Supplemental Digital Content is Available in the Text.
A pilot study assessing whether psychophysiologic symptom relief therapy can reduce nonspecific back pain compared with usual care and mindfulness-based stress reduction.
Keywords: Chronic pain, Psychosomatic medicine, Mind–body therapies, Back pain, Randomized control trial
Abstract
Introduction:
Chronic back pain is the leading cause of disability in the United States. Based on the hypothesis that nonspecific back pain may be rooted in a psychophysiologic etiology, we propose a new approach to chronic back pain.
Objectives:
A pilot study was conducted to assess whether psychophysiologic symptom relief therapy (PSRT) can reduce disability and back pain bothersomeness for patients with chronic back pain.
Methods:
This was a three-armed, randomized trial for adults with nonspecific chronic back pain that compared PSRT with usual care and an active comparator (mindfulness-based stress reduction [MBSR]). Psychophysiologic symptom relief therapy–randomized participants received a 12-week (36 hours) course based on the psychophysiological model of pain. All groups were administered validated questionnaires at baseline and at 4, 8, 13, and 26 weeks. The primary outcome was the reduction in pain disability measured by the Roland–Morris Disability Questionnaire.
Results:
The mean Roland–Morris Disability Questionnaire score for the PSRT group (n = 11) decreased from 9.5 (±4.3 SDs) to 3.3 (±5.1) after 26 weeks which was statistically significant compared with both MBSR (n = 12) (P = 0.04) and usual care (n = 12) (P = 0.03). Pain bothersomeness scores and pain-related anxiety decreased significantly over 26 weeks in PSRT compared with MBSR and usual care (data in manuscript). At 26 weeks, 63.6% of the PSRT arm reported being pain free (0/10 pain) compared with 25.0% and 16.7% in MBSR and usual care arms, respectively. Psychophysiologic symptom relief therapy attendance was 76%, and there was 100% follow-up of all groups.
Conclusion:
Psychophysiologic symptom relief therapy is a feasible and potentially highly beneficial treatment for patients with nonspecific back pain.
1. Introduction
Chronic back pain is the leading cause of disability worldwide. The global years lived with disability due to lower back pain have increased by 52.7% between 1990 (42.5 million) and 2017 (64.9 million).29 In addition to the devastating effects on life quality, there are major economic consequences of chronic pain.19 Annual healthcare costs attributable to pain range between $560 and $635 billion in the United States.11
The current paradigm of pain management focuses on treatment of a physical origin of pain, sometimes with adjunctive psychological support. However, as illuminated by the biopsychosocial model of pain, many chronic pain syndromes are not clearly linked to abnormal findings. In many cases of chronic back pain, a specific peripheral etiology for the pain cannot be identified, suggesting that central factors (including psychosocial processes) may play a predominant contributory role.6 Even when a potential source is identified (eg, disk bulge), the direct causation of pain remains unclear. To this end, repeated studies have found that patients with magnetic resonance imaging of abnormalities often report no pain, thus raising the question of whether many of these findings are associative (as opposed to causative) among patients with symptoms.1,10 In addition, commonly used therapies targeting the physical etiologies of pain, such as surgery and steroid injections, have not been clearly efficacious in randomized trials.5,27 Conversely, studies have found that risk factors for chronic back pain include psychological stress, depression, and psychosomatic factors, which presumably exert their effects primarily in the central nervous system.7,8,13 Taken together, there are limited data to support that many forms of chronic back pain have a strictly peripheral, physical origin (eg, joint inflammation).
Although controversial and yet unproven, previous reports propose that some forms of back pain may exhibit predominant psychological contributions. One of the first studies suggesting this association was published in 1946, where the author described a large cohort of young healthy males returning from the battlefield with back pain which was ultimately classified as psychosomatic.22 The author noted that explaining the concept to the soldiers resulted in pain relief for a number of them. More recently, a similar hypothesis was put forth by Sarno in a series of non–peer-reviewed books. His hypothesis was that chronic stress and other psychological factors (such as the repression of negative affective states) could result in a chronic pain syndrome and specifically back pain.23 The exact mechanism remains unclear, but an analogy could be made to other known effects of acute emotional states on acute physiological changes. For example, embarrassment (emotion) may result in vasodilation of the capillaries (physical response) or sudden traumatic news (strong negative emotion) may result in cardiogenic shock (ie, broken heart syndrome, physical response).16 Sarno's therapeutic approach mainly consisted of recognizing that pain was being amplified by underlying psychological stressors and that pain could be reduced by addressing emotional repression as well as increasing levels of physical activity (which had frequently become quite restricted in patients with chronic lower back pain). Based on these principles, Schechter et al.24 performed a case series in which 51 subjects received a mind–body treatment program that included office visits and educational materials. After completing the intervention, patients reported a 52% decrease in average pain and roughly 25% increase in physical day-to-day functioning as measured by the Short Form Survey (SF-12). Later, Schubiner's group modified Sarno's approach and developed an “affective self-awareness” program that led to significant improvement in pain severity and interference in patients with fibromyalgia.12 A related approach by Lumley et al. focusing on emotional expression (emotion awareness and expression therapy [EAET]) resulted in better outcomes for overall symptoms, widespread pain, physical functioning and negative affect compared with an education control group in a randomized trial with patients with fibromyalgia. In the same study, most pain outcomes with EAET did not differ from those in a group receiving cognitive behavioral therapy (CBT), but participants in the EAET group did report significantly lower overall symptoms and widespread pain compared with the CBT group.14 Another recent preliminary study showed that EAET may be more beneficial as compared to CBT.30 Additional randomized trials testing the efficacy of EAET for primary pain conditions (including fibromyalgia, irritable bowel syndrome, chronic pelvic pain, and nonspecific musculoskeletal pain) have been conducted. The results of these studies indicate that EAET is effective in reducing pain and other somatic symptoms and improving physical functioning.2,3,17,28,32
Based on these findings, our group coalesced the key components of these approaches into a novel, 12-week program of which the first 4 weeks were previously evaluated in a small feasibility study (#NCT02117921) and the later 8 weeks consisted of a mindfulness-based stress reduction (MBSR) program. Our program is based on a psychophysiological approach to understanding and treating pain as described above and is termed psychophysiologic symptom relief therapy (PSRT). Psychophysiologic symptom relief therapy is based on the hypothesis that nonspecific back pain is the symptomatic manifestation of a psychophysiological process that is substantively driven by stress, negative emotions, and other psychological processes.15,23 This intervention addresses underlying stressors and psychological contributors to persistent pain (including underlying stressful conflicts and aversive affective states), as well as conditioned pain responses and fear-avoidant behaviors. Treatment strategies in the first 4 weeks include psychophysiologic pain education, desensitization, and emotional expression. Given this focus during the first 4 weeks, our data collection and analysis plan allowed for assessment both at this juncture and beyond. The last 8 weeks of this program focused on mindfulness meditation (MBSR) whose goal is to provide the tools to better process current and future stressors while allowing for time to practice techniques learned earlier in the program.
