Highlights
-
•
Ketamine modulates cerebellar connectivity during response inhibition in depression.
-
•
Cerebellar–frontoparietal/sensory connectivity decreases in ketamine remitters.
-
•
Cerebellar-frontoparietal/salience connectivity predicts treatment outcome.
-
•
Cerebro-cerebellar loops serve as treatment biomarkers in major depression.
Keywords: Ketamine, Cerebellum, Large-scale networks, Response-inhibition, PPI
Abstract
Patients with major depressive disorder (MDD) exhibit impaired control of cognitive and emotional systems, including deficient response selection and inhibition. Though these deficits are typically attributed to abnormal communication between macro-scale cortical networks, altered communication with the cerebellum also plays an important role. Yet, how the circuitry between the cerebellum and large-scale functional networks impact treatment outcome in MDD is not understood. We thus examined how ketamine, which elicits rapid therapeutic effects in MDD, modulates cerebro-cerebellar circuitry during response-inhibition using a functional imaging NoGo/Go task in MDD patients (N = 46, mean age: 39.2, 38.1% female) receiving four ketamine infusions, and healthy controls (N = 32, mean age:35.2, 71.4% female). We fitted psychophysiological-interaction (PPI) models for a functionally-derived cerebellar-seed and extracted average PPI in three target functional networks, frontoparietal (FPN), sensory-motor (SMN) and salience (SN) networks. Time and remission status were then evaluated for each of the networks and their network-nodes. Follow-up tests examined whether PPI-connectivity differed between patient remitter/non-remitters and controls. Results showed significant decreases in PPI-connectivity after ketamine between the cerebellum and FPN (p < 0.001) and SMN networks (p = 0.008) in remitters only (N = 20). However, ketamine-related changes in PPI-connectivity between the cerebellum and the SN (p = 0.003) did not vary with remitter status. Cerebellar-FPN, -SN PPI values at baseline were also associated with treatment outcome. Using novel methodology to quantify the functional coupling of cerebro-cerebellar circuitry during response-inhibition, our findings highlight that these loops play distinct roles in treatment response and could potentially serve as novel biomarkers for fast-acting antidepressant therapies in MDD.
1. Introduction
Major depressive disorder (MDD) is one of the most common neuropsychiatric disorders and a leading cause of disability world-wide (WHO, 2019). The symptoms of MDD span multiple domains including emotional and mood disturbances, as well as psychomotor and cognitive impairments (WHO, 2019, Keller et al., 2019). Among these impairments, much evidence supports that disturbances in cognitive control, including difficulties in adjusting or inhibiting behavior, contribute to the pathophysiology of depression (Snyder, 2013, Rock et al., 2014). Specifically, response inhibition may play a role in the top-down control or regulation of emotion, which when compromised, could contribute to depressive symptoms (Langenecker et al., 2007, Park et al., 2019, Disner et al., 2011). Disruptions in goal directed attention and inhibitory control may lead to difficulties in day-to-day social and occupational function (Keller et al., 2019, Bennabi et al., 2013) and suicide risk in MDD (Moniz et al., 2017) and thus present important behavioral targets for intervention. However, the brain mechanisms and circuitry associated with response inhibition in MDD are incompletely understood and require further investigation.
Recent literature suggests that the cognitive and attentional deficits observed in MDD are not attributable to regionally localized brain abnormalities, but instead involve abnormal communication between large scale networks (LSNs) across spatially distributed brain regions or network nodes (Keller et al., 2019, Hamilton, 1960). Previous studies have emphasized disrupted communication in MDD between three sets of LSNs that include task-positive networks, such as the fronto-parietal network (FPN); the default mode network (DMN), involved in self-referential processes and internal attention; and the salience network (SN), which is involved in attention to salient stimuli and in the switching between DMN and FPN, during rest and functional tasks (Menon, 2011, Kaiser et al., 2015). Functional connectivity disruptions within DMN and between the dorsal-attention (DAN), sensory-motor (SMN) and visual (VN) networks have also been reported (Yan et al., 2019, Korgaonkar et al., 2019). Additionally, recent work suggests changes in connectivity within and between LSNs associate with remission to standard antidepressant therapy irrespective of type (Korgaonkar et al., 2019). However, it remains unclear how these networks are disrupted in MDD to impact particular domains of function such as inhibitory control and change with respect to treatment outcome (Yan et al., 2019).
To date, the main focus of neuroimaging research in MDD has been directed towards the cerebrum and the interactions amongst primary cortical hubs within the forebrain. Notably, it is now known that the cerebellum also plays an important role in emotion, cognitive control and executive function (Schmahmann, 2019, Noroozian, 2014). In particular, prior studies support the cerebellum’s involvement in the connectivity of most LSNs (Habas et al., 2009, Buckner et al., 2011, Ji et al., 2019) and reveal structural and functional (Guo et al., 2013, Liu et al., 2012) disruptions in cortico-cerebellar and cerebello-thalamo-cortico loops in patients with MDD (Lupo et al., 2019). Prefrontal-cerebellar loops have also been identified as key circuitry in motor learning and executive function, where the cerebellum acts to modulate the frontal cortex, regulating the start or end of actions by providing sensory feedback through cerebellar ‘internal models’, which are adjusted as a movement is repeated (Ito, 2008, Diedrichsen et al., 2019, Miquel et al., 2019). Further, the cerebellum’s contribution to response-inhibition (Stoodley et al., 2012, Mottolese et al., 2013, Kilteni and Ehrsson, 2020, Wynn et al., 2019) is emphasized by poor performance of patients with cerebellar impairments on Go/NoGo tasks (Stoodley et al., 2012) and is hypothesized to play an important role in post-error processing in communication with the prefrontal and anterior cingulate cortex via the thalamus (Lupo et al., 2019, Koziol et al., 2012, Gyurak et al., 2016). When disrupted, these circuitries are also found to be associated with cognitive and motor disturbances frequently observed in MDD (Bennabi et al., 2013, Sweeney et al., 1998). This research implicates the contribution of cerebro-cerebellar circuitries in MDD pathophysiology and the need to further investigate these circuitries and their role in executive control processes in depression and its treatment.
Ketamine is a non-competitive N-methyl-D-aspartate receptor (NMDAR) antagonist known to elicit fast-acting antidepressant effects in patients with MDD unresponsive to standard treatments (i.e., defined as having treatment resistant depression - TRD) (Iadarola et al., 2015, Zanos and Gould, 2018). Recently, ketamine therapy in MDD is also demonstrated to affect the brain at the systems level by disrupting the interaction between multiple networks during rest (Evans et al., 2018, Fleming et al., 2019) and task fMRI (Anticevic et al., 2012, Reed et al., 2018, Scheidegger et al., 2016, Sahib et al., 2020, Loureiro et al., 2020). For example, we have shown significant decreases in activation in the inhibitory control network and right cerebellum during a Go/NoGo task following ketamine treatment where regional changes in activation associated with clinical remission (Sahib et al., 2020). Notably, other imaging studies using ketamine in MDD have also reported blood-oxygenation-level-dependent (BOLD) changes in the cerebellum, as well as connectivity changes between the cerebellum and cortical networks (Barch et al., 2013, Downey et al., 2016). However, there are no studies specifically targeting ketamine’s modulation of cerebro-cerebellar systems. In the current investigation we thus sought to target the perturbation of cerebro-cerebellar circuitry using the Go/NoGo task previously shown to elicit treatment-related effects in response-inhibition networks in patients with TRD receiving serial ketamine (Sahib et al., 2020). Specifically, changes in target cortico-cerebellar networks were achieved by computing psychophysiological interaction (PPI) scores between a cerebellum-seed located in lobuleVIIb, involved in response-inhibition processes, and three main LSNs of the brain (FPN, SN and SMN) with respective nodes. This study had two primary goals: (1) to investigate how single and serial ketamine infusions modulate cerebro-cerebellar networks, and how these circuitries relate to treatment remission, and; (2) to examine whether significant ketamine-related PPI effects for cortico-cerebellar networks and network-nodes are associated with secondary clinical outcomes such as anxiety and anhedonia. Based on previous findings, we hypothesized that PPI connectivity between cerebellar seed and the SMN and FPN; and between SMN cerebellar seeds and DMN and FPN will be disrupted in TRD patients prior to treatment (Yan et al., 2019, Ionescu et al., 2018) and may distinguish treatment remitters from non-remitters (Rush et al., 2003).
