Abstract
In vivo phototoxicity testing is important for predicting drug-induced phototoxicity in humans. Currently, there is no internationally validated in vivo test method for the photosafety evaluation of pharmaceuticals. In this study, we evaluated the phototoxicity of systemically administered drugs using SD rats. We first determined the appropriate ultraviolet A (UVA) dose using 8-methoxypsoralen, a well-known phototoxic drug. Compared to lower and higher UVA doses, we found that a UVA dose of 10 J/cm2 allowed for the detection of phototoxic responses in both a dose- and time-dependent manner. We next performed a phototoxicity study using seven pharmaceutical drugs which included known phototoxic and non-phototoxic drugs using a UVA dose of 10 J/cm2. In order to improve the accuracy of our assessment, we evaluated both gross skin findings as well as histopathological findings. Using gross skin findings alone resulted in an accuracy of 85.7% which could be increased to 100% accuracy when the gross skin findings were combined with histopathological findings. This study suggests that the inclusion of histopathological findings increases the accuracy of the phototoxicity evaluation of systemically administered drugs in SD rats. In conclusion, we found that for studying drug-induced phytotoxicity, a 10 J/cm2 UVA dose serves as the optimal radiation dose, and that the inclusion of histopathological findings increases the accuracy of the phototoxicity evaluation of the drugs.
Keywords: Phototoxicity, In vivo test, UVA, Skin reaction, Histopathological finding
Introduction
Phototoxicity is an acute photo-induced skin irritation that occurs when photoreactive chemicals are applied topically or systemically [1]. Upon irradiation with sunlight, the absorbed light energy causes a molecular change in photoreactive chemical substances, causing phototoxicity [2]. Most phototoxic reactions are caused by UVA, because UVB does not penetrate the dermis as deeply as UVA, and UVC is mostly filtered by the atmosphere [3]. The most common clinical symptoms of phototoxic reactions are burning type reactions in which erythema, edema, blisters, and hyperpigmentation of the exposed areas occur [4].
Among pharmaceuticals, some anti-inflammatory analgesics, quinolone antibiotics, and tricyclic antidepressants have been reported to cause phototoxicity [5–10]. Since 2000, regulatory authorities have begun to recognize that the phototoxic potential of pharmaceuticals should be evaluated. In this regard, the US FDA, EU EMA, ICH, and other regulatory agencies now provide photo-safety guidelines that introduce test methods and evaluation strategies. The in vitro 3T3 neutral red uptake phototoxicity test (3T3 NRU PT, OECD TG432) is the only test method recommended in ICH S10, but has the disadvantage of having a high false positive rate [11, 12]. As mentioned in ICH S10, negative results in an appropriately conducted in vivo test can often appear as positive results in an in vitro phototoxicity test. Therefore, it is important that phototoxicity assessments are occasionally performed using in vivo phototoxicity tests to confirm any positive findings from the in vitro test. Currently, in vivo phototoxicity studies can be conducted using guinea pigs, rats, and mice [10, 13–19], but standardized in vivo assays have not been proposed in the ICH S10, OECD test guidelines, or other international guidelines.
These models use various irradiation doses of UVA and/or UVB. Although the type of light source and the irradiation dose have an impact on the phototoxic responses, little attention has been paid to the conditions used for light irradiation. In addition, in most of these models the phototoxic response is evaluated using visual observations and through scoring of a representative clinical phototoxic response. These methods are, therefore, limited by subjectivity and may be unable to detect toxicity depending on the method used for the gross examination of the skin.
The purpose of this study was to select the appropriate UVA dose for in vivo phototoxicity testing and to evaluate the phototoxicity of drugs by observing skin reactions. We selected seven drugs with well-known in vivo and clinical phototoxicity profiles. First, the dose of UVA was selected using the well-known phototoxic compound 8-MOP. The phototoxicity test was then performed using the selected UVA dose, and the phototoxicities of the seven drugs were determined both by gross examination of the skin and by histopathology.
