Abstract
For almost a century, researchers have puzzled over how the orbitofrontal cortex (OFC) contributes to behavior. Our understanding of the functions of this area have evolved as each new finding and piece of information is added to complete the larger picture. Despite this, the full picture of OFC function is incomplete. Here we begin by reviewing recent (and not so recent) theories of how OFC contributes to behavior. We then go onto highlight emerging work that has helped to broaden perspectives on the role that OFC plays in contingent learning, interoception, and social behavior. How OFC contributes to these aspects of behavior is not well understood. Here we argue that only by establishing where and how these and other functions fit within the puzzle of OFC, either alone or as part of larger brain-wide circuits, will we be able to fully realize the functions of this area.
Keywords: orbitofrontal cortex, prefrontal cortex, interoception, emotion, learning
INTRODUCTION
The precise functions of the orbitofrontal cortex (OFC, a prefrontal cortical region comprising Walker’s areas 11, 13, and 14) have been a puzzle for over a century. Initially, it was thought that OFC was important for inhibitory control of responses. Damage to this subregion of the prefrontal cortex is associated with impairments in foregoing prepotent responses as well as striking changes in affect (Butter, 1969; Fellows and Farah, 2003; Izquierdo and Murray, 2004). With the advent of more advanced methods, both behavioral and interventional, the idea that OFC is central to generally inhibiting ongoing behavior has faded, along with other ideas about somatic states. Instead a new set of ideas has emerged which emphasize a role for OFC as either the site of value computation or the representation of goal outcomes (Padoa-Schioppa and Schoenbaum, 2015).
The hypothesis that OFC is central to computing value was based largely on insights from neurophysiology studies that found that the activity of single neurons in OFC varied in step with the subjective value of rewards (Thorpe et al., 1983; Padoa-Schioppa and Assad, 2006). However, these findings are complicated by several factors. First, neurons selective for subjective reward are not unique to OFC and appear in other areas such as medial prefrontal cortex (MFC) and amygdala (Rudebeck et al., 2013a; Jezzini and Padoa-Schioppa, 2020). This potentially indicates that rather than being the site of reward valuation in the brain, OFC is part of a larger reward network (Baxter et al., 2000; Wellman et al., 2005; West et al., 2011). Second, there are questions about whether OFC is specifically involved in computing value per se or whether a role in value occurs as a result of signaling specific stimulus- or action-outcome associations (Holland and Gallagher, 2004).
A role for signaling outcomes has recently developed into the idea that OFC is a critical site for representing a map of ‘task states’ for ongoing behavior (Wilson et al., 2014). In the reinforcement learning literature, the underlying structure of a task is divided into multiple states, each associated with a reward when reached, and where transitions between states depend on actions (Niv, 2019; Bartolo and Averbeck, 2020). Information about future task states becomes vital when a human or animal has to consider the values of actions and conditioned associations during ongoing behavior. Notably, task states are hypothesized to involve circuit-level interactions between hippocampus, striatum, and OFC (McKenzie et al., 2016; Wikenheiser and Schoenbaum, 2016; Zhou et al., 2019). How state representations are organized in OFC is an open question, but some have speculated that abstract cognitive maps potentially use grid-like codes similar to what has been reported in entorhinal cortex and medial prefrontal cortex (Constantinescu et al., 2016; Behrens et al., 2018).
As the prior discussion emphasizes, the past half-century has seen a systematic narrowing of our understanding of what OFC does by ruling out what it does not do (for an excellent review see Stalnaker et al., 2015). To use the puzzle analogy, in order to see the broader picture of OFC functions, researchers have started to complete the borders of the OFC puzzle (Figure 1). Filling in the center - that is, functions for which the OFC is affirmatively involved in or required for – will be more complicated. In this review we discuss some sections of the puzzle we believe are needed to complete the full picture of the functions of OFC. In particular, we highlight areas of research on OFC that have received different levels of attention: contingent learning, interoception, and social valuation (Figure 1). We finish by speculating how these seemingly disparate functional pieces may actually interlock at the level of OFC as well as the next steps that could lead to completing the OFC puzzle.
Figure 1. The orbitofrontal puzzle.
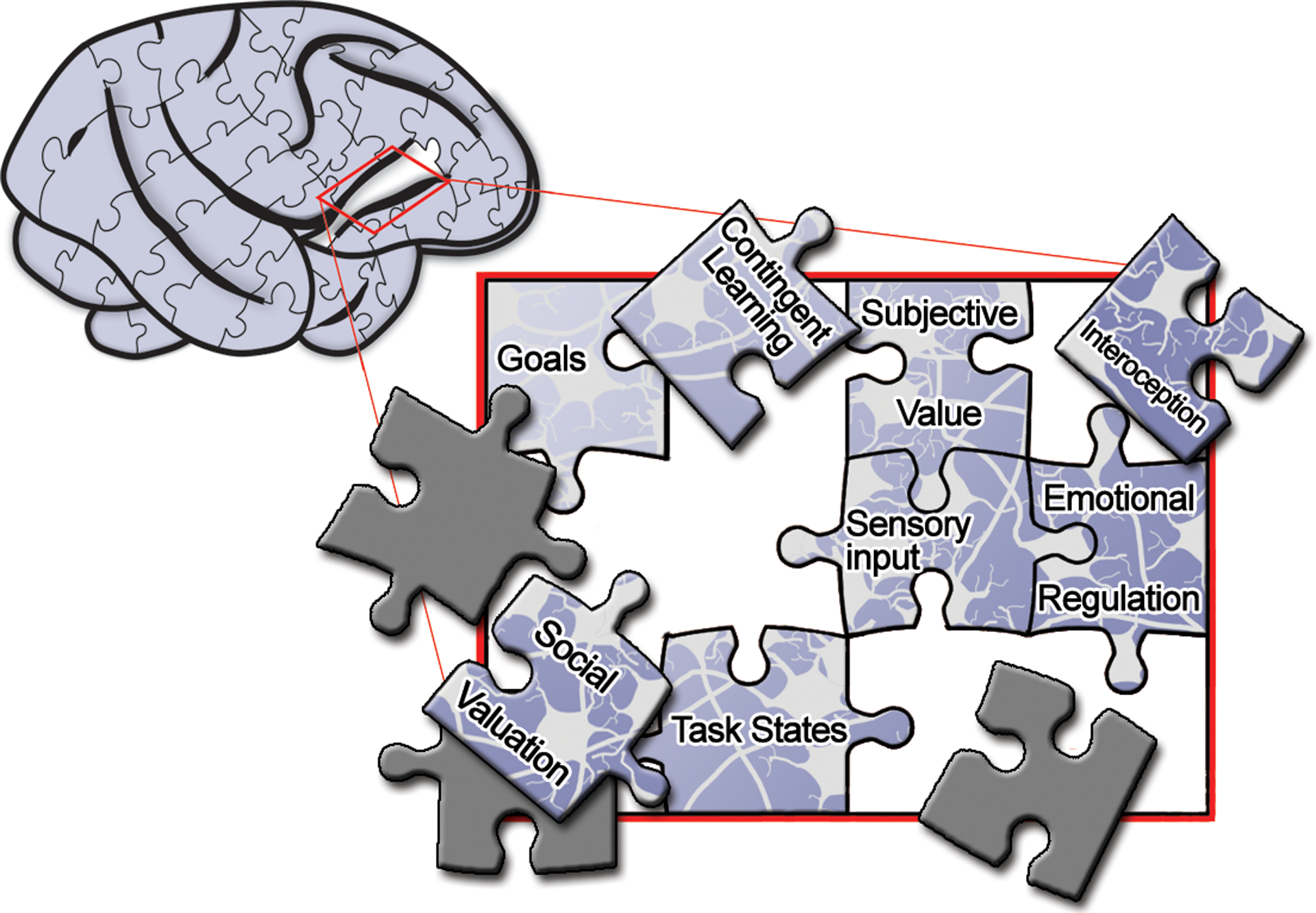
Whereas some edge pieces of the orbitofrontal puzzle are firmly in place; others remain to be placed correctly. Others are still undiscovered and thus are missing from the final picture.