2. Methods and materials
2.1. Participants and study design
This study included 46 MDD patients defined as TRD (i.e., failed ≥ 2 adequate antidepressant trials of adequate dose and duration, and had been continuously depressed for ≥ 6 months) and 32 healthy controls (HC). Patients eligible for ketamine treatment were recruited from clinician referral, targeted advertisements or clinicaltrials.gov (NCT02165449). Demographically similar HCs were recruited using advertisements from the same geographical area. The study cohort included subjects overlapping with those participating in a previous functional imaging study of response inhibition (Sahib et al., 2020). Patients had moderate to severe depressive symptoms prior to treatment as per the Hamilton Depression Rating Scale (HDRS), 17-item (Hamilton, 1960) (scores ≥ 17) [Table1] and were permitted to remain on approved monoaminergic antidepressant therapy, if unchanged in the preceding 6-weeks, for the study’s duration. Benzodiazepines were discontinued > 72 h prior to all study visits (e.g. scan sessions, ketamine infusion session). Exclusion criteria for all participants included any serious or unstable medical condition, currently or within the preceding 3-months, substance abuse or dependence (ascertained by laboratory testing), current or past history of psychosis, schizoaffective disorder or schizophrenia, developmental disorders, diagnosis of dementia of any type, and any contraindication to scanning.
Table 1.
Demographics and behavioral and clinical values by group and time point.
 |
Abbreviations: HC: healthy controls; MDD: major depressive disorder; std: standard deviation; T1: baseline; T2: 24 h after the first ketamine infusion; T3: 24 h after the fourth ketamine infusion; HDRS: Hamilton depressive rating scale; QIDS: quick inventory depressive scale; DASS: anxiety scale; N/A: not applicable.
Each patient received four serial intravenous infusions of racemic ketamine (Mylan Institutional, LLC). For each session a single sub-anesthetic dose (0.5 mg/kg) of ketamine diluted in 60 ml normal saline was delivered intravenously via pump, 2–3 times a week.
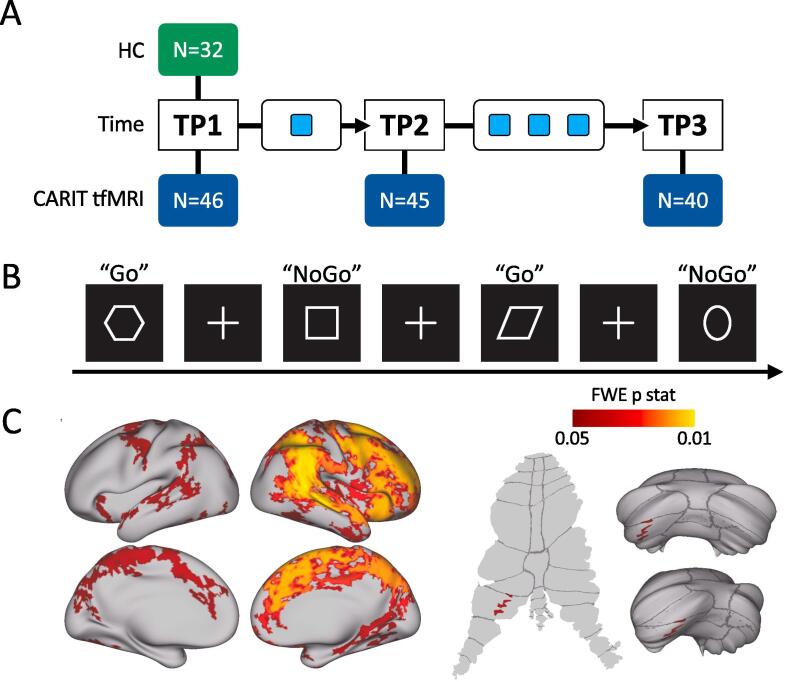
MRI scanning and clinical and behavioral data were acquired at three different timepoints: 1) pre-treatment baseline (T1), which occurred < 1 week before the first ketamine infusion; 2) 24 h after the first ketamine injection (T2) and; 3) 24 or 72 h after the last ketamine infusion (T3) [Fig. 1A]. The 72-hour follow-up at T3 occurred in 5 patients who received their final treatment on a Friday and their follow-up assessment on Monday. All subjects provided written informed consent following procedures approved by the UCLA Institutional Review Board (IRB).
Fig. 1.
A) Study design illustrating the three timepoints where MRI and behavioral data were acquired: TP1 refers to the first timepoint (baseline - before ketamine infusions), TP2 refers to the second timepoint (24 h after the first ketamine infusion), TP3 refers to the third timepoint (24 h after the fourth ketamine infusion). Light blue squares indicate ketamine infusions; B) Schematic of the task-fMRI block design; C) Zstat maps, for the cortical surface (top left), subcortical regions (bottom left), and cerebellum surface (right) obtained from the one sample t-test performed across HCs and MDD at TP1 for the NoGo > Go contrast, TFCE and FEW corrected p < 0.05. Upper right cerebellar surface is a posterior view (dorsal is up), and lower right cerebellar surface is a ventral view (posterior is up). Dorsal is up in the flattened cerebellar surface at left. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.2. Clinical data
At each time point, depression severity was assessed using the HDRS (Hamilton, 1960) as well as the Quick Inventory of Depressive Symptomatology (QIDS) (Lovibond, 1995), which provided self-assessments. Anxiety was measured with the Depression Anxiety Stress Scale (DASS) (Winter and Sheridan, 2014) [see Table 1]. Remitters were defined as patients with a HDRS score of ≤ 7 at T3.
2.3. MR methods
2.3.1. Go/NoGo functional imaging task
The Conditioned Approach Response Inhibition (CARIT) Task was adapted from (Harms et al., 2018), is used in the Human Connectome Project (HCP) Lifespan studies for Aging (Bookheimer et al., 2019, Andersson et al., 2003) as well as in a previous study by our group (Sahib et al., 2020). The task consisted of a block paradigm of geometrical shapes interleaved with resting periods marked with a fixation cross. Subjects were instructed to press a button with their right index finger each time they saw a shape (“GO”), but were instructed to withhold the response for circles and squares (“NO-GO”) [Fig. 1B]. Stimulus duration was 600 ms with 200 ms of fixation. Button presses were attributed to a given trial if they occurred within 800 ms of stimulus onset. No-Go and Go accuracy and reaction time were computed. For these three task-measures a general linear mixed model (GLMM) and 2-sample t-tests evaluated effects of time and time-by-remission and differences between HC, and MDD patients (remitters and non-remitters) at T1, respectively.
