Abstract
This study aims at investigating the protective effects of flavonoid fractions of diosmin and hesperidin in mitigating sub-chronic lead acetate-induced biochemical, oxidative stress, and histopathological alterations in adult male Wistar rats. Forty animals were randomly assigned into five groups, each consisting of eight animals. Group I animals was treated with deionised water only, group II, IV, and V were administered lead acetate 90 mg/Kg body weight (1/20th of the LD50), groups III, and IV was administered Daflon (100 mg/Kg), while group V was administered Daflon (200 mg/Kg), 30 min prior treatment with lead acetate. All treatments lasted for 42 days. Blood lead levels, electrolyte parameters, zinc protoporphyrin (ZPP) levels, activities of antioxidant enzymes, and histopathology of vital organs, were evaluated following standard practice. Sub-chronic lead acetate exposure induced a decrease in levels of serum electrolytes, and activities of antioxidant enzymes, while blood lead levels, ZPP, and malondialdehyde levels were increased. Lead exposure also instigated marked variation in histopathology of vital organs. Conversely, co-treatment with graded doses of daflon improved the levels of blood lead, electrolytes, ZPP, activities of antioxidant enzymes, and histopathology of vital organs. Data obtained from the current study indicate that rats exposed to sub-chronic doses of lead acetate show increased blood lead levels, electrolyte imbalance, alongside impairment in ZPP levels, activities of antioxidant enzymes, and histopathology, while pretreatment using daflon mitigated the ensued perturbations. This, therefore, suggests that consumption of foods enriched with flavonoid fractions of diosmin and hesperidin may be beneficial for individuals inhabiting lead-polluted environments.
Keywords: Hesperidin, Flavonoids, Diosmin, Biochemical, Zinc protoporphyrin, Oxidative stress
Introduction
Lead (Pb) is an industrial and environmental pollutant and the hazards of its exposure to man and animals remain a global health concern [1]. It occurs in nature as an oxide or salt and persists enduringly in environmental water, soil, dust, and lead-containing manufactured products [1]. Plants are usually exposed to lead through absorbed contaminated water, and animals can get exposed to it through the consumption of plants containing the lead residues [2]. In humans and animals, other routes of lead access into the body are via drinking water and inhalation of air-borne contaminated dust [3]. Once absorbed, lead diffuses rapidly and circulates to various organs, including the brain, kidney, liver, and highly calcified tissues [4, 5]. Plumbism and or saturnism is a term commonly used to describe the medical condition characterised by abnormally high systemic lead levels [5]. Episodes of lead poisonings occurred in Nigeria, which led to the deaths of 168 people between March and June 2010 in Zamfara State and 28 people in Niger state in May 2015 [6]. Lead has been reported to be one of the most ubiquitous and cumulative heavy metal toxicants of environmental and industrial origin. Agency for Toxic Substances and Disease Registry has placed lead second on the list of top 20 hazardous substances [7]. The side effects accompanying the use of chelating agents and their inability to alleviate lead toxicity have been a source of concern [8, 9], hence the need to explore safer alternatives in the treatment of lead toxicity. Natural products possessing both chelating and antioxidant activities could hold a great future in mitigating the adverse effects of lead toxicity, and therefore, need to be explored [10].
Lead-induced oxidative stress leading to tissue damage is one of the reported mechanisms of lead-associated pathologies [4, 11]. Oxidative stress is characterized by an imbalance between the production of reactive oxygen and nitrogen species (ROS and RNS), and the systemic capability to promptly detoxify the reactive intermediates [12, 13]. Antioxidants are known to mitigate the effects of ROS damage by mopping them up [14, 15]. Flavonoids have been reported to chelate metal ions consequently inhibiting the metal-mediated generation of reactive species, and hence shield target organs from oxidative stress [16, 17]. Interestingly, the administration of exogenous antioxidants is known to mitigate the effects of ROS damage by mopping them up [14]. Daflon (Daflon-500®) is an ultrarefined flavonoid fraction of Rutaceae aurantiae, comprising 90% diosmin (450 mg) and 10% hesperidin (50 mg) presented as film-coated tablets [18, 19]. It is a flavonoid, containing compound which has hepato-protective, anti-apoptotic, anti-inflammatory, vasoprotective, and antioxidant potentials [20, 21]. It is classed alongside other gamma-benzopyrone drugs, produced mostly from plants but can also be of synthetic origin [22].
There is an increasing need to develop an antioxidant-based prophylactic and therapeutic protocol as alternatives to the conventional drugs used in lead poisoning [23]. Previous experiments carried out in our laboratory [21] have demonstrated that sub-chronic doses of Daflon mitigate lead-induced changes in delta-aminolevulinic acid dehydratase activity and haematological parameters in Wistar rats. In light of these findings, the current study aimed to evaluate the effects of flavonoid fractions of diosmin and hesperidin in mitigating sub-chronic lead acetate induced biochemical, oxidative stress, and histopathological alterations in Wistar rats.
Materials and methods
Experimental animals
Forty (40) adult male Wistar rats varying between 190 and 250 g were utilized in this experiment. All animals were acquired from the National Veterinary Research Institute (NVRI), Jos Nigeria, and housed in metal cages in accordance with standard laboratory stipulations. The rats were carefully handled to minimize undue stress during the experiments. All animals were fed standard commercially-prepared rat chow (TOPFEEDS®, Jos, Nigeria) and clean water ad libitum. The rats were acclimatized to the laboratory for two weeks before the experiment commenced. The research was approved by the Ahmadu Bello University Committee on Animal Use and Care (ABUCAUC; ABU/2018/7183) and it was conducted in compliance with the National Institute of Health Guide for Care and Use of Laboratory Animals [24].
Sub-chronic toxicity studies
The rats were randomly assigned into five groups, each consisting of eight rats. Group I animals was treated with deionised water (2 mL/kg) only, group II, IV, and V were administered lead acetate 90 mg/Kg body weight (1/20th of the LD50), group III was administered with Daflon (100 mg/Kg body weight), while groups IV and V were administered Daflon (100 mg/Kg and 200 mg/Kg respectively) 30 min prior administration of lead acetate. The selected dosage was based on the previous report of Kobo et al. [25]. All treatments were administered orally for 42 days, during which animals were monitored for toxicity signs. After the experimental period, surviving animals were sacrificed by jugular venesection following the initial Pentobarbitone anesthesia, after which blood samples were collected and analysed.
