Abstract
Microplastics (MPs) have been recently recognized as a global environmental threat and its exposure as a risk factor to human health. Health effects through MPs exposure have been recently reported, especially through oral route of exposure. Since MPs could be exposed to humans through routes other than oral, this study was designed to evaluate whether MPs exposed through the inhalation route could be delivered to fetal mice and exhibit systemic toxicity. Polyethylene (PE) with 10–45 µm diameter were administered at 0 (distilled water, vehicle control), 6 (low administration), and 60 (high administration) µg/mouse/day to 3 pregnant dams per group from gestational day 9 to postnatal day (PND) 7 through intratracheal instillation. Dams and neonates were sacrificed at PND 7 and blood was collected. Various neonatal organs including brain, lung, heart, stomach, intestine, kidneys, and ovaries were collected for histopathological observation and weight measurement. No influence of PE-MPs administration was observed on the number of offsprings born, but the body and organs’ weight were heavier overall in the high administration group of dams and neonates than the other groups with statistical significance achieved in the heart and spleen weight. Level of serum acetylcholinesterase and glutathione peroxidase activity was decreased in the high administration group of dams and neonates compared with the other groups. Lung was the organ with highest number of PE-MPs present in the both administration groups of dams, and PE-MPs were also detected in liver and intestine of the high administration dams. Whereas, PND7 neonates showed accountable numbers of PE-MPs only in kidneys of the high administration group. Overall, the present study indicates that PE-MPs instilled intratracheally could be delivered to neonates from dams. Even though adverse effects from PE-MPs exposure during pregnant and lactational period are less prominent on both dam and neonate, potential of second-generation toxicity could be considered for further investigation.
Keywords: Polyethylene, Microplastics, Second-generation toxicity, Intratracheal instillation
Introduction
In recent years, the use of plastic products has raised intensely and plastics with smaller sizes are of great concern for environmental conservation as well as animal health. Microplastics (MPs), the term coined by Richard C. Thomson, refers to the plastic size < 5 mm [1, 2]. MPs get to the environment from the release of plastics used in cosmetics, detergents, sunscreens, and vector drugs or due to the fragmentation of plastic objects by UV-radiation, mechanical abrasion, and biological degradation [3, 4]. MPs get transported over long distances by wind, river, or ocean currents [5]. MPs intake has been associated with physical or chemical effects in marine species [6, 7]. Moreover, MP is thought to carry additional endogenous chemical additives and various pollutants during transport causing additional chances of toxicity to the exposed ones [8].
Studies revealed that MPs consumed by marine species get accumulated and distributed to body parts such as gills and gut of crab, circulatory system of mussels, and liver of zebrafish [9, 10]. It is well documented that MPs ≤ 20 µm can enter organs and smaller MPs with size 1–10 µm can cross through the cell membrane, blood–brain barrier, and placenta, leading to transfer to the next generation [8, 9, 11, 12]. Decrease of growth rate, fertility and longevity in plankton and algae; increased oxidative stress, neurotoxicity, liver damage and developmental toxicity in marine organisms and mice are some of the effects of MP ingestion [13–15].
Food consumption is one pathway for humans to get exposed to MPs since MPs have been isolated from marine invertebrates, crustaceans, and fish [16, 17]. MPs were also found in bottled water, commercial salt, and some canned food [18–20]. Another prominent route of exposure to MPs is inhalation, as the presence of MPs in atmospheric fallout, in sludge byproducts used for agricultural proposes and in air pollution caused by wearing out of rubber tires has been reported several times [8, 21]. MPs have been isolated from human lungs and nylon fiber workers exposed to fibrous MPs were associated with respiratory irritation, interstitial lung disease, breathlessness, and reduced lung capacity [8].
Most of the studies have focused on the toxic effects of MPs on aquatic organisms and demonstrated various adverse effects of MPs’ tissue accumulation. Also, few studies were oriented to assess the toxicity of oral administration or intratracheal instillation of polystyrene (PS) or polyvinyl chloride (PVC) MPs on mice or rats. It was proved that oral administration of polystyrene MPs (PS-MPs) led to the accumulation of MPs in tissues including liver, kidney, gut, and caused oxidative stress or metabolic changes [15, 22]. Another study indicated that oral exposure to PS-MPs led to gut microbiota dysbiosis, gut barrier dysfunction, and altered lipid metabolism in male mice [23, 24]. Only a study, dealing with the intergenerational transfer of MPs, revealed that oral administration of 0.5 µm and 5 µm PS-MPs to pregnant mice altered the amino acid metabolism and carnitine metabolism homeostasis of their offspring [25].
