Abstract
Although effective drugs have been developed, including 5-fluorouracil (5-FU), advanced colorectal cancer (CRC) shows low therapeutic sensitivity resulting from the development of 5-FU resistance. Thymidylate synthase (TS) is a target protein of 5-FU, and elevated TS lowers the 5-FU sensitivity of CRC cells. Here, we tested the efficacy of several candidate phytochemicals against human CRC-derived HCT116 cells expressing wild-type tumor suppressor protein P53 and HT29 cells expressing mutant P53. Among them, we found that apigenin enhanced the inhibitory effect of 5-FU on cell viability. In addition, apigenin inhibited the upregulation of TS induced by 5-FU. Apigenin also potentiated 5-FU-induced apoptosis of HCT116 cells and enhanced cell cycle disruption. Furthermore, apigenin increased reactive oxygen species production, intracellular and intramitochondrial Ca2+ concentrations, and mitochondrial membrane potential upon cotreatment with 5-FU. Knockdown of forkhead box protein M, a transcription factor modulating 5-FU sensitivity, enhanced the potentiation of apoptosis by apigenin in HCT116 cells. Moreover, apigenin suppressed TS expression and inhibited the viability of 5-FU-resistant HCT116 cells. Therefore, apigenin may improve the therapeutic efficacy of 5-FU against CRC by suppressing TS, but apoptosis induction is mainly dependent on functional P53.
Keywords: Apigenin, Thymidylate synthase, P53, Colon cancer, Chemoresistance
Highlights
-
•
Apigenin inhibits the upregulation of TS induced by 5-FU for apoptosis of CRC.
-
•
FOXM1 silencing enhances the potentiation of apoptosis by apigenin.
-
•
Suppressing TS and promoting P53 activity by apigenin reduce acquired 5-FU resistance.
1. Introduction
Colorectal cancer (CRC) is the second most common cause of cancer-related death in the United States [1]. Currently, 5-fluorouracil (5-FU) is one of the most effective chemotherapeutic agents for early-stage CRC. This antitumor efficacy results from the inhibition of thymidylate synthase (TS), an enzyme essential for DNA replication by catalyzing the conversion of deoxyuridine monophosphate (dUMPs) to deoxythymine monophosphate for deoxynucleotide biosynthesis [2], which in turn leads to DNA damage, S-phase arrest, and apoptosis [3]. Moreover, higher TS expression in CRC is associated with lower 5-FU sensitivity [4]; therefore, targeting TS is a rational evidence-based therapeutic strategy for enhancing 5-FU cytotoxicity and antitumor efficacy [5]. In addition, some ribosomal proteins play important roles in the therapeutic mechanism by 5-FU in cancer cells [6]. 5-FU-induced nucleolar stress results in the release of ribosomal proteins from the ribosomes, which activate the P53 pathway [7]. However, the clinical use of 5-FU is limited by drug resistance, and it is necessary to discover supplements for overcoming resistance to multiple drugs, including 5-FU, CPT-11, and oxaliplatin, and to identify mechanisms for improving drug sensitivity in CRC.
Apigenin (4′,5,7-trihydroxyflavone) is a plant flavone found in a wide range of fruits and vegetables with multiple documented biological activities, including anticancer properties. Compared with other flavonoids, apigenin can selectively induce cell cycle arrest and apoptosis of cancer cells with low mutagenicity and toxicity against normal cells and thus is gaining attention as a promising anticancer adjuvant [[8], [9], [10]]. Moreover, the structure and chemical properties of its protein binding site suggests that apigenin can inhibit the catalytic TS reaction by hydrogen bonding to the pyrimidine carbonyl and hydroxyl groups of deoxyribose dUMP [11]. Furthermore, several studies have suggested potential therapeutic efficacy in CRC cells, but it is still unclear whether apigenin can improve 5-FU sensitivity and regulate TS expression in CRC cells.
Drug sensitivity of CRC varies depending on the mutation status of the tumor suppressor protein P53 [12]. Because P53 can be specifically inhibited at the translational level by TS, these proteins are important factors for predicting therapeutic efficacy against CRC [13]. In addition, forkhead box protein M1 (FOXM1), a member of the forkhead box transcription factor family, plays an important role in tumorigenesis, organogenesis, and aging through proliferation-related transcriptional regulation, and recent studies have reported that the FOXM1–TS axis is involved in the development of 5-FU resistance by cancer cells [14,15]. Therefore, in this study, several phytochemicals including apigenin were selected as candidate adjuvants for 5-FU, and the individual efficacies for improving 5-FU-mediated inhibition of cell viability and for regulating the expression of P53 and TS were analyzed in CRC cells. In addition, the contributions of P53 were assessed by comparing the effects of combined 5-FU and apigenin on HCT116 cells expressing wild-type P53 and HT29 cells expressing mutant P53. Moreover, we investigated the effects of FOXM1 silencing on apoptosis induction and cell cycle modulation by apigenin.
2. Materials and methods
2.1. Chemicals
Apigenin, 5-FU, carvacrol, chrysin, coumestrol, formononetin, naringenin, osthole, quercetin, silibinin, stigmasterol, CPT-11, and oxaliplatin were purchased from Sigma-Aldrich, Inc (St. Louis, MO, USA), curcumin from HWI Analytik Gmbh (Rülzheim, Germany), and delphinidin from Indofine Chemical Company (Somerville, NJ, USA). Antibodies against P53, TS, poly-(ADP-ribose) polymerase (PARP), BAX, BCL-2, and CCND1 were purchased from Cell Signaling Technology (Beverly, MA, USA) and antibodies against α-tubulin (TUBA) and P21 from Santa Cruz Biotechnology Inc (Santa Cruz, CA, USA).
2.2. Cell culture
The human CRC-derived cell lines HCT116 and HT29 were obtained from the Korean Cell Line Bank (Seoul, Republic of Korea). To establish colon cancer cells resistant to 5-FU (5-FUR), HCT116 cells were cultured for at least 6 months with progressively increasing 5-FU concentrations starting at 0.5 μM.
2.3. Cell viability and proliferation test
Cell viability was measured using the Cell Proliferation Kit I (MTT) (Roche, Basel, Switzerland). Cells cultured in 96-well plates were incubated with 10 μL of MTT labeling reagent at 37°C in the dark. After 4 h, a solubilization solution was dispensed and incubated at 37°C overnight. The optical density was determined at 560 and 650 nm using Epoch™ microplate spectrophotometer (BioTek Instruments, Winooski, VT, USA). Cell proliferation was examined using the BrdU ELISA Kit (Roche). Treated cells were cultured in BrdU solution for 2 h to allow incorporation into genomic DNA and then in anti-BrdU-peroxidase solution for 90 min. Reaction products were quantified by measuring absorbance at 370 and 492 nm using Epoch™ microplate spectrophotometer.
2.4. Western blot analysis
Western blotting was performed to estimate the expression levels of proteins extracted from CRC cells. Proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes, and exposed to the indicated antibodies overnight. Membranes were then exposed to peroxidase-conjugated secondary antibody for 1 h, and band images were acquired using ChemiDoc equipment (Bio-Rad, Hercules, CA, USA).
2.5. Annexin V & propidium iodide staining
Apoptosis was analyzed using the Fluorescein isothiocyanate annexin V apoptosis detection kit I (BD Biosciences, Franklin Lakes, NJ, USA). To measure apoptosis, treated cells were harvested, stained with annexin V and propidium iodide (PI) for 15 min, and then analyzed using flow cytometry as described in a previous study [16].
2.6. 3D culture
Based on the previously reported hanging drop method, CRC spheroids were prepared [17]. Briefly, cells diluted in growth medium to a concentration of 1 × 105/mL were dropped into the lid of an inverted 60-mm culture dish in a volume of 25 μL containing 2500 cells in each drop. The bottom of the culture dish was filled with PBS, which served as the hydration chamber. Cells were treated with 20 μM of 5-FU, 20 μM of apigenin, or their combination for 3 days. Changes in spheroid morphology were observed using a DM3000 microscope (Leica, Wetzlar, Germany). Average colony area and colony counts were quantified using ImageJ. In addition, 96-well plates with satellite wells were prepared for the assessment of chemical effects in Matrigel. Briefly, micropatterned culture chips were fabricated by injection molding of polystyrene (K-RESIN, Chevron Phillips Chemical, TX, United States), made hydrophilic by air plasma treatment (CUTE-MP, Femto Science, Republic of Korea), and sterilized using ethylene oxide gas. Matrigel and growth medium were mixed at a ratio of 1:1 and solidified with cells at 37°C. Next, cells were treated by injecting the indicated chemicals into the satellite wells. Average colony area was examined in the Matrigel using a microscope before and after 48 h of chemical treatment.
2.7. Cell cycle assay
Cells were treated with RNase A and PI for 30 min, and the distribution of cells in SubG1, G0/G1, S, and G2/M phases was examined by flow cytometry as described in a previous study [16].
2.8. ROS assay
Intracellular accumulation of ROS as an index of oxidative stress was measured using DCFH-DA (Sigma-Aldrich, Inc). Briefly, cells were loaded with DCFH-DA for 30 min, treated with the indicated reagents for 1 or 24 h, and then harvested. The green fluorescence from oxidized DCF was measured using a flow cytometer (BD Biosciences).
2.9. Intracellular and intramitochondrial Ca2+ measurements
Intracellular and intramitochondrial calcium concentrations were measured using the fluorescent dyes Fluo-4 (Invitrogen, Carlsbad, CA, USA) and Rhod-2 (Invitrogen), respectively. After loading with Fluo-4 at 37°C for 20 min or Rhod-2 at 4°C for 30 min, cells were treated as indicated and fluorescence emission intensity measured using a flow cytometer.
2.10. Quantitative RT-PCR
To quantify gene expression, total RNA was extracted from cells using Trizol reagent (Invitrogen), and complementary DNAs (cDNAs) were synthesized using RT premix. Gene expression was then quantified using SYBR dye and primer pairs for TP53 (F: 5’-CCTCACCATCATCACACTGG-3’, R: 5’-TCTTGCGGAGATTCTCTTCC-3’) and FOXM1 (F: 5’-GGGTTTTCTCCTTTGCTTCC-3’, R: 5’-ATGGGTCTCGCTAAGTGTGG-3’). Relative mRNA levels were calculated using the 2−ΔΔCT method based on CT values. The expression of the GAPDH gene was measured for normalization.
2.11. Immunofluorescence staining for P53
Immunofluorescence was used to analyze the expression patterns of P53 in cells. Briefly, treated cells were permeabilized by incubation with 100% methanol at 4°C for 10 min. Next, cells were rinsed with PBS, blocked with goat serum for 2 h, incubated with P53 primary antibody overnight at 4°C, and then incubated with Alexa488-conjugated secondary antibody (Invitrogen) for 1 h. Nuclei were counterstained with DAPI for 5 min, and fluorescence images were acquired using a confocal microscope. Minimum three images were used for the quantification of fluorescence intensity by Metamorph Offline software (Molecular Devices).
