Abstract
Patients with essential tremor (ET) frequently develop concurrent dementia, which is often assumed to represent co-morbid Alzheimer disease (AD). Autopsy studies have identified a spectrum of tau pathologies in ET and tau isoforms have not been examined in ET. We performed immunoblotting using autopsy cerebral cortical tissue from patients with ET (n = 13), progressive supranuclear palsy ([PSP], n = 10), Pick disease ([PiD], n = 2), and AD (n = 7). Total tau in ET samples was similar to that in PSP and PiD but was significantly lower than that in AD. Abnormal tau levels measured using the AT8 phospho-tau specific (S202/T205/S208) monoclonal antibody in ET were similar to those in PSP but were lower than in PiD and AD. In aggregates, tau with 3 microtubule-binding domain repeats (3R) was significantly higher in AD than ET, while tau with 4 repeats (4R) was significantly higher in PSP. Strikingly, the total tau without N-terminal inserts in ET was significantly lower than in PSP, PiD, and AD, but total tau with other N-terminal inserts was not. Monomeric tau with one insert in ET was similar to that in PSP and PiD was lower than in AD. Thus, ET brains exhibit an expression profile of tau protein isoforms that diverges from that of other tauopathies.
Keywords: Alzheimer disease, Essential tremor, Neurodegeneration, Neurofibrillary tangle, Pick disease, Progressive supranuclear palsy, Tau isoform, Tauopathy
INTRODUCTION
Essential tremor (ET) is a progressive neurological disease characterized by kinetic tremor, its hallmark feature, but other types of tremor are often also present (1). The prevalence of ET is estimated to be 4% (age ≥40 years) and exceeding 20% in advanced age (>90 years) (2). Although the etiology of the disease is poorly understood, involvement of susceptibility genes and/or environmental factors has been proposed (3, 4).
An increasing number of independent studies have demonstrated that ET patients have poorer cognitive performance and are more susceptible to the development of progressive and severe cognitive impairment than age-matched controls without ET (5–7). In several population-based studies, both the odds and risk of dementia are elevated in ET cases compared to age-matched controls indicating that ET is a risk factor for dementia (8, 9). The pathophysiology of ET is not fully understood but a cluster of neurodegenerative morphological changes have been identified in the cerebellum, predominantly centered in/around Purkinje cells (10, 11). Building on earlier work linking ET to possible tau dysregulation (12–16), a recent study found greater tau tangle pathology in the neocortex of non-demented ET patients compared to non-demented controls, implying that ET might cause dysregulation of tau proteostasis (17).
Tau is a highly abundant microtubule (MT)-associated protein found in neuronal and glial cells (18). One of its best described functions is to facilitate MT assembly (19). In the human brain, tau predominantly exists as 6 isoforms derived from alternative pre-mRNA splicing of exons 2, 3, and 10 (20). Alternative splicing of exons 2 and 3 results in isoforms with 0, 1, or 2 inserts of the exon-derived sequences in the N-terminal region (0N, 1N, 2N), while alternative splicing of exon 10 yields tau with 3 or 4 MT-binding repeats in the C-terminal MT-binding domain (3R, 4R). In the adult human brain, the overall ratio of 3R and 4R tau isoform levels is roughly equal (21), although this ratio can change in different brain regions and under various pathological conditions (22). Autosomal dominant mutations in splice sites near exon 10, usually causing increased inclusion of this exon and an increase in 4R isoforms, are causal in a subset of patients with frontotemporal lobar degeneration (23). The 17q21.31 MAPT H1 haplotype, a large structural genomic inversion and common risk factor for sporadic tauopathy may also be associated with increased expression of 4R tau isoforms (24), but this is controversial (25). The functional consequences of the resultant imbalance in tau isoforms that lead to neurodegeneration remain unclear but 4R tau is more prone to aggregate and may be more toxic than 3R tau (26). How tau isoform imbalances may induce neurodegeneration is unclear. 4R tau isoforms show greater affinity for MTs and increased MT assembly compared to 3R tau in vitro (27, 28). Although the functions of the N-terminal inserts are not well understood, 0N, 1N, and 2N isoforms exhibit distinct subcellular localization and there are also regional differences in the expression of these isoforms in the brain (29, 30). Co-immunoprecipitation studies using mouse brain lysate demonstrate that 0N, 1N, and 2N isoforms differentially interact with distinct sets of proteins (31).
Given the central role of imbalances in tau isoforms in disease mechanisms, their relative abundance in brain tissue serves as a valuable diagnostic feature, marking involvement of specific tauopathic processes (32). Alzheimer disease (AD), chronic traumatic encephalopathy, and primary age-related tauopathy (PART) are characterized by mixed accumulation of both 3R and 4R tau (33–35). 4R predominant tauopathies include progressive supranuclear palsy (PSP), corticobasal degeneration, and argyrophilc grain disease (36–40). Pick disease (PiD), while very rare, is the only 3R predominant tauopathy and its existence raises the possibility that a selective toxicity of 4R is not the only pathological trigger, and that imbalances in tau isoforms alone are pathogenic. Importantly, tau isoforms have not been directly measured in ET, which is associated with tau accumulation and dementia.
Here, we conducted an exploratory study, comparing the tau isoform expression profile in a cohort of ET patients compared to patients with other tauopathies to ascertain the extent to which the cortical tau pathology differs from these other diseases. This was performed by measuring the levels of all 6 brain enriched tau isoforms, including monomeric and oligomerized forms, from postmortem brain tissue from a cohort of 13 ET postmortem brains and then comparing the results to those from patients with AD and 2 amyloid-independent primary tauopathies, PSP and PiD.
