Abstract
This study aimed to investigate the potential of Mangifera indica L. seed kernel extract, which is highly discarded by the global food processing industry, as a multifunctional bioactive ingredient for nutraceutical and cosmeceutical applications. Different extracting solvents were utilized, the extracts were then tested for their antioxidant activities using DPPH, ABTS radical scavenging assays, and inhibition of lipid peroxidation. Additionally, total phenolic content (TPC), total flavonoid content (TFC), and gallic acid content were elucidated using Folin–Ciocalteu and aluminum chloride colorimetric assays, as well as high performance liquid chromatography. The hydroethanolic extract (KMHE) exhibited the highest percentage yield, with the highest antioxidant activity owing to its high phenolic content. KMHE consisted of 773.66 ± 9.42 mg GAE/g extract in TPC, 36.20 ± 4.20 mg RU/g extract in TFC. Additionally, gallic acid was shown to be a major constituent of KMHE. KMHE was investigated for anti-tyrosinase, anti-hyaluronidase, anti-MMP-2, and anti-MMP-9 activities. Moreover, the anti-inflammatory effects of KMHE were studied in RAW 264.7 cells induced by nitric oxide and KMHE was shown to prevent DNA damage, indicating an inhibitory effect on cellular aging. KMHE showed outstanding anti-tyrosinase activity and was as potent an anti-hyaluronidase as gallic acid. Additionally, our results reveal notable anti-MMP-2 and anti-MMP-9 effects that were not significantly different from those of gallic acid. Furthermore, KMHE demonstrated 61.54 ± 2.39% nitric oxide inhibition, with no cytotoxic effects, in RAW264.7 cells, and also prevented DNA damage in the human fibroblast BJ cell line with no cytotoxic effects. Therefore, KMHE could be a promising, natural multifunctional bioactive compound for nutraceutical and cosmeceutical applications.
Keywords: Mangifera indica L. kernel, Matrix metalloproteinases, Anti-tyrosinase, Anti-hyaluronidase, Anti-inflammatory , DNA damage
Introduction
Skin aging has been reported to have an enormous influence on psychological status and quality of life [1]. Various factors participate in skin aging; the clinical manifestations of intrinsic skin aging, in which degenerative physiological processes are inherently involved, include dry and pale skin with the presence of fine wrinkles, while the manifestations of extrinsic aging or photoaging, mainly attributed to exposure to UV radiation and pollution, include deep wrinkles, irregular pigmentation, and the loss of elasticity with a rough appearance, known as sagging [1–3]. Free radicals or reactive oxygen species (ROS) are considered one of the most important initiators of both intrinsic and extrinsic skin aging [3, 4]. Indeed, numerous ROS are fundamentally generated through diverse sources during intrinsic aging, such as chain reactions within mitochondria, Fenton reactions, and several enzyme reactions [1]. Furthermore, harmful ROS are primarily generated during exposure to UV-irradiation or environmental pollution [4]. Subsequently, various physiological tissue reactions in which ROS participate are highly propagated, including lipid peroxidation, DNA damage, inflammation, hyperpigmentation, and the upregulation of degradative enzymes, including matrix metalloproteinases (MMP) and hyaluronidase (HAase), all of which contribute to the clinical signs of skin deterioration [1–3]. Accordingly, when attempting to establish the anti-aging capability of compounds, performing in-depth evaluations of all these corresponding reactions is greatly important. Currently, the use of naturally occurring anti-aging compounds is substantially increasing, as these products are thought to be safer than synthetic substances and possess vivid effectiveness [5].
Mango (Mangifera indica L.), a tropical fruit belonging to the Anacardiaceae family, is widely consumed around the world. The vast diversity of mango cultivars is attributed to the unique growth environment characteristics of each cultivar [6, 7]. Mango and processed mango products are the third most exported product of Thailand, with exportation accounting for 209.6 million US dollars in 2016–2017 [8]. The Kaew-Kamin cultivar, which originated from Cambodia, is widely consumed and grown in all regions of Thailand as the fruits can be harvested throughout the year. Additionally, industrial investments in the processing of this cultivar have been substantially increased by ASEAN, contributing great reinforcement from the Thai government. As a result of this increased processing, huge amounts of seed kernel are discarded each year. Although mango seed kernel is a waste-product derived from the mango processing industry, it has been recognized as a valuable source of antioxidant components, especially polyphenols, which confer several beneficial human health effects [9–11]. Our previous study reported free-radical scavenging and anti-inflammatory effects of mango seed kernels of the Kiew-Moragot cultivar, with potential to alleviate the progression of acne vulgaris [11]. However, thus far, no evidence of biological activity relating to anti-aging effects has been identified from mango seed kernels derived from the Kaew-Kamin cultivar.
Consequently, the aims of the present study were to evaluate the biological activities and chemical constituents of mango seed kernel extract from the Kaew-Kamin cultivar relating to the prevention of skin aging, including free radical scavenging ability, attenuation of lipid peroxidation, and anti-enzymatic activities against tyrosinase, HAase, and MMPs. Moreover, the seed kernel extract was studied for anti-inflammatory effects and ability to prevent DNA damage in skin cell lines, as well as cytotoxicity. These findings could identify a food by-product with promising multifunctional bioactive ingredients for nutraceutical and cosmeceutical applications.
Materials and methods
Chemical materials
The solvents, used for extraction and analysis, including hexane, ethyl acetate, ethanol, and dimethylsulfoxide (DMSO), were of analytical grade. The solvents used for high performance thin layer chromatography (HPLC), such as acetonitrile, methanol, and formic acid, were HPLC grade. 1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2,2′-Azino-bis (3-ethyl benzothiazoline-6-sulphonic acid) (ABTS), linoleic acid, 2,2′-Azobis (2-amidonopropane) dihydrochloride (AAPH), ammonium thiocyanate, tyrosinase from mushroom, and l-tyrosine were purchased from Fluka (Buchs, Switzerland). Hyaluronidase derived from bovine testis (E.C.3.2.1.3.5), gallic acid (GA), l-ascorbic acid, and Trolox were purchased from Sigma-Aldridge Inc. (Schnelldorf, Germany). Dulbecco’s modified eagle medium (DMEM), Roswell Park Memorial Institute (RPMI) 1640 Medium, 10% Fetal bovine serum (FBS), and 1% penicillin–streptomycin (100 U/ml) were purchased from Gibco® (Grand Island, NY, USA).
Plant materials
Mango seed kernels of Kaew-Kamin cultivars were provided by a mango processing industry in Phayao province, Thailand. The seed shells covering the kernels were completely removed and the kernels were then dried overnight at 50 °C using a hot-air oven prior to extraction. Next, the dried kernels were ground into a fine powder and the moisture content, solvent extractive value, total ash, and acid insoluble ash were authenticated. We found that the quality of the crude kernels was acceptable according to the standards stated in the Thai Herbal Pharmacopoeia 2018 [12].
Plant extraction
Two different procedures, traditional maceration and solvent fractionation, were performed for extracting the dried mango seed kernels. Briefly, prior to the extraction, wax components were disposed through the seed kernel using hexane maceration for 48 h in three cycles. Next, the wax-free residue was macerated separately in 95% ethanol and 50% ethanol for 48 h in three cycles. The 95% ethanol filtrate was evaporated using a rotary evaporator (Buchi® Rotavapor R-300, Thailand) to give KME (crude ethanolic extract). Meanwhile, the 50% ethanolic extract solution was initially evaporated to remove the ethanol and then spray-dried in a Buchi® Mini Spray dryer B-290 (Thailand) using a small amount of maltodextrin as a carrier, with inlet and outlet temperatures of 140 °C and 100 °C, respectively, to give KMHE (hydroethanolic extract).