2.12. Transfection
Knockdown of FOXM1 expression was performed by transfection of siFOXM1 using Lipofectamine 2000 (Sigma-Aldrich) according to the manufacturer’s instructions (Bioneer, Daejeon, Republic of Korea). The sequence of FOXM1 that siFOXM1 targets is 5’-AGTTTCCAGCTGGGATCAA-3’. The sequence of siFOXM1 is 5’-AGUUUCCAGCUGGGAUCAATT-3’ for sense and 5’-TTUCAAAGGUCGACCCUAGUU-3’ for antisense strand. A non-specific siRNA was used as the control (siControl). All siRNAs were purchased from Bioneer based on a genome-wide predesigned siRNA library. SiFOXM1 used for transfection had three different siRNA IDs (2305–1, 2305–2, and 2305–3) and was specified to be designed based on National Center for Biotechnology Information reference sequences (NM_001243088.1, NM_001243089.1, NM_021953.3, NM_202002.2, NM_202003.2).
2.13. Statistics
All statistical calculations were conducted using analysis of variance according to the general linear model (PROC-GLM) of the SAS statistical software 9.4 (SAS Institute, Cary, NC, USA). All experiments were repeated at least three times. A p value of <0.05 (two-tailed) was considered statistically significant for all tests.
3. Results
3.1. Phytochemicals potentiated the inhibition of CRC cell viability by 5-FU
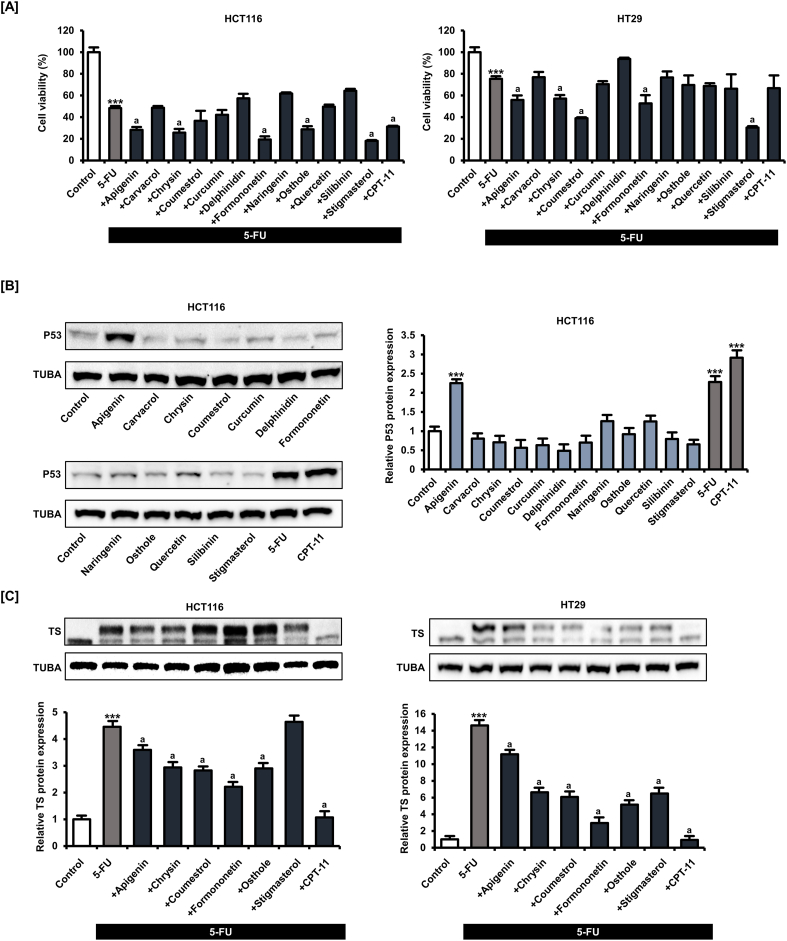
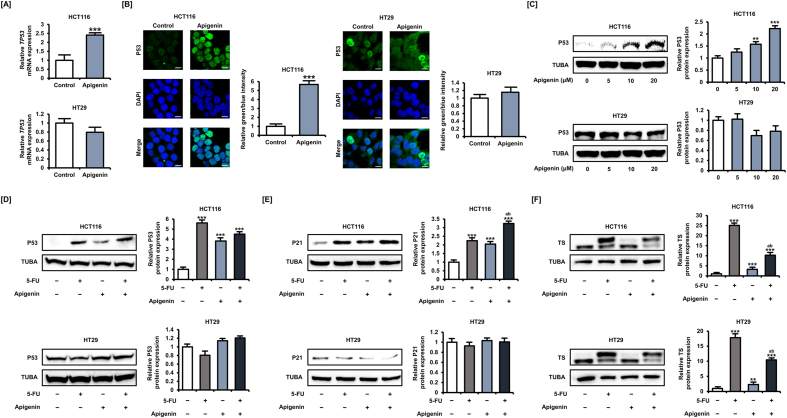
When applied alone for 48 h, 5-FU (1, 2, 4, 8, 16, 32, and 64 μM) dose-dependently inhibited the viability and proliferation of HCT116 and HT29 cells (Fig. S1), consistent with its known anticancer effect. We then examined whether any of the following natural compounds, apigenin [18], carvacrol [19], chrysin [20], coumestrol [21], curcumin [22], delphinidin [23], formononetin [24], naringenin [25], osthole [26], quercetin [27], silibinin [28], and stigmasterol [29], at 20 μM potentiated the anticancer effect of 5-FU (20 μM) for 48 h (Fig. 1A). We selected a concentration of 20 μM of phytochemicals based on the average concentration found in our previous studies to induce cancer cell death. To compare the effects of each phytochemical on cell viability in CRC cells, all phytochemicals were treated at the same concentration and for the same period. In each assay, the conventional anticancer drug CPT-11 (20 μM) was used as the positive control. Apigenin, chrysin, formononetin, osthole, and stigmasterol demonstrated the greatest potentiating effects of 5-FU on HCT116 cell viability, whereas apigenin, chrysin, coumestrol, formononetin, and stigmasterol showed the largest potentiating effects of 5-FU on HT29 cell viability.
Fig. 1.
Multiple phytochemicals, including apigenin potentiated the suppressive effect of 5-FU on colorectal cancer (CRC) cell viability. [A] The viability of human CRC lines HCT116 and HT29 following treatment with 5-FU (20 μM) alone or 5-FU plus the indicated phytochemical (20 μM) for 48 h as measured by MTT assay. [B] Expression of P53 by HCT116 and HT29 cells following phytochemical (20 μM) treatment for 24 h as measured by western blot. [C] Expression of thymidylate synthase (TS) by HCT116 and HT29 cells following treatment with 5-FU alone or 5-FU plus the indicated phytochemical (20 μM) for 24 h as measured by western blot. Upper bands represent bound TS, and lower bands represent unbound TS. Data are presented as representatives of the results of three independent experiments. Asterisk indicates a statistically significant difference compared to untreated control cells (***p < 0.001). The symbol 'a' indicates a significant effect of combination treatment compared to 5-FU alone (p < 0.05).
Next, we examined the changes in wild-type P53 expression in HCT116 cells after phytochemical and conventional anticancer drug treatment at 20 μM for 24 h (Fig. 1B) and found that among phytochemicals, only apigenin increased P53 expression by 2.3 times (p < 0.001) similar to 5-FU (2.3-fold, p < 0.001) and CPT-11 (2.9-fold, p < 0.001). We also found that 5-FU (20 μM) treatment for 24 h enhanced upper band expression of TS in HCT116 and HT29 cells, suggesting the formation of classic complexes of TS; therefore, we next investigated whether the phytochemicals that potentiated 5-FU activity, namely, apigenin, chrysin, coumestrol, formononetin, osthole, and stigmasterol, also suppressed TS (Fig. 1C). All tested phytochemicals except stigmasterol inhibited TS expression in HCT116 cells. In HT29 cells as well, all tested phytochemicals and CPT-11 (positive control) inhibited TS expression. These results suggest that phytochemicals potentiate the effects of 5-FU, possibly by reversing the 5-FU-induced increase in TS. Among the phytochemicals, apigenin increased P53 expression, suggesting that the application of apigenin is a novel strategy for CRC treatment. We therefore focused on the effects of apigenin in all subsequent experiments.
3.2. Apigenin enhanced 5-FU-mediated CRC cell growth suppression and apoptosis induction
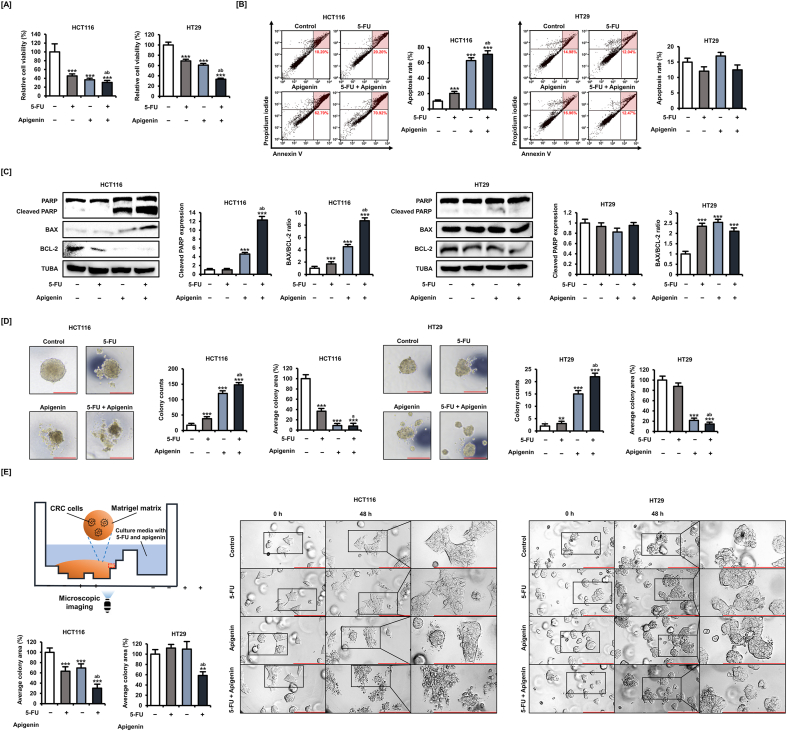
Apigenin dose-dependently inhibited the viability of both CRC cells for 48 h and was particularly effective against HCT116 cells (Fig. S2A). In addition, apigenin dose-dependently induced the apoptosis of HCT116 cells for 48 h (Fig. S2B). Based on these dose–response results, we set 20 μM as the optimal apigenin concentration for subsequent experiments. First, we confirmed that the addition of apigenin (20 μM) to 5-FU (20 μM) for 48 h induced greater reductions (69.3% reduction in HCT116 cells and 66.4% reduction in HT29 cells, p < 0.001) in HCT116 and HT29 cell viability than 5-FU alone (55% reduction in HCT116 cells and 31.1% reduction in HT29 cells, p < 0.001) (Fig. 2A). Further, annexin V and PI staining revealed that 5-FU dose-dependently induced late apoptosis of HCT116 cells (Fig. S3). Moreover, the addition of apigenin (20 μM) to 5-FU (20 μM) for 48 h increased the apoptosis rate (70.92%, p < 0.001) of HCT116 cells compared with 5-FU treatment alone (20.20%, p < 0.001) (Fig. 2B). On the other hand, apoptosis of HT29 cells was induced by 5-FU only at concentrations of ≥32 μM, whereas apigenin addition did not have a demonstrable potentiating effect, suggesting that this potentiating effect is dependent on (wild-type) P53 function. The cleavage of PARP is an important measure of DNA damage in cancer cells [30]. apigenin (20 μM) for 24 h significantly increased the expression of cleaved PARP in HCT116 cells, and the addition of 5-FU (20 μM) further enhanced the increasing effect (Fig. 2C). Interestingly, 5-FU alone, apigenin alone, or the combined treatment of 5-FU and apigenin did not significantly affect the expression of cleaved PARP compared to the control group in HT29 cells. These results are consistent with the result that 5-FU and apigenin did not show apoptosis-inducing effect in HT29 cells. The ratio of pro-apoptotic protein BAX to anti-apoptotic protein BCL-2 is a predictable indicator of mitochondrial-mediated apoptosis in cancer cells [31]. Treatment with 5-FU alone, apigenin alone, or 5-FU plus apigenin significantly increased the ratio of BAX/BCL-2 in HCT116 cells. In HT29 cells, treatment with 5-FU alone, apigenin alone, or 5-FU plus apigenin decreased the expression of BCL-2, resulting in an increase in the ratio of BAX/BCL-2, although it had no effect on the expression of BAX.