To evaluate PSRT, we conducted a single-center pilot randomized control trial to assess whether PSRT can reduce or eliminate the functional limitations, pain bothersomeness, and pain-related anxiety in patients with nonspecific chronic back pain when compared with MBSR (active comparator) and usual care (control group). Mindfulness-based stress reduction is an active comparator and a component of the PSRT program that has been shown to be more effective than usual care for low back pain in a large, randomized trial.4
2. Methods
2.1. Study design and setting
This was a prospective, three-armed, randomized controlled trial of a mind–body intervention for adults with nonspecific back pain compared with MBSR program and usual care. We originally planned and began a larger, phase III trial. However, we converted the original study to a pilot study to fully optimize the design and delivery of the intervention, the participant population, and the features of the trial.
The study was conducted at a tertiary medical center, Beth Israel Deaconess Medical Center in Boston, Massachusetts, from January 2019 to April 2020. The study was approved by the local institutional review board, and all subjects provided written informed consent. The study was registered on clinicaltrials.gov (NCT 04039139). Participants were recruited through physician referrals, flyers posted in the Boston metropolitan area, and social media.
2.2. Inclusion criteria
We included adults 18 to 67 years old with chronic back pain that lacked a clear organic etiology (eg, malignancy or infection). Chronic back pain was defined as occurring at least 3 days a week for the 3 months before enrollment. Participants had to be willing to consider a mind–body intervention (assessed during a screening interview with an investigator).
2.3. Exclusion criteria
Participants were excluded from the study if they (1) were >67 years (excluded because of an increased risk of underlying organic etiology of pain); (2) had diagnosed organic disease as cause of pain, such as malignancy, neurologic disorder (eg, amyotrophic lateral sclerosis and cauda equina syndrome); (3) had vertebral disk disease with neurological impairment; (4) had a diagnosis of significant psychiatric comorbidities such as schizophrenia, dementia, and bipolar disorder; and/or (5) were not willing to participate for the full duration of the study. Participants with depression were not excluded. Pain in patients with disk disease was not an exclusion unless there were neurological impairments. During the first 3 months of enrollment, some participants were included who had moderate to severe spinal stenosis but this was subsequently determined to fall into the category of potential organic disease and subsequent participants with these findings were not included.
2.4. Randomization and blinding
On enrollment into the trial, participants were randomized in a 1:1:1 ratio to the usual care arm, the active comparator arm, or the intervention arm in blocks with random sizes of 3 or 6. An independent statistician created the randomization list using a random number generator. Sealed envelopes were used to conceal the treatment allocation for each participant. Envelopes were opened after enrollment of a subject and in the presence of 2 members of the investigative team.
The trial was partially blinded; patients in the active comparator group (ie, MBSR) were not aware that they were in a comparator arm. Pain-related questionnaires (data) were collected through an online data collection tool without interaction from study staff. All questionnaires were completed by the participants at the time of randomization which occurred no longer than 1 week before the beginning of the interventions (or usual care). The trial statistician was not involved with any data collection. The intervention was conducted in groups of 1 to 3 participants at a time.
2.5. Study arms
2.5.1. Mindfulness-based stress reduction (active comparator)
Participants randomized to the active comparator arm underwent an 8-week group-based mindfulness-based stress reduction (MBSR) program taught by a trained investigator. Sessions were 2 hours in length, once per week for a total of 8 weeks with one full day session/retreat lasting 6 hours (total 22 hours, 9 sessions). Our program was modeled after the Mindfulness-Based Stress Reduction Authorized Curriculum Guide 2017 published by the University of Massachusetts Medical School, which established MBSR in 1979 (Santorelli, 2017). The MBSR classes were delivered by instructors with formal training in mindfulness-based interventions (2 female and 1 male) with experience spanning 3, 20, and 24 years.
2.5.2. Usual care (control)
Participants randomized to the usual care arm continued their prescribed treatment regimens under guidance of their physicians and without influence from the study team.
2.5.3. Psychophysiologic symptom relief therapy (intervention)
Participants randomized to the PSRT arm had a brief one-on-one overview of the course explained to them by an investigator and then participated in a 12-week (38 hours) course. During the first 4 weeks, participants participated in a PSRT program based on Sarno's psychophysiologic model of chronic back pain23 and included use of the books Healing Back Pain,23 Unlearn Your Pain,25 and Worry Less, Live More: The Mindful Way Through Anxiety Workbook.20 These sessions were held twice a week, 2 hours per session, for 4 weeks (total 16 hours, 8 sessions). The intervention was delivered through a combination of physician (author M.W.D.) and mind–body expert with 20 years of experience in patient support or counselling, supported by consultation or training from experts in the field of chronic pain. An in-depth description of this portion of the intervention is described in detail in the Appendix (available at http://links.lww.com/PR9/A129) and briefly as follows:
The overall program was constructed around 4 components: psychophysiologic pain education, returning to physical activity/desensitization, emotional expression, and stress reduction. The psychophysiologic pain education allowed participants to explore their pain history and learn about the clinical presentation of psychophysiological syndromes with the goal of achieving acceptance of the psychological origins of their pain (full details provided in the Appendix, available at http://links.lww.com/PR9/A129). After completing the educational component, desensitization techniques were used with the goal of reversing the conditioned response to a physical stimulus that was not the primary cause of the pain. Desensitization techniques included imagining doing a physical activity with visual motor imagery (eg, bending to pick up heavy books) that would typically elicit pain. The experience that visualization alone can elicit pain allows for the two-fold therapeutic approach of reinforcing that their pain was psychophysiologic but also serves as a means of then desensitizing from this pain by repeated visualization with the knowledge that they were safe. After pain could no longer be elicited by visualization, participants were encouraged to gradually resume physical activity similar to levels undertaken before pain onset (see Appendix, available at http://links.lww.com/PR9/A129 for full details). Finally, participants established techniques for appropriate emotional expression while discouraging emotional repression.
After the first 4 weeks, the course introduced the concept of mindfulness with a cognitive component incorporated into the classic MBSR program, with 2-hour sessions once a week for the remaining 8 weeks including one full day session of 6 hours and an hour long orientation (22 hours, 9 sessions). The component of stress reduction was achieved through the MBSR course, now performed in the context of subjects understanding the origins of their pain and having already achieved improvements in functional activity and reductions in pain.
2.6. Outcomes
2.6.1. Demographics and medical history
Demographics were patient-reported using standardized assessment forms. The medical history was assessed during an interview conducted by the study physician or principal investigator.
2.6.2. Primary outcome
The primary clinical outcome of this study was reduction in functional limitations or disability that was assessed using the Roland–Morris Low Back Pain and Disability Questionnaire (RDQ). The RDQ score values range from 0 to 24—with higher numeric scores indicating higher disability. The RDQ has been validated in patients with back pain and is widely used.21 Change from baseline RDQ was assessed at 4, 8, 13, and 26 weeks (primary outcome).
2.6.3. Secondary outcomes
Our secondary outcomes included back pain bothersomeness and pain-related anxiety.
2.6.3.1. Back pain bothersomeness
Back pain bothersomeness in the past week was measured by a 0 to 10 scale, with higher numeric scores indicating higher back pain bothersomeness. Baseline scores were compared with questionnaire results at 4, 8, 13, and 26 weeks.
2.6.3.2 Pain-related anxiety
Changes in pain-related anxiety were assessed using the responses to the Pain Anxiety Symptom Scale (PASS) short form.18 The PASS is a 20-item scale, and the responses to each question can take on numeric values from 0 to 5, with higher values indicating higher pain anxiety. Scores were summed across all questions, resulting in a PASS score range between 0 and 100, with higher numeric values indicating higher pain-related anxiety. Baseline PASS scores were compared with results at 4, 8, 13, and 26 weeks after the initial interview.