MATERIALS AND METHODS
Subjects and Tissue Samples
Fresh frozen postmortem ET brain tissue (temporal cortex, Brodmann area 37) was obtained from the Essential Tremor Centralized Brain Repository at Columbia University (New York, NY). These donors were enrolled prospectively and followed with motor and cognitive assessments, with 10 of 13 also enrolled in an ongoing longitudinal, prospective study of cognitive function in ET (Clinical Pathological Study of Cognitive Impairment in Essential Tremor, NINDS R01NS086736) which has been described elsewhere (41, 42). ET diagnoses were assigned by a senior movement disorder neurologist (E.D.L.) utilizing 3 sequential methods (43). Briefly, the clinical diagnosis of ET was initially assigned by treating neurologists, and secondly, confirmed by E.D.L. using questionnaires, review of medical records, and review of Archimedes spirals. Third, a detailed, videotaped, neurological examination was performed, action tremor was rated, and a total tremor score assigned (range: 0–36 [maximum]). Combined with the questionnaire data, the final diagnosis of each ET case was re-examined, using previously published diagnostic criteria, which have been shown to be both reliable and valid (43). None of the ET cases had a history of traumatic brain injury, exposure to medications with associated cerebellar toxicity (e.g. chemotherapeutic agents) or heavy ethanol use (43). The next-of-kin provided written consent for participation and brain donation. Institutional Review Board approval for collection of clinical data was approved at Yale University and Columbia University Medical Center (CUMC). Diagnoses of mild cognitive impairment (MCI) and dementia among the ET cases were assigned by review of cognitive assessment data by a neuropsychologist (S.C.).
For ET cases, the neuropathological workup was performed at the Essential Tremor Centralized Brain Repository. Frozen tissues (dorsolateral prefrontal cortex) from cases with PSP and PiD were obtained from University of California San Diego, University of Pittsburgh, Emory University, and University of California Irvine. AD cases were obtained from the NeuroBioBank (University of Maryland). The cases represented a convenience sample with available tissue; ET cases were further selected based on the presence of sufficient data on cognitive status. All methods were carried out in accordance with the relevant guidelines, laws and regulations. AD neuropathological assessments were performed using the current consensus guidelines (44–47).
Immunohistochemistry
Portions of frozen tissue were thawed and fixed in 10% neutral-buffered formalin, paraffin embedded, cut at 4–5 µm, placed on charged slides, and baked overnight at 70°C. Immunohistochemistry (IHC) was performed on a Ventana Benchmark XT (Roche Diagnostics, Indianapolis, IN). Antigen retrieval was done using CC1 buffer (Tris/Borate/EDTA buffer, pH 8.0–8.5; Roche Diagnostics, Indianapolis, IN) for 1 hour followed by primary antibody incubation for 30 min (clone AT8, 1:1000, Invitrogen/Thermo Fisher Scientific, Waltham, MA). Slides were imaged using an Olympus BX40 brightfield microscope with an Olympus DP27 camera and CellSens software (Olympus, Center Valley, PA).
Biochemical Studies
Western blotting was performed as previously described (48). Briefly, fresh-frozen brain tissue was homogenized with a glass-Teflon homogenizer at 500 rpm in 10 volumes (wt/vol) of ice-cold tissue homogenization buffer containing 20 mM Tris pH 7.4, 250 mM sucrose, 1 mM EDTA, 1 mM EGTA, and Halt protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific). For each sample, 30 μg of protein were boiled in Laemmili sample buffer (Bio-Rad, Hercules, CA) for 5 minutes, run with tau protein ladder (rPeptide, Watkinsville, GA) on 10% PROTEAN TGX Precast Gels (Bio-Rad), blotted to nitrocellulose membranes, and stained with anti-Tau (clone HT7, 1:3000, Invitrogen/Thermo Fisher Scientific), anti-pTau (clone AT8, 1:1000, Invitrogen/Thermo Fisher Scientific), anti-3R tau (clone 8E6/C11, 1:2000, Sigma-Aldrich, St. Louis, MO), anti-4R tau (clone 1E1/A6, 1:500, Sigma-Aldrich), anti-0N tau (clone EPR21726, 1:1000, Abcam, Cambridge, UK), anti-1N tau (clone 4H5.B9, 1:1000, Biolegend, San Diego, CA), anti-2N tau (clone 71C11, 1:1000, Biolegend), and anti-GAPDH (clone 6C5, 1:20000, Abcam) (Table 1). HRP-labeled secondary anti-mouse or rabbit antibody (1:20000, Vector Labs, Burlingame, CA) was detected by SuperSignal West Femto Maximum Sensitivity Substrate or Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific). Chemiluminescence was measured in an LAS-4000 Intelligent Dark Box imager (Fuji Film, Valhalla, NY), and relative optical densities (A.U., arbitrary units) were determined with AlphaEaseFC software, version 4.0.1 (Alpha Innotech, San Leandro, CA), normalized to total protein loaded (GAPDH). Signals above 75 kDa were measured as oligomers, from 45 kDa to 75 kDa as monomers, and 45 kDa and above or combined monomeric and oligomeric as total as previously described (49).
TABLE 1.
Antisera
| Dilution |
||||
|---|---|---|---|---|
| Antigen | Clone | Source | WB | IHC |
| Total tau | HT7 | Invitrogen | 1:3000 | — |
| p-tau | AT8 | Invitrogen | 1:1000 | 1:1000 |
| 3R tau | E6/C11 | Sigma-Aldrich | 1:2000 | — |
| 4R tau | 1E1/A6 | Sigma-Aldrich | 1:500 | — |
| 0N tau | EPR21726 | Abcam | 1:1000 | — |
| 1N tau | 4H5.B9 | Biolegend | 1:1000 | — |
| 2N tau | 71C11 | Biolegend | 1:1000 | — |
| GAPDH | 6C5 | Abcam | 1:20000 | — |
IHC, immunohistochemistry; WB, Western blot.
