Abstract
Developmental myelination is a protracted process that extends well into postnatal life. Cell-intrinsic mechanisms operate in myelin-forming oligodendrocytes, as well as microenvironmental interactions that guide and modulate every aspect of myelination, from oligodendrocyte precursor cell migration to oligodendrocyte differentiation and the formation of stable myelin internodes. During development and throughout adult life, neuron-oligodendroglial interactions shape activity and experience-dependent myelin adaptations to fine tune neural circuit dynamics and promote healthy neurological function.
Introduction
Myelin is a late evolutionary acquisition of the vertebrate nervous system. With increasing body size, the need arises for faster conduction of action potentials to sustain nervous system functions. Myelin in the central nervous system is formed by oligodendrocytes and is comprised of compact, lipid-rich membranes wrapped around axons. These axonal regions of myelin wrapping are called internodes and alternate with unmyelinated nodes of Ranvier where voltage-gated sodium channels are clustered. Myelin provides metabolic support to axons (Fünfschilling et al., 2012; Saab et al., 2016) and also functions as an electrical insulator, increasing the resistance and reducing the capacitance across axonal membrane. Myelin thereby facilitates rapid saltatory conduction of action potentials, with opening of voltage-gated sodium channels at the nodes of Ranvier and rapid action potential conduction along the axon from node to node. Various factors affect conduction speed, including the diameter of the axon, the thickness of the myelin sheath relative to the wrapped axon (g-ratio), and length and spacing of the internodes. Precision in coordinated neural network function is accomplished by differential regulation of these factors that alter conduction speed and - in the healthy brain - optimize neural circuit dynamics (Moore et al., 2020). Although initially considered innately determined during development, recent discoveries have elucidated the principle that myelination of certain axons can be modulated by neuronal activity long into adulthood. This phenomenon of neuronal activity-regulated adaptive myelination represents a component of nervous system plasticity that is emerging as crucial for higher brain functions, such as learning and memory.
Oligodendrocyte precursor cells (OPCs) are the actively renewing stem cell population which differentiate into myelin-forming oligodendrocytes. Oligodendroglial lineage cellular dynamics, from OPCs to myelinating oligodendrocytes, are regulated by both intrinsic and extrinsic factors. Distinct microenvironments across the brain and spinal cord exert different influences on the oligodendroglial lineage. As well, intrinsic heterogeneity exists in oligodendroglial cells in different regions of the central nervous system. In this review, we discuss these microenvironmental factors influencing diverse oligodendroglial cells in central nervous system, and consider the effects of neuronal activity-regulated myelination at the circuit level.
Oligodendroglial Heterogeneity and the Myelin Microenvironment
Myelination is shaped by both intrinsic and extrinsic cues that must guide oligodendrocytes to their target axons. Oligodendrocytes possess an intrinsic capacity for myelination of any correctly sized fiber; oligodendrocytes can myelinate paraformaldehyde-fixed axons or polymer filaments of appropriate diameter, and form internodes proportionate to the diameter of filaments (Bechler et al., 2015; Lee et al., 2013; Redmond et al., 2016). Yet only axons - but not glial cell processes, vascular structures, neuronal dendrites nor cell bodies - become myelinated. The cell-intrinsic capacity for myelination is modulated by environmental cues. For example, inhibitory cues on non-axonal structures exist to prevent myelination, and Jam2 is one such regulator preventing myelination on neuronal soma and dendrites (Redmond et al., 2016). In white matter tracts, correctly sized axons are not always myelinated (Hildebrand et al., 1993; Olivares et al., 2001; Saliani et al., 2017) or in layer II-III of the cortex, completely unmyelinated pyramidal neurons exist (Tomassy et al., 2014). The intrinsic myelination capacity and characteristics of oligodendrocytes varies both across different central nervous system regions and within oligodendrocyte populations of a given region.
Differential myelination capacity of distinct brain regions, varying vulnerabilities against demyelinating insults, and functional differences between grey and white matter suggest diverse OPC function and regulation. In the early postnatal period, OPCs throughout the brain exhibit similar transcriptional and electrophysiological phenotypes (Marques et al., 2018; Spitzer et al., 2019). But as OPCs differentiate into mature oligodendrocytes, they acquire diverse characteristics diverging into six mature oligodendrocyte states across the brain (Marques et al., 2016). Given the initial similarities, it is likely that the local microenvironment OPCs are exposed to during differentiation regulates acquisition of this diversity.
Multiple different studies demonstrate diversity in oligodendroglial lineage cells. Spinal cord oligodendrocytes generate longer myelin sheaths than cortical oligodendrocytes on synthetic fibers and in neuron co-cultures, which are consistent with the variations in their in vivo myelin profiles, indicating heterogeneity amongst intrinsic myelination programs of distinct oligodendrocyte populations from different regions (Bechler et al., 2015). Furthering this regional heterogeneity, mounting evidence suggests functional heterogeneity amongst OPCs as well. White matter OPCs exhibit increased differentiation capacity, even when transplanted to grey matter (Viganò et al., 2013). Recent findings from developing zebrafish spinal cord indicate two functionally distinct OPC subgroups; a neuron-rich regional subgroup that frequently divides and differentiates, while the axon-dendritic regional subgroup stays more quiescent and synaptically connected (Marisca et al., 2020). These zebrafish spinal cord OPC subgroups also show distinct morphologies and process motility, and different calcium signaling activity. Intriguingly, the local microenvironment in which the OPC cell body resides affects acquisition of these distinct proliferative or quiescent subgroup characteristics. OPCs retain these roles and do not switch their functions, which suggests different OPC subgroups rather than transitional cell states (Marisca et al., 2020). Indeed, OPCs become functionally heterogenous with age and across brain regions, acquiring different ion channel expression profiles (Spitzer et al., 2019). These changes also correlate with the differentiation potential of OPCs; NMDA receptors remain active in myelinating brain areas while non-myelinating areas lose NMDA receptor function (Spitzer et al., 2019). As discussed later in this review, the response of OPCs to environmental cues such as neuronal activity also varies in different white matter tracts, reflecting either heterogeneity in OPCs, or in the neurons whose axons constitute these tracts, or both. Heterogeneity of oligodendrocytes also occurs within anatomically-defined central nervous system regions. In the neocortex, some oligodendrocytes almost exclusively myelinate excitatory neurons, others myelinate preferentially inhibitory axons, and a third group of oligodendrocytes myelinate both (Zonouzi et al., 2019). Even though this myelination preference may simply arise from the neuronal composition of their microenvironment, the bias of individual oligodendrocytes towards a certain axon type seems to be independent of local axon availability (Zonouzi et al., 2019). The potential preference of myelinating excitatory or inhibitory neurons could be intrinsic to oligodendrocyte subpopulations; however, it is not yet clear how oligodendrocytes recognize different neuronal subtypes.
On the backdrop of the oligodendrocyte-intrinsic factors regulating myelination is a plastic process that can be regulated by dynamic external microenvironmental cues (Figure 1). We will examine some of these signals and evaluate their effect on oligodendroglial cells and myelination during development, throughout adulthood and in the context of disease states.
Figure 1. Microenvironmental interactions influencing myelination.
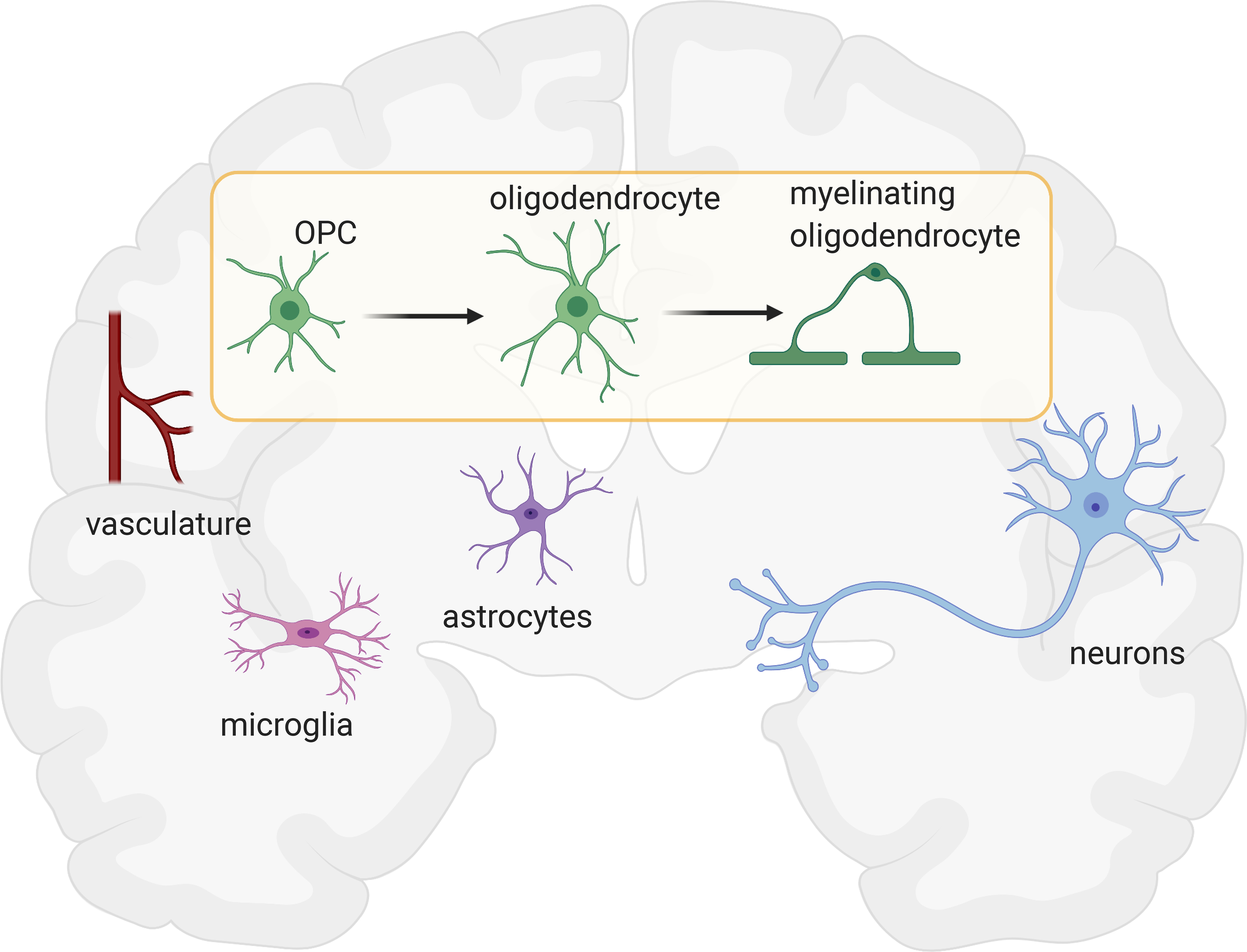
Oligodendroglial cells and myelin development are regulated by microenvironmental interactions. These interactions influence the dynamics of oligodendroglial cells such as proliferation of OPCs, differentiation into oligodendrocytes and myelination. Vasculature governs the migration route of OPCs by secreting Wnt signals. OPCs also respond to hypoxia by stimulating angiogenesis during development. Microglia are pivotal for oligodendrogenesis and myelination. A certain subset of microglia populating axon tracts mediate myelination during brain development. Disruptions in microglial-oligodendroglial interactions causes dysmyelination and maldevelopment. Reactive microglia in disease states can impair oligodendrogenesis or conversely can promote remyelination after injury. Astrocytes influence myelin thickness and nodal gap length via thrombin protease secretion. The gap junctions between astrocytes and oligodendrocytes contribute to the metabolic support of both glial cells, and myelination. During development, neuronal activity regulates myelin sheath dynamics and axon selection for myelination. Neuronal activity also promotes OPC proliferation, differentiation, and myelination, which is a process that continues to shape myelin during adulthood. OPCs form synapses with both excitatory and inhibitory neurons. Neurotransmitters including glutamate and GABA can regulate OPC proliferation and differentiation. Several neurotrophins and growth factors including NT3, PDGF, and CNTF are critical for OPC survival, while neuronal activity-regulated BDNF mediates oligodendroglial cell dynamics and OPC proliferation. Figure created in BioRender.
