Abstract
Background
Despite its low efficacy, chemotherapy with dacarbazine remains an option in metastatic melanoma patients after failure of immune checkpoint inhibitors (ICI) ± targeted therapy. Some observations suggested an increased efficacy of chemotherapy in melanoma or lung cancer patients previously treated with ICI; we aimed to evaluate the efficacy of dacarbazine in a controlled-group study of patients pre-treated or not with ICI.
Methods
We retrospectively collected data from all consecutive patients treated with dacarbazine for advanced cutaneous melanoma without brain metastasis, in our skin cancer centre between June 2006 and September 2019. The primary endpoint was progression-free survival (PFS); secondary endpoints were overall response rates (ORR), overall survival (OS) and safety of dacarbazine.
Results
Among 72 patients, 17 (23.6%) received dacarbazine after ICI and 55 (76.3%) without prior ICI. Despite less favourable prognostic factors in patients ICI-pre-treated, median PFS was 4.27 months (range 0.89–43.69) in this group versus 2.04 months (range 1.25–39.25) P = 0.03 in non-ICI-pre-treated patients; ORR were 35.3% and 12.7%, respectively. The median OS and the occurrence of adverse events were similar in both groups.
Conclusion
Dacarbazine seems to offer a short-lived benefit in patients with progressive advanced disease despite ICI (±targeted therapy), and could be an alternative before considering best supportive care.
Subject terms: Melanoma, Immunization
Introduction
New therapies such as immune checkpoint inhibitors (ICI) and targeted therapy have considerably improved survival of patients with advanced melanoma [1]. Targeted therapy, with BRAF and MEK inhibitors, has the advantage of inducing rapid responses [2–4] while ICI, with anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4; ipilimumab) and anti-programmed cell death protein 1 (PD-1; nivolumab, pembrolizumab) monoclonal antibodies (mAb) induces durable responses [5–7]. As BRAF plus MEK inhibitors showed similar efficacy in second and first lines whereas anti-PD-1 mAb efficacy is lower in second line [8, 9], ICI are now being recommended as first-line therapy in all patients with BRAF wild-type melanoma and in most patients with BRAFV600-mutant melanoma [10]. However, a large proportion of patients will progress: the 5-year overall survival (OS) rates are 34%, 39% and 52% with targeted therapy [2], pembrolizumab alone [11] and nivolumab plus ipilimumab [6], respectively.
In the absence of clinical trials, few therapeutic options are offered for patients failing these therapies. Despite low overall response rates (ORR) (10–15%), dacarbazine may still be used, in the absence of brain metastasis [12]. A few studies in melanoma or lung cancer patients [13–15] have even suggested an improved efficacy of salvage chemotherapy through a delayed effect of a prior treatment with ICI, but control groups are lacking.
Our hypothesis was that prior ICI treatment could improve efficacy of dacarbazine in advanced melanoma patients without brain metastasis. Our aim was to compare PFS, OS and response rates of dacarbazine with or without a previous treatment with ICI in patients with advanced melanoma, and to compare the tolerance of dacarbazine in both groups.
Patients and methods
Design and population
We conducted a retrospective study in our skin cancer referral centre. From the Pharmacy Department list of all consecutive patients treated with dacarbazine from June 2006 to September 2019, we included patients who had received ≥2 infusions of dacarbazine for an unresectable stage IIIC, IIID or IV cutaneous (or unknown) melanoma (uveal and mucosal melanoma excluded) according to American Joint Committee on Cancer (AJCC) 8th edition [16]. Patients with brain metastases before or at first day of dacarbazine were not included. Patients who received an adjuvant treatment with interferon α could be included, but no with ICI or BRAF plus MEK inhibitors.
Characteristics of included patients, data of primary melanoma and of dacarbazine tolerance were collected. Two groups of patients, according to whether they had received prior ICI therapy (ipilimumab, nivolumab and/or pembrolizumab) (ICI group) or not (non-ICI group, patients treated before the era of immunotherapy) were defined. Response to dacarbazine was assessed every 2 months by thoraco-abdominopelvic computerised tomography scans (CT-scans), brain CT-scans or magnetic resonance imaging according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) and classified in four categories: complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD) [17]. Adverse events of dacarbazine and ICI-induced adverse events were collected and graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Prior lines of treatment (and response data) with ICI (immune RECIST [18]: PD confirmed with 2 consecutive CT-scan 4 weeks apart), BRAF and/or MEK inhibitors (RECIST 1.1), radiotherapy or adjuvant therapy with interferon α were also collected. According to French Law, this study abided by standard medical practices and did not require a written informed consent. However, consent was obtained orally from all patients. In addition, patients gave written informed consent to participate in national French prospective cohorts of advanced melanoma (MelBase: NTC028228202, RIC-Mel: NCT03315468, MelanCohort). The study was conducted according to the principles of the declaration of Helsinki[19].