Fig. 2.
Apigenin potentiated CRC cell growth inhibition and apoptosis induction by 5-FU in 2D and 3D culture. [A] The viability of HCT116 and HT29 cells following treatment with apigenin (20 μM) plus 5-FU (20 μM) for 48 h analyzed using MTT assay. [B] Apoptotic death of HCT116 and HT29 cells following apigenin (20 μM) or 5-FU (20 μM) plus apigenin treatment for 48 h as estimated by dual annexin V/propidium iodide (PI) staining and flow cytometry. The upper right quadrant indicates cells in late apoptosis used for the quantification of cell death. [C] Expression of PARP, BAX, and BCL-2 in HCT116 and HT29 cells following treatment with 5-FU (20 μM) alone, apigenin (20 μM) alone, or apigenin plus 5-FU (20 μM) for 24 h as estimated by western blot. [D] The 3D structure of HCT116 and HT29 cell spheroids following 5-FU (20 μM) plus apigenin (20 μM) treatment for 3 days as quantified by ImageJ. The scale bar represents 300 μm. [E] Schematic diagram of our method for observing the morphology of cell/colonies in matrigel following 5-FU plus apigenin treatment in satellite wells. Comparison of cell/colony morphology before treatment and after 48 h of treatment with apigenin (20 μM) alone or apigenin plus 5-FU (20 μM) as quantified by ImageJ. The scale bar represents 200 μm. Data are presented as representatives of the results of three independent experiments. Asterisk indicates a statistically significant difference compared to untreated controls (***p < 0.001). The symbol 'a' indicates a significant difference between combination treatment and 5-FU treatment alone (p < 0.05). The symbol 'b' indicates a significant difference between combination treatment and apigenin treatment alone (p < 0.05).
For antitumor efficacy, candidates must suppress cell viability in the 3D tissue environment. Therefore, we next analyzed the effects of 5-FU (20 μM) and apigenin (20 μM) for 3 days on CRC cell spheroids (Fig. 2D). Apigenin increased the colony counts (from 17 colonies in the control group to 120 colonies in HCT116 cells and from 2 colonies in the control group to 15 colonies in HT29 cells, p < 0.001), decreased the average colony area (92.1% in HCT116 cells and 78.5% in HT29 cells), and potentiated the effects of 5-FU on both cell types. Combination treatment with 5-FU and apigenin significantly decreased spheroid formation compared with 5-FU alone treatment. To further confirm the potentiation of 5-FU efficacy in the 3D environment, we analyzed the effect on cells immobilized in Matrigel (Fig. 2E). Consistent with results in 2D and spheroid culture, the combination of 5-FU (20 μM) and apigenin (20 μM) administered from satellite wells for 48 h prevented large colony formation in HCT116 cells. The average colony area reduced by 36.8% (p < 0.001) with 5-FU alone, 30.5% (p < 0.001) with apigenin alone, and 69.9% (p < 0.001) with 5-FU plus apigenin compared with the control group in HCT116 cells. In HT29 cells, the combination treatment of 5-FU and apigenin significantly reduced the average colony area by 41.5% (p < 0.01), although treatment with each of them alone did not. Collectively, these results suggest that apigenin potentiates 5-FU effects on cell growth and survival of CRC cells.
3.3. Apigenin alone and in combination with 5-FU shifted the cell cycle stage distribution of CRC cells toward SubG1
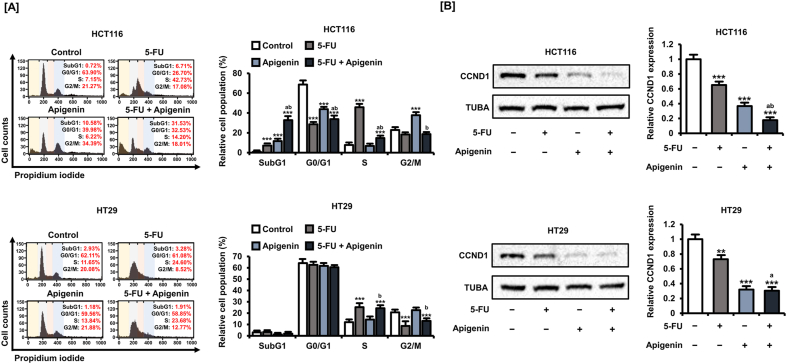
It is well known that 5-FU treatment causes S-phase arrest of CRC cells; therefore, we investigated whether apigenin further facilitates the shift in cell cycle stage distribution (Fig. 3A). Apigenin (20 μM) did not alter the cell cycle distribution of HT29 cells either alone or in combination with 5-FU (20 μM), whereas apigenin alone (11.6%, p < 0.001), 5-FU alone (7.2%, p < 0.001), and the combination (32,8%, p < 0.001) for 48 h increased the proportion of HCT116 cells in the SubG1 phase, implying entry into the apoptotic pathway.
Fig. 3.
Apigenin potentiated cell cycle disruption by 5-FU, potentially by influencing CCND1 expression. [A] Cell cycle distribution was determined by propidium iodide (PI) staining and flow cytometry and expressed as the proportions of cells in the SubG1, G1, S, and G2/M phases after treatment with 5-FU and/or apigenin for 48 h. [B] Expression of CCND1 in HCT116 and HT29 cells following treatment with 5-FU (20 μM) alone, apigenin (20 μM) alone, or apigenin plus 5-FU (20 μM) for 24 h as estimated by western blot. Data are presented as representatives of the results of three independent experiments. Asterisks indicate statistically significant differences compared to untreated controls (***p < 0.001; **p < 0.01). The symbol 'a' indicates a significant difference between combination treatment and 5-FU treatment alone (p < 0.05). The symbol 'b' indicates a significant difference between combination treatment and apigenin treatment alone (p < 0.05).
Next, we analyzed the changes in the expression of the key cell cycle regulator cyclin D1 (CCND1) by western blotting (Fig. 3B). In both CRC cell types, 5-FU (20 μM) alone (34.8% reduction in HCT116 cells, p < 0.001, and 28.0% reduction in HT29 cells, p < 0.01), apigenin (20 μM) alone (63.4% reduction in HCT116 cells, p < 0.001, and 68.0% reduction in HT29 cells, p < 0.001), and the combination (82.3% reduction in HCT116 cells, p < 0.001, and 79.6% reduction in HT29 cells, p < 0.001) for 48 h reduced CCND1 expression. Thus, apigenin can regulate the cell cycle of CRC cells both alone and in combination with 5-FU, possibly by suppressing CCND1. Moreover, these effects are dependent on wild-type P53.
3.4. Apigenin alone and in combination with 5-FU induced ROS production and mitochondrial dysfunction in CRC cells
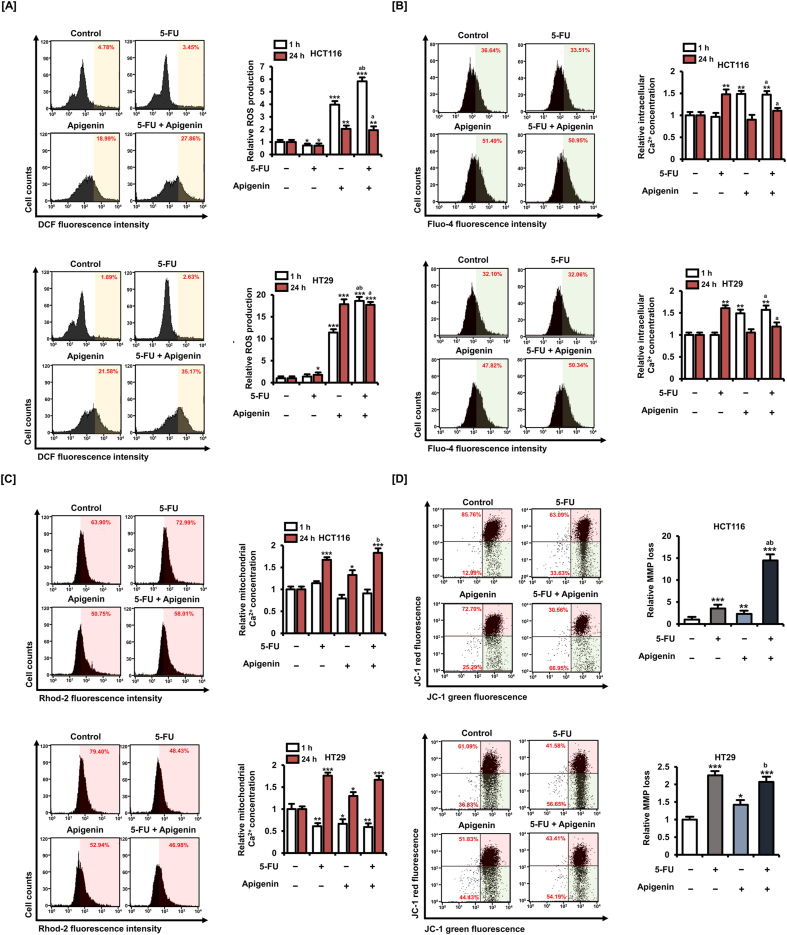
Oxidative stress is a major cell death mechanism of some conventional anticancer agents and therapeutic adjuvants; therefore, we investigated the effects of 5-FU (20 μM) alone, apigenin (20 μM) alone, and 5-FU plus apigenin for 1 and 24 h on ROS production as measured by 2′,7′-dichlorofluorescin diacetate (DCFH-DA) conversion to fluorescent 2′,7′-dichlorofluorescein (DCF) (Fig. 4A). Representative plot images of cell population treated for 1 h are presented in the left panel. Treatment with 5-FU alone for 1 h did not cause significant ROS accumulation in HT29 cells. Conversely, apigenin alone (4.0-fold in HCT116 cells and 11.4-fold in HT29 cells, p < 0.001) and in combination with 5-FU (5.8-fold in HCT116 cells, p < 0.001 and 18.6-fold in HT29 cells) promoted ROS production in both CRC cell lines within 1 h.