2.6.4. Additional outcomes
In addition to the main 3 outcomes as indicated above (disability, back pain bothersomeness, and pain-related anxiety), we evaluated the following outcomes:
2.6.4.1. Pain relief
Pain relief was defined as the percentage of participants who indicated that they were pain free by having a score of 0 of 10 in reference to back pain bothersomeness.
2.6.4.2. Functional recovery
Functional recovery was defined as the percentage of the participants who had a score of 0 on the RDQ.
2.6.4.3. Feasibility
Study feasibility was measured by evaluating the attendance at program classes in the intervention (PSRT) and MBSR arms, the completion of questionnaires in all arms, and adherence to the program in PSRT and MBSR arms. Adherence to the program was assessed by examining the number of hours spent in the past week spent working on skills learned in the program. This response variable was categorical with 6 levels (0 hours, <1 hour, 1–2 hours, 2–4 hours, 4–6 hours, and >6 hours).
2.7. Statistical analysis
Based on baseline RDQ mean and standard deviation data from Cherkin et al. and using an estimate of treatment effects from a previously unpublished feasibility study, we estimated that with an RDQ of approximately 11 and a standard deviation of 5, we would need a minimum of 11 subjects per group to detect a 50% reduction in RDQ (absolute reduction of 5.5 with a standard deviation of 3.5) with a power of 80%. The primary outcome in this trial was the change in the RDQ score from baseline to subsequent weeks (4, 8, 13, and 26), and the key secondary outcomes were the change in back pain bothersomeness (scale 0–10) and pain-related anxiety. The primary and secondary outcomes were analyzed using paired t-tests for within-group comparisons and a linear mixed-effects model for between-group comparisons with an autoregressive variance–covariance matrix with a lag 1 matrix to account for the correlation of within-patient repeated measures. Covariates in the model included treatment group, time (as a categorical variable with 5 levels, defined as baseline, 4 weeks, 8 weeks, 13 weeks, and 26 weeks), and the interaction between treatment group and time. Linear contrasts were used to estimate the mean difference between treatment arms for each time point. For a given pair of treatment groups at each follow-up time point, the Cohen d effect size was calculated using the difference between the means as the numerator and the within-groups pooled standard deviation as the denominator. All patients were included in the longitudinal model. The proportion of patients with functional recovery and pain relief was compared between PSRT and MBSR and PSRT and usual care groups using Fisher exact tests at each time point. For descriptive statistics, continuous data are presented as mean ± standard deviation or as counts (and percentages), based on the distribution of the data. In addition, median scores for the primary and key secondary outcomes for each randomization group at each individual time point are presented in the supplement (available at http://links.lww.com/PR9/A129).
3. Results
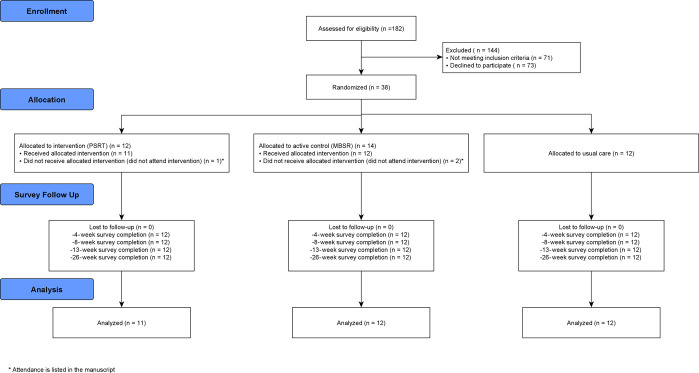
Figure 1 depicts participant flow through the study. Among 182 individuals assessed for eligibility, 38 were enrolled and randomized. Of the 38 enrolled patients, 3 patients did not attend a single session and were therefore not included in the modified intent-to-treat analysis. Among the 35 patients available for analysis, 11 (31.4%) were in the PSRT arm, 12 (34.2%) in the MBSR arm, and 12 (34.2%) in the usual care arm (Fig. 1). The survey completion across all time points was 100%. For the PSRT arm, the median number of sessions attended was 13 of 17 (76%). For the MBSR arm, the median number of sessions attended was 7 of 9 (78%). Baseline sociodemographic characteristics were generally well-matched between the groups (Table 1).
Figure 1.
Consort diagram.
Table 1.
Baseline cohort characteristics.*
| Characteristic | PSRT (N = 11) | MBSR (N = 12) | Usual care (N = 12) |
|---|---|---|---|
| Age, y (mean, SD) | 38.4 (12.8) | 39.3 (14.4) | 43.1 (13.0) |
| n (%) male | 5 (45.5%) | 6 (50.0%) | 3 (25.0%) |
| Race (n, %) | |||
| Asian | 2 (18.2%) | 0 (0.0%) | 0 (0.0%) |
| White | 5 (45.5%) | 6 (50.0%) | 10 (83.3%) |
| Others | 3 (27.3%) | 3 (25.0%) | 1 (8.3%) |
| African American | 1 (9.1%) | 3 (25.0%) | 1 (8.3%) |
| Ethnicity (n, %) | |||
| n (%) Hispanic† | 1 (12.5%) | 1 (10%) | 3 (27.3%) |
| Education‡ (n, %) | |||
| College graduate | 3 (27.3%) | 6 (50.0%) | 4 (36.4%) |
| Master's degree or higher | 4 (36.4%) | 2 (16.7%) | 4 (36.4%) |
| Some college/vocational school | 4 (36.4%) | 4 (33.3%) | 3 (27.3%) |
| Medical history (n, %) | |||
| Heart disease | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Cancer | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Diabetes | 0 (0.0%) | 1 (8.3%) | 2 (16.7%) |
| Hypertension | 1 (9.1%) | 1 (8.3%) | 1 (8.3%) |
| Liver disease | 0 (0.0%) | 0 (0.0%) | 1 (8.3%) |
| Kidney disease | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Anxiety | 6 (54.5%) | 6 (50.0%) | 2 (16.7%) |
| Depression | 3 (27.3%) | 5 (41.7%) | 2 (16.7%) |
| Previous diagnosis related to pain (n, %) | |||
| Radiculopathy | 4 (36.4%) | 4 (33.3%) | 4 (33.3%) |
| Musculoskeletal disease | 2 (18.2%) | 3 (25.0%) | 2 (16.7%) |
| Piriformis syndrome | 0 (0.0%) | 1 (8.3%) | 0 (0.0%) |
| Osteoarthritis | 3 (27.3%) | 1 (8.3%) | 0 (0.0%) |
| Previous pain interventions (n, %) | |||
| Spinal injections | 3 (27.3%) | 8 (66.7%) | 5 (41.7%) |
| Surgical intervention | 1 (9.1%) | 2 (16.7%) | 3 (25.0%) |
| Physical therapy | 8 (72.7%) | 9 (75.0%) | 10 (83.3%) |
| Chiropractor | 4 (36.4%) | 6 (50.0%) | 5 (41.7%) |
| Other therapies | 4 (36.4%) | 7 (58.3%) | 5 (41.7%) |
Missing values excluded from calculations of the counts and percentages.
Six patients (3 in PSRT, 2 in MBSR, and 1 in usual care arm) missing ethnicity information.