However, the data on tissue accumulation and further toxicity after tracheal instillation of MPs in mice are very limited, and a study on the adverse effect of MPs on offspring after exposure of pregnant and lactating mice is yet to be carried out. Therefore, this study aims to study the toxic effect of polyethylene microplastics (PE-MPs) on F1 offspring of female mice exposed to intratracheal instillation during pregnancy and lactating period. PE-MP was chosen for this study since PE has been reported as one of the most common types of plastic found in river water and riverine fish in Korea [26].
Materials and methods
Animals
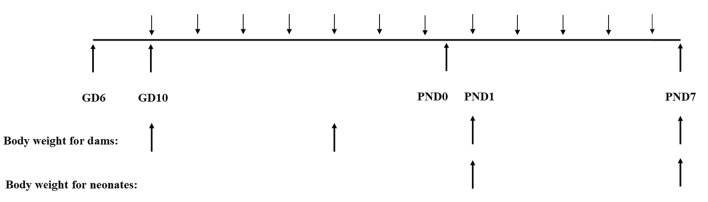
Six-day pregnant female mice, purchased from KOATECH (Pyeongtaek, Korea), were acclimatized for 3 days before the experiment. The mice were housed in an animal room maintained at a room temperature of 22 ± 3 ℃ with a 50 ± 20% relative humidity and 12-h light: dark cycle. All mice had ad libitum access to standard rodent food and autoclaved filtered water. Bodyweight of dams has been measured at the day of first and fifth MP administration, and the first day and the seventh day of lactation, and neonates weight has been measured after a day of birth and postnatal day (PND) 7 (Fig. 1). The numbers of offspring were counted a day after birth. All the maintenance and experimental procedures were according to the approval of Daegu Catholic University’s IACUC (IACUC-2019-022).
Fig. 1.
Schematic diagram for experimental processes. Microplastics were administered at gestational day (GD) 10 following 6 more administrations and 5 times more from postnatal day (PND) 1 to PND7 to dams. Body weight of dams has been measured at the first and fifth day of administration, and PND1 and 7. Body weight of neonates has been measured and PND1 and 7
Test substance and exposure to mice
Fluorescent red polyethylene microsphere (UVPMS-BR-0.995), particle size ranging from 10 to 45 µm, was purchased from Cospheric (Santa Barbara, CA, USA). The emission and excitation wavelength of MPs was 330 and 550 nm, respectively with a peak at 605 nm. The particles were suspended in distilled water and sonicated 30 min before administration to mice. Test groups were divided into 0 (control), 6 (low exposure), and 60 (high exposure) µg/mouse/administration groups, each group containing 3 pregnant mice. Seven intratracheal administration of MPs were carried out during pregnancy and additional five administration after delivery (Fig. 1). The administration was carried out by dripping 50 µl of suspension into intra-trachea using a mouse video instillator (Sejongbio, Daejeon, Korea), while the mice were under anesthesia with isoflurane (Hanapharm, Hwaseong, Korea). The concentration and instillation volume of PE-MP suspension were similar to the dose and volume used in previous studies [27, 28].
Histopathological evaluation
All the mice underwent sacrifice after CO2 asphyxiation. The weight of brain, lung, heart, stomach, kidney, intestine, and ovary of dams and neonates was taken. One dam and 5 neonates from each group were used for histopathological evaluation. Organs were fixed in 10% neutral formalin, embedded in paraffin, sectioned at 4 µm thickness, and stained with Hematoxylin and eosin for microscopic evaluation of histopathological changes. The histopathological evaluation was done by a toxicological pathology specialist certified.
Measurement of various biological markers
Heart blood from each mouse was collected and stored overnight at 4 ℃. Serum was collected after centrifuging the blood at 4000 rpm for 10 min at 4 ℃. The sera were used to assess the alteration of biomarkers such as acetylcholinesterase (AChE) or glutathione peroxidase (GSH-Px) activity, and triglyceride (TG) level. These markers were measured using commercially available kits (Abcam, Cambridge, UK) following the manufacturer’s instructions.
