Abstract
Background:
Visceral leishmaniasis (VL) is the most severe form of leishmaniasis. Correct identification of infected patients and reservoirs is vital to control the spread of VL. One important step in the control of Zoonotic Visceral leishmaniasis (ZVL) is the identification of infected dogs, which are the main domestic reservoir hosts of Leishmania infantum. We aimed to prepare and evaluate a new recombinant antigen using Bioinformatics tools for diagnosis of ZVL in domestic dogs.
Methods:
The present study was carried out in Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran during 2015–2018. Three L. infantum (JPCM5 strain) proteins were analyzed as follows: Nucleotide sequences of the surface proteins, putative amastin-like surface protein (P1), surface antigen protein 2 precursor (P2) and surface antigen-like protein (P3). The epitopes were predicted by several different bioinformatics servers using different methods. The predicted epitopes were selected with the highest immunogenic potential (P1P2P3) linked to each other with linkers (Gly, Se) and synthesized. Then the expression and protein purification were performed. In total, 114 serum samples were collected at 7 months. Positive and negative sera were confirmed using direct agglutination test (DAT). These recombinant antigens from L. infantum were used by indirect ELISA.
Results:
Considering the cut-off point of 0.23, the test showed a sensitivity of 98% (95%CI=89.50%–99.90%) and a specificity of 95.31% (95%CI=87.10%–98.72%). Kappa analysis indicated very good agreement (kappa=0.831) between ELISA and DAT (P<0.05).
Conclusion:
ELISA using the recombinant protein P1P2P3 has great potential for the diagnosis of canine visceral leishmaniasis (CVL).
Keywords: Visceral leishmaniasis, Leishmania infantum, Multi-epitope, Domestic dog
Introduction
Leishmaniasis is a complex of infectious diseases caused by protozoan parasites of the genus Leishmania and transmitted by Phlebotominae vectors. Visceral leishmaniasis (VL) is one of the most important clinical forms of leishmaniasis in humans as well as canines with high levels of morbidity and mortality. VL is usually caused by L. donovani and L. infantum (1). In Iran and the Middle East, VL is caused by L. infantum. Ardabil, East Azerbaijan, Fars, Bushehr, Kerman, Qom and North Khorasan provinces of Iran are endemic areas of VL (2,3).
Early and accurate diagnosis of VL is important to reduce mortality because the disease is fatal if left untreated (4). One of the important steps in control of canine visceral leishmaniasis (CVL) is the identification of infected dogs, which are the main reservoir of L. infantum (5).
The clinical symptoms of CVL vary from asymptomatic forms to restricted and deadly infection. Moreover, the incubation period lasts from several months to several years, depending on the virulence of the parasite and host genetic characteristics (6, 7). Thus, development of precise diagnostic methods for canine infection is essential for VL surveillance programs, as well as to understand immunological responses in resistant or susceptible animals (8, 9).
Improving serological tests for the diagnosis of VL is important because they are quick, easy, non-invasive, allow the screening of multiple samples and are capable of early detection, before the formation of lesions in VL (10). False-positive results especially in crude antigens are often seen in the sera of humans and dogs infected with L. infantum. Furthermore, the different strains of parasites and the protocols used for the preparation of crude antigens cause variations that can affect the sensitivity of this method (11).
Trying to obtain high sensitivities and specificities in testing, an alternative method is the use of multiepitope recombinant proteins. The use of synthetic peptides has low costs, high specificity and increases the features of safety tests relative to crude antigens. Bioinformatics and consequently identification of B-cell epitopes could play a major role in the development of synthetic vaccines, diagnostic tests and therapeutic products.
The present study reported for the first time the use of three surface antigens of L. infantum (P1,P2,P3).