Materials and methods
Chemicals
8-Methoxypsoralen (8-MOP), chlorpromazine (CPZ), ketoprofen (KPF), lomefloxacin (LMFX), nalidixic acid (NA), sparfloxacin (SPFX), gatifloxacin (GTFX), distilled water (DW) and corn oil were purchased from Sigma-Aldrich (St. Louis, MO, USA). Pentobarbital, an anesthetic, was obtained from Hanlim Pharm. Co., Ltd. (Seoul, Korea). All other reagents were purchased from commercial suppliers.
Animals
Female Sprague–Dawley (SD) rats (6 weeks old, Specific Pathogen Free) were purchased from Koatech (Pyeongtaek, Korea) and kept at an animal facility in the Ministry of Food and Drug Safety. Korea (Certification Number: 1501MFDS08) in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International Animal Care Policies (Accredited Unit, MFDS: Unit No. 001492). The animals were given free access to solid pelletized diets (Purina Mills Inc., Seoul, Korea) and sterilized water ad libitum. The rats were housed in an animal room maintained at a temperature of 22 ± 1 °C and a relative humidity of 50 ± 10%. The room was lit by artificial light for 12 h per day. The rats were acclimated for at least 5 days before experiments. In the study, all rats were 7–8 weeks of age. Weight variations did not exceed 20% of the mean weight. Any rats with abnormal observations were not used. The rats were randomly allocated to the test groups according to body weight (three rats per group).
Irradiation conditions
A UV irradiation device (Bio-Spectra, Vilber lourmat, Germany) equipped with a UV tube (T-40.L, Vilber lourmat, Germany) was used as a light source. The UV intensity at 365 nm (UVA) was measured using a UV intensity meter (UVP UVX radiometer: UVX-36 sensor; Analytik jena, Jena, Germany). UVA intensity was adjusted to 6 mW/cm2 and the animals were anesthetized and immobilized prior to constant light irradiation.
Selection of UVA irradiation dose
UVB-induced phototoxicity is rarely a problem for pharmaceuticals with systemic exposure since UVB minimally penetrates beyond the epidermis [11]. Therefore, only UVA, a long wavelength radiation that penetrates deep into the skin, was used. A UVA dose selection test was performed to select the UVA dose over the range of 5–20 J/cm2 suggested by ICH S10. The dose selection test was conducted using a well-known positive compound (8-MOP). 8-MOP was orally administered to the rats at 1, 5 and 10 mg/kg and then hair was removed from the animals back after anesthesia. The backs of the animals were irradiated with UVA at 5, 10, 15 and 20 J/cm2 at 2 × 5 cm2 irradiation site. After 24, 48, and 72 h of UVA irradiation, the skin reaction was evaluated and scored according to Draize’s criteria by gross examination of the skin (Table 1) [20].
Table 1.
