Abstract
Naltrexone (NTX) is a well-tolerated drug with a wide safety margin and mechanism of action that affords use across a wide variety of indications in adults and children. By antagonizing the opioid reward system, NTX can modulate behaviors that involve compulsivity or impulsivity, such as substance use, obesity, and eating disorders. Evidence regarding the disposition and efficacy of NTX is mainly derived from adult studies of substance use disorders and considerable variability exists. Developmental changes, plausible disease-specific alterations and genetic polymorphisms in NTX disposition, and pharmacodynamic pathways should be taken into consideration when optimizing the use of NTX in the pediatric population. This review highlights the current state of the evidence and gaps in knowledge regarding NTX to facilitate evidence-based pharmacotherapy of mental health conditions, for which few pharmacologic options exist.
Keywords: adolescent, disposition, naltrexone, ontogeny, pediatric, psychopharmacology, substance use
Introduction
Naltrexone (NTX) is an opioid antagonist initially developed in the 1960s that received FDA approval for treatment of adult opioid addiction in 1984.1 A decade later, it received approval for adult alcohol use disorder.1 More recently, NTX has been used for additional conditions across the lifespan, leading to a steady increase in overall use.2,3 In children and adolescents, NTX is used off-label in the treatment regimen of compulsive and impulsive behavior disorders driven by the opioid reward circuit, such as binge eating, impulsiveness, and non-suicidal self-injury.4–6 Unfortunately, there remains a paucity of data related to NTX safety and efficacy in children and adolescents to inform optimal pediatric dosing recommendations. These recommendations are ideally based on pediatric-specific data involving the dose-exposure-response relationship necessitating the conduct of prospective studies designed that will generate these data. Absent such data, a thorough review of the NTX disposition and response pathways is necessary to identify where ontogeny and genetic variation may have the largest impact on NTX disposition and response to inform future trial design and dosing recommendations.
Pharmacology
Naltrexone is a synthetic opioid antagonist that has a chemical structure similar to that of oxymorphone, with molecular substitution of cyclopropylmethyl for methyl group. Naltrexone also closely resembles the chemical structure of naloxone, a parenterally administered opioid antagonist, yet NTX is more potent, has increased oral bioavailability, and has a longer half-life.7 6-β-naltrexol (6βN) is the primary metabolite (Figure 1) and has 50% to 80% opioid receptor antagonist activity (Table 1). Mechanistically, opioid receptor antagonism prevents activation of the reward pathway (Figure 2) and subsequent dopamine surge responsible for the euphoria associated with opioid administration (e.g., morphine, heroin), pleasure seeking (e.g., food consumption), and compulsive behavior (e.g., gambling, binge eating). Naltrexone can be administered as an oral tablet (Revia, Barr Pharmaceuticals Inc, Pamona, NY) or intramuscular (XR-NTX, Vivitrol, Alkermes Inc, Waltham, MA) injection.8 Of note, studies in children focus on the use of the oral formulation. Intramuscular injection of a depot formulation of NTX is used to treat opioid and alcohol addiction when daily adherence presents a significant barrier to treatment. Intranasal NTX is currently being investigated as a longer-acting alternative to naloxone for acute opioid overdose.9,10 Naltrexone has been well tolerated in adult patients without an increase in serious adverse events compared with placebo, despite a wide dosing range.11–14 Limited data in children related to eating disorders15 and autism16–18 additionally demonstrate no serious adverse events. Nausea is the most common side effect (10%–20%), but it is generally relieved with food or slow titration.15,19,20 Of potential mechanistic concern due to opioid receptor blockade, mood (e.g., anxiety, depression) and sleep disturbances have not been associated with NTX use in adults.3,21
Figure 1.
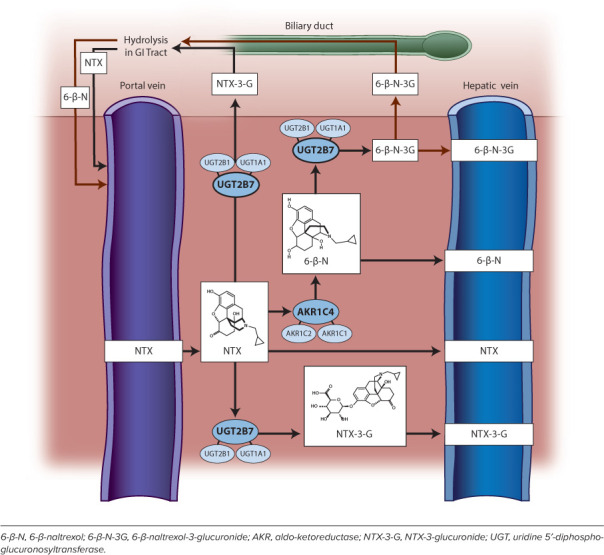
The naltrexone (NTX) disposition pathway in the liver.
Table 1.
| Opioid Receptor | Endogenous Ligand(s) | Primary Effects | NTX Binding Affinity | Relative 6βN Binding Affinity |
|---|---|---|---|---|
| μ | β-endorphin | Euphoria | Referent (0.0825–1 nM) | 35%–50% of NTX (0.74–2.1 nM) |
| κ | Dysnorphin A and B Neoendorphine | Dysphoria Stress Negative affect | 15%–25% of μ (0.509–3.9 nM) | ~50% of NTX (2.0–7.4 nM) |
| δ | Met-enkephalin Leu-enkephalin | Anxiolysis Positive affect | ≤1% of μ (8.02–149 nM) | ~25% of NTX (29–213 nM) |
6βN, 6-β-naltrexol
Figure 2.
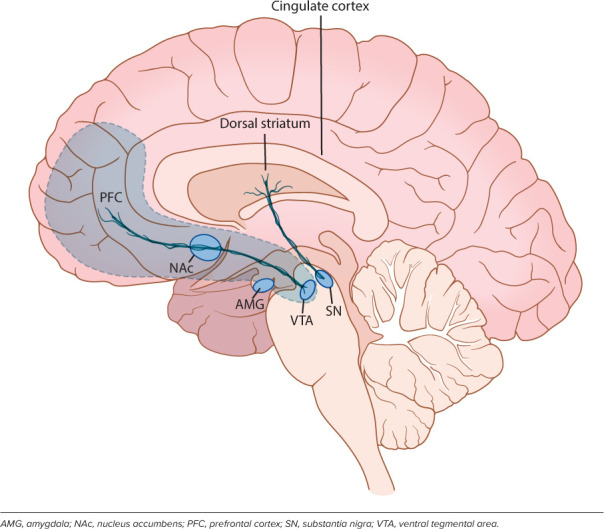
Reward circuitry. Opioid receptors are richly distributed within the reward circuit that includes the mesocorticolimbic pathway (depicted in gray area) and nigrostriatal pathway.
Efficacy in Adults
Clinical trials of NTX in adults have focused primarily on opioid and alcohol use disorders (Tables 2 and 3). For these indications, the long-acting intramuscular formulation (XR-NTX) has outperformed the oral tablet in clinical trials, likely because of adherence challenges with daily oral therapy. Beyond opioid and alcohol use disorder, NTX's use in other behavioral disorders has been investigated, and these limited data are summarized in Tables 4 to 7.
Table 2.
Summary of Naltrexone (NTX) Efficacy and Safety in Adult Trials for Opioid Use
| Reference | Sample Size (Study Design) | Drug: Dosing and Duration | Outcomes |
|---|---|---|---|
| Curran115 | 38 (PCT) | NTX: 6 times a wk for 2 mo, then 3 times a wk for 7 mo | No difference in study completion or treatment effects compared with PLB SAE: none |
| Cornish116 | 51 (PCT) | NTX: 2 times a wk for 6 mo | UDS opioid positive: NTX: 8%; PLB: 30% Probation revoked—return to prison: NTX: 26%; PLB: 56% SAE: none |
| Hollister117 | 192 (PCT) | NTX: 50 mg/day on Monday–Friday and 100 mg/day on Saturday for 8 wk. Then 100 mg on Monday and Wednesday and 150 mg on Friday for 9 mo | Craving score: NTX: −38.0; PLB: −12.9 UDS opioid positive: NTX: 10%; PLB: 33% SAE: none |
| Rawson122 | 132 (PCT) | NTX: 50 mg/day for 2 wk, then 50 mg/day on Monday–Friday and 100 mg on Saturday for 6 wk, then 100 mg on Monday and Wednesday and 150 mg on Friday for 16 wk | UDS opioid positive: NTX: 5.9%; PLB: 28% SAE: none |
| San124 | 50 (PCT) | NTX: 350 mg/wk for up to 1 yr | Treatment completion: NTX: 14.3%; PLB: 36.4% SAE: none |
| Lerner118 | 31 (PCT) | NTX: 350 mg/wk for 2 mo | Opioid free at 1 yr: NTX: 53%; PLB: 38% SAE: none |
| Shufman119 | 32 (PCT) | NTX: 25 mg 2 times a wk for 2 wk, then 50 mg 3 times a wk for 10 wk | Opioid free at 12 wk: NTX: 36%; PLB: 19% Treatment completion: NTX: 50%; PLB: 56% SAE: none |
| Guo128 | 302 (PCT) | NTX: 50 mg/day for 6 mo | Abstinence rate: NTX: 28.6%; PLB: 7.1% SAE: none |
| Krupitsky129 | 52 (PCT) | NTX: 50 mg/day for 6 mo | Relapse rate: NTX: 30%; PLB: 72% Freedom from relapse at 6 mo: NTX: 44%; PLB: 16% SAE: none |
| Krupitsky130 | 280 (PCT) | NTX: 50 mg/day for 6 mo, coadministered with FXT | Relapse rate: NTX + FXT: 30%; NTX: 31%; PLB: 60% SAE: none |
| Stella131 | 56, 4 arms: (PLB, NTX, NTX + PLB, NTX + PZP) | NTX: 50 mg/day ± 10 mg, coadministered PZP for 6 mo | Opioid free at 6 mo: NTX: 43%; NTX + PLB: 43%; NTX + PZM: 86%; PLB: 21% SAE: none |
| Schottenfeld132 | 126 (PCT) | NTX: 350 mg/wk or BPN up to 84 mg/wk or PLB for 24 wk | Freedom from heroin relapse, days (range): BPN: 79 (61–98); NTX: 64 (44–84); PLB: 39 (25–53) SAE: none |
| Krupitsky133 | 250 (PCT) | XR-NTX IM: 380 mg monthly for 24 wk | Abstinent rate (range): XR-NTX: 90% (70%–92%); PLB: 35% (11%–64%) SAE: none |
| Sullivan134 | 60 (ROL) | XR-NTX IM: 380 mg monthly for 24 wk NTX PO: 50 mg/day for 24 wk | Treatment retention rate: XR-NTX: 57%; NTX: 28% SAE: none |
BPN, buprenorphine; FXT, fluoxetine; IM, intramuscular; PCT, placebo-controlled trial; PLB, placebo; PO, orally; PZP, prazepam; ROL, randomized, open label; SAE, serious adverse event; UDS, urine drug screen; XR-NTX, naltrexone intramuscular depot injection
Table 3.
Summary of Naltrexone (NTX) Efficacy and Safety in Adult Trials for Alcohol Use
| Reference | Sample Size (Study Design) | Drug Dosing and Duration | Outcomes |
|---|---|---|---|
| Volpicelli135 | 70 (PCT) | NTX: 50 mg/day for 12 wk | Craving score: NTX: 1.41; PLB: 3.42 SAE: none |
| Oslin136 | 221 (PCT, genotype controlled for variant rs1799971) | NTX: 50 mg/day for 12 wk | Heavy drinking OR: Wildtype: 0.69 (95% CI: 0.41–1.18); Variant: 1.10 (95% CI: 0.52–2.31) SAE: none |
| O’Malley137 | 97 (PCT) | NTX: 50 mg/day plus supportive therapy or coping skills therapy for 12 wk | Abstinence rate (supportive group): NTX: 61%; PLB: 19% SAE: none |
| Anton138 | 1383 (PCT) | NTX: 100 mg/day for 16 wk ± medical management | Days abstinent: NTX: 80% (CV = 33%); PLB: 74% Good clinical outcome (medical management group): NTX: 74%, PLB 58%; NNT: NTX (n = 6) SAE: One possibly related to NTX (not further described) |
| Garbutt139 | 624 (PCT) | XR-NTX: 190 mg or 380 mg IM monthly for 6 mo | Heavy drinking days (relative to PLB): High dose: −25%; Low dose: −17% SAE: NTX group (eosinophilic pneumonia, interstitial pneumonia) |
| Kranzler140 | 315 (PCT) | XR-NTX: IM monthly for 3 mo | Absence of heavy drinking: NTX: 23%, PLB: 16% Abstinence rate: NTX: 18%, PLB: 10% SAE: none |
CV, coefficient of variation; IM, intramuscular; NNT, numbers needed to treat; PCT, placebo-controlled trial; PLB, placebo; PO, orally; SAE, severe adverse effect
Table 4.
