Abstract
Background
Readmissions in patients with congestive heart failure are common and often preventable. Limited data suggest that patients discharged to a less intensive postacute care setting than recommended are likely to readmit. We examined whether postacute setting discordance (discharge to a less intensive postacute setting than recommended by a physical and occupational therapist) was associated with hospital readmission in patients with congestive heart failure. We also assessed sociodemographic and clinical predictors of setting discordance.
Methods and Results
Retrospective analysis of administrative claims and electronic health record data was conducted on 25 500 adults with a discharge diagnosis of congestive heart failure from 12 acute care hospitals in Western Pennsylvania. Generalized linear mixed models were estimated to examine the association between postacute setting discordance and 30‐day hospital readmission and to identify predictors of setting discordance. The 30‐day readmission and postacute setting discordance rates were high (23.7%, 20.6%). While controlling for demographic and clinical covariates, patients in discordant postacute settings were more likely to be readmitted within 30 days (adjusted odds ratio [OR], 1.12; 95% CI, 1.04–1.20). The effect was also seen in the subgroup of patients with low mobility scores (adjusted OR, 1.20; 95% CI, 1.08–1.33). Factors associated with setting discordance were lower‐income, higher comorbidity burden, therapist recommendation disagreement, and midrange mobility limitations.
Conclusions
Postacute setting discordance was associated with an increased readmission risk in patients hospitalized with congestive heart failure. Maximizing concordance between therapist recommended and actual postacute discharge setting may decrease readmissions. Understanding factors associated with post‐acute setting discordance can inform strategies to improve the quality of the discharge process.
Keywords: heart failure, occupational therapy, physical therapy, postdischarge rehabilitation, readmission
Subject Categories: Heart Failure, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- ADL
activities of daily living
- AM‐PAC
Activity Measure for Post‐Acute Care
- IRF
inpatient rehabilitation facility
- OT
occupational therapist
- PT
physical therapist
- SNF
skilled nursing facility
Clinical Perspective
What Is New?
A total of 25 500 patients with primary or secondary discharge diagnoses of congestive heart failure and evaluations from rehabilitation therapists had 30‐day all‐cause readmission rates of 23.7%, and 1 in 5 patients received less intensive postacute care rehabilitation than recommended by the therapists.
Patients who discharged to a discordant postacute rehabilitation setting were associated with a higher risk of readmission, and a subgroup with lower physical function scores had an even greater risk.
There was less adherence to therapists’ discharge setting recommendation if patients had a lower income, more comorbidities, midrange mobility limitations, and conflicting recommendations from both therapists.
What Are the Clinical Implications?
Thirty‐day readmission rates are high for patients with congestive heart failure, as are nonadherence rates to therapists’ postacute rehabilitation recommendations.
The quality of the discharge process may improve when incorporating clear and consistent therapist postacute setting discharge recommendations with multidisciplinary discharge planning.
Mediating risk factors for discordant discharges is a potential strategy to increase healthcare value and patient outcomes.
A staggering public health problem, congestive heart failure (CHF) affects over 6 million Americans and is associated with substantial mortality, morbidity, and healthcare expenditures, costing over $39 billion a year.1 CHF is the leading cause of hospitalizations in older people (≥65 years), accounting for over 1 million hospitalizations annually.2 Unplanned readmissions are astoundingly common, with nearly 1 in 4 patients with CHF being readmitted within 30 days of hospital discharge.3 Many of these readmissions are considered preventable.4 In 2013, the Centers for Medicare and Medicaid Services’ Hospital Readmissions Reduction Program5 initiated financial penalties for hospitals with high 30‐day risk‐standardized readmission rates for patients hospitalized for acute CHF.
One key factor that may decrease the risk of hospital readmission and improve patient outcomes is successful coordination of discharge recommendations made by the patient’s care team.6 For example, helping the patient and family make informed and suitable decisions about postacute care may prevent readmission.6 Physical therapists (PTs) and occupational therapists (OTs) play key roles in determining the most appropriate postacute care setting for patients discharged from the acute care hospital with physical and cognitive impairments. Therapists use a complex decision‐making process for discharge planning based on the patient’s impairments and fall risk, the patient’s capacity to perform basic activities of daily living (ADL) within their individual environment (eg, bed mobility, walking, transfers), the ability of the caregiver to provide physical and social support as needed, and patient and family preference.7, 8
There are varying levels of postacute rehabilitation care. Inpatient rehabilitation facilities (IRFs) abide by Centers for Medicare and Medicaid Services guidelines providing at least 3 hours of rehabilitation a day for 5 days a week.9 Patients discharged to an IRF must be able to tolerate the intensity of the rehabilitation. Skilled nursing facilities (SNFs) do not have specific rehabilitation intensity guidelines but are considered a “less intensive” setting relative to IRFs; patients in SNFs may be seen 5 days a week for rehabilitation but for less time. The frequency and duration of community‐based home health rehabilitation varies but on average is about 2 to 3 times per week, with patients often given an exercise program to follow on the days not seen by a therapist. Because exercise intensity in the CHF population yields better outcomes, getting patients to the appropriate rehabilitation discharge setting where exercise is adequately dosed is important.10, 11
Limited evidence on small, heterogeneous samples suggests that readmission risk increases when postacute rehabilitation recommendations by therapists are not followed.12, 13 Our study extends prior work on the association between setting discordance (ie, disagreement between the recommended versus actual discharge setting) and risk of hospital readmission by focusing on CHF, a high‐priority discharge diagnosis; examining a larger sample of patients across multiple geographically diverse hospitals; using recommendations from both PTs and OTs; and including important covariates in our analyses representing the patients’ clinical and functional status. The objectives of our study were to (1) describe the degree of postacute care setting discordance (defined as the patient being discharged to a less intensive postacute setting than recommended by the PT and OT) for patients discharged from the acute care setting with a diagnosis of CHF; (2) examine the association between setting discordance and 30‐day all‐cause hospital readmission; and (3) identify sociodemographic and clinical predictors of setting discordance. We hypothesized that patients discharged to a lower‐intensity setting than recommended by the therapist would have increased odds of hospital readmission when compared with those who were discharged to the same or higher‐intensity setting than recommended. We also hypothesized that both sociodemographic and clinical characteristics would be associated with postacute setting discordance.