2.3.2. Image acquisition and preprocessing
Imaging of all subjects was performed on a Siemens 3 T Prisma MRI system at UCLA’s Brain Mapping Center using a 32-channel head coil. Image acquisition sequences were identical to the HCP Lifespan studies (https://www.humanconnectome.org). Here, structural sequences included a T1-weighted, and a T2-weighted acquisition, both with real-time motion correction. Functional scans included: one run of a Go/NoGo task-fMRI using a multi-band EPI sequence (MB accl. factor = 8, acquisition time = 6:15 min), and two sets of spin echo images that were used for distortion correction of the functional images using FSL’s topup (Glasser et al., 2013).
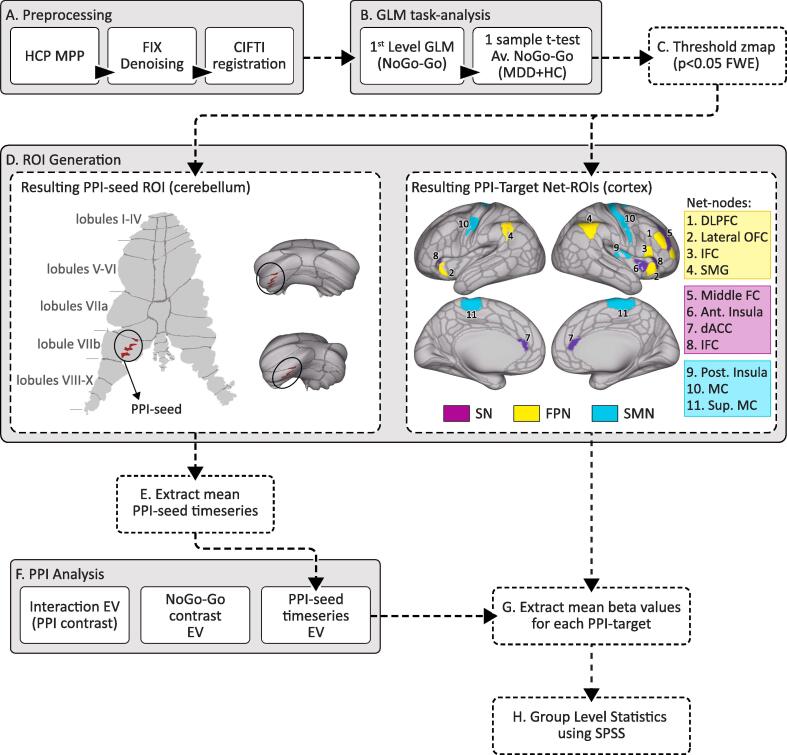
FMRI task data was preprocessed using the HCP minimal processing pipelines (Gorgolewski et al., 2017) implemented within the BIDS-App (Marcus et al., 2013) as previously described elsewhere (Loureiro et al., 2020) [Fig. 2A). After preprocessing, the functional images were further denoised using FSL’s FIX (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIX). Smoothing of 5 mm was applied to the preprocessed images using the grayordinates based approach (HCP). The quality of the functional images was assessed using relative and absolute motion plots. Subjects that moved > 3 mm and/or images that exhibited artifacts after FIX were excluded. One subject at T1 and three subjects at T2 (n = 4), were excluded due to motion artifacts (9). For visualization we used the Connectome Workbench platform (Woolrich et al., 2001).
Fig. 2.
Flow chart of the preprocessing and post-processing pipeline. A) Preprocessing pipeline: after processing the data through the Human Connectome Project (HCP) minimal preprocessing pipeline (MPP), data was denoised using FSL’s FIX and registered to MNI using CIFTI format; B) Task-fMRI analysis (NoGo > Go contrast): 1) general linear models (GLM) were fit for each subject at TP1 for HCs and MDD patients (first level analysis); 2) these maps were used for a one sample t-test; C) The resulting average map was TFCE corrected and thresholded and binarized at p < 0.05 FWE to be used as a mask for further analysis. D) ROI generation for the psychophysiological interaction (PPI) analysis: The thresholded map obtained from (C) (mapped in the cerebrum and cerebellar surfaces) was then overlaid with a large scale network (LSN) atlas (obtained from (Ji et al., 2019). The resulting overlaid map of the cerebellum was used to generate the cerebellar PPI-seed ROI (left) and the resulting overlaid map of the cerebrum was used to generate PPI-target ROIs (right). E) Timeseries were extracted from PPI-seed ROI (described in D). F) GLM-PPI analysis for the for each subject at each timepoint. Three explanatory variables (EVs) are required for the PPI analysis: 1) Extracted timeseries from the PPI-seed; 2) the NoGo > Go contrast text file and 3) the PPI interaction (between the NoGo > Go contrast and the extracted timeseries from the PPI-seed). G) Target-PPI masks generated from (C) were used to extract mean PPI beta values from the maps generated in (F). H) Extracted mean values were fed into SPSS for group-level analysis: general linear mixed models (GLMMs) evaluated time, remission and network-node effects of the PPI-connectivity changes between the PPI-seed and each of the main LSNs.
2.3.3. Average BOLD activation for NoGo > Go
To restrict PPI analysis brain regions involved in response-inhibition, we computed an average map for the NoGo > Go contrast (Sahib et al., 2020) for all the subjects at baseline (T1). The average activation map was thresholded after using threshold-free cluster enhancement (TFC) at p < 0.05, family-wise error (FWE) corrected for multiple comparisons [Fig. 1C]. The resulting p-map was then used to select PPI-seed and target ROIs, in the cerebellum and cortex respectively, for subsequent PPI analysis [Fig. 2].
2.3.4. PPI-Seed and -target ROIs generation
The cerebellum PPI-seed and target ROIs were generated by overlapping the NoGo > Go average activation map with an LSN atlas (Ji et al., 2019). We targeted three LSNs that are known to be involved in response-inhibition processes and also known to be disrupted in MDD (including the FPN, SN and SMN). A significant cluster in the cerebellum in lobule-VIIb in a dorsal-attention part of the cerebellum was defined as the PPI-seed, whereas the regions overlapping regions in the cortex were defined as PPI-target ROIs [Fig. 2D]. For each of the three networks we selected 3–4 target ROIs. The FPN target-nodes included the lateral orbitofrontal cortex (OFC) the inferior frontal cortex (IFC), the supramarginal gyrus (SMG) and dorsolateral prefrontal cortex (DLPFC); the SN target-ROIs included the anterior insula, the dorsal anterior cingulate (dACC) and the middle frontal cortex (mFC); the SMN target-ROIs included the posterior insula, the precentral and the superior paracentral [Fig. 2D].