Statistics and Data Analysis
Immunohistochemical AT8-positive p-tau burden was assessed in a semiquantitative manner. For mild cases, rare positive was observed on less than 10% of the stained tissue fragments. For tissues categorized as moderate, the preparation had significantly more than mild cases but did not exceed 50%. Severe cases had staining on a majority of the fragments (>50%). For biochemical analyses, the 13 ET cases were divided into the following 2 groups based on biochemical AT8 levels, ET (high AT8), and ET (low AT8). A cutoff value of low AT8 levels obtained from immunoblots was set to 10 since the highest value was 9 in the cases with negative p-tau burden assessed by IHC. Given this heterogeneity of the presence of p-tau aggregates among our ET samples, and the exploratory nature of this study, we analyzed these 2 groups separately and additionally as a combined group. Densitometry data were compared using one-way ANOVA with post-hoc Tukey’s HSD test for pair-wise comparisons of groups. The vast majority of data elements were normally distributed; hence, a parametric approach was used for data analysis. Using correlation coefficients, we also assessed whether age was associated with values of each tau isoform variable. Statistical analysis and graph production were done in GraphPad Prism (GraphPad Software, San Diego, CA).
RESULTS
In this study, we compared the tau pathology, including burden of abnormal AT8-positive phosphorylated tau and isoform ratios, in the brains from 13 ET patients with 10 PSP, 2 PiD, and 7 AD (Table 2). These ET cases were a convenience sample derived from the Essential Tremor Centralized Brain Repository. The average and median age of death for the ET cases was 90.2 and 90 years old, respectively (range: 83–99) with 2 males and 11 females. Six were demented, 4 had MCI, and 3 were cognitively normal (Table 3). Following comprehensive histopathological assessments for neurodegenerative disease, 8 had AD neuropathologic change as the primary neuropathological dementia diagnosis, 2 had hippocampal sclerosis, 1 had PART, 1 had mild Lewy body pathology (mild and involving the dorsal motor nucleus of the vagus and substantia nigra, but sparing the locus coeruleus and therefore not following a neuropathological stage of Parkinson disease), and the final had corticobasal degeneration. The latter 2 cases had ET for 23 and 58 years, respectively, and neither exhibited parkinsonism during life. These findings are consistent with the previous findings of additional heterogeneous neurodegenerative pathologies in elderly ET cases.
TABLE 2.
Patient Data
| ET | PSP | PiD | AD | |
|---|---|---|---|---|
| n | 13 | 10 | 2 | 8 |
| Age of death (years) | ||||
| 50–59 | 0 | 1 | 0 | 0 |
| 60–69 | 0 | 3 | 1 | 0 |
| 70–79 | 0 | 1 | 1 | 0 |
| 80–89 | 6 | 4 | 0 | 7 |
| 90–99 | 7 | 0 | 0 | 0 |
| Unreported | 0 | 1 | 0 | 0 |
| Average | 90.2 | 72.8 | 71 | 82.6 |
| Median | 90 | 78 | 71 | 81 |
| Sex (male/female) | 2/11 | 3/7 | 1/1 | 6/1 |
AD, Alzheimer disease; ET, essential tremor; PiD, Pick disease; PSP, progressive supranuclear palsy.
TABLE 3.
Tau and Other Pathologies in Essential Tremor
| ADC |
p-Tau (AT8) Burden* |
|||||||
|---|---|---|---|---|---|---|---|---|
| Age | Sex | Congnitive Status | 1° NP Dx | Thal Phase | Braak NFT | CERAD | IHC | WB (AU) |
| 99 | F | Dementia | HPS | III | V–VI | 0 | ++ | 12.6 |
| 89 | F | Dementia | ADC | IV–V | V–VI | +++ | +++ | 45.6 |
| 90 | F | Dementia | ADC | III | V–VI | ++ | ++ | 18.7 |
| 90 | F | Dementia | ADC | IV–V | V–VI | ++ | +++ | 35.1 |
| 85 | F | Dementia | ADC | IV–V | V–VI | +++ | ++ | 8.0 |
| 91 | F | Dementia | HPS | III | III–IV | +++ | + | 8.2 |
| 94 | F | MCI | ADC | III | III–IV | ++ | +++ | 11.8 |
| 94 | F | MCI | CBD | 0 | V–VI | 0 | ++ | 8.6 |
| 87 | M | MCI | LBD | 0 | III–IV | 0 | 0 | 7.0 |
| 94 | M | MCI | PART | I–II | III–IV | 0 | 0 | 7.4 |
| 83 | F | Normal | ADC | III | V–VI | ++ | + | 7.9 |
| 89 | F | Normal | ADC | III | III–IV | + | 0 | 8.5 |
| 88 | F | Normal | ADC | III | III–IV | 0 | 0 | 9.0 |
Temporal cortex (Brodmann area 37).
ADC, Alzheimer disease neuropathologic change; AU, arbitrary units; CBD, corticobasal degeneration; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease neuritic plaque burden; HPS, hippocampal sclerosis; IHC, immunohistochemistry; LBD, Lewy body disease; MCI, mild cognitive impairment; NFT, neurofibrillary tangle; 1° NP, Dx primary neuropathological diagnosis; PART, primary age-related tauopathy; WB, Western blot.
0, none; +, mild; ++, moderate; +++, severe.
