Abstract
Premature mothers present more anxiety and stress after delivery, which may be caused by mother–infant separation while hospitalised. Skin‐to‐skin contact (SSC), a mitigating factor for mother–infant separation, can benefit infants and mothers in many ways, but few studies focused on its efficacy on maternal anxiety and stress states. Therefore, this review aims to evaluate the effect of SSC on anxiety and stress. Comprehensive research was conducted in nine databases. Meta‐analysis was conducted to investigate the effect of SSC, and subgroup analyses were performed to explain the sources of heterogeneity. Eight randomised controlled trials with 728 participants were included, and SSC significantly reduced the level of anxiety ([standardised mean difference, SMD] −0.72; 95% CI −1.08 to −0.35) and stress state ([SMD] −0.84; 95% CI −1.59 to −0.09). One subgroup analysis revealed that SSC can relieve anxiety if performing SSC no less than 1 h per day ([SMD] −0.94; 95% CI −1.34 to −0.53). Another subgroup analysis suggested that applying SSC repeatedly and lasting less than 1 week ([SMD] −1.49; 95% CI −2.31 to −0.66) or for 1 week to 2 weeks ([SMD] −1.04; 95% CI −1.29 to −0.79) can significantly reduce maternal anxiety level but no significance if lasting over 2 weeks ([SMD] −0.33; 95% CI −0.67 to 0.01). SSC can effectively improve anxiety and stress states among premature mothers after delivery, and not definitive finding presents that only SSC that was performed no less than 60 min could improve postpartum anxiety states, while SSC alone was not as effective when carried out over 2 weeks.
Keywords: anxiety, postpartum period, premature birth, skin to skin contact, stress
Key messages.
Anxiety and stress states among postpartum women of premature infants require necessary attention, in terms of severity and frequency.
Our meta‐analysis suggests that skin‐to‐skin contact can effectively relieve anxiety and stress states among postpartum women of premature infants.
Our findings yield significant but still limited evidence that skin‐to‐skin contact for no less than 1 h per day has a protective effect on anxiety states, but this individual effect may be weakened by infants' physical condition, economic levels and other existent factors if the intervention exceeds 2 weeks. More evidence is needed to prove to come.
1. INTRODUCTION
Pregnancy and childbirth are two critical periods in a woman's life, but women are more prone to psychiatric illness in these two periods (Simavli et al., 2014). It is estimated that about 30% of primiparous women experienced anxiety, depression and stress in pregnancy (Nakić Radoš et al., 2018). The rate of serious anxiety in postpartum women is about 17%, and even higher, reaching 20% at 6‐week postpartum (Nakić Radoš et al., 2018), while 14.3% of postpartum women are suffering from general stress (Lau et al., 2017). Anxiety, depression and stress, which could interact with each other, are the major mental health problem affecting women during pregnancy or after delivery (Fairbrother et al., 2015). However, anxiety and stress states were overlooked because more attention was paid to depressive states among postpartum women. But in fact, anxiety and stress are more frequent than depression (Gao et al., 2019; Jordan & Minikel, 2019), and some women will worsen over time, accompanied by severe physical symptoms (Shimada et al., 2018). Furthermore, severe and lasting anxiety and stress can lead to postpartum depression (PPD) and family disharmony sometimes (Biaggi et al., 2016; Tedgård et al., 2020). It even has long‐term adverse consequences for some families and infants, such as leading to children's learning disabilities or cognitive difficulties and poor parent–child relationships (Ali et al., 2013; Hoffman et al., 2017; Tedgård et al., 2020), so it cannot be ignored.
Anxiety and stress of postpartum women are affected by many factors, and the main factors are infant states, family environment and maternal characteristics (Biaggi et al., 2016; Tedgård et al., 2020). Among them, infant states were perceived as an essential risk factor for anxiety and stress states. It was reported that the levels of stress and anxiety significantly increased in mothers of preterm infants compared to that of full‐term infants (Treyvaud et al., 2019), which means attention should be paid to them. This is why we focus on this group. In clinical practice, depending on local/national policies as well as hospital protocols and other factors, such as infant health complications and birth weight, many preterm infants are admitted to the neonatal intensive care unit (NICU)/paediatric ward, which to some extent negatively influences early parent–child bonding due to mother–infant separation (Baía et al., 2016; Ionio et al., 2017). While maternal–infant bonding is a protective factor of anxiety and stress. Consequently, skin‐to‐skin contact (SSC), which can establish maternal–infant bonding (Moore et al., 2016), has been promoted clinically in order to relieve psychological distress.
SSC refers to the exposure of newborn babies on the mother's bare chest for contact, while kangaroo care (KC) is often used to describe SSC in the Neonatal Intensive Care Units (NICU) (Stevens et al., 2014). Now, robust evidence supports that SSC shows benefit for both mothers and newborns in many ways. For the mother, SSC could promote the production of oxytocin (Vittner et al., 2018) and reduce postpartum haemorrhage (Saxton et al., 2015). For newborns, it can relieve stress (Bystrova et al., 2003), reduce neonatal crying (Widström et al., 2019) and stabilise physiological functions (such as better temperature regulation together with stable heart rate, breathing and blood oxygen saturation) (Beiranvand et al., 2014; Moore et al., 2016). It also can promote neurobehavioral development (Feldman & Eidelman, 2003) and reduce neonatal pain response or improve pain tolerance (Disher et al., 2017; Johnston et al., 2017). Moreover, the Cochrane Review in 2016 has confirmed that early or immediate SSC can promote breastfeeding (Moore et al., 2016).
In terms of psychology, the evidence of protective effects of SSC on distress has shown that SSC can reduce the standardised depression scores comparing to routine care, despite a small effect (Scime et al., 2019). However, compared to the effectiveness of SSC on depression, the efficacy of SSC on anxiety and stress among postpartum women can be diverse on account of more influencing factors together with a higher incidence of anxiety and stress. Currently, some studies have investigated the relationship between SSC and anxiety and stress and have yielded positive results (Badiee et al., 2014; Coşkun & Günay, 2020), just like Vittner D et al. (Vittner et al., 2018) suggested that SSC can effectively reduce the mother's anxiety level and even affect the father's stress. But others obtained negative results (Miles et al., 2006; Norouzi et al., 2013; Samra et al., 2015). A systematic review has investigated the effect of SSC on maternal mood including depression, anxiety and stress, but the data have not been quantitatively merged, and some of the included studies employed nonvalidated outcome measuring tools (Athanasopoulou & Fox, 2014). Validated measuring tools are essential in studies of psychological problems in case of mistaking other moods for anxiety and stress. Furthermore, the impact of SSC on PPD among mothers of preterm or low birth weight infants has been probed into in 2019 by Scime et al. (Scime et al., 2019), which demonstrated that SSC has a small protective effect on maternal depressive scores.
Therefore, there is a need to systematically review RCTs in the field of anxiety and stress, and provide evidence on the effect of SSC on them. Through this review, we will determine whether SSC has a protective effect on anxiety and stress among postpartum women of premature infants compared with routine care.
