Abstract
Type 2 deiodinase (Dio2) amplifies levels of 3,5,3′-L-triiodothyronine (T3), the active form of thyroid hormone, and is essential for cochlear maturation and auditory development. However, cellular routes for endocrine signaling in the compartmentalized, anatomically complex cochlea are little understood. Dio2 generates T3 from thyroxine (T4), a more abundant thyroid hormone precursor in the circulation, and is dramatically induced in the cochlea before the onset of hearing. The evidence implies that specific Dio2-expressing cell types critically mediate T3 signaling but these cell types are poorly defined because Dio2 is expressed transiently at low levels. Here, using a Dio2CreERt2 knockin that activates a fluorescent reporter, we define Dio2-expressing cochlear cell types at high resolution in male or female mice. Dio2-positive cells were detected in vascularized supporting tissues but not in avascular internal epithelia, indicating segregation of T3-generating and T3-responding tissues. In the spiral ligament and spiral limbus, Dio2-positive fibrocytes clustered around vascular networks that convey T4 into cochlear tissues. In the otic capsule, Dio2-positive osteoblasts localized at cartilage surfaces as the bony labyrinth matures. We corroborated the identities of Dio2-positive lineages by RNA-sequencing of individual cells. The results suggest a previously unrecognized role for fibrocytes in mediating hormonal signaling. We discuss a model whereby fibrocytes mediate paracrine-like control of T3 signaling to the organ of Corti and epithelial target tissues.
Keywords: thyroid hormone, auditory system, single cell transcriptome, neurodevelopment, selenoprotein
Thyroid hormone is critical for the development of hearing (1, 2). Human hearing loss is associated with endemic iodine deficiency (3), congenital hypothyroidism (4, 5), and mutations in the THRB thyroid hormone receptor gene (6, 7). Studies in model species indicate that the cochlea is a major target of thyroid hormone. Hypothyroid (8, 9) or Thrb-deficient rodents (10, 11) display deafness with defects in the organ of Corti, malfunction of the mechanosensory hair cells (10-13), partial loss of hair cells (12, 14), and deformity of the tectorial membrane (8, 15).
In addition to sufficient thyroid hormone in the circulation, auditory development requires type 2 deiodinase (Dio2), a thyroid hormone-activating enzyme that is highly induced in the postnatal mouse cochlea (16). Dio2 generates the active T3 form of thyroid hormone by deiodination of T4, a more abundant, tetra-iodinated form of the hormone (17, 18). Dio2-deficient mice display deafness with hypothyroid-like cochlear defects despite circulating T4 and T3 levels that would normally support auditory development (19). In situ hybridization detected Dio2 mRNA in lateral wall and modiolus areas (16) suggesting that supporting tissues amplify T3 levels. However, Dio2-expressing cell types are poorly defined because of the transient expression and short half-life of Dio2 protein (20) and lack of specific antibodies for immunodetection.
A major unknown about endocrine signaling in the cochlea concerns the route by which hormones in the circulation reach internal sensory tissues given the lack of direct blood flow to the organ of Corti (21). The major arterial system branches laterally as radiating arterioles into capillary networks located in 2 major fibrocyte zones of the cochlea in the lateral wall and the medial spiral limbus. Both the arterial and returning venous networks bypass the sensory and internal epithelia. The compartmentalized, bone-encased cochlea presents unique physical obstacles to hormonal transport in addition to a blood-labyrinth barrier that controls uptake of blood-borne substances at the cellular level (22, 23). Similar uncertainties exist regarding access routes for pharmaceutical hormonal preparations used in treating inner ear disorders (24).
During the period when Dio2 is induced, many cochlear tissues progress through terminal differentiation, raising questions about which cell types express Dio2 and whether these lineages suggest a link between the circulation and internal tissues. Using a sensitive Dio2CreERt2 model, we investigated the identity, anatomical location, and transcriptome signatures of Dio2-expressing cell types. The findings suggest that fibrocyte and osteoblast lineages mediate T3 signaling within the cochlea. We discuss implications for paracrine-like control between connective and internal epithelial tissues in cochlear development.
Materials and Methods
Dio2 CreERt2 Knockin and Mouse Strains
A CreERt2 cassette was fused at the ATG start codon of the endogenous Dio2 gene using flanking homology arms of (5′) 2900 and (3′) 5963 bp, respectively. The cassette was inserted by homologous recombination in C57BL/6 embryonic stem cells; founders were crossed onto Rosa26Flpe-deleter mice (C57BL/6 background) to remove the Neo selection gene (Ozgene Pty Ltd, Bentley DC, Western Australia). Founders were back-crossed to C57BL/6J (JAX #000664) to remove Flpe, then crossed to Rosa26Ai6 reporter mice which carry a Cre-dependent Ai6 (Zsgreen1) reporter at the Rosa26 locus (25) (JAX #007906; congenic C57BL/6J background). Analyses were performed on a largely congenic C57BL/6J background.
For all studies (with the exception below), the Dio2CreERt2 allele was maintained as heterozygous and Rosa26Ai6 allele as homozygous. Male and female mice were analyzed without obvious differences in results. Dio2-deletion was studied in mice with combined Dio2CreERt2 and Dio2 null (Dio2-) (17) alleles. Dio2CreERt2/-;Ai6 mice (knockout) were compared with Dio2CreERt2/+;Ai6 (control) mice, keeping a constant single CreERt2 allele in each genotype.
The Dio2CreERt2 allele was genotyped by polymerase chain reaction (PCR) using 3 primers: F-wt 5′-GAA TTG ATG GGT ACA CTC CAA CTG- 3′; R-common 5′-GGA GAA AAA GAC TGG CAG GAT CTG-3′; F-ki 5′-ATG GAG CAT CTG TAC AGC ATG AAG-3′, giving a wild-type band of 299 bp and knockin band of 597 bp. The Dio2 null allele (17) was genotyped as described (19) and Rosa26Ai6 allele was genotyped according to Jackson Lab protocols (https://www.jax.org/Protocol?stockNumber = 007906&protocolID = 28544). Thrbb1-lacz knockin mice were genotyped as described (14).
Tamoxifen (Sigma-Aldrich T-5648) was dissolved in corn oil (Sigma-Aldrich C-8267) at a stock concentration of 10 mg/mL. Neonatal or postnatal mice up to postnatal day 12 (P12) were injected subcutaneously with 20 μL of tamoxifen for 2 (or occasionally 3) consecutive days, then analyzed at least 3 or 4 days later, to allow time for stimulation of CreERt2 activity and activation of Ai6 reporter. At older ages, tamoxifen was injected intraperitoneally with 100 μL of 10 mg/mL stock (3 mg/40 g body weight) for 2 (occasionally 3) days. Stages of injection and analysis for each experiment are stated in figures and legends. Analyses compared tamoxifen or corn oil vehicle treatment or no treatment at different ages. Controls included comparison with Ai6 reporter (no CreERt2 present) and with wild-type mice (no CreERt2 and no Ai6 reporter) as recommended by The Jackson Laboratory (https://www.jax.org/strain/007906) since the Ai6 reporter allele may show low-level, Cre-independent expression in some tissues as indicated in the Allen Brain Atlas (http://connectivity.brain-map.org/transgenic/experiment/81034309).
Experiments were conducted following protocols and guidelines that were approved by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Animal Care and Use Committee at the National Institutes of Health.
Immunostaining
Cochleae were dissected, fixed in 4% paraformaldehyde (PFA) for 3 hours at 4 °C, decalcified in 100 mM EDTA/ phosphate buffered saline (PBS) for 3 to 7 days, then cryoprotected in 30% sucrose overnight at 4 °C. Cochleae were embedded in optimum cutting temperature compound (OCT, Tissue-Tek). Cryosections (12 μm thick) were blocked with antibody buffer (1.5% goat serum, 0.1% bovine serum albumin [BSA], 0.4% Triton X-100 in PBS) for 30 minutes at room temperature then incubated with primary antibody overnight at room temperature, then with secondary antibodies: Alexa Fluor 568-conjugated goat anti-rabbit (1:500, A-11011, Invitrogen) (26) or goat anti-rat antibody (1:500, A-11077, Invitrogen) (27) for 1 hour at room temperature. Primary antibodies used: Alf1 (1:500, AB178846, Abcam) (28); Aqp1 (1:500, AB2219, Millipore) (29); CD31 (1:50, 550274, BD Pharmingen) (30); Gjb2 (1:500, 51–2800, Invitrogen) (31); Myo6 (1:500, M-5187, Sigma-Aldrich) (32); NKCC1 (1:500, Ab3560p, Millipore) (33); RUNX2 (1:500, Ab192256, Abcam) (34); Sp7 (1:500, Ab209484, Abcam) (35); S100b (1:500, PA5–78161, Invitrogen) (36); TMEM 119 (1:500, Ab209064, Abcam) (37).
