Abstract
Background:
Dogs play an important role in transmission of parasites and zoonotic diseases, especially in developing countries. We aimed to investigate the prevalence of internal parasites in stray and pet dogs in Palestine.
Methods:
Fecal samples were collected during the period between Jan to May 2019. A total of 150 fecal samples were collected and tested for parasites using sedimentation and flotation techniques. The targeted dogs were both pet dogs and stray dogs and were grouped according to gender and age.
Results:
Although there was no significant difference in infestation between males and females, the prevalence rate of infestation in males was 63.1% compared to females 72.4%. Dogs of ages under one year had similar rate of infestation compared to older dogs with a rate of 67.3% and 67.4% respectively. Infestations were significantly higher (P<0.05) in stray dogs 81.4% compared to pet dogs 48.4%. The highest infestation rate was with Toxocara canis 46.0% followed by Dipylidium caninum 23.0%, Echinococcus spp. 14.0% ,Isosopora canis 9.0%, Ancylostoma caninum 8.0%, Giarda spp. 5.0%, Strongyloides spp. 4.0%, Trichuris vulpis 3.0%, and Cryptosporidium spp. 3.0%.
Conclusion:
Local dogs in Palestine, especially stray dogs, are infested with different types of intestinal parasites that may cause many common and non-common diseases to humans. To prevent the spread of these parasites, future public health should be proposed and applied by authorities to achieve a healthy status of the residents in the country. Health awareness spread among people about the seriousness of the diseases transmitted by dogs must also be activated.
Keywords: Gastrointestinal parasites, Stray dogs, Pet dogs, Zoonoses, Palestine
Introduction
Dogs play an important role in the transmission of diseases and infestations to humans. There are more than 60 zoonotic diseases transmitted from dogs to human. Dogs are both intermediate and final host in many parasitic infestations especially that are associated with the intestinal parasites (1).
The possible danger of dogs lies in the fact that they are among the most common animals that communicate with humans through their rearing in homes and their direct and indirect contact with humans. Dogs are the main source of transmission of many zoonotic parasitic diseases to humans such as Echinococcus spp. that lead to formation of the hydatid cysts, that is an important example. Many other parasites and protozoa can also be encountered such as the round worm Toxocara canis, the tape worm Dipylidium caninum, the hook worm Ancylostoma spp., and protozoa such as Giardia spp. and Cryptosporidium spp. (2–6).
The different parasites have negative impacts on dogs’ health and wellbeing such as blood loss and gastrointestinal tract hemorrhages that lead to anemia. Some other parasites will result in vomiting, fever, weight loss, dull hair coat and death (1, 5). The transmission of parasites to human is by direct and indirect contact with the dogs’ excreta that contains different stages of the parasites such as eggs, larvae, and oocyst, which can stay in the surrounding environment for long periods and become a potential source of parasitic infection through direct contact. In addition contaminated food and water can be a vehicle to transmit the infestation.
As indicated by several studies, developing countries suffer from more parasitic infections than other countries. One reason can be attributed to the poor living condition and lack of money to take care for their pets, in particular dogs (5, 7–9). There is no information about dogs’ parasites and its effects on human health in Palestine. Therefore, we aimed to investigate the prevalence of gastrointestinal parasites in dogs in Palestine.
Materials and Methods
Fecal samples
A total of 150 fecal samples, from which 92 males and 58 females (Table 1), were collected from local dogs at different locations, ages and housings. Fifty five samples were taken from dogs younger than one year old, and 95 samples were taken from older dogs (Table 2). Sixty four samples were taken from pet dogs and 86 from stray dogs (Table 3).
