Abstract
Recycled materials are found in many consumer products as part of a circular economy, however the chemical content of recycled products is generally uncharacterized. A suspect screening analysis using two-dimensional gas chromatography time-of-flight mass spectrometry (GCxGC-TOFMS) was applied to 210 products (154 recycled, 56 virgin) across 7 categories. Chemicals in products were tentatively identified using a standard spectral library or confirmed using chemical standards. A total of 918 probable chemical structures identified (112 of which were confirmed) in recycled materials versus 587 (110 confirmed) in virgin materials. Identified chemicals were characterized in terms of their functional use and structural class. Recycled paper products and construction materials contained greater numbers of chemicals than virgin products; 733 identified chemicals had greater occurrence in recycled compared to virgin materials. Products made from recycled materials contained greater numbers of fragrances, flame retardants, solvents, biocides, and dyes. The results were clustered to identify groups of chemicals potentially associated with unique chemical sources, and identified chemicals were prioritized for further study using high-throughput hazard and exposure information. While occurrence is not necessarily indicative of risk, these results can be used to inform the expansion of existing models or identify exposure pathways currently neglected in exposure assessments.
Keywords: recycling, consumer products, human exposure modeling, consumer exposure, ExpoCast, non-targeted analysis, suspect screening
Graphical Abstract

INTRODUCTION
Characterizing the risks associated with commercial chemicals requires an understanding of their corresponding exposure sources and pathways. Consumer exposures are of high interest in risk assessment; consumer uses of chemicals have been correlated with increased exposures in biomonitoring studies1, 2. There is an increasing desire by both manufacturers and consumers to develop sustainable approaches to the production and re-use of consumer products. However, within such a circular economy there may be an increased potential for exposures associated with chemicals in recycled consumer products. The chemical composition of a recycled product can be influenced by the original formulation of the source material, contamination during its original use, introduction of chemicals during disposal, or chemical modifications or additions during the recycling process. Although contaminants may be removed at some point during the recycling process via washing, filtering, vaporization, solvation, or other processes3, 4, some may remain in feedstock and become part of the solid matrix of any recycled product. Potentially harmful chemicals have been identified in recycled products such as toys5, 6, décor items5, paper products7, and appliances8. Recycled materials such as paper, plastics, and fabrics may contain components such as additives, glues, dyes, and fragrances, that were not intended to be present in products made from recycled goods 9. Classes of chemicals found in recycled consumer products include polycyclic aromatic hydrocarbons (PAHs),10 phthalates,6, 10 brominated and other flame retardants,6, 11, 12 and VOCs.13 These chemical classes include many compounds with potential for ultimate human exposure, as demonstrated by their measurement and detection in human biomonitoring studies.14, 15 The variety of recycled products available on the market and their potential for containing contamination introduced over different material lifestages suggests a need for a method to screen products for large numbers of compounds to characterize chemical sources and potential exposures.
Non-targeted analysis (NTA) and suspect screening analysis (SSA) have been used to determine the presence of numerous chemicals in house dust, water, food, blood, and consumer goods 16–23. NTA is an analytical chemistry technique for identifying unanticipated chemicals in samples of interest without the use of standards or defined suspect lists. SSA is similar to NTA in terms of the equipment used and overall objectives (e.g., chemical characterization without the use of standards), but focuses on the identification of chemical suspects contained within existing libraries/databases (generally with reference spectral data).24 Given these definitions, NTA is better suited for true chemical discovery, whereas SSA is better suited for the detection of known analytes having little real measurement or monitoring data. Using NTA or SSA, thousands of chemicals can be investigated in any given sample set. These methods are therefore a necessary compliment to traditional targeted analytical chemistry methods that focus on the quantitation of relatively few compounds for which authentic standards are relatively available. Phillips et al. 25 performed SSA on a variety of consumer products, including both formulations (i.e., chemical mixtures such liquids, pastes, or powders) and articles (manufactured items formed to a specific shape). Over 1600 chemicals were tentatively identified using matching to a spectral library; the majority of these chemicals were previously uncatalogued in EPA’s consumer product databases26, 27, which were primarily developed from safety data sheets (SDS). Many of these chemicals were associated with articles, which do not require SDS sheets in the U. S. under the Occupational Health and Safety Administration’s Hazard Communication 28. Chemical structure-based Quantitative Structure Use Relationship (QSUR) models29 were used to predict the likely chemical functions of the identified chemicals. Based on the results, the chemicals were likely of both intentional and unintentional origin.
SSA has the potential to identify chemicals in samples originating from a variety of sources that were introduced over the lifecycle of the product or its component parts. Understanding these sources (and their associated risks) across the supply chain is a major challenge facing product manufacturers 30. Co-occurring chemicals collectively associated with a common source may be present in multiple samples. Co-occurring chemicals collectively associated with a common source may be present in multiple samples. Unsupervised data mining techniques, such as hierarchical clustering, can be applied to SSA data to identify these co-occurring chemicals and develop unique source signatures. Here, a “source signature” represents a unique combination of chemicals that occur together in multiple samples. Hierarchical clustering has been used successfully to identify patterns of chemical occurrence (source signatures) for the purposes of identifying adulterated cooking oils31, assigning flavor profiles to alcoholic beverages32, and determining origin of cow milk samples33. The identification of such signatures can provide evidence of potential exposure sources associated with consumer products, including sources associated with recycled materials.
In this study, we use SSA to characterize the chemical composition of 210 household articles manufactured using either recycled or virgin materials. We summarize identified chemicals in terms of their chemical structural classes, known or predicted functional roles, and reported uses in commerce. We then use hierarchical cluster analysis to identify unique groups of chemicals co-occurring within subsets of products, and further investigate the commercial uses of the chemicals within clusters for evidence of chemical source (and thus potential exposure pathway). While the detection of a chemical within a particular product does not necessarily imply that any exposure or risk is present, it does provide information that can inform the identification (and mitigation) of previously unknown chemical sources and selection of chemicals for further study via targeted methods. Screening-level risk metrics (useful for prioritizing chemicals for further study) were calculated for each identified chemical using abundance and detection frequency results, along with available high-throughput exposure and bioactivity information. The research presented here demonstrates an integrated application of analytical chemistry, data mining, and cheminformatics to inform exposure analyses.
METHODS
Product Selection
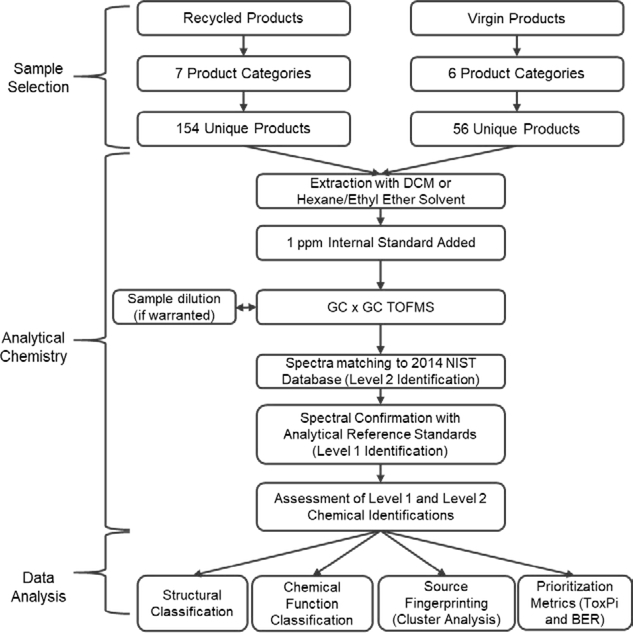
Consumer products manufactured using either recycled or virgin materials were purchased directly from local retail stores in San Antonio, Texas or ordered from online retailers. These products fell into one of seven categories: plastic children’s toys (e.g., bath toys, teethers) or play mats; paper products (e.g., construction paper, copy paper); fabric-containing clothing and home goods (e.g., t-shirts, rugs); plastic food contact materials (e.g., boards, sandwich bags); non-culinary use plastic household items (e.g., hangers, dog bowls); construction materials, including vinyl flooring, lumber, and boat-board (a thick plastic sheeting used in marine and vehicle applications); and residential products manufactured using recycled tire-derived material (e.g., turf mats, rubber mulches). A product was considered recycled only if was labeled as containing at least 50% recycled materials. The products analyzed and their classification (recycled or virgin) are given in Supplemental Table S1. The selection of products and subsequent analysis (described below) are summarized in Figure 1.
Figure 1.