Statistical analysis
The primary endpoint was PFS, defined as the time from the beginning of treatment with dacarbazine to date of progression or death, whatever the cause. Secondary endpoints were OS, ORR defined as the proportion of patients who achieved a PR or CR as best overall response rate (BORR). BORR was defined as the best response recorded from the start of the treatment until the PD or death. Quantitative data were expressed as mean ± standard deviation, median and range, qualitative data as frequency and percentages. Comparisons of means were performed using the Student’s t-test and the Wilcoxon rank sum test as appropriate. Comparison of median was performed using the Mood’s Median test. Comparisons of frequencies were performed using the Chi-square test and the Fisher’s exact test as appropriate. Survival curves of PFS and OS were estimated for each group, considered separately, using the Kaplan–Meier method and compared statistically using the log rank test. For all tests, a P-value <0.05 was considered significant. Statistical analyses were performed using R software version 3.2.3 (http://www.r-project.org).
Results
Characteristics of patients
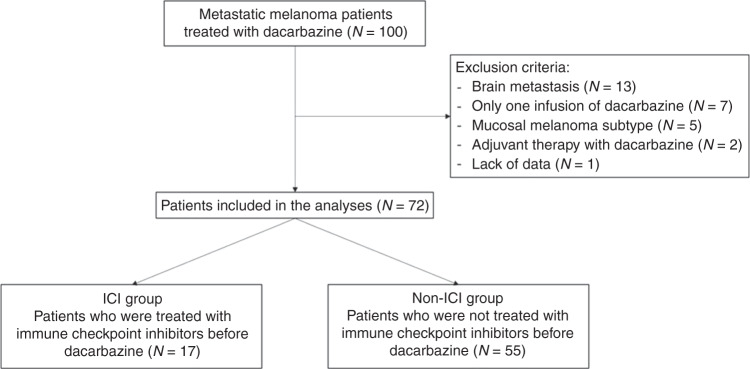
Among 100 consecutive patients treated for advanced melanoma with dacarbazine during the study period, 72 have been included in this study (Fig. 1). Characteristics of patients at the first day of dacarbazine are described in Table 1.
Fig. 1. Study flowchart.
ICI immune checkpoint inhibitors.
Table 1.
Characteristics of primary melanoma and of patients at Day 1 of dacarbazine.
| Total N = 72 | ICI group N = 17 | Non-ICI group N = 55 | P-values | |
|---|---|---|---|---|
| Breslow (mm, mean ± SD) | 3.8 ± 3.6 | 5.0 ± 3.2 | 3.5 ± 3.7 | 0.19 |
| Histological subtype | ||||
| SSM | 26 (36.1) | 4 (23.5) | 22 (40.0) | SSM, nodular or LMM versus other subtypes: 0.20 |
| Nodular melanoma | 14 (19.4) | 3 (17.6) | 11 (20.0) | |
| ACL | 6 (8.3) | 1 (5.9) | 5 (9.1) | |
| LMM | 2 (2.8) | 0 (0.0) | 2 (3.6) | |
| Unclassifiable | 4 (5.6) | 3 (17.6) | 1 (1.8) | |
| Unknown, no primitive | 20 (27.8) | 6 (35.3) | 14 (25.5) | |
| Age (years, mean ± SD) | 68.7 ± 17.8 | 66.7 ± 13.4 | 69.3 ± 19.0 | 0.55 |
| Patients of female gendre | 19 (26.4) | 6 (35.3) | 13 (23.6) | 0.36 |
| Stage AJCC 8th edition | ||||
| IIIC | 3 (4.2) | 0 (0.0) | 3 (5.5) | M1C versus other stages: 0.11 |
| IIID | 2 (2.8) | 1 (5.9) | 1 (1.8) | |
| IV M1A | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| IV M1B | 24 (33.3) | 3 (17.6) | 21 (38.2) | |
| IV M1C | 43 (59.7) | 13 (76.5) | 30 (54.5) | |
| LDH plasma level | ||||
| Normal | 29 (40.3) | 10 (58.8) | 19 (34.5) | Normal versus >ULN: 0.009 |
| >ULN | 18 (25.0) | 6 (35.3) | 12 (21.8) | |
| Not reported | 25 (34.7) | 1 (5.9) | 24 (43.6) | |
| ECOG performance status | ||||
| 0 | 47 (65.3) | 9 (52.9) | 38 (69.1) | ECOG > 1 versus ≤1: 0.47 |
| 1 | 13 (18.1) | 4 (23.5) | 9 (16.4) | |
| ≥2 | 12 (16.7) | 4 (23.5) | 8 (14.5) | |
| Number of metastases distant sites | ||||
| 0 | 5 (6.9) | 1 (5.9) | 4 (7.3) | ≥3 versus <3 sites: 0.03 |
| 1 | 15 (20.8) | 1 (5.9) | 14 (25.5) | |
| 2 | 17 (23.6) | 2 (11.7) | 15 (27.0) | |
| ≥3 | 35 (48.6) | 13 (76.5) | 22 (40.0) | |
| Distant metastases site | ||||
| Cutaneous or muscular | 21 (29.2) | 7 (41.2) | 14 (25.5) | |
| Node | 27 (37.5) | 12 (70.6) | 15 (27.3) | |
| Lung | 55 (76.4) | 13 (76.5) | 42 (76.4) | |
| Liver | 31 (43.1) | 8 (47.1) | 23 (41.8) | |
| Gastro-intestinal | 20 (27.8) | 8 (47.1) | 12 (21.8) | |
