Abstract
BACKGROUND
Patients with inflammatory bowel disease (IBD) are associated with increased cardiovascular risk and have increased overall cardiovascular burden. On the other hand, urotensin II (UII) is one of the most potent vascular constrictors with immunomodulatory effect that is connected with a number of different cardiometabolic disorders as well. Furthermore, patients with ulcerative colitis have shown increased expression of urotensin II receptor in comparison to healthy controls. Since the features of IBD includes chronic inflammation and endothelial dysfunction as well, it is plausible to assume that there is connection between increased cardiac risk in IBD and UII.
AIM
To determine serum UII levels in patients with IBD and to compare them to control subjects, as well as investigate possible associations with relevant clinical and biochemical parameters.
METHODS
This cross sectional study consecutively enrolled 50 adult IBD patients (26 with Crohn’s disease and 24 with ulcerative colitis) and 50 age and gender matched controls. Clinical assessment was performed by the same experienced gastroenterologist according to the latest guidelines. Ulcerative Colitis Endoscopic Index of Severity and Simple Endoscopic Score for Crohn’s Disease were used for endoscopic evaluation. Serum levels of UII were determined using the enzyme immunoassay kit for human UII, according to the manufacturer’s instructions.
RESULTS
IBD patients have significantly higher concentrations of UII when compared to control subjects (7.57 ± 1.41 vs 1.98 ± 0.69 ng/mL, P < 0.001), while there were no significant differences between Crohn’s disease and ulcerative colitis patients (7.49 ± 1.42 vs 7.65 ± 1.41 ng/mL, P = 0.689). There was a significant positive correlation between serum UII levels and high sensitivity C reactive peptide levels (r = 0.491, P < 0.001) and a significant negative correlation between serum UII levels and total proteins (r = -0.306, P = 0.032). Additionally, there was a significant positive correlation between serum UII levels with both systolic (r = 0.387, P = 0.005) and diastolic (r = 0.352, P = 0.012) blood pressure. Moreover, serum UII levels had a significant positive correlation with Ulcerative Colitis Endoscopic Index of Severity (r = 0.425, P = 0.048) and Simple Endoscopic Score for Crohn’s Disease (r = 0.466, P = 0.028) scores. Multiple linear regression analysis showed that serum UII levels retained significant association with high sensitivity C reactive peptide (β ± standard error, 0.262 ± 0.076, P < 0.001) and systolic blood pressure (0.040 ± 0.017, P = 0.030).
CONCLUSION
It is possible that UII is involved in the complex pathophysiology of cardiovascular complications in IBD patients, and its purpose should be investigated in further studies.
Keywords: Inflammatory bowel disease, Crohn’s disease, Ulcerative colitis, Urotensin II, Cardiovascular risk, Endoscopic activity
Core Tip: Urotensin II (UII) is a potent vasoconstrictor with an immunomodulatory effect that is connected to various cardiovascular disorders. On the other hand, patients with inflammatory bowel disease (IBD) have increased cardiovascular burden as well as increased expression of UII receptors. It is plausible that UII is involved in the complex pathophysiology of IBD complications. In the current study, we investigated UII levels in the IBD population and compared it to matched control subjects, as well as connection of UII with relevant clinical and biochemical parameters. The results of this study showed that serum UII levels are higher in IBD patients in comparison with the control group.
INTRODUCTION
Inflammatory bowel disease (IBD) is a relapsing chronic inflammation of the gastrointestinal tract with an unpredictable course[1]. It can be divided into two disorders: Crohn’s disease (CD) and ulcerative colitis (UC). Although the two disorders have a similar clinical manifestation, they differ in the location and the depth of the inflammation. The etiology of the disease is considered to be multifactorial as a complex interrelation between extrinsic factors, genetic predisposition, immunological imbalance and microbiota disturbances. Furthermore, IBD can exhibit a wide range of extraintestinal manifestations that affect the kidneys, eyes, joints, liver, skin, heart and blood circulation[2-6].
Urotensin II (UII) is a pleotropic peptide originally found 40 years ago in the neurosecretory system of the teleost fish, while in the meantime its activity was also found in humans. UII is considered to be the most potent vasoconstrictor discovered so far, with the effect 10-fold stronger than that of endothelin-1[7]. Furthermore, its expression was found distributed in most organs and tissues, including both the central nervous and cardiovascular systems, as well as the lungs, kidneys, spleen, hypophysis, adrenal glands, stomach, pancreas, ovaries and liver[8,9]. UII activity is regulated through the urotensin receptor (UTR), which after activation induces calcium mobilization in cellular plasma, smooth muscle cells proliferation and collagen synthesis[10]. Due to the wide range of functions, UII has an extensive effect on most of the organ systems in the body, and consequently it is also associated with numerous diseases and disorders[11-13]. Moreover, recent studies are pointing to possible UII immunomodulatory effects, as it was shown that UII is involved in the regulation of the inflammation process[14,15].