Chemical acquisition and preparation
Lead acetate of analytical grade was purchased from Sigma Aldrich (Cat No. 10142; St. Louis, MO, USA). Daflon (500 mg tablet; Batch No. MR 110528; SNS, France) was purchased from a certified pharmaceutical store and each was reconstituted in 5 mL of deionised water to form a 100 mg/mL stock solution.
Blood and tissue sample collection
At the end of the treatment period, all the experimental rats were sacrificed by jugular venesection following light pentobarbitone anaesthesia. Blood (5 mL) was then collected from individual rats, of which 2.5 mL was dispensed into a sample bottle containing heparin and used for haematological analyses. The remaining blood (2.5 mL) was transferred into plain test-tubes and allowed to stand for one hour. The clotted blood in test-tubes was then centrifuged at 1000 × g for 10 min. Thereafter, serum was transferred into serum vials and stored at − 4 °C until needed for subsequent assay of electrolytes and markers of oxidative stress. Postmortem examination was carried out on rats that died or were euthanised in the course of the experiment.
Determination of serum electrolyte parameters
The serum concentrations of electrolytes (K+, Na+, Cl−, HCO3−) were assayed using an automated haematologic analyser (Selectra Junior Spinlab 100, Vital Scientific, Dieren, Netherlands) in line with manufacturers’ recommendations. Standard controls were run prior to each detection, and the obtained values for the different electrolyte parameters were constantly within the normal limits.
Blood lead level detection
Levels of blood lead were evaluated using a microwave plasma atomic emission spectrometer (MP-AES) following microwave-assisted acid digestion as previously explained by Erick et al. [26]. Briefly, the glasswares were wiped with a 10% (v/v) HNO3 solution and then rinsed with milli-Q water. 10 μL of each blood sample was digested with 4.0 mL of 65% (v/v) HNO3 and 0.5 mL of 35% (v/v) hydrogen peroxide in polytetrafluoroethylene (PTFE) vessels. The vessels were transferred into the microwave system (MARS 5, CEM). Lead concentration was detected in samples and isotope 208Pb + was identified. All samples were analysed in duplicate assessed in triplicate to increase robustness.
Determination of zinc protoporphyrin
Zinc protoporphyrin (ZPP) was assayed using the ProtoFluoro-Z Haematoflurometer (Model 2060, Aviv, Lakewood, USA) and a calibrated glass slide by using varying concentration calibrator solutions as described by Grandjean [27]. The instrument excites blood samples pretreated by ProtoFluor-Z reagent containing potassium cyanide. Briefly, fifty microliters of whole blood was diluted with 200 mL of distilled water. Fifty microliters of the diluted blood was then added to 1.0 mL of ethyl acetic acid. The mixture was centrifuged for 3 min at 1500 × g, and supernatant mixed with 1.0 mL of 1.5 mol/L hydrochloric acid solution in a glass tube. Zinc protoporphyrin (ZPP) was then measured using a spectrofluorometer at excitation and emission wavelengths of 408 nm and 662 nm respectively.
Determination of superoxide dismutase activity (SOD)
The activity of SOD was assessed by the Northwest Life Science Specialties (NWLSS™) using the superoxide dismutase activity assay kits. The method is based on detecting the auto-oxidation rate of haematoxylin as previously explained by Martin et al. [28], with a slight modification to harness robustness. Serum SOD activity was measured by determining the ratio of auto-oxidation rates with and without the sample and expressed as “cytochrome C” units. Briefly, 230 µL of serum was added to each well, followed by the addition of 10 µL of serum (for blank). The wells were shaken to allow for proper mixing before incubation for 2 min. Haematoxylin reagent (10 µL) was added to begin the reaction. The instrument’s shaker function was used to stir the solution, and the absorbance immediately recorded at 560 nm every 10 s for five minutes using a microtitre plate reader.
Determination of glutathione peroxidase activity (GPx)
Glutathione peroxidase (GPx) activity was assayed at the Veterinary Physiology laboratory, Ahmadu Bello University Zaria using Palgia and Valentine’s [29] spectrophotometry method, based on Northwest Life Science Specialties (NWLSS™) glutathione peroxidase assay kits protocol. Glutathione peroxidase catalyzes the reduction of hydrogen peroxide (H2O2), oxidizing reduced glutathione (GSH) to form oxidized glutathione (GSSG). Briefly, 50 µL of diluted serum was added to each well followed by the addition of 50 µL of working NADPH to the wells. Fifty (50) µL of working H2O2 was then added to each well and the absorbance was recorded at 340 nm for 5 min using a spectrophotometer with a 30 s recording interval. The GPx activity was then calculated from the net rate.
Determination of catalase activity (CAT)
The activity of catalase (CAT) was determined spectrophotometrically according to the methods of Beers and Sizer [30], with modifications to increase robustness and convenience using the Northwest Life Science Specialties (NWLSS™) CAT activity assay kits protocol. It was carried out at the Veterinary Physiology laboratory, Ahmadu Bello University Zaria. Catalase activity was measured by monitoring the diminution of H2O2 substrate at 240 nm. Briefly, 15 µL of the diluted sample was added to a clean UV microplate well. Thereafter, 290 µL of assay cocktail was added to each well and mixed as quickly as possible using a reader shaker. The absorbance was recorded immediately at 240 nm every two seconds using a microplate reader.
Evaluation of serum malondialdehyde (MDA) concentration
Serum MDA concentration was measured as previously described by Draper and Hadley [31] and modified by Altuntas et al. [32]. Briefly, 2.5 mL of 100 g/L trichloroacetic acid solution was combined with 0.5 mL of serum and incubated for 15 min in a boiling water bath. Upon cooling, the mixture was centrifuged at 1000 × g for 10 min, and 2 mL of the supernatant was combined to 1 mL of 6.7 g/L thiobarbituric acid and the tube and incubated for 15 min in a boiling water bath. Upon cooling, the absorbance of the solution was detected at 532 nm, using a UV spectrophotometer (Jenway, 6405 Model, Japan). One milliliter of 10% trichloroacetic acid (TCA) and one milliliter of 0.67% thiobarbituric acid (TBA) served as blank.
Histopathological Examinations
At postmortem examination, the brain of each rat was examined for histopathological lesions. Tissue samples were collected from the organs and prepared as previously described by Luna [33]. Briefly, the tissue samples were fixed in Bouin’s solution, embedded in paraffin and graded alcohol, and then the tissues were cut at 8 µm using a microtome. The sections were stained with haematoxylin–eosin (H and E) prior to examination under a light microscope at different magnifications (× 100, x 250, and x 400). Lesions observed were recorded. Pictures of the slides were taken after optical focus using a digital camera (Casio®, EX-Z80, 8.1 MP, S/N 44315714B, China).
