Abstract
Objective:
To determine the incidence of psoriatic arthritis (PsA) in a US population and describe trends in incidence and mortality over 5 decades.
Methods:
The previously identified population-based cohort of Olmsted County, Minnesota residents ≥18 years of age who fulfilled PsA criteria during 1970–1999 was extended to include patients with incident PsA in 2000–2017. Age- and sex-specific incidence rates and point prevalence, adjusted to 2010 US white population, were reported.
Results:
There were 164 incident cases of PsA in 2000–17, with a mean age of 46.4 (SD=12.0) years and 47% females. The overall age- and sex-adjusted annual incidence of PsA per 100,000 population was 8.5 (95% CI 7.2–9.8), and higher in males (9.3, 95% CI 7.4–11.3) than females (7.7, 95% CI 5.9–9.4) in 2000–2017. Overall incidence was highest in the age range 40–59 years. The incidence rate was relatively stable in 2000–2017 with no evidence of increase overall or in males, but a modest increase of 3% per year in females, compared to 1970–1999 where a 4%/yr rise in incidence was observed. Point prevalence was 181.8 per 100,000 (95% CI 156.5–207.1) in 2015. The percentage of females increased from 39% in 1970–1999 and 41% in 2000–2009 to 54% in 2010–2017 (p=0.08). Overall survival in PsA did not differ from general population (SMR= 0.85, 95% CI 0.61–1.15).
Conclusion:
The incidence of PsA in this predominantly white US population was stable in 2000–2017 in contrast to previous years. However, an increasing proportion of females was found in this study.
Keywords: Psoriatic arthritis, spondyloarthritis, incidence, prevalence, mortality
INTRODUCTION
Psoriatic arthritis (PsA) is a chronic, progressive inflammatory musculoskeletal disease that can lead to serious joint damage and disability. There is significant variability in the reported prevalence and incidence rates of PsA across studies. The estimated prevalence of PsA ranges from 20 to 670 per 100,000 in Sweden (1) and Norway (2) respectively, and the incidence varies from 0.1 to 43.1 per 100,000 in Japan (3) and Norway (2) respectively. Variability in the estimates may also be related to differences in case ascertainment. While the lowest estimates were derived from studies using International Classification of Disease ninth edition (ICD-9) codes to identify PsA cases, the highest prevalence was described in studies that used self-reported diagnosis of PsA. (4,5) Although the development of ClASsification of Psoriatic ARthritis (CASPAR) criteria in 2006 has created some uniformity in epidemiological studies, (6) not all observational studies have used the criteria. Moreover, there are few population-based studies on the epidemiology of PsA, and temporal trends in the incidence of PsA in the US are unclear. While a previous study from Olmsted County reported increasing incidence of PsA from 1970 to 1999, (7) studies of other populations in more recent years have reported discrepancies in trends of PsA incidence. Knowing the recent epidemiology of PsA will help predict the actual burden of disease in the US and allocation of resources.
The objectives of our study were to 1) assess the annual incidence of PsA in 2000–2017 and examine time trends in incidence of PsA in 1970–2017 in Olmsted County, Minnesota, US; 2) estimate the point prevalence of PsA in 2015; and 3) assess mortality rates in patients with PsA.
METHODS
Study design.
This was a retrospective, population-based study from the Olmsted County, Minnesota using the data resources of the Rochester Epidemiology Project (REP).
Setting.
The population of Olmsted County, Minnesota, in which resides the city of Rochester, is well suited for investigation of the epidemiology of PsA as comprehensive medical records for all residents seeking medical care for over five decades are available. A record linkage system allows ready access to the medical records from all healthcare providers for the local population, including the Mayo Clinic, the Olmsted Medical Center and their hospitals, local nursing homes, and the few private practitioners. (8,9) This system ensures virtually complete ascertainment of all clinically recognized cases of PsA among the residents of Olmsted County, Minnesota. The population of Olmsted County in 2010 was 144,248 with 74.7% being adults of age ≥18 years. Patients who denied authorization to use their medical records for research were excluded. The study was approved by the Mayo Institutional Review Board (IRB) and the Olmsted Medical Center IRB (18-010851 and 051-OMC-18).
Study population and case ascertainment.
PsA cases were defined as patients ≥18 years fulfilling CASPAR criteria for PsA (sensitivity of 91.4% and specificity of 98.7%). (6) ICD-9/10 diagnostic codes for arthralgias, arthritis, monoarthritis, oligoarthritis, polyarthritis, spondylitis, ankylosing spondylitis, arthropathy, psoriatic arthropathy, spondyloarthropathy, and seronegative spondyloarthropathy were used to screen for patients with PsA. Medical record review of all potential cases was performed to ascertain fulfillment of the CASPAR criteria from January 1, 2000 to December 31, 2017. Questionable cases were resolved by mutual agreement between study investigators. Date of fulfillment of CASPAR criteria was considered as the incidence date of PsA. All patients were followed until December 31, 2019. The previously described 1970–1999 PsA cohort also used the CASPAR criteria. (7)
Data collection.
Complete medical records from all healthcare providers were identified and reviewed for each patient using a standardized, pre-tested data abstraction form (with the same definitions as used in the previous study from REP (7) for consistency). Information regarding demographics, clinical characteristics, laboratory data, and radiographic features was collected. Psoriasis was defined by documentation in the medical records either by a dermatologist or rheumatologist. Date of psoriasis diagnosis was taken as the date of established diagnosis of psoriasis by dermatology or rheumatology.
Statistical analysis.