Materials and Methods
The present study was carried out in Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran during 2015–2018. Surface proteins are major targets of diagnosis research due to their utility as cellular markers and their extracellular accessibility for diagnosis intervention. The surface proteins comprising epitopes are potentially able to more interaction with B-cells than intracellular proteins, in addition to induce antibodies against them. For this reason, we chose three surface antigens. Three L. infantum [JPCM5 strain] proteins were analyzed as follows: Nucleotide sequences of the surface proteins, putative amastin-like surface protein (P1) (GI: 134072905), surface antigen protein 2 precursor (P2) (GI: 339897242) and surface antigen-like protein (P3) (GI: 146076154), were obtained from the National Centre for Biotechnology Information (NCBI) Nucleotide Database (www.ncbi.nlm.nih.gov). The L. infantum JPCM5 strain was used in this study. The L. infantum JPC(MCAN/ES/98/LLM-724) strain was isolated in the WHO Collaborating Centre for Leishmaniasis, ISCIII, Madrid, Spain from the spleen of a naturally-infected dog resident in the area in 1998 (12).
Multiepitope protein prediction
To predict B-cell epitopes in the P1, P2, and P3 protein, the IEDB, BepiPred, ABCpred, BcePred, and CBTOPE web servers were used. Physicochemical properties were analyzed by using the ExPASy’s ProtParam server (http://web.expasy.org/protparam/). As well as signal peptide were analyzed. Evaluation of the secondary structure was analyzed by SOPMA server. The predicted epitopes were analyzed and were selected as the highest immunogenic potential. For the P1, P2, and P3 proteins, 19, 46, and 48 amino acids respectively were selected and used for in silico concatenation. Afterwards, they were fused to each other with proper linkers. The recombinant protein P1P2P3 contains 148 amino acids. The tertiary structure of final construct was modeled by I-TASSER server. The predicted model was validated with ProSA, VERIFY3D and Ramachandran plot. The modeling quality values showed the quality of the modeled as satisfactory. The sequence was synthesized and cloned into the expression vector pET-28a by SBS Genetech Co. (China) to produce recombinant expression plasmid P1P2P3.
Expression and purification of the recombinant polypeptide
The recombinant plasmid was transformed into E. coli BL21 (DE3). The single colonies of E. coli BL21 (DE3) containing the recombinant plasmid were cultured overnight in Luria-Bertani broth with 30 μg/ml kanamycin in a shaker incubator (200 rpm) at 37 °C. Then it was subcultured in LB medium and incubated with a shaker at 37 °C. One mM of Isopropyl-β-D-thiogalactopyranoside (IPTG) was added when the OD600 was around 0.6–0.8 and cultures were grown for a further 2, 4 and 6 h at 37 °C with agitation (200 rpm). Bacterial cells were centrifuged at 8,000× g for 5 min. The induced cells were lysed in lysis buffer and sonication. The induced samples were analyzed by SDS-PAGE with 15% resolving gel, followed by Coomassie Brilliant Blue G-250 staining. Then the expression of recombinant protein was confirmed by Western blotting. The recombinant protein purification was carried out by Ni-NTA column as specified by the manufacturer’s instructions (Novagen, USA). The purified recombinant protein was confirmed by Western blot. Protein concentration measured using the Bradford method.
Ethical approval
This study was approved by the Ethical Committee of Shahid Beheshti University of Medical Sciences (Ethical code no IR.SBMU.SM.REC.1394.66) in accordance with the Helsinki Declaration.
Samples
For ELISA assays 100 serum samples of two different clinical groups from the School of Public Health, Tehran University of Medical Sciences, Tehran, Iran were prepared. Fifty sera from L. infantum naturally infected dogs and 50 sera of healthy dogs (control group) were used. Additionally, in order to evaluate cross-reactivity on ELISA assays, we also used 14 samples of dogs sera infected by other infections including cutaneous leishmaniasis (n=3), toxocariasis (n=3), babesiosis (n=2), toxoplasmosis (n=2), brucellosis (n=1), and pneumonia (n=3) collected from the Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran. All collected samples were tested by using DAT (Gold standard) in the leishmaniasis Laboratory of the School of Public Health, Tehran University of Medical Sciences, Tehran, Iran for Anti-Leishmania antibodies. In total, 114 serum samples were collected during 7 months. The infected and uninfected dogs were confirmed by DAT (13, 14).
