Abstract
BACKGROUND
Gastric cancer is a prevalent malignant cancer with a high incidence and significantly affects the health of modern people globally. Cisplatin (DDP) is one of the most common and effective chemotherapies for patients with gastric cancer, but DDP resistance remains a severe clinical challenge.
AIM
To explore the function of M2 polarized macrophages-derived exosomal microRNA (miR)-588 in the modulation of DDP resistance of gastric cancer cells.
METHODS
M2 polarized macrophages were isolated and identified by specific markers using flow cytometry analysis. The exosomes from M2 macrophages were identified by transmission electron microscopy and related markers. The uptake of the PKH67-labelled M2 macrophages-derived exosomes was detected in SGC7901 cells. The function and mechanism of exosomal miR-588 from M2 macrophages in the modulation of DDP resistance of gastric cancer cells was analyzed by CCK-8 assay, apoptosis analysis, colony formation assay, Western blot analysis, qPCR analysis, and luciferase reporter assay in SGC7901 and SGC7901/DDP cells, and by tumorigenicity analysis in nude mice.
RESULTS
M2 polarized macrophages were isolated from mouse bone marrow stimulated with interleukin (IL)-13 and IL-4. Co-cultivation of gastric cancer cells with M2 polarized macrophages promoted DDP resistance. M2 polarized macrophages-derived exosomes could transfer in gastric cancer cells to enhance DDP resistance. Exosomal miR-588 from M2 macrophages contributed to DDP resistance of gastric cancer cells. miR-588 promoted DDP-resistant gastric cancer cell growth in vivo. miR-588 was able to target cylindromatosis (CYLD) in gastric cancer cells. The depletion of CYLD reversed miR-588 inhibition-regulated cell proliferation and apoptosis of gastric cancer cells exposed to DDP.
CONCLUSION
In conclusion, we uncovered that exosomal miR-588 from M2 macrophages contributes to DDP resistance of gastric cancer cells by partly targeting CYLD. miR-588 may be applied as a potential therapeutic target for the treatment of gastric cancer.
Keywords: Gastric cancer, Cisplatin resistance, M2 polarized macrophages, Exosome, miR-588, Cylindromatosis
Core Tip: In this study, we uncovered that exosomal miR-588 from M2 macrophages contributes to cisplatin resistance of gastric cancer cells by partly targeting cylindromatosis. miR-588 may be applied as a potential therapeutic target for the treatment of gastric cancer.
INTRODUCTION
Gastric cancer belongs to the most common malignant tumors and is the third cause of cancer-related death throughout the world[1]. Patients diagnosed with gastric cancer are usually at an advanced or late stage, which makes chemotherapy a prevalent strategy over the past decades[2]. Among the clinical chemotherapy agents, cisplatin (DDP) is capable of disrupting DNA repairment, which further causes DNA damage and apoptosis of cancer cells[3]. However, the developed therapeutic resistance makes the chemotherapy far less satisfactory in clinical application, which makes it urgent to decipher the mechanisms underlying this chemoresistance[4].
Tumor-associated macrophage (TAMs) are macrophages that participate in the tumor microenvironment function, and play a critical role in tumor development, including growth, metastasis, angiogenesis, and therapeutic resistance[5]. Recent studies have proposed that TAMs were capable of communicating with neighboring or distant cells via secreting exosomes[6,7]. Exosomes are membrane-derived transporting vesicles secreted by almost all types of cells, usually defined with a diameter ranging from 40 nm to 100 nm[8]. It is widely recognized that exosomes act as a communicator between cells through transporting various cargos such as mRNA, lipid, proteins, as well as microRNAs (miRNAs), and hence are related to various functions of targeted cells[9,10]. miRNAs are endogenous short noncoding RNAs that play pivotal roles in the regulation of cancer development by directly targeting mRNA[11]. Besides, miRNAs are recently proposed to be involved in drug resistance of cancer cells[12-14]. Among which, miR-588 is upregulated in human prostate cancer with prognostic significance and notably facilitates the proliferation of prostate cancer cells[15]. Importantly, it has been reported that miR-588 serves as a promising prognostic marker for gastric cancer[16]. However, the role of miR-588 in DDP resistance of gastric cancer cells is still unknown. Accordingly, we were interested in the correlation of TAM-derived exosomes with miR-588 and its role in the modulation of DDP resistance of gastric cancer cells.
Cylindromatosis (CYLD) is a representative deubiquitinating enzyme that could counteract the E3 ubiquitin ligases-mediated protein ubiquitination[17]. It plays an important role in multiple cellular processes during tumorigenesis, such as cell apoptosis, cell cycle, cell migration, and DNA damage[17]. Mechanistically, CYLD is profoundly related to multiple critical signaling transduction pathways, especially the NF-κB signaling pathway, and acts as a tumor suppressor[18]. It has been extensively demonstrated that CYLD could negatively regulate the activation of NF-kB through deubiquitinating its upstream K63, which further inhibits the activation of NF-kB-related pro-survival signaling and promotes caspase-8-induced cell apoptosis[19]. It is noteworthy that recent studies also proposed that loss of CYLD could trigger DDP resistance in several cancers, including gastric cancer and oral squamous cell carcinoma[20,21].
In this study, we adopted a M2-polarized macrophage model to mimic TAM function, and testified that TAM-derived exosomes conferred DDP resistance in gastric cancer. We showed that the exosomes secreted by TAM contained miR-588, which directly interacts with the 3’ untranslated region of CYLD to suppress its expression and its tumor-suppressing function. This process subsequently hindered cell apoptosis and promoted the proliferation as well as DDP resistance of gastric cancer cells. Our study illustrated the connection between TAM and DDP resistance of gastric cancer cells, providing a potential target for gastric cancer therapy.
MATERIALS AND METHODS
Cell culture and treatment
Gastric cancer cell line SGC7901, purchased from ATCC, was maintained in DMEM containing 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin (Sigma, United States) at 37 °C with 5% CO2. DDP-resistant SGC7901/DDP cells were developed from the parental SGC7901 cells that were subjected to persistent gradient exposure to DDP for about 12 mo, through increasing DDP concentration from 0.05 μg/mL until the cells acquired resistance to 1 μg/mL[22]. Mouse bone marrow cells (MBMCs) were purchased from Chinese Academy of Sciences Cell Bank of Type Culture Collection, and cultured in a normal DMEM:F12 medium containing 10% FBS and macrophage colony-stimulating factor (Sigma). To achieve polarization to M2, MBMCs were stimulated with interleukin (IL)-4 and IL-13 (30 ng/mL, Sigma) for 2 d. A transwell chamber assay was adopted to perform co-culture experiments, in which polarized macrophages were placed in the upper tanswell chamber and gastric cancer cells were cultured in the bottom well.