Slides were washed in PBS and mounted in VECTASHIELD medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA). Images were acquired using a Leica TCS SPE laser scanning confocal microscope. Fluorescent signals were quantified on 9 to 10 section views of mid-basal cochlear turns (n = 3 mice/genotype) using confocal microscopy. Signals were projected to maximum intensity to aid the viewing. The areas of the spiral limbus and spiral ligament were outlined, then fluorescent signals quantified using Image J program. Specific signals were normalized to signal from the acellular scala media and presented as mean ± standard error of the mean (SEM). For pairwise comparisons of significance, a 2-tailed Student t test was used.
X-gal Staining
Cochleae were fixed in 2% PFA for 1 hour and decalcified. Cryosections were incubated with substrate (5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside, 1mg/mL) from the β-Galactosidase Reporter Gene Staining Kit (Sigma), as described (14).
In Situ Hybridization
Cochleae were fixed in 4% PFA overnight. Cryosections (10 μm) were hybridized with antisense and control sense digoxigenin-labeled riboprobes generated from a mouse Dio2 cDNA (base coordinates, 590–1383), with colorimetric detection (Roche Diagnostics, Indianapolis, IN), as described (38).
Single Cell Transcriptome Analysis
Mice were given tamoxifen at P3/P4, then euthanized at P10. Cells were isolated from 3 preparations (1 male, 2 females). Most of the bony capsule and modiolus (auditory nerve and spiral ganglion) were removed. Pieces of lateral cochlea (spiral ligament, stria vascularis, part of basilar membrane, residual otic capsule) and medial cochlea (spiral limbus, osseous spiral lamina, parts of modiolus and inner sulcus) were dissociated in ice-cold PBS, incubated in preactivated papain (5 unit/mL, Calbiochem, cat# 5125) in PBS at 37 °C for 10 minutes and triturated by pipetting. The suspension was diluted 10-fold in Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum and filtered through a 35-µm cell-strainer (Corning, Glendale, AZ, cat #352235). One mL of suspension was added to the center of a 55 mm glass bottom dish (In Vitro Scientific, Sunnyvale, CA cat# D60–30–0-N) and placed on ice for 15 minutes to allow cells to settle. Fluorescent cells were identified on a Zeiss Axiovert 200M microscope and collected in a pulled glass microcapillary tube (Sutter Instrument Company, Novato, CA, #BF100–50–10) using an Eppendorf micromanipulator and suction pipette. Each cell was transferred into lysis solution (Takara Bio USA, Mountain View, CA, cat# 634890) in a PCR 8-strip tube (0.2 mL) for single cell RNA library preparation. Dishes and capillaries were pretreated with silicone oil (Sigma, St. Louis, MO, cat# 378321, viscosity 10 cst). This manual approach allowed visual verification of cell morphology, cell integrity, and absence of adherent cells. Total time from dissection to cell lysis was less than 80 minutes.
Synthesis of cDNA was begun immediately after cell lysis using a SMART-Seq v4 Ultra Low Input RNA Kit (Takara Bio USA, cat# 634890), following manufacturer’s instructions, then amplified using 21 PCR cycles and purified with AMPure XP beads (Beckman Coulter, Indianapolis, IN, cat# A63881). The quality of cDNA was assessed using a 2100 Bioanalyzer and high sensitivity DNA kit (Agilent, Santa Clara, CA, cat #5067–4626). Two µg of cDNA (20 µL vol) was sheared in 8-strip AFA tubes (Covaris, Woburn, MA, cat# 520275) using a Covaris ME220 Focused-ultrasonicator, giving fragments in a 200 to 500 bp range. Five µL (~0.5 µg) of fragmented cDNA was used to make each low input library using a ThruPLEX DNA-Seq Kit (Takara Bio USA, cat# R400676). Forty-eight single-cell libraries were quantified using a Qubit 2.0 fluorometer and Qubit dsDNA HS Assay kit (Invitrogen, Waltham, MA, cat# Q32854), then multiplex sequenced in 1 lane using an Illumina HiSeq3000 Sequencer at NIDDK Genomics Facility. For each library, single-end, 50 base reads were collected and converted by bcl2fastq (version 2) into fastq files and aligned on (GRCm38/mm10) using STAR (version 2.7.3a) (39). Output bam files were analyzed with Genomatix programs with gene transcript levels calculated as counts per million mapped reads (CPM). Forty-six of 48 libraries passed quality control and met a threshold of >2 million total reads (average reads/library 6.7 million; range, 3.7-13.9 million).
Single Cell Consensus Clustering, Heatmap, and Gene Ontology Analysis
Cluster analysis of single cell RNA-seq data was initially performed using R package SC3 (40) based on default parameters including correction for multiple testing by nonparametric Kruksal-Wallis test for differentially expressed genes (P < 0.01). Further analysis of differentially expressed genes across clusters revealed enrichment for established marker genes for osteoblasts, fibrocytes, and macrophages in the major clusters. Based on these marker genes, heatmap clustering was performed using R package Pheatmap. All R analyses were performed in R software version 4.0.2 (https://www.r-project.org/). Gene ontology (GO) was analyzed using NCBI DAVID with the whole mouse genome as background reference and selection of GO terms with P value < 10–3 per category.
Results
A Dio2CreERt2 Driver Allele to Identify Dio2-Expressing Cell Types
The Dio2CreERt2 knockin allele expressed CreERt2 instead of Dio2 protein from the endogenous Dio2 gene (Fig. 1A). CreERt2 recombinase activity was selectively induced with tamoxifen (41), usually administered on 2 consecutive days (Fig. 1B), which activated fluorescent signals from a Rosa26Ai6 reporter (encoding zsGreen1 protein) (25). Studies were performed on Dio2CreERt2/+;Rosa26Ai6/Ai6 mice (abbreviated in simpler form as Dio2CreERt2;Ai6) because heterozygous loss of Dio2 does not cause overt cochlear defects whereas homozygous loss results in organ of Corti abnormalities and deafness (19). Homozygous Dio2CreERt2/CreERt2 mice, like Dio2-/- (null) mice, have auditory impairment (thresholds for auditory-evoked brainstem responses to a click stimulus for homozygous and wild-type mice: 63 ± 1 and 43 ± 1 dB sound pressure level, respectively; mean ± SEM, n = 4, P < 0.001, Student t test).
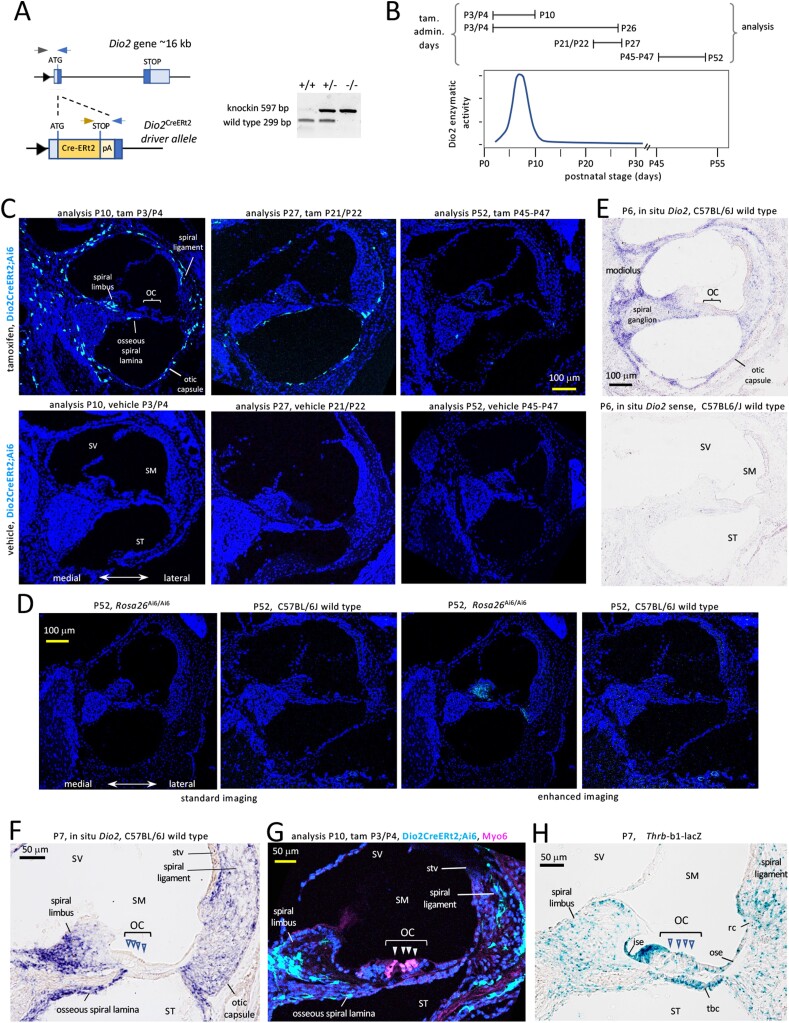
Figure 1.
