Abstract
Coccidiosis is a potentially severe enteritis caused by species of obligate intracellular parasites of the genus Eimeria. These parasites cause significant economic losses to the poultry industry, predominantly due to compromised efficiency of production as well as the cost of control. These losses were recently estimated to cost chicken producers approximately £10.4 billion worldwide annually. High levels of Eimeria infection cause clinical coccidiosis which is a significant threat to poultry welfare, and a pre-disposing contributory factor for necrotic enteritis. Control of Eimeria parasites and coccidiosis is therefore an important endeavour; multiple approaches have been developed and these are often deployed together. This review summarises current trends in strategies for control of Eimeria, focusing on three main areas: good husbandry, chemoprophylaxis and vaccination. There is currently no “perfect solution” and there are advantages and limitations to all existing methods. Therefore, the aim of this review is to present current control strategies and suggest how these may develop in the future.
Introduction
Coccidiosis is an enteric disease caused by obligate intracellular protozoa of the genus Eimeria, highly host-specific apicomplexan parasites closely related to the causative agents of many other human and animal diseases including species of: Babesia, Besnoitia, Cryptosporidium, Cystoisospora, Neospora, Plasmodium, Sarcocystis, Theileria, and Toxoplasma. Seven species of Eimeria that infect domestic chickens (Gallus gallus domesticus) (Reid et al. 2014) are recognised as globally ubiquitous (E. acervulina, E. brunetti, E. maxima, E. mitis, E. necatrix, E. praecox and E. tenella). Additionally, three cryptic Eimeria operational taxonomic units (OTUs) have been detected in chickens from across several continents (Cantacessi et al. 2008; Clark et al. 2016; Hinsu et al. 2018; Hauck et al. 2019), and on the basis of genotypic and phenotypic properties these were recently proposed to be previously unrecognised parasite species and given the names Eimeria lata, Eimeria nagambie and Eimeria zaria (Blake et al. 2021).
All species of Eimeria that infect chickens can cause coccidiosis, but four of these (E. acervulina, E. maxima, E. necatrix and E. tenella) are generally considered most important due to their pathogenicity, global prevalence and overall economic impact. The emergence of what appear to be previously undetected Eimeria species suggests that there is potential for additional pathogenic and economic threats in the future. All three of the newly described species have negative impacts on chicken production parameters and the live vaccines currently available to control coccidiosis confer very low or no protection against them, most likely because of the species-specific nature of immune protection induced by Eimeria infection (Fornace et al. 2013; Blake et al. 2021).
Eimeria infection of chickens is initiated by ingestion of sporulated oocysts from the environment (e.g., faeces and contaminated litter) leading to invasion of epithelial cells lining the intestinal tract by released sporozoites. Each Eimeria species exhibits marked tropism for specific regions of the gut, (see Table 1; Lai et al. 2011). The lifecycle of wild-type Eimeria species in chickens comprises a stable number of rounds of asexual reproduction (schizogony), typically three or four depending on species (McDonald and Rose 1987; Walker et al. 2013), with new enterocytes invaded for each round, before a sexual phase termed gametogony ensures. Following fertilisation, progeny oocysts are excreted and these sporulate in the external environment, becoming infectious to new hosts. The pathology associated with each Eimeria species varies, with infection occurring in different sections of the intestine and causing either malabsorptive (E. acervulina, E. maxima, E. mitis and E. praecox) or haemorrhagic (E. brunetti, E. necatrix and E. tenella) disease, Table 1 (Williams 1998; Blake and Tomley 2014; Burrell et al. 2020).
Table 1.
Pathogenicity, type of disease caused and region of development of the seven recognised species of Eimeria that cause coccidiosis in chickens (Blake and Tomley 2014; Cisman et al. 2020; Horton-Smith and Long 1965; Joyner 1958; Joyner and Davies 1960; Long, 1967; 1968; Reid et al. 2014; Williams 1998)
| Eimeria Species | Gross pathological lesions | Haemorrhagic disease | Malabsorptive disease | Region of development |
|---|---|---|---|---|
| E. brunetti | ✓ | ✓ | ✕ | Lower intestine |
| E. necatrix | ✓ | ✓ | ✕ | Mid-intestine and caeca |
| E. tenella | ✓ | ✓ | ✕ | Caeca |
| E. acervulina | ✓ | ✕ | ✓ | Duodenum |
| E. maxima | ✓ | ✕ | ✓ | Mid-intestine |
| E. mitis | ✕ | ✕ | ✓ | Mid-intestine |
| E. praecox | ✕ | ✕ | ✓ | Duodenum |
Coccidiosis varies significantly in its severity and impact on individual chicken health and flock productivity. Depending on parasite species, infectious dose, age and immune status of the host, infected chickens may show few, if any, clinical signs or can suffer effects ranging from reduction in expected weight gain, feed conversion or egg-production, failure to thrive due to malabsorption or diarrhoea, to severe enteritis and death. At flock level, an important consideration is the overall economic burden imposed by coccidiosis and the ongoing cost of its control. In 1995, the global cost was estimated at ~ £38 million annually, with 98% of that cost attributed to broilers (Williams 1999; Kadykalo et al. 2018). Today that figure has been recalculated as ~ £10.4 billion annually, taking account of current global poultry production and disease prevalence (Blake et al. 2020). The vast increase in cost is likely multifactorial and includes massive expansion of the industry in the past 25 years, increased broiler growth rates resulting in reduced growing periods (from ~ 45 days in 1995 to ~ 31–37 days today for intensive systems (Williams 1999; Kadykalo et al. 2018), and hence less time for birds to develop immunity and recover losses in weight gain.
Due to the significant economic and animal welfare impacts of coccidiosis, the need for ongoing management and control of Eimeria parasites remains essential. Management and control strategies can be broadly categorised into three main areas: animal husbandry, chemoprophylaxis and vaccination. In this review, the development, advantages and limitations of each approach will be discussed, together with a summary of the alternative strategies available.
Husbandry
Good husbandry is essential for effective control of clinical and subclinical coccidiosis. Key factors include consideration of the flock environment, such as litter quality, ventilation rate and humidity, as well as stocking density (Long et al. 1976; Bumstead and Millard 1992; Kim et al. 2008; Williams et al. 2009; Bacciu et al. 2014; Blake et al. 2005, 2015). In a broader context, the impact of host genetics can be beneficial, choosing lines or selectively breeding for individuals that are more resistant to Eimeria and the consequences of coccidiosis (Palafox et al. 1949; Champion 1954; Rosenberg et al. 1954; Jeffers et al. 1970; Swaggerty et al. 2011; Boulton et al. 2018a,b).
Impact of climatic factors
Eimeria oocysts have a tough multi-layered wall rendering them relatively resistant to most disinfectants. However, high temperatures (> 50 °C) and ammonia can disrupt oocyst integrity (Fish 1931; Horton-Smith et al. 1940; Williams 1997; Allen and Fetterer 2002). Humidity levels in the immediate environment affect the rate and efficiency of oocyst sporulation as well as subsequent longevity. Damp conditions in poultry houses can be advantageous for Eimeria survival, with examples such as water spillages or high rainfall resulting in humidity in excess of 60% (Anderson et al. 1976; Khan et al. 2006; Nematollahi et al. 2009; Awais et al. 2012). Open-house poultry rearing is practiced in many tropical and subtropical areas and is common in backyard production systems. In these external environments, under optimal conditions (25–30 °C, ~ 75% humidity with aeration), sporulated oocysts can survive for up to 602 days (Farr and Wehr 1949; Edgar 1955; Graat et al. 1994; Waldenstedt et al. 2001; Fatoba and Adeleke 2018). Under drier conditions and lower temperatures sporulation has been observed to be delayed (Musa et al. 2010).
Ambient temperatures of ~ 25 °C favour Eimeria oocyst sporulation; however, oocysts can survive temperatures as low as 4 °C (Anderson et al. 1976; Fayer 1980). In tropical settings it has been reported that oocyst sporulation and survival is favoured during and directly following rainy seasons with higher prevalence of Eimeria infection observed, for example in: Egypt during winter (rainy season December-February), Ethiopia after the rainy season in October, and the Kashmir valley, India, between September and November (Oikawa et al. 1979; Dar and Anwar 1981; Khan et al. 2006; Haug et al. 2008; Al-Gawad et al. 2012; Awais et al. 2012; Luu et al. 2013; Ahad et al. 2015; Sharma et al. 2015). However, higher temperatures are inhibitory, limiting replication. For example, the highest prevalence of coccidiosis in Pakistan was detected towards the end of the monsoon season as the ambient temperature decreased to ~ 25 °C (Awais et al. 2012), in common with previous studies of ambient temperature and season (Anderson et al. 1976; Dar and Anwar 1981; Khan et al. 2006).
While it appears that oocyst sporulation and survival is favoured in environments with higher humidity levels, especially following the main rainy seasons, it is not possible to solely ascribe high or low Eimeria prevalence exclusively to climatic factors. A lack of awareness of transmission and control; and limited resources are also key factors. These are often observed in the poultry management practices of low and middle income countries (Williams 1998; Lawal et al. 2016).
Poultry housing
In poultry houses, Eimeria oocysts can accumulate in the litter, feeders and drinkers (Gross 1985; Khan et al. 2006). Where there is high stocking density, the faecal-oral transmission of sporulated oocysts may increase rapidly within a short period of time (Williams 1995; Trees et al. 2001). Reducing Eimeria infection of chickens can be achieved by limiting oocyst sporulation in the environment, primarily by maintaining dry litter and improving ventilation to a poultry house (Stayer et al. 1995; Etuk et al. 2004). It has also been observed that oocyst viability starts to decline in broiler house litter after approximately 3 weeks, likely due to high environmental ammonia levels (Horton-Smith et al. 1940; Williams 1995). Minimising exposure to common stressors including overcrowding, high temperatures, debeaking, restriction of feed and dietary deficiencies can strengthen chicken immune responses to Eimeria (Williams 1998). Coccidiosis control may also be achieved through reducing water, wind, invertebrate, vermin and other mechanical dispersal of Eimeria oocysts by litter changes (Fayer 1980).
Chicken breed resistance and susceptibility
It has long been recognised that different chicken breeds can exhibit varied levels of “susceptibility” or “resistance” to Eimeria species, including tolerance to infection and rate of recovery from the pathological consequences of infection (Palafox et al. 1949; Champion 1954; Rosenberg et al. 1954; Jeffers et al. 1970; Bishop and Woolliams 2014; Boulton et al. 2018a). Differences in susceptibility to Eimeria infection have been found between and within outbred and inbred chicken breeds/lines, with reports of more than two-fold variations in overall susceptibility to Eimeria species (Bumstead and Millard 1992; Zhu et al. 2003; Pinard-van der Laan et al. 2009) for example, differences in response to E. tenella challenge have been identified between the relatively resistant Egyptian Fayoumi and more susceptible White Leghorn breeding lines (Pinard-van der Laan et al. 1998, 2009). Variation in phenotypes such as pathology and body weight gain during Eimeria infection has been used in genetic mapping studies to identify quantitative trait loci (QTL) regions associated with E. maxima and E. tenella resistance in chickens (Pinard-van der Laan et al. 2009; Bacciu et al. 2014; Hamzić et al. 2015). It is however notable that an inverse relationship has been observed between susceptibility to E. tenella and susceptibility to other Eimeria species such as E. maxima (Bumstead and Millard 1992), possibly limiting opportunities for breed improvement. Nonetheless, improved understanding of the genetic basis of coccidiosis resistance/tolerance/susceptibility traits through identification of genetic markers could be used to influence chicken breeding decisions, aiding future control of coccidiosis (Pinard-Van Der Laan et al. 1998; Boulton et al. 2018a). Genetic selection is a long-term approach that must be implemented throughout generations of chickens (Hamzić et al. 2015). However, in the long term it might prove more cost-effective, with fewer host and environmental effects, than chemoprophylactic and vaccinal methods of control.
Chemoprophylaxis
Control of coccidiosis by chemical prophylaxis has been practised in poultry production since 1948 (Grumbles et al. 1948; Chapman 2009; Kadykalo et al. 2018). Chemical intervention includes the use of two categories of anticoccidial compound: organic or synthetic. Organic compounds used for coccidiosis control are typically produced from fermentation reactions whereas synthetic compounds arise from chemical synthesis (Osweiler 2011; Noack et al. 2019). Ionophores, named due to their ion bearing properties, are a group of organic compounds that bind and transport ions across biological membranes, the majority of those used for control of coccidiosis are produced from fermentation reactions by Streptomyces species (Berger et al. 1951; Ryley and Wilson 1975; Remnant 2007; Osweiler 2011; Dorne et al. 2013; Clarke et al. 2014; Noack et al. 2019).