Summary of Naltrexone (NTX) Efficacy and Safety in Adult Trials for Obesity and Eating Disorders
| Reference | Sample Size (Study Design) | Drug: Dosing and Duration | Outcomes |
|---|---|---|---|
| Overweight and/or obesity | |||
| Apovian141 | 1496 (PCT) | NTX: 32 mg/day + bupropion 360 mg/day up to 56 wk | Weight: NB: −6.4%, PLB: −1.2% 5% weight loss: NB: 50.5% (CV = 124%); PLB: 17.1% SAE: NB: 2.1% (1 myocardial infarction, 1 seizure), PLB: 1.4% |
| Kolotkin142 | 3362 (PCT) | NTX: 32 mg/day + bupropion 360 mg/day for 56 wk | Weight: NB: −7.0% (CV = 129%); PLB: −2.3% Weight-loss associated QoL score: NB: +11.9, PLB: +8.2 SAE: none |
| Hollander143 | 505 (PCT) | Overweight/obese with type 2 diabetes. NTX: 32 mg/day + bupropion 360 mg/day for 56 wk | Weight: NB: −5.0% (CV = 98%), PLB: −1.8% 5% weight loss: NB: 44.5%; PLB: 18.9% SAE: 3.9% NB vs 4.7% PLB (similar profile to non-diabetic patients) |
| Wadden144 | 793 (PCT) | NTX: 32 mg/day + bupropion 360 mg/day for 56 wk + behavioral modification | Weight: NB: −9.3% (CV 94%), PLB: −5.1% 5% weight loss: NB: 66.4%, PLB: 42.5% (CV = 94%) SAE: none |
| Greenway145 | 1742 (PCT) | NTX: 16 or 32 mg/day + bupropion 360 mg/day for 56 wk | Weight: NB16: −4.9% (CV = 133%); NB32: −6.1% (CV = 107%); PLB: −1.4% 5% weight loss: NB16: 39%; NB32: 48%; PLB: 16% (CV = 133%) SAE: none |
| Malcolm151 | N = 41 (PCT) | Obesity – NTX: 200 mg/day × 8 wk | Weight loss, kg: NTX: 1.8 (CV = 200%), PLB: 1.5 Female: NTX 1.5, PLB 1.5 Male: NTX: 2.6, PLB: 1.4 SAE: NTX: 3 patients had liver transaminases 2 × ULN |
| Mason152 | N = 44 (PCT with crossover) | Obese females – Day 1: PLB; Day 4: NTX 25 mg; Day 7: PLB; Day 10: NTX 50 mg; Day 38: NTX 50 mg | NTX blunted association between reward-based eating drive and food craving (50 mg vs PLB) SAE: none |
| Eating disorders | |||
| Mitchell146 | 16 (PCT with crossover) | BN with normal weight – NTX: 50 mg/day for 3 wk | Binge days/wk: NTX: 4.9 (CV = 106%); PLB: 5.7 Vomit days/wk: NTX: 7.0 (CV = 143%), PLB: 7.6 SAE: none |
| Alger147 | Obese BED: 4; NL weight BN: 28 (PCT) | NTX: Titrate up to 50 mg thrice daily for 6 wk | Bingeing: Obese BED: −70% (SIQR: 21.4%) BN: −30% (SIQR: 15.6%) SAE: none |
| Marrazzi148 | N = 19 (PCT with crossover) | AN and BN – NTX: 100 mg twice daily for 6 wk | Binge and purge symptoms: Reduction in 95% of participants SAE: none |
| Jonas149 | N = 10 (open-label) | Antidepressant-resistant BN – NTX: 300 mg/day for 6 wk | Bulimic symptoms: Reduction of ≥75% in 70% of participants |
| SAE: none | |||
| Jonas150 | N = 16 (Open- label, randomized dosing scheme) | BN – NTX: ≤100 mg/day vs ≥200 mg/day for 6 wk | Binge days: High dose: −5.1 (CV = 150%); low dose: −1.9 (CV = 35%) Purge days: High dose: −3.9; low dose: −1.5 SAE: none |
AN, anorexia nervosa; BED, binge eating disorder; BN, bulimia nervosa; CV, coefficient of variation; NB, NTX/bupropion; NB16, NB + 16 mg of NTX; NB32, NB + 32 mg of NTX; NL, normal PCT, placebo-controlled trial; QoL, quality of life; SIQR, semi-interquartile range; ULN, upper limits of normal
Table 7.
Summary of Naltrexone (NTX) Efficacy and Safety in Adult Trials for Gambling and Other Behavioral Disorders
| Reference | Sample Size (Study Design) | Disease: Dosing and Duration | Outcomes |
|---|---|---|---|
| Ward171 | 14 (case series) | “Problem gamblers” – NTX: 50 mg/day | Gambling behavior/urge: − ≥83% SAE: none |
| Bosco172 | 3 (case series) | Pathologic gambling in Parkinson disease – NTX: 50 mg/day for 6–8 mo | Remission of pathologic gambling: 100% SAE: none |
| Grant173 | 25 (PCT) | Kleptomania – NTX: Titrate up to 150 mg/day for 8 wk | K-YBOCS: NTX: 3.83 (CV = 75%); PLB: 11.46 SAE: none |
| Grant174 | 77 (PCT) | Pathologic gambling – NTX: Up to 150 mg/day for 18 wk | PG-YBOCS: NTX: 9.7 (CV = 84%); PLB: 12.9 Abstinent at 1 mo: NTX: 40%; PLB: 11% SAE: none |
| Kovanen175 | 101 (PCT) | Pathologic gambling – NTX: 50 mg as needed (advised to take if urge to gamble or 30–60 min prior to gambling) for 20 wk | PG-YBOCS: NTX: 10.3 (CV = 74%); PLB: 13.1 SAE: none |
| Papay176 | 50 (PCT) | Impulsive compulsive disorder in Parkinson disease – NTX: 50–100 mg/day for 8 wk | QUIP-RS ICD Δ: NTX: −14.9 (95% CI: −9.9 to −19.9); PLB, −7.5 SAE: none |
| Grant177 | 51 (PCT) | Trichotillomania – NTX: up to 150 mg/day for 8 wk | MGH-PHS score: NTX: 12.2 (CV = 51%); PLB: 13.6 SAE: none |
| Toneatto178 | 52 (PCT) | Alcohol abusing and pathologic gambling – NTX: up to 250 mg/day for 11 wk (following 1-wk placebo run-in) | Gambling frequency: NTX: 11.4 (CV = 101%); PLB: 10.8 SAE: none |
CV, coefficient of variation; K-YBOCS, Yale-Brown Obsessive Compulsive Scale adapted for Kleptomania; MGH-PHS score, Massachusetts General Hospital Hair-Pulling Scale; PCT, placebo-controlled trial; PG-YBOCS, Yale-Brown Obsessive Compulsive Scale adapted for Pathological Gambling; PLB, placebo; QUIP-RS ICD: Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease-Rating Scale; SAE, serious adverse event
Table 5.
Summary of Naltrexone (NTX) Efficacy and Safety in Adult Trials for Self-injurious Behavior (SIB)
| Reference | Sample Size (Study Design) | Disease: Dosing and Duration | Outcomes |
|---|---|---|---|
| Sandman153 | 24 (randomized) | SIB – NTX: 0.5, 1, and 2 mg/kg | SIB: − >50% in >50% participants SAE: none |
| Sonne154 | 5 (open-label) | Females with borderline personality disorder – Wk 1: baseline; Wk 2: NTX 50–100 mg/day; Wk 3: post-NTX | YBOCS (modified for SIB): −48% (CV = 48%) SAE: none |
| Willemsen-Swinkels155 | 33 (PCT with crossover) | Adolescents and adults with intellectual disability – NTX: Titrate up to 150 mg/day for 4 wk | Stereotypic behavior: autistic +3% (CV = 47%); non-autistic +35% (37%) Global function: 50 mg +28%; 150 mg +21% SAE: none |
| Sandman156 | 4 (DB, PCT with crossover) | Males – NTX: 0, 25, 50, 100 mg twice weekly for 4 wk | SIB: −50%; No SIB at 100 mg in 75% SAE: none |
| Kars157 | 6 (PCT with crossover) | Males with intellectual disability – NTX: 50 mg/day for 3 wk | SIB: 40% experienced reduction SAE: none |
| Symons158 | 4 PCT with crossover) | Adults with intellectual disability – NTX: 1.5 mg/kg for 2 wk | SIB: reduction of ≥33% in 75% of participants SAE: none |
| Roth159 | 7 (open-label) | Females with SIB – NTX: 50 mg/day | SIB: cessation in 85% of participants SAE: none |
| Zingarelli160 | 8 (PCT with crossover) | Adults with autism – NTX: 50 mg/day for 3 wk | SIB: +22% over baseline SAE: none |
| Symons161 | 4 (PCT with crossover) | Adult males with intellectual disability – NTX: 1.5 mg/kg/day for 10 wk | SIB: −33% to −54% SAE: none |
| Thompson162 | 8 (PCT with crossover) | Adults with intellectual disability – NTX: 50 mg/day and 100 mg/day x for wk | Head-banging: 67%–77% of participants had reduction Self-biting: 100% of participants had reduction SAE: none |
| Sandman109 | 31 (PCT with crossover) | Adults with intellectual disability – NTX: 0.5, 1.0, 2.0 mg/kg/wk | SIB: participants experiencing ≥25% reduction: 0.5 mg/kg: 47.4% ≥1 mg/kg: 52.6% SAE: none |
PCT, placebo-controlled trial; PLB, placebo; SAE, serious adverse event; SIB, self-injurious behavior; YBOCS, Yale-Brown Obsessive Compulsive Scale
Table 6.
Summary of Naltrexone (NTX) Efficacy and Safety in Adult Trials for Pruritis
| Reference | Sample Size (Study Design) | Disease: Dosing and Duration | Outcomes |
|---|---|---|---|
| Wolfhagen163 | 16 (PCT) | Chronic cholestatic pruritis – NTX: 50 mg/day for 4 wk | Day itching (VAS): NTX: −54% (range, <5–55); PLB: −8% Night itching (VAS): NTX: −44% (range, <5–50); PLB: −7% SAE: none |
| Mansour-Ghanaei164 | 34 (PCT) | Cholestatic pruritis – NTX: 50 mg/day for 1 wk | Day pruritis (VAS): NTX: 7.54 (CV = 52%); PLB: 4.91 Night pruritis (VAS): NTX: 8.29 (CV = 45%); PLB: 5.54 SAE: none |
| Peer165 | 15 (PCT with crossover) | Uremic hemodialysis patients – NTX: 50 mg/day for 7 days | Pruritis (VAS): Baseline: 9.9; NTX: 2.1 (IQR: 1.5–2.15) SAE: none |
| Pauli-Magnus166 | 23 (PCT with crossover) | Uremic hemodialysis and peritoneal dialysis – NTX: 50 mg/day for 4 wk | Pruritis (VAS): NTX: −29.2% (95% CI: 18.7–39.6); PLB: −16.9% SAE: none |
| Malekzad167 | 38 (PCT) | Atopic dermatitis – NTX: 50 mg/day for 2 wk | Pruritis (VAS): NTX: 1.3 (CV = 107%); PLB: 4.5 Remission: NTX: n = 6; PLB: n = 0 SAE: none |
| Legroux-Crespel168 | 52 (RCT) | Uremic hemodialysis – NTX: 50 mg/day for 2 wk; LOR: 10 mg/day for 2 wk | Pruritis score: NTX: 27% of NTX patients had reduction with >3 VAS points SAE: none |
| Terg169 | 20 (PCT with crossover) | Cholestatic pruritis – NTX: 50 mg/day for 2 wk | Day pruritis (VAS): NTX: −56% (CV = 67%) Night pruritis: NTX: −40% (CV = 68%) Pruritus score: 45% of patients had >50% decrease SAE: none |
| Ajayi170 | 12 (RCT) | Chloroquine-induced pruritis – NTX: 50 mg pretreatment for 1 dose; PMZ: 25 mg pretreatment for 1 dose | Parasitic pruritogenic index: NTX: 9.1 (CV = 69%), PMZ: 12.1 SAE: none |
CV, coefficient of variation; LOR, loratadine; PCB, placebo; PCT, placebo-controlled trial; PMZ, promethazine; RCT, randomized controlled trial; SAE, serious adverse event; VAS, visual analog scale
Efficacy in Children
Currently, there are no FDA-approved pediatric indications for NTX, yet off-label use occurs in numerous conditions to target symptoms associated with the opioid reward pathway (Tables 8–11). These conditions range from self-harm to disordered eating to nonopioid or non-alcohol addictive behavior. The clinical indications for NTX are vast, with off-label use in many of these pediatric subpopulations increasing. Despite use of NTX across many indications, response rates are variable. When used in autism, a wide range (40%–80%) of patients reported behavioral benefits.22–25 In adolescents with eating disorders, a little more than 60% demonstrated reduction in binge/purge behaviors.15 Variable response rates are similar for self-injury based on currently available data. One potential contributing factor to variability in response is variable systemic exposure. Indeed, systemic exposure varies up to 10-fold in adults. Pharmacokinetic data in children do not yet exist. With the number of pediatric patients who could potentially benefit from NTX increasing, we need to understand how developmental and genetic factors may alter NTX disposition, thereby contributing to greater variability in systemic exposure and therapeutic response. In addition, it is important to understand disease-specific alterations in pharmacokinetics and pharmacodynamics that may impact the therapeutic outcome. The following sections will discuss these considerations based on available evidence to date.
Table 8.
Summary of Naltrexone (NTX) Efficacy and Safety in Pediatric Trials Involving Substance and Sexual Addiction.
| Reference | Sample Size (Study Design) | Dosing and Duration | Outcome |
|---|---|---|---|
| Miranda179 | 22 (PCT with crossover) | 15- to 19-yr–old adolescents with problem drinking – NTX: 50 mg/day for 8–10 days | Drinking days: NTX: 2.4 (CV = 58%); PLB: 3.1 Heavy drinking days: NTX: 1.1 (CV = 90%); PLB: 1.6 SAE: none |
| Deas19 | 5 (open-label) | Treatment-seeking adolescents with alcohol dependence – NTX: 50 mg/day up to 6 wk | Drinks per drinking days compared with baseline: −7.61 (CV = 13%) SAE: none |
| Hulse180 | 8 (retrospectivecase series) | 15- to 19-yr–old opioid dependent – NTX: 50 mg/day oral followed by NTX implant | Opioid overdose/yr: Implant: 0.19 (SE = 0.13); Oral: 1.9 (SE = 0.74) Baseline: 8.9 SAE: none |
| Fishman181 | 16 (Retrospective case series) | Opioid-dependent adolescents and young adults – NTX: implant | Retained in treatment ≥4 mo: 63% “good” outcome defined as substantially decreased opioid use: 56% SAE: none |
| Ryback183 | 21 (open-label) | 13- to 17-yr adolescents with sexual addiction – NTX: up to 200 mg/day for an average of 12 months (range 4.5–21 months) | Responders: 71% Relapse: occurred in n=13 when NTX tapered ≤50 mg/day |
CV, coefficient of variation; PCT, placebo-controlled trial; PLB, placebo; SAE, serious adverse event
Table 11.
