Summary
Hepatic encephalopathy (HE) is a neurological complication of hepatic dysfunction and portosystemic shunting. It is highly prevalent in patients with cirrhosis and is associated with poor outcomes. New insights into the role of peripheral origins in HE have led to the development of innovative treatment strategies like faecal microbiota transplantation. However, this broadening of view has not been applied fully to perturbations in the central nervous system. The old paradigm that HE is the clinical manifestation of ammonia-induced astrocyte dysfunction and its secondary neuronal consequences requires updating. In this review, we will use the holistic concept of the neurogliovascular unit to describe central nervous system disturbances in HE, an approach that has proven instrumental in other neurological disorders. We will describe HE as a global dysfunction of the neurogliovascular unit, where blood flow and nutrient supply to the brain, as well as the function of the blood-brain barrier, are impaired. This leads to an accumulation of neurotoxic substances, chief among them ammonia and inflammatory mediators, causing dysfunction of astrocytes and microglia. Finally, glymphatic dysfunction impairs the clearance of these neurotoxins, further aggravating their effect on the brain. Taking a broader view of central nervous system alterations in liver disease could serve as the basis for further research into the specific brain pathophysiology of HE, as well as the development of therapeutic strategies specifically aimed at counteracting the often irreversible central nervous system damage seen in these patients.
Keywords: NGVU, Ammonia, Systemic inflammation, Cirrhosis, Acute Liver Failure, Energy metabolism, Blood-brain barrier, Brain edema, Oxidative stress, Neuroinflammation, Glymphatic system
Abbreviations: ABC, ATP-binding cassette; ACLF, acute-on-chronic liver failure; AD, acute decompensation; ALF, acute liver failure; AOM, azoxymethane; AQP4, aquaporin 4; BBB, blood-brain barrier; BDL, bile duct ligation; BCRP, breast cancer resistance protein; CCL, chemokine ligand; CCR, C-C chemokine receptor; CE, cerebral oedema; CLD, chronic liver disease; CLDN, claudin; CNS, central nervous system; CSF, cerebrospinal fluid; GS, glutamine synthetase; HE, hepatic encephalopathy; HO-1, heme oxygenase 1; IL-, interleukin; mPT, mitochondrial pore transition; MMP-9, matrix metalloproteinase 9; MRP, multidrug resistance associated protein; NGVU, neurogliovascular unit; NKCC1, Na-K-2Cl cotransporter 1; OCLN, occludin; ONS, oxidative and nitrosative stress; P-gp, P-glycoprotein; PSS, portosystemic shunt; PCA, portacaval anastomosis; S1PR2, sphingosine-1-phosphate receptor 2; SUR1, sulfonylurea receptor 1; TAA, thioacetamide; TGFβ, transforming growth factor beta; TJ, tight junction; TNF, tumour necrosis factor; TNFR1, tumour necrosis factor receptor 1; ZO, zonula occludens
Key points.
-
-
Hepatic encephalopathy is a common and debilitating condition associated with often irreversible neurological deficits and a poor prognosis.
-
-
Ammonia remains central to the pathogenesis. However, inflammation has been shown to be similarly important.
-
-
Hepatic encephalopathy is characterised by multicellular dysfunction in the functional base unit of the brain, the neurogliovascular unit.
-
-
Both supply of energy and nutrients to the brain and energy production are impaired in HE.
-
-
Tight junction structure is disrupted in hepatic encephalopathy, allowing for increased influx of neurotoxins into the brain.
-
-
Efflux transporter activity at the blood-brain barrier is altered in hepatic encephalopathy, but the functional consequences are uncertain.
-
-
Ammonia and toxin accumulation leads to astrocyte swelling, oxidative and nitrosative stress, as well astrocyte senescence. Senescence might present an irreversible consequence of hepatic encephalopathy, as well as influence neuroinflammation.
-
-
Peripheral inflammation induces neuroinflammation through cytokines, chemokines and bile acids. Targeting neuroinflammation might prove beneficial in the future.
-
-
Impaired glymphatic drainage increases neuroinflammation in hepatic encephalopathy. This presents a therapeutic target.
Introduction
Hepatic encephalopathy (HE) is one of the most common and severe complications of both chronic and acute liver disease. It is a neuropsychiatric condition, with symptoms ranging from subtle attention deficits to coma. HE is associated with a poor prognosis, with a 1-year mortality rate of 50%.1 Although epidemiological data are scarce, the estimated cumulative prevalence goes up to 30 to 40% in patients with cirrhosis.2 HE is traditionally considered a reversible entity. However, evidence is mounting that HE episodes are associated with residual cognitive deficits after resolution or even liver transplantation. This suggests that certain alterations are irreversible, possibly through neurodegeneration.3,4 Obviously, HE poses an important healthcare challenge and therefore novel therapeutic strategies are needed.
The classical pathophysiological concept of HE is based on hepatocellular dysfunction and/or portosystemic shunting (PSS), resulting in high blood and brain ammonia levels. Elevated ammonia concentrations in the central nervous system (CNS) disturb homeostasis and result in cognitive defects.5 However, this model has proven to be incomplete. Evidence has identified systemic inflammation as a key driver in the development and clinical course of acutely decompensated (AD) cirrhosis, acute-on-chronic liver failure (ACLF)6,7 and acute liver failure (ALF).8 Accordingly, in HE, inflammatory mediators correlate with HE development and severity, regardless of ammonia levels.9 Hence, synergism between ammonia toxicity and inflammation is nowadays considered to be the driver of HE development.
Recent evidence equally shows that HE is not merely a disease of the liver and brain, but rather the result of multi-organ dysfunction. For example, gut microbiota composition and gut barrier permeability are altered in HE, allowing for higher production and influx of ammonia and inflammatory mediators into the body.10 The muscle is an important buffer for excess ammonia. Muscle mass loss, a hallmark of cirrhosis, additionally leads to elevated systemic ammonia concentrations and is associated with cognitive decline.11 Identification of extrahepatic contributing factors has led to the development of innovative treatment strategies. For example, branched chain amino acid supplementation is a treatment aimed at restoring muscle mass and evidence suggests it can ameliorate HE symptomatology.12 More recently, faecal microbiota transplantation has been shown to have a favourable safety profile and ammonia-lowering capacity.13
However, this broadening of view has not been applied fully to the pathophysiological perturbations occurring in the CNS. Accordingly, no specific brain-targeted therapy has been developed. Classically, HE is seen as a gliopathy, a disease of support cells with secondary repercussions for neuronal functioning and survival. As ammonia is solely metabolised in astrocytes14 and morphological changes in astrocytes are characteristic of HE,15 the bulk of research has focused on the contribution of astrocyte dysfunction to HE development, as well as astrocytes’ influence on neuronal signalling.
Recent evidence has however uncovered that brain injury in HE is actually the result of multicellular dysfunction within the CNS microenvironment. In Fig. 1, we give an overview of the alterations that have been described in different CNS compartments in HE. Homeostasis of the CNS microenvironment is essential for normal neuronal functioning and survival, and is regulated by a variety of cell types, including glial (microglia, astrocytes) and vascular cells (e.g. endothelial cells). The neurogliovascular unit (NGVU) is the functional integration of these cells and provides a framework through which CNS diseases can be studied and understood. In this unit, each cell type exhibits distinct functions, but cross-talk between the cell types influences the function of the NGVU as a whole.16,17 The blood-brain barrier (BBB) is also part of the NGVU.16,18 Disruption of cell-cell communication in the NGVU not only results in CNS injury, but restoration of NGVU functionality aids in CNS repair, making this an interesting therapeutic target in neurodegenerative disorders.17
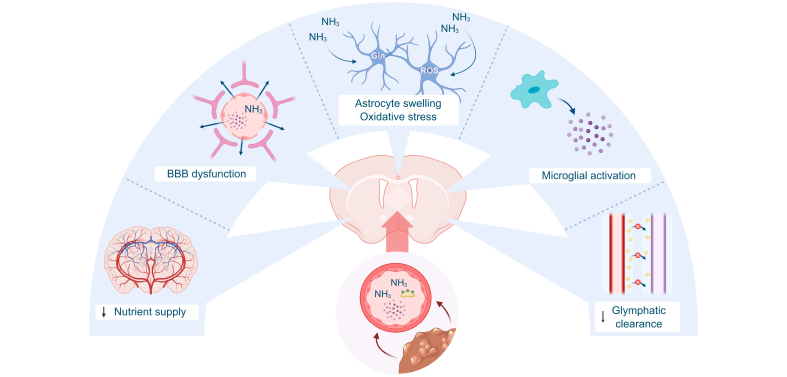
Fig. 1.
The neurogliovascular unit in hepatic encephalopathy.
In patients with decompensated cirrhosis, circulating levels of cytokines, ammonia and bile acids are elevated and reach the brain. In patients with hepatic encephalopathy, cerebral blood flow and nutrient supply are impaired. The BBB becomes leaky, allowing toxins like ammonia to enter the brain. Ammonia induces astrocyte swelling and oxidative stress, impairing normal functioning and eventually resulting in brain oedema. Microglia are activated and produce inflammatory mediators, resulting in a neuroinflammatory environment, further aggravating neurogliovascular dysfunction. Finally, impaired glymphatic clearance results in the accumulation of neurotoxic compounds. BBB, blood-brain barrier; Gln, glutamine; NH3, ammonia; ROS, reactive oxygen species.
In this review, we aim to describe HE as a combined dysfunction of multiple NGVU components, as has been done for other CNS diseases. Specific focus is placed on glial cells within the NGVU, namely microglia, endothelial cells and astrocytes, as well as the BBB. Additionally, we highlight knowledge gaps and potential novel therapeutic targets within the NGVU.
The neurogliovascular unit plays a key role in neurodegenerative disorders
Originally, the neurovascular unit concept was developed to stimulate a more integrated approach to neurological diseases.19 Nowadays, this concept has been further updated to the term NGVU, to underscore the importance of glial cells. As this manuscript focuses on support cells, (secondary) alterations in neurotransmission, neuronal functioning and survival are beyond the scope of this review. Below, we elaborate further on the different elements of the NGVU that are known to contribute to HE development.
Endothelial cells line the vasculature of the brain, with tight junctions (TJs) in between linking them firmly together, limiting bulk paracellular transport and preventing the uncontrolled influx of pathogens, cells and circulating water-soluble neurotoxins. TJs are made up of transmembrane proteins like claudins (CLDN)1,3,5,12 and occludin (OCLN), which are linked to cytoskeletal fragments through zonula occludens (ZO-)1, 2, and 3.18 Additionally, endothelial cells express transport proteins that tightly regulate the in- and outflow of substances into the brain. Together with the surrounding pericytes and endfeet from astrocytes, this forms a barrier called the BBB.20 The BBB ensures a tightly controlled neuronal environment that is necessary for adequate functioning.
Astrocytes modulate the barrier properties of endothelial cells through their endfeet, thereby influencing both BBB formation and maintenance20,21 Moreover, astrocytes regulate water transport across the BBB through expression of the water channel aquaporin 4 (AQP4) at their endfeet.22 Thirdly, astrocytes regulate blood flow and nutrient supply to different brain regions through secretion of vasoactive substances.16 As they link the vasculature to neurons in the so-called neurovascular coupling, astrocytes can regulate blood flow based on local neuronal needs.17 They additionally connect with neurons to provide trophic support,17 and aid in neurotransmitter reuptake and synapse regulation.21,23
Microglia are the resident CNS immune cells. In homeostatic conditions, they continuously scan the local environment for synaptic activity, pathogens and injury.23 Additionally, their housekeeping function consists of phagocytosis of dead/dying cells and debris, as well as synaptic remodelling and myelin homeostasis.24 In pathological conditions, microglia are important in the development of neuroinflammation, which is characterised by microglial activation, where microglia transform from a ramified into an amoeboid phenotype, coinciding with increased production of cytokines and chemokines.24
More recently, the role of the glymphatic system in the maintenance of the CNS microenvironment has been uncovered. In this system, interstitial fluid from the parenchymal CNS drains into the paravascular space and then the meningeal lymphatics, before finally reaching the systemic circulation.25 This provides a means of waste clearance beyond active efflux through the BBB.