Workflow of product categorization and suspect screening analysis for 210 consumer products. Product samples were extracted with either dichloromethane (DCM); for three samples, a 94:6 mixture of hexanes:ethyl ether was used in place of DCM due to sample degradation. An internal standard was added, and each extraction was then analyzed via GC x GC/TOF-MS to obtain its mass spectra. The spectra were then matched to the 2014 NIST database and a workflow implemented to score chemical identifications. Analytical standards were used to confirm a subset of the chemical identifications.
GCxGC-TOFMS Analysis
Sample extractions were performed in batches of 20 samples, with one sample per batch chosen for a duplicate extraction and analysis. Details of the extraction methods are provided in the Supplemental Information (SI). Two-dimensional gas chromatography (GCxGC) time-of-flight mass spectrometry (TOFMS) analysis was performed using an Agilent 7890 gas chromatograph coupled to a LECO PEGASUS 4D-TOF (LECO, St. Joseph, MI). Injection volume was 1.0 μL. The inlet temperature was 275°C and the inlet mode was splitless with a 1 min purge. Chromatographic separation was achieved using two columns. The primary column (1st dimension) was an RXi-1MS (30 m × 0.25 mm × 0.25 μm; Restek, Bellefonte, PA) and the second column (2nd dimension) was an RXi-17SilMS, (1.3 m × 0.18 mm × 0.18 μm; Restek, Bellefonte, PA). The first column was held at 45 °C for 3 minutes, ramped to 330 °C at a rate of 8°C/min, and held for 5.0 minutes. The second column and modulator were offset by 5.0 and 20 degrees Celsius, respectively. Helium carrier flow was set to constant flow at 1.0 mL/min. The transfer line temperature was set at 300 °C. The modulation period was 5 seconds (1.25 s hot, 1.25 s cold with 2 cycles per modulation period) through entire run. The mass spectrometer was operated using electron ionization (EI) at 70eV. The ion source temperature was set at 225 °C. Spectra were collected from 45–650 m/z with a scan time of 100 spectra/sec. Sensitivity was checked by verifying a signal to noise ratio of 10 with 2 pg of hexachlorobenzene on-column according to the manufacturer’s specification.
A solution containing multiple deuterated internal standards (including 1,4-dichlorobenzene-D4, naphthalene-D8, acenaphthene-D10, phenanthrene-D10, chrysene-D12 and perylene-D12) was spiked into the calibration standards, sample extracts, and system blanks at a concentration of 1.0 ppm prior to analysis. These standards were used for quality control; the peak shape, retention time and abundance of the internal standards were monitored throughout the batch for conditions that would have required corrective action. Specifically, retention time criteria were plus or minus two modulation periods in the first dimension and 0.1s in the second dimension. Some drift in abundance was allowed given the heavy matrix present in specific samples, but generally was not allowed to exceed plus or minus 50%. Peak shape changes in the form of excessive tailing was an indication of degraded injector conditions. Corrective action typically consisted of injector maintenance and column trimming or replacement followed by re-tuning and verification of a leak-free system and minimum sensitivity according to manufacturer’s specifications. A set of 184 chemical standards were selected for use as reference standards from the ToxCast chemical library34 based on availability, quality level, and likelihood of environmental presence. These chemical standards were assayed with each batch and used for confirmation. These chemicals are listed in Supplemental Table S2, along with their approximate retention times.
Multiple standards were analyzed in each analytical sequence. Additionally, system1/solvent blanks were analyzed throughout. A 1.0 mL aliquot of both sample dilutions (i.e., the 150 mL and 10 mL extracts) was transferred to an autosampler vial, spiked with internal standard, and analyzed. Each data file was assessed for overloading and/or adequate response to determine if further dilution or concentration of the sample extract and/or re-analysis was warranted. Dilution was applied for samples with significant overloading in one or more regions of the chromatogram, as determined by an approximate abundance threshold of 5e6.
Analytical data acquired from each sample batch were processed separately. The data were processed using LECO’s ChromaTOF software (version 4.71.0.0) to integrate peaks and to identify them based on confirmation standards or a search of the National Institute of Standards and Technology 2014 Mass Spectral Library (NIST 2014 v.2.2.07–2014), using a workflow previously described25. Features were processed against the reference standards and the NIST library simultaneously (with precedence give to a match to a standard). Details of the data processing and spectra matching (including scoring thresholds, QA procedures, and standard confirmation) are given in the SI. Identifications were classified according to levels defined by Schymanski et al. 35 Identified spectral library matches were classified as “tentative candidates” (Level 3 identifications) if the spectrum was indistinguishable from that of potential isomers or if the exact carbon chain length of the identification was unknown; otherwise they were classified as “probable structures” (Level 2 identifications). Chemicals that were identified via a reference standard were termed “confirmed structures” (Level 1) identifications.
Characterization of Identified Chemicals
The number of confirmed (Level 1) and probable (Level 2) identifications (product-chemical occurrences) in recycled and virgin products were evaluated overall and within product category. Differences were assessed using the nonparametric Mann-Whitney-Wilcoxon test (implemented via the wilcox.test function in R).
Confirmed and probable chemicals were also characterized according to their structure and potential uses. Recent work by described a structure-based chemical taxonomy (ClassyFire) whereby chemicals are categorized via an unambiguous nomenclature. Here, we determined the chemical taxonomy for each identified chemical by CASRN using the ClassyFire web application available at http://classyfire.wishartlab.com. Identified chemicals were also cross-referenced with chemical class lists compiled by EPA in the CompTox Chemicals Dashboard (https://comptox.epa.gov/dashboard), including lists for polycyclic aromatic hydrocarbons (PAHs), flame retardants, azo dyes, pesticide actives, and bisphenols. To characterize use of identified chemicals, the function (i.e. the role a chemical performs in the product) of each chemical was explored. The functional use database (FUse)37 within the Chemical and Products Database (CPDat)26, consists of function information for >75,000 chemicals found in consumer products. Confirmed chemicals were matched to those in FUse/CPDat using chemical abstract service registry numbers (CASRNs); for clarity here all reported functions in FUse were harmonized to function identifiers developed by the Organization for Economic Cooperation and Development (OECD) for use in chemical reporting. In addition, quantitative structure use relationship (QSUR) models29 were used to provide evidence of function if no reported information was available. Finally, more general chemical use information (such as sector of use) for the identified chemicals was obtained from EPA’s CPCat39 database and EPA’s Chemical Data Reporting (CDR, https://www.epa.gov/chemical-data-reporting).
Cluster Analysis
We applied hierarchical clustering analysis to the chemical identifications to characterize the differences between recycled and virgin products and to identify chemical groups of interest (e.g., co-occurring groups of chemicals present in multiple products that may be representative of unique sources). A binary array was created to represent the presence of each identified chemical in each product. Each array element was assigned a value of one if a product sample contained the identified chemical or zero if it was absent. Initial filtering was performed to remove chemicals that had homogeneous occurrence patterns over all products (i.e., were present in or absent from 95% of the products). The pairwise distance between each two chemicals’ presence in products was then calculated using the Jaccard distance metric40. These distances were then clustered using divisive hierarchical clustering, using the diana function of the cluster package within the R programming language43. An optimal number of clusters was selected that balanced the total within-cluster sum of squares (wss, a measure of cluster compactness) with the total number of clusters. The optimal number of clusters was selected by plotting wss for different number of clusters N, where N varies from 1 to the number of chemicals. In addition, average cluster silhouette width (a measure of both cluster compactness and intra-cluster separation) was also examined. An optimal N was selected that corresponded to an “elbow” in wss and a local maximum in silhouette width.
Prioritization of Identified Chemicals using Occurrence, Exposure, and Bioactivity Data
Chemical occurrence and abundance in samples and screening-level exposure and toxicity information were used to score the Level 1 and Level 2 chemical identifications for potential further study or additional confirmation. The scoring approaches used included the “ToxPi” calculation developed by Rager et al.45 and a bioactivity-to-exposure ratio (BER) similar to that used by Paul-Friedman et al.46 The calculation of ToxPi score and BER are described in detail in the SI. Briefly, the ToxPi score was based on the detection frequency in the product samples, the mean observed peak area (abundance), an exposure category (1–8) developed from the ExpoCast third-generation Systematic Empirical Evaluation of Models (SEEM3) consensus model,47 and the fraction of positives in available high-throughput assays for bioactivity in the Tox2148 program. ToxPi scores based solely on detection frequency and abundance were calculated for all chemicals; an enhanced score that included bioactivity and exposure was calculated when these data were available. The BER was calculated as the ratio of an administered equivalent dose (AED) associated with a lower-bound bioactive concentration from ToxCast to an upper-bound exposure estimate. Here the lower-bound bioactivity was the 10th percentile of the distribution of 50% maximal activity concentration (AC50) values from the available assays and the upper-bound exposure was the 95th percentile credible interval of the population median exposure rate from SEEM3. The lower-bound bioactivity was converted to AED using high-throughput toxicokinetic (HTTK) information from the httk R package49.
