These findings demonstrate that depression and anxiety worsened while clinical pain improved during the State-at-Home period of the pandemic suggesting a shift in symptoms perception.
Keywords: Mood disorder, Temporomandibular disorder, Expectations, Illness behaviors, Nocebo
ABSTRACT
Introduction:
The unknown and uncontrollable situation of the coronavirus disease 2019 (COVID-19) pandemic may have triggered changes in pain, anxiety, and depression along with a perception of nonspecific COVID-19 symptoms.
Objectives:
We determined how anxiety, depression, and pain outcomes varied during the “Stay-at-Home” order compared with the prepandemic period and whether nonspecific COVID-19 symptoms would occur.
Methods:
We conducted an online survey to opportunistically reassess clinical anxiety, depression, pain intensity, and pain interference while controlling for somatic symptom severity during the prepandemic and Stay-at-Home order period. During the Stay-at-Home period, anxiety, depression, pain intensity, and pain interference were reassessed. Coping strategies were assessed as a critical factor influencing pain behaviors. In addition, we explored the occurrence of nonspecific COVID-19 symptoms with an ad hoc survey referencing the Centers for Disease Control and Prevention publicly available COVID-19 symptoms.
Results:
We observed a significant increase in depression and anxiety levels during the Stay-at-Home period. Coping strategy changes (eg, increased exercise) were linked to lower pain severity and interference which improved overall. Participants who self-reported nonspecific COVID-19 symptoms had higher prepandemic depression. Among the 72 participants not diagnosed with COVID-19, 70.8% of the participants experienced symptoms resembling those associated with COVID-19.
Conclusion:
We suggest the parallel between pain outcome improvement and worsening anxiety and depression during the Stay-at-Home order might reflect a shift in symptoms, indicating that those patients with underlying mood disorders may require more help than they did before the pandemic.
1. Introduction
The pandemic of coronavirus disease 2019 (COVID-19)24,42 is one of the most serious outbreaks over the past century. It has induced significant distress and anxiety for patients55 and providers.7,62 Stress is the acute response to something fearful, unpredictable, and uncontrollable; it can also potentiate anxiety, an adaptive response that promotes harm avoidance.33 However, circumstances producing sustained distress, such as the COVID-19 pandemic, can result in overwhelming, excessive anxiety.31
The symptomatology of COVID-19 has evolved from only flu-like symptoms to a variety of symptoms that have been continuously updated by the Centers for Disease Control and Prevention (CDC).21 The daily threat of being exposed to COVID-19 or, worse, infected, could trigger the occurrence of nonspecific symptoms mimicking mild COVID-19–related symptoms that are not actually caused by the virus.
The Stay-at-Home order in the United States, State of Maryland refers to the condition for which residents were required to stay at home except for necessary life supplies and medical reasons from March 30 to June 9, 2020. During this short window of time, we focused on this opportunistic study to determine how anxiety, depression, and pain outcomes change along with the occurrence of COVID-19 symptoms that we considered “nonspecific” symptoms given that they were reported by people who either tested negative or were not aware of having been infected in accordance with previous definitions.6 Based on the recent media coverage on COVID-19 and worldwide emotional and physiological distress,32,59 our research question was to determine how personality factors23 and prepandemic levels of anxiety and depression3,4,7,10 could have affected anxiety, depression, and pain outcomes during the stressful Stay-at-Home order. To further explore the role of psychosocial factors, we assessed chronic pain coping strategies37 before and during the Stay-at-Home order and anticipated that effective adjustments in lifestyle would have resulted in less severe pain and interference despite the higher levels of anxiety and depression. Based on these hypotheses, we propose a framework that describes a shift in illness behaviors (ie, individual's responses to their health status56) as characterized by higher anxiety and depression and changes in pain and coping strategies to understand patients' behaviors within the context of the self-isolation during the COVID-19 pandemic.
2. Methods and Materials
We conducted this opportunistic study in a cohort of patients with chronic pain and healthy controls with whom we had already conducted before the outbreak an in-person and in-depth assessment of pain intensity, pain interference, anxiety, and depression. The Stay-at-Home order in the United States and, namely, in the State of Maryland started March 30 and ended June 9. During this time, we received institutional review board approval, and from May 12 to June 1, 2020, we enrolled 74 participants who were required to self-isolate during the lockdown.
We restricted the time for conducting the study to a 21-day window of the Stay-at-Home order in the State of Maryland. Study participants included 57 adults suffering from chronic pain and 17 healthy participants (Demographics are presented in Table 1). The study was approved by the local Institutional Review Board Committee at the University of Maryland. Participants were already phenotyped before the pandemic (21.09 ± 11.77 months) at the University of Maryland Schools of Nursing and/or the Brotman Orofacial Clinic at the School of Dentistry (for the patients with chronic facial pain).
Table 1.
Demographics of patients with chronic pain and healthy participants.
| Participants with chronic pain (n = 57) | Healthy participants (n = 17) | |
|---|---|---|
| Age (years) | 44.58 (12.91) | 33.12 (9.60) |
| Sex | ||
| Women | 48 | 11 |
| Men | 9 | 6 |
| Race | ||
| White | 42 | 9 |
| Non-White | 15 | 8 |
| Education | ||
| High school | 4 | 0 |
| College | 30 | 5 |
| Postgraduate level | 23 | 12 |
| Marital status | ||
| Married or living as married | 21 | 5 |
| Single or living as single | 36 | 12 |
| Annual income | ||
| $0–$59,999 | 27 | 8 |
| $60,000–$99,999 | 14 | 7 |
| $100,000 and above | 16 | 2 |
| Baseline clinical characteristics | ||
| Baseline chronic pain intensity | 47.70 (22.98) | n/a |
| Baseline chronic pain interference | 23.45 (26.43) | n/a |
| Baseline TMD duration (months) | 176.91 (141.16) | n/a |
| Before/during the Stay-at-Home order clinical assessments | ||
| Anxiety* | 40.30 (11.39)/45.61 (4.42) | 35.24 (6.75)/45.86 (4.48) |
| Depression† | 10.30 (8.70)/11.64 (9.80) | 4.06 (3.70)/8.20 (8.24) |
| Graded chronic pain‡ | 1.86 (1.08)/1.36 (0.97) | n/a |
| Prepandemic DASS anxiety§ | 6.11 (6.67) | 1.76 (3.53) |
| Prepandemic somatization║ | 7.09 (4.48) | n/a |
| During the Stay-at-Home order fear of COVID-19¶ | 15.68 (6.16) | 13.65 (5.05) |
| Concurrent overlapping pain conditions | ||
| Back pain | 19 | n/a |
| Migraine/Headaches | 11 | n/a |
| Irritable bowel syndrome | 3 | n/a |
| Fibromyalgia | 3 | n/a |
Data presented are expressed as mean ± SD.
Tools used to assess anxiety, depression, pain, somatization, and fear are as follows:
Anxiety was measured using STAI (State-Trait Anxiety Inventory).
Depression was measured using BDI (Beck Depression Inventory).
Graded chronic pain was assessed using GCPS (Graded Chronic Pain Scale).
DASS = Depression Anxiety Stress Scale.
Somatization was assessed using PHQ-15 (Patient Health Questionnaire-15).
Fear of COVID-19 was assessed using FCV-19S (Fear of COVID-19 Scale).
