Abstract
Glucocorticoid causes hyperglycemia, which is common in patients with or without diabetes. Prolonged hyperglycemia can be experienced even after the discontinuation of glucocorticoid use. In the present study, we examined the time course of blood glucose level in hospital patients who received transient glucocorticoid treatment. In addition, the mechanism of prolonged hyperglycemia was investigated by using dexamethasone (Dexa)-treated mice and cultured cells. The blood glucose level in glucose tolerance tests, level of insulin and glucagon-like peptide 1 (GLP-1), and the activity of dipeptidyl peptidase 4 (DPP-4) were examined during and after Dexa loading in mice, with histone acetylation level of the promoter region. Mice showed prolonged hyperglycemia during and after transient Dexa loading accompanied by persistently lower blood GLP-1 level and higher activity of DPP-4. The expression level of Dpp-4 was increased in the mononuclear cells and the promoter region of Dpp-4 was hyperacetylated during and after the transient Dexa treatment. In vitro experiments also indicated development of histone hyperacetylation in the Dpp-4 promoter region during and after Dexa treatment. The upregulation of Dpp-4 in cultured cells was significantly inhibited by a histone acetyltransferase inhibitor. Moreover, the histone hyperacetylation induced by Dexa was reversible by treatment with a sirtuin histone deacetylase activator, nicotinamide mononucleotide. We identified persistent reduction in blood GLP-1 level with hyperglycemia during and after Dexa treatment in mice, associated with histone hyperacetylation of promoter region of Dpp-4. The results unveil a novel mechanism of glucocorticoid-induced hyperglycemia, and suggest therapeutic intervention through epigenetic modification of Dpp-4.
Keywords: glucocorticoid induced hyperglycemia, dexamethasone, GLP-1, DPP-4, histone acetylation, memory phenomenon
Glucocorticoids are a class of steroid hormone that regulate glucose and lipid metabolism. They are used in various clinical situations to suppress immunological responses in autoimmune diseases, inflammatory diseases, and after organ transplantation; however, the adverse effects sometimes cause negative clinical outcomes (1-3). Glucocorticoid-induced hyperglycemia (GIH) is one of the most frequent adverse effects that is often difficult to control with ordinary lifestyle modification and caloric restrictions (4). Although discontinuation of glucocorticoid use should result in the disappearance of GIH, we experience cases of prolonged hyperglycemia even after the discontinuation.
The Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) is a large-scale clinical trial held in 1990s, in which patients with type 1 diabetes were subjected to either conventional or intensive treatment for 6.5 years (DCCT) and subsequent observation period for 12 years without initial intervention (EDIC) (5). The unexpected finding of DCCT/EDIC was “metabolic memory,” which refers to persistence of the beneficial effect of an intensive treatment on prevention of cardiovascular events, which endures even after the discontinuation. Natarajan et al. reported that the persistence of epigenetic modifications in monocytes resulting from intensive treatment causes metabolic memory of the intensive treatment (6, 7). The suppression of histone lysine-9 acetylation (H3K9Ac) in the promoter region of inflammatory genes by the intensive treatment accounts for the downregulation of inflammatory genes, including TNF-α and IL-1A. These findings suggest the significant roles for epigenetic modification of chromatin in the later development of cardiovascular diseases.
In the present study, to examine the prevalence of prolonged hyperglycemia after discontinuation of the treatment, we examined the time course of blood glucose level in the patients who received transient glucocorticoid treatment. This prolonged hyperglycemia has been traditionally described as “metasteroid diabetes,” has been recognized for a long time (8), and has been proposed to involve reduced peripheral insulin sensitivity associated with weight gain, stimulated hepatic gluconeogenesis (reviewed in (9)), and direct effects of the destruction of pancreatic cells leading to β-cell dysfunction (10); however, little is known about the actual status and pathogenesis. Therefore, we investigated the mechanism by using mice and cultured cells transiently treated with a potent and long-lasting glucocorticoid, dexamethasone (Dexa). To simulate transient steroid therapy in human, mice were temporarily loaded with Dexa and the time course of diabetes-related parameters were examined during and after treatment. We identified persistent reduction in GLP-1 level during and after transient Dexa-loading in mice, associated with histone hyperacetylation of the promoter region of the Dpp-4 gene. The epigenetic modification of Dpp-4 may account for memory phenomenon after transient Dexa loading, which causes metasteroid diabetes.
Research Design and Methods
Clinical Study Population
A single center-based retrospective study of metasteroid diabetes was conducted. The candidates (395 patients) for this study were consecutive patients diagnosed with steroid diabetes in the Division of Endocrinology, Metabolism and Nephrology, Department of Internal Medicine at Keio University Hospital between June 2001 and October 2017. Subjects who received transient glucocorticoid treatment and had a glycated hemoglobin (HbA1c) level of ≥6.5% or treated with antidiabetic drugs during glucocorticoid treatment were included in this study (48 patients). Patients for whom sufficient clinical information was not present were excluded. Patients’ HbA1c levels during and after glucocorticoid treatment at pretreatment baseline period, 1 year, and 6 months before the point of discontinuation of the glucocorticoid treatment, and 6 months, 1 year, and 2 years after discontinuation was retrospectively examined and associated with mean number of oral medications, insulin dose, and body weight. This study was approved by the ethics committee at the Keio University School of Medicine and conducted in accordance with the Declaration of Helsinki.
Animal Experiments
Animal experiments were performed in accordance with the animal care guidelines of Keio University. Age-matched male C57bl/6 mice (Nihon Clea, Tokyo, Japan) were maintained under specific pathogen-free conditions. Dexa pellets (15 mg/21-day release) were implanted subcutaneously when the mice were 12 weeks old. For the study of hypoglycemic agents, the mice were given sitagliptin (10 mg/kg), metformin (250 mg/kg), or dapagliflozin (10 mg/kg) by gavage for 7 days. The mice were sacrificed at the end of the experiments; tissue samples were harvested, crushed, and frozen at -80°C until analysis. Mononuclear cells were isolated from mice blood using commercially available lymphocyte separation solution with a density of 1.077 g/mL (Nacalai Tesuque, Kyoto, Japan).
Glucose Tolerance Test, Quantification of Serum Insulin and GLP-1 Level, and Serum DPP-4 Enzyme Activity Assay
For the glucose tolerance test, the mice were fasted for 15 hours and orally or IP injected with glucose at 1.0 g/kg body weight. Blood samples were collected from the tail vein, and the blood glucose level was immediately determined using the glucose dehydrogenase method (ACCU-CHEK Aviva, Roche Diagnostics, Tokyo, Japan). For the serum glucagon-like peptide 1 (GLP-1) and insulin level measurement, blood was collected from the orbital venous plexus, transferred to tubes containing DPP-4 inhibitor, and kept on ice. The serum GLP-1 concentration was measured with a GLP-1 ELISA kit (Immuno-Biological Laboratories Co, Gunma, Japan; RRID: AB_2892225; https://antibodyregistry.org/search.php?q=AB_2892225) (11), and the serum insulin concentration was measured with a mouse insulin ELISA kit (Morinaga Institute of Biological Science, Inc, Yokohama, Japan; RRID: AB_2811268; https://antibodyregistry.org/search.php?q=AB_2811268) (12). The serum DPP-4 enzyme activity was measured with a DPP-4 activity kit (Sigma-Aldrich, St Louis, MO, USA) in the fasting condition.
Cell Culture Experiments
In vitro experiments were performed using CRL-2586 hemangioendothelioma (EOMA) cells (ATCC, Manassas, VA, USA; RRID: CVCL_3507; https://web.expasy.org/cellosaurus/CVCL_3507) (13), which are derived from mice hematopoietic cells and are often used for experiments involving mesoderm cells. The cells were grown to 80% confluency in DMEM (#11995-065, Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum and treated in a 12-well dish with or without Dexa (Sigma-Aldrich). For the histone acetyltransferase (HAT) inhibition study, L002 (10 µM, Sigma-Aldrich) was administered to the cell-cultured medium during Dexa treatment. For sirtuin activation, nicotinamide mononucleotide (NMN) (100 µM, Oriental Yeast, Tokyo, Japan) was administered to the medium after Dexa loading. Dose-dependent efficacy of NMN was verified at the concentration of 0.1 to 100 µM after Dexa loading. Cell viability was not decreased in these experiments. Unless otherwise indicated, total RNA was extracted from the cells after 48 hours of treatment.