| Bone | 17 (23.6) | 5 (29.4) | 12 (21.8) | |
| Mutational status | ||||
| BRAFV600 | 10 (13.9) | 6 (35.3) | 4 (7.3) | |
| NRASQ61 | 10 (13.9) | 5 (29.4) | 5 (9.1) | |
| Wild-type | 14 (19.4) | 5 (29.4) | 9 (16.4) | |
| Not reported | 38 (52.2) | 1 (5.9) | 37 (67.3)a | |
| Dacarbazine line therapy | ||||
| First | 50 (69.4) | 0 (0.0) | 50 (90.9) | First line versus other lines: <0.0001 |
| Second | 9 (12.5) | 5 (29.4) | 4 (7.3) | |
| Third and more | 13 (18.1) | 12 (70.6) | 1 (1.8) | |
| Delay between Day 1 of dacarbazine and Day 1 of first-line therapy (months, median [range]) | 0 [0–48] | 12.5 [2.8–48.1] | 0 [0–14.3] | <0.0001 |
Unless specified, data are numbers (percentage).
SSM superficial spreading melanoma, ACL acral lentiginous melanoma, LMM lentigo maligna melanoma, SD standard deviation, LDH lactate dehydrogenase, ULN upper limit of normal, ECOG Eastern Cooperative Oncology Group.
aNot performed (before targeted therapy area).
In the ICI group, 17 patients (23.6%) received dacarbazine after one line (nine patients (52.9%): nivolumab or pembrolizumab monotherapy (N = 7), nivolumab plus ipilumumab (N = 2)) or two lines of ICI therapy (eight patients (47.1%): ipilimumab monotherapy and nivolumab or pembrolizumab (N = 7), nivolumab and then rechallenge with pembrolizumab after failure of targeted therapy (N = 1)). ICI were discontinued because of PD (N = 16) or toxicity (N = 1).
In the non-ICI group, 55 patients (76.3%) received dacarbazine without prior ICI therapy, in first-line in the majority of patients (N = 50; 90.9%), while it was in second (N = 5; 29.4%) or ≥third line (N = 12; 70.5%) in post-ICI patients (P < 0.0001) (Table 1). Prior treatments received before dacarbazine in both groups are summarised in Table 2. The median time from the Day 1 of the first-line therapy to the Day 1 of dacarbazine was 12.5 months [2.8–48.1] in ICI group versus 0.0 months [0.0–14.3] in non-ICI group (P < 0.0001). Significantly more patients from the ICI group had LDH superior to upper normal limit and ≥3 metastatic sites than from the non-ICI group (35.3% versus 21.8%, P = 0.009 and 76.5% versus 40.0%, P = 0.03, respectively. The proportion of patients with other pejorative prognostic factors was not statistically different in both groups (Table 1). The median delay between the last dose of ICI and the first dose of dacarbazine was 0.9 months [0.5–18.8]. To note, among 247 patients with advanced melanoma treated with ICI in our centre over the study period, 6.9% (17/247) had subsequent dacarbazine treatment.
Table 2.
Prior treatments received before dacarbazine in both groups.
| Total N = 72 | ICI group N = 17 | Non-ICI group N = 55 | |
|---|---|---|---|
| Adjuvant therapy | |||
| Interferon α | 12 (16.7) | 5 (29.4) | 7 (12.7) |
| Systemic metastatic therapy | |||
| Targeted therapy | |||
| BRAF+MEK inhibitors | 7 (9.7) | 5 (29.4)a | 2 (3.6) |
| BRAF inhibitor monotherapy | 2 (2.8) | 1 (5.9) | 1 (1.8) |
| MEK inhibitor monotherapy | 4 (5.6) | 2 (11.8) | 2 (3.6) |
| ICI | |||
| Nivolumab | 9 (12.5) | 9 (52.9) | 0 (0.0) |
| Pembrolizumab | 7 (9.7) | 7 (41.2) | 0 (0.0) |
| Ipilimumab | 7 (9.7) | 7 (41.2) | 0 (0.0) |
| Nivolumab + ipilimumab | 2 (2.8) | 2 (11.8) | 0 (0.0) |
| Radiotherapy | |||
| Hypofractionated | 16 (22.2) | 11 (64.7) | 5 (9.1) |
| Palliative | 6 (8.3) | 1 (5.9) | 5 (9.1) |
| Delay between radiotherapy and J1 Dacarbazine (months, median [range]) | 9,52 [2.01–24.73] | 8.42 [2.01–20.45] | 11.13 [3.03–24.73] |
Unless specified, data are numbers (percentage).