COVID-19, coronavirus disease 2019; TMD, temporomandibular disorder.
2.1. Survey tool and psychological questionnaires
Participants in this cohort were either healthy volunteers or patients who were diagnosed with temporomandibular disorder(s) pain (according to the Axis I Diagnostic Criteria for TMD [DC/TMD]) at the Brotman Facial Pain Clinic, School of Dentistry University of Maryland.54 The healthy volunteer group consisted of participants who did not use pain medication and did not have pain of any nature, neurological disorders, or psychiatric disorders.
In addition, patients with chronic pain had been evaluated in person to confirm the diagnosis of temporomandibular disorder (TMD),54 pain severity using the Graded Chronic Pain Scale (GCPS),60 and other overlapping chronic pain conditions46 (eg, migraine, low back pain, irritable bowel syndrome, and fibromyalgia; also refer to Table 1).
2.1.1. Psychological tools
A 6-item online Health Insurance Portability and Accountability Act–compliant survey through Research Electronic Data Capture34 was used. The survey briefly inquired about being diagnosed with COVID-19 and having experienced one or more symptoms of COVID-19, as per the CDC's publicly available list of symptoms in May 2020. The participants were first asked whether they were currently suffering from COVID-19 symptoms and whether they had been tested for COVID-19. If they had been, they were asked to indicate the result of the test. Participants were then asked to self-report whether they had experienced any symptoms from a structured checklist of CDC COVID-19 symptoms. Participants were also asked whether they had close contact with someone who had COVID-19. The last item asked if participants were diagnosed with COVID-19 and, if yes, how it was managed.
Psychological measurements were also collected (Table 2). Before the COVID-19 pandemic, all participants had been assessed in person for anxiety, depression, and chronic pain severity. Anxiety and depression had been assessed by using the Depression Anxiety Stress Scale,58 State-Trait Anxiety Inventory (STAI-II),5,61 and Beck Depression Inventory (BDI).9 Participants had also been characterized for personality factors using the Neuroticism, Extroversion, Openness Five-Factor Inventory.22 For this study, we focused on the extroversion component, anticipating that those who had an attitude-type characterized by interests in the external objects could have benefited (eg, less pain and nonspecific COVID-19 symptoms) during the Stay-at-Home isolation.
Table 2.
Clinical and psychological tools.
| Categories | Questionnaire | Description | Time points* | |
|---|---|---|---|---|
| Before | During | |||
| Anxiety and depression | STAI-II5,57 | The STAI-II is a 20-item measurement that assesses the anxiety levels that are distinguishable from depression symptoms. | ✓ | ✓ |
| BDI9 | The BDI is a 21-item self-reported inventory designed to assess the level of depressive symptomology. It is composed of items associated with depressive symptoms such as hopelessness and irritability; cognitive aspects such as guilty or feeling of being punished; and physical symptoms such as fatigue and weight loss. | ✓ | ✓ | |
| DASS54 | The DASS is a 21-item tool designed to measure the ubiquitous and clinically significant emotional states of depression, anxiety, and stress. In the current study, the DASS was used to assess the severity of the core symptoms of depression, anxiety, and stress. The advantage of DASS is that it distinguishes between symptoms of physical arousal and symptoms of generalized anxiety such as tension or agitation. | ✓ | — | |
| Personality factors | NEO-FFI19 | The NEO-FFI is a 60-item inventory that provides quick and accurate profiles of the 5 domains of personality including neuroticism, extraversion, openness, agreeableness, and conscientiousness. | ✓ | ✓ |
| Clinical factors | PHQ-1536 | The PHQ-15 is a valid tool for detection of patients at risk of somatoform disorders. The somatic symptoms listed in PHQ-15 overlap with panic disorder, generalized anxiety disorder, depressive disorders, or illness anxiety disorder. In the current study, the PHQ-15 served as a continuous measure of somatic symptoms severity. | ✓ | — |
| Pain-related factors | GCPS56 | The GCPS is a multidimensional measure that tests 2 dimensions of chronic pain severity: pain intensity and pain-related interference. | ✓ | ✓ |
| CPCI34 | The CPCI is a 65-item inventory that is designed to assess the strategies used by patients to cope with chronic pain. The inventory is composed of 2 categories including illness-focused coping scales (ie, guarding, resting, and ask for assistance) and wellness-focused coping scales (exercise/stretch, relaxation, task persistence, coping self-statement, and seeking social support). | ✓ | ✓ | |
| COVID-19–related factors | FCV-19S1 | The FCV-19S is a 7-item tool to assess the fear of COVID-19 | — | ✓ |
| COVID-19 survey | A 6-item compliant survey was created to assess COVID-19 symptomology. The survey briefly inquired about being diagnosed with COVID-19 and having experienced one or more symptoms of COVID-19, as per the CDC's publicly available list of symptoms | — | ✓ | |
The assessments were conducted before the Stay-at-Home order (before January 2020) and during the Stay-at-Home order (May 2020).
BDI, Beck Depression Inventory; CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; CPCI, Chronic Pain Coping Inventory; DASS, Depression Anxiety Stress Scale; FCV-19S, Fear of COVID-19 Scale; GCPS, Graded Chronic Pain Scale; NEO-FFI, Neuroticism, Extroversion, Openness Five-Factor Inventory; PHQ-15, Patient Health Questionnaire-15; STAI-II, State-Trait Anxiety Inventory.
For participants with TMD, chronic pain severity and pain interference and pain-related coping strategies were measured using the GCPS.60 Severity of somatic symptoms was measured using the Patient Health Questionnaire-15 (PHQ-15)39 and coping strategies with the Chronic Pain Coping Inventory (CPCI).37 Specifically, the characteristic pain scores from GCPS were calculated as the mean of the intensity ratings for current pain, worst pain, and average pain during the past 1 month to represent the chronic pain severity. The pain-related interference scores from GCPS were calculated as the mean of the ratings for difficulties performing social and work-related activities.
Because we expected that participants' COVID-19 symptoms, or their perception of having had these symptoms, could have been affected by their prepandemic psychological characteristics and mood disorders, we re-evaluated the extent and severity of their anxiety and depression at the time of the survey. To lessen the burden of taking the online survey, we limited the reassessments to anxiety, depression, personality characteristics, and chronic pain severity as well as coping strategy using the STAI-II,5 BDI,9 GCPS,60 and CPCI37 tools, respectively. We also added the recently created Fear of COVID-19 Scale1, a 7-item scale that quantifies the sensation of feeling anxious and scared about COVID-19.
2.2. Statistics
2.2.1. Outcomes
Primary outcomes were pain intensity and pain interference assessed by GCPS.60 Secondary outcomes were anxiety assessed by STAI-II,5 depression assessed by BDI,9 and self-reported perception of COVID-19 symptomology. Explorative outcomes were somatic symptoms measured using the PHQ-1539 and coping strategies assessed with the CPCI.37
For the anxiety, depression, and chronic pain outcomes, we first conducted repeated-measures analysis of covariance (ANCOVA) to examine the changes in those primary and secondary outcomes during the prepandemic and Stay-at-Home order. The time (before vs after the pandemic) was treated as the within-subjects factor, and group (chronic pain vs healthy controls) was set as the between-subjects factor. The time range between the 2 time-point assessments was treated as covariate.