Quantitative real-time PCR
The total RNA was extracted with a commercially available kit (74104, RNeasy mini kit, QIAGEN) from the mononuclear cells, small intestine, and cultured cells. cDNA was prepared by reverse transcription (RR037A, Primescript RT reagent kit, Takara Bio, Ohtsu, Japan). Subsequent real-time PCR was performed by using the Applied Biosystems 7500 Fast Real-Time PCR system and a premix reagent (RR820A, SYBR Premix Ex Taq II, Takara Bio), according to the manufacturer’s instructions. The mRNA levels of Proglucagon, PC1/3, Dpp-4, P300, CBP, HDAC1, HDAC5, Sirt1, Sirt3, and Sirt6 were examined and the results were adjusted by the level of 18S rRNA (for primer sequences see Supplementary Table 1) (14).
HAT, HDAC, and Sirtuin Activity Assay
HAT, HDAC, and sirtuin activities were measured using a commercially available kit according to the manufacturer’s instructions (56100 for HAT activity and 56200 for HDAC and sirtuin activity; Active Motif, Carlsbad, CA, USA). Sirtuin activity was measured by adding 250 µM nicotinamide adenine dinucleotide in the HDAC assay kit.
Chromatin Immunoprecipitation Assay
Chromatin immunoprecipitation (ChIP) assay was performed by using the Simple ChIP Plus Kit according to the manufacturer’s instructions (9005, Cell Signaling Technology Japan, Tokyo, Japan). The DNA–protein complexes were immunoprecipitated using ChIP-grade antibodies against histone H3 acetylation (H3Ac; RRID: AB_2614978; https://antibodyregistry.org/search.php?q=AB_2614978) (15), histone H3 lysine-9 acetylation (H3K9Ac; RRID: AB_2616593; https://antibodyregistry.org/search.php?q=AB_2616593) (16), histone H4 acetylation (H4Ac; RRID: AB_2793550; https://antibodyregistry.org/search.php?q=AB_2793550) (17), and histone H4 lysine-16 acetylation (H4K16Ac; RRID: AB_2636968; https://antibodyregistry.org/search.php?q=AB_2636968) (18) (Active motif). Levels of DNA in the immunoprecipitants were analyzed by quantitative PCR and normalized to the inputs. PCR primers were designed to amplify target sequences within 500 base pair upstream of the transcription start site of Dpp-4, where augmentation of histone acetylation has been reported.
Statistical Analyses
Statistical analyses were performed using SPSS 23 software (IBM, Armonk, NY, USA). All data are expressed as mean ± SE. Comparison of the means between 2 groups was performed by Student t test. Spearman correlation coefficient was used to assess the correlation between 2 parameters. P value < 0.05 was considered statistically significant.
Results
Transient Glucocorticoid Treatment in Human Subjects Causes Prolonged Hyperglycemia Even After Discontinuation
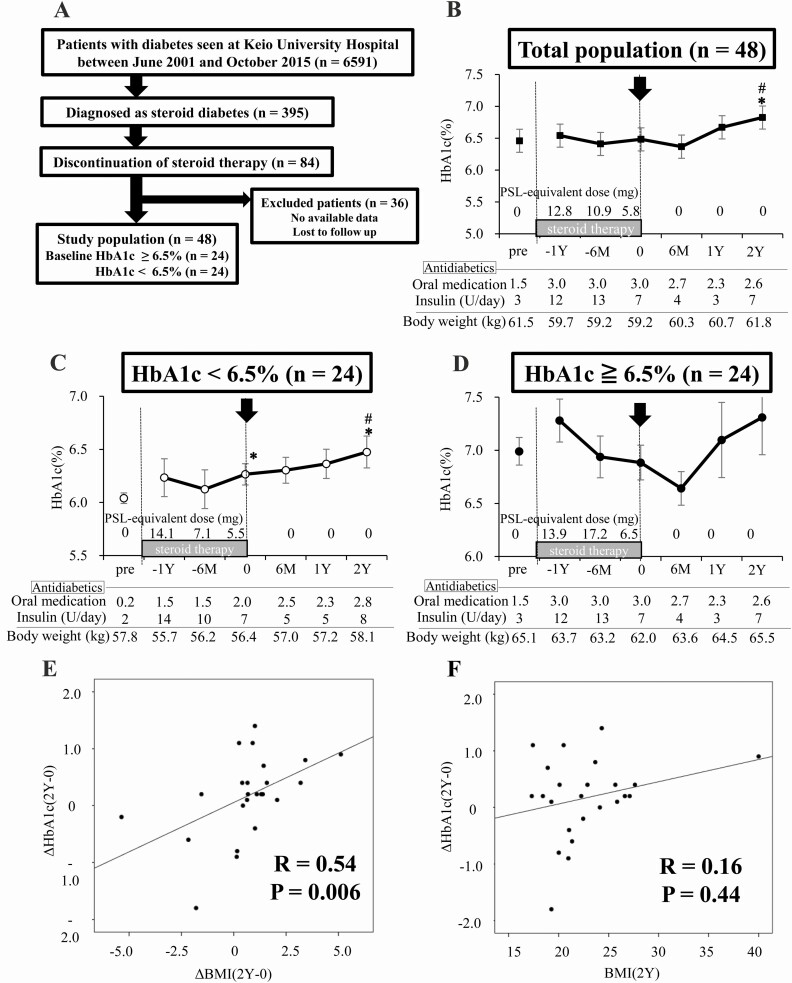
To elucidate the time course of metasteroid diabetes, patients’ HbA1c levels were retrospectively examined during and after glucocorticoid treatment. Subjects who received transient glucocorticoid treatment and were diagnosed with steroid diabetes, showing HbA1c level at 6.5% or higher or using antidiabetic drugs during the treatment, were included in this study. A total of 48 patients were enrolled (Fig. 1A). In the total study population (Fig. 1B), the HbA1c level during the treatment were equivalent to the pretreatment level, though the amount of antidiabetic drugs was increased during the treatment period. Glycemic control was not improved and hyperglycemia prolonged even after discontinuation of the treatment. The HbA1c level at 2 years after discontinuation (2Y) elevated significantly than that at the point of discontinuation, without decreasing the dose of antidiabetic drugs. Body weight was unchanged during glucocorticoid treatment; however, it increased in the 2 years after treatment discontinuation. We hypothesized that blood glucose level during and after transient glucocorticoid treatment might be deteriorated by the presence of insulin resistance and β-cell dysfunction; thus, the patients were divided into 2 groups based on the pretreatment baseline HbA1c level: HbA1c < 6.5% or ≧ 6.5% (Fig. 1C and D, respectively). The basal clinical characteristics of the 2 groups are summarized in Table 1. Mean pretreatment HbA1c levels were 6.0% and 6.9% in each group. The percentage of people with a body mass index (BMI) of 25 and greater or using insulin was significantly higher in HbA1c ≧ 6.5% group than in HbA1c < 6.5% group. Contrary to our expectation, in the subjects whose pretreatment HbA1c level was < 6.5% (Fig. 1C), the glycemic control gradually deteriorated during glucocorticoid treatment, and the HbA1c level at the point of discontinuation increased significantly compared with that at the pretreatment state. After discontinuation of glucocorticoid treatment, HbA1c level further elevated and reached a significantly higher value compared with that at the point of discontinuation, without decreasing the dose of antidiabetic drugs. In the subjects whose pretreatment HbA1c level was ≧ 6.5% (Fig. 1D), HbA1c level did not decrease and hyperglycemia prolonged even after discontinuation of glucocorticoid treatment. There was no significant difference in the time course of HbA1c level between males and females in both groups.
Figure 1.
Transient glucocorticoid treatment in human subjects causes prolonged hyperglycemia even after discontinuation. (A) Flow chart of patient enrollment; 48 patients are included. (B) HbA1c level time course of the total patients during and after glucocorticoid treatment. (C) HbA1c level time course of the patients with baseline HbA1c < 6.5 %. (D) HbA1c level time course of the patients with baseline HbA1c ≧6.5%. (E) Correlation between ΔHbA1c(2Y-0) and ΔBMI(2Y-0). (F) Correlation between ΔHbA1c(2Y-0) and BMI(2Y). Black lines in Fig. 1A-C indicate the HbA1c level during and after glucocorticoid treatment (%, as shown in the y-axis on the left side). M indicates month and Y indicates year. BMI(2Y) indicates posttreatment body mass index (BMI) at 2 years after the discontinuation. ΔBMI(2Y-0) indicates the change of BMI level from the point of discontinuation to 2 years later; ΔHbA1c(2Y-0), the change of HbA1c level from the point of discontinuation of glucocorticoid to 2 years later; HbA1c, glycated hemoglobin; PSL, prednisolone. *P < 0.05 vs the HbA1c level before glucocorticoid treatment (baseline HbA1c). #P < 0.05 vs the HbA1c level before discontinuation of glucocorticoid treatment.