In the last few decades, the extraintestinal manifestations and complications of IBD are a major issue that is increasingly investigated for an improvement of both diagnostic and treatment criteria. It is well-established that patients with IBD are associated with a high cardiovascular risk, and it was shown that they have a higher prevalence cardiovascular diseases[16-18]. However, a Danish cohort study showed that patients with IBD have a lower prevalence of the traditional cardiovascular risk factors in comparison to the general population, while on the other hand they had a higher cardiovascular burden[19]. This ambiguity is well-established, yet it is unclear what are the factors that contribute to high cardiovascular risk in patients with IBD. Since UII is one of the most potent vasoconstrictors known, and it is well-known that it is associated with cardiovascular diseases, it is reasonable to presume that there is a possible connection between cardiovascular risk in IBD patients and UII[20,21]. Moreover, seeing that recent studies are pointing to UII immunomodulatory effect and since the hallmark of IBD is the chronic inflammation, this further suggests the need to investigate clinically the possible association between them[14,15]. Additionally, a recent study conducted on patients with UC showed that they have an increased expression of UTR compared to healthy controls[22]. Moreover, that expression was found to be increased in both disease lesions and healthy tissue biopsies.
Hence, the aim of this study was to evaluate serum UII levels in patients with IBD and to compare them with healthy, gender and age matched controls. Moreover, we further investigated the possible association between UII levels and the relevant clinical and biochemical parameters.
MATERIALS AND METHODS
Study design and ethical considerations
This cross-sectional study was conducted at the University Hospital of Split and the University of Split School of Medicine during the period from January 2018 to March 2019.
Before the start of the study, every participant was informed about the aim, course and procedures involved, and they all signed an informed written consent. The study was conducted in accordance with all ethical principles of the Seventh Revision of the Helsinki Declaration, and it was approved by the Ethics Committee of University Hospital of Split (date of approval: November 23, 2017).
Subjects
This study included 50 adult patients with pre-diagnosed IBD (24 patients with UC and 26 patients with CD) and 50 healthy, age and gender matched controls. The diagnosis of UC and CD was established on clinical, radiological, endoscopic and histological traits in accordance with the European Consensus on Crohn’s Disease and Ulcerative Colitis[23]. Inclusion criteria were: Disease duration of at least 1 year, stable disease activity in the past 3 mo and age between 18 and 65 years. Exclusion criteria were: Diabetes, obesity, arterial hypertension, use of statins, cardiovascular disorders, therapy with corticosteroids during 3 mo prior to study onset, substance abuse and consumption of alcohol more than 40 g/d.
Additionally, we checked medical records of the control subjects regarding gastrointestinal conditions, and additionally we performed screening for abdominal pain presence, symptoms related to defecation and change in frequency and form of stool according to the Rome IV criteria for irritable bowel syndrome[24], as well as any other symptoms that could suggest gluten and lactose intolerance. If any of these conditions were present, we excluded the subject from the control group. Furthermore, all potential control group subjects underwent detailed physical examination along with laboratory analysis of the complete blood count and biochemical parameters. We excluded all participants who showed any sign of inflammation in any of these steps.
Disease severity assessment
Disease activity evaluation was performed using clinical and endoscopic indices. The assessment was conducted by the same experienced gastroenterologist according to the latest guidelines, and the colonoscopy needed for the evaluation of the disease activity was performed within 2 wk of blood sampling. We used endoscopic indices for the evaluation of the disease activity since they have an advantage before clinical indices according to the European Consensus on Crohn’s Disease and Ulcerative Colitis guidelines[25]. Moreover, all IBD patients had their high sensitivity C reactive peptide (hsCRP) and fecal calprotectin evaluated to assess further the activity of the disease.
Ulcerative Colitis Endoscopic Index of Severity (UCEIS) is a quantitative grading system used for the evaluation of UC activity. Depending on the score, there are four possible grades for disease activity: (0-1)–remission; (2-4)–mild; (5-6)–moderate and (7-8)–severe activity[26].
Simple Endoscopic Score for CD (SES-CD) is a quantitative grading system used for the evaluation of CD activity. According to the majority of studies, the threshold values for interpretation of the results are: (0-2)–remission; (3-6)–mild activity; (7-15)–moderate activity and (≥ 16)–severe disease[27].
Blood sampling and laboratory analysis
All blood samples were taken from the cubital vein after 12-h fasting. After extraction, all samples were processed in the same day except for the UII samples, which were centrifuged and stored at -80 °C for further analysis. All the procedures were conducted according to the international standards, in the same laboratory and by the same experienced medical biochemist who was blinded to the subjects group in the study. Serum levels of UII were determined using the enzyme immunoassay kit for human UII (Phoenix Pharmaceuticals, Burlingame, CA, United States), according to the manufacturer’s instructions. Concentration of the analyzed quality control sample that arrived from the manufacturer was within predefined acceptable deviation. The linear range of the assay was 0.06-8.2 ng/mL, and sensitivity was 0.06 ng/mL. Coefficient of variation within the probe was less than 10% and between probes was less than 15%. Other biochemical parameters were analyzed according to standard laboratory procedures.
Stool samples were received by a trained laboratory technician in sterile containers within 3 d of sampling. Fecal extraction and analyses were performed by an experienced medical biochemist.
Anthropometric and clinical assessment
All participants were subjected to detailed anamnesis, physical examination and measurements of anthropometric characteristics - body weight, body height and body mass index (BMI). For measurement of body weight and height, a calibrated medical scale with built-in heights (Seca, Birmingham, United Kingdom) was used. BMI was calculated according to the formula = [body weight (kg)]/[height per square (m2)].