From a regulatory perspective, there is an important difference in the classification of anticoccidial drugs across different regions of the globe. For example, in Europe ionophores are classified as feed additives, whereas in the USA they are instead classified as polyether ionophorous antibiotics (Chapman 2001, 2005). It is important to note that compounds not classified as feed additives can still be administered in feed. Anticoccidial drugs in feed have been regulated in the EU since the 1970’s (Hafez 2008), with 11 compounds currently licenced in the EU as feed additives, detailed in Table 2 (Goetting et al. 2011; Peek and Landman 2011; Dorne et al. 2013; EU 2021). Of these, some are also licenced for use in other production systems such as ruminants (lasalocid, monensin, halofuginone, diclazuril and decoquinate), pigs (semduramicin and narasin), turkeys (lasalocid, monensin, diclazuril, halofuginone, narasin, nicarbazin and salinomycin) and rabbits (diclazuril) (Mooney et al. 2020; NOAH 2021). Some other compounds are licenced for therapeutic interventions against coccidiosis, including toltrazuril, but these are classified as pharmaceuticals.
Table 2.
The 11 anticoccidial compounds authorised as feed additives in the European Union (Abbas et al. 2011a; Anadón and Martínez-Larrañaga 2014; AnimalDrugs@FDA 2021; Belanger et al. 2013; Bozkurt et al. 2013; Castanon 2007; Chapman 1997; Clarke et al. 2014; Dorne et al. 2013; Dubey 2019; EU 2021; Gerhold 2014; Goetting et al. 2011; Hafez 2008; Harder and Haberkorn 1989; Kant et al. 2013; Noack et al. 2019; NOAH 2021; Salisch 1989)
| Compound | Category | Effective Conc. ppm | Mode of action | Example products and Withdrawal period (days)a | Year of introduction |
|---|---|---|---|---|---|
| Lasalocid | Divalent ionophore | 75–125 | Disruption of ion balance across biological membranes and therefore membrane potential, stimulation of mitochondrial ATPase activity | Avatec—5 | 1977 |
| Maduramicin | Monovalent glycosidic ionophore | 5–6 | Disruption of ion balance across biological membranes by formation of complexes with lipid soluble cations | Cygro—5 | 1989 |
| Monensin | Monovalent ionophore | 100–110 | Combines with sodium and potassium cations to form lipid-soluble complexes causing NA+ influx and disruption of membrane permeability to result in osmotic cell lysis | Elancoban—1 | 1971 |
| Coxidin—1 | |||||
| Monimax (with nicarbazin)—0 | |||||
| Narasin | Monovalent ionophore | 60–80 | Forms dynamically reversible lipid soluble complexes with cations which alters transmembrane ion gradients and electrical potentials. Commonly used in combination with nicarbazin | Monteban—0 | 1986 |
| Maxiban (with nicarbazin)—0 | |||||
| Salinomycin | Monovalent ionophore | 44–66 | Complexly binding monovalent cations, particularly potassium ions, promoting efflux into the cell mitochondria and cytoplasm | Sacox—0 | 1983 |
| Semduramicin | Monovalent glycosidic ionophore | 25 | Disruption of ion balance across biological membranes by formation of complexes with lipid soluble cations | Aviax—5 | 1995 |
| Decoquinate | Synthetic | 30 | Inhibition of the parasite mitochondrial electron transport and therefore respiration | Deccox—3 | 1967 |
| Diclazuril | Synthetic | 1 | Interference in nucleic acid and parasite wall synthesis producing thickened incomplete oocyst walls, disruption of mitochondrial transmembrane potential | Coxiril—0 | 1990 |
| Clinacox—5 | |||||
| Halofuginone | Synthetic | 3 | Unknown | Stenorol—5 | 1975 |
| Nicarbazin | Synthetic | 125 | Acts as a calcium ionophore by increasing lipoprotein lipase activity, interferes with cholesterol metabolism and the formation of vitelline membrane. Commonly used in combination with narasin | Maxiban (with narasin)—0 | 1955 |
| Monimax (with monensin)—0 | |||||
| Robenidine | Synthetic | 33 | Respiratory chain phosphorylation, ATPase and oxidative phosphorylation inhibition and energy metabolism interference, prevention of merozoite development | Robenz—5 | 1972 |
aWithdrawal periods vary between production systems with the majority of products not licenced for use in egg laying birds
Advantages of chemoprophylaxis
Advantages of chemoprophylactic control of coccidiosis include the ease of administration. The majority of anticoccidial drugs are incorporated into milled feed or dispensed in the drinking water, providing direct and quick administration with no requirement for extra labour costs. Where chemoprophylactics are successfully used there is no need for treated birds to compete for energy with the parasite, therefore energy can be focussed into production gains. Reduced Eimeria cycling also reduces the risk of enteric dysbiosis and specific secondary bacterial enteritis, including for example necrotic enteritis for which uncontrolled infection with Eimeria species, especially E. maxima is a known predisposing factor (Al-Sheikhly and Al-Saieg 1980; Williams et al. 2003; Williams 2005; Adhikari et al. 2020). Furthermore, ionophores have been shown to have antimicrobial activity against gram positive bacteria including Clostridium perfringens, the causative agent of necrotic enteritis (Liu et al. 1976; Al-Sheikhly and Al-Saieg 1980; Williams et al. 2003; Williams 2005; Chapman et al. 2010; Lanckriet et al. 2010; Peek and Landman 2011; Adhikari et al. 2020). Finally, all seven established Eimeria species can be targeted with most chemoprophylactics, and it is likely that the three newly described Eimeria species will be equally susceptible.
Disadvantages of chemoprophylaxis
Disadvantages of chemoprophylactic control most significantly include the widespread occurrence of anticoccidial drug resistance. First identified in the 1950s, resistance is now recognised in Eimeria against all current anticoccidial drugs where it can be defined as reduced effectiveness in comparison to efficacy at introduction, discussed further below (Cuckler and Malanga 1955; Joyner 1970; Braunius 1982; Chapman 1997; Chapman et al. 2013). Withdrawal periods are also a disadvantage for many products. Withdrawal of chemoprophylaxis is required during the period of greatest weight gain immediately before slaughter, leaving chickens vulnerable to uncontrolled infection. Additionally, consumer concerns over chemical residues in produce and consumer pressure for “drug-free”, particularly antibiotic free, production is a further disadvantage (Williams 1998; Jenkins 2004; Peek and Landman 2011; Kadykalo et al. 2018). Regulatory classification differences, mentioned earlier, poses another complication. For example, in the US ionophores are classified as antibiotics and are regulated as such, rather than as feed additives. In other countries, such as Sweden, prophylactic administration is banned for use in production systems (Remnant 2007; Swaggerty et al. 2011; Blake and Tomley 2014).
Finally, it is important to note the potential for environmental residues of anticoccidial drugs to pose toxicity risks to non-target-organisms. Research into this topic is based on data obtained from accidental ingestion or incorrect dosage ingestion case studies (Dorne et al. 2013; Mooney et al. 2020). Pathologically, ionophore toxicity causes increased mitochondrial uptake, and cardiac and peripheral muscle cell necrosis and clinical signs in animals and humans include muscle weakness, acute rhabdomyolysis and mucoid insufficiencies (Caldeira et al. 2001; Kouyoumdjian et al. 2001; Dorne et al. 2013). Synthetic anticoccidial drug toxicity, where studied, is variable depending on the drug and dose with effects ranging from vomiting (observed with decoquinate) to liver enlargement (observed with robenidine) and maternal and developmental toxicity (observed with halofuginone and nicarbazin) (Dorne et al. 2013; EFSA 2008a, b, c; 2019a; b).
Development of resistance to anticoccidial drugs
Widespread use of anticoccidial drugs has increased Eimeria exposure to the compounds, providing multiple opportunities for resistance development. The World Health Organization (WHO) defines parasite resistance as: ‘the ability of a parasite strain to survive and/or multiply despite the administration and absorption of a drug given in doses equal to or higher than those usually recommended but within the limits of tolerance of the subject’ (WHO 1965; Abbas et al. 2011a; Peek and Landman 2011). Inherited resistance to anticoccidial drugs is a genetic adaptation to survive selection pressure applied by the specific mode of action of the compound(s) in an anticoccidial product. Acquiring partial (toleration of low concentrations) or complete resistance is complex and is defined by the degree of loss of sensitivity (Abbas et al. 2011a). Based on the characterisation of field strains it is thought that resistance to most anticoccidial drugs requires mutations at multiple loci, as opposed to arising from single point mutations, except for resistance to quinolones (Chapman 1997). However, the precise genetic basis of resistance is not known for any current anticoccidial drug. Cross-resistance has also been reported for some anticoccidial drugs, for example between the ionophores salinomycin, monensin, narasin, lasalocid and maduramicin, and between the synthetic compounds diclazuril and toltrazuril (Chapman 1997; Stephan et al. 1997; Abbas et al. 2008) which have closely related modes of action (Abbas et al. 2011a). The administration of inappropriate, specifically low, dosages are a significant factor in the development of resistance as this provides selection for partially resistant strains that become more prevalent in the absence of competition from susceptible strains, reducing efficacy of control and contributing to the step-wise development of completely resistant which then rapidly become the dominant population (Cuckler and Malanga 1955; Chapman 1997; Swaggerty et al. 2011). Incorrect dosing can sometimes result from accidental exposure of chickens to environmental residues. For example, close proximity to poultry farming activity has been found to increase the likelihood of detection of environmental drug residues in groundwater, potentially originating from excretion or spreading manure (Mooney et al. 2020). Additionally, poor poultry house hygiene can play a significant role in resistance development, as control of high level parasitaemia is difficult and resistance can spread rapidly once it arises.
Future control by chemoprophylaxis
It is a testament to the poultry industry that anticoccidial chemoprophylactics are still effective, at any degree, based on the time elapsed since introduction and the high prevalence of resistance. Part of that success could be attributed to rotational use of different compounds between flocks, including selection of compounds with different modes of action to improve the likelihood of effective control against pre-existing resistant strains in the environment (Braunius 1982; Chapman 1997, 2007; Chapman et al. 2010; Noack et al. 2019). However, resistance remains a problem, demanding a range of alternative and combined approaches to control such as: vaccines and good husbandry measures, in addition to rotational use of anticoccidial drugs (Vegad 2004).
Research into new compounds for the control of coccidiosis has slowed in recent decades, predominantly due to lack of broad spectrum activity and genotoxicity, legislative restrictions, speed of resistance development and consumer concerns over chemical residues in food, all of which reduce incentive to discover and develop new anticoccidial compounds (Jenkins 2004; Biftu et al. 2006; Liang et al. 2007; Scribner et al. 2007, 2008; Peek and Landman 2011; Chapman et al. 2013; Kadykalo et al. 2018; Noack et al. 2019). When investigating potential new candidate compounds, an important consideration is parasite target stage, for example to target early asexual stages reducing pathology or by targeting gametes, preventing production of viable oocysts to reduce transmission.
Current research includes investigation of the anticoccidial effects of aminomizuril and ethanamizuril, both metabolites of nitromezuril; a triazine compound in the same family as toltrazuril and diclazuril. Both compounds were found to be effective against E. tenella, E. necatrix, E. acervulina and E. maxima with suggested action affecting transcription and protein metabolism including significant downregulation of GPI-linked surface antigen (SAG), proteins on the surface membranes of invasive sporozoites and merozoites thought to be related to host cell adhesion (Lal et al. 2009; Li et al. 2019; Noack et al. 2019; Zhang et al. 2019; Wang et al. 2020). The apparent lack of observed toxicity to the host and cross resistance with toltrazuril and diclazuril, encourage further development of nitromezuril and ethanamizuril as potential novel anticoccidials (Fei et al. 2013; Li et al. 2019; Zhang et al. 2020a,b). Future development and discovery of anticoccidial drugs is likely to lie closely with advances in genome annotation and understanding of parasite biology. An improved genome annotation and understanding of protein pathways could therefore provide opportunities for the identification of drug targets to interfere with parasite metabolism, survival and reproduction as a form of chemoprophylactic control.
Vaccination
Anticoccidial vaccination aims to induce protective immunity against coccidiosis, traditionally viewed as the prevention of parasite replication and absence of clinical signs in birds challenged with Eimeria (Rose 1963; Beattie 1997). It has been known for decades that exposure to Eimeria oocysts, most notably multiple doses termed a “trickle infection”, can induce a robust protective immune response (Joyner and Norton 1976). Moreover, it is recognised that in the field for most species of Eimeria full flock immunity occurs only after chickens have experienced two or more cycles of infection (Chapman 1999). These observations form the basis of currently available live anticoccidial vaccines.