In the case of solvent fractionation, the wax-free residues were initially soaked in ethyl acetate followed by 95% ethanolic solution using the same procedure described previously for maceration. The rotary evaporator was also used to generate concentrated extracts of KMEA (ethyl acetate fraction) and KMEF (ethanolic fraction).
Determination of total phenolic content
Total phenolic content of the extracts was examined using Folin-Ciocalteu’s method and expressed in terms of mg gallic acid equivalents (GAE)/g extract. According to the previous procedure of Poomanee et al. [11], each extract in ethanolic solution was mixed homogeneously with Folin-Ciocalteu’s reagent and 7.5% w/v sodium carbonate (Na2CO3) and incubated for 30 min at room temperature. The absorbance of each sample was measured using a UV–VIS spectrophotometer (Shimadzu, UV-2450, Japan) at 765 nm. The gallic acid calibration curve, as a correlation between absorbances of gallic acid (Y) and concentrations (X), demonstrated an equation of Y = 0.00746X + 0.00309 with an excellent coefficient of determination (R2) of 0.9995.
Determination of total flavonoid content
The modified procedure of Poomanee et al. [11] was conducted for determination of total flavonoid content. Briefly, the extract in ethanolic solution (1 ml) was mixed with DI water (10 ml), 5% w/v sodium nitrite (NaNO2) (0.3 ml), and 10% aluminum chloride (AlCl3). The reaction mixture was then incubated for 5 min, afterwhich, 1 M sodium hydroxide (NaOH) (2 ml) was added. The absorbance of the mixtures was then examined at 510 nm and calculated in terms of mg rutin equivalents (RE)/g extract. The rutin calibration curve, as a correlation between absorbances of rutin (Y) and concentrations (X), showed an equation of Y = 0.0022X + 0.0166 with an excellent coefficient of determination (R2) of 0.9971.
Determination of antioxidant activities
DPPH radical scavenging assay
The free radical scavenging ability of the extracts were determined using DPPH assay following the previous method of Poomanee et al. [11]. Each extract, as well as the positive control, was dissolved in ethanolic solution and serially diluted to yield five final concentrations in the range of 3.125–100 µg/ml. The reaction mixture, consisting of the extract solution with 120 mM DPPH solution in a dilution factor of 1:10, was light-protected and incubated at room temperature for 30 min. Subsequently, the absorbance was measured using a microplate reader (SpectroSTAR Nano®, Ortenberg, Germany) at 520 nm. The scavenging ability was expressed to terms of percent (%) inhibition and 50% inhibitory concentration (IC50) as compared to gallic acid and Trolox as positive controls.
ABTS radical scavenging assay
Additionally, an ABTS scavenging assay was performed to ensure precise reporting of the free radical scavenging properties of the extracts in a term of Trolox equivalent antioxidant capacity (TEAC) [13]. ABTS assay was performed according to the methods of Poomanee et al. [11]. Briefly, ABTS radicals were initially generated through the addition of 7 mM ABTS solution to 140 mM potassium persulfate (K2S2O8) and incubating the mixture at room temperature for 16 h in the dark. The ethanolic solutions of each extract, in concentration ranges from 3.00 to 49.50 µg/ml, were mixed with the diluted ABTS radical solution in a ratio of 1:100 and incubated at room temperature for 6 min. The absorbance of each sample was measured using a microplate reader (SpectroSTAR Nano®, Ortenberg, Germany) at 734 nm. The TEAC of each extract was calculated according to a Trolox equivalent curve constructed using the relationship between absorbances (Y) and Trolox concentrations (X).
Inhibition of lipid peroxidation by linoleic acid thiocyanate method
The inhibitory effects on lipid peroxidation of the extracts were evaluated using the linoleic acid thiocyanate method, following the method of Poomanee et al. [11]. Each extract was serially diluted using 70% v/v methanolic solution to give five final concentrations in the range of 0.09–1.50 mg/ml. The lipidic mixture consisted of 1.3% w/v linoleic acid, phosphate buffer (PBS) pH 7.0, DI water, and the extract solution. An oxidative reaction was then generated by adding 46.35 mM AAPS solution into the lipidic mixture and the solution was incubated in the dark for 4 h at 45 °C. Subsequently, the inhibitory effects on linoleic acid peroxidation of the extracts were determined using the ferric thiocyanate method; results were expressed in terms of % inhibition and IC50 via comparison to gallic acid and Trolox as positive controls.
Determination of gallic acid content of the extract
The fingerprint of each extract, including its active compound content, were quantified using HPLC following a previously described procedure [11]. In brief, the Inertsil ODS-3 reverse phase C-18 column (5 µm, 4.6 × 250 mm, GL Science Inc., USA) served as a stationary phase at a modified column temperature of 25 °C. Gradient elution of the mobile phase, consisting of 0.5% acetic acid:acetonitrile (1:1) (solvent A) and 1% acetic acid (solvent B) in DI water was conducted as follows: 0–2 min, 5% of A; 2–15 min, 5–26% of A; 15–21 min, 26–35% of A; 21–30 min, 35–44% of A, using a wavelength detection of 280 nm. The extract was dissolved in absolute methanol to give a final concentration of 2500 mg/l. A gallic acid standard curve describing the area under the curve (mAU: Y) as a function of gallic acid concentrations (X) was constructed to calculate the gallic acid content of the extracts, which was expressed in terms of percentage of gallic acid using the following equation: Y = 68.54X − 12.08; R2 = 0.9994.
The utilized HPLC procedure was formerly validated for ability to verify limit of detection (LOD) and limit of quantitation (LOQ) of gallic acid, as well as % recovery and relative standard deviation (%RSD), tests which define the accuracy and precision of the procedure. The % recovery of gallic acid was determined in three levels, 50, 100, and 150 µg/ml, by adding a standard gallic acid solution to the placebo. The % recovery of between 85 and 115% and %RSD of less than 2% over 10 replicates of test samples represent acceptable intra-assay precision under the acceptance criteria [14, 15].
Determination of anti-enzymatic activities related to the skin aging process
Determination of anti-tyrosinase activity
Anti-tyrosinase activity was investigated following the procedure of Poomanee et al. [16]. In brief, the extract was serially diluted by 20% polysorbate 20 in DI water to give five final concentrations in the range of 0.31–5 mg/ml. The reaction mixture, in which extract solution (70 µl), PBS (pH 6.8, 70 µl), and 1.66 mM mushroom tyrosinase (Sigma-Aldrich, Singapore) in PBS (70 µl) were mixed, was incubated for 10 min at room temperature. Substrates of the tyrosinase enzyme, specifically 0.85 mM tyrosine or DOPA (3,4-dihydroxyphenylalanine) (70 µl), were added to the mixture and incubated for 20 min. The absorbance of each sample was measured using a microplate reader (SpectroSTAR Nano®, Ortenberg, Germany) at 492 nm. Anti-tyrosinase activity of the extract was reported in terms of % inhibition and IC50. In this experiment, alpha-arbutin served as a positive control.
Determination of MMP-2 and MMP-9 inhibition by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)
Cell culture
Albino Swiss Mouse embryonic fibroblasts 3T3 cell culture (BCRC 60071; ATCC® CCL92) and RAW 264.7 cells (ATCC-TIB-71) were purchased from the American Type Culture Collection (Rockville, MD, USA). Cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin–streptomycin at 37 °C in 5% CO2.