Table 1.
Patient characteristics in the clinical study
| HbA1c < 6.5% (n = 24) | HbA1c ≧ 6.5% (n = 24) | |
|---|---|---|
| Age (y) | 65.0 ± 2.4 | 65.0 ± 3.1 |
| Male (n, %) | 13 (54.2) | 17 (70.8) |
| BMI (kg/m2) | 22.1 ± 1.0 | 23.9 ± 0.8 |
| Maximum steroid amount | 51.2 ± 13.5 | 46.8 ± 5.7 |
| Duration of steroid therapy (month) | 21 ± 8.0 | 18.7 ± 6.8 |
| HbA1c at baseline (%) | 6.0 ± 0.1 | 6.9 ± 0.1** |
| BMI ≧ 25 kg/m2 (%) | 12.5 | 41.7** |
| Medication usage | ||
| Insulin (%) | 41.7 | 62.5* |
| OHA (%) | 37.5 | 41.7 |
The patients are divided into 2 groups based on the pretreatment baseline HbA1c level.
Abbreviations: BMI, body mass index; HbA1c, glycated hemoglobin; OHA, oral hypoglycemic agent.
*P < 0.05.
**P < 0.01 vs. HbA1c < 6.5% group.
We investigated the correlation between the change in HbA1c level between the point of discontinuation and 2 years later (ΔHbA1c(2Y-0)), and posttreatment BMI at 2 years later (BMI(2Y)), the change of BMI (ΔBMI(2Y-0)), drug amount, age, or duration of glucocorticoid treatment in the subjects whose pretreatment HbA1c level was < 6.5% (Table 2A), together with the correlation between the HbA1c level at 2 years later (HbA1c(2Y)) and the previously mentioned parameters (Table 2B). Significant positive correlations were found between ΔHbA1c(2Y-0) and ΔBMI(2Y-0) (R = 0.54, P = 0.006; Fig. 1E), but not with BMI(2Y) (R = 0.16, P = 0.44; Fig. 1F). Meanwhile, posttreatment HbA1c (HbA1c(2Y)) and BMI (BMI(2Y)) were positively correlated (R = 0.48, P = 0.017; Table 2B). These results indicate that transient glucocorticoid treatment causes prolonged hyperglycemia even after discontinuation and that weight gain after discontinuation affects glycemic control.
Table 2.
Correlation coefficients in the subjects whose pretreatment HbA1c level was < 6.5%
| A | |
|---|---|
| ΔHbA1c(2Y-0) | |
| BMI(2Y) | 0.164 |
| ΔBMI(2Y-0) | 0.541** |
| Drug amount | 0.098 |
| Age | -0.188 |
| Sex | 0.030 |
| Duration | -0.251 |
| B | |
| HbA1c(2Y) | |
| BMI(2Y) | 0.484* |
| ΔBMI(2Y-0) | 0.473* |
| Drug amount | 0.456* |
| Age | 0.159 |
| Sex | 0.121 |
| Duration | -0.135 |
(A) Spearman correlation coefficients were analyzed between the change of HbA1c from the point of discontinuation of the glucocorticoid treatment to 2 years later (ΔHbA1c(2Y-0)) and posttreatment BMI at 2 years later of the discontinuation (BMI(2Y)), the change of BMI from the point of discontinuation to 2 years later (ΔBMI(2Y-0)), drug amount, age, or duration of glucocorticoid treatment. We examined each variable by multiple regression analysis and found no confounding among these variables.
(B) Correlation coefficients were analyzed between posttreatment HbA1c at 2 years later of discontinuation of the treatment (HbA1c(2Y)) and BMI(2Y), ΔBMI(2Y-0), drug amount, age, or duration of GC treatment.
Abbreviations: BMI, body mass index; HbA1c, glycated hemoglobin.
*P < 0.05.
**P < 0.01.
Transient Dexa Loading in Mice Causes Prolonged Hyperglycemia Associated With Lower GLP-1 level and Higher DPP-4 Activity
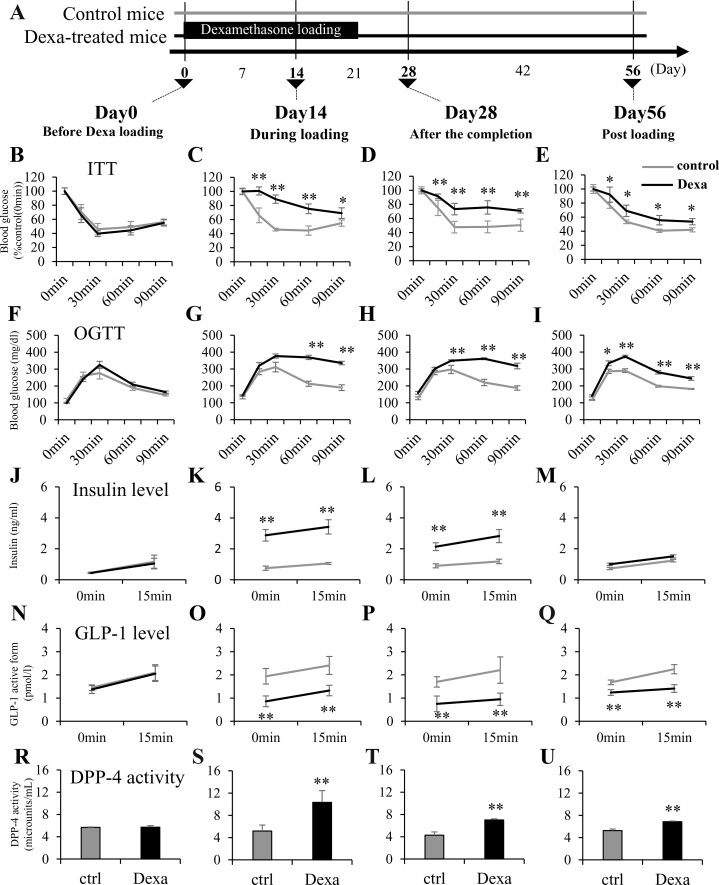
To examine GIH and metasteroid diabetes, we established experimental models using Dexa-loading pellets in mice. As shown in Fig. 2A, the effect of the 21-day-release Dexa pellet was monitored on day 14 (during loading), day 28 (after the completion), and day 56 (after loading). Insulin tolerance test (ITT) (Fig. 2B-E) and oral glucose tolerance test (OGTT) were performed at days 0, 14, 28, and 56 (Fig. 2F-I). The blood insulin levels (Fig. 2J-M), GLP-1 levels (Fig. 2N-Q), and DPP-4 activities (Fig. 2R-U) were examined. Despite the existence of insulin resistance in ITT at day 56, the insulin level of Dexa-treated mice did not exceed the control, suggesting a relative reduction in insulin secretion. Therefore, we examined blood level of insulin, GLP-1, and DPP-4 activity. Dexa-treated mice exhibited impaired glucose tolerance at 14 days after loading (Fig. 2G); this was associated with higher blood insulin level (Fig. 2K), lower blood GLP-1 level (Fig. 2O), and higher DPP-4 activity (Fig. 2S). The Dexa-treated mice also showed glucose intolerance at day 28 (Fig. 2H), when Dexa loading had been completed. The lower blood GLP-1 level and higher DPP-4 activity were sustained in this phase (Fig. 2P and T). On day 56, even after the completion of Dexa loading, both insulin resistance (Fig. 2E) and glucose intolerance (Fig. 2I) were persistent. Although insulin resistance was seen, the blood insulin level did not exceed the control (Fig. 2M), which is associated with lower GLP-1 level and higher DPP-4 activity (Fig. 2Q and U) and implicates relative impairment of insulin secretion in Dexa-treated mice at day 56.
Figure 2.