Dio2 CreERt2 allele and its expression in the cochlea. A, The CreERt2 knockin displaces the first Dio2 coding exon. Triangle, promoter; dark blue. boxes, coding exons; light blue, untranslated regions; pA, poly(A) site after CreERt2 stop. Arrowheads denote genotyping primers; gel shows genotyping results. B, Examples of tamoxifen (tam) treatments relative to a reference diagram of cochlear Dio2 activity, modified with permission from our data in ref #16 (16); Copyright (2000) National Academy of Sciences, USA. C, Specific fluorescent cells (pale blue) in the otic capsule and in fibrocyte areas (spiral limbus, spiral ligament) in Dio2CreERt2;Ai6 mice at P10 after administration of tam at P3/P4 (mid-cochlear turn). Later administration at P21/P22 (mid-basal turn) and at P45, P46 and P47 (mid-turn) yielded very few Dio2+ cells. Corn oil vehicle or no treatment gives no specific signal at any stage tested. DAPI (dark blue), general nuclear stain. D, Lack of specific fluorescent signals in Rosa26Ai6/Ai6 or wild-type C57BL6/J control mice without treatment at P52, using standard confocal imaging (equivalent to panel C) (cochlear mid-turns). Enhanced imaging reveals weak background in the spiral limbus in Rosa26Ai6/Ai6 but not wild-type mice, suggesting low-level expression of Ai6 reporter in the absence of the Dio2CreERt2 allele. E, In situ hybridization analysis of Dio2 RNA location using antisense (top) and sense control (bottom) probes, in wild-type mice at P6. F, Magnified view of Dio2 in situ hybridization signals at P7 showing correlation with Dio2+ fluorescent cells in panel G. G, Magnified view of Dio2+ cells in the spiral ligament, spiral limbus, the otic capsule and osseous spiral lamina. No Dio2+ cells were detected in the organ of Corti (OC) and adjacent epithelia. Myo6 labels hair cells (arrowheads). H, Thrb receptor gene expression in organ of Corti and adjacent tissues that lack Dio2 expression. Abbreviations: ise, inner sulcus epithelium; OC, organ of Corti; ose, outer sulcus epithelium; rc, root cell area; SM, scala media; ST, scala tympani; stv, stria vascularis; SV, scala vestibuli; tbc, tympanic border cells.
Tamoxifen was given at several time points relative to the peak of cochlear Dio2 activity, which rises sharply from postnatal day 2 (P2) to P8 then falls substantially by ~P14 with minimal levels at more mature ages (16). This transient activity peak is paralleled by a prominent rise and fall of Dio2 RNA levels as shown previously by northern blot (38) and quantitative PCR analyses (42). Tamoxifen treatment induced strong, specific cellular signals in Dio2CreERt2;Ai6 mice compared with vehicle-treated or untreated mice (Fig. 1C). Analysis of Dio2CreERt2;Ai6 mice at P10 after tamoxifen treatment at P3 and P4, a period spanning the Dio2 peak (Fig. 1B), identified Dio2-positive (Dio2+) cells in the otic or bony capsule and in fibrocyte areas of the spiral limbus and spiral ligament. Each region is investigated in detail in later sections. In accord with the early peak of Dio2 expression, tamoxifen treatment at P21/P22 resulted in a sparser pattern of Dio2+ cells and treatment at older ages resulted in very few detectable Dio2+ cells. The specificity of results was established at each stage by comparison with treatment with corn oil vehicle or with no treatment, both of which gave no specific signal compared with strong tamoxifen-induced signals in Dio2CreERt2;Ai6 mice (Fig. 1C). Further controls for the specificity of induced signals included analysis of the Ai6 reporter without CreERt2 and no treatment (Fig. 1D). Rosa26Ai6/Ai6 mice showed little or no background except for weak fluorescence in the spiral limbus detected by enhanced imaging at older ages, in contrast to the strong signals induced by tamoxifen during the period of Dio2 expression in Dio2CreERt2;Ai6 mice.
The specific fluorescence pattern detected in Dio2CreERt2;Ai6 mice correlated closely with the in situ hybridization pattern for Dio2 mRNA detected in wild-type mice (Fig. 1E), thus validating the Dio2CreERt2;Ai6 model. Consistent with our previous findings (16, 42), Dio2 RNA signal localized to the immature otic capsule, including in the osseous spiral lamina, and in fibrocyte areas of the spiral limbus and spiral ligament (higher magnification in Fig. 1F).Together, the above studies demonstrate that the Dio2CreERt2 allele drives specific fluorescent signals that reflect the temporal and cellular expression pattern of the Dio2 gene.
In Dio2CreERt2;Ai6 mice, Dio2 + cells were not detected in known T3-responsive tissues in the organ of Corti and adjacent epithelia, nor in root cells or tympanic border cells (Fig. 1G), which are major sites of expression of the Thrb receptor gene (Fig. 1H) (14, 43). Dio2+ cells were not located in the stria vascularis or any epithelia around the cochlear duct (ie, the endolymph-filled scala media).
Dio2+ Fibrocytes in the Spiral Ligament and Spiral Limbus
Cochlear fibrocytes are incompletely defined supporting cell types that derive from otic mesenchyme (44, 45) and reside in lateral (spiral ligament) and medial (spiral limbus) locations, as indicated by fibrocyte marker S100b (Fig. 2A). Dio2+ cells co-localized with S100b in both locations.
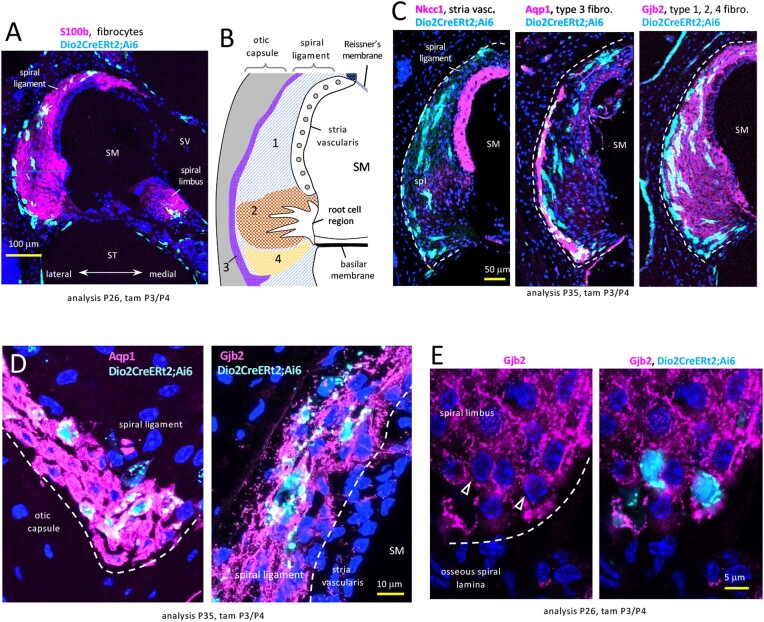
Figure 2.
Dio2+ fibrocytes in the spiral ligament (lateral) and spiral limbus (medial) in Dio2CreERt2;Ai6 mice. A, Fibrocyte regions indicated by S100b stain; cochlear mid-turn. B, Diagram of zones of fibrocyte types 1–4 in the spiral ligament. C, Lateral wall sections reveal that Dio2+ cells (pale blue) are not in the stria vascularis (NKCC1) but stain with markers of type 3 (Aqp1) and other fibrocyte types (Gjb2) (magenta). Double-positive cells appear whitish-blue. D, Magnified views of Dio2+ fibrocytes stained for Aqp1 (type 3, lower region) and Gjb2 (type 1, near stria vascularis). E, Spiral limbus Dio2+ fibrocytes stained for Gjb2 (arrowheads). DAPI (dark blue), general nuclear stain in panels A, B, D, E. Abbreviations: SM, scala media; ST, scala tympani; SV, scala vestibuli.