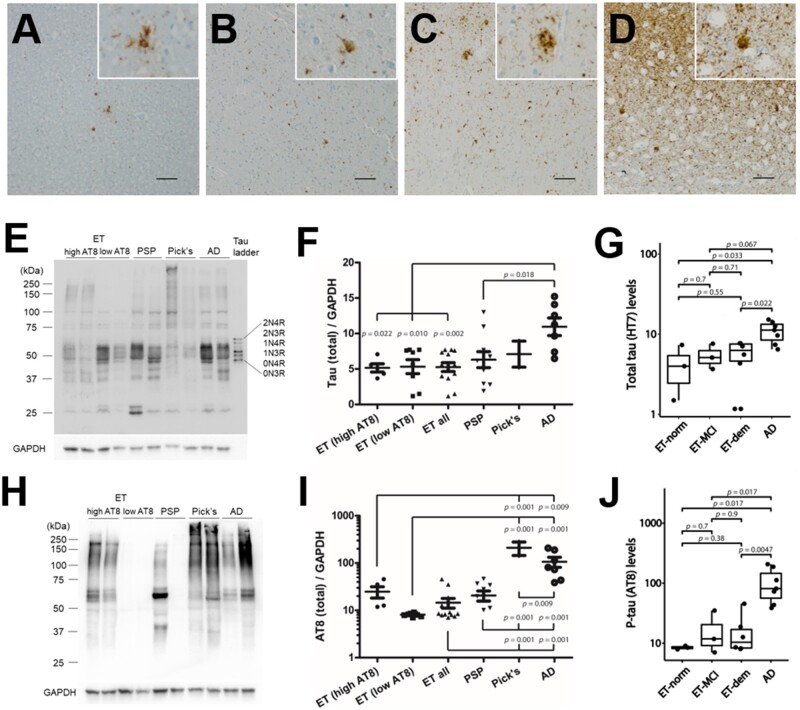
We started by performing an immunohistochemical study to assess our frozen ET tissue samples, which were from the contralateral hemisphere to that which was previously evaluated histologically, to confirm and document the presence of the AT8 phosphoepitope (p-tau) and measure their burden. By immunohistochemistry, we observed a varying burden of p-tau, with some ET cases completely devoid of p-tau pathology or with only scattered thread pathology (Fig. 1A, B; Table 3). These findings correlated well with the neuropathological assessments performed on fixed tissues derived from the contralateral hemisphere. Other ET cases had more severe p-tau burden, with robust neuronal and glial accumulation (Fig. 1C, D; Table 3).
FIGURE 1.
Biochemical assessment of tau pathology in essential tremor compared to Alzheimer disease and sporadic tauopathies. (A–D) Immunohistochemical studies were performed in parallel samples derived from the fresh frozen temporal cortex tissue block (Brodmann area 37) used for biochemical analysis to assess the presence of tau pathology using antisera recognizing tau phosphorylated at Ser202, Thr205, and Ser208 (p-tau, AT8). This analysis revealed a spectrum from low tau pathology consisting of minimal to scattered thread pathology (A, B), to high levels of tau pathology, consisting of marked neuronal pathology including neurons and dystrophic neurites (C) and/or glial tau pathology (D). Immunoblot using antisera targeting total tau (E–G) or p-tau (H–J) on total protein lysates. ET cases were stratified into high and low p-tau (AT8) groups from biochemical assessments (F, I) and cognitive status (G, J). Measurements were normalized to GAPDH. Two data points of PSP cases in (I) are outside the axis limits (the values are both 0). Scale bar = 100 µm.
Next, we used quantitative immunoblot analysis on the same samples to assess the total and pathological tau burden. The ET cases, including those with dementia, had significantly lower total tau than the AD cases (Fig. 1E, F). Although not statistically significant, total tau levels were numerically higher in ET cases that were cognitively normal versus those with MCI and dementia (Fig. 1G). Yet, the ET normal and dementia cases did have significantly less total tau than the AD group. With respect to p-tau, we observed a similar variability to that which we saw with our immunohistochemical analysis, with negligible to low levels in some of the ET cases and high in others (Fig. 1H, I; Table 3). The highest levels of p-tau were seen in AD and PiD. The levels of total tau and p-tau were more variable in the ET and PSP groups, which were not significantly different. The levels of p-tau in ET cases with MCI and dementia were higher than ET with normal cognitive status (Fig. 1J). Given this heterogeneity in the presence of p-tau aggregates among our ET samples, the 13 ET cases were dichotomized based on biochemical AT8 levels, ET (high AT8), and ET (low AT8). The groups were analyzed both separately and combined. Biochemical AT8 levels were similar to the semiquantitative AT8-positive assessments by immunohistochemistry (Table 3).
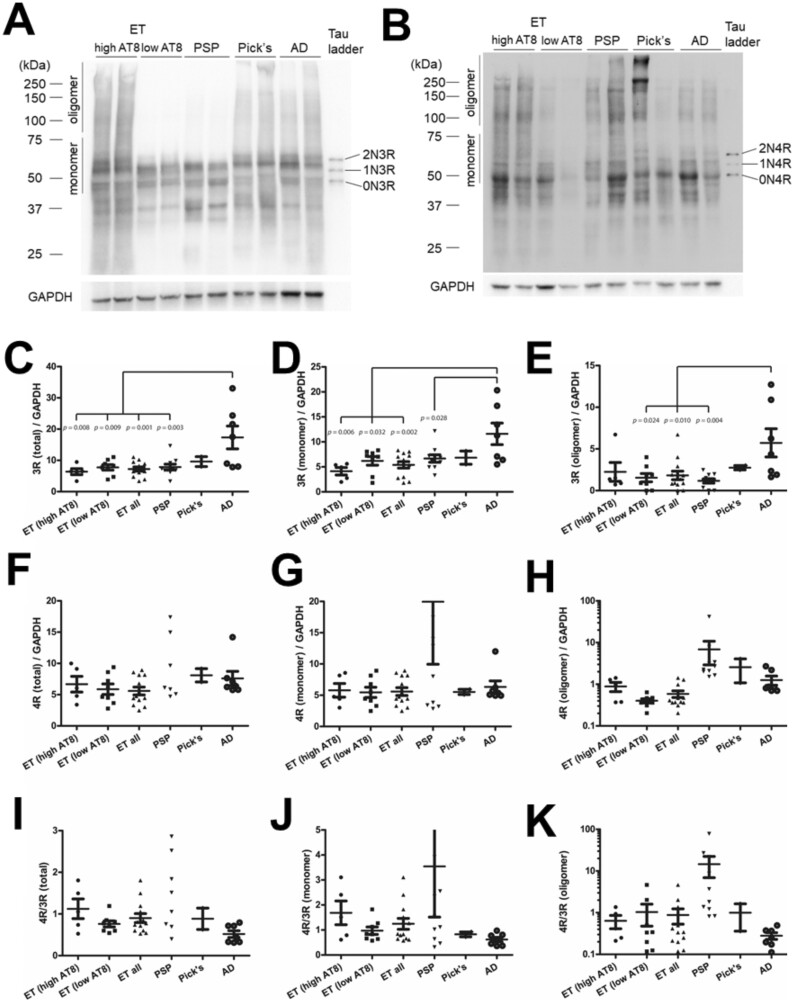
Burden of AT8-reactive phosphorylated tau is a robust and reproducible marker of pathology, but it is less useful for diagnostic subclassification on its own. Therefore, we assessed differences in tau isoforms derived from alterative pre-mRNA splicing of exon 10, which reflects divergent underlying pathology, by performing quantitative immunoblots using isoform-specific antisera that recognize 3R or 4R isoforms (Fig. 2A, B). In each sample, we assessed the total levels of the isoforms as well as monomeric and high molecular weight assemblies over 75 kDa (“oligomers”). Total, monomeric, and oligomeric 3R levels in ET were not different from those in PSP and PiD, but significantly lower in AD (Fig. 2C–E). Total, monomeric, and oligomeric 4R levels in ET cases were not statistically different from PSP, PiD, or AD (Fig. 2F–H). Total 4R to 3R ratios in ET were near 1 regardless of biochemical AT8 levels as is observed in mixed 3R/4R tauopathies (Fig. 2I).