One patient in usual care group missing education information.
MBSR, mindfulness-based stress reduction; PSRT, psychophysiologic symptom relief therapy.
3.1. Primary outcome
3.1.1. Roland–Morris Disability Questionnaire
The differences in RDQ scores between baseline and subsequent time points within a group are noted in Table 2.
Table 2.
Within-group mean differences (95% CI) of the summed score from the Roland–Morris Disability Questionnaire (RDQ), the summed score from the Pain Anxiety Symptom Scale Questionnaire (PASS), and the back pain bothersomeness score (pain bothersomeness) at each subsequent time point compared with baseline.*
| Variable | Group | 4 wk | 8 wk | 13 wk | 26 wk |
|---|---|---|---|---|---|
| RDQ | PSRT | −7.7 (95% CI: −10.4, −5.0), P < 0.01 | −8.5 (95% CI: −11.8, −5.3), P < 0.01 | −7.5 (95% CI: −9.9, −5.2), P < 0.01 | −6.2 (95% CI: −9.4, −3.0), P < 0.01 |
| MBSR | −2.7 (95% CI: −5.0, −0.3), P = 0.03 | −2.5 (95% CI: −7.6, −1.4), P < 0.01 | −5.1 (95% CI: −8.5, −1.6), P < 0.01 | −4.4 (95% CI: −7.4, −1.5), P < 0.01 | |
| Usual care | −1.2 (95% CI: −2.9, 0.6), P = 0.18 | 1.1† (95% CI: −2.3, 4.5), P = 0.50 | −0.2 (95% CI: −1.7, 1.3), P = 0.81 | −0.2 (95% CI: −2.8, 2.5), P = 0.89 | |
| Pain bothersomeness | PSRT | −3.5 (95% CI: −5.0, −1.9), P < 0.01 | −3.8 (95% CI: −5.6, −2.1), P < 0.01 | −4.5 (95% CI: −6.2, −2.7), P < 0.01 | −4.2 (95% CI: −6.9, −1.4), P < 0.01 |
| MBSR | −1.9 (95% CI: −4.4, 0.6), P = 0.12 | −2.4 (95% CI: −4.7, −0.1), P = 0.04 | −1.3 (95% CI: −3.9, 1.2), P = 0.27 | −2.4 (95% CI: −4.7, −0.1), P = 0.04 | |
| Usual care | −1.3 (95% CI: −2.4, −0.2), P = 0.02 | −0.6 (95% CI: −2.1, 0.9), P = 0.40 | −1.0 (95% CI: −2.6, 0.6), P = 0.20 | −1.8 (95% CI: −3.5, 0.0), P = 0.05 | |
| PASS | PSRT | −25.9 (95% CI: −41.8, −10.0), P < 0.01 | −35.0 (95% CI: −49.1, −20.9), P < 0.01 | −34.2 (95% CI: −48.6, −19.8), P < 0.01 | −33.6 (95% CI: −48.3, −19.0), P < 0.01 |
| MBSR | −5.5 (95% CI: −15.8, 4.8), P = 0.26 | −13.5 (95% CI: −23.1, −3.8), P = 0.01 | −17.3 (95% CI: −31.3, −3.4), P = 0.02 | −16.8 (95% CI: −26.1, −7.4), P < 0.01 | |
| Usual care | −7.5 (95% CI: −16.0, 1.0), P = 0.08 | −4.8 (95% CI: −15.1, 5.4), P = 0.32 | −6.0 (95% CI: −14.9, 2.9), P = 0.16 | −0.3 (95% CI: −8.5, 8.0), P = 0.95 |
Larger and more negative values indicate larger decrease compared with baseline (greater improvement).
Positive values indicate increase from baseline.
MBSR, mindfulness-based stress reduction; PSRT, psychophysiologic symptom relief therapy.
Between baseline and 4 weeks, there was a statistically significant decrease in RDQ scores in the PSRT and MBSR groups (PSRT mean reduction: 7.7, 95% CI: 5.0–10.4, P < 0.01, MBSR mean reduction: 2.7, 95% CI: 0.3–5.0, P = 0.03). There was no statistically significant change between baseline and 4 weeks in the usual care group (mean reduction 1.2, 95% CI: −0.6 to 2.9, P = 0.18) (Table 2).
Between baseline and 26 weeks, there was a statistically significant reduction in RDQ scores for the PSRT and MBSR groups, (PSRT mean reduction: 6.2, 95% CI: 3.0–9.4, P < 0.01) (MBSR mean reduction: 4.4, 95% CI: 1.5–7.4, P < 0.01). There was no statistically significant change between baseline and 26 weeks in the usual care group (mean reduction 0.2, 95% CI: −2.5 to 2.8, P = 0.89) (Table 2).
The baseline and the serial RDQ measurements between the 3 groups are noted in Table 3.
Table 3.
Summary statistics of the summed score from the Roland–Morris Disability Questionnaire (RDQ), the summed score from the Pain Anxiety Symptom Scale Questionnaire (PASS), and the back pain bothersomeness score (pain bothersomeness) for each group, with comparisons of means between groups at each time point and associated Cohen d statistic.
| Variable | Time point (wk) | PSRT mean ± SD |
MBSR mean ± SD |
Usual care mean ± SD |
Comparison (PSRT and MBSR)* | Comparison (PSRT and UC)* |
|---|---|---|---|---|---|---|
| RDQ | 0 | 9.5 ± 4.3 | 12.6 ± 5.8 | 8.7 ± 6.4 | PSRT = MBSR, t(84) = 1.35, P = 0.18, d = −0.57† | PSRT = UC, t(84) = −0.32, P = 0.75, d = 0.15 |
| 4 | 1.7 ± 2.9 | 9.9 ± 6.0 | 7.5 ± 5.4 | PSRT < MBSR, t(84) = 3.54, P < 0.01, d = −1.09 | PSRT < UC, t(84) = 2.38, P = 0.02, d = −0.96 | |
| 8 | 0.9 ± 2.1 | 8.1 ± 7.4 | 9.8 ± 7.9 | PSRT < MBSR, t(84) = 3.10, P < 0.01, d = −0.96 | PSRT < UC, t(84) = 3.64, P < 0.01, d = −1.03 | |
| 13 | 1.9 ± 4.0 | 7.5 ± 7.1 | 8.5 ± 6.6 | PSRT < MBSR, t(84) = 2.42, P = 0.02, d = −0.80 | PSRT < UC, t(84) = 2.71, P < 0.01, d = −0.92 | |
| 26 | 3.3 ± 5.1 | 8.2 ± 6.7 | 8.5 ± 7.6 | PSRT < MBSR, t(84) = 2.12, P = 0.04, d = −0.71 | PSRT < UC, t(84) = 2.15, P = 0.03, d = −0.70 | |
| Pain bothersomeness | 0 | 5.9 ± 1.7 | 6.7 ± 2.2 | 7.1 ± 2.3 | PSRT = MBSR, t(84) = 0.66, P = 0.51, d = −0.37 | PSRT = UC, t(84) = 1.10, P = 0.27, d = −0.54 |
| 4 | 2.5 ± 2.5 | 4.8 ± 3.3 | 5.8 ± 2.3 | PSRT < MBSR, t(84) = 2.00, P = 0.05, d = −0.69 | PSRT < UC, t(84) = 3.09, P < 0.01, d = −0.98 | |
| 8 | 2.1 ± 2.4 | 4.3 ± 3.1 | 6.5 ± 2.8 | PSRT = MBSR, t(84) = 1.88, P = 0.06, d = −0.69 | PSRT < UC, t(84) = 4.13, P < 0.01, d = −1.08 | |
| 13 | 1.5 ± 2.3 | 5.3 ± 3.2 | 6.1 ± 2.2 | PSRT < MBSR, t(84) = 3.38, P < 0.01, d = −0.99 | PSRT < UC, t(84) = 4.34, P < 0.01, d = −1.15 | |
| 26 | 1.7 ± 3.1 | 4.3 ± 3.3 | 5.3 ± 3.2 | PSRT < MBSR, t(84) = 2.20, P = 0.03, d = −0.70 | PSRT < UC, t(84) = 3.38, P < 0.01, d = −0.90 | |
| PASS | 0 | 41.6 ± 19.5 | 41.3 ± 24.9 | 40.0 ± 23.1 | PSRT = MBSR, t(84) = −0.05, P = 0.96, d = 0.02 | PSRT = UC, t(84) = −0.19, P = 0.85, d = 0.07 |
| 4 | 15.7 ± 18.3 | 35.8 ± 21.1 | 32.5 ± 22.3 | PSRT < MBSR, t(84) = 2.43, P = 0.02, d = −0.83 | PSRT = UC, t(84) = 1.90, P = 0.06, d = −0.71 | |