ELISA
In order to get the best results from ELISA assay, all ELISA procedures were optimized with regard to antigen concentrations, dilutions of sera and conjugated antibody by checkerboard titration. The wells of 96-well ELISA flat bottom microplates (Greiner) were coated with 1 μg/well of the purified protein diluted in 100 μl PBS buffer and incubated overnight at 4 °C. All the samples were analyzed by ELISA (15).
The optical density (OD) was measured at 450 nm using ELISA reader. Positive and negative serum samples of Anti-Leishmania infantum IgG antibodies were tested by using Indirect ELISA method. The results of ELISA method were analyzed by statistical analysis.
Statistical analysis
All the statistical analyses were processed with GraphPad Prism release 7.0, and SPSS (Chicago, IL, USA) software version 16.0. A cut off point for optimal sensitivity and specificity was determined using ROC analysis. The cut-off was obtained according to the point, which provides the maximum of sensitivity and specificity (16). Test was calculated for sensitivity (SE), specificity (SP), positive predictive value (PPV), negative predictive value (NPV), area under curve (AUC), accuracy, validity, and Kappa. Kappa values were determined with a confidence interval of 95% to determine the degree of agreement between the ELISA result in comparison to DAT. Kappa values classified according to the Fleiss scale: 0.00–0.20, poor; 0.21–0.40, fair; 0.41– 0.60, moderate; 0. 61–0.80, good; 0.81–0.99, very good and 1.00, perfect. A P-value of less than 0.05 was considered to indicate statistical significance.
Results
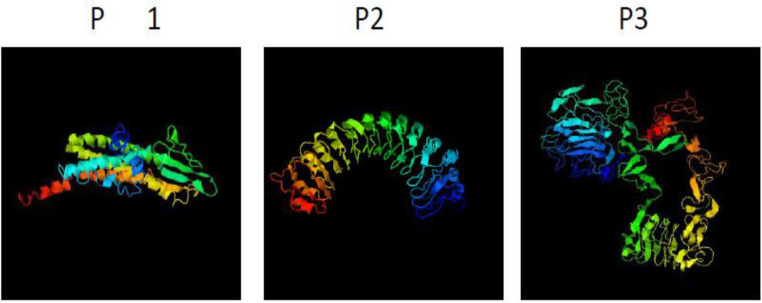
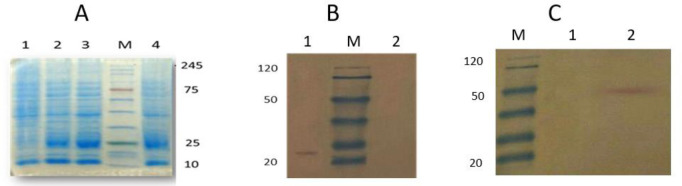
Based on bioinformatics analysis, B-cell linear and conformational epitopes of P1, P2, and P3 antigens were performed. Evaluations of the Secondary & Tertiary Structure were analyzed by several servers (Fig. 1). The recombinant plasmid was confirmed by PCR and restriction enzyme digestion (Fig. 2). In SDS-PAGE of recombinant protein a band of approximately 22 kDa was detected, the recombinant protein and purified recombinant protein were confirmed by Western blot (Fig. 3). All sera were tested for the presence of anti-leishmanias IgG antibodies by polyantigenic protein (Table 1,2). The results of ELISA were analyzed by statistical analysis.