FIGURE 2.
Biochemical assessment of 3 and 4 repeat tau isoforms in essential tremor compared to AD and sporadic tauopathies. Representative immunoblots using isoform specific antisera targeting 3R (A) or 4R (B) on total protein lysates. The relative levels of 3R (C–E) and 4R (F–H) as well as the ratios of 4R : 3R (I–K) normalized to GAPDH are shown. Three data points of PSP cases in (F), 2 in (G), 2 in (I), and 1 in (J) are outside the axis limits (the values are 20.6, 34.6, 148.9 in [F]; 26.7, 107.1 in [G]; 5.0, 21.6 in [I]; 21.5 in [J]). PSP in (F), 26.7 ± 14.6 (SEM); PSP in (I), 3.8 ± 2.1 (SEM).
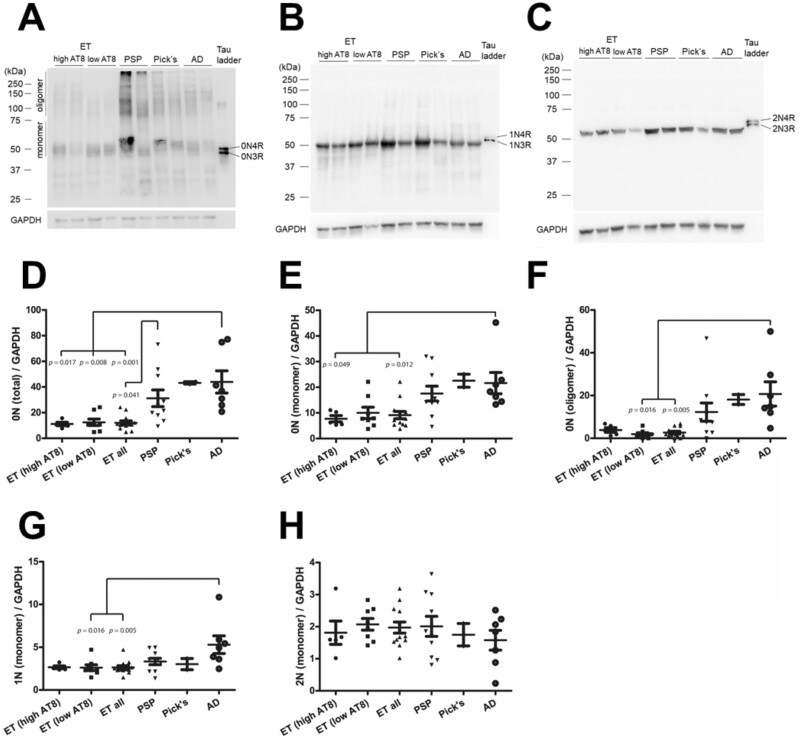
Finally, we assessed differences in N-terminal exons of tau (Fig. 3A–C). Total 0N levels in ET, regardless of AT8 level, were significantly lower than AD, and lower than PSP in the combined ET group (Fig. 3D–F). 0N monomers were significantly lower in the high AT8 and combined ET groups, but the low AT8 ET group was trending down. Conversely, 0N oligomers were significantly lower in the low AT8 and combined ET groups, and the high AT8 ET group was also similarly trending down. Monomeric 1N levels in ET were similar to PSP and PiD but significantly lower than AD when comparing the low AT8 and combined groups (Fig. 3G). Monomeric 2N levels in ET were not significantly different from PSP, PiD, or AD (Fig. 3H). Oligomeric 1N and 2N tau levels were very low or below the threshold of detection and were not quantified.
FIGURE 3.
Biochemical assessment of N-terminal tau isoforms in essential tremor compared to AD and sporadic tauopathies. Representative immunoblots using isoform-specific antisera targeting 0N (A), 1N (B), and 2N (C) on total protein lysates. The relative levels of tau species are shown normalized to GAPDH (D–H).
DISCUSSION
ET is increasingly viewed as a degenerative movement disorder (50–61) that might be variably associated with dementia (7–9). The mechanisms underlying this association are not clear. ET patients may have unique vulnerabilities that differentiate them from other patient populations as ET involves distinct brain systems. Here, we asked whether tau proteostasis in ET differs from other tauopathies by biochemically measuring abnormal cortical p-tau burden and tau isoform ratios compared to other primary and secondary tauopathies. We found that at autopsy, the neuropathological degenerative changes with respect to tau pathology in ET patients are variable and diverge considerably from those with AD. Specifically, the total overall p-tau burden in ET was significantly lower than AD and PiD, albeit these levels were highly variable which is similar to what we observed in PSP. The most striking finding, however, was in the N-terminal isoforms, with markedly lower levels of 0N tau in ET compared to all other groups. Together, these findings provide further evidence that the mechanisms underlying cognitive impairment in ET are divergent from AD and other neurodegenerative disorders.