Statistical analysis
Collected data were analyzed with statistical software MedCalc (version 17.4.1; MedCalc Software, Ostend, Belgium,). Quantitative data were expressed as mean ± SD or median and interquartile range, while qualitative data were expressed as whole number and percentage. Kolmogorov-Smirnov test was used to estimate the normality of data distribution. Comparison of serum UII levels and other parameters between patients with IBD and control subjects was done by Student t-test for independent samples or Mann-Whitney U test. For comparison of qualitative variables, Chi-squared test was used. Pearson’s correlation or Spearman’s correlation was used to estimate the correlation between biochemical, anthropometric and clinical parameters with serum UII levels. Furthermore, multiple linear regression analysis was used to determine significant independent predictors of serum UII levels, with reporting corresponding P values with unstandardized β-coefficients, standard error and t-values. The level of statistical significance was set at P < 0.05.
RESULTS
Baseline characteristics of the study population
There were no statistically significant differences regarding age, gender and anthropometric features between the IBD patients and healthy controls (Table 1). Laboratory analyses showed that the IBD group compared to the control group had significantly lower erythrocytes (4.7 ± 0.5 vs 5.0 ± 0.4 × 1012/L, P = 0.020), hemoglobin (140.4 ± 17.3 vs 148.1 ± 13.7 g/L, P = 0.015) and albumins (39.5 ± 5.1 vs 43.7 ± 2.4 g/L, P < 0.001), while they had significantly higher hsCRP levels (3.4 ± 2.6 vs 1.2 ± 1.1 mg/L, P < 0.001) (Table 2).
Table 1.
Baseline characteristics of the inflammatory bowel disease group and the control group
|
Parameter
|
IBD group (n = 50)
|
Control group (n = 50)
|
P
value1
|
| Male gender, n (%) | 31 (62) | 29 (58) | 0.838 |
| Age (yr) | 44.3 ± 14.8 | 40.6 ± 12.3 | 0.181 |
| Body weight (kg) | 78.4 ± 14.0 | 81.1 ± 15.0 | 0.356 |
| Body height (cm) | 176.9 ± 9.8 | 179.6 ± 7.9 | 0.130 |
| Body mass index (kg/m2) | 23.9 ± 3.7 | 24.9 ± 3.4 | 0.136 |
| SBP (mmHg) | 119.5 ± 11.2 | 116.6 ± 9.2 | 0.156 |
| DBP (mmHg) | 77.7 ± 8.3 | 75.0 ± 8.6 | 0.112 |
| Smoking, n (%) | 12 (24.0) | 9 (18.4) | 0.660 |
| Disease duration (yr)2 | 6.0 (3.0-11.0) | - | - |
| UCEIS (score) | 6.0 (5.0-7.0) | - | - |
| SES-CD (score) | 9.2 (6.6-12.0) | - | - |
| Aminosalycilates | 32 (64.0%) | - | - |
| DMARD | 15 (30.0%) | - | - |
| Monoclonal antibodies | 29 (58.0%) | - | - |
Chi-square test or t-test for independent samples.
Time period since the initial diagnosis.
DBP: Diastolic blood pressure; DMARD: Disease-modifying antirheumatic drug; SBP: Systolic blood pressure; SES-CD: Simple endoscopic score for Crohn’s disease; UCEIS: Ulcerative colitis index of severity. Data are presented as whole number (percentage), mean ± SD or median (interquartile range).
Table 2.
Laboratory parameters of the inflammatory bowel disease group and the control group
|
Parameter
|
IBD group (n = 50)
|
Control group (n = 50)
|
P
value1
|
| Erythrocytes (× 1012/L) | 4.7 ± 0.5 | 5.0 ± 0.4 | 0.020 |
| Hemoglobin (g/L) | 140.4 ± 17.3 | 148.1 ± 13.7 | 0.015 |
| Fasting glucose (mmol/L) | 5.1 ± 0.9 | 5.0 ± 0.6 | 0.653 |
| Urea (mmol/L) | 4.7 ± 1.5 | 5.6 ± 1.6 | 0.004 |
| Creatinine (μmol/L) | 71.4 ± 15.3 | 75.9 ± 14.7 | 0.134 |
| Total proteins (g/L) | 71.2 ± 6.6 | 72.1 ± 3.9 | 0.394 |
| Albumins (g/L) | 39.5 ± 5.1 | 43.7 ± 2.4 | < 0.001 |
| hsCRP (mg/L) | 3.4 ± 2.6 | 1.2 ± 1.1 | < 0.001 |
| Triglycerides (mmol/L) | 1.3 ± 0.9 | 1.1 ± 0.6 | 0.311 |
| Total cholesterol (mmol/L) | 4.8 ± 1.3 | 5.2 ± 1.2 | 0.091 |
| HDL cholesterol (mmol/L) | 1.3 ± 0.4 | 1.4 ± 0.3 | 0.447 |
| LDL cholesterol (mmol/L) | 2.6 (2.1-3.4) | 3.2 (2.4-3.9) | 0.018 |
| FC (μg/g) | 231 (61.5-619.2) | - | - |
t-test for independent samples or Mann-Whitney U test.
FC: Fecal calprotectin; HDL: High-density lipoprotein; HsCRP: High sensitivity C-reactive protein; LDL: Low-density lipoprotein. Data are presented as mean ± SD and median (interquartile range).