2. METHODS
2.1. Search strategy
This review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) Guidelines, and the PICOs framework was used when we performed the review question. Studies reported up to January 2021 were elected by searching PubMed, Embase, Cochrane Library, Psyc INFO (Ovid), CINAHL, Web of Science, CNKI, WanFang Database and Sinomed. Besides, the reference was manually searched to obtain relevant studies. Appendix 1 demonstrated the search term and a specific search strategy. All retrieved documents were imported into Endnote X9 (Thomas Reuters 2019) for subsequent literature screening in line with the inclusion and exclusion criteria.
2.2. Inclusion and exclusion criteria
Studies would be enrolled in the meta‐analysis if they met the PICOS criteria as follows:
Participants: Postpartum women with premature infants
Intervention: SSC, by definition, is placing the bare baby prone on the mother's naked chest at birth or afterward.
Comparison: Other types of care
Outcomes: Studies containing one of the following indicators were included, such as (a) primary outcome: anxiety, (b) secondary outcome: stress. All research data must be continuous variables, that is, mean ± standard error of the mean (SEM).
Study design: RCTs
Studies would be excluded if (1) not comprising the necessary information or data, (2) using nonstandardised measuring tools, (3) not written in Chinese or English and (4) unable to access the full text, such as study protocols and abstracts.
2.3. Study selection
Two authors independently screened the title and abstract and assigned ‘included’ or ‘no’ in line with inclusion and exclusion criteria. If one author considered that the article could be included, it would be reviewed through full‐text reading. Finally, all articles included in this study should be agreed upon by two authors through consensus or refer to a third author for consent.
2.4. Data extraction
The first author extracted data and recorded them on the spreadsheet designed for this analysis, and the second author verified it. Items extracted for analysis included the following: author and years, country, sample sizes, the details of the intervention and the main outcomes containing measuring tools and time.
2.5. Quality assessment
The quality of the included studies was evaluated by two authors using the Cochrane Collaboration Risk of Bias tool, independently. To be specific, authors mainly focused on the following elements: (a) generating random sequence, (b) allocation concealment, (c) blinding of implementers and participators, (d) blinding of outcome evaluator, (e) incomplete outcome information, (f) selective reports and (g) other biases. Then the studies were assessed as ‘low risk’, ‘high risk’ and ‘unclear risk’ of bias. In terms of any disagreement, they were resolved through consensus.
2.6. Data analysis
Data integration was conducted with the Review Manager (RevMan V.5.3; Cochrane Collaboration, Oxford, UK). The standardised mean difference (SMD) with their corresponding 95% CI was adopted for continuous variables which reflects the same outcome but measured with different methods. P < 0.05 was considered statistically significant. Heterogeneity among the included studies was examined by I 2 statistics. If I 2 ≤ 50%, the fixed‐effects model was adopted, while I 2 > 50%, the random‐effects model was available. The subgroup analysis was performed to explore the source of heterogeneity. Two subgroup analyses of the primary outcome were performed following the daily intervention time and the total intervention weeks, respectively.
3. RESULTS
3.1. Study selection
A total of 767 records were identified through an initial systematic search, of which 334 articles were removed due to duplicated citations. Then 429 remaining articles were reviewed by abstracts and titles, and our inclusion and exclusion criteria resulted in 41 results for the full‐text reviewing stage. By retrieving full‐text articles, 33 studies were excluded for the reason shown in Figure 1. Finally, eight studies were included in the final meta‐analysis.
FIGURE 1.
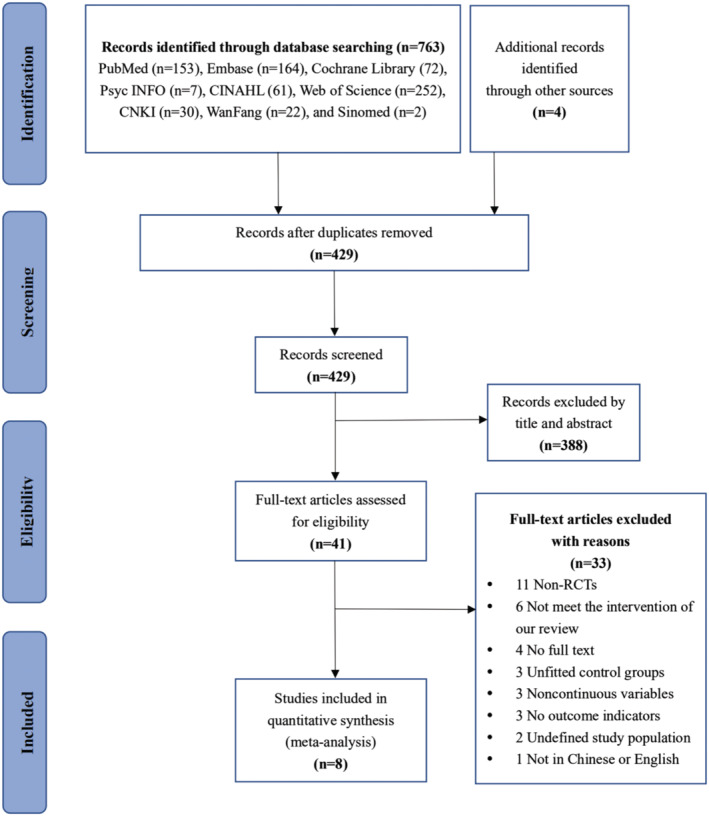
Flowchart of the study selection
3.2. Study characteristics
The eight included studies were conducted between 2006 and 2020 with a total of 728 participants (Table 1). All trials were performed across six countries, including China (Lai et al., 2006; Wang et al., 2020), Iran (Badiee et al., 2014; Norouzi et al., 2013), the United Kingdom (Miles et al., 2006), Israel (Feldman et al., 2014), the United States (Samra et al., 2015) and Turkey (Coşkun & Günay, 2020). Seven RCTs compared SSC with standard care, while one study provided mothers holding babies wrapped in blankets as a comparator (Samra et al., 2015). In the aspect of measure time, five studies measured immediately after the intervention, whereas two studies (Badiee et al., 2014; Miles et al., 2006) measured at discharge and one study (Feldman et al., 2014) measured range from birth to 6 months. Six studies reported the levels of maternal anxiety, which were evaluated by three different tools, including the State Anxiety Inventory Scale (Feldman et al., 2014; Lai et al., 2006; Miles et al., 2006; Norouzi et al., 2013), Parental Stress Scale: Neonatal Intensive Care Unit (Wang et al., 2020) and the General Health Questionnaire (Badiee et al., 2014). Four studies reported the levels of maternal stress, which were evaluated by two different tools, including Parenting Stress Index‐Short Form (Wang et al., 2020) and Parental Stress Scale: Neonatal Intensive Care Unit (Coşkun & Günay, 2020; Miles et al., 2006; Samra et al., 2015). Moreover, the specific implementation methods of SSC in eight studies will be introduced in more detail below.
TABLE 1.