Methods
Study Design and Sample
This retrospective cohort study examined electronic health records and administrative claims data from a large healthcare system in Western Pennsylvania. Data from January 1, 2016, to March 30, 2018, were examined. The data used in this study are proprietary, and per UPMC policy, data disclosure would require a suitable data use agreement requested to UPMC Quality Review Committee at AskQRC@upmc.edu. Details on the creation of our analytic data set and the statistical programming are available by the corresponding author upon request.
We identified patients admitted to 1 of 12 acute care hospitals located in urban and rural settings with a primary or secondary CHF diagnosis based on International Classification of Diseases, Tenth Revision (ICD‐10) codes (Table S1). Patients were included if they were aged ≥18 years, survived their acute care stay, and received at least one PT or OT visit during their stay. Patients were excluded if they transferred to another hospital, died within 30 days after discharge, had missing discharge destinations, or had missing or unclear postacute care recommendations by the therapist.
Study Variables
Our outcome variable was 30‐day all‐cause, within health system readmission. Our exposure variable was postacute care setting discordance, defined as being discharged to a less intensive rehabilitation setting than recommended by the therapist.6, 13 The patients’ discharge destinations were extracted from billing data and categorized as: home, home with home health therapy, or postacute care facility (SNF or IRF). Discharge to a SNF or IRF may depend upon availability14 and patient insurance15; therefore, we combined these 2 categories to represent facility‐based postacute care.16, 17, 18, 19 PT and OT postacute care recommendations were extracted from the electronic health record discharge planning section and included the following options: home without therapy, home with outpatient therapy, home with home health, or postacute facility (ie, SNF or IRF). In instances when the PT and OT recommendations did not agree (15.5%), we assigned the PT recommendation because PTs, on average, had more visits with the patients relative to OTs. Because of limitations in the data, we could not verify if patients were discharged with an outpatient therapy referral. Therefore, we combined therapists’ recommendation of “home without therapy” and “home with outpatient therapy” as 1 category, “home (with or without outpatient therapy).”
Postacute care setting discordance occurred when (1) the therapist recommended home health and the patient went home with no home health or (2) the therapist recommended a postacute care facility and the patient went home with or without home health (Table 1).
Table 1.
Therapist Recommended Postacute Setting Versus Actual Discharge Setting (n=25 500)
| Therapist Recommended | Actual Discharge Setting | ||
|---|---|---|---|
| Home, n (%) | Home Health Care, n (%) | Postacute Care Facility, n (%) | |
| Home | 1139 (42.8) | 1333 (50.1) | 190 (7.1) |
| Home with home health | 1914 (25.3) | 5006 (66.2) | 640 (8.5) |
| Postacute care facility | 1142 (7.5) | 2201 (14.4) | 11 935 (78.1) |
Grey shading: actual discharge setting is discordant when setting is less intensive than therapist recommendation.
No shading: actual discharge setting is concordant when setting is equal to or more intensive than therapist recommendation.
Covariates included demographic (eg, sex, race, age, marital status, primary insurance type, income by ZIP code) and clinical variables (eg, length of stay, intensive care unit use, risk of mortality,20 severity of illness,20 comorbidities,21 total number of therapist visits, and functional status).18 The risk of mortality (minor, moderate, major, extreme) and severity of illness variables (minor, moderate, major, extreme) were created using All Patient Refined Diagnosis Related Group algorithms.20 These variables have been used for cost adjustment within hospital systems as well as risk adjustment in claims data research.22 Comorbidities were represented by total count as well as the presence of the following relevant comorbidities: peripheral vascular disease, chronic pulmonary disease, diabetes mellitus (complicated), renal failure, liver disease, coagulopathy, obesity, blood loss anemia, alcohol abuse, drug abuse, depression, neurological disorder, and cancer.
Functional status was measured with the Activity Measure for Post‐Acute Care (AM‐PAC) “6‐clicks,” a validated instrument that measures basic mobility and ADL.18 The AM‐PAC basic mobility scale and ADL scale range from 6 to 24, with lower scores indicating more difficulty performing the task. Nursing assessed patient mobility upon admission using the AM‐PAC basic mobility scale (eg, bed mobility, sitting down/standing up from a chair, ambulation, stair negotiation) and the AM‐PAC ADL scale (eg, bathing, dressing, toileting). Because of the skewed distribution of the AM‐PAC data for both mobility and ADL scales, we categorized the measures as total assistance (AM‐PAC=6), major limitations (7–13), moderate limitations (14–18), minor limitations (19–23), and total independence (AM‐PAC=24). The minor, moderate, and major categories were created on the basis of the tertile distribution of the data. All variable definitions are provided in Table S2.
We imputed the following missing variables using the median or mode: for missing race (1.5%), we imputed White race; for missing marital status (3.0%), we imputed married; for missing risk of mortality (0.03%) and severity of illness (0.02%), we imputed major; and for missing median household income (0.3%), we imputed the median value.
Statistical Analysis
We first generated descriptive statistics to describe the degree of discordance between therapist‐recommended versus actual postacute care discharge setting and then examined the demographic and clinical characteristics of the sample stratified by setting discordance/concordance.
To examine the association between setting discordance and 30‐day hospital readmission, we used generalized linear mixed models with a random intercept for hospital, controlling for sociodemographic and clinical factors. Because AM‐PAC ADL scores were highly correlated with the AM‐PAC mobility scores (Spearman rho=0.86), we excluded this measure from our analysis. We assessed the association between setting discordance and readmission for the full sample and the subgroup of individuals with low mobility (≤16) and high mobility (>16) scores based on the median split.