Tissue accumulation of MPs in mice
For confirmation of transfer of PE-MPs from dams to offsprings, two neonates from each dam were sacrificed at PND1. All the organs were collectively homogenized in 50 ml polystyrene tube with 2 ml buffer containing 20 mM Tris (Tris(hydroxymethyl aminomethane), 100 mM NaCl, 2 mM NA2EDTA (sodium ethylenediaminetetraacetate), and 1% Igepal CA-630. The supernatant was collected after a quick spin at 3000 rpm for 1 min. 10 µl of supernatant was loaded to a hemocytometer and evaluated through a fluorescence microscope with 100X magnification (Eclipse 80i, Nikon, Japan) equipped with a 450–490 nm filter. Dams and remaining neonates were sacrificed at PND 7. Two dams and 12 neonates from each group were used for counting MPs numbers. Brain, liver, lung (including thyroid and parathyroid glands), and intestine from dams were homogenized with 5 ml of buffer whereas thymus, heart, stomach, spleen, kidneys, and ovaries were homogenized with 2 ml of buffer. Neonates' livers, lungs, and intestines were homogenized in 0.5 ml of buffer and brain, thymus, heart, stomach, spleen, and kidneys were homogenized with 0.2 ml buffer. The quantification of MPs was done similarly as mentioned above.
Statistical analysis
Statistical analysis was done using SPSS 19.0 K (SPSS Inc., Chicago, IL, USA). Data were expressed as mean ± standard error of the mean (SEM) or mean ± standard deviation. Data that passed normality tests were analyzed using a one-way analysis of variance (ANOVA). When data were significantly different, Scheffé’s test or Dunnet method was used as post-hoc analysis depending upon the equal or unequal variance. Kruskal–Wallis’ test by ranks was used as a non-parametric comparison test and a significant difference was further confirmed by Mann–Whitney U test. A p value of < 0.05 was considered to be statistically significant.
Results
No significant alterations in the number of offspring and body weight gain through oral administration of PE-MPs
PE-MPs administration to pregnant dams for 7 days during pregnancy had no significant effect on the numbers of offspring (control: 12, 15, 11 neonates; low: 17, 15, 13 neonates; high: 12, 12, 12 neonates from each dam). Even though administration of PE-MPs did not significantly influence on the body weight gains of dams during the gestational and the lactational period, the high PE-MPs administration group showed a higher body weight gain than both the control and the low PE-MPs administration group during lactational period (Table 1). This tendency was also exhibited in the body weight gains of neonates with significantly higher gain in the high PE-MPs administration group than the other two groups.
Table 1.
Body weight gains (g) of dams and neonates
| Control | Low | High | |
|---|---|---|---|
| Dams | |||
| Gestational period | 8.2 ± 1.3 | 7.1 ± 2.3 | 7.3 ± 2.0 |
| Lactational period | 4.7 ± 1.2 | 3.0 ± 1.1 | 6.3 ± 1.3 |
| Neonates | 2.2 ± 0.3 | 2.0 ± 0.4 | 2.6 ± 0.4* |
Bodyweight of dams has been measured at the day of first and fifth microplastics administration (0: control, low: 6 µg/mouse/administration, high: 60 µg/mouse/administration) during gestational period, and the PND1 and 7 during lactational period. Body weight of neonates has been measured at PND1 and 7. Data are expressed as means ± standard deviations. *p < 0.05, compared to the control and the low administration group
PE-MPs administration dose dependent variations in neonatal organ weights
Measurement of neonatal organs’ weight was done after sacrifice at PND7. All the organs weighed heavier in the high PE-MPs administration group than both the control and the low administration group and all the organs except heart and stomach weighed lighter in the low MPs administration group than both the control and the high PE-MPs administration group (Table 2). The weights of both kidneys, lung, heart, spleen, liver, and intestine were significantly lower in the low dose administered group than the high administration group. The weights of heart and spleen were significantly heavier in the high PE-MPs administration group than the other two groups.
Table 2.
