Abstract
Background:
Risky alcohol consumption is on the rise among older adults. Biomarkers such as phosphatidylethanol (PEth) have been used to evaluate the correspondence between an objective, laboratory-based biomarker and self-report of alcohol consumption. This study examined the relationship between PEth, self-report of alcohol consumption, and health indices in a sample of community-dwelling older to middle-age adults (aged 35 to 89) with healthy and risky levels of alcohol consumption.
Methods:
Self-reports of alcohol consumption were collected using the Alcohol Use Disorders Identification Test (AUDIT) and Form 30. In addition, indices of health along with a blood sample to determine PEth values were collected (N = 183).
Results:
PEth was correlated with age, AUDIT-C, AUDIT total, alcohol consumption, mood, and liver function measures but not with medical comorbidity or body mass index. Alcohol consumption over the past 30 days measured with Form 30 was the strongest predictor of PEth levels for both middle-age and older adults, with age a small contributing predictor. General alcohol consumption patterns for amount of alcohol consumed over a 30-day period revealed middle-age adults consumed larger amounts of alcohol compared with older adults, but older adults consumed alcohol on more days than middle-age adults. Middle-age participants evidenced higher PEth levels than older adults at comparable drinking rates.
Conclusions:
Overall, findings suggest a strong relationship between alcohol consumption and PEth levels with age a small but contributing factor to predicting PEth levels.
Keywords: Phosphatidylethanol (PEth), Older Adult, Aging, Ethanol, Biomarker, Alcohol
PHOSPHATIDYLETHANOL (PETH), AN ethanol (EtOH) biomarker, has been frequently used and characterized in the context of groups of individuals who consume unhealthy amounts of alcohol such as those attending alcohol treatment facilities and human immunodeficiency virus (HIV) clinics. There is increasing knowledge regarding the utility of PEth in the context of community-dwelling adults (Helander et al., 2019; Neumann et al., 2020; Nguyen et al., 2018; Reisfield et al., 2020; Schrock et al., 2017; Ulwelling and Smith, 2018). However, less is known for older adults for whom alcohol consumption can have a different, often more detrimental, impact on health (Moos et al., 2005). Unhealthy alcohol use in older adults can impact cognition, balance, and increase the risk for falls (Pergolizzi et al., 2008; Shimp, 1998; Woods et al., 2016), all of which can threaten independent living.
Breath testing, which is used to estimate recent alcohol consumption from the blood alcohol concentration (BAC) at the moment of sampling, or other blood-based measures such as ethyl glucuronide and ethyl sulfate can detect very recent alcohol ingestion (Halter et al., 2008; Mastrovito and Strathmann, 2020; Neumann et al., 2020; Reisfield et al., 2020). Other common blood- and urine-based measures used in a clinical setting include carbohydrate-deficient transferrin (CDT) and serum γ-glutamyltransferase (GGT; Litten et al., 2010; Mastrovito and Strathmann, 2020; Neumann et al., 2020; Reisfield et al., 2020). However, CDT and GGT may be influenced by other factors besides alcohol intake, including smoking, gender, age, and various diseases (Andresen-Streichert et al., 2018; Conigrave et al., 2003; Neumann et al., 2020). The sensitivity of CDT for detecting recent alcohol use may be as low as 50 to 70%, and false positives for CDT, though rare, can be caused by genetic variants of transferrin, severe liver disease, and pregnancy (Bortolotti, Sorio et al., 2018).
PEth is a more specific and sensitive alcohol biomarker acquired through blood samples. PEth is formed by phospholipase D, which incorporates EtOH into phospholipids in cell membranes after alcohol intake (Helander and Zheng, 2009). PEth exists in several forms, and there have been efforts by researchers to standardize PEth measurement and interpretation and some suggestion that combining 2 or more forms may increase clinical accuracy (Helander et al., 2019; Helander and Zheng, 2009; Zheng et al., 2011). Recognizing individual variation in PEth clearance, in general, PEth may be detected in blood for up to 10 days after a single episode of alcohol consumption and can be detected up to 3 weeks after repeated consumption and may be stable in preserved samples for years (Helander et al., 2019; Hill-Kapturczak et al., 2018; Javors et al., 2016; Lakso et al., 2019; Lopez-Cruzan et al., 2018; Schröck et al., 2017). Because PEth accumulates in the body after repeated drinking and because the elimination period varies from days to weeks according to amount and frequency ingested, it can be useful for providing information on alcohol consumption particularly when combined with additional biomarkers (Gnann et al., 2012; Neumann et al., 2020; Reisfield et al., 2020; Ulwelling and Smith, 2018). PEth is better than traditional biomarkers as it appears to be uninfluenced by liver disease (Andresen-Streichert et al., 2018; Stenton et al., 2019; Ulwelling and Smith, 2018), although more research in this area is needed (Hakim et al., 2019; Nguyen et al., 2018).
There is a growing literature comparing PEth with self-report of drinking levels, both moderate and heavy. Numerous studies have focused on samples of patients living with HIV, with several studies showing a good association between PEth and heavy and moderate drinking as measured with interview-style assessment of alcohol consumption such as the Timeline Followback (TLFB; Andresen-Streichert et al., 2018; Ferguson et al., 2020; Helander et al., 2019; Miller and Del Boca, 1994; Neumann et al., 2020). Other studies have attempted to relate PEth levels with drinking behavior in adolescent and young to middle-age adults, assessed using the Alcohol Use Disorders Identification Test (AUDIT), a self-report questionnaire-style measure of alcohol consumption, with findings of strong association between AUDIT and PEth levels in young, binge-drinking adults and typical consumption levels of community-dwelling adults with various health conditions including pregnant women and the critically ill (Afshar et al., 2017; Piano et al., 2015; Schrock et al., 2017). Most recently, dermal monitors have complimented self-report to characterize synthesis and elimination of PEth to experimentally determined volumes of alcohol (Hill-Kapturczak et al., 2018). The association of PEth with low or minimal alcohol consumption may be weaker or more variable (Helander et al., 2019; Helander et al., 2012; Kechagias et al., 2015; Neumann et al., 2020; Reisfield et al., 2020).
Our study was undertaken to evaluate the relationship between PEth and self-reported alcohol consumption of community-dwelling older adults with heavy and healthy alcohol consumption characterized using several established assessment measures. Middle-aged adults with similar consumption levels were included to provide a comparison group. In particular, we attempted to understand whether age may have any impact on the relationship between PEth levels and self-reported alcohol consumption.
