Abstract
Severe COVID-19 patients demonstrate hypercoagulability, necessitating thromboprophylaxis. However, less is known about the haemostatic profile in mild COVID-19 patients. We performed an age and gender-matched prospective study of 10 severe and 10 mild COVID-19 patients. Comprehensive coagulation profiling together with Thromboelastography and Clot Waveform Analysis were performed. FBC, PT, APTT, D-dimer, fibrinogen and CWA were repeated every 3 days for both groups and repeat TEG was performed for severe patients up till 15 days. On recruitment, severe patients had markers reflecting hypercoagulability including raised median D-dimer 1.0 μg/mL (IQR 0.6, 1.4) (p = 0.0004), fibrinogen 5.6 g/L (IQR 4.9, 6.6) (p = 0.002), Factor VIII 206% (IQR 171, 203) and vWF levels 265.5% (IQR 206, 321). Mild patients had normal values of PT, aPTT, fibrinogen and D-dimer, and slightly elevated median Factor VIII and von Willebrand factor (vWF) levels. Repeated 3-day assessments for both groups showed declining trends in D-dimer and Fibrinogen. CWA of severe COVID-19 group demonstrated hypercoagulability with an elevated median values of aPTT delta change 78.8% (IQR 69.8, 85.2) (p = 0.001), aPTT clot velocity (min1) 7.8%/s (IQR 6.7, 8.3) (p = 0.001), PT delta change 22.4% (IQR 19.4, 29.5) (p = 0.004), PT min1 7.1%/s (IQR 6.3, 9.0) (p = 0.02), PT clot acceleration (min 2) 3.6%/s2 (IQR 3.2, 4.5) (p = 0.02) and PT clot deceleration (max2) 2.9%/s2 (IQR 2.5, 3.5) (p = 0.02). TEG of severe patients reflected hypercoagulability with significant increases in the median values of CFF MA 34.6 mm (IQR 27.4,38.6) (p = 0.003), CRT Angle 78.9° (IQR 78.3, 80.0) (p = 0.0006), CRT A10 67.6 mm (IQR 65.8, 69.6) (p = 0.007) and CFF A10 32.0 mm (IQR 26.8, 34.0) (p = 0.003). Mild COVID-19 patients had absent hypercoagulability in both CWA and TEG. 2 severe patients developed thromboembolic events while none occurred in the mild COVID-19 group. Mild COVID-19 patients show absent parameters of hypercoagulability in global haemostatic tests while those with severe COVID-19 demonstrated parameters associated with hypercoagulability on the global haemostatic tests together with raised D-Dimer, fibrinogen, Factor VIII and vWF levels.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11239-021-02575-4.
Keywords: Thrombelastography, COVID-19, SARS-CoV-2, Hypercoagulability, Venous thromboembolism
Highlights
The haemostatic profile of mild hospitalized COVID-19 patients is not well defined.
Mild COVID-19 patients demonstrate mildly elevated markers of coagulation (Factor VIII and vWF)
Mild COVID-19 patients show absent parameters of hypercoagulability in global haemostatic tests.
Global haemostatic tests may lead to increased sensitivity in the diagnosis of COVID-19 associated hypercoagulability.
Introduction
The COVID-19 pandemic remains a serious global health threat, with more virulent new variants emerging. While most COVID-19 infections are mild, a subset progress to severe or critical illness, characterized by acute respiratory distress syndrome, often accompanied with multiorgan failure. With the lungs as the thrombotic epicenter causing pulmonary intravascular coagulopathy, severe COVID-19 is characterized by a high incidence of macro and microvascular thromboembolic events due to COVID-19 associated coagulopathy (CAC) [1, 2]. This may be attributed to platelet activation, endothelial dysfunction, neutrophil extracellular traps and activation of the coagulation cascade, which are reflective of an uncontrolled proinflammatory response with immune dysregulation and rarely, cytokine storm, in these severe or critically ill patients with sepsis. While hypercoagulability [3] and a high incidence of thrombosis [4, 5] in severely ill COVID-19 patients has been well described, less is known about the haemostatic profile in patients with mild COVID-19 infection who are non-hypoxic with normal chest imaging.
CAC can be dynamic with some patients being pro-thrombotic while others can be at risk of bleeding. The coagulation derangements are more marked in those who are severely or critically ill, likely reflective of a higher burden of micro thrombosis which may precede overt organ failure. Early recognition of CAC and the prompt initiation of thromboprophylactic therapy may lower risk of thromboembolic disease and improve outcomes. While standard coagulation tests are useful as a baseline assessment of coagulation profile, they are not sensitive enough to detect hypercoagulable and mild hypocoagulable states. These tests also do not provide sufficient information to diagnose and treat patients timely and according to their phenotype. Global tests of haemostasis [6–8] such as clot waveform analysis (CWA) and viscoelastic testing using thromboelastography (TEG) [9] or rotational thromboelastometry (ROTEM) [10] are helpful in the dynamic assessment of haemostasis in acutely ill COVID-19 patients and can improve this assessment [11].
The ISTH [12] and latest CHEST guidelines [13] on VTE and management in hospitalized COVID-19 patients have recommended a universal strategy of routine thromboprophylaxis with standard-dose UFH or LMWH after careful assessment of bleed risk, with the ISTH suggesting intermediate-dose LMWH may also be considered. While the ACTIV-4, REMAP-CAP and ATTACC multicentre trials [14] studied therapeutic anticoagulation dose heparin versus standard prophylaxis in COVID-19 patients in the ICU or those who were moderately ill in the general ward, mildly ill COVID-19 patients who are hospitalized are underrepresented in ongoing trials with a lack of high-quality evidence on the use of routine thromboprophylaxis in such patients. Moreover, direct comparison of the haemostatic profiles of mildly ill COVID-19 patients and severely ill COVID-19 patients is lacking.
To address this gap, we performed this study with the aim to describe and clinically correlate haemostatic profile of COVID-19 infection in severely ill patients compared with mildly ill patients matched for age and sex, by evaluating longitudinally, their basic coagulation parameters as well as performing global haemostatic tests using Thromboelastography and Clot Waveform analysis, with the secondary objective of evaluating outcomes of thrombosis in these 2 groups of patients.
Methods
Study design and setting
This was a prospective cohort study conducted at the National Centre for Infectious Diseases, Singapore. The study was approved by the National Healthcare Group Domain Specific Research Board (DSRB) and written informed consent obtained. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for cohort studies in the preparation of this manuscript.
Participants
Due to prevailing public health policy in Singapore, all diagnosed COVID-19 patients (including those with asymptomatic or mild COVID-19) are quarantined in hospitals or community care facilities. Patients ≥ 21 years of age who were admitted to the National Centre for Infectious Diseases, Singapore from June 2020 to January 2021 and met clinical criteria for COVID-19 with laboratory-confirmed SARS-CoV-2 infection as confirmed by PCR were screened. Patients who had either one of the following: PaO2/FiO2 ratio < 300 at screening (severe COVID-19) or SpO2 > 95% on room air at screening without abnormal chest X ray findings (mild COVID-19), were prospectively enrolled and analysed. These criteria were adapted from the Clinical Spectrum of SARS-CoV-2 Infection [15] by the National Institutes of Health (NIH), which defines severe illness as individuals who have SpO2 < 94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) < 300 mm Hg, respiratory frequency > 30 breaths/min, or lung infiltrates > 50%, and critical COVID-19 as individuals who have respiratory failure, septic shock, and/or multiple organ dysfunction. Patients with mild illness can exhibit a variety of symptoms such as fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste and smell; they do not have shortness of breath, dyspnea on exertion, or abnormal chest imaging.
Exclusion criteria included patients with the following conditions: congenital bleeding diathesis or thrombophilia, active bleeding at screening, established chronic liver failure, haemoglobin less than 7.5 g/dl within the previous 48 h, on long term anti-coagulants (patients on anti-platelet agents were not excluded) or those who were started on therapeutic anti-coagulation during their admission. However, patients who were initiated on standard dose pharmacological thromboprophylaxis i.e., subcutaneous low molecular weight heparin (LMWH) or unfractionated heparin (UFH), were not excluded. Patients had their blood samples taken on the day of recruitment as well as every 72 h till discharge or up to day 15 for both the critically ill and non-critically ill, non-oxygen dependent patients. Clinical data of a total of 20 patients recruited from June 2020 to Jan 2021 (10 severe patients compared with 10 mild patients matched for age and sex based on the above inclusion and exclusion criteria) was obtained.