Serum urotensin II levels
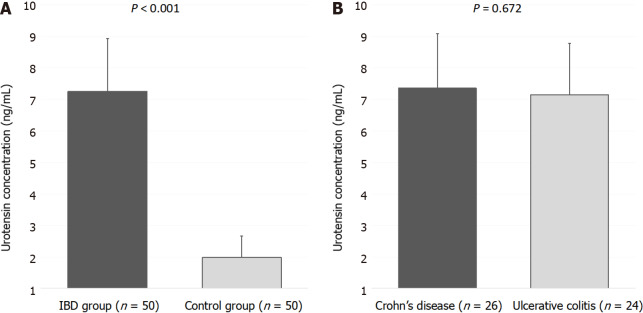
Serum UII levels were significantly higher in the IBD group compared to the control group (7.26 ± 1.67 vs 1.98 ± 0.68 ng/mL, P < 0.001) (Figure 1A). Furthermore, there were no statistically significant differences in serum UII levels between the patients with UC and patients with CD (7.15 ± 1.63 vs 7.36 ± 1.73 ng/mL, P = 0.672) (Figure 1B).
Figure 1.
Serum urotensin II levels. A: Comparison between the inflammatory bowel disease group and the control group. B: Comparison between patients with Crohn’s disease and patients with ulcerative colitis.
Correlations between urotensin II and other biochemical, anthropometric and clinical parameters
There was a significant positive correlation between serum UII levels and hsCRP levels (r = 0.491, P < 0.001) and a significant negative correlation between serum UII levels and total proteins (r = -0.306, P = 0.032). There were no significant correlations with other biochemical parameters (Table 3).
Table 3.
Correlation analysis between serum urotensin II levels and different biochemical, anthropometric and clinical parameters in the inflammatory bowel disease group (n = 50)
|
Parameter
|
r
1
|
P
value
|
| hsCRP (mg/L) | 0.491 | < 0.001 |
| Total proteins (g/L) | -0.306 | 0.032 |
| Albumins (g/L) | -0.182 | 0.210 |
| Triglycerides (mmol/L) | 0.057 | 0.698 |
| Total cholesterol (mmol/L) | -0.114 | 0.439 |
| HDL (mmol/L) | -0.153 | 0.298 |
| LDL (mmol/L) | -0.1033 | 0.487 |
| Urea (mmol/L) | 0.013 | 0.928 |
| Creatinine (μmol/L) | 0.133 | 0.356 |
| Age (yr) | -0.072 | 0.614 |
| Body mass index (kg/m2) | -0.037 | 0.800 |
| SBP (mmHg) | 0.387 | 0.005 |
| DBP (mmHg) | 0.352 | 0.012 |
| IBD duration (yr)2 | 0.0453 | 0.751 |
| FC (μg/g) | 0.0483 | 0.812 |
| UCEIS (score) | 0.4253 | 0.048 |
| SES-CD (score) | 0.4663 | 0.028 |
Pearson’s correlation coefficient.
Time period before the initial diagnosis.
Spearman’s rank correlation coefficient.
DBP: Diastolic blood pressure; FC: Fecal calprotectin; hsCRP: High sensitivity C-reactive protein; SBP: Systolic blood pressure; SES-CD: Simple endoscopic score for Crohn’s disease; UCEIS: Ulcerative colitis index of severity.
There was a significant positive correlation between serum UII levels with both systolic (r = 0.387, P = 0.005) and diastolic (r = 0.352, P = 0.012) blood pressure. Moreover, serum UII levels had a significant positive correlation with UCEIS (r = 0.425, P = 0.048) and SES-CD (r = 0.466, P = 0.028) scores (Table 3).
Multiple linear regression
Multiple linear regression analysis showed that serum UII levels retained significant association with hsCRP (β ± standard error, 0.262 ± 0.076, P < 0.001) and systolic blood pressure (0.040 ± 0.017, P = 0.030) after model adjustment for age, gender, BMI and diastolic blood pressure, with serum UII levels as a dependent variable (Table 4).
Table 4.
Multiple linear regression model of independent predictors for serum urotensin II levels
|
Variable
|
Β1
|
SE
|
t
value
|
P
value
|
| Age | 0.009 | 0.015 | 0.570 | 0.571 |
| Gender | -0.740 | 0.405 | -1.825 | 0.075 |
| BMI | -0.106 | 0.059 | -1.793 | 0.080 |
| SBP | 0.040 | 0.017 | 2.243 | 0.030 |
| DBP | 0.039 | 0.025 | 1.535 | 0.132 |
| hsCRP | 0.262 | 0.076 | 3.469 | < 0.001 |
Unstandardized coefficient β.
BMI: Body mass index; DBP: Diastolic blood pressure; hsCRP: High sensitivity C-reactive protein; SBP: Systolic blood pressure; SE: Standard error.
DISCUSSION
The results of this study showed that serum UII levels are higher in patients with IBD compared to the healthy controls, while there was no significant difference between patients with UC and patients with CD. To the best of our knowledge, this is the first clinical study to investigate serum UII levels in both UC and CD.
Association between UII and IBD was only explored in a recent experimental pilot study that investigated expression of the UII receptor UTR in patients with UC[22]. They measured UTR expression from biopsies of the UC lesions and healthy colon tissue, and their outcomes determined that UTR expression was significantly higher in both the UC lesions and healthy tissue of the UC patients compared to the control group biopsies. Furthermore, a Chinese animal study conducted on mice with dextran sulfate sodium induced colitis explored the mechanisms of UTR in colonic inflammation[28]. They administrated the mice with urantide, a special antagonist of UTR that consequently alleviated rectal bleeding, tissue injury and production of interleukin (IL)-17 and tumor necrosis factor alpha (TNF-α). Furthermore, they showed that the inhibition of UTR reduced the activation of the nuclear factor-κB both in vitro and in vivo. Similarly, a study conducted on mice with induced acute liver failure assessed UTR expression and mechanisms involved[15]. They found that mice treated with urantide and consequent UTR downregulation expressed prevention of proinflammatory cytokines increase. TNF-α, IL-1β and interferon-γ were significantly lower compared to the mice with induced acute liver failure that were not treated with urantide. Additionally, a recent study explored UTR effects in acute liver failure by using Kupffer cells as a model system[29]. They found that UTR mediates production and release of proinflammatory cytokines TNF-α, IL-1β and interferon-γ by using the inflammatory pathway nuclear factor-κB. All of these aforementioned studies point to the possibility that UII, besides the already well-established vasoconstriction, is also involved in the development and enhancement of the inflammatory response.