MATERIALS AND METHODS
Participants
Participants were community-dwelling middle-age and older adults who met the following study criteria. Inclusion criteria were as follows: (i) age 35 to 95 inclusive; (ii) self-report of ongoing alcohol consumption; (iii) willingness to refrain from alcohol for 24 hours prior to study visit; (iv) self-report of no up to mild or moderate chronic pain without severe functional limitations as indicated by a graded chronic pain scale (GCPS) score of 1 to 3 inclusive; and (v) cognitive, physical, and psychomotor ability to complete study visit and questionnaires. Exclusion criteria were as follows: (i) history of alcohol withdrawal symptoms including seizures or shakes, disorientation, or hallucinations (APA, 2013); (ii) abstinence or no reported alcohol use. This was included to minimize an excessive number of negative or nonquantifiable PEth assays since EtOH is a prerequisite for PEth synthesis; and (iii) major medical comorbidity that could significantly impact ability to participate in study tests and questionnaires (e.g., cerebral vascular accident in the prior 6 months, active cancer requiring current treatment, or possible or probable dementia or mild cognitive impairment).
Procedures
Participants were enrolled as part of a larger study examining alcohol consumption and pain medications. Participants responded to print advertisements or fliers and were given a phone screening to determine initial eligibility. This included questions regarding demographics, alcohol consumption (daily and weekly consumption), presence of alcohol withdrawal symptoms, current medications, and a pain questionnaire. All measures are described below. Participants who were eligible based on the phone screen were asked to come to the University of Washington Medical Center (UWMC) or the Veterans Administration Puget Sound Health Care System (VAPSHCS) for a study visit that included written informed consent along with completion of questionnaires and a blood draw.
For the study visit, participants were asked to refrain from drinking alcohol 24 hours in advance of the study visit and were administered a generic breath alcohol test at the start of the visit. Participants with a (BAC equivalent) level above 0.001 were excluded. Consent and procedures were approved by the University of Washington and VAPSHCS institutional review boards.
Whole-blood samples for PEth were collected in an EDTA vial and transferred to a tube for storage at −80°C until processing. A second tube with clot activator was collected, spun, aliquoted, and sent to the UWMC or VAPSHCS clinical laboratory for hepatic panel of tests including direct and total bilirubin, serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT), serum GGT, and alkaline phosphatase.
In addition to questionnaires detailed below, demographic information including age, education, gender, and occupation was recorded. Data were entered into an electronic database REDcap (Research Electronic Data Capture; Harris, Taylor et al., 2009), hosted by the University of Washington, imported into SPSS statistical software v.26 (IBM, 2019) and R (R_Core_Team, 2019), and double-checked for accuracy.
Questionnaires
AUDIT and AUDIT-C.
The 10-item AUDIT is a screening questionnaire developed by the World Health Organization (WHO) to identify harmful or hazardous alcohol consumption (Higgins-Biddle and Babor, 2018). Response options for each item range from 0 to 4, resulting in a total possible score of 40. The AUDIT-C is a shortened, 3-item version of the 10-item AUDIT, focused primarily on alcohol consumption habits of the last year (Bush et al., 1998). It consists of the first 3 AUDIT questions concerning alcohol consumption habits such as quantity, frequency, and binge drinking, and scores range from 0 to 12. In addition to the total score, AUDIT-C scores were categorized according to the WHO guidelines for alcohol consumption in the “at-risk” range (a score of 6 or greater for the sum of questions 1 to 3) and in the “healthy” range (less than 6).
Form 30.
To obtain more information on drinking habits, participants were asked to recall their alcohol consumption over the prior 30-day period using a calendar-based method and total drinks over 30 days were recorded (Miller and Del Boca, 1994). Standardized equivalents for drinks were used according to the National Institute on Alcohol, Abuse and Alcoholism (NIAAA) guidelines. Information from the Form 30 was used to calculate summary variables including total number of drinks over the past 30 days, total number days in which alcohol was consumed (total number of drinking days), and total number of heavy drinking days (6 or more for men, 4 or more for women) and average drinks per day and average drinks per drinking day. Participants were asked to refrain from consuming alcohol for the 24 hours in advance of their screening visit.
Medical Comorbidity Questionnaire (MCQ).
MCQ is a brief, checklist-style measure of medical conditions. Participants indicate whether they have a health condition (e.g., diabetes) and to what degree it interferes with their daily functioning. Scores range from 0 to 145, and higher score indicates more conditions and greater activity interference (Bayliss et al., 2005).
Patient Health Questionnaire (PHQ-9).
PHQ is a self-report, symptom measure for which a higher score indicates higher severity and more symptoms of depression. Scores range from 0 to 27 (Wittkampf et al., 2007).
Beck Anxiety Inventory (BAI).
BAI is a self-report anxiety symptom measure in which a higher score indicates endorsement of more and/or more severe anxiety symptoms. Scores range from 0 to 63 (Steer et al., 1997).
Blood Peth Analysis
Whole-blood samples were prepared for LC/MS/MS analysis using a modification of the method described by Zheng and colleagues (2011); see details in “Supplemental Information”.
Statistical Evaluation
Middle age was defined as 35 to 59 years, and older age as 60 years and over. Analyses comparing middle-age versus older adults and risky versus healthy alcohol consumption were conducted using Student’s t-tests. Initial analyses included separate tests for PEth 16:0/18:1 and 16:0/18:2, and the molar sum of the 2 analytes. However, since PEth 16:0/18:1 and 16:0/18:2 did not reveal differential results and were highly correlated with each other, the molar sum of PEth 16:0/18:1 and 16:0/18:2 was used for regression analyses. For regression analyses, the following variables were log10-transformed to more closely approximate a normal distribution: AUDIT total, Form-30 total drinks per day, and PEth total. For log10 transformation of variables that contained any values of zero or near-zero value, the number 1 was added to avoid undefined values from log transformation. The Pearson product–moment correlation analysis was conducted between PEth total and variables of interest (age, body mass index [BMI], GCPS, medical comorbidity, Form-30 subscales, liver function measures (bilirubin total, AST, ALT, GGT), mood measures (PHQ, BAI), AUDIT total, and AUDIT-C. Tables include raw (nontransformed) values of PEth 16:0/18:1 and 16:0/18:2 and PEth total in molar values.