In the lateral wall, fibrocytes occur as several subtypes, defined by regional location within the spiral ligament and by marker expression (46, 47) (Fig. 2B). Co-staining showed that Dio2+ fibrocytes represent the major type 1, 2 and 4 populations (positive for Gjb2, connexin 26) and the type 3 population (positive for Aqp1, aquaporin 1) that borders the otic capsule (Fig. 2C, 2D). In the medial spiral limbus, fibrocytes are less well defined but Dio2+ fibrocytes stained with established markers including Gjb2 (Fig. 2E). These findings based on both anatomical location and marker expression indicate that Dio2 is expressed in most if not all known populations of cochlear fibrocytes and suggest the potential of fibrocytes to mediate hormonal signaling.
Dio2+ Fibrocytes in Proximity to Cochlear Vasculature
The role of Dio2 in converting T4 to T3 implies that Dio2+ cells would take up T4 substrate from the circulation. In the cochlea, both lateral and medial fibrocyte zones represent major destinations for incoming blood flow and are associated with dense capillary networks (21). We therefore investigated the location of Dio2+ fibrocytes relative to the cochlear vasculature (diagram, Fig. 3A). In whole-mounted lateral wall tissue, staining for CD31 revealed the vascular network in the spiral ligament (Fig. 3B and 3C) and clustering of Dio2+ fibrocytes near these vessels. Magnified views (Fig. 3D) show a parallel alignment of Dio2+ fibrocytes with the orientation of vessels suggesting a coordinated arrangement of fibrocytes and vessels.
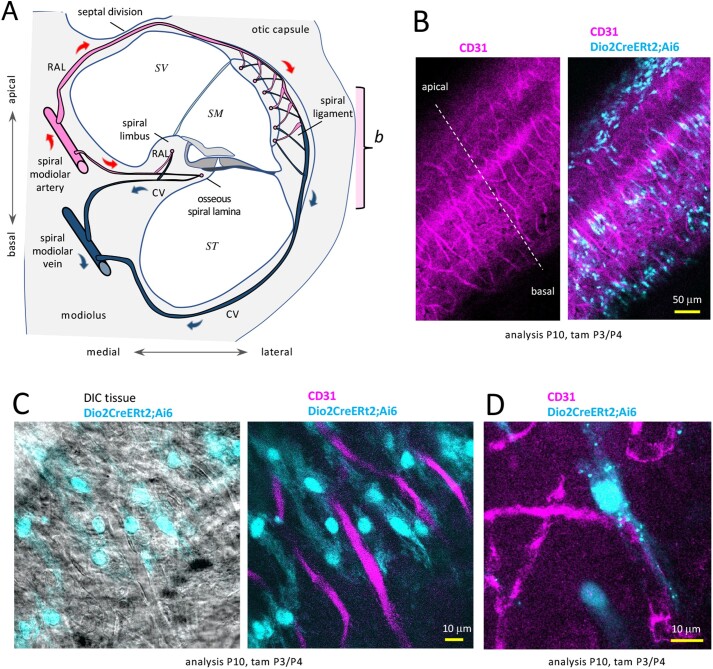
Figure 3.
Dio2+ fibrocytes in proximity to lateral vascular networks in Dio2CreERt2;Ai6 mice. A, Diagram of cochlear vasculature (mid-turn section). Radiating arterioles (RAL) branch in a short loop to the limbus and lamina and a longer loop through the bony septal division to capillary networks in the spiral ligament and stria vascularis. CV, collecting venules. Bracket “b” refers to view in panel B. B, Wholemount side view of Dio2+ fibrocytes (pale blue) interspersed in the vascular network (CD31, magenta) in the spiral ligament. C, Magnified wholemount view of Dio2+ fibrocytes, which align with vessel orientations; DIC, differential interference contrast view showing tissue context. D, Magnified view of a Dio2+ fibrocyte and vessels. Abbreviations: CV, collecting venule; RAL, radiating arteriole; SM, scala media; ST, scala tympani; SV, scala vestibuli.
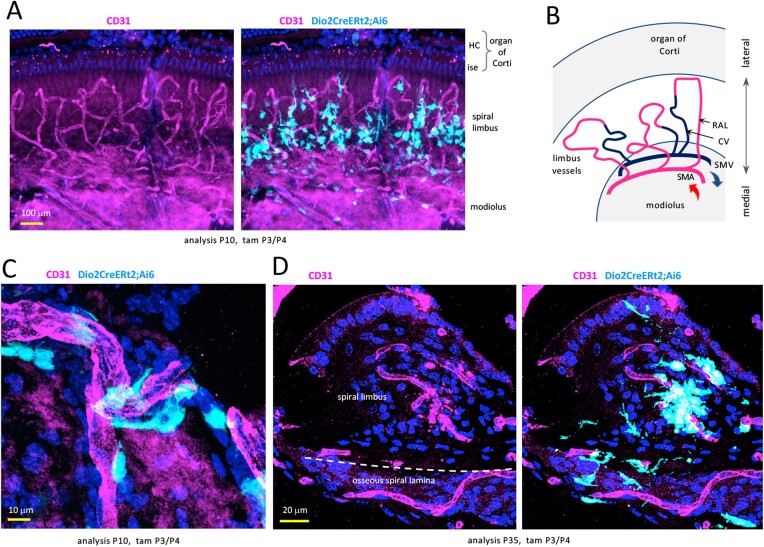
In the medial cochlea, vessels from the spiral modiolar artery form short loops and capillary networks in the spiral limbus and osseous spiral lamina (21) (Fig. 4A and 4B). Surface and sectional views revealed Dio2+ fibrocytes with branched morphology interspersed around both limbus (Fig. 4A, 4C, and 4D) and lamina vessels (Fig. 4D).
Figure 4.
Dio2+ fibrocytes in proximity to medial vascular networks in Dio2CreERt2;Ai6 mice. A, Wholemount surface view showing Dio2+ fibrocytes (pale blue) clustered around vessels (CD31, magenta) in the spiral limbus. DAPI, general nuclear stain (dark blue). B, Simplified diagram (surface view) of vasculature in spiral limbus region. RAL, radiating arterioles extend into capillary networks in the spiral limbus and spiral lamina (below, not seen in this view); CV, collecting venules. C, Magnified surface view of Dio2+ fibrocytes with projections around limbus vessels. D, Section showing Dio2+ fibrocytes around limbus and lamina vessels. Abbreviations: CV, collecting venule; HC, hair cells; ise, inner sulcus epithelium; RAL, radiating arteriole; SMA, spiral modiolar artery; SMV, spiral modiolar vein.
Dio2+ Osteoblasts in the Cochlea
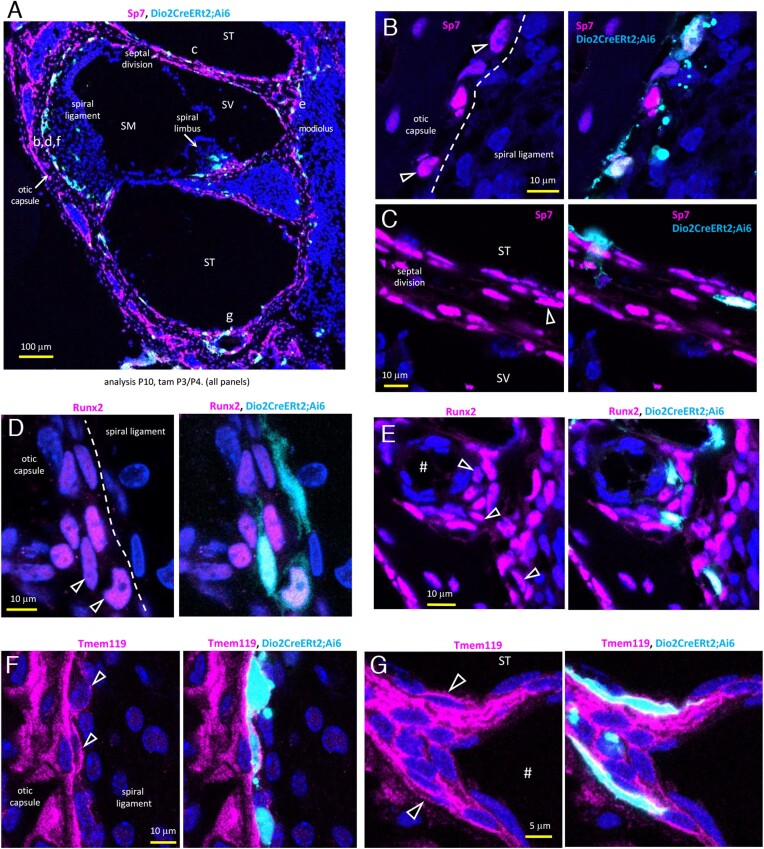
Otic mesenchyme forms cartilaginous condensates that eventually form the ossified otic capsule and modiolus (48, 49). Cochlear osteoblast differentiation is poorly defined. However, by staining for key osteogenic factors (Sp7, Runx2, Tmem119) (50), we detected Dio2+ osteoblasts at the surfaces of the otic capsule and modiolus at immature stages (P10) when Dio2 expression peaks. Fig. 5A shows an overview of staining for Sp7 (Osterix) differentiation factor. Magnified views show examples of Dio2+;Sp7 + osteoblasts in the lateral otic capsule bordering the spiral ligament (Fig. 5B) and the bony septal division between turns of the cochlea (Fig. 5C).