RESULTS AND DISCUSSION
Chemical Identifications in Recycled and Virgin Products
Samples from 154 recycled and 56 virgin products were extracted and analyzed via gas chromatography time-of-flight mass spectrometry following the workflow in Figure 1. Using the Chromatof software, identified spectral features were compared to the NIST 2014 database to determine the similarity to other known spectra. Across all samples, there were 84,002 individual spectra with high enough similarity scores (score>650) to be considered Level 2 (probable structure) or 3 (tentative candidate) identifications; the majority of these could only be identified as tentative candidates (e.g., isomers could not be distinguished) and were removed from further analyses, leaving a total of 13,644 Level 2 IDs (chemical-in-product combinations). Of these Level 2 IDs, 8,821 were confirmed (Level 1 ID) with one of the 184 reference standards (noting that individual chemicals were often confirmed in multiple products, thus yielding the 8,821 confirmed IDs). There were a total of 1,123 unique chemicals with a Level 2 ID (587 in virgin products and 918 in recycled products) and 112 were confirmed (101 in virgin and 112 in recycled). Note that the larger overall number of chemicals identified in recycled compared to virgin products is at least in part due to the larger number of recycled product samples. All probable (Level 2) and confirmed (Level 1) chemical IDs are provided in Supplemental Table S3.
The number of confirmed and probable chemicals in samples ranged from a low of 11 for a virgin plastic food contact item, to a high of 168 for a recycled paper product. There were 45 semi-ubiquitous chemicals present in at least half of all products, whereas there were 525 chemicals that were unique to a single product. We should note most chemicals identified as being ubiquitous across products were those determined using chemical standards, while those unique to one product were only tentatively identified and could perhaps be misidentified. A summary table of all the unique chemicals identified and the number of virgin and recycled products in which they were found is provided in Supplemental Table S4. While several of the analyses provided herein focus on chemicals unique to recycled products, ubiquitous chemicals could potentially be of interest from a risk perspective, as they might be associated with higher exposure.
The number of probable and confirmed chemical IDs per sample is summarized in Table 1. There was a significantly greater number of chemicals per sample in recycled construction materials (Mann-Whitney-Wilcoxon test; p-value = 0.0283) and paper products (p-value = 0.041) compared to virgin products. It is true that the products within a category differ in other ways beyond recycling status; some differences within categories are likely due to expected differences in product type, overall composition, and manufacturing process. However, given the diversity of both the recycled and virgin products in the different categories this still suggests that the circular nature of the recycling economy may have the potential to introduce additional chemicals into products. It is worthy to note that the differences are insignificant in categories that are more highly regulated (children’s products and food contact materials). The largest magnitude differences between the number of chemicals observed occurred in paper products. This is consistent with the fact that paper is recycled approximately 3.5 times before being removed from the material cycle whereas it has been estimated that only 10% of plastic has been recycled more than once51. Such differences across product categories could also be due to differences in recycling processes. Paper recycling can include steps (e.g., deinking and bleaching) that use significant numbers and types of chemicals52 whereas many plastic recycled items are generated via primarily mechanical (or “secondary”) processes.53
Table 1.
Number of chemicals per sample with a probable or confirmed identification in recycled and virgin consumer products. Nrecycled and Nvirgin refer to the number of recycled and virgin products that were tested.
| Category | Nrecycled | Meanrecycled | Medianrecycled | Nvirgin | Meanvirgin | Medianvirgin | p-value |
|---|---|---|---|---|---|---|---|
| All Products | 154 | 67.0 | 63 | 56 | 59.3 | 55 | 0.017 |
| Paper Products | 23 | 96.6 | 86 | 8 | 71.5 | 66.50 | 0.041 |
| Children's Products | 20 | 59.8 | 53.50 | 15 | 68.1 | 57 | 0.828 |
| Fabric Products | 17 | 82.4 | 89 | 14 | 64.1 | 63 | 0.242 |
| Recycled Tire Products | 22 | 66.5 | 66.50 | - | - | - | |
| Food Contact Materials | 22 | 59.9 | 60 | 11 | 56.6 | 54 | 0.417 |
| Construction Materials | 35 | 54.9 | 55 | 8 | 46.0 | 46.50 | 0.0283 |
| Plastic Home/Auto Products | 15 | 53.5 | 55 | 20 | 49.2 | 36.5 | 0.054 |
Characterization of Chemicals Identified
The Classyfire taxonomic tool was used to characterize chemical classes associated with each chemical identification. Chemical taxonomies and list presences are included in Supplemental Table S3. The Classyfire superclass, class, and subclass levels were analyzed. The counts of unique identified chemicals in each taxonomic level in recycled and virgin products overall is given in Supplemental Table S5. There were 13 structural superclasses represented with the highest number of identifications of benzenoids, hydrocarbons, and lipids and lipid-like molecules. Supplemental Figure S1 illustrates the overall differences in identifications per sample in recycled versus virgin products of the 20 most prevalent chemical classes; in recycled products there was significantly greater occurrence (Mann-Whitney-Wilcoxon test, p<0.05) of saturated hydrocarbons, benzene and substituted derivatives, fatty acyls, lactones, benzothiazoles, phenanthrenes and derivatives, and isoindoles and derivatives. Supplemental Figure S2 illustrates the chemical subclasses with the largest differences in fraction of occurrence in recycled and virgin products for each product type. Terpenoids (including mono-, di-, and sesqui- compounds) were more frequent in recycled materials in several categories, perhaps indicating contamination of products with fragrances (which have ubiquitous use in commerce). Recycled food contact materials had increased occurrence of cinnamic acid esters and triazoles, perhaps reflecting contamination of food-contact based feedstocks with naturally-occurring compounds or fungicides. Both recycled paper and fabric products had higher occurrence of cresols, which might reflect either dye or disinfectant presence.
Identified chemicals were also cross-referenced with chemical class lists compiled by EPA in the CompTox Chemicals Dashboard; results are summarized (overall and by category) in Supplemental Table S6. There were more unique chemicals found in recycled products for PAHs, pesticide actives (the compounds within pesticide formulations that kill pests), bisphenols, and flame retardants (although these higher counts are likely at least partially due to the larger number of recycled products analyzed). There was only one azo dye identified in samples (in a recycled fabric product), likely due to incompatibility of these compounds with GC (less than 10% of the listed azo dyes were even present in the NIST library). Notably, 16 unique flame retardants were found in recycled paper products compared to only 6 compounds in virgin paper. There were 31 occurrences of pesticide actives in recycled materials (9 unique chemicals) compared to 4 occurrences in virgin products (2 unique chemicals).
In this study, we observed a variety of compounds that were previously highlighted as potentially occurring in recycled consumer products of various types. Eighteen chemicals identified in recycled paper here (six phthalates, four phenols, two parabens, and six miscellaneous compounds [including resins and solvents]) were previously included on a list of compounds of interest for recycled paper7 (Supplemental Table S7). While phthalate plasticizers were (generally) identified in both virgin and recycled products; di(2-ethylhexyl) phthalate (which has been proposed as an indicator of phthalate contamination in recycled plastic) was found in a single virgin plastic home/auto product but in seven recycled products. Brominated flame retardants have been noted as potentially occurring in items made from recycled black plastic8, 11, 54; here three such compounds (1,2-Bis(2,4,6-tribromophenoxy) ethane, 2,2’,3,4,4’,5’,6-Heptabromodiphenyl ether, 3,3’,5,5’-Tetrabromobisphenol A) were found in a black recycled plastic clothing hanger. In addition to the brominated compounds, Leslie et al. identified other flame retardants in recycled plastic waste, including 2-ethylhexyl diphenyl phosphate, triphenyl phosphate, and tris(2-chloroethyl) phosphate; at least one of these compounds was identified in 37 recycled products, including food contact materials, children’s products, construction materials, and plastic home/auto products. Finally, Diekmann et al.55 compiled a list of PAHs present in products made from recycled rubber; we identified eight of the listed PAHs in recycled tire products, as well as five additional PAHs (see Supplemental Table S3).