To understand the relationship between PEth total (dependent variable) and independent variables: demographics (age, BMI, sex) and alcohol consumption measures (AUDIT-C, Form-30 subscores [see descriptions above]), linear regression analyses were conducted in R using the package censReg (R_Core_Team, 2019). CensReg, which accommodates censored data in calculating linear regressions using a maximum-likelihood procedure, was used because 16.4% of PEth values fell below the lower limit of quantification (LLOQ). For Form 30, the total number of standard drinks consumed over 30 days was used in the regression as this consumption index of the Form 30 was strongly correlated with PEth total. To determine other factors that best predicted PEth, a backward stepwise regression analysis was conducted. For each iteration of the censReg backward stepwise regression, the Akaike information criterion (AIC) for the full model was compared against the AIC omitting each of the variables one by one. With each step, any variable whose omission would decrease the AIC was then omitted, and the procedure was repeated until omitting further variables would only increase the AIC (see additional materials for details). To directly compare PEth total values at specified levels of drinks per day between middle-age and older adults, an ANOVA with log-transformed PEth total (see above) as dependent variable and age group and drinks per day categories was conducted. Average drinks per day from Form 30 were categorized as less than 1 (0.99 and lower), 1 (1 to 1.99 drinks per day), 2 (2 to 2.99 drinks per day), 3 (3.0 to 3.99), and 4 (4.0 to highest).
RESULTS
One hundred and eighty-three volunteers aged 35 to 89 years, 121 male and 62 female, 2.2% Hispanic or latino, 92.9% non-Hispanic/Latino, 4.9% unknown/not reported and 2.2% American Indian, 4.4% Asian, 9.3% black or African American, 3.3% more than one race, and 80.9% non-Hispanic white, completed the study visit. Student’s t-tests between middle-age and older adults within the at-risk group revealed significantly higher levels of PEth 16:0/18:2, PEth total, MCQ, PHQ-9, total AUDIT, total number of drinking days, average drinks per drinking day, heavy drinking days, and percent of drinking days that were heavy in the direction of higher scores for the MA group with the exception of MCQ and total number of drinking days, which were higher in the OA group. Within the healthy grouping MA and OA differed, total number of drinking days, average drinks per day, average drinks per drinking day, percent of drinking days that were heavy from the Form 30 in the direction of middle-age higher than older adults with the exception of MCQ and total number of drinking days (see Table 1; Fig. 1).
Table 1.
Demographics and Drinking Characteristics for At-Risk and Healthy Groups (Mean, Standard Deviation)
| At-risk | Healthy | |||
|---|---|---|---|---|
| Middle-age | Older adult | Middle | Older adult | |
| PEth total (μM) | 1.23 (1.38) | 0.68 (0.71) | 0.21 (0.38) | 0.14 (0.16) |
| PEth 16:0/18:2 (μM) | 0.59 (0.67) | 0.30 (0.26) | 0.10 (0.18) | 0.07 (0.08) |
| PEth 16:0/18:1 (μM) | 0.63 (0.74) | 0.38 (0.45) | 0.11 (0.20) | 0.07 (0.08) |
| Total DRs 30 days | 94.19 (58.61) | 77.06 (41.23) | 24.8 (22.36) | 33.80 (22.14) |
| Total # DR days | 19.14 (6.89) | 22.62 (7.11) | 11.1 (8.62) | 18.68 (9.05) |
| Total # HDD | 7.93 (8.05) | 3.62 (5.96) | 0.62 (1.77) | 0.21 (0.86) |
| Avg. # DRs/d | 3.16 (1.95) | 2.56 (1.37) | 0.82 (0.74) | 1.12 (0.74) |
| Avg. # DRs/DD | 5.07 (3.04) | 3.49 (1.51) | 2.14 (1.19) | 1.75 (0.66) |
| % Heavy DDs | 41.49 (36.5) | 19.29 (29.16) | 6.16 (15.37) | 0.88 (3.83) |
| AUDIT Total | 13.21 (5.48) | 10.62 (4.18) | 4.82 (2.36) | 4.62 (1.69) |
| AUDIT-C | 7.76 (1.40) | 7.00 (1.41) | 3.68 (1.24) | 3.85 (0.98) |
| Bilirubin Total (mg/dl) | 0.64 (0.31) | 0.58 (0.21) | 0.54 (0.30) | 0.70 (0.94) |
| AST (U/L) | 30.55 (25.2) | 23.90 (7.48) | 20.16 (6.9) | 23.77 (7.17) |
| ALT(U/L) | 28.48 (22.41) | 22.29 (8.57) | 22.14 (13.51) | 22.75 (12.42) |
| GGT (U/L) | 42.93 (46.70) | 47.60 (73.18) | 26.71 (25.44) | 23.86 (18.75) |
| Age | 45.79 (7.57) | 65.69 (5.03) | 48.14 (7.88) | 69.85 (6.72) |
| BMI | 26.93 (3.88) | 27.45 (3.94) | 28.86 (4.98) | 25.33 (4.06) |
| MCQ | 4.49 (5.38) | 11.18 (10.4) | 6.03 (5.61) | 8.09 (6.74) |
| PHQ-9 | 4.76 (4.69) | 2.81 (2.93) | 2.58 (2.75) | 1.75 (2.17) |
| BAI | 6.23 (7.88) | 3.41 (3.90) | 3.21 (3.93) | 2.16 (3.89) |
| N | 47 | 32 | 51 | 53 |
| Gender m/f | 31/16 | 26/6 | 32/19 | 32/21 |
PEth = phosphatidylethanol, PEth total is the sum of the molar blood concentrations of PEth 16:0/18:1 and 16:0/18:2; Total DRs 30 days = total drinks consumed in past 30 days, obtained with Timeline Followback (TLFB; Form 30), which also provided the next 5 variables; Total # DR days = number of days in the past 30 days that participant drank alcohol; Total # HDD = total number of heavy drinking days (≥4 for women and ≥6 for men); Avg. # DRs/day = average number of drinks consumed per day; Avg. # DRs/DD = average number of drinks consumed on a drinking day; % Heavy DDs, percent of drinking days that were heavy; AUDIT, Alcohol Use Disorders Identification Test, score range 0 to 30, higher scores indicate greater alcohol misuse; AUDIT-C, consumption portion of the AUDIT, score range 0 to 12, higher scores indicate riskier drinking; AST, aspartate transaminase; ALT, alanine transaminase; GGT, gamma-glutamyl transpeptidase; BMI, body mass index; MCQ, medical comorbidity questionnaire, score range 0 to 145, higher scores indicate more medical conditions and greater adverse impact; PHQ-9, Patient Health Questionnaire, score range 0 to 27, higher scores indicate more depression symptoms; BAI, Beck Anxiety Index, score range 0 to 63, higher scores indicate more anxiety symptoms.