Figure 5.
Dio2+ osteoblast lineage cells in Dio2CreERt2;Ai6 mice. A, Dio2+ cells (pale blue) stained with Sp7 osteogenic marker (magenta) in the immature bony capsule. Lower case letters (b-g) indicate approximate areas of magnified views in panels B-G. In all panels, nuclei are stained dark blue (DAPI). In all panels, analysis was at P10, after tamoxifen administration at P3/P4. Mid-basal turn sections. B, C, Examples of Dio2+ osteoblasts (arrowheads) stained with Sp7 in the otic capsule (B) and bony septal division (C). D, E, Dio2+ osteoblasts stained with early-stage factor Runx2 in the otic capsule (D) and near a vessel canal (#) in the modiolus (E). F, G, Dio2+ osteoblasts outlined by Tmem119 staining in the lateral otic capsule (F) and cells lining the bone near a vessel canal (#) at the base of the cochlear turn (G). Abbreviations: SM, scala media; ST, scala tympani; SV, scala vestibuli.
The early postnatal peak of Dio2 activity, when the otic capsule is minimally calcified, suggested that Dio2 is induced in early-stage osteoblasts, which we confirmed by co-staining for Runx2, a factor upstream of Sp7 in the osteogenic pathway. Examples of Dio2+;Runx2 + osteoblasts are shown in the otic capsule (Fig. 5D) and modiolus near a vessel canal (Fig. 5E).
Dio2+ cells also stained for Tmem119 (Obif, osteoblast induction factor), a critical factor for osteogenic maturation (51), as shown in examples in the otic capsule (Fig. 5F) and in flattened cells, possibly bone lining-like cells, near a vessel canal at the base of the cochlear turn (Fig. 5G). Cochlear bone lining cells, although little studied, are mesenchymal cell types on surfaces of the cochlear bony chambers (the perilymph-filled scala vestibuli, SV, and scala tympani, ST) and otic capsule (52).
Transcriptome Identities of Dio2+ Fibrocytes and Osteoblasts
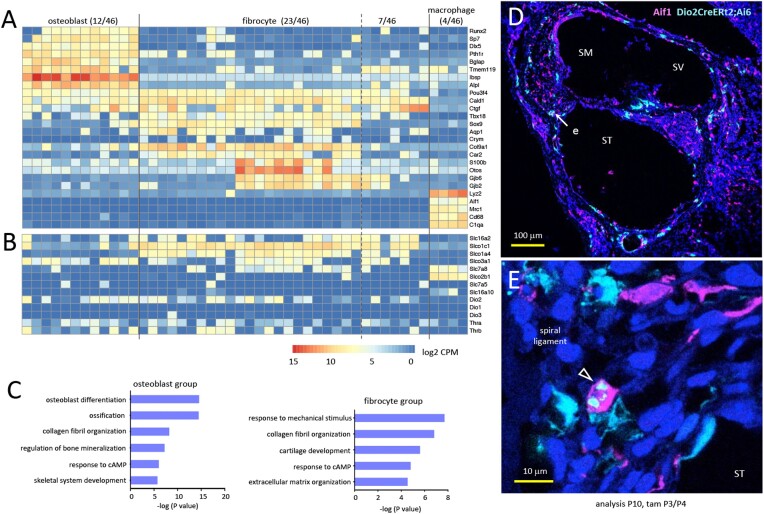
The preceding analyses of anatomical location and morphology of Dio2+ cells, corroborated by staining analyses for established markers indicate that fibrocytes and osteoblasts are the major Dio2-expressing cell types in the cochlea. We further confirmed the identity of Dio2+ cell types by high resolution RNA-sequencing of individual Dio2+ fluorescent cells isolated from immature cochlea, at P10, around the peak Dio2 expression. Given the lack of detailed transcriptome data of cochlear fibrocytes and osteoblasts, our approach of targeted selection of Dio2+ cells yielded fine resolution data (average 6.7 million reads/library) with the advantage of high definition of cell type identities (Fig. 6A, 6B, and 6C). A heatmap analysis of 46 cells (Fig. 6A) with analysis for established marker genes shows 2 major groups representing fibrocyte (23/46), and osteoblast (12/46) lineages, thus confirming the cell staining results of Dio2+ cell identities as osteoblasts (Fig. 5) and fibrocytes (Fig. 2). The osteoblast group expressed key osteogenic genes (eg, Runx2, Sp7) and later markers (eg, Tmem119, Ibsp), consistent with a status in terminal differentiation.
Figure 6.
Transcriptome analysis of individually isolated cochlear Dio2+ cell types. A, Heatmap of representative, differentially expressed genes identifying major osteoblast and fibrocyte groups in single cell datasets from mice heterozygous for the Dio2CreERt2 allele (Dio2CreERt2;Ai6). Tamoxifen was given at P3/P4 and cells analyzed at P10. A few cells (7/46) lacked consistent osteoblast or fibrocyte markers and may represent less-differentiated mesenchyme-derived cell types based on early markers (eg, Pou3f4). Four macrophages were identified. Data shown as log2 (CPM + 1). B, Candidate membrane transporters for thyroid hormone. Most fibrocytes express Slco1c1 and often Slc16a2 and Slco1a4; osteoblasts most consistently express Slco3a1. Most cells lacked Dio3 or Dio1 but expressed some level of Dio2, as expected. C, Gene ontology analysis of selected top categories for osteoblast and fibrocyte groups. D, Aif1 (Iba1) staining (magenta) shows macrophages in a dispersed pattern over most cochlear tissues compared with the restricted pattern of Dio2+ cells (pale blue) (D). E, Rare double-positive cell (arrowhead) in the spiral ligament (area “e” in panel D). In Aif1 + cells, Dio2CreERt2;Ai6 signal tended to clump rather than fill the cell. DAPI (dark blue), general nuclear stain.
The fibrocyte group expressed early markers such as Sox9 and Tbx18 (49, 53) and heterogeneous patterns of later known markers including Gjb2, Gjb6, Aqp1, Otos, and Crym, a gene that encodes a cytosolic protein with T3 binding activity (54). The results are consistent with fibrocytes at varied stages of terminal differentiation as expected at this immature stage when Dio2 expression peaks. A small subgroup (7/46) with fewer consistent osteoblast or fibrocyte markers nonetheless expressed mesenchymal markers such as Pou3f4 (48) and may represent less-differentiated fibrocyte or mesenchyme-derived cell types. Apart from the predicted osteoblast and fibrocyte lineages, a few macrophages (4/46) were identified. Staining with Aif1 (Iba1) (55) identified a dispersed pattern of macrophages compared with the more restricted pattern of Dio2+ cells with only rare cells showing co-localization (Fig. 6D and E). Dio2 functions have been reported for bone marrow macrophages (56).
Plasma Membrane Transporters for Thyroid Hormone
We investigated the single cell RNA-sequencing datasets to support the hypothesis that Dio2+ cell types would express membrane transporters for uptake of T4 and transfer of T3 to other cell types (57-59). In the cochlea, using in situ hybridization, we previously detected Slco1c1 (Oatp1c1) and Slc16a2 (Mct8) mRNA in fibrocyte areas and in the otic capsule (42). Heatmap analysis of Dio2+ cells (Fig. 6B) confirmed that most fibrocytes express Slco1c1 and additional transporters including Slc16a2 and Slco1a4. In contrast, osteoblasts most consistently express Slco3a1, but low levels of other transporters found in fibrocytes suggesting differential transporter signatures in osteoblasts and fibrocytes.