Clinical and laboratory data
Baseline patient characteristics included age, sex, ethnic group, comorbidities, and day of illness at point of enrolment. Disease severity upon recruitment was classified using the Sequential Organ Failure Assessment (SOFA) and PaO2/FiO2 ratio. Clinical data were prospectively collected including laboratory investigations, length of hospital and ICU stay, thrombotic and bleeding events and survival outcomes where available from clinical testing. Recorded interventions included the use of antivirals, systemic corticosteroids, respiratory support, and anticoagulants. Patients that survived to hospital discharge were considered survivors for the purposes of these analyses.
On recruitment (Day 0), venous blood was collected into Ethylenediaminetetraacetic acid (EDTA) tubes (Becton–Dickinson, New Jersey, USA) for baseline full blood count, and 3.2% trisodium citrate tubes for clotting factor levels, von Willebrand Factor antigen, Protein C, Protein S, anti-thrombin III. Anti-phospholipid screening was only performed for patients with a prolonged aPTT at baseline. Serial full blood count and DIC screen (comprising of PT, PTT, Fibrinogen, D-dimer), serial TEG (only for critically ill patients) and serial CWA (all patients) were performed at 3-day (± 1 day) intervals till Day 15 of recruitment (see supplementary material, Study protocol: Study schedule Table 1).
Table 1.
Demographic and clinical characteristics of Covid-19 patients
| Mildly ill COVID-19 patients (n = 10) | Severely ill COVID-19 patients (n = 10) | p-value | |
|---|---|---|---|
| Age (years); median (IQR) | 60 (50, 65) | 60 (49, 64) | 0.84 |
| Gender; n (%) | >0.99 | ||
| Male | 8 (80.0) | 8 (80.0) | |
| Female | 2 (20.0) | 2 (20.0) | |
| Ethnic group; n (%) | 0.44 | ||
| Chinese | 3 (30.0) | 2 (20.0) | |
| Malays | 2 (20.0) | 5 (50.0) | |
| Indians | 4 (40.0) | 1 (10.0) | |
| Others | 1 (10.0) | 2 (20.0) | |
| Comorbidities; n (%) | |||
| Hypertension | 7 (70.0) | 5 (50.0) | 0.65 |
| Hyperlipidaemia | 6 (60.0) | 3 (30.0) | 0.37 |
| COPD | 2 (20.0) | 2 (20.0) | >0.99 |
| Ischemic heart disease | 2 (20.0) | 1 (10.0) | >0.99 |
| Diabetes mellitus | 4 (40.0) | 5 (50.0) | >0.99 |
| Renal impairment | 2 (20.0) | 2 (20.0) | >0.99 |
| Day of illness at point of haemostatic assessment; median (IQR) | 6.5 (5, 8) | 9.5 (6, 13) | 0.24 |
| PADUA Score on assessment; median (IQR) | 3 (3, 4) | 5 (5, 5) | 0.0001 |
| SOFA score on ICU admission; median (IQR) | – | 2 (2, 4.5) | – |
| PaO2/ FiO2 on ICU admission median (IQR) | – | 194.5 (174, 241) | – |
| Highest oxygen requirement; n (%) | < 0.001 | ||
| Invasive ventilation | 0 (0.0) | 3 (30.0) | |
| High flow oxygen (≥4L/min) | 0 (0.0) | 6 (60.0) | |
| Low flow oxygen (< 4L/min) | 0 (0.0) | 1 (40.0) | |
| No supplemental oxygen | 10 (100.0) | 0 (0.0) | |
| Anti-coagulation; n (%) | |||
| Prophylactic | 0 (0.0) | 9 (90.0) | <0.001 |
| Experimental medication; n (%) | >0.99 | ||
| Baricitinib | 0 (0.0) | 2 (20.0) | |
| Dexamethasone | 0 (0.0) | 6 (60.0) | |
| Remdesivir | 2 (20.0) | 7 (70.0) | |
| Thrombotic complications; n(%) | 0 (0.0) | 2 (20.0) | 0.47 |
| Bleeding complications; n (%) | 0 (0.0) | 1 (10.0) | >0.99 |
| Outcome; n (%) | |||
| Death | 0 (0.0) | 1 (10.0) | |
| Discharged | 9 (90.0) | 9 (90.0) | >0.99 |
| Transferred | 1 (10.0) | 0 (0.0) | |
| Length of ICU stay (Days); median (IQR) | – | 8 (5, 10.5) | – |
| Length of hospital stay (Days); median (IQR) | 7 (6, 11) | 17 (13, 19) | 0.02 |
Materials
Tests of haemostasis
Coagulation tests were performed on the STA R Max Series coagulation analyzer (Diagnostica Stago, France). PT was quantified with STA Neoplastine CI Plus 10, aPTT with STACephascreen 10, fibrinogen (modified Clauss) with STA Liquid FIB, D-dimer with STA Liatest D-Di and thrombin clotting time with STA Thrombin 10. Clotting factor levels (Factors II, V, VII, VIII, IX, X, XI) were measured with STA Deficient II, V, VII, VIII, IX, X, and XI respectively. Von Willebrand factor (vWF) antigen was assayed with immunoturbidimetric method using STA Liastest vWF: Ag kit. Both assays for protein C and anti-thrombin III are functional chromogenic assays using STA Stachrom protein C and STA Stachrom ATIII kits respectively. The Protein S assay used is a functional clotting assay using a STA Staclot protein S kit. Lupus anticoagulant was performed using STA Staclot DRVV Screen, STA Staclot DRVV Confirm and PTT-LA. Anti-cardiolipin IgM and IgG were quantified using Inova test kits, and anti-B2-glycoprotein-1, Euroimmun test kit, both were performed on the Inova Quanta-lyser 3000 analyzer.
Global haemostatic tests
Thromboelastography was performed using (TEG) (Haemonetics, TEG6s). The TEG-6 is a microfluidic automated cartridge-based assay, where the citrated multichannel assay measures platelet–fibrin clot strength (maximal amplitude), reaction time (R time), kinetics (K, measure of time to reach 20 mm of clot strength from R) and angle (representative of the velocity of clot strength generation). Clot waveform analysis (CWA) was performed with Sysmex CN-6000 automated coagulation analyser (Sysmex Corporation, Kobe, Japan) with Dade Actin FSL (Siemens Healthcare, Marburg, Germany) for aPTT CWA and Innovin (Siemens Healthcare, Marburg, Germany) for PT CWA, as per International Society of Haemostasis and Thrombosis (ISTH) Scientific and Standardization Committee recommendation [16]. Four quantitative parameters were recorded: “Delta change” (difference between initial maximum and final maximum values of light transmittance), “Min1” (maximum velocity), “Min2” (maximum acceleration), and “Max2” (maximum deceleration).
Statistical analysis
Descriptive analyses were used to summarise the baseline characteristics and laboratory findings among the severely ill and mildly ill COVID-19 patients. For categorical data, frequencies and percentages were presented. Tests of association were performed by the Pearson’s chi-square test if expected counts in all cells were 5 or more, or by the Fisher’s exact test if one of the expected counts was fewer than 5. For continuous data, median and interquartile range (IQR) were presented due to its skewed distribution. Tests of association between mild COVID-19 patients and severe COVID-19 patients were performed using the Wilcoxon rank-sum test. A 2-sided p-value < 0.05 was statistically significant. All analyses were done using STATA 16.1. (Reference: StataCorp. 19. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.)
Results
Demographic and clinical characteristics
This prospective cohort study included 20 COVID-19 patients (10 severely or critically ill with PF ratio < 300, 10 mild COVID-19 patients) with a median age 60 (interquartile range (IQR) 49.5–64.5). The patients were predominantly male (16 out of 20 patients) with a multi-ethnic background (Chinese, Indian, Malay and Others). The patients had comorbidities of hypertension (60%), hyperlipidemia (45%), diabetes (45%) and chronic obstructive pulmonary disease (20%), with a slightly higher number of co-morbidities in mildly ill patients. The median day of haemostatic assessment of their illness was slightly earlier in mildly ill COVID-19 patients at Day 6.5 (IQR 5–8) of illness compared with severely ill patients at Day 9.5 (IQR 6–13) of illness, which is consistent with the progression of COVID-19 infection, where the onset of respiratory failure commences after the first week of illness.