Organ weight (mg) of neonates
| Organ | Control | Low | High |
|---|---|---|---|
| Kidney (left) | 22.4 ± 5.4 | 19.7 ± 3.4 | 24.9 ± 3.0* |
| Kidney (right) | 22.2 ± 6.4 | 21.0 ± 5.2 | 24.4 ± 2.4* |
| Thymus | 13.8 ± 4.9 | 13.0 ± 3.3 | 15.5 ± 3.8 |
| Lung | 87.5 ± 13.6 | 75.7 ± 12.8† | 94.0 ± 6.4 |
| Heart | 18.5 ± 1.7 | 18.5 ± 3.4 | 22.7 ± 2.9§ |
| Spleen | 19.3 ± 3.4 | 17.6 ± 4.8 | 24.2 ± 5.3§ |
| Brain | 226.0 ± 26.1 | 223.0 ± 31.2 | 230.0 ± 32.4 |
| Liver | 115.0 ± 20.2 | 104.0 ± 19.0 | 123.0 ± 28.1* |
| Stomach | 23.5 ± 7.0 | 23.5 ± 4.6 | 36.4 ± 43.3 |
| Intestine | 183.0 ± 56.1 | 179.0 ± 30.1 | 206.0 ± 19.1* |
Organ weight of neonates was measured at the time of sacrifice, PND7. Data are expressed as means ± standard deviations from 17 neonates for each group
Statistically significant differences (p < 0.05): *compared to the low administration group; †compared to the control and the high administration group; §compared to the control and the low administration group
No significant alterations in biological markers of dams and neonates through oral administration of PE-MPs
Levels of serum triglyceride were measured for monitoring an effect of PE-MPs on lipid metabolism [15, 25]. The PE-MPs administration did not induce a significant alteration on the serum TG levels of dams and neonates compared with those of the control group (Table 3). AChE activity was evaluated for screening a potential neurotoxicity [15, 25] and was lowered in the high PE-MPs administration group of dams without a statistical significance when compared with that of the control and the low PE-MPs administration group. This observation was also made for the neonatal serum AChE activity levels. The serum GSH-Px activity was determined to estimate an oxidative stress induced through PE-MPs administration [15, 25]. Even though no statistical significance, the GSH-Px activity was lower in the high PE-MPs administration group of dams than the other two groups, and a similar finding was observed with the neonatal groups.
Table 3.
Serum levels of triglyceride, and acetylcholinesterase or glutathione peroxidase activity
| Biological markers | Control | Low | High |
|---|---|---|---|
| Dams | |||
| Triglyceride (mM) | 0.621 ± 0.112 | 0.720 ± 0.205 | 0.719 ± 0.093 |
| Acetylcholinesterase activity (U/mL) | 36.4 ± 5.4 | 36.9 ± 3.0 | 28.8 ± 2.1 |
| Glutathione peroxidase activity (nmol/mL) | 949 ± 161 | 948 ± 379 | 784 ± 170 |
| Neonates | |||
| Triglyceride | 0.646 ± 0.248 | 0.610 ± 0.199 | 0.656 ± 0.275 |
| Acetylcholinesterase activity | 16.4 ± 2.6 | 15.5 ± 1.3 | 15.2 ± 1.3 |
| Glutathione peroxidase activity | 688 ± 80 | 805 ± 140 | 669 ± 230 |
Data are expressed as means ± standard deviations from 3 dams and 17 neonates for each group
Accumulation and distribution of PE-MPs’ in mice tissues
The high PE-MPs administration group of dams showed maximum accumulation of PE-MPs in lungs followed by heart, liver, and intestine (Table 4). A similar trend was observed in the low administration group of dams with no detection of PE-MPs in liver and intestine. Whereas the neonates sacrificed on PND7 were screened for PE-MPs’ presence in individual organs and the neonates from the high administration group showed traces of PE-MPs in the homogenates of kidneys only. No PE-MPs were detected in the other organs of neonates.
Table 4.