Summary of Naltrexone (NTX) Efficacy and Safety in Pediatric Trials in Prader-Willi syndrome, Crohns Disease, and Complex Regional Pain Syndrome
| Reference | Sample Size (Study Design) | Disease – Dosing and Duration | Outcome |
|---|---|---|---|
| Banga191 | 1 (case report) | Prader-Willi syndrome in a 15-yr-old male – NTX: 50 mg/day for 15 mo | Cessation of skin-picking. Resumption of behavior when NTX stopped and ceased again when NTX was restarted. SAE: none |
| Benjamin192 | 1 (case report) | Prader-Willi syndrome in a 9-yr-old male – NTX: up to 50 mg/day | Reduced food-seeking behavior and skin-picking. Resumption of behaviors occurred when NTX stopped and reduced again when restarted. SAE: none |
| Puri193 | 1 (case report) | Prader-Willi syndrome in a 13-yr-old female – NTX/bupropion: 32 mg/360 mg per day for 6 wk | Weight: 4% loss Aggression and eating habits: reduced BMI: baseline: 33.9; NTX: 32.7 SAE: none |
| Zlotkin194 | 4 (PCT) | Prader-Willi syndrome in a 13 to 17-yr–old obese – NTX: 50 mg twice daily for 7 days | Weight: 1.05-kg gain (CV = 65%) No change in attentiveness, alertness, mood, or nutrient intake. SAE: none |
| Smith195 | 14 (R, PCT) | Crohns Disease in 8- to 17-yr–old with– NTX: 0.1 mg/kg (max 4.5 mg/day) for 8 wk | PCDAI: baseline: 34.2; NTX: 21.7 (CV = 15) Remission: 25% achieved; improved: 67% SAE: none |
| Chopra196 | 1 (case report) | Complex regional pain syndrome in a 12-yr-old – NTX: up to 4.5 mg/day for 18 mo | Pain scores: baseline: 7–10/10 (baseline); NTX: 3–5/10 SAE: none |
BMI, body mass index; CV, coefficient of variation; PCDAI, Pediatric Crohn's Disease Activity Index;; PCT, placebo-controlled trial; PLB, placebo; R, randomized; SAE, serious adverse event
Table 9.
Summary of Naltrexone (NTX) Efficacy and Safety in Pediatric Trials in Self-injury and Autism
| Reference | Sample Size (Study Design) | Dosing and Duration | Outcome |
|---|---|---|---|
| Casner182 | 56 (retrospective case series) | School-aged children in Texas – NTX: 25–300 mg/day for 3–87 mo | Therapy maintenance: 57% SIB: −74% in 25% of objective responders SAE: none |
| Campbell184 | 41 (PCT) | 2.9- to 7.8-yr–old with autism – NTX: Up to 1 mg/kg/day for 3 wk | Hyperactivity score: NTX: −0.22, PLB: −0.36 SAE: none |
| Barrett185 | 1 (PCT) | 12-yr-old with autism and self-injury – NTX dose not reported | “Near zero rate of self-injury” SAE: none |
| Feldman23 | 24 (PCT with crossover) | 3.0- to 8.3-yr–old – NTX: 1.0 mg/kg for 2 wk | Median words produced: NTX: 55 (CV 153%); PLB: 64 Mean words produced: NTX: 12; PLB: 9.5 SAE: none |
| Kolmen25 | 11 (PCT with crossover) | 3- to 8-yr–old – NTX: 1.0 mg/kg | Parent CGI-I: NTX: 3.0 (CV 30%); PLB: 4.0 Teacher CGI-I: NTX: 3.4 (CV 24%); PLB: 4.0 SAE: none |
| Willemsen-Swinkels22 | 23 (PCT with crossover) | 3- to 7-yr–old – NTX: 0.7–1.2 mg/kg/day for 4 wk | Parent CGI-I: 45% favored NTX vs PLB Teacher CGI-I: 65% favored NTX vs PLB 35% deemed “individual drug responders” SAE: none |
| Bouvard186 | 10 (PCT with crossover) | 5- to 14-yr–old – 0.5 mg/kg/day for 1 mo | “Strong” response: 40% No response: 30%–40% SAE: none |
| Campbell187 | 18 (PCT) | 3- to 8-yr–old – NTX: up to 1 mg/kg/day for 21 days | Marked/moderate improvement: NTX: 67%; PLB: 11% SAE: none |
| Leboyer188 | 4 (PCT with crossover) | 4- to 19-yr–old – NTX: 0.5, 1.0, 2.0 mg/kg/day for 7 days | Global improvement and reduced SIB: 75% displayed at small and large doses SAE: none |
| Gonzalez189 | 41 (PCT) | 2.9 to 7.8-yr–old – NTX: up to 1 mg/kg/day for 3 wk | Global Clinical Consensus rating: NTX: marked/moderate improvement in 70% Mean weight change, kg: NTX: −0.16; PLB: −0.02 SAE: none |
| Kolmen24 | 13 (PCT with crossover) | 3- to 8-yr–old – NTX – 1 mg/kg/day × 2 wk | Parent CGI-I: NTX: 3.0 (CV 37%); PLB: 4.3 Teacher CGI-I: NTX: 3.4 (CV 26%), PLB: 4.1 SAE: none |
| Scifo190 | 12 (PCT with crossover) | 7- to 15-yr–old – NTX: 0.5, 1.0, 1.5 mg/kg every 48 hr for 15 wk | Symptoms (based on BSE): −27% (CV 52%) SAE: none |
BSE, Behavioral Summarized Evaluation; CGI-I, Clinical Global Impressions-Improvement; CV, coefficient of variation; PCT, placebo-controlled trial; PLB, placebo; SAE, serious adverse event
Table 10.
Summary of Naltrexone (NTX) Efficacy and Safety in Pediatric Trials in Eating Disorders
| Reference | Sample Size (Study Design) | Dosing and Duration | Outcome |
|---|---|---|---|
| Chatoor93 | 1 (case study, PCT) | 15-yr–old with BN – NTX: Up to 100 mg/day for 8 days | Urge scores: NTX: 1.5 (CV 33%); PLB: 4.5 SAE: none |
| Stancil15 | 33 (retrospective case series) | Adolescents with AN-BP and BN –NTX: up to 100 mg/day, mean duration 129 days | Reduced urges and behaviors: 67% of participants CGI-I score: mean: 2.7 (CV 48%) SAE: none |
| Raingeard12 | 10 (open-label) | Mean age, 22 yr; range, 17–29 yr with type 1 diabetes and BN/binge eating – NTX: 200 mg twice daily up to 1 yr | Purge behaviors: reduced 75% (range, 52%–100%) Weekly binge-eating events: reduced 86% (range, 29%–94%) SAE: none |
AN-BP, anorexia nervosa binge-purge subtype; BN, bulimia nervosa; CGI-I, Clinical Global Impressions-Improvement; CV, coefficient of variation; PCT, placebo-controlled trial; PLB, placebo; SAE, serious adverse event
Efficacy in Non-behavioral Conditions
Low-dose NTX (typically doses ranging from 10- to 50-fold lower than the FDA-approved oral dose for adult opioid and alcohol use disorder) has been trialed for a wide range of conditions, including chronic pain, inflammatory bowel disease, inflammatory skin disease (e.g., Hailey-Hailey, Sjogren syndrome), and multiple sclerosis in adults,26–28 and it appears to be safe and well-tolerated. Table 11 lists studies evaluating the efficacy and safety of naltrexone in non-behavioral conditions in children. Low-dose NTX is hypothesized to act like a Toll-like receptor 4 antagonist to alter immune system response and exhibit anti-inflammatory properties.26,29,30 Prospective, placebo-controlled trials evaluating efficacy are lacking yet are particularly important given high rates of placebo response in pain conditions. Future studies may more clearly elucidate the mechanism of action and efficacy of low-dose NTX.
NTX Disposition
Given the numerous potential indications in the pediatric population, a more comprehensive understanding of NTX's disposition pathway is necessary before widespread adoption occurs in children and adolescents. The following section will discuss the developmental and pharmacogenetic factors that could influence the dose-exposure profile in the pediatric patient, with much of this discussion extrapolated from in vitro and existing adult data. The disposition pathway is summarized in Figure 1.
Physicochemical Considerations. The physicochemical properties of NTX are an important determinant of its disposition and pharmacodynamic effects. Naltrexone is a strong base that is highly lipophilic. Naltrexone's octanol to water partition coefficient and distribution coefficient suggest the ability to readily translocate across the cellular membrane and blood-brain barrier,31,32 thus contributing to an onset of action that is rapid and a duration of action that is prolonged. As a Biopharmaceutics Drug Disposition Classification System Class I drug, high solubility, extensive metabolism, and a minimal role of gut and liver transport-mediated distribution are expected.33
Absorption. With NTX predominantly administered orally in children, the extent of systemic exposure is influenced by drug (e.g., physicochemical properties), biologic (e.g., mechanism of intestinal transport), and patient (e.g., pathophysiologic state of the gastrointestinal tract) factors, which can be influenced by development, genetics, and disease.
Naltrexone undergoes rapid and near complete absorption after oral administration.34,35 This suggests that translocation from the gut lumen to the portal venous circulation occurs via passive diffusion as opposed to transporter-mediated influx and is consistent with predictions based on physiochemical properties.33 6βN, a less lipophilic byproduct of NTX resulting from hepatic reduction by cytosolic aldo-keto reductases,36 could theoretically be subject to transporter-mediated reabsorption during enterohepatic recycling. However, there is currently a paucity of data related the degree of 6βN recirculation or its affinity for transporter-mediated translocation. Given 6βN's contribution to opioid antagonism, its candidacy for transporter-mediated cellular translocation (in both intestine and brain) must be investigated as a potential additional source of variability in NTX response.
Despite nearly complete absorption, the absolute bioavailability of NTX is low (~5%–40%) because of a high hepatic extraction ratio and extensive first-pass metabolism.34,35 Naltrexone is not a substrate for enterocyte efflux transporters, such as P-glycoprotein, breast cancer resistance protein, or multidrug resistance–associated protein (MDR2), known to attenuate absorption.37–39 Developmental and genetic factors may contribute to variability in bioavailability through their impact on metabolizing enzymes responsible for first-past metabolism.
Oral absorption of NTX may be impacted by the baseline physiologic state or pathophysiologic state it is treating. For example, delayed gastric emptying and total gut transit time, known to reduce drug absorption,40 occur in patients with eating disorders.41 Yet, the impact of these gastrointestinal alterations on NTX absorption has not yet been investigated in an eating disorder population. An altered microbiome associated with anorexia nervosa, obesity, and autism spectrum disorder42–44 may affect the extent of enterohepatic recycling. It is unclear whether microbiome alterations lead to clinically meaningful changes in systemic exposure because no pharmacokinetic data in this patient population are available.
Distribution. Naltrexone is widely distributed throughout the body, as evidenced by an average volume of distribution (Vd; ~1350 L) that greatly exceeds intravascular volume and total body water stores.1 Based on the physicochemical properties of NTX, hepatic uptake and peripheral tissue distribution likely occur by passive diffusion. Naltrexone easily passes the blood-brain barrier with a partition coefficient that exceeds that of morphine31 and evidence of μ receptor (MOR) occupancy in the human brain by <8 hours after a single oral dose and persisting for >48 hours.11,45 Substantial interindividual variability in NTX Vd has been demonstrated and may be partially explained by its lipophilic properties. Although conventional wisdom assumes that highly lipophilic drugs are more widely sequestered in conditions of relative high body fat (e.g., obesity), no consistent relationship between Vd and body fat percentage has been appreciated. This may be due in part to sequestering of the drug in body fat with unpredictable release back into the plasma, leading to an inconsistent alteration in systemic exposure.46 Developmental changes in total body fat relative to total body water may contribute to variability in NTX distribution in young children. The total body water to fat ratio is much higher in infants and normalizes around 2 to 3 years of age.47,48 It remains unclear whether the gradual increase in total body fat as a result of development leads to a correlative increase in NTX Vd. Disease-specific changes in total body fat may be dynamic (e.g., anorexia nervosa) and compound the challenges of understanding the relationship between total body fat and Vd. Low body fat seen in acute anorexia nervosa resolves with weight restoration and may contribute to intervariability and intravariability in the Vd49–51; however, the influence of disease state on NTX Vd in children and adolescents remains unknown. The impact of total body weight on the Vd is also not well understood, but some insight may be gleaned from studies of structurally similar compounds (e.g., morphine). A study of intravenous morphine administered to morbidly obese adults and healthy-weight controls demonstrated that obesity was associated with variability in morphine Vd but did not impact systemic exposure or elimination.52 Collectively, many drug and patient factors could alter Vd, but it is unclear if this variability in exposure impacts the NTX response at the level of the individual pediatric patient.