Flow cytometry
The M2 polarization was determined by staining for critical biomarkers and detection by flow cytometry. Briefly, cells were collected and incubated with fluorescence probe-conjugated antibodies against CD86, CD68, CD11b, CD80, CD206, CD163, and F4/80 at 4 °C for 30 min. Next, the cells were washed with PBS and analyzed by flow cytometry (BD Biosciences, United States). The antibodies used in this experiment were purchased from Abcam (United States).
For cell apoptosis, cells were processed with an apoptosis detection kit (Solarbio, China). Briefly, cells were collected and suspended in binding buffer, followed by staining with PI and Annexin V at room temperature for 20 min in dark. Next, the stained cells were collected and resuspended in binding buffer for analysis by flow cytometry.
Exosome isolation and analysis
Macrophages were cultured in medium containing 10% exosome-free FBS for 48 h. The medium was collected for extraction of exosomes with an exoEasy Maxi Kit (QIAGEN, Germany) following the manufacturer’s instruction.
To determine exosome internalization, the exosomes were labeled with PKH67 (Sigma) according to the manufacturer’s protocol. Subsequently, the labeled exosomes were used to incubate with cells in each experiment, and observed using a confocal microscope (Carl Zeiss, Germany).
Transmission electron microscopy
The images of exosomes were captured by transmission electron microscopy (TEM). In brief, the samples were dropped on cleaned cooper grids coated with carbon, and washed with Milli-Q water to remove extra samples, followed by 1-min staining with uranyl acetate and air dry. Then, the samples were observed and pictured with a transmission electron microscope (Carl Zeiss).
Cell transfection
SGC7901 cells were placed in 6-well plate at a density of 3 × 105 cells per well. After incubation for 12 h, miR-588 mimic (RiboBio, China), miR-588 inhibitor (RiboBio, China), or negative control (RiboBio, China) was mixed with lipofectamine 200 (Invitrogen, United States) in Opti-MEM medium (Sigma, United States) for 20 min and added into cell culture medium. Eight hours after transfection, the medium was replaced with fresh DMEM containing 10% FBS to incubate for another 24 h. Then, the cells were used for further experiments.
Cell viability and colony formation assay
Cell viability was determined with a CCK-8 kit (Beyotime, China). MFC cells and SGC7901 cells were seeded in 96-well plates at a density of 3 × 103 cells/well and processed accordingly. Twenty-four hours after incubation, the CCK-8 reagent was added into each well. The absorbance values were detected at 450 nm at 24 h, 48 h, and 72 h, respectively.
For colony formation experiment, SGC7901 cells transfected with miR-588 inhibitor or negative control were placed in a 6-well plate (1000 cells per well), and incubated in complete medium for 2 wk to form visible colonies. Subsequently, the colonies were washed, stained with crystal violet, and captured with a microscope (Carl Zeiss).
RNA extraction and quantitative real-time PCR
Total RNA was extracted with TRIzol reagent (Takara, Japan) and reversely transcribed to cDNA with an EasyScript Reverse Transcriptase kit (TransGen, China). The quantification of cDNA was performed by using a TransStart Green qPCR SuperMix kit (TransGen), and PCR was performed on a Real-Time PCR System (BD bioscience). For detection of miR-588, the extracted RNA was processed with a poly(A) tailing kit (Invitrogen) before reverse transcription. For normalization of mRNA and miRNA, GAPDH and U6 were used as the internal controls, respectively. The quantification of cDNA was analyzed by the 2–ΔΔCt method. The sequences of primers are as follows: GAPDH sense, 5’- ACAACTTTGGTATCGTGGAAGG-3’ and antisense, 5’-GCCATCACGCCACAGTTTC-3’; U6 sense, 5’-AGTAAGCCCTTGCTGTCAGTG-3’ and antisense, 5’-CCTGGGTCTGATAATGCTGGG-3’; Arg1 sense, 5’-GTGGAAACTTGCATGGACAAC-3’ and antisense, 5’-AATCCTGGCACATCGGGAATC-3’; IL-10 sense, 5’-GTACCACAGCAATGGCTACC-3 and antisense, 5’-TGTTGGTGACGGTCCAGTTG-3’; TGF-β sense, 5’-CTAATGGTGGAAACCCACAACG-3’ and antisense, 5’-TATCGCCAGGAATTGTTGCTG-3’; miR-588, 5′-CCGCTATTGCACATTACTAAGTTGCA-3
Western blot analysis
SGC7901 cells were placed in a 6-well plate at a density of 3 × 105 cells per well and transfected with miR-588 inhibitor. Total protein was extracted by using ice-cold RIPA buffer, separated by SDS-PAGE, and transferred to a nitrocellulose membrane. The membrane was then blocked by 5% non-fat milk at room temperature for 1 h, and incubated with specific primary antibodies against CD63 (1:1000, Abcam), CD9 (1:1000, Abcam), CD81 (1:1000, Abcam), HSP70 (1:500, Abcam), GAPDH (1:1000, Proteintech), Bax (1:1000, CST), pro-caspase-3 (1:2000, CST), cleaved caspase-3 (1:1000, CST), pro-caspase-9 (1:1000, CST), cleaved caspase-9 (1:1000, CST), CYLD (1:1000, Proteintech), and β-actin (1:2000, Proteintech) at 4 °C overnight. Next day, the blots were washed and incubated with secondary antibodies at room temperature for 2 h. The blots were visualized by using an ECL reagent and a gel imaging system (BD Bioscience).
Xenograft tumor model
C57 nude mice aged 5 wk were purchased from Shanghai Laboratory Animal Center of China. SGC7901 cells were transfected with miR-588 inhibitor or negative control, and suspended in saline at a density of 1 × 107 cells/mL. A total of 200 μL of SGC7901 cell suspension was subcutaneously injected to the left back of mice. A week after tumor cell inoculation, the mice were intraperitoneally injected with 15 mg/kg DDP. The mice were euthanized at day 30, and the tumors were isolated, weighted, and processed for subsequent analysis. Tumor size was measured every 5 d and calculated using the following formula: Width2 × length/2.
