Abstract
Background
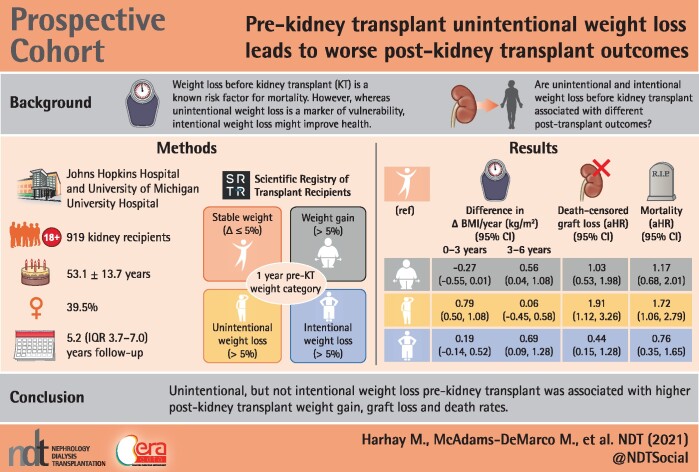
Weight loss before kidney transplant (KT) is a known risk factor for weight gain and mortality, however, while unintentional weight loss is a marker of vulnerability, intentional weight loss might improve health. We tested whether pre-KT unintentional and intentional weight loss have differing associations with post-KT weight gain, graft loss and mortality.
Methods
Among 919 KT recipients from a prospective cohort study, we used adjusted mixed-effects models to estimate post-KT BMI trajectories, and Cox models to estimate death-uncensored graft loss, death-censored graft loss and all-cause mortality by 1-year pre-KT weight change category [stable weight (change ≤ 5%), intentional weight loss (loss > 5%), unintentional weight loss (loss > 5%) and weight gain (gain > 5%)].
Results
The mean age was 53 years, 38% were Black and 40% were female. In the pre-KT year, 62% of recipients had stable weight, 15% had weight gain, 14% had unintentional weight loss and 10% had intentional weight loss. In the first 3 years post-KT, BMI increases were similar among those with pre-KT weight gain and intentional weight loss and lower compared with those with unintentional weight loss {difference +0.79 kg/m2/year [95% confidence interval (CI) 0.50–1.08], P < 0.001}. Only unintentional weight loss was independently associated with higher death-uncensored graft loss [adjusted hazard ratio (aHR) 1.80 (95% CI 1.23–2.62)], death-censored graft loss [aHR 1.91 (95% CI 1.12–3.26)] and mortality [aHR 1.72 (95% CI 1.06–2.79)] relative to stable pre-KT weight.
Conclusions
This study suggests that unintentional, but not intentional, pre-KT weight loss is an independent risk factor for adverse post-KT outcomes.
Keywords: kidney transplant, obesity, weight loss
GRAPHICAL ABSTRACT
KEY LEARNING POINTS
What is already known about this subject?
Weight loss is common before kidney transplant (KT) and research has shown that substantial pretransplant weight loss is associated with higher posttransplant weight gain, graft loss and mortality risks than maintaining a stable pretransplant body weight.
Renal dietitians have reported that the absence of time to support end-stage kidney disease (ESKD) patients who desire weight loss and the lack of ESKD-specific weight loss guidelines are barriers to achieving healthy intentional weight loss in this population.
Although weight loss is commonly required of transplant candidates with obesity to achieve access to transplant, few studies have examined long-term posttransplant health outcomes of intentional weight loss in this population.
What this study adds?
This study is the first to examine whether the intentionality of weight loss before KT is associated with differences in posttransplant health outcomes.
The study shows that although KT recipients with pretransplant intentional weight loss have similar early posttransplant BMI trajectories, graft loss and mortality risks compared with those with stable pretransplant weight, they also have larger BMI increases after 3 years posttransplant.
The results indicate that unintentional weight loss is an independent risk factor for adverse outcomes post-transplant, including among those transplant recipients who were obese before transplant.
What impact this may have on practice or policy?
The results of this study strongly support close surveillance of KT candidates for unintentional weight loss, including those who are overweight or obese, and the testing of interventions to mitigate or reverse the health effects of unintentional weight loss.
ESKD-specific nutritional guidelines for healthy weight loss and weight loss maintenance are needed in both pre- and post-KT settings.
INTRODUCTION
Weight loss is an established risk factor for death among people with end-stage kidney disease (ESKD) [1–5]. Findings from registry data have been consistent across studies of those with nondialysis-dependent chronic kidney disease (CKD) [6], those who are dialysis-dependent [2] and those who received a kidney transplant (KT) [7–9]. However, by classifying weight loss as a single risk factor, existing research has provided limited information on the potential differences in post-KT outcomes between those patients with unintentional and intentional weight loss.
Unintentional weight loss is a component of the physical frailty phenotype [10], a syndrome of reduced resilience to health stressors that is common among ESKD patients [9]. Unintentional weight loss is typically a later marker of frailty [11, 12] and may be accompanied by signs of increased inflammation, malnutrition and sarcopenia [13]. Frailty is associated with a higher risk of adverse outcomes, including death, in populations with ESKD and KT [14–20].
Compared with unintentional weight loss, less is known about the outcomes of people with ESKD who lost weight intentionally. In the absence of ESKD-specific weight loss nutritional guidelines, experts have expressed concern that even intentional weight loss could lead to harmful health effects [3, 21], particularly if it portends lower nutritional stores and muscle mass [22–26]. Conversely, weight loss might improve access to KT for candidates with obesity, as many KT programs will not accept patients who are above their specified ‘BMI threshold’ [21, 27]. The majority of dialysis patients with obesity report that they desire weight loss, particularly to satisfy KT program requirements [21, 28]. Therefore it is important to determine whether the known associations between weight loss and adverse outcomes after KT are consistent among those who lose weight intentionally.