| 8 | 6.6 ± 8.1 | 27.8 ± 23.9 | 35.2 ± 24.3 | PSRT < MBSR, t(84) = 2.56, P = 0.01, d = −0.90 | PSRT < UC, t(84) = 3.24, P < 0.01, d = −1.04 | |
| 13 | 7.5 ± 11.2 | 23.9 ± 22.8 | 34.0 ± 25.5 | PSRT < MBSR, t(84) = 2.00, P = 0.05, d = −0.77 | PSRT < UC, t(84) = 3.01, P < 0.01, d = −0.97 | |
| 26 | 8.0 ± 11.5 | 24.5 ± 22.6 | 39.8 ± 28.4 | PSRT < MBSR, t(84) = 2.00, P = 0.05, d = −0.77 | PSRT < UC, t(84) = 3.60, P < 0.01, d = −1.00 |
For both comparisons (PSRT vs MBSR and PSRT vs UC), the “<” and the “>” indicate lower and higher RDQ/back pain bothersomeness scores or PASS scores for PSRT compared with the other groups. The “=” indicates a nonstatistically significant difference from the mixed model comparing PSRT with the other groups at each time point.
d indicates value of Cohen d (standardized mean difference) between the 2 groups.
MBSR, mindfulness-based stress reduction; PSRT, psychophysiologic symptom relief therapy.
There was a statistically significant interaction of treatment group and time (P = 0.02) when the PSRT arm was compared with the MBSR group, with a greater reduction in RDQ scores in the PSRT arm. As noted in Table 3, the mean RDQ scores for PSRT were statistically significantly lower than those of MBSR at all time points including the 4-week and 26-week time point (4 weeks: Cohen d = −1.09, P < 0.01, 26 weeks: Cohen d = −0.71, P = 0.04). When the PSRT arm was compared with the usual care arm, there was a statistically significant interaction of treatment group and time (P < 0.01), with a greater reduction in RDQ scores in the PSRT arm. As noted in Table 3, the mean RDQ scores for PSRT were statistically significantly lower than those of usual care at all time points, including the 4-week and the 26-week time points (4 weeks: Cohen d = −0.96, P = 0.02, 26 weeks: Cohen d = −0.70, P = 0.03).
The largest amount of mean percent decrease between weeks in the RDQ scores was 83% (95% CI: 65%–100%) and occurred between baseline and the 4-week mark in the PSRT group. In MBSR, the largest amount of mean percent decrease between weeks in RDQ scores was 32% (95% CI: 3%–62%) and occurred between 4 and 8 weeks. In usual care, the largest amount of mean percent decrease between weeks in the RDQ scores was 11% (95% CI: −10% to 41%, negative value indicating increase) and occurred between baseline and 4 weeks.
3.2. Secondary outcomes
3.2.1. Back pain bothersomeness
The differences in back pain bothersomeness scores between baseline and subsequent time points within a group are noted in Table 2.
Between baseline and 4 weeks, there was a statistically significant decrease in back pain bothersomeness scores in the PSRT and usual care groups (PSRT mean reduction: 3.5, 95% CI: 1.9–5.0, P < 0.01, usual care mean reduction: 1.3, 95% CI: 0.2–2.4, P = 0.02). There was no statistically significant change between baseline and 4 weeks in the MBSR group (mean reduction 1.9, 95% CI: −0.6 to 4.4, P = 0.12) (Table 2).
Between baseline and 26 weeks, there was a statistically significant reduction in the back pain bothersomeness scores for the PSRT and MBSR groups (PSRT mean reduction: 4.2, 95% CI: 1.4–6.9, P < 0.01, MBSR mean reduction: 2.4, 95% CI: 0.1–4.7, P = 0.04). There was no statistically significant change between baseline and 26 weeks in the usual care group (mean reduction 1.8, 95% CI: 0.0–3.5, P = 0.05) (Table 2).
The baseline and serial back pain bothersomeness measurements between the 3 groups are noted in Table 3.
There was no statistically significant interaction of treatment group and time (P = 0.14) when the PSRT arm was compared with the MBSR group. However, as noted in Table 3, the mean back pain bothersomeness scores for PSRT were statistically significantly lower than those of MBSR at 4 weeks, 13 weeks, and 26 weeks. Specifically, at the 4-week and 26-week time point, the Cohen d between the 2 groups were d = −0.69, P = 0.05 (rounded up to 2 decimal places) and d = −0.70, P = 0.03, respectively. When the PSRT arm was compared with usual care, there was a statistically significant interaction of treatment group and time (P = 0.04), with more reduction in patient-reported back pain bothersomeness in the PSRT arm. As noted in Table 3, the mean pain bothersomeness scores for PSRT were statistically significantly lower than those of usual care at all time points, including the 4-week and 26-week time points (4 weeks: Cohen d = −0.98, P < 0.01, 26 weeks: Cohen d = −0.90, P < 0.01).
The largest amount of mean percent decrease between weeks in back pain bothersomeness scores was 60% (95% CI: 34%–86%) and occurred between baseline and 4 weeks in the PSRT group. In MBSR, the largest amount of mean percent decrease between weeks in back pain bothersomeness scores was 19% (95% CI: −16% to 54%, negative value indicating mean increase) and occurred between 13 and 26 weeks. In usual care, the largest amount of mean percent decrease between weeks in back pain bothersomeness scores was 18% (95% CI: −1% to 35%, negative value indicating increase) and occurred between baseline and 4 weeks.
3.2.2. Pain-related anxiety
The differences in PASS scores between baseline and subsequent time points within a group are noted in Table 2.
Between baseline and 4 weeks, there was a statistically significant decrease in PASS scores in the PSRT group (PSRT mean reduction: 25.9, 95% CI: 10.0–41.8, P < 0.01). There was no statistically significant change between baseline and 4 weeks in the MBSR group and the usual care group (MBSR mean reduction 5.5, 95% CI: −4.8 to 15.8, P = 0.26, usual care mean reduction 7.5, 95% CI: −1.0 to 16.0, P = 0.08).