With minimal protein binding of NTX (~20%),1,11 age-and disease-related changes in plasma proteins (e.g., albumin, α-1-acid glycoprotein) and/or binding affinity are not expected to have a meaningful impact on the observed variability in disposition and systemic exposure. Plasma proteins increase in the quantity and binding affinity after birth, approaching adult levels within 6 months to 3 years of life.53–55 The impact of pediatric disease states on plasma protein concentration is controversial. For example, in anorexia nervosa, systemic albumin-binding and sex hormone–binding globulin concentrations were not significantly different compared with healthy controls.56,57 However, albumin was notably reduced in a subset of anorexia nervosa patients with the lowest body mass index, suggesting there is a threshold at which albumin declines. In a study of hospitalized pediatric patients, approximately 10% had moderate to severe protein-energy imbalance, and a quarter of patients had serum albumin levels <30 g/L.58 Although changes in plasma proteins are not likely to affect NTX distribution, the protein-binding affinity of the active metabolite, 6βN, is unknown and requires further elucidation.
Metabolism. Naltrexone undergoes extensive bio-transformation into the major metabolite 6βN and the minor metabolites 2-hydroxy-3-O-methyl-6-β-naltrexol and 3-O-methyl-6-β-naltrexol, 2-hydroxy-3-methyl-naltrexone.1,59–61 Conjugated forms of NTX and metabolites are also observed and exceed the concentration of unconjugated NTX in plasma and urine.35,59
First-pass metabolism after oral administration leads to reduced bioavailability of 5% to 40% of the parent drug. The maximum plasma concentration of NTX is highly variable (~10-fold) in adults.13,34,62 Plasma concentrations of the primary metabolite, 6βN, exceed that of the parent by approximately 10- to 40-fold, with less variability demonstrated (~4-fold).63 When NTX is administered in an intramuscular depot formulation (thus bypassing first-pass metabolism), 6βN levels more closely approach the parent,13 suggesting a significant impact of first pass on 6βN formation.
6βN is formed through hepatic metabolism. In vitro, 6βN formation was restricted to the cytosol and was not detected in the liver microsomal fraction, suggesting no contribution from cytochrome p450.36 Naltrexone use in heroin addicts did not affect antipyrine metabolism, a non-specific probe of CYP450 enzymes, compared with baseline and healthy controls.64 The ability of NTX to affect medications metabolized through specific CYP450 enzymes has been investigated in vitro. Evidence suggests that NTX may have a modest inhibitory effect (~30%) on CYP2C9, CYP2D6, and CYP3A4, and no effect on CYP1A2.65 CYP2D6 and CYP2C19 are involved in the metabolism of many psychiatric medications, such as selective serotonin reuptake inhibitors, selective norepinephrine reuptake inhibitors, and antipsychotics, that may be taken concomitantly with NTX and are not likely to affect NTX metabolism, but these effects have not been investigated in human trials.
In the presence of the ketone reductase inhibitor menadione, 6βN formation was greatly diminished.66 Further analysis identified the aldo-keto reductase 1C (AKR1C) family, specifically AKR1C4, as the major enzyme responsible for 6βN formation, followed by minor contributions from AKR1C1 and AKR1C2.67,68
The AKR1C family is a group of cytosolic enzymes, formerly known as dihydrodiol dehydrogenases, also involved in endogenous steroid biotransformation (e.g., testosterone). AKR1C4 is liver specific, whereas AKR1C2 and AKR1C1 are found in other tissues, including the brain and reproductive organs.69,70 Developmental alterations in this metabolic pathway may contribute to variability in exposure in children and adolescents; however, the ontogenic pattern of AKR1C enzymes in humans is currently unknown. The utility of ontogenic data from animal studies is limited by increased plasticity and catalytic activity in the single-isoform AKR1C9 compared with individual human AKR1C isoforms.71 Although murine AKR1C9 was found to have activity in fetal rat osteo-blasts, it is difficult to interpret the human relevance.72 Although exceedingly variable among adult patients (128-fold), stable 6βN/NTX ratios in urine over time argue against autoinduction of metabolism.11,36,73 Liver X Receptor (LXRα), a nuclear receptor activated by bile acid metabolites, induces AKR1C4 gene expression.74 AKR1C enzymes are inhibited by sex hormones (e.g., testosterone), although it is unlikely that any clinically relevant inhibition exists given the high concentrations required to achieve an effect (e.g., >10-fold higher than the upper limit of normal in males).36 This suggests against a sex hormone–dependent effect on NTX metabolism.75 After oral administration, no sex-related difference in NTX clearance has been observed.13 In a small study in healthy adults receiving long-acting intramuscular NTX, the maximum plasma concentrations of both NTX and 6βN were 30% lower in females, whereas area under the curve ranged from 15% lower to 30% higher compared with males. Sex differences in muscle capillary density have not been appreciated.76 The mechanism responsible for possible reduced exposure XR-NTX in females remains unclear. Of note, sex differences in children, particularly comparing those that are prepubertal vs postpubertal, have not been explored.
The impact of AKR1C genetic variability on NTX variability is largely unknown. Two single-nucleotide polymorphisms (rs17134592 and rs3829125) leading to the missense mutations L311V and S145C, in AKR1C4 show decreased catalytic activity toward NTX when expressed in recombinant enzymes in vitro.77 L311V and S145C mutations are fairly common in the population sharing the mean allele frequencies of 10% to 51%.78,79 It remains unclear whether polymorphisms impact NTX biotransformation in vivo, but this merits further elucidation.
Phase II UDP-glucuronosyl transferase (UGT)–catalyzed conjugation is the mechanism by which the water solubility of NTX and 6βN is increased to enhance renal elimination.80,81 UGT2B7 appears to be the predominant isoform responsible for NTX and 6βN glucuronidation, with minor contributions from UGT1A1 and UGT2B1.80,82,83 The ontogenic pattern of UGT2B7 has been characterized using the probe substrates morphine84 and naloxone, a structurally similar oxymorphone analogue,85 where drug clearance is diminished in neonates compared with adults. Expression of UGT2B7 in pediatric liver tissue appears to be age dependent. There is a rapid increase in expression through infancy into young childhood, achieving 50% abundance of adult levels at approximately 3 years of age.86 Expression is not significantly different in early childhood through adolescence, but there is a 2.5-fold increase in activity in adults compared with adolescents.86 It remains unknown whether the ontogeny of UGT2B7 significantly affects the disposition of NTX clinically; however, application of ontogeny to morphine physiologically based pharmacokinetic models to children older than 1 year did not significantly alter morphine exposure prediction, suggestive of minimal changes to UGT2B7 expression in children ages 1 to 18 years.86 Commonly occurring genetic variants in UGT2B7 have not demonstrated a significant impact on protein abundance or activity in vitro.86 Genotype-informed in vivo studies that use specific UGT2B7 probe substrates will be needed to determine whether meaningful differences in activity exist.
Body weight–related changes on UGT2B7 activity are not well described. Looking again at the structurally similar compound, morphine, it appears that increasing total body weight (in morbidly obese adults) appears to only minimally impact formation of UGT2B7-dependent conjugates. It is unclear if this is clinically meaningful or will prove to be mirrored with NTX.52
Adults with severe liver disease, specifically those who were Child-Pugh Classes B and C, had 5- and 10-fold higher systemic exposure (i.e., area under the curve), respectively, than healthy adults.1 Interestingly, systemic exposure to 6βN did not differ among these groups. This suggests the need for caution and potential dose adjustment if NTX is required in the setting of chronic liver disease or cirrhosis.
Excretion. Most of NTX and metabolites is renally cleared (>95%).11 Naltrexone (free and conjugated) represents 5% to 20% of the total analyte recovered in the urine, with 6βN comprising the majority (~35%–60%). The parent is primarily excreted in its conjugated form (~95%), whereas 6βN is primarily excreted unconjugated (~65%).87 The minor metabolite, 2-hydroxy-3-methoxy 6-β-naltrexol, accounts for ~10% of the total drug excreted in the urine.35,59 Renal clearance of unconjugated NTX appears to occur primarily through glomerular filtration.87 Tubular secretion is involved in excretion of the metabolites 6βN, NTX-conjugate, and 6βN-conjugate. Glomerular filtration rate, a marker of renal clearance, is reduced in neonates and infants, but it approaches adult values by 1 to 2 years of age.88 If used in very young children (ages <2 years), as has been reported in a patient with Prader-Willi syndrome, the impact of reduced renal function on NTX elimination should be considered when selecting dose and dosing interval. Naltrexone has not been well studied in patients with renal impairment; thus, caution is advised in older children and adolescents with known renal disease. One small study in adults with end-stage renal disease requiring hemodialysis described ~5-fold increase in maximal exposure compared with historical healthy controls.89 The effect of concomitant drugs that alter tubular secretion (e.g., probenecid, methotrexate, indomethacin, chlorothiazide) on total NTX excretion is not known.90
Renal transporters are not known to play a significant role in NTX excretion. Investigations to date have evaluated organic cation transporter (OCTs) 1 to 3, OCTN1-2, and found that NTX is not a substrate. Naltrexone is also not a substrate for the efflux transporters breast cancer resistance protein MDR2.9,37,39 Less than 5% of the dose is recovered in stool at 24 hours after acute or chronic use.35 It is unclear whether laxative use associated with purge behavior in adolescents with eating disorders would impact excretion of NTX, although it is not likely given the minimal fraction recovered in fecal matter.
NTX Pharmacodynamics
Mechanism of Action. Although the variable response rates observed in children may be due to developmental or genetic factors influencing disposition and systemic exposure, it is also important to consider developmental alterations or genetic variation at the site of action, which may contribute to variable response.91,92 Naltrexone exerts its primary mechanism of action by blocking opioid receptors non-selectively. There are 3 main types of opioid receptors: μ (MOR), κ (KOR), and δ (DOR), each with a distinct set of downstream effects (Table 1) located throughout the brain and nervous system, particularly concentrated in the mesocorticolimbic pathway (Figure 2).
Naltrexone is most potent at the MOR (Table 1). Duration of MOR antagonism is dose dependent.1,93,94 A linear correlation of NTX and 6βN systemic exposure and MOR occupancy has not been observed in humans.45 Long residence times for NTX or 6βN at the MOR may contribute to the lack of correlation between plasma concentrations and receptor occupancy. The plasma exposure-response relationship for NTX remains unclear in adults and is unknown in children. The active metabolite 6βN may be a more potent antagonist peripherally (e.g., affecting gastrointestinal motility) than centrally (e.g., affecting analgesia and pupil constriction),95,96 suggesting the ability to rescue opioid-induced delay in gut transit time without significant interference with analgesia. Insight of MOR ontogeny is limited to murine models, which show MOR expression at birth with a gradual increase in receptor expression and binding capacity through adulthood.97–99 The clinical implications of MOR ontogeny on NTX response remain unclear and require prospective evaluation.
The impact of genetic variation in OPRM1, encoding the MOR, in predicting response to NTX is limited to alcohol use disorders. A single-nucleotide polymorphism, rs1799971 (sometimes referred to as OPRM1 A118G), decreases MOR expression100 and alters the binding capacity of β-endorphins.101 Clinically, rs1799971 was associated with the decreased daily consumption of alcohol in those administered NTX, but it did not affect long-term metrics of sobriety and relapse.102 Conversely, another evaluation in more than 600 adults with alcohol use disorder found that only carriers of rs1799971 prescribed NTX were more likely to have a good clinical outcome that exceeded placebo response.103 Currently, there are no data to ascertain the prognostic or predictive performance of opioid receptor single-nucleotide polymorphisms in NTX efficacy beyond addiction, including the aforementioned pediatric indications.
Although less studied, alterations in other opioid receptors may play a role in NTX's response. Naltrexone is less potent at the KOR and DOR compared with MOR (Table 1). Ontogenic patters in murine models demonstrate the presence of KOR at birth, whereas the DOR is detectable 2 weeks postnatal.97 Similarly to the ontogenic pattern in MOR, there is a gradual increase in receptor binding for DOR in rats from infancy to adolescence, followed by a plateau extending to adulthood. The κ opioid receptor has an even more gradual incline, with a peak in adolescence and then a tailing off to adulthood.97,98 The impact of genetic variation in OPRK1 and OPRD1, the genes encoding for KOR and DOR, respectively, on NTX response is limited. OPRK1 (rs963549; rs997917) and OPRD1 (rs678849; rs4654327) alone or in combination have been associated with various outcomes in NTX-treated alcohol use disorder, such as days to relapse and alcohol craving104,105; however, further studies are needed to characterize the role of OPRK1 and OPRD1 in modulating NTX response.
Exposure Targets and Biomarkers. Having established pharmacokinetic exposure targets can inform the dosing strategy and reduce interindividual variability. To date, data regarding exposure targets for NTX rest solely in the adult addiction literature. Naltrexone plasma concentrations of ≥2 ng/mL provide sufficient opioid blockade to heroin challenge.11 6βN plasma concentrations of >40 ng/mL prevent relapse in adult alcoholics.63 Certainly, more studies are needed to characterize the relationship between NTX exposure and clinical response including and beyond the addiction space. This may prove particularly challenging given that the action at the opioid receptor appears to exceed duration detectable in the plasma.
Pharmacodynamic biomarkers that shed light on the probability of response hold promise to aid clinicians in choosing the right drug for the right patient, particularly when response is variable among individuals with the same indication. Given NTX's mechanism of action, it is reasonable to speculate that levels of endogenous endorphins (e.g., β-endorphin, enkephalin) may vary at baseline and could provide insight into treatment sensitivity or response. As expected, NTX alters β-endorphin in plasma and cerebral spinal fluid,93,106,107 and limited data suggest an association between NTX response and β-endorphin levels.108–110 Opioid receptors also play a role in stress response, particularly cortisol release. Naltrexone alters cortisol levels, an effect potentially modulated by OPRM1 A118G genotype and sex.111 At this time, no established or widely accepted biomarkers of NTX response exist. Future research is needed to characterize the utility of biomarkers in identifying patients that may benefit from NTX.