Next, we determined how prepandemic personality characteristics and fear of COVID-19 could have influenced anxiety and depression during the Stay-at-Home order. To test this, separate hierarchical regressions were conducted with baseline anxiety and depression entered in block 1. Personality characteristics (neuroticism, extraversion, openness to experiences, agreeableness, and conscientiousness—prepandemic), fear of COVID-19 (during the pandemic), and group (chronic pain vs healthy participants) were treated as independent variables in block 2.
Within the chronic pain cohort, we tested how changes in pain coping strategies, prepandemic personality characteristics, current anxiety, depression, and fear of COVID-19 may have contributed to the chronic pain severity and interference during the pandemic. To test this, separate hierarchical regression analyses were conducted with chronic pain severity and interference as dependent variables. To determine outcome variations during the pandemic, baseline somatic symptom severity was entered in block 1 of the hierarchical regression. Personality characteristics, anxiety, and depression (assessed at baseline), fear of COVID-19, and changes in pain coping strategies (delta scores of before and during the pandemic) were treated as independent variables in block 2 of each of the regression model. This part of analysis was limited to participants with chronic pain.
2.2.2. Coronavirus disease 2019 symptomatology
To determine how participants with chronic pain differed from healthy participants in the occurrence of nonspecific COVID-19 symptoms (yes or no), χ2 tests were used to compare the proportion of participants who had COVID-19 nocebo-like symptoms. Moreover, to examine how anxiety and depression influenced the perception of nonspecific COVID-19 symptoms, repeated-measures ANCOVAs were conducted (1) to compare anxiety and depression between the participants who showed nonspecific COVID-19 symptoms and those who did not perceive symptoms and (2) to examine the changes of anxiety and depression before and during the Stay-at-Home order period. The 2 time points (before vs during the pandemic) were set as within-subjects factors. The perception of COVID-19 symptoms occurrence (yes vs no) and group (chronic pain vs healthy control) were set as between-subjects factors. The time range between the 2 measurements was set as covariate. Moreover, two-way ANCOVAs were conducted to compare prepandemic personality factors assessed by Neuroticism, Extroversion, Openness Five-Factor Inventory22 between participants who had nonspecific COVID-19 symptoms and those without the symptoms. The perception of COVID-19 symptoms and group were treated as between-subjects factors while the time range between the 2 measurements was treated as covariate. Finally, Spearman correlations were conducted to examine the associations among prepandemic NEO personality factors, anxiety, depression, fear of COVID-19, and number of COVID-19 symptoms.
Cohen d and 95% confidence intervals (CIs) are reported for all the results. Outliers in the number of nonspecific COVID-19 symptoms were also considered by using the Tukey formula: Upper = Q3 + (2.2 × (Q3 − Q1)); Lower = Q1 − (2.2 × (Q3 − Q1)). Q1 and Q 3 equal 25% and 75% percentiles, respectively.
All analyses were conducted using SPSS version 26, and the level of significance was set at P < 0.05.
3. Results
3.1. Anxiety and depression
When comparing mood and depression between prepandemic and Stay-at-Home time points, we found that anxiety and depression worsened in both healthy participants and participants with chronic pain (anxiety: main effect of time: F1,69 = 21.88, P < 0.001, main effect of group: F1,69 = 1.88, P = 0.175; depression: main effect of time: F1,69 = 5.65, P = 0.020, group: F1,69 = 6.46, P = 0.013, Fig. 1A, B).
Figure 1.
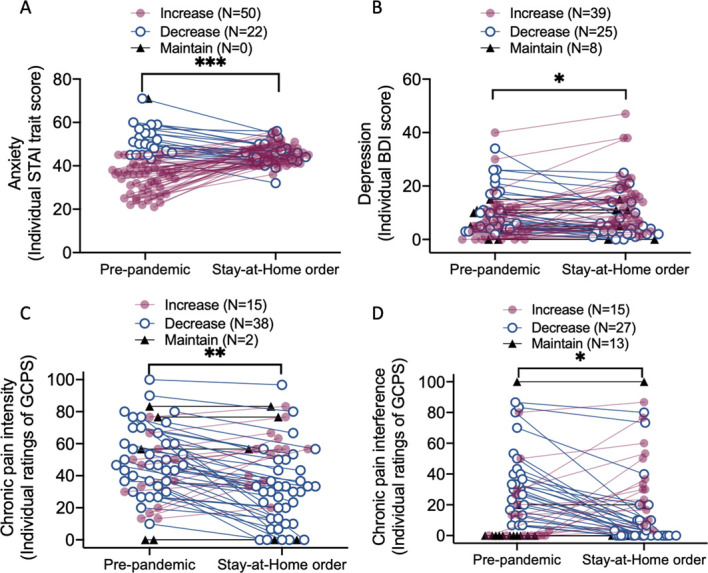
Anxiety, depression, and chronic pain severity or interference in the prepandemic and during the Stay-at-Home periods. (A) Anxiety level significantly increased during the Stay-at-Home order as compared with the prepandemic period. Fifty participants showed increases in anxiety while only 20 participants showed anxiety reductions. (B) There were significant increases in depression during the Stay-at-Home order when compared with the prepandemic phase. Although 25 participants showed depression reductions and 8 participants maintained the same level of depression, the remaining 39 participants showed increases in depression levels. (C) There were significant reductions of chronic pain severity during the Stay-at-Home order period compared with the prepandemic period. Although 15 participants showed increases in chronic pain severity and 2 participants showed the same chronic pain severity during the pandemic, most participants with chronic pain (N = 38) experienced reductions of chronic pain during the pandemic as compared with the baseline period. (D) The participants with chronic pain had significant reductions of chronic pain interference during the pandemic as compared with the prepandemic period. Fifteen participants experienced increases in chronic pain interference, 13 participants' pain interference level maintained the same, and 27 participants had experienced reductions of chronic pain interference. Individual participants' data are presented. Participants who showed increases in chronic pain severity and interference are presented in red; participants who showed decreasing pattern are presented in blue; and participants who maintained the same are presented in gray. Data from one participant were omitted from the figure because of a missing value at baseline. *P < 0.05; **P < 0.01; ***P < 0.001. BDI, Beck Depression Inventory; GCPS, Graded Chronic Pain Scale; STAI, State-Trait Anxiety Inventory
Prepandemic neuroticism (β = 0.57, P = 0.003) and openness to experiences (β = 0.27, P = 0.013) emerged as significant predictors of higher levels of anxiety during the Stay-at-Home order across participants with chronic pain and healthy participants. Baseline prepandemic anxiety was not significantly associated with anxiety during the pandemic (Table 3). For depression, neither fear of COVID-19 symptoms nor personality factors were significantly associated with depression during the Stay-at-Home order (all P > 0.141), despite the result that greater baseline depression was a significant predictor of higher depression level during the Stay-at-Home order phase (β = 0.66, P < 0.001).
Table 3.