Bold type indicates significant difference, p < 0.01, and italics indicates significant difference, p < 0.05, between middle-age and older age groups within the “at-risk” and “healthy” drinking groups for Student’s t-test.
Fig. 1.
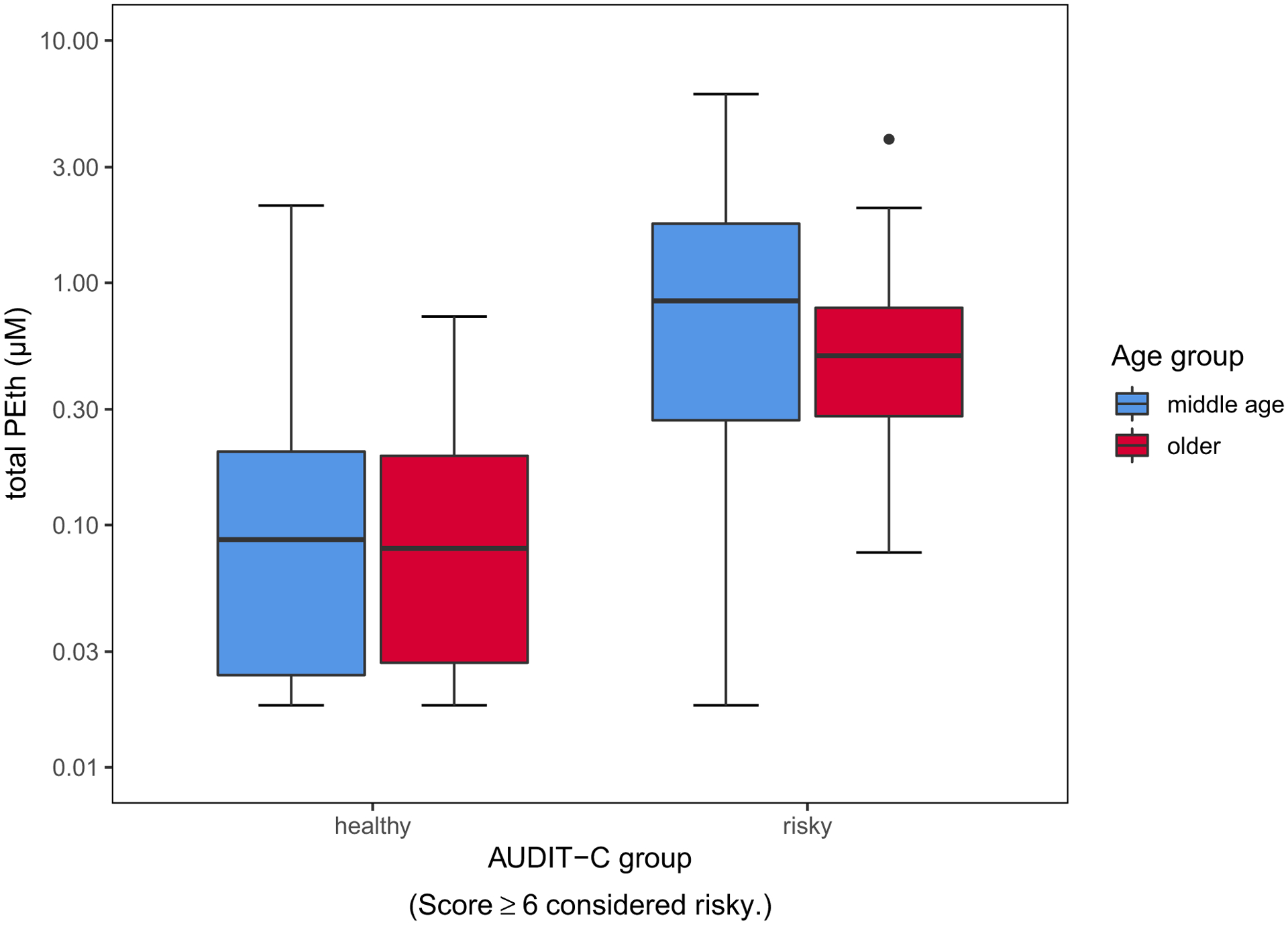
Boxplots of mean (line inside boxes) and standard deviations (vertical top and bottom of boxes) total blood PEth for middle-age (blue) and older adults (red) with healthy versus risky alcohol consumption as defined by AUDIT-C plotted onto a y-axis of log10-transformed values. Within the at-risk group, middle-age adults had significantly higher PEth levels compared with older adults (p < 0.05).
Older adults had more drinking days on average than middle-age participants independent of AUDIT-C grouping, and similarly, middle-age adults had higher average drinks per drinking day and percent of heavy drinking days independent of risk grouping. For subjects in the healthy-drinking range, PEth ranged from below the LLOQ to 2 μM with 73% of values in the quantifiable range for both PEth 16:0/18:1 and 16:0/18:2; for subjects in the risky or heavy consumption group, PEth ranged from below the LLOQ to 6 μM, with 98% of samples above the quantifiable range for both analytes. The range and standard deviation for middle-age and older adults were comparable within healthy versus risky groups. ANOVA results indicated middle-age group demonstrated higher PEth levels overall for drinks per day categories, F(1, 173) = 7.19, p < 0.01, which was significant at 3 drinks per day, F(1, 173) = 7.66, p < 0.01 (see Table 2).
Table 2.
Mean Total PEth (μM) (SD) by Age Group and Questionnaire Score or Value
| Form-30 average drinks per day | AUDIT-C score | |||
|---|---|---|---|---|
| Middle-age adults | Older adults | ↔ | Middle-age adults | Older adults |
| 0.185 (0.3) | 0.151 (0.4) | <1 | – | – |
| 0.534 (1.2) | 0.249 (0.2) | 1 | 0.207 (0.3) | – |
| 0.874 (1.0) | 0.463 (0.3) | 2 | 0.050 (0.1) | 0.039 (0.03) |
| 1.66 (1.6)** | 0.574 (0.3) | 3 | 0.273 (0.6) | 0.051 (0.04) |
| 1.23 (1.1) | 0.619 (0.1) | 4 | 0.167 (0.2) | 0.147 (0.2) |
| 1.04 (0.7) | – | 5 | 0.280 (0.4) | 0.236 (0.1) |
| 1.21 (0.8) | – | 6 | 1.012 (1.8) | 0.638 (0.5) |
| – | – | 7 | 0.40 (0.4) | 0.393 (0.3) |
| 2.14 (0.3) | 3.912 | 8 | 1.74 (1.8) | 0.596 (0.3) |
| – | – | 9 | 1.73 (0.7) | 0.437 (0.2) |
| – | – | 10 | 1.60 (0.8) | 2.33 (2.2) |
| – | – | 11 | – | – |
Center column indicates a value corresponding to questionnaire score: average drinks per day (left side) or AUDIT-C score (right side). Mean total PEth (μM) for each age group and questionnaire value is shown in columns 1, 2, 4, and 5.
p < 0.01 for t-test between age groups at a given questionnaire score.