Sensitivity of Dio2+ Cell Types to Dio2-Deficiency
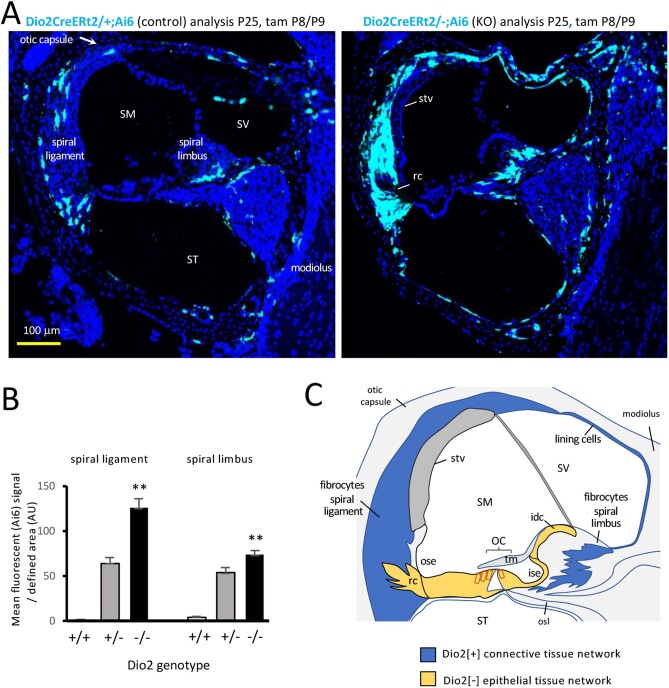
Hypothyroidism evokes increases of Dio2 gene expression in certain tissues, reflecting a means to generate T3 locally in the face of decreasing T3 availability in the circulation (60). We investigated the response of Dio2 gene expression to local hypothyroidism in the cochlea resulting from Dio2-deficiency (Fig. 7A and B). Dio2 knockouts (Dio2CreERt2/-; Rosa26Ai6/Ai6) displayed elevated fluorescent signals in lateral and medial fibrocyte areas compared with control (Dio2CreERt2/+;Rosa26Ai6/Ai6) mice. The response was specific to fibrocytes, osteoblasts, and the cells lining the bony capsule and the bony roof of the scala vestibuli. The response was not generalized to other cell types, such as cells of the stria vascularis (stv) and root cells (rc) (Fig. 7A, right panel). The results indicate that Dio2+ cell types are sensitive to thyroid hormone status and suggest the importance of Dio2 in generating T3 in specific cochlear tissues.
Figure 7.
Response of Dio2+ cell types to Dio2-deficiency. A, Elevated Ai6 reporter expression in Dio2 knockouts (Dio2CreERt2/-) compared with control (Dio2CreERt2/+) mice. Signals increased in spiral ligament and spiral limbus fibrocytes, osteoblasts, and cells around the otic capsule and modiolus surfaces. Sections of mid-basal cochlear turns. B, Ai6 (Zsgreen1) fluorescent signal in fibrocyte areas in Dio2 knockouts (n = 3 mice; 9 or 10 views/group). Heterozygous and homozygous genotypes carry an equivalent, single Dio2CreERt2 allele. Pairwise comparison of -/- and +/+ groups, ** P < 0.001, Student t test; (+/+ control shows minimal background signal). C, Model for fibrocyte-mediated, paracrine-like control of T3 signaling. Dio2+ cells within the “connective tissue gap junctional network” (blue) may amplify T3 to transfer across the cellular boundary to the “epithelial tissue gap junctional network” (yellow) including T3-sensitive target cells in the vicinity of the organ of Corti. Hair cells (red outline) lack gap junctions with surrounding epithelial tissue. Dio2+ osteoblasts are not indicated in this simplified diagram. Abbreviations: idc, interdental cells; ise, inner sulcus epithelium; OC, organ of Corti; ose, outer sulcus epithelium; osl, osseous spiral lamina; rc, root cells; SM, scala media; ST, scala tympani; stv, stria vascularis; SV, scala vestibuli; tm, tectorial membrane.
A Connective Tissue-Epithelial Tissue Boundary Defined by Dio2 Expression
The network of Dio2-sensitive fibrocytes and cells lining the bony roof of the scala vestibuli correlates closely with the “connective tissue gap junctional network” that has been proposed to mediate ionic transport in the cochlea (44, 46) (Fig. 7C). In contrast, an internal network of Dio2-negative cell types that includes known T3 target cells (see Fig. 1G and 1H), correlates with an “epithelial tissue gap junctional network” (46). The epithelial network consists of support cells of the organ of Corti and nearby epithelia (interdental cells, inner and outer sulcus epithelia, and root cells). The connective and epithelial tissue networks are separated by tight junctional boundaries (61) thereby requiring internetwork transport. The exclusion of Dio2+ cells from the epithelial network suggests a cellular boundary for control of T3 signaling to internal tissues.
Discussion
Fibrocytes and Paracrine-Like Control of Hormonal Signaling
Cochlear fibrocytes are poorly understood cell types that are considered to be important for ionic and metabolic homeostasis in the cochlea (44, 45). Our results suggest a previously unrecognized role for cochlear fibrocytes in hormonal signaling. Fibrocytes are appropriately situated to act as a local generator of T3 within the cochlea, meeting criteria expected for an intermediary that transfers T3 to avascular, internal tissues: (i) we showed that Dio2+ fibrocytes reside in vascularized regions of the cochlea where they could take up T4 from the circulation for conversion to T3; (ii) fibrocytes reside within a previously reported “connective tissue gap junctional network” that allows shuttling of solutes within the network but forms a barrier with an internal “epithelial tissue network” (44, 46). The internal network includes T3-sensitive cells in the organ of Corti and adjacent epithelia (8-10). Accordingly, fibrocytes provide a plausible cellular link between the circulation and T3-sensitive internal tissues.
The interface between the connective and epithelial tissue networks has previously been discussed primarily in terms of potassium ion transport (61), which is necessary for auditory transduction by the hair cells. We suggest that this cellular interface also provides the potential for paracrine-like control of T3 signals based on the segregation of T3-generating (Dio2+ fibrocytes) and T3-responding tissues (Dio2-negative epithelia) (Fig. 7C). Why would a regulated cellular barrier to T3 signaling be beneficial? This barrier could sensitively control amplification or constraint of T3 signaling as necessary during development. Constraint is important because immature tissues are highly sensitive to T3: we have reported that excessive T3 in neonatal mice causes cochlear abnormalities and deafness (38, 62). Paracrine-like control by Dio2+ fibrocytes in the cochlea, although anatomically complex, may be analogous to the control of neuronal responses by Dio2+ astrocytes at the blood-brain barrier (63, 64).
The cellular uptake of T4 and transfer of Dio2-generated T3 to internal tissues is probably mediated by trans-membrane thyroid hormone transporters belonging to solute carrier families. Transporters with varying affinities for T4, T3 and additional substrates have been identified based on in vitro and in some cases in vivo evidence (57-59). We detected Slco1c1 and Slc16a2 in fibrocytes by transcriptome analysis, corroborating in situ hybridization results (42). We previously demonstrated the requirement for membrane transport of T3 in the cochlea by deletion of Slc16a2 and Slc16a10, which resulted in hypothyroid-like cochlear abnormalities and deafness in mice (65). Only the combined deletions cause overt cochlear defects indicating complexity in the transporters and possibly cellular routes involved. Human SLCO1C1 (66) and SLC16A2 mutations (59) are associated with neurological impairment but hearing loss is rarely mentioned (67). Another transporter of amino acids and thyroid hormone, SLC7A8 (Lat2) localizes in fibrocytes and is associated with deafness (68).
The proposed paracrine-like control by Dio2+ fibrocytes broadens the concept of cell-cell signaling in cochlear development. In other examples, epithelia of the cochlear duct release factors that influence differentiation of nearby tissues (69) and otic mesenchyme influences spiral ganglion neurons (70). Also, retinoic acid-metabolizing enzymes in tissues adjacent to the early otic cup regulate the anterior-posterior organization of inner ear compartments (71).
Other hormones are thought to influence cochlear function (24, 72, 73) and synthetic steroid preparations are often used to treat inner ear disorders such as idiopathic sudden hearing loss, Meniere’s disease, or acoustic trauma (74). However, cellular routes of hormonal transport in the cochlea, whether natural or pharmacological, are poorly understood. Rational therapeutic approaches would benefit from better understanding of cellular transport routes. A speculation arising from our findings is that fibrocytes represent a cellular intermediary for signaling by other hormones as well as T3. We note that other means of hormone transport probably exist in the specialized tissues of the cochlea. For example, Megalin (Lrp2) is an endocytic receptor for lipophilic substrates, including 17β-estradiol, and localizes to the marginal cells of the stria vascularis (75), a tissue that does not express Dio2. Megalin-deficiency is reported to lead to progressive hearing loss.