Evaluation of skin reactions (Draize’s criteria: 1959)
| Score for erythema/eschar formation: | |
|---|---|
| 0; | No erythema |
| 1; | Very slight erythema (barely perceptible) |
| 2; | Well defined erythema |
| 3; | Moderate to severe erythema |
| 4; | Severe erythema (beet redness) to slight eschar formation (injuries in depth) |
| Score for edema formation: | |
| 0; | No edema |
| 1; | Very slight edema (barely perceptible) |
| 2; | Slight edema (edges of area well defined by definite raising) |
| 3; | Moderate edema (raised approximately 1 mm) |
| 4; | Severe edema (raised more than 1 mm and extending beyond area of exposure) |
Skin phototoxicity assessment for systemically administered drugs
This study used six known phototoxic drugs (8-MOP, LMFX, NA, CPZ, SPFX, KPF) and one known non-phototoxic drug (GTFX) which were chosen based on clinical and in vivo test data [18]. DW and corn oil were used as the solvent, and the concentration at which the test drug was maximally dissolved was used. 8-MOP and NA were dissolved in corn oil (10 mg/mL for 8-MOP and 1000 mg/mL for NA) and LMFX, CPZ, SPFX, KPF and GTFX were dissolved in distilled water (100 mg/mL for LMFX, 100 mg/mL for CPZ, 500 mg/mL for SPFX, 60 mg/mL for KPF, and 500 mg/mL for GFTX). The drugs were orally administered to rats at a dosing volume of 10 mL/kg. Rats were sedated with pentobarbital (22.5 mg/kg) via intraperitoneal injection before irradiation. The entire back of each animal was epilated, and a piece of aluminum foil in a rectangular shape (2 × 5 cm2) was placed over the back prior to light irradiation. The irradiation start time was set based on Tmax. The Tmax for each drug was obtained from previous reports [17–19]. The times used were: 0.5 h for NA and KPF; 1 h for 8-MOP, LMFX, SPFX, and GTFX; 2 h for CPZ. Before light irradiation, the UVA intensity was measured using a UV intensity meter. Animals were irradiated for about 0.5 h at an intensity of 6 mW/cm2 to irradiate with UVA at 10 J/cm2. After 24, 48, and 72 h of UVA irradiation, the skin reaction was evaluated by gross examination of the skin according to Draize’s criteria and by histopathology (Table 1) [20].
Skin reaction evaluations
Gross skin examination
The most sensitive early signs of compound-induced phototoxicity are usually erythema followed by edema [11]. Draize’s method, commonly used to observe the formation of erythema and edema, was used to evaluate phototoxicity. After 24, 48, and 72 h of irradiation, phototoxic skin reactions (erythema and edema formation) were evaluated and scored according to Draize’s criteria (Table 1) [20]. For each test group, the skin reaction scores (erythema/eschar and edema) from individual animals were summed for each site and the mean score was calculated according to the following equation:
A test drug was judged to have a phototoxic potential if the mean score of the UVA-irradiated group was higher than that of the non-irradiated group during any observation period. In this study, no statistical analysis was performed.
Histopathology
Irradiated skin was collected immediately after euthanasia with CO2 from animals that received 8-MOP, LMFX, NA, CPZ, SPFX, KPF, GTFX, and vehicle (corn oil or distilled water). The skin lesions were examined at 72 h after light irradiation. Skin samples were attached to thick paper in a flat orientation using a stapler and fixed with 4% PFA, embedded in paraffin, sectioned, stained with hematoxylin and eosin (H&E), and examined microscopically by a certified pathologist.
Results
Selection of the UVA irradiation dose
ICH S10 states that the identified skin phototoxicity response should be evaluated in terms of dose and time dependence. Based on this, a UVA dose selection test was performed. Rats were irradiated with UVA at 5, 10, 15, and 20 J/cm2 after being orally administered with the well-known phototoxic drug 8-MOP, the results of which are shown in Fig. 1. The concentrations of 8-MOP used in the UVA dose selection test were selected as 1, 5, and 10 mg/kg based and the results of previous studies [18]. Figure 1a shows the skin response score at 24 h after irradiation in the group administered 8-MOP. No skin changes were observed in the solvent control (corn oil) group and the group administered 1 mg/kg 8-MOP. When UVA 5 J/cm2 was irradiated, only slight edema was observed in 10 mg/kg 8-MOP, and there was no response at other concentrations. With the exception of the group administered UVA at 5 J/cm2 there was a tendency toward dose dependent increases in both erythema and edema in the groups irradiated with UVA at 10, 15 and 20 J/cm2. Figure 1b shows the Draize skin score over time in the groups administered 5 mg/kg 8-MOP When UVA was irradiated with 5 J/cm2, the edema increased up to 48 h and then subsided between 48 and 72 h, showing no time dependence. Eschar formation was observed from 48 h following irradiation with UVA at 15 and 20 J/cm2. There was tendency towards a time dependent formation for both erythema and edema following irradiation with UVA at 10 J/cm2. Based on these findings, the most appropriate UVA dose for in vivo phototoxicity testing to minimize excessive skin reactions and obtain time and concentration dependent results was determined to be 10 J/cm2.