Characteristics of the included studies
| Authors (year) | Country | Sample size, n | SSC group intervention | Control group intervention | Measure time | Main outcome | ||
|---|---|---|---|---|---|---|---|---|
| Total length | Duration | Frequency | ||||||
| Lai et al. (2006) | China | 30 | 3 days | 60 min | Daily | Standard care | Immediately after intervention | Anxiety: STAI Form Y‐1 |
| Norouzi et al. (2013) | Iran | 60 | NR | 30 min | NR | Standard care | Immediately after intervention | Anxiety: STAI Form Y‐1 |
| Badiee et al. (2014) | Iran | 50 | 1 week | 60 min | 3 times/day | Standard care | Upon discharge | Anxiety: GHQ |
| Wang et al. (2020) | China | 240 | 13 days | 2 h | Daily | Standard care | Immediately after intervention |
Anxiety: PSS: NICU Stress: PSI‐SF |
| Miles et al. (2006) | UK | 78 | 4 weeks | 20 min | Daily | Standard care | Upon discharge |
Anxiety: STAI Stress: PSS: NICU |
| Feldman et al. (2014) | Israel | 146 | 14 days | 60 min | NR | Standard care | Birth–6 months | Anxiety: STAI |
| Samra et al. (2015) | USA | 40 | Mean 13 days | 50 min | 3 times/week | Hold baby wrapped in a blanket | Immediately after intervention | Stress: PSS: NICU |
| Coşkun and Günay (2020) | Turkish | 84 | 3 weeks | 15–20 min | Daily | Standard care | Immediately after intervention | Stress: PSS: NICU |
Abbreviations: GHQ, General Health Questionnaire; NR, not reported; PSI‐SF, Parenting Stress Index‐Short Form; PSS: NICU, Parental Stress Scale: Neonatal Intensive Care Unit; State–Trait Anxiety Inventory Scale (Form Y‐1); UK, the United Kingdom; USA, the United States; STAI (Form Y‐1).
3.3. Descriptions of SSC
The interventions in the SSC group among eight studies varied in total length, session duration and frequency (Table 1). To be specific, the total length of SSC ranged from 3 days (Lai et al., 2006) to 4 weeks (Miles et al., 2006). The duration of SSC each time differed to include 15–20 min (Coşkun & Günay, 2020), 20 min (Miles et al., 2006), 30 min (Norouzi et al., 2013), 50 min (Samra et al., 2015), 60 min (Badiee et al., 2014; Feldman et al., 2014; Lai et al., 2006) and 2 h (Wang et al., 2020). In terms of frequency, two RCTs did not specify (Feldman et al., 2014; Norouzi et al., 2013). Whereas in other reported studies, intervention measures were carried out once per day (Coşkun & Günay, 2020; Lai et al., 2006; Miles et al., 2006; Wang et al., 2020), 3 times a day (Badiee et al., 2014) or 3 times a week (Samra et al., 2015).
3.4. Risk bias in the study
According to the Cochrane Collaboration Risk of Bias tool, the quality assessment of eight selected studies is displayed in Figure 2 and Appendix 2. In all, most of the studies reported random sequence generation and concealment of allocation, except for three studies (Badiee et al., 2014; Feldman et al., 2014; Miles et al., 2006) that did not specifically explain the methods of sequence generation and four studies (Badiee et al., 2014; Feldman et al., 2014; Miles et al., 2006; Wang et al., 2020) without the details of concealment of allocation. Then given that the outcome measures were self‐reporting, and the quality of the blindness of outcome assessment depends on the blindness of the participants, we evaluated the performance and detect bias together. As result, only one study (Samra et al., 2015) reported that blinding was not possible due to the nature of the intervention and was considered to be at high risk of performance and detect bias. Another seven studies were rated as ‘unclear risk’ of bias in these terms because they did not explain whether the participants were blinded. Five studies were assessed as a low risk of bias for ‘incomplete outcome data’, while two studies (Miles et al., 2006; Samra et al., 2015) were rated as high because of the imbalance in the loss of subjects between the two groups, and one study (Feldman et al., 2014) was rated as unclear because it did not provide information about the details. One study (Feldman et al., 2014) did not outline the data on stress scores, with a high risk of selective reporting, and the other seven studies have a low risk of bias. All of the studies have a low risk of other biases.
FIGURE 2.
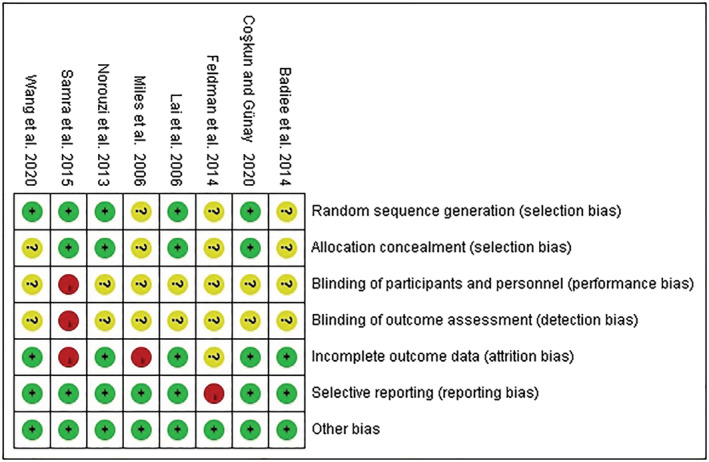
Quality assessment of included studies
3.5. Efficacy of SSC on anxiety state
Six studies (Badiee et al., 2014; Feldman et al., 2014; Lai et al., 2006; Miles et al., 2006; Norouzi et al., 2013; Wang et al., 2020) with 585 participants examined the efficacy of SSC on maternal anxiety by comparing with other types of care. SMD was applied to refer to the effect size, and a negative value indicates that the SSC group has a lower level of anxiety, which depends on the fact that the higher the score of the scale, the more severe the anxiety. Our results show that the heterogeneity between studies was high (I 2 = 75%), so we adopted the random‐effects model. The overall effect (Z = 3.81, P = 0.0001; Figure 3a) was significant, which reflected the fact that the efficacy of SSC on anxiety was superior to other types of care ([SMD] −0.72; 95% CI −1.08 to −0.35).
FIGURE 3.