According to these results, it could be hypothesized that in IBD, among other mechanisms, higher UII levels and consequently greater UTR activity can be associated with elevated TNF-α concentrations, which cause the development of the aberrant inflammatory response[30]. It is well established that IBD is linked with elevated TNF-α levels in the mucosa of the gastrointestinal tract and consequently an abnormal inflammatory response that is associated with the dysregulation of mucosal immune cells and tissue injury[31]. Moreover, TNF-α contributes to inflammation through disruption of the epithelial barrier, stimulation of villous epithelial cells apoptosis and secretion of chemokines from intestinal epithelium[32]. This possibility is further supported by our results that UII is in a significant positive association with hsCRP, which was additionally supported with multiple linear regression, and clinical indices of IBD activity as well. These results implicate that disease activity could be closely related to UII levels.
Moreover, it is important to highlight that the previous studies have shown that high TNF-α levels play a major role in the disruption of macro and microvascular circulation[33]. It induces the production of reactive oxygen species, which results in endothelial dysfunction, while several studies presented that the administration of anti-TNF-α therapy to patients with IBD results in a significant improvement of endothelial dysfunction[32,34]. It is possible that functional and structural changes of the vascular endothelium due to chronic inflammation consequently results in higher cardiovascular risk in IBD patients[35]. Furthermore, with more severe disease activity and consequent greater inflammation, the resulting endothelial dysfunction that accompanies these changes is more advanced. It was presented by two recent studies that heart dysfunction, as well as fibrosis and cell hypertrophy, were significantly decreased in experimental heart models treated with UTR antagonists[36,37]. Moreover, a recent study showed that elevated UII levels are associated with the severity of cardiovascular risk factors[38].
In the last 2 decades, it has become clear that chronic systemic inflammation plays a major part in the initiation and progression of atherosclerosis[39,40]. Circulating UII was reported to promote increase of reactive oxygen species levels, which are important molecules in the initiation of atherosclerosis[41]. In a Chinese animal study, urantide administration reduced the proportion of the macrophage lesion area as well as improved the plaque characteristics in hyperlipidemic rabbits by increasing the collagen content[42]. Even though UTR antagonist downregulated proinflammatory cytokines, it did not significantly change the lipid profile. In summary, although the UTR antagonist did not change the progression of atherosclerosis, it significantly affected composition of atherosclerotic plaque. These results imply that UII is associated with the process of atherosclerosis, but the downregulation of its receptor UTR only affects the properties of the atherosclerotic plaque, while it does not stop its actual progression.
Our results also determined a significant positive correlation between serum UII levels with systolic and diastolic blood pressure. This is in alignment with several other clinical and experimental studies that have found an association between UII and arterial pressure, probably present due to its established vasoconstrictive effect[43,44]. Moreover, studies have shown that IBD patients have a lower incidence of some of the traditional risk factors for cardiovascular diseases, including hypertension[19]. In a current scenario, it is still questionable why IBD patients have lower incidence of hypertension, although UII levels are elevated in comparison with healthy subjects. It is hard to hypothesize from our results what are the possible reasons for this ambiguity, and further studies are needed to elaborate this issue. However, it is possible that UII vasoconstriction effect is diminished by other factors that are present in IBD patients.
This study had several limitations. It was a single center study with a cross-sectional design. Moreover, our sample size was relatively low, and we were not able to eliminate completely all possible confounding effects.
CONCLUSION
In conclusion, this study showed that patients with IBD have a higher serum level of UII compared to the control group. This implied association with IBD was further supported with the positive correlation between UII and hsCRP, UCEIS and SES-CD. All of these results suggest that UII could be involved in the pathophysiology of IBD, especially in the inflammation severity and disease activity. However, future studies need to clarify these connections.
ARTICLE HIGHLIGHTS
Research background
Patients with inflammatory bowel disease (IBD) are associated with increased cardiovascular risk and have increased overall cardiovascular burden. On the other hand, urotensin II (UII) is one of the most potent vascular constrictors with immunomodulatory effect that is connected with a number of different cardiometabolic disorders as well. Since the features of IBD includes chronic inflammation and endothelial dysfunction, it is plausible to assume that there is connection between increased cardiac risk in IBD and UII.
Research motivation
While a recent study showed that patients with ulcerative colitis (UC) have increased expression of urotensin II receptor in comparison to healthy controls, a larger clinical study regarding UII serum levels in patients with IBD is still missing.
Research objectives
The aim of this study was to compare serum levels of UII between patients with IBD and healthy controls. The additional goal was to investigate the association of serum UII levels with the anthropometric, clinical and biochemical parameters.
Research methods
This study included 50 adult patients with pre-diagnosed IBD (24 patients with UC and 26 patients with Crohn’s disease (CD) and 50 healthy, age and gender matched controls. Serum levels of UII were determined using the enzyme immunoassay kit for human UII, according to the manufacturer’s instructions. Other parameters were analyzed according to the standard laboratory procedures.