The goal of this study was to test whether post-KT BMI trajectories and other outcomes differ between KT recipients with intentional and unintentional weight loss in a diverse cohort of KT recipients from two KT programs. We also assessed whether the intentionality of weight loss was associated with differences in post-KT graft loss and mortality. Finally, we examined whether post-KT BMI trajectory, graft loss and mortality differed in the subgroup of overweight and obese recipients who had intentional and unintentional pre-KT weight loss.
MATERIALS AND METHODS
Study design
We leveraged a two-center prospective cohort study of 919 adult KT recipients in which study participants with weight loss in the year prior to KT were asked if the weight loss was intentional. Participants were enrolled at admission for KT from the Johns Hopkins Hospital (December 2008–January 2020; n = 867) and the University of Michigan Hospital (March 2015–November 2016; n = 52). Inclusion criteria were age ≥18 years and the ability to speak English. KT recipients reported their height and weight at KT admission and at routine clinical follow-ups at ∼1, 3 and 6 months, 1 year and annually thereafter post-KT. BMI at each time point was calculated from reported height and weight. All participants were linked to the Scientific Registry of Transplant Recipients (SRTR) to ascertain graft loss and death outcomes. Participant characteristics at KT admission were self-reported (sex, race, ethnicity, education and dialysis modality) and calculated (age, time on dialysis). Additional participant comorbidities (cardiac, pulmonary, gastrointestinal and cancer diagnoses) were abstracted from medical records and supplemented with patient self-reports. At the time of KT, most participants also underwent complete frailty testing, including assessments of exhaustion, low physical activity, low grip strength and slow gait speed [10]. We determined participant urban/rural residence type by linking the self-reported zip code to US Census Bureau data [29]. Additional characteristics ascertained from the SRTR included diabetes status, insurance type and cause of ESKD. Participants who were alive in March 2020 were administratively censored. The institutional review boards of Johns Hopkins and the University of Michigan approved the study. All participants provided written informed consent. The protocol was in accordance with the Declaration of Helsinki. The clinical and research activities being reported were consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Exposure: pre-KT weight change and intentionality of weight loss
The primary exposure was weight loss intentionality in the year prior to KT. At KT admission, participants were asked about their current height and weight and weight 1 year prior to admission. If the admission weight was less than the weight 1 year prior to admission, the participants were asked to respond yes or no to the following question: ‘Did you intentionally try to lose weight within the last year?’ We calculated the relative weight change (in percent) as [(current body weight − previous body weight)/(previous body weight)]*100%. We then classified the pre-KT weight change into four categories: (i) stable weight = relative weight change ≤5%; (ii) weight gain = relative weight gain >5%; (iii) unintentional weight loss = relative weight loss >5% with a ‘no’ to the question for intention; and (iv) intentional weight loss = relative weight loss >5% with a ‘yes’ to the question for intention. We also conducted a validation study of our self-reported weight change measure among 63 recipients with a clinic visit between 11 and 13 months before KT in which we compared 1-year pre-KT weights reported at the time of KT to weights that were recorded during the pre-KT visits.
Descriptive analyses
We generated percentages for categorical characteristics, means [standard deviations (SDs)] for normally distributed continuous variables and medians and interquartile ranges (IQRs) for nonnormally distributed continuous variables. Differences in characteristics by pre-KT weight change category were tested using Fisher’s exact test for categorical variables, analysis of variance for normally distributed continuous variables and the Kruskal–Wallis test for nonnormally distributed continuous variables. We examined the distribution of relative pre-KT weight change using a histogram.
Post-KT trajectories of BMI
We examined unadjusted BMI trajectories during the 6 years after KT using quadratic prediction plots by pre-KT weight change categories. We then compared BMI trajectories by pre-KT weight change using a mixed-effects model with random slope (time) and random intercept (person) with an unstructured correlation structure to generate the best possible model fit. Based on exploratory data analysis using locally weighted scatterplot smoothing (LOWESS), we included a spline at 3 years post-KT to account for the nonlinear relationship over different time periods (0–3 years and 3–6 years post-KT). We adjusted for the following potential confounders: recipients’ sociodemographic factors (age at KT, sex, Black race, residence and insurance type), health factors (diabetes status and cause of ESKD), pre-KT years on dialysis, living or deceased donor type and 1-year pre-KT BMI. Additionally we tested whether the estimated yearly post-KT change of BMI (outcome) differed by pre-KT weight change category (exposure) using interaction terms by follow-up time and the spline term with stable pre-KT weight as the reference group. A Wald test was used for testing the significance of the interactions.
Mortality and graft loss rates by pre-KT change category
We estimated cumulative incidences of death-uncensored graft loss (i.e. graft loss for all causes including death), death-censored graft loss (i.e. death with a functioning graft treated as a censoring event) and all-cause mortality using the Kaplan–Meier method and log-rank test to compare unadjusted survival curves by pre-KT weight loss. Proportional hazard assumptions were confirmed by visually inspecting log–log plots. We then estimated the hazard ratios (HRs) for all three outcomes by pre-KT weight loss using Cox proportional hazards models: Model 1 generated the crude HRs (cHRs), Model 2 partially adjusted for recipient factors (age at KT, sex, Black race, donor type, diabetes status, cause of ESKD and 1-year pre-KT BMI) and Model 3 adjusted for variables in Model 2 and additional potential confounders (pre-KT dialysis time, urban/rural residence and insurance type).
All analyses were performed using Stata version 15 (StataCorp, College Station, TX, USA). Two-sided P-values <0.05 were considered statistically significant.
Missing data
Model covariates and outcomes were nonmissing with a few exceptions (Table 1). Therefore we used complete case analysis for adjusted models. To account for the possibility of differential attrition, in a sensitivity analysis we generated estimates of BMI trajectories by pre-KT weight change using multiple imputation (MI) and inverse-probability weighting (IPW), adjusting for the same covariates as in our primary model.
Table 1.