Table 1:
Prevalence of gastrointestinal parasites in dogs in Palestine according to sex
| Sex | Number examined | Number positive | % |
|---|---|---|---|
| Male | 92 | 59 | 64.1a |
| Female | 58 | 42 | 72.4a |
| Total | 150 | 101 | 68.3 |
Data with the same letter are significantly different (P<0.05)
Table 2:
Prevalence of gastrointestinal parasites of dogs in Palestine according to age
| Age | Number examined | Number positive | % |
|---|---|---|---|
| Young <1yr | 55 | 37 | 67.3a |
| Adult >1yr | 95 | 64 | 67.4a |
| Total | 150 | 101 | 67.3 |
Data with the same letter are significantly differ (P<0.05)
Table 3:
Prevalence of gastrointestinal parasites of dogs according to housing
| Housing | Number examined | Number of positive | % |
|---|---|---|---|
| Pet dogs | 64 | 31 | 48.4b |
| Stray dogs | 86 | 70 | 81.4a |
| Total | 150 | 101 | 64.9 |
Data with the same letter are significantly differ (P<0.05)
Collection of fecal Samples
Fecal samples were collected during the period from Jan to May 2019 from pet and stray dogs, in the northern West Bank of Palestine. Samples were taken directly from rectum of some dogs or soon after defecation from ground. Samples were placed in tight plastic bags, identified for location, age, gender and the dog living condition. Samples were transported to the Faculty of Agriculture and Veterinary Medicine-labs and stored at 4 °C for later inspection.
Fecal Examination
Samples were inspected within 48 h after collection. An initial microscopic examination was performed to monitor sample texture, color, presence of blood or mucus, helminthes and proglottids of cestodes. Samples were examined by flotation and sedimentation techniques (10, 11). Flotation was done by mixing 3 g fecal sample with 10 ml normal saline solution. The sample was poured through a tea strainer into a beaker or fecal cup, then it was centrifuged at 1,200 rpm for 10 min. A light microscope was then used to examine the centrifuged samples at 10X and 40X. In the sedimentation technique, a small volume of the sediment was put onto a slide with a long coverslip sediments and was examined under the light microscope at 10X and 40X (10, 11).
Statistical analysis
Chi-square tests (contained in the SPSS statistics software package: Release 16.0 standard version, SPSS Inc., Chicago, IL) were used to test for statistical significance of differences in parasite prevalence among population. The Descriptive statistical significance of percentage differences of parasite between subgroups was calculated by analysis of variance (ANOVA; also contained in SPSS) and Tukey’s for Multiple Comparisons. The differences were considered statistically significant when P<0.05.
Results
The rate of infestation was 64.1% in males and 72.4% in females (Table 1). The infestation in young dogs (< 1 yr) was 67.3% and a similar rate was observed in dogs with age above one yr 67.4% (Table 2). The infection in pet dogs was 48.4%, compared to infections of stray dogs which was 81.4% (Table 3).
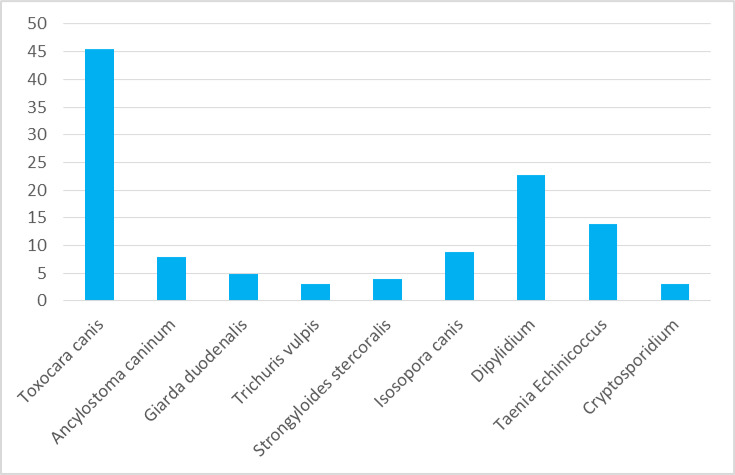
The highest infection was with T. canis 46.0%, followed by D. caninum 23.0%, then Echinococcus spp. 14.0%, I. canis 9.0%, A. caninum 8.0%, Giarda spp. 5.0%, Strongyloides spp. 4.0%, T. vulpis 3.0%, and Cryptosporidium spp. 3.0% (Fig. 1).
Fig. 1:
Prevalence rate of various gastrointestinal parasites in dogs in Palestine
Types and numbers of different gastrointestinal parasites of dogs in Palestine according to sex, age and housing are shown in Table 4.