MMP-2 and MMP-9 determination by SDS-PAGE
Briefly, each sample was dissolved in DI water and serially diluted to give four final concentrations of 1, 2.5, 5, and 10 mg/ml. The medium (180 µl) was mixed with each sample (20 µl) in a 96-well flat plate using aseptic technique. DI water served as a negative control, whereas l-ascorbic acid and gallic acid served as positive controls. After a 48 h incubation, each mixture was collected and then mixed with loading buffer containing 4% SDS and 0.04% Bromophenol blue in a ratio of 1:1. Gel separation, in which gelatin was used as a substrate, was performed. Each sample or control (20 µl) was separately loaded into each well located on the stacking gel. After electrophoresis, the gel page was soaked in reaction buffer at 37 °C for 24 h to remove the SDS–polyacrylamide gel. Then, the gel page was stained with 0.5% w/v Coomassie blue and destained to visualize the markers.
The area of each band on the gel was visualized using a gel documentation system and calculated in terms of relative MMP content using ImageJ software version 8.0. The percentage inhibition of each sample was compared with the negative control (100%) and calculated according to Eq. 1; additionally, the IC50 of each sample was reported.
| 1 |
Determination of anti-hyaluronidase (anti-HAase) activity by SDS-PAGE
The protocol of Chaiyana et al. [17] was employed for determination of anti-HAase activity, with some modifications. Sample dilution was analogous to the protocol for anti-MMP activity. The reaction mixture (50 µl), which consisted of 0.13 M NaCl, HAase, and sample (5 µl), was incubated at 37 °C. DI water served as a negative control, whereas l-ascorbic acid and gallic acid served as positive controls. After 48 h of incubation, the mixture was collected and then mixed with loading buffer containing 4% SDS and 0.04% Bromophenol blue prior to further experimentation. A separating gel was prepared by loading 20 µl of sample, containing 0.17 mg/ml hyaluronic acid (HA). After running the electrophoresis, the gel page was soaked in washing buffer (pH 7.2) at 37 °C for 1 h. Next, the gel page was left in the reaction buffer (0.02 M sodium acetate-acetic acid buffer) for 18 h. Staining buffer (Alcian buffer 8GX) was used to stain the gel page. Finally, the gel page was destained in 50% v/v methanol and 10% v/v acetic acid for 2 h.
The area of each band on the gel was visualized using a gel documentation system and calculated in terms of relative HAase content using ImageJ version 8.0 software (NIH, Bethesda, MD, USA). The % inhibition of each sample was compared to that of the negative control (100%) according to Eq. 2; additionally, the IC50 of each sample was reported.
| 2 |
Determination of anti-inflammatory activity via nitric oxide (NO) inhibition assay
Cell viability assay
Prior to evaluation of NO inhibition, the cytotoxicity of the selected extracts were evaluated in RAW 264.7 cells (ATCC-TIB-71). Cells were seeded at a density of 1 × 105 cells/ml in sterile 96-well plates for 24 h and then treated with either extract or gallic acid in serial concentrations of 0.1, 1, 10, 100, and 1000 µg/ml in 100 μl of medium for 48 h. Control cultures received identical amounts of DMSO, as did the treated cultures that served as vehicle controls. Subsequently, the medium was replaced with new medium and cell viability was analyzed using a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)-based cell growth determination kit (Promega, Madison, WI). Absorbance values were calculated according to Eq. 3 and presented as a percentage relative to the vehicle control.
| 3 |
where ‘Abs of sample’ refers to the absorbance of cells treated with the sample and ‘Abs of vehicle control’ is the absorbance of DMSO-treated cells.
NO inhibition assay
RAW 264.7 cells were seeded into a sterile 96 well-plate at 5 × 104 cells/well and cultured at 37 °C with 5% CO2/95% relative humidity (RH) for 24 h. Cells were then washed twice with PBS, pH 7.4. Each sample, in concentrations ranging from 0 to 100 µg/ml (200 µl), or the positive control (0.1 mg/ml of diclofenac sodium) were added to cell culture wells in RPMI 1640 medium containing 100 ng/ml of Lipopolysaccharides (LPS). RPMI 1640 medium served as a negative control. Following exposure for 24 h, 100 µl of the supernatant solution was mixed with 100 µl Griess reagent containing 1% w/w sulfanilamide and 0.1% w/w N-1-[naphthyl] ethylenediamine dihydrochloride in 2.5% w/w phosphoric acid. After 10 min, the absorbance of the solution was determined using a Varioskan flash microplate reader (Thermo Fisher, Finland) at 550 nm. The results were reported as % NO reduction, which was calculated according to Eq. 4.
| 4 |
where ‘Abs of control’ is the absorbance of cells treated with medium containing LPS; ‘Abs of sample’ and ‘Abs of blank’ are the absorbances of cells treated with the extract plus medium either containing or not-containing LPS, respectively.
Determination of ability to prevent DNA damage in the fibroblast BJ cell line by DNA fragmentation assay
Cell viability assay
Prior to the DNA fragmentation assay, the MTT cell viability assay was performed to detect any cytotoxic effects of the selected extracts on the human fibroblast BJ cell line (BJ). One hundred microliters containing BJ cells at a density of 4 × 104 cells/ml in 10% FBS-EMEM medium containing 0.1 mg/ml penicillin and 0.1 mg/ml streptomycin (complete medium) was added to each well of a 96-well flat plate, which was then incubated overnight at 37 °C under 5% CO2 atmosphere. Seed kernel extract was added to each well in medium (100 µl) in serial concentrations of 0, 125, 250, 500, and 1000 µg/ml. Each concentration was performed in triplicate. Medium, both with and without PBS, was used as a vehicle control and cell control, respectively. The cells were incubated at 37 °C under 5% CO2 atmosphere for 48 h. The colorimetric MTT assay was employed as mentioned previously. Due to the presence of maltodextrin in the extract, the cytotoxicity of maltodextrin was also investigated.
DNA fragmentation assay
Three milliliters of medium containing fibroblast BJ cells at a cell density of 4 x 104 cells/ml was added to 35 mm-plates. The cells were incubated at 37 °C under 5% CO2 atmosphere overnight. After incubation, the culture medium was removed and 3 ml of 1000 µg/ml KMHE in medium was added to the plates. The complete medium containing maltodextrin and PBS served as controls. Cells were incubated at 37 °C under 5% CO2 atmosphere for 48 h. Culture medium was then removed and 1.2 mM H2O2 (3 ml) in complete medium was added to the plate. Cells were then further incubated at 37 °C under 5% CO2 atmosphere for 3 h. Subsequently, the cells were harvested and subjected to DNA extraction using a DNA extraction kit (NucleoSpin® Tissue; MACHEREY–NAGEL GmbH & Co. KG, Germany), according to the manufacturer’s instructions. The extracted DNA was quantified using a NanoDrop 1000 spectrophotometer (NanoDrop, Wilmington, USA). Equal amounts of DNA samples were electrophoresed on a 1.5% agarose gel containing 1 µl/100 ml SYBR-Safe DNA gel stain (Invitrogen, Thermo Fisher Scientific Inc., MA, USA). The gel was examined and photographed under an ultraviolet gel documentation system.
Statistical analysis
All experiments were performed separately in triplicate. The results of all experiments are shown as mean ± SD. Differences in total phenolic content, total flavonoid content, and free radical scavenging abilities between each sample were statistically analyzed via one-way ANOVA with Tukey’s multiple comparison analysis using SPSS statistics software, version 17.7 (IBM co. Ltd., NY, USA). Differences in the anti-inflammatory and anti-enzymatic effects between the extracts and positive controls were statistically examined using the Independent T test. p values less than 0.05 (p < 0.05) were regarded as significantly different.