Role of OFC in contingent stimulus-reward learning
Contingent learning is the process of acquiring information to make predictions about the outcomes, either aversive or rewarding, that will likely occur as a result of selecting a particular course of action (Chudasama and Robbins, 2003; Schoenbaum et al., 2003; Izquierdo et al., 2004; Hampton et al., 2007; Rygula et al., 2010; Sternberg and McClelland, 2012). The term contingent learning incorporates many forms of learning (Bindra, 1974; Payne, 1982). Here we specifically focus on the process of learning whether a stimulus is predictive of a reward, irrespective of the identity or sensory qualities of the reward. This process is sometimes referred to as model-free learning and is in contrast to outcome-specific forms of learning, sometimes referred to as model-based learning, that incorporate the sensory qualities of reward into associations. Consequently, we focus on results from probabilistic reward-learning tasks as they have provided additional insight into contingent stimulus-reward learning in non-human primates.
Ventral PFC and interconnected parts of the limbic system are essential for contingent learning between stimuli and rewards. Lesions of OFC in humans, monkeys, mice, and other species are associated with marked deficits in stimulus-reward learning, as well as emotional responding (Rolls, 2000; Rudebeck et al., 2008). More recent studies exploring the effect of aspiration lesions of the entire OFC (Walker’s Areas 11, 13, and 14) in humans and macaques found that subjects were also impaired in a task where they had to learn and track which of three stimuli was associated with the highest probability of receiving a food reward (Walton et al., 2010; Noonan et al., 2017). These deficits were associated with an inability to make contingent associations between stimulus choices and reward or non-reward. Without an OFC, both humans and macaques rely on either their history of rewards or choices to guide future choices in the task.
This role for OFC in stimulus-reward contingency learning has, however, been called into question by a recent series of studies of macaques with excitotoxic lesions of the whole OFC (Rudebeck et al., 2013b). Unlike aspiration lesions, excitotoxic lesions spare white matter tracts adjacent to the lesion. In contrast to the effects of aspiration lesions, excitotoxic OFC lesions do not affect either reversal learning or emotional responses to anxiogenic stimuli, two of the classic effects associated with damage to OFC (Izquierdo et al., 2004, 2005). Similarly, excitotoxic lesions of OFC do not impact subjects’ performance in the aforementioned probabilistic learning task (Rudebeck et al., 2017b). Instead, the laterally adjacent ventrolateral PFC (Walker’s Areas 12, 45, and ventral 46), not OFC, appears to be critical for contingent stimulus-reward learning.
The results discussed above indicate that OFC does not contribute to contingent stimulus-reward learning and therefore the previously reported effects after aspiration lesions were caused by damage to white matter fibers. One reason for thinking that such a conclusion may not tell the whole story comes from a recent study showing that subtotal lesions of OFC have different behavioral effects than lesions that encompass the whole OFC. Specifically, Pujara and colleagues found that subtotal excitotoxic lesions of either medial OFC (corresponding to Walkers area 14) or lateral OFC (corresponding to Walkers areas 11 and 13) heightened defensive emotional responses to snakes (Pujara et al., 2019). This pattern of effects parallels what has been seen in marmosets after subtotal OFC lesions (Shiba et al., 2015) and potentially helps to explain the link between ventral PFC dysfunction and heightened emotional responding in clinical populations (Killgore et al., 2014).
While altered affective responding after subtotal lesions of OFC may not seem relevant to a discussion on contingent stimulus-reward learning, it is important to realize that nearly every study that has reported deficits in stimulus-reward learning after OFC damage has also reported alterations in emotional responding (for instance, Izquierdo et al., 2004, 2005; Rudebeck et al., 2006b, 2007). Indeed, in humans with damage to OFC there is a direct relationship between impairment in stimulus-reward learning and changes in emotion (Rolls et al., 1994). Note, that large excitotoxic OFC lesions are without effect on stimulus-reward learning and emotional responding in macaques (Rudebeck et al., 2013b). If subtotal lesions of OFC impact affective responding, then larger lesions must have obscured the effects of smaller lesions. It also potentially indicates that subtotal lesions of OFC could differentially contribute to contingent stimulus reward learning. Indeed, prior studies in rodents (Dalton et al., 2016), macaques (Noonan et al., 2010b; Murray and Rudebeck, 2018), and humans (Noonan et al., 2017) have reported that there are regional differences in OFC function. Furthermore, the effects of subtotal lesions being masked by larger lesions is not without precedent; the effects of infralimbic (IL) lesions on fear conditioning were often obscured by larger lesions that included IL and prelimbic cortex (Giustino and Maren, 2015).
How might excitotoxic lesions of either medial (Walker’s area 14) or lateral (Walker’s areas 11 and 13) OFC impact contingent stimulus-reward learning? Because aspiration and excitotoxic lesions of OFC have such different effects on behavior, it is questionable as to whether subtotal aspiration lesions of OFC will provide useful pointers (Noonan et al., 2010b). Neurophysiology studies indicate that there are marked differences in stimulus-reward encoding between the two areas (Rich and Wallis, 2014). However, the vast majority of these studies assessed single neuron responses after learning and those that did assess responding during learning only recorded within lateral OFC (for instance, Rudebeck et al., 2017a). Given the lack of available data, differences in anatomical connectivity might provide a clue. One prominent distinction between medial and lateral OFC is that each receives different levels of input from hippocampus and amygdala; medial OFC receives more input from hippocampus (Barbas and Blatt, 1995) whereas lateral OFC, especially caudo-lateral OFC, receives stronger input from amygdala (Aggleton et al., 2015). This difference between amygdala and hippocampal connections to OFC is largely conserved across humans, macaques, and rodents (McDonald, 1987; Jay and Witter, 1991; Neubert et al., 2014, 2015; Murphy and Deutch, 2018).
Recently, Costa and colleagues showed that lesions of amygdala are associated with deficits in learning probabilistic stimulus-reward associations (Costa et al., 2016). Similar deficits are apparent in deterministic learning settings as well (Schoenbaum et al., 2003; Rudebeck et al., 2017a). Following this, studies in rodents reported that interaction between amygdala and OFC is necessary during reward-related learning. In particular, Groman and colleagues probed the role of the connections between amygdala and whole OFC using diphtheria toxin to specifically lesion OFC projections to and from amygdala (Groman et al., 2019). Destruction of projections from amygdala to OFC caused a deficit in probabilistic learning, whereas destruction of projections from OFC to amygdala was associated with improved performance, especially after reversals. Thus, contingent learning between stimuli and rewards appears to be dependent on communication between OFC and amygdala in rodents. Within this circuit, projections from the amygdala to OFC are necessary for forming contingent associations between stimuli and rewards, while the projection from the OFC to amygdala is involved in maintaining previously learned value associations.
How Groman and colleagues’ findings from rodents translate to non-human primates is unclear as the ventral PFC in primates is expanded and differentiated compared to rodents (Preuss, 1995). fMRI studies in non-human primates indicate that interaction between amygdala and ventrolateral PFC, (specifically Walkers area 12) and not lateral OFC, is engaged during contingent stimulus-reward learning (Chau et al., 2015). Integrating these two findings indicates that interaction between non-human primate ventrolateral PFC and amygdala is required for contingent stimulus-reward learning, whereas lateral OFC is not. Thus, lesions of lateral OFC should be without effect on contingent learning. Instead, lateral OFC in non-human primates (Walkers areas 11 and 13) may be specialized for representing the specific sensory qualities of the reward that will follow a stimulus (Rudebeck and Murray, 2011; West et al., 2011; Murray et al., 2015), representations that are known to depend on interaction with amygdala (Baxter et al., 2000; Fiuzat et al., 2017).