The ratio of 4R to 3R tau isoforms that result from alternative pre-mRNA splicing of exon 10 are critical diagnostic markers of tauopathy subtypes. One observation was that ET and the primary tauopathies had considerably less 3R tau than AD patients. While AD is generally not thought to shift away from 1 in the tau 4R:3R isoform ratios, this has been previously observed by others (33, 62). We also found that ET and the other disease subtypes did not demonstrate the increase in 4R tau that is observed in PSP. While PSP had a marked elevation of 4R oligomers and monomers, there was a high degree of variability that overlapped with ET.
We further examined differences in tau isoform structure in the N-terminal region, which arises from alternative pre-mRNA splicing of exons 2 and 3. These isoforms, which are of limited use diagnostically, are less well studied. Remarkably, differences in the levels of N-terminal inserts showed the most striking difference. In ET, there were markedly lower levels of 0N tau isoforms compared to both PSP and AD. We also detected significantly lower levels of 1N monomers compared to AD, but not PSP. The significance of this finding remains unclear. N-terminal exons are outside the MT binding domain and have different binding partners, indicating that it has different function (63–65). The N-terminal region contains intrinsically disordered regions with evolutionary pressure that interact with proteins that play a role in membrane organization (66). The human and murine N-terminus also has important sequence divergence that might underly species-specific differences (67). N-terminal truncation of tau is hypothesized to be a pathogenic event in AD that drives toxicity (68, 69). 0N tau is the fetal isoform and its increase may represent reactivation of developmental pathways that are absent in ET (48). Further efforts to better understand the functional significance of the N-terminal region of tau in neurodegeneration are warranted.
This exploratory study had several notable limitations. First, the samples studied represent a small set of convenience samples from different sources and only include disease-associated tissue. Non-disease associated sample controls are not included, which limits conclusions from this study. It will be critical to conduct a larger study that fully incorporates a broader range of potential covariates. Nevertheless, while we recognize that several of our diagnostic groups differed by age, yet with rare exceptions, age was not associated with tau isoform data, and therefore could not have served as a confounding factor. The immunoblots and immunohistochemistry both rely on specificity of antisera and certain epitopes may be missed due to specific protein conformation and structures that influence antibody binding (70). Also, we focused on the temporal neocortex, an important part of the brain with vulnerability in multiple diseases, but further studies across multiple brain regions are essential to put these findings into the full context or regional vulnerability to tauopathy.
In conclusion, we found that ET patients might exhibit a unique expression signature of tau isoforms that is different from other tauopathies such as PSP, PiD, and AD. Especially, low total 0N tau levels, including oligomers, may be useful for separating ET from the other tauopathies. Levels of AT8-reactive phospho-tau (or corresponding severity of tau tangle pathology), which were not as high as in PiD or AD, does not seem to affect overall tau isoform expression patterns in ET. Additionally, nearly equal levels of 3R and 4R tau isoforms were found in ET, suggesting that ET is more similar to the 3R/4R tauopathies, but there is a high degree of variability that requires further investigation. Previous studies showed that non-demented ET patients had a higher Braak stage and higher number of NFT-positive neurons in the neocortex but not in the medial temporal lobe compared to age- and CERAD score-matched controls, suggesting a predisposition to tau pathology in the ET (15, 17). Taken together, the current findings are further evidence that clinically relevant tau pathology occurs in ET which represents a cerebral tauopathy with an increased susceptibility to cognitive decline.
ACKNOWLEDGMENTS
We are deeply grateful to the patients and staff of the contributing biorepositories, including the NIH NeuroBioBank at the University of Maryland, Columbia University (the Essential Tremor Centralized Brain Repository), the University of California San Diego, University of Pittsburgh, Emory University, and the University of California Irvine.
The authors report no conflicts of interest.
This research was funded by National Institutes of Health (R01AG054008 and R01NS095252 [JFC], R01NS086736 [SC/EDL], F32AG056098 [KF], P30 AG062429 and P50 AG005131 [University of California San Diego], P50 AG005133 [University of Pittsburg], P30 NS055077 and P50 AG025688 [Emory University], P50 AG016573 [University of California Irvine]), Alzheimer’s Association (NIRG-15-363188 [JFC]), the Tau Consortium, and an Alexander Saint-Amand Fellowship (JFC).
REFERENCES
- 1.Louis ED.Tremor. Continuum (Minneap Minn) 2019;25:959–75 [DOI] [PubMed] [Google Scholar]
- 2.Louis ED, Ferreira JJ.. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord 2010;25:534–41 [DOI] [PubMed] [Google Scholar]
- 3.Clark LN, Louis ED.. Challenges in essential tremor genetics. Rev Neurol (Paris) 2015;171:466–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis ED.Essential tremor then and now: How views of the most common tremor diathesis have changed over time. Parkinsonism Relat Disord 2018;46 Suppl 1:S70–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benito-Leon J, Louis ED, Bermejo-Pareja F; on behalf of the Neurological Disorders in Central Spain (NEDICES) Study Group. Population-based case-control study of cognitive function in essential tremor. Neurology 2006;66:69–74 [DOI] [PubMed] [Google Scholar]
- 6.Louis ED, Benito-Leon J, Vega-Quiroga S, et al. ; Neurological Disorders in Central Spain (NEDICES) Study Group. Faster rate of cognitive decline in essential tremor cases than controls: A prospective study. Eur J Neurol 2010;17:1291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis ED, Joyce JL, Cosentino S.. Mind the gaps: What we don't know about cognitive impairment in essential tremor. Parkinsonism Relat Disord 2019;63:10–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bermejo-Pareja F, Louis ED, Benito-Leon J; Neurological Disorders in Central Spain (NEDICES) Study Group. Risk of incident dementia in essential tremor: A population-based study. Mov Disord 2007;22:1573–80 [DOI] [PubMed] [Google Scholar]