Fig. 1.
Draize skin score in the positive control compound (8-MOP) groups irradiated with UVA at 5, 10, 15, and 20 J/cm2. UVA intensity was adjusted to 6 mW/cm2 prior to UVA irradiation. a Dose-dependent skin reaction after 24 h of UVA irradiation b Time-dependent skin reaction in the group administered 5 mg/kg 8-MOP
Skin phototoxicity assessment by gross examination
The Draize skin scores recorded in the phototoxicity assessment of systemically administered drugs are shown in Table 2 and Fig. 2. Representative photographs of the irradiated skin that had been administered with 10 mg/kg 8-MOP, 100 mg/kg LMFX, 1000 mg/kg NA, 100 mg/kg CPZ, 500 mg/kg SPFX, 60 mg/kg KPF, and 500 mg/kg GTFX are shown in Fig. 2a. The Draize skin scores of the irradiated groups are shown in Fig. 2b. For all drugs no phototoxic reaction was observed at any dose in the non-irradiated group (data not shown). Among the phototoxic skin reactions, erythema was observed in the groups administered 10 mg/kg 8-MOP, 100 mg/kg LMFX, 1000 mg/kg NA, 100 mg/kg CPZ, and 500 mg/kg SPFX, and edema was observed only in the groups administered 10 mg/kg 8-MOP, 1000 mg/kg NA, and 500 mg/kg SPFX. In the groups administered 60 mg/kg KPF and 500 mg/kg GTFX, no phototoxic skin reaction (erythema, edema) was observed at any time point. 10 mg/kg 8-MOP, 100 mg/kg LMFX, 1000 mg/kg NA, 100 mg/kg CPZ, and 500 mg/kg SPFX were judged to be phototoxic substances because skin reactions were observed in the irradiated group compared to the non-irradiated group, and 60 mg/kg KPF and 500 mg/kg GTFX, which had no reaction in the irradiated group, were determined as non-toxic substances (Table 3).
Table 2.
Skin scores of SD rats after UVA irradiation
| Group | Substance | Time | Dose | Mean score | |
|---|---|---|---|---|---|
| (hr) | (mg/kg) | Erythema | Edema | ||
| UV(+) | 8-MOP | 24 | 10 | 2 | 1 |
| LMFX | 100 | 1.67 | 0 | ||
| NA | 1000 | 2.00 | 1 | ||
| CPZ | 100 | 1.33 | 0 | ||
| SPFX | 500 | 1.00 | 0 | ||
| KPF | 60 | 0.00 | 0 | ||
| GTFX | 500 | 0.00 | 0 | ||
| UV(+) | 8-MOP | 48 | 10 | 4 | 2.33 |
| LMFX | 100 | 2.00 | 0 | ||
| NA | 1000 | 3.33 | 2 | ||
| CPZ | 100 | 0.67 | 0 | ||
| SPFX | 500 | 1.00 | 0.33 | ||
| KPF | 60 | 0.00 | 0 | ||
| GTFX | 500 | 0.00 | 0 | ||
| UV(+) | 8-MOP | 72 | 10 | 4 | 2.33 |
| LMFX | 100 | 2.00 | 0 | ||
| NA | 1000 | 4.00 | 1 | ||
| CPZ | 100 | 1.33 | 0 | ||
| SPFX | 500 | 1.00 | 0 | ||
| KPF | 60 | 0.00 | 0 | ||
| GTFX | 500 | 0.00 | 0 | ||
There were three SD rats per group. No phototoxic response (erythema, edema) was observed in the non-irradiation site at all time
Fig. 2.
Phototoxic reactions to test drugs and vehicles. Positive drugs: 8-MOP, LMFX, NA, CPZ, SPFX, and KPF, Negative drug: GFTX, Vehicle control: DW, corn oil a Representative photographs showing the phototoxic response (erythema/eschar, edema) for each drug after UVA 10 J/cm2 (intensity: 6 mW/cm2) irradiation b Plot of the Draize skin score for each irradiated group
Table 3.