Number of microplastics in each dam and neonate
| Organs | Control | Low | High |
|---|---|---|---|
| Dams | |||
| Lung | 0, 0 | 4000, 500 | 9000, 4000 |
| Heart | 0, 0 | 0, 200 | 0, 1400 |
| Liver | 0, 0 | 0, 0 | 0, 500 |
| Intestine | 0, 0 | 0, 0 | 0, 500 |
| Neonates | |||
| Kidney | ND | ND | 60, 40, 40, 40, 40, 40, 20 |
Data are obtained from 2 dams and 12 PND7 neonates for each group. Among the PND7 neonates, microplastics were detected only in kidneys of 7 neonates
ND not detected from all 12 PND7 neonates
Histopathological observation
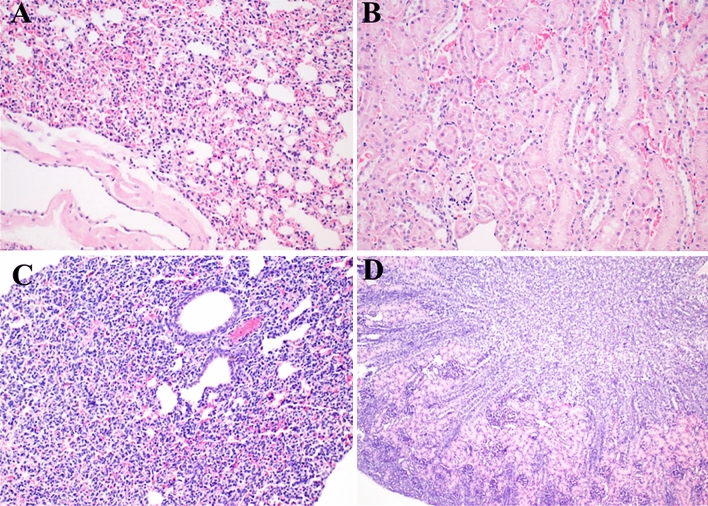
No specific observation on inflammation, necrosis, or lipid droplets was examined in brains, lungs, hearts, stomachs, kidneys, intestines, and ovaries of both dams and offsprings. Therein, photomicrographs were presented for lung and kidney from one dam and its neonate of the control group (Fig. 2).
Fig. 2.
Histopathologic features of lung and kidney from dam (a, b, respectively) and from neonate (c, d, respectively). Organs were stained with Hematoxylin and eosin, and photomicrographs are presented with × 200 (a–c) and × 100 (d) magnification
Discussion
This study is an attempt to address the gap in a scientific study on the effects of inhaled MPs and its effect on directly exposed mice and their offsprings. This study, with few numbers of experimental mice and other limitations, has been able to recognize the inhalation route as one of the important routes of exposure from mothers to F1 offsprings and confirming that inhalation of MPs from the environment could cause intergenerational toxicity in newborns. This study used the PE-MPs with size ranging 10–45 µm, which was one of the most commercially available PE-MPs conjugated with fluorescent dye and with diameter size capable of penetration into organs or crossing through the cell membrane and placenta [8, 9, 11, 12].
Although previous studies on aquatic organisms have stated about the effects of MPs on the survival of F1 offsprings, in this study, exposure of PE-MPs did not affect the survival of F1 generations. The finding of this study is in concordance to previous research carried, where oral administration of polystyrene MPs (PS-MP) to female mice in the gestational period did not alter the survival rates and sex ratio of offsprings [25]. The body weight gained during the gestational and lactational period were higher in the high PE-MPs administration group of dams and neonates. Increased body weight in neonates from the dams with high PE-MPs administration during lactation is observed for the first time in this study. The alteration in body weight in test groups depicted the potential of MPs to have intergenerational effects on body weight. Regarding the neonates’ organ weight, high dose of PE-MPs administration to dams tended to increase the absolute weight of organs with a significant effect on the kidneys, lung, heart, spleen, and liver. This could implicate the chance of PE-MP having effects on various organs of F1 offspring, however a study with a longer duration of exposure could help to depict the actual scenario of effects.
In this study, a reduction in serum level AChE activity was found in dams with similar change in neonates, suggesting that PE-MPs at a higher concentration exposure could cause neurotoxicity through suppressing the catalytic capacity of AChE. A similar result was observed in marine animals, such as brine shrimp exposed to PS-MP, mussels exposed to PE or PS-MP, common goby exposed to PE-MPs [29–31], and mice exposed to 10 µg/day and 100 µg/day for 28 days showed a marked decrease in AChE activity [16]. The activity level of GSH-Px, an important enzyme having a role against oxidative stress, was seemingly lowered in the high PE-MPs administration group of dams and neonates. A similar and significant result was observed with a higher dose of MPs in another study where MPs administered mice showed a markedly lowered level of serum GSH-Px after exposure to 100 and 500 µg/day [15]. Previous studies demonstrated concentration-dependent decreased serum or hepatic triglyceride in mice exposed to MPs and their offsprings, suggesting the disturbance of lipid metabolism by the exposure of MPs [15, 24, 25]. However, in this study MPs did not alter the serum TG level. The difference in route of exposure, amount of MPs exposed, and duration could have been a reason for no alteration of serum TG level in dams and F1 offsprings.