Data analyses
Obtained data were expressed as mean ± SEM and analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s posthoc multiple comparison test. GraphPad Prism version 6, San Diego, CA, USA (http://www.graphpad.com) was used for the analyses. Values of p < 0.05 were considered significant.
Results
Sub-chronic administration of daflon and lead acetate on markers of lead toxicity and oxidative stress
There was a significant decrease in the blood lead levels of rats in the DW (p < 0.01), DAF (p < 0.001), 100 mg/kg DAF + Pb (p < 0.05), and 200 mg/kg DAF + Pb (p < 0.01) treated groups compared to the Pb treated group which had the highest concentration (8.20 ± 0.7). No significant difference in blood lead levels was observed between rats in the DW group and those in the 200 mg/kg DAF + Pb group, however, there was a significant difference between 100 mg/kg DAF + Pb, and 200 mg/kg DAF + Pb treated groups (p < 0.001). The least blood lead level was observed in the DAF treated rat group (Fig. 1A).
Fig. 1.
Effect of treatment with daflon and lead acetate on markers of lead toxicity and lipid peroxidation. a Effect of daflon and lead acetate on blood lead concentration. b Effect of daflon and lead acetate on serum malondialdehyde concentration. c Effect of daflon and lead acetate on blood zinc protoporphyrin levels. Values are presented as Mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, is statistically different compared to the control group (Pb). Key: DW (Distilled Water); Pb (Lead Acetate 90 mg/Kg); DAF (Daflon 100 mg/kg) 100 mg/Kg DAF + Pb (100 mg/Kg Daflon + Lead Acetate 90 mg/Kg); 200 mg/kg DAF + Pb (200 mg/Kg Daflon + Lead Acetate 90 mg/Kg)
Additionally, there was a significant decrease in the blood zinc protoporphyrin levels of rats in the DW (p < 0.01), DAF (p < 0.01), 100 mg/kg DAF + Pb (p < 0.05), and 200 mg/kg DAF + Pb (p < 0.01) treated groups compared to the Pb treated group which had the highest concentration (161.0 ± 8.14). No significant difference in blood zinc protoporphyrin levels was observed between rats in the DW, DAF and 200 mg/kg DAF + Pb treated groups. There was also no significant difference between 100 mg/kg DAF + Pb, and 200 mg/kg DAF + Pb treated groups. The least blood zinc protoporphyrin level was observed in the DAF treated rats (Fig. 1B).
Similarly, a significant decrease in serum malondialdehyde level was observed in the DW (p < 0.01), DAF (p < 0.01), 100 mg/kg DAF + Pb (p < 0.05), and 200 mg/kg DAF + Pb (p < 0.01) treated groups compared to the Pb treated group which had the highest concentration (1.08 ± 0.15). No significant difference in serum malondialdehyde concentration was recorded in rats in the DW, DAF, and 200 mg/kg DAF + Pb treated groups. Similarly, no significant difference was observed between 100 mg/kg DAF + Pb, and 200 mg/kg DAF + Pb treated groups. The least serum malondialdehyde concentration was observed in the 200 mg/kg DAF + Pb treated rats (Fig. 1C).
Effect of treatment with daflon and lead acetate on serum electrolyte levels
There was a significant (p < 0.05) decrease in the concentrations of serum sodium, potassium, chloride, and bicarbonate ions, in the Pb group compared to the DW, DAF, and 200 mg/kg DAF + Pb groups. However, the concentrations of serum potassium and chloride ions of rats in the Pb group were not significantly (p < 0.05) lower compared to the 100 mg/kg DAF + Pb group. There was no significant change (p < 0.05) in the concentrations of serum sodium, potassium, chloride, and bicarbonate ions of DW and 200 mg/kg DAF + Pb groups. The lowest electrolyte concentrations were observed in the group treated with Pb while the peak concentration was observed in the DAF treated rats (Fig. 2).
Fig. 2.
Effect of treatment with daflon and lead acetate on serum electrolyte levels. Serum concentrations of electrolytes were determined using an automated haematologic analyser. a Effects of treatment on Na+ concentration. b Effects of treatment on K+ concentration. c Effects of treatment on Cl− concentration. d Effects of treatment on HCO3 concentration. Values are presented as Mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, is statistically different compared to the control group (Pb). Key: DW (Distilled Water); Pb (Lead Acetate 90 mg/Kg); DAF (Daflon 100 mg/kg) 100 mg/Kg DAF + Pb (100 mg/Kg Daflon + Lead Acetate 90 mg/Kg); 200 mg/kg DAF + Pb (200 mg/Kg Daflon + Lead Acetate 90 mg/Kg)
Effect of treatment with daflon and lead acetate on activities of serum antioxidant enzymes
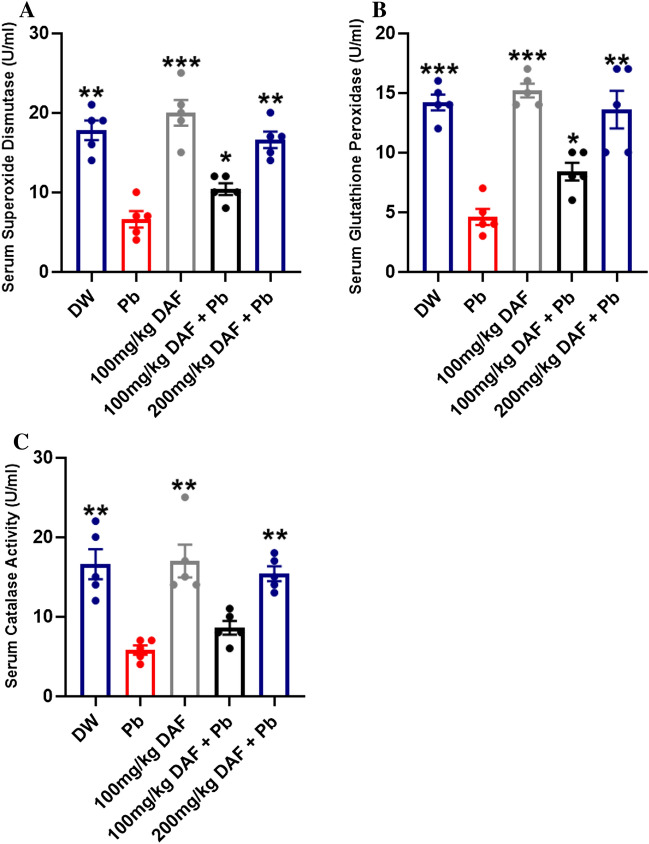
The activity of serum SOD was significantly elevated in rats treated with DW (p < 0.01), DAF (p < 0.001), 100 mg/kg DAF + Pb (p < 0.05), and 200 mg/kg DAF + Pb (p < 0.01) compared to the Pb treated group. There was no significant difference between the serum superoxide dismutase activities of rats in the DW group and those in the 200 mg/kg DAF + Pb group. However, there was a significant difference between 100 mg/kg DAF + Pb, and 200 mg/kg DAF + Pb treated groups (p < 0.01). The least superoxide dismutase activity was observed in the Pb treated group while the highest activity was recorded in rats treated with DAF only (Fig. 3A).