Proper cell-cell communication within the NGVU is vital for maintaining tissue homeostasis. On the other hand, NGVU dysfunction is a hallmark of multiple acute and chronic neurological disorders. For example, BBB dysfunction is an important part of neuronal toxicity in Alzheimer’s disease, wherein transporter alterations lead to an increased influx of amyloid beta into the brain. This results in increased levels of amyloid beta, which promotes microglial activation. Through cytokine release, activated microglia are subsequently responsible for astrocyte activation. These activated astrocytes then produce a neurotoxic factor that finally elicits the neuronal cell death responsible for the clinical phenotype.16,26 This nicely illustrates that the different NGVU components, from the BBB to microglia and astrocytes communicate with each other and in concert produce the circumstances for CNS pathology.
Hyperammonaemia and systemic inflammation are key to the development of HE
Ammonia (referring to both ionised NH4+ and non-ionic NH3 throughout the review) is primarily produced in the gut as a by-product of the digestion of nitrogen-containing compounds.5 In physiological conditions, nearly 100% of the ammonia that passes the liver is transformed into urea via the urea cycle and thereafter excreted in the kidneys.27 In liver disease, urea cycle effectiveness is greatly reduced. Furthermore, the hepatic circulation is bypassed by both intrahepatic and extrahepatic shunts.5 Sarcopenia, associated with cirrhosis, additionally limits the extrahepatic capacity to metabolise ammonia.5,11 Hence, the systemic capacity for ammonia detoxification is limited in liver disease, leading to increased CNS exposure. Once in the CNS, the detoxification of ammonia is solely carried out by astrocytes, where it is metabolised into glutamine via glutamine synthetase (GS).14 Ammonia has many direct toxic effects on cells, including inhibition of Krebs cycle enzymes, the mitochondrial respiratory chain and the malate-aspartate shuttle,28 as well as leading to the production of free radicals.29,30 Additionally, its metabolite glutamine is osmotically active, attracting water and resulting in cell swelling (Fig. 4A).31 We will elaborate further on these toxic characteristics in further paragraphs.
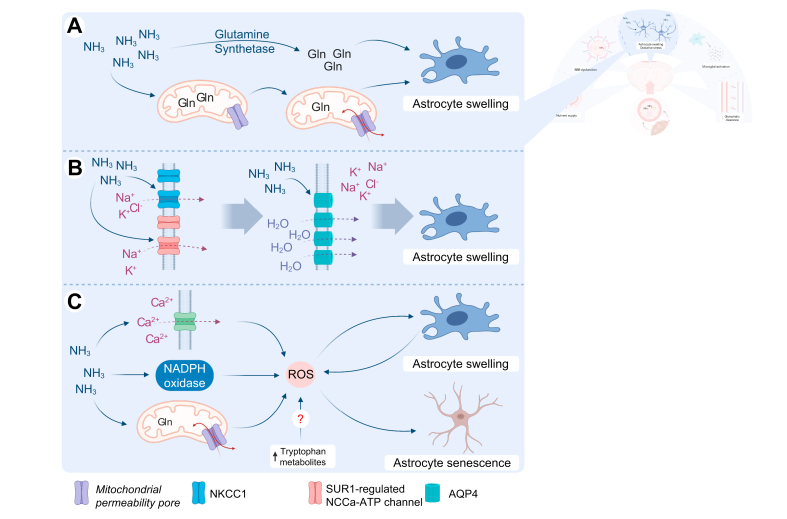
Fig. 4.
Astrocytes swell and are subject to oxidative stress in hepatic encephalopathy.
(A) Ammonia is metabolised into glutamine by glutamine synthetase. Glutamine acts as an osmolyte. Alternatively, glutamine is shuttled into the mitochondria, leading to mitochondrial pore transition and oxidative stress. Both mechanisms contribute to astrocyte swelling and cerebral oedema. (B) Astrocyte exposure to excess ammonia leads to overactivation of NKCC1 and the SUR1-regulated NCCa-ATP channel, resulting in influx of ions into the cell. Water follows the gradient created through increased AQP4 water channels, finally resulting in astrocyte swelling and cerebral oedema. (C) Ammonia leads to Ca2+ influx, activation of NADPH oxidase and mitochondrial pore transition. Tryptophan metabolites might also induce free radical production. These mechanisms increase levels of ROS, which enters a positive feedback loop with astrocyte swelling. Additionally, it leads to astrocyte senescence, possibly explaining the irreversible symptoms of HE. AQP4, aquaporin 4; Gln, glutamine; HE, hepatic encephalopathy; NADPH, nicotinamide adenine dinucleotide phosphate; NH3, ammonia; NKCC1, Na-K-2Cl cotransporter 1; ROS, reactive oxygen species; SUR1, sulfonylurea receptor 1.
Ammonia levels have diagnostic and prognostic value in HE. They not only correlate with disease severity and predict mortality but a decrease is also correlated with HE resolution.32,33 Normal ammonia levels have a high negative predictive value, and should prompt the search for an alternative cause of neurological symptoms.2 Also, exposure of animal models and in vitro astrocyte cell cultures to increased ammonia levels reproduces the clinical, biochemical and morphological alterations seen in patients.34 Ammonia-lowering strategies also remain the basis of current pharmacological therapy. Hence, ammonia toxicity is still a cornerstone of our understanding of HE development.35 However, ammonia does not correlate with HE severity in individual patients, with considerable overlap of levels across the spectrum of disease severity. Ammonia elevation is also often found in patients with cirrhosis without HE (up to 69%).32,36 Moreover, patients with severe HE can even exhibit normal blood ammonia levels.32,33 These data collectively suggest that ammonia is only part of the story.
In ALF, patients with higher levels of systemic inflammation have more rapid progression of HE and poor prognosis.8 In CLD, precipitating factors associated with a systemic inflammatory response, such as an infection, often underlie HE development.32 Recently, it was discovered that systemic inflammation not only correlates well with the clinical phenotype in AD and ACLF, but it additionally predicts the future clinical course.6,7 Also, the pattern of systemic inflammation correlates with AD phenotype. Specifically, AD with HE and/or renal dysfunction is characterised by the elevation of oxidative stress and tumour necrosis factor (TNF) levels. Interestingly, this phenotype is also associated with significantly elevated inflammation compared to AD without organ failures. This suggests that inflammation itself, and in particular this inflammatory profile, is responsible for the AD with HE and/or renal dysfunction phenotype.37 Finally, in patients with HE, the degree of systemic inflammation correlates well with HE severity, regardless of ammonia levels.9 Taken together, these data suggest that systemic inflammation is a key driver in HE.38
In summary, in acute and chronic liver disease, ammonia levels rise due to a combination of liver failure, PSS and decreased peripheral detoxification. Increased CNS exposure to ammonia has several toxic effects. Systemic inflammation present in both ALF and AD synergises with ammonia to elicit HE.
CNS dysfunction in hepatic encephalopathy, from nutrient influx to efflux of toxins
Energy supply and production is impaired
The brain has a high energy need and is almost exclusively dependent on glucose for its demands. Through neurovascular coupling, astrocytes tightly regulate cerebral blood flow, and hence nutrient and oxygen supply to the CNS, adapting this to localised neuronal needs.20,39 In conditions of lower availability of oxygen, astrocytes provide nutritional support to neurons17 by stimulating glycolysis and producing lactate, which can serve as a supplementary neuronal energy source.17,40 As such, the increased cerebral lactate concentrations observed in patients with ALF and CLD are often seen as a marker of energy failure in the brain.41 The significant decrease in ATP levels in patients with HE and in ATP/ADP ratios in bile duct ligated (BDL) rats, a model for HE in CLD, supports this notion.42 Moreover, metabolomic profiling of cerebrospinal fluid (CSF) of patients with HE showed evidence of disturbed energy metabolism, with accumulation of acetylated compounds, suggesting Krebs cycle defects.43
The cause of these energy disturbances is likely multifactorial. Several studies show that in HE associated with ALF and CLD, and even in subclinical HE, cerebral blood flow and oxygen metabolism are reduced (Fig. 2A).44,45 Secondly, ammonia can directly inhibit the Krebs cycle enzymes α-ketoglutarate dehydrogenase, isocitrate dehydrogenase and pyruvate dehydrogenase, limiting the effectiveness of oxidative phosphorylation (Fig. 2B).28 Finally, it has recently been uncovered that systemic inflammation in AD is closely correlated with a metabolic shift, with energy demands of the activated innate immune system being prioritised over peripheral organs. In this state, characterised by increased glycolysis, lipolysis, proteolysis and impaired mitochondrial oxidative phosphorylation and β-oxidation, glucose is reallocated to innate immune cells and away from peripheral organs, to allow for production of inflammatory cells and mediators. Upon glucose deprivation, the brain is uniquely dependent on ketone bodies for its energy supply. Interestingly however, β-hydroxybutyrate could not be detected in the blood of patients with decompensated cirrhosis, suggesting defective hepatic synthesis (Fig. 2C).46,47 Moreover, decreased levels of ketone bodies have been found in patients with (subclinical) HE compared to cirrhotic controls, suggesting this mechanism is of specific importance in HE.48,49
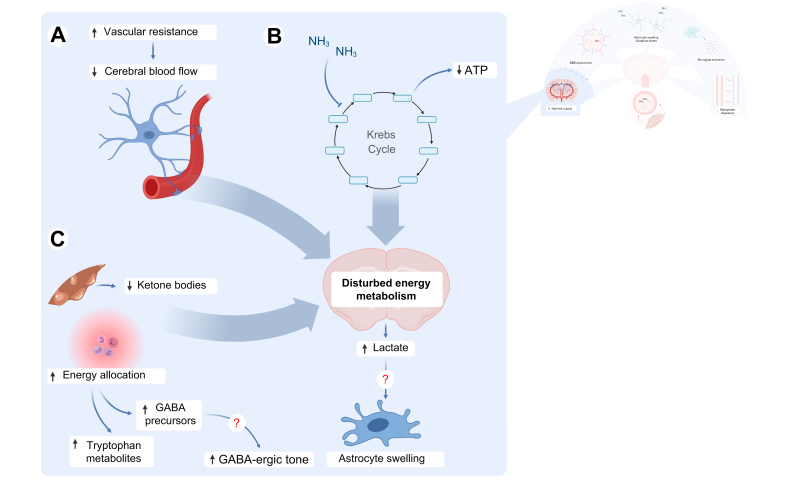
Fig. 2.
Energy metabolism is impaired.
(A) In hepatic encephalopathy, vascular resistance is increased in the cerebral circulation, leading to decreased blood flow and thus supply of oxygen and nutrients to the brain. (B) Additionally, ammonia inhibits key Krebs cycle enzymes like alpha-ketoglutarate dehydrogenase, leading to ATP depletion. (C) Inflammation leads to preferential energy and nutrient allocation towards the innate immune system. Ketone body production by the cirrhotic liver as an alternative energy source for the brain is insufficient. Additionally, toxic compounds like kynurenines and GABA precursors accumulate secondary to increased amino acid metabolism. Lactate, the end product of glycolysis, accumulates in the brain and might act as an osmolyte, leading to astrocyte swelling and brain oedema. GABA, gamma amino butyric acid; NH3, ammonia.
Accumulation of lactate in the cell may create an osmotic gradient, leading to cell swelling and cerebral oedema (CE).41 Cytotoxic CE and brain herniation are often seen in HE in ALF and are associated with a poor prognosis.50 In CLD, the presence and significance of CE are disputed.51,52 However, recent imaging studies show evidence for low grade oedema without accompanying intracranial hypertension.52 Although one group confirmed the correlation between brain lactate and brain swelling in BDL rats,53 this has been challenged recently.51,54
The increased energy needs of the activated immune system in AD additionally lead to preferential amino acid allocation through peripheral proteolysis. This has been shown to be associated with the accumulation of possible neurotoxins. Two systems are of particular interest. First, tryptophan metabolites like quinolinic acid, known to induce N-methyl-D-aspartate receptor overactivation and free radical production, accumulate through the tryptophan-kynurenine pathway. Levels of tryptophan metabolites correlate with the presence of HE in AD and are found in increased concentrations in the CSF of patients with HE46,47,55 Secondly, precursors of gamma amino butyric acid (GABA), the major inhibitory neurotransmitter in the brain, have been found to be elevated in blood and CSF samples of patients with AD and HE, respectively, correlating with HE severity and possibly contributing to an increased GABA-ergic tone.43,47 A recent trial reported on the beneficial cognitive effects of GABA antagonism in patients with HE.56
In conclusion, brain energy production is impaired through impaired blood flow, the direct effect of excess ammonia on energy production and metabolic reprogramming shuttling energy away from the brain. The resulting lactate accumulation might contribute to cell swelling and CE. Moreover, accumulation of neurotoxic compounds through this shuttling might influence brain function.