Baseline characteristics overall and by pre-KT weight change category
| Characteristics | Total (N = 919) | Weight change during the year prior to KT |
||||
|---|---|---|---|---|---|---|
| Stable weight | Weight gain | Unintentional weight loss | Intentional weight loss | P-value | ||
| (n = 570) | (n = 137) | (n = 124) | (n = 88) | |||
| Age at KT, mean (SD) | 53.1 (13.7) | 53.8 (13.7) | 51.1 (14.4) | 51.1 (13.8) | 54.4 (12.6) | 0.05 |
| Sex (female), % | 39.5 | 36.0 | 50.4 | 46.0 | 36.4 | 0.006 |
| Race, % | ||||||
| White | 52.3 | 54.0 | 43.1 | 51.6 | 56.8 | 0.25 |
| Black | 38.3 | 36.0 | 46.0 | 39.5 | 39.8 | |
| Hispanic | 3.3 | 3.3 | 5.1 | 2.4 | 1.1 | |
| Other | 6.1 | 6.7 | 5.8 | 6.5 | 2.3 | |
| Education, % | ||||||
| Below high school | 5.0 | 6.1 | 2.9 | 4.0 | 2.3 | 0.38 |
| High school | 34.8 | 34.2 | 40.1 | 33.1 | 33.0 | |
| Above high school | 59.8 | 59.1 | 56.9 | 62.9 | 64.8 | |
| Missing | 0.3 | 0.5 | 0.0 | 0.0 | 0.0 | |
| Diabetes, % | 30.3 | 30.9 | 27.7 | 25.0 | 37.5 | 0.23 |
| Cause of ESKD, % | ||||||
| Glomerular diseases | 24.4 | 22.3 | 24.1 | 32.3 | 27.3 | 0.17 |
| Diabetes | 15.8 | 16.0 | 17.5 | 12.1 | 17.0 | |
| Hypertension | 31.9 | 31.8 | 28.5 | 31.5 | 38.6 | |
| Others | 27.7 | 29.6 | 29.9 | 24.2 | 17.0 | |
| Missing | 0.2 | 0.4 | 0.0 | 0.0 | 0.0 | |
| Pre-KT dialysis modality, % | ||||||
| Pre-emptive KT | 18.7 | 19.8 | 12.4 | 17.7 | 22.7 | 0.21 |
| Hemodialysis | 62.0 | 62.5 | 61.3 | 62.9 | 59.1 | |
| Peritoneal dialysis | 16.5 | 15.1 | 22.6 | 17.7 | 14.8 | |
| Missing | 2.7 | 2.6 | 3.6 | 1.6 | 3.4 | |
| Years on dialysis, median (IQR) | 2.3 (0.4–5.3) | 2.6 (0.5–5.5) | 2.5 (0.9–5.7) | 1.5 (0.3–5.1) | 0.7 (0.1–3.0) | <0.001 |
| Residence, % | ||||||
| Rural | 3.0 | 3.0 | 2.2 | 3.2 | 4.5 | 0.79 |
| Urban | 95.4 | 95.4 | 95.6 | 96.8 | 93.2 | |
| Missing | 1.5 | 1.6 | 2.2 | 0.0 | 2.3 | |
| Insurance type, % | ||||||
| Public | 51.0 | 53.3 | 54.0 | 43.5 | 42.0 | 0.06 |
| Private | 49.0 | 46.7 | 46.0 | 56.5 | 58.0 | |
| 1-year pre-KT BMI, % | ||||||
| Underweight | 4.2 | 3.9 | 10.9 | 1.6 | 0.0 | <0.001 |
| Normal weight | 31.0 | 32.6 | 40.1 | 32.3 | 4.5 | |
| Overweight | 32.4 | 33.9 | 32.1 | 32.3 | 23.9 | |
| Obese | 32.3 | 29.6 | 16.8 | 33.9 | 71.6 | |
| Cardiac diseasea, % | ||||||
| Yes | 10.2 | 9.8 | 10.9 | 12.9 | 8.0 | 0.63 |
| No | 89.0 | 89.3 | 89.1 | 85.5 | 92.0 | |
| Missing | 0.8 | 0.9 | 0.0 | 1.6 | 0.0 | |
| Pulmonary disease, % | ||||||
| Yes | 4.8 | 4.7 | 1.5 | 6.5 | 8.0 | 0.11 |
| No | 94.5 | 94.4 | 98.5 | 91.9 | 92.0 | |
| Missing | 0.8 | 0.9 | 0.0 | 1.6 | 0.0 | |
| Gastrointestinal disease, % | ||||||
| Yes | 2.9 | 2.6 | 2.9 | 5.6 | 1.1 | 0.21 |
| No | 96.3 | 96.5 | 97.1 | 92.7 | 98.9 | |
| Missing | 0.8 | 0.9 | 0.0 | 1.6 | 0.0 | |
| History of metastatic cancer, % | 0.4 | 0.5 | 0.7 | 0.0 | 0.0 | 0.85 |
| Low grip strengthb, % | ||||||
| Yes | 43.0 | 41.4 | 43.1 | 50.0 | 43.2 | 0.34 |
| No | 49.0 | 50.9 | 45.3 | 42.7 | 51.1 | |
| Missing | 8.1 | 7.7 | 11.7 | 7.3 | 5.7 | |
| Low gait speedb, % | ||||||
| Yes | 12.4 | 11.1 | 15.3 | 14.5 | 13.6 | 0.34 |
| No | 68.3 | 70.0 | 62.0 | 64.5 | 72.7 | |
| Missing | 19.3 | 18.9 | 22.6 | 21.0 | 13.6 | |
| Low physical activityb, % | ||||||
| Yes | 45.6 | 43.2 | 47.4 | 58.1 | 40.9 | 0.03 |
| No | 51.3 | 53.3 | 49.6 | 40.3 | 55.7 | |
| Missing | 3.2 | 3.5 | 2.9 | 1.6 | 3.4 | |
| Exhaustionb, % | ||||||
| Yes | 28.0 | 24.6 | 35.8 | 37.9 | 23.9 | 0.003 |
| No | 71.2 | 74.4 | 63.5 | 61.3 | 76.1 | |
| Missing | 0.9 | 1.1 | 0.7 | 0.8 | 0.0 | |
Intentionality of weight loss was measured among recipients who experienced >5% relative weight loss in the 12 months prior to KT; stable weight was defined as ≤5% relative weight change in the 12 months prior to KT. BMI category is defined as underweight, BMI <18.5 kg/m2; normal, BMI ≥18.5–<25.0 kg/m2; overweight, BMI ≥25.0–<30.0 kg/m2; obese, BMI ≥30.0 kg/m2.