An “occurrence ratio” (OR) was calculated for every identified chemical (Figure 2 and Supplemental Table S8), equal to the incidence in recycled products divided by the incidence in virgin products. This global summary metric allowed us to identify chemicals with increased occurrence in recycled products as compared to virgin materials. This analysis was not performed within category, due to relatively small sample sizes, which result in discrete incidences (which makes interpretation difficult). Of the 1,123 chemicals identified in products, 733 (65%) had higher OR in recycled products (with 535 found only in recycled products) and 390 (35%) had higher OR in virgin products (with 203 found only in virgin products, although most in only a few products). Eleven chemicals had ORs greater than 4; six of these chemicals were present in at least five product categories. These chemicals included 1- and 2-methylnapthalene (used in chemical synthesis or as solvent dye carriers), benzoic acid (used as a preservative and in chemical production, including as a plasticizer precursor), diphenylamine (a fungicide), fluoranthene (a PAH), and 2,2-dimethoxy-1,2-diphenylethanone (a photoinitiator). Photoinitiators and fluoranthene have previously been identified as associated with recycled food contact materials56.
Figure 2.
Occurrence ratios (OR) for 1121 chemicals measured in virgin and recycled products. OR is defined as the fraction of recycled products with occurrence divided by the fraction of virgin products with occurrence. Size of the points indicates the number of individual products with occurrence; the color indicates the number of product categories overall with at least one occurrence. Chemicals with a discrete occurrence ratio > 4 are labeled. Points at the very top and bottom of the graph are chemicals with an OR of infinity (no occurrence in virgin products) and 0 (no occurrence in recycled materials) respectively; these chemicals occurred in relatively few products and categories. Chemicals are categorized by chemical structural superclass as provided the ClassyFire taxonomy.
Functional Uses of Confirmed Chemicals
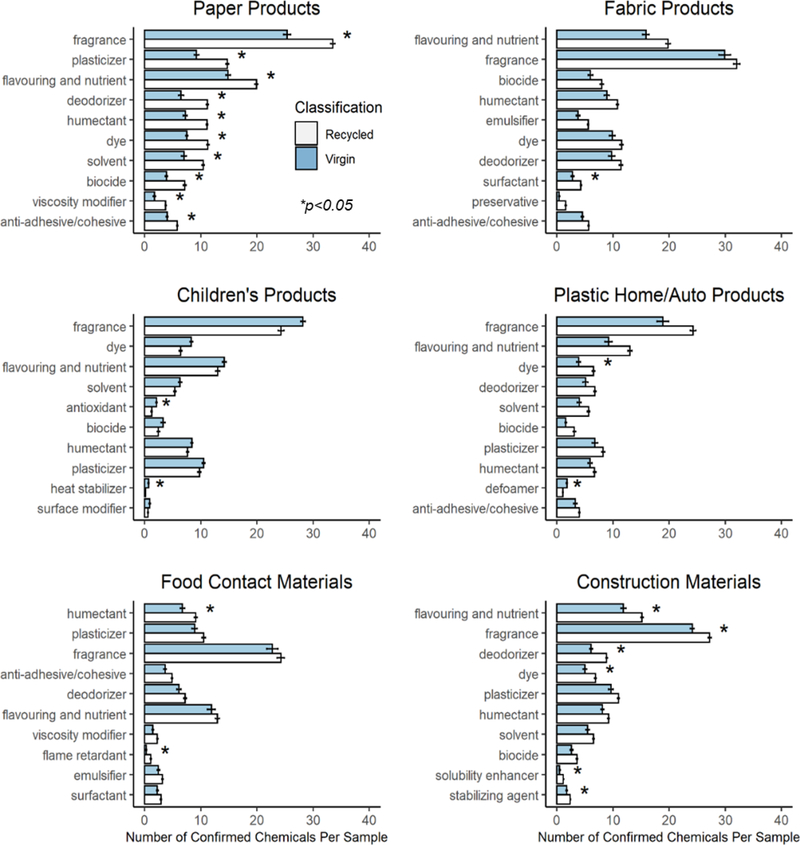
The functional uses of chemicals with confirmed identifications were investigated. There were 96 chemicals (out of 112 confirmed) with functional use data; some chemicals had multiple functions reported. The 21 most prevalent functional uses and their presence in recycled and virgin products are shown in Supplemental Figure S3; overall, there were significantly larger counts per sample of fragrances, flavorants (which were often fragrances as well), plasticizers, deodorizers, humectants, dyes, biocides (pesticides), anti-adhesives, and emulsifiers in recycled as compared to virgin materials. Plasticizers (including phthalates)9, 57, pesticides, dyes7, and fragrances59 have all previously been reported in recycled materials; deodorizers can be used in the recycling process. The occurrence within individual product categories of chemicals having various functional uses is illustrated in Figure 3. Many chemicals identified as fragrances were found in most product categories. This is likely due to multiple factors, including the fact that many chemicals in EPA’s use databases may be labeled as a “fragrance” in some data sources even if they are a ubiquitous chemical included in a fragrance formulation. In addition, many products may in fact contain intentionally-added masking agents that are also reported as fragrances. Finally, some fragrance chemicals may be occurring in products in trace amounts due to their general ubiquity in commerce. As with chemical occurrence overall, the greatest differences between recycled and virgin products were observed in paper products. Larger numbers of chemicals of many functions occurred in recycled paper, including fragrances, solvents, dyes, and biocides. Differences were also observed in construction products, including higher counts of fragrances and flavorants, deodorizers, and dyes. Recycled plastic household items also contained a larger number of dyes. In fabric products, larger numbers of surfactants were observed in recycled products, which may be due to washing activities associated with the manufacture of synthetic fabrics from recycled plastic items. Interestingly, there were larger numbers of humectants found in recycled food contact materials, perhaps indicative of food-based compounds present in feedstock derived from other food contact materials (product labeling indicated this was the case for at least some products). For example, both caffeine and oleic acid fell in this function category. There were also significantly more flame retardants in food contact materials, although the absolute counts of chemicals were low; it is difficult to develop a hypothesis for this result beyond unintentional contamination of recycled material stock during storage or transport. There were no functions with higher counts in recycled children’s products, again likely reflecting the greater regulation of these products.
Figure 3.
Functional uses of confirmed chemicals in recycled and virgin products. For each category, the 10 functional uses with the greatest difference in number of chemicals per sample (between recycled and virgin products) are illustrated. The Y-axis provides the mean number of chemicals per sample; error bars indicate standard error in counts. Asterisks indicate significant differences between virgin and recycled products (Mann-Whitney-Wilcoxon test, p<0.05).
Source Fingerprinting: Hierarchical Cluster Analysis
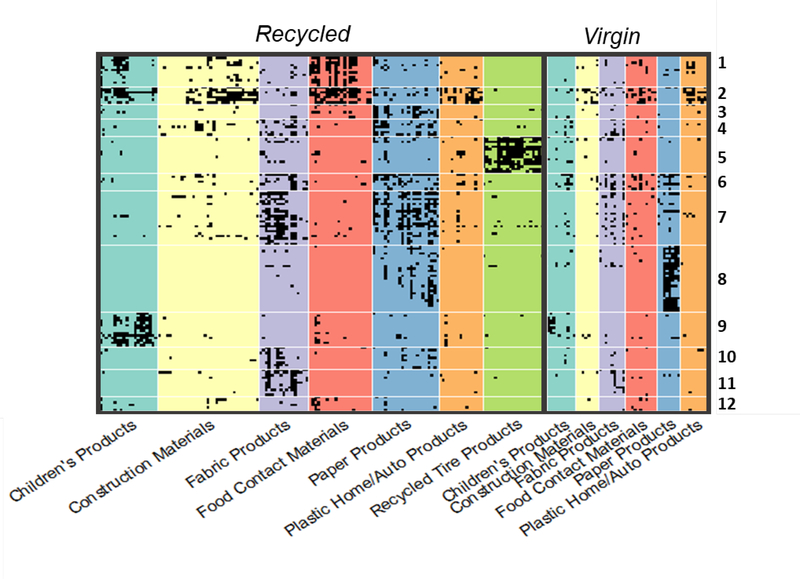
Hierarchical cluster analysis (HCA) of the occurrence of chemicals in products was performed to identify groups of co-occurring chemicals; sets of chemicals co-occurring in multiple products within or among categories may be indicative of a specific chemical source. As described in the Methods, an optimal number of clusters N was obtained via examination of wss and a local maximum in average cluster silhouette width; N=46 was selected. Twelve clusters of interest containing at least 5 chemicals were selected; one cluster was ignored as it contained over 60 chemicals present in many different products (with no discernable patterns of occurrence within product classification or category). The product category and classification (recycled or virgin) distribution within each cluster is illustrated in Supplemental Figure S4. The specific uses of the co-occurring chemicals in each of these 12 clusters were investigated using EPA’s CPDat database (including general use terms and reported functional uses), and EPA’s Chemical Data Reporting information. In addition, QSUR model predictions of function were used to provide evidence of function when no reported information was available. While many chemicals had multiple uses, commonly reported uses, sectors of use, or functions could be identified within each cluster. These common uses are summarized in Table 2. The content of the clusters of interest is visualized in Figure 4; the chemicals present in each cluster and their CPDat and CDR data are included in Supplemental Table S9.