Blood-brain barrier structure and function are altered
The brain is protected from the influx of toxic compounds by barriers, the most studied of which is the BBB. Breakdown of the BBB can lead to indiscriminate influx of toxins like ammonia and xenobiotics, as has been shown in HE.43,57 BBB breakdown can additionally lead to vasogenic CE and might thus be an additive or alternative explanation for CE development in HE.52 However, BBB permeability changes are not consistently observed in HE58 and early studies in patients with ALF and animal models showed no BBB ultrastructural abnormalities.59 Still, altered BBB permeability can also occur without directly visible ultrastructural alterations.
More recent studies show evidence of significant damage to the BBB in preclinical HE models and with it, increased levels of permeability markers and TJ alterations (Fig. 3A). In the galactosamine, azoxymethane (AOM) and thioacetamide (TAA) mouse models of ALF, BBB permeability is increased.[59], [60], [61] ZO-2 downregulation even precedes permeability changes in AOM-treated mice, suggesting that TJ disruption might occur before BBB permeability changes.62 Resolution of these changes is associated with a reduction in brain water in AOM mice, suggesting that at least in ALF, vasogenic oedema might contribute to the development of CE.63 In the rat BDL model of chronic HE, increased brain water content, extravasation of tracer molecules and loss of TJ proteins OCLN, ZO-1 and CLDN5 are observed,[64], [65], [66] although these findings are inconsistent.[67], [68], [69], [70] OCLN downregulation is seen as early as 2 days post-surgery in BDL rats, indicating that, as in ALF, BBB disruption could be an early phenomenon in CLD.66,71 Differences in experimental set-up could explain the contradictory observations. More research is necessary to determine the role of TJ alterations and BBB permeability changes in HE, especially in the context of CLD.
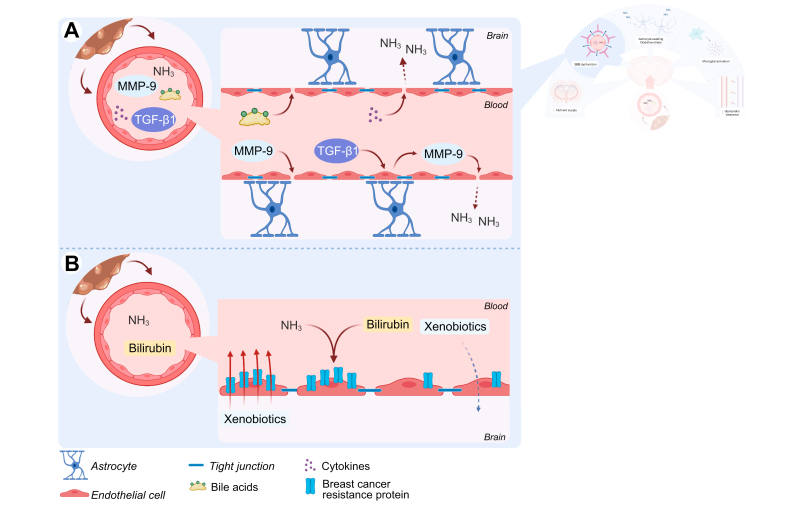
Fig. 3.
Blood-brain barrier structure and function is altered in hepatic encephalopathy.
(A) In (chronic) liver disease, levels of circulating ammonia, bile acids, cytokines, MMP-9 and TGFβ1 are increased. MMP-9 degrades TJ proteins like occludin and claudin-5. TGFβ1 leads to additional MMP-9 production by cerebral endothelial cells. Bile acids lead to deficient formation of TJs. Finally, cytokines like TNF decrease expression of TJ proteins like occludin. TJ breakdown leads to indiscriminate influx of toxins like ammonia. (B) Efflux transporters like BCRP protect the brain from drugs and other xenobiotics. Ammonia and bilirubin, which accumulate in conditions of HE, reduce expression and functionality of BCRP. This increases brain concentrations of xenobiotics and other ligands of BCRP and makes patients more susceptible to these compounds. BBB, blood-brain barrier; BCRP, breast cancer resistance protein; HE, hepatic encephalopathy; MMP-9, matrix metalloproteinase 9; NH3, ammonia; TGFβ, transforming growth factor beta; TJ, tight junction; TNF, tumour necrosis factor.
Mechanistically, TJ degradation through matrix metalloproteinase 9 (MMP-9) seems important, at least in ALF. Nguyen et al. showed that liver and cerebral endothelial cell-derived MMP-9 can cleave TJ proteins, and MMP-9 antagonism attenuates BBB permeability changes in ALF mice.63,72 McMillin et al. additionally postulate that in AOM mice, liver-derived transforming growth factor beta (TGFβ) 1 induces MMP-9 production in cerebral endothelial cells, leading to CLDN5 degradation.73 In CLD, Dhanda et al. similarly suggest that MMP-9 induced TJ breakdown in the brain of BDL rats.64 Alternatively, bile acids are proposed to permeabilise the BBB in BDL rats by stimulating OCLN phosphorylation, leading to deficient interactions with TJ proteins, such as ZO-1 and -2.66 As anti-TNF antibody treatment restores ultrastructural abnormalities, tracer extravasation and OCLN protein expression at the BBB in animal models of ALF, peripheral inflammation might also play a role in BBB breakdown in HE.74,75 In conclusion, TJ breakdown seems multifactorial in origin (Fig. 3A). However, more research is needed to elucidate which factors are relevant inducers of this phenomenon in HE.
Changes in transport systems are an alternative mechanism of BBB dysfunction. Interestingly, in the CSF of patients with HE, drugs that are substrates for the ATP-binding cassette (ABC) family of transporters seem to accumulate.43 ABC transporters like P-glycoprotein (P-gp), breast cancer resistance protein (BCRP) and multidrug resistance associated proteins 1-5 (MRP 1-5) regulate active efflux of xenobiotics to prevent accumulation in the CNS.18 ABC transporters can also export a variety of endogenous ligands, including cytokines, lipids and steroid hormones76 In vitro exposure of primary rat brain endothelial cells to ammonia, as well as in vivo acute ammonia intoxication, increases P-gp and MRP2 levels and their function.77 However, in preclinical models of HE, conflicting findings regarding P-gp levels are observed,78 possibly through an effect of chenodeoxycholic acid, a bile acid that can decrease the P-gp concentration in endothelial cell cultures.79 The evidence regarding BCRP is quite robust. In acute hyperammonaemic rats, in TAA-induced ALF and in the BDL rat model, activity and protein levels of BCRP are reduced.69,79,80 Finally, microarray data from post-mortem patients with HE showed an upregulation of MRP4 and a downregulation of MRP1.81 In conclusion, BBB efflux transporters are affected in HE (Fig. 3B). However, besides obvious consequences for CNS uptake of drugs, the precise consequences on CNS distribution of endogenous neuroactive substances and neurological functioning remain to be determined.
In summary, evidence supports different types of disturbances of the BBB in HE. While full-blown breakdown of the BBB is unlikely to occur in CLD as in ALF, decreased TJ functionality and the subsequent indiscriminate influx of substances (and thus possibly ammonia57) from the blood are apparent. Accordingly, evidence has shown that TJ breakdown is associated with CE, suggesting that oedema in ALF is of combined vasogenic and cytotoxic origin. However, in CLD, reports vary and no firm conclusion can be made. Secondly, active transport over the BBB is impaired, with alterations in ABC efflux transporters. This alters brain susceptibility to neuroactive drugs, although the precise effect on neurophysiology in the absence of xenobiotics is yet to be determined. As ionised ammonia can enter the brain through potassium transporters, alterations in these transport systems could be subject to future investigation.82 An overview of current knowledge is presented in Fig. 3.
Astrocytes swell upon ammonia exposure
Astrocytes are the only cells in the brain that express GS; they are thus particularly affected by ammonia elevation.14 Moreover, astrocytic morphology is markedly changed in HE, with cellular and nuclear enlargement, prominent nucleoli, a depleted cytoplasm and glycogen inclusions. This is known as Alzheimer type II astrocytosis and can be reproduced in animal and in vitro models.15,[83], [84], [85]
Exposure of cultured astrocytes to ammonia induces cellular swelling.84,86 These findings are nicely reproduced in animal models of both ALF and CLD.61,83,85 Glutamine accumulation, water and ion channel disturbances and oxidative and nitrosative stress (ONS) have been explored extensively as possible causes. Additionally, lactate accumulation might also play a role.
Astrocytic ammonia detoxification results in glutamine accumulation, which is osmotically active. This elicits cytoplasmic water accumulation and eventually CE (Fig. 4A).31 Glutamine concentrations are invariably elevated in brains and CSF of animal models and patients.43,87 In CLD models and in patients, other osmolytes, most notably myoinositol, choline and taurine, decrease, suggesting compensatory changes when hyperammonaemia is prolonged.51,54,88,89 The finding of low grade CE in CLD suggests that this compensation is insufficient.52 Interestingly, brain glutamine levels, indicative of brain ammonia exposure, correlate with HE severity, suggesting that brain ammonia levels rather than blood ammonia levels are important in HE development.89
Alternative/additive mechanisms for astrocyte swelling have also been proposed. The observation that glutamine can induce mitochondrial pore transition (mPT) and subsequent astrocyte swelling in vitro led to the so-called ‘Trojan Horse hypothesis’: ‘Shuttling of glutamine to the mitochondria leads to excess levels of mitochondrial ammonia, mPT, ONS and finally astrocyte swelling’ (Fig. 4A).90 Alternatively, increased intracellular ion concentrations and subsequent increased water influx could present another mechanism. The most important astrocytic ion importers are the Na-K-2Cl cotransporter 1 (NKCC1) and the sulfonylurea receptor-1 (SUR1)-regulated NCCa-ATP channel. NKCC1 overactivation occurs upon in vitro exposure of astrocytes to ammonia and, in vivo, in preclinical models of ammonia intoxication, ALF and CLD. NKCC1 deficiency or inhibition effectively rescues neurological dysfunction, astrocyte swelling and CE (Fig. 4B).[91], [92], [93], [94] Additionally, both in vitro and in the TAA model, SUR1, which is a marker for NCCa-ATP activity, was upregulated. Blockade of SUR1 results in decreased CE (Fig. 4B).95
AQP4 is the main water channel in astrocytes and allows for the flow of water along an osmotic gradient.96 An increase in AQP4 levels thus renders cells more susceptible to osmotic shifts. Importantly, AQP4 expression is induced upon the stimulation of cultured astrocytes by ammonia (Fig. 4B)97 and in preclinical models of ALF98 and CLD.51,64,65 Moreover, a localised increase in AQP4 has been observed in patients who died from ALF.99 Interestingly, AQP4 deficiency reduces brain water content after TAA administration.100 AQP4 dysregulation, in which the location changes from perivascular to a more even distribution within the astrocyte, is associated with CE in stroke and trauma.96 In a recent study in BDL rats, decreased vascular coverage of AQP4 was discovered, which begs the question does a similar phenomenon occur in HE.101
In conclusion, increased ammonia in both ALF and CLD leads to the creation of an osmotic gradient, either through glutamine (and/or lactate) or ionic imbalances. Astrocytes are more susceptible to these changes because of increased AQP4 expression. Finally, breakdown of the BBB might introduce a component of vasogenic oedema, at least in ALF. This eventually leads to clinically apparent and potentially fatal CE in ALF. In CLD, oedema is also present, but only detectable with specific imaging techniques. The clinical relevance of this mild CE is still debated.52
Astrocytes are subject to oxidative and nitrosative stress
ONS is explicitly present in experimental and clinical HE. Irreversibly oxidised albumin was specifically elevated in AD patients with HE and/or renal dysfunction without ACLF when compared to other forms of decompensation, suggesting this is a specific disease mechanism.37 In patients with HE, oxidative stress response genes like heme oxygenase-1 (HO-1) are upregulated in the cerebral cortex.102,103 Ammonia can directly induce ONS, as exposure of cultured astrocytes to ammonium salts results in the production of free radicals.29,30 Alternatively, tryptophan metabolites could induce ONS.55 Acutely hyperammonaemic rats display increased RNA oxidation104 and TAA-induced ALF results in increased lipid peroxidation and decreased antioxidant enzyme activity in the cerebral cortex, even at the pre-coma stage, implying that ONS is important in disease propagation.105,106 In chronic HE, evidence generally points towards the presence of both peripheral and central ONS. However, Rose et al. did not observe altered levels of reactive oxygen species, antioxidant enzymes nor end products like malondialdehyde in the brain of BDL rats or rats following portacaval anastomosis (PCA),68 while other studies did.42 Differences in experimental set-up can account for these seemingly contradictory observations. Interestingly, this seems to be an early phenomenon in the BDL model, as lipid peroxidation can be detected in the brain of BDL rats as early as 10 days postoperatively.107 Finally, antioxidant therapy ameliorates motor performance in BDL rats, coinciding with a normalisation of HO-1, suggesting that targeting ONS could be of value in HE.108
Multiple putative mechanisms for generating ONS in HE are suggested, namely overactivation of N-methyl-D-aspartate receptors,109,110 activation of NAPDH oxidase30,111 and the aforementioned Trojan Horse hypothesis (Fig. 4A,C).90 The pathophysiological consequences of reactive oxygen/nitrogen species generation are numerous.[111], [112], [113] ONS has also been linked to pro-inflammatory signalling (reviewed in114). Importantly, ONS can induce cell swelling and vice-versa, suggesting a positive feedback loop is at play (Fig. 4C).115 Interestingly, microarray gene expression analysis of brain cortex samples in patients with HE reveals that markers of cellular senescence are upregulated.116 Senescence is a non-proliferative cell state, in which cells obtain a specific, markedly more inflammatory, secretory profile in a process called ‘inflammaging’.117 Multiple papers from Görg et al. show that ammonia can induce astrocyte senescence in an ONS-dependent manner.111,116 Moreover, in vitro evidence suggests that ammonia could accelerate the inflammaging phenotype in adult astrocytes (Fig. 4C).117 As senescence is regarded as an irreversible process, this could account for some of the irreversible changes observed in HE.