Results
Plant extraction
Initially, the mango seed kernel extraction procedure was optimized. KMHE gave the highest percentage yield (28.98 ± 2.47%), followed by KMEF (22.01 ± 2.36%), KME (16.52 ± 2.85%), and KMEA (1.28 ± 0.40%), which gave the lowest yield. The majority of wax compounds inherently found in the mango seed kernel [11] were excluded by hexane extraction, which demonstrated a waxy, white characteristic. KMEA contained waxy compounds with a slight pinkish color. Additionally, it is worth noting that the white wax was found only with KME; there was no residue wax within KMEF, indicating that ethyl acetate fractionation may extract the residue wax prior to the ethanol fractionation.
Total phenolic and flavonoid contents of mango seed kernel extracts
The obtained extracts were subsequently quantified for their chemical constituents, including polyphenols and flavonoids, as these sorts of natural substances are thought to express antioxidant, anti-inflammatory, and anti-aging capabilities [18]. As shown in Fig. 1, the major constituents of mango seed kernel extracts were phenolic compounds, which were approximately 10-fold higher than flavonoids. Among all the extracts, the highest content of phenolics was shown in KMEF.
Fig. 1.
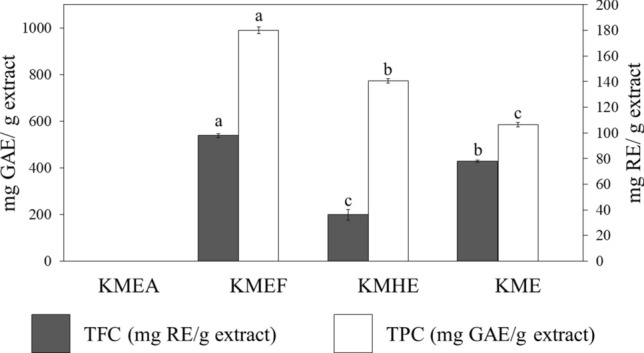
Total phenolic (TPC) and total flavonoid contents (TFC) of mango seed kernel extracts; Differences in alphabets (a, b, and c) imply significant differences (p < 0.05) between the mango seed kernel extracts of each experiment
Antioxidant capacities of the mango seed kernel extracts
Evaluation of the free radical scavenging properties of the extracts was initially conducted via DPPH and ABTS assays, which were chosen to mimic physiological ROS conditions. According to Table 1, KMHE, KMEF, and KME exerted strong free radical scavenging effects, which were comparable to Trolox, a potent water-soluble form of vitamin E. Noticeably, the scavenging capacities were not significantly different among all extracts. Meanwhile, KMEA demonstrated a moderate free radical scavenging effect, potentially indicating that some types of lipophilic polyphenols in the mango seed kernel are extracted by ethyl acetate. According to the results from the linoleic acid peroxidation assay, KMHE and KME exhibited notable effects comparable to Trolox and superior to both l-ascorbic acid, a typical water-soluble antioxidant used in cosmetology, and the standard gallic acid.
Table 1.
Free radical scavenging abilities and attenuating property on linoleic acid peroxidation of the obtained mango seed kernel extracts
| Samples | Free radical scavenging IC50 (µg/ml) | TEAC** (mg Trolox/mg extract) | Linoleic acid peroxidation IC50 (mg/ml) | |
|---|---|---|---|---|
| DPPH | ABTS | |||
| KMEA1 | 180.38 ± 14.74b | ND | 0.04 ± 0.00b | ND |
| KMEF2 | 8.13 ± 0.90a | 3.23 ± 0.46a | 0.63 ± 0.06a | 1.12 ± 0.15c |
| KMHE3 | 7.35 ± 0.35a | 3.39 ± 0.29a | 0.60 ± 0.03a | 0.62 ± 0.21a |
| KME4 | 10.07 ± 0.16a | 2.69 ± 0.46a | 0.61 ± 0.05a | 0.64 ± 0.06a,c |
| Standards | ||||
| Trolox | 5.37 ± 0.69 | 1.60 ± 0.01 | – | 0.46 ± 0.02 |
| Gallic acid | 1.84 ± 0.02 | 0.85 ± 0.06 | 1.32 ± 0.01 | 1.22 ± 0.11 |
| l-ascorbic acid | 4.4 ± 0.03 | 0.40 ± 0.01 | 4.28 ± 0.02 | 0.94 ± 0.03 |
Differences in superscripts a, b, and cimply significant differences (p < 0.05) between mango seed kernel extracts within the same column
ND not detectable
**TEAC indicates the Trolox equivalent antioxidant concentration determined by ABTS assay
1KMEA: Ethyl acetate fraction, 2KMEF: Ethanol fraction, 3KMHE: Hydro-ethanolic extract, 4KME: Ethanolic extract
Taking into consideration the excellent antioxidant efficacy of KMHE, along with its extraction method using less organic solvent, this extract was selected for further, in-depth investigation into its anti-aging and anti-inflammatory capabilities, as well as its ability to prevent DNA damage.
Gallic acid content determination
According to the HPLC validation, the percent recovery of gallic acid in the three tested concentrations of 50, 100, and 150 mg/l were in the ranges of 88.76–95.56%, 88.32–93.49%, and 91.31–97.00%, respectively, implying desirable accuracy. Additionally, the %RSD of the tested concentrations were 1.94%, 1.92%, and 1.84%, conferring good precision [14, 15]. The LOD, the minimum concentration at which gallic acid could be detected, was 3.5 mg/l, whereas the LOQ, the minimum concentration at which quantification of gallic acid could be performed with acceptable accuracy and precision, was 30 mg/ml.
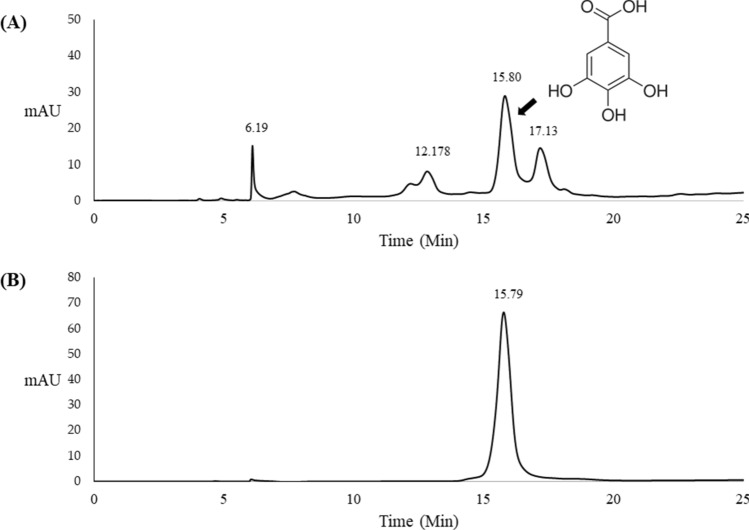
Figure 2 illustrates the HPLC fingerprint of KMHE, in which gallic acid represents a major compound at the retention time of 15.75 min, coinciding with the gallic acid standard. The percent gallic acid of the KMHE was 2.22 ± 0.01% w/w.
Fig. 2.