ICI immune checkpoint inhibitors.
aTwo patients received two lines of BRAF plus MEK inhibitors.
Progression-free survival
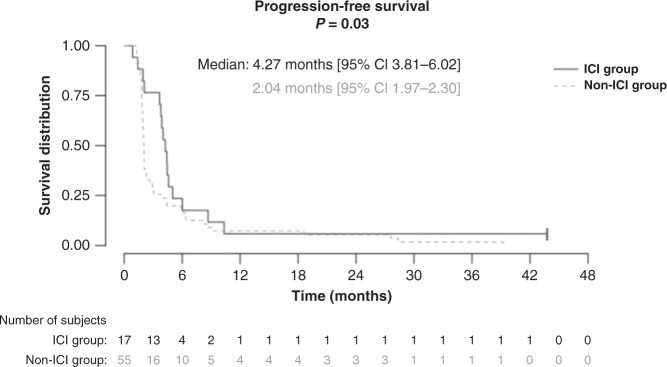
The median PFS was longer in the ICI group (4.27 months [95% CI 3.81–6.02]) than in the non-ICI group (2.04 months [95% CI 1.97–2.30], P = 0.03), (Fig. 2). The median number of dacarbazine cycles performed was 6 [2–23] in the ICI group versus 3 [2–25] in the non-ICI group (P = 0.13).
Fig. 2.
Progression-free survival of dacarbazine in metastatic melanoma patients pre-treated with immune checkpoint inhibitors (ICI group) or not (non-ICI group).
Secondary endpoints
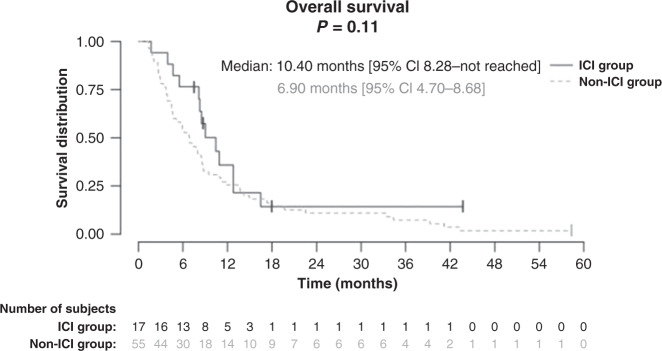
Median OS was not significantly different in both groups: 10.40 months [95% CI 8.28–not reached] and 6.90 months [95% CI 4.70–8.68], respectively, in the ICI group and non-ICI group (P = 0.11), (Fig. 3). The ORR was 35.3% in the ICI group versus 12.7% in the non-ICI group (P = 0.0007) (Table 3).
Fig. 3.
Overall survival of dacarbazine in metastatic melanoma patients pre-treated with immune checkpoint inhibitors (ICI group) or not (non-ICI group).
Table 3.
Response to dacarbazine in both groups.
| ICI group N = 17 | Non-ICI group N = 55 | P-values | |
|---|---|---|---|
| Best response | |||
| Complete response | 1 (5.9) | 2 (3.6) | |
| Partial response | 5 (29.4) | 5 (9.1) | |
| Stable disease | 7 (41.2) | 7 (12.7) | |
| Progressive disease | 4 (23.5) | 41 (74.5) | |
| Overall response rate | |||
| (complete + partial responses) | 6 (35.3) | 7 (12.7) | 0.0007 |
Unless specified, data are numbers (percentage).
ICI immune checkpoint inhibitors.
No difference was observed in the occurrence of adverse event in both groups (Table 4). We did not find any factor associated with a better PFS in the ICI group, such as the presence of severe immune-related events (≥grade 3 CTCAE 5.0) (P = 0.80) or objective response to ICI (P = 1) (data not shown).
Table 4.
Most common adverse events of dacarbazine in both groups.