Statistical analysis
All experiments in this study were performed at least three times independently. All data are presented as the mean ± SD and analyzed with GraphPad Prism 6.0 software. The statistical difference between two groups or multiples groups was analyzed by Student’s t-test or one-way ANOVA, and was considered significant at P < 0.05.
RESULTS
M2 polarized macrophages-derived exosomes can transfer in gastric cancer cells to enhance DDP resistance
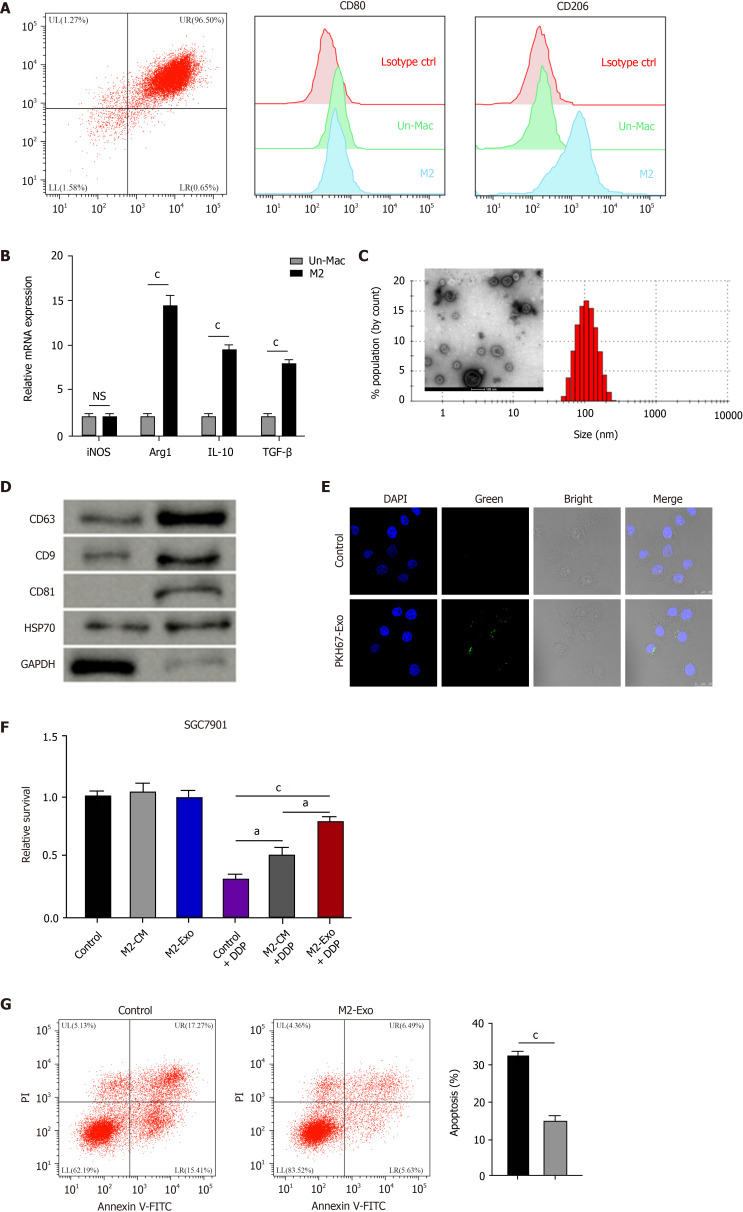
To evaluate the effect of M2 polarized macrophages on DDP resistance of gastric cancer cells, M2 polarized macrophages were isolated from murine bone marrow induced with IL-13 and IL-4 and were identified by the M2 specific marker CD206 (Figure 1A). Meanwhile, we found that the expression of M1 polarized marker iNOS was not chanced but the M2 markers, such as Arg1, IL-10, and TGF-β, were enhanced in M2 polarized macrophages compared with inactivated macrophages (Figure 1B). Then, the exosomes were isolated from M2 polarized macrophages and analyzed by TEM and identified by the expression of CD63, CD9, CD81, and HSP70 (Figure 1C and D). Moreover, the delivery of PKH67-labelled exosomes from M2 polarized macrophages was found in SGC7901 cells (Figure 1E). Significantly, M2 macrophages-derived conditioned medium and exosomes enhanced the cell survival of SGC7901 cells exposed to DDP (Figure 1F). The exosomes from M2 polarized macrophages repressed DDP-treated SGC7901 cell apoptosis (Figure 1G).
Figure 1.
M2 polarized macrophages-derived exosomes can transfer in gastric cancer cells to enhance cisplatin resistance. A and B: Flow cytometry for identifying the M2 polarized macrophages isolated from murine bone marrow induced with interleukin (IL)-13 and IL-4. The M2 specific marker CD206 was identified. Un-Mac represents inactivated macrophages and M2 represents macrophages activated by IL-13 and IL-4. The mRNA expression of iNOS, Arg1, IL-10, and TGF-β was detected by qPCR in Un-Mac and M2 macrophages; C: Identification of exosomes from M2 macrophages by transmission electron microscopy; D: Detection of expression of CD63, CD9, CD81, HSP70, and GAPDH by Western blot analysis; E: Detection of uptake of the PKH67-labelled M2 exosomes (M2-Exo) in SGC7901 cells; F: Analysis of cell viability by CCK-8 assay. M2 macrophages-derived conditioned medium and M2-Exo were used to treat SGC7901 cells exposed to cisplatin (DDP) for 48 h; G: Analysis of cell apoptosis by flow cytometry SGC7901 cells were co-treated with DDP and M2-Exo. Data shown are the mean ± SD. aP <0.05; cP < 0.001. DDP: Cisplatin; Un-Mac: Inactivated macrophages; M2-Exo: M2 exosomes.