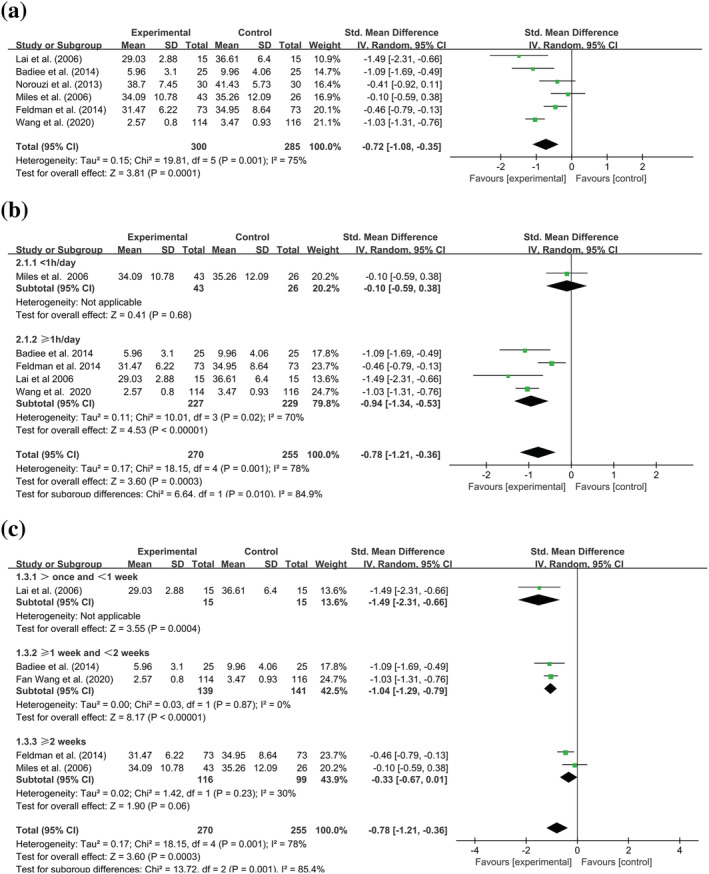
Forest plot of standard mean difference (with 95% CI) of the effect of skin‐to‐skin contact (SSC) on anxiety states among postpartum women with premature infants. (a) Meta‐analysis of anxiety comparing SSC with other types of care. (b) Subgroup analysis of anxiety based on the daily intervention time of SSC. (c) Subgroup analysis of anxiety based on the total length of SSC. Experimental group: skin‐to‐skin contact; Control group: other types of care; Outcome: anxiety states post‐intervention; Std: standard
The subgroup analysis was first based on the daily intervention time, and the studies were classified into two groups, like performing SSC < 1 h per day and ≥1 h per day. As results (Figure 3b), the anxiety level of the SSC group is not significantly lower than that of the control group when performing SSC less than 1 h per day ([SMD] −0.10; 95% CI −0.59 to 0.38; P = 0.68), while the efficacy was significant if performing SSC no less than 1 hour per day ([SMD] −0.94; 95% CI −1.34 to −0.53; P < 0.00001). Notably, the heterogeneity in the subgroup of ≥1 h per day is still high (I 2 = 70%).
Moreover, considering consistent and repeating SSC having an impact on maternal anxiety, we did subgroup analysis for the second time and chosen total intervention weeks as another subgroup factor. Based on this standard, five studies were classified into three subgroups including >once and <1 week, ≥1 week and <2 weeks and ≥2 weeks. The results demonstrated that it was significant in applying SSC repeatedly and lasting less than 1 week ([SMD] −1.49; 95% CI −2.31 to −0.66; P = 0.0004) or no less than 1 week and less than 2 weeks ([SMD] −1.04; 95% CI −1.29 to −0.79; P < 0.00001) for reducing maternal anxiety level, but it was not significant for lasting no less than 2 weeks([SMD] −0.33; 95% CI −0.67 to 0.01; P = 0.06) (Figure 3c).
3.6. Efficacy of SSC on the stress state
Data on stress were reported in four studies (Coşkun & Günay, 2020; Miles et al., 2006; Samra et al., 2015; Wang et al., 2020), with 218 subjects in the SSC group and 191 subjects in the control group. A meta‐analysis including these four studies was performed, yielding a significant effect of SSC (Z = 2.20, P = 0.03), which means stress reduction after the intervention. The results exerted that SMD was −0.84 accompanied by 95% CI −1.59 to −0.09. However, the heterogeneity was high with I 2 = 90% (Figure 4).
FIGURE 4.
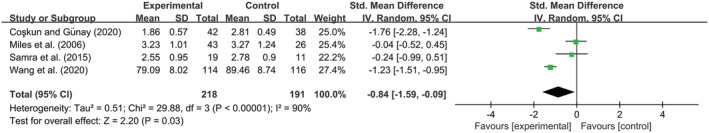
Forest plot of standard mean difference (with 95% CI) of the effect of skin‐to‐skin contact (SSC) on stress states among postpartum women with premature infants. Experimental group: skin‐to‐skin contact; Control group: other types of care; Outcome: stress states postintervention; Std: standard
3.7. Publication bias of studies
The numbers of studies for anxiety are six, while that for stress is four in this review. Therefore, we did not illustrate the publication bias of studies because no more than 10 articles were included.
4. DISCUSSION
This review aims to investigate the effect of SSC on anxiety and stress states among postpartum women. Through searching nine databases, eight articles involving 728 subjects were included in this study. The results of the meta‐analysis indicate that SSC can effectively reduce the anxiety and stress states among postpartum women in comparison with other types of care.
Anxiety state is a common problem affecting postpartum women. Many studies have applied SSC after delivery and explored the role of it on women's anxiety, but the evidence was not strong enough because the number of trials was limited and the studies were of poor methodological quality (Badiee et al., 2014; Miles et al., 2006; Norouzi et al., 2013). This meta‐analysis shows that that SSC yields a reduction of maternal anxiety in comparison with other types of care. Yet now, the mechanisms of SSC to relieve maternal anxiety are not unified. The establishment of mother–infant bonding is one of the significant mechanisms (Ahn et al., 2010; Cho et al., 2016; Feldman et al., 1999; Nyström & Axelsson, 2002). Literature has indicated that mothers of infants living in NICU often feel anxiety (Segre et al., 2014), because early and sustained maternal separation after delivery makes them feel worried about their infants and more prone to anxiety (Nyström & Axelsson, 2002). Therefore, SSC can reduce the prevalence of anxiety by providing mothers with the opportunity to get close to their infants and grasp information about their states. Building a deeper connection is another main mechanism (Efinger et al., 2019). When mothers undergo SSC, they focus more on infants and try their best to understand their infants' behaviour and give a response, which causes their attention to other things existing in limited supply and helps them build a deeper parent–child relationship. Furthermore, confidence‐building also provides a reasonable explanation for the mechanism of SSC relieving anxiety (Maastrup et al., 2018). In postpartum women, distrust and a sense of guilt towards their infants (Nyström & Axelsson, 2002) worry them whether they are capable of raising their children. SSC can usually build up their confidence and inspire mothers' sense of responsibility and care (Huang et al., 2019), thereby alleviating their anxiety. All in all, the above provides a reasonable illustration of the underlying mechanism of SSC to relieve anxiety, but additional research on the specific association between them is still required.
Considering the primary source of the considerable heterogeneity in our article could be the timing of SSC, so we performed a subgroup analysis in line with it and divided these studies into two groups, <1 h/day and ≥ 1 h/day. The result shows that it is meaningful for easing anxiety when intervening for no less than 1 hour per day, but the heterogeneity within the group did not reduce well. This finding is inconsistent with Moore et al.'s study (Moore et al., 2016), which put forward that there is no significant difference in outcome comparing more than 60 min of contact with less than 60 min of contact. It may be because this meta‐analysis included fewer articles and the population is mothers of full‐term newborns. However, due to the huge heterogeneity in our results, our conclusions also need to be interpreted with caution. In addition, we speculate that the heterogeneity may also come from the length of SSC, so we will further analyse it. Notably, it also reminds researchers to report more details, such as intervention start time, hospital stays and economic factors.