Age- and sex-specific incidence rates were calculated by using the number of incident cases as the numerator and REP census estimates as the denominator. Overall incidence rates were age- and sex-adjusted to the 2010 white population of the US. In order to compute 95% confidence intervals (95% CI) for incidence and prevalence rates, it was assumed that the number of incident cases followed a Poisson distribution. Trends in incidence rates were examined using Poisson regression methods with smoothing splines for age and calendar year. The annual incidence rates were graphically illustrated using a 3-year, centered, moving average to reduce the random fluctuations over time. The point prevalence of PsA in 2015 was determined using the number of prevalent cases on January 1, 2015 as the numerator and the Olmsted County population based on the REP census in 2015 as the denominator. Mortality rates following the diagnosis of PsA were estimated using Kaplan-Meier methods, and were compared to the expected survival rates in the Minnesota white population. The standardized mortality ratio (SMR) was estimated as the ratio of the observed and expected number of deaths. Ninety-five percent confidence intervals for the SMR were calculated assuming that the expected rates are fixed and the observed rates followed a Poisson distribution. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
A total of 484 Olmsted County residents with a potential diagnosis of PsA were identified. Among them, 164 patients aged ≥18 years of age fulfilled CASPAR criteria between January 1, 2000 to December 31, 2017. Remaining cases were excluded for the following reasons: did not fulfill CASPAR criteria (175, 36%), prevalent PsA subjects with disease onset outside of Olmsted County or disease onset before the study period (113, 23%), individuals who were not residents of Olmsted County (7, 1.5%), refusal of authorization to used medical records for research (6, 1.2%), missing history (9, 2%), PsA diagnosis after study period (2, 0.4%), and other arthritis diagnoses, n=8 (2%). Among those that fulfilled the CASPAR criteria (N=164), two patients did not have a physician diagnosis of PsA. A rheumatologist made the diagnosis of PsA for 160 patients, and the remaining two patients received a confirmatory diagnosis of PsA by internal medicine physicians. Both of them clearly met CASPAR criteria and had characteristic DIP erosions on radiographs. Among the patients that did not fulfill CASPAR criteria, none had a confirmatory diagnosis of PsA as per rheumatology evaluation.
Mean age of PsA patients in this cohort was 46.4 (±12.0) years, and 47% were females (Table 1). The majority of patients were Caucasians (87%), and 42% patients had a college degree. The percentage of females increased from 39% in 1970–1999 and 41% in 2000–2009 to 54% in 2010–2017 (p=0.08). The mean (SD) body mass index (BMI) of PsA patients was 30.9 (7.1) (Table 1).
Table 1.
Baseline characteristics of incident psoriatic arthritis patients between January 1, 2000, and December 31, 2017
| Characteristic | Total (N=164) |
|---|---|
| Age at incidence, Mean (±SD) yrs | 46.4 (12.0) |
| Sex, female | 77 (47%) |
| Race | |
| Black | 4 (2%) |
| Asian | 8 (5%) |
| White | 141 (87%) |
| Hispanic | 9 (6%) |
| Other/Mixed | 1 (1%) |
| Unknown | 1 |
| Education Level | |
| < High school graduate | 3 (2%) |
| High school graduate/<4yr college | 92 (56%) |
| ≥4yr college degree | 68 (42%) |
| Missing | 1 |
| BMI (kg/m 2 ), Mean (±SD) | 30.9 (7.1) |
| Missing | 9 |
| MSK symptom duration in years before physician diagnosis, median (IQR) | 2.5 (0.5–7.3) |
| Inflammatory joint pain distribution | |
| Upper limbs only | 58 (36%) |
| Lower limbs only | 40 (25%) |
| Upper & lower limbs | 64 (40%) |
| Missing | 2 |
| PsA joint symmetry at first diagnosis | |
| Asymmetrical | 133 (82%) |
| Symmetrical | 29 (18%) |
| Missing | 2 |
| DIP involvement | 53 (32%) |
| Enthesopathy | 50 (30%) |
| Dactylitis | 72 (44%) |
| Inflammatory back pain | 18 (11%) |
| Uveitis | 7 (4%) |
| Inflammatory bowel disease | 1 (1%) |
| Psoriasis (Current or personal) | |
| Current psoriasis | 150 (91%) |
| Personal history of psoriasis | 7 (4%) |
| No psoriasis | 7 (4%) |
| Family history of psoriasis | 60 (45%) |
| Missing | 31 |
| Psoriatic nail dystrophy (Current or past) | 75 (50%) |
| Missing | 15 |
| Rheumatoid factor negative | 139 (96%) |
| Not performed | 19 |
| Radiographic damage | 49 (30%) |
| Erosions at DIP joint | 21 (13%) |
| Joint erosions at sites other than DIP | 10 (6%) |
| Periosteal reaction | 11 (7%) |
| Juxta-articular bony proliferation | 8 (4%) |
| Symmetric sacroiliitis | 9 (5%) |
| Unilateral sacroiliitis | 2 (1%) |
| Osteolysis | 2 (1%) |
Clinical characteristics of incident PsA.
Among the 164 incident PsA patients, there was predominantly asymmetric joint involvement (82%), and distal interphalangeal joint (DIP) involvement was seen in 53 (32%) patients. A total of 50 (30%) patients had enthesopathy, 72 (44%) dactylitis, and 18 (11%) inflammatory back pain at or prior to the diagnosis of PsA. The most common sites of enthesopathy were plantar fascia (18%), lateral epicondyle (6%) and Achilles tendon (8%). A few patients presented with enthesopathy at multiple sites (7%). The median musculoskeletal symptom duration before physician diagnosis (162 patients) was 2.5 (IQR 0.5–7.3) years.
Psoriasis was present in 150 (91%) patients at diagnosis, 7 (4%) had a personal history of psoriasis, and 60 (45%) had a family history of psoriasis. Psoriatic nail dystrophy at or before the diagnosis of PsA was present in 75 (50%) patients. Seven (4%) patients had a history of uveitis, and 1 (1%) patient had inflammatory bowel disease.
Rheumatoid factor (RF) was negative in 139 (96%) of 145 patients in whom this was tested. Radiographic damage was noted in 49 (30%) patients; 21 (13%) had erosions at DIP, 11 (7%) had periosteal reaction, 10 (6%) had joint erosions at sites other than DIP, 11 (7%) had sacroiliitis, 8 (4%) had juxta-articular bony proliferation, and osteolysis was seen in 2 (1%) patients.
Incidence of PsA in the general population.
The overall age- and sex-adjusted annual incidence of PsA in 2000–2017 per 100,000 population was 8.5 (95% CI 7.2–9.8), and higher in males (9.3, 95% CI 7.4–11.3) than females (7.7, 95% CI 5.9–9.4) (Table 2). Overall incidence was highest in the age range 40–59 years. Among females, the annual incidence was highest in 50–59 years of age and no incident cases were seen after the sixth decade. The number of incident cases declined significantly after the sixth decade in males as well.
Table 2.