Dio2 in Osteoblasts
Dio2 is induced in cochlear osteoblasts during terminal differentiation before the onset of hearing. Unlike fibrocytes which have a plausible cellular route connecting the circulation to internal sensory tissues, this is less obvious for Dio2+ osteoblasts, which are more remote from the organ of Corti. Potentially, Dio2+ osteoblasts serve a distinct role in their immediate environment, producing T3 for bone or connective tissues. T3 serves varied functions in skeletal maturation, for example in long bones (76) and in the middle ear ossicles (77). Dio2 activity has been identified in osteoblasts in postnatal mouse calvarial explants (78). We have not observed gross defects in the otic capsule or bony labyrinth in Dio2-deficient mice. However, subtler defects are not excluded for example, in long term homeostasis as suggested by a report of brittle long bones and excessive mineralization in Dio2-deficient mice (79).
The cell types that line cochlear bone have been little studied (52) but our results identifying Dio2+ cells at the surfaces of the cochlear bony chambers suggest a possible role in the amplification and transfer of T3. It has been reported that mesenchymal cells lining the roof of the scala vestibuli form gap junctions with each other and with fibrocytes in the spiral limbus and spiral ligament, suggesting possible routes of communication (46) (Fig. 7C).
Finally, we note that Dio2-generated T3 may serve additional functions in nonepithelial and other supporting tissues of the cochlea, although these remain to be investigated.
Acknowledgments
This work was supported by the intramural research program at National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at the National Institutes of Health. Sequencing is supported by the Genomics Core, Dr. Sijung Yun, and Dr. Harold Smith, at NIDDK.
Glossary
Abbreviations
- CPM
counts per million mapped reads
- DAPI
4′,6-diamidino-2-phenylindole
- Dio2
type 2 deiodinase
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- P#
postnatal day #
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PFA
paraformaldehyde
- T3
3,5,3′-L-triiodothyronine
- T4
thyroxine
Additional Information
Disclosures : The authors have nothing to disclose and declare that there is no conflict of interest.
Data Availability
Single cell datasets are available on the GEO database with accession number GSE181057 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE181057).
References
- 1. Sohmer H, Freeman S. The importance of thyroid hormone for auditory development in the fetus and neonate. Audiol Neurootol. 1996;1(3):137-147. [DOI] [PubMed] [Google Scholar]
- 2. Ng L, Kelley MW, Forrest D. Making sense with thyroid hormone–the role of T(3) in auditory development. Nat Rev Endocrinol. 2013;9(5):296-307. [DOI] [PubMed] [Google Scholar]
- 3. DeLong GR, Stanbury JB, Fierro-Benitez R. Neurological signs in congenital iodine-deficiency disorder (endemic cretinism). Dev Med Child Neurol. 1985;27(3):317-324. [DOI] [PubMed] [Google Scholar]
- 4. Rovet J, Walker W, Bliss B, Buchanan L, Ehrlich R. Long-term sequelae of hearing impairment in congenital hypothyroidism. J Pediatr. 1996;128(6):776-783. [DOI] [PubMed] [Google Scholar]
- 5. Lichtenberger-Geslin L, Dos Santos S, Hassani Y, Ecosse E, Van Den Abbeele T, Léger J. Factors associated with hearing impairment in patients with congenital hypothyroidism treated since the neonatal period: a national population-based study. J Clin Endocrinol Metab. 2013;98(9):3644-3652. [DOI] [PubMed] [Google Scholar]
- 6. Brucker-Davis F, Skarulis MC, Pikus A, et al. Prevalence and mechanisms of hearing loss in patients with resistance to thyroid hormone. J Clin Endocrinol Metab. 1996;81(8):2768-2772. [DOI] [PubMed] [Google Scholar]
- 7. Ferrara AM, Onigata K, Ercan O, Woodhead H, Weiss RE, Refetoff S. Homozygous thyroid hormone receptor β-gene mutations in resistance to thyroid hormone: three new cases and review of the literature. J Clin Endocrinol Metab. 2012;97(4):1328-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deol MS. An experimental approach to the understanding and treatment of hereditary syndromes with congenital deafness and hypothyroidism. J Med Genet. 1973;10(3):235-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uziel A. Periods of sensitivity to thyroid hormone during the development of the organ of Corti. Acta Otolaryngol Suppl. 1986;429:23-27. [DOI] [PubMed] [Google Scholar]
- 10. Rusch A, Ng L, Goodyear R, et al. Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors. J Neurosci. 2001;21(24):9792-9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dettling J, Franz C, Zimmermann U, et al. Autonomous functions of murine thyroid hormone receptor TRα and TRβ in cochlear hair cells. Mol Cell Endocrinol. 2014;382(1):26-37. [DOI] [PubMed] [Google Scholar]
- 12. Mustapha M, Fang Q, Gong TW, et al. Deafness and permanently reduced potassium channel gene expression and function in hypothyroid Pit1dw mutants. J Neurosci. 2009;29(4):1212-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sundaresan S, Balasubbu S, Mustapha M. Thyroid hormone is required for the pruning of afferent type II spiral ganglion neurons in the mouse cochlea. Neuroscience. 2016;312:165-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ng L, Cordas E, Wu X, Vella KR, Hollenberg AN, Forrest D. Age-related hearing loss and degeneration of cochlear hair cells in mice lacking thyroid hormone receptor β1. Endocrinology. 2015;156(10):3853-3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knipper M, Richardson G, Mack A, et al. Thyroid hormone-deficient period prior to the onset of hearing is associated with reduced levels of beta-tectorin protein in the tectorial membrane: implication for hearing loss. J Biol Chem. 2001;276(42):39046-39052. [DOI] [PubMed] [Google Scholar]
- 16. Campos-Barros A, Amma LL, Faris JS, Shailam R, Kelley MW, Forrest D. Type 2 iodothyronine deiodinase expression in the cochlea before the onset of hearing. Proc Natl Acad Sci U S A. 2000;97(3):1287-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol. 2001;15(12):2137-2148. [DOI] [PubMed] [Google Scholar]
- 18. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23(1):38-89. [DOI] [PubMed] [Google Scholar]
- 19. Ng L, Goodyear RJ, Woods CA, et al. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci U S A. 2004;101(10):3474-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006;116(10):2571-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Axelsson A. Comparative anatomy of cochlear blood vessels. Am J Otolaryngol. 1988;9(6):278-290. [DOI] [PubMed] [Google Scholar]
- 22. Salt AN, Hirose K. Communication pathways to and from the inner ear and their contributions to drug delivery. Hear Res. 2018;362:25-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nyberg S, Abbott NJ, Shi X, Steyger PS, Dabdoub A. Delivery of therapeutics to the inner ear: the challenge of the blood-labyrinth barrier. Sci Transl Med. 2019;11(482):eaao0935. doi:10.1126/scitranslmed.aao0935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meltser I, Canlon B. Protecting the auditory system with glucocorticoids. Hear Res. 2011;281(1-2):47-55. [DOI] [PubMed] [Google Scholar]
- 25. Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. RRID:AB_143157. https://scicrunch.org/resources/Any/search?q=AB_143157&l=AB_143157 [Google Scholar]
- 27. RRID:AB_141874. https://scicrunch.org/resources/Any/search?q=AB_141874&l=AB_141874 [Google Scholar]
- 28. RRID:AB_2636859. https://scicrunch.org/resources/Any/search?q=AB_2636859&l=AB_2636859 [Google Scholar]
- 29. RRID:AB_1163380. https://scicrunch.org/resources/Any/search?q=AB_1163380&l=AB_1163380 [Google Scholar]
- 30. RRID:AB_393571. https://scicrunch.org/resources/Any/search?q=AB_393571&l=AB_393571 [Google Scholar]
- 31. RRID:AB_2533903. https://scicrunch.org/resources/Any/search?q=AB__2533903&l=AB__2533903 [Google Scholar]
- 32. RRID:AB_260563. https://scicrunch.org/resources/Any/search?q=260563&l=260563 [Google Scholar]
- 33. RRID:AB_91514. https://scicrunch.org/resources/Any/search?q=AB__91514%2C&l=AB__91 [Google Scholar]
- 34. RRID:AB_2713945. https://scicrunch.org/resources/Any/search?q=AB__2713945&l=AB__2713945 [Google Scholar]
- 35. RRID:AB_2892207. https://scicrunch.org/resources/Any/search?q=2892207&l=2892207 [Google Scholar]
- 36. RRID:AB_2736549. https://scicrunch.org/resources/Any/search?q=2736549&l=2736549 [Google Scholar]
- 37. RRID:AB_2800343. https://scicrunch.org/resources/Any/search?q=2800343&l=2800343 [Google Scholar]