Between baseline and 26 weeks, there was a statistically significant reduction in the PASS scores for the PSRT and MBSR groups (PSRT mean reduction: 33.6, 95% CI: 19.0–48.3, P < 0.01, MBSR mean reduction: 16.8, 95% CI: 7.4–26.1, P < 0.01). There was no statistically significant change between baseline and 26 weeks in the usual care group (mean reduction 0.3, 95% CI: −8.0 to 8.5, P = 0.95).
There was a statistically significant interaction between treatment group and time (P = 0.03) when the PASS score for the PSRT group was compared with the MBSR arm, with a greater decrease in the scores in the PSRT group. As noted in Table 3, the mean PASS scores for PSRT were statistically significantly lower than those of MBSR at all time points, including the 4-week and 26-week time point (4 weeks: Cohen d = −0.83, P = 0.02, 26 weeks: Cohen d = −0.77, P = 0.05 [rounded up to 2 decimals]). When the PSRT group was compared with the usual care arm, the interaction between treatment group and time was significant (P < 0.01), favoring a greater decrease in the PASS scores for the PSRT group. As noted in Table 3, the mean PASS scores for PSRT were statistically significantly lower than those of usual care at weeks 8, 13, and 26, but not at week 4. Specifically, at the 4-week and 26-week time point, the Cohen d between the 2 groups were d = −0.71, P = 0.06, and d = −1.00, P < 0.01, respectively.
The largest amount of mean percent decrease between weeks in PASS scores was 59% (95% CI: 30%–88%) and occurred between baseline and 4 weeks in the PSRT group. In MBSR, the largest amount of mean percent decrease between weeks in PASS scores was 27% (95% CI: 1%–54%) and occurred between 4 and 8 weeks. In usual care, the largest amount of mean percent decrease between weeks in PASS scores was 19% (95% CI: 1%–38%) and occurred between baseline and 4 weeks.
3.3. Additional outcomes
3.3.1. Functional recovery
Figure 2 illustrates the percent of functionally recovered participants at each time point. There were statistically significant differences between PSRT and MBSR or usual care at all time points except at the 26-week time point, when 5 of the patients (45.5%) in the PSRT arm were classified as functionally recovered, compared with 1 patient (8.3%) in the MBSR group (P = 0.07). Of note, no patients (0%) in the usual care group obtained functional recovery at any time point during the 26-week period.
Figure 2.
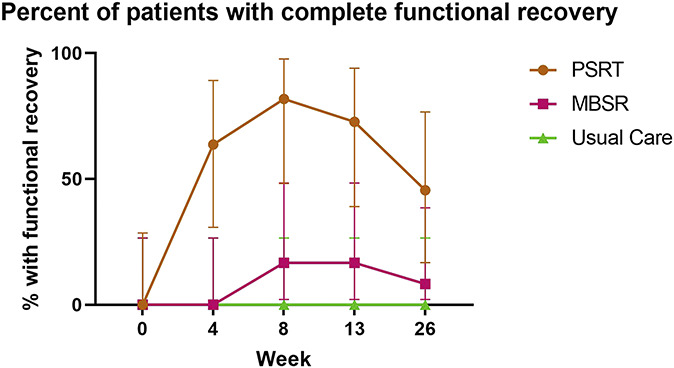
Functional recovery over time. Figure shows the percentage of patients (with the 95% confidence interval) in each group that were considered completely functionally recovered (0 of 24 on RDQ) at each time point, with 0 indicating baseline time. MBSR, mindfulness-based stress reduction; PSRT, psychophysiologic symptom relief therapy.
3.3.2. Pain relief
Figure 3 illustrates the percent of participants who indicated that they were pain free at each time point. At the 26-week time point, 7 patients (63.6%) in the PSRT group were classified as being pain free, compared with 3 patients (25.0%) in the MBSR arm and 2 patients (16.7%) in the usual care group. The difference in the proportion of patients who were pain free was not significant between the PSRT and the MBSR arms (P = 0.10) but was significant between the PSRT and the usual care groups (P = 0.04).
Figure 3.
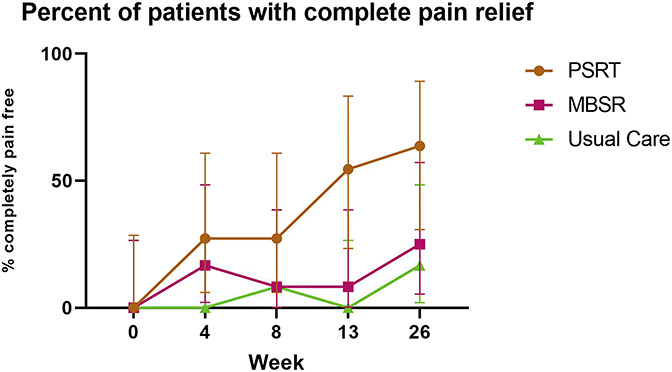
Pain relief over time. Figure shows, for every time point, the percentage of patients (with the 95% confidence interval) in each group who were pain free (0 out of 10 for pain bothersomeness) at that time, with 0 indicating baseline time. MBSR, mindfulness-based stress reduction; PSRT, psychophysiologic symptom relief therapy.
3.4. Adherence
Supplementary Table 1 (available at http://links.lww.com/PR9/A129) indicates the counts and percentages of patients for each of the 6 levels of hours spent working on skills learned in the program, for the PSRT and MBSR groups. Most patients spend between 1 and 6 hours per week practicing skills taught during the intervention (70% in PSRT; 73% in MBSR), with 1 to 2 hours being the most common response in PSRT (chosen 27% of the time) and 2 to 4 hours the most common in MBSR (chosen 42% of the time).
4. Discussion
Our results demonstrate that PSRT for chronic back pain was feasible and seemed to be potentially highly beneficial when compared with MBSR and usual care. Within 4 weeks, differences between PSRT, MBSR, and usual care were apparent across multiple domains and specifically for the primary outcome measure of functional disability. These effects on functional disability persisted through the 26-week monitoring period. Session attendance was high for both PSRT and MBSR arms, and there was 100% compliance with the completion of questionnaires from participants at each time point of interest, providing further evidence of feasibility for a larger future randomized control trial.
One previous case series in 51 patients used a similar approach to ours and suggested a reduction in back pain; however, this study was limited by a lack of a control group.24 In this randomized trial, we compared PSRT with both usual care and an active comparator (MBSR). MBSR was chosen as an active comparator for 2 reasons. First, one previous large, randomized trial showed that MBSR and CBT were superior to usual care for improving pain-related disability and reducing back pain.4 Thus, comparing PSRT to a currently accepted treatment that was previously found to be as effective as CBT for chronic low back pain4 allows for a better assessment of the effectiveness of this intervention. Second, MBSR is used in the later portions of the PSRT program. Comparisons of PSRT with MBSR thus allow us to evaluate the potential benefits of the PSRT components based on Sarno's psychophysiological model of pain. Thus, by using MBSR as an active comparator, we were able to both assess the independent efficacy of this treatment and compare it with a method with established efficacy. Of note, PSRT does not introduce MBSR until after the fourth week, and therefore, the outcomes at 4 weeks may be particularly informative between interventions. To that end, the largest improvements in all 3 parameters (pain-related disability, back pain bothersomeness, and pain-related anxiety) were noted in the PSRT arm at 4 weeks.