Annual incidence (per 100,000) of psoriatic arthritis by age and sex between January 1, 2000, and December 31, 2017, in Olmsted County, Minnesota.
| Male | Female | Total | ||||
|---|---|---|---|---|---|---|
| Age Group, yrs | N | Rate | N | Rate | N | Rate |
| 18–29 | 10 | 4.6 | 4 | 1.6 | 14 | 3.0 |
| 30–39 | 21 | 11.7 | 15 | 7.8 | 36 | 9.7 |
| 40–49 | 24 | 13.9 | 25 | 13.4 | 49 | 13.7 |
| 50–59 | 21 | 13.5 | 27 | 15.6 | 48 | 14.6 |
| 60–69 | 7 | 6.9 | 6 | 5.3 | 13 | 6.1 |
| 70–79 | 3 | 5.0 | 0 | 0.0 | 3 | 2.2 |
| 80+ | 1 | 3.0 | 0 | 0.0 | 1 | 1.1 |
| Total (95% CI) | 87 | 9.3 (7.4–11.3)† | 77 | 7.7 (5.9–9.4)† | 164 | 8.5 (7.2–9.8)†† |
Age-adjusted to the 2010 US White population.
Age- and sex-adjusted to the 2010 US White population
The overall incidence rate was relatively stable in the years 2000–2017 compared to 1970–1999. In the years 1970–1999, a significant increase in PsA incidence of 4% per year (RR 1.04, 95%CI 1.02–1.06) was seen. This rise in incidence was similar in both sexes (interaction p=0.81). In 2000–2017, there was no evidence of increase in PsA incidence overall (RR 1.01, 95% CI: 0.98–1.04) or in males (RR 0.98, 95% CI: 0.94–1.02), but in females a 3% per year increase in incidence (RR 1.03, 95% CI: 0.99–1.08) was observed that did not reach statistical significance (Figure 1). Likewise, the test for a sex difference in the incidence trends for PsA did not reach statistical significance (interaction p=0.10). However, significant age by calendar year trends in incidence rates were noted for females (p=0.004), but not for males (p=0.37). Among females, the incidence of PsA increased over time primarily in ages 40–49 and 50–59 years, declined in ages 70–79 and 80+ years, and was relatively stable over time in the remaining age groups (Figure 2A). The peak age at incidence for females was 50–59 years beginning in 1990. Among males, the increase in PsA incidence prior to 2000 was primarily in the 30–39, 40–49 and 50–59 year age groups. The peak age at incidence for males shifted from 30–39 years in 1980 to 40–49 years in 2010. The change in age at diagnosis over time is further demonstrated in Figure 3 wherein linear relationships between calendar year and age were noted for both sexes with similar increases in the mean age at diagnosis of PsA in both sexes of 1.9 years per decade of calendar time (p=0.009). Similarly, there was no significant change in overall BMI or BMI in males or females from 2000–2017 (Figure 4).
Figure 1.
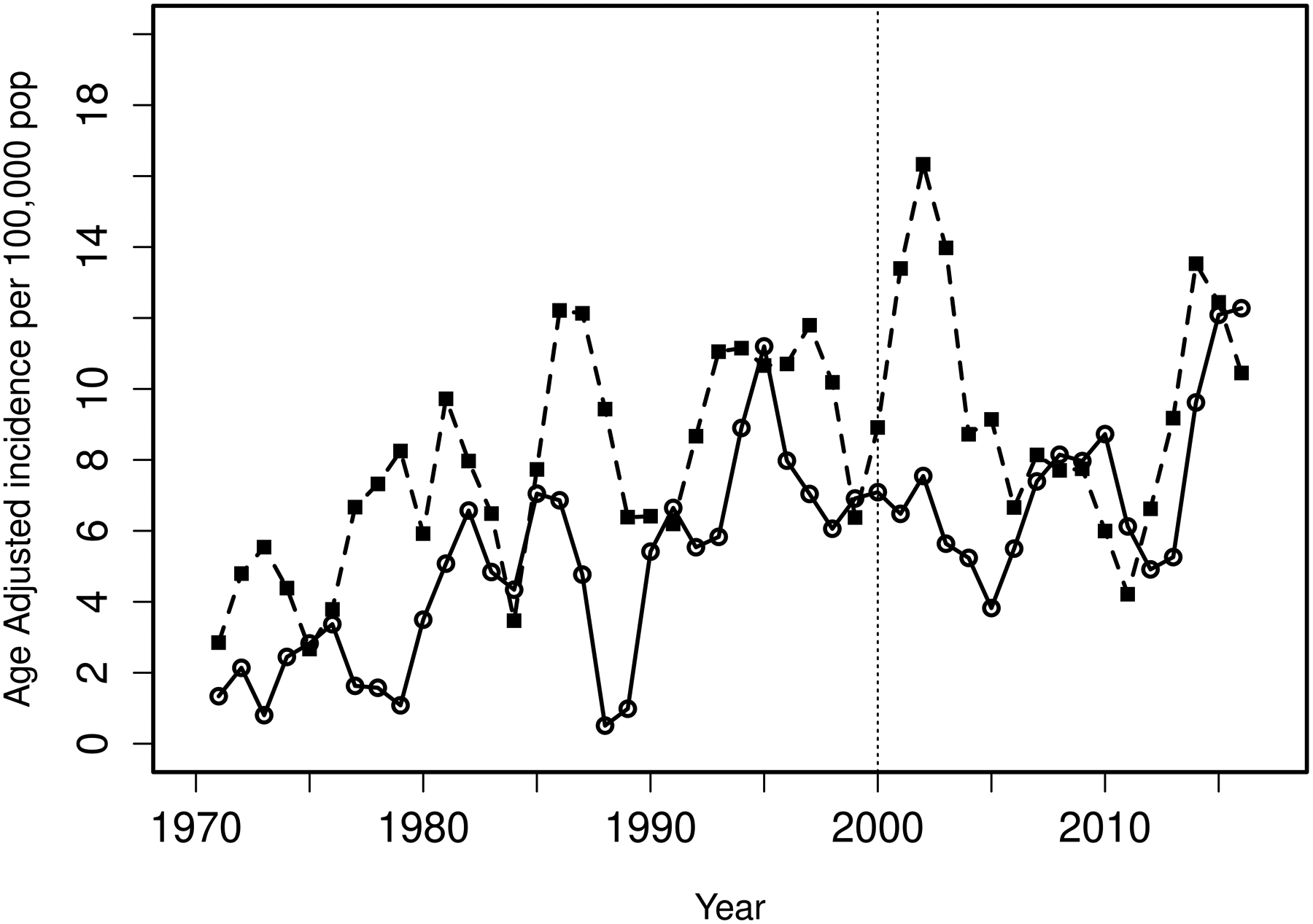
Age-adjusted incidence (per 100,000) over calendar year for psoriatic arthritis from 1970–2017 using 3 year moving averages by sex (males=dashed line and black squares, females=solid line and open circles) based on data from 299 incident cases (170 males and 129 females) of psoriatic arthritis in 1970–17.
Figure 2.