Fig. 3.
Effect of treatment with daflon and lead acetate on activities of serum antioxidant enzymes. a Effect of treatment with daflon and lead acetate on serum superoxide dismutase activity. b Effect of treatment with daflon and lead acetate on serum Glutathione peroxidase activity c Effect of treatment with daflon and lead acetate on serum catalase activity. Values are presented as Mean ± SEM. *p < 0.05, ** p < 0.01, ***p < 0.001, is statistically different compared to the control group (Pb). Key: DW (Distilled Water); Pb (Lead Acetate 90 mg/Kg); DAF (Daflon 100 mg/kg) 100 mg/Kg DAF + Pb (100 mg/Kg Daflon + Lead Acetate 90 mg/Kg); 200 mg/kg DAF + Pb (200 mg/Kg Daflon + Lead Acetate 90 mg/Kg)
The activity of serum glutathione peroxidase was significantly higher in rats in the DW (p < 0.001), DAF (p < 0.001), 100 mg/kg DAF + Pb (p < 0.05), and 200 mg/kg DAF + Pb (p < 0.01) groups when compared to the Pb group. There was no significant difference between the serum glutathione peroxidase activities of rats in the DW group and those in the DAF treated group. Notably, there was a significant difference between 100 mg/kg DAF + Pb, and 200 mg/kg DAF + Pb treated groups (p < 0.01). The least glutathione peroxidase activity was observed in the Pb treated group while the highest activity was recorded in rats treated with DAF only (Fig. 3B).
The activity of catalase was significantly higher in rats in the DW (p < 0.01), DAF (p < 0.01), 100 mg/kg DAF + Pb (p < 0.05), and 200 mg/kg DAF + Pb (p < 0.01) groups compared to the Pb group. There was no significant difference between the catalase activities of rats in the DW group and those in the 200 mg/kg DAF + Pb group. Besides, there was a significant difference between 100 mg/kg DAF + Pb, and 200 mg/kg DAF + Pb treated groups (p < 0.01). The least catalase activity was observed in the Pb treated group while the highest activity was recorded in rats treated with DAF only (Fig. 3C).
Relationship between MDA concentration and blood lead levels following sub-chronic administration of daflon
A strong correlation was observed between the concentration of MDA and blood lead levels. Pearson’s correlation coefficient of r = 0.5786 and p = 0.0024 was observed (Fig. 4).
Fig. 4.
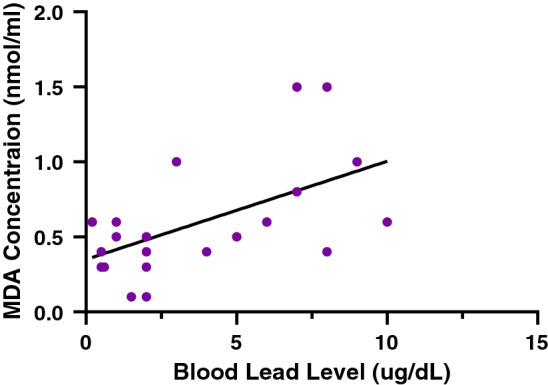
Relationship between MDA concentration and blood lead levels following sub-chronic administration of daflon. r = Pearson’s correlation coefficient, p < 0.05 are considered significant
Effects of daflon and lead acetate on histo-architecture of organs
Effects of daflon and lead acetate on histo-architecture of the cerebral cortex
The normal architecture of the cerebral cortex of the brain was revealed in distilled water treated rats with normal molecular cell layers, pyramidal cell layer, and granular cell layers (Fig. 5A). Rats treated with lead acetate only showed degenerated neurons with irregular contours, vacuoles, and depletion of cells in the successive layers of the cerebral cortex. Thus, the delamination of the cerebral cortex layers (Fig. 5B). Rats treated with Daflon (100 mg/kg) showed perfect arrangement in the molecular cell layer, granular cell layer, and pyramidal cell layer (Fig. 5C). Rats pretreated with Daflon (100 mg/kg and 200 mg/kg) followed by lead acetate (90 mg/kg) showed a well-arranged molecular cell layer, granular cell layer with slight a distortion of pyramidal cell layer and slightly degenerated neurons (Fig. 5D and 5E respectively).
Fig. 5.
Effects of daflon and lead acetate on histo-architecture of the cortex. a DW; b Pb; c DAF; d 100 mg/kg DAF + Pb; E 200 mg/kg DAF + Pb. (H and E × 250). Key: DW (Distilled Water); Pb (Lead Acetate 90 mg/Kg); DAF (Daflon 100 mg/kg) 100 mg/Kg DAF + Pb (100 mg/Kg Daflon + Lead Acetate 90 mg/Kg); 200 mg/kg DAF + Pb (200 mg/Kg Daflon + Lead Acetate 90 mg/Kg). V-Vacuoles; N-Normal Neurons; D-Degenerated Neurons
Effects of daflon and lead acetate on histo-architecture of the liver
The normal architecture of liver sinusoid with Kupffer cell, central vein, and viable hepatocyte was noted in rats treated with distilled water (Fig. 6A). Rats that were treated with lead acetate exhibited interrupted liver parenchyma with indications of hyperaemia in the liver sinusoids and a congested central vein. There was also focal necrosis of some hepatocytes which appeared vacuolated (Fig. 6B). Rats treated with daflon (100 mg/kg) showed a preserved liver parenchyma, hepatocytes appeared viable (Fig. 6C). Rats pretreated with Daflon (100 mg/kg and 200 mg/kg) followed by lead acetate (90 mg/kg) showed preserved liver parenchyma, hepatocytes appear viable, and prominent kupffer cells were seen in sinusoids (Fig. 6D and 6E respectively).