Summary of the phototoxicity test for drugs
| Substance | Dose | CAS.NO. | Clinicala | Judgement | |
|---|---|---|---|---|---|
| (mg/kg) | Gross skin examination | Histopathologic examination | |||
| 8-MOP | 10 | 298-81-7 | Phototoxic | Phototoxic | Phototoxic |
| LMFX | 100 | 98079-52-8 | Phototoxic | Phototoxic | Phototoxic |
| NA | 1000 | 389-08-2 | Phototoxic | Phototoxic | Phototoxic |
| CPZ | 100 | 69-09-0 | Phototoxic | Phototoxic | Phototoxic |
| SPFX | 500 | 110871-86-8 | Phototoxic | Phototoxic | Phototoxic |
| KPF | 60 | 22071-15-4 | Photoallergic | Non-Phototoxic | Phototoxic |
| GTFX | 500 | 112811-59-3 | Non-phototoxic | Non-phototoxic | Non-phototoxic |
aData from Yonezawa et al. [18]
Skin phototoxicity assessment by histopathology
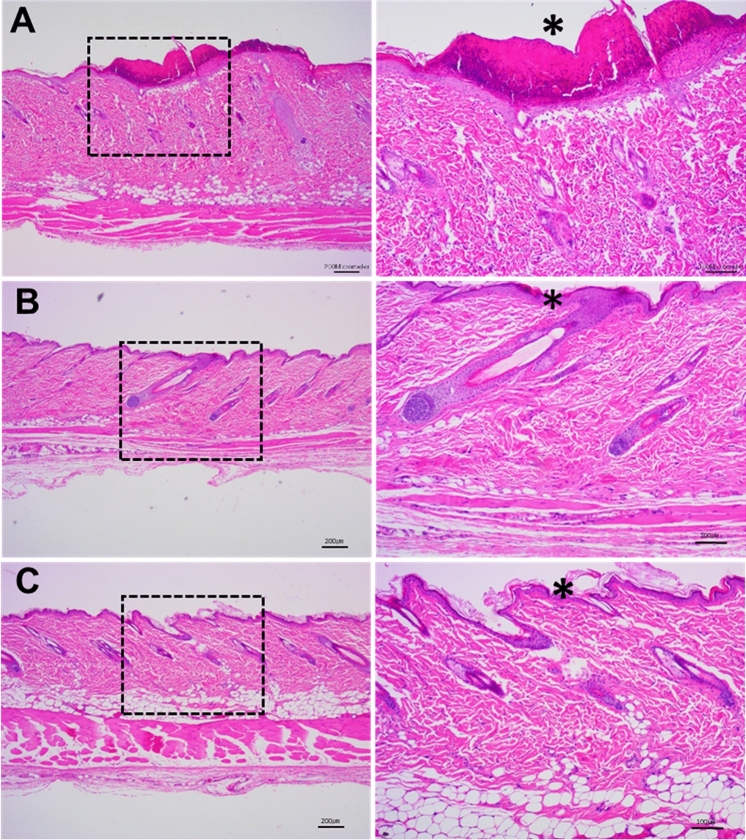
For all drugs, no specific histopathological lesions were observed in the non-radiation group, and the normal structure of the epidermis and dermis was well maintained (data not shown). Representative histopathologic photographs of the irradiation groups that had been administered 10 mg/kg 8-MOP, 60 mg/kg KPF, and 500 mg/kg GTFX are shown in Fig. 3. In the rats administered 10 mg/kg 8-MOP, severe necrosis of the epidermal layer and severe erosion and ulceration due to necrosis products in the keratin were observed. Necrotic findings with inflammatory cells were observed in rats administered 100 mg/kg LMFX. In rats administered 1000 mg/kg NA, very severe erosion and ulceration and severe necrosis were observed. Mild spongiosis and necrosis were observed in rats administered 100 mg/kg CPZ. In rats administered 500 mg/kg SPFX, in some of the epidermal layers mild spongiform and necrotic findings were observed. Peeling of the weak keratin layer was observed in rats administered 60 mg/kg KPF. No pathological findings were observed in rats treated with 500 mg/kg GTFX, which is known to be a non-toxic drug. Therefore, 10 mg/kg 8-MOP, 100 mg/kg LMFX, 1000 mg/kg NA, 100 mg/kg CPZ, and 500 mg/kg SPFX were judged to be phototoxic drugs and 500 mg/kg GFTX was judged to be a non-phototoxic drug (Table 3).