| Total N = 72 | ICI group N = 17 (23.6) | Non-ICI group N = 55 (76.3) | P-values (All grades) | |
|---|---|---|---|---|
| Anemia | ||||
| All grades | 19 (26.4) | 7 (41.2) | 12 (21.8) | 0.13 |
| Grades 3/4 | 3 (4.2) | 1 (5.9) | 2 (3.6) | |
| Lymphopenia | ||||
| All grades | 17 (23.6) | 6 (35.3) | 11 (20.0) | 0.21 |
| Grades 3 | 5 (6.9) | 3 (17.6) | 2 (3.6) | |
| Neutropenia | ||||
| All grades | 16 (22.2) | 5 (29.4) | 11 (20.0) | 0.51 |
| Grades 3/4 | 7 (9.7) | 4 (23.5) | 3 (5.5) | |
| Thrombocytopenia | ||||
| All grades | 19 (26.4) | 5 (29.4) | 14 (25.5) | 0.76 |
| Grades 3/4 | 1 (1.4) | 0 (0.0) | 1 (1.8) | |
| Fatigue | ||||
| All grades | 27 (37.5) | 9 (52.9) | 18 (32.7) | 0.42 |
| Grades 3/4 | 8 (11.1) | 2 (11.8) | 6 (10.9) | |
| Nausea | ||||
| All grades | 17 (23.6) | 5 (29.4) | 12 (21.8) | 0.53 |
| Grades 3/4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Vomiting | ||||
| All grades | 12 (16.7) | 5 (29.4) | 7 (12.7) | 0.14 |
| Grades 3/4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Cholestasis | ||||
| All grades | 12 (16.7) | 5 (29.4) | 7 (12.7) | 0.14 |
| Grades 3/4 | 5 (6.9) | 1 (5.9) | 4 (7.3) | |
| Diarrhoea | ||||
| All grades | 5 (6.9) | 2 (11.8) | 3 (5.5) | 0.58 |
| Grades 3/4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
Unless specified, data are numbers (percentage).
ICI immune checkpoint inhibitors.
Discussion
Our study found a significantly better PFS from Day 1 of dacarbazine treatment in advanced melanoma patients without brain metastasis previously treated with ICI, as compared to control patients not previously treated with ICI. The ORR was also significantly higher in the ICI group despite a higher proportion of patients with poor prognostic factors (high plasma LDH level, number of metastatic sites) in ICI-pre-treated patients. Thus, a positive impact of ICI pre-treatment on anti-tumour efficacy of dacarbazine suggests an immune-related anti-tumour response of dacarbazine, which would be enhanced by a delayed effect of the immune therapy.
In clinical practice, chemotherapy is offered, despite the lack of evidence-based data in the literature, to patients with no other therapeutic options, still alive after a previous treatment with ICI (±BRAF plus MEK inhibitors), discontinued because of PD or toxicity.
In non-small cell lung cancer, Park et al. found in a controlled study better ORR in 73 patients receiving chemotherapy administered after anti-PD-1 or anti-PD-L1 mAb as compared to chemotherapy administered previously (53.4% versus 34.9%, respectively, P = 0.03) [12]. In melanoma, a study including 18 advanced melanoma patients treated with chemotherapy (including 10 with dacarbazine) after a previous treatment with ICI found a median OS of 12 months and a median PFS of 5.4 months. These figures are better than survivals usually reported with dacarbazine in this population of patients [15]. Also, a non-comparative German study found a disease control rate (=rate of CR+PR+SD) in 58 patients treated between 2007 and 2017 with chemotherapy (mostly dacarbazine) after ICI near 25%, this rate was superior to the one expected classically with dacarbazine [14]. In a non-comparative retrospective multicenter study, 463 metastatic melanoma patients treated with chemotherapy (including dacarbazine for 25%) after failing ICI were analysed, with a median PFS of 2.7 months and a median OS of 7.1 months. These results seem poorer than ours and may be explained by a higher delay between the last dose of ICI and the first dose of chemotherapy: 1.9 months versus 0.9 months in our study, and by unfavorable baseline patient characteristics (with 58% of ECOG > 1) [20]. It is important to note that in the non-ICI group, 43.6% of patients did not have reported LDH level recorded (versus 5.9% in the ICI group); thus, the significance of the observed difference may be questionable. However, a number ≥3 metastatic sites, another major prognostic factor, was also significantly more frequent in the ICI group than in the non-ICI group, without any missing data, suggesting that the significant result with higher LDH is likely to be admissible.
However, these series of reported cases lack a control group. Recently, Hadash-Bengad et al. found better outcomes in 35 metastatic melanoma patients receiving chemotherapy (including dacarbazine) when they were pre-treated with ICI, with a median PFS of 5.2 months versus 2.5 months, squaring with our results. Also, they focused on a chemotherapy-responsive patient, and analyzed his immune cell population: they showed an increased proportion of CD8+ cells, with elevated PD-1 and CD69 expression, during chemotherapy, as compared to ICI treatment time, suggesting immune-activation [21].
To our knowledge, our study is the first to compare chemotherapy efficacy, focusing on dacarbazine, in melanoma metastatic patients according to prior ICI use. In our study, the median PFS of 2.0 months found in the non-ICI group, with a majority treated as first line, was similar to the PFS reported with first-line dacarbazine in clinical trials [12, 22].