2.3.5. PPI-GLM analysis
To evaluate connectivity changes between the cerebellum and intrinsic networks during the NoGo > Go condition, we conducted a PPI analysis using the cerebellum-seed generated from the average NoGo-Go activation using FSL FEAT (IBM SPSS, 2019). This analysis generated one zstat map per subject and timepoint (T1, T2, and T3). The design matrix included seven explanatory variables (EVs): EV1) NoGo; EV2) Go; EV3) NoGo error; EV4) Go error; EV5) the contrast of interest (NoGo > Go); EV6) the average time-course of the PPI-seed ROI and; EV7) the interaction term between the PPI contrast and the PPI-seed ROI time-course. The interaction term (EV7) is defined as the scalar product of the task contrast time course (EV5) and the average time course of the PPI-seed ROI (EV6) (defined through FEAT by selecting the “interaction” option on the “Basic shape” section on the EV tab: https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/PPIHowToRun). The first five EVs are binary and represent the block design for each condition and for the contrast of interest (NoGo > GO), and the last two EVs are continuous. To evaluate the change in connectivity between the cerebellum PPI-seeds and cortical networks and nodes, beta values obtained for the PPI-interaction contrast were extracted and averaged from target LSNs and node ROIs (for significant networks only) for each subject and timepoint.
2.3.6. PPI group analysis
Group level analyses were conducted using SPSS software (Stoodley and Schmahmann, 2009) and designed to test two primary hypotheses. First, we tested ketamine’s modulation of PPI-connectivity between the cerebellum and LSNs. Specifically, we computed general linear mixed models (GLMMs), using time and node ROIs as within-subject measures for each LSN examined separately. PPI betas extracted from the target ROIs served as the dependent variables, and time and remission as fixed effects. We were interested in main effects of time, time-by-remission, time-by-node and time-by-remission-by-node interactions. Follow-up analyses of significant interactions effects were performed as appropriate. For the GLMMs, p < 0.017 was used as the threshold of significance (Bonferroni corrected for the three LSNs). Secondly, we evaluated clinical associations with PPI-connectivity. That is, if significant main effects observed in the omnibus GLMMs described above we investigated: a) whether PPI values differed between remitters, non-remitters and HCs at baseline (T1) using GLMs; and b) whether PPI changes, or PPI baseline values are associated with clinical outcome in patients, including the QIDS, HDRS and anxiety (DASS) using Pearson’s correlations. For all cross-sectional comparisons between independent groups (patient remitters, non-remitters and controls), age and sex were included as covariates.
3. Results
3.1. Demographic and clinical results
Demographics and clinical variables for the HCs and MDD patients are presented in Table 1. Two sample t-tests considering HCs and MDD patients at baseline (T1), revealed no significant differences for age (t = −1.42, p = 0.16), and education (t = 0.72, p = 0.47) and a trending difference for sex (χ2 = 3.845, p = 0.061). GLMMs revealed a significant effect of time for all 4 clinical scales: HDRS (F(2) = 83.09, p < 0.001); QIDS (F(2) = 85.49, p < 0.001); DASS (F(2) = 29.01, p < 0.001) and SHAPS (F(2) = 31.39, p < 0.001). Two sample t-tests and a chi-square test comparing remitters and non-remitters at T1 revealed no significant differences for age (t = −0.81, p = 0.42), education (t = −1.03, p = 0.30), HDRS (t = 0.45, p = 0.48) and sex (χ2 = 0.538, p = 0.531).
3.2. Go/NoGo functional imaging task measures
Go accuracy (t = −1.036, p = 0.304), NoGo accuracy (t = −0.123, p = 0.901) and reaction time (t = 1.620, p = 0.110) did not significantly differ at baseline (T1) between patients and HCs. GLMMs revealed a main effect of time for NoGo accuracy (F(2) = 7.255, p = 0.001) where both remitters and non-remitters showed an increase in NoGo accuracy after single and serial ketamine infusions. However, control subjects (n = 17) who were scanned twice 2–4 weeks apart, did not show an increase in NoGo accuracy over time, arguing against possible practice effects. There were no significant main effects or interactions for Go accuracy and reaction time, and therefore these measures were not used as covariates in subsequent models.
3.3. Average BOLD activation for NoGo > Go
The average activation map for the NoGo > Go contrast revealed mostly right hemisphere increases in BOLD (positive z-scores) in the prefrontal cortex (PFC), supplementary motor area (SMA), anterior insula (AI), superior frontal cortex (SFC), anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), precuneus, and in the left cerebellum-lobuleVIIb [Fig. 1C].
3.4. Ketamine modulation of PPI between cerebellum and LSNs
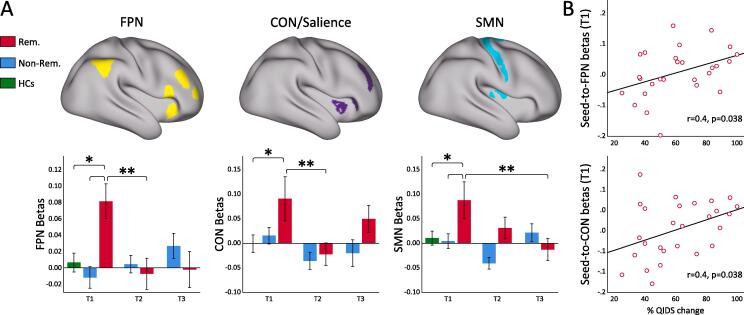
GLMMs revealed a significant time-by-remission effect for PPI changes between the cerebellum and the FPN (F(2,765.59) = 10.83, p < 0.001), and the SMN (F(2,769.48) = 4.86, p = 0.008), where only remitters showed a significant decrease in PPI after ketamine in follow-up analysis of simple effects as plotted in Fig. 3. GLMMs also revealed a main effect of time for PPI between the cerebellum and the SN (F(2,656.74) = 5.89, p = 0.003), though there was no significant interaction with remitter status [Fig. 3]. Within each LSN, there were no significant interactions between time or remitter status for the node ROIs. Finally, at baseline (T1) the connectivity for the remitters across the three networks was significantly different than HCs as also shown in Fig. 3.
Fig. 3.
Significant PPI-connectivity changes with ketamine. A) PPI-seed; B) PPI between PPI-seed (left lobule VIIb) and frontoparietal (FPN) (in the left); the salience (SN) (middle) and somatomotor network (SMN).
3.5. Clinical correlates of PPI changes
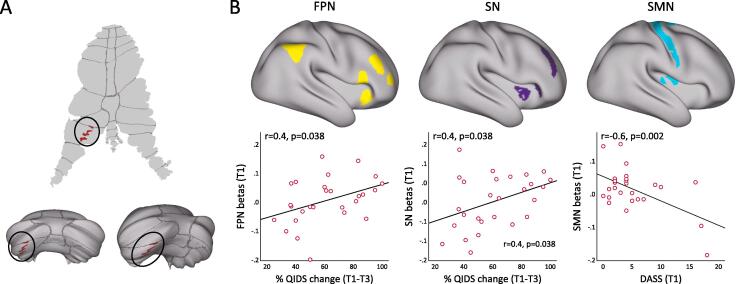
Baseline (T1) PPI values between the cerebellum and FPN (Pearson’s r = 0.41, p = 0.036); and between the cerebellum and the SN (r = 0.45, p = 0.019) significantly correlated with %QIDS change across treatment [Fig. 4]. At baseline (T1) PPI-connectivity between the cerebellum and the SMN was associated with anxiety severity measured by DASS (r = −0.558, p = 0.002). There were no associations between change in PPI values for any of the networks with change in clinical outcome measures.
Fig. 4.
Correlations with clinical measures. A) PPI-seeds; B) Correlation of PPI at baseline (T1) between cerebellum and frontoparietal (FPN) with QIDS improvements (left); correlation of PPI at baseline (T1) between cerebellum and salience (SN) with QIDS improvements (middle) and; correlation of PPI at baseline (T1) between cerebellum and somatomotor (SMN) with anxiety (DASS) at baseline (T1) (right).