- 38. Ng L, Hernandez A, He W, et al. A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function. Endocrinology. 2009;150(4):1952-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kiselev VY, Kirschner K, Schaub MT, et al. SC3: consensus clustering of single-cell RNA-seq data. Nat Methods. 2017;14(5):483-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Indra AK, Warot X, Brocard J, et al. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;27(22):4324-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sharlin DS, Visser TJ, Forrest D. Developmental and cell-specific expression of thyroid hormone transporters in the mouse cochlea. Endocrinology. 2011;152(12):5053-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bradley DJ, Towle HC, Young WS 3rd. Alpha and beta thyroid hormone receptor (TR) gene expression during auditory neurogenesis: evidence for TR isoform-specific transcriptional regulation in vivo. Proc Natl Acad Sci U S A. 1994;91(2):439-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol. 2006;576(Pt 1):11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Furness DN. Forgotten fibrocytes: a neglected, supporting cell type of the cochlea with the potential to be an alternative therapeutic target in hearing loss. Front Cell Neurosci. 2019;13:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kikuchi T, Kimura RS, Paul DL, Adams JC. Gap junctions in the rat cochlea: immunohistochemical and ultrastructural analysis. Anat Embryol. 1995;191(2):101-118. [DOI] [PubMed] [Google Scholar]
- 47. Spicer SS, Schulte BA. The fine structure of spiral ligament cells relates to ion return to the stria and varies with place-frequency. Hear Res. 1996;100(1-2):80-100. [DOI] [PubMed] [Google Scholar]
- 48. Phippard D, Lu L, Lee D, Saunders JC, Crenshaw EB 3rd. Targeted mutagenesis of the POU-domain gene Brn4/Pou3f4 causes developmental defects in the inner ear. J Neurosci. 1999;19(14):5980-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trowe MO, Shah S, Petry M, et al. Loss of Sox9 in the periotic mesenchyme affects mesenchymal expansion and differentiation, and epithelial morphogenesis during cochlea development in the mouse. Dev Biol. 2010;342(1):51-62. [DOI] [PubMed] [Google Scholar]
- 50. Salhotra A, Shah HN, Levi B, Longaker MT. Mechanisms of bone development and repair. Nat Rev Mol Cell Biol. 2020;21(11):696-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mizuhashi K, Kanamoto T, Ito M, et al. OBIF, an osteoblast induction factor, plays an essential role in bone formation in association with osteoblastogenesis. Dev Growth Differ. 2012;54(4):474-480. [DOI] [PubMed] [Google Scholar]
- 52. Chole RA, Tinling SP. Bone lining cells of the mammalian cochlea. Hear Res. 1994;75(1-2):233-243. [DOI] [PubMed] [Google Scholar]
- 53. Trowe MO, Maier H, Schweizer M, Kispert A. Deafness in mice lacking the T-box transcription factor Tbx18 in otic fibrocytes. Development. 2008;135(9):1725-1734. [DOI] [PubMed] [Google Scholar]
- 54. Suzuki S, Suzuki N, Mori J, Oshima A, Usami S, Hashizume K. micro-Crystallin as an intracellular 3,5,3’-triiodothyronine holder in vivo. Mol Endocrinol. 2007;21(4):885-894. [DOI] [PubMed] [Google Scholar]
- 55. Kishimoto I, Okano T, Nishimura K, Motohashi T, Omori K. Early development of resident macrophages in the mouse cochlea depends on yolk sac hematopoiesis. Front Neurol. 2019;10:1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van der Spek AH, Surovtseva OV, Jim KK, et al. Regulation of intracellular triiodothyronine is essential for optimal macrophage function. Endocrinology. 2018;159(5):2241-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Heuer H, Visser TJ. The pathophysiological consequences of thyroid hormone transporter deficiencies: insights from mouse models. Biochim Biophys Acta. 2013;1830(7):3974-3978. [DOI] [PubMed] [Google Scholar]
- 58. Schweizer U, Köhrle J. Function of thyroid hormone transporters in the central nervous system. Biochim Biophys Acta. 2013;1830(7):3965-3973. [DOI] [PubMed] [Google Scholar]
- 59. Groeneweg S, van Geest FS, Peeters RP, Heuer H, Visser WE. Thyroid hormone transporters. Endocr Rev. 2020;41(2):146-201. [DOI] [PubMed] [Google Scholar]
- 60. Peeters R, Fekete C, Goncalves C, et al. Regional physiological adaptation of the central nervous system deiodinases to iodine deficiency. Am J Physiol Endocrinol Metab. 2001;281(1):E54-E61. [DOI] [PubMed] [Google Scholar]
- 61. Zhao HB, Kikuchi T, Ngezahayo A, White TW. Gap junctions and cochlear homeostasis. J Membr Biol. 2006;209(2-3):177-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Peeters RP, Ng L, Ma M, Forrest D. The timecourse of apoptotic cell death during postnatal remodeling of the mouse cochlea and its premature onset by triiodothyronine (T3). Mol Cell Endocrinol. 2015;407:1-8. doi:10.1016/j.mce.2015.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Guadaño-Ferraz A, Obregón MJ, St Germain DL, Bernal J. The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proc Natl Acad Sci U S A. 1997;94(19):10391-10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bernal J, Guadaño-Ferraz A, Morte B. Thyroid hormone transporters–functions and clinical implications. Nat Rev Endocrinol. 2015;11(7):406-417. [DOI] [PubMed] [Google Scholar]
- 65. Sharlin DS, Ng L, Verrey F, et al. Deafness and loss of cochlear hair cells in the absence of thyroid hormone transporters Slc16a2 (Mct8) and Slc16a10 (Mct10). Sci Rep. 2018;8(1):4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stromme P, Groeneweg S, Lima de Souza EC, et al. Mutated thyroid hormone transporter OATP1C1 associates with severe brain hypometabolism and juvenile neurodegeneration. Thyroid. 2018;28(11):1406-1415. [DOI] [PubMed] [Google Scholar]
- 67. Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74(1):168-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Espino Guarch M, Font-Llitjos M, Murillo-Cuesta S, et al. Mutations in L-type amino acid transporter-2 support SLC7A8 as a novel gene involved in age-related hearing loss. eLife. 2018;7:e31511. doi:10.7554/eLife.31511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wu DK, Kelley MW. Molecular mechanisms of inner ear development. Cold Spring Harb Perspect Biol. 2012;4(8):a008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brooks PM, Rose KP, MacRae ML, et al. Pou3f4-expressing otic mesenchyme cells promote spiral ganglion neuron survival in the postnatal mouse cochlea. J Comp Neurol. 2020;528(12):1967-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bok J, Raft S, Kong KA, Koo SK, Dräger UC, Wu DK. Transient retinoic acid signaling confers anterior-posterior polarity to the inner ear. Proc Natl Acad Sci U S A. 2011;108(1):161-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Meltser I, Tahera Y, Simpson E, et al. Estrogen receptor beta protects against acoustic trauma in mice. J Clin Invest. 2008;118(4):1563-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Graham CE, Vetter DE. The mouse cochlea expresses a local hypothalamic-pituitary-adrenal equivalent signaling system and requires corticotropin-releasing factor receptor 1 to establish normal hair cell innervation and cochlear sensitivity. J Neurosci. 2011;31(4):1267-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Salt AN, Plontke SK. Pharmacokinetic principles in the inner ear: Influence of drug properties on intratympanic applications. Hear Res. 2018;368:28-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. König O, Rüttiger L, Müller M, et al. Estrogen and the inner ear: megalin knockout mice suffer progressive hearing loss. Faseb J. 2008;22(2):410-417. [DOI] [PubMed] [Google Scholar]
- 76. Bassett JH, Williams GR. Role of Thyroid Hormones in Skeletal Development and Bone Maintenance. Endocr Rev. 2016;37(2):135-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cordas EA, Ng L, Hernandez A, Kaneshige M, Cheng SY, Forrest D. Thyroid hormone receptors control developmental maturation of the middle ear and the size of the ossicular bones. Endocrinology. 2012;153(3):1548-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Williams AJ, Robson H, Kester MHA, et al. Iodothyronine deiodinase enzyme activities in bone. Bone. 2008;43(1):126-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bassett JH, Boyde A, Howell PG, et al. Optimal bone strength and mineralization requires the type 2 iodothyronine deiodinase in osteoblasts. Proc Natl Acad Sci U S A. 2010; 107(16):7604-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Single cell datasets are available on the GEO database with accession number GSE181057 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE181057).