Live and live attenuated vaccines
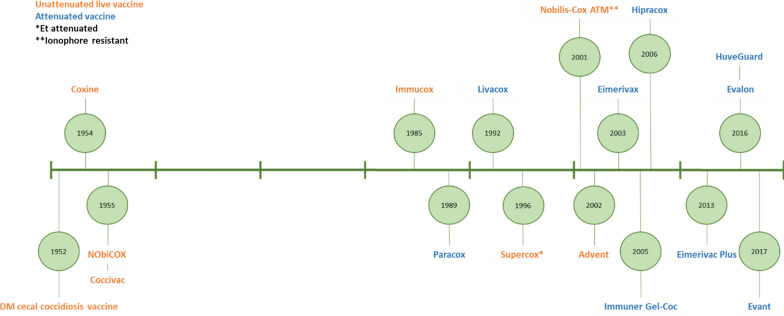
The first generation of vaccines against coccidiosis comprised admixtures of wild-type isolates of Eimeria oocysts and induced homologous immune protection against those species included in the mixture. Many wild-type vaccines have been developed, mainly using locally derived strains of parasites without any modification that changes their natural virulence. Thus, these vaccines remain fully virulent and are considered to be non-attenuated. Coccivac® containing E. tenella oocysts was launched as the first commercial coccidiosis vaccine in the US in 1952 under the trade name ‘DM Cecal Coccidiosis Vaccine’ (Edgar, 1958), Fig. 1. Thereafter, first-generation vaccines were developed to incorporate additional Eimeria species and have been widely utilized, particularly in North America (Soutter et al. 2020). Coccivac® has gone through many reformulations over the past six decades with variants of the original product: CocciVac-B, CocciVac-D, and most recently CocciVac D2. In 1985, Dr. Eng-Hong Lee (Lee 1987) developed Immucox, consisting of sporulated oocysts of E. acervulina, E. tenella and E. maxima with or without E. necatrix and E. brunetti. This was first marketed in Canada, but updated formulations are now used in more than 40 countries (Akanbi and Taiwo 2020).
Fig. 1.
Timeline of vaccine development for use in chickens
Second generation vaccines contain live oocysts from attenuated lines of Eimeria parasites. Heat treatment and x-irradiation were used in attempts to attenuate Eimeria, but neither were fully successful to induce robust and reproducible preparations (Mielke 1993; Jungmann and Mielke 1989). In most cases, attenuation has been achieved by selection for a more rapidly completed life cycle. Repeatedly harvesting the earliest “precocious” oocysts produced at the beginning of the patent period can be used to select for stable Eimeria populations that have shorter life-cycles, fewer endogenous stages and reduced pathogenicity (Jeffers 1975). Importantly, these precocious parasites remain highly immunogenic. This approach has underpinned development of most commercial attenuated anticoccidial vaccines such as Paracox®, Eimerivax®, Hipracox®, Eimerivac Plus® and Immuner Gel-Coc®. Although less common, attenuation has also been achieved by serial parasite passage through embryonated chicken eggs, for example selecting an E. tenella line that is included in the live attenuated Livacox® vaccine range (McDougald and Jeffers 1976; Bedrnik et al. 1989; Shirley and Bedrník 1997).
Recent innovations in live anticoccidial vaccination include the development of vaccine series such as HuveGuard®, where single vaccination using the formulation MMAT (including E. maxima, E. mitis, E. acervulina and E. tenella) can induce protection against species that are especially relevant to broiler production. Subsequently, vaccination using HuveGuard® NB from 14 days of age onwards can be used to vaccinate against E. necatrix and E. brunetti, less fecund species with longer lifecycles that are more relevant in older chickens. Alternatively, the vaccines Evalon® and Evant® produced by Hipra include a montanide-based adjuvant named Hipramune-T to enhance vaccine efficacy. In studies of E. acervulina and E. tenella profilin subunit antigen vaccines, montanide adjuvants have been shown to enhance protective immunity against avian coccidiosis by observed stimulation of IL-2, IL-10, IL-17A and IFN-γ gene transcription and increased CD8 + lymphocyte infiltration at the site of immunization (Jang et al. 2010,2011a,b).
In contrast to the live non-attenuated vaccines, live attenuated anticoccidial vaccines have a far greater safety margin, even if administered at a ten-fold overdose. Nonetheless, both vaccine types are effective and can induce a significant degree of immune protection against Eimeria challenge. Currently, only live attenuated anticoccidial vaccines are licenced in Europe, in contrast to much of the rest of the World where live non-attenuated vaccines are more common. To date there is little evidence of parasite evolution towards resistance against vaccination, likely influenced by host exposure to the large and complex array of antigens expressed by Eimeria throughout their endogenous lifecycle (Shirley et al. 2005; Reid et al. 2014).
Vaccination limitations
A significant drawback of live anticoccidial vaccines is that their production depends on in vivo growth of vaccinal parasites using chickens, as oocysts cannot be produced efficiently in vitro (Marugan-Hernandez et al. 2020). This is especially challenging for live-attenuated vaccine lines that have lowered reproductive capacity compared to non-attenuated equivalents, hence very large numbers of chickens are needed for vaccine production. Attenuated vaccines cost between two and six times more than non-attenuated alternatives (Blake et al. 2020). Immunity induced by live vaccination, as for natural infection, is exquisitely species-specific so effective vaccines have to include many different Eimeria species, and in some examples strains, each requiring independent amplification in chickens (Blake and Tomley 2014).
Differences between chicken production systems make it necessary to adapt vaccine formulations depending on the target animal. Those vaccines intended for use in intensively produced broiler chickens that are reared for only 5–7 weeks are likely to contain between three and five Eimeria species/strains, whereas vaccines for laying birds need to be more comprehensive and may contain all seven species of Eimeria. A critical inclusion for laying birds and breeding stock is E. necatrix, which can be a major cause of coccidiosis around the time when egg laying begins (McDougald et al. 1990).
Another complexity with these vaccines is the relative antigenic diversity that is observed in geographically distinct species or even strains of Eimeria. There is a risk of introducing an undesirable Eimeria species/strain present in vaccines into the farm environment. Strain-specific variation in E. maxima, the most antigenically diverse Eimeria species that infects chickens, has been reported. For example, it was found that the E. maxima parasites present in Immucox® were unable to protect against an indigenous E. maxima strain isolated from a peninsula in the Eastern Shore of Maryland, USA (Danforth et al. 1997; Long and Millard 1979). In response, some formulations such as Paracox® include two antigenically distinct E. maxima strains. Nonetheless, vaccines such as Paracox® and Immucox® are effective globally, indicating that antigenic diversity is not a common problem. However, no vaccine currently on the market includes any of the three Eimeria species described recently (Blake et al. 2021). These Eimeria species were first detected during an investigation of persistent vaccine failure (Morris et al. 2007), and all three have been shown to escape immunity induced by vaccination using at least one current vaccine (Blake et al. 2021). Careful evaluation is required when formulating live anticoccidial vaccines and those formulations may need to be fine-tuned according to experiences gained after implementation in new regions.
Application of non-attenuated live vaccines can pose safety issues if administered unevenly or to immune-suppressed chickens, resulting in compromised performance, clinical coccidiosis and even mortality (Anderson et al. 1976). Recent innovations in administration ameliorate this problem, as discussed below. Other limitations of live vaccines include the necessity for detailed quality control of the efficacy of each vaccine batch that can only be achieved in vivo, as well as a short shelf life and the requirement for a cold chain (Soutter et al. 2020). Another major challenge is inability to rapidly differentiate vaccinal Eimeria from field isolates, hindering quality control of vaccine administration, as well as diagnosis of vaccine-related problems and vaccine breaks.
Vaccine administration
Several approaches are used to administer anticoccidial vaccines to chickens. Most common methods include spraying oocysts directly onto newly hatched chicks so that oocysts are ingested during preening (Chapman 1996; Albanese et al. 2018), spraying onto food, incorporating oocysts within peckable gels that are given to newly hatched chicks (Danforth et al. 1997; Danforth 1998) or by dispersal in drinking water with a viscous agent that keeps oocysts in suspension (Williams 1999). Oocyst vaccines can also be administered via eye-drop inoculation at the hatchery (Chapman 1996).
Alternatives include in ovo injection on the 18th day of egg incubation. In ovo vaccination is common for a range of viral vaccines and products such as Inovocox have been developed for in ovo vaccination against coccidiosis, currently used in the US. Such an approach is attractive for mass administration of an Eimeria vaccine (Watkins et al. 1995), offering the opportunity for efficient pre-hatch delivery prior to exposure to environmental challenge (Chapman et al. 2002). The requirement to introduce complex formulations of sterile oocysts has been challenging, but not unsustainable (Shirley et al. 2005).
Accurate and even vaccine application is important. Asynchronous exposure can result in significant variation in the number of oocysts ingested, causing uncontrolled variation in immune status and the possibility of a coccidiosis outbreak in chickens with low previous exposure and immune responses (Williams 1998). Poor litter management can exacerbate the problem, preventing ingestion of a sufficient number of vaccine oocysts and limiting oocyst cycling. Chickens may be subjected to relatively high challenge doses of non-attenuated oocysts, resulting in high pathogenicity, or even mortality, necessitating the use of therapeutic anticoccidial drugs following vaccination (Lightowlers 1994; Reid 1990). Mitigations of this problem have included the development of bioshuttle programmes where chickens are vaccinated with a non-attenuated drug-susceptible anticoccidial vaccine at or around day of hatch. Vaccinated chicks then receive a drug-free starter diet, permitting efficient vaccine replication, followed by routine chemoprophylactic supplementation of the grower diet to limit uncontrolled parasite reproduction. Thus, the efficacy and safety of live anticoccidial vaccines could be enhanced by careful adaptation of administration methods and housing techniques available to promote and then control oocyst cycling.
Benefits associated with use of live anticoccidial vaccines include reducing the selective pressure on parasites that favours anticoccidial drug resistance. Incorporating between three and five rounds of vaccination using drug-susceptible vaccine strains in an integrated coccidiosis control programme, interrupts the routine application of anticoccidial drugs, and can restore sensitivity to drugs such as salinomycin (Chapman and Jeffers 2015). Thus, rotating between anticoccidial control using live parasite vaccines and chemoprophylaxis can improve the longevity of current control measures.
Future control by vaccination
Current limitations associated with chemoprophylaxis and vaccination for Eimeria have encouraged efforts to develop new control strategies, including a range of candidate recombinant vaccines. Since the first attempts to develop recombinant vaccines in the 1980’s several candidate antigens have been tried and tested (Vermeulen 1998; Blake and Tomley 2014), although no recombinant vaccine has reached the market. Nonetheless, efforts to develop recombinant vaccines are thought to be feasible because of the evident robust protective immune response achieved following natural Eimeria infection. Identification of antigens that induce natural immune protection can provide a rational basis to vaccine development. Several reviews focused on the identification, testing and delivery of immunoprotective antigens have been published in recent years (Blake et al. 2017; Venkatas and Adeleke 2019), so only brief details will be included here.
At least twenty five antigens have been defined and tested as vaccine candidates in Eimeria species, with varying levels of success (Blake et al. 2017). The results obtained from these studies typify the challenges faced by scientists trying to identify a “golden bullet” antigen or antigen cocktail to induce complete protection against complex pathogens such as Eimeria. One major issue is posed by the complexity of the Eimeria large genomes, with ~ 6000–10,000 protein coding genes, dependant on species (ToxoDB 2021), making it difficult to predict genuinely protective antigens that stimulate an efficacious immune response. Vaccine development for other apicomplexan parasites such as P. falciparum and T. gondii have suffered similar frustrations (Takala and Plowe 2009; Arnott et al. 2014; Gedik et al. 2016). One explanation for this could be that, unlike live vaccines, recombinant vaccines expressing one or a small number of antigens induce a more focused and less reproducible or efficacious immune response. Recognising that natural anticoccidial immune responses are species-specific, and in some examples strain-specific, it is likely that multiple antigens will be required in a future recombinant vaccine. If one to three antigens are required to protect against a single Eimeria species (Blake et al. 2017), it is likely that a recombinant anticoccidial vaccine for broilers may require six or more antigens to protect against key species such as E. acervulina, E. maxima and E. tenella, incurring significant challenges for manufacture.