We conducted 4 sensitivity analyses. We first conducted our analysis including individuals who died within the first 30 days after discharge without a hospital readmission preceding the event (N=26 798). Our dependent variable for this analysis was death or readmission within 30 days. Our second sensitivity analysis excluded all records with missing data (ie, records with imputed values). Third, we examined setting discordance on the basis of a 4‐level measure of discharge setting (ie, home, home with home health, IRF, SNF). Finally, we conducted our analysis on the subgroup of patients who had had a primary diagnosis of CHF (N=4480) (Table S1 and S3).
We also used a generalized linear mixed model to examine the sociodemographic and clinical predictors of setting discordance. To understand the predictive value of sociodemographic and clinical factors, versus controlling for these factors, we created a more parsimonious set of variables, eliminating those that were collinear with each other. Specifically, we eliminated the severity of illness and risk of mortality measures that were highly correlated with the comorbidity index, the AM‐PAC ADL measure that was highly correlated with the AM‐PAC mobility measure, and the individual comorbidities that were correlated with each other and the overall comorbidity index. We also created a dichotomous variable to indicate when PT and OT discharge setting recommendations were in agreement. This study was reviewed by the university’s Institutional Review Board and was classified as exempt. All analyses were performed using STATA version 16.1 (StataCorp, College Station, TX).
Results
The sample consisted of 25 500 (39.6%) adult patients (Figure S1). Of patients who had at least one PT or OT visit (n=39 220), 9906 (25.3%) were missing postacute rehabilitation recommendations, and 2516 (6.4%) had unclear postacute rehabilitation recommendations. Patients with missing recommendations were generally younger and had fewer comorbidities (Table S4). Patients with unclear recommendations were generally older, women, and less ill (Table S4).
Overall, the sample was 55% women and 89% White, and 80% were aged >65 years old (Table 2). The median hospital length of stay was 6.7 days and the median number of comorbidities was 7. Most patients had mobility (64%) and ADL (53%) limitations that were moderate or greater as measured by the AM‐PAC. The setting discordance rate was 20.6%, and the 30‐day readmission rate was 23.7%. There were differences between groups based on discordance (Table 2). For example, therapists’ recommendations were more likely followed when patients were in the highest category for risk of mortality and severity of illness measurements, had longer intensive care unit stays, had severe limitations in AM‐PAC mobility and ADL scores, and had more visits with therapists.
Table 2.
Patient Demographic and Clinical Characteristics by Post‐Acute Discharge Setting Concordance/Discordance (n=25 500)
|
Setting Concordance, N (%) N=20 243 (79.4%) |
Setting Discordance, N (%) N=5257 (20.6%) |
Total n=25 500 |
|
|---|---|---|---|
| Age, y (%) | |||
| 18–55 | 1177 (5.8) | 315 (6.0) | 1492 (5.9) |
| 56–65 | 2870 (14.2) | 861 (16.4) | 3731 (14.6) |
| 66–75 | 4875 (24.1) | 1314 (25.0) | 6189 (24.3) |
| 76–85 | 6172 (30.5) | 1556 (29.6) | 7728 (30.3) |
| 86+ | 5149 (25.4) | 1211 (23.0) | 6360 (24.9) |
| Sex, n (%) | |||
| Male | 9204 (45.5) | 2374 (45.2) | 11 578 (45.4) |
| Female | 11 039 (54.5) | 2883 (54.8) | 13 922 (54.6) |
| Race, n (%) | |||
| White | 18 064 (89.2) | 4620 (87.9) | 22 684 (89.0) |
| Black | 1990 (9.8) | 586 (11.2) | 2576 (10.1) |
| Other‡ | 189 (0.9) | 51 (1.0) | 240 (0.9) |
| Marital status, n (%) | |||
| Married | 9115 (45.0) | 2343 (44.6) | 11 458 (44.9) |
| Divorced/Widowed | 7929 (39.2) | 2066 (39.3) | 9995 (39.2) |
| Single | 3199 (15.8) | 848 (16.1) | 4047 (15.9) |
| Insurance, n (%) | |||
| Commercial | 4217 (20.8) | 1016 (19.3) | 5233 (20.5) |
| Medicare | 14 705 (72.6) | 3845 (73.1) | 18 550 (72.8) |
| Medicaid | 1061 (5.2) | 313 (6.0) | 1374 (5.4) |
| Self‐pay | 29 (0.1) | 12 (0.2) | 41 (0.2) |
| Other | 231 (1.1) | 71 (1.4) | 302 (1.2) |
| Median income by ZIP code, mean (SD) |
47 949.7 (15 738.0) |
46 988.8 (15 838.9) |
47 751.6 (15 763.3) |
| Hospital LOS, median (IQR) | 6.9 (4.4–10.9) | 5.7 (3.8–8.8) | 6.7 (4.2–8.8) |
| % ICU use (%) | 6210 (30.7) | 1268 (24.1) | 7478 (29.3) |
| Mean (SD) ICU days* | 5.1 (7.1) | 3.7 (4.0) | 4.8 (6.7) |
| AM‐PAC mobility, n (%) | |||
| 6 (total assistance) | 1297 (6.4) | 244 (4.6) | 1541 (6.0) |