Transient dexamethasone loading in mice causes prolonged hyperglycemia associated with lower GLP-1 level and higher DPP-4 activity. Mice were subcutaneously implanted with dexamethasone (Dexa) pellets designed for a 21-day continuous release. Blood glucose level of Dexa-treated mice persistently increased in the ITT and OGTT. Decreased GLP-1 level and increased DPP-4 activity of Dexa-treated mice during and after Dexa-loading were revealed. (A) Schematic representation of the transient Dexa loading in the experimental mice. (B-E) Blood glucose levels of Dexa-treated mice during the ITT. (F-I) Those during the OGTT. (J-M) Plasma insulin levels of Dexa-treated mice during the OGTT. (N-Q) Plasma GLP-1 levels of Dexa-treated mice during the OGTT. (R-U) Plasma DPP-4 activity of Dexa-treated mice during the OGTT. (B, F, J, N, R) Before Dexa-loading (day 0), (C, G, K, O, S) during Dexa-loading (day 14), (D, H, L, P, T) 1 week after the completion of Dexa loading (after the completion: day 28), and (E, I, M, Q, U) 5 weeks after the completion of Dexa loading (postloading: day 56). DPP-4, dipeptidyl peptidase 4; GLP-1, glucagon-like peptide 1; ITT, insulin tolerance test; OGTT, oral glucose tolerance test. Gray lines indicate control mice; black lines represent Dexa-treated mice. *P < 0.05, **P < 0.01 vs control. N = 8 per group.
A DPP-4 Inhibitor, Sitagliptin, Effectively Improves Hyperglycemia Induced by Dexa Loading in Mice
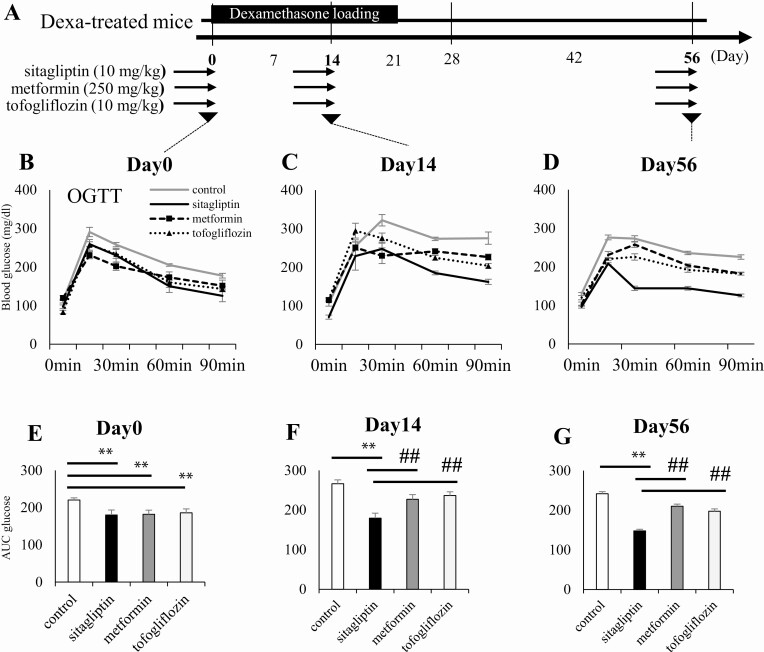
To examine whether the DPP-4 inhibitor could effectively reverse glucose intolerance in the Dexa-treated mice, we administered representative antidiabetic drugs, namely, sitagliptin (10 mg/kg), metformin (250 mg/kg), and tofogliflozin (10 mg/kg), in the mice after Dexa treatment (Fig. 3A-D). Each drug had the same potential to lower the blood glucose level before Dexa loading (day 0, Fig. 3B and E). The Dexa-treated mice were administered each drug before days 14 and 56 for a week and were tested for glucose tolerance. Among these drugs, sitagliptin improved glucose intolerance more effectively than other drugs during and after Dexa loading (Fig. 3F and G). The result suggests that the increased Dpp-4 activity underlies the prolonged glucose intolerance induced by transient Dexa-loading.
Figure 3.
A DPP-4 inhibitor, sitagliptin, effectively improves hyperglycemia induced by dexamethasone loading in mice. Mice were subjected to sitagliptin (10 mg/kg), metformin (250 mg/kg) and tofogliflozin (10 mg/kg) by gavage for 7 days during and after Dexa loading. Blood glucose level in the OGTT of Dexa-treated mice (days 14 and 56) was effectively improved by sitagliptin. (A) Schematic representation of the drug treatment in the experimental mice. (B, E) Blood glucose level in the OGTT before Dexa loading (day 0), (C, F) during Dexa loading (day 14), and postloading (day 56). Dexa, dexamethasone; DPP-4, dipeptidyl peptidase 4; OGTT, oral glucose tolerance test. Gray lines represent drug free Dexa-treated mice, whereas black lines, broken lines, and dotted lines represent sitagliptin, metformin, and tofogliflozin treatment in Dexa-treated mice, respectively. *P < 0.05, **P < 0.01 vs control, ##P < 0.01 vs sitagliptin treatment group. N = 8 per group.
Increased Expression of Dpp-4 gene After Dexamethasone Loading in Mice Is Associated With Histone Hyperacetylation
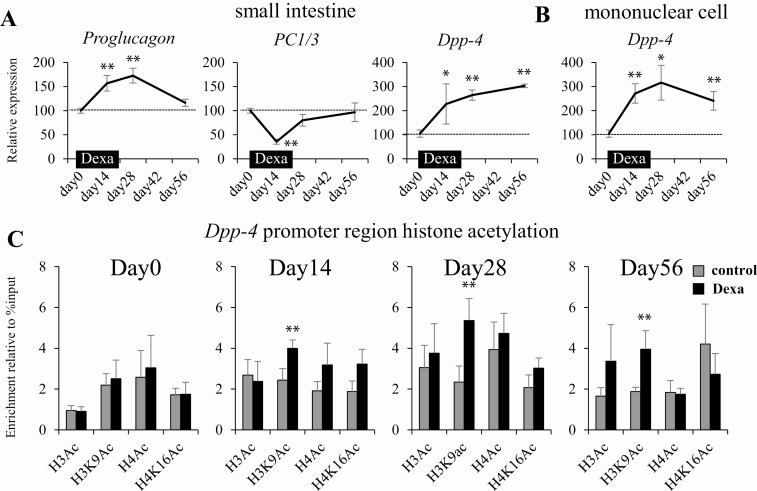
To examine the mechanism of the lowered GLP-1 level after Dexa loading in mice, we performed quantitative PCR analysis of genes involved in the production of GLP-1. GLP-1 is produced from proglucagon in the L cells located in the lower small intestine and the rate-limiting enzyme for the production is PC1/3. DPP-4 cleaves active GLP-1 to an inactive form and is universally expressed in the systemic organs. We chose the small intestine and mononuclear cells as the main productive sites of Dpp-4 because the expression level is higher. We evaluated the expression of Proglucagon, PC1/3, and Dpp-4. In accordance with the DPP-4 activity, transient Dexa loading induced persistent upregulation of Dpp-4 in the small intestine and mononuclear cells (Fig. 4A and B). The expression level of PC1/3 transiently diminished and that of Proglucagon increased during Dexa loading. After the completion of Dexa loading, the expression of Proglucagon and PC1/3 returned to a normal level. The persistent change in gene expression after completion of intervention may be caused by epigenetic modification of the target gene. Because histone hyperacetylation is a main factor promoting gene expression, we examined histone acetylation of the Dpp-4 promoter region in the mononuclear cells by ChIP assay during and after Dexa loading. We analyzed the H3Ac (pan-acetylated histone), H3K9Ac, H4Ac, and H4K16Ac levels in the promoter region of Dpp-4. During and after Dexa loading, significant and sustained hyperacetylation of H3K9 in the Dpp-4 promoter region was identified in the mononuclear cell (Fig. 4C). Taken together, the histone hyperacetylation of Dpp-4 induced by transient Dexa loading contributes to the maintenance of the increased DPP-4 activity and prolonged hyperglycemia after completion of glucocorticoid treatment.
Figure 4.
Increased expression of Dpp-4 gene after dexamethasone loading in mice is associated with histone hyperacetylation. (A) Expression of Proglucagon, PC1/3, and Dpp-4 in the small intestine. (B) Expression of Dpp-4 in the mononuclear cells collected from the serum. (C) Time course of histone acetylation in the promoter region of Dpp-4 gene in the mononuclear cells. H3Ac, histone H3 acetylation; H3K9Ac, histone H3 lysine-9 acetylation; H4Ac, histone H4 acetylation; H4K16Ac, histone H4 lysine-16 acetylation. *P < 0.05, **P < 0.01 vs control. N = 8 per group.