In conclusion, ONS is a vital part of HE pathophysiology and ammonia toxicity in astrocytes. While the focus has been on its importance in cell swelling and CE, consequences are more far reaching, e.g. senescence. As antioxidant therapy has proven beneficial in rats with HE, this is a valid potential therapeutic target.
Microglia are activated and promote an inflammatory brain environment in HE
There is ample evidence in HE that inflammation is not only peripheral, but also involves neuroinflammation, characterised by cytokine production and microglial activation. In patients with ALF, a net efflux of cytokines from the brain was discovered, suggesting active cerebral cytokine production.118 Multiple studies have since found evidence of microglial activation and proliferation in post-mortem samples of patients with HE.119,120 Gene and protein expression analysis of post-mortem cortex samples from patients with HE invariably show an upregulation of microglial markers, like ionised calcium-binding adapter molecule 1, compared to cirrhotic controls. Interestingly, both upregulation of pro-inflammatory and anti-inflammatory markers are observed in cortical samples of patients with HE, suggesting that both microglial phenotypes coexist simultaneously.103,119,121,122
These findings are confirmed by preclinical HE models. In the TAA, AOM and hepatic devascularisation animal models for HE in ALF, there is extensive evidence for microglial activation in different brain regions.[123], [124], [125], [126], [127], [128], [129], [130] Microglial activation is a late event in disease development and only occurs at the time of CE and herniation, suggesting these events might be interlinked.106,123 In the rat PCA model of PSS, upregulation of interleukin (IL)-6, inducible nitric oxide synthetase, cyclooxygenase131 and microglial activation in the cerebellum and hippocampus are observed.132,133 Although one research group could not find any,134 most studies point towards ubiquitously activated microglia in the brain of BDL rodents.122,[135], [136], [137], [138] This is associated with an increased presence of pro-inflammatory mediators like IL-1β, TNF and chemokine ligand (CCL) 2.122,137
The above findings cannot simply be explained by an effect of ammonia. Indeed, ammonia does not induce production of pro-inflammatory cytokines in microglia in vitro, under basal conditions,119,121 or after lipopolysaccharide stimulation,121 suggesting that the synergism between ammonia and inflammation does not occur directly in microglia. In vivo, acute ammonia intoxication cannot activate microglia.119,123 On the contrary, in rats fed an ammonium diet, activated microglia are observed.136,137,139,140 More research into the direct influence of ammonia on microglia is necessary to explain this apparent contradiction.
Systemic inflammation is known to induce microglial activation and neuroinflammation.141 Interestingly, a significant correlation is seen between C-reactive protein levels and inflammatory gene markers in the cortex of patients with HE, suggesting such a link.103 Systemic anti-inflammatory treatment with ibuprofen restores behaviour and microglial morphology in BDL and hyperammonaemic rats.137 Moreover, treatment with minocycline ameliorates neurological function and microglial activation in ALF.128 This suggests that systemic inflammation rather than ammonia is at least partially involved in the development of neuroinflammation in HE.
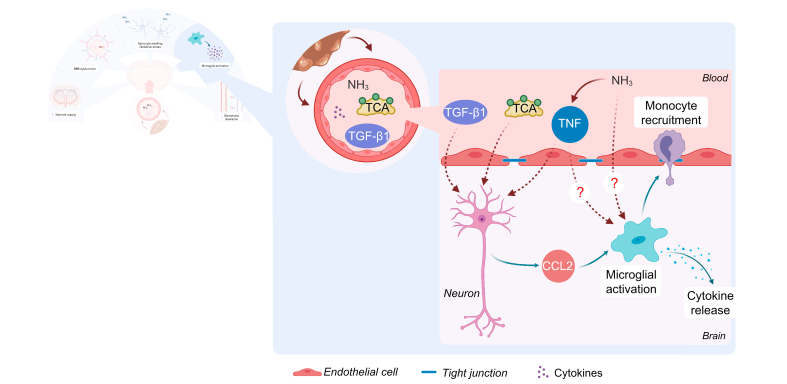
Signal transduction from the periphery to the CNS occurs in multiple ways. Most evidence points towards a role for peripheral TNF, possibly through TNF receptor 1 (TNFR1). Treatment of PCA rats with infliximab, an anti-TNF antibody that is not able to cross the BBB and thus only operates peripherally, improves balance function and microglial activation.133 Interestingly, infliximab can reverse both the behavioural deficits and the inflammatory changes in microglia caused by pure chronic hyperammonaemia, suggesting that ammonia can elicit a peripheral inflammatory response, which induces microglial activation through TNF. This finding seemingly reconciles the limited effects of ammonia on microglial cultures with the presence of neuroinflammation in rats fed an ammonium diet.142 TNFR1-deficient mice show decreased CE and increased time to coma after AOM exposure143 and decreased levels of activated microglia after BDL.135 Interestingly, TNF is specifically elevated in AD patients with single renal dysfunction and/or low grade HE, further supporting its importance.37 Additionally, McMillin et al. suggested a direct interaction between TGFβ1 and the neuronal TGFβ receptor 2 in eliciting neuroinflammation in AOM mice.144 Interestingly, TGFβ upregulation is also observed in BDL rat brains.134 Next, non-cytokine-related factors are also implicated in this humoral communication from the periphery to the CNS. Notable in this regard are bile acids.124,126,145 Bile acids accumulate in the brain in both acute and chronic liver failure,43 and are associated with HE and future decompensation.146 In AOM mice, microglial activation through the bile acid receptors sphingosine-1-phosphate receptor 2 (S1PR2) and G protein-coupled bile acid receptor 1 signalling has been shown.124,126 Finally, activated peripheral immune cells can translocate to the CNS to activate microglia. Research by D’Mello et al. nicely shows that in BDL mice, microglia produce CCL2, which acts as a chemoattractant for peripheral monocytes.135 Inhibiting monocyte infiltration improves sickness behaviour in these mice.135 Whether similar immune cell infiltration occurs in other models and clinical HE remains an open question.
Evidence regarding signalling cascades in the CNS that lead to microglial activation, as well as the functional consequences of microglial activation, is limited. However, multiple independent reports underline the importance of the CCL2/C-C chemokine receptor type 2 (CCR2) axis. Bile acid accumulation in AOM mouse brains results in neuronal S1PR2 activation and CCL2 production. Through the CCR2/CCR4 receptors, CCL2 activates microglia. Conversely, intracerebroventricular administration of an S1PR2 antagonist reduces microglial activation in the AOM model.124,125,130 p38 MAPK and IL-1β have similarly been implicated. In PCA and hyperammonaemic rats, intracerebroventricular administration of p38 MAPK inhibitors and IL-1R antagonists improves microglial activation and behavioural performance.140,147,148 The fact that direct intracerebral administration of therapeutics has a clinical effect underscores the importance of CNS alterations in the development of HE, regardless of peripheral alterations.
In summary, these data suggest a role for local CNS inflammation in both acute and chronic HE. Peripheral cytokines/chemokines/bile acids rather than ammonia seem to be instrumental in directly eliciting neuroinflammation. Interestingly, targeting both central and peripheral signalling cascades has proven beneficial and both seem to be valid targets for therapeutic development. Large knowledge gaps still exist with regards to the stimuli that induce microglial activation and the underlying molecular pathways involved in neuroinflammation in HE. Insight into the functional consequences of these processes is also lacking. Expanding on this might provide insights that lead to the identification of novel therapeutic targets. A summary of current knowledge is presented in Fig. 5.
Fig. 5.
Mechanisms and consequences of microglial activation in hepatic encephalopathy.
In (chronic) liver disease, circulating levels of ammonia, TCA, TGFβ1 and cytokines like TNF are increased. TGFβ1, TNF and TCA bind to their respective neuronal receptors, which respond with CCL2 production. CCL2 binds to CCR2/4 on microglia, resulting in an activated phenotype. Additionally, hyperammonaemia can induce peripheral TNF increases, which induces microglial activation through the TNFR1, although whether this effect is direct or indirect is not known. Whether compounds like bile acids and cytokines have a direct influence on microglia in HE is unknown. The direct effect of hyperammonaemia on microglia is still an open question. Microglial activation results in cytokine production and recruitment of peripheral immune cells, in particular monocytes. This constitutes a neuroinflammatory environment, which is detrimental to brain functioning. CCL, chemokine ligand; CCR, C-C chemokine receptor; NH3, ammonia; TCA, taurocholic acid; TGFβ, transforming growth factor beta; TNF, tumour necrosis factor; TNFR1, TNF receptor 1.
Efflux of solutes and toxins towards meningeal lymphatics is reduced, further aggravating neuroinflammation
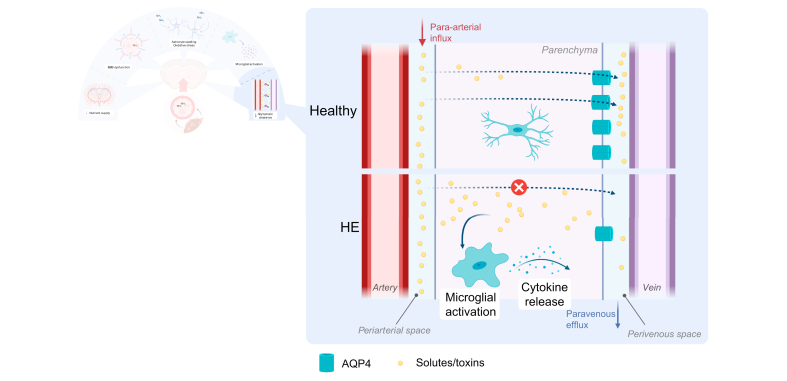
Recent evidence suggests that efflux impairment might also contribute to the accumulation of neurotoxic substances. Interestingly, in BDL rats, glymphatic clearance is impaired in the prefrontal cortex, hippocampus and olfactory bulb. This was associated with decreased vessel coverage of AQP4, which is suggested to play an important role in glymphatic clearance.101 While this contradicts earlier observations that AQP4 levels were increased in HE, the observation that AQP4 is redistributed away from perivascular sites and towards an even distribution in astrocytes in patients dying from ALF might reconcile these observations.99 In addition to that, Hsu et al. recently discovered that promoting meningeal lymphangiogenesis through injection of vascular endothelial growth factor-C ameliorated neuroinflammation and motor function alterations in BDL rats. This not only indicates that inadequate clearance of substances from the interstitial fluid is important in the development of neuroinflammation, but also that this might be an interesting therapeutic target (Fig. 6).122 More research is necessary to elucidate the precise mechanics of this glymphatic dysfunction in HE.
Fig. 6.
Glymphatic dysfunction leads to accumulation of toxins and neuroinflammation.