Table 2.
Summary of use information for of chemicals co-occurring in multiple products. Twelve clusters of interest containing between 5 and 50 chemicals are described.
| Cluster ID | Number of Chemicals | Primary Classification | Primary Categories of Occurrence1 | Frequently Occurring Uses, Sectors, or Functions2 | Example Chemicals |
|---|---|---|---|---|---|
| 1 | 13 | Recycled | Children’s products, construction products, food contact materials | Pesticide actives and inerts | Permethrin, bifenthrin, chlorpyriphos |
| 2 | 7 | Both | Children’s products, construction materials, food contact materials, plastic home/auto products | Plastics and plastics manufacturing (including intermediates), polymer additives (UV stabilizer, antioxidant, odor agent) | Tris(2,4-di-tert-butylphenyl) phosphite, octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate, 2-(phenylmethylene)octanal |
| 3 | 6 | Recycled | paper products | Manufacture of ink, paints/coatings, or paper surface treatments; pesticides | 2,2-Dimethoxy-1,2-diphenylethanone, Propylbenzene, DEET, p,p'-methoxychlor olefin |
| 4 | 7 | Both | Construction materials, fabric products, and paper products, fabric products | Manufacture of ink, paints, or dyes; use in ink, toner, and colorant products | 2-(2-Butoxyethoxy)ethanol, (1-hydroxycyclohexyl)(phenyl)methanone, phthalic anhydride |
| 5 | 15 | Recycled | Recycled tire products | Intermediates, rubber components, and processing aids used in the manufacture of rubber products or rubber tires, or in rubber recycling | Aniline, diphenylamine, dicyclohexylamine, phthalimide |
| 6 | 7 | Both | Fabric and paper products, children’s products, food contact materials | Manufacture of plastics, including plasticizers or plasticizer precursors and other polymer additives. | Triethyl citrate, dimethyl phthalate, benzaldehyde |
| 7 | 22 | Both | Paper products and fabric products | Cleaning product, ink, and apparel manufacturing; solvents, fragrances, biocides, dyes, flame retardants | 1-Phenoxy-2-propanol, p-cresol, tris(2-chloroisopropyl)phosphate |
| 8 | 27 | Both | Paper products | Dyes and dye manufacturing, fragrances, pigments and pigment manufacturing | Leucomalachite green, Michler's ketone, dehydroabietic acid |
| 9 | 14 | Both | Children’s products | An alternative plasticizer used in children’s products due to its low toxicity; adhesives, colorants, and chemicals used in their production | Bis(2-ethylhexyl) terephthalate, tetradecanoic acid, 1,4-bis(2-hydroxy-2-propyl)benzene |
| 10 | 9 | Recycled | Fabric and paper products | Fragrances, flavorants, manufacturing of chemicals, cleaning and washing | Methyl benzoate, triclosan, dimethyl succinate |
| 11 | 11 | Both | Fabric products | Flame retardants, fragrances, apparel manufacturing | 2-Butyl-1H-isoindole-1,3(2H)-dione, octrizole, biphenyl phosphate |
| 12 | 6 | Both | Food contact materials | Polymer additives (e.g., odor agent, stabilizers); intermediates | 2-Hydroxy-4-methoxybenzophenone, hexyl salicylate, 3,5-di-tert-butyl-4-hydroxyhydocinnamic acid |
See Supplemental Figure S4 for product category distributions.
See Supplemental Table S5 for available use information.
Figure 4.
Clusters of co-occurring probable and confirmed chemicals in recycled and virgin products. This figure illustrates the occurrence patterns of chemicals within products, clustered so that chemicals with similar patterns are grouped. The black cells indicate occurrence of a chemical in a product. Chemicals and clusters vary vertically and product category vary horizontally. Twelve clusters of interest containing between 5 and 50 chemicals are pictured. Some clusters (e.g., cluster 2) identified groups of chemicals occurring in products across multiple categories, while others (e.g., clusters 5 and 9) identified chemicals concentrated in unique categories. The uses of chemicals within each cluster were investigated to provide evidence of chemical source (see Table 2).
The cluster analysis of chemicals observed across products can elucidate potential chemical sources. The resulting clusters identified sets of co-occurring chemicals of probable intentional and unintentional origin, based on the available chemical use information. For example, clusters 11 and 12 contained chemicals present in both virgin and recycled materials that can reasonably be associated with the manufacture of fabric products (flame retardants and apparel manufacturing chemicals) and food contact materials (polymer additives), respectively. However, other clusters (e.g., the pesticides of cluster 1 or the manufacturing, cleaning and washing, and fragrance chemicals of cluster 10) may identify potential contaminants, as their uses were not reasonably associated with intentional addition and/or they were only associated with recycled products. These results can aid in the development of hypotheses around chemical source. For example, the pesticides in cluster 1 are an interesting example. Several of the products associated with the chemicals in this cluster were potentially made from recycled plastic from milk jugs and/or other food packaging. Non-organic milk has been found to contain chlorpyriphos and permethrin at significant detection rates60; migration of these compounds from milk into packaging is thus a hypothetical source (which of course would need to be confirmed with additional studies). Identification of source can potentially identify candidate materials, processes, lifecycle stages, or issues for targeting for chemical mitigation activities.
The analysis presented here is a proof-of-concept; a more representative analysis of products within product category could provide more detailed information regarding likely source. Larger product sample sizes will also allow for the potential implementation of co-clustering algorithms such as those used in the evaluation of microarray data to identify genes that are expressed under a subset of conditions. This will allow for the identification of clusters of co-occuring chemicals that are found in some, but not all, products within a category. This will be especially useful as products within categories were quite diverse. Co-clustering methods are quite computationally intensive when data are noisy, so improvements in the ability to identify chemicals within a sample will also make this approach more tenable.
Identification of co-occurring chemicals is only the first step in any “forensic” analysis of chemicals found in products or in environmental media. Additional information on chemical structure and use is required as further evidence of source. Here we have made use of new high-throughput tools (a taxonomy and a suite of predictive models) for investigating chemical structures and functions. These tools enable us to form hypotheses regarding potential sources. In addition, we have attempted to catalog and investigate additional, specific information about chemical use where available. EPA’s ExpoCast project has recently developed an informatics framework for collecting and curating thousands of additional documents on chemical use, further expanding our existing databases. The data being developed here will further support workflows for characterizing chemical identifications and potential sources.
Prioritization of Chemicals for Further Study Using Screening-Level Risk Metrics
This project was initiated under the Office of Research and Development’s Chemical Safety for Sustainability Program to examine in an innovative, high-throughput manner chemicals that may appear in a variety of recycled consumer articles with the goal of identifying and prioritizing potential chemical exposure sources. Quantitative risk-based metrics (ToxPi and BER scores) were calculated for each of the chemicals identified in this study with the goal of informing prioritization of chemicals for further study (e.g., additional confirmation in the current sample extracts; studies of larger representative sets of product samples; or studies of chemical emission, leaching, or exposure).
Exposure and bioactivity data were available for 1,010 and 528 of the 1,123 unique identified chemicals, respectively; 524 chemicals had both and for those an enhanced ToxPi could be calculated. BER could be calculated for 446 chemicals with available bioactivity, toxicokinetic, and exposure information. All occurrence, abundance, exposure, and bioactivity variables and final ToxPi and BER scores are provided in Supplemental Table S10; the distributions of the prioritization metrics are illustrated in Figure S5.
The five chemicals with the highest enhanced ToxPi scores were 2,4-Di-tert-butylphenol (DTXSID2026602), 2,4-Bis(1-methyl-1-phenylethyl)phenol (DTXSID7029241), N-(1,3-Dimethylbutyl)-N’-phenyl-p-phenylenediamine (DTXSID9025114), 2,2’-Methylenebis(4-methyl-6-tert-butylphenol) (DTXSID4020870), and 4-(1,1,3,3-Tetramethylbutyl)phenol (DTXSID9022360). Of the chemicals without bioactivity and exposure data, the highest scores occurred for several alkanes, for which standards were available and thus confirmed in a large number of samples. Fourteen of twenty chemicals with the highest enhanced ToxPi (Figure S6) had an occurrence ratio>1 (higher prevalence in recycled products). These chemicals had high ToxPi scores due to a mix of high abundance and high bioactivity.