Another significant task remains identification of an efficient delivery system for optimised panels of vaccinal antigens. Observations that cellular immunity may be key to successful immune protection against Eimeria challenge suggest that vaccination should elicit a T-lymphocyte response (Lillehoj et al. 2005). DNA vaccination could be considered based on descriptions of the mode of immune stimulation (Kalinna 1997), although scalable delivery to large numbers of chickens remains problematic. Alternatives include vectored vaccine approaches, with examples including Salmonella strains (Konjufca et al. 2006) or various yeasts such as Saccharomyces cerevisiae, which can survive the gastrointestinal tract of the host and give rise to a mucosal immune response (Sun et al. 2014). Genetically modified Eimeria strains have been tested as vaccine vectors, inspired by the opportunity to deliver antigens to the target gut compartment in a relevant biological context. Although some studies have suggested promising results and feasibility, such as using transgenic E. tenella to deliver an immunogenic antigen of E. maxima to produce partial protective immunity against E. maxima challenge (Tang et al. 2018), there is, however, still some way to go before Eimeria can be used as an efficient vaccine vector (Pastor-Fernández et al. 2018). Ultimately, progress towards novel vaccines is likely to depend on a combination of a deeper understanding of the mechanisms of immunity induced by Eimeria, helping to select appropriate parasite antigens that can cover a wider range of strains, and an improvement of genetic and culture manipulation tools.
Alternative strategies for control of coccidiosis
Probiotic supplementation
Challenges to effective control of coccidiosis posed by resistance to chemoprophylaxis or limited availability to cost-effective vaccines have prompted exploration for alternative strategies (Gaggìa et al. 2010; Giannenas et al. 2012; Ritzi et al. 2014), identifying a range of probiotics and dietary supplements such as essential oils or other herbal products (Guo et al. 2004; Quiroz-Castañeda and Dantán-González 2015).
Probiotic additives are live non-pathogenic microorganisms that are considered to have a health benefit when administered to chickens, commonly via their diet, usually with the aim of improving and maintaining a healthy gut microbiome (Gaggìa et al. 2010; Giannenas et al. 2012; Ritzi et al. 2014). The school of thought behind the use of probiotic supplementation in the control of coccidiosis is that a healthy microbiota can play a role in host immune system enhancement and protection against some intestinal pathogens (Dalloul et al. 2005; Ritzi et al. 2014). The most commonly used probiotics in the livestock industry, including in poultry are: Bacillus, Bifidobacteria, Enterococcus, Lactobacillus and Saccharomyces (Gaggìa et al. 2010). The efficacy of probiotics in protection against pathological lesions caused by Eimeria species has not been definitively proven (Giannenas et al. 2012). Some studies have shown that treatment with probiotics, such as Lactobacillus salivarius and L. acidophilus, associates with reduced oocyst shedding, while supplementation with a Bacillus has been associated with lower lesion scores compared to untreated controls (Dalloul et al. 2003, 2005; Tierney et al. 2004; El-Dakhly et al. 2006; Lee et al. 2010b; Giannenas et al. 2012). Typically, studies into the efficacy of probiotics conclude that they can alleviate the effects of coccidiosis when anticoccidial drugs are not in use however when performance is directly compared, anticoccidial drugs tend to outperform probiotic treatment, particularly at peak infection and in measures such as oocyst shedding or feed conversion ratio (Giannenas et al. 2012; Bozkurt et al. 2014; Ritzi et al. 2014).
Essential oil and organic acid supplementation
Several essential oils have also been suggested as alternatives for the control of coccidiosis, commonly due to their reported antiparasitic action. Some, such as oregano, thyme and garlic, have been associated with reduced disease burden in terms of improved body weight gain, reduced oocyst shedding following challenge and fewer pathological lesions (Giannenas et al. 2003; Küçükyilmaz et al. 2012; Abou-Elkhair et al. 2014). The efficacy of essential oils is not, however, well characterised. Generally, the use of anticoccidial drugs outperforms treatment with essential oils and in some cases toxicity of essential oils used can result in poor performance, indicating the importance of establishing an effective concentration (Giannenas et al. 2003; Christaki et al. 2004; Oviedo-Rondón et al. 2005, 2006; Küçükyilmaz et al. 2012). Essential oils may therefore be a useful supplement for chicken diets to provide some anticoccidial effects and improve host intestinal health, although they are unlikely to replace anticoccidial drugs.
Dietary supplementation with organic acids such as acetic and butyrate acid have also been suggested for the control of coccidiosis due to observed improved weight gain, feed conversion ratio and reduced oocyst shedding and lesion scores, in addition to their growth-promoting, antimicrobial and immune stimulating properties (Abbas et al. 2011b; Ali et al. 2014). In some studies reduced feed intake has been reported due to reduced palatability of feed supplemented with organic acids (Cave 1984; Ali et al. 2014), therefore when supplementing feeds it is important to consider the effect on the feed acidity and odour and minimise adverse changes that would reduce feed intake or body weight gain.
Future control by alternative measures
For many of the alternative measures suggested for the control of coccidiosis there is a lack of understanding of the full mechanism of action against the parasite. Additionally, in most cases the greatest positive effects against infection with Eimeria were observed when the alternative measure was used in combination with anticoccidial drugs or alongside vaccination (Abbas et al. 2011b; Giannenas et al. 2012; Ali et al. 2014; Bozkurt et al. 2014; Ritzi et al. 2014). It is therefore important to note that further investigation is required into these alternative measures before conclusions can be drawn about their cost effectiveness in comparison to other current measures for control.
Conclusions
Successful control of coccidiosis is multifactorial. Good animal husbandry is a key cornerstone in this endeavour and involves strict biosecurity measures, commonly supplemented with chemoprophylaxis and/or vaccination (Awais et al. 2012; Reid et al. 2014; Lawal et al. 2016; Morgan and Godwin 2017). The outcome of control by chemoprophylaxis or vaccination is influenced by factors such as chicken age, type of production system and genetic capacity for tolerance to subclinical infection.
Alternative control measures including probiotics and a range of food supplements are becoming increasingly popular, however, evidence of their efficacy remains limited. Improved control can be supported through better education and management of potential risk factors, for example: temperature, humidity, accumulation of sporulated oocysts on litter and rotational use of chemoprophylactics to reduce resistance emergence.
Immunity induced by current anticoccidial vaccines is Eimeria species-specific, and vaccine composition must be tailored to each geographical region and chicken production system (Graat et al. 1994), therefore, accurately identifying geographical prevalence and genetic diversity within Eimeria species and strains is important for successful control (Morris and Gasser 2006; Lee et al. 2010a; Ogedengbe et al. 2011; Györke et al. 2013).
In the future, improved and more readily scalable vaccines can be expected to make a bigger contribution to control of coccidiosis, together with complementary strategies such as rotational use of anticoccidial drugs with differing modes of action and selective breeding for improved resistance to the parasite. As the research community continues to increase understanding of Eimeria species parasites and host immunity, control measures will develop through identification of anticoccidial drug and vaccine candidate targets. These efforts can improve chicken welfare and reduce economic losses incurred by the poultry industry as a whole, including in low- and middle-income countries vulnerable to the economic burden of coccidiosis.
Acknowledgements
Not applicable.
Authors’ contributions
EA and GS were the most significant contributors to the manuscript, MJ contributed to drafting the manuscript and DX, VM, DB and FT contributed to the editing and revision of the manuscript. All authors read and approved the final manuscript.
Funding
The Royal Veterinary College, UK.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elizabeth Attree and Gonzalo Sanchez-Arsuaga contributed equally to this work
Contributor Information
Elizabeth Attree, Email: eattree18@rvc.ac.uk.
Gonzalo Sanchez-Arsuaga, Email: gsanchezarsuaga@rvc.ac.uk.
References
- Abbas R, Iqbal Z, Sindhu Z-D, Khan M, Arshad M. Identification of cross-resistance and multiple resistance in Eimeria tenella field isolates to commonly used anticoccidials in Pakistan. J Appl Poult Res. 2008;17(3):361–368. doi: 10.3382/japr.2008-00027. [DOI] [Google Scholar]
- Abbas R, Iqbal Z, Blake D, Khan M, Saleemi M. Anticoccidial drug resistance in fowl coccidia: the state of play revisited. Worlds Poult Sci J. 2011;67(2):337–350. doi: 10.1017/S004393391100033X. [DOI] [Google Scholar]
- Abbas RZ, Munawar SH, Manzoor Z, Iqbal Z, Khan MN, Saleemi MK, Zia MA, Yousaf A. Anticoccidial effects of acetic acid on performance and pathogenic parameters in broiler chickens challenged with Eimeria tenella. Pesqui Vet Brasil. 2011;31(2):99–103. doi: 10.1590/S0100-736X2011000200001. [DOI] [Google Scholar]
- Abou-Elkhair R, Gaafar KM, Elbahy N, Helal MA, Mahboub HD, Sameh G. Bioactive effect of dietary supplementation with essential oils blend of oregano, thyme and garlic oils on performance of broilers infected with Eimeria species. Glob Vet. 2014;13(6):977–985. [Google Scholar]
- Adhikari P, Kiess A, Adhikari R, Jha R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J Appl Poult Res. 2020;29(2):515–534. doi: 10.1016/j.japr.2019.11.005. [DOI] [Google Scholar]
- Ahad S, Tanveer S, Malik TA. Seasonal impact on the prevalence of coccidian infection in broiler chicks across poultry farms in the Kashmir valley. J Parasit Dis. 2015;39(4):736–740. doi: 10.1007/s12639-014-0434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanbi OB, Taiwo VO. The effect of a local isolate and Houghton strain of Eimeria tenella on clinical and growth parameters following challenge in chickens vaccinated with IMMUCOX® and LIVACOX® vaccines. J Parasit Dis. 2020 doi: 10.1007/s12639-020-01202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese GA, Tensa LR, Aston EJ, Hilt DA, Jordan BJ. Evaluation of a coccidia vaccine using spray and gel applications. Poult Sci. 2018;97(5):1544–1553. doi: 10.3382/ps/pey011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Gawad AA, Mahdy OA, El-Massry AA, Al-Aziz MS. Studies on coccidia of Egyptian Balady breed chickens. Life Sci J. 2012;9(3):568–576. [Google Scholar]
- Ali A, Seddiek SA, Khater H. Effect of butyrate, clopidol and their combination on the performance of broilers infected with Eimeria maxima. Br Poult Sci. 2014;55(4):474–482. doi: 10.1080/00071668.2014.920488. [DOI] [PubMed] [Google Scholar]
- Allen PC, Fetterer R. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin Microbiol Rev. 2002;15(1):58–65. doi: 10.1128/CMR.15.1.58-65.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sheikhly F, Al-Saieg A. Role of coccidia in the occurrence of necrotic enteritis of chickens. Avian Dis. 1980 doi: 10.2307/1589700. [DOI] [PubMed] [Google Scholar]
- Anadón A, Martínez-Larrañaga M. Veterinary drugs residues: coccidiostats. In: Motarjemi Y, editor. Encyclopedia of food safety; 2014;3:63-75.
- Anderson WI, Reid WM, Johnson JK. Effects of high environmental temperatures on cecal coccidiosis. Poult Sci. 1976;55(4):1429–1435. doi: 10.3382/ps.0551429. [DOI] [PubMed] [Google Scholar]
- AnimalDrugs@FDA. US food and drug. 2021. https://animaldrugsatfda.fda.gov/adafda/views/#/home/searchResult.