In chronic liver disease, AQP4 vessel coverage is decreased. This impairs the flow of solutes and toxins from the periarterial to the perivenous space and further on to meningeal lymphatic vessels. Subsequently, these compounds accumulate in the brain parenchyma, activating microglia and promoting a neuroinflammatory microenvironment, which disturbs normal brain functioning. AQP4, aquaporin 4; HE, hepatic encephalopathy.
To conclude, outflow of solutes and waste products is impaired through a dysfunctional glymphatic system. In HE, inflow of neurotoxins into the CNS is increased, while outflow is impaired, resulting in the accumulation of neurotoxins and subsequent neuroinflammation, which then contributes to disease development.
Brain disturbances in ALF and CLD are distinct, but overlapping
As evidenced by this manuscript, the pathophysiology of HE in the context of ALF and CLD possesses many overlapping characteristics. However, it should equally be stressed that both syndromes exhibit unique characteristics that modulate the relative contribution of the described pathways. Cirrhosis is characterised by portal hypertension, microcirculatory dysfunction and reduced organ perfusion as well as immune dysfunction, with both paralysis and hyperactivation occurring.149 Moreover, the more chronic nature of hyperammonaemia allows for compensatory mechanisms to develop (e.g. myoinositol decrease).51 In ALF, on the other hand, HE is characterised by an acute and more extreme rise in ammonia levels, precluding compensatory changes, and thereby frequently leading to CE.50 Moreover, the inflammatory response is different, as the immune modulation that defines cirrhosis is not present in these patients. Finally, it should be noted that there is a paucity of animal models suited to study HE in the context of CLD. Particularly, current models for AD with HE are unsatisfactory. Thus, many mechanistical insights into the specific brain pathophysiology of HE are derived from preclinical studies using ALF models, and are not necessarily applicable in cirrhosis.150 In Table 1, similarities and differences of HE in ALF and CLD are listed.
Table 1.
Overlapping and differing characteristics of hepatic encephalopathy in ALF and CLD.
| NGVU disturbance | ALF/CLD overlap | ALF/CLD difference |
|---|---|---|
| Disturbed energy metabolism | CLD: Deficient ketone body production in the cirrhotic liver, insufficient to compensate for glucose allocation to the immune system[46], [47], [48], [49]; Lactate elevation is inconsistent51,54 | |
| Blood-brain barrier disruption | ALF: Barrier integrity disruption results in vasogenic brain oedema50,52; MMP9 mediates breakdown of tight junctions63,72,73 | |
| CLD: Evidence regarding BBB permeability, structure and tight junction alterations is inconsistent[67], [68], [69], [70] | ||
| Astrocyte swelling | ALF: Cellular swelling results in clinically significant brain oedema50,52; Hyperactivation of the ion channels NKCC1 and SUR1 creates ionic imbalances across the cell membrane and attracts water into the cell[93], [94], [95] | |
| CLD: Compensatory decrease of myoinositol and other osmolytes51,54,89; Low grade cerebral oedema of unclear clinical significance52 | ||
| Oxidative stress | CLD: Possible disconnect, with only systemic oxidative stress in the absence of cerebral oxidative stress68; Senescence only studied in vitro and in patients with CLD111,113,116 | |
| Neuroinflammation |
|
ALF: TGFβ1144 and bile acids124 can contribute to microglial activation in mouse models of ALF |
| Glymphatic clearance | CLD: Decreased glymphatic clearance leads to neuroinflammation in BDL rats101,122 |
ALF, acute liver failure; AQP4, aquaporin 4; BBB, blood-brain barrier; BCRP, breast cancer resistance protein; BDL, bile duct ligated; CLD, chronic liver disease; MMP9, matrix metalloproteinase 9; NKCC1, Na-K-2Cl cotransporter 1; SUR1, sulfonylurea receptor-1; TGFβ1, transforming growth factor β1; TNF, tumour necrosis factor
Conclusions and future perspectives
HE is a frequent and inadequately understood complication of acute and chronic liver failure. Its pathophysiology is very complex. Historically, the focus has primarily been on hyperammonaemia and subsequent astrocytic swelling as causal factors. While part of the answer, this view proved incomplete. Peripheral stimuli other than hyperammonaemia are equally important in disease development, with cytokines, chemokines and bile acids all having a role to play. Effects on astrocytes are more far reaching than osmotic changes and cell swelling. These include oxidative stress, energy disturbances and cellular senescence. Finally, and most importantly, HE is a disease that involves multiple components of the NGVU beyond astrocytic changes. Liver failure induces a pro-inflammatory environment in the CNS with increased cytokine and chemokine production, as well as an influx of peripheral immune cells. BBB disruption increases the influx of neurotoxic substances and water, while glymphatic dysfunction impairs their clearance. Cerebral blood flow is impaired, as is energy/nutrient supply to the brain, resulting in lactate and toxin accumulation. These factors all contribute to disease development. However, the functional consequences of these individual perturbations are often unclear. More research is needed to elucidate the precise causes and consequences of these perturbations.
These mechanisms are intertwined and the individual contribution of each one to the total pathology is hard to distinguish. While significant advances have been made in the past years, the exact interplay between different factors have been largely unexplored. Given the fact that the complexity of HE pathogenesis is likely derived from the interaction and synergism between the aforementioned mechanisms, more work has to be done to identify common origins and effects of NGVU dysfunction. How and where ammonia neurotoxicity and systemic inflammation intersect and influence disease development is an important topic for future research. This will be instrumental in identifying new therapeutic targets that would allow for the development of therapies specifically aimed at the CNS perturbations in HE.
Financial support
WC is supported by a grant from the Research Foundation – Flanders (11A6420N). SL is supported by a grant from the Research Foundation – Flanders (12R0321N). HVV and AG are senior clinical researchers of the Research Foundation – Flanders.
Authors’ contributions
WC performed the literature review, drafted the manuscript and visualized the research. LVH, AG, REV and CVS supervised the research. SL, XV, HVV, HDG and LD provided important intellectual input. LVH, SL, AG, XV, HVV, HDG, LD, REV and CVS reviewed and edited the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
All figures were created with BioRender.com. The authors thank Stephanie Depuydt for assistance in designing the figures.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100352.
Supplementary data
The following is the supplementary data to this article:
References
- 1.Bustamante J., Rimola A., Ventura P.-J., Navasa M., Cirera I., Reggiardo V. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999 May;30(5):890–895. doi: 10.1016/s0168-8278(99)80144-5. [DOI] [PubMed] [Google Scholar]
- 2.Vilstrup H., Amodio P., Bajaj J., Cordoba J., Ferenci P., Mullen K.D. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European association for the study of the liver and the American association for the study of liver diseases. J Hepatol. 2014 Sep;61(3):642–659. doi: 10.1016/j.jhep.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Franco O., Morin J., Cortes-Sol A., Molina-Jimenez T., Del Moral D.I., Flores-Munoz M. Cognitive impairment after resolution of hepatic encephalopathy: a systematic review and meta-analysis. Front Neurosci. 2021;15:11. doi: 10.3389/fnins.2021.579263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ochoa-Sanchez R., Tamnanloo F., Rose C.F. Hepatic encephalopathy: from metabolic to neurodegenerative. Neurochem Res. 2021 Jun 15 doi: 10.1007/s11064-021-03372-4. [DOI] [PubMed] [Google Scholar]
- 5.Tapper E.B., Jiang Z.G., Patwardhan V.R. Refining the ammonia hypothesis. Mayo Clin Proc. 2015 May;90(5):646–658. doi: 10.1016/j.mayocp.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Clària J., Stauber R.E., Coenraad M.J., Moreau R., Jalan R., Pavesi M. Systemic inflammation in decompensated cirrhosis: characterization and role in acute-on-chronic liver failure. Hepatology. 2016 Oct;64(4):1249–1264. doi: 10.1002/hep.28740. [DOI] [PubMed] [Google Scholar]
- 7.Trebicka J., Fernandez J., Papp M., Caraceni P., Laleman W., Gambino C. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020 Oct;73(4):842–854. doi: 10.1016/j.jhep.2020.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Rolando N., Wade J., Davalos M., Wendon J., Philpott-Howard J., Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000 Oct;32(4):734–739. doi: 10.1053/jhep.2000.17687. [DOI] [PubMed] [Google Scholar]
- 9.Shawcross D.L., Sharifi Y., Canavan J.B., Yeoman A.D., Abeles R.D., Taylor N.J. Infection and systemic inflammation, not ammonia, are associated with Grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J Hepatol. 2011 Apr;54(4):640–649. doi: 10.1016/j.jhep.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z., Ruan J., Li D., Wang M., Han Z., Qiu W. The role of intestinal bacteria and gut–brain Axis in hepatic encephalopathy. Front Cell Infect Microbiol. 2021 Jan 21;10:595759. doi: 10.3389/fcimb.2020.595759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lattanzi B., D’Ambrosio D., Merli M. Hepatic encephalopathy and sarcopenia: two faces of the same metabolic alteration. J Clin Exp Hepatol. 2019 Jan;9(1):125–130. doi: 10.1016/j.jceh.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gluud L.L., Dam G., Les I., Marchesini G., Borre M., Aagaard N.K. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017 May 18;5(5):CD001939. doi: 10.1002/14651858.CD001939.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassouneh R., Bajaj J.S. Gut microbiota modulation and fecal transplantation: an overview on innovative strategies for hepatic encephalopathy treatment. J Clin Med. 2021 Jan 18;10(2):330. doi: 10.3390/jcm10020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayakumar A.R., Norenberg M.D. Glutamine synthetase: role in neurological disorders. Adv Neurobiol. 2016;13:327–350. doi: 10.1007/978-3-319-45096-4_13. [DOI] [PubMed] [Google Scholar]
- 15.Norenberg M.D. The role of astrocytes in hepatic encephalopathy. Neurochem Pathol. 1987 Apr;6(1–2):13–33. doi: 10.1007/BF02833599. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad A., Patel V., Xiao J., Khan M.M. The role of neurovascular system in neurodegenerative diseases. Mol Neurobiol. 2020 Nov;57(11):4373–4393. doi: 10.1007/s12035-020-02023-z. [DOI] [PubMed] [Google Scholar]
- 17.Huang L., Nakamura Y., Lo E.H., Hayakawa K. Astrocyte signaling in the neurovascular unit after central nervous system injury. Int J Mol Sci. 2019 Jan 11;20(2):282. doi: 10.3390/ijms20020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweeney M.D., Zhao Z., Montagne A., Nelson A.R., Zlokovic B.V. Blood-brain barrier: from physiology to disease and back. Physiol Rev. 2019 Jan;99(1):21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Bock M., Decrock E., Wang N., Bol M., Vinken M., Bultynck G. The dual face of connexin-based astroglial Ca2+ communication: a key player in brain physiology and a prime target in pathology. Biochim Biophys Acta BBA - Mol Cell Res. 2014 Oct;1843(10):2211–2232. doi: 10.1016/j.bbamcr.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Abbott N.J., Rönnbäck L., Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006 Jan;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 21.Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol (Berl) 2010 Jan;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J., Kong H., Hua X., Xiao M., Ding J., Hu G. Altered blood–brain barrier integrity in adult aquaporin-4 knockout mice. NeuroReport. 2008 Jan;19(1):1–5. doi: 10.1097/WNR.0b013e3282f2b4eb. [DOI] [PubMed] [Google Scholar]