In total, 170 chemicals had a BER of < 1; this indicates that when uncertainty is considered, it is possible that exposure may exceed bioactive dose. The chemicals with the lowest BER values were Octadecanoic acid (DTXSID8021642), 1,2-Propylene glycol (DTXSID0021206), Terephthalic acid (DTXSID6026080), Octanoic acid (DTXSID3021645), Ethylene glycol (DTXSID8020597); these chemicals had both relatively low estimated bioactive doses, and high exposures in the SEEM framework. Fifteen of the 20 chemicals with lowest BER had higher occurrence ratio in recycled products, including 9 chemicals that were only found in recycled products. The BER values provide a general prioritization metric comparing conservative, lower bound bioactive doses with upper bound population exposures. Note that these exposures incorporate data from multiple exposure pathways, and thus are not necessarily reflective of exposures associated with the use of these products. However, they do provide for the ranking of chemicals based on an overall screening-level risk basis. The BER and ToxPi scores do not represent the only way of assessing the priority of chemicals. Other prioritization metrics could be developed that include additional criteria, including additional risk indicators (e.g., bioaccumulation or persistence metrics, or inclusion of chemicals on regulatory lists of interest) or practical factors such as method amenability or availability of standards.
Limitations of the Current Study
In this study, all samples designated “recycled” were chosen based on product packaging specifically stating the presence of at least 50% recycled material. Each sample lacking a statement indicating the inclusion of recycled material was designated “virgin”. As there are no specific requirements for labeling, it is possible that some virgin products could contain recycled material. In addition, product packaging could also be derived from recycled material that may contaminate the surface of a product with compounds not associated with it. Because of this, the potential inclusion of recycled material in virgin products cannot be ruled out.
The number of chemicals detected in this study is certain to be an underestimate of the actual quantity present in the samples. No single analytical method will detect every chemical in a sample. In this case, only chemicals amenable to gas chromatography or present as single substances in the NIST spectral library could be distinguished. For example, Pivnenko et al.7 reported several mineral oil hydrocarbons (MOHs) that are potentially found in recycled paper. However, only one of the MOHs reported was found in the NIST spectral library, as some of those reported were mixtures, and others are more likely to be identified using liquid chromatography. Furthermore, sample extraction was restricted to either the polar dichloromethane solvent or the non-polar hexane/ethyl ether solvent. Other solvents could potentially extract other chemicals, or the same chemicals with greater efficiency (affecting eventual concentration estimates). In addition, the extraction process itself may have been inconsistent across samples. While the samples were cut into small pieces prior to extraction, it is possible that some samples may have been only extracted at the surface. This could cause bias if the materials were not homogeneous. There is also the possibility of excluding chemicals that co-elute with the solvent.
This study utilized a low-resolution GCxGC-TOFMS rather than a high-resolution instrument. A high-resolution instrument would provide more confidence in identification. However, when reviewing full mass spectra (and not just a single ion) sufficient signal is needed to have enough confidence in the identification, and we have found deconvolution algorithms for high resolution data tend to produce errors for low level peaks due to poor ion statistics. Therefore, high-resolution data does not automatically afford an advantage in sensitivity. However, automated recursive high-resolution workflows are being developed which aim to first identify the best, representative mass spectra across a sample set then iteratively process the data using a specified, mass accurate ion at the corresponding retention time. Furthermore, both low and high resolution GCxGC-TOFMS rely principally on low resolution library (e.g., NIST) / pattern search of the fragmentation spectrum.
The current study was designed to investigate only the presence of chemicals in recycled products and develop approaches for investigating and/or attributing chemical sources. Exposures due to chemical presence could eventually be predicted if quantitative estimates of concentrations in product samples could be estimated. Quantitative characterization of chemical concentrations within extracted samples is challenging given the limited availability of authentic standards for probable chemicals. Additional uncertainty is introduced when estimating the concentration in the original sample from that in the extracted sample (given varying extraction efficiencies across unique chemicals, sampled media, and extraction conditions). Considering these limitations, concentrations of individual chemicals in extracted and original samples were not estimated here (although quantitative NTA is a developing area of research within the ExpoCast project). Furthermore, chemical presence in a product matrix (as identified after soxhlet extraction) does not imply leaching or emission of that chemical from the products, nor does it imply direct or indirect exposure to the consumer. Properties such as water-octanol partition coefficients and vapor pressure (Table S10) impact the likelihood that a chemical will emit from a product under normal conditions and migrate into household media such as air or dust, resulting in the potential for exposure. For example, semivolatile compounds known to emit from consumer products and partition into air and dust include phthalates62 like benzyl butyl phthalate63 (found here in 122 products) and flame retardants such as tris(2-ethylhexyl) phosphate64 (26 products). Other compounds may be firmly bound within the product matrix and thus do not migrate. Additional studies are needed that measure and eventually predict (based on product matrix and chemical properties) leachability and emission characteristics. Such models and eventual quantification of concentrations of observed chemicals in select products will enable higher-tier exposure and risk characterization beyond the screening-level prioritization metrics reported here.
Supplementary Material
Figure S1. ClassyFire class categorization of chemicals in recycled and virgin products.
Figure S2. ClassyFire subclass categorization of chemicals in recycled and virgin products
Figure S3. Chemicals with reported functional uses in recycled and virgin products.
Figure S4. Distribution of product chemical occurrence in the key clusters of interest.
Figure S5. Contribution of abundance, detection frequency, bioactivity, and exposure to ToxPi scores for the 20 chemicals with the highest enhanced ToxPi scores.
Figure S6. Contribution of abundance, detection frequency, bioactivity, and exposure to ToxPi scores for the 20 chemicals with the highest enhanced ToxPi scores.
Table S1. Products analyzed and classification (recycled or virgin).
Table S2. Reference standards used in this study, including ToxCast chemicals.
Table S3. Probable and confirmed chemical identifications with chemical list presence information and ClassyFire categories.
Table S4. Product occurrence of unique chemicals identified.
Table S5. Summary of ClassyFire chemical taxonomic categories for recycled and virgin chemicals.
Table S6. Summary of chemical list presence for recycled and virgin chemicals.
Table S7. Reported function and use information for identified chemicals.
Table S8. Occurrence ratios for identified chemicals.
Table S9. Chemical cluster content and reported function and use information.
Table S10. Prioritization metrics (ToxPis and BER) for all identified chemicals.
Synopsis:
Mass spectrometry-based suspect screening methods were used to identify hundreds of chemicals in consumer products made from recycled and virgin materials. This analysis can inform characterization of the chemical sources and human exposures associated with circular product lifecycles.
DISCLAIMER/ACKNOWLEDGEMENT
This research was supported in part by an appointment to the Research Participant Program at the National Exposure Research Laboratory, administered by the Oak Ridge Institute for Science and Education through Interagency Agreement No. 92431601 between the U.S. Department of Energy and the U.S. Environmental Protection Agency. The views expressed in this manuscript are solely those of the authors and do not represent the policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products should not be interpreted as an endorsement by the U.S. Environmental Protection Agency.