Fig. 6.
Effects of daflon and lead acetate on histo-architecture of the liver. a DW; b Pb; c DAF; d 100 mg/kg DAF + Pb; E) 200 mg/kg DAF + Pb. (H and E × 250). Key: DW (Distilled Water); Pb (Lead Acetate 90 mg/Kg); DAF (Daflon 100 mg/kg) 100 mg/Kg DAF + Pb (100 mg/Kg Daflon + Lead Acetate 90 mg/Kg); 200 mg/kg DAF + Pb (200 mg/Kg Daflon + Lead Acetate 90 mg/Kg). S-liver sinusoid; CV-central vein (CV); H-hepatocyte
Discussion
Blood and or its constituents are the primary indicators of internal lead exposure. Good biochemistry of lead toxicity is crucial to understanding alterations in serum electrolytes observed in lead-exposed subjects. This is imperative to offering alternatives to diagnosis, and management of plumbism. The result from this study revealed a significant reduction in the mean serum electrolyte levels in the group of rats treated with Pb acetate. One of the critical mechanisms of lead toxicity on the molecular machinery of animals is its covalent binding to the electrolyte carrier proteins albumin and calmodulin, oxidative damage to cell membranes [34].
Importantly, lead has been demonstrated to inhibit the transformation of coproporphyrinogen III to protoporphyrin IX resulting in a diminution in haemoglobin generation and a shortened life span of erythrocytes [35]. The activity of the ferrochelatase enzyme which catalyses iron incorporation into protoporphyrin IX is also impaired by lead. This impairment causes zinc-protoporphyrin formation, which has also been used as a biomarker of lead toxicity [36]. Moreover, lead has been reported to induce oxidative damage to biological membranes by the accumulation of oxidant metabolites such as free protoporphyrins, and also by direct or indirect inhibition of antioxidant enzymes, thereby reducing the total antioxidant protection of the cell, affecting membrane structure and function and altering physiological processes of organs and tissues [37]. Daflon was able to improve the zinc protoporphyrin levels in the administered groups by forming a molecular complex, which prevented the availability of lead and thus hindered lead entry and accumulation in the tissues of the rats.
Exposure to lead may alter antioxidant defense mechanisms, resulting in an imbalance between radical-generating and radical-scavenging activities, and consequently, the generation of oxidation products. Malondialdehyde (MDA) has been reported to be a major end-product of lipid peroxidation, and its concentration is determined by the magnitude of MDA in blood/tissue samples [38]. The alterations on the surface of erythrocyte membranes caused by lead intoxication could be responsible for the increased MDA concentration obtained in the lead acetate-exposed group, and hence, indicates an increased formation of free radicals by the heavy metal. The finding of elevated MDA concentration in the lead acetate-exposed animals is consistent with results obtained in previous studies [39–42], verifying that exposure to lead correlates with the increase in MDA concentration. The group pretreated with daflon, however, exhibited considerably lower MDA levels; hence, suggesting the ability of daflon to function as an effective antioxidant in preventing lead-induced oxidative damage.
The physiological system of electrolyte balance regulates serum and intracellular concentrations, and likewise, the optimum mineral contents in tissues. Our result shows that imbalances in electrolyte levels were most evidenced in the lead acetate treated group. This imbalance may impair homeostasis and alter physiological activities maintained via coordinated interaction of organs including the intestines, blood, kidney, and the site of net absorption and excretion. These findings demonstrate that lead toxicity is the foremost culprit in the observed electrolyte imbalance. Our results corroborate that of Liu et al. [34] who showed that exposure to lead had a considerable effect on serum electrolyte levels. Our results also showed that daflon counteracted the poisonous effects of lead in the exposed subjects. It is suggested that daflon exerted its protective effect by preventing oxidative damage to cell membranes [34].
Similarly, lead acetate was noted to cause alterations in serum activities of antioxidant enzymes (SOD, CAT, and GPx) in the lead acetate-subjected animals, and this can be linked to the possible involvement of oxidative stress in the pathophysiology of lead poisoning [43].
The finding of decreased serum activities of antioxidant enzymes in the lead acetate exposed subjects when compared to the control and the groups pretreated with graded doses of daflon is in line with the work of previous researchers [42, 44], who reported similar results as that obtained in this study. One of the possible explanations for this finding is ascribed to the auto-oxidation of over accumulated aminolevulinic acid due to the inhibition of aminolevulinic acid dehydratase, which might have culminated in the generation of superoxide and hydrogen peroxide [21, 45].
Similarly, under physiological conditions, cells possess both enzymatic and non-enzymatic defense to cope with free radicals [46], however, when oxidative stress ensues, these mechanisms are distorted and require the introduction of exogenous antioxidants in some instances, as was the case in this study. Oxidative damage, however, may occur when the antioxidant potential is decreased leading to an increase in oxidative stress [47]. These observations give credence to lead as having a high affinity for sulfhydryl (SH) groups and can alter the antioxidant activities of SOD, CAT, GPx, and glucose-6-phosphate dehydrogenase (G6PD) by inhibiting functional SH groups and forming less stable mercaptide complexes [48].
Apart from targeting the sulfhydryl groups, lead can also replace the zinc ions that serve as important co-factors for antioxidant enzymes and inactivate them [49]. Increased susceptibility of cells to oxidative stress may arise because lead toxicity affects the uptake of important trace elements (Se, Zn, and Cu) required by antioxidant enzymes (GPx, CAT, and SOD) for proper molecular structure and activity. The dose-dependent significant difference observed in the serum activities of these antioxidant enzymes in the group pretreated with daflon when compared to the lead acetate exposed group indicates the ameliorative potentials of the flavonoid mixture.
The finding in the current study of the disorganisation of cells in the successive layers of the cerebral cortex, typified by disruption of cell layer arrangement in the lead acetate-treated rats may be attributable to depletion of cells in the granular layer of the cerebral cortex. The findings in the brain of lead acetate-treated rats in this study agree with some previous reports [50, 51]. These changes could be consequent effects of lead toxicity on oxygen and sulfur-containing bioligands, leading to oxidative stress that is detrimental to the survival of cells [52]. It has been reported that the brain is susceptible to peroxidase damage because of several factors such as high oxygen tension, low mitotic rate, high lipid content, and as well as low antioxidant concentration [53]. These factors make the brain more vulnerable to lead toxicity than any other organ of the exposed rats.