Fig. 4.
Apigenin enhances ROS production, Ca2+ dysregulation, and mitochondrial membrane potential (MMP) depolarization in 5-FU-treated CRC cells. [A] ROS generation after 5-FU (20 μM), apigenin (20 μM) and 5-FU plus apigenin treatment for 1 or 24 h as measured by DCF fluorescence. [B] Intracellular Ca2+ concentration increases after 5-FU (20 μM), apigenin (20 μM), and 5-FU plus apigenin treatment for 1 or 24 h as measured by Fluo-4 staining. [C] Mitochondrial Ca2+ concentration increases after 5-FU (20 μM), apigenin (20 μM), and 5-FU plus apigenin treatment for 1 or 24 h as measured by Rhod-2 staining. [D] Loss of MMP after 5-FU (20 μM), apigenin (20 μM), and 5-FU plus apigenin treatment for 24 h as measured by JC-1 staining and flow cytometry. The degree of mitochondrial membrane depolarization was determined by the proportion of cells in the lower area of the plot. Data are presented as representatives of the results of three independent experiments. Histogram plots in the left panel from [A] to [C] are representative images for the 1 h treatment group. Asterisks indicate statistically significant differences compared with untreated controls (***p < 0.001; **p < 0.01; *p < 0.05). The symbol 'a' indicates a significant difference between combination treatment and 5-FU treatment alone (p < 0.05). The symbol 'b' indicates a significant difference between combination treatment and apigenin treatment alone (p < 0.05).
Oxidative stress from ROS accumulation is associated with both calcium dysregulation, mitochondria calcium accumulation, and induction of the mitochondrial apoptosis pathway. Therefore, we measured the effects of 5-FU (20 μM) and apigenin (20 μM) on intracellular and intramitochondrial Ca2+ concentrations using the calcium-sensitive fluorescent dyes Fluo-4 and Rhod-2, respectively (Fig. 4B–C). While treatment with 5-FU increased the intracellular Ca2+ concentration after 24 h (1.5-fold in HCT116 cells and 1.6-fold in HT29 cells, p < 0.01), apigenin increased the Ca2+ concentration after only 1 h (1.5-fold in HCT116 cells and 1.6-fold in HT29 cells, p < 0.01) (Fig. 4B). Conversely, mitochondrial Ca2+ level was not affected by a 1-h treatment with 5-FU alone, apigenin alone, or the combination in HCT116 cells (Fig. 4C). Meanwhile, 5-FU alone, apigenin alone, or combination treatment reduced the mitochondrial Ca2+ levels in HT29 cells. However, all three treatments for 24 h increased the mitochondrial Ca2+ concentration. Combination treatment of 5-FU with apigenin for 24 h increased the mitochondrial Ca2+ levels by 1.8-fold (p < 0.001) in HCT116 cells and 1.7-fold (p < 0.001) in HT29 cells compared with controls. Representative plot images for cell populations treated for 1 h are presented in the left panel of Fig. 4B and C. These results suggest that an early increase in cytoplasmic Ca2+ induced by apigenin enhances the rate of mitochondrial calcium uptake, ultimately resulting in greater mitochondria dysfunction and high apoptosis rate.
Energy production by mitochondria depends on the maintenance of a transmembrane potential between the inner membrane and matrix. Thus, to examine possible mitochondrial dysfunction directly, we measured this mitochondrial membrane potential (MMP) in CRC cells using the fluorescent MMP indicator JC-1 (Fig. 4D). Consistent with reduced cell viability and increased apoptosis rate, treatment with 5-FU (20 μM), apigenin (20 μM), and combined 5-FU plus apigenin for 24 h induced mitochondrial depolarization, with greater effects on HCT116 cells than on HT29. Further, combination treatment induced greater MMP loss than either agent alone. Combination treatment of 5-FU and apigenin for 24 h increased the relative MMP loss by 14.5-fold (p < 0.001) in HCT116 cells and 2.1-fold (p < 0.001) in HT29 cells compared with controls. These results suggest that apigenin cotreated with 5-FU enhances oxidative stress and mitochondrial dysfunction within CRC cells, thereby increasing the cell death rate.
3.5. Apigenin upregulated P53 and downregulated TS in CRC cells
We next examined the effects of apigenin on the expression of the putative targets P53 and TS. Apigenin treatment (20 μM) for 24 h upregulated the expression of TP53 by 2.4-fold (p < 0.001) in HCT116 cells, but did not affect expression in HT29 cells based on quantitative RT-PCR analysis (Fig. 5A). Apigenin (20 μM) for 24 h significantly also increased the expression of P53 in the nuclei of HCT116 cells, but again had no effect on P53 expression in HT29 cells by immunofluorescence analysis (Fig. 5B). The increase in P53 expression in HCT116 cells was dose-dependent (Fig. 5C); but in contrast to others, apigenin (20 μM) treatment for 24 h did not potentiate the increase induced by 5-FU (20 μM) (Fig. 5D). We analyzed the expression of P21 as a target protein of P53, which is involved in cell cycle arrest and apoptosis (Fig. 5E). Interestingly, treatment with 5-FU (20 μM), apigenin (20 μM), and combined 5-FU plus apigenin for 24 h significantly increased the expression of P21 only in HCT116 cells, not in HT29 cells. In both CRC cell lines, however, apigenin (20 μM) treatment for 24 h reversed the 5-FU-induced elevation in TS expression (Fig. 5F). Combination treatment with 5-FU and apigenin reduced upper expression of TS by 58.2% (p < 0.001) in HCT116 cells and 41.6% (p < 0.001) in HT29 cells compared with 5-FU alone treatment. Moreover, the intensity of the lower band representing unbounded TS also reduced by the additional treatment of apigenin. These results suggest that apigenin enhances the anticancer effect of 5-FU by mitigating the associated increase in TS, even in P53 mutant cells, but does not directly activate the P53-regulated apoptosis pathway.
Fig. 5.
Apigenin upregulates P53 expression and reverses the 5-FU-induced upregulation of thymidylate synthase (TS) in CRC cells. [A] Expression of TP53 in HCT116 and HT29 cells following apigenin (20 μM) treatment for 24 h was analyzed using quantitative RT-PCR. [B] Regulation of P53 expression by apigenin (20 μM) for 24 h was analyzed by immunofluorescence (green). Nuclei were counterstained with DAPI (blue). The scale bars represent 40 μm, [C] Expression of P53 in HCT116 and HT29 cells following apigenin (20 μM) treatment for 24 h as estimated by western blot. [D] Expression of P53 in HCT116 and HT29 cells following apigenin (20 μM) plus 5-FU (20 μM) treatment for 24 h was analyzed by western blot. [E] Expression of P21 in HCT116 and HT29 cells following apigenin (20 μM) plus 5-FU (20 μM) treatment for 24 h was analyzed by western blot. [F] Expression of TS in HCT116 and HT29 cells following apigenin (20 μM) plus 5-FU (20 μM) treatment for 24 h was analyzed by western blot. Upper bands represent bound TS, and lower bands represent unbound TS. Data are presented as representatives of the results of three independent experiments. Asterisks indicate statistically significant differences compared with untreated controls (***p < 0.001; **p < 0.01). The symbol 'a' indicates a significant difference between combination treatment and 5-FU treatment alone (p < 0.05). The symbol 'b' indicates a significant difference between combination treatment and apigenin treatment alone (p < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
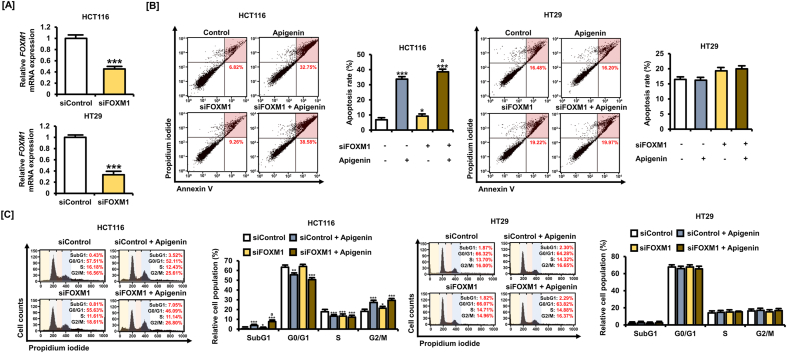
3.6. Potentiation of 5-FU efficacy by apigenin involves FOXM1 silencing
Previous studies have demonstrated that the P53-modulated transcription factor FOXM1 regulates the sensitivity of cancer cells to 5-FU [14,32]. To examine the contributions of FOXM1 to the effects of apigenin alone and to the potentiation of 5-FU responses, we examined drug response in FOXM1 knockdown cells established by transfection with a targeted small interfering (si)RNA (siFOXM1) (Fig. 6A). siFOXM1 transfection at 10 nM reduced FOXM1 gene expression by 54.7% (p < 0.001) in HCT116 cells and 66.7% (p < 0.001) in HT29 cells. Knockdown of FOXM1 in HCT116 cells enhanced late apoptosis rate compared with control siRNA (from 6.82% to 9.26%) and potentiated the effects of apigenin (20 μM) alone from 32.75% to 38.58% (Fig. 6B). In HT29 cells, siFOXM1 (10 nM) transfection for 5 h did not cause a significant change in apoptosis rate, although it slightly increased the rate of late apoptosis. Transfection with siFOXM1 (10 nM) also increased the proportion of apigenin-treated HCT116 cells in SubG1 phase from 3.52% to 7.05% (Fig. 6C), consistent with the increased activation of the apoptotic pathway. However, FOXM1 knockdown did not affect the cell cycle distribution of HT29 cells, suggesting that the action of FOXM1 is under the influence of (wild-type) P53. These results further suggest that FOXM1 downregulation in CRC cells enhances the anticancer effect of apigenin by facilitating effects on the cell cycle and apoptosis and that this facilitation relies on P53 expression and function.
Fig. 6.
FOXM1 silencing contributes to apigenin-induced apoptosis and cell cycle modulation. [A] Knockdown of FOXM1 following siFOXM1 (10 nM) transfection for 5 h of HCT116 and HT29 cells was analyzed by quantitative RT-PCR. [B] Death rates of HCT116 and HT29 cells following siFOXM1 (10 nM) transfection for 5 h and apigenin (20 μM) treatment for 48 h as measured by dual Annexin V/propidium iodide (PI) staining and flow cytometry. The cells in the upper right quadrant are in late apoptosis. [C] Cell cycle distribution following siFOXM1 (10 nM) transfection for 5 h and apigenin (20 μM) treatment for 48 h determined by PI staining and flow cytometry and expressed as the proportions of cells in SubG1, G1, S, and G2/M phases. Data are presented as representatives of the results of three independent experiments. Asterisk indicates a statistically significant difference compared with untreated controls (***p < 0.001; **p < 0.01; *p < 0.05). The symbol 'a' indicates a significant difference between combination treatment and apigenin alone (p < 0.05).