Persistent and repetitive interventions may be another source of heterogeneity. So we performed subgroup analysis according to the length of SSC, including >1 time and <1 week, ≥1 week and <2 weeks and ≥ 2 weeks. The heterogeneity in ≥1 week and <2 weeks and ≥ 2 weeks groups was significantly lower than before. The consequence demonstrated a significant reduction in anxiety among postpartum women when performing SSC for less than 2 weeks but without statistical significance when lasting more than 2 weeks. This is to some extent related to the prolonged hospital stay. In Miles et al.'s research (Miles et al., 2006), all subjects carried out SSC for 4 weeks, which means their babies must stay in NICU for a longer time. Therefore, anxiety caused by financial stress and other complex reasons may not be offset by SSC (Liang et al., 2019; Ramakrishna et al., 2019). In addition, the population in Miles et al.'s study is mothers of extremely preterm infants. The extremely preterm infant is considerably more immature and sick, which brings great anxiety to mothers and may cover up the role of SSC (Rogers et al., 2013). Therefore, it is necessary to pay attention to this group of mothers. However, due to the small number of studies, whether the invalid effect of SSC lasting more than 2 weeks is related to the length of hospitalisation or the mother of extremely premature infants still needs further study.
Except for anxiety, SSC is also effective for stress in women after delivery. Many potential mechanisms by which SSC may impact stress are as follows. First, in physiology, investigators found that women with high anxiety have a higher level of salivary cortisol, and women in the SSC group had a greater reduction in saliva cortisol than those in the control group (Bigelow et al., 2012). Salivary cortisol is a hormone product produced by the limbic‐hypothalamic‐pituitary adrenocortical (L‐HPA) axis, which is a stress response system participating in emotion regulation (Bigelow et al., 2012). However, specific neurobiological mechanisms still need to be explored. Moreover, SSC can increase the mother's oxytocin, which is important for generating positive maternal emotions. Secondly, in psychology, the process of SSC is accompanied by parental role transition. When mothers conducted SSC, they often have positive maternal feelings and develop a mother–infant bonding relationship (Coşkun & Günay, 2020). Whereas the considerable level of heterogeneity between studies will limit the strength of the evidence for the efficacy of SSC on stress even leads to mistaken results. So it reinforced the need for further well‐designed studies inquiring into the effect of SSC on postpartum stress.
The strengths of our study are the meta‐analysis that was conducted according to PRISMA Guidelines, and our included studies were all RCTs. Simultaneously, it is one of the few meta‐analyses to explore the effects of SSC on postpartum women's anxiety and stress states. Moreover, we focused on the efficacy of SSC frequency and duration time and found significant results, like 60 min of SSC is essential, which is worth exploring further.
However, there are still some limitations to our study. First, a total of eight articles were involved in our study despite our effort to use a comprehensive search strategy, which may restrict the strength of our evidence. Secondly, since some of the included studies were of poor methodological quality and did not report in line with guidelines, further analysing the source of stress heterogeneity cannot be performed.
5. CONCLUSION
In conclusion, our findings support the beneficial effects of SSC on anxiety and stress states among postpartum mothers of premature infants. Additionally, limited evidence shows that only performing SSC no less than 60 min per day can make great effects on anxiety, and individual SSC has little efficacy on anxiety when carried out over 2 weeks and the possible reason may be the efficacy of it may be weakened by infants' physical condition, economic levels and other existent factors. However, considering that the limited number of studies included and high heterogeneity were found in our meta‐analysis, this finding needs to be further verified. Hence, studies should report infants' physical condition, family economic levels or other complicated factors in the future. Simultaneously, the exact details of SSC, such as initiation time, frequency and duration, are also need to be ascertained for good clinical practice. Moreover, the most suitable timing of SSC for alleviating anxiety and stress states still needs to be investigated.
CONFLICT OF INTEREST
The authors report no conflict of interest.
CONTRIBUTIONS
AZ and XF conceptualized and designed the study, secured funding, supervised data collection, and critically reviewed the manuscript. SC, RW, LS, and XS acquire the data. ZZ, HZ, YL, SC, RW, and XS analyzed and interpreted the data. SC, RW, and XS drafted the article. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
ACKNOWLEDGEMENTS
We acknowledge the support of the National Center for Women and Children's Health, Maternal and Child Nutrition and Health Research Project (2020FYH012).
APPENDIX A. Some examples of the search strategy
| PubMed | |
|---|---|
| #1 | ((((‘Kangaroo‐Mother Care Method’[Mesh]) OR (Skin‐to‐skin contact)) OR (Kangaroo mother care)) OR (Kangaroo care)) OR (Early Essential Newborn Care) |
| #2 | (‘Postpartum Period’[Mesh]) OR (((((((((postnatal) OR (perinatal)) OR (peripartum)) OR (post birth))) OR (after delivery)) OR (after birth)) OR (puerperium)) OR (puerperal)) OR (postpartum)) |
| #3 | (((‘Anxiety’[Mesh]) OR ‘Anxiety Disorders’[Mesh]) OR ‘Stress, Psychological’[Mesh]) OR (((((Hypervigilance) OR (Nervousness)) OR (stress)) OR (pressure)) OR (tension)) |
| #4 | #1 AND #2 AND #3 |
| CINAHL | |
|---|---|
| #1 | (MH ‘Kangaroo Care+’) OR Skin‐to‐skin contact OR Kangaroo mother care OR Kangaroo‐Mother Care Method OR Early Essential Newborn Care |
| #2 | (MH ‘Postnatal Period+’) OR postnatal OR perinatal OR peripartum OR post birth OR after delivery OR after birth OR puerperium OR puerperal OR postpartum |
| #3 | ( (MH ‘Stress, Psychological+’) OR (MH ‘Stress+’)) OR ((MH ‘Anxiety+’) OR (MH ‘Anxiety Disorders+’)) OR Hypervigilance OR Nervousness OR stress OR pressure OR tension |
| #4 | #1 AND #2 AND #3 |
| EMBASE | |
|---|---|
| #1 | 'skin to skin contact'/exp |
| #2 | 'kangaroo care'/exp |
| #3 | 'kangaroo‐mother care method' |
| #4 | 'skin‐to‐skin contact' |
| #5 | 'kangaroo mother care' |
| #6 | 'early essential newborn care' |
| #7 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 |
| #8 | 'puerperium'/exp |
| #9 | 'perinatal period'/exp |
| #10 | 'postnatal' |
| #11 | 'perinatal' |
| #12 | 'peripartum' |
| #13 | 'post birth' |
| #14 | 'after delivery' |
| #15 | 'after birth' |
| #16 | 'puerperal' |
| #17 | 'postpartum' |
| #18 | #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 |
| #19 | 'anxiety'/exp |
| #20 | 'anxiety disorder'/exp |
| #21 | 'stress'/exp |
| #22 | 'mental stress'/exp |
| #23 | 'hypervigilance' |
| #24 | 'nervousness' |
| #25 | 'stress' |
| #26 | 'pressure' |
| #27 | 'tension' |
| #28 | #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 |
| #29 | #7 AND #18 AND #28 |
| Web of Science | |
|---|---|
| #1 | TS=(Kangaroo‐Mother Care Method OR Skin‐to‐skin contact OR Kangaroo mother care OR Kangaroo care OR Early Essential Newborn Care) |
| #2 | TS=(Postpartum Period OR postnatal OR perinatal OR peripartum OR post birth OR after delivery OR after birth OR puerperium OR puerperal OR postpartum) |
| #3 | TS=(Anxiety Disorders OR Anxiety OR Hypervigilance OR Nervousness OR stress OR Stress, psychological OR pressure OR tension) |
| #4 | #1 AND #2 AND #3 |
APPENDIX B. The justification for the risk of bias assessments.