The final model regression analysis in comparing the relative strength of questionnaires in predicting PEth was Form-30 total drinks and age (see Fig. 2).
Fig. 2.
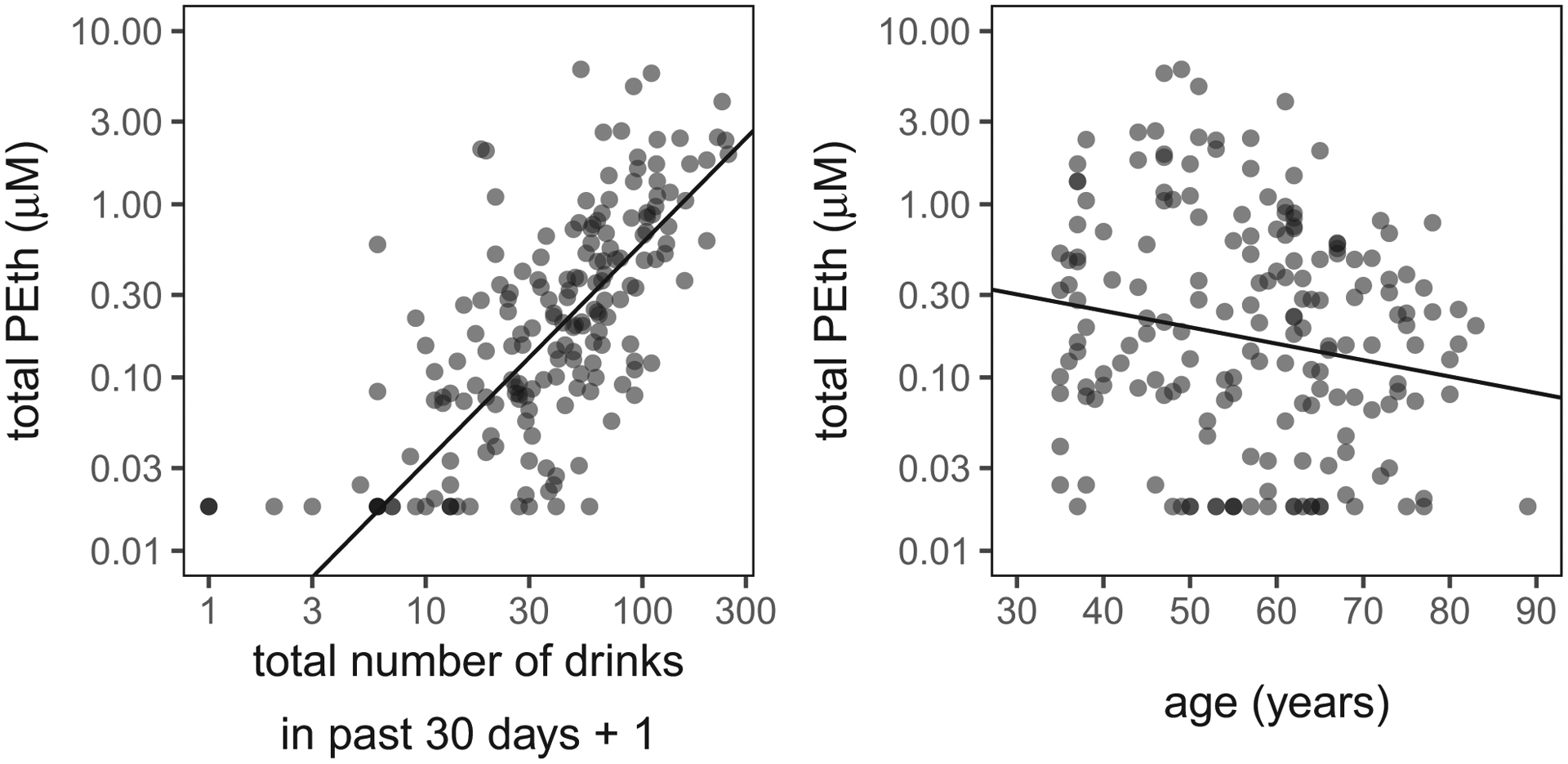
Blood PEth concentrations compared with Form-30 total number of drinks consumed over the past 30 days and also compared with subject age. Because many observations overlap, points are semi-transparent, and darker points indicate more overlapping data. The best-fit regression line from the R package censReg shown in black. The y-axis values are log10-transformed values.
DISCUSSION
The aim of this study was to characterize the relationship between age and self-report of alcohol drinking patterns with PEth, a blood-based biomarker of alcohol consumption, in a community-dwelling population of middle-age and older adults with moderate-to-risky alcohol consumption.
A comparison between age groups within the at-risk consumption category (defined by AUDIT-C) revealed that middle-age adults had higher total blood PEth concentrations and total number of drinks when compared to older adults. Regression analyses revealed age to be a significant although small (compared to alcohol consumption) contributor to PEth levels. Previous studies that included age as a variable in the analysis have not found it to be a significant predictor of PEth (Afshar et al., 2017; Hahn et al., 2012). A recent case–control study of middle-age adults (age 25 to 74, mean age 55 years) examined PEth combined with self-report of alcohol consumption in relation to stroke risk and found PEth levels to be a significant risk factor in addition to hypertension (Johansson et al., 2020). However, most prior studies have not included a large number of adults in the older age range as the present study does. Older adults tend to process alcohol at a slower rate compared with younger adults (Vestal et al., 1977). There is variation in PEth levels due to a number of factors in humans, for which studies have primarily included younger adults (Helander et al., 2019; Hill-Kapturczak et al., 2018; Lopez-Cruzan et al., 2018; Stenton et al., 2019). While we are not aware of any difference between younger and older adults for phospholipase D, the enzyme responsible for incorporating EtOH into phospholipids, studies examining variability of PEth formation and elimination in older adults have not been conducted.