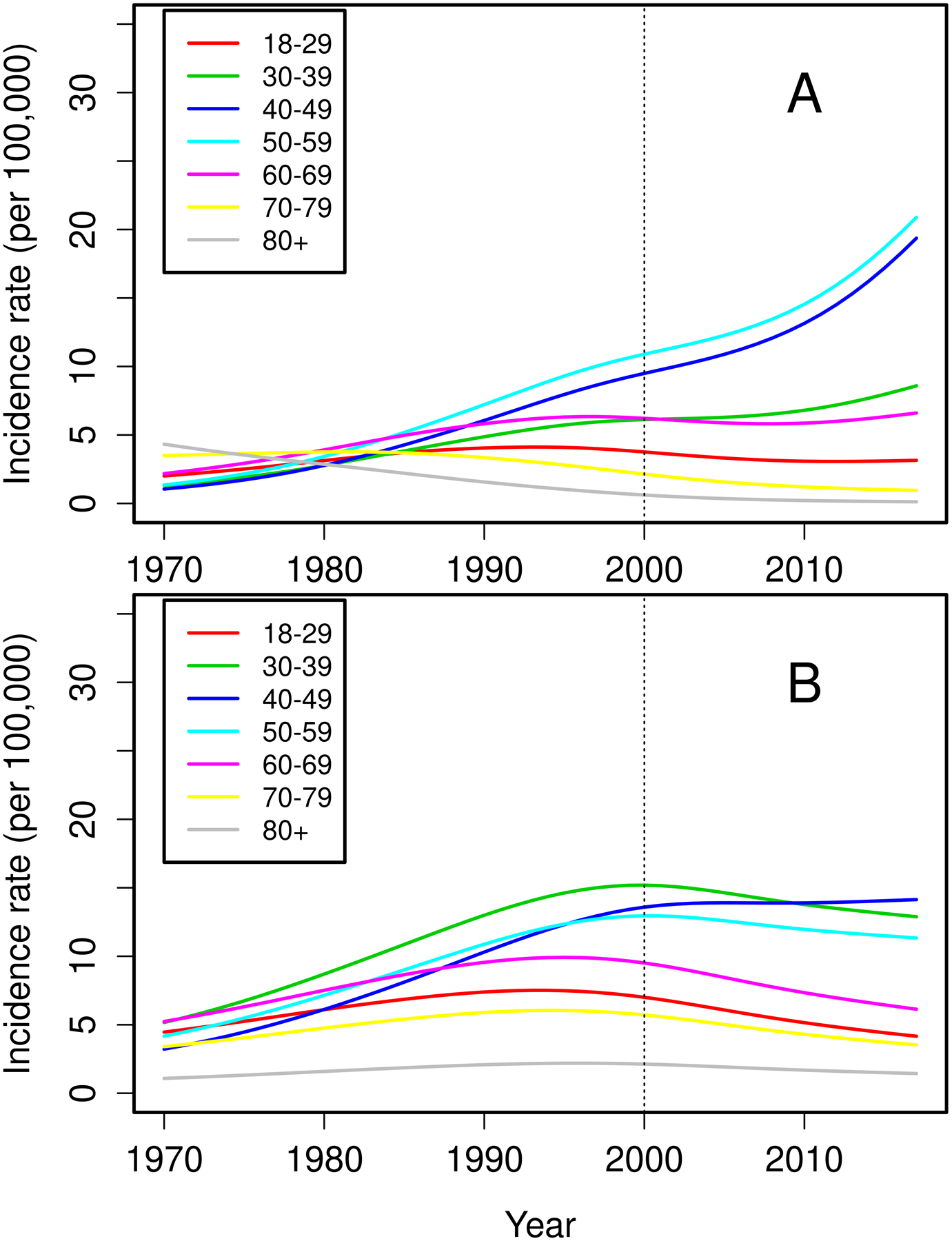
Trends in incidence of psoriatic arthritis among residents of Olmsted County, Minnesota in 1970–2017 for 129 females (panel A) and 170 males (panel B) according to age groups.
Figure 3.
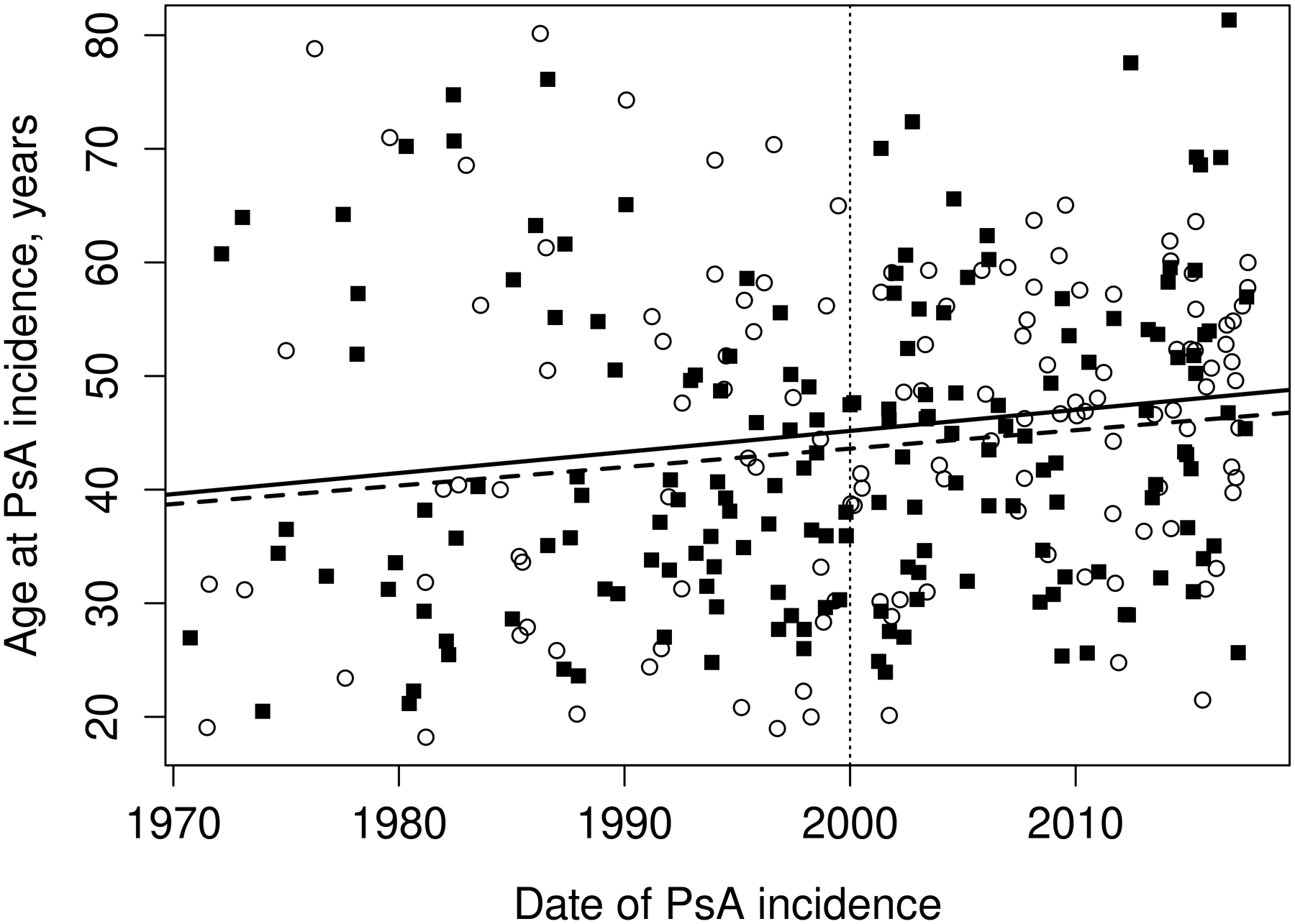
Trends in age at diagnosis of psoriatic arthritis among residents of Olmsted County, Minnesota in 1970–2017 by sex: males (dashed line and black squares) and females (solid line and open circles).