Interactions of Oligodendroglial Cells with Vasculature
The nascent vasculature of the central nervous system provides a scaffolding upon which OPCs migrate to their destinations in the brain and spinal cord. More than a structural route for migration, the vascular endothelial cells secrete Wnt signals that guide the extensive migration of OPCs from ventricular zone to distant parenchyma (Tsai et al., 2016). Vascular-oligodendroglial interactions are bidirectional and maturation of cerebral vasculature and myelination are complementary developmental processes. OPC and endothelial-tip cell interactions mediate vasculature and myelin communication. OPC density and angiogenic signaling regulate vascular remodeling in white matter during development via Wnt7a/7b, which in turn affects myelination (Chavali et al., 2020). OPCs expression of hypoxia-inducible factor 1/2a (HIF1/2a) activates OPC-derived secretion of Wnt7a/7b. Autocrine signaling of Wnt7a/7b inhibits OPC differentiation and paracrine action of Wnt7a/7b concomitantly stimulates angiogenesis in the early postnatal brain. In this way, OPCs respond to hypoxia by stimulating angiogenesis and blocking the metabolically expensive process of myelination until vascular maturation provides sufficient oxygen supply (Yuen et al., 2014).
Interactions of Oligodendroglial Cells with Microglia and Astrocytes
Microglia and astrocytes, the other glial cells of the brain, contribute in important ways to myelination. A balance of healthy glial cell populations is crucial for myelin development, homeostasis and plasticity. Microglia are critical for oligodendrogenesis and myelin development and promote myelination through secretion of IGF1 (Hagemeyer et al., 2017; Wlodarczyk et al., 2017). A subset of microglia, which are CD11c+ and abundant in white matter regions, supply IGF1 for early postnatal myelination and its loss causes thinner myelin and decreases myelin-associated proteins including Plp, MBP, Mag, and Mog (Wlodarczyk et al., 2017). These microglia exhibit a unique gene expression profile that is different than adult microglia (Hagemeyer et al., 2017; Wlodarczyk et al., 2017). Single cell RNA sequencing in mice elucidates this subset as a class of microglia, referred as axon-tract microglia, which only appears prior to developmental myelination along axon tracts of the corpus callosum and cerebellum (Hammond et al., 2019). The temporal and spatial restriction of this highly specialized subpopulation underscores a leading role for microglia during early developmental myelination. Concordantly, a striking case report reveals the critical role for microglia during human brain development; an infant with homozygous mutations in colony stimulating factor 1 receptor (CSF1R) gene, resulting in congenital absence of microglia, exhibited periventricular leukoencephalopathy and developmental malformations of white matter structures including agenesis of the corpus callosum (Oosterhof et al., 2019). Microglia also phagocytose excess myelin sheaths during development in the zebrafish, and increased neuronal activity limits this myelin pruning function of microglia (Hughes and Appel, 2020). Thus, microglia both support and sculpt myelination during development. In demyelination and dysmyelinating diseases, microglial state can alternatively promote or impair effective oligodendrocyte generation (Miron et al., 2013; Gibson et al., 2019). Microglia activated in a neurotrophic state can promote remyelination after demyelinating injury through growth factor secretion (Miron et al., 2013). In a more neurotoxic state of activation in disease states, microglia can instead initiate a complex cascade of microenvironmental perturbations, including induction of neurotoxic astrocyte reactivity, a reduction in OPC numbers, a blockade in oligodendrocyte maturation and dysmyelination (Gibson et al., 2019).
Astrocytes, another class of glia that exhibits molecular and regional heterogeneity and that can assume neurotrophic or neurotoxic states (Batiuk et al., 2020; Liddelow et al., 2017; Zhang and Barres, 2010), also regulate myelination in important ways. Astrocytes regulate myelin thickness and nodal gap length by exocytosis of thrombin protease inhibitors at the node of Ranvier (Dutta et al., 2018). Neurofascin155 is a cell adhesion molecule that attaches myelin to the axon, and thrombin proteases cleave this protein detaching myelin sheaths but astrocytes inhibit this action. This reversible process occurs in adulthood indicating astrocytic contribution to myelin remodeling; however, it is not yet clear whether this function may be regulated by experience or neuronal activity. The coupling of astrocytes and oligodendrocytes may be critical for the metabolic support between these two glial cells, and for myelination. Disruption of gap junctions between astrocytes and oligodendrocytes by loss of connexins Cx47 and Cx30 causes myelin deficits including vacuole formation and thinner myelin (Orthmann-Murphy et al., 2007; Tress et al., 2012). Furthermore, oligodendrocytes require external lipids for myelin production, which is supplied by astrocytes (Camargo et al., 2017). However, in disease states, astrocytes activated to a neurotoxic state by the influence of reactive microglia can induce cell death of oligodendrocytes (Liddelow et al., 2017). Taken together, these findings demonstrate pivotal roles for both microglia and astrocytes in supporting myelination in health, and conversely contributing importantly to myelin disease. With the continued exploration of distinct glial cell functions, microenvironmental regulators of myelin in health and disease will be better understood and more targetable for therapy in a range of neurological diseases.
Interactions of Oligodendroglial Cells with Neurons
Do neurons themselves influence the extent to which their axons are myelinated during development or beyond, and does this occur in an activity-regulated manner? A wealth of time-lapse in vivo imaging studies in developing zebrafish spinal cord has revealed how neuronal activity may regulate OPC and myelin sheath dynamics. Contact-mediated signaling between OPC processes enables the identification of nearby cells and their environment. With this sensing mechanism OPCs migrate to the spaces that are unoccupied by other OPCs, thereby achieving a uniform distribution (Kirby et al., 2006). After differentiation, oligodendrocytes hold a limited time window to generate nascent myelin sheaths which is followed by slower paced sheath retractions, extensions or stabilization (Czopka et al., 2013; Hines et al., 2015). Neuronal activity is implicated in both myelin sheath dynamics and axon selection for myelination. Inhibiting neuronal activity either by overexpressing the Kir2.1 inward rectifying K+ channel or by expressing tetanus neurotoxin light chain to inhibit synaptic vesicle release, reduces the number of myelinated axons (Hines et al., 2015). Neuronal activity-dependent vesicle secretion biases oligodendrocyte axon selection towards active axons for myelination. Synaptic vesicles accumulate at the sites of myelin ensheathment, potentially marking the axonal regions to be myelinated (Hines et al., 2015). Inhibiting synaptic vesicle release decreases myelin sheath length, and number of myelin sheaths produced by individual oligodendrocytes (Hines et al., 2015; Mensch et al., 2015). Taken together, these findings suggest that neuronal activity regulates both sheath dynamics and the myelination capacity of oligodendrocytes during development. Determining the contributions of different neuronal activity components, such as ion flux, synaptic vesicle release and activity-dependent secretion of various proteins, will elucidate how neurons regulate oligodendroglial cell interactions with axons at different stages of development and in distinct regions of the nervous system.