Co-culture of gastric cancer cells with M2 polarized macrophages promotes DDP resistance
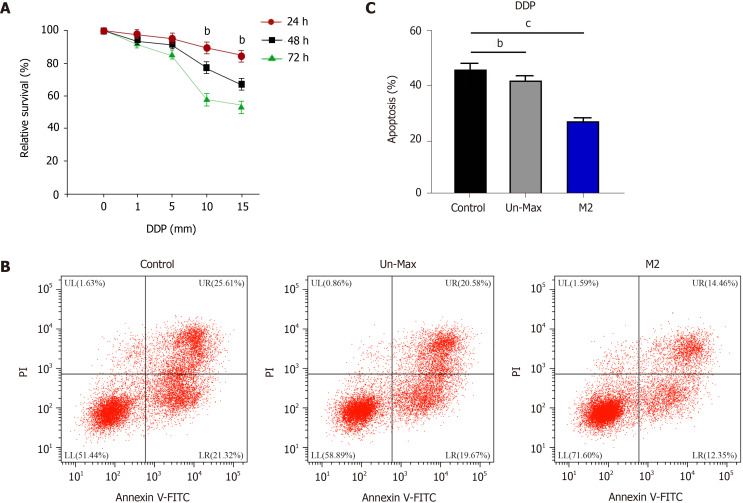
Next, we found that DDP treatment reduced the proliferation of SGC7901 cells in a time-dependent manner (Figure 2A). The co-culture of inactivated macrophages inhibited apoptosis of DDP-treated SGC7901 cells, while the co-culture of M2 polarized macrophages further reduced the cell apoptosis (Figure 2B and C).
Figure 2.
Co-culture of gastric cancer cells with M2 polarized macrophages promotes cisplatin resistance. A: Detection of cell viability by CCK-8 assay in SGC7901 cells treated with cisplatin (DDP); B and C: Analysis of cell apoptosis by flow cytometry in SGC7901 cells co-cultured with inactivated macrophages and M2 macrophages exposed to DDP for 48 h. Data shown are the mean ± SD. bP < 0.01; cP <0.001. DDP: Cisplatin.
Exosomal miR-588 from M2 macrophages contributes to DDP resistance of gastric cancer cells
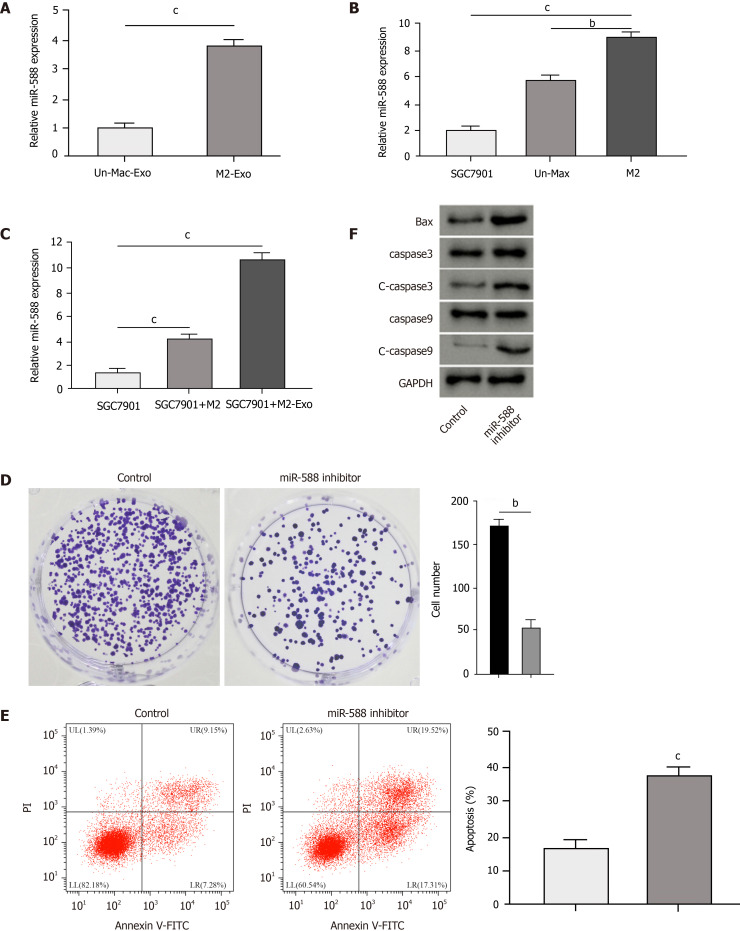
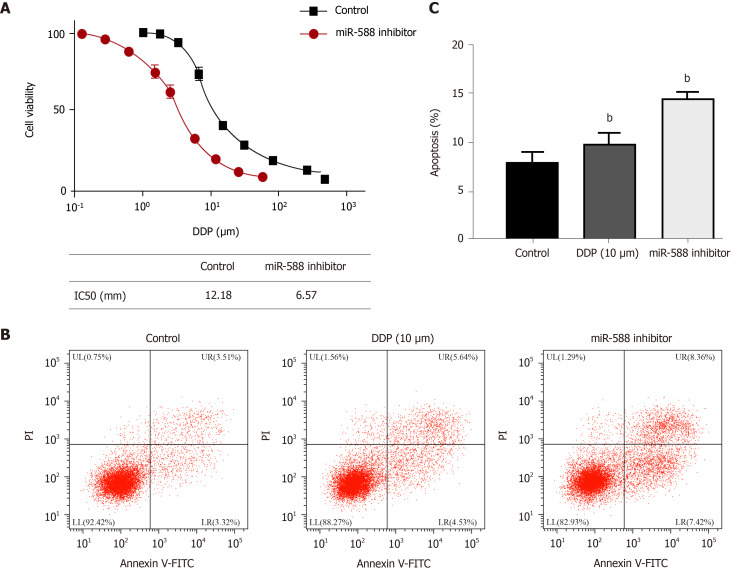
We then observed that the expression of miR-588 was elevated in the exosomes from M2 macrophages compared with inactivated macrophages (Figure 3A). The miR-588 expression was enhanced in macrophages relative to SGC7901 cells, especially in the M2 polarized macrophages (Figure 3B). Meanwhile, the expression of miR-588 was increased in the DDP-resistant SGC7901 cells co-cultured with M2 macrophages or exosomes from M2 macrophages (Figure 3C). Moreover, we found that the inhibition of miR-588 by inhibitor repressed colony formation of DDP-resistant SGC7901 cells (Figure 3D). The repression of miR-588 induced DDP-resistant SGC7901 cell apoptosis (Figure 3E). The expression of Bax, cleaved caspase-3, and cleaved caspase-9 was enhanced by miR-588 inhibitor in DDP-resistant SGC7901 cells (Figure 3F). Moreover, the IC50 of DDP in the inhibition of SGC7901 cell viability was inhibited by miR-588 inhibitor (Figure 4A). Treatment with DDP induced apoptosis of SGC7901 cells, in which miR-588 inhibitor reinforced this effect (Figure 4B and C).
Figure 3.