Figure 4.
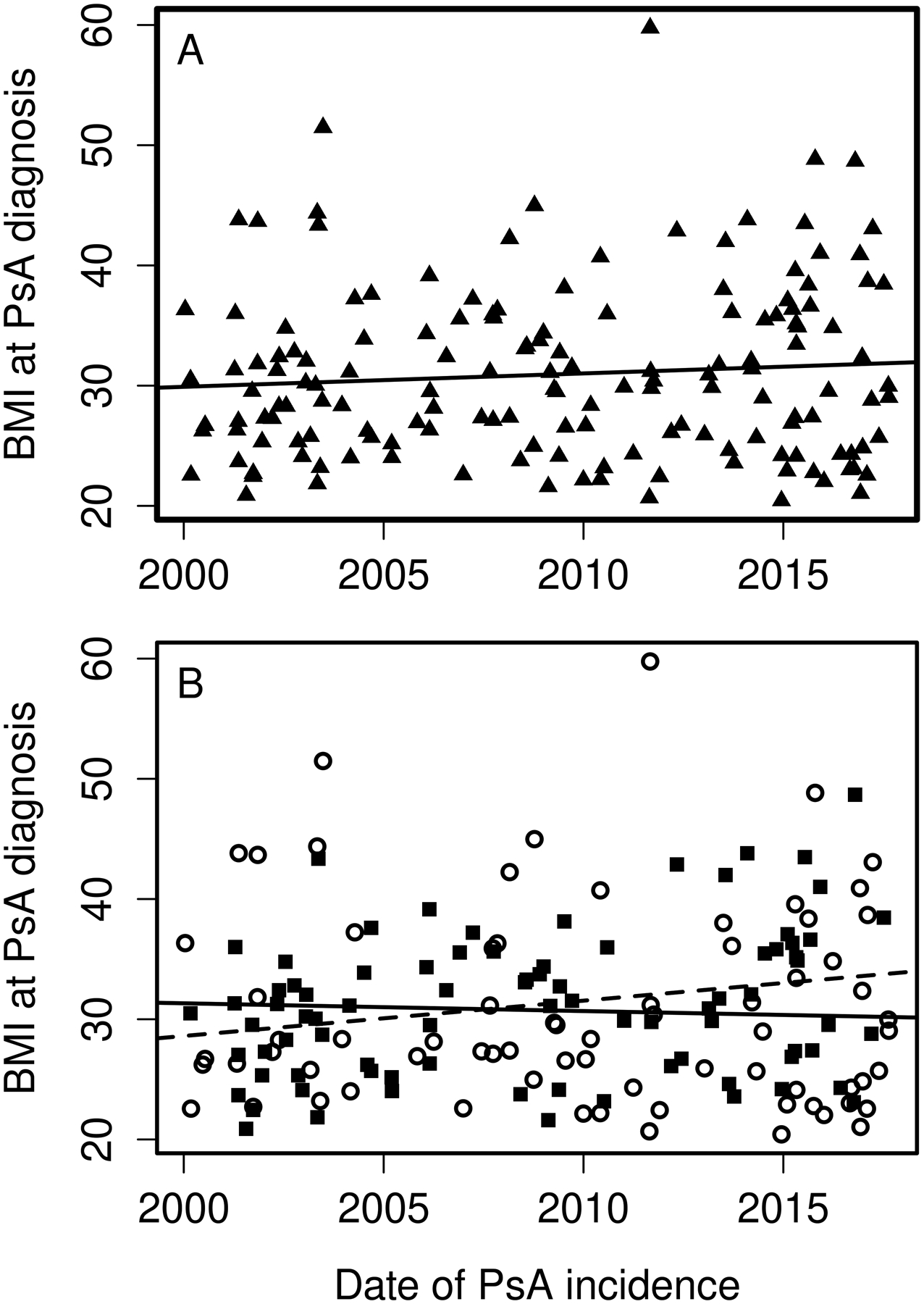
Trends in body mass index (BMI) at PsA diagnosis from 2000–2017 overall (panel A) and by sex (panel B): males (dashed line and black squares) and females (solid line and open circles).
Prevalence of PsA in the general population.
There were 200 Olmsted County residents (116 males and 84 females) with prevalent PsA on January 1, 2015.The overall estimated point prevalence per 100,000 was 181.8 (95% CI 156.5–207.1). The prevalence per 100,000 was 225.5 (95% CI 184.2–266.7) for males and 140.2 (95% CI 110.1–170.3) for females.
Mortality in PsA.
During a median of 13 years of follow-up (4607 total person-years), 40 patients with incident PsA in 1970–2017 died. Overall survival in PsA patients did not differ from that of the general population, with a standardized mortality ratio of 0.85 (95% CI 0.61–1.15). No significant changes in mortality over time were observed.
DISCUSSION
Our study showed an estimated incidence 8.5 per 100,000 over the study period (2000–2017) and a prevalence of 181.8 per 100,000 (Jan 1, 2015). In contrast to the previous increasing trends seen from 1970–1999, the incidence of PsA was stable in the years 2000–2017; and the proportion of women increased over time.