Included history of myocardial infarction and congestive heart failure.
Low grip strength, low gait speed, low physical activity and exhaustion were defined according to the physical frailty phenotype definition [10].
Secondary analysis: subgroup with overweight and obese BMI
We examined whether the estimated BMI trajectories by pre-KT weight change were consistent in the subgroup of KT recipients who were overweight or obese (BMI ≥25 kg/m2) 1 year prior to KT. Using Cox proportional hazards models, we also estimated the risks of death-uncensored graft loss, death-censored graft loss and all-cause mortality in the overweight/obese KT recipient subgroup by pre-KT weight change category.
Sensitivity analysis
In adjusting for pre-KT BMI, we expected that there was a possibility for collider stratification bias, leading to inflated effect estimates [30]. Therefore we also replicated our main analyses without adjustment for pre-KT BMI. Finally, to distinguish other causes of unintentional weight loss from weight loss due to fluid removal in the first year of dialysis [2], we examined whether results were robust among KT recipients with at least 2 years of dialysis at the time of KT.
RESULTS
The study population included 919 patients who received KT between December 2008 and January 2020; 867 from Johns Hopkins Hospital and 52 from the University of Michigan Hospital. They were followed-up for an average of 2.4 years (SD 2.0) and had an average number of 4.0 (SD 2.7) follow-up visits over the 6 years post-KT. The mean age was 53.1 years (SD 13.7), 39.5% were female and 38.3% were Black. The average relative body weight change in the year prior to KT was −1.1% (SD 8.0) (Figure 1), with 40% of the cohort having some weight loss in the pre-KT year. Pre-KT relative weight loss was similar when comparing the unintentional and intentional weight loss groups (median relative weight loss 9.5% versus 9.2%; P = 0.58) (Supplementary data, Figure S1). In the subgroup with a clinical visit 11–13 months prior to KT, there was a median difference of 0 kg (IQR −3.64–2.27) between 1-year pre-KT weight reported at the time of KT and at the pre-KT clinic. There were no differences in the validity of self-reported weight change across pre-KT weight change categories.
FIGURE 1.

Distribution of relative body weight change in the year prior to KT among 919 KT recipients. Positive weight change represents weight gain and negative weight change represents weight loss. The mean percent of weight change was −1.1 (SD 8.0).
The majority of participants (62%) were categorized as having stable pre-KT weight, whereas 15% had weight gain, 14% had unintentional weight loss and 10% had intentional weight loss. Compared with those with stable pre-KT weight, recipients with intentional weight loss were older, had less dialysis exposure and were more likely to have pre-KT obesity (Table 1). There were no statistically significant differences in cardiovascular, gastrointestinal, pulmonary or cancer diagnoses between weight change groups (P > 0.05 for all). Compared with those with stable weight, self-reported exhaustion and low physical activity were more common in the unintentional weight loss group, whereas the proportions with low grip strength and slow gait speed were similar. Follow-up time and number of follow-up visits were similar when comparing pre-KT weight change groups (P = 0.10 and 0.73, respectively).
Post-KT BMI trajectories by pre-KT weight change
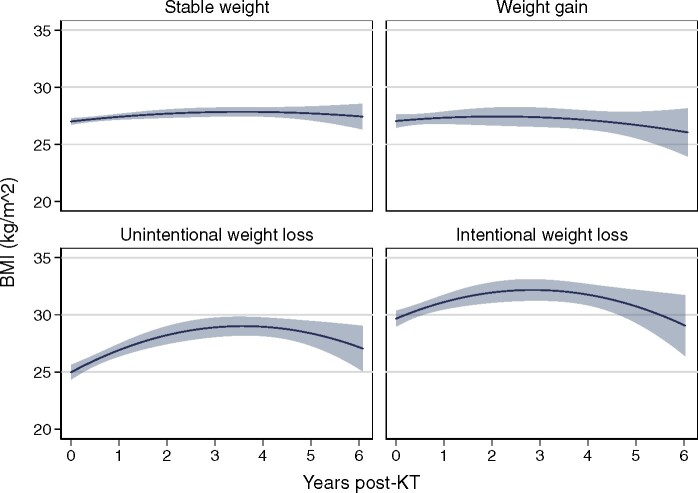
In unadjusted analyses, those with stable weight or weight gain pre-KT had relatively stable BMI over the post-KT follow-up period, whereas those with intentional and unintentional weight loss had larger early increases in BMI followed by BMI decreases (Figure 2). In the fully adjusted model, KT recipients with stable weight in the year prior to KT had increases in BMI of 0.54 kg/m2/year [95% confidence interval (CI) 0.42–0.67]in the 3 years post-KT and decreases in BMI of 0.43 kg/m2/year in post-KT Years 3–6. Compared with those with stable weight, those with intentional weight loss had similar post-KT BMI trajectories in Years 0–3 and higher increases in BMI in Years 3–6, whereas those with unintentional pre-KT weight loss had higher increases in BMI in post-KT Years 0–3 (difference 0.79 kg/m2/year; P < 0.001) (Table 2). Those with pre-KT weight gain had post-KT BMI trajectories similar to those with stable weight in Years 0–3 and greater increases in BMI in Years 3–6 (difference +0.56 kg/m2/year; P = 0.03).