Also unknown is how lesions of medial OFC, which receives a prominent input from hippocampus, might impact stimulus-reward learning in macaques. Recently, using a combination of neural recordings and closed-loop electrical stimulation Knudsen and Wallis (2020) showed the importance of functional interaction between OFC and hippocampus for stimulus-reward learning. Neural activity was recorded in both structures simultaneously while macaques performed a probabilistic learning task. Using a generalized partial directed coherence analysis, they found that that learning-related theta oscillations in OFC are driven by input from hippocampus. Further, disrupting theta oscillations in either OFC or hippocampus impacted learning. Thus, hippocampal-OFC functional interactions are essential for normal patterns of contingent learning. However, in this study micro-stimulation was largely targeted to parts of lateral, not medial OFC. This points to a general mechanism being engaged between all of OFC and hippocampus during learning, but leaves open the question of what role specifically medial OFC plays.
At present, no single neuron recordings in medial OFC and hippocampus during stimulus-reward learning exist in macaques, but here results from rodents and lesion studies provide insight. Emerging research suggests that interaction between OFC and hippocampus is essential for reward-guided behavior by allowing representations of task states and task structure to be integrated into on-going reward-guided behavior and guide learning (Farovik et al., 2015; Wikenheiser et al., 2017). Hippocampal lesions in macaques disrupt the use of task structure during stimulus-reward reversal learning; in particular, macaques with lesions of hippocampus require more trials than controls after a reversal before switching their choices to the now rewarded option (Jang et al., 2015). While the same analysis failed to find any effect of medial OFC lesions, it is possible that ceiling effects obscured the impact of the lesion on the use of task structure to guide choices. Based on these results and our understanding of the underlying neuroanatomy, medial OFC lesions would be expected to impair macaques’ ability to switch to choosing a new option following a reversal.
In summary, it is currently unclear how distinct parts of OFC contribute to the different processes engaged during contingent stimulus reward-learning. While direct experiments testing their unique functions are required, extant data indicate that ventrolateral PFC, through interaction with amygdala, is the circuit that is essential for learning contingent relationships between stimuli and rewards (model-free learning; Murray and Rudebeck, 2018). The contributions of OFC areas are less clear, but we hypothesize that medial OFC through connections from hippocampus may be vital for representing task structure, representations that are critical for shaping future learning (Wikenheiser and Schoenbaum, 2016). By contrast, we speculate that lateral OFC is not required for contingent stimulus reward learning. Instead through interaction with amygdala lateral OFC may be specialized for learning and representing specific stimulus-outcome associations (i.e. model-based learning; Baxter et al., 2000; Fiuzat et al., 2017). Put another way, ventrolateral PFC- and lateral OFC-amygdala circuits are engaged to represent information about the end state of an action or association, whereas medial OFC-hippocampal pathways represent the structure of the environment, essentially how to transition to different states (Rushworth et al., 2011; Bradfield and Hart, 2020).
Interoception and the OFC
Interoception is the sensation and representation of internal bodily states (Craig et al., 2000; Craig, 2002). While insula cortex is seen as the central hub for interoception in the brain, a growing literature shows that OFC also plays a key role in integrating bodily states such as satiety and bodily arousal into ongoing behavior (García-Cordero et al., 2016). In healthy individuals, hemodynamic activity in OFC reflects current bodily arousal, as indexed by heart rate, as well as cortisol level during stressful tasks (Wang et al., 2005). Maladaptive processing of interoceptive states such as bodily arousal is a key symptom of anxiety disorders and has been linked to dysfunction within OFC (for a review see Paulus and Stein, 2010). Similarly, altered OFC representations of bodily state are speculated to contribute to major depressive disorder (Cusi et al., 2012).
Maladaptive processing of bodily states has also been reported in people and animals with OFC damage and indicate that OFC is a modulator, not a driver, of affective responding (Stalnaker et al., 2015). For instance, excitotoxic lesions of OFC in marmosets alters the relationship between overt behavior and cardiovascular arousal during reward guided behavior (Reekie et al., 2008). People with damage to OFC have diminished skin-conductance responses (SCR) in response to emotionally charged images (Damasio et al., 1990). In addition, electrical stimulation of OFC is associated with changes in heart rate, pupil dilation, and breathing rate (Kaada et al., 1949). Thus, OFC potentially has dual functions; it represents the current bodily state, but may also influence it.
Despite the known role for OFC in interoception, few studies have explored the single-neuron level mechanisms underlying representations of bodily arousal in OFC. This is in part due to the challenging nature of recording neural activity as well as monitoring measures of bodily arousal such as heart rate from awake animals during ongoing behaviors. Recently, we recorded the activity of single neurons in lateral OFC (specifically, Walker’s areas 11 and 13) and dorsal anterior cingulate cortex (dACC; Walker’s areas 9/24) while we also monitored heart rate (HR) in macaque monkeys. Recordings were made while monkeys made reward-guided decisions between stimuli that they had extensively-learned would lead to different amounts of reward (Fujimoto et al., 2021). To track the spontaneous changes in bodily arousal during the task, we specifically analyzed HR during the fixation period on each trial when monkeys were waiting for cues to be presented. In this period, HR is not influenced by anticipated reward, choice direction, or breath holding associated with consuming rewards and therefore is a proxy of spontaneous fluctuations in bodily arousal.
In this setting we found that the firing rate of a substantial proportion of lateral OFC neurons and similar proportion of dACC neurons tracked trial-to-trial fluctuations in HR. Because we explicitly dissociated HR from other task components, such as reward-value and movement direction, the encoding of HR within lateral OFC and dACC directly reflects bodily arousal, independent of other factors. It is important to note that we found a significant proportion of HR coding neurons in these parts of frontal cortex even during the fixation period. Based on current data, interoceptive representations in OFC likely incorporate multiple factors which contribute to HR including reward, satiety, and fatigue during ongoing behavior. We conclude that interoceptive representations in OFC as well as dACC reflect spontaneous fluctuations of bodily arousal regardless of task context. A role for OFC in interoception would be consistent with ideas about OFC as representing a cognitive map of task space, since interoceptive representations would provide internal state information during a task.
In addition, we were able to assess how representations of HR in lateral OFC and dACC are impacted when bodily arousal is tonically increased. This was possible as recordings were made both before and after bilateral amygdala lesions, which cause a tonic increase in HR (Mitz et al., 2017; Fujimoto et al., 2021). Bilateral amygdala lesions significantly reduced HR coding within lateral OFC, as well as reward value coding. This effect was unique to lateral OFC as HR coding in dACC was actually increased after lesions. Because bilateral amygdala lesions enhanced HR both at rest and during the task, this suggests that the effects seen in lateral OFC are the direct consequence of eliminating amygdala input to the OFC. In this view, OFC representations of bodily arousal are dependent on input from amygdala. Thus, dysfunctional interoceptive mechanisms in OFC seen in psychiatric disorders may be related to larger network-level dysfunction including amygdala-to-OFC projections (Paulus and Stein, 2010).