Neuronal activity also shapes rodent myelination during adolescence, at the tail end of brain development. Optogenetic stimulation of neuronal activity in the mouse premotor cortex strongly promotes OPC proliferation, oligodendrogenesis and myelination, which is accompanied by an improvement in motor function that depends upon new oligodendrocyte generation (Gibson et al., 2014). Cortical projection neuronal activity stimulates oligodendrogenesis and myelination in the deep layers of premotor cortex and cortico-callosal white matter projections. In contrast, no oligodendrogenesis was observed along the corresponding cortico-fugal projections in the corticospinal tract, indicating either differences amongst OPCs across these brain regions or differences in the oligodendroglial interactions of distinct neuronal subtypes, such as corticocallosal and corticofugal projection neurons. This activity-regulated modulation of myelin continues during adulthood, with a similar degree of new oligodendrocyte generation but reduced magnitude of OPC proliferation compared to the juvenile period (Gibson et al., 2014). Studies using chemogenetic or experience-dependent (whisker tickling) stimulation of somatosensory neuronal activity similarly found activity-regulated OPC proliferation, oligodendrogenesis and myelination of somatosensory cortical axons (Hughes et al., 2018; Mitew et al., 2018); more active somatosensory neurons exhibit thicker myelin, emphasizing that the relative activity of individual neurons refines myelination (Mitew et al., 2018).
Taken together, these findings demonstrate the principle that neuronal activity can regulate myelination and oligodendroglial lineage cell dynamics in select circuits. In the following sections, we will explore what is known about the molecular mechanisms governing adaptive neuron-oligodendroglial interactions and the emerging roles for myelin plasticity in brain functions (Figure 2).
Figure 2. Myelin adapts with changing neuronal activity and this plasticity is required for various brain functions.
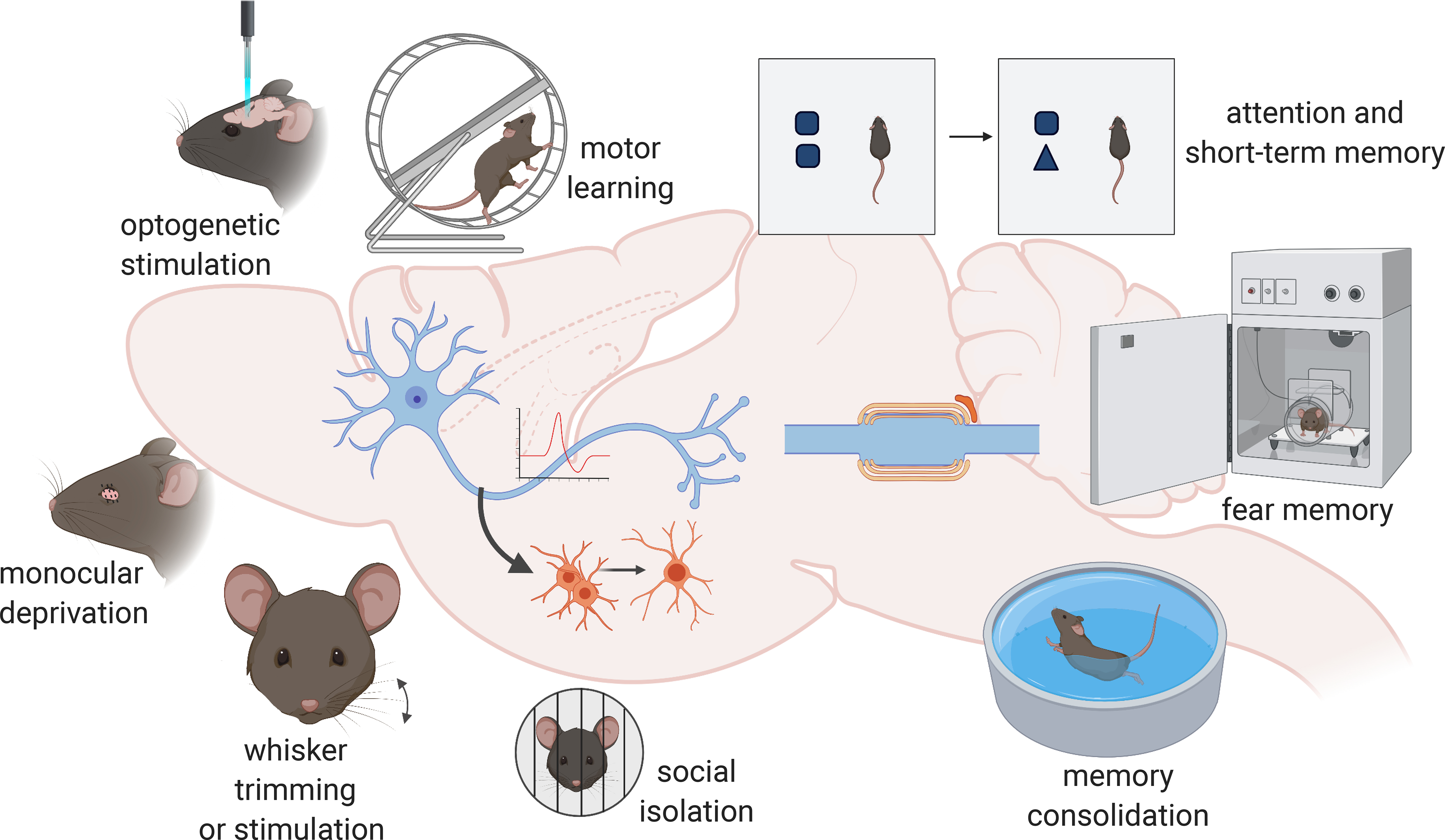
Neuronal activity regulates myelin adaptations throughout adulthood, and this process is necessary for a diverse array of behaviors. Clockwise from top left: Optogenetic stimulation of secondary motor cortex projection neurons promotes OPC proliferation, oligodendrogenesis and myelination of the corresponding projections through the corpus callosum which in turn influences motor function. Motor skill learning requires new oligodendrocytes, and preventing OPC differentiation disrupts the ability of mice to learn to run on a complex wheel. Blocking the neuronal activity regulated-response of OPCs impairs short-term memory and attention function, and mice fail to discriminate between new and familiar objects. New oligodendrocyte generation is also necessary for fear memory and spatial memory function, because oligodendrogenesis mediates consolidation of memories. Social isolation causes myelin abnormalities in medial prefrontal cortex and disrupts working memory. Whisker trimming in rodents perturbs OPC proliferation and survival, whereas stimulation expands the myelination of associated barrel cortex. Monocular deprivation affects myelin remodeling specifically on parvalbumin neurons, adapting myelin to new neural circuit arrangements. Figure created in BioRender.
Neurotransmitters in neuron-oligodendroglial interactions
OPCs form bona fide synapses with both excitatory and inhibitory neurons (Bergles et al., 2000; Káradóttir et al., 2005; Lin and Bergles, 2004). Axon-glial synapses form in grey matter (Bergles et al., 2000; Lin and Bergles, 2004) and en passant synapses form in white matter tracts (Kukley et al., 2007; Ziskin et al., 2007). This discovery stoked great interest in neuron-OPC synapses as a potential route of communication that may contribute to neuronal activity-regulated adaptive myelination. In the mature mouse brain, OPCs exhibit comprehensive synaptic access to brain-wide neural projections as revealed by monosynaptic rabies tracing studies (Mount et al., 2019). Cortical OPCs, including secondary motor cortex and primary somatosensory cortex, and white matter OPCs in corpus callosum receive inputs from both local neurons and functionally interconnected thalamic neurons, thereby “listening” to different components of a given circuit. Strikingly, the ratio of glutamatergic to GABAergic afferents an OPC receives are consistent across these three regions, suggesting a regulated input balance from excitatory and inhibitory neurons. Most studies of OPC afferents focus on glutamatergic and GABAergic connectivity, so whether OPCs receive synapses from other neuronal subtypes, such as dopaminergic or serotonergic neurons, is yet to be determined.
Glutamate is the most abundant excitatory neurotransmitter in the CNS and mediates both synaptic and non-synaptic signaling (Kula et al., 2019). OPCs express a variety of ionotropic and metabotropic glutamate receptors (Barres et al., 1990; Borges et al., 1994). Initial in vitro studies suggested that glutamatergic signaling through ionotropic glutamate receptors, α-aminio-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors inhibited OPC proliferation and differentiation (Gallo et al., 1996; Yuan et al., 1998). However, genetic mouse models studied in the absence of experimental modulation of neuronal activity indicate that OPC-selective deletion of OPC AMPA receptor subunits in vivo does not affect OPC proliferation but does influence the survival of newly-differentiating oligodendrocytes (Kougioumtzidou et al., 2017). Similarly, genetic deletion of the obligatory NR1 subunit of the N-methyl-D-aspartate (NMDA) receptor does not influence OPC proliferation or density nor developmental oligodendrogenesis (De Biase et al., 2011). OPCs are electrophysiologically different than oligodendrocytes. With differentiation into mature oligodendrocytes, OPCs eliminate their synapses and downregulate AMPA and NMDA receptors and voltage-gated sodium channels (NaV) (De Biase et al., 2010). Glutamate release from cultured neurons promotes in vitro myelination by increasing the local synthesis of myelin basic protein (MBP) (Wake et al., 2011). However, this glutamate signaling between axons and OPCs may be non-synaptic, and neuron-OPC synapses may not be required for myelination (Wake et al., 2015). The specific functions of neuron-OPC synapses remain to be fully elucidated.
GABAergic interneurons form synaptic connections with OPCs (Lin and Bergles, 2004; Mount et al., 2019). Similar to immature neurons and other stem-like cells, GABA release from interneurons causes a depolarization in OPCs, because OPCs express Na+-K+-Cl− co-transporter 1 (NKCC1) and maintain a high level of intracellular chloride ion concentration (Lin and Bergles, 2004). GABAA receptor antagonist bicuculline treatment, or deletion of NKCC1, during mouse postnatal development increases OPC proliferation but decreases the number of mature oligodendrocytes in cerebellar white matter (Zonouzi et al., 2015), indicating that GABA and voltage-sensitive mechanisms regulate cerebellar OPC lineage progression. Collectively, OPCs possess the necessary characteristics to respond to different neurotransmitters and in turn regulate cellular dynamics and function, although much work remains to fully elucidate the effects of various neurotransmitters on heterogeneous oligodendroglial lineage cell subpopulations in different developmental states.