While there is limited data on incidence of PsA in the US for comparison, the incidence in our study is consistent with that reported in a recent meta-analysis (8.26 per 100,000). (10) Our study results are similar to the incidence estimates of 6.0 to 8.0 per 100,000 from most European countries. (11–15) However, the reported incidence varies widely with the geographic region. Compared to our study, incidence (per 100,000) was lower in Greece (3.02) (16) and Czech-republic (3.60) (17), and higher in Israel (10.9) (18), Canada (13–15) (19), Finland (23.10) (20) and Norway (43.10) (2). The overall incidence trend was stable from 2000–2017 in our study, which is consistent with that seen in Ontario, Canada (2008–2015) (19) and Israel (2006–2015) (18). In contrast, studies from Denmark (1997–2011) (21) and Taiwan (2000–2013) (22) report increasing incidence during slightly earlier years. A similar increasing incidence was noted in the years 1970–1999 in our population. (7) It is unclear if there has been a similar change in incidence rates in more recent years in these countries. The initial increase in reported estimates could have been secondary to increased recognition of disease over time, after which the rates have been steady. However, an actual change in rates over time is possible, and it is unclear if changes in therapies of psoriasis over the last decade have impacted the incidence of PsA.
Similarly, limited data exists on the age- and sex-stratified incidence estimates. We observed increases in the mean age at diagnosis of PsA over time in both sexes. Interestingly, our study also showed a modest increase in incidence among women during recent years, specifically in age range of 40–59 years. A similar increase in the proportion of women was also noted in Denmark (21) and Taiwan (22). Moreover, a similar rise in incidence in women from 40–59 years was observed in Denmark. (23) In Israel, however, both sexes had a similar degree of increase in incidence over time (2006–2015). (18) Higher proportion of females (56%) was noted in our study in the years 2010–2017. While Denmark (1998–2010) (21) had a similar female predominance; higher incidence in men was observed in Norway (1978 −1996) (14), Argentina (2000 –2006) (15), and Czech republic (2002–2003) (17). Data from different time periods might have led to the disparate results. In fact, previous study from Olmsted County (1970–1999) also reported a higher incidence of PsA in men (61%). (7) Since the population of Olmsted County is relatively stable, without any major changes in the demographics or major population shifts, the differences are unlikely to be secondary to changes in population characteristics. (8,9)
The prevalence of PsA seen in our study was higher than that reported in the meta-analysis (133 per 100,000). (10) Data from earlier years (1961 to 2012) in the meta-analysis might have contributed to the difference. The reported prevalence also varies widely with the geographic location. In the US, prevalence estimates range from 6 per 100,000 in a study using International Classification of Disease (ICD-9) codes (5) to 25 per 100,000 in studies using self-reported diagnosis of PsA (4). In Europe, the prevalence ranges from 50 to 210 per 100,000 in Turkey (24) and Sweden (25), respectively. Prevalence is much lower in Asia: 0.1, 2 and 4 per 100,000 in Japan (3), China (26) and Taiwan (22), respectively. Our findings of increased overall prevalence (15% increase from 2000 (7) to 2015) are consistent with recent studies from Canada and Asia. (18,19,22) Increased awareness of PsA, introduction of CASPAR criteria in 2006, and increased use of advanced imaging (e.g., ultrasound and magnetic resonance imaging) could have contributed to the increased prevalence. The prevalence of PsA was higher in men in 2015. Similar higher male prevalence was also observed in Norway (1978 to 1996) (14) and Argentina (2000 to 2006) (15). In contrast, female predominance was noted in Denmark (59% in 2010) (21) and Czech republic (2002–2003) (17). Therefore, while studies agree on the increasing prevalence over time, there is disparity in the proportion of male and females.
The difference in incidence and prevalence estimates across different geographic regions could be due to different data collection periods, underdiagnoses (in Asia), or genetic and environmental differences. (10,27) Geographic region and ethnicity have been shown to have an impact on the prevalence, clinical manifestation, and prognosis of SpA. (28) Differences in distribution of human leukocyte-associated antigens (HLA) and other genetic determinants across ethnic groups, could account for the disparity even within the same subcontinent. (29,30) Being Scandinavian in ancestry, the estimates from Olmsted County may be closer to the higher estimates from the Nordic countries, and higher than other parts of the US. (25,31,32) Prevalence of psoriasis also differ across different geographical regions, and higher the number of psoriasis cases higher the expected prevalence of PsA. (33) Similarly, higher prevalence of obesity, hyperlipidemia, and smoking, which are strong risk factors for PsA, could account for higher prevalence of PsA in North America. (34)
Additionally, methodological differences among studies including use of different criteria sets, ICD codes, self-reported patient diagnosis, likely account for the differences. (4,5) Most studies used diagnostic coding algorithms and the presence of arthritis in patients with psoriasis as case definition of PsA. Others used a diagnosis of psoriasis plus arthritis, and older criteria such as the European Spondyloarthropathy Study Group (ESSG), Moll and Wright, and Vasey and Espinoza, which have shown inadequate sensitivity and specificity for PsA. (10,35) Only a few studies, including the previous study from Olmsted County, used CASPAR criteria. (2,7,15,26,36)
Overall mortality in PsA was similar to the general population, with no significant changes in mortality over time. These results are consistent with data from population-based studies, including the previous Olmsted County study (7) and recent data from the UK The Health Improvement Network (37). Increased mortality risk seen in some of the previous clinic and hospital-based studies could reflect selection bias capturing more active or severe PsA. (38)
The strengths of our study include the unique record linkage system of the REP, allowing near complete ascertainment of all clinically recognizable PsA cases in a well-defined population. Furthermore, case ascertainment used the validated CASPAR criteria with detailed review of the medical records. The study includes trends of PsA over nearly half a century; and provides a unique picture of how the epidemiology of PsA has changed over time in a stable population-based setting. Our study also provides information on the clinical and radiographic features of PsA at diagnosis. Radiographs were collected in all patients at baseline. Radiographic joint damage was similar to that described in early PsA patients (39) and slightly lower than in the Toronto PsA cohort (mean disease duration= 9 years) (40).