| 7–15 (major limitations) | 5812 (28.7) | 1301 (24.8) | 7113 (27.9) |
| 16–19 (moderate limitations) | 5996 (29.6) | 1718 (32.7) | 7714 (30.3) |
| 20–23 (minor limitations) | 3766 (18.6) | 1193 (22.7) | 4959 (19.5) |
| 24 (total independence) | 2274 (11.2) | 576 (11.0) | 2850 (11.2) |
| Missing, n (%) | 1098 (5.4) | 225 (4.3) | 1323 (5.2) |
| AM‐PAC ADLs, n (%) | |||
| 6 (total assistance) | 1154 (5.7) | 228 (4.3) | 1382 (5.4) |
| 7–15 (major limitations) | 4876 (24.1) | 1094 (20.8) | 5970 (23.4) |
| 16–19 (moderate limitations) | 4775 (23.6) | 1262 (24.0) | 6037 (23.7) |
| 20–23 (minor limitations) | 4312 (21.3) | 1353 (25.7) | 5665 (22.2) |
| 24 (total independence) | 4028 (19.9) | 1095 (20.8) | 5123 (20.1) |
| Missing, n (%) | 1098 (5.4) | 225 (4.3) | 1323 (5.2) |
| Diagnoses, n (%) | |||
| Peripheral vascular disease | 5401 (26.7) | 1385 (26.4) | 6786 (26.6) |
| Chronic pulmonary disease | 9027 (44.6) | 2541 (48.3) | 11 568 (45.4) |
| Diabetes mellitus (complicated) | 6751 (33.4) | 1849 (35.2) | 8600 (33.7) |
| Renal failure | 8250 (40.8) | 2249 (42.8) | 10 499 (41.2) |
| Liver disease | 1399 (6.9) | 364 (6.9) | 1763 (6.9) |
| Coagulopathy | 2403 (11.9) | 527 (10.0) | 2930 (11.5) |
| Obesity | 4907 (24.2) | 1348 (25.6) | 6255 (24.5) |
| Blood loss anemia | 349 (1.7) | 85 (1.6) | 434 (1.7) |
| Alcohol abuse | 661 (3.3) | 192 (3.7) | 853 (3.4) |
| Drug abuse | 389 (1.9) | 122 (2.3) | 511 (2.0) |
| Depression | 5008 (24.7) | 1286 (24.5) | 6294 (24.7) |
| Neurological | 3529 (17.4) | 802 (15.3) | 4331 (17.0) |
| Cancer | 1646 (8.1) | 421 (8.0) | 2067 (8.1) |
| Severity of illness, n (%) | |||
| Minor | 298 (1.5) | 56 (1.1) | 354 (1.4) |
| Moderate | 4136 (20.4) | 1133 (21.6) | 5269 (20.7) |
| Major | 11 068 (54.7) | 3140 (59.7) | 14 208 (55.7) |
| Extreme | 4741 (23.4) | 928 (17.7) | 5669 (22.2) |
| Risk of mortality, n (%) | |||
| Minor | 75 (0.4) | 32 (0.6) | 107 (0.4) |
| Moderate | 5946 (29.4) | 1603 (30.5) | 7549 (29.6) |
| Major | 9804 (48.4) | 2716 (51.7) | 12 520 (49.1) |
| Extreme | 4418 (21.8) | 906 (17.2) | 5324 (20.9) |
| Elixhauser comorbidity index, mean (SD) | 6.8 (2.2) | 6.8 (2.1) | 6.8 (2.1) |
| Median (IQR) | 7 (5–8) | 7 (5–8) | 7 (5–8) |
| Number of therapist visits, n (%) | |||
| Low (1–3) | 5085 (25.1) | 1867 (35.5) | 6952 (27.3) |
| Med (4–6) | 7564 (37.4) | 2122 (40.4) | 9686 (38.0) |
| High (7+) | 7594 (37.5) | 1268 (24.1) | 8862 (34.8) |
| Visits by therapists, mean (SD) | |||
| PT total visits | 4.3 (3.3) | 3.6 (2.5) | 4.2 (3.2) |
| OT total visits | 2.6 (2.8) | 1.9 (2.3) | 2.5 (2.7) |
| 30‐day readmission, n (%) | 4719 (23.1) | 1322 (25.2) | 6041 (23.7) |
| Days to readmission†, mean (SD) | 12.7 (8.6) | 12.9 (8.8) | 12.8 (8.7) |
ADLs indicates activities of daily living; AM‐PAC, Activity Measure for Post‐Acute Care; ICU, intensive care unit; IQR, interquartile range; LOS, lemgth of stay; OT, occupational therapist; and PT, physical therapist.
Conditional on ICU use.
(n=6041).
Other indicates American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander; Unknown
Of those recommended to go home with home health, 25.3% went home with no home health. Of those recommended to a postacute care facility, 7.5% went home without home health, and 14.4% went home with home health (Table 1).
Postacute Setting Discordance and 30‐Day Readmission
Figure 1 illustrates the association between setting discordance and 30‐day readmission for the full sample and the high and low mobility subgroups (AM‐PAC mobility ≤16 or >16). Setting discordance was associated with greater odds of readmission (adjusted odds ratio, 1.12; 95% CI, 1.04–1.20; P=0.002). The point estimate for those classified in the lower mobility group (AM‐PAC mobility ≤16) was greater (adjusted odds ratio, 1.20; 95% CI, 1.08–1.33; P=0.001) than that for the high mobility group (adjusted odds ratio, 1.10; 95% CI, 0.99–1.22; P=0.064), though the confidence intervals for these point estimates overlapped and the odds ratio for the high mobility group was nonsignificant. Our full model results are presented in Table S5.
Figure 1. Multilevel analysis of the association between setting discordance and 30‐day all‐cause readmission.
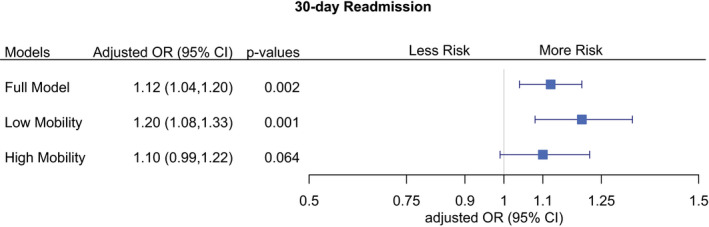
Mixed‐effects model with random intercept for hospital, controlling for demographics, insurance, median income, comorbidities, length of stay, intensive care use, mortality risk, illness severity, AM‐PAC mobility score only, discharge destination, total visits from physical and occupational therapy. Full model: n=25 500. Low mobility (AM‐PAC ≤16): n=11 972. High mobility (AM‐PAC >16): n=12 205. OR, odds ratio.