Research results
Analysis has shown that IBD patients have significantly higher concentrations of UII when compared to control subjects (7.57 ± 1.41 vs 1.98 ± 0.69 ng/mL, P < 0.001), while there were no significant differences between CD and UC patients (7.49 ± 1.42 vs 7.65 ± 1.41 ng/mL, P = 0.689). There was a significant positive correlation between serum UII levels and high sensitivity C reactive peptide levels (r = 0.491, P < 0.001), UC Endoscopic Index of Severity (r = 0.425, P = 0.048) and Simple Endoscopic Score for CD (r = 0.466, P = 0.028) scores.
Research conclusions
Our clinical results suggest that UII could be involved in the pathophysiology of IBD, especially in the inflammation severity and disease activity.
Research perspectives
Future larger scale multicenter studies need to clarify the connection between UII and IBD.
ACKNOWLEDGEMENTS
The authors would like to thank to Behmen D, MA for her careful language assistance.
Footnotes
Institutional review board statement: The study was approved by the Ethics Committee of University Hospital of Split, No. 500-03/17-01/86.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: There are no conflict of interest to report.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Invited manuscript
Peer-review started: April 27, 2021
First decision: June 13, 2021
Article in press: August 19, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sassaki LY S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Yuan YY
Contributor Information
Damir Alicic, Department of Gastroenterology, University Hospital of Split, Split 21000, Croatia.
Dinko Martinovic, Department of Pathophysiology, University of Split School of Medicine, Split 21000, Croatia.
Doris Rusic, Department of Pharmacy, University of Split School of Medicine, Split 21000, Croatia.
Piero Marin Zivkovic, Department of Gastroenterology, University Hospital of Split, Split 21000, Croatia.
Ivana Tadin Hadjina, Department of Gastroenterology, University Hospital of Split, Split 21000, Croatia.
Marino Vilovic, Department of Pathophysiology, University of Split School of Medicine, Split 21000, Croatia.
Marko Kumric, Department of Pathophysiology, University of Split School of Medicine, Split 21000, Croatia.
Daria Tokic, Department of Anesthesiology and Intensive care, University Hospital of Split, Split 21000, Croatia.
Daniela Supe-Domic, Department of Health Studies, University of Split, Split 21000, Croatia.
Slaven Lupi-Ferandin, Department of Maxillofacial Surgery, University Hospital of Split, Split 21000, Croatia.
Josko Bozic, Department of Pathophysiology, University of Split School of Medicine, Split 21000, Croatia. josko.bozic@mefst.hr.
Data sharing statement
No additional data are available.
References
- 1.Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91–99. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brnic D, Martinovic D, Zivkovic PM, Tokic D, Vilovic M, Rusic D, Tadin Hadjina I, Libers C, Glumac S, Supe-Domic D, Tonkic A, Bozic J. Inactive matrix Gla protein is elevated in patients with inflammatory bowel disease. World J Gastroenterol. 2020;26:4866–4877. doi: 10.3748/wjg.v26.i32.4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brnić D, Martinovic D, Zivkovic PM, Tokic D, Tadin Hadjina I, Rusic D, Vilovic M, Supe-Domic D, Tonkic A, Bozic J. Serum adropin levels are reduced in patients with inflammatory bowel diseases. Sci Rep. 2020;10:9264. doi: 10.1038/s41598-020-66254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fragoulis GE, Liava C, Daoussis D, Akriviadis E, Garyfallos A, Dimitroulas T. Inflammatory bowel diseases and spondyloarthropathies: From pathogenesis to treatment. World J Gastroenterol. 2019;25:2162–2176. doi: 10.3748/wjg.v25.i18.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filimon AM, Negreanu L, Doca M, Ciobanu A, Preda CM, Vinereanu D. Cardiovascular involvement in inflammatory bowel disease: Dangerous liaisons. World J Gastroenterol. 2015;21:9688–9692. doi: 10.3748/wjg.v21.i33.9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng K, Faye AS. Venous thromboembolism in inflammatory bowel disease. World J Gastroenterol. 2020;26:1231–1241. doi: 10.3748/wjg.v26.i12.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svistunov AA, Tarasov VV, Shakhmardanova SA, Sologova SS, Bagaturiya ET, Chubarev VN, Galenko-Yaroshevsky PA, Avila-Rodriguez MF, Barreto GE, Aliev G. Urotensin II: Molecular Mechanisms of Biological Activity. Curr Protein Pept Sci. 2018;19:924–934. doi: 10.2174/1389203718666170829162335. [DOI] [PubMed] [Google Scholar]
- 8.Federico A, Zappavigna S, Dallio M, Misso G, Merlino F, Loguercio C, Novellino E, Grieco P, Caraglia M. Urotensin-II Receptor: A Double Identity Receptor Involved in Vasoconstriction and in the Development of Digestive Tract Cancers and other Tumors. Curr Cancer Drug Targets. 2017;17:109–121. doi: 10.2174/1568009616666160621101248. [DOI] [PubMed] [Google Scholar]