Neuronal factors in neuron-oligodendroglial interactions
Many microenvironmental cues secreted by neurons, including neurotrophins, were identified as important factors for oligodendroglial cell survival and maintenance during the efforts to purify and culture these cells. Neurotrophin 3 (NT3) and PDGF are crucial for OPC survival and proliferation, and ciliary neurotrophic factor (CNTF) supports oligodendrocyte survival in vitro (Barres et al., 1993).
Brain-derived neurotrophic factor (BDNF) is another microenvironmental cue which is neuronal activity-regulated and can regulate oligodendroglial cell dynamics. The effects of BDNF appears to be mediated through TrkB receptors on OPCs (Geraghty et al., 2019; Vondran et al., 2010). BDNF plays important roles in both developmental myelination and activity-regulated myelin plasticity. In development, homozygous deletion of Bdnf is embryonic lethal but heterozygous loss results in ~40% decreased Bdnf protein levels and reduced numbers of OPCs and decreased myelin-associated protein levels without a change in oligodendrocyte numbers (Vondran et al., 2010). Consistently, conditional deletion of TrkB receptors in mature oligodendrocytes does not change oligodendrocyte numbers, but does reduce the myelin sheath thickness in the CNS (Wong et al., 2013). Activity-regulated Bdnf release from cortical projection neurons is required for activity-regulated myelination of cortical projections, and OPC-specific deletion of the BDNF receptor TrkB entirely abrogates activity-dependent myelination of cortical projection neuron axons (Geraghty et al., 2019). In addition to its direct effects on oligodendroglial cells, BDNF increases OPC responsivity to glutamate by increasing functional expression of NMDA receptors (Lundgaard et al., 2013). Neuregulin, also appears to operate this switch, suggesting that multiple neurotrophins may impact the response of OPCs to neurotransmitter signals (Lundgaard et al., 2013). Therefore, neuronal factors, acting either directly on oligodendroglial lineage cells or altering other interactions in the brain microenvironment, regulate the interplay between neurons and myelin. Many other factors, including Nrg1, Jagged1, F3/contacin, L1, TAG-1, PSA-NCAM, Lingo1, Fractalkine, laminin-α2, and certain cadherins regulate axon-oligodendrocyte interactions at the various stages of axon selection and myelination (for review please see Almeida, 2018) during development and beyond.
Neuronal Activity-Regulated Adaptive Myelination and Behavior
Initial pioneering studies investigating the contribution of neuronal activity demonstrated that transection of a developing optic nerve or intraocular injection of tetrodotoxin (TTX) during development significantly reduced the rate of OPC proliferation, oligodendrocyte survival, and optic nerve myelination (Barres and Raff, 1993; Demerens et al., 1996). These findings lead to the subsequent studies that directly demonstrated neuronal activity-dependent OPC proliferation, oligodendrogenesis and myelination (Gibson et al., 2014; Hughes et al., 2018; Mitew et al., 2018). A wealth of studies now supports the conclusion that neuronal activity shapes oligodendroglial cell dynamics and modulates plastic changes in myelination of neural circuits, consequently altering the neural function. As each oligodendrocyte usually makes between 20–50 myelin sheaths (Bacmeister et al., 2020; Bechler et al., 2015; Czopka et al., 2013; Hughes et al., 2018), just a few new oligodendrocytes can substantially change the myelination of a neural circuit. Small changes in myelin thickness or internode length can lead to significant increases in conduction velocity. Neural circuits require precisely timed coordination to support proper circuit functions. Highly variable and discontinuous myelin profiles of cortical neurons, and the extent of unmyelinated axonal territory in the adult neocortex present substantial capacity for myelin alterations, which could be tailored to distinct patterns of neuronal activity (Tomassy et al., 2014). Computational and mathematical modelling studies also support the concept that activity-dependent myelin plasticity enables adaptation of conduction velocity to promote oscillatory synchrony (Noori et al., 2020; Pajevic et al., 2014). Concordantly, mounting evidence demonstrates that neuronal activity-regulated adaptive myelination is required for healthy learning and memory function, discussed in detail below.
Sensory Experience
Myelin is critical for proper processing of sensory experience, and achieving the precise temporal coincidence for receiving signals from different components in the auditory system. This precision is achieved by differential regulation of myelination and axon caliber along two different collaterals of a bifurcating axon in the brainstem (Seidl and Rubel, 2016; Seidl et al., 2014). The contralateral projections display longer internodes and bigger axon caliber compared to the ipsilateral projections, thus transmitting signals faster and equalizing the conduction times of two projections to the neuronal soma. Remarkably, these differences between two projections are established before the onset of hearing, underscoring intrinsic developmental mechanisms.
Experience-dependent myelin plasticity affects both visual and sensory systems. Monocular deprivation of one eye in adult rats decreases markers of myelination in the related hemisphere of the visual cortex while the non-deprived hemisphere exhibits the opposite (Murphy et al., 2020). This suggests that myelin adapts to the changing balance of cortical neuronal activity. Strikingly, this myelin adaptation to monocular deprivation occurs in a neuron class-specific manner; myelination of cortical projection neurons remains unchanged while the myelin profiles of inhibitory parvalbumin neurons remodel, and consequently ion channels at the Nodes of Ranvier are redistributed (Yang et al., 2020). The highly specific nature of adaptive myelination indicates that myelin plasticity is part of a reconfiguration process that adjusts neural circuits to changing sensory experience.
In somatosensory cortex, removal of whiskers from birth causes increased proliferation of OPCs by P6 (Mangin et al., 2012) and reduces the myelinated axon density at P60 in barrel cortex (Barrera et al., 2013). Although these results seem counterintuitive at first glance considering the effect of neuronal activity on OPC proliferation and differentiation, whisker trimming also increases the rate of apoptosis in these newly generated oligodendrocyte precursors (Hill et al., 2014). The total number of mature oligodendrocytes are reduced in this setting suggesting that the initial increase in OPC proliferation is a homeostatic response to the reduction of mature and differentiating oligodendrocytes. Therefore, a critical time window between the OPC cell division and differentiation determines the survival of these cells based on the changes in the microenvironment and neuronal activity. Sensory enhancement can also reshape myelin during adulthood. Three weeks of exposure to a sensory-enriched environment (whisker stimulation) robustly increased oligodendrogenesis in and myelination of the barrel cortex (Hughes et al., 2018). Longitudinal tracking of oligodendrocyte formation in the adult somatosensory cortex revealed that only a fraction of the differentiated oligodendrocytes achieves stable integration while the rest die. Remarkably, once integrated, oligodendrocytes remain remarkably stable throughout life (Hughes et al., 2018; Tripathi et al., 2017; Yeung et al., 2014). This emphasizes a critical window for the survival of newly generated oligodendrocytes that is determined by microenvironment in the somatosensory cortex during adulthood. Overall, increased neuronal activity mediates myelin changes to adaptively tune neural circuit dynamics.
Social Experience
Social experience modulates myelin structure in relevant anatomical regions. People who were raised in orphanages with severe social deprivation and neglect display altered myelination in limbic white matter tracts and these structural changes are associated with lasting cognitive impairments (Chugani et al., 2001; Eluvathingal, 2006). Subsequent to these human imaging and neuropsychological studies, a critical period for myelination was identified in the early juvenile period following weaning in mice (Makinodan et al., 2012). Animals that are socially isolated after weaning (P21), showed decreased sociability and working memory together with thinner myelin in medial prefrontal cortex (mPFC), shorter and fewer internodes, and reduced processes length for mature oligodendrocytes. Strikingly, the myelin abnormalities caused by social isolation at P21 were not reversed by social re-integration at P35, highlighting the juvenile period as a critical time for myelination and oligodendroglial development relevant to social function. Prolonged periods of social isolation in adult mice also caused reduced myelin thickness in mPFC, and social withdrawal (Liu et al., 2012). Remarkably, social re-integration reversed these disturbances in adults, indicating that experience-dependent myelin plasticity of adulthood may be different or differently influence social behavior-related circuit function compared to a critical window in the juvenile period.
What are the molecular mechanisms that underlie social experience-mediated changes in mPFC myelination? Neuregulin 1 (NRG1)-ErbB3 signaling is one signaling pathway implicated in social-experience regulated myelin plasticity (Makinodan et al., 2012; Roy et al., 2007). ErbB3 loss from oligodendrocytes mimics the effects of social isolation causing thinner myelin sheaths, together with decreased sociability. Reduced expression of type III NRG1 in mPFC neurons during social isolation can cause reduced oligodendrocyte ErbB3 signaling, leading to defects in myelination (Makinodan et al., 2012). Another line of evidence links the blood vessels to myelin plasticity in mPFC, with vasoactive peptide endothelin (EDN) signaling (Swire et al., 2019). Increased neuronal activity enhances blood flow, which in turn promotes EDN expression in endothelial cells. Social isolation dampens EDN expression and leads to disrupted mPFC myelination, which can be rescued by an agonist for EDN receptor B that activates EDN signaling.
Taken together, these findings support the conclusions that experience-dependent myelination in mPFC is regulated by neuron-oligodendrocyte communication and contributes to social behavior. The data also highlight juvenile brain development as a critical period for healthy cognition and behavior that is vulnerable to irreversible myelin deficits.
Motor Learning
Evidence for experience-dependent myelin plasticity in human brain has been revealed by structural imaging studies which demonstrated white matter alterations after motor learning. People who learn playing piano or a complex visuo-motor skill like juggling show structural changes in white matter tracts (Bengtsson et al., 2005; Scholz et al., 2009; Steele et al., 2013). These myelin changes are observed in brain regions that are involved in executing the motor task. Rats trained in a single-pellet reaching task showed myelin changes in the external capsule and cingulum, both of which are white matter areas connecting motor related regions (Sampaio-Baptista et al., 2013). Link between motor learning and myelin changes became the focus of following studies which investigated the relationship between neuronal activity and oligodendroglial cells.