Fig. 1a:
Tertiary structure B-cell constructs proteins P1, P2, and P3 predicted by I-TASSER server
Fig. 2:
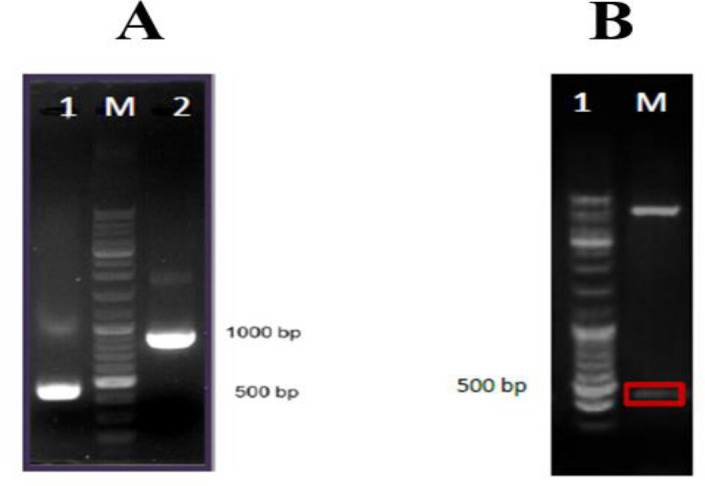
A: Confirmation of the recombinant plasmid by the PCR. Lane 1, PCR product without fragment plasmid; Lane M, DNA1kb marker; Lane 2, PCR product; B: Confirmation of the recombinant plasmid by restriction enzyme; Lane 1: PET28a / P1P2P3 plasmid digested with Sac1 and HindIII enzymes; Lane M, DNA1kb marker
Fig. 3:
A: SDS-PAGE of recombinant protein P1P2P3 : lane 1, BL21 (negative control); lane 2, induced cells for 2 h; lane3, induced cells for 4 h; Lane M, protein size marker (SMOBIO); lane 4, induced cells for 6 h by 1 mM IPTG was separated in 15% SDS-PAGE. B: Western blot analysis of recombinant protein P1P2P3 with anti His-tag antibody. Lane 1 recombinant protein; Lane M, protein size marker (Fermentas); Lane 2, BL21 (negative control). C: Western blot analysis of recombinant purified protein P1P2P3 with anti His-tag antibody. Lane M, protein size marker (Fermentas); Lane 1, BL21 (negative control); Lane 2, recombinant purified protein
Table 1:
Results of positive serum for anti-Leishmania IgG antibody using recombinant P1P2P3 antigen
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0.20 | 0.49 | 0.26 | 0.45 | 0.57 | 0.69 | 0.61 | 0.91 | 0.81 | 0.70 | 0.54 | 0.69 |
| B | 0.24 | 0.82 | 0.75 | 0.62 | 0.51 | 0.55 | 0.81 | 0.70 | 0.47 | 0.95 | 0.41 | 0.79 |
| C | 0.25 | 0.81 | 0.79 | 0.42 | 0.72 | 0.77 | 0.43 | 0.72 | 0.82 | 0.42 | 0.54 | 0.61 |
| D | 0.52 | 0.61 | 0.46 | 0.98 | 0.52 | 0.41 | 0.64 | 0.75 | 0.55 | 0.98 | 0.59 | 0.99 |
| E | 0.51 | 0.39 | 0.03 | 0.04 |
A1-E2: positive serums
E11, E12: blank
Fig. 1b:
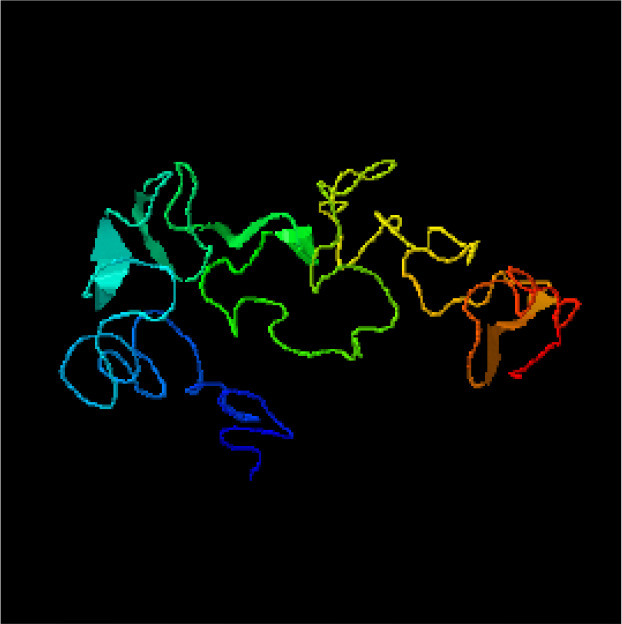
Tertiary structure B-cell construct Recombinant protein P1 P2 P3 predicted by I-TASSER server
Table 2:
Results of negative serum for anti-leishmania IgG antibody using recombinant P1P2P3 antigen
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0.01 | 0.17 | 0.14 | 0.03 | 0.05 | 0.14 | 0.15 | 0.02 | 0.19 | 0.20 | 0.16 | 0.18 |
| B | 0.07 | 0.02 | 0.17 | 0.07 | 0.20 | 0.17 | 0.23 | 0.18 | 0.01 | 0.21 | 0.19 | 0.17 |
| C | 0.12 | 0.17 | 0.05 | 0.01 | 0.12 | 0.01 | 0.02 | 0.17 | 0.19 | 0.14 | 0.15 | 0.19 |
| D | 0.19 | 0.09 | 0.24 | 0.09 | 0.04 | 0.06 | 0.18 | 0.14 | 0.24 | 0.15 | 0.20 | 0.12 |
| E | 0.24 | 0.05 | 0.12 | 0.15 | 0.02 | 0.04 | 0.18 | 0.05 | 0.14 | 0.20 | 0.19 | 0.02 |
| F | 0.07 | 0.09 | 0.17 | 0.14 | 0.02 | 0.05 |
A1-E2: negative serums
E3-F4: negative serums (other infections)
F11, F12: blank