- Arnott A, Wapling J, Mueller I, Ramsland PA, Siba PM, Reeder JC, Barry AE. Distinct patterns of diversity, population structure and evolution in the AMA1 genes of sympatric Plasmodium falciparum and Plasmodium vivax populations of Papua New Guinea from an area of similarly high transmission. Malar J. 2014;13(1):233. doi: 10.1186/1475-2875-13-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awais MM, Akhtar M, Iqbal Z, Muhammad F, Anwar MI. Seasonal prevalence of coccidiosis in industrial broiler chickens in Faisalabad, Punjab, Pakistan. Trop Anim Health Prod. 2012;44(2):323–328. doi: 10.1007/s11250-011-0024-x. [DOI] [PubMed] [Google Scholar]
- Bacciu N, Bed’Hom B, Filangi O, Romé H, Gourichon D, Répérant J-M, Le Roy P, Pinard-van der Laan M-H, Demeure O. QTL detection for coccidiosis (Eimeria tenella) resistance in a Fayoumi×Leghorn F2 cross, using a medium-density SNP panel. Genet Sel Evol. 2014;46(1):14. doi: 10.1186/1297-9686-46-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie SE. Immunity to and transport of sporozoites of Eimeria species in the domestic fowl, Gallusdomesticus. Canada: University of Guelph; 1997. [Google Scholar]
- Bedrnik P, Kucera J, Firmanova A, Jurkovic P. Field vaccination of broilers against coccidiosis. Avian Pathol. 1989;18(2):255–264. doi: 10.1080/03079458908418600. [DOI] [PubMed] [Google Scholar]
- Belanger A, Haydon K, Keffaber K, Marsteller T. The science and mechanisms behind ionophores for pigs and poultry. Greenfield Indiana: Elanco Animal Health; 2013. [Google Scholar]
- Berger J, Rachlin A, Scott W, Sternbach L, Goldberg M. The isolation of three new crystalline antibiotics from streptomyces1. J Am Chem Soc. 1951;73(11):5295–5298. doi: 10.1021/ja01155a084. [DOI] [Google Scholar]
- Biftu T, Feng D, Fisher M, Liang G-B, Qian X, Scribner A, Dennis R, Lee S, Liberator PA, Brown C. Synthesis and SAR studies of very potent imidazopyridine antiprotozoal agents. Bioorg Med Chem Lett. 2006;16(9):2479–2483. doi: 10.1016/j.bmcl.2006.01.092. [DOI] [PubMed] [Google Scholar]
- Bishop SC, Woolliams JA. Genomics and disease resistance studies in livestock. Livest Sci. 2014;166:190–198. doi: 10.1016/j.livsci.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DP, Tomley FM. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 2014;30(1):12–19. doi: 10.1016/j.pt.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Blake DP, Hesketh P, Archer A, Carroll F, Shirley MW, Smith AL. The influence of immunizing dose size and schedule on immunity to subsequent challenge with antigenically distinct strains of Eimeria maxima. Avian Pathol. 2005;34(6):489–494. doi: 10.1080/03079450500368292. [DOI] [PubMed] [Google Scholar]
- Blake DP, Clark EL, Macdonald SE, Thenmozhi V, Kundu K, Garg R, Jatau ID, Ayoade S, Kawahara F, Moftah A. Population, genetic, and antigenic diversity of the apicomplexan Eimeria tenella and their relevance to vaccine development. Proc Natl Acad Sci. 2015;112(38):E5343–E5350. doi: 10.1073/pnas.1506468112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DP, Pastor-Fernández I, Nolan MJ, Tomley FM. Recombinant anticoccidial vaccines-a cup half full? Infect Genet Evol. 2017;55:358–365. doi: 10.1016/j.meegid.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Blake DP, Knox J, Dehaeck B, Huntington B, Rathinam T, Ravipati V, Ayoade S, Gilbert W, Adebambo AO, Jatau ID. Re-calculating the cost of coccidiosis in chickens. Vet Res. 2020;51(1):1–14. doi: 10.1186/s13567-020-00837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D, Vrba V, Xia D, Danladi Jatau I, Spiro S, Nolan MJ, Underwood G, Tomley F. Genetic and biological characterisation of three cryptic Eimeria operational taxonomic units that infect chickens (Gallus gallus domesticus) Int J Parasitol. 2021 doi: 10.1016/j.ijpara.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton K, Nolan MJ, Wu Z, Psifidi A, Riggio V, Harman K, Bishop SC, Kaiser P, Abrahamsen MS, Hawken R. Phenotypic and genetic variation in the response of chickens to Eimeria tenella induced coccidiosis. Genet Sel Evol. 2018;50(1):1–12. doi: 10.1186/s12711-018-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton K, Nolan MJ, Wu Z, Riggio V, Matika O, Harman K, Hocking PM, Bumstead N, Hesketh P, Archer A. Dissecting the genomic architecture of resistance to Eimeria maxima parasitism in the chicken. Front Genet. 2018;9:528. doi: 10.3389/fgene.2018.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt M, Giannenas I, Küçükyilmaz K, Christaki E, Florou-Paneri P. An update on approaches to controlling coccidia in poultry using botanical extracts. Br Poult Sci. 2013;54(6):713–727. doi: 10.1080/00071668.2013.849795. [DOI] [PubMed] [Google Scholar]
- Bozkurt M, Aysul N, Küçükyilmaz K, Aypak S, Ege G, Catli A, Akşit H, Çöven F, Seyrek K, Çınar M. Efficacy of in-feed preparations of an anticoccidial, multienzyme, prebiotic, probiotic, and herbal essential oil mixture in healthy and Eimeria spp.-infected broilers. Poult sci. 2014;93(2):389–399. doi: 10.3382/ps.2013-03368. [DOI] [PubMed] [Google Scholar]
- Braunius W. Coccidiosis in broilers: the effective use of anticoccidial drugs. Worlds Poult Sci J. 1982;38(3):176–185. doi: 10.1079/WPS19820013. [DOI] [Google Scholar]
- Bumstead N, Millard B. Variation in susceptibility of inbred lines of chickens to seven species of Eimeria. Parasitology. 1992;104(3):407–413. doi: 10.1017/S0031182000063654. [DOI] [PubMed] [Google Scholar]
- Burrell A, Tomley FM, Vaughan S, Marugan-Hernandez V. Life cycle stages, specific organelles and invasion mechanisms of Eimeria species. Parasitology. 2020;147(3):263–278. doi: 10.1017/S0031182019001562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira C, Neves WS, Cury PM, Serrano P, Baptista MA, Burdmann EA. Rhabdomyolysis, acute renal failure, and death after monensin ingestion. Am J Kidney Dis. 2001;38(5):1108–1112. doi: 10.1053/ajkd.2001.28618. [DOI] [PubMed] [Google Scholar]
- Cantacessi C, Riddell S, Morris GM, Doran T, Woods WG, Otranto D, Gasser RB. Genetic characterization of three unique operational taxonomic units of Eimeria from chickens in Australia based on nuclear spacer ribosomal DNA. Vet Parasitol. 2008;152(3–4):226–234. doi: 10.1016/j.vetpar.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Castanon J. History of the use of antibiotic as growth promoters in European poultry feeds. Poult Sci. 2007;86(11):2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- Cave N. Effect of dietary propionic and lactic acids on feed intake by chicks. Poult Sci. 1984;63(1):131–134. doi: 10.3382/ps.0630131. [DOI] [PubMed] [Google Scholar]
- Champion LR. The inheritance of resistance to cecal coccidiosis in the domestic fowl. Poult Sci. 1954;33(4):670–681. doi: 10.3382/ps.0330670. [DOI] [Google Scholar]
- Chapman HD. Administration of a coccidiosis vaccine to day-old turkeys via the eye and development of immunity to Eimeria species. Poult Sci. 1996;75(12):1496–1497. doi: 10.3382/ps.0751496. [DOI] [PubMed] [Google Scholar]
- Chapman H. Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol. 1997;26(2):221–244. doi: 10.1080/03079459708419208. [DOI] [PubMed] [Google Scholar]
- Chapman H. The development of immunity to Eimeria species in broilers given anticoccidial drugs. Avian Pathol. 1999;28(2):155–162. doi: 10.1080/03079459994885. [DOI] [PubMed] [Google Scholar]
- Chapman H. Use of anticoccidial drugs in broiler chickens in the USA: analysis for the years 1995 to 1999. Poult Sci. 2001;80(5):572–580. doi: 10.1093/ps/80.5.572. [DOI] [PubMed] [Google Scholar]
- Chapman HD. Perspectives for the control of coccidiosis in poultry by chemotherapy and vaccination. Guelph: Proceedings of the Ninth International Coccidiosis Conference; 2005. [Google Scholar]
- Chapman H. Rotation programmes for coccidiosis control. Int Poult Product. 2007;15(1):7–9. [Google Scholar]
- Chapman H. A landmark contribution to poultry science—prophylactic control of coccidiosis in poultry. Poult Sci. 2009;88(4):813–815. doi: 10.3382/ps.2008-00316. [DOI] [PubMed] [Google Scholar]
- Chapman H, Jeffers T. Restoration of sensitivity to salinomycin in Eimeria following 5 flocks of broiler chickens reared in floor-pens using drug programs and vaccination to control coccidiosis. Poult Sci. 2015;94(5):943–946. doi: 10.3382/ps/pev077. [DOI] [PubMed] [Google Scholar]
- Chapman H, Cherry T, Danforth H, Richards G, Shirley M, Williams R. Sustainable coccidiosis control in poultry production: the role of live vaccines. Int J Parasitol. 2002;32(5):617–629. doi: 10.1016/S0020-7519(01)00362-9. [DOI] [PubMed] [Google Scholar]
- Chapman H, Jeffers T, Williams R. Forty years of monensin for the control of coccidiosis in poultry. Poult Sci. 2010;89(9):1788–1801. doi: 10.3382/ps.2010-00931. [DOI] [PubMed] [Google Scholar]
- Chapman HD, Barta JR, Blake D, Gruber A, Jenkins M, Smith NC, Suo X, Tomley FM. A selective review of advances in coccidiosis research. Adv Parasitol. 2013;83(83):93–171. doi: 10.1016/B978-0-12-407705-8.00002-1. [DOI] [PubMed] [Google Scholar]
- Christaki E, Florou-Paneri P, Giannenas I, Papazahariadou M, Botsoglou NA, Spais AB. Effect of a mixture of herbal extracts on broiler chickens infected with Eimeria tenella. Anim Res. 2004;53(2):137–144. doi: 10.1051/animres:2004006. [DOI] [Google Scholar]
- Cisman M, Ahmed Z, Mohamoud H. Scope specification of coccidiosis in the poultry on researchers. Int J Avian Wildl Biol. 2020;5(2):32–37. doi: 10.15406/ijawb.2020.05.00171. [DOI] [Google Scholar]
- Clark EL, Macdonald SE, Thenmozhi V, Kundu K, Garg R, Kumar S, Ayoade S, Fornace KM, Jatau ID, Moftah A. Cryptic Eimeria genotypes are common across the Southern but not Northern hemisphere. Int J Parasitol. 2016;46(9):537–544. doi: 10.1016/j.ijpara.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L, Fodey TL, Crooks SR, Moloney M, O'Mahony J, Delahaut P, O’Kennedy R, Danaher M. A review of coccidiostats and the analysis of their residues in meat and other food. Meat Sci. 2014;97(3):358–374. doi: 10.1016/j.meatsci.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Cuckler AC, Malanga CM. Studies on drug resistance in coccidia. J Parasitol. 1955;41(3):302–311. doi: 10.2307/3274212. [DOI] [PubMed] [Google Scholar]
- Dalloul R, Lillehoj H, Shellem T, Doerr J. Enhanced mucosal immunity against Eimeria acervulina in broilers fed a Lactobacillus-based probiotic. Poult Sci. 2003;82(1):62–66. doi: 10.1093/ps/82.1.62. [DOI] [PubMed] [Google Scholar]