| Badiee et al. (2014) | ||
|---|---|---|
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors only mentioned that participants were randomly selected, but did not report the details of random sequence generation and allocation concealment. |
| Allocation concealment (selection bias) | Unclear risk | |
| Blinding of participants and personnel (performance bias) | Unclear risk | Whether the participants were blinded was not explained in the article. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome measures were self‐reporting, while the blinding of participants and personnel is not clear. |
| Incomplete outcome data (attrition bias) | Low risk | The number of participants pre‐intervention and postintervention was consistent. |
| Selective reporting (reporting bias) | Low risk | The expected outcome indicators were reported. |
| Other bias | Low risk | |
| Coşkun and Günay (2020) | ||
|---|---|---|
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quoting sentences from the article ‘The premature infants were selected by random sampling method. After the names of the premature infants who were written and put in an envelope, they were divided into the kangaroo care group and standard care group.’ |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) | Unclear risk | Whether the participants were blinded was not explained in the article. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome measures were self‐reporting, while the blinding of participants and personnel is not clear. |
| Incomplete outcome data (attrition bias) | Low risk | The number of participants and the reason of loss follow‐up were clarified. |
| Selective reporting (reporting bias) | Low risk | The expected outcome indicators were reported. |
| Other bias | Low risk | |
| Feldman et al. (2014) | ||
|---|---|---|
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quoting sentences from the article ‘53 mother–infant dyads were randomly selected…’. But this article did not report the details of random sequence generation and allocation concealment. |
| Allocation concealment (selection bias) | Unclear risk | |
| Blinding of participants and personnel (performance bias) | Unclear risk | Whether the participants were blinded was not explained in the article. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome measures were self‐reporting, while the blinding of participants and personnel is not clear. |
| Incomplete outcome data (attrition bias) | Unclear risk | The number of participants in the results was not reported. |
| Selective reporting (reporting bias) | High risk | The outcome measure parenting stress was not reported in results. |
| Other bias | Low risk | |
| Lai et al. (2006) | ||
|---|---|---|
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quoting sentences from the article ‘Permuted block randomization with sealed envelopes stratified ongender was used to assign participants to either thetreatment or control group’. |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) | Unclear risk | Whether the participants were blinded was not explained in the article. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome measures were self‐reporting, while the blinding of participants and personnel is not clear. |
| Incomplete outcome data (attrition bias) | Low risk | The number of participants pre‐and post‐intervention was consistent. |
| Selective reporting (reporting bias) | Low risk | The expected outcome indicators were reported. |
| Other bias | Low risk | |
| Miles et al. (2006) | ||
|---|---|---|
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors only mentioned that participants were randomly selected, but did not report the details of random sequence generation and allocation concealment. |
| Allocation concealment (selection bias) | Unclear risk | |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quoting sentences from the article ‘… not require nursing staff to provide care for the same infant … have served to minimise any possibility of bias towards either STS or …’ However, whether the participants were blinded was not explained in the article. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome measures were self‐reporting while blinding of participants is not clear. |
| Incomplete outcome data (attrition bias) | High risk | The number of people lost to follow‐up in the two groups was unbalanced. |
| Selective reporting (reporting bias) | Low risk | The expected outcome indicators were reported. |
| Other bias | Low risk | |
| Norouzi et al. (2013) | ||
|---|---|---|
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quoting sentences from the article ‘1. … participants were randomly allocated into…; 2.The drawings were prepared by a different person who was blind to the order of group assignment.’ |
| Allocation concealment (selection bias) | Low risk | Quoting sentences from the article ‘Cards of three numbers indicating group assignment were randomly placed in opaque sealed envelopes’. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Whether the participants were blinded was not explained in the article. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome measures were self‐reporting while blinding of participants is not clear. |
| Incomplete outcome data (attrition bias) | Low risk | The number of participants pre‐and post‐intervention was consistent. |
| Selective reporting (reporting bias) | Low risk | The expected outcome indicators were reported. |
| Other bias | Low risk | |
| Samra et al. (2015) | ||
|---|---|---|
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quoting sentences from the article ‘A simple randomisation scheme using opaque sequentially numbered envelopes to conceal upcoming subject allocation was used. … a study assistant who was not involved in recruitment developed an equal number of study cards … and placed one card inside each opaque envelope before sealing it.’ |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) | High risk | Quoting sentences from the article ‘Blinding was not possible for the intervention.’ |
| Blinding of outcome assessment (detection bias) | High risk | Outcome measures were self‐reporting, while the subjects were not blinded. |
| Incomplete outcome data (attrition bias) | High risk | The number of people lost to follow‐up in the intervention group and control group was unbalanced. |
| Selective reporting (reporting bias) | Low risk | The expected outcome indicators were reported. |
| Other bias | Low risk | |
| Wang et al. (2020) | ||
|---|---|---|
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quoting sentences from the article ‘Participants were divided into intervention group and control group using random number table’. |
| Allocation concealment (selection bias) | Unclear risk | This detail was not referred to. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Whether the participants were blinded was not explained in the article. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome measures were self‐reporting, while the blinding of participants and personnel is not clear. |
| Incomplete outcome data (attrition bias) | Low risk | The number of participants and the reason of loss follow‐up were clarified. |
| Selective reporting (reporting bias) | Low risk | The expected outcome indicators were reported. |
| Other bias | Low risk | |
Cong, S., Wang, R., Fan, X., Song, X., Sha, L., Zhu, Z., Zhou, H., Liu, Y., & Zhang, A. (2021). Skin‐to‐skin contact to improve premature mothers' anxiety and stress state: A meta‐analysis. Maternal & Child Nutrition, 17(4), e13245. 10.1111/mcn.13245
Shengnan Cong, Rui Wang, Xuemei Fan and Xiaowei Song contributed equally to this work.
Funding information National Center for Women and Children's Health, Maternal and Child Nutrition and Health Research Project, Grant/Award Number: 2020FYH012
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study can be obtained from the text, table, figures, supporting information and the following references (Badiee et al., 2014; Coşkun & Günay, 2020; Feldman et al., 2014; Lai et al., 2006; Miles et al., 2006; Norouzi et al., 2013; Samra et al., 2015; Wang et al., 2020).