FIGURE 2.
Unadjusted estimates of post-KT BMI trajectories by pre-KT weight change in the year prior to KT (N = 919). Estimated BMI with 95% CIs are presented from quadratic prediction plots. Intentionality of weight loss was measured among recipients who experienced weight loss >5% within 1 year prior to KT and stable weight was defined as weight change in 1 year prior to KT ≤5%.
Table 2.
Adjusted estimates of post-KT BMI trajectories by pre-KT weight change category (N = 885)
| Subgroup | Estimated yearly change of BMI, kg/m2 (95% CI) |
|||
|---|---|---|---|---|
| 0–3 years post-KT |
P-value (interaction) |
3–6 years post-KT |
P-value (interaction) |
|
| Stable weight [a] | 0.54 (0.42–0.67) | – | −0.43 (−0.67 to −0.20) | – |
| Weight gain [b] | 0.27 (0.02–0.53) | – | 0.13 (−0.33–0.59) | – |
| Difference [b – a] | −0.27 (−0.55–0.01) | 0.06 | 0.56 (0.04–1.08) | 0.03 |
| Unintentional weight loss [c] | 1.34 (1.08–1.60) | – | −0.37 (−0.83–0.09) | – |
| Difference [c – a] | 0.79 (0.50–1.08) | <0.001 | 0.06 (−0.45–0.58) | 0.81 |
| Intentional weight loss [d] | 0.74 (0.43–1.04) | – | 0.25 (−0.29–0.80) | – |
| Difference [d – a] | 0.19 (−0.14–0.52) | 0.25 | 0.69 (0.09–1.28) | 0.02 |
Estimated yearly changes in BMI with 95% CIs are presented from adjusted mixed-effects models. Associations that are statistically significant at P < 0.05 are in bolded. All models adjusted for age at KT, sex, Black race, donor type, diabetes status, cause of ESKD, time on dialysis at KT, residence, insurance and 1-year pre-KT BMI.
Pre-KT weight change and post-KT graft loss
In unadjusted analyses, the cumulative incidences of death-uncensored and death-censored graft losses were highest among those with unintentional weight loss and lowest among those with intentional weight loss (P = 0.02 for both outcomes) (Figure 3A and B). In Cox models adjusted for potential confounders including pre-KT BMI (Model 3), only unintentional pre-KT weight loss, relative to stable weight, was associated with higher hazards of death-censored graft loss [adjusted HR (aHR) 1.91 (95% CI 1.12–3.26)] and death-uncensored graft loss [aHR 1.80 (95% CI 1.23–2.62)] (Table 3).
FIGURE 3.
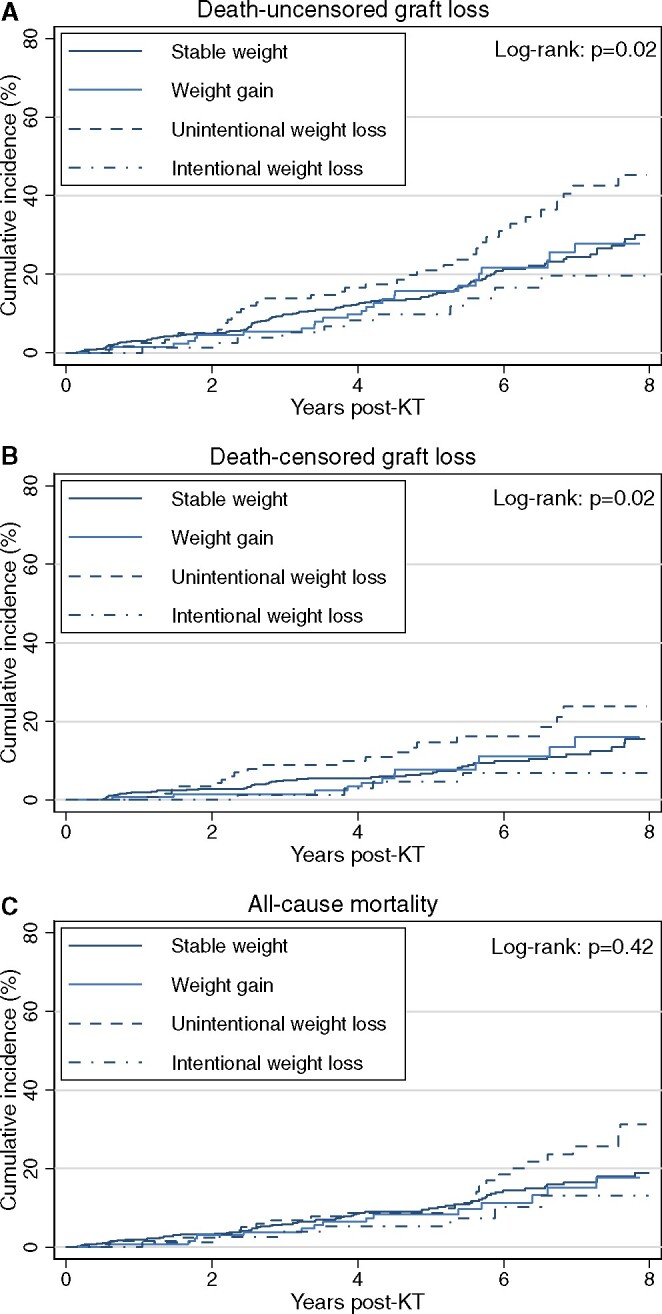
Unadjusted cumulative incidence of (A) death-uncensored graft loss, (B) death-censored graft loss and (C) all-cause mortality by pre-KT weight change category (N = 919). Intentionality of weight loss was assessed among recipients who experienced a relative weight loss >5% in the year prior to KT and stable weight was defined as ≤5% weight change in the year prior to KT.
Table 3.