Our study has several limitations. First, PsA cases presenting with minimal skin disease may have been missed and misclassified as undifferentiated arthritis or peripheral spondyloarthritis. Also patients with mild PsA may have never presented to the rheumatologist and subsequently never diagnosed as PsA, which may have resulted in an underestimation of PsA incidence. The sensitivity of CASPAR criteria is about 91%, and cases not fulfilling CASPAR criteria were excluded. (6) Community physicians may not appropriately characterize joint pain as inflammatory, which is required in the CASPAR criteria. However, due to the extended study period and availability of near-complete medical history in this population, we believe most PsA cases were ascertained. Second, axial radiographs were performed only if clinically indicated, and not routinely in all patients with PsA. Therefore, asymptomatic patients were likely missed and axial involvement is under-represented. Up to 42% of PsA patients had axial involvement on plain radiographs in a prospective cross-sectional study. (41) Similarly, a specific enthesitis index wasn’t collected. However, the percentage of patients with enthesopathy and dactylitis were similar to that reported in longitudinal PsA cohorts. (42,43) Third, being a retrospective study, the usual limitations regarding completeness of medical record documentation apply. Lastly, the population of Olmsted County, Minnesota is predominantly white (~90%), which may limit the generalizability of study results to other racial/ethnic groups.
In conclusion, our study found stable incidence of PsA in recent years. However, an increasing proportion of females was found in this study. Further work is needed to determine the role of sex hormones, gene expression and other mechanisms underlying these changes.
Funding info:
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676, and Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Karmacharya is supported by T32 AR56950 grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases for the Musculoskeletal Research Training Program, and Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) pilot research grant. Dr. Duarte-García is supported by the Centers for Disease Control and Prevention, Rheumatology Research Foundation Scientist Development Award, the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, the Women's Health Career Enhancement Award, and the Eaton Family Career Development Award.
Disclosure of interests:
Dr. Ogdie has served as a consultant for AbbVie, Amgen, BMS, Celgene, Corrona, Gilead, Janssen, Lilly, Novartis, Pfizer, and UCB (less than 10,000 each) and has received grants from Novartis and Pfizer to Penn and from Amgen to Forward (grants more than 10,000). Dr. Davis has received consulting fees and/or honoraria from AbbVie and Sanofi-Genzyme (less than $10,000 each) and research support from Pfizer. Dr. Ernste has received research support from Octapharma and Genentech (less than $10,000 each). No other disclosures relevant to this article.
Footnotes
Ethical approval information: The study was approved by the Mayo Institutional Review Board (IRB) and the Olmsted Medical Center IRB (18-010851 and 051-OMC-18).
Data sharing statement: Access to the complete de-identified data can be made available following approval. Requests for additional study related data can be sent to Paras Karmacharya at paraskarmacharya@gmail.com
REFERENCES
- 1.Hellgren L Association between rheumatoid arthritis and psoriasis in total populations. Acta Rheumatol Scand 1969;15:316–326. [DOI] [PubMed] [Google Scholar]
- 2.Hoff M, Gulati AM, Romundstad PR, Kavanaugh A, Haugeberg G. Prevalence and incidence rates of psoriatic arthritis in central Norway: data from the Nord-Trøndelag health study (HUNT). Ann Rheum Dis 2015;74:60–64. [DOI] [PubMed] [Google Scholar]
- 3.Hukuda S, Minami M, Saito T, Mitsui H, Matsui N, Komatsubara Y, et al. Spondyloarthropathies in Japan: nationwide questionnaire survey performed by the Japan Ankylosing Spondylitis Society. J Rheumatol 2001;28:554–559. [PubMed] [Google Scholar]
- 4.Gelfand JM, Gladman DD, Mease PJ, Smith N, Margolis DJ, Nijsten T, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol 2005;53:573. [DOI] [PubMed] [Google Scholar]
- 5.Asgari MM, Wu JJ, Gelfand JM, Salman C, Curtis JR, Harrold LR, et al. Validity of diagnostic codes and prevalence of psoriasis and psoriatic arthritis in a managed care population, 1996–2009. Pharmacoepidemiol Drug Saf 2013;22:842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–2673. [DOI] [PubMed] [Google Scholar]
- 7.Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Time trends in epidemiology and characteristics of psoriatic arthritis over 3 decades: a population-based study. J Rheumatol 2009;36:361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc 2012;87:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, Pankratz JJ, Brue SM, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scotti L, Franchi M, Marchesoni A, Corrao G. Prevalence and incidence of psoriatic arthritis: A systematic review and meta-analysis. Semin Arthritis Rheum 2018;48:28–34. [DOI] [PubMed] [Google Scholar]
- 11.Kaipiainen-Seppänen O, Aho K. Incidence of chronic inflammatory joint diseases in Finland in 1995. J Rheumatol 2000;27:94–100. [PubMed] [Google Scholar]
- 12.Söderlin MK, Börjesson O, Kautiainen H, Skogh T, Leirisalo-Repo M. Annual incidence of inflammatory joint diseases in a population based study in southern Sweden. Ann Rheum Dis 2002;61:911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen OBV, Svendsen AJ, Ejstrup L, Skytthe A, Junker P. The occurrence of psoriatic arthritis in Denmark. Ann Rheum Dis 2008;67:1422–1426. [DOI] [PubMed] [Google Scholar]
- 14.Nossent JC, Gran JT. Epidemiological and clinical characteristics of psoriatic arthritis in northern Norway. Scand J Rheumatol 2009;38:251–255. [DOI] [PubMed] [Google Scholar]
- 15.Soriano ER, Rosa J, Velozo E, Schpilberg M, Imamura PM, Diaz J, et al. Incidence and prevalence of psoriatic arthritis in Buenos Aires, Argentina: a 6-year health management organization-based study. Rheumatol Oxf Engl 2011;50:729–734. [DOI] [PubMed] [Google Scholar]