HPLC chromatograms of the hydroethanolic extract of mango seed kernel (KMHE; a) and b gallic acid (50 mg/l) using UV detection at a wavelength of 280 nm
Determination of attenuating properties on tyrosinase enzyme
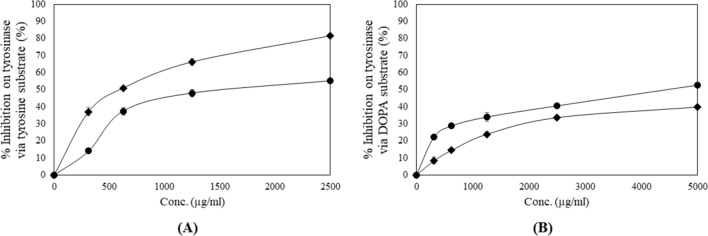
A promising attenuation effect on tyrosinase by KMHE was evidenced, as shown in Fig. 3, for both l-tyrosine and dihydroxyphenylalanine (DOPA) substrates, which demonstrated IC50 values of 1.09 ± 0.07 and 4.25 ± 0.26 mg/ml, respectively. It is worth noting that the potential of KMHE via the DOPA pathway was significantly stronger than that of alpha-arbutin, a widely used depigmenting ingredient in cosmetology.
Fig. 3.
Attenuating effects of mango seed kernel extract (KMHE) (filled circle) and alpha-arbutin (filled daimond) on the tyrosinase enzyme via tyrosine substrate (a) and 3,4-dihydroxyphenylalanine (DOPA) substrate (b)
Determination of attenuating properties on matrix metalloproteinases (MMP)-2 and -9
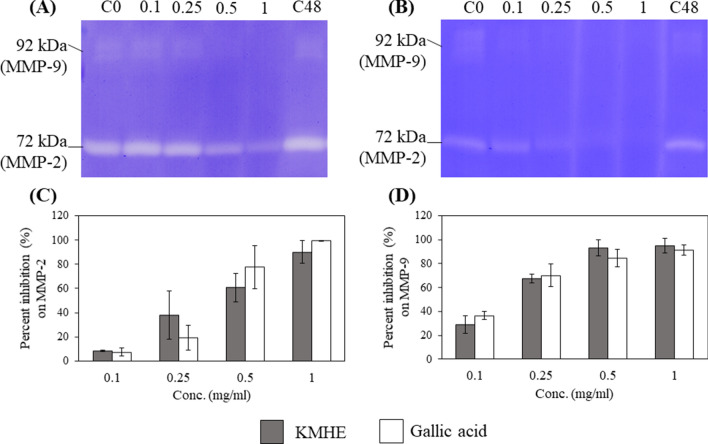
Our results show that KMHE demonstrates outstanding anti-MMP-2 and anti-MMP-9 effects, with IC50 values of 0.46 ± 0.20 and 0.11 ± 0.03 mg/ml, respectively; these results were not significantly different from those of gallic acid. As shown in Fig. 4c and d, there were no significant differences (p < 0.05) observed between any of the extracts in terms of percent inhibition of MMP-2 or MMP-9 when compared to gallic acid at all tested concentrations.
Fig. 4.
Inhibitory effects on Matrix metalloproteinase (MMP)-2 and MMP-9 by mango seed kernel extract (KMHE; a) and gallic acid b as shown by SDS-PAGE zymography; percent inhibitions of MMP-2 c and MMP-9 d by four different concentrations of KMHE and gallic acid, specifically 0.1, 0.25, 0.5, and 1 mg/ml, after a 48 h incubation. C0: control at the initial timepoint; C48: control after the 48 h incubation
Determination of anti-enzymatic activity on Hyaluronidase (HAase)
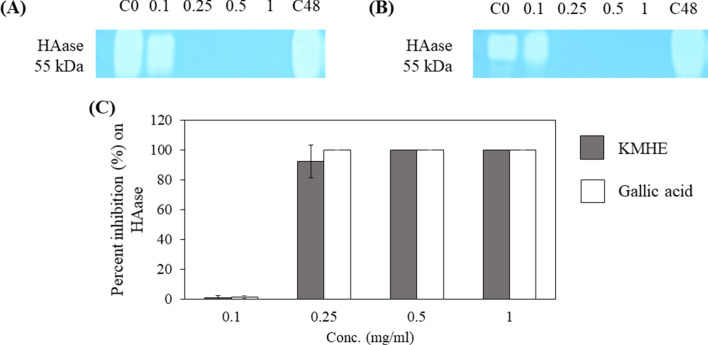
Figure 5 illustrates potent inhibitory effects by KMHE and gallic acid on HAase, with IC50 values of 0.20 ± 0.01 mg/ml and 0.19 ± 0.01 mg/ml, respectively.
Fig. 5.
Attenuating effects on hyaluronidase (HAase) by the mango seed kernel extract (KMHE; a) and gallic acid b, as shown by SDS-PAGE zymography and percent inhibition by each concentration c for four different concentrations of KMHE and gallic acid, specifically, 0.1, 0.25, 0.5, and 1 mg/ml, after 48 h of incubation. C0: control at the initial timepoint; C48: control after 48 h of incubation
Determination of nitric oxide (NO) inhibition
Initially, the cytotoxicity of KMHE and gallic acid were determined in RAW 264.7 cells, in order to avoid false positives regarding anti-inflammatory effects due to cell intoxication. Moreover, NO production can be optimally detected at percentage cell viabilities greater than 80% [19]. Figure 6a illustrates that KMHE, in a concentration range of 100-1000 µg/ml, has a slight toxic effect on the cells, as % cell viability was below 80%. Accordingly, both the extract and gallic acid were evaluated for NO inhibition in the concentration range of 0.1–10 µg/ml.
Fig. 6.
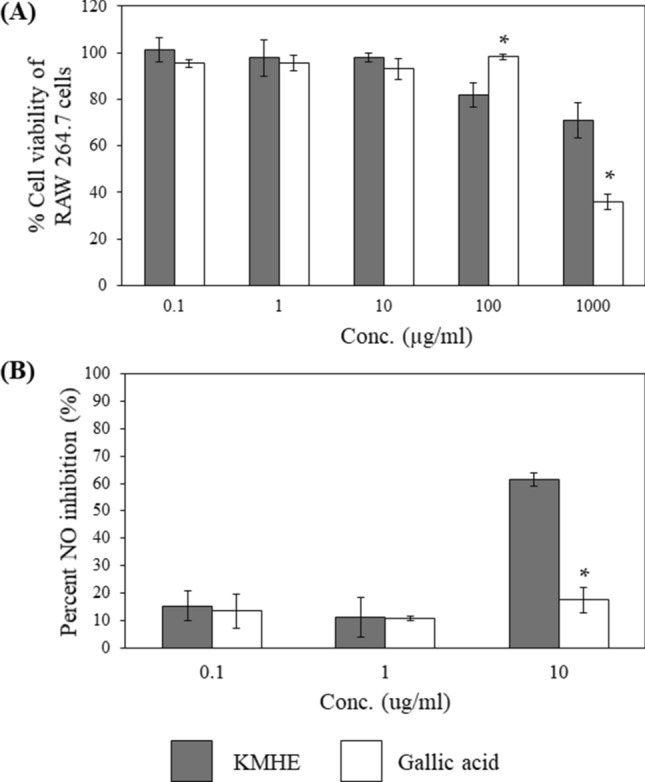
Percent cell viability of RAW 264.7 cells after exposure to the mango seed kernel extract (KMHE) and gallic acid at the indicated concentrations (a). Percent inhibition on nitric oxide (NO) (b). * implies a significant difference between KMHE and gallic acid at the same concentration (p < 0.05)
The inhibitory capabilities of KMHE and gallic acid on NO production are shown in Fig. 6b. The results clearly demonstrate a significantly stronger effect from the extract when compared to gallic acid at the tested concentration of 10 µg/ml, without any evidence of cell toxicity. Moreover, the % inhibition of diclofenac sodium (100 µg/ml), which served as a positive control, was 19.17%, less than the extract.