REFERENCES
- 1.Wambaugh JF; Setzer RW; Reif DM; Gangwal S; Mitchell-Blackwood J; Arnot JA; Joliet O; Frame A; Rabinowitz J; Knudsen TB; Judson RS; Egeghy P; Vallero D; Hubal EAC, High-Throughput Models for Exposure-Based Chemical Prioritization in the Expo Cast Project. Environmental science & technology 2013, 47, (15), 8479–8488. [DOI] [PubMed] [Google Scholar]
- 2.Wambaugh JF; Wang A; Dionisio KL; Frame A; Egeghy P; Judson R; Setzer RW, High Throughput Heuristics for Prioritizing Human Exposure to Environmental Chemicals. Environmental science & technology 2014, 48, (21), 12760–12767. [DOI] [PubMed] [Google Scholar]
- 3.Zhao YB; Lv XD; Ni HG, Solvent-based separation and recycling of waste plastics: A review. Chemosphere 2018, 209, 707–720. [DOI] [PubMed] [Google Scholar]
- 4.Ragaert K; Delva L; Van Geem K, Mechanical and chemical recycling of solid plastic waste. Waste Manag 2017, 69, 24–58. [DOI] [PubMed] [Google Scholar]
- 5.Miller GZ; Tighe ME; Peaslee GF; Peña K; Gearhart J, Toys, décor, and more: evidence of hazardous electronic waste recycled into new consumer products. Journal of Environmental Protection 2016, 7, (03), 341. [Google Scholar]
- 6.Ionas AC; Dirtu AC; Anthonissen T; Neels H; Covaci A, Downsides of the recycling process: Harmful organic chemicals in children’s toys. Environment International 2014, 65, 54–62. [DOI] [PubMed] [Google Scholar]
- 7.Pivnenko K; Eriksson E; Astrup TF, Waste paper for recycling: Overview and identification of potentially critical substances. Waste Management 2015, 45, 134–142. [DOI] [PubMed] [Google Scholar]
- 8.Turner A; Filella M, Bromine in plastic consumer products - Evidence for the widespread recycling of electronic waste. Science of the Total Environment 2017, 601, 374–379. [DOI] [PubMed] [Google Scholar]
- 9.Hahladakis JN; Velis CA; Weber R; Iacovidou E; Purnell P, An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater 2018, 344, 179–199. [DOI] [PubMed] [Google Scholar]
- 10.Celeiro M; Armada D; Dagnac T; de Boer J; Llompart M, Hazardous compounds in recreational and urban recycled surfaces made from crumb rubber. Compliance with current regulation and future perspectives. Science of the Total Environment 2021, 755. [DOI] [PubMed] [Google Scholar]
- 11.Guzzonato A; Puype F; Harrad SJ, Evidence of bad recycling practices: BFRs in children’s toys and food-contact articles. Environ Sci-Proc Imp 2017, 19, (7), 956–963. [DOI] [PubMed] [Google Scholar]
- 12.Leslie HA; Leonards PEG; Brandsma SH; de Boer J; Jonkers N, Propelling plastics into the circular economy - weeding out the toxics first. Environment International 2016, 94, 230–234. [DOI] [PubMed] [Google Scholar]
- 13.Cabanes A; Fullana A, New methods to remove volatile organic compounds from post-consumer plastic waste. Science of the Total Environment 2021, 758. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS), National Health and Nutrition Examination Survey Data. In U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, 2019. [Google Scholar]
- 15.Health Canada Biomonitoring Content Summary for the Canadian Health Measures Survey: Cycles 1–6 (2007–2019); Ottawa, ON, Canada., 2020. [Google Scholar]
- 16.Park YH; Lee K; Soltow QA; Strobel FH; Brigham KL; Parker RE; Wilson ME; Sutliff RL; Mansfield KG; Wachtman LM; Ziegler TR; Jones DP, High-performance metabolic profiling of plasma from seven mammalian species for simultaneous environmental chemical surveillance and bioeffect monitoring. Toxicology 2012, 295, (1–3), 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vergeynst L; Van Langenhove H; Joos P; Demeestere K, Suspect screening and target quantification of multi-class pharmaceuticals in surface water based on large-volume injection liquid chromatography and time-of-flight mass spectrometry. Anal Bioanal Chem 2014, 406, (11), 2533–2547. [DOI] [PubMed] [Google Scholar]
- 18.Hilton DC; Jones RS; Sjodin A, A method for rapid, non-targeted screening for environmental contaminants in household dust. J Chromatogr A 2010, 1217, (44), 6851–6856. [DOI] [PubMed] [Google Scholar]
- 19.Schymanski EL; Singer HP; Slobodnik J; Ipolyi IM; Oswald P; Krauss M; Schulze T; Haglund P; Letzel T; Grosse S; Thomaidis NS; Bletsou A; Zwiener C; Ibanez M; Portoles T; de Boer R; Reid MJ; Onghena M; Kunkel U; Schulz W; Guillon A; Noyon N; Leroy G; Bados P; Bogialli S; Stipanicev D; Rostkowski P; Hollender J, Non-target screening with high-resolution mass spectrometry: critical review using a collaborative trial on water analysis. Anal Bioanal Chem 2015, 407, (21), 6237–6255. [DOI] [PubMed] [Google Scholar]
- 20.Esslinger S; Riedl J; Fauhl-Hassek C, Potential and limitations of non-targeted fingerprinting for authentication of food in official control. Food Res Int 2014, 60, 189–204. [Google Scholar]
- 21.Riedl J; Esslinger S; Fauhl-Hassek C, Review of validation and reporting of non-targeted fingerprinting approaches for food authentication. Anal Chim Acta 2015, 885, 17–32. [DOI] [PubMed] [Google Scholar]
- 22.Sapozhnikova Y; Hoh E, Suspect Screening of Chemicals in Food Packaging Plastic Film by Comprehensive Two-Dimensional Gas Chromatography Coupled to Time-of-Flight Mass Spectrometry. Lc Gc Eur 2019, 32, (11), 578–591. [Google Scholar]
- 23.Wang YJ; Gao W; Wang YW; Jiang GB, Suspect screening analysis of the occurrence and removal of micropollutants by GC-QTOF MS during wastewater treatment processes. Journal of Hazardous Materials 2019, 376, 153–159. [DOI] [PubMed] [Google Scholar]
- 24.Pourchet M; Debrauwer L; Klanova J; Price EJ; Covaci A; Caballero-Casero N; Oberacher H; Lamoree M; Damont A; Fenaille F; Vlaanderen J; Meijer J; Krauss M; Sarigiannis D; Barouki R; Le Bizec B; Antignac JP, Suspect and non-targeted screening of chemicals of emerging concern for human biomonitoring, environmental health studies and support to risk assessment: From promises to challenges and harmonisation issues. Environment International 2020, 139. [DOI] [PubMed] [Google Scholar]
- 25.Phillips KA; Yau A; Fayela KA; Isaacs KK; McEachran A; Grulke C; Richard AM; Williams AJ; Sobus JR; Thomas RS; Wambaugh JF, Suspect Screening Analysis of Chemicals in Consumer Products. Environmental science & technology 2018, 52, (5), 3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dionisio KL; Phillips K; Price PS; Grulke C; Williams A; Biryol D; Hong T; Isaacs KK, The Chemical and Products Database, a resource for exposure-relevant data on chemicals in consumer products. Scientific Data 2018, 5, (180125), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldsmith MR; Grulke CM; Brooks RD; Transue TR; Tan YM; Frame A; Egeghy PP; Edwards R; Chang DT; Tornero-Velez R; Isaacs K; Wang A; Johnson J; Holm K; Reich M; Mitchell J; Vallero DA; Phillips L; Phillips M; Wambaugh JF; Judson RS; Buckley TJ; Dary CC, Development of a consumer product ingredient database for chemical exposure screening and prioritization. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 2014, 65, 269–79. [DOI] [PubMed] [Google Scholar]
- 28.CFR, Hazard Communication. In 2012. [Google Scholar]
- 29.Phillips KA; Wambaugh JF; Grulke CM; Dionisio KL; Isaacs KK, High-throughput screening of chemicals as functional substitutes using structure-based classification models. Green Chem 2017, 19, (4), 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scruggs CE; Ortolano L; Schwarzman MR; Wilson MP, The role of chemical policy in improving supply chain knowledge and product safety. Journal of Environmental Studies and Sciences 2014, 4, (2), 132–141. [Google Scholar]
- 31.Goodacre R; Vaidyanathan S; Bianchi G; Kell DB, Metabolic profiling using direct infusion electrospray ionisation mass spectrometry for the characterisation of olive oils. Analyst 2002, 127, (11), 1457–1462. [DOI] [PubMed] [Google Scholar]
- 32.Xiao ZB; Yu D; Niu YW; Chen F; Song SQ; Zhu JC; Zhu GY, Characterization of aroma compounds of Chinese famous liquors by gas chromatography-mass spectrometry and flash GC electronic-nose. J Chromatogr B 2014, 945, 92–100. [DOI] [PubMed] [Google Scholar]
- 33.Sacco D; Brescia MA; Sgaramella A; Casiello G; Buccolieri A; Ogrinc N; Sacco A, Discrimination between Southern Italy and foreign milk samples using spectroscopic and analytical data. Food Chem 2009, 114, (4), 1559–1563. [Google Scholar]
- 34.Richard AM; Judson RS; Houck KA; Grulke CM; Volarath P; Thillainadarajah I; Yang C; Rathman J; Martin MT; Wambaugh JF; Knudsen TB; Kancherla J; Mansouri K; Patlewicz G; Williams AJ; Little SB; Crofton KM; Thomas RS, ToxCast Chemical Landscape: Paving the Road to 21st Century Toxicology. Chem Res Toxicol 2016, 29, (8), 1225–51. [DOI] [PubMed] [Google Scholar]
- 35.Schymanski EL; Jeon J; Gulde R; Fenner K; Ruff M; Singer HP; Hollender J, Identifying small molecules via high resolution mass spectrometry: communicating confidence. Environmental science & technology 2014, 48, (4), 2097–8. [DOI] [PubMed] [Google Scholar]
- 36.Feunang YD; Eisner R; Knox C; Chepelev L; Hastings J; Owen G; Fahy E; Steinbeck C; Subramanian S; Bolton E, ClassyFire: automated chemical classification with a comprehensive, computable taxonomy. Journal of cheminformatics 2016, 8, (1), 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isaacs KK; Goldsmith MR; Egeghy P; Phillips K; Brooks R; Hong T; Wambaugh JF, Characterization and prediction of chemical functions and weight fractions in consumer products. Toxicology reports 2016, 3, 723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Organisation for Economic Co-operation and Development Internationally Harmonised Functional Product and Article Use Categories ENV/JM/MONO(2017)14; 2017. [Google Scholar]
- 39.Dionisio KL; Frame AM; Goldsmith MR; Wambaugh JF; Liddell A; Cathey T; Smith D; Vail J; Ernstoff AS; Fantke P; Jolliet O; Judson RS, Exploring consumer exposure pathways and patterns of use for chemicals in the environment. Toxicology reports 2015, 2, 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaccard P, The distribution of the flora in the alpine zone. 1. New phytologist 1912, 11, (2), 37–50. [Google Scholar]
- 41.Kaufman L; Rousseeuw PJ, Finding Groups in Data: An Introduction to Cluster Analysis. Wiley: New York, 1990. [Google Scholar]
- 42.Maechler M; Rousseeuw P; Struyf A; Hubert M; Hornik K cluster: Cluster Analysis Basics and Extensions.