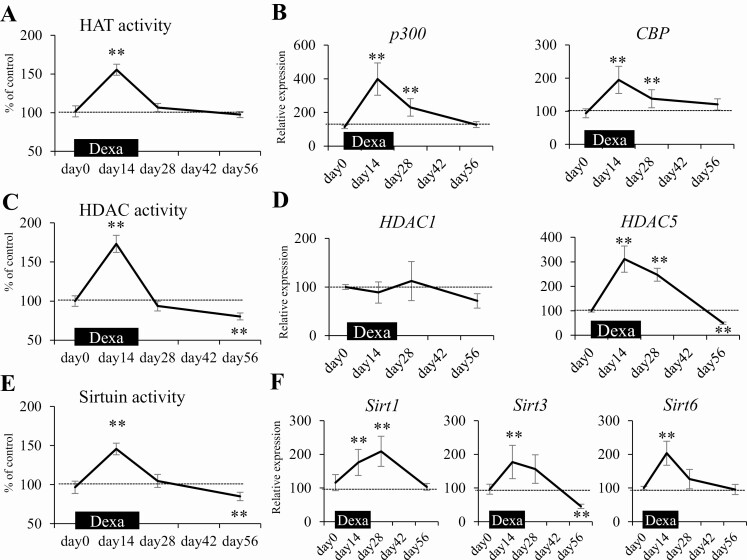
Alterations in HAT, HDAC, and Sirtuin Activity Are Associated With Histone Hyperacetylation of Dpp-4 Gene During and After Dexamethasone Loading in Mice
Because histone hyperacetylation in the Dpp-4 promoter region was involved in the pathogenesis of prolonged reduction of GLP-1 level and glucose intolerance after Dexa loading in mice, the molecular mechanism underlying the histone modification was explored. Histone acetylation is induced by glucocorticoids through activation of HATs, so we examined the time course of HAT activity and the expression patterns of the representative HAT genes p300 and CBP. During Dexa loading, when Dpp-4 expression increased, HAT activity (Fig. 5A) in the mononuclear cells as well as the expression of p300 and CBP significantly increased (Fig. 5B); meanwhile, they returned to the original level after the completion of Dexa loading. Therefore, we could not explain the maintenance of histone hyperacetylation of Dpp-4 promoter region after the completion of Dexa loading by enhanced HAT activity. To elucidate how the hyperacetylation state was maintained after completion, we additionally examined HDAC and sirtuin activities and gene expressions. The activities and gene expressions significantly increased during Dexa loading (Fig. 5C-F). Notably, both activities significantly reduced after the completion of Dexa loading when they are compared with those at the pretreatment level. We speculate that the reduction of HDAC and sirtuin activities after Dexa loading may contribute to the maintenance of hyperacetylation of Dpp-4. A kind of positive feedback regulation by which histone acetylation itself acts to fix a histone hyperacetylation state may be involved in the maintenance (19).
Figure 5.
Alterations in the activity of HATs, HDACs, and sirtuins are associated with histone hyperacetylation of Dpp-4 gene during and after dexamethasone loading in mice. HAT activity in mononuclear cells significantly increased during Dexa loading and returned to the original state. Meanwhile, HDAC and sirtuin activities increased during Dexa loading and significantly decreased after loading. (A) Time course of HAT activity during and after Dexa loading. (B) Time course of HAT gene expressions. (C) HDAC activity. (D) HDAC gene expressions. (E) Sirtuin activity. (F) Sirtuin gene expressions. Dexa, dexamethasone; HAT, histone acetyltransferase; HDAC, histone deacetyltransferase. *P < 0.05, **P < 0.01 vs control. N = 8 per group.
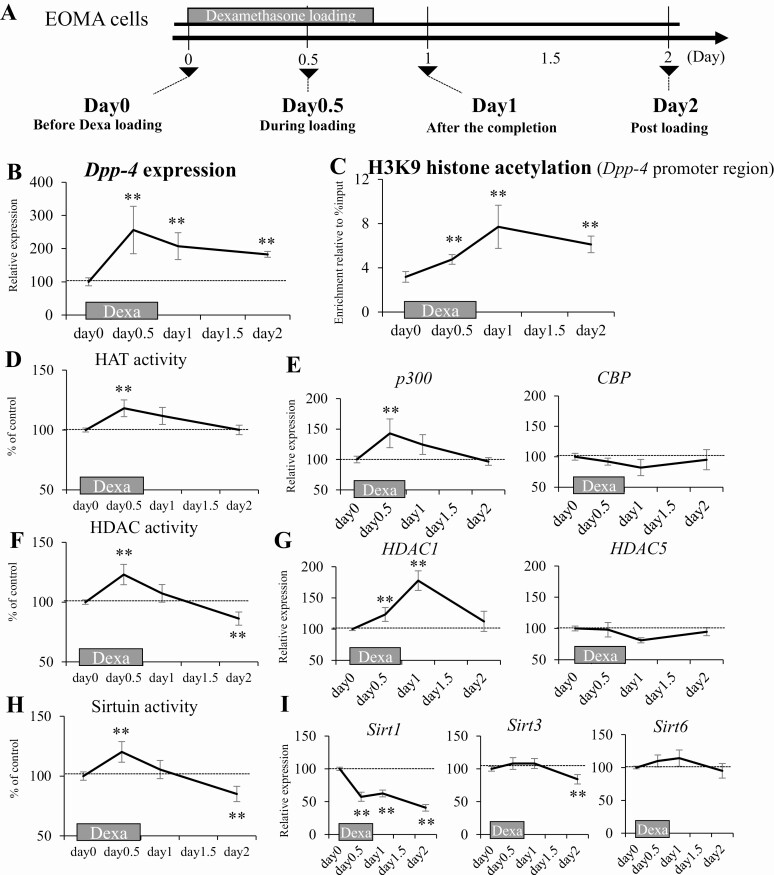
Persisted Histone Hyperacetylation and Increased Expression of Dpp-4 During and After Dexa Treatment Are Reproducible in the Cultured Cells
To examine the detailed mechanism of Dpp-4 hyperacetylation caused by dysregulation of the histone modification during and after Dexa treatment, we developed an in vitro experimental model using CRL2586 EOMA cells (Fig. 6A). We confirmed persistent increase in Dpp-4 expression by transient Dexa loading in the cultured cells, simulating the in vivo experiments (Fig. 6B). The persistent increase in H3K9 acetylation of the Dpp-4 promoter region during and after Dexa loading was similar to that in the Dexa-treated mice (Fig. 6C). The histone hyperacetylation of Dpp-4 during Dexa loading increased the HAT activity (Fig. 6D), which was associated with the upregulation of p300 (Fig. 6E). The histone hyperacetylation of Dpp-4 after Dexa loading completion was associated with decreased HDAC and sirtuin activities (Fig. 6F and H). As for gene expression, the reduction of HDAC and sirtuin activities was associated with the downregulation of Sirt1 and Sirt3 (Fig. 6G and I). The results indicate that the histone hyperacetylation of Dpp-4 during and after Dexa loading can be explained by the alteration of expression level of these histone modifier genes.
Figure 6.
Persistent histone hyperacetylation and increased expression of Dpp-4 during and after dexamethasone treatment are reproducible in the cultured cells. (A) Schematic representation of the in vitro study of Dexa treatment. (B) Dpp-4 expression. (C) H3K9 acetylation level of the promoter region of Dpp-4. (D) HAT activity. (E) HAT gene expressions. (F) HDAC activity. (G) HDAC gene expressions. (H) Sirtuin activity. (I) Sirtuin gene expressions. Dexa, dexamethasone; EOMA, hemangioendothelioma; HAT, histone acetyltransferase; HDAC, histone deacetyltransferase. *P < 0.05, **P < 0.01 vs control. N = 8 per group.