PE-MPs accumulated in both dams and offsprings. Dams from low and high exposure groups showed maximum accumulation in the lungs followed by the heart. The low dose exposed did not reveal any accumulation in the liver and intestine, but the high dose exposed mice showed the same level of PE-MPs’ accumulation in liver and intestine. This trend of PE-MPs distribution was different than a study where mice exposed to oral administration of MPs showed accumulation in gut > kidney > liver for 5 µm diameter MPs and kidney > gut > liver for 20 µm MPs [15]. The difference in accumulation and distribution of MPs may be an outcome of the different routes of exposure as intratracheal administration of MPs overloaded lung tissue and got distributed to the heart via blood from the lungs. More importantly, intergenerational accumulation of MPs to F1 offspring was confirmed by this study as the kideny homogenates of neonates from the high exposure dams showed the accumulation of PE-MPs. Similar to the aforementioned study claiming high overloading of 20 µm MPs in the kidney of mice, our study where 10–45 µm PE-MPs was used, neonates from high exposure dams showed PE-MPs accumulation in the kidney. No accountable numbers of PE-MPs were seen in other organs, this could be due to the short duration of exposure. Thus, it is speculated that increasing dose and exposure period can further increase the likelihood of detection in other organs. Through this study, a qualitative and quantitative evaluation of PE-MPs in tissue homogenates was conducted, however, there is a limitation of visual evaluation through a fluorescence microscope. Although evaluation criteria were established to compensate for the limitation, the use of fluorescence spectroscopy using a spectrophotometer is further recommended.
Histological examination of tissue sections from dams and neonates revealed normal morphology without noticeable pathological findings. Similar to our findings, oral administration of PS-MPs of different size to mice three times a week for 28 days, revealed no morphological changes in intestinal tissue sections [32]. In other studies, mice administered with PS-MPs 100 µg/day for 28 days and PE-MPs 600 µg/day for 5 weeks showed clear signs of inflammation in the liver and intestine, respectively [15, 33]. The major differences in these studies are the dose and duration of exposure. Therefore, it can be postulated that intratracheal instillation of MPs in a higher concentration for a longer duration could cause histopathological changes in mice and their offsprings as both dams and neonates showed accumulation of MPs in organs even at the concentration which is much lower than in aforementioned studies.
In conclusion, our results confirmed that PE-MPs instilled intratracheally causes accumulation of PE-MPs in various organs most prominently in the lungs followed by the heart, concluding different sites for accumulation of PE-MPs depending upon the exposure route. In addition, certain differences on neonatal body weight gain and organ weights were observed between the control and the PE-MPs exposure groups, whereas the present study resulted in no significant alterations in the biological changes and histopathological observation, leading to overall conclusion of no apparent toxicity in neonates through maternal inhalational exposure of PE-MPs. This study also demonstrated intergenerational transportation of PE-MPs from dams to their F1 offsprings, which could suggest the necessity of further systemic evaluation on MPs-mediated second-generation adverse health effects. A future study with different doses and duration of exposure with an efficient system of MPs quantification is recommended.
Acknowledgements
This research was supported by the Risk Assessment Program for Management of Microplastics Project, funded by Korea Ministry of Environment (MOE) (grant No. 2020003120002) and the educational training program for the management of information on the hazards and risk of chemical substances funded by the Ministry of Environment, Korea (entrusted to Korea Chemicals Management Association). This study was conducted as a part of the first author’s master’s thesis.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to disclose.
Footnotes
The original online version of this article was revised: In the Acknowledgements section of this article, the funding information was incorrect.
Ravi Gautam and Yong Heo contributed equally to this work.