Accumulating evidence in recent years underscores a role for activity-regulated oligodendrogenesis and myelination in a variety of behaviors, including acquisition of new motor skills. A conditional knockout model for myelin regulatory factor (Myrf), which can block OPC differentiation without disturbing the preexisting oligodendrocytes (Emery et al., 2009), demonstrated the necessity of myelination for motor skill learning. Mice learnt a new motor skill by running on a complex wheel with irregularly spaced rungs, and this complex motor learning task leads to increased OPC proliferation and oligodendrogenesis in the corpus callosum (McKenzie et al., 2014). Deficiency for new oligodendrocyte generation during this complex wheel learning task prevented Myrf conditional knockouts from mastering the new skill. However, deletion of Myrf after learning the skill did not affect their performance on the wheel later on, highlighting the importance of myelination for acquiring the motor skill. In wild type mice, new immature oligodendrocytes were identified in subcortical white matter within 2.5 hours of introduction to complex wheel, and within 4 hours this increase was also detectable in the motor cortex (Xiao et al., 2016). This rapid response of oligodendroglial cells to learning a new motor task indicates a close communication of these cells with the activity of related neural circuits and is concordant with the findings after optogenetic stimulation of motor cortical projection neurons (Gibson et al., 2014) described above.
Can activity-regulated myelination promote motor system remyelination and recovery after injury? Neuronal activity-dependent oligodendrogenesis is crucial for remyelination of the motor cortex, but the timing of learning is critical. Soon after demyelination, motor cortical neurons exhibit hyperexcitability hindering learning and preventing remyelination (Bacmeister et al., 2020). Although neuronal activity regulates oligodendroglial dynamics in general, the pattern or frequency of neuronal activity likely matters and this hyperexcitable environment is not favorable for oligodendrogenesis and remyelination, consistent with the previous observation that optogenetically-induced motor seizures do not induce OPC proliferation and oligodendrogenesis like physiomimetic, motor behavior-inducing, 20 Hz neuronal stimulation does (Gibson et al., 2014). Delaying motor training until after this post-injury hyperexcitable period improves motor cortex remyelination, which occurs through both new and surviving oligodendrocytes and enhances motor functional outcomes (Bacmeister et al., 2020).
Together these studies demonstrate that adaptive myelination contributes substantially to motor skill learning. Importantly, simply exercising a previously learnt skill, or non-physiological levels of neuronal activity do not promote, and may even inhibit, the same myelination response. This role of neuronal activity-regulated myelination in the adult brain highlights the importance of neuron-OPC interactions for learning and adaptive neural circuit function, a principle that is further supported by the studies discussed below.
Memory and Cognition
Cognition relies on coordinated neural circuit function. Activity-dependent myelination could adapt spike-time arrivals, oscillations and synchrony across different neural circuits, and fine-tuning myelin structure can thus contribute to learning and memory (Pajevic et al., 2014). Recent studies focusing on experience-induced oligodendrogenesis also demonstrate that myelin plasticity is a crucial component of these brain functions.
The molecular mechanisms that mediate neuronal activity-regulated myelination are likely complex and possibly heterogenous across different brain regions and neuronal types. In cortical projection neurons, BDNF-TrkB signaling is a critical mechanistic component (Geraghty et al., 2019). Disruption of this signaling axis, either by blocking neuronal activity-induced BDNF release or deleting TrkB receptors from OPCs, abrogates activity-dependent oligodendrogenesis and myelination while homeostatic oligodendrogenesis is unaffected. Loss of the Bdnf receptor TrkB from OPCs after development in the juvenile period or in adulthood causes deficits evident in a cognitive behavioral test of short-term (< 5 min) memory and attention; mice lacking TrkB fail to discriminate between familiar and new objects in the short-interval novel object recognition test performed 4 weeks after OPC-specific TrkB deletion (Geraghty et al., 2019). This indicates that ongoing adaptive myelination contributes to the optimal functioning of the distributed networks that underlie attention and/or short-term memory function.
Fear learning behavior involves multiple brain regions, such as mPFC, basolateral amygdala, anterior cingulate cortex and hippocampus. Remarkably, fear learning induces proliferation and subsequent differentiation of OPCs in mPFC only, increasing the number of myelinated axons (Pan et al., 2020). Basolateral amygdala also exhibits an initial increase in OPC proliferation but this new population of cells fails to survive and differentiate into myelinating oligodendrocytes in the following weeks (Pan et al., 2020). Although the same experience activates multiple brain regions, it does not evoke a similar oligodendroglial response in each of these areas. Seemingly, a differential regulation of neuronal activity-induced oligodendrogenesis exists across the brain, concordant with observations that different neuronal types differentially exhibit activity-regulated myelin changes (Gibson et al., 2014; Yang et al., 2020). Suppressing production of mature oligodendrocytes with conditional Myrf knockout prevents remote fear memory recall 30 days after the fear conditioning session, but recent memory recall in 24 hours remains intact (Pan et al., 2020). Together these data suggest that production of new myelin which occurs over weeks is required for the remote fear memory recall. However, oligodendroglial changes happening within hours is dispensable for recent fear memory recall. Memory consolidation is a long-term process by which recent experiences are stored as enduring memories, and it involves reorganization of neural circuits at both synaptic and circuit levels. Initial hippocampus-dependent short-term fear memories are gradually distributed to cortical regions, and the generation of new myelin is critical for this process. Blocking oligodendrogenesis inhibits fear memory consolidation by preventing oscillatory coupling of hippocampal sharp wave ripples and cortical spindles (Steadman et al., 2020).
Similarly, spatial learning and/or memory formation induces oligodendrogenesis and increases the number of myelinated axons in cortical and associated white matter regions (Steadman et al., 2020). Deletion of Myrf impairs spatial memory recall in 28 days, but not in 24 hours, suggesting that oligodendrogenesis is necessary in the post-learning period for the consolidation of spatial memories. Therefore, neuronal activity-induced myelination is crucial for the process of memory consolidation supporting a critical role for myelin in synchronizing activity across neural circuits.
Myelin Dysregulation in Disease
Chemotherapy
Cancer chemotherapy drugs can alter the brain microenvironment and adversely affect cognitive functions. This syndrome of neurological deficits, known as chemotherapy-related cognitive impairment (CRCI), includes impaired attention, memory, executive function and decline in speed of information processing. Methotrexate (MTX), an anti-metabolite chemotherapy drug, causes long-term depletion of white matter OPCs, disrupts differentiation, and impairs myelination (Gibson et al., 2019). This white-mater specific vulnerability indicates the differential regulation of oligodendroglial cells in different brain microenvironments. Strikingly, when MTX-naïve OPCs are transplanted into MTX-exposed brains, they also exhibit increased differentiation, highlighting the effect of perturbed microenvironment on oligodendroglial cell dynamics. MTX directly induces a reactive microglial state specifically in white matter microglia, which in turn induces neurotoxic astrocyte reactivity and together these reactive glia impair oligodendroglial lineage function and myelination (Gibson et al., 2019). This MTX mouse model recapitulates a plethora of chemotherapy-related neurological deficits; impairments in motor speed, increased anxiety, and deficits in short-term memory and attention. Even 6 months after the MTX exposure these cognitive and myelin deficits remains, demonstrating the persistent effects of chemotherapy. Moreover, adaptive myelination is impaired after MTX treatment, and neuronal activity-regulated OPC proliferation, oligodendrogenesis and myelination are abrogated (Geraghty et al., 2019). MTX-induced microglial activation is central to this loss of adaptive myelination through microglial-dependent depletion of cortical neuronal Bdnf expression; stimulating TrkB signaling rescues cognitive function after MTX exposure, but only if OPCs express TrkB (Geraghty et al., 2019). Similarly, depleting microglia through pharmacological CSF1R blockade restores neuronal Bdnf expression, reverses these MTX-induced myelin deficits and rescues cognitive performance (Geraghty et al., 2019; Gibson et al., 2019). These findings underscore a sensitive balance between the interactions of glial cell types in the brain, and how a glial dysregulation can exert persistent impairments in neurological function.
Neurodegenerative Diseases
Numerous studies have demonstrated prominent white matter abnormalities in neurodegenerative diseases such as Alzheimer’s, Huntington’s, and Parkinson’s using brain imaging techniques in humans (Agosta et al., 2011; Bourbon-Teles et al., 2019; Dean et al., 2016). Are the myelin deficits a secondary effect of neurodegeneration or are they a part of the cause? A common hallmark of these degenerative diseases is axonal loss. Conceivably, dying neurons affect their environment and cause secondary myelin impairments, gliosis, and inflammatory response, all of which in turn can lead to the white matter abnormalities. Long axons are particularly metabolically vulnerable structures that are supported by myelin and oligodendrocytes. Especially in disease conditions, this oligodendroglial support can influence the survival or susceptibility of neurons thus contributing to the onset and/or progression of neurodegeneration (Nave and Ehrenreich, 2014). Even though it is unlikely that oligodendroglial cell impairments are the primary source of pathology in these neurodegenerative diseases, they are involved in the multiple factors contributing to selective vulnerability of neurons and microenvironmental toxicity. Furthermore, given the prominent activation of microglia in neurodegenerative diseases and the influence certain states of neuroinflammation can exert on myelin plasticity (Geraghty et al., 2019), a loss of plasticity of myelin may contribute to the cognitive impairment that is characteristic of neurodegenerative diseases. A comprehensive perspective that includes glial biology is necessary in the investigation of systems-level brain diseases which involve complex cellular and molecular mechanisms.