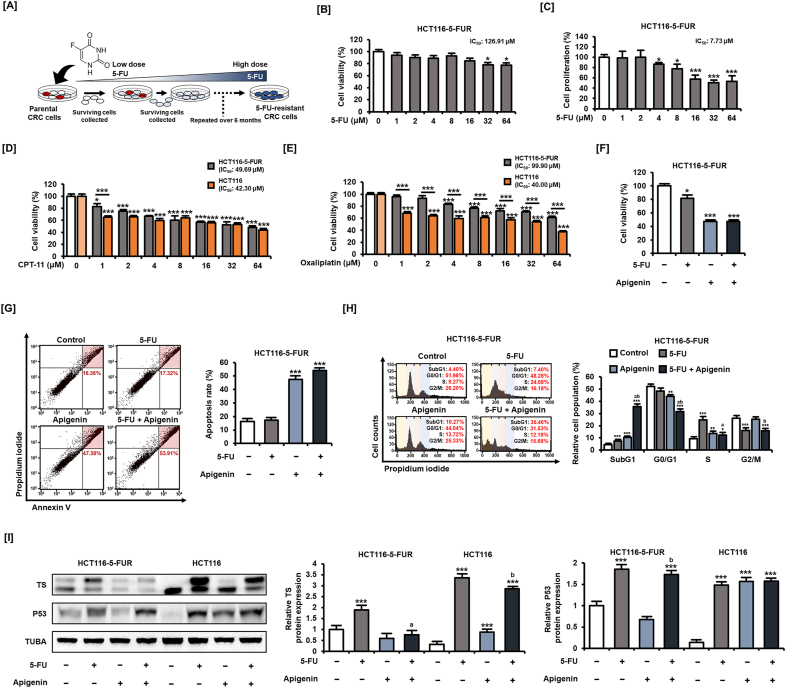
3.7. Apigenin alleviated induced 5-FU resistance of CRC cells
The development of tumor cell resistance to primary chemotherapeutic agents results in tumor regrowth, metastasis, and poor outcome; therefore, agents that can prevent resistance are of high clinical value. We tested the capacity of apigenin to reverse 5-FU resistance using a CRC cell line established by treating HCT116 cells with gradually increasing 5-FU concentrations for approximately 6 months (Fig. 7A). These HCT116-5-FUR cells exhibited a marked increase in 5-FU resistance as indicated by an increase in IC50 to 126.91 μM from 32.30 μM in HCT116 cells, as shown in Fig. S1 (Fig. 7B). In addition, these cells showed enhanced resistance to the inhibition of proliferation by 5-FU for 48 h (Fig. 7C). Next, we examined whether HCT116-5-FUR cells had multidrug resistance to CPT-11 and oxaliplatin, the conventional anticancer drugs administered together with 5-FU for the treatment of CRC. Compared with HCT116 cells, HCT116-5-FUR cells showed no difference in cell viability inhibitory effect except for a low concentration of 1 μM for CPT11 treatment for 48 h (Fig. 7D). On the other hand, the results of cell viability changes following the dose-dependent treatment of oxaliplatin for 48 h suggest that the sensitivity to oxaliplatin is lowered in HCT116-5-FUR cells compared to HCT116 cells (Fig. 7E). These results suggest that 5-FU resistance may also be involved in the therapeutic efficacy of other anticancer drugs. Meanwhile, apigenin (20 μM) treatment for 48 h reduced the cell viability of HCT116-5-FUR cells by 52.6% (p < 0.001) to a level similar to that of parental HCT116 cells (Fig. 7F) and enhanced the rate of HCT116-5-FUR cell apoptosis when combined with 5-FU (53.91%) compared with 5-FU alone (17.32%) for 48 h (Fig. 7G). Addition of apigenin (20 μM) for 48 h also enhanced the proportion of HCT116-5-FUR cells in S-phase arrest compared with 5-FU (20 μM) treatment alone (Fig. 7H) and increased the proportion of 5-FU-treated HCT116-5-FUR cells entering the SubG1 phase from 7.40% to 35.46%.
Fig. 7.
Apigenin restores 5-FU sensitivity to CRC cells. [A] Schematic representation of the method for establishing 5-FU-resistant CRC cells (HCT116-5-FUR cells). [B] Viability of HCT116-5-FUR cells following 5-FU treatment for 48 h was analyzed by MTT assay. [C] Proliferation of HCT116-5-FUR cells following 5-FU treatment for 48 h was analyzed using BrdU ELISA. [D] Viability of HCT116-5-FUR and HCT116 cells following CPT-11 treatment for 48 h was analyzed by MTT assay. [E] Viability of HCT116-5-FUR and HCT116 cells following oxaliplatin treatment for 48 h was analyzed by MTT assay. [F] Viability of HCT116-5-FUR cells following 5-FU (20 μM) plus apigenin (20 μM) treatment for 48 h was analyzed using MTT assay. [G] Apoptotic death rate of HCT116-5-FUR cells following 5-FU (20 μM) plus apigenin (20 μM) treatment for 48 h as estimated by dual Annexin V/PI staining and flow cytometry. Cells in the upper right quadrant are in late apoptosis. [H] Effect of 5-FU (20 μM) plus apigenin (20 μM) for 48 h on HCT116-5-FUR cell cycle distribution as measured by PI staining and flow cytometry, and expressed by the proportions in SubG1, G1, S, and G2/M phases. [I] Expression of TS in HCT116-5-FUR cells following 5-FU (20 μM) plus apigenin (20 μM) treatment for 24 h was analyzed using western blotting. Upper bands represent bound TS, and lower bands represent unbound TS. Data are presented as representatives of the results of three independent experiments. Asterisks indicate statistically significant differences compared with untreated controls (***p < 0.001; **p < 0.01; *p < 0.05). The symbol 'a' indicates a significant difference between combination treatment and 5-FU treatment alone (p < 0.05). The symbol 'b' indicates a significant difference between combination treatment and apigenin treatment alone (p < 0.05).
We then examined whether these effects are associated with the regulation of TS and P53 as in parental HCT-116 cells by western blotting (Fig. 7I). The amount of classical complexes of TS was higher in untreated HCT116-5-FUR cells than untreated HCT116 cells. Conversely, 5-FU (20 μM) for 24 h increased both bounded (upper) and unbounded (lower) TS expression to a greater degree in HCT116 cells. These findings suggest that high TS complex expression in HCT116 cells increases resistance to 5-FU. Furthermore, apigenin (20 μM) treatment attenuated the increased expression of both bounded and unbounded TS in HCT116-5-FUR cells. However, apigenin influenced P53 only in control HCT116 cells but not in HCT116-5-FUR cells. In other words, apigenin may also increase the therapeutic efficacy of 5-FU in 5-FU-resistant CRC cells through regulation of TS expression but independently of P53.
4. Discussion
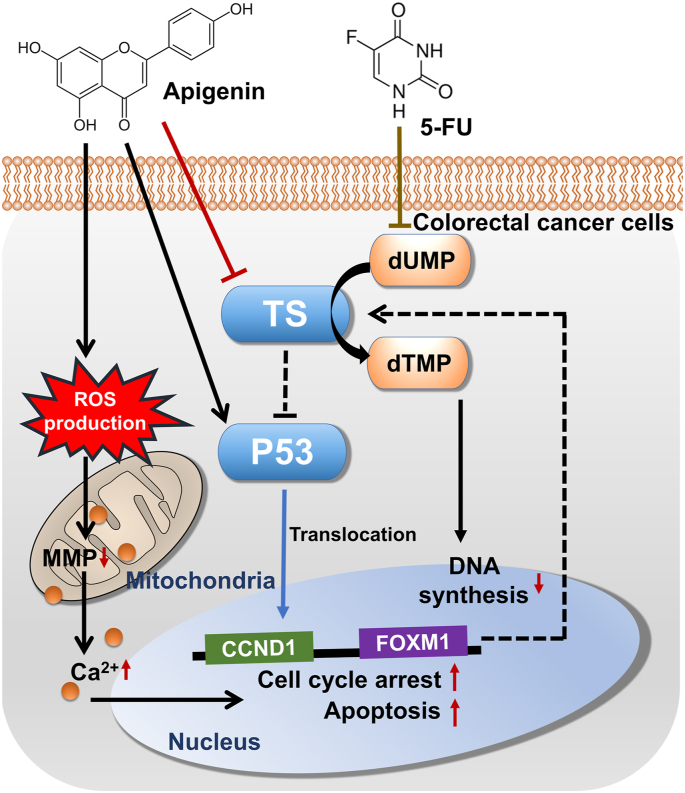
The 5-year survival rate of patients with advanced CRC is <10%, mainly owing to the development of drug resistance and ensuing tumor recurrence [33]. 5-FU is the primary first-line drug used for the treatment of numerous cancer types, including CRC. However, in patients with advanced CRC, 5-FU treatment response rate is only 10%–15%; therefore, it is applied in combination with other cytotoxic drugs such as CPT-11 and oxaliplatin [34,35]. In addition, phytochemicals with low cytotoxicity in normal cells are valuable adjuncts to conventional therapeutics. In this study, we found that among the various phytochemicals with documented anticancer effects in our previous studies, several, including apigenin, enhanced the capacity of 5-FU to reduce CRC cell viability (Fig. 8).
Fig. 8.
Schematic representation of apigenin effects on CRC cells when added with 5-FU. Thymidylate synthase (TS) expression is increased by 5-FU, whereas apigenin reverses this increase. Inhibition of TS expression induces DNA damage in CRC cells, leading to apoptosis. Apigenin upregulates the expression of the antitumor protein P53, which may further induce cell cycle arrest and apoptosis under 5-FU cotreatment. Apigenin also induces ROS production, intracellular Ca2+ dysregulation, and MMP loss in CRC cells. Silencing of FOXM1 further enhances apigenin-induced cell death. Dotted bars indicate mechanisms that have been reported in previous studies but have not been validated in the present study.