The middle-age risky group evidenced higher depression and anxiety scores and higher clinical liver indices (AST, ALT) than the middle-age healthy group. It is unlikely that depression or anxiety scores impact PEth directly; however, mood factors have been associated with heavy alcohol consumption, which may be helpful to clinicians looking for a convergence of data to interpret PEth values inconsistent with self-report of alcohol consumption.
The average PEth total for the middle-age at-risk group tended to be higher than that of the older adult at-risk group (mean difference of middle-age and older: 0.55 μM, p < 0.05), whereas the healthy groups had comparable values (mean difference: 0.07 μM, ns). This finding likely reflects the absolute number of drinks consumed in the month prior to assessment, as the middle-age risky group did differ significantly on the absolute number of drinks compared with risky older adults. The difference between the 2 age groups may also reflect more variability in drinking patterns. Within the risky group, middle-aged participants had significantly higher AUDIT total score compared with the risky older age group. The AUDIT total score includes questions reflective of problems related to alcohol consumption in contrast to the AUDIT-C, which is restricted entirely to consumption questions. This suggests that other factors related to alcohol consumption beyond the actual consumption level may be more prevalent in the middle-age risky group. In regard to health risks, as noted above, alcohol consumption as indicated by PEth has been shown to be a risk factor for stroke (Johansson et al., 2020).
The reported number of drinks consumed over the past month obtained from the Form 30 was the strongest predictor of PEth. In comparison with other brief measures such as the AUDIT-C, the Form 30 requires more time and staff effort to complete. However, the interview style used for the Form 30 to obtain past consumption information may provide more precise information and better prediction of PEth levels. Both Form-30 and AUDIT-C measures were comparable with regard to correlation with PEth (Pearson’s r for Form-30 total number of drinks = 0.53, AUDIT total = 0.58, and AUDIT-C = 0.51). Other studies have also reported a stronger relationship between PEth and amount of alcohol consumed over 14 to 30 days obtained using a TLFB method of obtaining information compared with AUDIT or AUDIT-C. Schrock and colleagues (2017) reported PEth correlation with amount of alcohol consumed obtained with TLFB of r = 0.70 and r = 0.68 for AUDIT score in a group of community-dwelling volunteers, and Ferguson and colleagues (2020) reported a PEth correlation of r = 0.67 with TLFB and r = 0.53 with AUDIT score, similar to other studies (Kechagias et al., 2015; Schrock et al., 2017; Stewart et al., 2014). A strong association between PEth and amount of alcohol consumed has resulted in attempts to develop guidelines for interpretation of test results with regard to drinking levels (e.g., low to abstinent, moderate, heavy, or risky; Helander and Hansson, 2013; Kummer et al., 2016; Ulwelling and Smith, 2018). U.S. NIAAA guidelines indicate that the same level of alcohol consumption may have more adverse impact on older adults, which is reflected in the age adjustment for categorization of healthy versus risky alcohol consumption (Satre et al., 2018). Similarly, guidelines for the range of PEth levels corresponding to moderate versus heavy alcohol consumption may also need to be adjusted according to age and possibly gender.
The general pattern of alcohol consumption was different for middle-age and older adults on the Form 30. This was particularly evident when comparing middle-age and older adults, within the “at-risk” consumption group (defined by AUDIT-C). Older adults reported overall less alcohol consumed over a longer period of time (higher number of drinking days on Form 30), suggesting a regular pattern of steady consumption of fewer drinks consumed per day and per drinking day. In contrast, the middle-age risky drinking group had much higher average drinks per drinking day and fewer drinking days overall, suggesting heavy drinking per drinking day and possibly a binge-drinking pattern. Percent of drinking days categorized as heavy was significantly higher in the middle-age group compared with older adults.
Studies of PEth levels in younger adults have characterized drinking patterns as risky in comparison with moderate or light patterns with a strong relationship between PEth and alcohol consumption, particularly for risky alcohol consumption with slightly weaker or more variable associations between PEth and moderate-to-light drinking (Andresen-Streichert et al., 2018; Francis et al., 2015; Hahn et al., 2012; Helander et al., 2019; Helander et al., 2012; Reisfield et al., 2020; Schrock et al., 2017; Walther et al., 2015). Piano and colleagues (2015) examined a sample of young (18 to 30 years old) drinkers using specific criteria for binge consumption. PEth was correlated with alcohol consumption in both the binge-drinking and healthy-drinking groups. However, as the amount of alcohol consumption was greater in the binge group compared with the moderate group, the pattern of binge consumption likely does not influence PEth more than amount consumed. Stewart and colleagues (2014) found a difference in the strength of relationship between PEth and alcohol consumption with a median split for time since last drink reported using TLFB. This would suggest that a binge-drinking pattern may have the potential for variability in PEth levels given that the time since last drink is likely variable. However, we did not find in our sample that Form-30 time since last drink contributed to PEth prediction in a model where Form-30 total number of drinks and time since last drink were both included. The Form-30 total drinks over 30 days was the strongest predictor of PEth levels among Form-30 subscales.
Self-reported alcohol consumption information in the present study was coded (i.e., confidential), and participants were not seeking alcohol treatment, which may increase confidence that the self-report information regarding alcohol consumption may be an accurate reflection of actual consumption. There were statistical outliers in both age and consumption groups. For the moderate (healthy) drinking group, 73% of samples were in the quantifiable range, and in the risky or heavy consumption group, 98% of samples were above the quantifiable range. For the percent of samples in the quantifiable range, this sample is similar or slightly above other samples with moderate-to-low alcohol consumption, which ranged from 48 to 70% (Afshar et al., 2017; Johansson et al., 2020; Neumann et al., 2020; Schrock et al., 2017).
Limitations of this study include reliance on self-report of alcohol use and focus on a sample of community-dwelling adults in which older adults and men are over-represented and adults younger than 35 years were excluded. Despite active attempts to enroll older adults with heavy alcohol consumption, there were equal numbers of participants in the heavy alcohol range between the middle-age and older adult age groups. The sample does not represent participants with extremely high alcohol consumption levels as the presence of alcohol withdrawal symptoms was an exclusion criteria. Future studies should continue to include samples that represent older adults to allow for confirmation of our findings and to further appreciate the relationship between age, PEth, and alcohol consumption.
In summary, findings suggest a strong relationship between amount of alcohol consumption and PEth levels with age a small but contributing factor to predicting PEth levels. Middle-aged adults had higher PEth levels than older adults at comparable drinking levels. PEth levels were most strongly associated with amount of alcohol consumed rather than number of drinking days for information reflecting the last 30 days (Form 30).