Of course, these initial insights into how HR is encoded in lateral OFC lead to further questions; how is the encoding of bodily arousal in OFC associated or integrated with (or not) other forms of affect-related representations? As discussed above, lateral OFC receives the majority of amygdala-OFC projections (Aggleton et al., 2015); is HR encoding localized to lateral OFC or do medial OFC neurons show similar functions? How do interoceptive signals in OFC bias representations of outcomes or costs that might be used to guide decision-making? While we found that single neuron encoding of bodily arousal and other variables were largely separate, we speculate that interoceptive representations may come to bias other representations such as rewards and punishments in OFC when bodily arousal profoundly fluctuates in response to affective events. This is because there is a non-linear relationship between arousal and performance (Yerkes and Dodson, 1908; Hebb, 1955), and thus interoceptive representations in OFC may influence the encoding of other variables in non-linear manner. In this view, interaction between the reward-value coding and interoceptive representation in OFC may bias decisions toward emotionally-charged objects, stimuli, or outcomes. Ultimately, to reveal the full picture of how OFC represents and contributes to bodily arousal and how this influences decision-making will require examining OFC activity in a variety of task settings that incorporate aspects of both learning and risk.
Role of OFC in valuation beyond the economic: social influences and social behavior
As discussed above, our current understanding of OFC places it firmly as a piece within the broader picture of valuation - minimally as providing estimates of future states that could enhance an organism’s fitness. OFC’s communication with amygdala, as well as other aspects of limbic circuitry, makes it an important contributor to affective responding but also to understanding associations between stimuli and outcomes as well as adjusting to different environments. In both human and non-human primates, social interactions are essential to survival, and appropriate social behaviors can enhance fitness. Thus, determinations of social value, such as assessing the face of a stranger as part of an approach-avoid determination, likely falls under the purview of OFC.
Lesion studies in humans were some of the first investigations into the role of OFC in social behaviors. Patients with damage to ventromedial frontal cortex (VMPFC), including medial parts of OFC, show impairment in social behavior, but no deficits in executive function such as objective problem-solving (Bechara et al., 2000; Rolls et al., 1994). At the time, these impairments in social decision making, including an inability to identify and respond appropriately to others’ emotions, were interpreted as a result of the OFC’s influence on somatic states (Damasio et al., 1990). Damasio and colleagues suggest that these patients possess all the knowledge about the social situation they might need to effectively navigate it, but they are unable to either apply the knowledge of the broader social landscape to the specific interaction in order to respond appropriately, or they are unable to effectively choose the socially appropriate response.
As our techniques have advanced, investigations of the OFC have been extended via neuroimaging and neurostimulation. Imaging studies have revealed that humans possess face-sensitive patches in OFC (Tsao et al., 2008a; Troiani et al., 2016). These regions have been defined as those that during functional MRI show increased activation when faces are presented as opposed to non-face objects. Accordingly, part of the difficulty that patients with OFC lesions may have in responding appropriately to social situations is that they have difficulty identifying facial expressions (Hornak et al., 2003; Heberlein et al., 2008). OFC lesion patients also appear to rely on ‘first judgments’ about a person rather than integrating different sources of information when making social decisions. For instance, people with lesions of lateral OFC made choices on politicians based only on facial attractiveness, as compared to controls who took into account both attractiveness and judgments of competency (Xia et al., 2015). This indicates a specific role of OFC in integrating different sources of information to provide accurate estimates of value.
Imaging studies in intact participants have further confirmed the vital role of OFC in discriminating between the value of different faces. For example, OFC responds preferentially to infant faces over adult faces (for review see Parsons et al., 2013) and to attractive adult faces over average-rated faces (Aharon et al., 2001; Ishai, 2007; Winston et al., 2007). Knowledge about other individual’s emotional state can help to guide choices or contribute to socially appropriate behavior. OFC seems to play a role in holding onto information about facial expression in working memory (LoPresti et al., 2008; Ross et al., 2013). Conversely, stimulation of OFC via tDCS can increase efficiency and speed of facial expression recognition (Willis et al., 2015). Total OFC volume predicts, in humans, both social network size and subjective rating of social well-being, a scale of values including social integration, contribution, actualization, and coherence (Powell et al., 2012; Kong et al., 2019). Taken together, these findings suggest a key role for OFC in recognition and subjective valuation of faces.
Similar findings have been reported in neuroimaging studies in macaques: three face-sensitive patches (prefrontal orbital, lateral, and anterior; PO, PL, PA, Figure 2) have been identified in the frontal cortex, and six in the temporal cortex (Tsao et al., 2003, 2008b; Janssens et al., 2014; Haile et al., 2019; Taubert et al., 2020). However, few studies have focused on face patches in frontal cortex or specifically OFC (Patch PO) (Ó Scalaidhe et al., 1999; Tsao et al., 2008b; Russ and Leopold, 2015). A functional connectivity study of face patches in macaques showed that area PO had strong functional connectivity with several temporal face patches, including areas MF (middle fundus), ML (middle lateral), and AM (anterior medial), as well as with broader regions like prefrontal, premotor, inferior parietal, temporal, and visual cortices (Schwiedrzik et al., 2015). That all face patches identified in this study were functionally connected to amygdala, OFC, insula, and hippocampus suggests a broader functional network for emotional responses to faces and facial expression which incorporates the OFC value networks.
Figure 2. Temporal and frontal lobe face patches in the macaque brain.
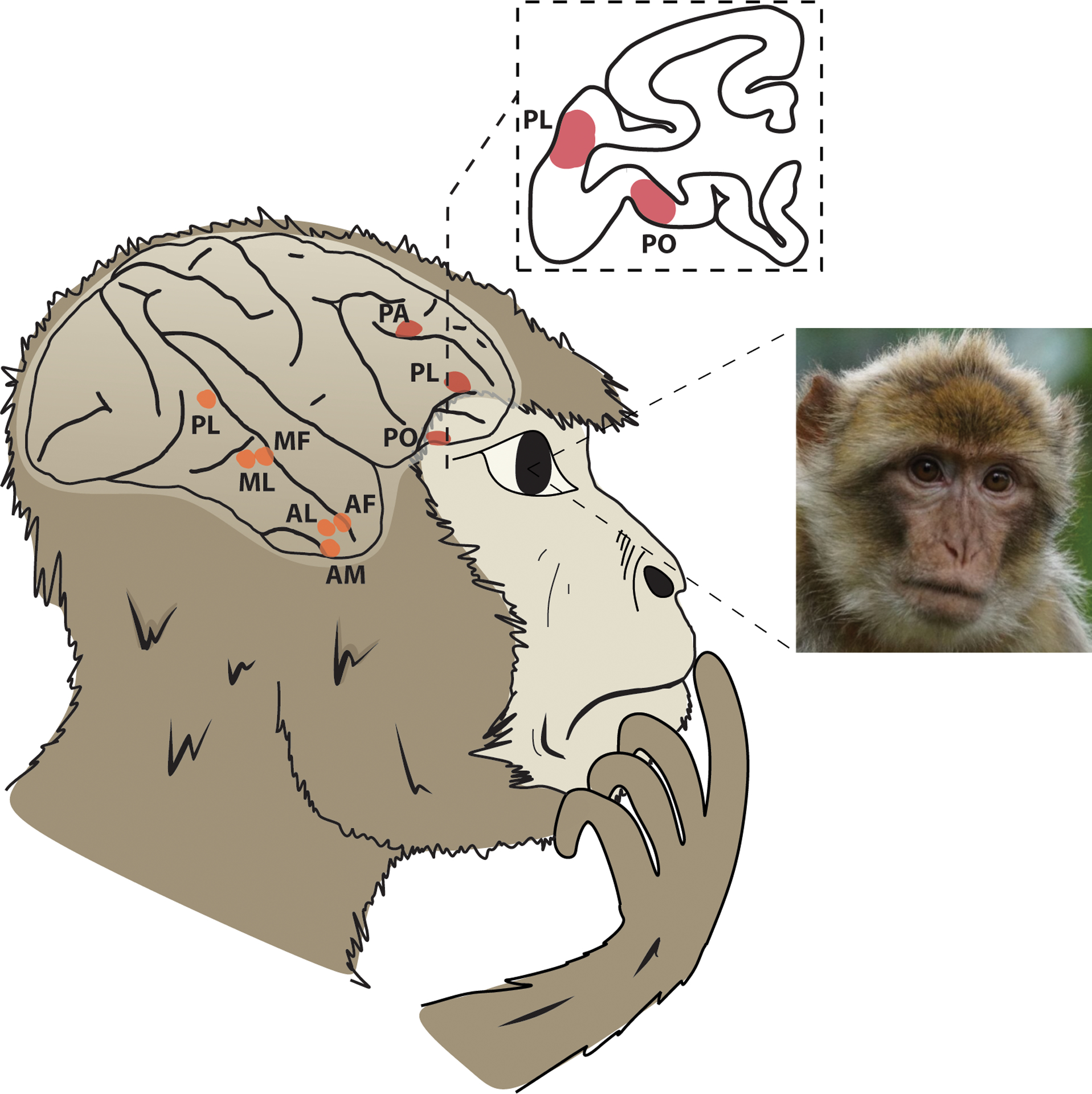
Schematic lateral view of the macaque brain showing the location of frontal face patches PA, PL, and PO in red (dark grey), and temporal face patches PL, ML, MF, AL, AF, and AM in orange (light gray). Inset figure shows a coronal section the macaque brain depicting the frontal face patches PL and PO in red. PO is located within the orbitofrontal cortex.