Apigenin is one of the most widely found flavones in plants. Apigenin is primarily found in Asteraceae; parsley, chamomile, celery, and oregano are also major sources of apigenin [36,37]. The therapeutic potential of apigenin has been widely reported in various diseases, including cancer [36]. Apigenin inhibits the survival and growth of choriocarcinoma and endometriosis cells [18,38]. Numerous studies have also shown that various plant flavonoids, including apigenin, can enhance the therapeutic efficacy of 5-FU [[39], [40], [41]]. For instance, curcumin improved the sensitivity of breast cancer cells to 5-FU by inhibiting the upregulation of TS expression following 5-FU exposure [39]. Quercetin, a flavonoid contained in fruits and vegetables, potentiates 5-FU-induced DNA fragmentation and pro-apoptotic effects in a P53-dependent manner in CRC cells [42]. Another flavonoid, luteolin, inhibits CRC cell growth by regulating the expression of proteins related to apoptosis, including P53, BAX, and BCL-2, when combined with 5-FU [43]. In addition, S-adenosyl-l-methionine, a naturally occurring sulfur-containing nucleoside, induces cell cycle arrest and ROS accumulation in P53-deficient CRC cells, reducing cell viability along with 5-FU [44]. Moreover, apigenin enhanced the apoptosis-inducing effect of 5-FU in breast cancer cells [40]. Apigenin also synergized with 5-FU to promote G2/M arrest and increase ROS production by neck squamous cell carcinoma cells, leading to apoptosis [45]. Similarly, apigenin enhanced the anticancer effect of 5-FU against hepatocellular carcinoma cells in vivo and in vitro by promoting ROS production, mitochondrial membrane disruption, and apoptosis [46]. Several past studies have suggested that apigenin has polypharmacological properties in inducing apoptosis in CRC cells. Lee and colleagues found that apigenin induced autophagy in HCT116 cells and that the inhibition of autophagy enhanced apigenin-induced apoptosis [47]. In addition, apigenin induces a senescence phenotype in CRC cells, mitigating tumor formation [48]. Moreover, a recent study revealed that apigenin inhibits the growth of CRC cells mediated by miRNA-215-5p [49]. These studies suggest that the apoptosis-inducing mechanism of apigenin revealed in this study is part of the broad intracellular physiological regulation mechanism of apigenin in CRC cells. However, it remains uncertain whether apigenin can enhance the anticancer effect of 5-FU in CRC cells. To our knowledge, this study revealed for the first time that apigenin can induce oxidative stress, Ca2+ dysregulation, and mitochondrial dysfunction, thereby potentiating the cytotoxicity of 5-FU. Several chemotherapeutic drugs induce cancer cell apoptosis through the production of excessive ROS [50], which in turn induces mitochondrial dysfunction and activates mitochondrial apoptosis signals. Therefore, apigenin may increase the sensitivity of tumors to 5-FU by activating the mitochondria-mediated apoptosis pathway. In this study, there is a limitation that a dose-dependent combination treatment was not performed to calculate a value such as a combination index that can quantify whether apigenin can cause a synergistic effect with 5-FU. A recently published study revealed that apigenin caused synergistic effects with 5-FU in inhibiting cell viability [49]. In the study, analysis of the combination index on cell viability after concomitant treatment with 20, 40, and 80 μM of apigenin and 10, 20, and 50 μM of 5-FU in HCT116 cells clearly shows that 5-FU and apigenin have a strong synergistic effect [49]. In addition, our study leads us to speculate that 5-FU and apigenin may have a synergistic effect on apoptosis of CRC cells, although we confirmed the effects of both 5-FU and apigenin at a single concentration of 20 μM. Our results also suggest that both 5-FU and apigenin share a functional P53-dependent mechanism for inducing apoptosis in CRC cells.
Similar to the conventional anticancer drug CPT-11, apigenin also upregulated the expression of the antitumor protein P53. The P53 protein acts as an initiator of DNA repair in response to damage and triggers the apoptosis of cells with extensive DNA damage to prevent the propagation of mutations [51]. Mutant P53 is associated with shorter overall CRC survival [52], and cancer cells with mutant P53 are less sensitive to 5-FU [53]. Moreover, loss of P53 function in cancer cells may reduce therapeutic sensitivity to 5-FU [54]. In esophageal squamous cells, apigenin induced G2/M arrest and P53-independent mitochondria-mediated apoptosis [55]. The present study also suggests that apigenin may act in both a P53-dependent and P53-independent manner in CRC cells. P53-dependent mechanism by apigenin in CRC cells appears to be closely related to apoptosis, mainly represented by increased expression of cleaved PARP and P21. On the other hand, it is speculated that the mechanism of apigenin acting independently of P53 is mainly related to redox imbalance, represented by generation of ROS and Ca2+ imbalance. The present study revealed a clear difference in the effects of apigenin and 5-FU on CRC cells with wild-type P53 (HCT116) compared with those in cells with mutant P53 (HT29), implying that the apoptosis-inducing effect of apigenin relies on normal P53 function. Therefore, further research is required for identifying ways to increase the treatment efficiency in CRC cells, which are mutated or deficient in P53. As an example of a study in this context, 5-FU and free ribosomal protein encapsulated in polymer nanoparticles can enhance apoptosis through induction of nucleolar stress in P53-deficient cancer cells [56]. Evidence has suggested that 5-FU-induced nucleolar stress in cancer cells mediates ribosomal proteins to activate P53 and its target, P21 [6,7,31]. It was also found that 5-FU induces mitochondrial-mediated apoptosis by targeting ribosomal proteins in P53-deficient CRC cells, suggesting that P53-independent apoptosis may be induced [57]. In cancer cells lacking P53, overexpression of ribosomal proteins leads to cell cycle arrest and activation of P21, resulting in apoptosis [58]. Therefore, overexpressing ribosomal proteins is considered an approach that can enhance the cytotoxicity of 5-FU. Furthermore, in cancer cells, ribosomal proteins influence the activity of P21 independent of P53 and may be involved in multidrug resistance [59]. In our study, single treatment of 5-FU or apigenin, and the combination of 5-FU and apigenin increased the expression of P21 only in HCT116 cells but not in HT29 cells, just like the expression of P53. Although further analysis is required, it can be speculated that P53 and its downstream proteins are closely related to the mechanism by which apigenin induces apoptosis in CRC cells. The presence of functional P53 in CRC cells may play a decisive role in the extent of the apoptosis-inducing effect of apigenin. PARP cleavage is a useful marker of programmed cell death in CRC [30]. Moreover, the ratio of BAX/BCL-2 is an important measure to evaluate the severity of mitochondrial-mediated apoptosis following drug treatment in cancer cells [57]. The expression of cleaved PARP, BAX, and BCL-2 regulated by apigenin in combination with 5-FU in CRC cells complements the apoptosis-inducing effect of apigenin in response to the presence of functional P53. In addition. further studies need to verify whether apigenin can be involved in the regulation of ribosomal proteins to improve drug resistance in CRC cells. Previous studies have suggested that resistance to one drug in cancer cells may be accompanied by resistance to other anticancer drugs [59,60]. Lung cancer cells resistant to 5-FU have also been shown to be resistant to other anticancer drugs, including 5'-deoxyl-5-fluorouridine, oxaliplatin, and cisplatin [59]. In the present study, CRC cells resistant to 5-FU were only resistant at low concentration following CPT-11 treatment, but were resistant to oxaliplatin treatment at all concentrations analyzed. These results suggest that further studies are needed to determine whether apigenin may be involved in the overcoming of multidrug resistance in CRC.
We also found modest anticancer effects of apigenin alone, consistent with several previous studies. For instance, apigenin was reported to inhibit CRC cell proliferation with an IC50 of 1.8 μM [61]. Turktekin and colleagues (2011) also reported that apigenin increased the expression of the pro-apoptotic effectors CASP3 and CASP8 and decreased the expression of mTOR and CCND1 in HT29 cells [62]. In addition, apigenin promoted circular chemorepellent-induced defects in CRC cells, thus inhibiting spheroid formation [63] and suggesting efficacy against early tumorigenesis. Moreover, Sen and colleagues (2019) reported that simultaneous delivery of apigenin and 5-FU in the same liposomes inhibited the proliferation and increased the apoptosis rate in a CRC xenograft mouse model [64]. Although the potential therapeutic efficacy of apigenin against CRC cells has been demonstrated in various preclinical models, this is the first study to show that apigenin can potentiate the anticancer effect of 5-FU, including against cells with induced 5-FU resistance, at least in part through modulation of P53 and TS expression.
TS is a folate-dependent enzyme responsible for the production of thymidylate, an essential intermediate for DNA biosynthesis [65]. The active metabolite of 5-FU binds to the active site of dUMP to form an inactive TS complex, thereby preventing the conversion of dUMP to dTMP. In vitro and in vivo studies have shown that high TS expression is closely related to the development of 5-FU chemoresistance [66,67]. Furthermore, TS gene and protein expression levels as well as enzymatic activity are inversely correlated with the 5-FU sensitivity of CRC cells [68]. Due to its chemical structure, apigenin is an effective inhibitor of TS, and we speculate that apigenin induces CRC cell death and cell cycle disruption through downregulation of TS activity and concomitant upregulation of P53 [11]. In CRC, P53 expression is downregulated by TS. Therefore, cells overexpressing TS will be less sensitive to cell cycle arrest by DNA-damaging agents [13]. Li and colleagues (2020) reported the development of a new TS inhibitor that can inhibit non–small-cell lung cancer growth by upregulating P53 expression [69]. Moreover, TS inhibition facilitates DNA damage, induces MMP depolarization, and ultimately activates apoptotic signaling pathways. Continuous exposure to conventional chemotherapeutic agents upregulates pro-survival signaling pathways and multidrug resistance genes, thereby establishing resistance. This resistance can be modeled in vitro by prolonged exposure to increasing chemotherapeutic drug concentrations, and such cells have proven useful for screening potential adjuvants that can reduce drug resistance in CRC [70]. Compared with 5-FU-sensitive cell lines, resistant CRC cells expressed higher levels of TS [71], and we demonstrate that adjunct apigenin can induce apoptosis even in 5-FU-resistant (5-FUR) CRC cells by downregulating TS independently of P53. In immunoblots analysis, the upper band of TS indicates the formation of a ternary complex of drug and TS and the lower band indicates unmodified TS [72,73]. We found that additional treatment with apigenin suppressed the expression of both bounded and unbounded TS forms in CRC cells. These results suggest that apigenin inhibits protein translation of TS rather than posttranslational modification in CRC cells.
FOXM1 is a transcription factor involved in the progression and chemoresistance of numerous carcinomas [74]. It is highly expressed in drug-resistant cells, whereas FOXM1 deficiency enhances the sensitivity of cancer cells to genotoxic agents [75,76]. Much evidence suggests that FOXM1 contributes to the survival and growth of CRC [77]. Further, 5-FU increased the expression of FOXM1 in CRC, whereas FOXM1 knockdown increased the sensitivity to 5-FU [15]. In addition, FOXM1 can bind to the promoter region of TS and induces 5-FU resistance of CRC cells by upregulating TS expression. Moreover, Intuyod and colleagues (2018) suggested that the FOXM1–TS axis is a useful diagnostic and therapeutic target to predict and improve 5-FU resistance. We found that FOXM1 knockdown potentiated the apoptosis-inducing effect of apigenin as evidenced by the increased entry of CRC cells into SubG1 phase.
5. Conclusions
Apigenin improved the inhibitory effects of 5-FU on cell viability and upregulated P53 expression. It increased the generation of ROS, dysregulation of Ca2+, cell cycle arrest, and depolarization of MMP in the colon cancer cells. Suppression of FOXM1 expression enhanced the potentiation of cell death by apigenin. Furthermore, apigenin inhibited TS expression and blocked the viability of 5-FU-resistant colon cancer cells. A limitation of this study is that we have not identified how the FOXM1–TS axis regulates the apoptosis of CRC cells under apigenin or combined 5-FU plus apigenin treatment. Nonetheless, this study is the first to demonstrate that apigenin can potentiate the efficacy of 5-FU against CRC and reduce acquired 5-FU resistance. Through the application of apigenin, an appropriate combination can be adopted under various conditions for the development of an integrated treatment strategy that can ultimately alleviate the high toxicity of 5-FU. Studies on the extraction and formulation from natural sources that can maximize the therapeutic potential of apigenin in CRC are warranted.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2021R1C1C1009807 and 2021R1A2C2005841).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.102144.
Contributor Information
Gwonhwa Song, Email: ghsong@korea.ac.kr.