Table 4:
Types and numbers of gastrointestinal parasites recovered from dogs in Palestine, distributed according to sex, age and housing
| Vriable | Number examined | Type of parasites detected | Number of each parasite | Total number positive dogs |
|---|---|---|---|---|
| Sex | ||||
| Male | 92 | T. canis | 28 | 59 (4 cases with multiple parasites) |
| D. caninum | 14 | |||
| Echinococcus spp. | 7 | |||
| I. canis | 2 | |||
| A. caninum | 4 | |||
| Giarda spp. | 2 | |||
| Strongyloides spp. | 2 | |||
| T. vulpis | 2 | |||
| Cryptosporidium spp. | 2 | |||
| Female | 58 | T. canis | 18 | 42 (10 cases with multiple parasites) |
| D. caninum | 9 | |||
| Echinococcus spp. | 7 | |||
| I. canis | 7 | |||
| A. caninum | 4 | |||
| Giarda spp. | 3 | |||
| Strongyloides spp. | 2 | |||
| T. vulpis | 1 | |||
| Cryptosporidium spp. | 1 | |||
| Age | ||||
| Young <1year | 55 | T. canis | 21 | 37 (3 cases with multiple parasites) |
| D. caninum | 5 | |||
| Echinococcus spp. | 4 | |||
| I. canis | 6 | |||
| Giarda spp. | 1 | |||
| T. vulpis | 1 | |||
| Cryptosporidium spp. | 2 | |||
| Adult >1year | 95 | T. canis | 25 | 64 (10 cases with multiple parasites) |
| D. caninum | 18 | |||
| Echinococcus spp. | 10 | |||
| I. canis | 3 | |||
| A. caninum | 7 | |||
| Giarda spp. | 4 | |||
| Strongyloides spp. | 4 | |||
| T. vulpis | 2 | |||
| Cryptosporidium spp. | 1 | |||
| Housing | ||||
| Household | 64 | T. canis | 13 | 31 (3 cases with multiple parasites) |
| D. caninum | 5 | |||
| Echinococcus spp. | 4 | |||
| I. canis | 3 | |||
| A. caninum | 1 | |||
| Giarda spp. | 2 | |||
| Strongyloides spp. | 3 | |||
| Cryptosporidium spp. | 3 | |||
| Stray | 86 | T. canis | 33 | 70 (11 cases with multiple parasites) |
| D. caninum | 18 | |||
| Echinococcus spp. | 10 | |||
| I. canis | 6 | |||
| A. caninum | 7 | |||
| Giarda spp. | 3 | |||
| Strongyloides spp. | 1 | |||
| T. vulpis | 3 | |||
| Total | 150 | 344 | 101 | |
Discussion
The Results showed that there was no significant difference between the rate of parasitic infestations between male and female dogs, which were 64.1% and 71.4%, respectively. The living conditions and behavior may explain this result. Living in herds expose dogs to similar conditions and infection. The higher infections in females can be explained by contacts with several males during estrus and the stress might increase the incidence of infestation through negatively affecting the females’ immune system (3, 9). Similar findings were reported from Nigeria, Cuba and Poland where similar infections were detected between male and female dogs (5, 9, 12).
Similar infestation rates were observed in the two age groups, where they were 67.3% and 67.4% for young dogs and old dogs respectively. Similar living condition and same food available to dogs may explain these results (4, 8). The results of this study are in agreement with the results from Cuba and Nigeria where dogs’ age had no effect on infestation rate (5, 9). Results of this study are also in agreement with a previous study in the West Bank (13), where age of dogs had no effects on the infestation with T. canis parasite. In contrast, younger animals are more susceptible to infestations with intestinal parasites more than older dogs (14).
Stray dogs had more infestations compared to household dogs (81.4% and 48.4%) respectively. This could be explained that stray dogs live around garbage dumps which is the main source of feed as well as the consumption of dead animal and rodents that increase the chance of internal parasitic infestations in dogs, which makes stray dogs more vulnerable to infestation because these animals are an intermediate host for many intestinal parasites (2, 10).
As for the kinds of the parasites found, the results of this study are in agreement with previous reports (6, 8, 9, 15), where the infestations of dogs were due to T. canis, D. caninum, Echinicoccus spp., I. canis, A. caninum, Giarda spp., Strongyloides spp., T. vulpis and Cryptosporidium spp. The highest infestation rate with T. canis found in this study was also reported in many studies (7, 9, 16–20).