Practical Issues for Off-Label NTX Use in Children and Adolescents
Although a legitimate need for NTX exists in the pediatric population, access to the drug remains mired in complexity.112–114 Payors have implemented various measures to ensure judicious use of drugs labeled for alcohol and opioid use disorders. These criteria have been reframed and refined in response to the Mental Health Parity and Addiction Equity Act legislation, Affordable Care Act coverage mandates, and the more recent opioid epidemic.115–117 Between 2010 and 2018, NTX prescribing has increased 8.5-fold, from 64,000 to 549,000 prescriptions, and XR-NTX prescribing nearly 30-fold, from 7474 to 216,561 prescriptions.118,119 Notably, the indications for substance use disorder also shape the criteria under which these drugs can be accessed. Across the Medicaid system, NTX and XR-NTX carry preferred status in 44 and 34 US states and territories, respectively; prior authorization requirements are required in 8 and 19 US states and territories for NTX and XR-NTX, respectively; and at least 5 and 16 US states and territories impose quantity or dosing limitations for NTX and XR-NTX, respectively, although data for this latter statistic were missing for at least one third of states.117 These dosing limits are of particular relevance for the treatment of eating disorders where the doses that are demonstrated to be effective are more than double those approved for treating alcohol and drug dependence.
Access to NTX and XR-NTX through private insurance is more heterogenous.120 Although spending for inpatient substance abuse treatment has dropped substantially among private payors, there has not been a commensurate increase in outpatient spending, and the uptake of medication-assisted treatment, under which NTX and XR-NTX fall, has been slow.121,122 In fact, studies published from 2010 to 2015 report that only 17.3% of national substance use disorder treatment centers offered NTX, 9.1% offered XR-NTX, and their corresponding use rates are substantially lower.123,124 Accordingly, pediatric providers are likely to incur challenges when attempting to access these medications for their patients. Definitive pediatric dosing guidance for NTX is lacking. However, Table 3 summarizes the dosing regimens previously used in various pediatric populations. Given the limited number of controlled trials, there remains a knowledge gap on optimal dosing within the pediatric population.
Pediatric-friendly NTX formulations are currently nonexistent, with only 50-mg tablets readily available. For individuals who require lower or more flexible dosing or for those who are unable to swallow pills, there are some reports of successful compounding of NTX into a liquid formulation. Naltrexone powder or commercial tablets have been compounded with preservatives (e.g., ascorbic acid, sodium benzoate) or a commercially available taste-masking suspension agent (SyrSpend SF PH4 liquid, Fagron US, St Paul, MN), with stability up to 90 days if stored at 4°C in the dark.125,126 There is one report of crushing commercially available tablets with orange juice to yield a 1 mg/mL solution; however, stability data for this particular formulation are not available.127 Without taste-masking, compounded NTX is described as bitter and gritty; thus, formulation palatability should not be forgotten, particularly for pediatric patients.
Conclusion
Naltrexone is a well-tolerated drug with a wide safety margin and mechanism of action that affords use across a wide variety of indications in adults and children. In addition to substance use disorders, NTX shows promise in treating conditions for which few therapeutic options exist, such as obesity, compulsivity, and eating disorders. To date, evidence regarding the disposition and efficacy of NTX is mainly derived from adult studies of substance use disorders, and considerable variability exists. Developmental changes, plausible disease-specific alterations, and genetic polymorphisms in NTX disposition and pharmacodynamic pathways should be taken into consideration when optimizing the use of NTX in the pediatric population.
In this review, the current state of the evidence has been detailed to inform the clinician. Gaps in knowledge have been highlighted to support opportunities for future research. Taken together, the information reviewed will facilitate evidence-based pharmacotherapy of mental health conditions with complex etiologies and multifaceted treatment.
Acknowledgments
The authors would like to thank Brenda L. Bunch for working with us to create the illustrations for Figure 1 and Figure 2.
ABBREVIATIONS
- 6βN
6-β-naltrexol
- AKR
aldo-keto-reductase
- DOR
δ opioid receptor
- FDA
US Food and Drug Administration
- KOR
κ opioid receptor
- MOR
μ opioid receptor
- NTX
naltrexone
- UGT
UDP-glucuronosyl transferase
- Vd
volume of distribution
- WT
wild type
Footnotes
Disclosures. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. Stephani L. Stancil is supported by a grant from the Eunice Kennedy Shriver National Institute for Child Health and Human Development (T32 ND069038).
Ethical Approval and Informed Consent. Given the nature of this study, the work was exempt from institution review board/ethics committee review.
References
- 1.Pomona, NY: Barr Pharmaceuticals Inc; 2013. Naltrexone Revia [package insert] Accessed September 17, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/018932s017lbl.pdf. [Google Scholar]
- 2.Hadland SE, Wharam JF, Schuster MA et al. Trends in receipt of buprenorphine and naltrexone for opioid use disorder among adolescents and young adults, 2001–2014. JAMA Pediatr. 2017;171(8):747–755. doi: 10.1001/jamapediatrics.2017.0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latif ZE, Solli KK, Opheim A et al. No increased pain among opioid-dependent individuals treated with extended-release naltrexone or buprenorphine-naloxone: a 3-month randomized study and 9-month open-treatment follow-up study. Am J Addict. 2019;28(2):77–85. doi: 10.1111/ajad.12859. [DOI] [PubMed] [Google Scholar]
- 4.Frank GK. Altered brain reward circuits in eating disorders: chicken or egg? Curr Psychiatry Rep. 2013;15(10):396. doi: 10.1007/s11920-013-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heal DJ, Hallam M, Prow M et al. Dopamine and mu-opioid receptor dysregulation in the brains of binge-eating female rats--possible relevance in the psychopathology and treatment of binge-eating disorder. J Psychopharmacol. 2017;31(6):770–783. doi: 10.1177/0269881117699607. [DOI] [PubMed] [Google Scholar]
- 6.Selleck RA, Baldo BA. Feeding-modulatory effects of mu-opioids in the medial prefrontal cortex: a review of recent findings and comparison to opioid actions in the nucleus accumbens. Psychopharmacology (Berl) 2017;234(9–10):1439–1449. doi: 10.1007/s00213-016-4522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin WR, Jasinski DR, Mansky PA. Naltrexone, an antagonist for the treatment of heroin dependence: effects in man. Arch Gen Psychiatry. 1973;28(6):784–791. doi: 10.1001/archpsyc.1973.01750360022003. [DOI] [PubMed] [Google Scholar]
- 8.Sudakin D. Naltrexone: not just for opioids anymore. J Med Toxicol. 2016;12(1):71–75. doi: 10.1007/s13181-015-0512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krieter P, Chiang CN, Gyaw S et al. Pharmacokinetic interaction between naloxone and naltrexone following intranasal administration to healthy subjects. Drug Metab Dispos. 2019;47(7):690–698. doi: 10.1124/dmd.118.085977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krieter P, Gyaw S, Chiang CN et al. Enhanced intranasal absorption of naltrexone by dodecyl maltopyranoside: implications for the treatment of opioid overdose. J Clin Pharmacol. 2019;59(7):947–957. doi: 10.1002/jcph.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verebey K. The clinical pharmacology of naltrexone: pharmacology and pharmacodynamics. NIDA Res Monogr. 1981;28:147–158. [PubMed] [Google Scholar]
- 12.Raingeard I, Courtet P, Renard E, Bringer J. Naltrexone improves blood glucose control in type 1 diabetic women with severe and chronic eating disorders. Diabetes Care. 2004;27(3):847–848. doi: 10.2337/diacare.27.3.847. [DOI] [PubMed] [Google Scholar]
- 13.Dunbar JL, Turncliff RZ, Dong Q et al. Single- and multiple-dose pharmacokinetics of long-acting injectable naltrexone. Alcohol Clin Exp Res. 2006;30(3):480–490. doi: 10.1111/j.1530-0277.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- 14.Bolton M, Hodkinson A, Boda S et al. Serious adverse events reported in placebo randomised controlled trials of oral naltrexone: a systematic review and meta-analysis. BMC Med. 2019;17(1):10. doi: 10.1186/s12916-018-1242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stancil SL, Adelman W, Dietz A, Abdel-Rahman S. Naltrexone reduces binge eating and purging in adolescents in an eating disorder program. J Child Adolesc Psychopharmacol. 2019;29(9):721–724. doi: 10.1089/cap.2019.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herman BH, Asleson GS, Powell A et al. Cardiovascular and other physical effects of acute administration of naltrexone in autistic children. J Child Adolesc Psychopharmacol. 1993;3(3):157–168. doi: 10.1089/cap.1993.3.157. [DOI] [PubMed] [Google Scholar]
- 17.Herman BH, Hammock MK, Egan J et al. Role for opioid peptides in self-injurious behavior: dissociation from autonomic nervous system functioning. Dev Pharmacol Ther. 1989;12(2):81–89. [PubMed] [Google Scholar]
- 18.Campbell M, Overall JE, Small AM et al. Naltrexone in autistic children: an acute open dose range tolerance trial. J Am Acad Child Adolesc Psychiatry. 1989;28(2):200–206. doi: 10.1097/00004583-198903000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Deas D, May MP, Randall C et al. Naltrexone treatment of adolescent alcoholics: an open-label pilot study. J Child Adolesc Psychopharmacol. 2005;15(5):723–728. doi: 10.1089/cap.2005.15.723. [DOI] [PubMed] [Google Scholar]
- 20.Yeomans MR, Gray RW. Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev. 2002;26(6):713–728. doi: 10.1016/s0149-7634(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 21.Latif ZE, Šaltyte Benth J, Solli KK et al. Anxiety, depression, and insomnia among adults with opioid dependence treated with extended-release naltrexone vs buprenorphine-naloxone: a randomized clinical trial and follow-up study. JAMA Psychiatry. 2019;76(2):127–134. doi: 10.1001/jamapsychiatry.2018.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willemsen-Swinkels SH, Buitelaar JK, van Engeland H. The effects of chronic naltrexone treatment in young autistic children: a double-blind placebo-controlled crossover study. Biol Psychiatry. 1996;39(12):1023–1031. doi: 10.1016/0006-3223(95)00297-9. [DOI] [PubMed] [Google Scholar]
- 23.Feldman HM, Kolmen BK, Gonzaga AM. Naltrexone and communication skills in young children with autism. J Am Acad Child Adolesc Psychiatry. 1999;38(5):587–593. doi: 10.1097/00004583-199905000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Kolmen BK, Feldman HM, Handen BL, Janosky JE. Naltrexone in young autistic children: a double-blind, placebo-controlled crossover study. J Am Acad Child Adolesc Psychiatry. 1995;34(2):223–231. doi: 10.1097/00004583-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Kolmen BK, Feldman HM, Handen BL, Janosky JE. Naltrexone in young autistic children: replication study and learning measures. J Am Acad Child Adolesc Psychiatry. 1997;36(11):1570–1578. doi: 10.1016/S0890-8567(09)66567-9. [DOI] [PubMed] [Google Scholar]
- 26.Patten DK, Schultz BG, Berlau DJ. The safety and efficacy of low-dose naltrexone in the management of chronic pain and inflammation in multiple sclerosis, fibromyalgia, Crohn's disease, and other chronic pain disorders. Pharmacotherapy. 2018;38(3):382–389. doi: 10.1002/phar.2086. [DOI] [PubMed] [Google Scholar]
- 27.Jaros J, Lio P. Low dose naltrexone in dermatology. J Drugs Dermatol. 2019;18(3):235–238. [PubMed] [Google Scholar]
- 28.Ekelem C, Juhasz M, Khera P, Mesinkovska NA. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155(2):229–236. doi: 10.1001/jamadermatol.2018.4093. [DOI] [PubMed] [Google Scholar]
- 29.Hutchinson MR, Zhang Y, Brown K et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28(1):20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Zhang Y, Peng Y et al. Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br J Pharmacol. 2016;173(5):856–869. doi: 10.1111/bph.13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porter SJ, Somogyi AA, White JM. In vivo and in vitro potency studies of 6beta-naltrexol, the major human metabolite of naltrexone. Addict Biol. 2002;7(2):219–225. doi: 10.1080/135562102200120442. [DOI] [PubMed] [Google Scholar]
- 32.Mazák K, Hosztafi S, Noszál B. Species-specific lipophilicity of morphine antagonists. Eur J Pharm Sci. 2015;78:1–7. doi: 10.1016/j.ejps.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 33.Benet LZ, Broccatelli F, Oprea TI. BDDCS applied to over 900 drugs. AAPS J. 2011;13(4):519–547. doi: 10.1208/s12248-011-9290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer MC, Straughn AB, Lo MW et al. Bioequivalence, dose-proportionality, and pharmacokinetics of naltrexone after oral administration. J Clin Psychiatry. 1984;45(9, pt 2):15–19. [PubMed] [Google Scholar]