Whasun Lim, Email: wlim@kookmin.ac.kr.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Goding Sauer A., Fedewa S.A., Butterly L.F., Anderson J.C. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 2.Bathe O.F., Franceschi D., Livingstone A.S., Moffat F.L., Tian E., Ardalan B. Increased thymidylate synthase gene expression in liver metastases from colorectal carcinoma: implications for chemotherapeutic options and survival. Cancer J Sci Am. 1999;5:34–40. [PubMed] [Google Scholar]
- 3.Longley D.B., Boyer J., Allen W.L., Latif T., Ferguson P.R., Maxwell P.J. The role of thymidylate synthase induction in modulating p53-regulated gene expression in response to 5-fluorouracil and antifolates. Cancer Res. 2002;62:2644–2649. [PubMed] [Google Scholar]
- 4.Johnston P.G., Lenz H.J., Leichman C.G., Danenberg K.D., Allegra C.J., Danenberg P.V. Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res. 1995;55:1407–1412. [PubMed] [Google Scholar]
- 5.Lee J.H., Park J.H., Jung Y., Kim J.H., Jong H.S., Kim T.Y. Histone deacetylase inhibitor enhances 5-fluorouracil cytotoxicity by down-regulating thymidylate synthase in human cancer cells. Mol. Canc. Therapeut. 2006;5:3085–3095. doi: 10.1158/1535-7163.MCT-06-0419. [DOI] [PubMed] [Google Scholar]
- 6.Pecoraro A., Pagano M., Russo G., Russo A. Ribosome biogenesis and cancer: overview on ribosomal proteins. Int. J. Mol. Sci. 2021:22. doi: 10.3390/ijms22115496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun X.X., Dai M.S., Lu H. 5-fluorouracil activation of p53 involves an MDM2-ribosomal protein interaction. J. Biol. Chem. 2007;282:8052–8059. doi: 10.1074/jbc.M610621200. [DOI] [PubMed] [Google Scholar]
- 8.Way T.D., Kao M.C., Lin J.K. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. J. Biol. Chem. 2004;279:4479–4489. doi: 10.1074/jbc.M305529200. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S., Afaq F., Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem. Biophys. Res. Commun. 2001;287:914–920. doi: 10.1006/bbrc.2001.5672. [DOI] [PubMed] [Google Scholar]
- 10.Czeczot H., Tudek B., Kusztelak J., Szymczyk T., Dobrowolska B., Glinkowska G. Isolation and studies of the mutagenic activity in the Ames test of flavonoids naturally occurring in medical herbs. Mutat. Res. 1990;240:209–216. doi: 10.1016/0165-1218(90)90060-f. [DOI] [PubMed] [Google Scholar]
- 11.Siragusa L., Luciani R., Borsari C., Ferrari S., Costi M.P., Cruciani G. Comparing drug images and repurposing drugs with BioGPS and FLAPdock: the thymidylate synthase case. ChemMedChem. 2016;11:1653–1666. doi: 10.1002/cmdc.201600121. [DOI] [PubMed] [Google Scholar]
- 12.Abu El Maaty M.A., Strassburger W., Qaiser T., Dabiri Y., Wolfl S. Differences in p53 status significantly influence the cellular response and cell survival to 1,25-dihydroxyvitamin D3-metformin cotreatment in colorectal cancer cells. Mol. Carcinog. 2017;56:2486–2498. doi: 10.1002/mc.22696. [DOI] [PubMed] [Google Scholar]
- 13.Ju J., Pedersen-Lane J., Maley F., Chu E. Regulation of p53 expression by thymidylate synthase. Proc. Natl. Acad. Sci. U. S. A. 1999;96:3769–3774. doi: 10.1073/pnas.96.7.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Intuyod K., Saavedra-Garcia P., Zona S., Lai C.F., Jiramongkol Y., Vaeteewoottacharn K. FOXM1 modulates 5-fluorouracil sensitivity in cholangiocarcinoma through thymidylate synthase (TYMS): implications of FOXM1-TYMS axis uncoupling in 5-FU resistance. Cell Death Dis. 2018;9:1185. doi: 10.1038/s41419-018-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Varghese V., Magnani L., Harada-Shoji N., Mauri F., Szydlo R.M., Yao S. FOXM1 modulates 5-FU resistance in colorectal cancer through regulating TYMS expression. Sci. Rep. 2019;9:1505. doi: 10.1038/s41598-018-38017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim W., Yang C., Bazer F.W., Song G. Chrysophanol induces apoptosis of choriocarcinoma through regulation of ROS and the AKT and ERK1/2 pathways. J. Cell. Physiol. 2017;232:331–339. doi: 10.1002/jcp.25423. [DOI] [PubMed] [Google Scholar]
- 17.Foty R. A simple hanging drop cell culture protocol for generation of 3D spheroids. JoVE. 2011;51 doi: 10.3791/2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim W., Park S., Bazer F.W., Song G. Apigenin reduces survival of choriocarcinoma cells by inducing apoptosis via the PI3K/AKT and ERK1/2 MAPK pathways. J. Cell. Physiol. 2016;231:2690–2699. doi: 10.1002/jcp.25372. [DOI] [PubMed] [Google Scholar]
- 19.Lim W., Ham J., Bazer F.W., Song G. Carvacrol induces mitochondria-mediated apoptosis via disruption of calcium homeostasis in human choriocarcinoma cells. J. Cell. Physiol. 2019;234:1803–1815. doi: 10.1002/jcp.27054. [DOI] [PubMed] [Google Scholar]
- 20.Lim W., Ryu S., Bazer F.W., Kim S.M., Song G. Chrysin attenuates progression of ovarian cancer cells by regulating signaling cascades and mitochondrial dysfunction. J. Cell. Physiol. 2018;233:3129–3140. doi: 10.1002/jcp.26150. [DOI] [PubMed] [Google Scholar]
- 21.Lim W., Jeong M., Bazer F.W., Song G. Coumestrol inhibits proliferation and migration of prostate cancer cells by regulating AKT, ERK1/2, and JNK MAPK cell signaling cascades. J. Cell. Physiol. 2017;232:862–871. doi: 10.1002/jcp.25494. [DOI] [PubMed] [Google Scholar]
- 22.Lim W., Jeong M., Bazer F.W., Song G. Curcumin suppresses proliferation and migration and induces apoptosis on human placental choriocarcinoma cells via ERK1/2 and SAPK/JNK MAPK signaling pathways. Biol. Reprod. 2016;95:83. doi: 10.1095/biolreprod.116.141630. [DOI] [PubMed] [Google Scholar]
- 23.Lim W., Jeong W., Song G. Delphinidin suppresses proliferation and migration of human ovarian clear cell carcinoma cells through blocking AKT and ERK1/2 MAPK signaling pathways. Mol. Cell. Endocrinol. 2016;422:172–181. doi: 10.1016/j.mce.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Park S., Bazer F.W., Lim W., Song G. The O-methylated isoflavone, formononetin, inhibits human ovarian cancer cell proliferation by sub G0/G1 cell phase arrest through PI3K/AKT and ERK1/2 inactivation. J. Cell. Biochem. 2018;119:7377–7387. doi: 10.1002/jcb.27041. [DOI] [PubMed] [Google Scholar]
- 25.Park S., Lim W., Bazer F.W., Song G. Naringenin suppresses growth of human placental choriocarcinoma via reactive oxygen species-mediated P38 and JNK MAPK pathways. Phytomedicine. 2018;50:238–246. doi: 10.1016/j.phymed.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Bae H., Lee J.Y., Song J., Song G., Lim W. Osthole interacts with an ER-mitochondria axis and facilitates tumor suppression in ovarian cancer. J. Cell. Physiol. 2021;236:1025–1042. doi: 10.1002/jcp.29913. [DOI] [PubMed] [Google Scholar]
- 27.Lim W., Yang C., Park S., Bazer F.W., Song G. Inhibitory effects of quercetin on progression of human choriocarcinoma cells are mediated through PI3K/AKT and MAPK signal transduction cascades. J. Cell. Physiol. 2017;232:1428–1440. doi: 10.1002/jcp.25637. [DOI] [PubMed] [Google Scholar]
- 28.Ham J., Lim W., Bazer F.W., Song G. Silibinin stimluates apoptosis by inducing generation of ROS and ER stress in human choriocarcinoma cells. J. Cell. Physiol. 2018;233:1638–1649. doi: 10.1002/jcp.26069. [DOI] [PubMed] [Google Scholar]
- 29.Bae H., Song G., Lim W. Stigmasterol causes ovarian cancer cell apoptosis by inducing endoplasmic reticulum and mitochondrial dysfunction. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12060488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitacre C.M., Zborowska E., Willson J.K., Berger N.A. Detection of poly(ADP-ribose) polymerase cleavage in response to treatment with topoisomerase I inhibitors: a potential surrogate end point to assess treatment effectiveness. Clin. Canc. Res. 1999;5:665–672. [PubMed] [Google Scholar]
- 31.Russo A., Saide A., Cagliani R., Cantile M., Botti G., Russo G. rpL3 promotes the apoptosis of p53 mutated lung cancer cells by down-regulating CBS and NFkappaB upon 5-FU treatment. Sci. Rep. 2016;6:38369. doi: 10.1038/srep38369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qu K., Xu X., Liu C., Wu Q., Wei J., Meng F. Negative regulation of transcription factor FoxM1 by p53 enhances oxaliplatin-induced senescence in hepatocellular carcinoma. Canc. Lett. 2013;331:105–114. doi: 10.1016/j.canlet.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Dahan L., Sadok A., Formento J.L., Seitz J.F., Kovacic H. Modulation of cellular redox state underlies antagonism between oxaliplatin and cetuximab in human colorectal cancer cell lines. Br. J. Pharmacol. 2009;158:610–620. doi: 10.1111/j.1476-5381.2009.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douillard J.Y., Cunningham D., Roth A.D., Navarro M., James R.D., Karasek P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 35.Giacchetti S., Perpoint B., Zidani R., Le Bail N., Faggiuolo R., Focan C. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 2000;18:136–147. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 36.Salehi B., Venditti A., Sharifi-Rad M., Kregiel D., Sharifi-Rad J., Durazzo A. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shankar E., Goel A., Gupta K., Gupta S. Plant flavone apigenin: an emerging anticancer agent. Curr Pharmacol Rep. 2017;3:423–446. doi: 10.1007/s40495-017-0113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park S., Lim W., Bazer F.W., Song G. Apigenin induces ROS-dependent apoptosis and ER stress in human endometriosis cells. J. Cell. Physiol. 2018;233:3055–3065. doi: 10.1002/jcp.26054. [DOI] [PubMed] [Google Scholar]
- 39.Vinod B.S., Antony J., Nair H.H., Puliyappadamba V.T., Saikia M., Narayanan S.S. Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis. 2013;4:e505. doi: 10.1038/cddis.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi E.J., Kim G.H. 5-Fluorouracil combined with apigenin enhances anticancer activity through induction of apoptosis in human breast cancer MDA-MB-453 cells. Oncol. Rep. 2009;22:1533–1537. doi: 10.3892/or_00000598. [DOI] [PubMed] [Google Scholar]