Neurophysiology recordings in macaques corroborate, and expand on, the findings from neuroimaging studies. Individual neurons within OFC have been observed to respond preferentially to socially-relevant visual stimuli such as unfamiliar conspecific faces (Thorpe et al., 1983; Rolls et al., 2005; Watson and Platt, 2012). A recent study in macaques recorded neural activity from OFC neurons in the presumed location of the face patch area PO observed in neuroimaging studies (Barat et al., 2018). This study only recorded from neurons that changed firing rate when the monkeys were presented with a face. These face-sensitive cells responded differentially to social categories and features such as eyes/body vs face, female vs male, and threat/grin expression vs neutral face. The neurons’ responses were not influenced by either vocal stimuli, pairing of facial and vocal stimuli, or associating faces with reward or punishments. Although other areas in OFC may respond to both of these types of inputs, it appears that the majority of face responsive neurons within PO are selective only for faces under the conditions tested. This finding is important as it means that face representations in PO and potentially other parts of OFC may not be integrated with other modalities, setting up a dedicated module for face related valuation. Notably, the site selected for recording in the study by Barat, and indeed the location of PO as described in many imaging studies is within lateral OFC. This potentially suggests that the valuation of face is localized to lateral OFC. Against this, other neural recording studies have reported no differences in response to social valuation across medial or lateral OFC neurons, suggesting this valuation function is integrated across the whole OFC (Watson and Platt, 2012).
OFC contains cells sensitive to faces, but what role does it play in social behavior? As discussed previously, OFC is anatomically connected to many cortical areas across the brain (most heavily to temporal and frontal cortex) as well as to the amygdala, hippocampus, thalamus, and other subcortical areas (Cavada et al., 2000). The anterior cingulate cortex (ACC) is a region of particular interest given the role of both OFC and ACC in rewarded behaviors (Rushworth et al., 2007; Khani, 2014). Aspiration lesions of ACC gyrus (ACCg) produce deficits in social behavior; ACCg lesion animals spent less time looking at social videos of other macaques and were faster to retrieve food items in the presence of social stimuli (Rudebeck et al., 2006a). Interestingly, lesions containing both lateral orbital and ventral prefrontal cortex, or lesions of medial OFC, produced no change in responsiveness to social stimuli, although they were associated with increased aggressive behaviors (Rudebeck et al., 2006a; Noonan et al., 2010a). This would appear to indicate that OFC alone, or at least not medial OFC, is not essential for the valuation of social stimuli, although it may form a part of a larger social valuation network. In some ways, these results are reminiscent of early OFC lesion studies in humans; perhaps, like human lesion patients, monkeys with medial OFC lesions still recognize and value social stimuli but respond inappropriately, i.e. with increased aggression.
The question of whether OFC itself contributes to social behavior has been further probed using novel paradigms where monkeys decide whether to give rewards to themselves, a conspecific, or both. In these so called vicarious reward tasks, OFC neurons respond most strongly when the animal chooses to reward only itself, not a conspecific (Azzi et al., 2012). This initial study by Azzi and colleagues additionally documented activity in lateral OFC in response to different facial identities among conspecifics, with a stronger response to more highly-ranked social partners. A later study by Chang and colleagues that used a similar design also found that OFC neurons, both medial and lateral, appear to respond to stimuli that predict self-reward rather than to partner alone or to an empty chair (Chang et al., 2013). In contrast, ACC sulcus (ACCs) neurons responded only to reward to the partner alone or to no one, while ACCg neurons responded to all three stimuli. Thus, evidence from neurophysiology studies indicate that although OFC neurons are primarily concerned with rewards as they pertain to the self, they also track the social environment, including hierarchical information about a group, and information about the value of outcomes to others.
OFC appears to integrate information about facial identity and expression, a function that gives it a role in making judgments about social interactions. The weight of evidence suggests that rather than being the sole site for social value computation itself, OFC is an important component of larger social valuation networks and may feed forward information to other regions, such as ACC, that integrate and produce responses to social information. Consistent with this idea, a neuroimaging study in humans examined the relationship between OFC and ACC during immersive social interactions (Beyer et al., 2015). The design examined neural activity in both regions in response to punishments (in the form of loud noises) from virtual partners with neutral or angry expressions. OFC responses to the partner’s angry expression correlated negatively with the expression of aggressive behavior, while ACC activation to the same stimulus correlated positively with aggressive behavior. This indicates that OFC and ACC work in concert to identify social information and produce appropriate responses. In macaques, free-viewing of naturalistic social interactions indicated that OFC, and specifically face patch PO, responded to videos of animals interacting with objects in their environments or to videos of two animals socially interacting (Sliwa and Freiwald, 2017). The amygdala and ACC were also strongly responsive to videos of social interaction, suggesting a functional interaction among these regions.
In summary, OFC is an important component of social valuation. However, we have yet to determine what OFC is necessary for, rather than to what it contributes. OFC clearly plays a role in conceiving and navigating complex social hierarchies and changing social environments to enable the most advantageous action to be taken, but what its specific role is remains an outstanding question. By following up on these issues, we can determine how OFC plays a role in the computation of value beyond appetitive and aversive outcomes. What is needed now are answers to a number of key questions. For example, is the information about social identity and context that OFC provides to downstream areas such as ACC necessary for appropriate social behaviors? Lesions of OFC indicate that this is the case, but clear evidence is still lacking, especially now that there are questions about how lateral parts of OFC, where PO is located, contribute to affective behavior (Pujara et al., 2019).
Integration of OFC functions and conclusions
Our understanding of the functions of the orbitofrontal cortex has changed over the years, as more sophisticated and precise methods have debunked previously held ideas and made way for new theories (Stalnaker et al., 2015). Nonetheless, the complete picture of all its functions remains obscured. In this review we have focused on three areas of research related to OFC function about which we believe the field still has much to discover, namely contingent stimulus-reward learning, interoception, and social valuation. While these processes may appear to be disparate, we believe that these parts of the OFC puzzle are heavily interlinked.