- 23.Sheeler C., Rosa J.-G., Ferro A., McAdams B., Borgenheimer E., Cvetanovic M. Glia in neurodegeneration: the housekeeper, the defender and the perpetrator. Int J Mol Sci. 2020 Dec 2;21(23):9188. doi: 10.3390/ijms21239188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hickman S., Izzy S., Sen P., Morsett L., El Khoury J. Microglia in neurodegeneration. Nat Neurosci. 2018 Oct;21(10):1359–1369. doi: 10.1038/s41593-018-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen M.K., Mestre H., Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018 Nov;17(11):1016–1024. doi: 10.1016/S1474-4422(18)30318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017 Jan;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oja S.S., Saransaari P., Korpi E.R. Neurotoxicity of ammonia. Neurochem Res. 2017 Mar;42(3):713–720. doi: 10.1007/s11064-016-2014-x. [DOI] [PubMed] [Google Scholar]
- 28.Rama Rao K.V., Norenberg M.D. Brain energy metabolism and mitochondrial dysfunction in acute and chronic hepatic encephalopathy. Neurochem Int. 2012 Jun;60(7):697–706. doi: 10.1016/j.neuint.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murthy C.R.K., Rao K.V.R., Bai G., Norenberg M.D. Ammonia-induced production of free radicals in primary cultures of rat astrocytes. J Neurosci Res. 2001;66(2):282–288. doi: 10.1002/jnr.1222. [DOI] [PubMed] [Google Scholar]
- 30.Reinehr R., Görg B., Becker S., Qvartskhava N., Bidmon H.J., Selbach O. Hypoosmotic swelling and ammonia increase oxidative stress by NADPH oxidase in cultured astrocytes and vital brain slices. Glia. 2007;55(7):758–771. doi: 10.1002/glia.20504. [DOI] [PubMed] [Google Scholar]
- 31.Norenberg M.D., Bender A.S. Astrocyte swelling in liver failure: role of glutamine and benzodiazepines. Acta Neurochir Suppl (Wien) 1994;60:24–27. doi: 10.1007/978-3-7091-9334-1_6. [DOI] [PubMed] [Google Scholar]
- 32.Ong J.P., Aggarwal A., Krieger D., Easley K.A., Karafa M.T., Van Lente F. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003 Feb;114(3):188–193. doi: 10.1016/s0002-9343(02)01477-8. [DOI] [PubMed] [Google Scholar]
- 33.Shalimar, Sheikh M.F., Mookerjee R.P., Agarwal B., Acharya S.K., Jalan R. Prognostic role of ammonia in patients with cirrhosis. Hepatology. 2019 Sep;70(3):982–994. doi: 10.1002/hep.30534. [DOI] [PubMed] [Google Scholar]
- 34.Jayakumar A.R., Rama Rao K.V., Norenberg M.D. Neuroinflammation in hepatic encephalopathy: mechanistic aspects. J Clin Exp Hepatol. 2015 Mar;5:S21–S28. doi: 10.1016/j.jceh.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadjihambi A., Arias N., Sheikh M., Jalan R. Hepatic encephalopathy: a critical current review. Hepatol Int. 2018 Feb;12(S1):135–147. doi: 10.1007/s12072-017-9812-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicolao F., Efrati C., Masini A., Merli M., Attili A.F., Riggio O. Role of determination of partial pressure of ammonia in cirrhotic patients with and without hepatic encephalopathy. J Hepatol. 2003 Apr;38(4):441–446. doi: 10.1016/s0168-8278(02)00436-1. [DOI] [PubMed] [Google Scholar]
- 37.Trebicka J., Amoros A., Pitarch C., Titos E., Alcaraz-Quiles J., Schierwagen R. Addressing profiles of systemic inflammation across the different clinical phenotypes of acutely decompensated cirrhosis. Front Immunol. 2019 Mar 19;10:476. doi: 10.3389/fimmu.2019.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arroyo V., Angeli P., Moreau R., Jalan R., Clària J., Trebicka J. The systemic inflammation hypothesis: towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol. 2020 Dec doi: 10.1016/j.jhep.2020.11.048. S0168827820338368. [DOI] [PubMed] [Google Scholar]
- 39.Bélanger M., Allaman I., Magistretti P.J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011 Dec;14(6):724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Pellerin L., Bouzier-Sore A.-K., Aubert A., Serres S., Merle M., Costalat R. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007 Sep;55(12):1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- 41.Bosoi C.R., Rose C.F. Elevated cerebral lactate: implications in the pathogenesis of hepatic encephalopathy. Metab Brain Dis. 2014 Dec;29(4):919–925. doi: 10.1007/s11011-014-9573-9. [DOI] [PubMed] [Google Scholar]
- 42.Dhanda S., Sunkaria A., Halder A., Sandhir R. Mitochondrial dysfunctions contribute to energy deficits in rodent model of hepatic encephalopathy. Metab Brain Dis. 2018 Feb;33(1):209–223. doi: 10.1007/s11011-017-0136-8. [DOI] [PubMed] [Google Scholar]
- 43.Weiss N., Barbier Saint Hilaire P., Colsch B., Isnard F., Attala S., Schaefer A. Cerebrospinal fluid metabolomics highlights dysregulation of energy metabolism in overt hepatic encephalopathy. J Hepatol. 2016 Dec;65(6):1120–1130. doi: 10.1016/j.jhep.2016.07.046. [DOI] [PubMed] [Google Scholar]
- 44.Bjerring P.N., Gluud L.L., Larsen F.S. Cerebral blood flow and metabolism in hepatic encephalopathy—a meta-analysis. J Clin Exp Hepatol. 2018 Sep;8(3):286–293. doi: 10.1016/j.jceh.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ponziani F.R., Funaro B., Lupascu A., Ainora M.E., Garcovich M., Caracciolo G. Minimal hepatic encephalopathy is associated with increased cerebral vascular resistance. A transcranial Doppler ultrasound study. Sci Rep. 2019 Dec;9(1):15373. doi: 10.1038/s41598-019-51867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreau R., Clària J., Aguilar F., Fenaille F., Lozano J.J., Junot C. Blood metabolomics uncovers inflammation-associated mitochondrial dysfunction as a potential mechanism underlying ACLF. J Hepatol. 2020 Apr;72(4):688–701. doi: 10.1016/j.jhep.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Zaccherini G., Aguilar F., Caraceni P., Clària J., Lozano J.J., Fenaille F. Assessing the role of amino acids in systemic inflammation and organ failure in patients with ACLF. J Hepatol. 2021 May;74(5):1117–1131. doi: 10.1016/j.jhep.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 48.Dabos K.J., Parkinson J.A., Sadler I.A., Plevris J.N., Hayes P.C. 1H nuclear magnetic resonance spectroscopy-based metabonomic study in patients with cirrhosis and hepatic encephalopathy. World J Hepatol. 2015;7(12):1701. doi: 10.4254/wjh.v7.i12.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiménez B., Montoliu C., MacIntyre D.A., Serra M.A., Wassel A., Jover M. Serum metabolic signature of minimal hepatic encephalopathy by 1H-nuclear magnetic resonance. J Proteome Res. 2010 Oct;9(10):5180–5187. doi: 10.1021/pr100486e. [DOI] [PubMed] [Google Scholar]
- 50.Bernal W., Lee W.M., Wendon J., Larsen F.S., Williams R. Acute liver failure: a curable disease by 2024? J Hepatol. 2015 Apr;62(1):S112–S120. doi: 10.1016/j.jhep.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 51.Braissant O., Rackayová V., Pierzchala K., Grosse J., McLin V.A., Cudalbu C. Longitudinal neurometabolic changes in the hippocampus of a rat model of chronic hepatic encephalopathy. J Hepatol. 2019 Sep;71(3):505–515. doi: 10.1016/j.jhep.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 52.Cudalbu C., Taylor-Robinson S.D. Brain edema in chronic hepatic encephalopathy. J Clin Exp Hepatol. 2019 May;9(3):362–382. doi: 10.1016/j.jceh.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bosoi C.R., Zwingmann C., Marin H., Parent-Robitaille C., Huynh J., Tremblay M. Increased brain lactate is central to the development of brain edema in rats with chronic liver disease. J Hepatol. 2014 Mar;60(3):554–560. doi: 10.1016/j.jhep.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 54.Rackayova V., Braissant O., McLin V.A., Berset C., Lanz B., Cudalbu C. 1H and 31P magnetic resonance spectroscopy in a rat model of chronic hepatic encephalopathy: in vivo longitudinal measurements of brain energy metabolism. Metab Brain Dis. 2016 Dec;31(6):1303–1314. doi: 10.1007/s11011-015-9715-8. [DOI] [PubMed] [Google Scholar]
- 55.Clària J., Moreau R., Fenaille F., Amorós A., Junot C., Gronbaek H. Orchestration of tryptophan-kynurenine pathway, acute decompensation, and acute-on-chronic liver failure in cirrhosis. Hepatology. 2019 Apr;69(4):1686–1701. doi: 10.1002/hep.30363. [DOI] [PubMed] [Google Scholar]
- 56.Montagnese S., Lauridsen M., Vilstrup H., Zarantonello L., Lakner G., Fitilev S. A pilot study of golexanolone, a new GABA-A receptor-modulating steroid antagonist, in patients with covert hepatic encephalopathy. J Hepatol. 2021 Jul;75(1):98–107. doi: 10.1016/j.jhep.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Lockwood A.H., Yap E.W., Wong W.H. Cerebral ammonia metabolism in patients with severe liver disease and minimal hepatic encephalopathy. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 1991 Mar;11(2):337–341. doi: 10.1038/jcbfm.1991.67. [DOI] [PubMed] [Google Scholar]
- 58.Keiding S., Sørensen M., Bender D., Munk O.L., Ott P., Vilstrup H. Brain metabolism of 13N-ammonia during acute hepatic encephalopathy in cirrhosis measured by positron emission tomography. Hepatol Baltim Md. 2006 Jan;43(1):42–50. doi: 10.1002/hep.21001. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen J.H. Blood–brain barrier in acute liver failure. Neurochem Int. 2012 Jun;60(7):676–683. doi: 10.1016/j.neuint.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cauli O., López–Larrubia P., Rodrigo R., Agusti A., Boix J., Nieto–Charques L. Brain region-selective mechanisms contribute to the progression of cerebral alterations in acute liver failure in rats. Gastroenterology. 2011 Feb;140(2):638–645. doi: 10.1053/j.gastro.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 61.Grant S., McMillin M., Frampton G., Petrescu A.D., Williams E., Jaeger V. Direct comparison of the thioacetamide and azoxymethane models of type A hepatic encephalopathy in mice. Gene Expr. 2018 Aug 22;18(3):171–185. doi: 10.3727/105221618X15287315176503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shimojima N., Eckman C.B., McKinney M., Sevlever D., Yamamoto S., Lin W. Altered expression of zonula occludens-2 precedes increased blood–brain barrier permeability in a murine model of fulminant hepatic failure. J Invest Surg. 2008 Jan;21(3):101–108. doi: 10.1080/08941930802043565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nguyen J.H., Yamamoto S., Steers J., Sevlever D., Lin W., Shimojima N. Matrix metalloproteinase-9 contributes to brain extravasation and edema in fulminant hepatic failure mice. J Hepatol. 2006 Jun;44(6):1105–1114. doi: 10.1016/j.jhep.2005.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dhanda S., Sandhir R. Blood-brain barrier permeability is exacerbated in experimental model of hepatic encephalopathy via MMP-9 activation and downregulation of tight junction proteins. Mol Neurobiol. 2018 May;55(5):3642–3659. doi: 10.1007/s12035-017-0521-7. [DOI] [PubMed] [Google Scholar]
- 65.Wright G., Soper R., Brooks H.F., Stadlbauer V., Vairappan B., Davies N.A. Role of aquaporin-4 in the development of brain oedema in liver failure. J Hepatol. 2010 Jul;53(1):91–97. doi: 10.1016/j.jhep.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 66.Quinn M., McMillin M., Galindo C., Frampton G., Pae H.Y., DeMorrow S. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig Liver Dis. 2014 Jun;46(6):527–534. doi: 10.1016/j.dld.2014.01.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wright G., Davies N.A., Shawcross D.L., Hodges S.J., Zwingmann C., Brooks H.F. Endotoxemia produces coma and brain swelling in bile duct ligated rats. Hepatology. 2007;45(6):1517–1526. doi: 10.1002/hep.21599. [DOI] [PubMed] [Google Scholar]