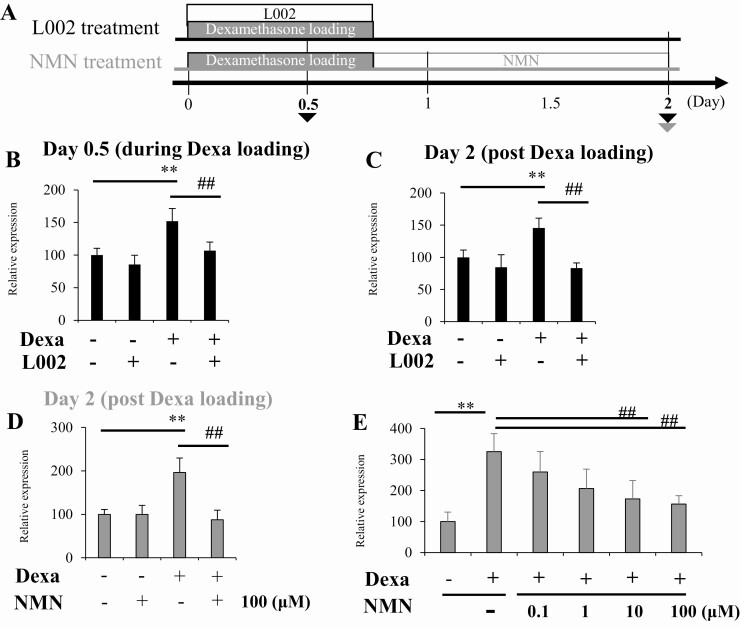
Histone Hyperacetylation by Dexa Treatment in the Cultured Cells Is Cancelled by a HAT inhibitor, L002, or a Sirtuin Activator, NMN
To explore the therapeutic approach, we used the p300 inhibitor L002 and the sirtuin activator NMN to correct the dysregulation of histone modification of Dpp-4 induced by Dexa loading (Fig. 7A). We added L002 to EOMA cells together with Dexa to suppress the upregulation of p300 during Dexa loading and assayed Dpp-4 gene expression. The treatment with L002 resulted in the suppression of Dpp-4 expression both during and after Dexa loading (Fig. 7B and C). To compensate for the downregulation of sirtuins, we added NMN, which can increase sirtuin activity and promote histone deacetylation to the cells after Dexa loading, and analyzed the Dpp-4 expression. The treatment with NMN resulted in the significant suppression of Dpp-4 expression in a dose-dependent manner (Fig. 7D and E). These results indicate that upregulation of Dpp-4 and histone hyperacetylation induced by Dexa treatment to cultured cells can be canceled by intervention to modify histone acetylation.
Figure 7.
Histone hyperacetylation by Dexa treatment in the cultured cells is cancelled by a HAT inhibitor, L002, or a sirtuin activator, NMN. (A) Schematic representation of the study. (B) Dpp-4 gene expression on day 0.5 and (C) day 2 of the cultured cells treated with L002. (C) Dpp-4 gene expression of the cultured cells treated with NMN. (E) Dose-dependent effect of NMN on Dpp-4 gene expression in the Dexa-loaded cells. *P < 0.05, **P < 0.01 vs control. n.s., not significant vs without Dexa loading. #P < 0.05, ##P < 0.01 vs without L002 or NMN. N = 8 per group. Dexa, dexamethasone; HAT, histone acetyltransferase; NMN, nicotinamide mononucleotide.
Discussion
Metasteroid diabetes refers to persistent hyperglycemia after discontinuation of transient treatment with glucocorticoids. Although it was first described in 1950s (5), the mechanism has not been elucidated. In the present study, we examined the actual status of metasteroid diabetes in the patients of our hospital and revealed that the prevalence (57%), defined as the patients whose glycemic control was worsened after discontinuation of glucocorticoids, was consistent with a previous report (45%). To elucidate the possible mechanism of metasteroid diabetes, we established transient Dexa-loading models using Dexa-treated mice and cultured EOMA cells and revealed persistent reduction in blood GLP-1 level after Dexa loading in mice, which is associated with histone hyperacetylation of the promoter region of Dpp-4.
The predominant mechanism responsible for hyperglycemia after administration of glucocorticoids is assumed to be insulin resistance (20), although both insulin resistance and impaired insulin secretion have been demonstrated. Glucocorticoids directly disrupt the signaling pathway of insulin, including insulin receptors, insulin receptor substrate-1/-2, phosphoinositide 3-kinase/Akt, and the downstream molecules, and thus cause insulin resistance (9). They impair the receptor binding affinity of insulin and decrease glucose uptakes reducing glucose transporter type 4 translocation in the skeletal muscle (21). These impairments in insulin action have been demonstrated at the level of both the liver and muscle. In addition, glucocorticoids directly inhibit insulin secretion from rat pancreatic islet cells by disturbing the activation of protein kinase C (22). As for metasteroid diabetes, a report indicated impairment of beta-cell function after completion of Dexa treatment in guinea pigs (23). Importantly, the ability to compensate for insulin resistance with an increase in insulin secretion determines the extent of hyperglycemia in response to glucocorticoid treatment (24). In the present experimental model, we revealed that Dexa-treated mice exhibited persistent hyperglycemia in both ITT and OGTT even after discontinuation of treatment (day 56). Despite the existence of insulin resistance in ITT at day 56, the insulin level of Dexa-treated mice did not exceed the control, suggesting a relative reduction in insulin secretion. Therefore, we precisely investigated insulin secretion and GLP-1 level as pathogenesis of hyperglycemia during and after glucocorticoid treatment. The blood insulin level of Dexa-treated mice appeared to be same as that of the control; however, it did not increase adequately to maintain a normal glucose level in OGTT at day 56; thus, we judged that persistent hyperglycemia from relative insulin deficiency remained and was associated with lowered blood GLP-1 level and elevated DPP-4 activity. Therefore, we assumed that the reduction in GLP-1 level plays significant roles in the pathogenesis of GIH and metasteroid diabetes.
GLP-1 is a representative incretin derived from the L cells in the lower small intestine and promotes insulin secretion in a glucose-dependent manner, and DPP-4 inactivates circulating GLP-1. Therefore, GLP-1 potentiates glucose-stimulated insulin secretion and does not trigger insulin secretion in the absence of nutrients. Although the origin of DPP-4 has not been fully clarified, mononuclear cells and endothelial cells, both of which are differentiated from mesodermal hematopoietic cells, are suggested to play pivotal roles in determining circulating level of DPP-4 (25). We examined expression of genes in the small intestine, which are related to GLP-1 production, and found that expression of the rate-limiting enzyme PC1/3, which processes proglucagon to GLP-1, decreased during Dexa loading, but it returned to the basal level after the completion. Meanwhile, the expression of Dpp-4 in the small intestine and mononuclear cells persistently increased during and after Dexa loading. A previous study has revealed reduction of GLP-1 level during short-term steroid treatment in normoglycemic participants (26); however, upregulation of Dpp-4 in GIH and the involvement in metasteroid diabetes are novel findings of the present study. Consistent with the findings, effectiveness of DPP-4 inhibitors and GLP-1 receptor agonists in the treatment of patients with GIH has been reported (27-29). A recent study also revealed that glucocorticoid treatment upregulates Dpp-4 transcriptionally in a human-derived monocyte/macrophage cell line, which is compatible with our findings (30). The optimized guidelines for GIH and metasteroid diabetes in human have not been established (31), but the present study supports the notion that DPP-4 inhibitors and GLP-1 receptor agonists have a potency to effectively improve glucose intolerance in both GIH and metasteroid diabetes.
The persistent upregulation of Dpp-4 gene after completion of Dexa loading is a kind of memory phenomenon. The memory phenomenon or the legacy effect refers to the late-onset effect of an intervention that becomes apparent after discontinuation of the intervention (32, 33). The concept is based on postinterventional analyses of large-scale randomized controlled trials, in which the superiority of a treatment persists even after the intervention is over. The most famous example is the “metabolic memory” in the DCCT/EDIC study that accounts for the late-onset reduction of cardiovascular events after discontinuation of the initial intensive glucose-lowering treatment for patients with type 1 diabetes. The similar concept of late-onset beneficial effects after completion of initial treatment for prevention of cardiovascular events has been demonstrated in clinical trials: for example, in the TROPHY study (34) and the WOSCOPS study (35). Although mechanism of memory phenomenon was unclear for decades, accumulating evidence suggests that epigenetic regulation of gene expression plays significant roles in the persistent effect of transient stimuli. DNA methylation and histone acetylation are representative epigenetic regulation of chromatin (36). Among them, hyperacetylation of histone H3K9 in monocytes is reported to be involved in the metabolic memory in DCCT/EDIC (7). Therefore, we hypothesized that histone hyperacetylation might account for the persistent upregulation of Dpp-4 after Dexa treatment. Histone acetylation profile of the Dpp-4 promoter region in the mononuclear cells of mice during and after Dexa loading was examined and persistent hyperacetylation of histone H3K9 in the region, which can account for upregulation of Dpp-4 was identified.