Serum samples were tested by ELISA, considering the cut-off point of 0.23, the test showed a sensitivity of 98% (95% CI=89.50%–99.90%) and a specificity of 95.31% (95% CI=87.10%–98.72%) The Indirect ELISA test with the 95% CI, showed PPV(94.2), NPV(98.4), Accuracy(96.5), and Validity(96.7). The results of comparison of ELISA tests with recombinant antigens against DAT test as a standard method can be seen in the (Table 3).Statistical analysis of the data showed the very good agreement (kappa=0.831) between ELISA and DAT. Considering this cutoff value, 49 samples of 50 cases were diagnosed correctly as positive and one was negative. Moreover, 61 samples of 64 cases of control and other infection groups were diagnosed as negative and three were positive by ELISA.
Table 3:
Comparision of results between direct agglutination test (DAT) (1: 80 threshold) and ELISA of recombinant antigen on 114 dog serum samples in the diagnosis of Leishmania infantum infection in dogs
| ELISA | |||
|---|---|---|---|
|
| |||
| DAT | Positive N(%) |
Negative N(%) |
Total N(%) |
| Positive(≤ 1:320) | 49 (94.2) | 1(1.6) | 50 (43.9) |
| Negative(>1:80) | 3 (5.8) | 61 (98.4) | 64 (56.1) |
| Total | 52 (100) | 62 (100) | 114 (100) |
Discussion
The early and correct diagnosis of leishmaniasis is essential for disease control and treatment. Important step in the control of VL is the identification of infected dogs, which are the main domestic reservoir of L. infantum (17, 18). Serological methods are strong tools in CVL diagnosis that often used for canine mass screening (19, 20).
When crude antigens are used, Increases the number of false-positive results and cross-reactivity with other canine pathogens (21, 22). The use of native antigens for diagnostic tests is expensive, time-consuming, and has many restrictions. To control these limitations recombinant antigen by using bioinformatics tools can standardize diagnostic methods. An alternative method to resolve these limitations is peptide synthesis by using bioinformatics tools. In recent years synthetic peptides used as antigens have shown high sensitivity and specificity as well as decrease in cross-reactivity (23–25).
Sensitivities and specificities found using synthetic peptides or recombinant proteins in ELISA were, respectively, 88% and 95% (20), 75% and 90% (26), 72.7% and 87.3% (27), and 100% and 98% (28).
In this study for the first time, a predicted recombinant protein from L.infantem B-cell epitopes of three surface antigens (P1, P2, and P3) was successfully expressed and analyzed for CVL diagnosis. We chose surface proteins because they contain epitopes that are potentially able to more interaction with B-cells than intracellular proteins and to induce antibodies against them. Appropriate levels of sensitivity and specificity are especially desirable to avoid false-negative and false-positive reactions. This problem can lead to disease transmission and unnecessary euthanasia of healthy dogs (29).