Change history
11/9/2021
A Correction to this paper has been published: 10.1007/s43188-021-00113-1
References
- 1.Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AWG, McGonigle D, Russell AE. Lost at sea: where is all the plastic? Science. 2004;304:838. doi: 10.1126/science.1094559. [DOI] [PubMed] [Google Scholar]
- 2.Moore CJ. Synthetic polymers in the marine environment: a rapidly increasing, long-term threat. Environ Res. 2008;108:131–139. doi: 10.1016/j.envres.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 3.Andrady AL. Microplastics in the marine environment. Mar Pollut Bull. 2011;62:1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 4.Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, Galloway T, Thompson R. Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ Sci Technol. 2011;45:9175–9179. doi: 10.1021/es201811s. [DOI] [PubMed] [Google Scholar]
- 5.Abbasi S, Keshavarzi B, Moore F, Turner A, Kelly FJ, Dominguez AO, Jaafarzadeh N. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environ Pollut. 2019;244:153–164. doi: 10.1016/j.envpol.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 6.Pedà C, Caccamo L, Fossi MC, Gai F, Andaloro F, Genovese L, Perdichizzi A, Romeo T, Maricchiolo G. Intestinal alterations in European sea bass Dicentrarchus labrax (Linnaeus, 1758) exposed to microplastics: preliminary results. Environ Pollut. 2016;212:251–256. doi: 10.1016/j.envpol.2016.01.083. [DOI] [PubMed] [Google Scholar]
- 7.Cole M, Lindeque P, Fileman E, Halsband C, Galloway TS. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ Sci Technol. 2015;49:1130–1137. doi: 10.1021/es504525u. [DOI] [PubMed] [Google Scholar]
- 8.Wright SL, Kelly FJ. Plastic and human health: a micro issue? Environ Sci Technol. 2017;51:6634–6647. doi: 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- 9.Browne MA, Dissanayake A, Galloway TS, Lowe DM, Thompson RC. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.) Environ Sci Technol. 2008;42:5026–5031. doi: 10.1021/es800249a. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Zhang Y, Deng Y, Jiang W, Zhao Y, Geng J, Ding L, Ren H. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ Sci Technol. 2016;50:4054–4060. doi: 10.1021/acs.est.6b00183. [DOI] [PubMed] [Google Scholar]
- 11.von Moos N, Burkhardt-Holm P, Köhler A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ Sci Technol. 2012;46:11327–11335. doi: 10.1021/es302332w. [DOI] [PubMed] [Google Scholar]
- 12.Hans B, Hollman PCH, Peters RJB. Potential health impact of environmentally released micro- and nanoplastics in the human food production chain: experiences from nanotoxicology. Environ Sci Technol. 2015;49:8932–8947. doi: 10.1021/acs.est.5b01090. [DOI] [PubMed] [Google Scholar]
- 13.Lee K-W, Shim WJ, Kwon OY, Kang J-H. Size-dependent effects of micro polystyrene particles in the marine copepod tigriopus japonicus. Environ Sci Technol. 2013;47:11278–11283. doi: 10.1021/es401932b. [DOI] [PubMed] [Google Scholar]
- 14.Nobre CR, Santana MFM, Maluf A, Cortez FS, Cesar A, Pereira CDS, Turra A. Assessment of microplastic toxicity to embryonic development of the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea) Mar Pollut Bull. 2015;92:99–104. doi: 10.1016/j.marpolbul.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 15.Deng Y, Zhang Y, Lemos B, Ren H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci Rep. 2017;7:46687. doi: 10.1038/srep46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Cauwenberghe L, Janssen CR. Microplastics in bivalves cultured for human consumption. Environ Pollut. 2014;193:65–70. doi: 10.1016/j.envpol.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Rochman CM, Tahir A, Williams SL, Baxa DV, Lam R, Miller JT, The FC, Werorilangi S, The SJ. Anthropogenic debris in seafood: plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci Rep. 2015;5:14340. doi: 10.1038/srep14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang D, Shi H, Li L, Li J, Jabeen K, Kolandhasamy P. Microplastic pollution in table salts from China. Environ Sci Technol. 2015;49:13622–13627. doi: 10.1021/acs.est.5b03163. [DOI] [PubMed] [Google Scholar]