- Dalloul RA, Lillehoj HS, Tamim NM, Shellem TA, Doerr JA. Induction of local protective immunity to Eimeria acervulina by a Lactobacillus-based probiotic. Comp Immunol Microbiol Infect Dis. 2005;28(5–6):351–361. doi: 10.1016/j.cimid.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Danforth HD. Use of live oocyst vaccines in the control of avian coccidiosis: experimental studies and field trials. Int J Parasitol. 1998;28(7):1099–1109. doi: 10.1016/S0020-7519(98)00078-2. [DOI] [PubMed] [Google Scholar]
- Danforth HD, Lee EH, Martin A, Dekich M. Evaluation of a gel-immunization technique used with two different Immucox vaccine formulations in battery and floor-pen trials with broiler chickens. Parasitol Res. 1997;83(5):445–451. doi: 10.1007/s004360050278. [DOI] [PubMed] [Google Scholar]
- Dar AS, Anwar AH. Incidence and pathogenesis of coccidiosis in chickens around Faisalabad. Pak Vet J. 1981;1(1):20–21. [Google Scholar]
- Dorne J, Fernández-Cruz M, Bertelsen U, Renshaw D, Peltonen K, Anadon A, Feil A, Sanders P, Wester P, Fink-Gremmels J. Risk assessment of coccidostatics during feed cross-contamination: animal and human health aspects. Toxicol Appl Pharmacol. 2013;270(3):196–208. doi: 10.1016/j.taap.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Coccidiosis in livestock, poultry, companion animals, and humans. Boca Raton: CRC Press; 2019. [Google Scholar]
- Edgar S. Sporulation of oocysts at specific temperatures and notes on the prepatent period of several species of avian coccidia. J Parasitol. 1955;41(2):214–216. doi: 10.2307/3273795. [DOI] [PubMed] [Google Scholar]
- EFSA, E. F. S. A. Cross-contamination of non-target feedingstuffs by decoquinate authorised for use as a feed additive-scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008;6(4):656. doi: 10.2903/j.efsa.2008.656. [DOI] [Google Scholar]
- EFSA, E. F. S. A. Cross-contamination of non-target feedingstuffs by halofuginone hydrobromide authorised for use as a feed additive-scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008;6(4):657. doi: 10.2903/j.efsa.2008.657. [DOI] [Google Scholar]
- EFSA, E. F. S. A. Cross-contamination of non-target feedingstuffs by nicarbazin authorised for use as a feed additive-scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008;6(4):690. doi: 10.2903/j.efsa.2008.690. [DOI] [Google Scholar]
- EFSA, E. F. S. A. Safety and efficacy of Deccox®(decoquinate) for chickens for fattening. EFSA J. 2019;17(1):e05541. doi: 10.2903/j.efsa.2019.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA, E. F. S. A. Safety and efficacy of Robenz® 66G (robenidine hydrochloride) for chickens for fattening and turkeys for fattening. EFSA J. 2019;17(3):e05613. doi: 10.2903/j.efsa.2019.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Dakhly KM, El-Sawah AA, Shalaby A, El-Nesr KA. The efficacy of Lactobacillus acidophilus and/or diclazuril for inhibition and control of Eimeria tenella infection in balady chicks. Kafrelsheikh Vet Med J. 2006;4(1):1–19. doi: 10.21608/kvmj.2006.109296. [DOI] [Google Scholar]
- Etuk E, Okoli I, Uko M. Prevalence and management issues associated with poultry coccidiosis in Abak agricultural zone of Akwa Ibom state, Nigeria. Int J Poult Sci. 2004;3(2):135–139. doi: 10.3923/ijps.2004.135.139. [DOI] [Google Scholar]
- EU . European Union Register of Feed Additives pursuant to Regulation (EC) No. 1831/2003, Annex I: list of additives. Luxembourg: Publications Office of the Europe an Union; 2021. p. 2021. [Google Scholar]
- Farr MM, Wehr EE. Survival of Eimeria acervulina, E. tenella, and E. maxima oocysts on soil under various field conditions. Ann NY Acad Sci. 1949;52(4):468–472. doi: 10.1111/j.1749-6632.1949.tb53932.x. [DOI] [Google Scholar]
- Fatoba AJ, Adeleke MA. Diagnosis and control of chicken coccidiosis: a recent update. J Parasit Dis. 2018;42(4):483–493. doi: 10.1007/s12639-018-1048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R. Epidemiology of protozoan infections: the coccidia. Vet Parasitol. 1980;6(1–3):75–103. doi: 10.1016/0304-4017(80)90039-4. [DOI] [Google Scholar]
- Fei C, Fan C, Zhao Q, Lin Y, Wang X, Zheng W, Wang M, Zhang K, Zhang L, Li T. Anticoccidial effects of a novel triazine nitromezuril in broiler chickens. Vet Parasitol. 2013;198(1–2):39–44. doi: 10.1016/j.vetpar.2013.08.024. [DOI] [PubMed] [Google Scholar]
- Fish F. The effect of physical and chemical agents on the oocysts of Eimeria tenella. Science. 1931;73(1889):292–293. doi: 10.1126/science.73.1889.292. [DOI] [PubMed] [Google Scholar]
- Fornace KM, Clark EL, Macdonald SE, Namangala B, Karimuribo E, Awuni JA, Thieme O, Blake DP, Rushton J. Occurrence of Eimeria species parasites on small-scale commercial chicken farms in Africa and indication of economic profitability. PLoS ONE. 2013;8(12):e84254. doi: 10.1371/journal.pone.0084254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggìa F, Mattarelli P, Biavati B. Probiotics and prebiotics in animal feeding for safe food production. Int J Food Microbiol. 2010;141:S15–S28. doi: 10.1016/j.ijfoodmicro.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Gedik Y, İz SG, Can H, Döşkaya AD, Gürhan SİD, Gürüz Y, Döşkaya M. Immunogenic multistage recombinant protein vaccine confers partial protection against experimental toxoplasmosis mimicking natural infection in murine model. Trials Vaccinol. 2016;5:15–23. doi: 10.1016/j.trivac.2015.11.002. [DOI] [Google Scholar]
- Gerhold RWJ. Overview of coccidiosis in poultry. 2014. https://www.msdvetmanual.com/poultry/coccidiosis/overview-of-coccidiosis-in-poultry. Accessed 12 July 2021.
- Giannenas I, Florou-Paneri P, Papazahariadou M, Christaki E, Botsoglou N, Spais A. Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Arch Anim Nutr. 2003;57(2):99–106. doi: 10.1080/0003942031000107299. [DOI] [PubMed] [Google Scholar]
- Giannenas I, Papadopoulos E, Tsalie E, Triantafillou E, Henikl S, Teichmann K, Tontis D. Assessment of dietary supplementation with probiotics on performance, intestinal morphology and microflora of chickens infected with Eimeria tenella. Vet Parasitol. 2012;188(1–2):31–40. doi: 10.1016/j.vetpar.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Goetting V, Lee K, Tell LA. Pharmacokinetics of veterinary drugs in laying hens and residues in eggs: a review of the literature. J Vet Pharmacol Ther. 2011;34(6):521–556. doi: 10.1111/j.1365-2885.2011.01287.x. [DOI] [PubMed] [Google Scholar]
- Graat E, Henken A, Ploeger H, Noordhuizen J, Vertommen M. Rate and course of sporulation of oocysts of Eimeria acervulina under different environmental conditions. Parasitology. 1994;108(5):497–502. doi: 10.1017/S0031182000077350. [DOI] [PubMed] [Google Scholar]
- Gross W. Effect of social environment and oocyst dose on resistance and immunity to Eimeria tenella challenge. Avian Dis. 1985;29(4):1018–1029. doi: 10.2307/1590455. [DOI] [PubMed] [Google Scholar]
- Grumbles L, Delaplane J, Higgins T. Continuous feeding of low concentrations of sulfaquinoxaline for the control of coccidiosis in poultry. Poult Sci. 1948;27(5):605–608. doi: 10.3382/ps.0270605. [DOI] [Google Scholar]
- Guo F, Kwakkel R, Williams B, Parmentier H, Li W, Yang Z, Verstegen M. Effects of mushroom and herb polysaccharides on cellular and humoral immune responses of Eimeria tenella-infected chickens. Poult Sci. 2004;83(7):1124–1132. doi: 10.1093/ps/83.7.1124. [DOI] [PubMed] [Google Scholar]
- Györke A, Pop L, Cozma V. Prevalence and distribution of Eimeria species in broiler chicken farms of different capacities. Parasite. 2013 doi: 10.1051/parasite/2013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafez HM. Poultry coccidiosis: prevention and control approaches. Archiv Fur Geflugelkd. 2008;72(1):2–7. [Google Scholar]
- Hamzić E, Buitenhuis B, Hérault F, Hawken R, Abrahamsen MS, Servin B, Elsen J-M, Pinard-van der Laan M-H, Bed’Hom B. Genome-wide association study and biological pathway analysis of the Eimeria maxima response in broilers. Genet Sel Evol. 2015;47(1):91. doi: 10.1186/s12711-015-0170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder A, Haberkorn A. Possible mode of action of toltrazuril: studies on two Eimeria species and mammalian and Ascaris suum enzymes. Parasitol Res. 1989;76(1):8–12. doi: 10.1007/BF00931064. [DOI] [PubMed] [Google Scholar]
- Hauck R, Carrisosa M, McCrea BA, Dormitorio T, Macklin KS. Evaluation of next-generation amplicon sequencing to identify Eimeria spp. of chickens. Avian Dis. 2019;63(4):577–583. doi: 10.1637/aviandiseases-D-19-00104. [DOI] [PubMed] [Google Scholar]
- Haug A, Gjevre A-G, Thebo P, Mattsson JG, Kaldhusdal M. Coccidial infections in commercial broilers: epidemiological aspects and comparison of Eimeria species identification by morphometric and polymerase chain reaction techniques. Avian Pathol. 2008;37(2):161–170. doi: 10.1080/03079450801915130. [DOI] [PubMed] [Google Scholar]
- Hinsu AT, Thakkar JR, Koringa PG, Vrba V, Jakhesara SJ, Psifidi A, Guitian J, Tomley FM, Rank DN, Raman M. Illumina next generation sequencing for the analysis of Eimeria populations in commercial broilers and indigenous chickens. Front Vet Sci. 2018;5:176. doi: 10.3389/fvets.2018.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton-Smith C, Long P. The development of Eimeria necatrix Johnson, 1930 and Eimeria brunetti Levine, 1942 in the caeca of the domestic fowl (Gallus domesticus) Parasitology. 1965;55(3):401–405. doi: 10.1017/S0031182000069109. [DOI] [Google Scholar]
- Horton-Smith C, Taylor E, Turtle E. Ammonia fumigation for coccidial disinfection. Vet Rec. 1940;52:829–832. [Google Scholar]
- Jang SI, Lillehoj HS, Lee SH, Lee KW, Park MS, Bauchan GR, Lillehoj EP, Bertrand F, Dupuis L, Deville S. Immunoenhancing effects of Montanide™ ISA oil-based adjuvants on recombinant coccidia antigen vaccination against Eimeria acervulina infection. Vet Parasitol. 2010;172(3–4):221–228. doi: 10.1016/j.vetpar.2010.04.042. [DOI] [PubMed] [Google Scholar]
- Jang SI, Lillehoj HS, Lee SH, Lee KW, Lillehoj EP, Bertrand F, Dupuis L, Deville S. Montanide™ ISA 71 VG adjuvant enhances antibody and cell-mediated immune responses to profilin subunit antigen vaccination and promotes protection against Eimeria acervulina and Eimeria tenella. Exp Parasitol. 2011;127(1):178–183. doi: 10.1016/j.exppara.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Jang SI, Lillehoj HS, Lee SH, Lee KW, Lillehoj EP, Bertrand F, Dupuis L, Deville S. Mucosal immunity against Eimeria acervulina infection in broiler chickens following oral immunization with profilin in Montanide™ adjuvants. Exp Parasitol. 2011;129(1):36–41. doi: 10.1016/j.exppara.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Jeffers T. Attenuation of Eimeria tenella through selection for precociousness. J parasitol. 1975 doi: 10.2307/3279381. [DOI] [PubMed] [Google Scholar]