- 9.Pereira-Castro J, Brás-Silva C, Fontes-Sousa AP. Novel insights into the role of urotensin II in cardiovascular disease. Drug Discov Today. 2019;24:2170–2180. doi: 10.1016/j.drudis.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Castel H, Desrues L, Joubert JE, Tonon MC, Prézeau L, Chabbert M, Morin F, Gandolfo P. The G Protein-Coupled Receptor UT of the Neuropeptide Urotensin II Displays Structural and Functional Chemokine Features. Front Endocrinol (Lausanne) 2017;8:76. doi: 10.3389/fendo.2017.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eyre HJ, Speight T, Glazier JD, Smith DM, Ashton N. Urotensin II in the development and progression of chronic kidney disease following ⅚ nephrectomy in the rat. Exp Physiol. 2019;104:421–433. doi: 10.1113/EP087366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan K, Albanese I, Yu B, Shalal Y, Al-Kindi H, Alaws H, Tardif JC, Gourgas O, Cerutti M, Schwertani A. Urotensin II, urotensin-related peptide, and their receptor in aortic valve stenosis. J Thorac Cardiovasc Surg. 2019 doi: 10.1016/j.jtcvs.2019.09.029. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Yin L, Jia WH, Wang NQ, Xu CY, Hou BY, Li N, Zhang L, Qiang GF, Yang XY, Du GH. Chronic Urotensin-II Administration Improves Whole-Body Glucose Tolerance in High-Fat Diet-Fed Mice. Front Endocrinol (Lausanne) 2019;10:453. doi: 10.3389/fendo.2019.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun SL, Liu LM. Urotensin II: an inflammatory cytokine. J Endocrinol. 2019 doi: 10.1530/JOE-18-0505. [DOI] [PubMed] [Google Scholar]
- 15.Liang DY, Liu LM, Ye CG, Zhao L, Yu FP, Gao DY, Wang YY, Yang ZW. Inhibition of UII/UTR system relieves acute inflammation of liver through preventing activation of NF-κB pathway in ALF mice. PLoS One. 2014;8:e64895. doi: 10.1371/journal.pone.0064895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bigeh A, Sanchez A, Maestas C, Gulati M. Inflammatory bowel disease and the risk for cardiovascular disease: Does all inflammation lead to heart disease? Trends Cardiovasc Med. 2020;30:463–469. doi: 10.1016/j.tcm.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Singh S, Kullo IJ, Pardi DS, Loftus EV Jr. Epidemiology, risk factors and management of cardiovascular diseases in IBD. Nat Rev Gastroenterol Hepatol. 2015;12:26–35. doi: 10.1038/nrgastro.2014.202. [DOI] [PubMed] [Google Scholar]
- 18.Zivkovic PM, Matetic A, Tadin Hadjina I, Rusic D, Vilovic M, Supe-Domic D, Borovac JA, Mudnic I, Tonkic A, Bozic J. Serum Catestatin Levels and Arterial Stiffness Parameters Are Increased in Patients with Inflammatory Bowel Disease. J Clin Med. 2020;9 doi: 10.3390/jcm9030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kristensen SL, Ahlehoff O, Lindhardsen J, Erichsen R, Jensen GV, Torp-Pedersen C, Nielsen OH, Gislason GH, Hansen PR. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death--a Danish nationwide cohort study. PLoS One. 2013;8:e56944. doi: 10.1371/journal.pone.0056944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed AH, Maulood IM. Endothelin-1 and angiotensin-II modulate urotensin-II vasoconstriction in rat aorta exposed to mercury. Bratisl Lek Listy. 2018;119:444–449. doi: 10.4149/BLL_2018_081. [DOI] [PubMed] [Google Scholar]
- 21.Jumaah S, Çelekli A, Sucu M. The role of human urotensin-II in patients with hypertrophic cardiomyopathy. J Immunoassay Immunochem. 2018;39:150–162. doi: 10.1080/15321819.2017.1344130. [DOI] [PubMed] [Google Scholar]
- 22.Gravina AG, Dallio M, Tuccillo C, Martorano M, Abenavoli L, Luzza F, Stiuso P, Lama S, Grieco P, Merlino F, Caraglia M, Loguercio C, Federico A. Urotensin II receptor expression in patients with ulcerative colitis: a pilot study. Minerva Gastroenterol Dietol. 2020;66:23–28. doi: 10.23736/S1121-421X.19.02602-3. [DOI] [PubMed] [Google Scholar]
- 23.Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, Langner C, Limdi JK, Pellino G, Zagórowicz E, Raine T, Harbord M, Rieder F European Crohn’s and Colitis Organisation [ECCO] Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11:649–670. doi: 10.1093/ecco-jcc/jjx008. [DOI] [PubMed] [Google Scholar]
- 24.Schmulson MJ, Drossman DA. What Is New in Rome IV. J Neurogastroenterol Motil. 2017;23:151–163. doi: 10.5056/jnm16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sturm A, Maaser C, Calabrese E, Annese V, Fiorino G, Kucharzik T, Vavricka SR, Verstockt B, van Rheenen P, Tolan D, Taylor SA, Rimola J, Rieder F, Limdi JK, Laghi A, Krustiņš E, Kotze PG, Kopylov U, Katsanos K, Halligan S, Gordon H, González Lama Y, Ellul P, Eliakim R, Castiglione F, Burisch J, Borralho Nunes P, Bettenworth D, Baumgart DC, Stoker J European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR] ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis. 2019;13:273–284. doi: 10.1093/ecco-jcc/jjy114. [DOI] [PubMed] [Google Scholar]