We cannot exclude that improved PFS observed in the ICI group is the consequence of an “immortal time bias”: [23] patients in our ICI group had a much longer follow-up from Day 1 of the first-line therapy to Day 1 of dacarbazine than those of the non-ICI group (P < 0.0001), because we selected patients still alive when they received dacarbazine. Indeed, a proportion of patients died before they could be included in this study: over the study period, among patients treated with ICI, with an estimated PD risk of 2/3 risk, only 6.9% were included on this study. Evolution kinetic profiles of patients could also be different between the two groups: patients from the ICI group had a much longer course from the diagnosis of metastatic disease than patients from the non-ICI group. We can assume that despite higher proportions of poor prognostic factors, patients from the ICI group had a slower progression profile that could explain better outcomes. Gaudy-Marqueste et al. reported that kinetics index, calculated from changes in total metastatic volume during the first three months of disease progression (before any treatment), is the best prognostic indicator in metastatic melanoma [24]. This value was not quantifiable in our study, due to its retrospective nature (no CT-scan three months before the first infusion of dacarbazine in first-line-treated patients). Thus, differences observed in evolution kinetic profiles in the two groups might explain the significant difference observed in PFS. However, we would also have expected a better OS if patients with slow kinetics were selected, but median OS was not significantly different in the two groups. Finally, we cannot rule out that the absence of significant difference might be due to the low number of patients, particularly in the group of patients pre-treated with ICI. Nevertheless, a possible difference in kinetic profiles between both groups cannot explain the best ORR rate observed in ICI-pre-treated patients. Ideally, prospective randomised data would be needed to answer to these questions. Moreover, the longer PFS observed in the ICI group in our study may be biased by the low number of patients in this group, and should be confirmed in a larger study.
We can hypothesise that the ORR and increased PFS observed represent a delayed effect of ICI mAB, but all patients except one, who withdraw ICI for toxicity, had switched to dacarbazine after experiencing PD on previous immune therapy, what goes against this hypothesis. Patients treated with ICI had a PD confirmed with 2 successive CT-scan 4 weeks apart before initiating dacarbazine, therefore the best PFS observed cannot be explained by pseudo-progression due to ICI. To note, 12 patients (70.6%) from the ICI group were treated with radiotherapy to enhance the efficacy of ICI [25, 26], and 10 patients in the non-ICI group (18.2%) also received radiotherapy several months before the first dose of dacarbazine (Table 2). In the ICI group the median delay between the last dose of ICI and the first dose of dacarbazine was 0.9 months [0.5–18.8]. We can hypothesise that ICI were still active when dacarbazine was introduced, the half-life of ICI being 25 days (nivolumab), 22 days (pembrolizumab) and 15 days (ipilimumab) resulting in an elimination time of 4.1 months, 3.6 months and 2.5 months, respectively.
The mechanisms linking chemotherapy with the immune system have been widely studied over the past decade. Chemotherapy is able to induce immunogenic cell death, which induces an expression of tumour antigens and the release of danger-associated molecular patterns in the tumour microenvironment, during cell death [27]. Therefore, the action of the immune system is essential to its effectiveness [28]. These discoveries have led to therapeutic advances: in patients with non-small cell lung cancer, the combination of platinum-containing chemotherapy and pembrolizumab is now used in first-line therapy, for certain histological subtypes [29]. It seems that this combination reduces the risk of hyperprogression but also benefits from a synergistic effect between chemotherapy and immunotherapy [30]. Other studies showed a benefit with the concomitant association of chemotherapy and immunotherapy in urothelial carcinomas and in head and neck cancers [31–33].
Concomitant combination of chemotherapy and immunotherapy has been rarely studied in first-line unresectable advanced melanoma. Indeed, hyperprogression seems to be infrequent in melanoma (around 10%) [34], the classical low efficacy of chemotherapy in melanoma patients may make it more difficult to highlight a synergistic effect. However, a phase 2 trial studying ipilimumab plus fotemustine combination on 86 advanced melanoma, including 20 patients with brain metastases, showed a disease control rate of 46.5% [35]. To our knowledge, the combination of chemotherapy and anti-PD-1 has never been studied in first-line metastatic melanoma.
Vera Aguilera et al. found, in a retrospective study about 60 patients with metastatic melanoma treated with chemo-immunotherapy after PD-1 inhibitor failure, a clinical outcomes improvement, compared to patients who received ICI or chemotherapy alone [36]. Nevertheless, combination of chemotherapy with immunotherapy should increase toxicity. In our study, as in the Vera Aguilera et al. study, we have not reported more severe adverse events in patients treated with the sequential treatment with ICI mAb followed by dacarbazine compared to patients treated with dacarbazine without a prior ICI therapy. To note, in our study, no hospitalisation for a severe adverse event with dacarbazine was observed in both groups. No randomised controlled trials have been conducted comparing a systemic therapy with placebo or best supportive care in metastatic cutaneous melanoma [37]. Thus, due to its acceptable tolerance profile, dacarbazine does not seem to affect quality of life, and can be offered to patients as an alternative to best supportive care when no clinical trial is available for ICI-resistant melanoma patients.