Supplementary Material
ACKNOWLEDGMENTS
This research was funded in part by R01 AG047979 from National Institute on Aging (NIA) and UL 1TR002319 and VAPSHCS, and University of Washington Department of Anesthesiology and Pain Management. The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria, participation in speakers bureaus, membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) in the subject matter or materials discussed in this manuscript.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
REFERENCES
- Afshar M, Burnham EL, Joyce C, Clark BJ, Yong M, Gaydos J, Cooper RS, Smith GS, Kovacs EJ, Lowery EM (2017) Cut-point levels of phosphatidylethanol to identify alcohol misuse in a mixed cohort including critically ill patients. Alcohol Clin Exp Res 41:1745–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen-Streichert H, Muller A, Glahn A, Skopp G, Sterneck M (2018) Alcohol biomarkers in clinical and forensic contexts. Dtsch Arztebl Int 115:309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA (2013) Diagnostic and Statistical Manual of Mental Disorders, 5th edn. American Psychiatric Association, Washington, DC. [Google Scholar]
- Bayliss EA, Ellis JL, Steiner JF (2005) Subjective assessments of comorbidity correlate with quality of life health outcomes: initial validation of a comorbidity assessment instrument. Health Qual Life Outcomes 3:51–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotti F, Sorio D, Bertaso A, Tagliaro F (2018) Analytical and diagnostic aspects of carbohydrate deficient transferrin (CDT): a critical review over years 2007–2017. J Pharm Biomed Anal 147:2–12. [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA (1998) The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 158:1789–1795. [DOI] [PubMed] [Google Scholar]
- Conigrave KM, Davies P, Haber P, Whitfield JB (2003) Traditional markers of excessive alcohol use. Addiction 98(Suppl. 2):31–43. [DOI] [PubMed] [Google Scholar]
- Ferguson TF, Theall KP, Brashear M, Maffei V, Beauchamp A, Siggins RW, Simon L, Mercante D, Nelson S, Welsh DA, Molina PE (2020) Comprehensive assessment of alcohol consumption in people living with HIV (PLWH): The New Orleans alcohol use in HIV study. Alcohol Clin Exp Res 44:1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis JM, Weiss HA, Helander A, Kapiga SH, Changalucha J, Grosskurth H (2015) Comparison of self-reported alcohol use with the alcohol biomarker phosphatidylethanol among young people in northern Tanzania. Drug Alcohol Depend 156:289–296. [DOI] [PubMed] [Google Scholar]
- Gnann H, Weinmann W, Thierauf A (2012) Formation of phosphatidylethanol and its subsequent elimination during an extensive drinking experiment over 5 days. Alcohol Clin Exp Res 36:1507–1511. [DOI] [PubMed] [Google Scholar]
- Hahn JA, Dobkin LM, Mayanja B, Emenyonu NI, Kigozi IM, Shiboski S, Bangsberg DR, Gnann H, Weinmann W, Wurst FM (2012) Phosphatidylethanol (PEth) as a biomarker of alcohol consumption in HIV-positive patients in sub-Saharan Africa. Alcohol Clin Exp Res 36:854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim F, Wiart JF, Menard O, Allorge D, Gaulier JM (2019) Phosphatidylethanol blood analysis. Ann Biol Clin 77:638–644. [DOI] [PubMed] [Google Scholar]
- Halter CC, Dresen S, Auwaerter V, Wurst FM, Weinmann W (2008) Kinetics in serum and urinary excretion of ethyl sulfate and ethyl glucuronide after medium dose ethanol intake. Int J Legal Med 122:123–128. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander A, Bottcher M, Dahmen N, Beck O (2019) Elimination characteristics of the alcohol biomarker phosphatidylethanol (PEth) in blood during alcohol detoxification. Alcohol Alcohol 54:251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander A, Hansson T (2013) National Harmonization of the alcohol biomarker PEth. Lakartidningen 110:1747–1748. [PubMed] [Google Scholar]
- Helander A, Hermansson U, Beck O (2019) Dose-response characteristics of the alcohol biomarker phosphatidylethanol (PEth)—a study of outpatients in treatment for reduced drinking. Alcohol Alcohol 54:567–573. [DOI] [PubMed] [Google Scholar]
- Helander A, Péter O, Zheng Y (2012) Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcohol 47:552–557. [DOI] [PubMed] [Google Scholar]
- Helander A, Zheng Y (2009) Molecular species of the alcohol biomarker phosphatidylethanol in human blood measured by LC-MS. Clin Chem 55:1395–1405. [DOI] [PubMed] [Google Scholar]
- Higgins-Biddle JC, Babor TF (2018) A review of the Alcohol Use Disorders Identification Test (AUDIT), AUDIT-C, and USAUDIT for screening in the United States: past issues and future directions. Am J Drug Alcohol Abuse 44:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Kapturczak N, Dougherty DM, Roache JD, Karns-Wright TE, Javors MA (2018) Differences in the synthesis and elimination of phosphatidylethanol 16:0/18:1 and 16:0/18:2 after acute doses of alcohol. Alcohol Clin Exp Res 42:851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM (2019) SPSS Statistics, Version 26. IBM Corporation, Armonk, NY. [Google Scholar]
- Javors MA, Hill-Kapturczak N, Roache JD, Karns-Wright TE, Dougherty DM (2016) Characterization of the pharmacokinetics of phosphatidylethanol 16:0/18:1 and 16:0/18:2 in human whole blood after alcohol consumption in a clinical laboratory study. Alcohol Clin Exp Res 40:1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson K, Johansson L, Pennlert J, Soderberg S, Jansson JH, Lind MM (2020) Phosphatidylethanol levels, as a marker of alcohol consumption, are associated with risk of intracerebral hemorrhage. Stroke 51:2148–2152. [DOI] [PubMed] [Google Scholar]
- Kechagias S, Dernroth DN, Blomgren A, Hansson T, Isaksson A, Walther L, Kronstrand R, K agedal B, Nystrom FH (2015) Phosphatidylethanol compared with other blood tests as a biomarker of moderate alcohol consumption in healthy volunteers: a prospective randomized study. Alcohol Alcohol 50:399–406. [DOI] [PubMed] [Google Scholar]