The hepatic changes observed in the lead acetate-treated rats may be reflective of damage to hepatocytes, as previously reported [54], possibly due to cycling of the heavy metal or possible interaction with proteins and enzymes of the hepatic parenchymal tissue, thereby interfering with the antioxidant defense mechanism. This is thought to result in the buildup of free radicals which eventually initiate inflammatory responses [55]. The observed focal necrosis of hepatocytes seen in the lead acetate-treated group, which were similarly reported by Ozkaya et al. [56], could also be a result of perivascular hypoxia due to a reduction in circulating red blood cell mass [21]. The milder hepatic damage observed in the rats pretreated with daflon followed by exposure to lead acetate may be due to the antioxidant effect of daflon [57] on possible lead acetate-induced oxidative stress.
The present study has demonstrated that sub-chronic lead acetate administration to male Wistar rats for 42 days caused lowered levels of serum electrolytes, and activities of SOD, GPx, and CAT, as well as higher values of blood lead levels, zinc protoporphyrin, and MDA when compared to all other treatment groups. The study also demonstrated histopathological alterations in the brain and liver of rats administered with lead acetate. On the other hand, pretreatment with graded doses of daflon protected against increased blood lead levels, electrolyte imbalance, oxidative stress, lipid peroxidation, and histopathological changes following sub-chronic lead acetate administration in male Wistar rats. It is therefore plausible that consumption of foods containing natural antioxidants, diosmin, and hesperidin may be recommended for individuals living in areas with high environmental lead contamination.
Acknowledgement
Authors are grateful to Mr. Denis Otie and Abdulwahab Hashimu of Veterinary Pharmacology and Toxicology Laboratory, Ahmadu Bello University Zaria for their technical inputs in the accomplishment of this project.
Funding
No external fund was received for this work.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s43188-024-00257-w
Change history
7/30/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1007/s43188-024-00257-w
References
- 1.Burki T (2020) Report says 815 million children have high blood lead levels. Lancet 396:370. 10.1016/S0140-6736(20)31684-6 10.1016/S0140-6736(20)31684-6 [DOI] [PubMed] [Google Scholar]
- 2.Hedayati A, Darabitabar F (2017) Lethal and sub-lethal impacts of lead on some hematological, biochemical and immunological indices in Caspian roach. Pollution 3:21–27. 10.22059/poll.2017.59567 10.22059/poll.2017.59567 [DOI] [Google Scholar]
- 3.Bah H, Bandeira MJ, Gomes-Junior EA, Anjos A, Rodrigues Y, Dos Santos NR, Martinez VO, Rocha R, Costa RG, Adorno EV, Menezes-Filho JA (2020) Environmental exposure to lead and hematological parameters in Afro-Brazilian children living near artisanal glazed pottery workshops. J Environ Sci Health 55:964–974. 10.1080/10934529.2020.1761738 10.1080/10934529.2020.1761738 [DOI] [PubMed] [Google Scholar]
- 4.Ayla O, Metin O (2015) Biochemistry of reactive oxygen and nitrogen species. Faculty of Veterinary Medicine, University of Kafkas, Turkey, Croatia, InTech, pp 37–58. 10.5772/61193
- 5.Yabe J, Nakayama SM, Nakata H, Toyomaki H, Yohannes YB, Muzandu K, Kataba A, Zyambo G, Hiwatari M, Narita D, Yamada D, Hangoma P, Munyinda NS, Mufune T, Ikenaka Y, Choongo K, Ishizuka M (2020) Current trends of blood lead levels, distribution patterns and exposure variations among household members in Kabwe, Zambia. Chemosphere 243:125412. 10.1016/j.chemosphere.2019.125412 10.1016/j.chemosphere.2019.125412 [DOI] [PubMed] [Google Scholar]
- 6.Wani AL, Ara A, Usmani JA (2015) Lead toxicity: a review. Interdiscip Toxicol 8:55–64. 10.1515/intox-2015-0009 10.1515/intox-2015-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agency for Toxic Substances and Disease Registry (ATSDR) (2020) Toxicological profile for Lead. U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA. 10.15620/cdc:95222
- 8.Ameh MP, Mohammed M, Ofemile YP, Mohammed MG, Gabriel A, Isaac AO (2020) Detoxifying Action of Aqueous Extracts of Mucuna pruriens Seed and Mimosa pudica Root Against Venoms of Naja nigricollis and Bitis arietans. Recent Pat Biotechnol 14:134–144. 10.2174/1872208313666191025110019 10.2174/1872208313666191025110019 [DOI] [PubMed] [Google Scholar]
- 9.Isaac AO, Joseph AO, Victor SO, Lamidi YI, Andrew AM (2017) Ameliorative effects of kaempferol and zinc gluconate on erythrocyte osmotic fragility and haematological parameters in Wistar rats exposed to noise stress. Insights Biomed 2:15. 10.21767/2572-5610.100031 10.21767/2572-5610.100031 [DOI] [Google Scholar]
- 10.Bokara KK, Brown E, McCormick R, Yallapragada PR, Rajanna S, Bettaiya R (2008) Lead-induced increase in antioxidant enzyme and lipid peroxidation products in developing rat brain. Biometals 21:9–16. 10.1007/s10534-007-9088-5 10.1007/s10534-007-9088-5 [DOI] [PubMed] [Google Scholar]
- 11.Wei W, Wu X, Bai Y, Li G, Feng Y, Meng H, Li H, Li M, Zhang X, He M, Guo H (2020) Lead exposure and its interactions with oxidative stress polymorphisms on lung function impairment: results from a longitudinal population-based study. Environ Res 187:109645. 10.1016/j.envres.2020.109645 10.1016/j.envres.2020.109645 [DOI] [PubMed] [Google Scholar]
- 12.Gems D, Patridge L (2008) Stress-response hormesis and aging: that which does not kill us makes us stronger. Cell Metab 7:200–203. 10.1016/j.cmet.2008.01.001 10.1016/j.cmet.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 13.Isaac A, Ibrahim Y, Andrew A, Edward D, Solomon A (2017) The cortisol steroid levels as a determinant of health status in animals. J Proteomics Bioinform 10:277–283. 10.4172/jpb.1000452 10.4172/jpb.1000452 [DOI] [Google Scholar]