- 43.The R Project for Statistical Computing Version 3.4.0; 2017.
- 44.Rousseeuw PJ, Silhouettes - a graphical aid to the interpretation and validation of cluster-analysis. Journal of Computational and Applied Mathematics 1987, 20, 53–65. [Google Scholar]
- 45.Rager JE; Strynar MJ; Liang S; McMahen RL; Richard AM; Grulke CM; Wambaugh JF; Isaacs KK; Judson R; Williams AJ; Sobus JR, Linking high resolution mass spectrometry data with exposure and toxicity forecasts to advance high-throughput environmental monitoring. Environ Int 2016, 88, 269–280. [DOI] [PubMed] [Google Scholar]
- 46.Paul Friedman K; Gagne M; Loo LH; Karamertzanis P; Netzeva T; Sobanski T; Franzosa JA; Richard AM; Lougee RR; Gissi A; Lee JJ; Angrish M; Dorne JL; Foster S; Raffaele K; Bahadori T; Gwinn MR; Lambert J; Whelan M; Rasenberg M; Barton-Maclaren T; Thomas RS, Utility of In Vitro Bioactivity as a Lower Bound Estimate of In Vivo Adverse Effect Levels and in Risk-Based Prioritization. Toxicol Sci 2020, 173, (1), 202–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ring CL; Arnot J; Bennett DH; Egeghy P; Fantke P; Huang L; Isaacs KK; Jolliet O; Phillips K; Price PS; Shin HM; Westgate JN; Setzer RW; Wambaugh JF, Consensus Modeling of Median Chemical Intake for the U.S. Population Based on Predictions of Exposure Pathways. Environmental science & technology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas RS; Paules RS; Simeonov A; Fitzpatrick SC; Crofton KM; Casey WM; Mendrick DL, The US Federal Tox21 Program: A strategic and operational plan for continued leadership. ALTEX 2018, 35, (2), 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pearce RG; Setzer RW; Strope CL; Sipes NS; Wambaugh JF, httk: R Package for High-Throughput Toxicokinetics. J Stat Softw 2017, 79, (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Confederation of European Paper Industries Sustainability Report 2013; Confederation of European Paper Industries: Brussels, Belgium, 2013. [Google Scholar]
- 51.Geyer R; Jambeck JR; Law KL, Production, use, and fate of all plastics ever made. Sci Adv 2017, 3, (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bajpai P, Recycling and Deinking of Recovered Paper. Elsevier: Waltham, MA, 2013. [Google Scholar]
- 53.Al-Salem SM; Lettieri P; Baeyens J, Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manage 2009, 29, (10), 2625–2643. [DOI] [PubMed] [Google Scholar]
- 54.Turner A, Black plastics: Linear and circular economies, hazardous additives and marine pollution. Environment International 2018, 117, 308–318. [DOI] [PubMed] [Google Scholar]
- 55.Diekmann A; Giese U; Schaumann I, Polycyclic aromatic hydrocarbons in consumer goods made from recycled rubber material: A review. Chemosphere 2019, 220, 1163–1178. [DOI] [PubMed] [Google Scholar]
- 56.Geueke B; Groh K; Muncke J, Food packaging in the circular economy: Overview of chemical safety aspects for commonly used materials. Journal of Cleaner Production 2018, 193, 491–505. [Google Scholar]
- 57.Pivnenko K; Eriksen MK; Martin-Fernandez JA; Eriksson E; Astrup TF, Recycling of plastic waste: Presence of phthalates in plastics from households and industry. Waste Manage 2016, 54, 44–52. [DOI] [PubMed] [Google Scholar]
- 58.Nerin C; Batlle R; Cacho J, Quantitative analysis of pesticides in postconsumer recycled plastics using off-line supercritical fluid extraction GC-ECD. Anal Chem 1997, 69, (16), 3304–3313. [Google Scholar]
- 59.Strangl M; Fell T; Schlummer M; Maeurer A; Buettner A, Characterization of odorous contaminants in post-consumer plastic packaging waste using multidimensional gas chromatographic separation coupled with olfactometric resolution. J Sep Sci 2017, 40, (7), 1500–1507. [DOI] [PubMed] [Google Scholar]
- 60.Welsh JA; Braun H; Brown N; Um C; Ehret K; Figueroa J; Boyd Barr D, Production-related contaminants (pesticides, antibiotics and hormones) in organic and conventionally produced milk samples sold in the USA. Public Health Nutr 2019, 22, (16), 2972–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhatia PS; Iovleff S; Govaert G, blockcluster: An R Package for Model-Based Co-Clustering. J Stat Softw 2017, 76, (9), 1–24. [Google Scholar]
- 62.Eichler CMA; Hubal EAC; Little JC, Assessing Human Exposure to Chemicals in Materials, Products and Articles: The International Risk Management Landscape for Phthalates. Environmental science & technology 2019, 53, (23), 13583–13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Just AC; Miller RL; Perzanowski MS; Rundle AG; Chen QX; Jung KH; Hoepner L; Camann DE; Calafat AM; Perera FP; Whyatt RM, Vinyl flooring in the home is associated with children’s airborne butylbenzyl phthalate and urinary metabolite concentrations. J Expo Sci Env Epid 2015, 25, (6), 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Langer S; Fredricsson M; Weschler CJ; Beko G; Strandberg B; Remberger M; Toftum J; Clausen G, Organophosphate esters in dust samples collected from Danish homes and daycare centers. Chemosphere 2016, 154, 559–566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. ClassyFire class categorization of chemicals in recycled and virgin products.
Figure S2. ClassyFire subclass categorization of chemicals in recycled and virgin products
Figure S3. Chemicals with reported functional uses in recycled and virgin products.
Figure S4. Distribution of product chemical occurrence in the key clusters of interest.
Figure S5. Contribution of abundance, detection frequency, bioactivity, and exposure to ToxPi scores for the 20 chemicals with the highest enhanced ToxPi scores.
Figure S6. Contribution of abundance, detection frequency, bioactivity, and exposure to ToxPi scores for the 20 chemicals with the highest enhanced ToxPi scores.
Table S1. Products analyzed and classification (recycled or virgin).
Table S2. Reference standards used in this study, including ToxCast chemicals.
Table S3. Probable and confirmed chemical identifications with chemical list presence information and ClassyFire categories.
Table S4. Product occurrence of unique chemicals identified.
Table S5. Summary of ClassyFire chemical taxonomic categories for recycled and virgin chemicals.
Table S6. Summary of chemical list presence for recycled and virgin chemicals.
Table S7. Reported function and use information for identified chemicals.
Table S8. Occurrence ratios for identified chemicals.
Table S9. Chemical cluster content and reported function and use information.
Table S10. Prioritization metrics (ToxPis and BER) for all identified chemicals.