4. Discussion
The primary findings from this study indicate that low-dose ketamine infusion therapy, a rapidly acting treatment for MDD, served to decrease the connectivity between the posterior cerebellum and both the FPN and SMN in patient remitters, but not non-remitters. However, the connectivity between the cerebellum and the SN decreased for both remitters and non-remitters, though there was a greater mean decrease for the remitters in comparison to non-remitters after both single and serial infusion. Additionally, our results showed that cerebellar-FPN and cerebellar-SN connectivity at baseline (T1) was associated with subjective clinical outcome as measured by the QIDs, suggesting cortico-cerebellar connections during response inhibition are relevant to successful treatment response and may have utility as biomarkers for successful ketamine response.
4.1. Ketamine and the cerebellum
The cerebellum plays a key role in cognition and executive control (Diedrichsen et al., 2019, Koziol et al., 2012, Hirose et al., 2014). In particular, cerebro-cerebellar loops have been associated with response-inhibition processes, and improvements in inhibitory performance has been associated with functional changes in this circuitry (Wynn et al., 2019, Villanueva, 2012). The interplay between LSNs and the cerebellum at rest or during task fMRI are shown to be impaired in several psychiatric disorders, and in MDD specifically (Miquel et al., 2019, Depping et al., 2018). Studies have also reported that the cerebellum and cortico-cerebellar circuitries are modulated by both standard antidepressant medications (Guo et al., 2013, Lupo et al., 2019, Tozzi et al., 2020, Khalili-Mahani et al., 2015) and single ketamine infusion, which is found to disrupt the connectivity within LSNs and cortico-cerebellar loops in HCs and in MDD (Reed et al., 2018, Rush et al., 2003, Woelfer et al., 2019). Further, increased levels of plasma brain-derived neurotrophic factor (BDNF) after single ketamine infusion have been linked with functional connectivity decreases between the dmPFC and posterior cerebellum, which suggests that the antidepressant effects of ketamine may be related to neurotrophic processes in these circuitries (Behan et al., 2014). Despite these findings, the current investigation is the first to explicitly address how ketamine, which elicits robust and rapid antidepressant effects, modulates the connectivity of cerebellum with cortical LSNs in MDD. As relevant for understanding ketamine’s therapeutic effects on functional systems, our results revealed that ketamine differentially affects the PPI-connectivity between distinct cortico-cerebellar pathways during a NoGo/Go response-inhibition task.
4.2. Ketamine modulation during NoGo-Go
Our analysis revealed a significant time-by-remission effect for PPI changes between the cerebellum and the FPN and SMN, where PPI decreased significantly only for remitters. Frontocerebellar circuitry is known to play a key role in executive function, and aberrant functional activity of these loops have been linked to increased impulsive behavior in various neuropsychiatric disorders including ADHD, bipolar and drug-abuse patients (Miquel et al., 2019, Jung et al., 2014, Alalade et al., 2011). Previous studies have also shown that disrupted connectivity between the posterior part of the cerebellum and components of the FPN and SMN is linked to the pathophysiology of MDD during rest and that these dysfunctions may contribute to the cognitive control and psychomotor retardation deficits encountered in depressed patients (Guo et al., 2013, Liu et al., 2012, Tozzi et al., 2020, Schrijvers et al., 2009). In particular, disruptions in the SMA were linked with higher levels of psychomotor retardation (Chen et al., 2018). Further, a network comprising of the lateral cerebellum, DLPFC, insula, SMA and M1, was identified and linked with the ability to switch between tasks, which facilitates making transitions in a constantly changing environment and is a core feature of executive function (Koziol et al., 2012). Even though FPN-cerebellar connectivity has been poorly studied in the context of treatment response in MDD, increased FPN connectivity during a NoGo/Go task has been associated with treatment response to the standard antidepressant sertraline (Khalili-Mahani et al., 2015). Concerning the effects of ketamine in these circuitries, ketamine is found to affect motor and cognitive functioning and to modulate somato-motor regions (Sahib et al., 2020, Rush et al., 2003, Muthukumaraswamy et al., 2015) and activity within the FPN (Sahib et al., 2020, Abdallah et al., 2017) as well as cerebellar and DLPFC global brain connectivity during rest (Rush et al., 2003, Clark et al., 2020) in line with our observations.
Our results also showed a significant main effect of time for PPI changes between the cerebellum and the SN, where PPI decreased for both remitters and non-remitters (with a greater decrease for remitters). Multiple studies have shown that the insula and regions of the salience network are involved in proactive control during response-inhibition processes, where it appears that these regions are closely related with attention and task-monitoring (Menon and Uddin, 2010). The salience network is also known to be responsible for facilitating attention to external stimuli and to mediate the anticorrelations between thee default mode network and task-positive networks (such as the FPN)(Evans et al., 2018). In MDD disruptions in the SN activity have been associated with both depression and somatic symptom severity (Avery et al., 2014, Geliebter et al., 2016) and connectivity disruptions between the FPN, SN and default mode network are also commonly reported in MDD patients, in agreement with the triple network dysfunction model (Menon, 2011). Further, heightened cerebellar-SN connectivity in binge-eaters was previously associated with the integration of conflict processing from external cues and motor learning and directed attention which has a direct effect in response-inhibition processes (Moreno-Rius and Miquel, 2017, Nugent et al., 2020). A decrease in the connectivity between the cerebellum and the SN after ketamine may thus be associated with alterations in the reward function and impulsive behavior, although these links were not explicitly examined here. In line with our results, reductions in the salience network connectivity after ketamine infusion have been observed in previous studies (Fleming et al., 2019, Adhikari et al., 2020, Kilts et al., 2006). However, further studies would be required to better understand this process as there are no studies to date, to our knowledge, evaluating the effects of ketamine in cerebellar-SN connectivity during response-inhibition processes. Finally, it is also worth noting that while some previous studies report decreased connectivity of cerebro-cerebellar networks in MDD during rest or other contexts (Tozzi et al., 2020), here we observed changes that occurred in the direction of normal controls, suggesting decreased connectivity between cerebro-cerebellar networks during response inhibition signifies a normalization of function.
4.3. Clinical and behavioral associations with PPI-connectivity during NoGo > Go
Our results revealed that PPI connectivity between the cerebellum and the FPN at baseline (T1), was associated with changes in QIDS after serial ketamine infusion. Additionally, remitters exhibited increased cerebellar-FPN and cerebellar-SMN PPI values at baseline in comparison to non-remitters and HCs. Fronto-parietal and fronto-limbic-cerebellar connectivity measures within the FPN network during response inhibition tasks, have been identified as predictors of MDD response to standard antidepressants (Langenecker et al., 2007, Gyurak et al., 2016). Intrinsic connectivity between the SMN and task-positive networks is also shown to differentiate responders from non-responders, where the former presented increased connectivity at baseline (Korgaonkar et al., 2019). Even though not significant, remitters showed increased cerebellar-SN PPI-connectivity at baseline in comparison to non-remitters and these values were associated with clinical outcome as measured by the QIDS after serial ketamine infusion. Accordingly, previous studies have reported a differential change in connectivity between the salience and DMN for responders and non-responders to ketamine, where the connectivity increased for responders and decreased for non-responders (Avery et al., 2014). We did not detect any correlations with HDRS measures. Even though QIDS and HDRS are correlated, our results suggest that patient’s perception of symptom improvement may be more sensitive. In summary, our study suggests that the antidepressant effects of ketamine are differentially associated with cerebellar-cortical loops including cerebellar-SN and cerebellar-FPN PPI-connectivity during response inhibition at baseline, and that these circuitries can potentially be used as biomarkers for ketamine treatment response.