- 14.Adinortey MB, Sarfo JK, Kwarteng J, Adinortey CA, Ekloh W, Kuatsienu LE, Kwadwo Nyarko A (2018) The Ethnopharmacological and Nutraceutical Relevance of Launaea taraxacifolia (Willd.) Amin ex C. Jeffrey. Evid Based Complement Alternat Med 2018:7259146. 10.1155/2018/7259146 10.1155/2018/7259146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akefe IO, Ayo JO, Sinkalu VO (2020) Kaempferol and zinc gluconate mitigate neurobehavioral deficits and oxidative stress induced by noise exposure in Wistar rats. PLoS ONE 15:e0236251. 10.1371/journal.pone.0236251 10.1371/journal.pone.0236251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodowska MK (2017) Natural flavonoids: classification, potential role, and application of flavonoid analogues. Eur J Biol Res 7:108–123. 10.5281/zenodo.545778 10.5281/zenodo.545778 [DOI] [Google Scholar]
- 17.Shubina VS, Shatalin YV (2017) Antioxidant and iron-chelating properties of taxifolin and its condensation product with glyoxylic acid. J Food Sci Technol 54:1467–1475. 10.1007/s13197-017-2573-0 10.1007/s13197-017-2573-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramlet AA (2001) Clinical benefits of Daflon 500 mg in the most severe stages of chronic venous insufficiency. Angiology 52:49–56. 10.1177/000331970105200107 10.1177/000331970105200107 [DOI] [PubMed] [Google Scholar]
- 19.Rizk SM, Sabri NA (2009) Evaluation of clinical activity and safety of Daflon 500 mg in type 2 diabetic female patients. Saudi Pharm J 17:199–207. 10.1016/j.jsps.2009.08.008 10.1016/j.jsps.2009.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prabhu VV, Sathyamurthy D, Ramasamy A, Das S, Anuradha M, Pachiappan S (2016) Evaluation of protective effects of diosmin (a citrus flavonoid) in chemical-induced urolithiasis in experimental rats. Pharm Biol 54:1513–1521. 10.3109/13880209.2015.1107105 10.3109/13880209.2015.1107105 [DOI] [PubMed] [Google Scholar]
- 21.Lamidi IY, Hudu MG, Akefe IO, Adamu S, Salihu SI (2020) Sub-chronic administration of flavonoid fraction Daflon improve lead-induced alterations in delta-aminolevulinic acid dehydratase activity, erythrocytic parameters, and erythrocyte osmotic fragility in Wistar rats. Comp Clin Pathol. 10.1007/s00580-020-03144-6 10.1007/s00580-020-03144-6 [DOI] [Google Scholar]
- 22.Eman G, Fawzi B (2020) Micronized flavonoid fraction Daflon 500 protects heart against ischemia–reperfusion injury: an old medicine for a new target. All Life 13:556-568. 10.1080/26895293.2020.1832921 10.1080/26895293.2020.1832921 [DOI] [Google Scholar]
- 23.Lamidi IY, Akefe IO (2017) Mitigate effects of antioxidants in lead toxicity. Clin Pharmacol Toxicol J 1:1–9 [Google Scholar]
- 24.Garber JC, Barbee RW, Bielitzki JT, Clayton LA, Donovan JC, Kohn DF, Lipman NS et al (2011) Guide for the care and use of laboratory animals, 8th edn. National Academies Press, Washington, DC [Google Scholar]
- 25.Kobo PI, Ayo JO, Aluwong T, Zezi AU, Maikai V, Ambali SF (2014) Flavonoid mixture ameliorates increase in erythrocyte osmotic fragility and malondialdehyde concentration induced by Trypanosoma brucei brucei-infection in Wistar rats. Res Vet Sci 96:139–142. 10.1016/j.rvsc.2013.10.005 10.1016/j.rvsc.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 26.Erick H, Yong W, Ian DB (2016) A novel methodology for rapid digestion of rare earth element ores and determination by microwave plasma-atomic emission spectrometry and dynamic reaction cell-inductively coupled plasma-mass spectrometry. Talanta 160:521–527. 10.1016/j.talanta.2016.07.067 10.1016/j.talanta.2016.07.067 [DOI] [PubMed] [Google Scholar]
- 27.Grandjean P (1979) Occupational lead exposure in Denmark: screening with a haematofluorimeter. Br J Ind Med 36:52–58. 10.1136/oem.36.1.52 10.1136/oem.36.1.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin JP Jr, Dailey M, Sugarman E (1987) Negative and positive assays of superoxide dismutase based on haematoxylin autoxidation. Arch Biochem Biophys 225:329–336. 10.1016/0003-9861(87)90400-0 10.1016/0003-9861(87)90400-0 [DOI] [PubMed] [Google Scholar]
- 29.Palgia DE, Valentine WN (1967) Studies on qualitative and quantitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169 [PubMed] [Google Scholar]
- 30.Beers FN, Sizer IW (1952) A spectrophotometric method for measuring the break-down of hydrogen peroxide by catalase. J Biol Chem 195:133–140 10.1016/S0021-9258(19)50881-X [DOI] [PubMed] [Google Scholar]
- 31.Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431. 10.1016/0076-6879(90)86135-i 10.1016/0076-6879(90)86135-i [DOI] [PubMed] [Google Scholar]
- 32.Altuntas I, Delibas N, Sutcu R (2002) The effects of organophosphate insecticide methidathion on lipid peroxidation and anti-oxidant enzymes in rat erythrocytes: role of vitamins E and C. Hum Exp Toxicol 21:681–685. 10.1191/0960327102ht304oa 10.1191/0960327102ht304oa [DOI] [PubMed] [Google Scholar]
- 33.Luna GH (1960) Manual of histologic staining method of armed forces institute of pathology, 35th edn. McGraw-Hill Book Company, New York, p 46 [Google Scholar]
- 34.Akefe IO, Yusuf IL, Adegoke VA (2019) C-glycosyl flavonoid orientin alleviates learning and memory impairment by radiofrequency electromagnetic radiation in mice via improving antioxidant defence mechanism. Asian Pac J Trop Biomed 9:518-523. 10.4103/2221-1691.271725
- 35.Suradkar SG, Ghodasara DJ, Vihol P, Patel J, Jaiswal V, Prajapati KS (2009) Haematobiochemical alterations induced by lead acetate toxicity in wistar rats. Vet World 2:429–439 [Google Scholar]