Previous reports have demonstrated that modifiers of histone acetylation, such as HATs, HDACs, and sirtuins, may regulate blood glucose level and diabetic complications. For example, p300 is upregulated in the high-glucose condition in endothelial cells and leads to vascular complications of diabetes (37). HDAC inhibition leads to glucose intolerance through dysregulation of insulin receptor substrate 2 in db/db mice (38). Among sirtuins, SIRT1 and SIRT6 have significant roles in insulin secretion and glycolysis (39-42). SIRT3 regulates energy consumption in mitochondria (43). In addition to these findings, Dexa induces histone modification by recruiting the CBP/p300 element and promoting HAT activity (44), and SIRT1 is involved in the transcription of genes, which are mediated by glucocorticoids (45). In the treatment of leukemia, Dexa is used to induce apoptosis in lymphoid cells, and its effect is due to epigenetic modification of pro-apoptotic genes (46, 47). Therefore, we assumed that these histone modifiers might participate in the induction of prolonged hyperacetylation in the Dpp-4 promoter region during and after Dexa treatment. Examination of activity and expression of these histone modifiers revealed that they independently alter in response to Dexa, and therefore, HATs, HDACs, and sirtuins would play different roles in the histone acetylation during the time course of Dexa treatment. Specifically, HAT activity in mononuclear cells was increased during Dexa treatment, and it was reasonable to assume that histone hyperacetylation in Dpp-4 was carried by HATs. However, as the enhancement of HAT activity was transient, it was difficult to explain the maintenance of the histone hyperacetylation after discontinuation of Dexa by the action of HATs. Therefore, we examined HDACs and sirtuins, both of which remove histone acetylation. Their activities transiently increased during Dexa treatment, which was similar to HATs; however, they were significantly reduced after Dexa treatment, implying the weakened release of histone acetylation. These results suggested that persistent histone hyperacetylation in the Dpp-4 promoter region is carried out by activation of HATs during Dexa treatment and is subsequently maintained by suppression of HDACs and sirtuins after discontinuation. Judging from the expression level, HAT activation during Dexa treatment can be attributable to upregulation of p300 and reduction of histone deacetylation after the discontinuation can be explained by suppression of Sirt3 expression.
Based on these findings, we hypothesized that the mitigation of altered activities of these histone modifiers during and after Dexa treatment would reverse histone hyperacetylation in the Dpp-4 promoter region. In vitro experiments were conducted with EOMA cells treated with a HAT inhibitor, L002, to decrease enhanced HAT activity during Dexa loading, or a sirtuin activator, NMN, to increase sirtuin activity after Dexa loading. Development of the histone hyperacetylation during Dexa treatment was significantly inhibited by L002. Moreover, the already developed histone hyperacetylation after Dexa treatment was reversed by NMN treatment. These findings suggest that Dexa-induced upregulation of Dpp-4 is reversible by interventions that modify epigenetic regulation of gene expression. Although epigenetic editing is still a developing research field (48), it is expected to consist of a new therapeutic approach for diabetes. For example, promotion of β-cell replication has been successfully demonstrated in vitro, by selective modification of DNA methylation at the CDKN1C locus (49).
Elevated DPP-4 activity leads to inactivation of GLP-1 or other incretins and subsequent reduction in insulin secretion. In addition to the ability to enhance glucose-induced insulin secretion, GLP-1 has a well-known effect of reducing hunger and body weight (50). Meta-analysis of 21 trials with 6411 participants revealed that treatment with GLP-1 receptor agonists in overweight patients for at least 20 weeks achieved 2.9 kg greater body weight reduction than the control group (51). In cohorts of the present clinical study, we revealed body weight gain after completion of transient glucocorticoid treatment, which is significantly related to the extent of HbA1c elevation in the metasteroid phase. Considering the finding that transient Dexa loading in mice caused consistently lowered blood GLP-1 level, we speculate that lowered GLP-1 level may underlie both hyperglycemia and body weight gain after glucocorticoid treatment discontinuation in the human clinical study. As far as we know, circulating GLP-1 level during and after glucocorticoid treatment has not been determined in humans. Further examination is expected to elucidate whether alteration in GLP-1 level and DPP-4 activity is found in human subjects with GIH or metasteroid diabetes.
We conclude that persistent Dpp-4 upregulation with histone hyperacetylation in the promoter region, which leads to a lowered blood GLP-1 level, plays critical roles in the development of GIH and subsequent metasteroid diabetes in mice. The sustained histone hyperacetylation induced by transient glucocorticoid treatment consists of the memory phenomenon of hyperglycemia. The results suggest a novel concept of intervention for GIH and metasteroid diabetes in human through GLP-1 agonism, DPP-4 inhibition, or epigenetic modification of Dpp-4 promoter region. Further study is expected to clarify the alteration in the DPP-4/GLP-1 cascade during and after glucocorticoid treatment in human subjects.
Acknowledgments
Financial Support: This work was supported by the Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research with grant numbers JP26460920 to K.M. and JP16K15471 to H.I.
Author Contributions: A.U. and K.M. designed and conducted the experiments, sample processing, and data analysis. All authors reviewed and edited the manuscript. H.I. supervised the project.
Glossary
Abbreviations
- 2Y
2 years
- BMI
body mass index
- ChIP
chromatin immunoprecipitation
- DCCT
Diabetes Control and Complications Trial
- Dexa
dexamethasone
- EDIC
Epidemiology of Diabetes Interventions and Complications
- EOMA
hemangioendothelioma
- GIH
glucocorticoid-induced hyperglycemia
- GLP-1
glucagon-like peptide 1
- H3K9Ac
histone H3 lysine-9 acetylation
- HAT
histone acetyltransferase
- HbA1c
glycated hemoglobin
- ITT
insulin tolerance test
- NMN
nicotinamide mononucleotide
- OGTT
oral glucose tolerance test
Contributor Information
Asuka Uto, Division of Endocrinology, Metabolism and Nephrology, Keio University, School of Medicine, Tokyo, 160-8582, Japan.
Kazutoshi Miyashita, Division of Endocrinology, Metabolism and Nephrology, Keio University, School of Medicine, Tokyo, 160-8582, Japan.
Sho Endo, Division of Endocrinology, Metabolism and Nephrology, Keio University, School of Medicine, Tokyo, 160-8582, Japan.
Masaaki Sato, Division of Endocrinology, Metabolism and Nephrology, Keio University, School of Medicine, Tokyo, 160-8582, Japan.
Masaki Ryuzaki, Division of Endocrinology, Metabolism and Nephrology, Keio University, School of Medicine, Tokyo, 160-8582, Japan.
Kenichiro Kinouchi, Division of Endocrinology, Metabolism and Nephrology, Keio University, School of Medicine, Tokyo, 160-8582, Japan.
Masanori Mitsuishi, Division of Endocrinology, Metabolism and Nephrology, Keio University, School of Medicine, Tokyo, 160-8582, Japan.
Shu Meguro, Division of Endocrinology, Metabolism and Nephrology, Keio University, School of Medicine, Tokyo, 160-8582, Japan.
Hiroshi Itoh, Division of Endocrinology, Metabolism and Nephrology, Keio University, School of Medicine, Tokyo, 160-8582, Japan.