Prepandemic predictors for anxiety, depression, and chronic pain characteristics during the Stay-at-Home order period.
| Hierarchical regression model on anxiety during the pandemic assessed by STAI-II | ||||
|---|---|---|---|---|
| Blocks | Predictors | Standardized coefficient | ||
| β | t-value | P | ||
| Block 1 | Baseline anxiety | 0.205 | 1.755 | 0.084 |
| Block 2 | NEO—neuroticism | 0.566 | 3.015 | 0.003 |
| NEO—extraversion | 0.206 | 1.664 | 0.101 | |
| NEO—openness to experiences | 0.274 | 2.543 | 0.013 | |
| NEO—agreeableness | −0.172 | −1.247 | 0.217 | |
| NEO—conscientiousness | 0.213 | 1.749 | 0.085 | |
| Fear of COVID-19 | 0.195 | 1.813 | 0.075 | |
| Participants with TMD vs HC | 0.020 | 0.166 | 0.869 | |
| Hierarchical regression model on depression during the pandemic assessed by BDI | ||||
|---|---|---|---|---|
| Blocks | Predictors | Standardized coefficient | ||
| β | t-value | P | ||
| Block 1 | Baseline depression | 0.662 | 7.393 | 0.000 |
| Block 2 | NEO—neuroticism | −0.019 | −0.151 | 0.880 |
| NEO—extraversion | 0.084 | 0.772 | 0.443 | |
| NEO—openness to experiences | 0.032 | 0.335 | 0.739 | |
| NEO—agreeableness | −0.179 | −1.495 | 0.140 | |
| NEO—conscientiousness | 0.009 | 0.086 | 0.932 | |
| Fear of COVID-19 | 0.112 | 1.173 | 0.245 | |
| Participants with TMD vs HC | −0.038 | −0.338 | 0.736 | |
| Hierarchical regression model on chronic pain intensity during the pandemic assessed by GCPS | ||||
|---|---|---|---|---|
| Blocks | Predictors | Standardized coefficient | ||
| β | t-value | P | ||
| Block 1 | PHQ-15 baseline somatic symptom severity | 0.514 | 4.192 | 0.000 |
| Block 2 | NEO—neuroticism | 0.183 | 1.308 | 0.199 |
| NEO—extraversion | −0.012 | −0.069 | 0.945 | |
| NEO—openness to experiences | 0.230 | 1.974 | 0.056 | |
| NEO—agreeableness | −0.158 | −1.123 | 0.268 | |
| NEO—conscientiousness | −0.044 | −0.334 | 0.740 | |
| Fear of COVID-19 | 0.067 | 0.530 | 0.599 | |
| Baseline anxiety | −0.099 | −0.522 | 0.605 | |
| Baseline depression | 0.418 | 2.361 | 0.023 | |
| Changes in asking assistance | 0.386 | 2.426 | 0.020 | |
| Changes in exercise | −0.352 | −2.578 | 0.014 | |
| Changes in social support | 0.277 | 2.204 | 0.035 | |
| Hierarchical regression model on chronic pain interference during the pandemic assessed by GCPS | ||||
|---|---|---|---|---|
| Blocks | Predictors | Standardized coefficient | ||
| β | t-value | P | ||
| Block 1 | PHQ-15 baseline somatic symptom severity | 0.412 | 3.161 | 0.003 |
| Block 2 | NEO—neuroticism | −0.193 | −1.374 | 0.178 |
| NEO—extraversion | −0.327 | −1.962 | 0.057 | |
| NEO—openness to experiences | 0.200 | 1.716 | 0.094 | |
| NEO—agreeableness | −0.168 | −1.189 | 0.242 | |
| NEO—conscientiousness | −0.259 | −1.979 | 0.055 | |
| Fear of COVID-19 | 0.145 | 1.132 | 0.265 | |
| Baseline anxiety | 0.219 | 1.152 | 0.257 | |
| Baseline depression | 0.324 | 1.821 | 0.076 | |
| Changes in asking assistance | 0.185 | 1.160 | 0.253 | |
| Changes in exercise | −0.283 | −2.063 | 0.046 | |
| Changes in social support | −0.004 | −0.028 | 0.977 | |
Significant results are marked as bold entries. BDI, Beck Depression Inventory; COVID-19, coronavirus disease 2019; GCPS, Graded Chronic Pan Scale; HC, healthy controls; NEO, Neuroticism, Extraversion, and Openness Five-Factor Inventory; PHQ-15, Patient Health Questionnaire-15; STAI-II, State-Trait Anxiety Inventory—Trait subscale; TMD, temporomandibular disorder.
3.2. Chronic pain intensity, interference, and pain coping strategies
Patients suffering from chronic pain reported a reduction of pain intensity (F1,53 = 10.52, P = 0.002), with 73% of patients reporting improved pain severity during the Stay-at-Home period (Fig. 1C). Similarly, the level of chronic pain interference during the pandemic was significantly lower (mean = 15.33, SEM = 3.66) than that before the pandemic (mean = 23.21, SEM = 3.78, F1,53 = 5.24, P = 0.026, Fig. 1D).
The pain coping strategies that patients used also changed significantly during the pandemic compared with the prepandemic period. In particular, patients reported higher use of asking for assistance (eg, “asked someone to do something for me,” F1,53 = 4.36, P = 0.017), exercise or stretch (eg, “stretch the muscles in my leg,” F1,51 = 5.07, P = 0.029), and seeking social support (eg, “made arrangement to see a friend or family member,” F1,51 = 8.53, P = 0.005) during the pandemic than during the prepandemic period (Fig. 2).
Figure 2.
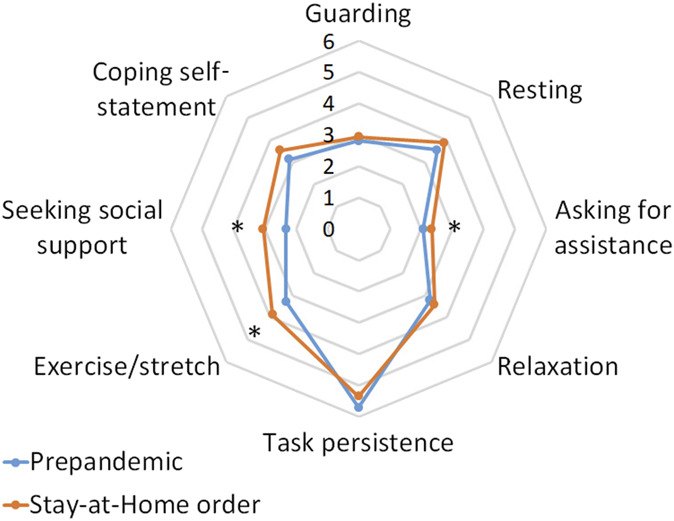
Radar plot of chronic pain coping strategies in the temporomandibular disorder cohort. Participants reported more use of asking for assistance (eg, “asked someone to do something for me”), exercise or stretch (eg, “stretch the muscles in my leg”), and seeking social support (eg, “made arrangement to see a friend or family member”) during the pandemic than before the pandemic period. Data from before the pandemic (before January 2020) are presented in light blue; data during the Stay-at-Home order (May 2020) are presented in orange. Chronic Pain Coping Inventory was adopted to measure the strategies that patient used to cope with chronic pain. It comprised subscales guarding (the extent that patients restrict the use of body part as a way to cope with pain), resting (the extent that patients use pain-contingent rest such as lying down to cope with pain), asking for assistance (the frequency when patients ask someone for help when they are in pain), relaxation (the frequency when patients use strategies such as imagination, listening to music, meditation, or self-hypnosis to relax), task persistence (the extent that patients continue daily activities despite pain), exercise or stretch (how many days per week the patients stretch their muscle and exercise), seeking social support (the frequency when patients seek out family members or friends for companion and support when in pain), and coping self-statement (the frequency when patients use adaptive cognition when they experience pain). *P < 0.05.