- 35.Verebey K, Volavka J, Mule SJ, Resnick RB. Naltrexone: disposition, metabolism, and effects after acute and chronic dosing. Clin Pharmacol Ther. 1976;20(3):315–328. doi: 10.1002/cpt1976203315. [DOI] [PubMed] [Google Scholar]
- 36.Porter SJ, Somogyi AA, White JM. Kinetics and inhibition of the formation of 6beta-naltrexol from naltrexone in human liver cytosol. Br J Pharmacol. 2000;50(5):465–471. doi: 10.1046/j.1365-2125.2000.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metcalf MD, Rosicky AD, Hassan HE et al. Opioids and efflux transporters, part 4: influence of N-substitution on P-glycoprotein substrate activity of noroxymorphone analogues. Bioorg Med Chem Lett. 2014;24(15):3592–3595. doi: 10.1016/j.bmcl.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanaan M, Daali Y, Dayer P, Desmeules J. P-glycoprotein is not involved in the differential oral potency of naloxone and naltrexone. Fundam Clin Pharmacol. 2009;23(5):543–548. doi: 10.1111/j.1472-8206.2009.00724.x. [DOI] [PubMed] [Google Scholar]
- 39.Mitra P, Venitz J, Yuan Y et al. Preclinical disposition (in vitro) of novel μ-opioid receptor selective antagonists. Drug Metab Dispos. 2011;39(9):1589–1596. doi: 10.1124/dmd.111.038588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nimmo WS. Drugs, diseases and altered gastric emptying. Clin Pharmacokinet. 1976;1(3):189–203. doi: 10.2165/00003088-197601030-00002. [DOI] [PubMed] [Google Scholar]
- 41.Zipfel S, Sammet I, Rapps N et al. Gastrointestinal disturbances in eating disorders: clinical and neurobiological aspects. Auton Neurosci. 2006;129(1–2):99–106. doi: 10.1016/j.autneu.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 42.Liu R, Hong J, Xu X et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23(7):859–868. doi: 10.1038/nm.4358. [DOI] [PubMed] [Google Scholar]
- 43.Seitz J, Trinh S, Herpertz-Dahlmann B. The microbiome and eating disorders. Psychiatr Clin North Am. 2019;42(1):93–103. doi: 10.1016/j.psc.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Rosenfeld CS. Microbiome disturbances and autism spectrum disorders. Drug Metab Dispos. 2015;43(10):1557–1571. doi: 10.1124/dmd.115.063826. [DOI] [PubMed] [Google Scholar]
- 45.Rabiner EA, Beaver J, Makwana A et al. Pharmacological differentiation of opioid receptor antagonists by molecular and functional imaging of target occupancy and food reward-related brain activation in humans. Mol Psychiatry. 2011;16(8):826–835. 785. doi: 10.1038/mp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kyler KE, Wagner J, Hosey-Cojocari C et al. Drug dose selection in pediatric obesity: available information for the most commonly prescribed drugs to children. Paediatr Drugs. 2019;21(5):357–369. doi: 10.1007/s40272-019-00352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Funk RS, Brown JT, Abdel-Rahman SM. Pediatric pharmacokinetics: human development and drug disposition. Pediatr Clin North Am. 2012;59(5):1001–1016. doi: 10.1016/j.pcl.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Wagner J, Abdel-Rahman SM. Pediatric pharmacokinetics. Pediatr Rev. 2013;34(6):258–269. doi: 10.1542/pir.34-6-258. [DOI] [PubMed] [Google Scholar]
- 49.El Ghoch M, Calugi S, Lamburghini S, Dalle Grave R. Anorexia nervosa and body fat distribution: a systematic review. Nutrients. 2014;6(9):3895–3912. doi: 10.3390/nu6093895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El Ghoch M, Calugi S, Milanese C et al. Body composition in men with anorexia nervosa: longitudinal study. Int J Eat Disord. 2017;50(7):856–860. doi: 10.1002/eat.22721. [DOI] [PubMed] [Google Scholar]
- 51.El Ghoch M, Milanese C, Calugi S et al. Regional fat distribution in adolescent and adult females with anorexia nervosa: a longitudinal study. Clin Nutr. 2015;34(6):1224–1232. doi: 10.1016/j.clnu.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 52.de Hoogd S, Välitalo PAJ, Dahan A et al. Influence of morbid obesity on the pharmacokinetics of morphine, morphine-3-glucuronide, and morphine-6-glucuronide. Clin Pharmacokinet. 2017;56(12):1577–1587. doi: 10.1007/s40262-017-0544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meistelman C, Benhamou D, Barre J et al. Effects of age on plasma protein binding of sufentanil. Anesthesiology. 1990;72(3):470–473. doi: 10.1097/00000542-199003000-00013. [DOI] [PubMed] [Google Scholar]
- 54.Holt DW, Hayler AM, Healey GF. Effect of age and plasma concentrations of albumin and alpha 1-acid glycoprotein on protein binding of disopyramide. Br J Clin Pharmacol. 1983;16(3):344–345. doi: 10.1111/j.1365-2125.1983.tb02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sethi PK, White CA, Cummings BS et al. Ontogeny of plasma proteins, albumin and binding of diazepam, cyclosporine, and deltamethrin. Pediatr Res. 2016;79(3):409–415. doi: 10.1038/pr.2015.237. [DOI] [PubMed] [Google Scholar]
- 56.Achamrah N, Coëffier M, Rimbert A et al. Micronutrient status in 153 patients with anorexia nervosa. Nutrients. 2017;9(3):225. doi: 10.3390/nu9030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosen E, Bakshi N, Watters A et al. Hepatic complications of anorexia nervosa. Dig Dis Sci. 2017;62(11):2977–2981. doi: 10.1007/s10620-017-4766-9. [DOI] [PubMed] [Google Scholar]
- 58.Hendricks KM, Duggan C, Gallagher L et al. Malnutrition in hospitalized pediatric patients. Current prevalence. Arch Pediatr Adolesc Med. 1995;149(10):1118–1122. doi: 10.1001/archpedi.1995.02170230072010. [DOI] [PubMed] [Google Scholar]
- 59.Wall ME, Brine DR, Perez-Reyes M. The metabolism of naltrexone in man. NIDA Res Monogr. 1981;28:105–131. [PubMed] [Google Scholar]
- 60.Brine GA, Brine DR, Welch CD et al. Carbon-13 nuclear magnetic resonance identification of 2-hydroxy-3-O-methyl-6beta-naltrexol as a minor naltrexone metabolite. Res Commun Chem Pathol Pharmacol. 1978;22(3):455–464. [PubMed] [Google Scholar]
- 61.Cone EJ, Gorodetzky CW, Darwin WD et al. The identification and measurement of two new metabolites of naltrexone in human urine. Res Commun Chem Pathol Pharmacol. 1978;20(3):413–433. [PubMed] [Google Scholar]
- 62.Mason BJ, Goodman AM, Dixon RM et al. A pharmaco-kinetic and pharmacodynamic drug interaction study of acamprosate and naltrexone. Neuropsychopharmacology. 2002;27(4):596–606. doi: 10.1016/S0893-133X(02)00368-8. [DOI] [PubMed] [Google Scholar]
- 63.McCaul ME, Wand GS, Rohde C, Lee SM. Serum 6-beta-naltrexol levels are related to alcohol responses in heavy drinkers. Alcohol Clin Exp Res. 2000;24(9):1385–1391. [PubMed] [Google Scholar]
- 64.Pini LA, Ferretti C, Trenti T et al. Effects of long-term treatment with naltrexone on hepatic enzyme activity. Drug Metabol Drug Interact. 1991;9(2):161–174. doi: 10.1515/dmdi.1991.9.2.161. [DOI] [PubMed] [Google Scholar]
- 65.AlRabiah H, Ahad A, Mostafa GAE, Al-Jenoobi FI. Effect of naltrexone hydrochloride on cytochrome P450 1A2, 2C9, 2D6, and 3A4 activity in human liver microsomes. Eur J Drug Metab Pharmacokinet. 2018;43(6):707–713. doi: 10.1007/s13318-018-0482-x. [DOI] [PubMed] [Google Scholar]
- 66.Wermuth B, Platts KL, Seidel A, Oesch F. Carbonyl reductase provides the enzymatic basis of quinone detoxication in man. Biochem Pharmacol. 1986;35(8):1277–1282. doi: 10.1016/0006-2952(86)90271-6. [DOI] [PubMed] [Google Scholar]
- 67.Ohara H, Miyabe Y, Deyashiki Y et al. Reduction of drug ketones by dihydrodiol dehydrogenases, carbonyl reductase and aldehyde reductase of human liver. Biochem Pharmacol. 1995;50(2):221–227. doi: 10.1016/0006-2952(95)00124-i. [DOI] [PubMed] [Google Scholar]
- 68.Breyer-Pfaff U, Nill K. Carbonyl reduction of naltrexone and dolasetron by oxidoreductases isolated from human liver cytosol. J Pharm Pharmacol. 2004;56(12):1601–1606. doi: 10.1211/0022357045020. [DOI] [PubMed] [Google Scholar]
- 69.Penning TM, Burczynski ME, Jez JM et al. Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351(pt 1):67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Penning TM, Jin Y, Steckelbroeck S et al. Structure-function of human 3 alpha-hydroxysteroid dehydrogenases: genes and proteins. Mol Cell Endocrinol. 2004;215(1–2):63–72. doi: 10.1016/j.mce.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 71.Penning TM, Jin Y, Heredia VV, Lewis M. Structure-function relationships in 3alpha-hydroxysteroid dehydrogenases: a comparison of the rat and human isoforms. J Steroid Biochem Mol Biol. 2003;85(2–5):247–255. doi: 10.1016/s0960-0760(03)00236-x. [DOI] [PubMed] [Google Scholar]
- 72.McCarthy TL, Hochberg RB, Labaree DC, Centrella M. 3-ketosteroid reductase activity and expression by fetal rat osteoblasts. J Biol Chem. 2007;282(47):34003–34012. doi: 10.1074/jbc.M707502200. [DOI] [PubMed] [Google Scholar]
- 73.Liu JC, Ma JD, Morello CM et al. Naltrexone metabolism and concomitant drug concentrations in chronic pain patients. J Anal Toxicol. 2014;38(4):212–217. doi: 10.1093/jat/bku019. [DOI] [PubMed] [Google Scholar]
- 74.Stayrook KR, Rogers PM, Savkur RS et al. Regulation of human 3 alpha-hydroxysteroid dehydrogenase (AKR1C4) expression by the liver X receptor alpha. Mol Pharmacol. 2008;73(2):607–612. doi: 10.1124/mol.107.039099. [DOI] [PubMed] [Google Scholar]
- 75.Stapelfeld C, Maser E. Sex hormones reduce NNK detoxification through inhibition of short-chain dehydrogenases/reductases and aldo-keto reductases in vitro. Chem Biol Interact. 2017;276:167–173. doi: 10.1016/j.cbi.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 76.Toft I, Lindal S, Bønaa KH, Jenssen T. Quantitative measurement of muscle fiber composition in a normal population. Muscle Nerve. 2003;28(1):101–108. doi: 10.1002/mus.10373. [DOI] [PubMed] [Google Scholar]
- 77.Kume T, Iwasa H, Shiraishi H et al. Characterization of a novel variant (S145C/L311V) of 3alpha-hydroxysteroid/dihydrodiol dehydrogenase in human liver. Pharmacogenetics. 1999;9(6):763–771. [PubMed] [Google Scholar]
- 78.Bains OS, Grigliatti TA, Reid RE, Riggs KW. Naturally occurring variants of human aldo-keto reductases with reduced in vitro metabolism of daunorubicin and doxorubicin. J Pharmacol Exp Ther. 2010;335(3):533–545. doi: 10.1124/jpet.110.173179. [DOI] [PubMed] [Google Scholar]
- 79.Sherry ST, Ward MH, Kholodov M et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29(1):308–311. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coffman BL, King CD, Rios GR, Tephly TR. The glucuronidation of opioids, other xenobiotics, and androgens by human UGT2B7Y(268) and UGT2B7H(268) Drug Metab Dispos. 1998;26(1):73–77. [PubMed] [Google Scholar]
- 81.King CD, Green MD, Rios GR et al. The glucuronidation of exogenous and endogenous compounds by stably expressed rat and human UDP-glucuronosyltransferase 1.1. Arch Biochem Biophys. 1996;332(1):92–100. doi: 10.1006/abbi.1996.0320. [DOI] [PubMed] [Google Scholar]
- 82.Green MD, Bélanger G, Hum DW et al. Glucuronidation of opioids, carboxylic acid-containing drugs, and hydroxylated xenobiotics catalyzed by expressed monkey UDP-glucuronosyltransferase 2B9 protein. Drug Metab Dispos. 1997;25(12):1389–1394. [PubMed] [Google Scholar]
- 83.King CD, Rios GR, Green MD et al. Comparison of stably expressed rat UGT1.1 and UGT2B1 in the glucuronidation of opioid compounds. Drug Metab Dispos. 1997;25(2):251–255. [PubMed] [Google Scholar]
- 84.McRorie TI, Lynn AM, Nespeca MK et al. The maturation of morphine clearance and metabolism. Am J Dis Child. 1992;146(8):972–976. doi: 10.1001/archpedi.1992.02160200094036. [DOI] [PubMed] [Google Scholar]
- 85.Moreland TA, Brice JE, Walker CH, Parija AC. Naloxone pharmacokinetics in the newborn. Br J Clin Pharmacol. 1980;9(6):609–612. doi: 10.1111/j.1365-2125.1980.tb01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhatt DK, Mehrotra A, Gaedigk A et al. Age- and genotype-dependent variability in the protein abundance and activity of six major uridine diphosphate-glucuronosyltransferases in human liver. Clin Pharmacol Ther. 2019;105(1):131–141. doi: 10.1002/cpt.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cone EJ, Gorodetzky CW, Yeh SY. The urinary excretion profile of naltrexone and metabolites in man. Drug Metab Dispos. 1974;2(6):506–512. [PubMed] [Google Scholar]
- 88.Stancil SL, Chapron A, Abdel-Rahman S. Neonatal and Pediatric Pharmacology Therapeutic Principles in Practice. 5th ed. Drug absorption, distribution, metabolism, excretion and transporters in newborns and children. [In Press] [Google Scholar]
- 89.Kambia NK, Dine T, Odou P et al. Pharmacokinetics and dialysability of naltrexone in patients undergoing hemodialysis. Eur J Drug Metab Pharmacokinet. 2004;29(4):225–230. doi: 10.1007/BF03190603. [DOI] [PubMed] [Google Scholar]
- 90.Nierenberg DW. Drug inhibition of penicillin tubular secretion: concordance between in vitro and clinical findings. J Pharmacol Exp Ther. 1987;240(3):712–716. [PubMed] [Google Scholar]