As per our Singapore NCID treatment guidelines for COVID-19 [17], we used pharmacological venous thromboembolism (VTE) prophylaxis for patients with critical or severe COVID-19, as well as risk stratified mild/moderate COVID-19 patients for requirement for thromboprophylaxis using the PADUA prediction score. The median Padua prediction score on assessment was raised for severely ill patients (5 points) compared with mildly ill patients (3 points), with a median SOFA score of 2 (IQR 2–4.5 points) on ICU admission. 9 of the 10 severe patients were initiated on pharmacological thromboprophylaxis with low molecular weight heparin (enoxaparin), with 1 patient excluded due to haemorrhagic conversion of ischaemic stroke. The other group of 10 mild patients had low Padua scores and were not placed on pharmacological thromboprophylaxis. 2 of the severe patients developed thrombosis, one with proximal lower limb thrombosis and the other with ischaemic stroke with haemorrhagic conversion. No other bleeding events were noted in the other patients. The SOFA score was low on ICU admission as patients were pre-emptively admitted to ICU for high flow oxygen, preceding a planned intubation if respiratory failure worsened. The median PaO2/FiO2 ratio on admission to ICU was 194.5 (IQR 174–241) with 3 patients requiring mechanical ventilation, with 1 developing intractable respiratory failure requiring extra corporeal membrane oxygenation and eventually death. The median length of ICU stay was 8 days (IQR 5–10.5 days) for severe patients. The median length of hospital stay was 17 days (IQR 13–19 days) for severe patients and a shorter 7 days (IQR 6–11 days) for mild patients. Table 1 summarizes the demographic and clinical characteristics of these 20 Covid-19 patients.
Laboratory and haemostatic tests
On Day 0 of recruitment, severe patients had median haemoglobin concentration of 13.1 g/dl (IQR 12.3, 13.7), with a normal median white blood cell count of 8.1 × 109/L (IQR 1.9, 6.2 g/dl), lymphopenia of 0.8 × 109/L (IQR 0.5, 1.2), and a raised median lactate dehydrogenase (LDH) of 840.5 U/L (IQR 604, 1418.5) and raised median C-reactive protein (CRP) of 88.7 mg/L (IQR 66.3, 172.0). In contrast, mild patients had on Day 0, median haemoglobin concentration of 15.0 g/dl (IQR 12.8, 15.6), with a normal median white blood cell count of 5.2 × 109/L (IQR 4.0, 6.0), absent lymphopenia, with absolute median lymphocyte count of 1.4 × 109/L (IQR 1.2, 1.6), a normal median lactate dehydrogenase of 433.0 U/L (IQR 354.0, 533.0) and normal median CRP 6.5 mg/L (IQR 3.8, 23.9). Lupus anticoagulant was present in 3 severe patients with 2 having elevated anti β2 glycoprotein 1. Repeated 3-day assessments till Day 15 for both groups of patients showed slight decline in median haemoglobin levels.
For haemostatic tests, mild patients on Day 0 had normal median values of PT, aPTT, fibrinogen and D-dimer, and demonstrated slightly elevated median Factor VIII levels 176% (IQR 157, 192) and elevated von Willebrand factor 225% (IQR 158, 237). Severe patients on Day 0 had a normal median PT, slightly elevated median aPTT 36.3 s (IQR 32.8, 41.6), as well as markers suggestive of hypercoagulability including raised median D-dimer 1.0 μg/mL (IQR 0.6, 1.4) (p = 0.0004), elevated median fibrinogen level 5.6 g/L (IQR 4.9, 6.6) (p = 0.002), raised median Factor VIII 206% (IQR 171, 230) and raised von Willebrand factor 265.5% (IQR 206, 321). Repeated 3-day assessments for both groups of patients showed declining trends in D-dimer and Fibrinogen values, with normalisation of D-dimer and fibrinogen levels towards Day 15. The median values for both mild and severe patients for Factors II, V, VII, IX, X and XI, Protein C, Protein S and anti-thrombin III were within the normal reference ranges. Tables 2 and 3 summarizes the multiple laboratory and coagulation parameters that were performed.
Table 2.
Comparisons of FBC, LDH, CRP and basic coagulation tests between mildly ill and severely ill COVID-19 Patients
| Laboratory tests (Reference ranges) | Mildly ill COVID-19 patients (n = 10) | Severely ill COVID-19 Patients (n = 10) | p-value |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Haemoglobin (g/dL)b | |||
| Baseline | 15.0 (12.8, 15.6) | 13.1 (12.3, 13.7) | 0.08 |
| Day 3 | 14.2 (13.6, 15.7) | 13.2 (11.7, 13.6) | 0.03 |
| Day 6 | 13.0 (12.8, 17.2) | 13.1 (11.8, 13.9) | 0.46 |
| Day 9 | 13.4 (13.4, 13.4)a | 12.4 (12.0, 12.9) | 0.50 |
| Day 12 | 13.6 (13.6, 13.6)a | 12.3 (11.5, 13.7) | 0.75 |
| Day 15 | 13.8 (13.8, 13.8)a | 12.4 (11.8, 13.8) | 0.80 |
| WBC (× 109/L) (4.0–9.6) | |||
| Baseline | 5.7 (3.7, 6.0) | 6.8 (6.2, 7.4) | 0.04 |
| Day 3 | 5.2 (3.9, 5.8) | 8.9 (6.0, 11.4) | 0.02 |
| Day 6 | 9.0 (4.7, 9.6) | 8.9 (7.9, 12.8) | 0.62 |
| Day 9 | 4.2 (4.2, 4.2)a | 7.6 (6.2, 17.7) | 0.50 |
| Day 12 | 4.1 (4.1, 4.1)a | 8.1 (6.1, 10.6) | 0.25 |
| Day 15 | 3.8 (3.8, 3.8)a | 8.4 (6.8, 10.8) | 0.40 |
| Lymphocytes (× 109/L) (1.1–3.1) | |||
| Baseline | 1.4 (1.2, 1.6) | 0.8 (0.5, 1.2) | 0.06 |
| Day 3 | 1.2 (0.7, 1.6) | 0.9 (0.6, 1.4) | 0.37 |
| Day 6 | 1.8 (0.7, 2.0) | 1.2 (1.1, 2.3) | 0.83 |
| Day 9 | 0.4 (0.4, 0.4)a | 1.5 (1.4, 1.8) | 0.25 |
| Day 12 | 0.8 (0.8, 0.8)a | 1.8 (1.3, 2.2) | 0.25 |
| Day 15 | 0.8 (0.8, 0.8)a | 2.0 (1.5, 2.1) | 0.40 |
| Platelets (× 109/L) (150–360) | |||
| Baseline | 234.0 (137.0, 250.0) | 290.5 (233.0, 364.0) | 0.07 |