We also observed that reduced PPI-connectivity between the cerebellum and the SMN is associated with higher levels of anxiety symptoms at baseline. This result is in line with previous studies which showed disrupted activity of the postcentral gyrus (Li et al., 2016, Li et al., 2019) and its connectivity with the left cerebellum was associated with impairments in the state of anxiety (Fales et al., 2008). This result supports that cerebellar-SMN circuitry can be used to differentiate particular features of depression as may be further examined in future studies.
4.4. Limitations and future perspectives
This study has some limitations that bear consideration. Firstly, since this study was not designed as a randomized clinical trial, it is not possible to eliminate possible placebo effects. However, we emphasize that our main goal is to understand the mechanistic effects of ketamine at the level of higher sensory, motor, and cognitive systems. Secondly, in this study, patients and controls did not differ in performance during administration of the Go/NoGo fMRI task at baseline. As noted, the current investigation was powered to detect within-subject changes in neural activity related to ketamine treatment rather than cross-sectional differences between patients and controls. However, it is important to note that fMRI may better detect alterations functional circuitry even if in the absence of performance differences (Khalili-Mahani et al., 2015, Wagner et al., 2006). Further, despite its importance to cognitive and executive control, there is very limited literature concerning the role of different regions of the cerebellum in response-inhibition processes and their disturbances in MDD and how this circuitry relates to treatment outcome after ketamine. Future studies are thus needed to further investigate the effects of ketamine, and other rapidly acting treatments for MDD, with regard to the cerebellum and cerebellar-cerebro loops and their relation to clinical outcomes.
5. Conclusion
In summary, our findings suggest that ketamine modulates the connectivity between the cerebellum and large-scale cortical networks including the FPN, SMN and SN, which have previously been implicated in the pathophysiology of depression. Additionally, cerebellar-FPN and cerebellar-SN connectivity at baseline appear associated with clinical outcome after serial ketamine therapy. These findings support that ketamine may regulate higher order function through cerebellar-cortico loops, which are involved in a number of functions including executive control, emotion regulation, motor learning and impulsive behavior in MDD. This study generates new insights into the neural mechanisms associated with response inhibition processes in MDD and the antidepressant effects of single and serial ketamine therapy, where cortico-cerebellar circuitry at the systems level may be used as ketamine biomarkers.
CRediT authorship contribution statement
Joana R.A. Loureiro: Conceptualization, Visualization, Data curation, Methodology, Formal analysis, Software, Writing – original draft. Ashish K. Sahib: Methodology, Software, Writing - review & editing. Megha Vasavada: Visualization, Writing - review & editing. Amber Leaver: Investigation, Writing - review & editing. Antoni Kubicki: Methodology, Investigation. Benjamin Wade: Methodology, Writing - review & editing. Shantanu Joshi: Methodology, Writing - review & editing. Gerhard Hellemann: Methodology, Validation, Conceptualization. Eliza Congdon: Validation, Resources. Roger P. Woods: Methodology, Writing - review & editing. Randall Espinoza: Funding acquisition, Writing - review & editing. Katherine L. Narr: Funding acquisition, Supervision, Conceptualization, Resources, Writing - review & editing.
Acknowledgments
Acknowledgements
This work was supported by the National Institute of Mental Health of the National Institutes of Health (Grant Nos. MH110008 [to KLN and RE], and MH102743 [to KLN]). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
The authors have nothing to disclose. There are no conflicts of interest.
References
- Abdallah C.G., Averill L.A., Collins K.A., Geha P., Schwartz J., Averill C. Ketamine Treatment and Global Brain Connectivity in Major Depression. Neuropsychopharmacology. 2017;42(6):1210–1219. doi: 10.1038/npp.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari B.M., Dukart J., Hipp J.F., Forsyth A., McMillan R., Muthukumaraswamy S.D. Effects of ketamine and midazolam on resting state connectivity and comparison with ENIGMA connectivity deficit patterns in schizophrenia. Hum. Brain Mapp. 2020;41(3):767–778. doi: 10.1002/hbm.24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alalade E., Denny K., Potter G., Steffens D., Wang L. Altered cerebellar-cerebral functional connectivity in geriatric depression. PLoS ONE. 2011;6(5):e20035. doi: 10.1371/journal.pone.0020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L., Skare S., Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Anticevic A., Gancsos M., Murray J.D., Repovs G., Driesen N.R., Ennis D.J. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2012;109(41):16720–16725. doi: 10.1073/pnas.1208494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery J.A., Drevets W.C., Moseman S.E., Bodurka J., Barcalow J.C., Simmons W.K. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol. Psychiatry. 2014;76(3):258–266. doi: 10.1016/j.biopsych.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D.M., Burgess G.C., Harms M.P., Petersen S.E., Schlaggar B.L., Corbetta M. Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage. 2013;80:169–189. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan B., Connolly C.G., Datwani S., Doucet M., Ivanovic J., Morioka R. Response inhibition and elevated parietal-cerebellar correlations in chronic adolescent cannabis users. Neuropharmacology. 2014;84:131–137. doi: 10.1016/j.neuropharm.2013.05.027. [DOI] [PubMed] [Google Scholar]
- Bennabi D., Vandel P., Papaxanthis C., Pozzo T., Haffen E. Psychomotor retardation in depression: a systematic review of diagnostic, pathophysiologic, and therapeutic implications. Biomed Res. Int. 2013;2013:158746. doi: 10.1155/2013/158746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S.Y., Salat D.H., Terpstra M., Ances B.M., Barch D.M., Buckner R.L. The Lifespan Human Connectome Project in Aging: An overview. Neuroimage. 2019;185:335–348. doi: 10.1016/j.neuroimage.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Krienen F.M., Castellanos A., Diaz J.C., Yeo B.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.H., Li C.T., Lin W.C., Hong C.J., Tu P.C., Bai Y.M. Persistent antidepressant effect of low-dose ketamine and activation in the supplementary motor area and anterior cingulate cortex in treatment-resistant depression: A randomized control study. J. Affect. Disord. 2018;225:709–714. doi: 10.1016/j.jad.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Clark S.V., King T.Z., Turner J.A. Cerebellar Contributions to Proactive and Reactive Control in the Stop Signal Task: A Systematic Review and Meta-Analysis of Functional Magnetic Resonance Imaging Studies. Neuropsychol. Rev. 2020 doi: 10.1007/s11065-020-09432-w. [DOI] [PubMed] [Google Scholar]