Toxocariasis has been reported as the second most common helminth infection in developed countries (21, 22). Worldwide studies about Toxocariasis in dogs revealed wide range prevalences, with 86 to 100% in puppies and 1 to 45% in adults (23–27)
Toxocara eggs are shed to the environment in the feces of infested dogs. It takes 2 to 4 week for Toxocara larvae to develop inside the eggs and become infectious (28). Toxocara eggs are very resistant to various environmental conditions due the strong protective layer surrounding them, which allows the eggs to survive in the environment for months or even years (28, 29).
D. caninum parasite took the second place in infestation rate with 23.0%. This can be attributed to the lack of sanitation and hygiene which increase the incidence of infection from the surrounding environment. Some insects as fleas that act as intermediate host which transmitted the infestation to dogs that are the final host (10, 17, 30). The infestation with Echinococcus spp., which was in the third place with 14.0% infestation rate, can be due to the large population of the intermediate hosts as herbivores. Stray Dogs (canidae) feed on the discarded viscera of these animals (sheep, goats) which are the source of the Scolices, which grow to mature worms inside the final host (dogs). Eggs of this worm can survive harsh condition and stay as a potential infection to herbivores and human (2, 31). Echinococcus spp. were also reported by many other studies and reports (7, 8, 14, 15, 31). Echinococcosis is one of the most widespread and important global helminth zoonoses (32). In Palestine, mainly due to the presence of stray dogs, the cases of echinoccosis in old sheep slaughtered was approximately 41.2% (33). This cestode is found in a wide spectrum of intermediate hosts such as humans, sheep, goats, camels, cattle, pigs and equine. Canids as the final host, shed the eggs in feces, contaminating vegetation which is then consumed by humans or other intermediate hosts. It is estimated that echinococcosis results in annual economic losses of several billion dollars in livestock sector due to low performance, morbidity and/or mortality of infected animals, and condemnation of infected organs of slaughtered animals (34).
The results of the rest of the intestinal parasites that were reported in this study including I. canis 9.0%, A. caninum 8.0%, Giarda spp. 5.0%, Strongyloides spp. 4.0%, T. vulpis 3.0%, Cryptosporidium spp. 3.0%, were similar to many international studies in terms of their infestation rates in dogs in different countries of the world with a variation in the incidence of infestation that depends on the conditions of dogs’ breeding, nutrition and the degree of care practiced for them, as well as their environment, as most of these factors differ from one country to another (4, 6, 7, 12, 15, 19, 20, 35–37).
Conclusion
This study showed clearly that local dogs in Palestine, especially stray dogs, are infected with different types of intestinal parasites that cause many common and non-common diseases. The most common types of parasites are T. canis, D. caninum, Echinococcus spp., and I. canis. The study showed that stray dogs of different races and ages pose a real threat to human health because they transmit zoonotic parasites between humans and other animals. To prevent the spread of these parasites, the role of public health must be activated, along with health awareness among people through education about the seriousness of the diseases transmitted by dogs, how they are transmitted and how to prevent them. As well as how to deal with stray dogs and limit their spread and movement in residential areas. Tight control over butchers and slaughterhouses to prevent them from throwing waste to the surrounding environment and to animals and dogs, that might act as a vehicle for the transmission of many diseases to humans. The owners of the dogs should be urged to inspect their dogs periodically and give anthelmintic if needed. The elimination of intermediate hosts such as rodents and insects is also highly advised. These hosts play an important role in completing the life cycles of some dangerous parasites that may affect the human health and life.
It is also necessary for specialists at the Ministry of Health and Environment and other governmental and non-governmental organization, to take random samples from the population and conduct the necessary laboratory tests to find out the extent of infestation with these parasites among the population in Palestine.