Our current understanding of contingent stimulus-reward learning is based primarily on studies of whole OFC lesions, which indicate that OFC may not be required for this process. As we discuss above, the role of OFC may be more nuanced. We highlight two OFC centered pathways, one that incorporates medial OFC and hippocampus which we hypothesize may contribute to stimulus reward learning through representing task structure. We conclude that contingent stimulus-reward learning, forming predictive links between stimuli and rewards, is likely the purview of circuits involving the ventrolateral PFC and amygdala. We note, however, that during contingent learning, choices and their correlated rewards are used not only to form contingent associations but also spread in time to influence temporally adjacent choices and their outcomes. Thorndike termed this non-contingent learning the “spread of effect” (Thorndike, 1933).
In a recent paper, Wittmann and colleagues showed that these non-contingent learning mechanisms may be dependent on anterior insula, potentially extending into parts of posterior lateral OFC (Wittmann et al., 2020). They found that during probabilistic reward learning, the recent history of rewards which they termed the ‘global reward state’ impacts contingent learning through an insula-linked mechanism. In this setting, global reward state approximates non-contingent learning. Through a combination of modeling and behavioral analysis, Wittman and colleagues showed that the global reward state directly impacts trial-unique prediction errors that are used to drive contingent stimulus-reward learning. While insula cortex has primarily been linked with interoceptive processing (Craig, 2002), we have recently found that neurons in the lateral OFC also track the current state of bodily arousal (Fujimoto et al., 2021). Based on this finding, tracking of global reward state could be related to the state of arousal that results from the history of reward. Disentangling representations of bodily states from centrally-mediated tracking of reward history will be an important question for future research. This question also highlights the potential interplay between contingent stimulus-reward learning and interoception at the level of OFC.
How might interactions between contingent learning and interoception relate to social processing in OFC? Notably, people and animals with damage to ventral PFC have impairments in social behavior, as well as contingent learning and arousal processing (Damasio et al., 1990; Bechara et al., 1999; Fellows and Farah, 2003). One possibility is that social impairments could in part be caused by disruption to OFC-linked functions; for instance, difficulty in updating values in rapidly changing social situations or a loss of interoceptive feedback during interpersonal communication could drive inappropriate social behavior. Aside from the effects of other functions of the OFC being disrupted, neurons within the OFC are also sensitive to faces and respond to facial identity or facial expression (Tsao et al., 2003; Barat et al., 2018). Still other neurons are sensitive to value within the context of a social interaction (Azzi et al., 2012; Chang et al., 2013; Barat et al., 2018). Because lesions of medial OFC in macaques do not alter social valuation (Noonan et al., 2010a), this again suggests that amygdala-lateral OFC interactions may, like contingent learning and interoception, be central to social valuations. Teasing apart how this shared circuitry mediates the interactions (or not) between these processes at the level of OFC is a key question and highlights the need for more investigation between social valuation and OFC function.
In summary, after decades of research, the broader functions of OFC are starting to come into focus. As we highlight above, progress in teasing apart the fine grain functionality of OFC will depend on understanding both the functions and connectivity of individual segments of the region, as well as the role of OFC as a whole in more global circuits. Thus, rather than seeing OFC as an isolated set of pieces to be fit together all on its own, we should see it as a section of a larger puzzle that connects to multiple systems through connectivity with areas such as amygdala, hippocampus, insula, and ACC. Only by determining the form, shape, and function of these connections will we be able to complete the OFC puzzle.
Acknowledgements:
CE, AF, JMF, FMS, BR and PHR are supported by grants from NIMH and the BRAIN initiative (R01MH110822 and RF1MH117040). BER is supported by grants from NIMH (R01MH111439) and NINDS (R01NS109498). AF is also supported by Overseas Research Fellowship from Takeda Science Foundation.
References
- Aggleton JP, Wright NF, Rosene DL, Saunders RC (2015) Complementary patterns of direct amygdala and hippocampal projections to the macaque prefrontal cortex. Cereb Cortex 25:4351–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC (2001) Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron 32:537–551. [DOI] [PubMed] [Google Scholar]
- Azzi JCB, Sirigu A, Duhamel JR (2012) Modulation of value representation by social context in the primate orbitofrontal cortex. Proc Natl Acad Sci U S A 109:2126–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barat E, Wirth S, Duhamel JR (2018) Face cells in orbitofrontal cortex represent social categories. Proc Natl Acad Sci U S A 115:E11158–E11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Blatt GJ (1995) Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus 5:511–533. [DOI] [PubMed] [Google Scholar]
- Bartolo R, Averbeck BB (2020) Prefrontal Cortex Predicts State Switches during Reversal Learning. Neuron 106:1044–1054.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CCC, Izquierdo AD, Murray EA (2000) Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci 20:4311–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR (2000) Emotion, Decision Making and the Orbitofrontal Cortex. Cereb Cortex 10:295–307. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP (1999) Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci 19:5473–5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Muller TH, Whittington JCR, Mark S, Baram AB, Stachenfeld KL, Kurth-Nelson Z (2018) What Is a Cognitive Map? Organizing Knowledge for Flexible Behavior. Neuron 100:490–509. [DOI] [PubMed] [Google Scholar]
- Beyer F, Münte TF, Göttlich M, Krämer UM (2015) Orbitofrontal cortex reactivity to angry facial expression in a social interaction correlates with aggressive behavior. Cereb Cortex 25:3057–3063. [DOI] [PubMed] [Google Scholar]
- Bindra D (1974) A motivational view of learning, performance, and behavior modification. Psychol Rev 81:199–213. [DOI] [PubMed] [Google Scholar]
- Bradfield LA, Hart G (2020) Rodent medial and lateral orbitofrontal cortices represent unique components of cognitive maps of task space. Neurosci Biobehav Rev 108:287–294. [DOI] [PubMed] [Google Scholar]
- Butter CM (1969) Impairments in selective attention to visual stimuli in monkeys with inferotemporal and lateral striate lesions. Brain Res 12:374–383. [DOI] [PubMed] [Google Scholar]
- Cavada C, Compañy T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suárez F (2000) The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex 10:220–242. [DOI] [PubMed] [Google Scholar]
- Chang SWC, Gariépy JF, Platt ML (2013) Neuronal reference frames for social decisions in primate frontal cortex. Nat Neurosci 16:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau BKH, Sallet J, Papageorgiou GK, Noonan MP, Bell AH, Walton ME, Rushworth MFS (2015) Contrasting Roles for Orbitofrontal Cortex and Amygdala in Credit Assignment and Learning in Macaques. Neuron 87:1106–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW (2003) Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: Further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci 23:8771–8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu AO, O’Reilly JX, Behrens TEJ (2016) Organizing conceptual knowledge in humans with a gridlike code. Science (80-) 352:1464–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa VD, Dal Monte O, Lucas DR, Murray EA, Averbeck BB (2016) Amygdala and Ventral Striatum Make Distinct Contributions to Reinforcement Learning. Neuron 92:505–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2002) How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3:655–666. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM (2000) Thermosensory activation of insular cortex. Nat Neurosci 3:184–190. [DOI] [PubMed] [Google Scholar]
- Cusi AM, Nazarov A, Holshausen K, MacQueen GM, McKinnon MC (2012) Systematic review of the neural basis of social cognition in patients with mood disorders. J Psychiatry Neurosci 37:154–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton GL, Wang NY, Phillips AG, Floresco SB (2016) Multifaceted contributions by different regions of the orbitofrontal and medial prefrontal cortex to probabilistic reversal learning. J Neurosci 36:1996–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H (1990) Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res 41:81–94. [DOI] [PubMed] [Google Scholar]