Unadjusted and adjusted associations between pre-KT weight change category and post-KT death-uncensored graft loss, death-censored graft loss and all-cause mortality
| Outcomes by pre-KT weight change category | Model 1 (n = 919) |
Model 2 (n = 917) |
Model 3 (n = 885) |
|---|---|---|---|
| cHR (95% CI) | aHR (95% CI) | aHR (95% CI) | |
| Death-uncensored graft lossa | |||
| Stable weight | Reference | Reference | Reference |
| Weight gain | 1.00 (0.66–1.51) | 0.98 (0.64–1.51) | 1.08 (0.70–1.65) |
| Unintentional weight loss | 1.62 (1.12–2.32) | 1.80 (1.24–2.60) | 1.80 (1.23–2.62) |
| Intentional weight loss | 0.69 (0.38–1.26) | 0.68 (0.37–1.27) | 0.72 (0.39–1.36) |
| Death-censored graft lossb | |||
| Stable weight | Reference | Reference | Reference |
| Weight gain | 0.97 (0.52–1.83) | 0.98 (0.51–1.89) | 1.03 (0.53–1.98) |
| Unintentional weight loss | 1.92 (1.15–3.21) | 1.96 (1.16–3.30) | 1.91 (1.12–3.26) |
| Intentional weight loss | 0.52 (0.19–1.44) | 0.44 (0.15–1.26) | 0.44 (0.15–1.28) |
| All-cause mortality | |||
| Stable weight | Reference | Reference | Reference |
| Weight gain | 0.93 (0.56–1.56) | 1.01 (0.59–1.71) | 1.17 (0.68–2.01) |
| Unintentional weight loss | 1.31 (0.82–2.07) | 1.63 (1.02–2.61) | 1.72 (1.06–2.79) |
| Intentional weight loss | 0.70 (0.34–1.44) | 0.72 (0.33–1.54) | 0.76 (0.35–1.65) |
cHRs and aHRs with 95% CIs are presented from unadjusted and adjusted Cox models, respectively. Associations that are statistically significant at P < 0.05 are in bold. Model 2 adjusted for age at KT, sex, Black race, donor type, diabetes status, cause of ESKD and 1-year pre-KT BMI. Model 3 adjusted for age at KT, sex, Black race, donor type, diabetes status, cause of ESKD, time on dialysis by KT, residence, insurance and 1-year pre-KT BMI.
Graft survival calculated from the date of transplant to graft failure, death or end of follow-up.
Graft survival calculated from the date of transplant to graft failure or end of follow-up, and death with a functioning graft treated as censoring.
Pre-KT weight change and post-KT all-cause mortality
During a median follow-up period of 5.2 years (IQR 3.7–7.0), 13.4% of the cohort (n = 123) died. In unadjusted analyses, the cumulative incidence of mortality was highest among those with unintentional weight loss and lowest among those with intentional weight loss (P = 0.42) (Figure 3C). In the fully adjusted Cox model (Model 3), unintentional pre-KT weight loss, relative to stable weight, was associated with a 1.72-fold higher hazard of mortality [aHR 1.72 (95% CI 1.06–2.79)], whereas pre-KT intentional weight loss and weight gain were not associated with differences in post-KT mortality (Table 3).
Secondary analysis among recipients with overweight and obese BMI
In the subgroup with overweight and obese BMIs in the year prior to KT, in a fully adjusted model, those with stable pre-KT weight had a 0.37 kg/m2 (95% CI 0.21–0.53)higher BMI per year in post-KT Years 0–3 and a 0.51 kg/m2 lower BMI per year during post-KT Years 3–6 (Supplementary data, Table S1). Those with pre-KT weight gain had greater BMI losses than those with stable weight in post-KT Years 0–3 (difference −0.49 kg/m2/year; P = 0.02) and had similar BMI trajectories in Years 3–6. Those with intentional weight loss had BMI trajectories similar to those with stable weight in Years 0–3 and greater increases in BMI [difference +0.71 kg/m2/year (95% CI 0.06–1.36)] in Years 3–6. Both death-censored and death-uncensored graft loss rates were >2-fold higher in those with pre-KT unintentional weight loss than in those with stable pre-KT weight, whereas graft loss rates were similar between those with stable weight and those with weight gain and intentional weight loss. There were no statistically significant differences in post-KT mortality when comparing the overweight/obese subgroup with stable weight pre-KT to their counterparts with weight gain and unintentional and intentional weight loss (Supplementary data, Table S2).
Sensitivity analyses
Findings on the post-KT BMI trajectories and graft loss and mortality outcomes were similar in models that excluded 1-year pre-KT BMI in the adjustment (Supplementary data, Tables S3 and S4). Results were also similar in models that accounted for differential attrition using MI and IPW (Supplementary data, Table S5). Results were robust in the subgroup of KT recipients with at least 2 years of dialysis exposure before KT (Supplementary data, Table S6).
DISCUSSION
In this study of a large, diverse, two-center cohort of KT recipients, we found that KT recipients with pre-KT intentional and unintentional weight loss have distinct post-KT courses with respect to BMI trajectories, graft loss and mortality risk. Compared with recipients with stable pre-KT BMI, those with unintentional weight loss had greater BMI increases in the first 3 years post-KT and the highest graft loss and mortality rates. These differences were consistent after adjustment for initial BMI and several other potential confounders. The findings suggest that, independent of BMI, findings of pre-KT weight loss should prompt queries about the intentionality of weight loss and additional assessments of vulnerability for those with unintentional weight loss.