Acknowledgements
No financial support was received for this study.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Khanteand GS, Khan LA, Bodhke AM, et al. Epidemiological survey of gastro-intestinal parasites of non-descript dogs in Nagpur City. Vet World. 2009; 2(1):22–23. [Google Scholar]
- 2.Al-Bajalan M. Prevalence of intestinal helminths in stray dogs of Kalar city/Sulaimani province. Iraqi Journal of Veterinary Medicine. 2010; 34(1):151–157. [Google Scholar]
- 3.Perera P, Rajapakse R, Rajakaruna R. Gastrointestinal parasites of dogs in Hantana area in the Kandy District. Journal of the National Science Foundation of Sri Lanka. 2013; 41 (2):81–91. [Google Scholar]
- 4.Alvarado-Esquivel C, Romero-Salas D, Aguilar-Domínguez M, et al. Epidemiological assessment of intestinal parasitic infections in dogs at animal shelter in Veracruz, Mexico. Asian Pacific Journal of Tropical Biomedicine. 2015; 5(1):34–39. [Google Scholar]
- 5.Mustapha F, Balami S, Malgwi S, et al. Prevalence of Gastrointestinal Parasites of Hunting Dogs in Maiduguri, Borno state, Nigeria. IOSR Journal of Agriculture and Veterinary Science. 2016; 9:39–42. [Google Scholar]
- 6.Puebla LEJ, Nunez FA, Rivero LR, et al. Prevalence of Intestinal Parasites in Dogs from Municipality La Lisa, Havana, Cuba. Journal of Veterinary Science & Technology. 2015; 6:5. [Google Scholar]
- 7.Hadi A, Ali A. Prevalence of gastrointestinal Helminthes and protozoa among stray dogs in Baghdad. Iraqi J of Vet Med. 2016; 40(1):1–4. [Google Scholar]
- 8.Mohamed T, Al barwary L. Prevalence of Intestinal Parasites in the Intestine of Dogs (Sheep-Keeper, Owned, Pet and Stray) in Duhok Province, Kurdistan Region. Journal of Veterinary Science & Technology. 2016; 7(6):1–4. [Google Scholar]
- 9.Luis Enrique JP, Moreno LR, Nunez FA, et al. Prevalence of intestinal parasitic infections in dogs from Havana, Cuba: risk of zoonotic infections to humans. Anim Husb Dairy Vet Sci. 2018; 2(3):1–5. [Google Scholar]
- 10.Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. 7th ed. London: Baillière Tindall, 1982. P. 119–127. [Google Scholar]
- 11.Demelash K, Abebaw M, Negash A, et al. A Review on Diagnostic Techniques in Veterinary Helminthlogy. Nat Sci. 2016; 14(7): 109–118. [Google Scholar]
- 12.Bartosik J, Dziwirek K, Łojek J, et al. Prevalence of intestinal parasite infection in dogs from selected rural areas of central and southern Poland. Scientific Annals of Polish Society of Animal Production. 2017; 13(1):61–69. [Google Scholar]
- 13.Othman R. Prevalence of Toxocara canis in Dogs, North West Bank of Palestine. Korean J Parasitol. 2011; 49(2):181–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sowemimo OA. The prevalence and intensity of gastrointestinal Parasites of dogs in Illefe, Nigeria J Helminth. 2009; 83(1):27–31. [DOI] [PubMed] [Google Scholar]
- 15.Sommer MF, Zdravković N, Vasić A, et al. Gastrointestinal parasites in shelter dogs from Belgrade, Serbia. Vet Parasitol Reg Stud Reports. 2017; 7:54–57. [DOI] [PubMed] [Google Scholar]
- 16.Al-Jassim KBN, Mahmmod YS, Salem ZM, et al. Epidemiological investigation of gastrointestinal parasites in dog populations in Basra province, Southern Iraq. J Parasit Dis. 2017; 41(4):1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abo-Shehada M, Ziyadeh Y. Prevalence of endoparasites in dog faecal deposits in Jordan. J Helminthol. 1991; 65(4):313–4. [DOI] [PubMed] [Google Scholar]
- 18.Little SE, Johnson EM, Lewis D, et al. Prevalence of intestinal parasites in pet dogs in the United States. Vet Parasitol. 2009; 166(1–2):144–52. [DOI] [PubMed] [Google Scholar]