Controlling for the level of somatic symptom severity assessed during the prepandemic evaluation, we found that an increase in exercise was associated with reduced chronic pain severity (Table 3). However, an increase in asking for assistance and an increase in social support were associated with a higher level of chronic pain severity (Table 3). Moreover, higher prepandemic depression was linked to greater chronic pain severity during the Stay-at-Home order (Table 3). Prepandemic depression and changes in coping strategies, when taken together, explained 31.5% of the variance of chronic pain severity during the Stay-at-Home order (F11,38 = 2.58, P = 0.015, R2 change = 0.315).
We found that increased exercise was associated with reduced pain interference after controlling for the prepandemic level of somatic symptom severity, explaining 40.6% of variance of chronic pain interference (F11,38 = 3.30, P = 0.003, R2 change = 0.406, Table 3).
Prepandemic NEO-related personality characteristics, baseline anxiety, and current fear of COVID-19 did not influence chronic pain severity (all P > 0.056) and chronic pain interference (all P > 0.055) during the pandemic.
3.3. Nonspecific coronavirus disease 2019 nocebo-like symptoms
We used the list of CDC symptoms for COVID-19 publicly available in May 2020. Current COVID-19 symptoms listed by the CDC have a few additional symptoms (eg, fatigue, nausea or vomiting, and COVID-19 tongue) that were unknown at the time the study was conducted (Fig. 3).
Figure 3.
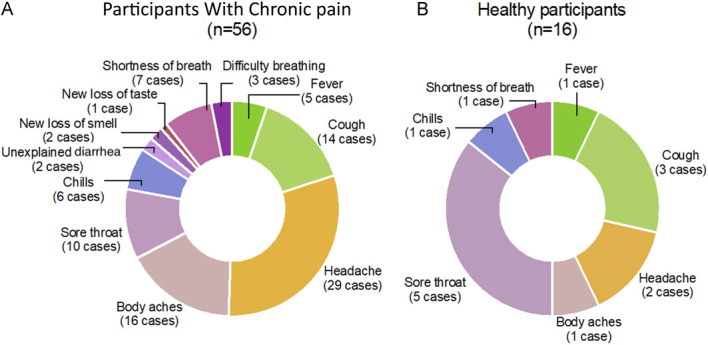
Distribution of COVID-19 nocebo-like symptoms. The survey inquired briefly about being diagnosed with COVID-19 and having experienced one or more symptoms of COVID-19, as per the CDC's publicly available list of symptoms in May 2020 (fatigue was not listed by then). They included fever or chills, cough, shortness of breath or difficulty breathing, muscle or body aches, headache, new loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting, and unexplained diarrhea. The pie on the left side shows the occurrence of perceived, self-reported nonspecific COVID-19 symptoms in patients suffering from chronic pain. Some reported symptoms overlap with TMD symptomatology (eg, headache). The pie on the right side displays the type of symptoms reported by health participants. Both patients with chronic pain and healthy participants were either tested negative or were not aware of having being infected. CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; TMD, temporomandibular disorder.
A total of 72 participants either tested negative or were not aware of being infected were included in the analyses. One of them was tested 4 times, and all results were negative. Three participants were tested twice, and both results were negative. Yet, when they were asked about having experienced COVID-19 symptoms, 51 of the 72 reported having experienced from 1 (n = 28, 38%, 95% CI = 28%–51%) to 10 symptoms (n = 1, 1%, 95% CI = 0%–4%), refer to Figure 3. Only 2 of the 74 participants responded “yes” to the question “have you been diagnosed with COVID-19?” (1 participant with chronic pain and 1 healthy participant). Both were excluded from these analyses.
We then compared participants who reported having experienced nonspecific COVID-19 symptoms with those who did not. There was a significant group (chronic pain vs healthy participants) by perceived COVID-19 (yes vs no) by time (pre vs during the pandemic) interaction (F1,62 = 4.83, P = 0.032) on depression. In particular, we found that participants with chronic pain who reported not being diagnosed with COVID-19 but having experienced nonspecific COVID-19 nocebo-like symptoms were characterized by higher baseline depression as compared with participants with asymptomatic chronic pain (P = 0.033, Cohen d = 0.832; 95% CI = 0.176–1.489), although those 2 groups showed similar levels of depression during the pandemic (P = 0.309). For prepandemic anxiety, we found no significant main effect of the perceived COVID-19 symptoms nor in its interaction with group or time (all P > 0.220).
Those who perceived symptoms had marginally lower scores for extroversion (prepandemic and during the pandemic) than those who did not experience nonspecific COVID-19 symptoms (F1,67 = 3.46, P = 0.067). Neuroticism and openness did not affect the occurrence of COVID-19 nocebo-like symptomatology (all P > 0.252).
For the number of COVID-19 nocebo-like symptomatology, higher baseline Depression Anxiety Stress Scale-anxiety (Spearman r = 0.26, P = 0.027), higher depression (Spearman r = 0.31, P = 0.009), and lower extroversion (Spearman r = −0.41, P < 0.001) were associated with a greater number of COVID-19 symptoms. Removing the outliers did not change the findings (anxiety: Spearman r = 0.28, P = 0.018; depression: Spearman r = 0.29, P = 0.014; extroversion: Spearman r = −0.38, P = 0.001).
In addition, the occurrence of nonspecific COVID-19 symptoms differed between healthy participants and patients with chronic pain (χ2 = 7.30, P = 0.007). Seventy-nine percent of those suffering from chronic pain reported having experienced at least one COVID-19 nocebo-like symptom (95% CI = 70%–88%) vs only 44% of the healthy participants (95% CI = 32%–56%). When we examined participants who showed worsening pain during the pandemic (increase pain vs decrease or sustained pain) and those who reported COVID-19 symptoms (yes vs no), we observed no overlapping between participants who showed worsening in pain and those who reported nonspecific COVID-19 symptoms (χ2 = 0.87, P = 0.351).
Age, sex, race, socioeconomic status (education, marital status, and annual income), and pain comorbidities (eg, irritable bower syndrome, Table 1) did not influence the reported occurrence of the nonspecific COVID-19 symptoms (all P > 0.175).
4. Discussion
This study monitored pain, anxiety, and depression outcomes in a cohort of study participants who had undergone a prepandemic in-depth clinical assessment and healthy controls who were required to self-isolate during the first Stay-at-Home lockdown in the United States.
Patients suffering from chronic pain reported a reduction of pain severity and interference, with 73% of self-reported improved pain severity during the lockdown. This positive result on pain severity and interference was in contrast with the occurrence or worsening in anxiety and depression symptoms in patients with chronic pain.
Our results of pain improvements are in contrast with pain experts' concerns positing a potential for pain worsening during the COVID-19 pandemic.14,25 A recent study reported a worsening of pain intensity and interference in 150 participants with fibromyalgia.35 Importantly, the study used a cross-sectional design and relied on memory of pain levels before the Stay-at-Home mandate35 (eg, memory biases for pain self-reports). These 2 aspects along with the different underlying pain disorders (fibromyalgia vs TMD) may explain the difference in findings with our results. Larger cohort studies are needed to draw definite answers about chronic pain–related symptoms and their fluctuations during the COVID-19 pandemic.