Additional Information
Disclosure Summary: There is no conflict of interest.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Hench PS, Slocumb CH, Polley HF, Kendal EC. Effect of cortisone and pituitary adrenocorticotropic hormone (ACTH) on rheumatic diseases. J Am Med Assoc. 1950;144(16):1327-1335. [DOI] [PubMed] [Google Scholar]
- 2. Goodwin WE, Kaufman JJ, Mims MM, et al. Human renal transplantation. I. Clinical experiences with six cases of renal homotransplantation. J Urol. 1963;89:13-24. [DOI] [PubMed] [Google Scholar]
- 3. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711-1723. [DOI] [PubMed] [Google Scholar]
- 4. Conn JW, Fajans SS. Influence of adrenal cortical steroids on carbohydrate metabolism in man. Metabolism. 1956;5(2):114-127. [PubMed] [Google Scholar]
- 5. Gold A. The treatment of diabetes mellitus with oral tolbutamide. Can J Biochem Physiol. 1957;35(11):953-960. [PubMed] [Google Scholar]
- 6. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care. 2016;39(5):686-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miao F, Chen Z, Genuth S, et al. ; DCCT/EDIC Research Group . Evaluating the role of epigenetic histone modifications in the metabolic memory of type 1 diabetes. Diabetes. 2014;63(5):1748-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Z, Miao F, Paterson AD, et al. ; DCCT/EDIC Research Group . Epigenomic profiling reveals an association between persistence of DNA methylation and metabolic memory in the DCCT/EDIC type 1 diabetes cohort. Proc Natl Acad Sci U S A. 2016;113(21):E3002-E3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Raalte DH, Ouwens DM, Diamant M. Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur J Clin Invest. 2009;39(2):81-93. [DOI] [PubMed] [Google Scholar]
- 10. Zhang X, Yong W, Lv J, et al. Inhibition of forkhead box O1 protects pancreatic beta-cells against dexamethasone-induced dysfunction. Endocrinology. 2009;150(9):4065-4073. [DOI] [PubMed] [Google Scholar]
- 11. RRID: AB_2892225. [Google Scholar]
- 12. RRID: AB_2811268. [Google Scholar]
- 13. RRID: CVCL_3507. [Google Scholar]
- 14.doi: 10.6084/m9.figshare.15162465. [DOI]
- 15. RRID: AB_2614978. [Google Scholar]
- 16. RRID: AB_2616593. [Google Scholar]
- 17. RRID: AB_2793550. [Google Scholar]
- 18. RRID: AB_2636968. [Google Scholar]
- 19. Yang H, Yan B, Liao D, Huang S, Qiu Y. Acetylation of HDAC1 and degradation of SIRT1 form a positive feedback loop to regulate p53 acetylation during heat-shock stress. Cell Death Dis. 2015;6:e1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15(5):469-474. [DOI] [PubMed] [Google Scholar]
- 21. Weinstein SP, Wilson CM, Pritsker A, Cushman SW. Dexamethasone inhibits insulin-stimulated recruitment of GLUT4 to the cell surface in rat skeletal muscle. Metabolism. 1998;47(1):3-6. [DOI] [PubMed] [Google Scholar]
- 22. Zawalich WS, Tesz GJ, Yamazaki H, Zawalich KC, Philbrick W. Dexamethasone suppresses phospholipase C activation and insulin secretion from isolated rat islets. Metabolism. 2006;55(1):35-42. [DOI] [PubMed] [Google Scholar]
- 23. Duncan LJ, Baird JD. Compounds administered orally in the treatment of diabetes mellitus. Pharmacol Rev. 1960;12:91-158. [PubMed] [Google Scholar]
- 24. Henriksen JE, Alford F, Ward GM, Beck-Nielsen H. Risk and mechanism of dexamethasone-induced deterioration of glucose tolerance in non-diabetic first-degree relatives of NIDDM patients. Diabetologia. 1997;40(12):1439-1448. [DOI] [PubMed] [Google Scholar]
- 25. Mulvihill EE, Varin EM, Gladanac B, et al. Cellular sites and mechanisms linking reduction of dipeptidyl peptidase-4 activity to control of incretin hormone action and glucose homeostasis. Cell Metab. 2017;25(1):152-165. [DOI] [PubMed] [Google Scholar]
- 26. Jensen DH, Aaboe K, Henriksen JE, et al. Steroid-induced insulin resistance and impaired glucose tolerance are both associated with a progressive decline of incretin effect in first-degree relatives of patients with type 2 diabetes. Diabetologia. 2012;55(5):1406-1416. [DOI] [PubMed] [Google Scholar]
- 27. Matsuo K, Nambu T, Matsuda Y, et al. Evaluation of the effects of exenatide administration in patients with type 2 diabetes with worsened glycemic control caused by glucocorticoid therapy. Intern Med. 2013;52(1):89-95. [DOI] [PubMed] [Google Scholar]
- 28. van Genugten RE, van Raalte DH, Muskiet MH, et al. Does dipeptidyl peptidase-4 inhibition prevent the diabetogenic effects of glucocorticoids in men with the metabolic syndrome? A randomized controlled trial. Eur J Endocrinol. 2014;170(3):429-439. [DOI] [PubMed] [Google Scholar]
- 29. El Ghandour S, Azar S. Incretin based therapy in the management of steroid induced diabetes mellitus. Curr Diabetes Rev. 2014;10(6):360-363. [DOI] [PubMed] [Google Scholar]
- 30. Diaz-Jimenez D, Petrillo MG, Busada JT, Hermoso MA, Cidlowski JA. Glucocorticoids mobilize macrophages by transcriptionally up-regulating the exopeptidase DPP4. J Biol Chem. 2020;295(10):3213-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Balla A, Chobanian M. New-onset diabetes after transplantation: a review of recent literature. Curr Opin Organ Transplant. 2009;14(4):375-379. [DOI] [PubMed] [Google Scholar]
- 32. Itoh H, Kurihara I, Miyashita K, Tanaka M. Clinical significance of ‘cardiometabolic memory’: a systematic review of randomized controlled trials. Hypertens Res. 2017;40(6):526-534. [DOI] [PubMed] [Google Scholar]
- 33. Itoh H, Kurihara I, Miyashita K. Organ memory: a key principle for understanding the pathophysiology of hypertension and other non-communicable diseases. Hypertens Res. 2018;41(10):771-779. [DOI] [PubMed] [Google Scholar]
- 34. Julius S, Nesbitt SD, Egan BM, et al. ; Trial of Preventing Hypertension (TROPHY) Study Investigators . Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354(16):1685-1697. [DOI] [PubMed] [Google Scholar]
- 35. Ford I, Murray H, Packard CJ, Shepherd J, Macfarlane PW, Cobbe SM; West of Scotland Coronary Prevention Study Group . Long-term follow-up of the West of Scotland Coronary Prevention Study. N Engl J Med. 2007;357(15):1477-1486. [DOI] [PubMed] [Google Scholar]
- 36. Kato M, Natarajan R. Diabetic nephropathy–emerging epigenetic mechanisms. Nat Rev Nephrol. 2014;10(9):517-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen S, Feng B, George B, Chakrabarti R, Chen M, Chakrabarti S. Transcriptional coactivator p300 regulates glucose-induced gene expression in endothelial cells. Am J Physiol Endocrinol Metab. 2010;298(1):E127-E137. [DOI] [PubMed] [Google Scholar]
- 38. Kawada Y, Asahara SI, Sugiura Y, et al. Histone deacetylase regulates insulin signaling via two pathways in pancreatic β cells. Plos One. 2017;12(9):e0184435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bordone L, Motta MC, Picard F, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. Plos Biol. 2006;4(2):e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moynihan KA, Grimm AA, Plueger MM, et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2(2):105-117. [DOI] [PubMed] [Google Scholar]
- 41. Cao SX, Dhahbi JM, Mote PL, Spindler SR. Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc Natl Acad Sci U S A. 2001;98(19):10630-10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhong L, D’Urso A, Toiber D, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140(2):280-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dittenhafer-Reed KE, Richards AL, Fan J, et al. SIRT3 mediates multi-tissue coupling for metabolic fuel switching. Cell Metab. 2015;21(4):637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang H, Wei W, Menconi M, Hasselgren PO. Dexamethasone-induced protein degradation in cultured myotubes is p300/HAT dependent. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R337-R334. [DOI] [PubMed] [Google Scholar]
- 45. Amat R, Solanes G, Giralt M, Villarroya F. SIRT1 is involved in glucocorticoid-mediated control of uncoupling protein-3 gene transcription. J Biol Chem. 2007;282(47):34066-34076. [DOI] [PubMed] [Google Scholar]
- 46. Jing D, Huang Y, Liu X, et al. Lymphocyte-specific chromatin accessibility pre-determines glucocorticoid resistance in acute lymphoblastic leukemia. Cancer Cell. 2018;34(6):906-921.e8. [DOI] [PubMed] [Google Scholar]
- 47. Lu BY, Thanawala SU, Zochowski KC, Burke MJ, Carroll WL, Bhatla T. Decitabine enhances chemosensitivity of early T-cell precursor-acute lymphoblastic leukemia cell lines and patient-derived samples. Leuk Lymphoma. 2016;57(8):1938-1941. [DOI] [PubMed] [Google Scholar]
- 48. Ling C, Rönn T. Epigenetics in human obesity and type 2 diabetes. Cell Metab. 2019;29(5):1028-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ou K, Yu M, Moss NG, et al. Targeted demethylation at the CDKN1C/p57 locus induces human β cell replication. J Clin Invest. 2019;129(1):209-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meier JJ, Gallwitz B, Schmidt WE, Nauck MA. Glucagon-like peptide 1 as a regulator of food intake and body weight: therapeutic perspectives. Eur J Pharmacol. 2002;440(2-3):269-279. [DOI] [PubMed] [Google Scholar]
- 51. Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. Bmj. 2012;344:d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.