| Day 3 | 222.5 (167.0, 263.0) | 382.0 (339.0, 490.0) | 0.002 |
| Day 6 | 283.0 (199.0, 378.0) | 409.0 (363.0, 509.0) | 0.09 |
| Day 9 | 199.0 (199.0, 199.0)a | 345.0 (253.0, 543.0) | 0.25 |
| Day 12 | 241.0 (241.0, 241.0)a | 281.0 (236.0, 493.0) | 0.75 |
| Day 15 | 226.0 (226.0, 226.0)a | 352.0 (266.0, 441.0) | 0.80 |
| LDH (U/L) (270–550) | |||
| Baseline | 433.0 (354.0, 533.0) | 840.5 (604.0, 1418.5) | 0.23 |
| Day 3 | 371.0 (363.0, 681.0) | 630.0 (573.0, 925.5) | 0.29 |
| Day 6 | 827.0 (827.0, 827.0) | 475.0 (466.0, 518.0) | 0.67 |
| Day 9 | – | 677.5 (480.0, 875.0) | – |
| Day 12 | – | – | – |
| Day 15 | – | – | – |
| CRP (mg/L) (0.0–7.0) | |||
| Baseline | 6.5 (3.8, 23.9) | 88.7 (66.3, 172.0) | 0.06 |
| Day 3 | 14.5 (1.1, 47.2) | 66.0 (10.3, 133.3) | 0.39 |
| Day 6 | – | 8.3 (3.2, 15.7) | – |
| Day 9 | – | 8.3 (7.9, 13.9) | – |
| Day 12 | – | 6.5 (1.9, 34.4) | – |
| Day 15 | – | 1.8 (0.9, 2.8) | – |
| PT (secs) (11.7–14.0) | |||
| Baseline | 12.8 (12.4, 12.9) | 14.0 (12.9, 15.7) | 0.005 |
| Day 3 | 12.3 (12.1, 12.7) | 14.6 (13.5, 15.6) | 0.0007 |
| Day 6 | 13.0 (13.0, 13.2) | 14.1 (13.4, 14.6) | 0.16 |
| Day 9 | 15.6a | 14.1 (13.5, 15.0) | 0.50 |
| Day 12 | 14.6a | 13.5 (13.4, 13.8) | 0.50 |
| Day 15 | 13.3a | 13.4 (12.8, 14.1) | 0.99 |
| aPTT (secs) (27.0–37.0) | |||
| Baseline | 29.0 (28.0, 30.5) | 36.3 (32.8, 41.6) | 0.005 |
| Day 3 | 28.7 (27.7, 32.3) | 30.9 (29.9, 38.8) | 0.21 |
| Day 6 | 29.5 (28.2, 31.4) | 31.5 (29.0, 33.9) | 0.64 |
| Day 9 | 41.0a | 32.2 (29.6, 35.0) | 0.25 |
| Day 12 | 38.4a | 32.5 (30.2, 34.9) | 0.25 |
| Day 15 | 34.4a | 31.2 (29.4, 33.8) | 0.80 |
| D-Dimer (FEU) (μg/mL) (<0.50) | |||
| Baseline | 0.3 (0.3, 0.4) | 1.0 (0.6, 1.4) | 0.0004 |
| Day 3 | 0.3 (0.3, 0.3) | 0.6 (0.4, 2.5) | 0.0005 |
| Day 6 | 0.3 (0.3, 0.3) | 0.8 (0.4, 3.1) | 0.03 |
| Day 9 | 0.5 (0.5, 0.5)a | 0.7 (0.4, 2.7) | 0.99 |
| Day 12 | 0.3 (0.3, 0.3)a | 0.4 (0.3, 2.4) | 0.99 |
| Day 15 | 0.3 (0.3, 0.3)a | 0.3 (0.3, 1.8) | 0.80 |
| Fibrinogen (g/L) (1.8–4.5) | |||
| Baseline | 4.1 (3.6, 4.4) | 5.6 (4.9, 6.6) | 0.002 |
| Day 3 | 4.0 (3.6, 4.8) | 5.0 (4.2, 6.8) | 0.08 |
| Day 6 | 4.4 (4.1, 6.3) | 4.8 (4.1, 5.4) | 0.76 |
| Day 9 | 4.3 (4.3, 4.3)a | 4.8 (4.5, 5.3) | 0.50 |
| Day 12 | 4.4 (4.4, 4.4)a | 4.3 (4.0, 5.2) | 0.99 |
| Day 15 | 4.0 (4.0, 4.0)a | 4.2 (3.8, 5.4) | 0.80 |
| TCT (secs) (15.0–18.0) | |||
| Baseline | 16.5 (16.0, 17.4) | 17.6 (16.5, 18.8) | 0.09 |
| Day 3 | 16.7 (16.4, 17.0) | 17.9 (17.3, 19.5) | 0.01 |
| Day 6 | 16.0 (15.4, 16.8) | 18.1 (17.4, 19.4) | 0.01 |
| Day 9 | 17.3 (17.3, 17.3)a | 18.8 (17.7, 19.3) | 0.50 |
| Day 12 | 17.6 (17.6, 17.6)a | 17.6 (17.0, 18.6) | 0.99 |
| Day 15 | 16.9 (16.9, 16.9)a | 16.6 (15.8, 18.0) | 0.99 |
aResults for 1 patient only, 9 others were discharged for isolation at community care facilities
bHaemoglobin for males: 13.6–16.6 g/dL, Haemoglobin for females 11.8–14.6 g/dL
Table 3.
Comparisons of coagulation results between mildly ill and severely ill Covid-19 and ICU Patients
| Laboratory tests (Reference ranges) | Mildly Ill COVID-19 patients (n = 10) | Severely Ill COVID-19 patients (n = 10) | p-value |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Factor II (%) (70–120) | 108.5 (99, 119) | 113.5 (87, 117) | 0.85 |
| Factor V (%) (70–120) | 116.5 (106, 128) | 109.5 (86, 151) | 0.63 |
| Factor VII (%) (55–170) | 118 (105, 145) | 100.5 (74, 128) | 0.17 |
| Factor VIII (%) (60–150) | 176 (157, 192) | 206 (171, 230) | 0.14 |
| Factor IX (%) (60–150) | 115 (95, 145) | 144.5 (126, 191) | 0.055 |
| Factor X (%) (70–120) | 105.5 (88, 124) | 103 (90, 124) | 0.93 |
| Factor XI (%) (60–150) | 109.5 (97, 127) | 119 (112, 163) | 0.18 |
| von Willebrand factor (%) (56–160) | 225 (158, 237) | 265.5 (206, 321) | 0.12 |
| Anti-thrombin III (%) (80–130) | 101.5 (93, 110.5) | 101 (84, 106) | 0.81 |
| Protein C (%) (70–150) | 103.5 (88.5, 113.5) | 92.5 (72, 99) | 0.09 |
| Protein S (%) (55–130) | 74 (66, 86.5) | 71 (64, 80) | 0.95 |
| Lupus Anticoagulant; n(%) | 0.50 | ||
| Absent | 1 (100.0) | 0 (0.0) | |
| Weakly present | 0 (0.0) | 3 (100.0) | |
| Anti-cardiolipin IgG (GPL units); n(%) | – | ||
| <20 | 1 (100.0) | 3 (100.0) | |
| Anti-cardiolipin IgM (MPL units); n(%) | – | ||
| <20 | 1 (100.0) | 3 (100.0) | |
| Anti B2 (RU/mL); n(%) | 0.99 | ||
| <2 | 1 (100.0) | 1 (33.3) | |
| ≥2 | 0 (0.0) | 2 (66.7) |
Global haemostatic tests
aPTT and PT clot waveform analysis (CWA) (Table 4)
Table 4.
Comparisons of clot waveform analysis results between mildly ill Covid-19 patients and severely ill COVID-19 patients
| Mildly ill COVID-19 patientsb (n = 8) | Severely ill COVID-19 Patients (n = 10) | p-value | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Delta change (aPTT) (%) (25.21–63.09) | |||
| Baseline | 55.2 (43.3, 62.4) | 78.8 (69.8, 85.2) | 0.001 |
| Day 3 | 55.1 (50.4, 60.6) | 65.8 (58.8, 85.6) | 0.06 |
| Day 6 | 61.7 (59.5, 64.6) | 67.9 (56.9, 73.6) | 0.63 |
| Day 9 | 62.2 (62.2, 62.2)a | 66.4 (62.7, 71.2) | 0.57 |
| Day 12 | 62.1 (62.1, 62.1)a | 62.6 (59.2, 72.0) | 0.99 |
| Day 15 | 56.9 (56.9, 56.9)a | 60.2 (54.2, 72.2) | 0.80 |
| Min 1 (aPTT) (%/s) (2.86–6.78) | |||
| Baseline | 5.7 (4.9, 6.1) | 7.8 (6.7, 8.3) | 0.001 |
| Day 3 | 5.9 (5.2, 6.2) | 6.8 (5.9, 8.1) | 0.08 |
| Day 6 | 6.5 (5.9, 6.6) | 7.0 (6.5, 7.7) | 0.19 |
| Day 9 | 6.1 (6.1, 6.1)a | 7.2 (4.7, 7.5) | 0.99 |
| Day 12 | 6.1 (6.1, 6.1)a | 7.2 (6.5, 7.8) | 0.29 |