We observed a worsening in self-reported anxiety and depression. The combined effect of the pandemic outbreak, forced Stay-at-Home, constant mass media coverage, and high stress may have caused the occurrence of distinct symptoms (eg, abnormal illness behavior27,51,52). The significant reduction of self-reported pain in patients with TMD during the forced lockdown may reflect a shift in attention from chronic pain to the time-framed situational perception of mood disorders (eg, anxiety and depression). Participants with higher baseline distressing states were more prone to experiencing higher anxiety and depression during the Stay-at-Home period. As the literature has shown in the past months, this is also true for other somatic symptoms we controlled for (eg, PHQ-1539).
In addition, the current study found, as an explorative outcome, that higher prepandemic extraversion was linked to lower anxiety and lower frequency of nonspecific COVID-19 nocebo-like symptoms during the Stay-at-Home order. Extraversion, defined as a personality character that is talkative, assertive, active, sociable, and energetic, has been generally linked to positive affect47,57 and higher levels of resilience to stress.11,44 More importantly, the associations between extraversion and positive affect are independent of an individual's social activity.45
Finally, we observed that 70.8% of the surveyed participants experienced some symptoms resembling the publicly available list of symptoms for COVID-19 and that those with higher anxiety and depression and lower extroversion scores were likely to report nonspecific nocebo COVID-19 symptoms. However, our study did not include in-person severe acute respiratory syndrome coronavirus 2 tests and some symptoms (eg, headache) overlap with the TMD symptomatology. Therefore, the occurrence of nonspecific COVID-19 symptoms is a finding that requires caution on being interpreted as a nocebo phenomenon.
Others have suggested that unspecific COVID-19 symptoms might represent nocebo responses.2 Nocebo effects have been linked to information disclosure about potential side effects of a treatment contributing to the occurrence of adverse effects.20 A treatment and an adequate control group are required to infer the nocebo effect as the cause of adverse events.17,18 Adverse events resulting from mass media and internet-based information have been reported as nocebo phenomena in the case of active drugs such as thyroxine,28 statins,49,50 and mass psychogenic illness after some (H1N1 influence and other) vaccinations.36,38,63 A recent article published during the pandemic investigated nocebo-prone behavior using the Q-No tool, a questionnaire used to predict the nocebo response,48 in participants with autoimmune rheumatic diseases amid the COVID-19 pandemic Stay-at-Home order period.29 Nocebo behaviors were detected in 51 of the 500 individuals (10.2%). Total Q-No scores were higher in the COVID-19 period compared with the pre-COVID-19 era. Among 78 patients with available Q-No questionnaires in the pre-COVID-19 era, 11 (14.1%) displayed nocebo behavior, which increased to 16 (20.5%) amid the COVID-19 pandemic. However, participants in that study were not tested for COVID-19.
This study has both limitations and strengths to acknowledge. First, the sample size is small although these results can be informative given the challenge of current pandemic. In this study, we used a sample of a small group of patients with chronic pain compared with healthy participants who agreed to participate to the study conducted within a short window (3 weeks) of the Stay-at-Home lockdown. Second, because of the paucity of testing at the time of the study (ie, limited access to testing sites and limited availability of kits at the sites), we cannot exclude the possibility that some participants were, in fact, infected with the severe acute respiratory syndrome coronavirus 2. Moreover, the patient database consists of patients with pain disorders (eg, headache or body aching) making it difficult to separate exacerbation of mood disorders from the underlying pain diseases. Third, the COVID-19 symptomology was limited at the time we conducted the study. The CDC suggested 3 classes of most common, less common, and serious symptoms; some of them (eg, in particular fatigue) were not included because they were not publicly available when we conducted this study.
In terms of strengths, it should be noted that the current study provided an important snapshot of the initial response to the COVID-19 pandemic and the first lockdown in the United States. The first lockdown extended for many months in some countries, and many countries have had repeated lockdowns and/or are currently in lockdown situations. This study was conducted when the pandemic was brand new and individual levels of uncertainty were an acute phenomenon and can inform the behavioral responses as the initial lockdowns continued while the global death toll mounted and the responses to subsequent lockdowns.
Overall, our findings illuminate the possibility that the lockdown might have caused a series of symptoms. The results on coping strategies indicate a shift in attention from chronic pain to anxiety and depression. This shift suggests a need to encourage behavioral changes in lifestyle and promote a more comprehensive illness symptom management. The tendency to seek more support underlies the fact that more disabled people need and seek most support. Therefore, while promoting and encouraging a more effective access to eHealth in the United States and across countries,14–16,25,26,64 it is relevant to understand that psychobehavioral treatments cannot be limited to pain severity and interference. Through a broader approach, particularly pain psychology together with the multidisciplinary pain therapy, we can potentially help prevent unwanted mood disorders.
The general population, patients, and healthcare providers should be aware of the disparate effects potentially caused by the worldwide distress12,40 such as the COVID-19 pandemic. Healthcare providers are in an ideal position to help educate both patients and their colleagues about the possibility of increase in anxiety and depression. People should be encouraged to practice a lifestyle that ensures regular physical activity30,53,65 and includes wisely selected media coverage and information sources41,43 to reduce worsening anxiety and unwanted potential nocebo-like responses while still adhering to the best safety practices regarding COVID-19. Patients, particularly those who tend to seek less social interactions, may benefit from social support.8,13,66 Importantly, amid the State-at-Home order of the COVID-19 pandemic, those vulnerable, especially those patients with underlying mood disorders, may require more help than they did before the pandemic.
Disclosures
L. Colloca reported having received grants from National Institutes of Health, grants from MPowering the State, and personal fees from Cleveland Clinic and Florida Atlantic University, outside the submitted work.
This research was supported by the National Institute of Dental Craniofacial Research (R01 DE025946, L.C.), National Center for Complementary and Integrative Health (R01AT01033, L.C.), and National Institute on Alcohol Abuse and Alcoholism (R13-AA028424). The funding agencies have no roles in the study. The views expressed here are the authors' own and do not reflect the position or policy of Maryland State and National Institutes of Health or any other part of the federal government.
Acknowledgments
The authors thank the study participants for their time. The authors also thank John Melnicki for his support and Rania Deranieh for her comments on the first version of this manuscript.
Author contributions: L. Colloca: study design, data analysis, and manuscript preparation; S. Thomas: study design and comment on manuscript; M. Yin: comment on the manuscript; N.R. Haycock: data collection and comment on manuscript; Y. Wang: data analysis, figures and table preparation, and comment on manuscript.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Sharon Thomas, Email: sthomas365@icloud.com.
Margaret Yin, Email: maggieyin2837@gmail.com.
Nathaniel R. Haycock, Email: nrh41@georgetown.edu.
Yang Wang, Email: yang.wang@umaryland.edu.