Conclusion
Our study shows, in a controlled setting, a modest but significant improvement of PFS and ORR in patients treated with dacarbazine after a previous treatment with ICI in comparison to the same chemotherapy regimen, without prior immunotherapy with ICI. These results confirm that dacarbazine, without any alternative available therapy, is still part of the therapeutic arsenal in order to treat patients with advanced melanoma after the failure of immune checkpoint blockers (±BRAF plus MEK inhibitors in BRAFV600-mutant melanoma), and could be offered before best supportive care in these late-stage melanoma patients. Moreover, this study highlights the hypothesis that ICI could enhance the efficacy of chemotherapy, which has been shown in other cancers but never in melanoma, classically resistant to chemotherapy. Our results have to be confirmed in larger studies, and prospective studies are required to clarify the mechanisms involved in the enhancement of chemotherapy efficacy thanks to the previous immunotherapy; the best sequence to achieve optimal anti-tumour effects remains to be studied.
Acknowledgements
Not applicable.
Author contributions
SB: Conceptualisation, Methodology, Data curation, Writing—Original draft preparation. AB: Formal analysis, Software. PS: Supervision. EFB: Conceptualisation, Methodology, Writing—Reviewing and Editing. LC, MF, TS, MC, LGL: investigation. CL, AB: Writing—review.
Funding information
None.
Data availability
The anonymised datasets analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
According to French Law, this study abided by standard medical practices and did not require a written informed consent. However, consent was obtained orally from all patients. In addition, patients gave written informed consent to participate in national French prospective cohorts of advanced melanoma (MelBase: NTC028228202, RIC-Mel: NCT03315468, MelanCohort). The study was conducted according to the principles of the declaration of Helsinki.
Competing interests
CL: Pierre Fabre, Novartis, Bristol-Myers Squibb and MSD (C/A). PS: Amgen, Bristol-Myers Squibb, MSD, Merck-Serono, Pfizer, Roche-Genentech, Pierre Fabre, and Novartis (C/A). EFB: Pierre Fabre, Novartis, Bristol-Myers Squibb and MSD (C/A). (C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Authors’ information (optional).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ugurel S, Röhmel J, Ascierto PA, Flaherty KT, Grob JJ, Hauschild A, et al. Survival of patients with advanced metastatic melanoma: the impact of novel therapies-update 2017. Eur J Cancer. 2017;83:247–57. doi: 10.1016/j.ejca.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 2.Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019;381:626–36. doi: 10.1056/NEJMoa1904059. [DOI] [PubMed] [Google Scholar]
- 3.Ascierto PA, Dummer R, Gogas HJ, Flaherty KT, Arance A, Mandala M, et al. Update on tolerability and overall survival in COLUMBUS: landmark analysis of a randomised phase 3 trial of encorafenib plus binimetinib vs vemurafenib or encorafenib in patients with BRAF V600-mutant melanoma. Eur J Cancer. 2020;126:33–44. doi: 10.1016/j.ejca.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Ribas A, Daud A, Pavlick AC, Gonzalez R, Lewis KD, Hamid O, et al. Extended 5-year follow-up results of a phase Ib study (BRIM7) of vemurafenib and cobimetinib in BRAF-mutant melanoma. Clin Cancer Res. 2020;26:46–53. doi: 10.1158/1078-0432.CCR-18-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30:582–8. doi: 10.1093/annonc/mdz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535–46. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 7.Maio M, Grob J-J, Aamdal S, Bondarenko I, Robert C, Thomas L, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol. 2015;33:1191–6. doi: 10.1200/JCO.2014.56.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreft S, Gesierich A, Eigentler T, Franklin C, Valpione S, Ugurel S, et al. Efficacy of PD-1-based immunotherapy after radiologic progression on targeted therapy in stage IV melanoma. Eur J Cancer. 2019;116:207–15. doi: 10.1016/j.ejca.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Schreuer MS, Chevolet IL, Jansen YJ, Seremet TC, Wilgenhof S, Liénard D, et al. Objective responses can be obtained by CTLA-4 inhibition in metastatic melanoma after BRAF inhibitor failure. Melanoma Res. 2015;25:68–74. doi: 10.1097/CMR.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 10.Seth R, Messersmith H, Kaur V, Kirkwood JM, Kudchadkar R, McQuade JL, et al. Systemic therapy for melanoma: ASCO Guideline. J Clin Oncol. 2020;38:3947–70. doi: 10.1200/JCO.20.00198. [DOI] [PubMed] [Google Scholar]
- 11.Robert C, Ribas A, Schachter J, Arance A, Grob J-J, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1239–51. doi: 10.1016/S1470-2045(19)30388-2. [DOI] [PubMed] [Google Scholar]
- 12.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 13.Park SE, Lee SH, Ahn JS, Ahn M-J, Park K, Sun J-M. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non–small cell lung cancer. J Thorac Oncol. 2018;13:106–11. doi: 10.1016/j.jtho.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Weichenthal M, Ugurel S, Leiter UM, Satzger I, Kähler KC, Welzel J, et al. Salvage therapy after failure from anti-PD-1 single agent treatment: a Study by the German ADOReg melanoma registry. J Clin Oncol. 2019;37:9505–9505. doi: 10.1200/JCO.2019.37.15_suppl.9505. [DOI] [Google Scholar]