| Day 15 | 4.4 (4.4, 4.4)a | 6.7 (6.0, 7.9) | 0.40 |
| Min 2 (aPTT) (%/s2) (0.46–1.10) | |||
| Baseline | 0.9 (0.8, 1.0) | 1.2 (0.9, 1.3) | 0.07 |
| Day 3 | 0.9 (0.9, 1.0) | 1.1 (1.0, 1.5) | 0.02 |
| Day 6 | 1.0 (0.9, 1.1) | 1.1 (1.1, 1.2) | 0.08 |
| Day 9 | 1.0 (1.0, 1.0)a | 1.3 (1.0, 1.8) | 0.25 |
| Day 12 | 0.9 (0.9, 0.9)a | 1.2 (1.0, 1.3) | 0.29 |
| Day 15 | 2.3 (2.3, 2.3)a | 1.1 (1.0, 2.8) | 0.80 |
| Max2 (aPTT) (%/s2) (0.37–0.93) | |||
| Baseline | 0.7 (0.7, 0.8) | 1.0 (0.7, 1.0) | 0.04 |
| Day 3 | 0.8 (0.7, 0.8) | 0.9 (0.8, 1.2) | 0.11 |
| Day 6 | 0.9 (0.7, 0.9) | 0.9 (0.9, 1.0) | 0.19 |
| Day 9 | 0.7 (0.7, 0.7)a | 1.1 (0.8, 1.4) | 0.25 |
| Day 12 | 0.7 (0.7, 0.7)a | 1.0 (0.9, 1.1) | 0.29 |
| Day 15 | 1.7 (1.7, 1.7)a | 0.9 (0.8, 2.3) | 0.80 |
| Delta change (PT) (%) (6.52–17.28) | |||
| Baseline | 14.9 (11.2, 16.7) | 22.4 (19.4, 29.5) | 0.004 |
| Day 3 | 14.8 (13.2, 17.0) | 16.7 (14.2, 27.6) | 0.28 |
| Day 6 | 16.8 (16.2, 19.4) | 18.3 (15.2, 19.5) | 0.92 |
| Day 9 | 17.4 (17.4, 17.4)a | 16.3 (14.9, 20.7) | 0.86 |
| Day 12 | 16.8 (16.8, 16.8)a | 15.8 (14.6, 20.8) | 0.86 |
| Day 15 | 14.2 (14.2, 14.2)a | 15.2 (13.0, 21.8) | 0.80 |
| Min 1 (PT) (%/s) (1.95–5.67) | |||
| Baseline | 5.3 (4.1, 5.4) | 7.1 (6.3, 9.0) | 0.02 |
| Day 3 | 4.8 (4.3, 5.4) | 5.0 (4.5, 8.5) | 0.49 |
| Day 6 | 5.4 (5.1, 6.4) | 5.5 (4.7, 5.9) | 0.78 |
| Day 9 | 5.1 (5.1, 5.1)a | 6.6 (4.8, 7.9) | 0.75 |
| Day 12 | 5.1 (5.1, 5.1)a | 5.1 (4.6, 6.7) | 0.99 |
| Day 15 | 5.8 (5.8, 5.8)a | 5.0 (4.2, 7.3) | 0.80 |
| Min 2 (PT) (%/s2) (0.97–2.93) | |||
| Baseline | 2.7 (2.1, 2.7) | 3.6 (3.2, 4.5) | 0.02 |
| Day 3 | 2.5 (2.2, 2.8) | 2.5 (2.3, 4.4) | 0.57 |
| Day 6 | 2.8 (2.5, 3.3) | 2.8 (2.4, 3.0) | 0.78 |
| Day 9 | 2.6 (2.6, 2.6)a | 2.4 (1.1, 4.0) | 0.99 |
| Day 12 | 2.5 (2.5, 2.5)a | 2.7 (2.4, 3.4) | 0.99 |
| Day 15 | 0.9 (0.9, 0.9)a | 2.1 (1.7, 2.6) | 0.40 |
| Max 2 (PT) (%/s2) (0.75–2.35) | |||
| Baseline | 2.2 (1.7, 2.2) | 2.9 (2.5, 3.5) | 0.02 |
| Day 3 | 2.0 (1.8, 2.2) | 1.9 (1.8, 3.3) | 0.66 |
| Day 6 | 2.2 (1.9, 2.6) | 2.2 (1.8, 2.3) | 0.63 |
| Day 9 | 1.9 (1.9, 1.9)a | 1.9 (0.9, 3.2) | 0.99 |
| Day 12 | 1.9 (1.9, 1.9)a | 2.1 (1.9, 2.7) | 0.86 |
| Day 15 | 0.7 (0.7, 0.7)a | 1.7 (1.3, 2.1) | 0.40 |
Reference intervals for clot waveform parameters were established locally based on 124 healthy controls in accordance with the Clinical and Laboratory Standards Institute guidelines
aResults for 1 patient only, 9 others were discharged to community care facilities
b2 COVID-19 patients progressed from mildly ill to a moderately ill state, hence their CWA parameters were excluded from the analysis
Plasma specimens from the 20 patients were evaluated by CWA every 3 days till Day 15 of recruitment. On Day 0 of assessment, CWA performed on aPTT from platelet poor plasma of severely ill patients showed a significant decrease in light transmission, represented by a high median delta change of 78.8% (IQR 69.8, 85.2) (p = 0.001) (Fig. 1). An increased clot velocity, represented by an elevated median Min1 of 7.7%/s (reference range 2.85–6.65%/s) (Fig. 2); increased clot acceleration, represented by elevated median Min2 of 1.2%/s2 (reference range 0.46–1.08%/s) and increased clot deceleration, represented by elevated median Max2 of 1.0%/s2 (reference range 0.37–0.91%/s) was observed. CWA performed on PT also showed similar elevated median Min1 of 6.9%/s (reference range 1.96–5.51%/s); elevated median min2 of 3.5%/s (reference range 0.98–2.84%/s), elevated median Max2 of 2.8%/s (reference range 0.74–2.28%/s), and elevated median delta change of 33.0 (reference range 6.6–16.9%).
Fig. 1.
Comparison of median delta change in aPTT (%/s) between mildly ill and severely ill COVID-19 patients
Fig. 2.
Comparison of median min1 in aPTT (%/s) between mildly ill and severely ill COVID-19 patients
For the mild patients, the median aPTT and PT clot waveform parameters were all within normal range throughout the 15 days of assessment. 2 mild patients who later developed consolidation on subsequent repeat chest X ray were excluded from CWA analysis, as their illness worsened, fulfilling criteria for moderate COVID-19 illness. These 2 patients demonstrated elevated delta change, clot velocity, clot acceleration and deceleration on CWA analysis. Comparison of CWA parameters between severe patients and mild patients on Day 0 of recruitment, showed in the severe group, a statistically significant higher (above the upper limit of the respective reference intervals) median aPTT delta change 78.8 (IQR 69.8, 85.2) (p = 0.003), median PT delta change 22.4 (IQR 19.4, 29.5) (p = 0.008), median aPTT clot velocity (min1) of 7.7%/s (IQR 6.4, 8.3) (p = 0.02), median PT clot velocity (min1) of 7.1%/s (IQR 6.3, 9.0), median PT clot acceleration (min 2) of 3.6%/s2 (IQR 3.2, 4.5) (p = 0.02) and clot deceleration (max2) 2.9 (IQR 2.5, 3.5) (p = 0.02) than in the mild group, which had normal median aPTT and PT delta change, clot velocity (min 1), clot acceleration (min2) and clot deceleration (max2).
Over the 6 time points of assessment spread over 15 days, there was an overall declining trend in the CWA parameters (aPTT and PT) for both severe and mild patients. There was no biphasic waveform pattern present on light transmission curves of the COVID-19 patients that could suggest an underlying DIC.
Thromboelastography (TEG) (Table 5)
Table 5.