- 19.Szwabe K, Blaszkowska J. Stray dogs and cats as potential sources of soil contamination with zoonotic parasites. Ann Agric Environ Med. 2017; 24(1):39–43. [DOI] [PubMed] [Google Scholar]
- 20.Curi N, Paschoal A, Massara RL, et al. Risk factors for gastrointestinal parasite infections of dogs living around protected areas of the Atlantic Forest: implications for human and wildlife health. Braz J Biol. 2017; 77(2): 388–395. [DOI] [PubMed] [Google Scholar]
- 21.Fisher M. Toxocara cati: an underestimated zoonotic agent. Trends Parasitol. 2003; 19(4):167–70. [DOI] [PubMed] [Google Scholar]
- 22.Radwan NA, Khalil AI, El Mahi RA. Morphology and occurrence of species of Toxocara in wild mammal populations from Egypt. Comparative Parasitology. 2009; 76:273–282. [Google Scholar]
- 23.Habluetzel A, Traldi G, Ruggieri S, et al. An estimation of Toxocara canis prevalence in dogs, environmental egg contamination and risk of human infection in the Marche region of Italy. Vet Parasitol. 2003; 113(3–4):243–52. [DOI] [PubMed] [Google Scholar]
- 24.Dai RS, Li ZY, Li F, et al. Severe infection of adult dogs with helminths in Hunan Province, China poses significant public health concerns. Vet Parasitol. 2009; 160(3–4):348–50. [DOI] [PubMed] [Google Scholar]
- 25.Soriano SV, Pierangeli NB, Roccia I, et al. A wide diversity of zoonotic intestinal parasites infects urban and rural dogs in Neuquén, Patagonia, Argentina. Vet Parasitol. 2010; 167(1):81–5. [DOI] [PubMed] [Google Scholar]
- 26.Schär F, Inpankaew T, Traub RJ, et al. The prevalence and diversity of intestinal parasitic infections in humans and domestic animals in a rural Cambodian village. Parasitol Int. 2014; 63(4):597–603. [DOI] [PubMed] [Google Scholar]
- 27.Fan CK, Holland CV, Loxton K, et al. Cerebral Toxocariasis: Silent Progression to Neurodegenerative Disorders? Clin Microbiol Rev. 2015; 28(3):663–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CDC (Centers for Disease Control and Prevention) . Parasites-Toxocariasis. Global Health, Division of Parasitic Diseases and Malaria. 2019; https://www.cdc.gov/parasites/toxocariasis/index.html
- 29.Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003; 16(2):265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amapu TY, Latu MY, Dapiya HS, et al. Occurrence of Gastrointestinal Parasitic Associated with Exotic Dogs in Commercial Breeding Mills in Jos Metropolis-Nigeria. Academic Journal of Life Sciences. 2019; 5(3), 15–22. [Google Scholar]
- 31.Sánchez Thevenet P, Alvarez HM, Torrecillas C, et al. Dispersion of Echinococcus granulosus eggs from infected dogs under natural conditions in Patagonia, Argentina. J Helminthol. 2019; 94:e29. [DOI] [PubMed] [Google Scholar]
- 32.Wernery U. Zoonoses in the Arabian Peninsula. Saudi Med J. 2014; 35(12):1455–1462. [PMC free article] [PubMed] [Google Scholar]
- 33.Abuseir S. Major Causes and associated economic losses of carcass and organ condemnation in cattle and sheep in the northern part of Palestine. Journal of World Poultry Research. 2019; 9(4):317–323. [Google Scholar]
- 34.Budke C, Deplazes P, Torgerson P. Global socioeconomic impact of cystic echinococcosis. Emerg Infect Dis. 2006; 12: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Llokmani A, Rapti D. The prevalence of Some Intestinal Parasites in Stray Dogs from Tetova, Fyr Macedonia. Europ Sci J. 2017; 13:21–25. [Google Scholar]
- 36.Palmer CS, Andrew Thompson RC, Traub RJ, et al. National study of the gastrointestinal Parasites of dogs and cats in Australia. Vet Parasitol. 2008; 151(2–4):181–90. [DOI] [PubMed] [Google Scholar]
- 37.Federica G, Antonio S, Luisa D, et al. Parasitic infections in dogs involved in animal-assisted interventions. Italian Journal of Animal Science. 2018; 17(1):269–272. [Google Scholar]