- 19.Karami A, Golieskardi A, Choo CK, Larat V, Karbalaei S, Salamatinia B. Microplastic and mesoplastic contamination in canned sardines and sprats. Sci Total Environ. 2018;612:1380–1386. doi: 10.1016/j.scitotenv.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Mason SA, Welch VG, Neratko J. Synthetic polymer contamination in bottled water. Front Chem. 2018;6:407. doi: 10.3389/fchem.2018.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dris R, Gasperi J, Saad M, Mirande C, Tassin B. Synthetic fibers in atmospheric fallout: a source of microplastics in the environment? Mar Pollut Bull. 2016;15:290–293. doi: 10.1016/j.marpolbul.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Deng Y, Zhang Y, Qiao R, Bonilla MM, Yang X, Ren H, Lemos B. Evidence that microplastics aggravate the toxicity of organophosphorus flame retardants in mice (Mus musculus) J Hazard Mater. 2018;5:348–354. doi: 10.1016/j.jhazmat.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Lu L, Luo T, Zhao Y, Cai C, Fu Z, Jin Y. Interaction between microplastics and microorganism as well as gut microbiota: a consideration on environmental animal and human health. Sci Total Environ. 2019;667:94–100. doi: 10.1016/j.scitotenv.2019.02.380. [DOI] [PubMed] [Google Scholar]
- 24.Lu L, Wan Z, Luo T, Zhengwei Fu, Jin Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci Total Environ. 2018;631–632:449–458. doi: 10.1016/j.scitotenv.2018.03.051. [DOI] [PubMed] [Google Scholar]
- 25.Luo T, Zhang Y, Wang C, Wang X, Zhou J, Shen M, Zhao Y, Fu Z, Jin Y. Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ Pollut. 2019;255:113–122. doi: 10.1016/j.envpol.2019.113122. [DOI] [PubMed] [Google Scholar]
- 26.Park TJ, Lee SH, Lee MS, Lee JK, Lee SH, Zoh KD. Occurrence of micropastics in the Han river and riverine fish in South Korea. Sci Total Environ. 2020;708:134535. doi: 10.1016/j.scitotenv.2019.134535. [DOI] [PubMed] [Google Scholar]
- 27.Poulsen SS, Jackson P, Kling K, Knudsen KB, Skaug V, Kyjovska ZO, Thomsen BL, et al. Multi-walled carbon nanotube physicochemical properties predict pulmonary inflammation and genotoxicity. Nanotoxicology. 2016;10:1263–1275. doi: 10.1080/17435390.2016.1202351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bendtsen KM, Brostrøm A, Koivisto AJ, Koponen I, Berthing T, Bertram N, Kling KI, et al. Airport emission particles: exposure characterization and toxicity following intratracheal instillation in mice. Part Fibre Toxicol. 2019;16:23. doi: 10.1186/s12989-019-0305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avio CG, Gorbi S, Milan M, Benedetti M, Fattorini D, D’Errico G, Pauletto M, Bargelloni L, Regoli F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ Pollut. 2015;198:211–222. doi: 10.1016/j.envpol.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 30.Gambardella C, Morgana S, Ferrando S, Bramini M, Piazza V, Costa E, Garaventa F, Faimali M. Effects of polystyrene microbeads in marine planktonic crustaceans. Ecotoxicol Environ Saf. 2017;145:25–257. doi: 10.1016/j.ecoenv.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 31.Fonte E, Ferreira P, Guilhermino L. Temperature rise and microplastics interact with the toxicity of the antibiotic cefalexin to juveniles of the common goby (Pomatoschistus microps): post-exposure predatory behaviour, acetylcholinesterase activity and lipid peroxidation. Aquat Toxicol. 2016;180:173–185. doi: 10.1016/j.aquatox.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Stock V, Böhmert L, Lisicki E, Block R, Cara-Carmona J, Pack LK, Selb R, et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch Toxicol. 2019;93:1817–1833. doi: 10.1007/s00204-019-02478-7. [DOI] [PubMed] [Google Scholar]
- 33.Li B, Ding Y, Cheng X, Sheng D, Xu Z, Rong Q, Wu Y, Zhao H, Ji X, Zhang Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere. 2020;244:125492. doi: 10.1016/j.chemosphere.2019.125492. [DOI] [PubMed] [Google Scholar]