- Farovik A, Place RJ, McKenzie S, Porter B, Munro CE, Eichenbaum H (2015) Orbitofrontal cortex encodes memories within value-based schemas and represents contexts that guide memory retrieval. J Neurosci 35:8333–8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ (2003) Ventromedial frontal cortex mediates affective shifting in humans: Evidence from a reversal learning paradigm. Brain 126:1830–1837. [DOI] [PubMed] [Google Scholar]
- Fiuzat EC, Rhodes SEV, Murray EA (2017) The role of orbitofrontal-amygdala interactions in updating action-outcome valuations in macaques. J Neurosci 37:2463–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto A, Murray EA, Rudebeck PH (2021) Interaction between decision-making and interoceptive representations of bodily arousal in frontal cortex. bioRxiv:2021.02.04.429750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cordero I et al. (2016) Feeling, learning from and being aware of inner states: Interoceptive dimensions in neurodegeneration and stroke. Philos Trans R Soc B Biol Sci 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino TF, Maren S (2015) The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front Behav Neurosci 9:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Keistler C, Keip AJ, Hammarlund E, DiLeone RJ, Pittenger C, Lee D, Taylor JR (2019) Orbitofrontal Circuits Control Multiple Reinforcement-Learning Processes. Neuron 103:734–746.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile TM, Bohon KS, Romero MC, Conway BR (2019) Visual stimulus-driven functional organization of macaque prefrontal cortex. Neuroimage 188:427–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AN, Adolphs R, Tyszka MJ, O’Doherty JP (2007) Contributions of the Amygdala to Reward Expectancy and Choice Signals in Human Prefrontal Cortex. Neuron 55:545–555. [DOI] [PubMed] [Google Scholar]
- Hebb DO (1955) Drives and the C. N. S. (conceptual nervous system). Psychol Rev 62:243–254. [DOI] [PubMed] [Google Scholar]
- Heberlein AS, Padon AA, Gillihan SJ, Farah MJ, Fellows LK (2008) Ventromedial frontal lobe plays a critical role in facial emotion recognition. J Cogn Neurosci 20:721–733. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M (2004) Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol 14:148–155. [DOI] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O’Doherty J, Bullock PR, Polkey CE (2003) Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain 126:1691–1712. [DOI] [PubMed] [Google Scholar]
- Ishai A (2007) Sex, beauty and the orbitofrontal cortex. Int J Psychophysiol 63:181–185. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA (2004) Combined Unilateral Lesions of the Amygdala and Orbital Prefrontal Cortex Impair Affective Processing in Rhesus Monkeys. J Neurophysiol 91:2023–2039. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA (2004) Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci 24:7540–7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA (2005) Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci 25:8534–8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang AI, Costa VD, Rudebeck PH, Chudasama Y, Murray EA, Averbeck BB (2015) The role of frontal cortical and medial-temporal lobe brain areas in learning a Bayesian prior belief on reversals. J Neurosci 35:11751–11760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens T, Zhu Q, Popivanov ID, Vanduffel W (2014) Probabilistic and single-subject retinotopic maps reveal the topographic organization of face patches in the macaque cortex. J Neurosci 34:10156–10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Witter MP (1991) Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol 313:574–586. [DOI] [PubMed] [Google Scholar]
- Jezzini A, Padoa-Schioppa C (2020) Neuronal Activity in the Primate Amygdala during Economic Choice. J Neurosci 40:1286–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaada BR, Pribram KH, Epstein JA (1949) Respiratory and vascular responses in monkeys from temporal pole, insula, orbital surface and cingulate gyrus; a preliminary report. J Neurophysiol 12:347–356. [DOI] [PubMed] [Google Scholar]
- Khani A (2014) Partially dissociable roles of OFC and ACC in stimulus-guided and action-guided decision making. J Neurophysiol 111:1717–1720. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Britton JC, Schwab ZJ, Price LM, Weiner MR, Gold AL, Rosso IM, Simon NM, Pollack MH, Rauch SL (2014) Cortico-limbic responses to masked affective faces across ptsd, panic disorder, and specific phobia. Depress Anxiety 31:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EB, Wallis JD (2020) Closed-Loop Theta Stimulation in the Orbitofrontal Cortex Prevents Reward-Based Learning. Neuron 106:537–547.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, Yang K, Sajjad S, Yan W, Li X, Zhao J (2019) Neural correlates of social well-being: Gray matter density in the orbitofrontal cortex predicts social well-being in emerging adulthood. Soc Cogn Affect Neurosci 14:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPresti ML, Schon K, Tricarico MD, Swisher JD, Celone KA, Stern CE (2008) Working memory for social cues recruits orbitofrontal cortex and amygdala: A functional magnetic resonance imaging study of delayed matching to sample for emotional expressions. J Neurosci 28:3718–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ (1987) Organization of amygdaloid projections to the mediodorsal thalamus and prefrontal cortex: A fluorescence retrograde transport study in the rat. J Comp Neurol 262:46–58. [DOI] [PubMed] [Google Scholar]
- McKenzie S, Keene CS, Farovik A, Bladon J, Place R, Komorowski R, Eichenbaum H (2016) Representation of memories in the cortical–hippocampal system: Results from the application of population similarity analyses. Neurobiol Learn Mem 134:178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitz AR, Chacko RV, Putnam PT, Rudebeck PH, Murray EA (2017) Using pupil size and heart rate to infer affective states during behavioral neurophysiology and neuropsychology experiments. J Neurosci Methods 279:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MJM, Deutch AY (2018) Organization of afferents to the orbitofrontal cortex in the rat. J Comp Neurol 526:1498–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Moylan EJ, Saleem KS, Basile BM, Turchi J (2015) Specialized areas for value updating and goal selection in the primate orbitofrontal cortex. Elife 4:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Rudebeck PH (2018) Specializations for reward-guided decision-making in the primate ventral prefrontal cortex. Nat Rev Neurosci 19:404–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Sallet J, Rushworth MFS (2015) Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. Proc Natl Acad Sci U S A 112:E2695–E2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Thomas AG, Sallet J, Rushworth MFS (2014) Comparison of Human Ventral Frontal Cortex Areas for Cognitive Control and Language with Areas in Monkey Frontal Cortex. Neuron 81:700–713. [DOI] [PubMed] [Google Scholar]
- Niv Y (2019) Learning task-state representations. Nat Neurosci 22:1544–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Chau BKH, Rushworth MFS, Fellows LK (2017) Contrasting effects of medial and lateral orbitofrontal cortex lesions on credit assignment and decision-making in humans. J Neurosci 37:7023–7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Sallet J, Rudebeck PH, Buckley MJ, Rushworth MF (2010a) Does the medial orbitofrontal cortex have a role in social valuation? Eur J Neurosci 31:2341–2351. [DOI] [PubMed] [Google Scholar]
- Noonan MP, Walton ME, Behrens TEJ, Sallet J, Buckley MJ, Rushworth MFS (2010b) Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc Natl Acad Sci U S A 107:20547–20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ó Scalaidhe SP, Wilson FAW, Goldman-Rakic PS (1999) Face-selective neurons during passive viewing and working memory performance of rhesus monkeys: Evidence for intrinsic specialization of neuronal coding. Cereb Cortex 9:459–475. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA (2006) Neurons in the orbitofrontal cortex encode economic value. 441:223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Schoenbaum G (2015) Dialogue on economic choice, learning theory, and neuronal representations. Curr Opin Behav Sci 5:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CE, Stark EA, Young KS, Stein A, Kringelbach ML (2013) Understanding the human parental brain: A critical role of the orbitofrontal cortex. Soc Neurosci 8:525–543. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB (2010) Interoception in anxiety and depression. Brain Struct Funct 214:451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JW (1982) Contingent decision behavior: A review and discussion of issues. Psychol Bull 92:382–402. [Google Scholar]