Determination of ability to prevent DNA damage
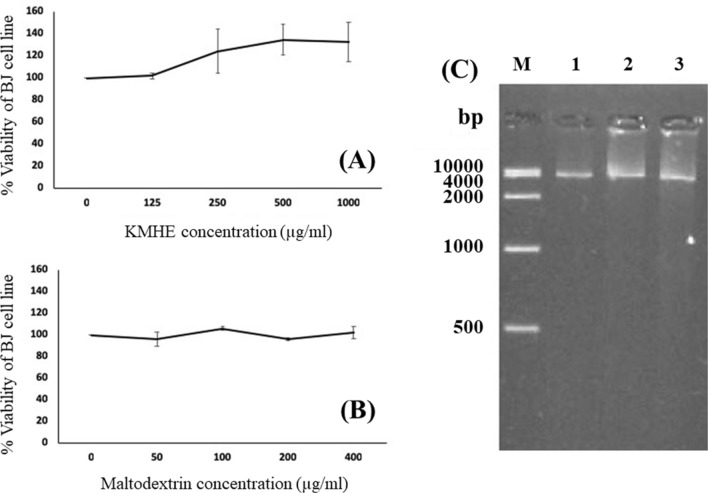
Prior to the evaluations of DNA damage, clarifying the cytotoxic effects of KMHE on human fibroblast BJ cells was important. Additionally, maltodextrin, which served as a carrier for KMHE in the spray-drying procedure, was investigated for cytotoxicity. As shown in Fig. 7a and b, KMHE and maltodextrin did not reduce cell viability nor induce cytotoxicity in BJ cells (p > 0.05).
Fig. 7.
Effect of KMHE (a) and maltodextrin (b) on cell viability in a human fibroblast BJ cell line; (c) Protective effects of (1) KMHE, (2) maltodextrin, and (3) phosphate buffered saline on DNA fragmentation in the human fibroblast BJ cell line
Human fibroblast BJ cells were pre-treated with 1000 µg/ml KMHE for 48 h prior to 3 h exposure to H2O2. Maltodextrin, which served as a carrier for the extract in the spray-drying process, was also evaluated for DNA-protective effects in order to avoid false positives. As shown in Fig. 7c, KMHE demonstrated small protective effects against DNA fragmentation resulting from H2O2 exposure in BJ cells, when compared to controls, maltodextrin, or PBS.
Discussion
In the current study, mango seed kernel extracts derived from the Kaew-Kamin cultivar were comprehensively evaluated for their potential ability to prevent skin aging. A range of research has reported mango seed kernels to be a rich source of polyphenols, which are the main contributor to this products promising range of diverse biological activities [6, 20, 21]. Chiefly, polyphenols have been regarded as functional ingredients in anti-aging products owing to several of their functions, including as metal chelators, reducing agents, anti-inflammatory agents, and inhibitors of lipid peroxidation due to their ability to rapidly donate a hydrogen atom to free radicals [3, 18]. Hence, the extraction methods and solvents were chosen in accordance with the following aspects: a high potential for extracting hydrophilic polyphenols, which were the target polyphenols, and being safe for human use [22, 23]. Indeed, differences in the solvent polarities particularly influenced the physical characteristics, quantities of polyphenols, and percentage yield of the obtained extracts [24]. Hydrophilic polyphenols were denoted to be generally extracted by semi-polar solvents, such as methanol and ethanol, and their mixture with water [18]. Our results demonstrate that maceration with 50% ethanol–water and 95% ethanol fractionation as extraction procedures for mango seed kernels provide a high percentage yield and polyphenols-rich extract.
On account of the complexities involved in the process of aging, the anti-aging properties of the obtained extracts were comprehensively elucidated through various methodologies. ROS has been reported as an important cellular initiator of aging contributing to a wide range of physiological tissue deterioration [1, 2]. A number of reports have denoted that ROS, generated from either cellular metabolism or environmental sources, has the potential to destroy cellular proteins, lipids, and genetic elements [25]. Furthermore, cell membrane peroxidation, which is a chain reaction involving oxidative stress of phospholipids, has been widely recognized as a consequence of the induction of ROS [2]. The mango seed kernel extracts exerted either a strong free radical scavenging capacity or a notable inhibitory effect on lipid peroxidation. However, among all the extracts, KMEF contained the highest total phenolic content. These findings suggest that the attenuating property on lipid peroxidation does not correspond to the amount of phenolic compounds found in the extracts. Chaiyana et al. [17] also reported a weak correlation between total phenolic content and the attenuating property on lipid peroxidation, as phenolic compounds, being hydrophilic substances, were not entirely dissolved in the experimental environment.
Gallic acid has been shown to be a major phenolic compound in kernel extracts from the Kaew Kamin cultivar. Our previous findings have denoted that mango seed kernel extract from the Kiew-Moragot cultivar mainly contain gallic acid [11]. Although gallic acid was regarded as a major phenolic acid detected at the wavelength of 280 nm, taking into account the high total phenolic content of the extract, other polyphenols may also be responsible for the antioxidant capacity, thus requiring further elucidation.
Along with the cell deterioration attributed to oxidative stress, skin hyperpigmentation, a clinical manifestation related to skin aging, can be accelerated in the presence of ROS through upregulation of the tyrosinase enzyme and its substrates [4]. Tyrosinase, a copper-containing glycoprotein, plays a pivotal role in melanogenesis via two major steps, comprising initial hydroxylation of l-tyrosine to 3,4-dihydroxyphenylalanine (DOPA) and subsequent oxidation of DOPA to DOPAquinone. Therefore, reduction of tyrosinase function potentially decreases cutaneous hyperpigmentation [26]. Molecularly, both tyrosine and DOPA consist of a hydroxyl moiety, which confers an H + to activate tyrosinase [27]. Similarly, gallic acid contains three hydroxyl groups in its backbone that can act as competitive inhibitors by reacting as substrates in the system. Alpha-arbutin, a botanical glucoside derived of hydroquinone, also acts as a competitive inhibitor, although with a milder effect than hydroquinone, due to the fact that it gradually releases hydroquinone molecules via breakage of glycosidic bonds [26, 28]. The significantly lower effects from alpha-arbutin, when compared to KMHE, in the DOPA oxidation pathway may be due to the less active hydroxyl groups of alpha-arbutin compared to those of gallic acid. Such results imply that KMHE may be a promising depigmenting extract for the prevention of atypical brown spots due to skin aging.
Our study is the first to illustrate the anti-enzymatic activities on age-related enzymes of mango seed kernel extracts. MMPs are typically produced by epithelial cells, fibroblasts, mast cells, and neutrophils and play an essential role in remodeling of the skin extracellular matrix (ECM). MMPs are commonly accelerated through the induction of ROS via the NF-kB and Activator Protein-1 (AP-1) pathways [29–31]. The skin ECM, which is mainly composed of collagens and elastins, primarily offers skin structural integrity. Accordingly, the degradation of ECM due to upregulation of MMPs predominantly leads to skin sagging and aging [29, 31]. Inhibitory effects on MMP-2 (72-kDa type IV collagenase) and MMP-9 (92-kDa type IV collagenase) have been widely examined, since the combined effects of these two MMPs generates a variety of skin deterioration [29, 30]. The strong inhibitory effects attributed to mango seed kernel extract (KMHE) are in correspondence with results by Calabriso et al. [32], which illustrate that polyphenols derived from grape skin also show inhibitory effects on the expression of MMP-2 and MMP-9 from inflammatory monocytes. It is worth noting that KMHE could alleviate the progression of skin deterioration and skin aging.