Several online software analysis methods such as IEDB, BepiPred, ABCpred, BcePred and CBTOPE were used to predict B-cell epitopes. Moreover the tertiary structure of the recombinant protein was predicted by I-TASSER server. Peptides that included regions rich in B-cell epitopes and had higher antigenicity were considered. Finally, to achieve the best immunization in construct, selected epitopes were fused to each other with appropriate linkers (Gly, Ser). The sequence was synthesized, and then cloned into the expression vector pET-28a to produce recombinant expression plasmid P1P2P3. Multiepitope recombinant plasmid was verified by PCR and restriction enzyme digestion. Afterward expression and purification of protein performed and the purified recombinant protein confirmed by Western blot. Potential of recombinant protein as diagnostic antigens for CVL was assessed by ELISA.
Statistical analysis of the data showed very good agreement (kappa=0.831) between ELISA and DAT. This finding showed different and higher density of immunodominant epitopes of recombinant protein P1P2P3 causing more sensitivity and better assay reproducibility.
Conclusion
We have produced and validated new recombinant antigens from L.infantem B-cell epitopes for CVL diagnosis. Our work suggested that synthetic peptide-based ELISA strategy may be useful for the development of a sensitive and highly specific serodiagnosis for CVL.
Acknowledgements
The present study is a part of the Ph.D. thesis of Parisa Yaghoubi and supported financially by a grant from the Research Deputy of School of Medicine of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant No. 6445). The Authors sincerely appreciate from Cellular and Molecular Biology Research Center, Department of Medical Parasitology and Mycology of Shahid Beheshti University of Medical Sciences, and leishmaniasis laboratory of the School of Public Health, Tehran University of Medical Sciences.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Abass E, Kang C, Martinkovic F, et al. Heterogeneity of Leishmania donovani parasites complicates diagnosis of visceral leishmaniasis: comparison of different serological tests in three endemic regions. PLoS One. 2015;10(3):e0116408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohebali M. Visceral leishmaniasis in Iran: Review of the Epidemiological and Clinical Features. Iran J Parasitol. 2013;8(3):348–358. [PMC free article] [PubMed] [Google Scholar]
- 3.Mohebali M, Moradi-Asl E, Rassi Y. Geographic distribution and spatial analysis of Leishmania infantum infection in domestic and wild animal reservoir hosts of zoonotic visceral leishmaniasis in Iran: A systematic review. J Vector Borne Dis. 2018;55(3):173–183. [DOI] [PubMed] [Google Scholar]
- 4.Bern C, Maguire JH, Alvar J. Complexities of assessing the disease burden attributable to leishmaniasis. PLoS Negl Trop Dis. 2008;2(10): e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maia C, Campino L. Cytokine and Phenotypic Cell Profiles of Leishmania infantum Infection in the Dog. J Trop Med. 2012;2012: 541571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohebali M, Edrissian GH, Shirzadi MR, et al. An observational study on the current distribution of visceral leishmaniasis in different geographical zones of Iran and implication to health policy. Travel Med Infect Dis. 2011;9(2):67–74. [DOI] [PubMed] [Google Scholar]
- 7.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–77. [DOI] [PubMed] [Google Scholar]
- 8.Dantas-Torres F, de Brito ME, Brandao-Filho SP. Seroepidemiological survey on canine leishmaniasis among dogs from an urban area of Brazil. Vet Parasitol. 2006;140(1–2):54–60. [DOI] [PubMed] [Google Scholar]
- 9.Moshfe A, Mohebali M, Edrissian G, et al. Canine visceral leishmaniasis: asymptomatic infected dogs as a source of L. infantum infection. Acta Trop. 2009;112(2):101–105 [DOI] [PubMed] [Google Scholar]