- 68.Bosoi C.R., Yang X., Huynh J., Parent-Robitaille C., Jiang W., Tremblay M. Systemic oxidative stress is implicated in the pathogenesis of brain edema in rats with chronic liver failure. Free Radic Biol Med. 2012 Apr;52(7):1228–1235. doi: 10.1016/j.freeradbiomed.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 69.Xu P., Ling Z., Zhang J., Li Y., Shu N., Zhong Z. Unconjugated bilirubin elevation impairs the function and expression of breast cancer resistance protein (BCRP) at the blood-brain barrier in bile duct-ligated rats. Acta Pharmacol Sin. 2016 Aug;37(8):1129–1140. doi: 10.1038/aps.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Y.-C., Sheen J.-M., Tain Y.-L., Chen C.-C., Tiao M.-M., Huang Y.-H. Alterations in NADPH oxidase expression and blood–brain barrier in bile duct ligation-treated young rats: effects of melatonin. Neurochem Int. 2012 Jun;60(8):751–758. doi: 10.1016/j.neuint.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 71.Faropoulos K., Chroni E., Assimakopoulos S.F., Mavrakis A., Stamatopoulou V., Toumpeki C. Altered occludin expression in brain capillaries induced by obstructive jaundice in rats. Brain Res. 2010 Apr;1325:121–127. doi: 10.1016/j.brainres.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 72.Chen F., Radisky E.S., Das P., Batra J., Hata T., Hori T. TIMP-1 attenuates blood–brain barrier permeability in mice with acute liver failure. J Cereb Blood Flow Metab. 2013 Jul;33(7):1041–1049. doi: 10.1038/jcbfm.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McMillin M.A., Frampton G.A., Seiwell A.P., Patel N.S., Jacobs A.N., DeMorrow S. TGFβ1 exacerbates blood–brain barrier permeability in a mouse model of hepatic encephalopathy via upregulation of MMP9 and downregulation of claudin-5. Lab Invest. 2015 Aug;95(8):903–913. doi: 10.1038/labinvest.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang W., Lv S., Zhou Y., Fu J., Li C., Liu P. Tumor necrosis factor-α affects blood–brain barrier permeability in acetaminophen-induced acute liver failure. Eur J Gastroenterol Hepatol. 2011 Jul;23(7):552–558. doi: 10.1097/MEG.0b013e3283470212. [DOI] [PubMed] [Google Scholar]
- 75.Lv S., Song H.-L., Zhou Y., Li L.-X., Cui W., Wang W. Tumour necrosis factor-α affects blood-brain barrier permeability and tight junction-associated occludin in acute liver failure: blood-brain barrier in liver failure. Liver Int. 2010 May 18;30(8):1198–1210. doi: 10.1111/j.1478-3231.2010.02211.x. [DOI] [PubMed] [Google Scholar]
- 76.Qosa H., Miller D.S., Pasinelli P., Trotti D. Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res. 2015 Dec;1628:298–316. doi: 10.1016/j.brainres.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang J., Zhang M., Sun B., Li Y., Xu P., Liu C. Hyperammonemia enhances the function and expression of P-glycoprotein and Mrp2 at the blood-brain barrier through NF-κB. J Neurochem. 2014 Dec;131(6):791–802. doi: 10.1111/jnc.12944. [DOI] [PubMed] [Google Scholar]
- 78.Jin S., Wang X.-T., Liu L., Yao D., Liu C., Zhang M. P-glycoprotein and multidrug resistance-associated protein 2 are oppositely altered in brain of rats with thioacetamide-induced acute liver failure. Liver Int. 2013 Feb;33(2):274–282. doi: 10.1111/j.1478-3231.2012.02862.x. [DOI] [PubMed] [Google Scholar]
- 79.Liu L., Miao M., Chen Y., Wang Z., Sun B., Liu X. Altered function and expression of ABC transporters at the blood–brain barrier and increased brain distribution of phenobarbital in acute liver failure mice. Front Pharmacol. 2018 Mar 6;9:190. doi: 10.3389/fphar.2018.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Y., Zhang J., Xu P., Sun B., Zhong Z., Liu C. Acute liver failure impairs function and expression of breast cancer-resistant protein (BCRP) at rat blood-brain barrier partly via ammonia-ROS-ERK1/2 activation. J Neurochem. 2016 Jul;138(2):282–294. doi: 10.1111/jnc.13666. [DOI] [PubMed] [Google Scholar]
- 81.Jördens M.S., Keitel V., Karababa A., Zemtsova I., Bronger H., Häussinger D. Multidrug resistance-associated protein 4 expression in ammonia-treated cultured rat astrocytes and cerebral cortex of cirrhotic patients with hepatic encephalopathy: mrp4 Expression Changes in Hepatic Encephalopathy. Glia. 2015 Nov;63(11):2092–2105. doi: 10.1002/glia.22879. [DOI] [PubMed] [Google Scholar]
- 82.Sørensen M. Update on cerebral uptake of blood ammonia. Metab Brain Dis. 2013 Jun;28(2):155–159. doi: 10.1007/s11011-013-9395-1. [DOI] [PubMed] [Google Scholar]
- 83.Jover R., Rodrigo R., Felipo V., Insausti R., Sáez-Valero J., García-Ayllón M.S. Brain edema and inflammatory activation in bile duct ligated rats with diet-induced hyperammonemia: a model of hepatic encephalopathy in cirrhosis. Hepatology. 2006;43(6):1257–1266. doi: 10.1002/hep.21180. [DOI] [PubMed] [Google Scholar]
- 84.Norenberg M.D., Baker L., Norenberg L.O., Blicharska J., Bruce-Gregorios J.H., Neary J.T. Ammonia-induced astrocyte swelling in primary culture. Neurochem Res. 1991 Jul;16(7):833–836. doi: 10.1007/BF00965694. [DOI] [PubMed] [Google Scholar]
- 85.Rivera-Mancía S., Montes S., Méndez-Armenta M., Muriel P., Ríos C. Morphological changes of rat astrocytes induced by liver damage but not by manganese chloride exposure. Metab Brain Dis. 2009 Jun;24(2):243–255. doi: 10.1007/s11011-009-9138-5. [DOI] [PubMed] [Google Scholar]
- 86.Back A., Tupper K.Y., Bai T., Chiranand P., Goldenberg F.D., Frank J.I. Ammonia-induced brain swelling and neurotoxicity in an organotypic slice model. Neurol Res. 2011 Dec;33(10):1100–1108. doi: 10.1179/1743132811Y.0000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chavarria L., Cordoba J. Magnetic resonance imaging and spectroscopy in hepatic encephalopathy. J Clin Exp Hepatol. 2015 Mar;5:S69–S74. doi: 10.1016/j.jceh.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cordoba J., Sanpedro F., Alonso J., Rovira A. 1H magnetic resonance in the study of hepatic encephalopathy in humans. Metab Brain Dis. 2003;15 doi: 10.1023/a:1021926405944. [DOI] [PubMed] [Google Scholar]
- 89.Zeng G., Penninkilampi R., Chaganti J., Montagnese S., Brew B.J., Danta M. Meta-analysis of magnetic resonance spectroscopy in the diagnosis of hepatic encephalopathy. Neurology. 2020 Mar 17;94(11):e1147–e1156. doi: 10.1212/WNL.0000000000008899. [DOI] [PubMed] [Google Scholar]
- 90.Rama Rao K.V., Norenberg M.D. Glutamine in the pathogenesis of hepatic encephalopathy: the trojan Horse hypothesis revisited. Neurochem Res. 2014 Mar;39(3):593–598. doi: 10.1007/s11064-012-0955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rangroo Thrane V., Thrane A.S., Wang F., Cotrina M.L., Smith N.A., Chen M. Ammonia triggers neuronal disinhibition and seizures by impairing astrocyte potassium buffering. Nat Med. 2013 Dec;19(12):1643–1648. doi: 10.1038/nm.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eefsen M., Vainer B., Cathrine Bisgaard H., Stolze Larsen F. Cerebral expression of NKCC1 in rats with acute and chronic hyperammonemia. J Liver Dis Transpl. 2012;01(02) [Google Scholar]
- 93.Jayakumar A.R., Liu M., Moriyama M., Ramakrishnan R., Forbush B., Reddy P.V.B. Na-K-Cl cotransporter-1 in the mechanism of ammonia-induced astrocyte swelling. J Biol Chem. 2008 Dec 5;283(49):33874–33882. doi: 10.1074/jbc.M804016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jayakumar A.R., Valdes V., Norenberg M.D. The Na–K–Cl cotransporter in the brain edema of acute liver failure. J Hepatol. 2011 Feb;54(2):272–278. doi: 10.1016/j.jhep.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 95.Jayakumar A.R., Valdes V., Tong X.Y., Shamaladevi N., Gonzalez W., Norenberg M.D. Sulfonylurea receptor 1 contributes to the astrocyte swelling and brain edema in acute liver failure. Transl Stroke Res. 2014 Feb;5(1):28–37. doi: 10.1007/s12975-014-0328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stokum J.A., Kurland D.B., Gerzanich V., Simard J.M. Mechanisms of astrocyte-mediated cerebral edema. Neurochem Res. 2015 Feb;40(2):317–328. doi: 10.1007/s11064-014-1374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rama Rao K.V., Chen M., Simard J.M., Norenberg M.D. Increased aquaporin-4 expression in ammonia-treated cultured astrocytes. Neuroreport. 2003 Dec;14(18):2379–2382. doi: 10.1097/00001756-200312190-00018. [DOI] [PubMed] [Google Scholar]
- 98.Rama Rao K.V., Jayakumar A.R., Tong X., Curtis K.M., Norenberg M.D. Brain aquaporin-4 in experimental acute liver failure. J Neuropathol Exp Neurol. 2010 Sep;69(9):869–879. doi: 10.1097/NEN.0b013e3181ebe581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thumburu K.K., Dhiman R.K., Vasishta R.K., Chakraborti A., Butterworth R.F., Beauchesne E. Expression of astrocytic genes coding for proteins implicated in neural excitation and brain edema is altered after acute liver failure. J Neurochem. 2014 Mar;128(5):617–627. doi: 10.1111/jnc.12511. [DOI] [PubMed] [Google Scholar]
- 100.Rama Rao K.V., Verkman A.S., Curtis K.M., Norenberg M.D. Aquaporin-4 deletion in mice reduces encephalopathy and brain edema in experimental acute liver failure. Neurobiol Dis. 2014 Mar;63:222–228. doi: 10.1016/j.nbd.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hadjihambi A., Harrison I.F., Costas-Rodríguez M., Vanhaecke F., Arias N., Gallego-Durán R. Impaired brain glymphatic flow in experimental hepatic encephalopathy. J Hepatol. 2019 Jan;70(1):40–49. doi: 10.1016/j.jhep.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Görg B., Qvartskhava N., Bidmon H.-J., Palomero-Gallagher N., Kircheis G., Zilles K. Oxidative stress markers in the brain of patients with cirrhosis and hepatic encephalopathy. Hepatology. 2010;52(1):256–265. doi: 10.1002/hep.23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Görg B., Bidmon H.-J., Häussinger D. Gene expression profiling in the cerebral cortex of patients with cirrhosis with and without hepatic encephalopathy. Hepatology. 2013 Jun;57(6):2436–2447. doi: 10.1002/hep.26265. [DOI] [PubMed] [Google Scholar]
- 104.Görg B., Qvartskhava N., Keitel V., Bidmon H.J., Selbach O., Schliess F. Ammonia induces RNA oxidation in cultured astrocytes and brain in vivo. Hepatology. 2008;48(2):567–579. doi: 10.1002/hep.22345. [DOI] [PubMed] [Google Scholar]
- 105.Singh S., Mondal P., Trigun S.K. Acute liver failure in rats activates glutamine-glutamate cycle but declines antioxidant enzymes to induce oxidative stress in cerebral cortex and cerebellum. PloS One. 2014 Apr 22;9(4) doi: 10.1371/journal.pone.0095855. Appanna VD, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Faleiros B.E., Miranda A.S., Campos A.C., Gomides L.F., Kangussu L.M., Guatimosim C. Up-regulation of brain cytokines and chemokines mediates neurotoxicity in early acute liver failure by a mechanism independent of microglial activation. Brain Res. 2014 Aug;1578:49–59. doi: 10.1016/j.brainres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 107.Chroni E., Patsoukis N., Karageorgos N., Konstantinou D., Georgiou C. Brain oxidative stress induced by obstructive jaundice in rats. J Neuropathol Exp Neurol. 2006 Feb;65(2):193–198. doi: 10.1097/01.jnen.0000200152.98259.4e. [DOI] [PubMed] [Google Scholar]
- 108.Wang Q.-M., Yin X.-Y., Duan Z.-J., Guo S.-B., Sun X.-Y. Role of the heme oxygenase/carbon monoxide pathway in the pathogenesis and prevention of hepatic encephalopathy. Mol Med Rep. 2013 Jul;8(1):67–74. doi: 10.3892/mmr.2013.1472. [DOI] [PubMed] [Google Scholar]