- Depping M.S., Schmitgen M.M., Kubera K.M., Wolf R.C. Cerebellar Contributions to Major Depression. Front. Psychiatry. 2018;9:634. doi: 10.3389/fpsyt.2018.00634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J., King M., Hernandez-Castillo C., Sereno M., Ivry R.B. Universal Transform or Multiple Functionality? Understanding the Contribution of the Human Cerebellum across Task Domains. Neuron. 2019;102(5):918–928. doi: 10.1016/j.neuron.2019.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner S.G., Beevers C.G., Haigh E.A., Beck A.T. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 2011;12(8):467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Downey D., Dutta A., McKie S., Dawson G.R., Dourish C.T., Craig K. Comparing the actions of lanicemine and ketamine in depression: key role of the anterior cingulate. Eur. Neuropsychopharmacol. 2016;26(6):994–1003. doi: 10.1016/j.euroneuro.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Evans J.W., Szczepanik J., Brutsche N., Park L.T., Nugent A.C., Zarate C.A., Jr. Default Mode Connectivity in Major Depressive Disorder Measured Up to 10 Days After Ketamine Administration. Biol. Psychiatry. 2018 doi: 10.1016/j.biopsych.2018.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J.W., Szczepanik J., Brutsche N., Park L.T., Nugent A.C., Zarate C.A., Jr. Default Mode Connectivity in Major Depressive Disorder Measured Up to 10 Days After Ketamine Administration. Biol. Psychiatry. 2018;84(8):582–590. doi: 10.1016/j.biopsych.2018.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales C.L., Barch D.M., Rundle M.M., Mintun M.A., Snyder A.Z., Cohen J.D. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol. Psychiatry. 2008;63(4):377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming L.M., Javitt D.C., Carter C.S., Kantrowitz J.T., Girgis R.R., Kegeles L.S. A multicenter study of ketamine effects on functional connectivity: Large scale network relationships, hubs and symptom mechanisms. Neuroimage Clin. 2019;22:101739. doi: 10.1016/j.nicl.2019.101739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geliebter A., Benson L., Pantazatos S.P., Hirsch J., Carnell S. Greater anterior cingulate activation and connectivity in response to visual and auditory high-calorie food cues in binge eating: Preliminary findings. Appetite. 2016;96:195–202. doi: 10.1016/j.appet.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Sotiropoulos S.N., Wilson J.A., Coalson T.S., Fischl B., Andersson J.L. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K.J., Alfaro-Almagro F., Auer T., Bellec P., Capota M., Chakravarty M.M. BIDS apps: Improving ease of use, accessibility, and reproducibility of neuroimaging data analysis methods. PLoS Comput. Biol. 2017;13(3) doi: 10.1371/journal.pcbi.1005209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Liu F., Xue Z., Gao K., Liu Z., Xiao C. Abnormal resting-state cerebellar-cerebral functional connectivity in treatment-resistant depression and treatment sensitive depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;44:51–57. doi: 10.1016/j.pnpbp.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Gyurak A., Patenaude B., Korgaonkar M.S., Grieve S.M., Williams L.M., Etkin A. Frontoparietal Activation During Response Inhibition Predicts Remission to Antidepressants in Patients With Major Depression. Biol. Psychiatry. 2016;79(4):274–281. doi: 10.1016/j.biopsych.2015.02.037. [DOI] [PubMed] [Google Scholar]
- Habas C., Kamdar N., Nguyen D., Prater K., Beckmann C.F., Menon V. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 2009;29(26):8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms M.P., Somerville L.H., Ances B.M., Andersson J., Barch D.M., Bastiani M. Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects. Neuroimage. 2018;183:972–984. doi: 10.1016/j.neuroimage.2018.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HCP, Connectome Workbench.
- Hirose S., Jimura K., Kunimatsu A., Abe O., Ohtomo K., Miyashita Y. Changes in cerebro-cerebellar interaction during response inhibition after performance improvement. Neuroimage. 2014;99:142–148. doi: 10.1016/j.neuroimage.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Iadarola N.D., Niciu M.J., Richards E.M., Vande Voort J.L., Ballard E.D., Lundin N.B. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther. Adv. Chronic Dis. 2015;6(3):97–114. doi: 10.1177/2040622315579059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM SPSS. 2019.
- Ionescu D.F., Felicione J.M., Gosai A., Cusin C., Shin P., Shapero B.G. Ketamine-Associated Brain Changes: A Review of the Neuroimaging Literature. Harv. Rev. Psychiatry. 2018;26(6):320–339. doi: 10.1097/HRP.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Control of mental activities by internal models in the cerebellum. Nat. Rev. Neurosci. 2008;9(4):304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- Ji J.L., Spronk M., Kulkarni K., Repovs G., Anticevic A., Cole M.W. Mapping the human brain's cortical-subcortical functional network organization. Neuroimage. 2019;185:35–57. doi: 10.1016/j.neuroimage.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y.C., Schulte T., Muller-Oehring E.M., Namkoong K., Pfefferbaum A., Sullivan E.V. Compromised frontocerebellar circuitry contributes to nonplanning impulsivity in recovering alcoholics. Psychopharmacology. 2014;231(23):4443–4453. doi: 10.1007/s00213-014-3594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A.S., Leikauf J.E., Holt-Gosselin B., Staveland B.R., Williams L.M. Paying attention to attention in depression. Transl. Psychiatry. 2019;9(1):279. doi: 10.1038/s41398-019-0616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili-Mahani N., Niesters M., van Osch M.J., Oitzl M., Veer I., de Rooij M. Ketamine interactions with biomarkers of stress: a randomized placebo-controlled repeated measures resting-state fMRI and PCASL pilot study in healthy men. Neuroimage. 2015;108:396–409. doi: 10.1016/j.neuroimage.2014.12.050. [DOI] [PubMed] [Google Scholar]
- Kilteni K., Ehrsson H.H. Functional Connectivity between the Cerebellum and Somatosensory Areas Implements the Attenuation of Self-Generated Touch. J. Neurosci. 2020;40(4):894–906. doi: 10.1523/JNEUROSCI.1732-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts C.D., Kelsey J.E., Knight B., Ely T.D., Bowman F.D., Gross R.E. The neural correlates of social anxiety disorder and response to pharmacotherapy. Neuropsychopharmacology. 2006;31(10):2243–2253. doi: 10.1038/sj.npp.1301053. [DOI] [PubMed] [Google Scholar]
- M.S. Korgaonkar A.N. Goldstein-Piekarski A. Fornito L.M. Williams Intrinsic connectomes are a predictive biomarker of remission in major depressive disorder Mol Psychiatry 2019. [DOI] [PMC free article] [PubMed]
- Koziol L.F., Budding D.E., Chidekel D. From movement to thought: executive function, embodied cognition, and the cerebellum. Cerebellum. 2012;11(2):505–525. doi: 10.1007/s12311-011-0321-y. [DOI] [PubMed] [Google Scholar]
- Langenecker S.A., Kennedy S.E., Guidotti L.M., Briceno E.M., Own L.S., Hooven T. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biol. Psychiatry. 2007;62(11):1272–1280. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Meng Y., Yuan M., Zhang Y., Ren Z., Zhang Y. Therapy for Adult Social Anxiety Disorder: A Meta-Analysis of Functional Neuroimaging Studies. J. Clin. Psychiatry. 2016;77(11):e1429–e1438. doi: 10.4088/JCP.15r10226. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang M., Li K., Zou F., Wang Y., Wu X. The Altered Somatic Brain Network in State Anxiety. Front. Psychiatry. 2019;10:465. doi: 10.3389/fpsyt.2019.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Zeng L.L., Li Y., Ma Q., Li B., Shen H. Altered cerebellar functional connectivity with intrinsic connectivity networks in adults with major depressive disorder. PLoS ONE. 2012;7(6):e39516. doi: 10.1371/journal.pone.0039516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro J.R.A., Leaver A., Vasavada M., Sahib A.K., Kubicki A., Joshi S. Modulation of amygdala reactivity following rapidly acting interventions for major depression. Hum. Brain Mapp. 2020;41(7):1699–1710. doi: 10.1002/hbm.24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond, S.H.L., 1995. P.F. Manual for the Depression Anxiety Stress Scales. Sydney: Psychology Foundation. 2nd ed., pp. 7334–1423-0.