- 15.Saint-Jean M, Fronteau C, Peuvrel L, Khammari A, Varey E, Quéreux G, et al. Chemotherapy efficacy after first-line immunotherapy in 18 advanced melanoma patients. Medicine. 2020;99:e21329. doi: 10.1097/MD.0000000000021329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gershenwald JE, Scolyer RA. Melanoma staging: American Joint Committee on Cancer (AJCC) 8th Edition and Beyond. Ann Surg Oncol. 2018;25:2105–10. doi: 10.1245/s10434-018-6513-7. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Hodi FS, Hwu W-J, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34:1510–7. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 20.Goldinger SM, Lo S, Hassel JC, Forschner A, McKean MA, Zimmer L, et al. The utility of chemotherapy after immunotherapy failure in metastatic melanoma: a multicenter case series. J Clin Oncol. 2018;36:e21588–e21588. doi: 10.1200/JCO.2018.36.15_suppl.e21588. [DOI] [Google Scholar]
- 21.Hadash-Bengad R, Hajaj E, Klein S, Merims S, Frank S, Eisenberg G, et al. Immunotherapy potentiates the effect of chemotherapy in metastatic melanoma—a retrospective study. Front Oncol. 2020;10:70. doi: 10.3389/fonc.2020.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hauschild A, Grob J-J, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 23.Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007;16:241–9. doi: 10.1002/pds.1357. [DOI] [PubMed] [Google Scholar]
- 24.Gaudy-Marqueste C, Archier E, Grob A, Durieux O, Loundou A, Richard M-A, et al. Initial metastatic kinetics is the best prognostic indicator in stage IV metastatic melanoma. Eur J Cancer. 2014;50:1120–4. doi: 10.1016/j.ejca.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Roger A, Finet A, Boru B, Beauchet A, Mazeron J-J, Otzmeguine Y, et al. Efficacy of combined hypo-fractionated radiotherapy and anti-PD-1 monotherapy in difficult-to-treat advanced melanoma patients. OncoImmunology. 2018;7:e1442166. doi: 10.1080/2162402X.2018.1442166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Funck-Brentano E, Baghad B, Fort M, Aouidad I, Roger A, Beauchet A, et al. Efficacy of late concurrent hypofractionated radiotherapy in advanced melanoma patients failing anti-PD-1 monotherapy. Int J Cancer. 2020;147:1707–14. doi: 10.1002/ijc.32934. [DOI] [PubMed] [Google Scholar]
- 27.Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L, et al. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008;15:3–12. doi: 10.1038/sj.cdd.4402269. [DOI] [PubMed] [Google Scholar]
- 28.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 29.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378:2078–92. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 30.Kim JY, Lee KH, Kang J, Borcoman E, Saada-Bouzid E, Kronbichler A, et al. Hyperprogressive disease during anti-PD-1 (PDCD1) / PD-L1 (CD274) therapy: a systematic review and meta-analysis. Cancers (Basel) 2019;11:1699. doi: 10.3390/cancers11111699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powles T, Gschwend JE, Loriot Y, Bellmunt J, Geczi L, Vulsteke C, et al. Phase 3 KEYNOTE-361 trial: pembrolizumab (pembro) with or without chemotherapy versus chemotherapy alone in advanced urothelial cancer. J Clin Oncol. 2017;35:TPS4590–TPS4590. doi: 10.1200/JCO.2017.35.15_suppl.TPS4590. [DOI] [Google Scholar]
- 32.Galsky MD, Necchi A, Sridhar SS, Ogawa O, Ruscica D, Angra N, et al. A phase III, randomized, open-label, multicenter, global study of first-line (1L) durvalumab in combination with standard of care (SoC) chemotherapy and durvalumab in combination with tremelimumab and soc chemotherapy versus soc chemotherapy alone in patients with unresectable locally advanced or metastatic urothelial cancer (UC) J Clin Oncol. 2019;37:TPS499–TPS499. doi: 10.1200/JCO.2019.37.7_suppl.TPS499. [DOI] [Google Scholar]
- 33.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–28. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 34.Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23:4242–50. doi: 10.1158/1078-0432.CCR-16-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Giacomo AM, Ascierto PA, Pilla L, Santinami M, Ferrucci PF, Giannarelli D, et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol. 2012;13:879–86. doi: 10.1016/S1470-2045(12)70324-8. [DOI] [PubMed] [Google Scholar]
- 36.Vera Aguilera J, Paludo J, McWilliams RR, Zhang H, Li Y, Kumar AB, et al. Chemo-immunotherapy combination after PD-1 inhibitor failure improves clinical outcomes in metastatic melanoma patients. Melanoma Res. 2020;30:364–75. doi: 10.1097/CMR.0000000000000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crosby T, Fish R, Coles B, Mason M. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev. 2000;CD001215. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymised datasets analysed during the current study are available from the corresponding author on reasonable request.