- Kummer N, Ingels AS, Wille SMR, Hanak C, Verbanck P, Lambert WEE, Samyn N, Stove CP (2016) Quantification of phosphatidylethanol 16:0/18:1, 18:1/18:1, and 16:0/16:0 in venous blood and venous and capillary dried blood spots from patients in alcohol withdrawal and control volunteers. Anal Bioanal Chem 408:825–838. [DOI] [PubMed] [Google Scholar]
- Lakso HA, Wuolikainen A, Sundkvist A, Johansson I, Marklund SL (2019) Long-term stability of the alcohol consumption biomarker phosphatidylethanol in erythrocytes at-80 degrees C. Clin Mass Spectrometry 11:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Bradley AM, Moss HB (2010) Alcohol biomarkers in applied settings: recent advances and future research opportunities. Alcohol Clin Exp Res 34:955–967. [DOI] [PubMed] [Google Scholar]
- Lopez-Cruzan M, Roache JD, Hill-Kapturczak N, Karns-Wright TE, Dougherty DM, Sanchez JJ, Koek W, Javors MA (2018) Pharmacokinetics of phosphatidylethanol 16:0/20:4 in human blood after alcohol intake. Alcohol Clin Exp Res 42:2094–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrovito R, Strathmann FG (2020) Distributions of alcohol use biomarkers including ethanol, phosphatidylethanol, ethyl glucuronide and ethyl sulfate in clinical and forensic testing. Clin Biochem 82:85–89. [DOI] [PubMed] [Google Scholar]
- Miller WR, Del Boca FK (1994) Measurement of drinking behavior using the Form 90 family of instruments. J Stud Alcohol Suppl 12:112–118. [DOI] [PubMed] [Google Scholar]
- Moos RH, Brennan PL, Schutte KK, Moos BS (2005) Older adults’ health and changes in late-life drinking patterns. Aging Ment Health 9: 49–59. [DOI] [PubMed] [Google Scholar]
- Neumann J, Beck O, Helander A, Bottcher M (2020) Performance of PEth compared with other alcohol biomarkers in subjects presenting for occupational and pre-employment medical examination. Alcohol Alcohol 55:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VL, Paull P, Haber PS, Chitty K, Seth D (2018) Evaluation of a novel method for the analysis of alcohol biomarkers: ethyl glucuronide, ethyl sulfate and phosphatidylethanol. Alcohol 67:7–13. [DOI] [PubMed] [Google Scholar]
- Pergolizzi J, Boger RH, Budd K, Dahan A, Erdine S, Hans G, Kress HG, Langford R, Likar R, Raffa RB, Sacerdote P (2008) Opioids and the management of chronic severe pain in the elderly: consensus statement of an international expert panel with focus on the six clinically most often used World Health Organization step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract 8:287–313. [DOI] [PubMed] [Google Scholar]
- Piano MR, Tiwari S, Nevoral L, Phillips SA (2015) Phosphatidylethanol levels are elevated and correlate strongly with AUDIT scores in young adult binge drinkers. Alcohol Alcohol 50:519–525. [DOI] [PubMed] [Google Scholar]
- R_Core_Team (2019) A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Reisfield GM, Teitelbaum SA, Opie SO, Jones J, Morrison DG, Lewis B (2020) The roles of phosphatidylethanol, ethyl glucuronide, and ethyl sulfate in identifying alcohol consumption among participants in professionals health programs. Drug Test Anal 12:1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satre DD, Bahorik AL, Mackin RS (2018) Alcohol and drug use among older adults: associations with widowhood, relationship quality, and physical health. J Gerontol B Psychol Sci Soc Sci 73:633–635. [DOI] [PubMed] [Google Scholar]
- Schröck A, Thierauf-Emberger A, Schürch S, Weinmann W (2017) Phosphatidylethanol (PEth) detected in blood for 3 to 12 days after single consumption of alcohol—a drinking study with 16 volunteers. Int J Legal Med. 131(1), 153–160. [DOI] [PubMed] [Google Scholar]
- Schrock A, Wurst FM, Thon N, Weinmann W (2017) Assessing phosphatidylethanol (PEth) levels reflecting different drinking habits in comparison to the alcohol use disorders identification test—C (AUDIT-C). Drug Alcohol Depend 178:80–86. [DOI] [PubMed] [Google Scholar]
- Shimp LA (1998) Safety issues in the pharmacologic management of chronic pain in the elderly. Pharmacotherapy 18:1313–1322. [PubMed] [Google Scholar]
- Steer RA, Ball R, Ranieri WF, Beck AT (1997) Further evidence for the construct validity of the Beck depression Inventory-II with psychiatric outpatients. Psychol Rep 80:443–446. [DOI] [PubMed] [Google Scholar]
- Stenton J, Walther L, Hansson T, Andersson A, Isaksson A (2019) Inter individual variation and factors regulating the formation of phosphatidylethanol. Alcohol Clin Exp Res 43:2322–2331. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Koch DG, Willner IR, Anton RF, Reuben A (2014) Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res 38:1706–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulwelling W, Smith K (2018) The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci 63:1634–1640. [DOI] [PubMed] [Google Scholar]
- Vestal RE, McGuire EA, Tobin JD, Andres R, Norris AH, Mezey E (1977) Aging and ethanol metabolism. Clin Pharmacol Ther 21:343–354. [DOI] [PubMed] [Google Scholar]
- Walther L, de Bejczy A, Löf E, Hansson T, Andersson A, Guterstam J, Hammarberg A, Asanovska G, Franck J, Söderpalm B, Isaksson A (2015) Phosphatidylethanol is superior to carbohydrate-deficient transferrin and γ-glutamyltransferase as an alcohol marker and is a reliable estimate of alcohol consumption level. Alcohol Clin Exp Res 39:2200–2208. [DOI] [PubMed] [Google Scholar]
- Wittkampf KA, Naeije L, Schene AH, Huyser J, van Weert HC (2007) Diagnostic accuracy of the mood module of the Patient Health Questionnaire: a systematic review. Gen Hosp Psychiatry 29:388–395. [DOI] [PubMed] [Google Scholar]
- Woods AJ, Porges EC, Bryant VE, Seider T, Gongvatana A, Kahler CW, de la Monte S, Monti PM, Cohen RA (2016) Current heavy alcohol consumption is associated with greater cognitive impairment in older adults. Alcohol Clin Exp Res 40:2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Beck O, Helander A (2011) Method development for routine liquid chromatography-mass spectrometry measurement of the alcohol biomarker phosphatidylethanol (PEth) in blood. Clin Chim Acta 412:1428–1435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