Results of the sensitivity analyses are presented in Table S6. The results were generally similar, though some findings were nonsignificant because of smaller sample sizes when including individuals who died within 30 days (n=1298); when using a 4‐level discharge categorization (ie, home, home health, SNF, IRF); when excluding records with missing data (n=992); and for those who had a primary CHF diagnosis (n=4480). Of the 6044 individuals who had a readmission within 30 days, ≈20% (N=1322) were discharged to a less intensive setting than recommended. Figure 2 presents data on the distribution of the readmissions with discordant discharges. Over 70% of the discordant discharges were instances when the patient was recommended for a postacute care facility but instead went home with or without home health (Figure 2).
Figure 2. Discordant recommendations and discharge settings (n=1322).
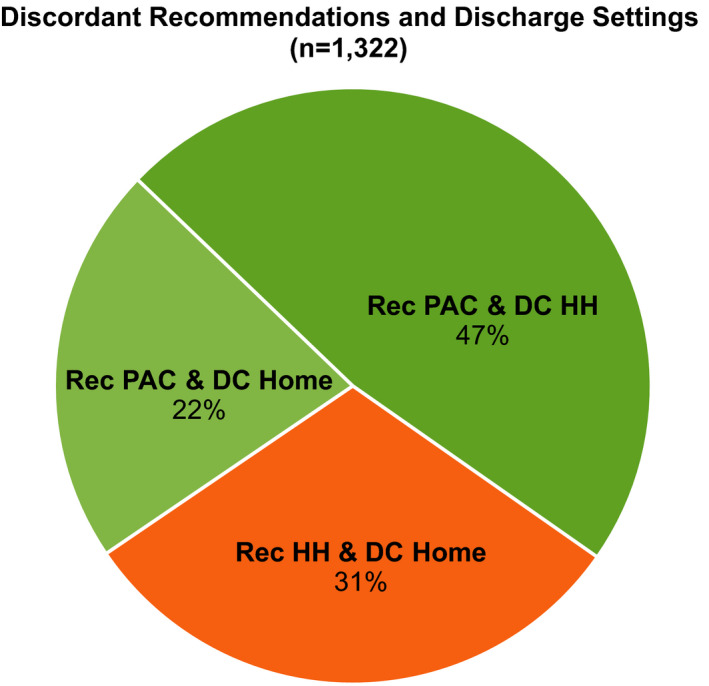
Percentage of discordant discharges with readmission within 30 days. Description: Majority of readmissions across 30 days were recommended for a postacute care facility but went home with or without home health services (mean: 69.2%) vs recommendations for home with home health and went home without services (mean, 30.8%). DC indicates discharge; HH, home health; PAC, postacute care facility; and Rec, therapist recommendation.
Predictors of Postacute Setting Discordance
Figure 3 presents the analysis examining sociodemographic and clinical factors associated with setting discordance. Relative to patients with total assist and major mobility limitations, patients with moderate to minor limitations were more likely to discharge to discordant settings. Patients with ≥8 comorbidities were also more likely to be in discordant settings. Based on the point estimate, there was some suggestion that patients on Medicaid, self‐pay, or other insurance (relative to commercial insurance) were more likely to have discordant discharges, though the findings were nonsignificant. Patients aged ≥76 years (relative to those aged 18–55 years), those with a higher median household income, longer hospital stays, more therapist visits, and the same setting recommendations from both therapists were more likely to discharge to the therapists’ recommended postacute setting with the same or higher rehabilitation intensity.
Figure 3. Sociodemographic and clinical factors associated with setting discordance (n=25 500).
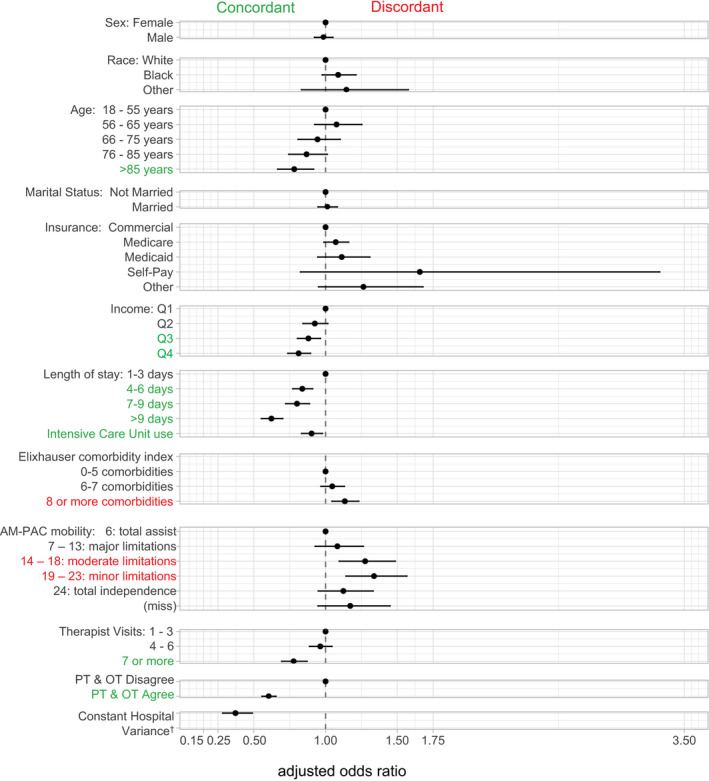
Controlling for demographics, insurance, median income, comorbidities, length of stay, ICU use, AM‐PAC mobility scores only, total visits from physical and occupational therapy; recommendation agreement between therapists.
†Hospital variance adjusted odds ratio, 0.06; 95% CI, 0.03–0.14). AM‐PAC, Activity Measure for Post‐Acute Care; OT, occupational therapist; PT, physical therapist; Q1, first quartile; Q2, second quartile; Q3, third quartile; and Q4, fourth quartile.