References
- [1].Ahorsu DK, Lin CY, Imani V, Saffari M, Griffiths MD, Pakpour AH. The Fear of COVID-19 Scale: development and initial validation. Int J Ment Health Addict 2020. doi: 10.1007/s11469-020-00270-8 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Amanzio M, Howick J, Bartoli M, Cipriani GE, Kong J. How do nocebo phenomena provide a theoretical framework for the COVID-19 pandemic? Front Psychol 2020;11:589884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Asmundson GJG, Paluszek MM, Landry CA, Rachor GS, McKay D, Taylor S. Do pre-existing anxiety-related and mood disorders differentially impact COVID-19 stress responses and coping? J Anxiety Disord 2020;74:102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bakioglu F, Korkmaz O, Ercan H. Fear of COVID-19 and positivity: mediating role of intolerance of uncertainty, depression, anxiety, and stress. Int J Ment Health Addict 2020. doi: 10.1007/s11469-020-00331-y [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barker HR, Jr, Wadsworth AP, Jr, Wilson W. Factor structure of the State-Trait Anxiety Inventory in a nonstressful situation. J Clin Psychol 1976;32:595–8. [DOI] [PubMed] [Google Scholar]
- [6].Barsky AJ, Saintfort R, Rogers MP, Borus JF. Nonspecific medication side effects and the nocebo phenomenon. JAMA 2002;287:622–7. [DOI] [PubMed] [Google Scholar]
- [7].Barzilay R, Moore TM, Greenberg DM, DiDomenico GE, Brown LA, White LK, Gur RC, Gur RE. Resilience, COVID-19-related stress, anxiety and depression during the pandemic in a large population enriched for healthcare providers. Transl Psychiatry 2020;10:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bavel JJV, Baicker K, Boggio PS, Capraro V, Cichocka A, Cikara M, Crockett MJ, Crum AJ, Douglas KM, Druckman JN, Drury J, Dube O, Ellemers N, Finkel EJ, Fowler JH, Gelfand M, Han S, Haslam SA, Jetten J, Kitayama S, Mobbs D, Napper LE, Packer DJ, Pennycook G, Peters E, Petty RE, Rand DG, Reicher SD, Schnall S, Shariff A, Skitka LJ, Smith SS, Sunstein CR, Tabri N, Tucker JA, Linden SV, Lange PV, Weeden KA, Wohl MJA, Zaki J, Zion SR, Willer R. Using social and behavioural science to support COVID-19 pandemic response. Nat Hum Behav 2020;4:460–71. [DOI] [PubMed] [Google Scholar]
- [9].Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 1988;8:77–100. [Google Scholar]
- [10].Boyraz G, Legros DN, Tigershtrom A. COVID-19 and traumatic stress: the role of perceived vulnerability, COVID-19-related worries, and social isolation. J Anxiety Disord 2020;76:102307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Campbell-Sills L, Cohan SL, Stein MB. Relationship of resilience to personality, coping, and psychiatric symptoms in young adults. Behav Res Ther 2006;44:585–99. [DOI] [PubMed] [Google Scholar]
- [12].Chaix B, Delamon G, Guillemassé A, Brouard B, Bibault J-E. Psychological distress during the COVID-19 pandemic in France: a national assessment of at-risk populations. Gen Psychiatry 2020;33:e100349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cheng P, Xia G, Pang P, Wu B, Jiang W, Li YT, Wang M, Ling Q, Chang X, Wang J, Dai X, Lin X, Bi X. COVID-19 epidemic peer support and crisis intervention via social media. Community Ment Health J 2020;56:786–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Clauw DJ, Hauser W, Cohen SP, Fitzcharles MA. Considering the potential for an increase in chronic pain after the COVID-19 pandemic. PAIN 2020;161:1694–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cohen SP, Baber ZB, Buvanendran A, McLean BC, Chen Y, Hooten WM, Laker SR, Wasan AD, Kennedy DJ, Sandbrink F, King SA, Fowler IM, Stojanovic MP, Hayek SM, Phillips CR. Pain management best practices from multispecialty organizations during the COVID-19 pandemic and public health crises. Pain Med 2020;21:1331–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cohen SP, Hooten WM, Phillips CR. Pain management during COVID-19 and steroids: striking a balance. Pain Med 2020;21:1731–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Colloca L. Nocebo effects can make you feel pain. Science 2017;358:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Colloca L, Barsky AJ. Placebo and nocebo effects. N Engl J Med 2020;382:554–61. [DOI] [PubMed] [Google Scholar]
- [19].Colloca L, Benedetti F. Nocebo hyperalgesia: how anxiety is turned into pain. Curr Opin Anaesthesiol 2007;20:435–9. [DOI] [PubMed] [Google Scholar]
- [20].Colloca L, Miller FG. The nocebo effect and its relevance for clinical practice. Psychosom Med 2011;73:598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Symptoms of COVID-19. Coronavirus disease 2019 (COVID-19), Vol. 2020, 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. [Google Scholar]
- [22].Costa PT, Jr, McCrae RR. The revised NEO Personality Inventory (NEO-PI-R). Thousand Oaks: Sage Publications, Inc, 2008. [Google Scholar]
- [23].Costa PT, Jr, McCrae RR. Domains and facets: hierarchical personality assessment using the revised NEO personality inventory. J Pers Assess 1995;64:21–50. [DOI] [PubMed] [Google Scholar]
- [24].Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, Aaron JG, Claassen J, Rabbani LE, Hastie J, Hochman BR, Salazar-Schicchi J, Yip NH, Brodie D, O'Donnell MR. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020;395:1763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Eccleston C, Blyth FM, Dear BF, Fisher EA, Keefe FJ, Lynch ME, Palermo TM, Reid MC, Williams ACC. Managing patients with chronic pain during the COVID-19 outbreak: considerations for the rapid introduction of remotely supported (eHealth) pain management services. PAIN 2020;161:889–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Emerick T, Alter B, Jarquin S, Brancolini S, Bernstein C, Luong K, Morrisseyand S, Wasan A. Telemedicine for chronic pain in the COVID-19 era and beyond. Pain Med 2020;21:1743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Escamilla EI, Ortiz LAE, Pargas JEA, Martinez AM, Botello BAL, Villa VDB, Villarreal JJ. Attitudes associated with hypochondria and abnormal behavior towards illness in health science students. Psychiatr Q 2020;91:921–8. [DOI] [PubMed] [Google Scholar]
- [28].Faasse K, Cundy T, Petrie KJ. Medicine and the Media. Thyroxine: anatomy of a health scare. BMJ 2009;339:b5613. [DOI] [PubMed] [Google Scholar]
- [29].Fragoulis GE, Evangelatos G, Arida A, Bournia VK, Fragiadaki K, Karamanakos A, Kravvariti E, Laskari K, Panopoulos S, Pappa M, Mitsikostas DD, Tektonidou MG, Sfikakis PP. Nocebo-prone behaviour in patients with autoimmune rheumatic diseases during the COVID-19 pandemic. Mediterr J Rheumatol 2020;31(suppl 2):288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gao Z, Lee JE, McDonough DJ, Albers C. Virtual reality exercise as a coping strategy for health and wellness promotion in older adults during the COVID-19 pandemic. J Clin Med 2020;9:1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Goldfarb EV. Participant stress in the COVID-19 era and beyond. Nat Rev Neurosci 2020;21:663–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gorman K, Engel-Rebitzer E, Ledoux A, Bovin M, Marx B. Peritraumatic experience and traumatic stress. Comprehensive guide to post-traumatic stress disorders. Cham: Springer International Publishing, 2016. p. 907–24. [Google Scholar]