Diminished skin hydration is another pathology involved in skin aging. The degradation of HA, an extracellular glycosaminoglycan, is a main participator in skin senescence due to the resultant loss in water absorbing capacity [33]. HA can either be destroyed through enzymatic degradation, principally by HAase, or partially non-enzymatic degradation, as a consequence of oxidative stress and inflammation [34, 35]. Undoubtedly, an extract that exerts antioxidant effects and attenuates the activity of HAase could delay the progression of skin aging and preserve skin hydration. Kolayli et al. [36] showed that anti-HAase positively correlates with the content of polyphenols in the extract, which is in correspondence with our results. Consequently, KMHE could be utilized as a hyaluronidase inhibitor for anti-skin aging purposes. Taken together, KMHE possesses outstanding anti-tyrosinase, anti-MMP, and anti-HAase effects, thereby conferring comprehensive attenuating properties against age-related enzymes. Physiologically, it is possible that the capabilities of this extract may be augmented through its potent ability to quench ROS, a major inducer of the aging process [1, 11].
The induction of oxidative stress through exposure to UV radiation and pollution are considered extrinsic causes that substantially potentiate skin inflammatory events leading to chronic skin aging [37]. NO is considered an important inflammatory mediator contributing to a wide range of degenerative physiological responses [38]. Excessive NO production also contributes to the propagation of tissue damage [19]. Our previous findings have reported that mango seed kernel extract derived from the Kiew-Moragot cultivar exerts anti-inflammatory properties via inhibition of interleukin (IL)-6 and IL-8 produced by LPS-stimulated RAW 264.7 cells [11]. Ippoushi et al. [39] also reported that a polyphenol derived from ginger strongly inhibits NO production in LPS-treated J774.1 macrophages in a dose-dependent manner. Additionally, the inhibitory effect on NO synthesis was positively correlated to the number of hydroxyl groups existing in the polyphenols [19]. Our study provides additional information regarding the anti-inflammatory effects of mango seed kernels.
The propagation of DNA damage owing to exposure to UV radiation and pollution has been widely established [1, 40]. Additionally, cellular DNA strands can be directly damaged via UV absorption, resulting in the erratic manipulation of nucleotides and, subsequently, skin aging [41]. For this reason, in the current study, we elucidate, for the first time, an in-depth analysis of the protective effects of KMHE against DNA damage. From the results, it can be seen that KMHE exerts a protective effect against the DNA damage induced by H2O2, a by-product of cellular respiration that has been regarded as one of the potent inducers of oxidative stress and aging [42]. The protective effect of KMHE may be due to its excellent free radical scavenging capacity, by which H2O2 and other related radicals are diminished. Additionally, the extract presented no toxicity to human BJ fibroblasts, thereby indicating it is a safe functional ingredient for human use, however, an irritation test in human volunteers will be necessary in further investigations. Accordingly, the hydroethanolic extract of mango seed kernels from the Kaew Kamin cultivar, which are mainly composed of gallic acid and phenolic compounds, exert multifunctional activities related to anti-aging capabilities while maintaining a good safety profile.
In conclusion, the chemical constituents and biological activities of mango seed kernels derived from the Kaew Kamin cultivar relating to anti-skin aging are reported for the first time. KMHE showed the highest percentage yield, equal to 28.98 ± 2.47% w/w, with the highest antioxidant activities in terms of both free radical scavenging effects and attenuating property against lipid peroxidation; a fact that correlated to the high phenolic content of KMHE. Utilizing HPLC analysis, gallic acid was identified as a main constituent that could be used as a marker for further quality control. KMHE also demonstrated good anti-tyrosinase activity and potent anti-enzymatic effects against the skin aging process, which were as potent as gallic acid against HAase, MMP-2 and MMP-9. Additionally, KMHE possessed moderate NO inhibitory effects, with no cytotoxic effects, on RAW264.7 cells and induced protective effects against DNA fragmentation in an H2O2-treated human fibroblast BJ cell line, also with no cytotoxicity. Therefore, KMHE is a promising, safe multifunctional bioactive ingredient that could be further applied in nutraceutical and cosmeceutical products. Nonetheless, clinical evaluation will also be important in further investigations.
Acknowledgements
This research was funded by the Agricultural Research and Development Agency (Public organization, ARDA) Thailand and partially supported by Chiang Mai University grant. The possible APC was funded by Innovation Center for Holistic Health, Nutraceuticals, and Cosmeceuticals, Faculty of Pharmacy, Chiang Mai University.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Worrapan Poomanee, Email: worrapan.p@cmu.ac.th.
Watcharee Khunkitti, Email: watkhu@kku.ac.th.
Wantida Chaiyana, Email: wantida.chaiyana@cmu.ac.th.
Nutjeera Intasai, Email: nutjeera.in@cmu.ac.th.
Wei-Chao Lin, Email: weilin@mail.cnu.edu.tw.
Shang-Chian Lue, Email: myluemy@mail.cnu.edu.tw.
Pimporn Leelapornpisid, Email: pimporn.lee@cmu.ac.th.
References
- 1.Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27:729–738. doi: 10.1177/0963-6897.1772.5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yaar M, Eller MS, Gilchrest BA. Fifty years of skin aging. Perspect Progr Cutaneous Biol. 2002;7:51–58. doi: 10.1046/j.1523-1747.2002.19636.x. [DOI] [PubMed] [Google Scholar]
- 3.Cao C, Xiao Z, Wu Y, Ge C. Diet and skin aging-from the perspective of food nutrition. Nutrients. 2020;12:870–895. doi: 10.3390/nu12030870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masaki H. Role of antioxidants in the skin: anti-aging effects. J Dermatol Sci. 2010;58:85–90. doi: 10.1016/j.jdermsci.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Tundis R, Loizzo MR, Bonesi M, Menichini F. Potential role of natural compounds against skin aging. Curr Med Chem. 2015;22:1515–1538. doi: 10.2174/0929867322666150227151809. [DOI] [PubMed] [Google Scholar]
- 6.Lauricella M, Emanuele S, Calvaruso G, Giuliano MD, Anneo A. Multifaceted health benefits of Mangifera indica L. (Mango): the inestimable value of orchards recently planted in Sicilian rural areas. Nutrients. 2017;9:1–14. doi: 10.3390/nu9050525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pravez M. Pharmacological activities of mango (Mangifera indica): a review. J Pharmacogn Phytochem. 2016;5:1–7. [Google Scholar]
- 8.Office of Agricultural Economics (2017) Agricultural import and export data. Ministry of Agriculture and Co-operation, The Royal Thailand Government. http://www.oae.go.th/assets/portals/1/files/jounal/2561/thailandtradestat2560.pdf. Accessed 18 June 2020