Discussion
This study is the first large‐scale analysis examining whether discordance between recommended and actual postacute care setting was associated with hospital readmission in patients with CHF. We found that setting discordance was associated with greater odds of 30‐day hospital readmission. We also found this effect was slightly larger in the subgroup of individuals with low mobility scores. Patients discharged to a less intensive setting than recommended are likely at greater risk of complications or events (eg, falls) that may lead to a rehospitalization. Those with greater mobility limitations may be particularly vulnerable. Several studies have identified a direct relationship between mobility limitations and risk of readmission.23, 24
Our work supports and extends prior literature.12, 13 In a retrospective cohort study involving 762 patients admitted to the medical/surgical unit of an academic hospital, Smith et al13 found that patients were 2.9 times more likely to be readmitted within 30 days when the physical therapists’ recommendations were not followed. Another study examined 322 patients discharged after an acute care hospitalization and reported an increased readmission risk when PT recommendations for postacute PT services were not met (adjusted odds ratio, 1.18; 95% CI, 1.08–3.03).12 Limitations of both studies include: small heterogeneous samples from a single hospital, lack of control for confounders such as illness severity, comorbidities, and mobility status of the patient, and exclusion of OT recommendations.
The majority of patients who had a readmission and a discordant discharge were recommended for a postacute facility (ie, IRF or SNF) but were discharged home with or without home health. Therapists typically recommend a post‐acute care facility if there are significant rehabilitation needs or if the patients’ safety and needs cannot be met at home. Insufficient social support at home can likely trigger adverse events requiring rehospitalization.12, 25, 26 Our results suggest this may have been the case for our sample.
We identified several predictors of discordant care. People in the midrange of mobility problems were more likely to be in discordant postacute settings relative to those with extreme mobility limitations (total assistance: AM‐PAC score of 6, chi‐square P=0.002) and those with no mobility limitations (total independence: AM‐PAC score of 24, chi‐square P=0.002). These findings may be related to a “disconnect” between the therapist’s evaluation and how the patient appears to other team members (eg, family, discharge planners, physicians). For example, despite the chronic nature of CHF, the therapist may recognize rehabilitation potential even in those patients who have multiple comorbidities in addition to CHF. Likewise, the therapist may uncover subtle impairments and environmental barriers in those with minor to moderate mobility limitations that would benefit from a more intensive postacute care setting.27
Older patients and those with higher household incomes were more likely to receive the recommended level of care or higher. The reasons behind these findings are less clear but possibly related to insurance and patient resources, which may facilitate discharge to the appropriate postacute care setting.28 While insurance was not a significant predictor of discordant care, the point estimates of insurance coverage (ie, Medicaid, self‐pay, other) was associated with discordant care. As might be expected, patients with more therapist visits and longer lengths of stay were more likely to be in concordant postacute settings. This is likely attributable to the additional time the therapist had with the patient,29, 30, 31 the patient’s family, and the care team at the hospital, leading to a more informed postacute rehabilitation recommendation by the therapist. Having the same recommendation between both therapists was one of the strongest significant predictors of concordant discharges. This is likely attributable, in part, to a clear and consistent message across disciplines. Consistent messages about discharge recommendations from PTs and OTs to patients and the care team may carry more weight with decision making.
Readmission rates for CHF are common, expensive, and often considered preventable.4, 32 Frailty and severe impairments across multiple domains of function, including strength, balance, mobility, and endurance, have been documented in the CHF population and are thought to contribute to readmissions.33 Data also suggest that improving physical function in this population may reduce readmission risk.34 Identification of social and physical function barriers at the point of care may lead to the timely targeting of appropriate resources, especially physical therapy–directed rehabilitative services, for at‐risk patients.
Discharge planning is a complex process that involves several key players including the patient, the patient’s family, PT and OT, nursing, the attending physician, social work, and the discharge coordinator. Successful discharge planning requires a coordinated effort from all acute team members to provide clear and consistent communication to the patient and decision makers. Discharge coordinators should also facilitate follow‐up with outpatient CHF care providers to improve continuity of care.35, 36 Potential reasons behind setting discordance are varied and may be attributable to patient preference, patient resources, insurance restrictions, or postacute care availability. The influence of the health care team members may also impact the patient’s postacute care choice.
The Institute of Medicine and Centers for Medicare and Medicaid Services recommends that hospitalization determinants (eg, health literacy, social support, and physical and cognitive function)37 be captured in electronic health records for value‐based care and population health management.38, 39, 40 Such information has rarely been captured and, if it is, has been used inconsistently to inform clinical decisions or care plans.40 Identifying appropriate social support is often a challenge for the care team during an acute hospitalization. Of the 2516 unclear therapist postacute setting recommendations in our study, 21.4% recommended a discharge to home contingent on the availability of social support; otherwise, a postacute facility would be the alternative recommendation. Future studies should acquire information on the availability of household support and verify the caregiver’s capability, as it is an important factor when providing adequate physical assistance and helping the patients’ medical and dietary compliance.41
Limitations
Our study is not without limitations. We used an observational design and cannot conclude a causal relationship between discordant recommendations and hospital readmission. There is also the potential for unmeasured confounding not captured by the variables in our model. Our analysis was also limited to a single health system, and we excluded patients without therapist visits reducing the external validity of our findings. In addition, >80% of our sample had a secondary rather than a primary diagnosis of CHF. Previous studies have recommended identifying CHF via secondary diagnoses attributable to readmission penalties, incentivizing hospitals to avoid putting CHF in the primary diagnosis position.42 We analyzed individuals who were hospitalized for surgical (30.8% of the sample) versus medical reasons based on Diagnosis Related Group codes, and our results were similar. Finally, we had incomplete information on some important variables including readmission outside the health system, reason for readmission, and secondary insurance.
Conclusions
Patients with CHF who were discharged to less intensive postacute settings than recommended were more likely to be readmitted to the hospital in 30 days. When stratifying by high and low mobility scores, the effect was slightly larger in the subgroup with low mobility scores. We also found that the majority of individuals who were readmitted and had discordant discharges were recommended for a postacute care facility but went home. Both clinical and sociodemographic factors such as high comorbidity burden, therapist disagreement on postacute discharge setting, and midrange physical functional limitations were associated with postacute setting discordance. Systematic assessments of social and functional health determinants at the point of clinical care may improve CHF management and readmission risk by timely identification of rehabilitation needs and community resources available to optimize patient self‐management for improved health, especially upon discharge.43, 44
Sources of Funding
None.