- [33].Grillon C, Duncko R, Covington MF, Kopperman L, Kling MA. Acute stress potentiates anxiety in humans. Biol Psychiatry 2007;62:1183–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hruschak V, Flowers KM, Azizoddin DR, Jamison RN, Edwards RR, Schreiber KL. Cross-sectional study of psychosocial and pain-related variables among patients with chronic pain during a time of social distancing imposed by the coronavirus disease 2019 pandemic. PAIN 2021;162:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Huang WT, Hsu CC, Lee PI, Chuang JH. Mass psychogenic illness in nationwide in-school vaccination for pandemic influenza A(H1N1) 2009, Taiwan, November 2009-January 2010. Euro Surveill 2010;15:19575. [DOI] [PubMed] [Google Scholar]
- [37].Jensen MP, Turner JA, Romano JM, Strom SE. The Chronic Pain Coping Inventory: development and preliminary validation. PAIN 1995;60:203–16. [DOI] [PubMed] [Google Scholar]
- [38].Kharabsheh S, Al-Otoum H, Clements J, Abbas A, Khuri-Bulos N, Belbesi A, Gaafar T, Dellepiane N. Mass psychogenic illness following tetanus-diphtheria toxoid vaccination in Jordan. Bull World Health Organ 2001;79:764–70. [PMC free article] [PubMed] [Google Scholar]
- [39].Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002;64:258–66. [DOI] [PubMed] [Google Scholar]
- [40].Levaot Y, Greene T, Palgi Y. The associations between media use, peritraumatic distress, anxiety and resilience during the COVID-19 pandemic. J Psychiatr Res 2020. doi: 10.1016/j.jpsychires.2020.11.018 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- [41].Limaye RJ, Sauer M, Ali J, Bernstein J, Wahl B, Barnhill A, Labrique A. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health 2020;2:e277–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of covid-19—studies needed. N Engl J Med 2020;382:1194–6. [DOI] [PubMed] [Google Scholar]
- [43].Llewellyn S. Covid-19: how to be careful with trust and expertise on social media. BMJ 2020;368:m1160. [DOI] [PubMed] [Google Scholar]
- [44].Lü W, Wang Z, Liu Y, Zhang H. Resilience as a mediator between extraversion, neuroticism and happiness, PA and NA. Personal Individual Differences 2014;63:128–33. [Google Scholar]
- [45].Lucas RE, Le K, Dyrenforth PS. Explaining the extraversion/positive affect relation: sociability cannot account for extraverts' greater happiness. J Pers 2008;76:385–414. [DOI] [PubMed] [Google Scholar]
- [46].Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping chronic pain conditions: implications for diagnosis and classification. J Pain 2016;17(9 suppl):T93–T107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].McNiel JM, Fleeson W. The causal effects of extraversion on positive affect and neuroticism on negative affect: manipulating state extraversion and state neuroticism in an experimental approach. J Res Personal 2006;40:529–50. [Google Scholar]
- [48].Mitsikostas DD, Deligianni CI. Q-No: a questionnaire to predict nocebo in outpatients seeking neurological consultation. Neurol Sci 2015;36:379–81. [DOI] [PubMed] [Google Scholar]
- [49].Moon J, Cohen Sedgh R, Jackevicius C. Examining the nocebo effect of statins through statin adverse events reported in the FDA Adverse Event Reporting System (FAERS). Circ Cardiovasc Qual Outcomes 2020;14:e007480. [DOI] [PubMed] [Google Scholar]
- [50].Pedro-Botet J, Climent E, Benaiges D. Muscle and statins: from toxicity to the nocebo effect. Expert Opin Drug Saf 2019;18:573–9. [DOI] [PubMed] [Google Scholar]
- [51].Pilowsky I. The concept of abnormal illness behavior. Psychosomatics 1990;31:207–13. [DOI] [PubMed] [Google Scholar]
- [52].Prior KN, Bond MJ. Patterns of 'abnormal' illness behavior among healthy individuals. Am J Health Behav 2017;41:139–46. [DOI] [PubMed] [Google Scholar]
- [53].Ranasinghe C, Ozemek C, Arena R. Exercise and well-being during COVID 19—time to boost your immunity. Expert Rev Anti Infect Ther 2020;18:1195–200. [DOI] [PubMed] [Google Scholar]
- [54].Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, List T, Svensson P, Gonzalez Y, Lobbezoo F, Michelotti A, Brooks SL, Ceusters W, Drangsholt M, Ettlin D, Gaul C, Goldberg LJ, Haythornthwaite JA, Hollender L, Jensen R, John MT, De Laat A, de Leeuw R, Maixner W, van der Meulen M, Murray GM, Nixdorf DR, Palla S, Petersson A, Pionchon P, Smith B, Visscher CM, Zakrzewska J, Dworkin SF; International RDC/TMD Consortium Network, International association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J Oral Facial Pain Headache 2014;28:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Selenica P, Alemar B, Matrai C, Talia KL, Veras E, Hussein Y, Oliva E, Beets-Tan RGH, Mikami Y, McCluggage WG, Kiyokawa T, Weigelt B, Park KJ, Murali R. Massively parallel sequencing analysis of 68 gastric-type cervical adenocarcinomas reveals mutations in cell cycle-related genes and potentially targetable mutations. Mod Pathol 2020;34:1213–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sirri L, Grandi S. Illness behavior. Adv Psychosom Med 2012;32:160–81. [DOI] [PubMed] [Google Scholar]
- [57].Smillie LD, DeYoung CG, Hall PJ. Clarifying the relation between extraversion and positive affect. J Personal 2015;83:564–74. [DOI] [PubMed] [Google Scholar]
- [58].Tran TD, Tran T, Fisher J. Validation of the Depression Anxiety Stress Scales (DASS) 21 as a screening instrument for depression and anxiety in a rural community-based cohort of northern Vietnamese women. BMC Psychiatry 2013;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Vance MC, Kovachy B, Dong M, Bui E. Peritraumatic distress: a review and synthesis of 15 years of research. J Clin Psychol 2018;74:1457–84. [DOI] [PubMed] [Google Scholar]
- [60].Von Korff M, Dworkin SF, Le Resche L. Graded chronic pain status: an epidemiologic evaluation. PAIN 1990;40:279–91. [DOI] [PubMed] [Google Scholar]
- [61].Wadsworth AP, Jr, Barker HR, Barker BM. Factor structure of the State-Trait Anxiety Inventory under conditions of variable stress. J Clin Psychol 1976;32:576–9. [DOI] [PubMed] [Google Scholar]
- [62].Wang Y, Duan Z, Peng K, Li D, Ou J, Wilson A, Wang N, Si L, Chen R. Acute stress disorder among frontline health professionals during the COVID-19 outbreak: a structural equation modelling investigation. Psychosom Med 2020;83:373–9. [DOI] [PubMed] [Google Scholar]
- [63].Yang TU, Kim HJ, Lee YK, Park YJ. Psychogenic illness following vaccination: exploratory study of mass vaccination against pandemic influenza A (H1N1) in 2009 in South Korea. Clin Exp Vaccine Res 2017;6:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Yasri S, Wiwanitkit V. Pain management during the COVID-19 pandemic. Pain Med 2020;21:2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yeo TJ. Sport and exercise during and beyond the COVID-19 pandemic. Eur J Prev Cardiol 2020;27:1239–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zysberg L, Zisberg A. Days of worry: emotional intelligence and social support mediate worry in the COVID-19 pandemic. J Health Psychol 2020. doi: 10.1177/1359105320949935[Epub ahead of print]. [DOI] [PubMed]