- Jeffers T, Challey J, McGibbon W. Response of several lines of fowl and their single-cross progeny to experimental infection with Eimeria tenella. Avian Dis. 1970 doi: 10.2307/1588464. [DOI] [PubMed] [Google Scholar]
- Jenkins M. Control of avian coccidiosis: drugs and vaccines. London: Miscellaneous Publishing Information Bulletin; 2004. p. 3. [Google Scholar]
- Joyner L. Experimental Eimeria mitis infections in chickens. Parasitology. 1958;48(1–2):101–112. doi: 10.1017/S0031182000021090. [DOI] [PubMed] [Google Scholar]
- Joyner L. Coccidiosis: problems arising from the development of anticoccidial drug resistance. Exp Parasitol. 1970;28(1):122–128. doi: 10.1016/0014-4894(70)90077-9. [DOI] [PubMed] [Google Scholar]
- Joyner L, Davies S. Detection and assessment of sublethal infections of Eimeria tenella and Eimeria necatrix. Exp Parasitol. 1960;9:243–249. doi: 10.1016/0014-4894(60)90031-X. [DOI] [PubMed] [Google Scholar]
- Joyner LP, Norton CC. The immunity arising from continuous low-level infection with Eimeria maxima and Eimeria acervulina. Parasitology. 1976;72(1):115–125. doi: 10.1017/S0031182000058534. [DOI] [PubMed] [Google Scholar]
- Jungmann R, Mielke D. Use of Eimeria tenella radiovaccine for immunoprophylaxis in fowl against coccidiosis. Monatshefte Fuer Vet. 1989;44(13):464–466. [Google Scholar]
- Kadykalo S, Roberts T, Thompson M, Wilson J, Lang M, Espeisse O. The value of anticoccidials for sustainable global poultry production. Int J Antimicrob Agents. 2018;51(3):304–310. doi: 10.1016/j.ijantimicag.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Kalinna BH. DNA vaccines for parasitic infections. Immunol Cell Biol. 1997;75(4):370–375. doi: 10.1038/icb.1997.58. [DOI] [PubMed] [Google Scholar]
- Kant V, Singh P, Verma PK, Bais I, Parmar MS, Gopal A, Gopal V. Anticoccidial drugs used in the poultry: an overview. Sci Int. 2013;1(7):261–265. doi: 10.17311/sciintl.2013.261.265. [DOI] [Google Scholar]
- Khan M, Irshad H, Anjum R, Jahangir M, Nasir U. Eimeriosis in poultry of Rawalpindi/Islamabad area. Pak Vet J. 2006;26(2):85–87. [Google Scholar]
- Kim D, Hong Y, Park D, Lamont S, Lillehoj H. Differential immune-related gene expression in two genetically disparate chicken lines during infection by emopenEimeriaemclose emopenmaximaemclose. In: Pinard MH, Gay C, Pastoret PP, Dodet B, editors. Animal genomics for animal health. Basel: Karger Publishers; 2008. pp. 131–40. [Google Scholar]
- Konjufca V, Wanda SY, Jenkins MC, Curtiss R., 3rd A recombinant attenuated Salmonella enterica serovar Typhimurium vaccine encoding Eimeria acervulina antigen offers protection against E. acervulina challenge. Infect Immun. 2006;74(12):6785–96. doi: 10.1128/IAI.00851-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouyoumdjian JA, Morita MDPA, Sato AK, Pissolatti AF. Fatal rhabdomyolysis after acute sodium monensin (Rumensin®) toxicity: case report. Arq Neuropsiquiatr. 2001;59(3A):596–598. doi: 10.1590/S0004-282X2001000400022. [DOI] [PubMed] [Google Scholar]
- Küçükyilmaz K, Bozkurt M, Selek N, Güven E, Eren H, Atasever A, Bintaş E, Çatlı AU, Çınar M. Effects of vaccination against coccidiosis, with and without a specific herbal essential oil blend, on performance, oocyst excretion and serum IBD titers of broilers reared on litter. Ital J Anim Sci. 2012;11(1):e1. [Google Scholar]
- Lai L, Bumstead J, Liu Y, Garnett J, Campanero-Rhodes MA, Blake DP, Palma AS, Chai W, Ferguson DJ, Simpson P. The role of sialyl glycan recognition in host tissue tropism of the avian parasite Eimeria tenella. PLoS Pathog. 2011;7(10):e1002296. doi: 10.1371/journal.ppat.1002296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal K, Bromley E, Oakes R, Prieto JH, Sanderson SJ, Kurian D, Hunt L, Yates JR, III, Wastling JM, Sinden RE. Proteomic comparison of four Eimeria tenella life-cycle stages: unsporulated oocyst, sporulated oocyst, sporozoite and second-generation merozoite. Proteomics. 2009;9(19):4566–4576. doi: 10.1002/pmic.200900305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanckriet A, Timbermont L, De Gussem M, Marien M, Vancraeynest D, Haesebrouck F, Ducatelle R, Van Immerseel F. The effect of commonly used anticoccidials and antibiotics in a subclinical necrotic enteritis model. Avian Pathol. 2010;39(1):63–68. doi: 10.1080/03079450903505771. [DOI] [PubMed] [Google Scholar]
- Lawal JR, Jajere SM, Ibrahim UI, Geidam YA, Gulani IA, Musa G, Ibekwe BU. Prevalence of coccidiosis among village and exotic breed of chickens in Maiduguri, Nigeria. Vet World. 2016;9(6):653. doi: 10.14202/vetworld.2016.653-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EH. Vaccination against coccidiosis in commercial roaster chickens. Can Vet J. 1987;28(7):434–436. [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Kim WH, Jeong J, Yoo J, Kwon Y-K, Jung BY, Kwon JH, Lillehoj HS, Min W. Prevalence and cross-immunity of Eimeria species on Korean chicken farms. J Vet Med Sci. 2010 doi: 10.1292/jvms.09-0517. [DOI] [PubMed] [Google Scholar]
- Lee K-W, Lillehoj HS, Jang SI, Li G, Lee S-H, Lillehoj EP, Siragusa GR. Effect of Bacillus-based direct-fed microbials on Eimeria maxima infection in broiler chickens. Comp Immunol Microbiol Infect Dis. 2010;33(6):e105–e110. doi: 10.1016/j.cimid.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Li X-Y, Liu L-L, Zhang M, Zhang L-F, Wang X-Y, Wang M, Zhang K-Y, Liu Y-C, Wang C-M, Xue F-Q. Proteomic analysis of the second-generation merozoites of Eimeria tenella under nitromezuril and ethanamizuril stress. Parasites Vectors. 2019;12(1):1–10. doi: 10.1186/s13071-019-3841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G-B, Qian X, Feng D, Fisher M, Brown CM, Gurnett A, Leavitt PS, Liberator PA, Misura AS, Tamas T. Synthesis and SAR studies of potent imidazopyridine anticoccidial agents. Bioorg Med Chem Lett. 2007;17(13):3558–3561. doi: 10.1016/j.bmcl.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Lightowlers M. Vaccination against animal parasites. Vet Parasitol. 1994;54(1–3):177–204. doi: 10.1016/0304-4017(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Lillehoj HS, Ding X, Quiroz MA, Bevensee E, Lillehoj EP. Resistance to intestinal coccidiosis following DNA immunization with the cloned 3–1E Eimeria gene plus IL-2, IL-15, and IFN-γ. Avian Dis. 2005;49(1):112–117. doi: 10.1637/7249-073004R. [DOI] [PubMed] [Google Scholar]
- Liu C-M, Ralph J, Fern L, Hermann T, Jenkins E, Liu M, Palleroni NJ, Prosser BL, Sello LH, Stempel A. Studies on a new polyether antibiotic, Ro 21-6150. J Antibiot. 1976;29(1):21–28. doi: 10.7164/antibiotics.29.21. [DOI] [PubMed] [Google Scholar]
- Long P. Studies on Eimeria praecox Johnson, 1930, in the chicken. Parasitology. 1967;57(2):351–361. doi: 10.1017/S0031182000072140. [DOI] [Google Scholar]
- Long P. The pathogenic effects of Eimeria praecox and E. acervulina in the chicken. Parasitology. 1968;58(3):691–700. doi: 10.1017/S0031182000028997. [DOI] [PubMed] [Google Scholar]
- Long PL, Millard BJ. Immunological differences in Eimeria maxima: effect of a mixed immunizing inoculum on heterologous challenge. Parasitology. 1979;79(3):451–457. doi: 10.1017/S0031182000053841. [DOI] [PubMed] [Google Scholar]
- Long PL, Millard B, Joyner L, Norton CC. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet Lat. 1976;6(3):201–217. [PubMed] [Google Scholar]
- Luu L, Bettridge J, Christley RM, Melese K, Blake D, Dessie T, Wigley P, Desta TT, Hanotte O, Kaiser P. Prevalence and molecular characterisation of Eimeria species in Ethiopian village chickens. BMC Vet Res. 2013;9(1):1–7. doi: 10.1186/1746-6148-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marugan-Hernandez V, Jeremiah G, Aguiar-Martins K, Burrell A, Vaughan S, Xia D, Randle N, Tomley F. The growth of Eimeria tenella: characterization and application of quantitative methods to assess sporozoite invasion and endogenous development in cell culture. Front Cell Infect Microbiol. 2020;10:579833. doi: 10.3389/fcimb.2020.579833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald V, Rose ME. Eimeria tenella and E. necatrix: a third generation of schizogony is an obligatory part of the developmental cycle. J parasitol. 1987 doi: 10.2307/3282145. [DOI] [PubMed] [Google Scholar]
- McDougald L, Jeffers T. Eimeria tenella (Sporozoa, Coccidia): gametogony following a single asexual generation. Science. 1976;192(4236):258–259. doi: 10.1126/science.1257765. [DOI] [PubMed] [Google Scholar]
- McDougald LR, Fuller AL, McMurray BL. An outbreak of Eimeria necatrix coccidiosis in breeder pullets: analysis of immediate and possible long-term effects on performance. Avian Dis. 1990;34(2):485–487. doi: 10.2307/1591441. [DOI] [PubMed] [Google Scholar]
- Mielke D. A new system for the quantitative evaluation of the effectiveness of an Eimeria tenella radiovaccine. Appl Parasitol. 1993;34(1):11–18. [PubMed] [Google Scholar]
- Mooney D, Richards K, Danaher M, Grant J, Gill L, Mellander P-E, Coxon C. An investigation of anticoccidial veterinary drugs as emerging organic contaminants in groundwater. Sci Total Environ. 2020;746:141116. doi: 10.1016/j.scitotenv.2020.141116. [DOI] [PubMed] [Google Scholar]
- Morgan JA, Godwin RM. Mitochondrial genomes of Australian chicken Eimeria support the presence of ten species with low genetic diversity among strains. Vet Parasitol. 2017;243:58–66. doi: 10.1016/j.vetpar.2017.05.025. [DOI] [PubMed] [Google Scholar]
- Morris G, Gasser R. Biotechnological advances in the diagnosis of avian coccidiosis and the analysis of genetic variation in Eimeria. Biotechnol Adv. 2006;24(6):590–603. doi: 10.1016/j.biotechadv.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Morris GM, Woods WG, Richards DG, Gasser RB. Investigating a persistent coccidiosis problem on a commercial broiler–breeder farm utilising PCR-coupled capillary electrophoresis. Parasitol Res. 2007;101(3):583–589. doi: 10.1007/s00436-007-0516-9. [DOI] [PubMed] [Google Scholar]
- Musa I, Sa’idu L, Jatau I, Adamu J, Otu M, Abdu P. Outbreak of coccidiosis in 5-day old commercial broiler breeder flock in Zaria, Nigeria. Int J Poult Sci. 2010;9(12):1112–1115. doi: 10.3923/ijps.2010.1112.1115. [DOI] [Google Scholar]
- Nematollahi A, Moghaddam G, Pourabad RF. Prevalence of Eimeria species among broiler chicks in Tabriz (Northwest of Iran) Mun Ent Zool. 2009;4(1):53–58. [Google Scholar]
- Noack S, Chapman HD, Selzer PMJPR. Anticoccidial drugs of the livestock industry. Parasitol Res. 2019;118(7):2009–2026. doi: 10.1007/s00436-019-06343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOAH. NOAH compendium—datasheets. 2021. https://www.noahcompendium.co.uk/datasheets. Accessed 14 July 2021.