The PSRT approach as provided is novel in that elements of Sarno's work are intertwined with MBSR, thus providing an optimal platform for treating the underlying disorder and testing our proposed hypothesis. Fundamental differences exist between PSRT, MBSR, and CBT. Cognitive behavioral therapy involves taking an active, structured approach to pain self-management based on the biopsychosocial model of pain; it focuses on behavioral modifications (eg, increases in activity and pacing) and cognitive restructuring (eg, challenging dysfunctional thoughts and increasing self-efficacy) as a means to reduce pain and distress and improve function in patients with a variety of chronic pain conditions.9 Mindfulness-based interventions aim to reduce pain through stress reduction and other multiple, unique neural mechanisms, irrespective of the etiology of the pain.31 By contrast, PSRT acknowledges and treats pain as a manifestation of a psychosomatic or psychophysiological disorder. This subtle but fundamental difference provides patients with a much different orientation to their pain. From this orientation, subjects are then exposed to the additional elements of the program including desensitization, emotional expression, and mindfulness as detailed in the supplementary material (available at http://links.lww.com/PR9/A129).
Apart from the previous case series as noted, there have been limited studies using similar approaches to ours for patients with chronic back pain. Previous work has investigated the efficacy of some related approaches, with promising results when targeting unresolved trauma, conflict, and relational disturbances in patients with musculoskeletal pain, fibromyalgia, headaches, pelvic pain, and irritable bowel syndrome.2,3,13,17,26,28,32 In addition, Burger et al. evaluated a similar mind–body approach in a preliminary, uncontrolled case series using emotional awareness and expression therapy for chronic musculoskeletal pain. This study also showed a significant reduction in pain, disability, depression, and stress at both 3-month and 6-month follow-up. Approximately, one-third of the patients improved by 70% in pain and other outcomes and two-thirds improved at least by 30%.2
The results of this trial indicate that a larger randomized controlled trial would be feasible using methods outlined in this article. The high session attendance (>70%), questionnaire survey completion (100%), and low drop-out rate (7%) suggest that PSRT is a feasible intervention for the treatment of patients with nonspecific back pain. The high questionnaire completion rate is potentially attributed to our compensation strategy where subjects were reimbursed per questionnaire instead of a lump sum for participation in general or for compensation related to class attendance. Importantly, PSRT is intended for individuals with nonspecific back pain. Although many pain syndromes (malignancy, fractures, and infection) are rooted in a physical source, a majority of currently classified back pain syndromes do not have a definitive organic cause.
5. Limitations
We conducted a single-center, pilot randomized control trial that would benefit from replication in larger trials. The findings of this study have to be seen in light of some limitations. First, the treatment length and total time spent were more in the intervention arms with the PSRT lasting 12 weeks (and 38 total hours) and the active comparator group (MBSR) lasting 8 weeks (and 22 total hours). The rationale for this difference was our aim to compare PSRT with MBSR, which requires the use of both curricula as intended; hence, we refrained from altering MBSR's established curriculum. However, concerns about the differential treatment durations are mitigated by the fact that the biggest treatment differences occurred at the 4-week mark for both treatments, before the participants received the MBSR portion of PSRT. That is, most of the treatment effect seems to be due to the unique first component of PSRT. Second, we initially intended on completing a definitive trial but converted to a pilot study for reasons noted in the methods. Third, we made minor adjustments to fully optimize the design and delivery of the intervention (ie, the use of a particular book and the addition of the full MBSR program to the treatment group, as opposed to a modified short version which we used initially) and the participant population (ie, minor adjustments to the exclusion criteria: we originally included subjects with spinal stenosis but later excluded this group out of concern that this could represent organic disease). The beginning of the PSRT course was largely provided by one of the authors (M.W.D.), and the impact of an individual's teaching or communication skills could potentially impact reception. The skills of the MBSR instructors could also have affected the outcome of that intervention. Fourth, the PSRT group was not blinded to their treatment allocation; however, the presence of the MBSR group (active comparator) somewhat mitigated this limitation because they were not informed that they were in an active comparator group and likely believed they were in the treatment arm. Fifth, we assessed pain bothersomeness as opposed to average pain. Sixth, the time commitment (4 hours weekly) might be a limiting factor when considering reimbursement by insurance payers. Although our results are suggestive of efficacy, further studies need to be performed to reproduce our findings taking into account these limitations.
6. Conclusion
Psychophysiologic symptom relief therapy is a feasible and potentially highly beneficial treatment for patients with nonspecific back pain. Furthermore, larger-scale studies are needed to examine the efficacy of PSRT.
Disclosures
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A129.
Supplementary Material
Acknowledgements
The study was funded by a philanthropic donation by Adam D'Angelo. The authors acknowledge Dr. Howard Schubiner for his consultative expertise and education of instructors before initiating the intervention. The authors would also like to acknowledge Francesca Montillo and Jeremy Silverman for their administrative support of the trial as well as editorial input on this manuscript.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
Contributor Information
Garrett S. Thompson, Email: gthompson594@gmail.com.
Shivani Mehta, Email: smehta3@bidmc.harvard.edu.
Myrella Paschali, Email: mpaschali@bwh.harvard.edu.
Patricia Howard, Email: phoward2@bidmc.harvard.edu.
Sofie B. Antonsen, Email: sofie.bjerring.antonsen@gmail.com.
Lakshman Balaji, Email: lbalaji@bidmc.harvard.edu.
Suzanne M. Bertisch, Email: SBERTISCH@PARTNERS.ORG.
Robert Edwards, Email: rredwards@bwh.harvard.edu.
Long H. Ngo, Email: lngo@bidmc.harvard.edu.
Anne V. Grossestreuer, Email: agrosses@bidmc.harvard.edu.