- 36.Sakai T (2000) Biomarkers of lead exposure. Ind Health 38:127–142. 10.2486/indhealth.38.127 10.2486/indhealth.38.127 [DOI] [PubMed] [Google Scholar]
- 37.Rendon-Ramirez A, Cerbon-Solorzano J, Maldonado-Vega M, Quintanar-Escorza MA, Calderon-Salinas JV (2007) Vitamin E reduces the oxidative damage on d-aminolevulinic dehydratase induced by lead intoxication in rat erythrocytes. Toxicol In Vitro 21:1121–1126. 10.1016/j.tiv.2007.04.019 10.1016/j.tiv.2007.04.019 [DOI] [PubMed] [Google Scholar]
- 38.Eze JI, Anene BM, Chukwu CC (2008) Determination of serum and organ malondialdehyde (MDA) concentration, a lipid peroxidation index in Trypanosoma bruceiinfected rats. Comp Clin Pathol 17:67–72. 10.1007/s00580-008-0722-6 10.1007/s00580-008-0722-6 [DOI] [Google Scholar]
- 39.Aksu DS, Didin M, Kayikci F (2012) The protective role of polyphenols on blood cells in rats exposed to lead. Rev Română Med Lab 20:233–243 [Google Scholar]
- 40.Nisar NA, Sultana M, Waiz HA, Para PA, Baba NA, Zargar FA, Raja WH (2013) Experimental study on the effect of vitamin C administration on lipid peroxidation and antioxidant enzyme activity in rats exposed to chlorpyriphos and lead acetate. Vet World 6:461–466. 10.5455/vetworld.2013.461-466 10.5455/vetworld.2013.461-466 [DOI] [Google Scholar]
- 41.Wang J, Zhu H, Yang Z, Liu Z (2013) Antioxidative effects of hesperetin against lead acetate-induced oxidative stress in rats. Indian J Toxicol 45:395–398. 10.4103/0253-7613.115015 10.4103/0253-7613.115015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okediran BS, Biobaku KT, Olaifa FH, Atata AJ (2017) Haematological and antioxidant enzyme response to lead toxicity in male Wistar rats. Ceylon J Sci 46:31–37. 10.4038/cjs.v46i2.7427 10.4038/cjs.v46i2.7427 [DOI] [Google Scholar]
- 43.Jalali MS, Seyedeh NH, Mousavi M (2017) Comparative effect of silymarin and D-penicillamine on lead induced hemotoxicity and oxidative stress in rat. Iranian J Toxicol 11:12–18. 10.29252/arakmu.11.3.11 10.29252/arakmu.11.3.11 [DOI] [Google Scholar]
- 44.Ujowundu CO, Okwu GN, Achilike JJ, Nwaogu LA, Iheme CI (2017) Lead-induced oxidative stress and chemoprotective role of dietary supplements on Wistar albino rats. Annu Res Rev Biol 13:1–14. 10.9734/ARRB/2017/33167 10.9734/ARRB/2017/33167 [DOI] [Google Scholar]
- 45.Gurer H, Ozgunes H, Neal R, Spitzand DR, Ercal N (1998) Antioxidant effects of N–acetyl cysteine and succimer in red blood cells from lead-exposed rats. Toxicology 128:181–189. 10.1016/j.tox.2004.07.006 10.1016/j.tox.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 46.Shalana M, Mostafab M, Hassounab M (2005) Amelioration of lead toxicity on rat liver with vitamin and silymarin supplements. Toxicology 206:1–15. 10.1016/j.tox.2004.07.006 10.1016/j.tox.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 47.Patra RCD, Swarup SK, Dwivedi AS (2001) Trace minerals in blood of young calves during exposure to lead. Indian J Animal Sci 71:507–510. 10.4061/2011/457327 10.4061/2011/457327 [DOI] [Google Scholar]
- 48.Patra RC, Rautray AK, Swarup D (2011) Oxidative stress in lead and cadmium toxicity and its amelioration. Vet Med Int 22:1–9. 10.4061/2011/457327 10.4061/2011/457327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flora SJ, Flora G, Saxena G, Mishra M (2007) Arsenic and lead induced free radical generation and their reversibility following chelation. Cell Mol Biol 53:26–47. 10.1170/T773 10.1170/T773 [DOI] [PubMed] [Google Scholar]
- 50.Sidhu P, Nehru B (2004) Lead intoxication: histological and oxidative damage in rat cerebrum and cerebellum. J Trace Elements Exp Med 17:45–53. 10.1002/jtra.10052 10.1002/jtra.10052 [DOI] [Google Scholar]
- 51.Kayode IMO, Olugbenga IE (2017) Lead acetate induced cerebral tissue damage; The effect of Phoenix dactylifera pits extract. Eur J Med Plants 21:1–9. 10.9734/EJMP/2017/37302 10.9734/EJMP/2017/37302 [DOI] [Google Scholar]
- 52.Lawton LJ, Donaldson WE (1991) Lead-induced tissue fatty acid alterations and lipid peroxidation. Biol Trace Elem Res 28:83–97. 10.1007/BF02863075 10.1007/BF02863075 [DOI] [PubMed] [Google Scholar]
- 53.Julka D, Pal R, Gill KD (1992) Neurotoxicity of dichlorvos: effect on antioxidant system in the rat central nervous system. Exp Mol Pathol 56:144–152. 10.1016/0014-4800(92)90031-6 10.1016/0014-4800(92)90031-6 [DOI] [PubMed] [Google Scholar]
- 54.Gajawat S, Sancheti G, Goyal P (2005) Vitamin C against concomitant exposure to heavy metal and radiation: a study on variations in hepatic cellular counts. Asian J Exposure Sci 19:53–58. 10.1007/s12291-013-0375-3 10.1007/s12291-013-0375-3 [DOI] [Google Scholar]
- 55.Johar D, Roth JC, Bay GH, Walker JN, Kroczak TJ, Los M (2004) Inflammatory response, reactive oxygen species, programmed (necrotic-like and apoptotic) cell death and cancer. Roczniki Akademia Medyczna Bialymstoku 49:31–39 [PubMed] [Google Scholar]
- 56.Ozkaya A, Sahin Z, Kuzu M, Selim Y, Mustafa S, Mirac O, Ertan U, Veysel Y, Ramazan C, Yologlu S (2017) Role of geraniol against lead acetate-mediated hepatic damage and their interaction with liver carboxylesterase activity in rats. Arch Physiol Biochem 17:1–8. 10.1080/13813455.2017.1364772 10.1080/13813455.2017.1364772 [DOI] [PubMed] [Google Scholar]
- 57.Yasim A, Ozbag D, Kilinc M, Ciralik H, Toru I (2011) The effects of diosmin-hesperidin combination treatment on the lipid profile and oxidative antioxidative systems in high-cholesterol diet-fed rats. Turkey Gorgus Kalp Damar Cerrahisi Dergisi 1:55–61. 10.5897/IJMMS.9000121 10.5897/IJMMS.9000121 [DOI] [Google Scholar]