Fig. 3.
Representative histopathological findings at 72 h after UVA 10 J/cm2 (intensity: 6 mW/cm2) irradiation. a Rat skin administered with 8-MOP; asterisk indicate severe necrosis containing necrotic cell debris, severe erosion and ulcer b Rat skin administered with KPF; asterisk indicate mild necrosis, hyperplasia and keratin sloughing c Rat skin administered with GFTX; asterisk indicate normal epidermis. Note the normal keratin layer
Discussion
Using a positive control phototoxic compound, 8-MOP, this study selected the appropriate UVA irradiation dose for the subsequent in vivo phototoxicity test. This test assessed drug phototoxicity using two skin reaction evaluation methods. In the test, a total of six phototoxic substances (8-MOP, LMFX, NA, CPZ, SPFX, and KPF) in humans and one non-toxic substance (GTFX) in humans were used [18].
In vivo phototoxicity tests for systemically administered compounds has been conducted in a variety of species, including guinea pig, mouse, and rat [11]. Although guinea pigs have been widely used in previous studies, rats and mice have only recently been used to assess the phototoxicity of systemically administered drugs. ICH S10 guidelines suggest that light irradiation should occur near the Tmax of the drug, which is the time when the administered drug reaches the maximum concentration in the blood and has the greatest response to UVA irradiation. Since rats are commonly used to examine the PK profile in the early stages of drug development, no further PK studies were required thereby reducing the number of rats used in the study.
The UVA irradiation dose to be used for the in vivo phototoxicity test was selected through a UVA dose selection test. In the ICH S10 guidelines, UVA at 5–20 J/cm2 is currently used successfully for both in vitro and in vivo phototoxicity assessments, which is a UVA dose similar to that obtained during long-term outdoor activities in the summer at noon. Several study groups have also evaluated the phototoxicity of test substances using UVA doses at 5–20 J/cm2 [15, 17, 19, 21], but did not comment on why the UVA dose was used. As a result of selecting the UVA dose using a well-known phototoxic drug (8-MOP), the UVA dose that was able to provide dose and time-dependent results while minimizing the pain to animals was 10 J/cm2.
As a result of gross examination using Draize’s criteria, with the exception of KPF, 8-MOP, LMFX, NA, CPZ, and SPFX were found to be phototoxic. Combining the results of the gross skin examination and histopathology, a total of six substances were determined to be phototoxic, and one was determined to be non-phototoxic. It is often difficult to evaluate relatively insignificant changes in skin reactions by gross examination of the skin alone. For example, KPF has been reported to cause photoallergic contact dermatitis when administered orally to humans [22], but in a study conducted in Yonezawa et al. [18] it was determined to be a non-phototoxic substance by gross examination of the skin alone. In this study based on histopathological findings from H&E staining, a clear peeling of the keratin layer was observed in the irradiated group administered KPF, whereas the non-irradiated group was normal. Consequently, KPF was determined to be a phototoxic drug. GTFX, judged to be non-phototoxic, was previously reported to have some phototoxicity [23, 24], but has been found to be a negative material in other studies [19]. The use of histopathology allows for the confirmation of phototoxic reactions by substances that are difficult to observe by gross examination, and so it appears that the accuracy of the evaluation of phototoxicity can be improved by including histopathology as an additional indicator.