- 16.Alamanos Y, Papadopoulos NG, Voulgari PV, Siozos C, Psychos DN, Tympanidou M, et al. Epidemiology of psoriatic arthritis in northwest Greece, 1982–2001. J Rheumatol 2003;30:2641–2644. [PubMed] [Google Scholar]
- 17.Hanova P, Pavelka K, Holcatova I, Pikhart H. Incidence and prevalence of psoriatic arthritis, ankylosing spondylitis, and reactive arthritis in the first descriptive population-based study in the Czech Republic. Scand J Rheumatol 2010;39:310–317. [DOI] [PubMed] [Google Scholar]
- 18.Eder L, Cohen AD, Feldhamer I, Greenberg-Dotan S, Batat E, Zisman D. The epidemiology of psoriatic arthritis in Israel - a population-based study. Arthritis Res Ther 2018;20:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eder L, Widdifield J, Rosen CF, Cook R, Lee K-A, Alhusayen R, et al. Trends in the prevalence and incidence of psoriasis and psoriatic arthritis in Ontario, Canada: A population-based study. Arthritis Care Res 2018. [DOI] [PubMed] [Google Scholar]
- 20.Savolainen E, Kaipiainen-Seppänen O, Kröger L, Luosujärvi R. Total incidence and distribution of inflammatory joint diseases in a defined population: results from the Kuopio 2000 arthritis survey. J Rheumatol 2003;30:2460–2468. [PubMed] [Google Scholar]
- 21.Egeberg A, Kristensen LE, Thyssen JP, Gislason GH, Gottlieb AB, Coates LC, et al. Incidence and prevalence of psoriatic arthritis in Denmark: a nationwide register linkage study. Ann Rheum Dis 2017;76:1591–1597. [DOI] [PubMed] [Google Scholar]
- 22.Wei JC-C, Shi L-H, Huang J-Y, Wu X-F, Wu R, Chiou J-Y. Epidemiology and Medication Pattern Change of Psoriatic Diseases in Taiwan from 2000 to 2013: A Nationwide, Population-based Cohort Study. J Rheumatol 2018;45:385–392. [DOI] [PubMed] [Google Scholar]
- 23.Egeberg A, Kristensen LE. Impact of age and sex on the incidence and prevalence of psoriatic arthritis. Ann Rheum Dis 2018;77:e19. [DOI] [PubMed] [Google Scholar]
- 24.Cakır N, Pamuk ÖN, Derviş E, Imeryüz N, Uslu H, Benian Ö, et al. The prevalences of some rheumatic diseases in western Turkey: Havsa study. Rheumatol Int 2012;32:895–908. [DOI] [PubMed] [Google Scholar]
- 25.Löfvendahl S, Theander E, Svensson Å, Carlsson KS, Englund M, Petersson IF. Validity of diagnostic codes and prevalence of physician-diagnosed psoriasis and psoriatic arthritis in southern Sweden--a population-based register study. PloS One 2014;9:e98024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li R, Sun J, Ren L-M, Wang H-Y, Liu W-H, Zhang X-W, et al. Epidemiology of eight common rheumatic diseases in China: a large-scale cross-sectional survey in Beijing. Rheumatol Oxf Engl 2012;51:721–729. [DOI] [PubMed] [Google Scholar]
- 27.Yang Q, Qu L, Tian H, Hu Y, Peng J, Yu X, et al. Prevalence and characteristics of psoriatic arthritis in Chinese patients with psoriasis. J Eur Acad Dermatol Venereol JEADV 2011;25:1409–1414. [DOI] [PubMed] [Google Scholar]
- 28.Lau CS, Burgos-Vargas R, Louthrenoo W, Mok MY, Wordsworth P, Zeng QY. Features of spondyloarthritis around the world. Rheum Dis Clin North Am 1998;24:753–770. [DOI] [PubMed] [Google Scholar]
- 29.Bowcock AM, Cookson WOCM. The genetics of psoriasis, psoriatic arthritis and atopic dermatitis. Hum Mol Genet 2004;13Spec No 1:R43–55. [DOI] [PubMed] [Google Scholar]
- 30.Tam L-S, Leung Y-Y, Li EK. Psoriatic arthritis in Asia. Rheumatol Oxf Engl 2009;48:1473–1477. [DOI] [PubMed] [Google Scholar]
- 31.Melton LJ. History of the Rochester Epidemiology Project. Mayo Clin Proc 1996;71:266–274. [DOI] [PubMed] [Google Scholar]
- 32.Madland TM, Apalset EM, Johannessen AE, Rossebö B, Brun JG. Prevalence, disease manifestations, and treatment of psoriatic arthritis in Western Norway. J Rheumatol 2005;32:1918–1922. [PubMed] [Google Scholar]
- 33.Parisi R, Symmons DPM, Griffiths CEM, Ashcroft DM, Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013;133:377–385. [DOI] [PubMed] [Google Scholar]
- 34.Scher JU, Ogdie A, Merola JF, Ritchlin C. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol 2019;15:153–166. [DOI] [PubMed] [Google Scholar]
- 35.Taylor WJ, Marchesoni A, Arreghini M, Sokoll K, Helliwell PS. A comparison of the performance characteristics of classification criteria for the diagnosis of psoriatic arthritis. Semin Arthritis Rheum 2004;34:575–584. [DOI] [PubMed] [Google Scholar]
- 36.Dönmez S, Pamuk ÖN, Akker M, Ak R. Clinical features and types of articular involvement in patients with psoriatic arthritis. Clin Rheumatol 2015;34:1091–1096. [DOI] [PubMed] [Google Scholar]
- 37.Ogdie A, Maliha S, Shin D, Love TJ, Baker J, Jiang Y, et al. Cause-specific mortality in patients with psoriatic arthritis and rheumatoid arthritis. Rheumatol Oxf Engl 2017;56:907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong K, Gladman DD, Husted J, Long JA, Farewell VT. Mortality studies in psoriatic arthritis: results from a single outpatient clinic. I. Causes and risk of death. Arthritis Rheum 1997;40:1868–1872. [DOI] [PubMed] [Google Scholar]
- 39.Anon. A Prospective, Clinical and Radiological Study of Early Psoriatic Arthritis: An Early Synovitis Clinic Experience - PubMed. Available at: https://pubmed.ncbi.nlm.nih.gov/14523223/.AccessedJuly 7, 2020. [DOI] [PubMed]
- 40.Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA)--an analysis of 220 patients. Q J Med 1987;62:127–141. [PubMed] [Google Scholar]
- 41.Jadon DR, Sengupta R, Nightingale A, Lindsay M, Korendowych E, Robinson G, et al. Axial Disease in Psoriatic Arthritis study: defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann Rheum Dis 2017;76:701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.A P, S L, V C, Dd G. Clinical Enthesitis in a Prospective Longitudinal Psoriatic Arthritis Cohort: Incidence, Prevalence, Characteristics, and Outcome. Arthritis Care Res 2017;69. Available at: https://pubmed.ncbi.nlm.nih.gov/27998023/?from_single_result=27998023&show_create_notification_links=False.AccessedJuly 7, 2020. [DOI] [PubMed] [Google Scholar]
- 43.Brockbank JE, Stein M, Schentag CT, Gladman DD. Dactylitis in psoriatic arthritis: a marker for disease severity? Ann Rheum Dis 2005;64:188–190. [DOI] [PMC free article] [PubMed] [Google Scholar]