- 10.Souza AP, Soto M, Costa JM, et al. Towards a more precise serological diagnosis of human tegumentary leishmaniasis using Leishmania recombinant proteins. PLoS One. 2013;8(6):e66110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caballero ZC, Sousa OE, Marques WP, Saez-Alquezar A, Umezawa ES. Evaluation of serological tests to identify Trypanosoma cruzi infection in humans and determine cross-reactivity with Trypanosoma rangeli and spp. Clin Vaccine Immunol. 2007;14(8):1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denise H, Poot J, Jimenez M, et al. Studies on the CPA cysteine peptidase in the Leishmania infantum genome strain JPCM5. BMC Mol Biol. 2006;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harith A, Kolk A, Kager P, et al. A simple and economical direct agglutination test for serodiagnosis and sero-epidemiological studies of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1986;80(4):583–536. [DOI] [PubMed] [Google Scholar]
- 14.Hosseininejad M, Mohebali M, Hosseini F, Karimi S, Sharifzad S, Akhoundi B. Seroprevalence of canine visceral leishmaniasis in asymptomatic dogs in Iran. Iranian Journal of Veterinary Research, Shiraz University. 2012; 13(1):1–4. [Google Scholar]
- 15.Rafati S, Nakhaee A, Taheri T, et al. Protective vaccination against experimental canine visceral leishmaniasis using a combination of DNA and protein immunization with cysteine proteinases type I and II of L. infantum. Vaccine. 2005; 23(28): 3716–3725. [DOI] [PubMed] [Google Scholar]
- 16.Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45(1–2):23–41. [DOI] [PubMed] [Google Scholar]
- 17.Molina R, Amela C, Nieto J, et al. Infectivity of dogs naturally infected with Leishmania infantum to colonized Phlebotomus perniciosus. Trans R Soc Trop Med Hyg. 1994;88(4):491–493. [DOI] [PubMed] [Google Scholar]
- 18.Mohebali M, Hajjaran H, Hamzavi Y, et al. Epidemiological aspects of canine visceral leishmaniosis in the Islamic Republic of Iran. Vet Parasitol. 2005;129(3–4):243–251. [DOI] [PubMed] [Google Scholar]
- 19.Barati M, Mohebali M, Alimohammadian MH, Khamesipour A, Akhoundi B, Zarei Z. Canine visceral leishmaniasis: seroprevalence survey of asymptomatic dogs in an endemic area of northwestern Iran. J Parasit Dis. 2015;39(2):221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faria AR, Costa MM, Giusta MS, et al. High-throughput analysis of synthetic peptides for the immunodiagnosis of canine visceral leishmaniasis. PLoS Negl Trop Dis. 2011;5(9):e1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanette MF, Lima VM, Laurenti MD, et al. Serological cross-reactivity of Trypanosoma cruzi, Ehrlichia canis, Toxoplasma gondii, Neospora caninum and Babesia canis to Leishmania infantum chagasi tests in dogs. Rev Soc Bras Med Trop. 2014;47(1):105–107. [DOI] [PubMed] [Google Scholar]
- 22.Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol. 2002;9(5):951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.EL‐Manzalawy Y, Dobbs D, Honavar V. Predicting linear B‐cell epitopes using string kernels. J Mol Recognit. 2008;21(4):243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skwarczynski M, Toth I. Peptide-based synthetic vaccines. Chem Sci. 2016;7(2):842–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen JEP, Lund O, Nielsen M. Improved method for predicting linear B-cell epitopes. Immunome Res. 2006;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chavez-Fumagalli MA, Martins VT, Testasicca MC, et al. Sensitive and specific serodiagnosis of Leishmania infantum infection in dogs by using peptides selected from hypothetical proteins identified by an immunoproteomic approach. Clin Vaccine Immunol. 2013;20(6):835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taran M, Mohebali M, Modaresi M, Mamishi S, Mahmoudi M, Mojarad M. Diagnosis of canine visceral leishmaniasis by ELISA using K39sub recombinant antigen. Iran. J Public Health. 2007;36(2):1–6. [Google Scholar]
- 28.Porrozzi R, Santos da Costa MV, Teva A, et al. Comparative evaluation of enzyme-linked immunosorbent assays based on crude and recombinant leishmanial antigens for serodiagnosis of symptomatic and asymptomatic Leishmania infantum visceral infections in dogs. Clin Vaccine Immunol. 2007;14(5):544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa DN, Codeco CT, Silva MA, Werneck GL. Culling dogs in scenarios of imperfect control: realistic impact on the prevalence of canine visceral leishmaniasis. PLoS Negl Trop Dis. 2013;7(8): e2355. [DOI] [PMC free article] [PubMed] [Google Scholar]