- 41.Meiyanto E., Hermawan A., Anindyajati A. Natural products for cancer-targeted therapy: citrus flavonoids as potent chemopreventive agents. Asian Pac. J. Cancer Prev. APJCP. 2012;13:427–436. doi: 10.7314/apjcp.2012.13.2.427. [DOI] [PubMed] [Google Scholar]
- 42.Xavier C.P., Lima C.F., Rohde M., Pereira-Wilson C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Canc. Chemother. Pharmacol. 2011;68:1449–1457. doi: 10.1007/s00280-011-1641-9. [DOI] [PubMed] [Google Scholar]
- 43.Erdogan M.K., Agca C.A., Askin H. Quercetin and luteolin improve the anticancer effects of 5-fluorouracil in human colorectal adenocarcinoma in vitro model: a mechanistic insight. Nutr. Canc. 2021:1–17. doi: 10.1080/01635581.2021.1900301. [DOI] [PubMed] [Google Scholar]
- 44.Mosca L., Pagano M., Pecoraro A., Borzacchiello L., Mele L., Cacciapuoti G. S-Adenosyl-l-Methionine overcomes uL3-mediated drug resistance in p53 deleted colon cancer cells. Int. J. Mol. Sci. 2020;22 doi: 10.3390/ijms22010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan L.P., Chou T.H., Ding H.Y., Chen P.R., Chiang F.Y., Kuo P.L. Apigenin induces apoptosis via tumor necrosis factor receptor- and Bcl-2-mediated pathway and enhances susceptibility of head and neck squamous cell carcinoma to 5-fluorouracil and cisplatin. Biochim. Biophys. Acta. 2012;1820:1081–1091. doi: 10.1016/j.bbagen.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Hu X.Y., Liang J.Y., Guo X.J., Liu L., Guo Y.B. 5-Fluorouracil combined with apigenin enhances anticancer activity through mitochondrial membrane potential (Delta Psi m)-mediated apoptosis in hepatocellular carcinoma. Clin. Exp. Pharmacol. Physiol. 2015;42:146–153. doi: 10.1111/1440-1681.12333. [DOI] [PubMed] [Google Scholar]
- 47.Lee Y., Sung B., Kang Y.J., Kim D.H., Jang J.Y., Hwang S.Y. Apigenin-induced apoptosis is enhanced by inhibition of autophagy formation in HCT116 human colon cancer cells. Int. J. Oncol. 2014;44:1599–1606. doi: 10.3892/ijo.2014.2339. [DOI] [PubMed] [Google Scholar]
- 48.Banerjee K., Mandal M. Oxidative stress triggered by naturally occurring flavone apigenin results in senescence and chemotherapeutic effect in human colorectal cancer cells. Redox Biol. 2015;5:153–162. doi: 10.1016/j.redox.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng Y., Han X., Mo F., Zeng H., Zhao Y., Wang H. Apigenin inhibits the growth of colorectal cancer through down-regulation of E2F1/3 by miRNA-215-5p. Phytomedicine. 2021;89:153603. doi: 10.1016/j.phymed.2021.153603. [DOI] [PubMed] [Google Scholar]
- 50.Lu G.D., Shen H.M., Chung M.C., Ong C.N. Critical role of oxidative stress and sustained JNK activation in aloe-emodin-mediated apoptotic cell death in human hepatoma cells. Carcinogenesis. 2007;28:1937–1945. doi: 10.1093/carcin/bgm143. [DOI] [PubMed] [Google Scholar]
- 51.Harwood F.G., Frazier M.W., Krajewski S., Reed J.C., Houghton J.A. Acute and delayed apoptosis induced by thymidine deprivation correlates with expression of p53 and p53-regulated genes in colon carcinoma cells. Oncogene. 1996;12:2057–2067. [PubMed] [Google Scholar]
- 52.Elsaleh H., Powell B., McCaul K., Grieu F., Grant R., Joseph D. p53 alteration and microsatellite instability have predictive value for survival benefit from chemotherapy in stage III colorectal carcinoma. Clin. Canc. Res. 2001;7:1343–1349. [PubMed] [Google Scholar]
- 53.Bunz F., Hwang P.M., Torrance C., Waldman T., Zhang Y., Dillehay L. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J. Clin. Invest. 1999;104:263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Longley D.B., Latif T., Boyer J., Allen W.L., Maxwell P.J., Johnston P.G. The interaction of thymidylate synthase expression with p53-regulated signaling pathways in tumor cells. Semin. Oncol. 2003;30:3–9. doi: 10.1016/s0093-7754(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Q., Zhao X.H., Wang Z.J. Cytotoxicity of flavones and flavonols to a human esophageal squamous cell carcinoma cell line (KYSE-510) by induction of G2/M arrest and apoptosis. Toxicol. Vitro. 2009;23:797–807. doi: 10.1016/j.tiv.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 56.Russo A., Maiolino S., Pagliara V., Ungaro F., Tatangelo F., Leone A. Enhancement of 5-FU sensitivity by the proapoptotic rpL3 gene in p53 null colon cancer cells through combined polymer nanoparticles. Oncotarget. 2016;7:79670–79687. doi: 10.18632/oncotarget.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pagliara V., Saide A., Mitidieri E., d'Emmanuele di Villa Bianca R., Sorrentino R., Russo G. 5-FU targets rpL3 to induce mitochondrial apoptosis via cystathionine-beta-synthase in colon cancer cells lacking p53. Oncotarget. 2016;7:50333–50348. doi: 10.18632/oncotarget.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Russo A., Esposito D., Catillo M., Pietropaolo C., Crescenzi E., Russo G. Human rpL3 induces G(1)/S arrest or apoptosis by modulating p21 (waf1/cip1) levels in a p53-independent manner. Cell Cycle. 2013;12:76–87. doi: 10.4161/cc.22963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russo A., Saide A., Smaldone S., Faraonio R., Russo G. Role of uL3 in multidrug resistance in p53-mutated lung cancer cells. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18030547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang A. Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Canc. 2011;71:3–10. doi: 10.1016/j.lungcan.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 61.Lee S.W., Lee J.T., Lee M.G., Lee H.W., Ahn S.J., Lee Y.J. In vitro antiproliferative characteristics of flavonoids and diazepam on SNU-C4 colorectal adenocarcinoma cells. J. Nat. Med. 2009;63:124–129. doi: 10.1007/s11418-008-0300-x. [DOI] [PubMed] [Google Scholar]
- 62.Turktekin M., Konac E., Onen H.I., Alp E., Yilmaz A., Menevse S. Evaluation of the effects of the flavonoid apigenin on apoptotic pathway gene expression on the colon cancer cell line (HT29) J. Med. Food. 2011;14:1107–1117. doi: 10.1089/jmf.2010.0208. [DOI] [PubMed] [Google Scholar]
- 63.Holzner S., Brenner S., Atanasov A.G., Senfter D., Stadler S., Nguyen C.H. Intravasation of SW620 colon cancer cell spheroids through the blood endothelial barrier is inhibited by clinical drugs and flavonoids in vitro. Food Chem. Toxicol. 2018;111:114–124. doi: 10.1016/j.fct.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 64.Sen K., Banerjee S., Mandal M. Dual drug loaded liposome bearing apigenin and 5-Fluorouracil for synergistic therapeutic efficacy in colorectal cancer. Colloids Surf. B Biointerfaces. 2019;180:9–22. doi: 10.1016/j.colsurfb.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 65.Carreras C.W., Santi D.V. The catalytic mechanism and structure of thymidylate synthase. Annu. Rev. Biochem. 1995;64:721–762. doi: 10.1146/annurev.bi.64.070195.003445. [DOI] [PubMed] [Google Scholar]
- 66.Chu E., Koeller D.M., Johnston P.G., Zinn S., Allegra C.J. Regulation of thymidylate synthase in human colon cancer cells treated with 5-fluorouracil and interferon-gamma. Mol. Pharmacol. 1993;43:527–533. [PubMed] [Google Scholar]
- 67.Swain S.M., Lippman M.E., Egan E.F., Drake J.C., Steinberg S.M., Allegra C.J. Fluorouracil and high-dose leucovorin in previously treated patients with metastatic breast cancer. J. Clin. Oncol. 1989;7:890–899. doi: 10.1200/JCO.1989.7.7.890. [DOI] [PubMed] [Google Scholar]
- 68.Peters G.J., Backus H.H.J., Freemantle S., van Triest B., Codacci-Pisanelli G., van der Wilt C.L. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Bba-Mol Basis Dis. 2002;1587:194–205. doi: 10.1016/s0925-4439(02)00082-0. [DOI] [PubMed] [Google Scholar]
- 69.Li X.Y., Wang D.P., Lu G.Q., Liu K.L., Zhang T.J., Li S. Development of a novel thymidylate synthase (TS) inhibitor capable of up-regulating P53 expression and inhibiting angiogenesis in NSCLC. J. Adv. Res. 2020;26:95–110. doi: 10.1016/j.jare.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boyer J., McLean E.G., Aroori S., Wilson P., McCulla A., Carey P.D. Characterization of p53 wild-type and null isogenic colorectal cancer cell lines resistant to 5-fluorouracil, oxaliplatin, and irinotecan. Clin. Canc. Res. 2004;10:2158–2167. doi: 10.1158/1078-0432.ccr-03-0362. [DOI] [PubMed] [Google Scholar]
- 71.Saga Y., Suzuki M., Mizukami H., Kohno T., Takei Y., Fukushima M. Overexpression of thymidylate synthase mediates desensitization for 5-fluorouracil of tumor cells. Int J Cancer. 2003;106:324–326. doi: 10.1002/ijc.11221. [DOI] [PubMed] [Google Scholar]
- 72.Brody J.R., Gallmeier E., Yoshimura K., Hucl T., Kulesza P., Canto M.I. A proposed clinical test for monitoring fluoropyrimidine therapy: detection and stability of thymidylate synthase ternary complexes. Cancer Biol Ther. 2006;5:923–927. doi: 10.4161/cbt.5.8.2976. [DOI] [PubMed] [Google Scholar]
- 73.Mori R., Yoshida K., Futamura M., Suetsugu T., Shizu K., Tanahashi T. The inhibition of thymidine phosphorylase can reverse acquired 5FU-resistance in gastric cancer cells. Gastric Cancer. 2019;22:497–505. doi: 10.1007/s10120-018-0881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Myatt S.S., Lam E.W. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 75.Khongkow P., Karunarathna U., Khongkow M., Gong C., Gomes A.R., Yague E. FOXM1 targets NBS1 to regulate DNA damage-induced senescence and epirubicin resistance. Oncogene. 2014;33:4144–4155. doi: 10.1038/onc.2013.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Millour J., de Olano N., Horimoto Y., Monteiro L.J., Langer J.K., Aligue R. ATM and p53 regulate FOXM1 expression via E2F in breast cancer epirubicin treatment and resistance. Mol. Canc. Therapeut. 2011;10:1046–1058. doi: 10.1158/1535-7163.MCT-11-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoshida Y., Wang I.C., Yoder H.M., Davidson N.O., Costa R.H. The forkhead box M1 transcription factor contributes to the development and growth of mouse colorectal cancer. Gastroenterology. 2007;132:1420–1431. doi: 10.1053/j.gastro.2007.01.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.