- 26.Travis SP, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, Feagan BG, Hanauer SB, Lichtenstein GR, Marteau PR, Reinisch W, Sands BE, Yacyshyn BR, Schnell P, Bernhardt CA, Mary JY, Sandborn WJ. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology. 2013;145:987–995. doi: 10.1053/j.gastro.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Daperno M, D'Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, Sostegni R, Rocca R, Pera A, Gevers A, Mary JY, Colombel JF, Rutgeerts P. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. doi: 10.1016/s0016-5107(04)01878-4. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Zhang J, Chen X, Wu T, Xu X, Cao G, Li H, Li Y. UII/GPR14 is involved in NF-κB-mediated colonic inflammation in vivo and in vitro. Oncol Rep. 2016;36:2800–2806. doi: 10.3892/or.2016.5069. [DOI] [PubMed] [Google Scholar]
- 29.Liu LM, Liang DY, Ye CG, Tu WJ, Zhu T. The UII/UT system mediates upregulation of proinflammatory cytokines through p38 MAPK and NF-κB pathways in LPS-stimulated Kupffer cells. PLoS One. 2015;10:e0121383. doi: 10.1371/journal.pone.0121383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lichtenstein GR. Comprehensive review: antitumor necrosis factor agents in inflammatory bowel disease and factors implicated in treatment response. Therap Adv Gastroenterol. 2013;6:269–293. doi: 10.1177/1756283X13479826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedrich M, Pohin M, Powrie F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity. 2019;50:992–1006. doi: 10.1016/j.immuni.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Schinzari F, Armuzzi A, De Pascalis B, Mores N, Tesauro M, Melina D, Cardillo C. Tumor necrosis factor-alpha antagonism improves endothelial dysfunction in patients with Crohn's disease. Clin Pharmacol Ther. 2008;83:70–76. doi: 10.1038/sj.clpt.6100229. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Park Y, Wu J, Chen Xp, Lee S, Yang J, Dellsperger KC, Zhang C. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009;116:219–230. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zanoli L, Inserra G, Cappello M, Ozturk K, Castellino P. Aortic Stiffness in Patients With Inflammatory Bowel Disease Reduced After Anti-Tumor Necrosis Factor Therapy. J Am Coll Cardiol. 2019;73:981–982. doi: 10.1016/j.jacc.2018.12.032. [DOI] [PubMed] [Google Scholar]
- 35.Cibor D, Domagala-Rodacka R, Rodacki T, Jurczyszyn A, Mach T, Owczarek D. Endothelial dysfunction in inflammatory bowel diseases: Pathogenesis, assessment and implications. World J Gastroenterol. 2016;22:1067–1077. doi: 10.3748/wjg.v22.i3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park CH, Lee JH, Lee MY, Lee BH, Oh KS. A novel role of G protein-coupled receptor kinase 5 in urotensin II-stimulated cellular hypertrophy in H9c2UT cells. Mol Cell Biochem. 2016;422:151–160. doi: 10.1007/s11010-016-2814-y. [DOI] [PubMed] [Google Scholar]
- 37.Oh KS, Lee JH, Yi KY, Lim CJ, Park BK, Seo HW, Lee BH. A novel urotensin II receptor antagonist, KR-36996, improved cardiac function and attenuated cardiac hypertrophy in experimental heart failure. Eur J Pharmacol. 2017;799:94–102. doi: 10.1016/j.ejphar.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Demirpence M, Guler A, Yilmaz H, Sayin A, Pekcevik Y, Turkon H, Colak A, Ari EM, Aslanipour B, Kocabas GU, Calan M. Is elevated urotensin II level a predictor for increased cardiovascular risk in subjects with acromegaly? J Endocrinol Invest. 2019;42:207–215. doi: 10.1007/s40618-018-0905-1. [DOI] [PubMed] [Google Scholar]
- 39.Chistiakov DA, Grechko AV, Myasoedova VA, Melnichenko AA, Orekhov AN. The role of monocytosis and neutrophilia in atherosclerosis. J Cell Mol Med. 2018;22:1366–1382. doi: 10.1111/jcmm.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koelwyn GJ, Corr EM, Erbay E, Moore KJ. Regulation of macrophage immunometabolism in atherosclerosis. Nat Immunol. 2018;19:526–537. doi: 10.1038/s41590-018-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohammadi A, Najar AG, Khoshi A. Effect of urotensin II on apolipoprotein B100 and apolipoprotein A-I expression in HepG2 cell line. Adv Biomed Res. 2014;3:22. doi: 10.4103/2277-9175.124661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu QQ, Cheng DX, Xu LR, Li YK, Zheng XY, Liu Y, Li YF, Liu HL, Bai L, Wang R, Fan JL, Liu EQ, Zhao SH. Urotensin II and urantide exert opposite effects on the cellular components of atherosclerotic plaque in hypercholesterolemic rabbits. Acta Pharmacol Sin. 2020;41:546–553. doi: 10.1038/s41401-019-0315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe T, Kanome T, Miyazaki A, Katagiri T. Human urotensin II as a link between hypertension and coronary artery disease. Hypertens Res. 2006;29:375–387. doi: 10.1291/hypres.29.375. [DOI] [PubMed] [Google Scholar]
- 44.Debiec R, Christofidou P, Denniff M, Bloomer LD, Bogdanski P, Wojnar L, Musialik K, Charchar FJ, Thompson JR, Waterworth D, Song K, Vollenweider P, Waeber G, Zukowska-Szczechowska E, Samani NJ, Lambert D, Tomaszewski M. Urotensin-II system in genetic control of blood pressure and renal function. PLoS One. 2013;8:e83137. doi: 10.1371/journal.pone.0083137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.