Comparisons of thromboelastography results between mildly ill and severely ill COVID-19 Patients
| Mildly ill COVID-19 patients (n = 10) | Severely ill COVID-19 Patients (n = 10) | p-value | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| CK R (4.6–9.1 min) | |||
| Baseline | 6.3 (5.8, 7.5) | 6.4 (5.8, 7.9) | 0.78 |
| Day 3 | – | 6.3 (6.1, 6.9) | – |
| Day 6 | – | 5.8 (4.7, 7.2) | – |
| Day 9 | – | 6.0 (5.5, 8.5) | – |
| Day 12 | – | 7.9 (6.5, 9.6) | – |
| Day 15 | – | 7.2 (6.2, 9.4) | – |
| CK K (0.8–2.1 min) | |||
| Baseline | 1.6 (1.3, 2.1) | 1.1 (0.9, 1.2) | 0.008 |
| Day 3 | – | 0.8 (0.8, 1.3) | – |
| Day 6 | – | 0.8 (0.8, 1.1) | – |
| Day 9 | – | 1.0 (0.8, 1.2) | – |
| Day 12 | – | 1.1 (1.0, 1.3) | – |
| Day 15 | – | 1.2 (1.0, 1.8) | – |
| CK Angle (63°–78°) | |||
| Baseline | 69.1 (64.6, 74.5) | 75.3 (73.4, 77.2) | 0.01 |
| Day 3 | – | 76.8 (71.5, 78.2) | – |
| Day 6 | – | 77.6 (74.6, 80.5) | – |
| Day 9 | – | 75.7 (73.8, 78.1) | – |
| Day 12 | – | 73.0 (64.5, 74.6) | – |
| Day 15 | – | 74.4 (68.6, 77.8) | – |
| CK MA (52-69 mm) | |||
| Baseline | 58.8 (57.2, 59.9) | 66.6 (64.2, 69.6) | 0.009 |
| Day 3 | – | 68.2 (65.6, 69.3) | – |
| Day 6 | – | 67.8 (65.2, 70.5) | – |
| Day 9 | – | 68.5 (65.5, 69.9) | – |
| Day 12 | – | 67.2 (64.6, 71.3) | – |
| Day 15 | – | 64.6 (63.3, 67.8) | – |
| CRT R (0.3–1.1 min) | |||
| Baseline | 0.5 (0.5, 0.7) | 0.6 (0.4, 0.6) | 0.85 |
| Day 3 | – | 0.6 (0.5, 0.7) | – |
| Day 6 | – | 0.6 (0.4, 0.6) | – |
| Day 9 | – | 0.6 (0.4, 0.7) | – |
| Day 12 | – | 0.6 (0.5, 0.7) | – |
| Day 15 | – | 0.6 (0.6, 0.7) | – |
| CRT K (0.8–2.7 min) | |||
| Baseline | 1.5 (1.1, 1.6) | 0.8 (0.8, 0.8) | 0.0006 |
| Day 3 | – | 0.9 (0.7, 0.9) | – |
| Day 6 | – | 0.8 (0.7, 0.9) | – |
| Day 9 | – | 0.8 (0.7, 0.8) | – |
| Day 12 | – | 0.8 (0.8, 0.8) | – |
| Day 15 | – | 0.9 (0.8, 1.0) | – |
| CRT Angle (60°–78°) | |||
| Baseline | 74.1 (72.0, 75.2) | 78.9 (78.3, 80.0) | 0.002 |
| Day 3 | – | 78.4 (77.6, 81.0) | – |
| Day 6 | – | 79.5 (77.9, 81.6) | – |
| Day 9 | – | 79.8 (77.7, 81.2) | – |
| Day 12 | – | 79.7 (78.1, 80.0) | – |
| Day 15 | – | 77.5 (76.8, 79.6) | – |
| CRT MA (50-70 mm) | |||
| Baseline | 61.7 (60.0, 65.2) | 69.0 (68.1, 70.6) | 0.009 |
| Day 3 | – | 69.0 (68.2, 72.5) | – |
| Day 6 | – | 69.8 (67.3, 73.1) | – |
| Day 9 | – | 69.5 (67.7, 72.6) | – |
| Day 12 | – | 69.2 (67.8, 71.5) | – |
| Day 15 | – | 67.7 (66.1, 70.6) | – |
| CKH R (4.3–8.3 min) | |||
| Baseline | 6.8 (5.8, 7.3) | 6.6 (5.2, 7.7) | 0.99 |
| Day 3 | – | 5.9 (5.5, 6.7) | – |
| Day 6 | – | 6.1 (4.6, 7.3) | – |
| Day 9 | – | 5.9 (5.3, 8.6) | – |
| Day 12 | – | 7.1 (6.3, 8.3) | – |
| Day 15 | – | 6.9 (6.1, 7.8) | – |
| CKH K (0.8–1.9 min) | |||
| Baseline | 1.3 (1.3, 2.0) | 1.0 (0.8, 1.2) | 0.02 |
| Day 3 | – | 0.9 (0.8, 1.2) | – |
| Day 6 | – | 0.9 (0.8, 1.1) | – |
| Day 9 | – | 1.0 (0.8, 1.1) | – |
| Day 12 | – | 1.0 (1.0, 1.4) | – |
| Day 15 | – | 1.2 (1.0, 1.6) | – |
| CKH Angle (64°–77°) | |||
| Baseline | 71.3 (65.6, 74.4) | 75.9 (74.0, 78.2) | 0.01 |
| Day 3 | – | 76.6 (74.7, 77.9) | – |
| Day 6 | – | 77.4 (73.8, 79.8) | – |
| Day 9 | – | 76.4 (74.8, 77.1) | – |
| Day 12 | – | 74.9 (70.9, 76.1) | – |
| Day 15 | – | 74.2 (68.5, 77.0) | – |
| CKH MA (52–69 mm) | |||
| Baseline | 59.5 (58.3, 60.9) | 66.7 (64.9, 69.4) | 0.006 |
| Day 3 | – | 68.3 (65.8, 69.1) | – |
| Day 6 | – | 68.4 (65.8, 70.6) | – |
| Day 9 | – | 68.5 (66.1, 69.9) | – |
| Day 12 | – | 67.7 (64.0, 71.4) | – |
| Day 15 | – | 65.4 (64.7, 67.4) | – |
| CRT A10 (44-67 mm) | |||
| Baseline | 55.1 (52.6, 61.5) | 67.6 (65.8, 69.6) | 0.007 |
| Day 3 | – | 67.9 (66.0, 71.9) | – |
| Day 6 | – | 68.4 (65.3, 72.6) | – |
| Day 9 | – | 68.3 (65.7, 71.7) | – |
| Day 12 | – | 67.8 (65.7, 70.6) | – |
| Day 15 | – | 65.3 (62.7, 69.4) | – |
| CFF MA (15–32 mm) | |||
| Baseline | 19.6 (18.9, 21.4) | 34.6 (27.4, 38.6) | 0.003 |
| Day 3 | – | 35.1 (27.5, 43.8) | – |
| Day 6 | – | 37.3 (26.5, 46.0) | – |
| Day 9 | – | 37.6 (29.3, 40.3) | – |
| Day 12 | – | 35.4 (31.2, 40.6) | – |
| Day 15 | – | 27.7 (25.8, 36.7) | – |
| CFF A10 (15–30 mm) | |||
| Baseline | 19.3 (18.6, 23.5) | 32.0 (26.8, 34.0) | 0.003 |
| Day 3 | – | 32.6 (25.1, 38.5) | – |
| Day 6 | – | 32.0 (24.8, 42.3) | – |
| Day 9 | – | 34.6 (27.0, 35.8) | – |
| Day 12 | – | 31.1 (29.5, 36.7) | – |
| Day 15 | – | 25.0 (23.8, 34.0) | – |
| Y 30 | |||
| Baseline | 0 | 0 | – |
| Day 3 | – | 0 | – |
| Day 6 | – | 0 | – |
| Day 9 | – | 0 | – |
| Day 12 | – | 0 | – |
| Day 15 | – | 0 | – |
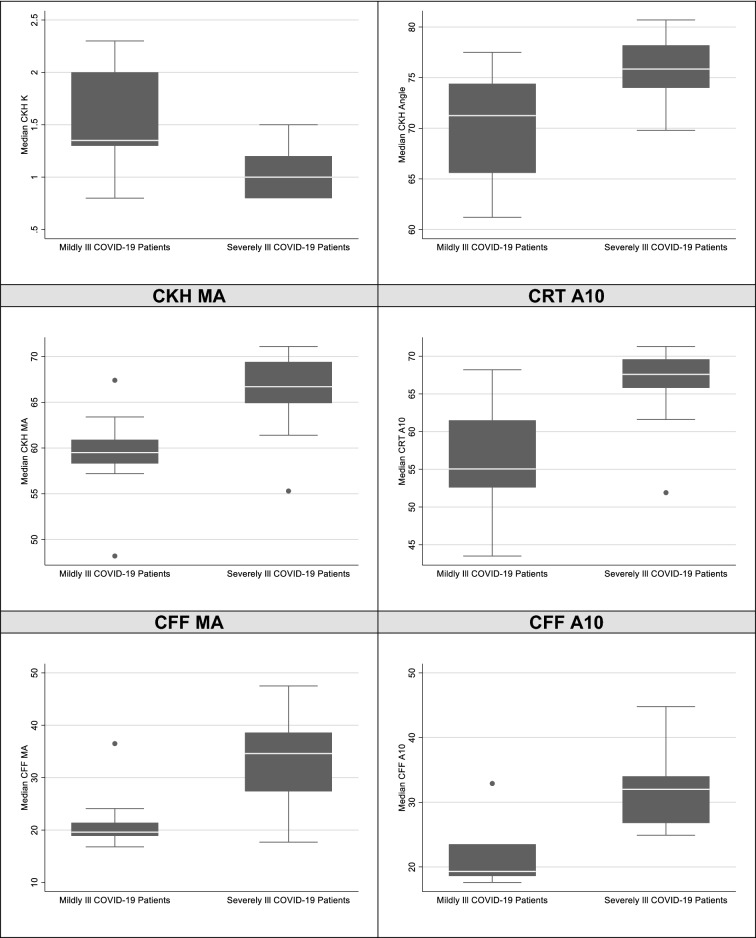
At Day 0 of assessment, in severely ill patients there was a statistically significant increase in the median maximal amplitude (MA) in the citrated functional fibrinogen (CFF) channel 34.6 mm (IQR 27.4, 38.6) (p = 0.003), elevated median Angle in the tissue factor and kaolin activated channel (CRT) of 78.9° (IQR 78.3, 80.0) (p = 0.002), increased CRT A10 67.6 mm (65.8, 69.6) (p = 0.007), and increased CFF A10 32.0 mm (IQR 26.8, 34.0) (p = 0.007) (Fig. 3). Mild COVID-19 patients had normal parameters for the Reaction Rate (R), Kinetics Time (K), Angle (α), Maximal amplitude (MA) for the 4 channels, namely the kaolin activated TEG channel (CK), tissue factor and kaolin activated channel (CRT), Kaolin with Heparinase TEG (CKH) and citrated functional fibrinogen channel (CFF). None of the severely ill or mild patients demonstrated any fibrinolysis with a LY30% of 0.