- 109.Haack N., Dublin P., Rose C.R. Dysbalance of astrocyte calcium under hyperammonemic conditions. PloS One. 2014 Aug 25;9(8) doi: 10.1371/journal.pone.0105832. Mongin AA, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lachmann V., Görg B., Bidmon H.J., Keitel V., Häussinger D. Precipitants of hepatic encephalopathy induce rapid astrocyte swelling in an oxidative stress dependent manner. Arch Biochem Biophys. 2013 Aug;536(2):143–151. doi: 10.1016/j.abb.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 111.Görg B., Karababa A., Schütz E., Paluschinski M., Schrimpf A., Shafigullina A. O-GlcNAcylation-dependent upregulation of HO1 triggers ammonia-induced oxidative stress and senescence in hepatic encephalopathy. J Hepatol. 2019 Nov;71(5):930–941. doi: 10.1016/j.jhep.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 112.Lu K., Zimmermann M., Görg B., Bidmon H.-J., Biermann B., Klöcker N. Hepatic encephalopathy is linked to alterations of autophagic flux in astrocytes. EBioMedicine. 2019 Oct;48:539–553. doi: 10.1016/j.ebiom.2019.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Görg B., Karababa A., Häussinger D. Hepatic encephalopathy and astrocyte senescence. J Clin Exp Hepatol. 2018 Sep;8(3):294–300. doi: 10.1016/j.jceh.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heidari R. Brain mitochondria as potential therapeutic targets for managing hepatic encephalopathy. Life Sci. 2019 Feb;218:65–80. doi: 10.1016/j.lfs.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 115.Görg B., Schliess F., Häussinger D. Osmotic and oxidative/nitrosative stress in ammonia toxicity and hepatic encephalopathy. Arch Biochem Biophys. 2013 Aug;536(2):158–163. doi: 10.1016/j.abb.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 116.Görg B., Karababa A., Shafigullina A., Bidmon H.J., Häussinger D. Ammonia-induced senescence in cultured rat astrocytes and in human cerebral cortex in hepatic encephalopathy. Glia. 2015 Jan;63(1):37–50. doi: 10.1002/glia.22731. [DOI] [PubMed] [Google Scholar]
- 117.Bobermin L.D., Roppa R.H.A., Gonçalves C.-A., Quincozes-Santos A. Ammonia-induced glial-inflammaging. Mol Neurobiol. 2020 Aug;57(8):3552–3567. doi: 10.1007/s12035-020-01985-4. [DOI] [PubMed] [Google Scholar]
- 118.Wright G., Shawcross D., Olde Damink S.W.M., Jalan R. Brain cytokine flux in acute liver failure and its relationship with intracranial hypertension. Metab Brain Dis. 2007 Oct 22;22(3–4):375–388. doi: 10.1007/s11011-007-9071-4. [DOI] [PubMed] [Google Scholar]
- 119.Zemtsova I., Görg B., Keitel V., Bidmon H.-J., Schrör K., Häussinger D. Microglia activation in hepatic encephalopathy in rats and humans. Hepatology. 2011 Jul;54(1):204–215. doi: 10.1002/hep.24326. [DOI] [PubMed] [Google Scholar]
- 120.Dennis C.V., Sheahan P.J., Graeber M.B., Sheedy D.L., Kril J.J., Sutherland G.T. Microglial proliferation in the brain of chronic alcoholics with hepatic encephalopathy. Metab Brain Dis. 2014 Dec;29(4):1027–1039. doi: 10.1007/s11011-013-9469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Karababa A., Groos-Sahr K., Albrecht U., Keitel V., Shafigullina A., Görg B. Ammonia attenuates LPS-induced upregulation of pro-inflammatory cytokine mRNA in Co-cultured astrocytes and microglia. Neurochem Res. 2017 Mar;42(3):737–749. doi: 10.1007/s11064-016-2060-4. [DOI] [PubMed] [Google Scholar]
- 122.Hsu S.-J., Zhang C., Jeong J., Lee S., McConnell M., Utsumi T. Enhanced meningeal lymphatic drainage ameliorates neuroinflammation and hepatic encephalopathy in cirrhotic rats. Gastroenterology. 2021 Mar;160(4) doi: 10.1053/j.gastro.2020.11.036. 1315-1329.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rangroo Thrane V., Thrane A.S., Chanag J., Alleluia V., Nagelhus E.A., Nedergaard M. Real-time analysis of microglial activation and motility in hepatic and hyperammonemic encephalopathy. Neuroscience. 2012 Sep;220:247–255. doi: 10.1016/j.neuroscience.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McMillin M., Frampton G., Grant S., Khan S., Diocares J., Petrescu A. Bile acid-mediated sphingosine-1-phosphate receptor 2 signaling promotes neuroinflammation during hepatic encephalopathy in mice. Front Cell Neurosci. 2017 Jul 5;11:191. doi: 10.3389/fncel.2017.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McMillin M., Frampton G., Thompson M., Galindo C., Standeford H., Whittington E. Neuronal CCL2 is upregulated during hepatic encephalopathy and contributes to microglia activation and neurological decline. J Neuroinflam. 2014;11(1):121. doi: 10.1186/1742-2094-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McMillin M., Frampton G., Tobin R., Dusio G., Smith J., Shin H. TGR5 signaling reduces neuroinflammation during hepatic encephalopathy. J Neurochem. 2015 Nov;135(3):565–576. doi: 10.1111/jnc.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McMillin M., Grant S., Frampton G., Andry S., Brown A., DeMorrow S. Fractalkine suppression during hepatic encephalopathy promotes neuroinflammation in mice. J Neuroinflam. 2016 Dec;13(1):198. doi: 10.1186/s12974-016-0674-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jiang W., Desjardins P., Butterworth R.F. Cerebral inflammation contributes to encephalopathy and brain edema in acute liver failure: protective effect of minocycline. J Neurochem. 2009 Apr;109(2):485–493. doi: 10.1111/j.1471-4159.2009.05981.x. [DOI] [PubMed] [Google Scholar]
- 129.Chastre A., Bélanger M., Beauchesne E., Nguyen B.N., Desjardins P., Butterworth R.F. Inflammatory cascades driven by tumor necrosis factor-alpha play a major role in the progression of acute liver failure and its neurological complications. PloS One. 2012 Nov 15;7(11) doi: 10.1371/journal.pone.0049670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang L., Tan J., Jiang X., Qian W., Yang T., Sun X. Neuron-derived CCL2 contributes to microglia activation and neurological decline in hepatic encephalopathy. Biol Res. 2017 Dec;50(1):26. doi: 10.1186/s40659-017-0130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cauli O., Rodrigo R., Piedrafita B., Boix J., Felipo V. Inflammation and hepatic encephalopathy: ibuprofen restores learning ability in rats with portacaval shunts. Hepatology. 2007;46(2):514–519. doi: 10.1002/hep.21734. [DOI] [PubMed] [Google Scholar]
- 132.Agusti A., Hernández-Rabaza V., Balzano T., Taoro-Gonzalez L., Ibañez-Grau A., Cabrera-Pastor A. Sildenafil reduces neuroinflammation in cerebellum, restores GABAergic tone, and improves motor in-coordination in rats with hepatic encephalopathy. CNS Neurosci Ther. 2017 May;23(5):386–394. doi: 10.1111/cns.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dadsetan S., Balzano T., Forteza J., Agusti A., Cabrera-Pastor A., Taoro-Gonzalez L. Infliximab reduces peripheral inflammation, neuroinflammation, and extracellular GABA in the cerebellum and improves learning and motor coordination in rats with hepatic encephalopathy. J Neuroinflammation. 2016 Sep 13;13(1):245. doi: 10.1186/s12974-016-0710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wright G.A.K., Sharifi Y., Newman T.A., Davies N., Vairappan B., Perry H.V. Characterisation of temporal microglia and astrocyte immune responses in bile duct-ligated rat models of cirrhosis. Liver Int. 2014;34(8):1184–1191. doi: 10.1111/liv.12481. [DOI] [PubMed] [Google Scholar]
- 135.D’Mello C., Le T., Swain M.G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factor signaling during peripheral organ inflammation. J Neurosci. 2009 Feb 18;29(7):2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen J.-R., Wang B.-N., Tseng G.-F., Wang Y.-J., Huang Y.-S., Wang T.-J. Morphological changes of cortical pyramidal neurons in hepatic encephalopathy. BMC Neurosci. 2014 Dec;15(1):15. doi: 10.1186/1471-2202-15-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rodrigo R., Cauli O., Gomez–Pinedo U., Agusti A., Hernandez–Rabaza V., Garcia–Verdugo J. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology. 2010 Aug;139(2):675–684. doi: 10.1053/j.gastro.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 138.D’Mello C., Almishri W., Liu H., Swain M.G. Interactions between platelets and inflammatory monocytes affect sickness behavior in mice with liver inflammation. Gastroenterology. 2017 Nov;153(5):1416–1428.e2. doi: 10.1053/j.gastro.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 139.Balzano T., Arenas Y.M., Dadsetan S., Forteza J., Gil-Perotin S., Cubas-Nuñez L. Sustained hyperammonemia induces TNF-a IN Purkinje neurons by activating the TNFR1-NF-κB pathway. J Neuroinflam. 2020 Dec;17(1):70. doi: 10.1186/s12974-020-01746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Taoro-González L., Cabrera-Pastor A., Sancho-Alonso M., Arenas Y.M., Meseguer-Estornell F., Balzano T. Differential role of interleukin-1β in neuroinflammation-induced impairment of spatial and nonspatial memory in hyperammonemic rats. FASEB J. 2019 Sep;33(9):9913–9928. doi: 10.1096/fj.201900230RR. [DOI] [PubMed] [Google Scholar]
- 141.Hoogland I.C.M., Houbolt C., van Westerloo D.J., van Gool W.A., van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflam. 2015 Dec;12(1):114. doi: 10.1186/s12974-015-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Balzano T., Dadsetan S., Forteza J., Cabrera-Pastor A., Taoro-Gonzalez L., Malaguarnera M. Chronic hyperammonemia induces peripheral inflammation that leads to cognitive impairment in rats: reversed by anti-TNF-a treatment. J Hepatol. 2020;73:12. doi: 10.1016/j.jhep.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 143.Bémeur C., Qu H., Desjardins P., Butterworth R.F. IL-1 or TNF receptor gene deletion delays onset of encephalopathy and attenuates brain edema in experimental acute liver failure. Neurochem Int. 2010 Jan;56(2):213–215. doi: 10.1016/j.neuint.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 144.McMillin M., Grant S., Frampton G., Petrescu A.D., Williams E., Jefferson B. Elevated circulating TGFβ1 during acute liver failure activates TGFβR2 on cortical neurons and exacerbates neuroinflammation and hepatic encephalopathy in mice. J Neuroinflam. 2019 Dec;16(1):69. doi: 10.1186/s12974-019-1455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xie G., Wang X., Jiang R., Zhao A., Yan J., Zheng X. Dysregulated bile acid signaling contributes to the neurological impairment in murine models of acute and chronic liver failure. EBioMedicine. 2018 Nov;37:294–306. doi: 10.1016/j.ebiom.2018.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Horvatits T., Drolz A., Roedl K., Rutter K., Ferlitsch A., Fauler G. Serum bile acids as marker for acute decompensation and acute-on-chronic liver failure in patients with non-cholestatic cirrhosis. Liver Int. 2017 Feb;37(2):224–231. doi: 10.1111/liv.13201. [DOI] [PubMed] [Google Scholar]
- 147.Agusti A., Cauli O., Rodrigo R., Llansola M., Hernandez-Rabaza V., Felipo V. p38 MAP kinase is a therapeutic target for hepatic encephalopathy in rats with portacaval shunts. Gut. 2011;60:1572–1579. doi: 10.1136/gut.2010.236083. [DOI] [PubMed] [Google Scholar]
- 148.Agusti A., Dziedzic J.L., Hernandez-Rabaza V., Guilarte T.R., Felipo V. Rats with minimal hepatic encephalopathy due to portacaval shunt show differential increase of translocator protein (18 kDa) binding in different brain areas, which is not affected by chronic MAP-kinase p38 inhibition. Metab Brain Dis. 2014 Dec;29(4):955–963. doi: 10.1007/s11011-013-9461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Engelmann C., Clària J., Szabo G., Bosch J., Bernardi M. Pathophysiology of decompensated cirrhosis: portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol. 2021 Jul;75:S49–S66. doi: 10.1016/j.jhep.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Demorrow S., Cudalbu C., Davies N., Jayakumar A., Rose C.F. 2021 ISHEN guidelines on animal models of hepatic encephalopathy. Liver Int. 2021 Jul;41(7):1474–1488. doi: 10.1111/liv.14911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.