Neuropsychiatric Diseases
Aberrant neural circuit dynamics, connectivity and synaptic impairments are central to neuropsychiatric diseases (Chen et al., 2018). In the recent years accumulating evidence reveals defects in oligodendroglial cells and myelin integrity in many psychiatric disorders including depression, schizophrenia, bipolar disorder, and autism (Chen et al., 2018). Although these studies are supported by neuropathological, brain imaging and genetic findings, the role of oligodendroglial cells in the disease pathology is yet to be uncovered. A combination of genetic predisposition and environmental factors underlie in the development of neuropsychiatric diseases. Most related genetic risk factors localize to non-coding regulatory regions causing differential splicing or expression of incorrect isoforms (Gandal et al., 2018). Integrating genomic level data and transcriptome-wide characterization across brain cell types can provide mechanistic insights into pathological effectors of these complex array of diseases.
The dynamic interplay between neural circuit activity and consequent myelin adaptations likely contributes to the pathophysiology of neurological and neuropsychiatric diseases. Possible myelin maladaptation to perturbed neural circuit dynamics may reinforce the disease pathology. For example, epilepsy syndromes are characterized by aberrantly synchronous neuronal activity and more recent evidence suggests myelin abnormalities in people with some forms of epilepsy (Drenthen et al., 2020). However, much work remains to determine if the various types of seizures can induce aberrant activity-regulated myelination, and if that aberrant myelin might contribute to maladaptive circuit function and even promote seizures. Moreover, it remains to be determined how activity-regulated myelination may contribute to pathophysiology in other disease states characterized by aberrant patterns of activity. For example, drugs of abuse alter neural circuit function and modify synaptic transmission of the reward circuitry (Lü Scher and Malenka, 2011) and long-term opiate users also exhibit myelin pathology (Bora et al., 2012). Conceivably, synaptic connectivity between neurons and OPCs might similarly be affected. Reward circuitry modifications caused by drugs of abuse might also involve myelin adaptations to adjust each aspect of circuit function. Elucidating if and how myelin alterations may reshape neural circuit dynamics maladaptively is critical to explore in neurological and neuropsychiatric conditions for which altered activity patterns are central (Figure 3).
Figure 3. Myelin dysregulation can arise from different stages of oligodendroglial lineage and myelin adaptation.
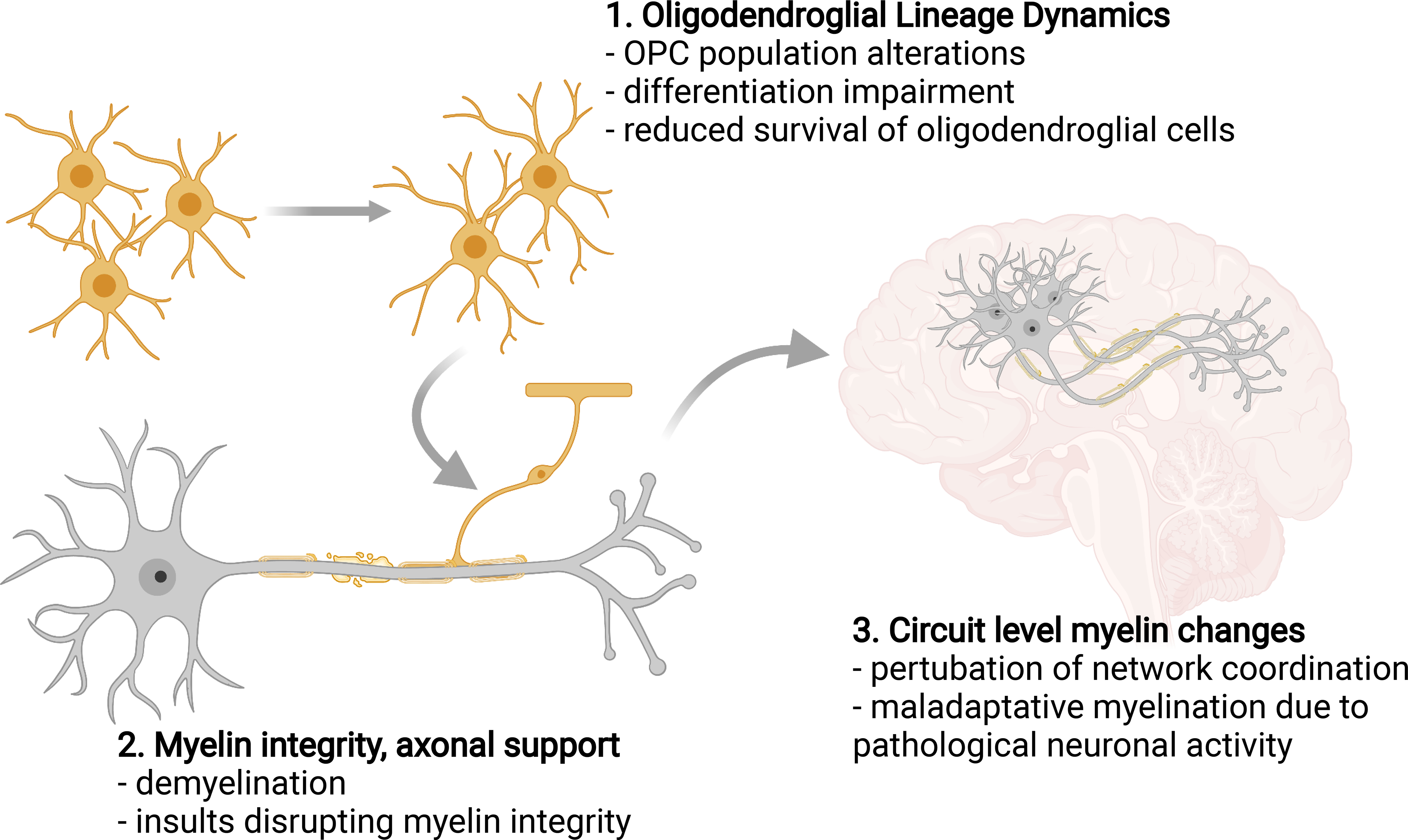
A variety disease states can influence different stages of myelin formation or adaptation and contribute to myelin dysregulation. 1. Oligodendroglial lineage dynamics: Pathologies affecting OPC populations and their dynamics can generate a knock-on effect on the rest of the lineage. These can vary from OPC population alterations in particular brain regions to differentiation blocks, and to overall reduction in the survival of oligodendroglial cells. For example, certain cancer therapies induce disruptions in white matter oligodendroglial cells, which also affects activity-regulated myelin plasticity and cognition. 2. Myelin integrity, axonal support: several debilitating diseases and conditions are characterized by demyelination, which leads to deterioration of neuronal functions. In addition to demyelinating disorders, neurodegenerative and neuropsychiatric diseases also exhibit white matter disruption. Microenvironmental toxicity or neuronal pathology can contribute to the decline of myelin and oligodendrocytes. 3. Circuit level myelin adaptations: neuronal-activity regulated myelination is adaptive in the healthy brain, and contributes to cognitive functions by fine-tuning neuronal network coordination. Abnormal patterns of neuronal activity might cause maladaptative myelination, which in turn could promote pathological neuronal network function, particularly in neuropsychiatric diseases. Cellular level disruptions in oligodendroglial cells (1) or abnormal myelination (2) can cause circuit level (3) myelin dysregulation, which can alter circuit function and cognition. Figure created in BioRender.
Next Questions
Communication between cell types in the brain, heterogeneity of microglia and astrocytes, neuronal composition of the microenvironment, secreted proteins, ion concentrations and neuronal activity all influence the functioning of an oligodendroglial cell. During postnatal development, transcriptionally similar OPCs acquire characteristics based on the microenvironment, which may also change with age. Thanks to mounting discoveries, the functional differences between OPC subpopulations are coming into light. Is differentiation into myelinating oligodendrocyte the only purpose of OPCs or do they have other functions? Considering the indicative molecular pathways, and the electrophysiological differences between OPCs, there may be an OPC subpopulation that is highly synaptically connected, tuning in for neuronal activity from different parts of a neural circuit, and infrequently dividing (Káradóttir et al., 2008; Marisca et al., 2020; Mount et al., 2019). Meanwhile, another OPC subpopulation which receives limited synaptic input but frequently divides to supply new mature oligodendrocytes may be the major source of new myelin (Bacmeister et al., 2020; Marisca et al., 2020). In the realm of adaptive myelination, it is not clear which of these OPC populations initially senses neuronal activity, but the dividing and differentiating OPC subpopulation is likely to be the respondents (Marisca et al., 2020). This oligodendroglial response to neuronal activity varies depending on the neural circuit indicating a possible functional heterogeneity amongst OPCs as well as differences between neuronal subtypes. Considering the activity-dependent regulation of myelination throughout life, it is conceivable that these neuron-oligodendroglial interactions may become maladaptive in disease states, aggravating neuropathological conditions. Elucidating the molecular mechanisms mediating diverse myelin responses throughout the nervous system and the effects of myelin plasticity at the circuit level will be pivotal to understanding the role of myelin plasticity in nervous system development, health and disease.
Acknowledgements:
The authors gratefully acknowledge support from the National Institute of Neurological Disorders and Stroke (R01NS092597), NIH Director’s Pioneer Award (DP1NS111132), Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation (M.M.)