References
- [1].Berg L, Hellum C, Gjertsen Ø, Neckelmann G, Johnsen LG, Storheim K, Brox JI, Eide GE, Espeland A; Norwegian Spine Study Group. Do more MRI findings imply worse disability or more intense low back pain? A cross-sectional study of candidates for lumbar disc prosthesis. Skeletal Radiol 2013;42:1593–602. [DOI] [PubMed] [Google Scholar]
- [2].Burger AJ, Lumley MA, Carty JN, Latsch DV, Thakur ER, Hyde-Nolan ME, Hijazi AM, Schubiner H. The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: a preliminary, uncontrolled trial. J Psychosom Res 2016;81:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Carty JN, Ziadni MS, Holmes HJ, Tomakowsky J, Peters K, Schubiner H, Lumley MA. The effects of a life stress emotional awareness and expression interview for women with chronic urogenital pain: a randomized controlled trial. Pain Med 2019;20:1321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cherkin DC, Sherman KJ, Balderson BH, Cook AJ, Anderson ML, Hawkes RJ, Hansen KE, Turner JA. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA 2016;315:1240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chou R, Baisden J, Carragee EJ, Resnick DK, Shaffer WO, Loeser JD. Surgery for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine (Phila Pa 1976) 2009;34:1094–109. [DOI] [PubMed] [Google Scholar]
- [6].Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol 2015;29:6–19. [DOI] [PubMed] [Google Scholar]
- [7].Croft PR, Papageorgiou AC, Ferry S, Thomas E, Jayson MI, Silman AJ. Psychologic distress and low back pain. Evidence from a prospective study in the general population. Spine (Phila Pa 1976) 1995;20:2731–7. [DOI] [PubMed] [Google Scholar]
- [8].Currie SR, Wang J. More data on major depression as an antecedent risk factor for first onset of chronic back pain. Psychol Med 2005;35:1275–82. [DOI] [PubMed] [Google Scholar]
- [9].Ehde DM, Dillworth TM, Turner JA. Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol 2014;69:153–66. [DOI] [PubMed] [Google Scholar]
- [10].el Barzouhi A, Vleggeert-Lankamp CLAM, Lycklama à Nijeholt GL, Van der Kallen BF, van den Hout WB, Jacobs WCH, Koes BW, Peul WC; Leiden-The Hague Spine Intervention Prognostic Study Group. Magnetic resonance imaging in follow-up assessment of sciatica. N Engl J Med 2013;368:999–1007. [DOI] [PubMed] [Google Scholar]
- [11].Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain 2012;13:715–24. [DOI] [PubMed] [Google Scholar]
- [12].Hsu MC, Schubiner H, Lumley MA, Stracks JS, Clauw DJ, Williams DA. Sustained pain reduction through affective self-awareness in fibromyalgia: a randomized controlled trial. J Gen Intern Med 2010;25:1064–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lumley MA, Cohen JL, Stout RL, Neely LC, Sander LM, Burger AJ. An emotional exposure-based treatment of traumatic stress for people with chronic pain: preliminary results for fibromyalgia syndrome. Psychotherapy (Chic) 2008;45:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lumley MA, Schubiner H, Lockhart NA, Kidwell KM, Harte SE, Clauw DJ, Williams DA. Emotional awareness and expression therapy, cognitive behavioral therapy, and education for fibromyalgia: a cluster-randomized controlled trial. PAIN 2017;158:2354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lumley MA, Schubiner H. Emotional awareness and expression therapy for chronic pain: rationale, principles and techniques, evidence, and critical review. Curr Rheumatol Rep 2019;21:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Maiti A, Dhoble A. Takotsubo cardiomyopathy. N Engl J Med 2017;377:e24. [DOI] [PubMed] [Google Scholar]
- [17].Maroti D, Ek J, Widlund R-M, Schubiner H, Lumley MA, Lilliengren P, Bileviciute-Ljungar I, Ljótsson B, Johansson R. Internet-administered emotional awareness and expression therapy for somatic symptom disorder–a preliminary efficacy trial. Front Psychiatry 2021;12:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McCracken LM, Dhingra L. A short version of the Pain Anxiety Symptoms Scale (PASS-20): preliminary development and validity. Pain Res Manag 2002;7:45–50. [DOI] [PubMed] [Google Scholar]
- [19].Mokdad AH, Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany E, Lee A, Khan AR, Ahmadi A, Ferrari AJ, Kasaeian A, Werdecker A, Carter A, Zipkin B, Sartorius B, Serdar B, Sykes BL, Troeger C, Fitzmaurice C, Rehm CD, Santomauro D, Kim D, Colombara D, Schwebel DC, Tsoi D, Kolte D, Nsoesie E, Nichols E, Oren E, Charlson FJ, Patton GC, Roth GA, Hosgood HD, Whiteford HA, Kyu H, Erskine HE, Huang H, Martopullo I, Singh JA, Nachega JB, Sanabria JR, Abbas K, Ong K, Tabb K, Krohn KJ, Cornaby L, Degenhardt L, Moses M, Farvid M, Griswold M, Criqui M, Bell M, Nguyen M, Wallin M, Mirarefin M, Qorbani M, Younis M, Fullman N, Liu P, Briant P, Gona P, Havmoller R, Leung R, Kimokoti R, Bazargan-Hejazi S, Hay SI, Yadgir S, Biryukov S, Vollset SE, Alam T, Frank T, Farid T, Miller T, Vos T, Bärnighausen T, Gebrehiwot TT, Yano Y, Al-Aly Z, Mehari A, Handal A, Kandel A, Anderson B, Biroscak B, Mozaffarian D, Dorsey ER, Ding EL, Park EK, Wagner G, Hu G, Chen H, Sunshine JE, Khubchandani J, Leasher J, Leung J, Salomon J, Unutzer J, Cahill L, Cooper L, Horino M, Brauer M, Breitborde N, Hotez P, Topor-Madry R, Soneji S, Stranges S, James S, Amrock S, Jayaraman S, Patel T, Akinyemiju T, Skirbekk V, Kinfu Y, Bhutta Z, Jonas JB, Murray CJL. The state of US health, 1990-2016: burden of diseases, injuries, and risk factors among US states. JAMA 2018;319:1444–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Orsillo SM, Roemer L. Worry less, live more: the mindful way through anxiety workbook. New York, NY: Guilford Publications, 2016. [Google Scholar]
- [21].Roland M, Fairbank J. The Roland-Morris disability questionnaire and the Oswestry disability questionnaire. Spine 2000;25:3115–24. [DOI] [PubMed] [Google Scholar]
- [22].Sargent M. Psychosomatic backache. N Engl J Med 1946;234:427–30. [DOI] [PubMed] [Google Scholar]
- [23].Sarno JE. The mindbody prescription: healing the body, healing the pain. New York, NY: Grand Central Publishing, 2001. [Google Scholar]
- [24].Schechter D, Smith AP, Beck J, Roach J, Karim R, Azen S. Outcomes of a mind-body treatment program for chronic back pain with no distinct structural pathology—a case series of patients diagnosed and treated as tension myositis syndrome. Altern Ther Health Med 2007;13:26–35. [PubMed] [Google Scholar]
- [25].Schubiner H, Betzold M. Unlearn your pain. Pleasant Ridge, MI: Mind Body Publishing, 2010. [Google Scholar]
- [26].Slavin-Spenny O, Lumley MA, Thakur ER, Nevedal DC, Hijazi AM. Effects of anger awareness and expression training versus relaxation training on headaches: a randomized trial. Ann Behav Med 2013;46:181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Staal JB, de Bie R, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev 2008;2008:Cd001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Thakur ER, Holmes HJ, Lockhart NA, Carty JN, Ziadni MS, Doherty HK, Lackner JM, Schubiner H, Lumley MA. Emotional awareness and expression training improves irritable bowel syndrome: a randomized controlled trial. Neurogastroenterol Motil 2017. doi: 10.1111/nmo.13143 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wu A, March L, Zheng X, Huang J, Wang X, Zhao J, Blyth FM, Smith E, Buchbinder R, Hoy D. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the Global Burden of Disease Study 2017. Ann Transl Med 2020;8:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yarns BC, Lumley MA, Cassidy JT, Steers WN, Osato S, Schubiner H, Sultzer DL. Emotional awareness and expression therapy achieves greater pain reduction than cognitive behavioral therapy in older adults with chronic musculoskeletal pain: a preliminary randomized comparison trial. Pain Med 2020;21:2811–22. [DOI] [PubMed] [Google Scholar]
- [31].Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci 2011;31:5540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ziadni MS, Carty JN, Doherty HK, Porcerelli JH, Rapport LJ, Schubiner H, Lumley MA. A life-stress, emotional awareness, and expression interview for primary care patients with medically unexplained symptoms: a randomized controlled trial. Health Psychol 2018;37:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A129.