- 91.Mulla H. Understanding developmental pharmacodynamics: importance for drug development and clinical practice. Paediatr Drugs. 2010;12(4):223–233. doi: 10.2165/11319220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 92.McLaughlin MJ, Wagner J, Shakhnovich V et al. Considerations for implementing precision therapeutics for children. Clin Transl Sci. 2019;12(2):140–150. doi: 10.1111/cts.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chatoor I, Herman BH, Hartzler J. Effects of the opiate antagonist, naltrexone, on binging antecedents and plasma beta-endorphin concentrations. J Am Acad Child Adolesc Psychiatry. 1994;33(5):748–752. doi: 10.1097/00004583-199406000-00016. [DOI] [PubMed] [Google Scholar]
- 94.Lee MC, Wagner HN, Jr, Tanada S et al. Duration of occupancy of opiate receptors by naltrexone. J Nucl Med. 1988;29(7):1207–1211. [PubMed] [Google Scholar]
- 95.Yancey-Wrona J, Dallaire B, Bilsky E et al. 6β-naltrexol, a peripherally selective opioid antagonist that inhibits morphine-induced slowing of gastrointestinal transit: an exploratory study. Pain Med. 2011;12(12):1727–1737. doi: 10.1111/j.1526-4637.2011.01279.x. [DOI] [PubMed] [Google Scholar]
- 96.Yancey-Wrona JE, Raymond TJ, Mercer HK et al. 6beta-naltrexol preferentially antagonizes opioid effects on gastrointestinal transit compared to antinociception in mice. Life Sci. 2009;85(11–12):413–420. doi: 10.1016/j.lfs.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 97.Spain JW, Roth BL, Coscia CJ. Differential ontogeny of multiple opioid receptors (mu, delta, and kappa) J Neurosci. 1985;5(3):584–588. doi: 10.1523/JNEUROSCI.05-03-00584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xia Y, Haddad GG. Ontogeny and distribution of opioid receptors in the rat brainstem. Brain Res. 1991;549(2):181–193. doi: 10.1016/0006-8993(91)90457-7. [DOI] [PubMed] [Google Scholar]
- 99.Clendeninn NJ, Petraitis M, Simon EJ. Ontological development of opiate receptors in rodent brain. Brain Res. 1976;118(1):157–160. doi: 10.1016/0006-8993(76)90852-0. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y, Wang D, Johnson AD et al. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280(38):32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- 101.Bond C, LaForge KS, Tian M et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A. 1998;95(16):9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hartwell EE, Feinn R, Morris PE et al. Systematic review and meta-analysis of the moderating effect of rs1799971 in OPRM1, the mu-opioid receptor gene, on response to naltrexone treatment of alcohol use disorder. Addiction. 2020;115(8):1426–1437. doi: 10.1111/add.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Anton RF, Oroszi G, O'Malley S et al. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65(2):135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gelernter J, Gueorguieva R, Kranzler HR et al. Opioid receptor gene (OPRM1, OPRK1, and OPRD1) variants and response to naltrexone treatment for alcohol dependence: results from the VA Cooperative Study. Alcohol Clin Exp Res. 2007;31(4):555–563. doi: 10.1111/j.1530-0277.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 105.Ashenhurst JR, Bujarski S, Ray LA. Delta and kappa opioid receptor polymorphisms influence the effects of naltrexone on subjective responses to alcohol. Pharmacol Biochem Behav. 2012;103(2):253–259. doi: 10.1016/j.pbb.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gordon RJ, Panigrahi SK, Meece K et al. Effects of opioid antagonism on cerebrospinal fluid melanocortin peptides and cortisol levels in humans. J Endocr Soc. 2017;1(10):1235–1246. doi: 10.1210/js.2017-00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kosten TR, Kreek MJ, Ragunath J, Kleber HD. A preliminary study of beta endorphin during chronic naltrexone maintenance treatment in ex-opiate addicts. Life Sci. 1986;39(1):55–59. doi: 10.1016/0024-3205(86)90437-6. [DOI] [PubMed] [Google Scholar]
- 108.Price RC, Christou NV, Backman SB et al. Opioid-receptor antagonism increases pain and decreases pleasure in obese and non-obese individuals. Psychopharmacology. 2016;233(23–24):3869–3879. doi: 10.1007/s00213-016-4417-4. [DOI] [PubMed] [Google Scholar]
- 109.Sandman CA, Touchette P, Lenjavi M et al. beta-Endorphin and ACTH are dissociated after self-injury in adults with developmental disabilities. Am J Ment Retard. 2003;108(6):414–424. doi: 10.1352/0895-8017(2003)108<414:EAAADA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 110.Sandman CA, Hetrick W, Taylor DV, Chicz-DeMet A. Dissociation of POMC peptides after self-injury predicts responses to centrally acting opiate blockers. Am J Ment Retard. 1997;102(2):182–199. doi: 10.1352/0895-8017(1997)102<0182:DOPPAS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 111.Lovallo WR, Enoch MA, Acheson A et al. Cortisol stress response in men and women modulated differentially by the mu-opioid receptor gene polymorphism OPRM1 A118G. Neuropsychopharmacology. 2015;40(11):2546–2554. doi: 10.1038/npp.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barry CL, Sindelar JL. Equity in private insurance coverage for substance abuse: a perspective on parity. Health Aff (Millwood) 2007;26(6):w706–w716. doi: 10.1377/hlthaff.26.6.w706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Andrews CM, Grogan CM, Smith BT et al. Medicaid benefits for addiction treatment expanded after implementation of the Affordable Care Act. Health Aff (Millwood) 2018;37(8):1216–1222. doi: 10.1377/hlthaff.2018.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grogan CM, Andrews C, Abraham A et al. Survey highlights differences in medicaid coverage for substance use treatment and opioid use disorder medications. Health Aff (Millwood) 2016;35(12):2289–2296. doi: 10.1377/hlthaff.2016.0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Curran S, Savage C. Patient response to naltrexone: issues of acceptance, treatment effects, and frequency of administration. NIDA Res Monogr. 1976;(9):67–69. doi: 10.1037/e497452006-012. [DOI] [PubMed] [Google Scholar]
- 116.Cornish JW, Metzger D, Woody GE et al. Naltrexone pharmacotherapy for opioid dependent federal probationers. J Subst Abuse Treat. 1997;14(6):529–534. doi: 10.1016/s0740-5472(97)00020-2. [DOI] [PubMed] [Google Scholar]
- 117.Clinical evaluation of naltrexone treatment of opiate-dependent individuals: report of the National Research Council Committee on Clinical Evaluation of Narcotic Antagonists. Arch Gen Psychiatry. 1978;35(3):335–340. [PubMed] [Google Scholar]
- 118.Lerner A, Sigal M, Bacalu A et al. A naltrexone double blind placebo controlled study in Israel. Isr J Psychiatry Relat Sci. 1992;29(1):36–43. [PubMed] [Google Scholar]
- 119.Shufman EN, Porat S, Witztum E et al. The efficacy of naltrexone in preventing reabuse of heroin after detoxification. Biol Psychiatry. 1994;35(12):935–945. doi: 10.1016/0006-3223(94)91240-8. [DOI] [PubMed] [Google Scholar]
- 120.Horgan CM, Reif S, Hodgkin D et al. Availability of addiction medications in private health plans. J Subst Abuse Treat. 2008;34(2):147–156. doi: 10.1016/j.jsat.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mark TL, Kassed CA, Vandivort-Warren R et al. Alcohol and opioid dependence medications: prescription trends, overall and by physician specialty. Drug Alcohol Depend. 2009;99(1–3):345–349. doi: 10.1016/j.drugalcdep.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rawson RA, Glazer M, Callahan EJ, Liberman RP. Naltrexone and behavior therapy for heroin addiction. NIDA Res Monogr. 1979;(25):26–43. doi: 10.1037/e497382006-005. [DOI] [PubMed] [Google Scholar]
- 123.Aletraris L, Bond Edmond M, Roman PM. Adoption of injectable naltrexone in U.S. substance use disorder treatment programs. J Stud Alcohol Drugs. 2015;76(1):143–151. [PMC free article] [PubMed] [Google Scholar]
- 124.San L, Pomarol G, Peri JM et al. Follow-up after a six-month maintenance period on naltrexone versus placebo in heroin addicts. Br J Addict. 1991;86(8):983–990. doi: 10.1111/j.1360-0443.1991.tb01859.x. [DOI] [PubMed] [Google Scholar]
- 125.Polonini HC, Silva SL, de Almeida TR et al. Compatibility of caffeine, carvedilol, clomipramine hydrochloride, folic acid, hydrochlorothiazide, loperamide hydrochloride, methotrexate, nadolol, naltrexone hydrochloride and pentoxifylline in SyrSpend SF PH4 oral suspensions. Eur J Hosp Pharm. 2016;23(6):352–358. doi: 10.1136/ejhpharm-2016-000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fawcett JP, Morgan NC, Woods DJ. Formulation and stability of naltrexone oral liquid for rapid withdrawal from methadone. Ann Pharmacother. 1997;31(11):1291–1295. doi: 10.1177/106002809703101102. [DOI] [PubMed] [Google Scholar]
- 127.Bronfenbrener R. Inexpensive compounding of low dose naltrexone (LDN) with orange juice. J Am Acad Dermatol. 2019;85(3):e139. doi: 10.1016/j.jaad.2019.03.067. [DOI] [PubMed] [Google Scholar]
- 128.Guo S, Jiang Z, Wu Y. Efficacy of naltrexone hydrochloride for preventing relapse among opiate-dependent patients after detoxification. Hong Kong J Psychiatry. 2001;11(4):2–8. [Google Scholar]
- 129.Krupitsky EM, Zvartau EE, Masalov DV et al. Naltrexone for heroin dependence treatment in St. Petersburg, Russia. J Subst Abuse Treat. 2004;26(4):285–294. doi: 10.1016/j.jsat.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 130.Krupitsky EM, Zvartau EE, Masalov DV et al. Naltrexone with or without fluoxetine for preventing relapse to heroin addiction in St. Petersburg, Russia. J Subst Abuse Treat. 2006;31(4):319–328. doi: 10.1016/j.jsat.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 131.Stella L, D'Ambra C, Mazzeo F et al. Naltrexone plus benzodiazepine aids abstinence in opioid-dependent patients. Life Sci. 2005;77(21):2717–2722. doi: 10.1016/j.lfs.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 132.Schottenfeld RS, Chawarski MC, Mazlan M. Maintenance treatment with buprenorphine and naltrexone for heroin dependence in Malaysia: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371(9631):2192–2200. doi: 10.1016/S0140-6736(08)60954-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Krupitsky E, Nunes EV, Ling W et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377(9776):1506–1513. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- 134.Sullivan MA, Bisaga A, Pavlicova M et al. A randomized trial comparing extended-release injectable suspension and oral naltrexone, both combined with behavioral therapy, for the treatment of opioid use disorder. Am J Psychiatry. 2019;176(2):129–137. doi: 10.1176/appi.ajp.2018.17070732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49(11):876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- 136.Oslin DW, Leong SH, Lynch KG et al. Naltrexone vs placebo for the treatment of alcohol dependence: a randomized clinical trial. JAMA Psychiatry. 2015;72(5):430–437. doi: 10.1001/jamapsychiatry.2014.3053. [DOI] [PubMed] [Google Scholar]
- 137.O'Malley SS, Jaffe AJ, Chang G et al. Naltrexone and coping skills therapy for alcohol dependence: a controlled study. Arch Gen Psychiatry. 1992;49(11):881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- 138.Anton RF, O'Malley SS, Ciraulo DA et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 139.Garbutt JC, Kranzler HR, O'Malley SS et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293(13):1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- 140.Kranzler HR, Wesson DR, Billot L. Naltrexone depot for treatment of alcohol dependence: a multicenter, randomized, placebo-controlled clinical trial. Alcohol Clin Exp Res. 2004;28(7):1051–1059. doi: 10.1097/01.alc.0000130804.08397.29. [DOI] [PubMed] [Google Scholar]
- 141.Apovian CM, Aronne L, Rubino D et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II) Obesity (Silver Spring) 2013;21(5):935–943. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kolotkin RL, Chen S, Klassen P et al. Patient-reported quality of life in a randomized placebo-controlled trial of naltrexone/bupropion for obesity. Clin Obes. 2015;5(5):237–244. doi: 10.1111/cob.12108. [DOI] [PubMed] [Google Scholar]
- 143.Hollander P, Gupta AK, Plodkowski R et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12):4022–4029. doi: 10.2337/dc13-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wadden TA, Foreyt JP, Foster GD et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011;19(1):110–120. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Greenway FL, Fujioka K, Plodkowski RA et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 146.Mitchell JE, Christenson G, Jennings J et al. A placebo-controlled, double-blind crossover study of naltrexone hydrochloride in outpatients with normal weight bulimia. J Clin Psychopharmacol. 1989;9(2):94–97. doi: 10.1097/00004714-198904000-00004. [DOI] [PubMed] [Google Scholar]
- 147.Alger SA, Schwalberg MD, Bigaouette JM et al. Effect of a tricyclic antidepressant and opiate antagonist on binge-eating behavior in normoweight bulimic and obese, binge-eating subjects. Am J Clin Nutr. 1991;53(4):865–871. doi: 10.1093/ajcn/53.4.865. [DOI] [PubMed] [Google Scholar]