- 9.Shah KA, Patel MB, Patel RJ, Parmar PK. Mangifera indica (mango) Pharmacogn Rev. 2010;4:42–48. doi: 10.4103/0973-7847.65325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jahurul MHA, Zaidul ISM, Ghafoor K, Al-juhaimi F, Nyam K, Norulaini NAN, et al. Mango (Mangiferin indica L.) by-products and their valuable components: a review. Food Chem. 2015;183:173–180. doi: 10.1016/j.foodchem.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 11.Poomanee W, Chaiyana W, Mueller M, Viernstein H, Khunkitti W, Leelapornpisid P. In-vitro investigation of anti-acne properties of Mangifera indica L. kernel extract and its mechanism of action against Propionibacterium acnes. Anaerobe. 2018;52:64–74. doi: 10.1016/j.anaerobe.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Department of Medical Sciences, Ministry of Public Health (2018) Thai Herbal Pharmacopoeia. 1st ed. Keawjawjom printing and Publishing Suan Sunandha Rajaphat University, Bangkok, Thailand, pp 522–532
- 13.Floegel A, Kim D, Chung S, Koo SI, Chun OK. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compos Anal. 2011;24:1043–1048. doi: 10.1096/fasebj.24.1_supplement.535.9. [DOI] [Google Scholar]
- 14.Shabir GA. Validation of high-performance liquid chromatography methods for pharmaceutical analysis, understanding the differences and similarities between validation requirements of the US Food and drug administration, the US pharmacopeia and the international conference on harmonization. J Chromatogr A. 2003;987:57–66. doi: 10.1016/S0021-9673(02)01536-4. [DOI] [PubMed] [Google Scholar]
- 15.Babu S, Sudhakar V, Murthy TEGK. Validated HPLC method for determining related substances in compatibility studies and novel extended release formulation for ranolazine. J Chromatogr Sep Tech. 2014;5:1–7. doi: 10.4172/2157-7064.1000209. [DOI] [Google Scholar]
- 16.Poomanee W, Chaiyana W, Intasai N, Leelapornpisid P. Biological activities and characterization of the pod extracts from Sompoi (Acacia concinna Linn.) grown in Northern Thailand. Int J Pharm Pharm Sci. 2015;7:237–241. doi: 10.1016/j.ajps.2017.03.001. [DOI] [Google Scholar]
- 17.Chaiyana W, Auchapreeda S, Punyoyai C, Neimkhum W, Lee K-H, Lin W-C, et al. Ocimum sanctum Linn. as a natural source of skin anti-ageing compounds. Ind Crop Prod. 2019;127:217–224. doi: 10.1016/j.indcrop.2018.10.081. [DOI] [Google Scholar]
- 18.Ajila CM, Brar SK, Verma M, Tyagi RD, Godbout S, Velero JR. Extraction and analysis of polyphenols: recent trends. Crit Rev Biotechnol. 2010 doi: 10.3109/07388551.2010.513677. [DOI] [PubMed] [Google Scholar]
- 19.Karunakaran T, Ismail IS, Ee GCL, Nor SMM, Palachandran K, Santhanam RK. Nitric oxide inhibitory and anti-Bacillus activity of phenolic compounds and plant extracts from Mesua species. Rev Bras Farmacogn. 2018;28:231–234. doi: 10.1016/j.bjp.2018.01.007. [DOI] [Google Scholar]
- 20.Sairam K, Hemalatha S, Kumar A, Venkataraman S. Evaluation of anti-diarrhoeal activity in seed extracts of Mangifera indica. J Ethnopharmacol. 2003;84:11–15. doi: 10.1016/S0378-8741(02)00250-7. [DOI] [PubMed] [Google Scholar]
- 21.Rajan S, Prave Thirunalasundari T, Jeeva S. Anti-enteric bacterial activity and phytochemical analysis of the seed kernel extract of Mangifera indica Linnaeus against Shigella dysenteriae (Shiga, corrig.) Castellani and Chalmers. Asian Pac J Trop Med. 2013;4:294–300. doi: 10.1016/S1995-7645(11)60089-8. [DOI] [PubMed] [Google Scholar]
- 22.Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, et al. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal. 2014;22:296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawar CR, Surana SJ. Optimizing conditions for gallic acid extraction from Caesalpinia decapetala wood. Pak J Pharm Sci. 2010;23:423–425. [PubMed] [Google Scholar]
- 24.Mojzer EB, Hrnˇciˇc MK, Škerget M, Knez Ž, Bren U. Polyphenols: extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules. 2016;21:901–939. doi: 10.3390/molecules21070901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53:75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- 26.Ebanks JP, Wickett RR, Boissy RE. Mechanisms regulating skin pigmentation: the rise and fall of complexion coloration. Int J Mol Sci. 2009;10:4066–4087. doi: 10.3390/ijms10094066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuo A, Dong H, Yu Y, Shu Q, Zheng L, Yu X, et al. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin Med. 2018;13:51–63. doi: 10.1186/s13020-018-0206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zolghadri S, Bahrami A, Khan MTH, Munoz-Munoz J, Garcia-Molina F, Garcia-Canovas F, et al. A comprehensive review on tyrosinase inhibitors. J Enzyme Inhib Med. 2019;34:279–309. doi: 10.1080/14756366.2018.1545767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philips N, Auler S, Hugo R, Conzalez S. Beneficial regulation of matrix metalloproteinases for skin health. Enzyme Res. 2011 doi: 10.4061/2011/427285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. 2016;17:868–888. doi: 10.3390/ijms17060868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan T, Qin Z, Xia W, Shao Y, Vorrhees JJ, Fisher GJ. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009;14:20–24. doi: 10.1038/jidsymp.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calabriso N, Massaro M, Scoditti E, Pellegrino M, Ingrosso I, Giovinazzo G, et al. Red grape skin polyphenols blunt matrix metalloproteinase-2 and -9 activity and expression in cell models of vascular inflammation: protective role in degenerative and inflammatory diseases. Molecules. 2016;21:1147–1165. doi: 10.20944/preprints201912.0030.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baumann L. Skin ageing and its treatment. J Pathol. 2007;211:241–251. doi: 10.1002/path.2098. [DOI] [PubMed] [Google Scholar]
- 34.Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermato-Endocrinology. 2012;4:253–258. doi: 10.4161/derm.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bralley E, Greenspan P, Hargrove JL, Hartle DK. Inhibition of hyaluronidase activity by Vitis rotundifolia. (Muscadine) berry seeds and skins. Pharm Biol. 2007;45:667–673. doi: 10.1080/13880200701545018. [DOI] [Google Scholar]
- 36.Kolayli S, Can Z, Yildiz O, Sahin H, Karaoglu SA. A comparative study of the antihyaluronidase, antiurease, antioxidant, antimicrobial and physicochemical properties of different unifloral degrees of chestnut (Castanea sativa Mill.) honey. J Enzyme Inhib Med Chem. 2016;31:96–104. doi: 10.1080/14756366.2016.1209494. [DOI] [PubMed] [Google Scholar]
- 37.Thornfeldt CR. Chronic inflammation is etiology of extrinsic aging. J Cosmet Dermatol. 2008;7:78–82. doi: 10.1111/j.1473-2165.2008.00366.x. [DOI] [PubMed] [Google Scholar]
- 38.Conforti F, Menichini F. Phenolic compounds from plants as nitric oxide production inhibitors. Curr Med Chem. 2011;18:1137–1145. doi: 10.2174/092986711795029690. [DOI] [PubMed] [Google Scholar]
- 39.Ippoushi K, Azuma K, Ito H, Horie H, Higashio H. [6]-Gingerol inhibits nitric oxide synthesis in activated J774.1 mouse macrophages and prevents peroxynitrite-induced oxidation and nitration reaction. Life Sci. 2003;73:3427–3437. doi: 10.1016/j.lfs.2003.06.022. [DOI] [PubMed] [Google Scholar]
- 40.DeMarini DM, Claxton LD. Outdoor air pollution and DNA damage. Occup Environ Med. 2006;63:227–229. doi: 10.1136/oem.2005.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panich U, Sittithumcharee G, Rathviboon N, Jirawatnotai S. Ultraviolet radiation-induced skin aging: the role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int. 2016 doi: 10.1155/2016/7370642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals. Nat Rev Mol Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]