REFERENCES
- Ahn, H. Y., Lee, J., & Shin, H.‐J. (2010). Kangaroo care on premature infant growth and maternal attachment and post‐partum depression in South Korea. Journal of Tropical Pediatrics, 56(5), 342–344. 10.1093/tropej/fmq063 [DOI] [PubMed] [Google Scholar]
- Ali, N. S., Mahmud, S., Khan, A., & Ali, B. S. (2013). Impact of postpartum anxiety and depression on child's mental development from two peri‐urban communities of Karachi, Pakistan: A quasi‐experimental study. BMC Psychiatry, 13, 274. 10.1186/1471-244x-13-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasopoulou, E., & Fox, J. R. (2014). Effects of kangaroo mother care on maternal mood and interaction patterns between parents and their preterm, low birth weight infants: A systematic review. Infant Mental Health Journal, 35(3), 245–262. 10.1002/imhj.21444 [DOI] [PubMed] [Google Scholar]
- Badiee, Z., Faramarzi, S., & MiriZadeh, T. (2014). The effect of kangaroo mother care on mental health of mothers with low birth weight infants. Advanced Biomedical Research, 3, 214. 10.4103/2277-9175.143262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baía, I., Amorim, M., Silva, S., Kelly‐Irving, M., de Freitas, C., & Alves, E. (2016). Parenting very preterm infants and stress in neonatal intensive care units. Early Human Development, 101, 3–9. 10.1016/j.earlhumdev.2016.04.001 [DOI] [PubMed] [Google Scholar]
- Beiranvand, S., Valizadeh, F., Hosseinabadi, R., & Pournia, Y. (2014). The effects of skin‐to‐skin contact on temperature and breastfeeding successfulness in full‐term newborns after cesarean delivery. International Journal of Pediatrics, 2014, 846486–7. 10.1155/2014/846486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biaggi, A., Conroy, S., Pawlby, S., & Pariante, C. M. (2016). Identifying the women at risk of antenatal anxiety and depression: A systematic review. Journal of Affective Disorders, 191, 62–77. 10.1016/j.jad.2015.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow, A., Power, M., MacLellan‐Peters, J., Alex, M., & McDonald, C. (2012). Effect of mother/infant skin‐to‐skin contact on postpartum depressive symptoms and maternal physiological stress. Journal of Obstetric, Gynecologic, and Neonatal Nursing, 41(3), 369–382. 10.1111/j.1552-6909.2012.01350.x [DOI] [PubMed] [Google Scholar]
- Bystrova, K., Widström, A. M., Matthiesen, A. S., Ransjö‐Arvidson, A. B., Welles‐Nyström, B., Wassberg, C., Vorontsov, I., & Uvnäs‐Moberg, K. (2003). Skin‐to‐skin contact may reduce negative consequences of “the stress of being born”: A study on temperature in newborn infants, subjected to different ward routines in St. Petersburg. Acta Paediatrica, 92(3), 320–326. 10.1080/08035250310009248 [DOI] [PubMed] [Google Scholar]
- Cho, E.‐S., Kim, S.‐J., Kwon, M. S., Cho, H., Kim, E. H., Jun, E. M., & Lee, S. (2016). The effects of kangaroo care in the neonatal intensive care unit on the physiological functions of preterm infants, maternal‐infant attachment, and maternal stress. Journal of Pediatric Nursing, 31(4), 430–438. 10.1016/j.pedn.2016.02.007 [DOI] [PubMed] [Google Scholar]
- Coşkun, D., & Günay, U. (2020). The effects of kangaroo care applied by Turkish mothers who have premature babies and cannot breastfeed on their stress levels and amount of milk production. Journal of Pediatric Nursing, 50, e26–e32. 10.1016/j.pedn.2019.09.028 [DOI] [PubMed] [Google Scholar]
- Disher, T., Benoit, B., Johnston, C., & Campbell‐Yeo, M. (2017). Skin‐to‐skin contact for procedural pain in neonates: Acceptability of novel systematic review synthesis methods and GRADEing of the evidence. Journal of Advanced Nursing, 73(2), 504–519. 10.1111/jan.13182 [DOI] [PubMed] [Google Scholar]
- Efinger, L., Thuillard, S., & Dan‐Glauser, E. S. (2019). Distraction and reappraisal efficiency on immediate negative emotional responses: Role of trait anxiety. Anxiety, Stress, and Coping, 32(4), 412–427. 10.1080/10615806.2019.1597859 [DOI] [PubMed] [Google Scholar]
- Fairbrother, N., Young, A. H., Janssen, P., Antony, M. M., & Tucker, E. (2015). Depression and anxiety during the perinatal period. BMC Psychiatry, 15, 206. 10.1186/s12888-015-0526-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, R., & Eidelman, A. I. (2003). Skin‐to‐skin contact (Kangaroo Care) accelerates autonomic and neurobehavioural maturation in preterm infants. Developmental Medicine and Child Neurology, 45(4), 274–281. 10.1017/s0012162203000525 [DOI] [PubMed] [Google Scholar]
- Feldman, R., Rosenthal, Z., & Eidelman, A. I. (2014). Maternal‐preterm skin‐to‐skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biological Psychiatry, 75(1), 56–64. 10.1016/j.biopsych.2013.08.012 [DOI] [PubMed] [Google Scholar]
- Feldman, R., Weller, A., Leckman, J. F., Kuint, J., & Eidelman, A. I. (1999). The nature of the mother's tie to her infant: Maternal bonding under conditions of proximity, separation, and potential loss. Journal of Child Psychology and Psychiatry, 40(6), 929–939. 10.1017/S0021963099004308 [DOI] [PubMed] [Google Scholar]
- Gao, M., Hu, J., Yang, L., Ding, N., Wei, X., Li, L., Liu, L., Ma, Y., & Wen, D. (2019). Association of sleep quality during pregnancy with stress and depression: A prospective birth cohort study in China. BMC Pregnancy and Childbirth, 19(1), 444. 10.1186/s12884-019-2583-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, C., Dunn, D. M., & Njoroge, W. F. M. (2017). Impact of postpartum mental illness upon infant development. Current Psychiatry Reports, 19(12), 100. 10.1007/s11920-017-0857-8 [DOI] [PubMed] [Google Scholar]
- Huang, X., Chen, L., & Zhang, L. (2019). Effects of paternal skin‐to‐skin contact in newborns and fathers after cesarean delivery. The Journal of Perinatal & Neonatal Nursing, 33(1), 68–73. 10.1097/jpn.0000000000000384 [DOI] [PubMed] [Google Scholar]
- Ionio, C., Lista, G., Mascheroni, E., Olivari, M. G., Confalonieri, E., Mastrangelo, M., Brazzoduro, V., Balestriero, M. A., Banfi, A., Bonanomi, A., Bova, S., Castoldi, F., Colombo, C., Introvini, P., & Scelsa, B. (2017). Premature birth: Complexities and difficulties in building the mother‐child relationship. Journal of Reproductive and Infant Psychology, 35(5), 509–523. 10.1080/02646838.2017.1383977 [DOI] [PubMed] [Google Scholar]
- Johnston, C., Campbell‐Yeo, M., Disher, T., Benoit, B., Fernandes, A., Streiner, D., Inglis, D., & Zee, R. (2017). Skin‐to‐skin care for procedural pain in neonates. Cochrane Database of Systematic Reviews, 2(2), Cd008435. 10.1002/14651858.CD008435.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, V., & Minikel, M. (2019). Postpartum anxiety: More common than you think. The Journal of Family Practice, 68(3), 165–174. [PubMed] [Google Scholar]