Exosomal miR-588 from M2 macrophages contributes to cisplatin resistance of gastric cancer cells. A: Detection of expression of miR-588 by qPCR in the exosomes from M2 macrophages and inactivated macrophages; B: Detection of expression of miR-588 by qPCR in cisplatin (DDP)-resistant SGC7901 cells, M2 macrophages, and inactivated macrophages; C: Detection of expression of miR-588 by qPCR in DDP-resistant SGC7901 cells co-cultured with M2 macrophages or exosomes from M2 macrophages; D-F: Analysis of cell proliferation by colony formation assay (D), cell apoptosis by flow cytometry (E), and expression of Bax, capase-3, caspase-9, cleaved caspase-3 (c-caspase-3), and cleaved caspase 9 (c-caspase-9) by Western blot analysis (F) in DDP-resistant SGC7901 cells treated with miR-588 inhibitor. Data shown are the mean ± SD, bP < 0.01; cP < 0.001.
Figure 4.
miR-588 contributes to cisplatin resistance of gastric cancer cells. A: Detection of cell viability by CCK-8 assay in SGC7901 cells treated with miR-588 inhibitor and exposed to cisplatin (DDP) at the indicated doses; B and C: Detection of cell apoptosis by flow cytometry in DDP-resistant SGC7901 cells co-treated with DDP and miR-588 inhibitor. Data shown are the mean ± SD, bP < 0.01. DDP: Cisplatin.
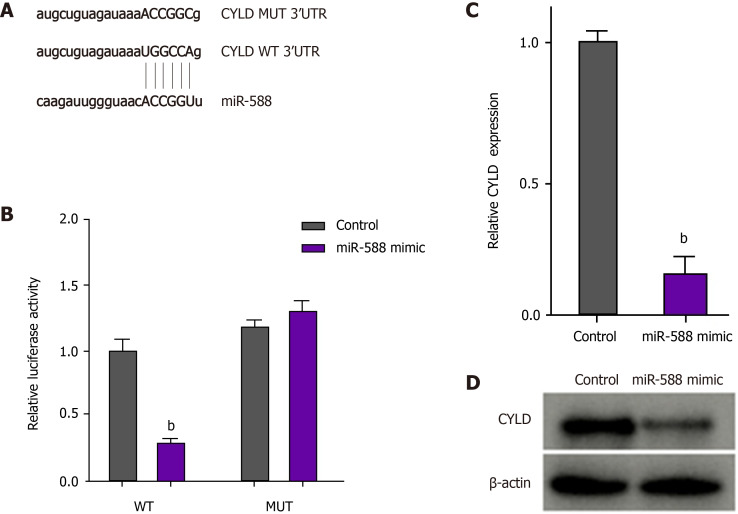
miR-588 can target CYLD in gastric cancer cells
We then identified the binding site for miR-588 in CYLD in the bioinformatic analysis (Figure 5A). The luciferase activity and mRNA expression of CYLD were significantly repressed by miR-588 mimic in the DDP-resistant SGC7901 cells (Figure 5B and C). Similarly, the protein levels of CYLD were inhibited by miR-588 mimic in DDP-resistant SGC7901 cells (Figure 5D).
Figure 5.
miR-588 can target cylindromatosis in gastric cancer cells. A: The binding site of miR-588 and cylindromatosis (CYLD) was predicted in TargetScan database; B-D: After cisplatin (DDP)-resistant SGC7901 cells were treated with miR-588 mimic, the luciferase activity of CYLD was measured by luciferase reporter assays (B); the expression of CYLD was determined by qPCR (C); and the expression of CYLD was measured by Western blot analysis (D). Data shown are the mean ± SD. bP < 0.01. CYLD: Cylindromatosis.
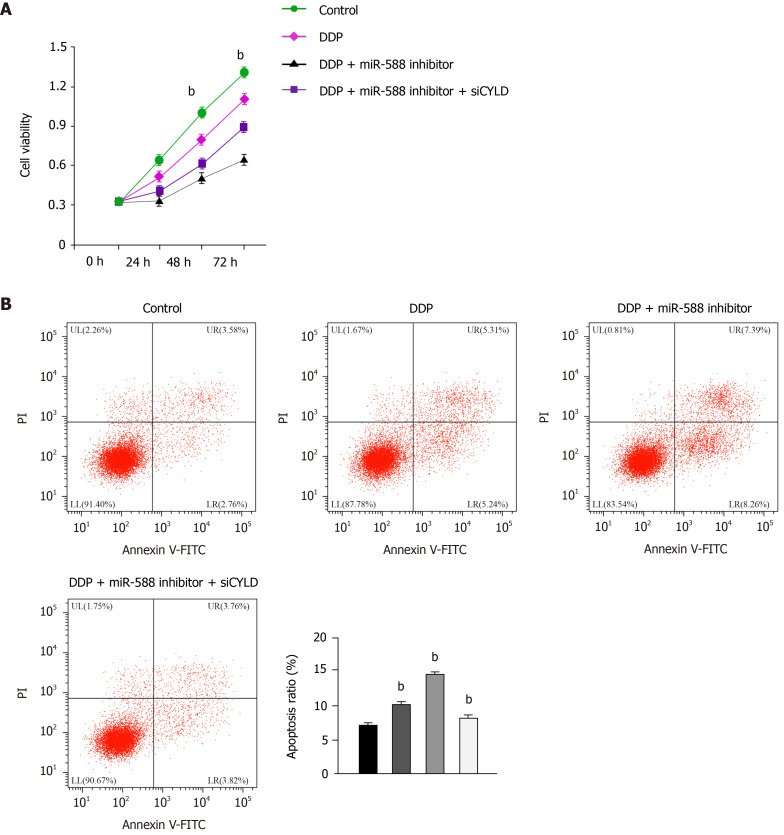
miR-588/CYLD axis regulates DDP resistance of gastric cancer cells
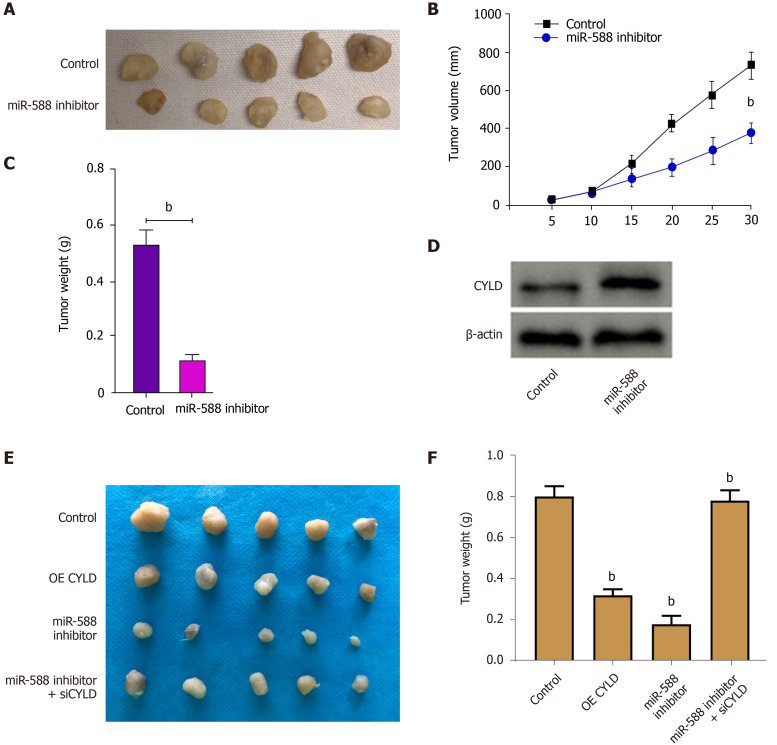
Next, we evaluated the effect of miR-588/CYLD axis on DDP resistance of gastric cancer cells. Our data showed that treatment with DDP inhibited SGC7901 cell proliferation; miR-588 inhibitor enhanced the function of DDP, while the depletion of CYLD by siRNA reversed the effect of miR-588 inhibition (Figure 6A). Moreover, treatment with DDP induced apoptosis of SGC7901 cells; miR-588 inhibitor further enhanced the cell apoptosis, while CYLD knockdown reversed the effect of miR-588 inhibitor (Figure 6B). Moreover, tumorigenicity analysis in nude mice demonstrated that miR-588 inhibitor suppressed the tumor growth in vivo (Figure 7A-D). Meanwhile, we validated that the overexpression of CYLD or treatment with miR-588 inhibitor repressed the tumor growth in nude mice, while the depletion of CYLD rescued miR-588 inhibitor-reduced tumor growth in the model (Figure 7E and F).
Figure 6.
miR-588/cylindromatosis axis regulates cisplatin resistance of gastric cancer cells. A: Cell viability detected by CCK-8 assay; B: Cell apoptosis analyzed by flow cytometry. The SGC7901 cells were treated with cisplatin (DDP), or co-treated with DDP and miR-588 inhibitor with or without cylindromatosis siRNA. Data shown are the mean ± SD. bP < 0.01. DDP: Cisplatin; CYLD: Cylindromatosis.
Figure 7.