- 148.Marrazzi MA, Bacon JP, Kinzie J, Luby ED. Naltrexone use in the treatment of anorexia nervosa and bulimia nervosa. Int Clin Psychopharmacol. 1995;10(3):163–172. doi: 10.1097/00004850-199510030-00005. [DOI] [PubMed] [Google Scholar]
- 149.Jonas JM, Gold MS. Treatment of antidepressant-resistant bulimia with naltrexone. Int J Pscyhiatry Med. 1986;16(4):305–309. doi: 10.2190/0cmp-4xxh-3641-nrdw. [DOI] [PubMed] [Google Scholar]
- 150.Jonas JM, Gold MS. The use of opiate antagonists in treating bulimia: a study of low-dose versus high-dose naltrexone. Psychiatry Res. 1988;24(2):195–199. doi: 10.1016/0165-1781(88)90062-5. [DOI] [PubMed] [Google Scholar]
- 151.Malcolm R, O'Neil PM, Sexauer JD et al. A controlled trial of naltrexone in obese humans. Int J Obes. 1985;9(5):347–353. [PubMed] [Google Scholar]
- 152.Cornford CS, Close HJ, Bray R et al. Contraceptive use and pregnancy outcomes among opioid drug-using women: a retrospective cohort study. PloS One. 2015;10(3):e0116231. doi: 10.1371/journal.pone.0116231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sandman CA, Hetrick WP, Taylor DV et al. Naltrexone reduces self-injury and improves learning. Exp Clin Psychopharmacol. 1993;1(1–4):242–258. [Google Scholar]
- 154.Sonne S, Rubey R, Brady K et al. Naltrexone treatment of self-injurious thoughts and behaviors. J Nerv Ment Dis. 1996;184(3):192–195. doi: 10.1097/00005053-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 155.Willemsen-Swinkels SH, Buitelaar JK, Nijhof GJ, van England H. Failure of naltrexone hydrochloride to reduce self-injurious and autistic behavior in mentally retarded adults: double-blind placebo-controlled studies. Arch Gen Psychiatry. 1995;52(9):766–773. doi: 10.1001/archpsyc.1995.03950210060011. [DOI] [PubMed] [Google Scholar]
- 156.Sandman CA, Barron JL, Colman H. An orally administered opiate blocker, naltrexone, attenuates self-injurious behavior. Am J Ment Retard. 1990;95(1):93–102. [PubMed] [Google Scholar]
- 157.Kars H, Broekema W, Glaudemans-van Gelderen I et al. Naltrexone attenuates self-injurious behavior in mentally retarded subjects. Biol Psychiatry. 1990;27(7):741–746. doi: 10.1016/0006-3223(90)90589-t. [DOI] [PubMed] [Google Scholar]
- 158.Symons FJ, Tapp J, Wulfsberg A et al. Sequential analysis of the effects of naltrexone on the environmental mediation of self-injurious behavior. Exp Clin Psychopharmacol. 2001;9(3):269–276. doi: 10.1037//1064-1297.9.3.269. [DOI] [PubMed] [Google Scholar]
- 159.Roth AS, Ostroff RB, Hoffman RE. Naltrexone as a treatment for repetitive self-injurious behaviour: an open-label trial. J Clin Psychiatry. 1996;57(6):233–237. [PubMed] [Google Scholar]
- 160.Zingarelli G, Ellman G, Hom A et al. Clinical effects of naltrexone on autistic behavior. Am J Ment Retard. 1992;97(1):57–63. [PubMed] [Google Scholar]
- 161.Symons FJ, Sutton KA, Bodfish JW. Preliminary study of altered skin temperature at body sites associated with self-injurious behavior in adults who have developmental disabilities. Am J Ment Retard. 2001;106(4):336–343. doi: 10.1352/0895-8017(2001)106<0336:PSOAST>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 162.Thompson T, Hackenberg T, Cerutti D et al. Opioid antagonist effects on self-injury in adults with mental retardation: response form and location as determinants of medication effects. Am J Ment Retard. 1994;99(1):85–102. [PubMed] [Google Scholar]
- 163.Wolfhagen FH, Sternieri E, Hop WC et al. Oral naltrexone treatment for cholestatic pruritus: a double-blind, placebo-controlled study. Gastroenterology. 1997;113(4):1264–1269. doi: 10.1053/gast.1997.v113.pm9322521. [DOI] [PubMed] [Google Scholar]
- 164.Mansour-Ghanaei F, Taheri A, Froutan H et al. Effect of oral naltrexone on pruritus in cholestatic patients. World J Gastroenterol. 2006;12(7):1125–1128. doi: 10.3748/wjg.v12.i7.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Peer G, Kivity S, Agami O et al. Randomised crossover trial of naltrexone in uraemic pruritus. Lancet. 1996;348(9041):1552–1554. doi: 10.1016/s0140-6736(96)04176-1. [DOI] [PubMed] [Google Scholar]
- 166.Pauli-Magnus C, Mikus G, Alscher DM et al. Naltrexone does not relieve uremic pruritus: results of a randomized, double-blind, placebo-controlled crossover study. J Am Soc Nephrol. 2000;11(3):514–519. doi: 10.1681/ASN.V113514. [DOI] [PubMed] [Google Scholar]
- 167.Malekzad F, Arbabi M, Mohtasham N et al. Efficacy of oral naltrexone on pruritus in atopic eczema: a double-blind, placebo-controlled study. J Eur Acad Dermatol Venereol. 2009;23(8):948–950. doi: 10.1111/j.1468-3083.2009.03129.x. [DOI] [PubMed] [Google Scholar]
- 168.Legroux-Crespel E, Clèdes J, Misery L. A comparative study on the effects of naltrexone and loratadine on uremic pruritus. Dermatology. 2004;208(4):326–330. doi: 10.1159/000077841. [DOI] [PubMed] [Google Scholar]
- 169.Terg R, Coronel E, Sordá J et al. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37(6):717–722. doi: 10.1016/s0168-8278(02)00318-5. [DOI] [PubMed] [Google Scholar]
- 170.Ajayi AA, Kolawole BA, Udoh SJ. Endogenous opioids, mu-opiate receptors and chloroquine-induced pruritus: a double-blind comparison of naltrexone and promethazine in patients with malaria fever who have an established history of generalized chloroquine-induced itching. Int J Dermatol. 2004;43(12):972–977. doi: 10.1111/j.1365-4632.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 171.Ward S, Smith N, Bowden-Jones H. The use of naltrexone in pathological and problem gambling: a UK case series. J Behav Addict. 2018;7(3):827–833. doi: 10.1556/2006.7.2018.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bosco D, Plastino M, Colica C et al. Opioid antagonist naltrexone for the treatment of pathological gambling in Parkinson disease. Clin Neuropharmacol. 2012;35(3):118–120. doi: 10.1097/WNF.0b013e31824d529b. [DOI] [PubMed] [Google Scholar]
- 173.Grant JE, Kim SW, Odlaug BL. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol Psychiatry. 2009;65(7):600–606. doi: 10.1016/j.biopsych.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 174.Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783–789. doi: 10.4088/jcp.v69n0511. [DOI] [PubMed] [Google Scholar]
- 175.Kovanen L, Basnet S, Castrén S et al. A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. Eur Addict Res. 2016;22(2):70–79. doi: 10.1159/000435876. [DOI] [PubMed] [Google Scholar]
- 176.Papay K, Xie SX, Stern M et al. Naltrexone for impulse control disorders in Parkinson disease: a placebo-controlled study. Neurology. 2014;83(9):826–833. doi: 10.1212/WNL.0000000000000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Grant JE, Odlaug BL, Schreiber LR, Kim SW. The opiate antagonist, naltrexone, in the treatment of trichotillomania: results of a double-blind, placebo-controlled study. J Clin Psychopharmacol. 2014;34(1):134–138. doi: 10.1097/JCP.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 178.Toneatto T, Brands B, Selby P. A randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of concurrent alcohol use disorder and pathological gambling. Am J Addict. 2009;18(3):219–225. doi: 10.1080/10550490902787007. [DOI] [PubMed] [Google Scholar]
- 179.Miranda R, Ray L, Blanchard A et al. Effects of naltrexone on adolescent alcohol cue reactivity and sensitivity: an initial randomized trial. Addict Biol. 2014;19(5):941–954. doi: 10.1111/adb.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hulse GK, Tait RJ. A pilot study to assess the impact of naltrexone implant on accidental opiate overdose in “high-risk” adolescent heroin users. Addict Biol. 2003;8(3):337–342. doi: 10.1080/13556210310001602257. [DOI] [PubMed] [Google Scholar]
- 181.Fishman MJ, Winstanley EL, Curran E et al. Treatment of opioid dependence in adolescents and young adults with extended release naltrexone: preliminary case-series and feasibility. Addiction. 2010;105(9):1669–1676. doi: 10.1111/j.1360-0443.2010.03015.x. [DOI] [PubMed] [Google Scholar]
- 182.Casner JA, Weinheimer B, Gualtieri CT. Naltrexone and self-injurious behavior: a retrospective population study. J Clin Psychopharmacol. 1996;16(5):389–394. doi: 10.1097/00004714-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 183.Ryback RS. Naltrexone in the treatment of adolescent sexual offenders. J Clin Psychiatry. 2004;65(7):982–986. doi: 10.4088/jcp.v65n0715. [DOI] [PubMed] [Google Scholar]
- 184.Campbell M, Anderson LT, Small AM et al. Naltrexone in autistic children: behavioral symptoms and attentional learning. J Am Acad Child Adolesc Psychiatr. 1993;32(6):1283–1291. doi: 10.1097/00004583-199311000-00024. [DOI] [PubMed] [Google Scholar]
- 185.Barrett RP, Feinstein C, Hole WT. Effects of naloxone and naltrexone on self-injury: a double-blind, placebo-controlled analysis. Am J Ment Retard. 1989;93(6):644–651. [PubMed] [Google Scholar]
- 186.Bouvard MP, Leboyer M, Launay JM et al. Low-dose naltrexone effects on plasma chemistries and clinical symptoms in autism: a double-blind, placebo-controlled study. Psychiatry Res. 1995;58(3):191–201. doi: 10.1016/0165-1781(95)02601-r. [DOI] [PubMed] [Google Scholar]
- 187.Campbell M, Anderson LT, Small AM et al. Naltrexone in autistic children: a double-blind and placebo-controlled study. Psychopharmacol Bull. 1990;26(1):130–135. [PubMed] [Google Scholar]
- 188.Leboyer M, Bouvard MP, Launay JM et al. Brief report: a double-blind study of naltrexone in infantile autism. J Autism Dev Disord. 1992;22(2):309–319. doi: 10.1007/BF01058158. [DOI] [PubMed] [Google Scholar]
- 189.Gonzalez NM, Campbell M, Small AM et al. Naltrexone plasma levels, clinical response and effect on weight in autistic children. Psychopharmacol Bull. 1994;30(2):203–208. [PubMed] [Google Scholar]
- 190.Scifo R, Cioni M, Nicolosi A et al. Opioid-immune interactions in autism: behavioural and immunological assessment during a double-blind treatment with naltrexone. Ann Ist Super Sanita. 1996;32(3):351–359. [PubMed] [Google Scholar]
- 191.Banga A, Connor DF. Effectiveness of naltrexone for treating pathologic skin picking behavior in an adolescent with Prader-Willi syndrome. J Child Adolesc Psychopharmacol. 2012;22(5):396–398. doi: 10.1089/cap.2012.0028. [DOI] [PubMed] [Google Scholar]
- 192.Benjamin E, Buot-Smith T. Naltrexone and fluoxetine in Prader-Willi syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32(4):870–873. doi: 10.1097/00004583-199307000-00025. [DOI] [PubMed] [Google Scholar]
- 193.Puri MR, Sahl R, Ogden S, Malik S. Prader-Willi syndrome, management of impulsivity, and hyperphagia in an adolescent. J Child Adolesc Psychopharmacol. 2016;26(4):403–404. doi: 10.1089/cap.2015.0240. [DOI] [PubMed] [Google Scholar]
- 194.Zlotkin SH, Fettes IM, Stallings VA. The effects of naltrexone, an oral beta-endorphin antagonist, in children with the Prader-Willi syndrome. J Clin Endocrinol Metab. 1986;63(5):1229–1232. doi: 10.1210/jcem-63-5-1229. [DOI] [PubMed] [Google Scholar]
- 195.Smith JP, Field D, Bingaman SI et al. Safety and tolerability of low-dose naltrexone therapy in children with moderate to severe Crohn's disease: a pilot study. J Clin Gastroenterol. 2013;47(4):339–345. doi: 10.1097/MCG.0b013e3182702f2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chopra P, Cooper MS. Treatment of Complex Regional Pain Syndrome (CRPS) using low dose naltrexone (LDN) J Neuroimmune Pharmacol. 2013;8(3):470–476. doi: 10.1007/s11481-013-9451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Butelman ER, Yuferov V, Kreek MJ. kappa-opioid receptor/dynorphin system: genetic and pharmaco-therapeutic implications for addiction. Trends Neurosci. 2012;35(10):587–596. doi: 10.1016/j.tins.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Valbrun LP, Zvonarev V. The opioid system and food intake: use of opiate antagonists in treatment of binge eating disorder and abnormal eating behavior. J Clin Med Res. 2020;12(2):41–63. doi: 10.14740/jocmr4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Raynor K, Kong H, Chen Y et al. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol. 1994;45(2):330–334. [PubMed] [Google Scholar]
- 200.Codd EE, Shank RP, Schupsky JJ, Raffa RB. Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: structural determinants and role in antinociception. J Pharmacol Exp Ther. 1995;274(3):1263–1270. [PubMed] [Google Scholar]
- 201.Wang D, Sun X, Sadee W. Different effects of opioid antagonists on mu-, delta-, and kappa-opioid receptors with and without agonist pretreatment. J Pharmacol Exp Ther. 2007;321(2):544–552. doi: 10.1124/jpet.106.118810. [DOI] [PubMed] [Google Scholar]
- 202.Pelotte AL, Smith RM, Ayestas M et al. Design, synthesis, and characterization of 6beta-naltrexol analogs, and their selectivity for in vitro opioid receptor subtypes. Bioorg Med Chem Lett. 2009;19(10):2811–2814. doi: 10.1016/j.bmcl.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 203.Ko MC, Divin MF, Lee H et al. Differential in vivo potencies of naltrexone and 6beta-naltrexol in the monkey. J Pharmacol Exp Ther. 2006;316(2):772–779. doi: 10.1124/jpet.105.094409. [DOI] [PubMed] [Google Scholar]