- Ogedengbe JD, Hunter DB, Barta JR. Molecular identification of Eimeria species infecting market-age meat chickens in commercial flocks in Ontario. Vet Parasitol. 2011;178(3–4):350–354. doi: 10.1016/j.vetpar.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Oikawa H, Kawaguchi H, Katagiri K, Nakamoto K. Incidence of chicken coccidia from broiler houses in Japan, 1973–1977. Zentralblatt fur bakteriologie, parasitenkunde, infektionskrankheiten und hygiene. Erste abteilung originale. Reihe A Med Mikrobiol Parasitol. 1979;244(2–3):339. [PubMed] [Google Scholar]
- Osweiler G. Ruminant toxicology, an issue of veterinary clinics: food animal practice—E-Book. Amsterdam: Elsevier Health Sciences; 2011. [Google Scholar]
- Oviedo-Rondón E, Clemente-Hernández S, Williams P, Losa R. Responses of coccidia-vaccinated broilers to essential oil blends supplementation up to forty-nine days of age. J Appl Poultry Res. 2005;14(4):657–664. doi: 10.1093/japr/14.4.657. [DOI] [Google Scholar]
- Oviedo-Rondón E, Hume M, Hernández C, Clemente-Hernández S. Intestinal microbial ecology of broilers vaccinated and challenged with mixed Eimeria species, and supplemented with essential oil blends. Poult Sci. 2006;85(5):854–860. doi: 10.1093/ps/85.5.854. [DOI] [PubMed] [Google Scholar]
- Palafox A, Alicata J, Kartman L. Breeding chickens for resistance to cecal coccidiosis1. Worlds Poult Sci J. 1949;5(2):84–87. doi: 10.1079/WPS19490017. [DOI] [Google Scholar]
- Pastor-Fernández I, Kim S, Billington K, Bumstead J, Marugán-Hernández V, Küster T, Ferguson DJ, Vervelde L, Blake DP, Tomley FM. Development of cross-protective Eimeria-vectored vaccines based on apical membrane antigens. Int J Parasitol. 2018;48(7):505–518. doi: 10.1016/j.ijpara.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Peek H, Landman W. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet Q. 2011;31(3):143–161. doi: 10.1080/01652176.2011.605247. [DOI] [PubMed] [Google Scholar]
- Pinard-Van Der Laan M, Monvoisin J, Pery P, Hamet N, Thomas M. Comparison of outbred lines of chickens for resistance to experimental infection with coccidiosis (Eimeria tenella) Poult Sci. 1998;77(2):185–191. doi: 10.1093/ps/77.2.185. [DOI] [PubMed] [Google Scholar]
- Pinard-van der Laan M-H, Bed'Hom B, Coville J-L, Pitel F, Feve K, Leroux S, Legros H, Thomas A, Gourichon D, Repérant J-M. Microsatellite mapping of QTLs affecting resistance to coccidiosis (Eimeria tenella) in a Fayoumi×White Leghorn cross. BMC Genom. 2009;10(1):31. doi: 10.1186/1471-2164-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz-Castañeda RE, Dantán-González E. Control of avian coccidiosis: future and present natural alternatives. BioMed Res Int. 2015 doi: 10.1155/2015/430610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid WM. History of avian medicine in the United States. X. Control of coccidiosis. Avian Dis. 1990;34(3):509–525. doi: 10.2307/1591239. [DOI] [PubMed] [Google Scholar]
- Reid AJ, Blake DP, Ansari HR, Billington K, Browne HP, Bryant J, Dunn M, Hung SS, Kawahara F, Miranda-Saavedra D, Malas TB, Mourier T, Naghra H, Nair M, Otto TD, Rawlings ND, Rivailler P, Sanchez-Flores A, Sanders M, Subramaniam C, Tay YL, Woo Y, Wu XK, Barrell B, Dear PH, Doerig C, Gruber A, Ivens AC, Parkinson J, Rajandream MA, Shirley MW, Wan KL, Berriman M, Tomley FM, Pain A. Genomic analysis of the causative agents of coccidiosis in domestic chickens. Genome Res. 2014;24(10):1676–1685. doi: 10.1101/gr.168955.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remnant JG. Veterinary dictionary for students, 162. London: Bloomsbury Publishing; 2007. [Google Scholar]
- Ritzi MM, Abdelrahman W, Mohnl M, Dalloul RA. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult Sci. 2014;93(11):2772–2778. doi: 10.3382/ps.2014-04207. [DOI] [PubMed] [Google Scholar]
- Rose ME. Some aspects of immunity to Eimeria infections. Ann NY Acad Sci. 1963;113(1):383–399. doi: 10.1111/j.1749-6632.1963.tb40677.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg MM, Alicata JE, Palafox AL. Further evidence of hereditary resistance and susceptibility to cecal coccidiosis in chickens. Poult Sci. 1954;33(5):972–980. doi: 10.3382/ps.0330972. [DOI] [Google Scholar]
- Ryley JF, Wilson RG. Laboratory studies with some recent anticoccidials. Parasitology. 1975;70(2):203–222. doi: 10.1017/S0031182000049672. [DOI] [PubMed] [Google Scholar]
- Salisch H. Recent developments in the chemotherapy of parasitic infections of poultry. Worlds Poult Sci J. 1989;45(2):115–124. doi: 10.1079/WPS19890009. [DOI] [Google Scholar]
- Scribner A, Dennis R, Hong J, Lee S, McIntyre D, Perrey D, Feng D, Fisher M, Wyvratt M, Leavitt P. Synthesis and biological activity of imidazopyridine anticoccidial agents: part I. Eur J Med Chem. 2007;42(11–12):1334–1357. doi: 10.1016/j.ejmech.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Scribner A, Dennis R, Lee S, Ouvry G, Perrey D, Fisher M, Wyvratt M, Leavitt P, Liberator P, Gurnett A. Synthesis and biological activity of imidazopyridine anticoccidial agents: part II. Eur J Med Chem. 2008;43(6):1123–1151. doi: 10.1016/j.ejmech.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Sharma S, Iqbal A, Azmi S, Mushtaq I, Wani ZA, Ahmad S. Prevalence of poultry coccidiosis in Jammu region of Jammu and Kashmir State. J Parasit Dis. 2015;39(1):85–89. doi: 10.1007/s12639-013-0286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley MW, Bedrník P. Live attenuated vaccines against avian coccidiosis: success with precocious and egg-adapted lines of Eimeria. Parasitol Today. 1997;13(12):481–484. doi: 10.1016/S0169-4758(97)01153-8. [DOI] [PubMed] [Google Scholar]
- Shirley MW, Smith AL, Tomley FM. The biology of avian Eimeria with an emphasis on their control by vaccination. Adv Parasitol. 2005;60:285–330. doi: 10.1016/S0065-308X(05)60005-X. [DOI] [PubMed] [Google Scholar]
- Soutter F, Werling D, Tomley FM, Blake DP. Poultry coccidiosis: design and interpretation of vaccine studies. Front Vet Sci. 2020;7:101. doi: 10.3389/fvets.2020.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stayer P, Pote L, Keirs R. A comparison of Eimeria oocysts isolated from litter and fecal samples from broiler houses at two farms with different management schemes during one growout. Poult Sci. 1995;74(1):26–32. doi: 10.3382/ps.0740026. [DOI] [PubMed] [Google Scholar]
- Stephan B, Rommel M, Daugschies A, Haberkorn A. Studies of resistance to anticoccidials in Eimeria field isolates and pure Eimeria strains. Vet Parasitol. 1997;69(1–2):19–29. doi: 10.1016/S0304-4017(96)01096-5. [DOI] [PubMed] [Google Scholar]
- Sun H, Wang L, Wang T, Zhang J, Liu Q, Chen P, Chen Z, Wang F, Li H, Xiao Y. Display of Eimeria tenella EtMic2 protein on the surface of Saccharomyces cerevisiae as a potential oral vaccine against chicken coccidiosis. Vaccine. 2014;32(16):1869–1876. doi: 10.1016/j.vaccine.2014.01.068. [DOI] [PubMed] [Google Scholar]
- Swaggerty CL, Genovese KJ, He H, Duke SE, Pevzner IY, Kogut MH. Broiler breeders with an efficient innate immune response are more resistant to Eimeria tenella. Poult Sci. 2011;90(5):1014–1019. doi: 10.3382/ps.2010-01246. [DOI] [PubMed] [Google Scholar]
- Takala SL, Plowe CV. Genetic diversity and malaria vaccine design, testing and efficacy: preventing and overcoming ‘vaccine resistant malaria’. Parasite Immunol. 2009;31(9):560–573. doi: 10.1111/j.1365-3024.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Liu X, Yin G, Suo J, Tao G, Zhang S, Suo X. A novel vaccine delivery model of the apicomplexan Eimeria tenella expressing Eimeria maxima antigen protects chickens against infection of the two parasites. Front Immunol. 2018;8:1982. doi: 10.3389/fimmu.2017.01982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney J, Gowing H, Van Sinderen D, Flynn S, Stanley L, McHardy N, Hallahan S, Mulcahy G. In vitro inhibition of Eimeria tenella invasion by indigenous chicken Lactobacillus species. Vet Parasitol. 2004;122(3):171–182. doi: 10.1016/j.vetpar.2004.05.001. [DOI] [PubMed] [Google Scholar]
- ToxoDB. Organisms: genome info and stats. 2021. https://toxodb.org/toxo/app/search/organism/GenomeDataTypes/result?filterTerm=eimeria. Accessed 14 July 2021.
- Trees AJ, Jordan F, Pattison M, Alexander D, Faragher T. Parasitic diseases. Poultry diseases. Philadelphia: Saunders; 2001. pp. 405–420. [Google Scholar]
- Vegad JL. Poultry diseases: a guide for farmers and poultry professionals. Luckmow: International Book Distributing Company; 2004. [Google Scholar]
- Venkatas J, Adeleke M. A review of Eimeria antigen identification for the development of novel anticoccidial vaccines. Parasitol Res. 2019;118(6):1701–1710. doi: 10.1007/s00436-019-06338-2. [DOI] [PubMed] [Google Scholar]
- Vermeulen A. Progress in recombinant vaccine development against coccidiosis a review and prospects into the next millennium. Int J Parasitol. 1998;28(7):1121–1130. doi: 10.1016/S0020-7519(98)00080-0. [DOI] [PubMed] [Google Scholar]
- Waldenstedt L, Elwinger K, Lunden A, Thebo P, Uggla A. Sporulation of Eimeria maxima oocysts in litter with different moisture contents. Poult Sci. 2001;80(10):1412–1415. doi: 10.1093/ps/80.10.1412. [DOI] [PubMed] [Google Scholar]
- Walker RA, Ferguson DJ, Miller CM, Smith NC. Sex and Eimeria: a molecular perspective. Parasitology. 2013;140(14):1701. doi: 10.1017/S0031182013000838. [DOI] [PubMed] [Google Scholar]
- Wang C, Liu Y, Zheng H, Li Y, He J, Wang X, Wang M, Zhang L, Xue F, Zhang K. Safety pharmacology assessment of ethanamizuril, a novel triazines coccidiostat. Res Vet Sci. 2020;132:271–278. doi: 10.1016/j.rvsc.2020.07.003. [DOI] [PubMed] [Google Scholar]
- Watkins KL, Brooks MA, Jeffers TK, Phelps PV, Ricks CA. The effect of in ovo oocyst or sporocyst inoculation on response to subsequent coccidial challenge. Poult Sci. 1995;74(10):1597–1602. doi: 10.3382/ps.0741597. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization technical report series no. 296. Geneva: WHO; 1965. p. 29. [PubMed] [Google Scholar]
- Williams R. Epidemiological studies of coccidiosis in the domesticated fowl (Gallus gallus): II. Physical condition and survival of Eimeria acervulina oocysts in poultry-house litter. Appl Parasitol. 1995;36(2):90–96. [PubMed] [Google Scholar]
- Williams R. Laboratory tests of phenolic disinfectants as oocysticides against the chicken coccidium Eimeria tenella. Vet Rec. 1997;141(17):447–448. doi: 10.1136/vr.141.17.447. [DOI] [PubMed] [Google Scholar]
- Williams R. Epidemiological aspects of the use of live anticoccidial vaccines for chickens. Int J Parasitol. 1998;28(7):1089–1098. doi: 10.1016/S0020-7519(98)00066-6. [DOI] [PubMed] [Google Scholar]
- Williams R. A compartmentalised model for the estimation of the cost of coccidiosis to the world’s chicken production industry. Int J Parasitol. 1999;29(8):1209–1229. doi: 10.1016/S0020-7519(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Williams R. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34(3):159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- Williams R, Marshall R, La Ragione R, Catchpole J. A new method for the experimental production of necrotic enteritis and its use for studies on the relationships between necrotic enteritis, coccidiosis and anticoccidial vaccination of chickens. Parasitol Res. 2003;90(1):19–26. doi: 10.1007/s00436-002-0803-4. [DOI] [PubMed] [Google Scholar]
- Williams R, Marshall R, Pages M, Dardi M, Del Cacho E. Pathogenesis of Eimeria praecox in chickens: virulence of field strains compared with laboratory strains of E. praecox and Eimeria acervulina. Avian Pathol. 2009;38(5):359–366. doi: 10.1080/03079450903186028. [DOI] [PubMed] [Google Scholar]
- Zhang M, Li X, Zhao Q, She R, Xia S, Zhang K, Zhang L, Wang X, Wang M, Liu Y. Anticoccidial activity of novel triazine compounds in broiler chickens. Vet Parasitol. 2019;267:4–8. doi: 10.1016/j.vetpar.2019.01.006. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wang C, Li Y, He J, Wang M, Wang X, Zhang L, Fei C, Zheng H, Liu Y. Rat two-generation reproductive toxicity and teratogenicity studies of a novel coccidiostat–ethanamizuril. Regul Toxicol Pharmacol. 2020;113:104623. doi: 10.1016/j.yrtph.2020.104623. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wang X, Wang M, Liu Y, Zhang L, Wang C, Fei C, Li J, Xue F. Rat 90-day oral toxicity study of a novel coccidiostat–ethanamizuril. Regul Toxicol Pharmacol. 2020;111:104550. doi: 10.1016/j.yrtph.2019.104550. [DOI] [PubMed] [Google Scholar]
- Zhu J, Lillehoj H, Allen P, Van Tassell C, Sonstegard T, Cheng H, Pollock D, Sadjadi M, Min W, Emara M. Mapping quantitative trait loci associated with resistance to coccidiosis and growth. Poult Sci. 2003;82(1):9–16. doi: 10.1093/ps/82.1.9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.