From the results of our study, we concluded that a UVA doe of 10 J/cm2 is appropriate in examining the in vivo phototoxicity of compounds. In addition, an evaluation of phototoxicity incorporating both gross examination of the skin and skin histopathology is more accurate than studies that rely solely on gross examination of the skin.
Acknowledgments
This research was supported by the Ministry of Food and Drug Safety of Korea in 2017-2018 (grant numbers 17181MFDS402). We would like to thank Editage (www.editage.co.kr) for English language editing.
Compliance with ethical standards
Conflict of interest
None declared.
Informed consent
Not applicable.
References
- 1.Lee YS, Yi JS, Lim HR, Kim TS, Ahn IY, Ko KY, et al. Phototoxicity evaluation of pharmaceutical substances with a reactive oxygen species assay using ultraviolet A. Toxicol Res. 2017;33:43–48. doi: 10.5487/TR.2017.33.1.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mujtaba SF, Srivastav AK, Agnihotry S, Anas M. Drug-induced phototoxic response. In: Ray R, Haldar C, Dwivedi A, Agarwal N, Singh J, editors. Photocarcinogenesis and photoprotection. Singapore: Springer; 2018. pp. 77–84. [Google Scholar]
- 3.Monteiro AF, Rato M, Martins C. Drug-induced photosensitivity: photoallergic and phototoxic reactions. Clin Dermatol. 2016;34:571–581. doi: 10.1016/j.clindermatol.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Bosca F, Marın ML, Miranda MA. Photoreactivity of the nonsteroidal anti-inflammatory 2-arylpropionic acids with photosensitizing side effects. Photochem Photobiol. 2001;75:637–655. doi: 10.1562/0031-8655(2001)0740637POTNAI2.0.CO2. [DOI] [PubMed] [Google Scholar]
- 5.Spielmann H, Balls M, Brand M, Doring B, Holzhutter HG, Kalweit S, et al. EEC/COLIPA project on in vitro phototoxicity testing: first results obtained with a Balb/c 3T3 cell phototoxicity assay. Toxicol In Vitro. 1994;8:793–796. doi: 10.1016/0887-2333(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 6.Spielmann H, Balls M, Dupuis J, Pape WJ, Pechovitch G, de Silva O, et al. The international EU/COLIPA in vitro phototoxicity validation study: results of phase II (blind trial). Part 1: the 3T3 NRU phototoxicity test. Toxicol In Vitro. 1998;12:305–327. doi: 10.1016/S0887-2333(98)00006-X. [DOI] [PubMed] [Google Scholar]
- 7.Spielmann H, Muller L, Averbeck D, Balls M, Brendler-Schwaab S, Castell JV, et al. The second ECVAM workshop on phototoxicity testing: the report and recommendations of ECVAM workshop 42. Altern Lab Anim. 2000;28:777–814. doi: 10.1177/026119290002800603. [DOI] [PubMed] [Google Scholar]
- 8.Moore DE. Drug-induced cutaneous photosensitivity: incidence, mechanism, prevention and management. Drug Saf. 2002;25:345–372. doi: 10.2165/00002018-200225050-00004. [DOI] [PubMed] [Google Scholar]
- 9.Jones PA, King AV. High throughput screening (HTS) for phototoxicity hazard using the in vitro 3T3 neutral red uptake assay. Toxicol In Vitro. 2003;17:703–708. doi: 10.1016/S0887-2333(03)00101-2. [DOI] [PubMed] [Google Scholar]
- 10.Neumann NJ, Blotz A, Wasinska-Kempka G, Rosenbruch M, Lehmann P, Ahr HJ, et al. Evaluation of phototoxic and photoallergic potentials of 13 compounds by different in vitro and in vivo methods. J Photochem Photobiol B. 2003;79:25–34. doi: 10.1016/j.jphotobiol.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 11.International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use (2013) ICH Guideline S10 Guidance on Photosafety evaluation of pharmaceuticals