- 9.Thawani SP, Schupf N, Louis ED.. Essential tremor is associated with dementia: Prospective population-based study in New York. Neurology 2009;73:621–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louis ED.Essential tremor: A common disorder of Purkinje neurons? Neuroscientist 2016;22:108–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis ED, Kerridge CA, Chatterjee D, et al. Contextualizing the pathology in the essential tremor cerebellar cortex: A patholog-omics approach. Acta Neuropathol 2019;138:859–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vilarino-Guell C, Soto-Ortolaza AI, Rajput A, et al. MAPT H1 haplotype is a risk factor for essential tremor and multiple system atrophy. (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't). Neurology 2011;76:670–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Martin E, Martinez C, Alonso-Navarro H, et al. H1-MAPT and the risk for familial essential tremor. PLoS One 2012;7:e41581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louis ED, Babij R, Ma K, et al. Essential tremor followed by progressive supranuclear palsy: Postmortem reports of 11 patients. J Neuropathol Exp Neurol 2013;72:8–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan JJ, Lee M, Honig LS, et al. Alzheimer's-related changes in non-demented essential tremor patients vs. controls: Links between tau and tremor? Parkinsonism Relat Disord 2014;20:655–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark LN, Liu X, Parmalee NL, et al. The microtubule associated protein tau H1 haplotype and risk of essential tremor. Eur J Neurol 2014;21:1044–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrell K, Cosentino S, Iida MA, et al. Quantitative assessment of pathological tau burden in essential tremor: A postmortem study. J Neuropathol Exp Neurol 2019;78:31–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo T, Noble W, Hanger DP.. Roles of tau protein in health and disease. Acta Neuropathol 2017;133:665–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiang L, Sun X, Austin TO, et al. Tau does not stabilize axonal microtubules but rather enables them to have long labile domains. Curr Biol 2018;28:2181–9 e4 [DOI] [PubMed] [Google Scholar]
- 20.Buee L, Bussiere T, Buee-Scherrer V, et al. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 2000;33:95–130 [DOI] [PubMed] [Google Scholar]
- 21.Arendt T, Stieler JT, Holzer M.. Tau and tauopathies. Brain Res Bull 2016;126:238–92 [DOI] [PubMed] [Google Scholar]
- 22.Majounie E, Cross W, Newsway V, et al. Variation in tau isoform expression in different brain regions and disease states. Neurobiol Aging 2013;34:1922 e7–e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niblock M, Gallo JM.. Tau alternative splicing in familial and sporadic tauopathies. Biochem Soc Trans 2012;40:677–80 [DOI] [PubMed] [Google Scholar]
- 24.Myers AJ, Pittman AM, Zhao AS, et al. The MAPT H1c risk haplotype is associated with increased expression of tau and especially of 4 repeat containing transcripts. (Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov't). Neurobiol Dis 2007;25:561–70 [DOI] [PubMed] [Google Scholar]
- 25.Hayesmoore JB, Bray NJ, Cross WC, et al. The effect of age and the H1c MAPT haplotype on MAPT expression in human brain. Neurobiol Aging 2009;30:1652–6 [DOI] [PubMed] [Google Scholar]
- 26.Schoch KM, DeVos SL, Miller RL, et al. Increased 4R-tau induces pathological changes in a human-tau mouse model. Neuron 2016;90:941–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goode BL, Chau M, Denis PE, et al. Structural and functional differences between 3-repeat and 4-repeat tau isoforms. Implications for normal tau function and the onset of neurodegenetative disease. J Biol Chem 2000;275:38182–9 [DOI] [PubMed] [Google Scholar]
- 28.Lu M, Kosik KS.. Competition for microtubule-binding with dual expression of tau missense and splice isoforms. Mol Biol Cell 2001;12:171–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu C, Gotz J.. Profiling murine tau with 0N, 1N and 2N isoform-specific antibodies in brain and peripheral organs reveals distinct subcellular localization, with the 1N isoform being enriched in the nucleus. PLoS One 2013;8:e84849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trabzuni D, Wray S, Vandrovcova J, et al. MAPT expression and splicing is differentially regulated by brain region: Relation to genotype and implication for tauopathies. Hum Mol Genet 2012;21:4094–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu C, Song X, Nisbet R, et al. Co-immunoprecipitation with tau isoform-specific antibodies reveals distinct protein interactions and highlights a putative role for 2N tau in disease. J Biol Chem 2016;291:8173–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovacs GG.Invited review: Neuropathology of tauopathies: Principles and practice. Neuropathol Appl Neurobiol 2015;41:3–23 [DOI] [PubMed] [Google Scholar]
- 33.Espinoza M, de Silva R, Dickson DW, et al. Differential incorporation of tau isoforms in Alzheimer's disease. J Alzheimers Dis 2008;14:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol 2014;128:755–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt ML, Zhukareva V, Newell KL, et al. Tau isoform profile and phosphorylation state in dementia pugilistica recapitulate Alzheimer's disease. Acta Neuropathol 2001;101:518–24 [DOI] [PubMed] [Google Scholar]
- 36.Flament S, Delacourte A, Verny M, et al. Abnormal Tau proteins in progressive supranuclear palsy. Similarities and differences with the neurofibrillary degeneration of the Alzheimer type. Acta Neuropathol 1991;81:591–6 [DOI] [PubMed] [Google Scholar]
- 37.Vermersch P, Robitaille Y, Bernier L, et al. Biochemical mapping of neurofibrillary degeneration in a case of progressive supranuclear palsy: Evidence for general cortical involvement. Acta Neuropathol 1994;87:572–7 [DOI] [PubMed] [Google Scholar]
- 38.Buee Scherrer V, Hof PR, Buee L, et al. Hyperphosphorylated tau proteins differentiate corticobasal degeneration and Pick's disease. Acta Neuropathol 1996;91:351–9 [DOI] [PubMed] [Google Scholar]