Footnotes
Yalçın and Monje present a Review discussing how microenvironmental interactions guide and modulate myelination, from oligodendrocyte precursor cell migration to oligodendrocyte differentiation and the formation of stable myelin internodes. During development and beyond, neuron-oligodendroglial interactions shape activity and experience-dependent myelin adaptations to fine-tune circuit dynamics and promote healthy neurological function.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agosta F, Pievani M, Sala S, Geroldi C, Galluzzi S, Frisoni GB, and Filippi M (2011). White matter damage in Alzheimer disease and its relationship to gray matter atrophy. Radiology 258, 853–863. [DOI] [PubMed] [Google Scholar]
- Almeida RG (2018). The rules of attraction in central nervous system myelination. Front. Cell. Neurosci 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacmeister CM, Barr HJ, McClain CR, Thornton MA, Nettles D, Welle CG, and Hughes EG (2020). Motor learning promotes remyelination via new and surviving oligodendrocytes. Nat. Neurosci 23, 819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera K, Chu P, Abramowitz J, Steger R, Ramos RL, and Brumberg JC (2013). Organization of myelin in the mouse somatosensory barrel cortex and the effects of sensory deprivation. Dev. Neurobiol 73, 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, and Raff MC (1993). Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361, 258–260. [DOI] [PubMed] [Google Scholar]
- Barres BA, Koroshetz WJ, Swartz KJ, Chun LLY, and Corey DP (1990). Ion channel expression by white matter glia: The O-2A glial progenitor cell. Neuron 4, 507–524. [DOI] [PubMed] [Google Scholar]
- Barres BA, Schmid R, Sendnter M, and Raff MC (1993). Multiple extracellular signals are required for long-term oligodendrocyte survival. Development 118, 283–295. [DOI] [PubMed] [Google Scholar]
- Batiuk MY, Martirosyan A, Wahis J, de Vin F, Marneffe C, Kusserow C, Koeppen J, Viana JF, Oliveira JF, Voet T, et al. (2020). Identification of region-specific astrocyte subtypes at single cell resolution. Nat. Commun 11, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechler ME, Byrne L, and Ffrench-Constant C (2015). CNS Myelin Sheath Lengths Are an Intrinsic Property of Oligodendrocytes. Curr. Biol 25, 2411–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, and Ullén F (2005). Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci 8, 1148–1150. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Roberts JDB, Somogyl P, and Jahr CE (2000). Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191. [DOI] [PubMed] [Google Scholar]
- de Biase LM, Kang SH, Baxi EG, Fukaya M, Pucak ML, Mishina M, Calabresi PA, and Bergles DE (2011). NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J. Neurosci 31, 12650–12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biase LM, Nishiyama A, and Bergles DE (2010). Excitability and Synaptic Communication within the Oligodendrocyte Lineage. J. Neurosci 30, 3600–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Yücel M, Fornito A, Pantelis C, Harrison BJ, Cocchi L, Pell G, and Lubman DI (2012). White matter microstructure in opiate addiction. Addict. Biol 17, 141–148. [DOI] [PubMed] [Google Scholar]
- Borges K, Ohlemeyer C, Trotter J, and Kettenmann H (1994). Ampa/kainate receptor activation in murine oligodendrocyte precursor cells leads to activation of a cation conductance, calcium influx and blockade of delayed rectifying K+ channels. Neuroscience 63, 135–149. [DOI] [PubMed] [Google Scholar]
- Bourbon-Teles J, Bells S, Jones DK, Coulthard E, Rosser A, and Metzler-Baddeley C (2019). Myelin Breakdown in Human Huntington’s Disease: Multi-Modal Evidence from Diffusion MRI and Quantitative Magnetization Transfer. Neuroscience 403, 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo N, Goudriaan A, van Deijk A-LF, Otte WM, Brouwers JF, Lodder H, Gutmann DH, Nave K-A, Dijkhuizen RM, Mansvelder HD, et al. (2017). Oligodendroglial myelination requires astrocyte-derived lipids. PLOS Biol. 15, e1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavali M, Ulloa-Navas MJ, Pérez-Borredá P, Garcia-Verdugo JM, McQuillen PS, Huang EJ, and Rowitch DH (2020). Wnt-Dependent Oligodendroglial-Endothelial Interactions Regulate White Matter Vascularization and Attenuate Injury. Neuron 108, 1130–1145.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Duan H, Xiao L, and Gan J (2018). Genetic and Epigenetic Alterations Underlie Oligodendroglia Susceptibility and White Matter Etiology in Psychiatric Disorders. Front. Genet 9, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhász C, Nagy F, and Chugani DC (2001). Local Brain Functional Activity Following Early Deprivation: A Study of Postinstitutionalized Romanian Orphans. Neuroimage 14, 1290–1301. [DOI] [PubMed] [Google Scholar]
- Czopka T, ffrench-Constant C, and Lyons DA (2013). Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths invivo. Dev. Cell 25, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC, Sojkova J, Hurley S, Kecskemeti S, Okonkwo O, Bendlin BB, Theisen F, Johnson SC, Alexander AL, and Gallagher CL (2016). Alterations of Myelin Content in Parkinson’s Disease: A Cross-Sectional Neuroimaging Study. PLoS One 11, e0163774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, and Lubetzki C (1996). Induction of myelination in the central nervous system by electrical activity. Proc. Natl. Acad. Sci. U. S. A 93, 9887–9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenthen GS, Backes WH, Aldenkamp AP, Vermeulen RJ, Klinkenberg S, and Jansen JFA (2020). On the merits of non-invasive myelin imaging in epilepsy, a literature review. J. Neurosci. Methods 338, 108687. [DOI] [PubMed] [Google Scholar]
- Dutta DJ, Woo DH, Lee PR, Pajevic S, Bukalo O, Huffman WC, Wake H, Basser PJ, SheikhBahaei S, Lazarevic V, et al. (2018). Regulation of myelin structure and conduction velocity by perinodal astrocytes. Proc. Natl. Acad. Sci 115, 11832–11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal TJ (2006). Abnormal Brain Connectivity in Children After Early Severe Socioemotional Deprivation: A Diffusion Tensor Imaging Study. Pediatrics 117, 2093–2100. [DOI] [PubMed] [Google Scholar]
- Emery B, Agalliu D, Cahoy JD, Watkins TA, Dugas JC, Mulinyawe SB, Ibrahim A, Ligon KL, Rowitch DH, and Barres BA (2009). Myelin Gene Regulatory Factor Is a Critical Transcriptional Regulator Required for CNS Myelination. Cell 138, 172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Möbius W, et al. (2012). Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, Mcbain CJ, Wright P, Knutson PL, and Armstrong2 RC (1996). Oligodendrocyte Progenitor Cell Proliferation and Lineage Progression Are Regulated by Glutamate Receptor-Mediated K+ Channel Block. [DOI] [PMC free article] [PubMed]
- Gandal MJ, Zhang P, Hadjimichael E, Walker RL, Chen C, Liu S, Won H, Van Bakel H, Varghese M, Wang Y, et al. (2018). Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science (80-. ). 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty AC, Gibson EM, Ghanem RA, Greene JJ, Ocampo A, Goldstein AK, Ni L, Yang T, Marton RM, Paşca SP, et al. (2019). Loss of Adaptive Myelination Contributes to Methotrexate Chemotherapy-Related Cognitive Impairment. Neuron 103, 250–265.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, et al. (2014). Neuronal Activity Promotes Oligodendrogenesis and Adaptive Myelination in the Mammalian Brain. Science (80-. ). 344, 1252304–1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, Greene JJ, Geraghty AC, Goldstein AK, Ni L, et al. (2019). Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell 176, 43–55.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeyer N, Hanft KM, Akriditou MA, Unger N, Park ES, Stanley ER, Staszewski O, Dimou L, and Prinz M (2017). Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. 134, 441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, Walker AJ, Gergits F, Segel M, Nemesh J, et al. (2019). Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 50, 253–271.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand C, Remahl S, Persson H, and Bjartmar C (1993). Myelinated nerve fibres in the CNS. Prog. Neurobiol 40, 319–384. [DOI] [PubMed] [Google Scholar]
- Hill RA, Patel KD, Goncalves CM, Grutzendler J, and Nishiyama A (2014). Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nat. Neurosci 17, 1518–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines JH, Ravanelli AM, Schwindt R, Scott EK, and Appel B (2015). Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci 18, 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AN, and Appel B (2020). Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci 23, 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Orthmann-Murphy JL, Langseth AJ, and Bergles DE (2018). Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. [DOI] [PMC free article] [PubMed]
- Káradóttir R, Cavelier P, Bergersen LH, and Attwell D (2005). NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438, 1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Káradóttir R, Hamilton NB, Bakiri Y, and Attwell D (2008). Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat. Neurosci. 11, 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, and Appel B (2006). In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat. Neurosci 9, 1506–1511. [DOI] [PubMed] [Google Scholar]
- Kougioumtzidou E, Shimizu T, Hamilton NB, Tohyama K, Sprengel R, Monyer H, Attwell D, and Richardson WD (2017). Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. Elife 6, 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, and Dietrich D (2007). Vesicular glutamate release from axons in white matter. [DOI] [PubMed]
- Kula B, Chen T-J, and Kukley M (2019). Glutamatergic signaling between neurons and oligodendrocyte lineage cells: Is it synaptic or non-synaptic? [DOI] [PubMed]
- Lee S, Chong SYC, Tuck SJ, Corey JM, and Chan JR (2013). A rapid and reproducible assay for modeling myelination by oligodendrocytes using engineered nanofibers. Nat. Protoc 8, 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, and Bergles DE (2004). Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci 7, 24–32. [DOI] [PubMed] [Google Scholar]