miR-588/cylindromatosis axis modulates cisplatin-resistant gastric cancer cell growth in vivo. A-D: The nude mice were injected with SGC7901 cells treated with miR-588 inhibitor, the tumor tissues (A), tumor volume (B), and tumor weight (C) were measured, and the expression of cylindromatosis (CYLD) was detected by Western blot analysis in the tumor tissues (D); E and F: The nude mice were injected with SGC7901 cells treated with CYLD overexpressing plasmid or miR-588 inhibitor, or co-treated with miR-588 inhibitor and CYLD siRNA, and the tumor tissues (E) and tumor weight (F) were measured. Data shown are the mean ± SD (n = 5). bP < 0.01. CYLD: Cylindromatosis.
DISCUSSION
Gastric cancer is a common malignant cancer with a high death rate and limited therapeutic strategies. DDP is widely applied in the treatment of gastric cancer patients with significant effectiveness, but DDP resistance remains a critical clinical issue. In this study, we identified that M2 polarized macrophages-derived exosomal miR-588 contributes to DDP resistance of gastric cancer cells.
Previous investigations have shown that M2 polarized macrophages-derived exosomes are involved in the modulation of cancer development. It has been reported that tumor-related macrophages-derived exosomes contribute to gastric cancer cell invasion via apolipoprotein E[23]. Macrophage-derived exosomal miR-21 regulates DDP resistance of gastric cancer cells[24]. M2 macrophages-derived exosomes promote metastasis of hepatocarcinoma by delivering αMβ2 integrin[25]. These studies have shown the critical function of macrophages-derived exosomal miRNAs in the modulation of cancer development. Meanwhile, miR-588 is a widely reported cancer regulator in previous studies. It has been found that miR-588 is enhanced in prostate cancer and is correlated with a poor prognosis[15]. miR-588 modulates epithelial-mesenchymal transition, migration, and invasion of gastric cancer cells by targeting EIF5A2 signaling[26]. The PICSAR/miR-588/EIF6 axis contributes to the regulation of hepatocellular carcinoma progression by targeting AKT/mTOR signaling[27]. Moreover, it has been reported that miR-588 serves as a prognosis biomarker for gastric cancer[16]. In previous reports, miR-588 has not been prominently implicated in gastric cancer through interaction between tumor and the surrounding microenvironment. We were interested in the function of M2 macrophages-derived exosomes in cancer development and noticed the potential function of miR-588 in gastric cancer. Therefore, we explored the correlation of M2 macrophage-derived exosomes with miR-588 and their function in gastric cancer. Our data showed that co-cultivation of gastric cancer cells with M2 polarized macrophages promoted DDP resistance. M2 polarized macrophages-derived exosomes could transfer in gastric cancer cells to enhance DDP resistance. Exosomal miR-588 from M2 macrophages contributed to DDP resistance of gastric cancer cells. miR-588 promoted DDP-resistant gastric cancer cell growth in vivo. These data not only provide new evidence of the critical function of M2 polarized macrophages-derived exosomes in regulating cancer progression, but also indicate the innovative role of miR-588 in gastric cancer. The correlation of M2 polarized macrophages-derived exosomal miR-588 and miR-588 in the cancer cells in regulating cancer development is an interesting scientific issue, which is needed to explore in the future. Besides, the function of other pivotal miRNAs that have been described as crucial regulators in exosomes-mediated cellular communication should be explored by more investigations. Meanwhile, the mechanisms of miR-588 transfer from macrophage to gastric cancer cells remain unclear. According to previous studies, the communications between macrophages and gastric cancer cells can be mediated by exosomes, which can engulf local tissues immediately or swarm into body fluid to affect distant target organs through endocytosis[7,24,28]. The mechanisms of miR-588 transfer from macrophage to gastric cancer cells should be confirmed by more complicated investigations in future.
