in heart failure with preserved ejection fraction: structure, function, and significance. Circ Heart Fail. 2014;7:1042–1049. DOI: 10.1161/CIRCHEARTFAILURE.114.001276. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, et al. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure‐Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006;47:1997–2004. DOI: 10.1016/j.jacc.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 5.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. DOI: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 6.Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63:493–505. DOI: 10.1016/j.jacc.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 7.Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, Cha SS, Seward JB. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006;47:1018–1023. DOI: 10.1016/j.jacc.2005.08.077. [DOI] [PubMed] [Google Scholar]
- 8.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. DOI: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE; I‐PRESERVE Investigators . Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. DOI: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 10.Rossi A, Cicoira M, Florea VG, Golia G, Florea ND, Khan AA, Murray STM, Nguyen JT, O'Callaghan P, Anand IS, et al. Chronic heart failure with preserved left ventricular ejection fraction: diagnostic and prognostic value of left atrial size. Int J Cardiol. 2006;110:386–392. DOI: 10.1016/j.ijcard.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko H, Koike A, Senoo K, Tanaka S, Suzuki S, Nagayama O, Sagara K, Otsuka T, Matsuno S, Funada R, et al. Role of cardiopulmonary dysfunction and left atrial remodeling in development of acute decompensated heart failure in chronic heart failure with preserved left ventricular ejection fraction. J Cardiol. 2012;59:359–365. DOI: 10.1016/j.jjcc.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, Chakrabarti S, Sauer AJ, Rich JD, Freed BH, et al. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7:288–299. DOI: 10.1161/CIRCHEARTFAILURE.113.000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community‐based study. J Am Coll Cardiol. 2009;53:1119–1126. DOI: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donal E, Lund LH, Oger E, Bosseau C, Reynaud A, Hage C, Drouet E, Daubert JC, Linde C; KaRen Investigators . Importance of combined left atrial size and estimated pulmonary pressure for clinical outcome in patients presenting with heart failure with preserved ejection fraction. Eur Heart J Cardiovasc Imaging. 2017;18:629–635. DOI: 10.1093/ehjci/jex005. [DOI] [PubMed] [Google Scholar]
- 15.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart. 2012;98:813–820. DOI: 10.1136/heartjnl-2011-301388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedberg P, Selmeryd J, Leppert J, Henriksen E. Left atrial minimum volume is more strongly associated with N‐terminal pro‐B‐type natriuretic peptide than the left atrial maximum volume in a community‐based sample. Int J Cardiovasc Imaging. 2016;32:417–425. DOI: 10.1007/s10554-015-0800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russo C, Jin Z, Homma S, Rundek T, Elkind MSV, Sacco RL, Di Tullio MR. LA phasic volumes and reservoir function in the elderly by real‐time 3d echocardiography: normal values, prognostic significance, and clinical correlates. JACC Cardiovasc Imaging. 2017;10:976–985. DOI: 10.1016/j.jcmg.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu VC, Takeuchi M, Kuwaki H, Iwataki M, Nagata Y, Otani K, Haruki N, Yoshitani H, Tamura M, Abe H, et al. Prognostic value of LA volumes assessed by transthoracic 3D echocardiography: comparison with 2D echocardiography. JACC Cardiovasc Imaging. 2013;6:1025–1035. DOI: 10.1016/j.jcmg.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Russo C, Jin Z, Liu R, Iwata S, Tugcu A, Yoshita M, Homma S, Elkind MSV, Rundek T, DeCarli C, et al. LA volumes and reservoir function are associated with subclinical cerebrovascular disease: the CABL (Cardiovascular Abnormalities and Brain Lesions) study. JACC Cardiovasc Imaging. 2013;6:313–323. DOI: 10.1016/j.jcmg.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thadani SR, Shaw RE, Fang Q, Whooley MA, Schiller NB. Left atrial end‐diastolic volume index as a predictor of cardiovascular outcomes: the Heart and Soul Study. Circ Cardiovasc Imaging. 2020;13:e009746. DOI: 10.1161/CIRCIMAGING.119.009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. DOI: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 22.Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–972. DOI: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O’Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, et al. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail. 2014;7:104–115. DOI: 10.1161/CIRCHEARTFAILURE.113.000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132:402–414. DOI: 10.1161/CIRCULATIONAHA.115.015884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos ABS, Roca GQ, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Fang JC, Zile MR, Pitt B, Solomon SD, et al. Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9:e002763. DOI: 10.1161/CIRCHEARTFAILURE.115.002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. DOI: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 27.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O’Meara E, Desai AS, Heitner JF, Li G, Fang J, et al. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014;7:740–751. DOI: 10.1161/CIRCHEARTFAILURE.114.001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Posina K, McLaughlin J, Rhee P, Li L, Cheng J, Schapiro W, Gulotta RJ, Berke AD, Petrossian GA, Reichek N, et al. Relationship of phasic left atrial volume and emptying function to left ventricular filling pressure: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2013;15:99. DOI: 10.1186/1532-429X-15-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fatema K, Barnes ME, Bailey KR, Abhayaratna WP, Cha S, Seward JB, Tsang TS. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study. Eur J Echocardiogr. 2009;10:282–286. DOI: 10.1093/ejechocard/jen235. [DOI] [PubMed] [Google Scholar]
- 30.Schaaf M, Andre P, Altman M, Maucort‐Boulch D, Placide J, Chevalier P, Bergerot C, Thibault H. Left atrial remodelling assessed by 2D and 3D echocardiography identifies paroxysmal atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2017;18:46–53. DOI: 10.1093/ehjci/jew028. [DOI] [PubMed] [Google Scholar]
- 31.Shin SH, Jang JH, Baek YS, Kwon SW, Park SD, Woo SI, Kim DH, Kwan J. Prognostic impact of left atrial minimal volume on clinical outcome in patients with non‐obstructive hypertrophic cardiomyopathy. Int Heart J. 2018;59:991–995. DOI: 10.1536/ihj.17-606. [DOI] [PubMed] [Google Scholar]
- 32.Hohendanner F, Messroghli D, Bode D, Blaschke F, Parwani A, Boldt LH, Heinzel FR. Atrial remodelling in heart failure: recent developments and relevance for heart failure with preserved ejection fraction. ESC Heart Fail. 2018;5:211–221. DOI: 10.1002/ehf2.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Issa O, Peguero JG, Podesta C, Diaz D, De La Cruz J, Pirela D, Brenes JC. Left atrial size and heart failure hospitalization in patients with diastolic dysfunction and preserved ejection fraction. J Cardiovasc Echogr. 2017;27:1–6. DOI: 10.4103/2211-4122.199064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramkumar S, Yang H, Wang Y, Nolan M, Negishi T, Negishi K, Marwick TH. Association of the active and passive components of left atrial deformation with left ventricular function. J Am Soc Echocardiogr. 2017;30:659–666. DOI: 10.1016/j.echo.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Morris DA, Takeuchi M, Krisper M, Kohncke C, Bekfani T, Carstensen T, Hassfeld S, Dorenkamp M, Otani K, Takigiku K, et al. Normal values and clinical relevance of left atrial myocardial function analysed by speckle‐tracking echocardiography: multicentre study. Eur Heart J Cardiovasc Imaging. 2015;16:364–372. DOI: 10.1093/ehjci/jeu219. [DOI] [PubMed] [Google Scholar]
- 36.Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D’Avila A, Dobrev D, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Heart Rhythm. 2017;14:e3–e40. DOI: 10.1016/j.hrthm.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta DK, Shah AM, Giugliano RP, Ruff CT, Antman EM, Grip LT, Deenadayalu N, Hoffman E, Patel I, Shi M, et al. Left atrial structure and function in atrial fibrillation: ENGAGE AF‐TIMI 48. Eur Heart J. 2014;35:1457–1465. DOI: 10.1093/eurheartj/eht500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obokata M, Negishi K, Kurosawa K, Tateno R, Tange S, Arai M, Amano M, Kurabayashi M. Left atrial strain provides incremental value for embolism risk stratification over CHA₂DS₂‐VASc score and indicates prognostic impact in patients with atrial fibrillation. J Am Soc Echocardiogr. 2014;27:709–716. DOI: 10.1016/j.echo.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Badano LP, Miglioranza MH, Mihăilă S, Peluso D, Xhaxho J, Marra MP, Cucchini U, Soriani N, Iliceto S, Muraru D. Left atrial volumes and function by three‐dimensional echocardiography: reference values, accuracy, reproducibility, and comparison with two‐dimensional echocardiographic measurements. Circ Cardiovasc Imaging. 2016;9:3004229. DOI: 10.1161/CIRCIMAGING.115.004229. [DOI] [PubMed] [Google Scholar]
- 40.Okamatsu K, Takeuchi M, Nakai H, Nishikage T, Salgo IS, Husson S, Otsuji Y, Lang RM. Effects of aging on left atrial function assessed by two‐dimensional speckle tracking echocardiography. J Am Soc Echocardiogr. 2009;22:70–75. DOI: 10.1016/j.echo.2008.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