Fig. 3.
Boxplots of TEG parameters which exhibited significant differences between mildly ill and severely ill COVID-19 patients
For the severely ill patients, the MA for the CK, CRT and CFF channels, CK and CRT angles, and CRT and CFF A10 showed a peak in values towards Day 9 of assessment, before a decreasing trend was noted towards Day 15. Tables 4 and 5 summarizes the multiple quantitative parameters for the global haemostatic tests (CWA and TEG) that were recorded.
Discussion
To our knowledge, this is the first prospective, matched study comparing conventional coagulation tests and global haemostatic tests between mild and severe COVID-19 patients. Currently there are no diagnostic criteria for COVID-19 associated coagulopathy (CAC), however three stages of CAC have been proposed by Thachil et al. [18] to aid early recognition and for early intervention. Stage 1 comprises of mild symptoms without oxygen supplementation, mild systemic inflammation, and mild systemic coagulopathy. Stage 2 comprises of severe symptoms, requiring oxygen supplementation, progressive pulmonary inflammation, increased incidence of microthrombi and local coagulopathy and critical care support with a hypercoagulable state. Stage 3, the critically ill patient with COVID-19 has respiratory failure which may require mechanical ventilatory support or extracorporeal membranous oxygenation support. The coagulation profile may have a high D-dimer levels 6 times above upper limit of normal, hyperfibrinogenaemia, thrombocytopenia, prolonged PT, a high incidence of pulmonary and venous thrombosis, and in rare cases DIC.
Our mild patients who remained in Stage 1 of CAC were non oxygen dependent, remained ambulant throughout their hospitalization and were discharged well. Upon initial assessment, they had mildly raised coagulation parameters (mildly raised median Factor VIII levels and vWF levels, with normal median D-dimer and fibrinogen levels) and normal global haemostatic tests (normal TEG and normal CWA parameters), with no elevation of median CWA parameters on repeated assessment and no elevation of baseline TEG on Day 0. They did not receive thromboprophylaxis with LMWH and no corresponding thrombotic or bleeding events were noted.
Conversely, our severe patients enrolled were in either stage 2 or 3 of CAC on their day of recruitment with the majority initiated on standard dose LMWH thromboprophylaxis. Upon initial assessment, they had markedly raised coagulation parameters (elevated median D-dimer, Fibrinogen, Factor VIII, vWF levels) indicative of a hypercoagulable state which correlated with a raised C-reactive protein (CRP) and raised white blood cell count that reflected an inflammatory state. 3 patients developed a lupus anticoagulant. Only 2 out of 10 critically ill patients developed thrombosis and 1 subsequently developed intracranial bleeding due to haemorrhagic conversion of ischaemic stroke. Our decreased rate of thrombosis observed in the severely ill group compared to higher published international rates [19] is consistent with the rates seen in a local Singapore multicentre ICU study which we performed [5], showing lower venous thromboembolism but higher arterial thrombosis. This may be due to regional and ethnic variations, where previous studies have shown patients of Asian lineage having a lower VTE risk compared to Western cohorts [20]. Our patients were also younger with fewer comorbidities with early admission to the ICU while not requiring mechanical ventilation and started early on interventions such as high dose dexamethasone, remdesivir and tocilizumab, which may mitigate the development of a significant thrombo-inflammatory state associated with severe COVID-19.
In recent studies on CWA and COVID-19 associated hypercoagulability, CWA parameters were significantly higher in severe COVID-19 [3, 21] as compared with mild disease [22, 23]. The analysis of aPTT and PT clot waveform parameters in our severe patients showed hypercoagulability with elevated delta change, clot velocity and clot acceleration and deceleration. An elevated delta change, which corresponds to decreased light transmission due to increased clot thickness, correlates with high levels of fibrinogen that contribute to clot strength and thickness. Elevated clot velocity (min1) reflects an increased thrombin burst and elevated clot acceleration/deceleration (min2, max2) reflects enhancement of prothrombinase activity [24], as well as the observed hyperfibrinogenaemia which enhances the speed and acceleration of clot formation. For CWA in mild COVID-19, there was no demonstrable increase in clot waveform parameters throughout the assessment. 2 patients in the mild group had their clot waveform analysis parameters excluded from analysis as they later developed chest X ray changes and desaturation (not requiring supplemental oxygenation), resulting in a progression of disease classification to moderate COVID-19. These patients had worsening of their COVID-19 illness on Day 3–6 of assessment, with elevated CWA aPTT and PT delta change, min 1, min2, max2 as well as increased fibrinogen levels, suggesting the development of clinically detectable hypercoagulability.
The use of TEG in the evaluation of CAC in critically ill COVID-19 patients was initially proposed by Panigada et al. [9], and later affirmed by a recent systematic review [25] that showed its efficacy in identifying and evaluating a hypercoagulable state in patients with COVID-19. TEG parameters demonstrated a hypercoagulable state with decreased K (kinetic) time, increased alpha angle, increased maximal amplitude (MA) and decreased LY30 (decreased fibrinolysis), with findings suggestive of decreased time to clot initiation, increased clot strength and decreased clot breakdown.
Our evaluation of TEG performed at Day 0 of assessment for severe patients demonstrated statistically significant elevations of median values of CFF MA, CRT Angle, CRT A10 and CFF A10 above the reference ranges, compared to mild patients who had normal values. While in severe patients the CK K, CK angle, CRT K, CRT angle, CRT MA, CKH K, CKH angle, and CKH MA remained within the normal reference range, they were significantly higher in the severely ill group compared to the mild group. A low lysis time (LY30) was observed to be less than 1% for all TEG performed in both groups. An increased CFF MA and CFF A10 quantifies fibrinogen contribution to clot strength and correlates with the increased fibrinogen levels seen in severely ill patients, supporting the involvement of fibrinogen in causing hypercoagulability in COVID-19. Over the 15-day assessment of TEG in severe patients, the rising CFF MA (which was noted on Day 0 of assessment) peaked at Day 9, reflective of associated increased hypercoagulability in the ICU and worsening respiratory function. An elevated CRT A10 and CRT angle which peaked at Day 6 and Day 9 respectively suggests increased fibrin activation and polymerization, reflective of increased speed of clot propagation, while reduced fibrinolytic activity was observed with all patients showing TEG that had LY30% of less than 1%, characteristic of fibrinolytic shutdown seen in COVID-19. Despite thromboprophylaxis, severely ill patients still exhibited hypercoagulability as shown by elevated CFF MA and CFF A10 reflecting a high platelet–fibrin clot strength from hyperfibrinogenaemia. This reflects an underdosing of anticoagulation, where the inadequate response to standard thromboprophylaxis has been demonstrated by Gurbel et al. [26, 27] in hospitalized COVID-19 patients that did not achieve the intended pharmacodynamic effect, given the persistent hypercoagulability shown on TEG despite thromboprophylaxis.
Despite recommendations by the ISTH and CHEST guidelines for a universal strategy of thromboprophylaxis for all hospitalized COVID-19 patients with standard LMWH or UFH after bleeding risk assessment, our prospective matched study has not shown evidence for sustained hypercoagulability nor the need for pharmacological thromboprophylaxis in our mild, non-hypoxic, ambulant group of COVID-19 patients who do not progress beyond Stage 1 of CAC. Universal thromboprophylaxis is not without the associated bleeding risks, and implementation of this strategy may not be suitable for all patients. Tests of coagulation and global haemostatic tests demonstrate the ability to identify hypercoagulability in critically ill patients and absence of hypercoagulability in mild patients compared to laboratory coagulation tests such as PT and aPTT, which were found to be within normal range in our patients (or slightly prolonged in other studies). Comparatively, point of care global haemostatic tests such as TEG have the advantage of a shorter turnaround time than laboratory testing for Factor VIII or vWF levels. In addition, careful monitoring with coagulation and platelet function tests [28] including platelet reactivity [29] appear beneficial for early identification of high risk COVID-19 patients and for optimization of antithrombotic therapy to reduce thrombotic risk during the critical phase of disease. Based on our findings, we propose a combination of coagulation parameters (Fibrinogen, Factor VIII and D-dimer levels) and global haemostatic tests paired with Padua scoring for VTE for a more individualized approach to thrombotic risk assessment and management.