- Lupo M., Siciliano L., Leggio M. From cerebellar alterations to mood disorders: A systematic review. Neurosci. Biobehav. Rev. 2019;103:21–28. doi: 10.1016/j.neubiorev.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Marcus D.S., Harms M.P., Snyder A.Z., Jenkinson M., Wilson J.A., Glasser M.F. Human Connectome Project informatics: quality control, database services, and data visualization. Neuroimage. 2013;80:202–219. doi: 10.1016/j.neuroimage.2013.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel M., Nicola S.M., Gil-Miravet I., Guarque-Chabrera J., Sanchez-Hernandez A. A Working Hypothesis for the Role of the Cerebellum in Impulsivity and Compulsivity. Front. Behav. Neurosci. 2019;13:99. doi: 10.3389/fnbeh.2019.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniz M., de Jesus S.N., Pacheco A., Goncalves E., Viseu J., Bras M. The Influence of Planning and Response Inhibition on Cognitive Functioning of Non-Psychotic Unipolar Depressed Suicide Attempters. Eur. J. Psychol. 2017;13(4):717–732. doi: 10.5964/ejop.v13i4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Rius J., Miquel M. The cerebellum in drug craving. Drug Alcohol. Depend. 2017;173:151–158. doi: 10.1016/j.drugalcdep.2016.12.028. [DOI] [PubMed] [Google Scholar]
- Mottolese C., Richard N., Harquel S., Szathmari A., Sirigu A., Desmurget M. Mapping motor representations in the human cerebellum. Brain. 2013;136(Pt 1):330–342. doi: 10.1093/brain/aws186. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D., Shaw A.D., Jackson L.E., Hall J., Moran R., Saxena N. Evidence that Subanesthetic Doses of Ketamine Cause Sustained Disruptions of NMDA and AMPA-Mediated Frontoparietal Connectivity in Humans. J. Neurosci. 2015;35(33):11694–11706. doi: 10.1523/JNEUROSCI.0903-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noroozian M. The role of the cerebellum in cognition: beyond coordination in the central nervous system. Neurol. Clin. 2014;32(4):1081–1104. doi: 10.1016/j.ncl.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Nugent A.C., Ballard E.D., Gilbert J.R., Tewarie P.K., Brookes M.J., Zarate C.A., Jr. The Effect of Ketamine on Electrophysiological Connectivity in Major Depressive Disorder. Front. Psychiatry. 2020;11:519. doi: 10.3389/fpsyt.2020.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C., Rosenblat J.D., Lee Y., Pan Z., Cao B., Iacobucci M. The neural systems of emotion regulation and abnormalities in major depressive disorder. Behav. Brain Res. 2019;367:181–188. doi: 10.1016/j.bbr.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Reed J.L., Nugent A.C., Furey M.L., Szczepanik J.E., Evans J.W., Zarate C.A., Jr. Ketamine normalizes brain activity during emotionally valenced attentional processing in depression. Neuroimage Clin. 2018;20:92–101. doi: 10.1016/j.nicl.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock P.L., Roiser J.P., Riedel W.J., Blackwell A.D. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol. Med. 2014;44(10):2029–2040. doi: 10.1017/S0033291713002535. [DOI] [PubMed] [Google Scholar]
- Rush A.J., Trivedi M.H., Ibrahim H.M., Carmody T.J., Arnow B., Klein D.N. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol. Psychiatry. 2003;54(5):573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Sahib A.K., Loureiro J.R., Vasavada M.M., Kubicki A., Wade B., Joshi S.H. Modulation of inhibitory control networks relate to clinical response following ketamine therapy in major depression. Transl. Psychiatry. 2020;10(1):260. doi: 10.1038/s41398-020-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger M., Henning A., Walter M., Boeker H., Weigand A., Seifritz E. Effects of ketamine on cognition-emotion interaction in the brain. Neuroimage. 2016;124(Pt A):8–15. doi: 10.1016/j.neuroimage.2015.08.070. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. The cerebellum and cognition. Neurosci. Lett. 2019;688:62–75. doi: 10.1016/j.neulet.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Schrijvers D., De Bruijn E.R., Maas Y.J., Vancoillie P., Hulstijn W., Sabbe B.G. Action monitoring and depressive symptom reduction in major depressive disorder. Int. J. Psychophysiol. 2009;71(3):218–224. doi: 10.1016/j.ijpsycho.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Snyder H.R. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol. Bull. 2013;139(1):81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Valera E.M., Schmahmann J.D. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59(2):1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney J.A., Strojwas M.H., Mann J.J., Thase M.E. Prefrontal and cerebellar abnormalities in major depression: evidence from oculomotor studies. Biol. Psychiatry. 1998;43(8):584–594. doi: 10.1016/s0006-3223(97)00485-x. [DOI] [PubMed] [Google Scholar]
- Tozzi L., Goldstein-Piekarski A.N., Korgaonkar M.S., Williams L.M. Connectivity of the Cognitive Control Network During Response Inhibition as a Predictive and Response Biomarker in Major Depression: Evidence From a Randomized Clinical Trial. Biol. Psychiatry. 2020;87(5):462–472. doi: 10.1016/j.biopsych.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva R. The cerebellum and neuropsychiatric disorders. Psychiatry Res. 2012;198(3):527–532. doi: 10.1016/j.psychres.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Wagner G., Sinsel E., Sobanski T., Kohler S., Marinou V., Mentzel H.J. Cortical inefficiency in patients with unipolar depression: an event-related FMRI study with the Stroop task. Biol. Psychiatry. 2006;59(10):958–965. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- WHO, World Health Organization, 2019. https://www.who.int/news-room/fact-sheets/detail/depression.
- Winter W., Sheridan M. Previous reward decreases errors of commission on later 'No-Go' trials in children 4 to 12 years of age: evidence for a context monitoring account. Dev. Sci. 2014;17(5):797–807. doi: 10.1111/desc.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelfer M., Li M., Colic L., Liebe T., Di X., Biswal B. Ketamine-induced changes in plasma brain-derived neurotrophic factor (BDNF) levels are associated with the resting-state functional connectivity of the prefrontal cortex. World J. Biol. Psychiatry. 2019;1–15 doi: 10.1080/15622975.2019.1679391. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Ripley B.D., Brady M., Smith S.M. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Wynn S.C., Driessen J.M.A., Glennon J.C., Brazil I.A., Schutter D. Cerebellar Transcranial Direct Current Stimulation Improves Reactive Response Inhibition in Healthy Volunteers. Cerebellum. 2019;18(6):983–988. doi: 10.1007/s12311-019-01047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G., Chen X., Li L., Castellanos F.X., Bai T.J., Bo Q.J. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc. Natl. Acad. Sci. U. S. A. 2019;116(18):9078–9083. doi: 10.1073/pnas.1900390116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P., Gould T.D. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatr. 2018;23(4):801–811. doi: 10.1038/mp.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]