Moreover, it has been reported that inhibition of CYLD enhances IFN-γ-regulated PD-L1 expression in thymic epithelial cancer[29]. LINC01260 serves as a tumor inhibitor by regulating the miR-562/CYLD axis in lung cancer[30]. CYLD enhances nasopharyngeal carcinoma cells apoptosis by modulating NDRG1[31]. MiR-454 contributes to oxaliplatin resistance and cell proliferation by targeting CYLD in gastric cancer[32]. These reports have identified the tumor suppressive function of CYLD in cancer progression. Our mechanical investigation showed that miR-588 targeted CYLD in gastric cancer cells and miR-588/CYLD axis contributed to the progression of gastric cancer. It presents a crucial mechanism underlying miR-588-mediated cancer development. CYLD may not be the only downstream factor underlying miR-588-mediatde tumorigenesis. Other regulators in miR-588-modulated cancer pathogenesis and their crosstalk may be investigated further in future. Targeting exosomal miR-588 from M2 macrophages may be a promising therapeutic strategy for gastric cancer and the clinical translation should be explored in future investigations.
CONCLUSION
In conclusion, we uncovered that exosomal miR-588 from M2 macrophages contributes to DDP resistance of gastric cancer cells by partly targeting CYLD. miR-588 may be applied as a potential therapeutic target for the treatment of gastric cancer.
ARTICLE HIGHLIGHTS
Research background
Gastric cancer is a prevalent malignant cancer with a high incidence and significantly affects the health of modern people globally. Cisplatin (DDP) is one of the most common and effective chemotherapies for patients with gastric cancer, but DDP resistance remains a severe clinical challenge.
Research motivation
To identify the function of M2 polarized macrophages-derived exosomal miR-588 in gastric cancer cells.
Research objectives
To explore the effect of M2 polarized macrophages-derived exosomal miR-588 on DDP resistance of gastric cancer cells.
Research methods
M2 polarized macrophages were isolated and identified by specific markers using flow cytometry analysis. The exosomes from M2 macrophages were identified by TEM and related markers. The uptake of the PKH67-labelled M2 macrophages-derived exosomes was detected in SGC7901 cells. The function and mechanism of exosomal miR-588 from M2 macrophages in the modulation of DDP resistance of gastric cancer cell was analyzed by CCK-8 assay, apoptosis analysis, colony formation assay, Western blot analysis, qPCR analysis, and luciferase reporter assay in SGC7901 and SGC7901/DDP cells, and by tumorigenicity analysis in nude mice.
Research results
Polarized macrophages were isolated from mouse bone marrow stimulated with interleukin (IL)-13 and IL-4. Co-culture of gastric cancer cells with M2 polarized macrophages promoted DDP resistance. M2 polarized macrophage-derived exosomes could transfer in gastric cancer cells to enhance DDP resistance. Exosomal miR-588 from M2 macrophages contributed to DDP resistance of gastric cancer cells. miR-588 promoted DDP-resistant gastric cancer cell growth in vivo. miR-588 was able to target cylindromatosis (CYLD) in gastric cancer cells. The depletion of CYLD reversed miR-588 inhibition-regulated cell proliferation and apoptosis of gastric cancer cells exposed to DDP.
Research conclusions
In conclusion, we uncovered that exosomal miR-588 from M2 macrophages contributes to DDP resistance of gastric cancer cells by partly targeting CYLD.
Research perspectives
miR-588 may be applied as a potential therapeutic target for the treatment of gastric cancer.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Zibo Central Hospital.
Institutional animal care and use committee statement: All animal experiments in this study were approved by the Zibo Central Hospital.
Conflict-of-interest statement: The authors declare no conflict of interest for this manuscript.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Manuscript source: Unsolicited manuscript
Peer-review started: April 23, 2021
First decision: May 27, 2021
Article in press: August 11, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanabe S, Yashiro M S-Editor: Wu YXJ L-Editor: Wang TQ P-Editor: Li JH
Contributor Information
Hai-Yan Cui, Department of Pathology, The Fourth People’s Hospital of Zibo City, Zibo 255000, Shandong Province, China.
Jian-Sheng Rong, Department of Pathology, Zibo Central Hospital, Zibo 255036, Shandong Province, China.
Ju Chen, Department of Ultrasound Medicine, Zibo Central Hospital, Zibo 255036, Shandong Province, China.
Jie Guo, Department of Health, The Fourth People’s Hospital of Zibo City, Zibo 255000, Shandong Province, China.
Jia-Qin Zhu, Department of Gastroenterology, The Fourth People’s Hospital of Zibo City, Zibo 255000, Shandong Province, China.
Mei Ruan, Department of Oncology, The Fourth People’s Hospital of Zibo City, Zibo 255000, Shandong Province, China.
Rong-Rong Zuo, Department of Pathology, The Fourth People’s Hospital of Zibo City, Zibo 255000, Shandong Province, China.
Shuang-Shuang Zhang, Department of Pathology, The Fourth People’s Hospital of Zibo City, Zibo 255000, Shandong Province, China.
Jun-Mei Qi, Department of Pathology, The Fourth People’s Hospital of Zibo City, Zibo 255000, Shandong Province, China.
Bao-Hua Zhang, Department of Pathology, Zibo Central Hospital, Zibo 255036, Shandong Province, China. zhangbaohua314@163.com.
Data sharing statement
No additional data are available.