- 39.Togo T, Sahara N, Yen SH, et al. Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol 2002;61:547–56 [DOI] [PubMed] [Google Scholar]
- 40.Buee L, Delacourte A.. Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick's disease. Brain Pathol 1999;9:681–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins K, Rohl B, Morgan S, et al. Mild cognitive impairment subtypes in a cohort of elderly essential tremor cases. J Int Neuropsychol Soc 2017;23:390–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rohl B, Collins K, Morgan S, et al. Daytime sleepiness and nighttime sleep quality across the full spectrum of cognitive presentations in essential tremor. J Neurol Sci 2016;371:24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Babij R, Lee M, Cortes E, et al. Purkinje cell axonal anatomy: Quantifying morphometric changes in essential tremor versus control brains. Brain 2013;136:3051–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montine TJ, Phelps CH, Beach TG, et al. ; Alzheimer’s Association. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: A practical approach. Acta Neuropathol 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirra SS.The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: A commentary. Neurobiol Aging 1997;18:S91–4 [DOI] [PubMed] [Google Scholar]
- 46.Braak H, Braak E, Bohl J.. Staging of Alzheimer-related cortical destruction. Eur Neurol 1993;33:403–8 [DOI] [PubMed] [Google Scholar]
- 47.Thal DR, Rub U, Orantes M, et al. Phases of A-deposition in the human brain and its relevance for the development of AD. Neurology 2002;58:1791–800 [DOI] [PubMed] [Google Scholar]
- 48.Hefti MM, Farrell K, Kim S, et al. High-resolution temporal and regional mapping of MAPT expression and splicing in human brain development. PLoS One 2018;13:e0195771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cherry JD, Kim SH, Stein TD, et al. Evolution of neuronal and glial tau isoforms in chronic traumatic encephalopathy. Brain Pathol 2020;30:913–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altunisik E, Baykan AH.. Comparison of the olfactory bulb volume and the olfactory tract length between patients diagnosed with essential tremor and healthy controls: Findings in favor of neurodegeneration. Cureus 2019;11:e5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Axelrad JE, Louis ED, Honig LS, et al. Reduced Purkinje cell number in essential tremor: A postmortem study. Arch Neurol 2008;65:101–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bagepally BS, Bhatt MD, Chandran V, et al. Decrease in cerebral and cerebellar gray matter in essential tremor: A voxel-based morphometric analysis under 3T MRI. J Neuroimaging 2012;22:275–8 [DOI] [PubMed] [Google Scholar]
- 53.Benito-Leon J, Alvarez-Linera J, Hernandez-Tamames JA, et al. Brain structural changes in essential tremor: Voxel-based morphometry at 3-Tesla. J Neurol Sci 2009;287:138–42 [DOI] [PubMed] [Google Scholar]
- 54.Caligiuri ME, Arabia G, Barbagallo G, et al. Structural connectivity differences in essential tremor with and without resting tremor. J Neurol 2017;264:1865–74 [DOI] [PubMed] [Google Scholar]
- 55.Louis ED, Faust PL.. Essential tremor pathology: Neurodegeneration and reorganization of neuronal connections. Nat Rev Neurol 2020;16:69–83 [DOI] [PubMed] [Google Scholar]
- 56.Louis ED, Faust PL, Vonsattel JP, et al. Older onset essential tremor: More rapid progression and more degenerative pathology. Mov Disord 2009;24:1606–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 2007;130:3297–307 [DOI] [PubMed] [Google Scholar]
- 58.Nicoletti G, Manners D, Novellino F, et al. Diffusion tensor MRI changes in cerebellar structures of patients with familial essential tremor. Neurology 2010;74:988–94 [DOI] [PubMed] [Google Scholar]
- 59.Novellino F, Nicoletti G, Cherubini A, et al. Cerebellar involvement in essential tremor with and without resting tremor: A Diffusion Tensor Imaging study. Parkinsonism Relat Disord 2016;27:61–6 [DOI] [PubMed] [Google Scholar]
- 60.Prasad S, Shah A, Bhalsing KS, et al. Clinical correlates of abnormal subcortical volumes in essential tremor. J Neural Transm (Vienna) 2019;126:569–76 [DOI] [PubMed] [Google Scholar]
- 61.Tak AZA, Şengül Y, Karadağ AS.. Evaluation of thickness of retinal nerve fiber layer, ganglion cell layer, and choroidal thickness in essential tremor: Can eyes be a clue for neurodegeneration? Acta Neurol Belg 2018;118:235–41 [DOI] [PubMed] [Google Scholar]
- 62.Uchihara T.Neurofibrillary changes undergoing morphological and biochemical changes—How does tau with the profile shift of from four repeat to three repeat spread in Alzheimer brain? Neuropathology 2020;40:450–9 [DOI] [PubMed] [Google Scholar]
- 63.Gauthier-Kemper A, Suárez Alonso M, Sündermann F, et al. Annexins A2 and A6 interact with the extreme N terminus of tau and thereby contribute to tau's axonal localization. J Biol Chem 2018;293:8065–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McInnes J, Wierda K, Snellinx A, et al. Synaptogyrin-3 mediates presynaptic dysfunction induced by tau. Neuron 2018;97:823–35 e8 [DOI] [PubMed] [Google Scholar]
- 65.Stefanoska K, Volkerling A, Bertz J, et al. An N-terminal motif unique to primate tau enables differential protein-protein interactions. J Biol Chem 2018;293:3710–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trushina NI, Bakota L, Mulkidjanian AY, et al. The evolution of tau phosphorylation and interactions. Front Aging Neurosci 2019;11:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hernández F, Merchán-Rubira J, Vallés-Saiz L, et al. Differences between human and murine tau at the N-terminal end. Front Aging Neurosci 2020;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amadoro G, Corsetti V, Atlante A, et al. Interaction between NH(2)-tau fragment and Abeta in Alzheimer's disease mitochondria contributes to the synaptic deterioration. Neurobiol Aging 2012;33:833.e1–25. [DOI] [PubMed] [Google Scholar]
- 69.Corsetti V, Amadoro G, Gentile A, et al. Identification of a caspase-derived N-terminal tau fragment in cellular and animal Alzheimer's disease models. Mol Cell Neurosci 2008;38:381–92 [DOI] [PubMed] [Google Scholar]
- 70.Ercan E, Eid S, Weber C, et al. A validated antibody panel for the characterization of tau post-translational modifications. Mol Neurodegener 2017;12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]