Disclosures
T. Euloth and B. Matcho are both regional directors of Inpatient Rehabilitation Services for the health system examined in this study. The remaining authors have no disclosures to report.
Supporting information
Table S1–S6
Figure S1
Acknowledgments
The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
(J Am Heart Assoc. 2021;10:e020425. DOI: 10.1161/JAHA.120.020425.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020425
For Sources of Funding and Disclosures, see page 10.
References
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;E139–E596. DOI: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Suter LG, Li S‐X, Grady JN, Lin Z, Wang Y, Bhat KR, Turkmani D, Spivack SB, Lindenauer PK, Merrill AR, et al. National patterns of risk‐standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29:1333–1340. DOI: 10.1007/s11606-014-2862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergethon KE, Ju C, DeVore AD, Hardy NC, Fonarow GC, Yancy CW, Heidenreich PA, Bhatt DL, Peterson ED, Hernandez AF. Trends in 30‐day readmission rates for patients hospitalized with heart failure: findings from the Get With the Guidelines–Heart Failure registry. Circ Heart Fail. 2016;9:e002594. DOI: 10.1161/CIRCHEARTFAILURE.115.002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428. DOI: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A, Allen LA, Bhatt DL, Cox M, DeVore AD, Heidenreich PA, Hernandez AF, Peterson ED, Matsouaka RA, Yancy CW, et al. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA cardiology. 2018;3:44–53. DOI: 10.1001/jamacardio.2017.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auerbach AD, Kripalani S, Vasilevskis EE, Sehgal N, Lindenauer PK, Metlay JP, Fletcher G, Ruhnke GW, Flanders SA, Kim C, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176:484–493. DOI: 10.1001/jamainternmed.2015.7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jette DU, Grover L, Keck CP. A qualitative study of clinical decision making in recommending discharge placement from the acute care setting. Phys Ther. 2003;83:224–236. DOI: 10.1093/ptj/83.3.224. [DOI] [PubMed] [Google Scholar]
- 8.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, Deruyter F, Eng JJ, Fisher B, Harvey RL, et al., American Heart Association Stroke Council, council on cardiovascular and stroke nursing, council on clinical cardiology, and council on quality of care and outcomes research . Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:e98–e169. DOI: 10.1161/STR.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Medicare & Medicaid Services . Medicare Cf, Services M . Medicare benefit policy manual. Chapter 1, section 110. 2010. Available at: https://www.cms.gov/Regulations‐and‐Guidance/Guidance/Manuals/downloads/bp102c01.pdf. Accessed July 1, 2021.
- 10.Haykowsky MJ, Daniel KM, Bhella PS, Sarma S, Kitzman DW. Heart failure: exercise‐based cardiac rehabilitation: who, when, and how intense? Can J Cardiol. 2016;32:S382–S387. DOI: 10.1016/j.cjca.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. DOI: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 12.Shoemaker MJ, Gutowski A, Mallgren M, Oliver L, Van Dam A, McLeod J, Mohney E. Physical therapist determination of discharge disposition in the acute care setting. J Acute Care Phys Ther. 2019;10:93–106. DOI: 10.1097/JAT.0000000000000099. [DOI] [Google Scholar]
- 13.Smith BA, Fields CJ, Fernandez N. Physical therapists make accurate and appropriate discharge recommendations for patients who are acutely ill. Phys Ther. 2010;90:693–703. DOI: 10.2522/ptj.20090164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacks GD, Hill C, Rogers SO Jr. Insurance status and hospital discharge disposition after trauma: inequities in access to postacute care. J Trauma Acute Care Surg. 2011;71:1011–1015. DOI: 10.1097/TA.0b013e3182092c27. [DOI] [PubMed] [Google Scholar]
- 15.Chan L, Doctor J, Temkin N, MacLehose RF, Esselman P, Bell K, Dikmen S. Discharge disposition from acute care after traumatic brain injury: the effect of insurance type. Arch Phys Med Rehabil. 2001;82:1151–1154. DOI: 10.1053/apmr.2001.24892. [DOI] [PubMed] [Google Scholar]
- 16.Hansen VJ, Gromov K, Lebrun LM, Rubash HE, Malchau H, Freiberg AA. Does the risk assessment and prediction tool predict discharge disposition after joint replacement? Clin Orthop Relat Res. 2015;473:597–601. DOI: 10.1007/s11999-014-3851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jehkonen M, Ahonen JP, Dastidar P, Koivisto AM, Laippala P, Vilkki J, Molnár G. Predictors of discharge to home during the first year after right hemisphere stroke. Acta Neurol Scand. 2001;104:136–141. DOI: 10.1034/j.1600-0404.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 18.Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. AM‐PAC “6‐clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94:1252–1261. DOI: 10.2522/ptj.20130359. [DOI] [PubMed] [Google Scholar]
- 19.Wee JY, Hopman WM. Stroke impairment predictors of discharge function, length of stay, and discharge destination in stroke rehabilitation. Am J Phys Med Rehabil. 2005;84:604–612. DOI: 10.1097/01.phm.0000171005.08744.ab. [DOI] [PubMed] [Google Scholar]
- 20.Averill RF, Goldfield N, Hughes JS, Bonazelli J, McCullough EC, Steinbeck BA, Mullin R, Tang AM, Muldoon J, Turner L. All Patient Refined Diagnosis Related Groups (APR‐DRGs) Version 20.0: Methodology Overview. Wallingford, CT: 3M Health Information Systems; 2003:91. [Google Scholar]
- 21.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. DOI: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Hackworth J, Askegard‐Giesmann J, Rouse T, Benneyworth B. The trauma registry compared to all patient refined diagnosis groups (APR‐DRG). Injury. 2017;48:1063–1068. DOI: 10.1016/j.injury.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Fisher SR, Kuo Y‐F, Sharma G, Raji MA, Kumar A, Goodwin JS, Ostir GV, Ottenbacher KJ. Mobility after hospital discharge as a marker for 30‐day readmission. J Gerontol A Biol Sci Med Sci. 2013;68:805–810. DOI: 10.1093/gerona/gls252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berian JR, Mohanty S, Ko CY, Rosenthal RA, Robinson TN. Association of loss of independence with readmission and death after discharge in older patients after surgical procedures. JAMA Surg. 2016;151:e161689. DOI: 10.1001/jamasurg.2016.1689. [DOI] [PubMed] [Google Scholar]