Prior studies have identified weight loss as common before KT and associated with higher risks of post-KT weight gain, graft loss and mortality [1, 7, 8, 31]. However, no studies have identified whether said outcomes diverge after distinguishing whether weight loss was intentional or unintentional. Reasons for both weight loss and gain are likely multifactorial, related to nutrition, inflammation and physical activity [32–34]. Weight gain might be protective if it reflects improving nutrition and muscle mass, whereas increased visceral adiposity could exacerbate comorbidities and immobility [22]. The results of our study confirmed previous findings that weight gain is common in the early post-KT course. However, those with unintentional weight loss before KT had the highest post-KT BMI increases and higher graft loss and death rates than those with pre-KT weight gain, intentional weight loss or stable weight. Studies are needed to determine whether interventions to modify post-KT weight gain might impact longer-term graft and survival outcomes.
We found that a single assessment of the intentionality of weight loss at the time of KT could be useful to identify recipients who are most vulnerable to adverse post-KT outcomes. These findings are relevant given prior evidence of a lack of consensus in the nephrology community on the importance of weight loss as a marker of vulnerability. For example, Delphi study results suggested that 29% of ESKD clinicians rated weight loss as an irrelevant frailty component in ESKD [35]. In a recent survey of US KT programs, unintentional weight loss was rated as a less important frailty marker than limitations in activities of daily living and performance on the sit-to-stand test [36]. However, our study showed that despite having similar comorbidity burdens, KT recipients who reported pre-KT unintentional weight loss were also more likely than their counterparts to endorse exhaustion and low physical activity, two other components of the physical frailty phenotype [10]. Therefore knowledge of pre-KT unintentional weight loss could be especially useful to prompt more resource-intensive frailty evaluations and targeted interventions such as prehabilitation [37–39], including both exercise [40] and nutritional components [21].
Obesity is common among people with ESKD and is a pervasive barrier to KT. However, studies that have examined weight loss as a single exposure have found that pre-KT weight loss among KT candidates with obesity is associated with increased mortality and graft loss risk [1, 7, 8], raising concerns that even intentional weight loss might be harmful for ESKD patients [3, 41]. However, in our subgroup analysis of KT recipients who were overweight or obese in the year prior to KT, those with intentional weight loss pre-KT had post-KT BMI trajectories, graft loss and mortality risks similar to those with stable pre-KT weight. In contrast, overweight and obese KT recipients with unintentional pre-KT weight loss had the highest post-KT BMI increases and were at a ˃2-fold higher risk of death-uncensored graft loss than those with stable weight. These findings suggest that among KT candidates with obesity, unintentional, but not intentional, weight loss is a signal of vulnerability.
To our knowledge, this is the first study to examine differences in post-KT outcomes when distinguishing unintentional and intentional weight loss rather than by examining weight loss as a single risk factor or unintentional weight loss alone [42]. The study strengths include its prospective design and its geographically and ethnically diverse patient population. However, the limitations of the study should also be considered. We could not account for potential confounders such as body composition and disease severity. We did not have access to dialysis records to confirm pre-KT dry weights, although our validation analysis suggested limited evidence of recall bias. We also did not have information on weight loss approaches [21], such as bariatric surgery, or on post-KT pregnancies. Finally, we did not have details on causes of graft loss and death that could elucidate mechanisms by which pre-KT untintentional weight loss might increase post-KT risks.
In summary, the findings of this prospective cohort study suggest that there are distinct post-KT clinical outcomes for recipients with weight loss, depending on whether weight loss was intentional or unintentional. Unintentional, but not intentional, weight loss before KT was associated with higher post-KT increases in BMI, graft loss and death rates. Findings were consistent among recipients who were overweight or obese before KT. KT programs should monitor patients for pre-KT unintentional weight loss. Interventions are needed to address the adverse health impacts of unintentional weight loss in ESKD.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
FUNDING
This study was funded by the National Institute on Aging (R01AG055781). The authors wish to acknowledge the National Institutes of Health funding [grants K24AI144954 (to DLS), K01AG064040 (to NMC), K23DK105207 (to MNH) and R01DK124388 (to MNH)].
AUTHORS’ CONTRIBUTIONS
M.N.H., N.M.C. and M.M.D. were responsible for study conception and design. M.N.H., X.C., N.M.C., S.P.N., D.L.S. and M.M.D. were responsible for the analysis and interpretation of data, drafting the article and revising it, providing intellectual content and approval of the final version.
CONLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest to disclose.
Supplementary Material
REFERENCES
- 1.Brilleman SL, Moreno-Betancur M, Polkinghorne KR. et al. Changes in body mass index and rates of death and transplant in hemodialysis patients: a latent class joint modeling approach. Epidemiology 2019; 30: 38–47 [DOI] [PubMed] [Google Scholar]
- 2.Chang TI, Ngo V, Streja E. et al. Association of body weight changes with mortality in incident hemodialysis patients. Nephrol Dial Transplant 2017; 32: 1549–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Detwiler RK.Con: weight loss prior to transplant: no. Nephrol Dial Transplant 2015; 30: 1805–1809 [DOI] [PubMed] [Google Scholar]
- 4.Harhay MN, Ranganna K, Boyle SM. et al. Association between weight loss before deceased donor kidney transplantation and posttransplantation outcomes. Am J Kidney Dis 2019; 74: 361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ku E, Kopple JD, Johansen KL. et al. Longitudinal weight change during CKD progression and its association with subsequent mortality. Am J Kidney Dis 2017; 71: 657–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ku E, Kopple JD, Johansen KL. et al. Longitudinal weight change during CKD progression and its association with subsequent mortality. Am J Kidney Dis 2018; 71: 657–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schold JD, Srinivas TR, Guerra G. et al. A “weight-listing” paradox for candidates of renal transplantation? Am J Transplant 2007; 7: 550–559 [DOI] [PubMed] [Google Scholar]