- 12.Lynch AM, Wilcox P. Review of the performance of the 3T3 NRU in vitro phototoxicity assay in the pharmaceutical industry. Exp Toxicol Pathol. 2011;63:209–214. doi: 10.1016/j.etp.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Wagai N, Yamaguchi F, Sekiguchi M, Tawara K. Phototoxic potential of quinolone antibacterial agents in Balb/c mice. Toxicol Lett. 1990;54:299–308. doi: 10.1016/0378-4274(90)90197-T. [DOI] [PubMed] [Google Scholar]
- 14.Horio T, Miyauchi H, Asada Y, Aoki Y, Harada M. Phototoxicity and photoallergenicity of quinololnes in guinea pigs. J Dermatol Sci. 1994;7:130–135. doi: 10.1016/0923-1811(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto N, Akimoto A, Kawashima H, Kim S. Comparative study of skin phototoxicity with three drugs by an in vivo mouse model. J Toxicol Sci. 2010;35:97–100. doi: 10.2131/jts.35.97. [DOI] [PubMed] [Google Scholar]
- 16.Seto Y, Inoue R, Kato M, Yamada S, Onoue S. Photosafety assessments on pirfenidone: photochemical, photobiological, and pharmacokinetic characterization. J Photochem Photobiol B. 2013;120:44–51. doi: 10.1016/j.jphotobiol.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Adachi T, Satou Y, Satou H, Shibata H, Miwa S, Iwase Y, et al. Assessment of 8-methosypsoralen, lomefloxacin, sparfloxacin, and pirfenidone phototoxicity in Long-Evans rats. Int J Toxicol. 2014;34:16–23. doi: 10.1177/1091581814559397. [DOI] [PubMed] [Google Scholar]
- 18.Yonezawa Y, Ohsumi T, Miyashita T, Kataoka A, Hashimoto K, Nejishima H, et al. Evaluation of skin phototoxicity study using SD rats by transdermal and oral administration. J Toxicol Sci. 2015;40:667–683. doi: 10.2131/jts.40.667. [DOI] [PubMed] [Google Scholar]
- 19.Kuga K, Yasuno H, Sakai Y, Harada Y, Shimizu F, Miyamoto Y, et al. The abdominal skin of female Sprague-Dawley rats is more sensitive than the back skin to drug-induced phototoxicity. J Pharmacol Toxicol Methods. 2017;88:46–55. doi: 10.1016/j.vascn.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Draize JH (1959) Dermal toxicity. In appraisal of the safety of chemicals in foods, drugs and cosmetics. The Association of Food and Drug Officials of the United States, Texas State Department of Health, Austin
- 21.Turnock S, Gerbeix C, Thirion-Delalande C, Pearson N, Forster R. Assessment of phototoxicity in pigmented Long-Evans rat: sparfloxacin and 8-methoxypsorlalen. Regul Toxicol Pharmacol. 2018;92:303–314. doi: 10.1016/j.yrtph.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Carterina F, Nicoletta C, Antonio VG, Gianni A. Photodermatitis caused by oral ketoprofen: two case reports. Contact dermatitis. 2011;64:181–183. doi: 10.1111/j.1600-0536.2010.01817.x. [DOI] [PubMed] [Google Scholar]
- 23.Reus AA, Usta M, Kenny JD, Clements PJ, Pruimboom-Brees I, Aylott M, et al. The in vivo rat skin photomicronucleus assay: phototoxicity and photogenotoxicity evaluation of six fluoroquinolones. Mutagenesis. 2012;27:721–729. doi: 10.1093/mutage/ges038. [DOI] [PubMed] [Google Scholar]
- 24.Seto Y, Inoue R, Ochi M, Gandy G, Yamada S, Onoue S. Combined use of in vitro phototoxic assessments and cassette dosing pharmacokinetic study for phototoxicity characterization of fluoroquinolones. AAPS J. 2011;13:482–492. doi: 10.1208/s12248-011-9292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]