While limitations of our study include firstly, the small number of patients recruited as a single centre cohort that may limit generalizability, and secondly, the drop out of the mild COVID-19 patients at Day 9 because they were discharged well to community care facilities for isolation, we believe our comparison data sufficiently demonstrates parameters suggestive of a hypercoagulable state reflected by global haemostatic tests and conventional markers of haemostasis (fibrinogen, Factor VIII, D-dimer), as we recruited patients within the same study period and the demographics between the 2 groups were comparable.
In conclusion, severely ill COVID-19 patients demonstrate coagulation parameters associated with hypercoagulability in both conventional coagulation tests and global tests of haemostasis, while mild COVID-19 patients had mildly elevated coagulation tests with absence of parameters of hypercoagulability in global tests of haemostasis. Further well-designed randomized control trials are urgently required to assess the haemostatic profile in the asymptomatic, non-hospitalized COVID-19 patients and hospitalized mild COVID-19 patients, and to evaluate the safety and efficacy of pharmacologic thromboprophylaxis in mildly ill hospitalized COVID-19 patients. Our findings suggest the use of global haemostatic tests may lead to increased sensitivity in the diagnosis of hypercoagulability and appears reasonable in assisting clinicians in the decision for thromboprophylaxis of COVID-19 patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors greatly appreciate the efforts of our fellow healthcare workers during this pandemic. Special thanks to Sysmex Corporation (Japan) and Transmedic Pte. Ltd. for their technical support.
Author contributions
BEF, DCL and CYW conceived the study. All authors contributed substantially to the acquisition, analysis and interpretation of data, critical revision of manuscript for important intellectual content.
Funding
This work was supported by a National Healthcare Group-National Centre for Infectious Diseases (NHG-NCID) COVID-19 Centre Grant (COVID19 CG0004).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the National Healthcare Group Domain Specific Review Board (DSRB ref: 2020/00633).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kiat Hoe Ong and Yew Woon Chia senior authors contributed equally.
References
- 1.Becker RC. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50:54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitra S, Ling RR, Yang IX, et al. Severe COVID-19 and coagulopathy: a systematic review and meta-analysis. Ann Acad Med Singap. 2021;50(4):325–335. doi: 10.47102/annals-acadmedsg.2020420. [DOI] [PubMed] [Google Scholar]
- 3.Fan BE, Ng J, Chan SSW, et al. COVID-19 associated coagulopathy in critically ill patients: a hypercoagulable state demonstrated by parameters of haemostasis and clot waveform analysis. J Thromb Thrombolysis. 2021;51:663–674. doi: 10.1007/s11239-020-02318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan CW, Fan BE, Teo WZY, et al. Low incidence of venous thrombosis but high incidence of arterial thrombotic complications among critically ill COVID-19 patients in Singapore. Thrombosis J. 2021;19:14. doi: 10.1186/s12959-021-00268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lance MD (2015) A general review of major global coagulation assays: thromboelastography, thrombin generation test and clot waveform analysis. Thrombosis J;13:1. http://www.thrombosisjournal.com/content/13/1/1 [DOI] [PMC free article] [PubMed]
- 7.Sevenet PO, Depasse F. Clot waveform analysis: Where do we stand in 2017? Int J Lab Hem. 2017;39:561–568. doi: 10.1111/ijlh.12724. [DOI] [PubMed] [Google Scholar]
- 8.Nair SC, Dargaud Y, Chitlur M, Srivastava A. Tests of global haemostasis and their applications in bleeding disorders. Haemophilia. 2010;16(Suppl 5):85–92. doi: 10.1111/j.1365-2516.2010.02304.x. [DOI] [PubMed] [Google Scholar]
- 9.Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Veenendaal N, Scheeren TWL, Meijer K, van der Voort PHJ. Rotational thromboelastometry to assess hypercoagulability in COVID-19 patients. Thromb Res. 2020;196:379–381. doi: 10.1016/j.thromres.2020.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan BE, Chia YW, Sum CLL, et al. Global haemostatic tests in rapid diagnosis and management of COVID-19 associated coagulopathy in acute limb ischaemia. J Thromb Thrombolysis. 2020;50:292–297. doi: 10.1007/s11239-020-02165-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: Chest guideline and expert panel report. Chest. 2020;158(3):1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leentjens J, van Haaps TF, Wessels PF, Schutgens REG, Middeldorp S. COVID-19-associated coagulopathy and antithrombotic agents-lessons after 1 year. Lancet Haematol. 2021;8(7):e524–e533. doi: 10.1016/S2352-3026(21)00105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical Spectrum of SARS-CoV-2 Infection (Last Updated: April 21, 2021) https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- 16.Shima M, Thachil J, Nair SC (2013) Srivastava a; scientific and standardization committee. Towards standardization of clot waveform analysis and recommendations for its clinical applications. J Thromb Haemost 11(7):1417–1420. 10.1111/jth.12287 [DOI] [PubMed]
- 17.Treatment Guidelines for COVID-19 (Version 6.0, dated 14 June 2021) https://www.ncid.sg/Health-Professionals/Diseases-and-Conditions/Pages/COVID-19.aspx
- 18.Thachil J, Cushman M, Srivastava A. A proposal for staging COVID-19 coagulopathy. Res Pract Thromb Haemost. 2020;4:731–736. doi: 10.1002/rth2.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Minno A, Ambrosino P, Calcaterra I, Di Minno MND. COVID-19 and Venous Thromboembolism: a Meta-analysis of Literature Studies. Semin Thromb Hemost. 2020;46(7):763–771. doi: 10.1055/s-0040-1715456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee LH, Gallus A, Jindal R, Wang C. Wu CC: incidence of venous thromboembolism in Asian populations: a systematic review. Thromb Haemost. 2017;117:2243–2260. doi: 10.1160/TH17-02-0134. [DOI] [PubMed] [Google Scholar]
- 21.Tan CW, Low JGH, Wong WH, Chua YY, Goh SL, Ng HJ. Critically ill COVID-19 infected patients exhibit increased clot waveform analysis parameters consistent with hypercoagulability. Am J Hematol. 2020;95(7):E156–E158. doi: 10.1002/ajh.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan CW, Tan JY, Wong WH, et al. Clinical and laboratory features of hypercoagulability in COVID-19 and other respiratory viral infections amongst predominantly younger adults with few comorbidities. Sci Rep. 2021;11:1793. doi: 10.1038/s41598-021-81166-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimura T, Kurano M, Kanno Y, et al. Clot waveform of APTT has abnormal patterns in subjects with COVID-19. Sci Rep. 2021;11:5190. doi: 10.1038/s41598-021-84776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wada H, Matsumoto T, Ohishi K, Shiraki K, Shimaoka M. Update on the clot waveform analysis. Clin Appl Thromb Hemost. 2020;26:1076029620912027. doi: 10.1177/1076029620912027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartmann J, Ergang A, Mason D, Dias JD (2021) The role of TEG analysis in patients with COVID-19-Associated coagulopathy: a systematic review. Diagnostics (Basel);11(2):172. Published 2021 Jan 26. 10.3390/diagnostics11020172 [DOI] [PMC free article] [PubMed]
- 26.Gurbel PA, Bliden KP, Rout A, et al. Bedside thromboelastography to rapidly assess the pharmacodynamic response of anticoagulants and aspirin in COVID-19: evidence of inadequate therapy in a predominantly minority population. J Thromb Thrombolysis. 2021;51(4):902–904. doi: 10.1007/s11239-021-02435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurbel PA, Bliden KP, Levy JH, et al (2021) Thrombogenicity markers for early diagnosis and prognosis in COVID-19: a change from the current paradigm? [published online ahead of print, 2021 Aug 6]. Blood Coagul Fibrinolysis. 10.1097/MBC.0000000000001069. doi:10.1097/MBC.0000000000001069 [DOI] [PMC free article] [PubMed]
- 28.Gurbel PA, Tantry US, Storey RF (2021) International COVID-19 thrombosis biomarkers colloquium: COVID-19 diagnostic tests [published online ahead of print, 2021 May 22]. J Thromb Thrombolysis;1–7. 10.1007/s11239-021-02465-9 [DOI] [PMC free article] [PubMed]
- 29.Bertolin AJ, Dalçóquio TF, Salsoso R, et al. Platelet Reactivity and Coagulation Markers in Patients with COVID-19. Adv Ther. 2021;38(7):3911–3923. doi: 10.1007/s12325-021-01803-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.