References
- 1.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 2.Johnston FM, Beckman M. Updates on Management of Gastric Cancer. Curr Oncol Rep. 2019;21:67. doi: 10.1007/s11912-019-0820-4. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh S. Cisplatin: The first metal based anticancer drug. Bioorg Chem. 2019;88:102925. doi: 10.1016/j.bioorg.2019.102925. [DOI] [PubMed] [Google Scholar]
- 4.Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev. 2012;64:706–721. doi: 10.1124/pr.111.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017;114:206–221. doi: 10.1016/j.addr.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, Zeh HJ, Kang R, Wang J, Tang D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16:2069–2083. doi: 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lan J, Sun L, Xu F, Liu L, Hu F, Song D, Hou Z, Wu W, Luo X, Wang J, Yuan X, Hu J, Wang G. M2 Macrophage-Derived Exosomes Promote Cell Migration and Invasion in Colon Cancer. Cancer Res. 2019;79:146–158. doi: 10.1158/0008-5472.CAN-18-0014. [DOI] [PubMed] [Google Scholar]
- 8.Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126:1208–1215. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;1871:455–468. doi: 10.1016/j.bbcan.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-Mediated Metastasis: Communication from a Distance. Dev Cell. 2019;49:347–360. doi: 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 12.Bach DH, Hong JY, Park HJ, Lee SK. The role of exosomes and miRNAs in drug-resistance of cancer cells. Int J Cancer. 2017;141:220–230. doi: 10.1002/ijc.30669. [DOI] [PubMed] [Google Scholar]
- 13.Bayraktar R, Van Roosbroeck K. miR-155 in cancer drug resistance and as target for miRNA-based therapeutics. Cancer Metastasis Rev. 2018;37:33–44. doi: 10.1007/s10555-017-9724-7. [DOI] [PubMed] [Google Scholar]
- 14.Gao ZQ, Wang JF, Chen DH, Ma XS, Yang W, Zhe T, Dang XW. Long non-coding RNA GAS5 antagonizes the chemoresistance of pancreatic cancer cells through down-regulation of miR-181c-5p. Biomed Pharmacother. 2018;97:809–817. doi: 10.1016/j.biopha.2017.10.157. [DOI] [PubMed] [Google Scholar]
- 15.Zhao N, Lin T, Zhao C, Zhao S, Zhou S, Li Y. MicroRNA-588 is upregulated in human prostate cancer with prognostic and functional implications. J Cell Biochem. 2017 doi: 10.1002/jcb.26417. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Zhang J, Gong W, Dai W, Xu X, Xu S. miR-588 is a prognostic marker in gastric cancer. Aging (Albany NY) 2020;13:2101–2117. doi: 10.18632/aging.202212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun SC. CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes. Cell Death Differ. 2010;17:25–34. doi: 10.1038/cdd.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lork M, Verhelst K, Beyaert R. CYLD, A20 and OTULIN deubiquitinases in NF-κB signaling and cell death: so similar, yet so different. Cell Death Differ. 2017;24:1172–1183. doi: 10.1038/cdd.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- 20.Suenaga N, Kuramitsu M, Komure K, Kanemaru A, Takano K, Ozeki K, Nishimura Y, Yoshida R, Nakayama H, Shinriki S, Saito H, Jono H. Loss of Tumor Suppressor CYLD Expression Triggers Cisplatin Resistance in Oral Squamous Cell Carcinoma. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20205194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Wang Q, Xu G, Meng N, Huang X, Jiang Z, Chen C, Zhang Y, Chen J, Li A, Li N, Zou X, Zhou J, Ding Q, Wang S. The long noncoding RNA CRAL reverses cisplatin resistance via the miR-505/CYLD/AKT axis in human gastric cancer cells. RNA Biol. 2020;17:1576–1589. doi: 10.1080/15476286.2019.1709296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu W, Chen Q, Wang Q, Sun Y, Wang S, Li A, Xu S, Røe OD, Wang M, Zhang R, Yang L, Zhou J. JWA reverses cisplatin resistance via the CK2-XRCC1 pathway in human gastric cancer cells. Cell Death Dis. 2014;5:e1551. doi: 10.1038/cddis.2014.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng P, Luo Q, Wang W, Li J, Wang T, Wang P, Chen L, Zhang P, Chen H, Liu Y, Dong P, Xie G, Ma Y, Jiang L, Yuan X, Shen L. Tumor-associated macrophages-derived exosomes promote the migration of gastric cancer cells by transfer of functional Apolipoprotein E. Cell Death Dis. 2018;9:434. doi: 10.1038/s41419-018-0465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, Ma Y, Shen L. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36:53. doi: 10.1186/s13046-017-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu J, Gao W, Tang Q, Yu Y, You W, Wu Z, Fan Y, Zhang L, Wu C, Han G, Zuo X, Zhang Y, Chen Z, Ding W, Li X, Lin F, Shen H, Tang J, Wang X. M2 Macrophage-Derived Exosomes Facilitate HCC Metastasis by Transferring αM β2 Integrin to Tumor Cells. Hepatology. 2021;73:1365–1380. doi: 10.1002/hep.31432. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Zhou X, Xu M, Guo Y, Ye L, Long L, Wang H, Tan P. MicroRNA-588 regulates invasion, migration and epithelial-mesenchymal transition via targeting EIF5A2 pathway in gastric cancer. Cancer Manag Res. 2018;10:5187–5197. doi: 10.2147/CMAR.S176954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z, Mo H, Sun L, Wang L, Chen T, Yao B, Liu R, Niu Y, Tu K, Xu Q, Yang N. Long noncoding RNA PICSAR/miR-588/EIF6 axis regulates tumorigenesis of hepatocellular carcinoma by activating PI3K/AKT/mTOR signaling pathway. Cancer Sci. 2020;111:4118–4128. doi: 10.1111/cas.14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binenbaum Y, Fridman E, Yaari Z, Milman N, Schroeder A, Ben David G, Shlomi T, Gil Z. Transfer of miRNA in Macrophage-Derived Exosomes Induces Drug Resistance in Pancreatic Adenocarcinoma. Cancer Res. 2018;78:5287–5299. doi: 10.1158/0008-5472.CAN-18-0124. [DOI] [PubMed] [Google Scholar]
- 29.Umemura S, Zhu J, Chahine JJ, Kallakury B, Chen V, Kim IK, Zhang YW, Goto K, He Y, Giaccone G. Downregulation of CYLD promotes IFN-γ mediated PD-L1 expression in thymic epithelial tumors. Lung Cancer. 2020;147:221–228. doi: 10.1016/j.lungcan.2020.07.018. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Lei Y, Lin J, Huang Y, Zhang J, Chen K, Sun S, Lin X. The LINC01260 Functions as a Tumor Suppressor via the miR-562/CYLD/NF-κB Pathway in Non-Small Cell Lung Cancer. Onco Targets Ther. 2020;13:10707–10719. doi: 10.2147/OTT.S253730. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Lin Y, Wang L, Luo W, Zhou X, Chen Y, Yang K, Liao J, Wu D, Cai L. CYLD Promotes Apoptosis of Nasopharyngeal Carcinoma Cells by Regulating NDRG1. Cancer Manag Res. 2020;12:10639–10649. doi: 10.2147/CMAR.S268216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang C, Liu J, Pan X, Peng C, Xiong B, Feng M, Yang X. miR-454 promotes survival and induces oxaliplatin resistance in gastric carcinoma cells by targeting CYLD. Exp Ther Med. 2020;19:3604–3610. doi: 10.3892/etm.2020.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.