- 25.Eastwood CA, Quan H, Howlett JG, King‐Shier KM. Factors associated with 7‐day rehospitalization after heart failure admission. J Cardiovasc Nurs. 2017;32:339–347. DOI: 10.1097/JCN.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz KA. Predictors of early hospital readmissions of older adults who are functionally impaired. J Gerontol Nurs. 2000;26:29–36. DOI: 10.3928/0098-9134-20000601-06. [DOI] [PubMed] [Google Scholar]
- 27.Doukky R, Mangla A, Ibrahim Z, Poulin MF, Avery E, Collado FM, Kaplan J, Richardson D, Powell LH. Impact of physical inactivity on mortality in patients with heart failure. Am J Cardiol. 2016;117:1135–1143. DOI: 10.1016/j.amjcard.2015.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burke RE, Juarez‐Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Patient and hospitalization characteristics associated with increased postacute care facility discharges from US hospitals. Med Care. 2015;53:492–500. DOI: 10.1097/MLR.0000000000000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews AW, Li D, Freburger JK. Association of rehabilitation intensity for stroke and risk of hospital readmission. Phys Ther. 2015;95:1660–1667. DOI: 10.2522/ptj.20140610. [DOI] [PubMed] [Google Scholar]
- 30.Corr S, Bayer A. Occupational therapy for stroke patients after hospital discharge—a randomized controlled trial. Clin Rehabil. 1995;9:291–296. DOI: 10.1177/026921559500900403. [DOI] [Google Scholar]
- 31.Kumar A, Resnik L, Karmarkar A, Freburger J, Adhikari D, Mor V, Gozalo P. Use of hospital‐based rehabilitation services and hospital readmission following ischemic stroke in the United States. Arch Phys Med Rehabil. 2019;100:1218–1225. DOI: 10.1016/j.apmr.2018.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lloyd‐Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, et al., American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Executive summary: heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. DOI: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 33.Reeves GR, Whellan DJ, Patel MJ, O'Connor CM, Duncan P, Eggebeen JD, Morgan TM, Hewston LA, Pastva AM, Kitzman DW. Comparison of frequency of frailty and severely impaired physical function in patients ≥60 years hospitalized with acute decompensated heart failure versus chronic stable heart failure with reduced and preserved left ventricular ejection fraction. Am J Cardiol. 2016;117:1953–1958. DOI: 10.1016/j.amjcard.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reeves GR, Whellan DJ, O’Connor CM, Duncan P, Eggebeen JD, Morgan TM, Hewston LA, Pastva A, Patel MJ, Kitzman DW. A novel rehabilitation intervention for older patients with acute decompensated heart failure: the REHAB‐HF pilot study. JACC Heart Fail. 2017;5:359–366. DOI: 10.1016/j.jchf.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merrill JA, Sheehan B, Carley KM, Stetson P. Transition networks in a cohort of patients with congestive heart failure: a novel application of informatics methods to inform care coordination. Appl Clin Inform. 2015;6:548. DOI: 10.4338/ACI-2015-02-RA-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAlister FA, Youngson E, Kaul P, Ezekowitz JA. Early follow‐up after a heart failure exacerbation: the importance of continuity. Circ Heart Fail. 2016;9:e003194. DOI: 10.1161/CIRCHEARTFAILURE.116.003194. [DOI] [PubMed] [Google Scholar]
- 37.Greysen SR, Cenzer IS, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med. 2015;175:559–565. DOI: 10.1001/jamainternmed.2014.7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Committee on the Recommended Social and Behavioral Domains and Measures for Electronic Health Records, Board on Population Health and Public Health Practice, Institute of Medicine . Capturing social and behavioral domains and measures in electronic health records: Phase 2. Washington (DC): National Academies Press (US). 2015. [PubMed]
- 39.Adler NE, Stead WW. Patients in context—EHR capture of social and behavioral determinants of health. N Engl J Med. 2015;372:698–701. DOI: 10.1056/NEJMp1413945. [DOI] [PubMed] [Google Scholar]
- 40.Aronson L, Bautista CA, Covinsky K. Medicare and care coordination: expanding the clinician's toolbox. JAMA. 2015;313:797–798. DOI: 10.1001/jama.2014.18174. [DOI] [PubMed] [Google Scholar]
- 41.Retrum JH, Boggs J, Hersh A, Wright L, Main DS, Magid DJ, Allen LA. Patient‐identified factors related to heart failure readmissions. Circ Cardiovasc Qual Outcomes. 2013;6:171–177. DOI: 10.1161/CIRCOUTCOMES.112.967356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blecker S, Herrin J, Li L, Yu H, Grady JN, Horwitz LI. Trends in hospital readmission of medicare‐covered patients with heart failure. J Am Coll Cardiol. 2019;73:1004–1012. DOI: 10.1016/j.jacc.2018.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pandey A, Kitzman D, Whellan DJ, Duncan PW, Mentz RJ, Pastva AM, Nelson MB, Upadhya B, Chen H, Reeves GR. Frailty among older decompensated heart failure patients: prevalence, association with patient‐centered outcomes, and efficient detection methods. JACC Heart Fail. 2019;7:1079–1088. DOI: 10.1016/j.jchf.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pastva AM, Hugenschmidt CE, Kitzman DW, Nelson MB, Brenes GA, Reeves GR, Mentz RJ, Whellan DJ, Chen H, Duncan PW. Cognition, physical function, and quality of life in older patients with acute decompensated heart failure. J Cardiac Fail. 2021;27:286–294. DOI: 10.1016/j.cardfail.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S6
Figure S1