- 8.Harhay MN, Ranganna K, Boyle SM. et al. Association between weight loss before deceased donor kidney transplantation and posttransplantation outcomes. Am J Kidney Dis 2019; 74: 361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harhay MN, Rao MK, Woodside KJ. et al. An overview of frailty in kidney transplantation: measurement, management and future considerations. Nephrol Dial Transplant 2020; 35: 1099–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fried LP, Tangen CM, Walston J. et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M157 [DOI] [PubMed] [Google Scholar]
- 11.Xue QL.The frailty syndrome: definition and natural history. Clin Geriatr Med 2011; 27: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stenholm S, Ferrucci L, Vahtera J. et al. Natural course of frailty components in people who develop frailty syndrome: evidence from two cohort studies. J Gerontol A Biol Sci Med Sci 2019; 74: 667–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McAdams-DeMarco MA, Ying H, Thomas AG. et al. Frailty inflammatory markers, and waitlist mortality among patients with end-stage renal disease in a prospective cohort study . Transplantation 2018; 102: 1740–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alfaadhel TA, Soroka SD, Kiberd BA. et al. Frailty and mortality in dialysis: evaluation of a clinical frailty scale. Clin J Am Soc Nephrol 2015; 10: 832–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao Y, Dalrymple L, Chertow GM. et al. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med 2012; 172: 1071–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansen KL, Chertow GM, Jin C. et al. Significance of frailty among dialysis patients. J Am Soc Nephrol 2007; 18: 2960–2967 [DOI] [PubMed] [Google Scholar]
- 17.McAdams-DeMarco MA, Law A, King E. et al. Frailty and mortality in kidney transplant recipients. Am J Transplant 2015; 15: 149–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAdams-DeMarco MA, Law A, Salter ML. et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc 2013; 61: 896–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker SR, Brar R, Eng F. et al. Frailty and physical function in chronic kidney disease: the CanFIT study. Can J Kidney Health Dis 2015; 2: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haugen CE, Gross A, Chu NM. et al. Development and validation of an inflammatory-frailty index for kidney transplantation. J Gerontol 2020; 76: 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suresh A, Robinson L, Milliron BJ. et al. Approaches to obesity management in dialysis settings: renal dietitian perspectives. J Renal Nutr 2020; 30: 561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Androga L, Sharma D, Amodu A. et al. Sarcopenia, obesity, and mortality in US adults with and without chronic kidney disease. Kidney Int Rep 2017; 2: 201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delgado C, Doyle JW, Johansen KL.. Association of frailty with body composition among patients on hemodialysis. J Renal Nutr 2013; 23: 356–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JC, Kalantar-Zadeh K, Kopple JD.. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol 2013; 24: 337–351 [DOI] [PubMed] [Google Scholar]
- 25.Malhotra R, Deger SM, Salat H. et al. Sarcopenic obesity definitions by body composition and mortality in the hemodialysis patients. J Renal Nutr 2017; 27: 84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afshinnia F, Wilt TJ, Duval S. et al. Weight loss and proteinuria: systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant 2010; 25: 1173–1183 [DOI] [PubMed] [Google Scholar]
- 27.Segev DL, Massie AB, Schold JD. et al. If you’re not fit, you mustn’t quit: observational studies and weighing the evidence. Am J Transplant 2011; 11: 652–653 [DOI] [PubMed] [Google Scholar]
- 28.Saeed Z, Janda KM, Tucker BM. et al. Personal attitudes toward weight in overweight and obese US hemodialysis patients. J Renal Nutr 2017; 27: 340–345 [DOI] [PubMed] [Google Scholar]
- 29.US Census Bureau. TIGER/Line Shapefiles. 2010. https://www.census.gov/geographies/mapping-files/time-series/geo/tiger-line-file.html
- 30.Glymour MM, Weuve J, Berkman LF. et al. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol 2005; 162: 267–278 [DOI] [PubMed] [Google Scholar]
- 31.Aksoy N.Weight gain after kidney transplant. Exp Clin Transplant 2016; 14: 138–140 [PubMed] [Google Scholar]
- 32.Heng AE, Montaurier C, Cano N. et al. Energy expenditure, spontaneous physical activity and with weight gain in kidney transplant recipients. Clin Nutr 2015; 34: 457–464 [DOI] [PubMed] [Google Scholar]
- 33.Zelle DM, Kok T, Dontje ML. et al. The role of diet and physical activity in post-transplant weight gain after renal transplantation. Clin Transplant 2013; 27: E484–E490 [DOI] [PubMed] [Google Scholar]
- 34.Cashion AK, Hathaway DK, Stanfill A. et al. Pre-transplant predictors of one yr weight gain after kidney transplantation. Clin Transplant 2014; 28: 1271–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Pilsum Rasmussen S, Konel J, Warsame F. et al. Engaging clinicians and patients to assess and improve frailty measurement in adults with end stage renal disease. BMC Nephrol 2018; 19: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAdams-DeMarco MA, Van Pilsum Rasmussen SE, Chu NM. et al. Perceptions and practices regarding frailty in kidney transplantation: results of a national survey. Transplantation 2020; 104: 349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng XS, Myers JN, Chertow GM. et al. Prehabilitation for kidney transplant candidates: is it time? Clin Transplant 2017; 31: e13020. [DOI] [PubMed] [Google Scholar]
- 38.McAdams-DeMarco MA, Ying H, Van Pilsum Rasmussen S. et al. Prehabilitation prior to kidney transplantation: results from a pilot study. Clin Transplant 2018; 33: e13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheshadri A, Johansen KL.. Prehabilitation for the frail patient approaching ESRD. Semin Nephrol 2017; 37: 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkinson TJ, McAdams-DeMarco M, Bennett PN.. Advances in exercise therapy in predialysis chronic kidney disease, hemodialysis, peritoneal dialysis, and kidney transplantation. Curr Opin Nephrol Hypertens 2020; 29: 471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navaneethan SD, Kirwan JP, Arrigain S. et al. Overweight, obesity and intentional weight loss in chronic kidney disease: NHANES 1999–2006. Int J Obes 2012; 36: 1585–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McAdams-DeMarco MA, Ying H, Olorundare I. et al. Individual frailty components and mortality in kidney transplant recipients. Transplantation 2017; 101: 2126–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.