- Lai, H. L., Chen, C. J., Peng, T. C., Chang, F. M., Hsieh, M. L., Huang, H. Y., & Chang, S. C. (2006). Randomized controlled trial of music during kangaroo care on maternal state anxiety and preterm infants' responses. International Journal of Nursing Studies, 43(2), 139–146. 10.1016/j.ijnurstu.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Lau, Y., Htun, T. P., Wong, S. N., Tam, W. S. W., & Klainin‐Yobas, P. (2017). Therapist‐supported internet‐based cognitive behavior therapy for stress, anxiety, and depressive symptoms among postpartum women: A systematic review and meta‐analysis. Journal of Medical Internet Research, 19(4), e138. 10.2196/jmir.6712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, L. A., Berger, U., & Brand, C. (2019). Psychosocial factors associated with symptoms of depression, anxiety and stress among single mothers with young children: A population‐based study. Journal of Affective Disorders, 242, 255–264. 10.1016/j.jad.2018.08.013 [DOI] [PubMed] [Google Scholar]
- Maastrup, R., Weis, J., Engsig, A. B., Johannsen, K. L., & Zoffmann, V. (2018). Now she has become my daughter': Parents' early experiences of skin‐to‐skin contact with extremely preterm infants. Scandinavian Journal of Caring Sciences, 32(2), 545–553. 10.1111/scs.12478 [DOI] [PubMed] [Google Scholar]
- Miles, R., Cowan, F., Glover, V., Stevenson, J., & Modi, N. (2006). A controlled trial of skin‐to‐skin contact in extremely preterm infants. Early Human Development, 82(7), 447–455. 10.1016/j.earlhumdev.2005.11.008 [DOI] [PubMed] [Google Scholar]
- Moore, E. R., Bergman, N., Anderson, G. C., & Medley, N. (2016). Early skin‐to‐skin contact for mothers and their healthy newborn infants. Cochrane Database of Systematic Reviews, 11(11), Cd003519. 10.1002/14651858.CD003519.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakić Radoš, S., Tadinac, M., & Herman, R. (2018). Anxiety during pregnancy and postpartum: Course, predictors and comorbidity with postpartum depression. Acta Clinica Croatica, 57(1), 39–51. 10.20471/acc.2017.56.04.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norouzi, F., Keshavarz, M., SeyedFatemi, N., & Montazeri, A. (2013). The impact of kangaroo care and music on maternal state anxiety. Complementary Therapies in Medicine, 21(5), 468–472. 10.1016/j.ctim.2013.07.006 [DOI] [PubMed] [Google Scholar]
- Nyström, K., & Axelsson, K. (2002). Mothers' experience of being separated from their newborns. Journal of Obstetric, Gynecologic, and Neonatal Nursing, 31(3), 275–282. 10.1111/j.1552-6909.2002.tb00049.x [DOI] [PubMed] [Google Scholar]
- Ramakrishna, S., Cooklin, A. R., & Leach, L. S. (2019). Comorbid anxiety and depression: A community‐based study examining symptomology and correlates during the postpartum period. Journal of Reproductive and Infant Psychology, 37(5), 468–479. 10.1080/02646838.2019.1578870 [DOI] [PubMed] [Google Scholar]
- Rogers, C. E., Kidokoro, H., Wallendorf, M., & Inder, T. E. (2013). Identifying mothers of very preterm infants at‐risk for postpartum depression and anxiety before discharge. Journal of Perinatology, 33(3), 171–176. 10.1038/jp.2012.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samra, H. A., Dutcher, J., McGrath, J. M., Foster, M., Klein, L., Djira, G., Hansen, J., Wallenburg, D., & Dowling, D. (2015). Effect of skin‐to‐skin holding on stress in mothers of late‐preterm infants: A randomized controlled trial. Advances in Neonatal Care, 15(5), 354–364. 10.1097/anc.0000000000000223 [DOI] [PubMed] [Google Scholar]
- Saxton, A., Fahy, K., Rolfe, M., Skinner, V., & Hastie, C. (2015). Does skin‐to‐skin contact and breast feeding at birth affect the rate of primary postpartum haemorrhage: Results of a cohort study. Midwifery, 31(11), 1110–1117. 10.1016/j.midw.2015.07.008 [DOI] [PubMed] [Google Scholar]
- Scime, N. V., Gavarkovs, A. G., & Chaput, K. H. (2019). The effect of skin‐to‐skin care on postpartum depression among mothers of preterm or low birthweight infants: A systematic review and meta‐analysis. Journal of Affective Disorders, 253, 376–384. 10.1016/j.jad.2019.04.101 [DOI] [PubMed] [Google Scholar]
- Segre, L. S., McCabe, J. E., Chuffo‐Siewert, R., & O'Hara, M. W. (2014). Depression and anxiety symptoms in mothers of newborns hospitalized on the neonatal intensive care unit. Nursing Research, 63(5), 320–332. 10.1097/NNR.0000000000000039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada, F., Ohira, Y., Hirota, Y., Ikegami, A., Kondo, T., Shikino, K., Suzuki, S., Noda, K., Uehara, T., & Ikusaka, M. (2018). Anxiety and depression in general practice outpatients: The long‐term change process. International Journal of General Medicine, 11, 55–63. 10.2147/ijgm.S130025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simavli, S., Kaygusuz, I., Gumus, I., Usluogulları, B., Yildirim, M., & Kafali, H. (2014). Effect of music therapy during vaginal delivery on postpartum pain relief and mental health. Journal of Affective Disorders, 156, 194–199. 10.1016/j.jad.2013.12.027 [DOI] [PubMed] [Google Scholar]
- Stevens, J., Schmied, V., Burns, E., & Dahlen, H. (2014). Immediate or early skin‐to‐skin contact after a Caesarean section: A review of the literature. Maternal & Child Nutrition, 10(4), 456–473. 10.1111/mcn.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedgård, E., Tedgård, U., Råstam, M., & Johansson, B. A. (2020). Parenting stress and its correlates in an infant mental health unit: A cross‐sectional study. Nordic Journal of Psychiatry, 74(1), 30–39. 10.1080/08039488.2019.1667428 [DOI] [PubMed] [Google Scholar]
- Treyvaud, K., Spittle, A., Anderson, P. J., & O'Brien, K. (2019). A multilayered approach is needed in the NICU to support parents after the preterm birth of their infant. Early Human Development, 139, 104838. 10.1016/j.earlhumdev.2019.104838 [DOI] [PubMed] [Google Scholar]
- Vittner, D., McGrath, J., Robinson, J., Lawhon, G., Cusson, R., Eisenfeld, L., Walsh, S., Young, E., & Cong, X. (2018). Increase in oxytocin from skin‐to‐skin contact enhances development of parent‐infant relationship. Biological Research for Nursing, 20(1), 54–62. 10.1177/1099800417735633 [DOI] [PubMed] [Google Scholar]
- Wang, F., Li, Y., Li, S. L., Liu, Y. H., Sun, C. X., & Hu, Y. F. (2020). Effects of kangaroo mother care on anxiety and parenting stress in premature mothers. Chinese Journal of Behavioural Medicine and Brain Sciences, 29(01), 74–78. 10.3760/cma.j.cn371468-20190819-00562 [DOI] [Google Scholar]
- Widström, A. M., Brimdyr, K., Svensson, K., Cadwell, K., & Nissen, E. (2019). Skin‐to‐skin contact the first hour after birth, underlying implications and clinical practice. Acta Paediatrica, 108(7), 1192–1204. 10.1111/apa.14754 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study can be obtained from the text, table, figures, supporting information and the following references (Badiee et al., 2014; Coşkun & Günay, 2020; Feldman et al., 2014; Lai et al., 2006; Miles et al., 2006; Norouzi et al., 2013; Samra et al., 2015; Wang et al., 2020).