- Powell J, Lewis PA, Roberts N, García-Fiñana M, Dunbar RIM (2012) Orbital prefrontal cortex volume predicts social network size: An imaging study of individual differences in humans. Proc R Soc B Biol Sci 279:2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM (1995) Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cogn Neurosci 7:1–24. [DOI] [PubMed] [Google Scholar]
- Pujara MS, Rudebeck PH, Ciesinski NK, Murray EA (2019) Heightened defensive responses following subtotal lesions of macaque orbitofrontal cortex. J Neurosci:2812–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reekie YL, Braesicke K, Man MS, Roberts AC (2008) Uncoupling of behavioral and autonomic responses after lesions of the primate orbitofrontal cortex. Proc Natl Acad Sci U S A 105:9787–9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich EL, Wallis JD (2014) Medial-lateral organization of the orbitofrontal cortex. J Cogn Neurosci 26:1347–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET (2000) The orbitofrontal cortex and reward. Cereb Cortex 10:284–294. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Critchley HD, Browning AS, Inoue K (2005) Face-selective and auditory neurons in the primate orbitofrontal cortex. Exp Brain Res 170:74–87. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J (1994) Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry 57:1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RS, Lopresti ML, Schon K, Stern CE (2013) Role of the hippocampus and orbitofrontal cortex during the disambiguation of social cues in working memory. Cogn Affect Behav Neurosci 13:900–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Bannerman DM, Rushworth MFS (2008) The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cogn Affect Behav Neurosci 8:485–497. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Buckley MJ, Walton ME, Rushworth MFS (2006a) A role for the macaque anterior cingulate gyrus in social valuation. Science (80-) 313:1310–1312. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Mitz AR, Chacko RV, Murray EA (2013a) Effects of amygdala lesions on reward-value coding in orbital and medial prefrontal cortex. Neuron 80:1519–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA (2011) Dissociable Effects of Subtotal Lesions within the Macaque Orbital Prefrontal Cortex on Reward-Guided Behavior. J Neurosci 31:10569–10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Ripple JA, Mitz AR, Averbeck BB, Murray EA (2017a) Amygdala contributions to stimulus–reward encoding in the macaque medial and orbital frontal cortex during learning. J Neurosci 37:2186–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Saunders RC, Lundgren DA, Murray EA (2017b) Specialized Representations of Value in the Orbital and Ventrolateral Prefrontal Cortex: Desirability versus Availability of Outcomes. Neuron 95:1208–1220.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA (2013b) Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat Neurosci 16:1140–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Millette BHP, Shirley E, Rushworth MFS, Bannerman DM (2007) Distinct contributions of frontal areas to emotion and social behaviour in the rat. Eur J Neurosci 26:2315–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MFS (2006b) Separate neural pathways process different decision costs. Nat Neurosci 9:1161–1168. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Behrens TEJ, Rudebeck PH, Walton ME (2007) Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci 11:168–176. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Noonan MAP, Boorman ED, Walton ME, Behrens TE (2011) Frontal Cortex and Reward-Guided Learning and Decision-Making. Neuron 70:1054–1069. [DOI] [PubMed] [Google Scholar]
- Russ BE, Leopold DA (2015) Functional MRI mapping of dynamic visual features during natural viewing in the macaque. Neuroimage 109:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R, Walker SC, Clarke HF, Robbins TW, Roberts AC (2010) Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J Neurosci 30:14552–14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M (2003) Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem 10:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiedrzik CM, Zarco W, Everling S, Freiwald WA (2015) Face Patch Resting State Networks Link Face Processing to Social Cognition. PLOS Biol 13:e1002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y, Kim C, Santangelo AM, Roberts AC (2015) Lesions of either anterior orbitofrontal cortex or ventrolateral prefrontal cortex in marmoset monkeys heighten innate fear and attenuate active coping behaviors to predator threat. Front Syst Neurosci 8:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwa J, Freiwald WA (2017) A dedicated network for social interaction processing in the primate brain. Science (80-) 356:745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Cooch NK, Schoenbaum G (2015) What the orbitofrontal cortex does not do. Nat Neurosci 18:620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg DA, McClelland JL (2012) Two mechanisms of human contingency learning. Psychol Sci 23:59–68. [DOI] [PubMed] [Google Scholar]
- Taubert J, Wardle SG, Ungerleider LG (2020) What does a “face cell” want?’. Prog Neurobiol 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike EL (1933) A proof of the law of effect. Science (80-) 77:173–175. [DOI] [PubMed] [Google Scholar]
- Thorpe SJ, Rolls ET, Maddison S (1983) The orbitofrontal cortex: Neuronal activity in the behaving monkey. Exp Brain Res 49:93–115. [DOI] [PubMed] [Google Scholar]
- Troiani V, Dougherty CC, Michael AM, Olson IR (2016) Characterization of face-selective patches in orbitofrontal cortex. Front Hum Neurosci 10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RBH (2003) Faces and objects in macaque cerebral cortex. Nat Neurosci 6:989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Moeller S, Freiwald WA (2008a) Comparing face patch systems in macaques and humans. Proc Natl Acad Sci U S A 105:19514–19519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Schweers N, Moeller S, Freiwald WA (2008b) Patches of face-selective cortex in the macaque frontal lobe. Nat Neurosci 11:877–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Behrens TEJ, Buckley MJ, Rudebeck PH, Rushworth MFS (2010) Separable Learning Systems in the Macaque Brain and the Role of Orbitofrontal Cortex in Contingent Learning. Neuron 65:927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA (2005) Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci U S A 102:17804–17809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KK, Platt ML (2012) Social signals in primate orbitofrontal cortex. Curr Biol 22:2268–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman LL, Gale K, Malkova L (2005) GABAA-Mediated Inhibition of Basolateral Amygdala Blocks Reward Devaluation in Macaques. J Neurosci 25:4577–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EA, DesJardin JT, Gale K, Malkova L (2011) Transient Inactivation of Orbitofrontal Cortex Blocks Reinforcer Devaluation in Macaques. J Neurosci 31:15128–15135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, Marrero-Garcia Y, Schoenbaum G (2017) Suppression of Ventral Hippocampal Output Impairs Integrated Orbitofrontal Encoding of Task Structure. Neuron 95:1197–1207.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, Schoenbaum G (2016) Over the river, through the woods: Cognitive maps in the hippocampus and orbitofrontal cortex. Nat Rev Neurosci 17:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis ML, Murphy JM, Ridley NJ, Vercammen A (2015) Anodal tDCS targeting the right orbitofrontal cortex enhances facial expression recognition. Soc Cogn Affect Neurosci 10:1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RC, Takahashi YK, Schoenbaum G, Niv Y (2014) Orbitofrontal cortex as a cognitive map of task space. Neuron 81:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, O’Doherty J, Kilner JM, Perrett DI, Dolan RJ (2007) Brain systems for assessing facial attractiveness. Neuropsychologia 45:195–206. [DOI] [PubMed] [Google Scholar]
- Wittmann MK, Fouragnan E, Folloni D, Klein-Flügge MC, Chau BKH, Khamassi M, Rushworth MFS (2020) Global reward state affects learning and activity in raphe nucleus and anterior insula in monkeys. Nat Commun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C, Stolle D, Gidengi E, Fellows LK (2015) Lateral orbitofrontal cortex links social impressions to political choices. J Neurosci 35:8507–8514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD (1908) The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol 18:459–482. [Google Scholar]
- Zhou J, Montesinos-Cartagena M, Wikenheiser AM, Gardner MPH, Niv Y, Schoenbaum G (2019) Complementary Task Structure Representations in Hippocampus and Orbitofrontal Cortex during an Odor Sequence Task. Curr Biol 29:3402–3409.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]