- Liu J, Dietz K, Deloyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, et al. (2012). Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü Scher C, and Malenka RC (2011). Review Drug-Evoked Synaptic Plasticity in Addiction: From Molecular Changes to Circuit Remodeling. Neuron 69, 650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans KA, Swire M, Volbracht K, Gautier HOB, Franklin RJM, ffrench-Constant C, et al. (2013). Neuregulin and BDNF Induce a Switch to NMDA Receptor-Dependent Myelination by Oligodendrocytes. PLoS Biol. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, and Corfas G (2012). A Critical Period for Social Experience-Dependent Oligodendrocyte Maturation and Myelination. Science (80-. ). 337, 1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin J-M, Li P, Scafidi J, and Gallo V (2012). Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nat. Neurosci 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marisca R, Hoche T, Agirre E, Hoodless LJ, Barkey W, Auer F, Castelo-Branco G, and Czopka T (2020). Functionally distinct subgroups of oligodendrocyte precursor cells integrate neural activity and execute myelin formation. Nat. Neurosci 23, 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques S, Zeisel A, Codeluppi S, van Bruggen D, Mendanha Falcao A, Xiao L, Li H, Haring M, Hochgerner H, Romanov RA, et al. (2016). Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science (80-. ). 352, 1326–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques S, van Bruggen D, Vanichkina DP, Floriddia EM, Munguba H, Väremo L, Giacomello S, Falcão AM, Meijer M, Björklund ÅK, et al. (2018). Transcriptional Convergence of Oligodendrocyte Lineage Progenitors during Development. Dev. Cell 46, 504–517.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, Paes de Faria J, Emery B, Tohyama K, and Richardson WD (2014). Motor skill learning requires active central myelination. Science (80-. ). 346, 318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, and Lyons DA (2015). Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci 18, 628–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, Van Wijngaarden P, Wagers AJ, Williams A, Franklin RJM, et al. (2013). M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci 16, 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitew S, Gobius I, Fenlon LR, McDougall SJ, Hawkes D, Xing YL, Bujalka H, Gundlach AL, Richards LJ, Kilpatrick TJ, et al. (2018). Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun 9, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Meschkat M, Ruhwedel T, Trevisiol A, Tzvetanova ID, Battefeld A, Kusch K, Kole MHP, Strenzke N, Möbius W, et al. (2020). A role of oligodendrocytes in information processing. Nat. Commun 11, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount CW, Yalçın B, Cunliffe-Koehler K, Sundaresh S, and Monje M (2019). Monosynaptic tracing maps brain-wide afferent oligodendrocyte precursor cell connectivity. Elife 8, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Mancini SJ, Clayworth KV, Arbabi K, and Beshara S (2020). Experience-Dependent Changes in Myelin Basic Protein Expression in Adult Visual and Somatosensory Cortex. Front. Cell. Neurosci 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA, and Ehrenreich H (2014). Myelination and oligodendrocyte functions in psychiatric diseases. JAMA Psychiatry 71, 582–584. [DOI] [PubMed] [Google Scholar]
- Noori R, Park D, Griffiths JD, Bells S, Frankland PW, Mabbott D, and Lefebvre J (2020). Activity-dependent myelination: A glial mechanism of oscillatory self-organization in large-scale brain networks. Proc. Natl. Acad. Sci. U. S. A 117, 13227–13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares R, Montiel J, and Aboitiz F (2001). Species differences and similarities in the fine structure of the mammalian corpus callosum. Brain. Behav. Evol 57, 98–105. [DOI] [PubMed] [Google Scholar]
- Oosterhof N, Chang IJ, Karimiani EG, Kuil LE, Jensen DM, Daza R, Young E, Astle L, van der Linde HC, Shivaram GM, et al. (2019). Homozygous Mutations in CSF1R Cause a Pediatric-Onset Leukoencephalopathy and Can Result in Congenital Absence of Microglia. Am. J. Hum. Genet 104, 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orthmann-Murphy JL, Freidin M, Fischer E, Scherer SS, and Abrams CK (2007). Two distinct heterotypic channels mediate gap junction coupling between astrocyte and oligodendrocyte connexins. J. Neurosci 27, 13949–13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajevic S, Basser PJ, and Fields RD (2014). Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 276, 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S, Mayoral SR, Choi HS, Chan JR, and Kheirbek MA (2020). Preservation of a remote fear memory requires new myelin formation. Nat. Neurosci 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond SA, Mei F, Eshed-Eisenbach Y, Osso LA, Leshkowitz D, Shen YAA, Kay JN, Aurrand-Lions M, Lyons DA, Peles E, et al. (2016). Somatodendritic Expression of JAM2 Inhibits Oligodendrocyte Myelination. Neuron 91, 824–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K, Murtie JC, El-Khodor BF, Edgar N, Sardi SP, Hooks BM, Benoit-Marand M, Chen C, Moore H, O’Donnell P, et al. (2007). Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc. Natl. Acad. Sci. U. S. A 104, 8131–8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab AS, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, Kusch K, Möbius W, Goetze B, Jahn HM, Huang W, et al. (2016). Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism. Neuron 91, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliani A, Perraud B, Duval T, Stikov N, Rossignol S, and Cohen-Adad J (2017). Axon and myelin morphology in animal and human spinal cord. Front. Neuroanat 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio-Baptista C, Khrapitchev AA, Foxley S, Schlagheck T, Scholz J, Jbabdi S, DeLuca GC, Miller KL, Taylor A, Thomas N, et al. (2013). Motor Skill Learning Induces Changes in White Matter Microstructure and Myelination. J. Neurosci 33, 19499–19503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TEJ, and Johansen-Berg H (2009). Training induces changes in white-matter architecture. Nat. Neurosci 12, 1370–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl AH, and Rubel EW (2016). Systematic and differential myelination of axon collaterals in the mammalian auditory brainstem. Glia 64, 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl AH, Rubel EW, and Barría A (2014). Differential Conduction Velocity Regulation in Ipsilateral and Contralateral Collaterals Innervating Brainstem Coincidence Detector Neurons. J. Neurosci 34, 4914–4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer SO, Sitnikov S, Kamen Y, and De Faria O (2019). Oligodendrocyte Progenitor Cells Become Regionally Diverse and Heterogeneous with Age. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steadman PE, Xia F, Ahmed M, Mocle AJ, Penning ARA, Geraghty AC, Steenland HW, Monje M, Josselyn SA, and Frankland PW (2020). Disruption of Oligodendrogenesis Impairs Memory Consolidation in Adult Mice. Neuron 105, 150–164.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CJ, Bailey JA, Zatorre RJ, and Penhune VB (2013). Early Musical Training and White-Matter Plasticity in the Corpus Callosum: Evidence for a Sensitive Period. J. Neurosci 33, 1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swire M, Kotelevtsev Y, Webb DJ, Lyons DA, and Ffrench-Constant C (2019). Endothelin signalling mediates experience-dependent myelination in the CNS. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassy GS, Berger DR, Chen H-H, Kasthuri N, Hayworth KJ, Vercelli A, Seung HS, Lichtman JW, and Arlotta P (2014). Distinct Profiles of Myelin Distribution Along Single Axons of Pyramidal Neurons in the Neocortex. Science (80-. ). 344, 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tress O, Maglione M, May D, Pivneva T, Richter N, Seyfarth J, Binder S, Zlomuzica A, Seifert G, Theis M, et al. (2012). Panglial gap junctional communication is essential for maintenance of myelin in the CNS. J. Neurosci 32, 7499–7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi RB, Jackiewicz M, Mckenzie IA, Kougioumtzidou E, Grist M, Richardson Correspondence WD, and Richardson WD (2017). Remarkable Stability of Myelinating Oligodendrocytes in Mice. Cell Rep. 21, 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H-H, Niu J, Munji R, Davalos D, Chang J, Zhang H, Tien A-C, Kuo CJ, Chan JR, Daneman R, et al. (2016). Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science (80-. ). 351, 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viganò F, Möbius W, Götz M, and Dimou L (2013). Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat. Neurosci 16, 1370–1372. [DOI] [PubMed] [Google Scholar]
- Vondran MW, Clinton-Luke P, Honeywell JZ, and Dreyfus CF (2010). BDNF +/− mice exhibit deficits in oligodendrocyte lineage cells of the basal forebrain. Glia 58, 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Lee PR, and Fields RD (2011). Control of Local Protein Synthesis and Initial Events in Myelination by Action Potentials. Science (80-. ). 333, 1647–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Ortiz FC, Woo DH, Lee PR, Angulo MC, and Fields RD (2015). Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat. Commun 6, 7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarczyk A, Holtman IR, Krueger M, Yogev N, Bruttger J, Khorooshi R, Benmamar-Badel A, Boer-Bergsma JJ, Martin NA, Karram K, et al. (2017). A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J. 36, 3292–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AW, Xiao J, Kemper D, Kilpatrick TJ, and Murray SS (2013). Oligodendroglial Expression of TrkB Independently Regulates Myelination and Progenitor Cell Proliferation. J. Neurosci 33, 4947–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Ohayon D, McKenzie IA, Sinclair-Wilson A, Wright JL, Fudge AD, Emery B, Li H, and Richardson WD (2016). Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat. Neurosci 19, 1210–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SM, Michel K, Jokhi V, Nedivi E, and Arlotta P (2020). Neuron class-specific responses govern adaptive myelin remodeling in the neocortex. Science 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung MSY, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, et al. (2014). Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159, 766–774. [DOI] [PubMed] [Google Scholar]
- Yuan X, Eisen AM, McBain CJ, and Gallo V (1998). A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development 125, 2901–2914. [DOI] [PubMed] [Google Scholar]
- Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SPJ, Zahed H, Maltepe E, and Rowitch DH (2014). Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell 158, 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, and Barres BA (2010). Astrocyte heterogeneity: An underappreciated topic in neurobiology. Curr. Opin. Neurobiol 20, 588–594. [DOI] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, and Bergles DE (2007). Vesicular release of glutamate from unmyelinated axons in white matter. [DOI] [PMC free article] [PubMed]
- Zonouzi M, Scafidi J, Li P, McEllin B, Edwards J, Dupree JL, Harvey L, Sun D, Hübner CA, Cull-Candy SG, et al. (2015). GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nat. Neurosci 18, 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonouzi M, Berger D, Jokhi V, Kedaigle A, Lichtman J, and Arlotta P (2019). Individual Oligodendrocytes Show Bias for Inhibitory Axons in the Neocortex. Cell Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]


